- 1Hangzhou Center for Disease Control and Prevention (Hangzhou Health Supervision Institution), Hangzhou, China
- 2The Affiliated Hospital of Hangzhou Normal University, Hangzhou, China
Objectives: This study aimed to investigate the epidemiological characteristics and risk factors associated with human psittacosis in Hangzhou city, eastern China.
Methods: The human psittacosis data from 2021 to 2024 were obtained from the China information system for diseases control and prevention infectious disease surveillance system. Epidemiological investigations were carried out on the patients' past medical history, clinical manifestations, chest CT results and treatment status. A community-based 1:3 matched case-control study was performed to investigate the risk factors associated with Chlamydia psittaci infection.
Results: During the study period, 137 confirmed cases of human psittacosis were identified through laboratory tests, of which 24 (17.52%) were classified as critical cases, including one fatality. The epidemic curve indicated that the majority of cases occurred between October and March. Among the cases, 48.91% were female, and the median age was 63 years. There were more female cases among those aged <60 years, while there were more male cases among those aged ≥60 years. Multivariate logistic regression analysis revealed that the presence of bird habitats within 500 m of the living area [odds ratio (OR) = 3.81, 95% confidence interval (CI) = 2.19–6.61], parrots kept (OR = 2.95, 95%CI = 1.10–7.89) and poultry kept (OR = 2.15, 95%CI = 1.02–4.53) remained significantly associated with the risk of disease infection.
Conclusions: Human psittacosis has become a notable public health concern in Hangzhou city, with an increase in psittacosis cases reported in recent years. Exposure to poultry, birds, or environments contaminated with Chlamydia psittaci was associated with infection. Urgent actions to reduce psittacosis cases and mitigate the impact of outbreaks are needed, including strengthening surveillance, raising public awareness, and promoting collaboration between the agricultural and health sectors.
1 Introduction
Chlamydia psittacosis (C. psittaci) is a zoonotic pathogen that affects humans, birds and various animal populations (1). C. psittaci is currently classified into 15 different outer membrane protein A (ompA) genotypes: A to F, E/B, WC, M56, 1V, 6N, Mat116, R54, YP54, and CPX0308 (2). Humans are susceptible to infection by any genotype of C. psittaci, but certain genotypes, such as genotype A, appear to be more frequently linked to severe illness in infected patients compared to others (3). A variety of clinical manifestations have been documented in humans with psittacosis, ranging from the more common subclinical or brief, self-resolving flu-like symptoms to the rarer but severe cases of fulminant psittacosis, characterized by multi-organ failure. When treated appropriately, the infection is rarely fatal (4, 5). Although direct human-to-human transmission is uncommon, the disease is primarily contracted by inhaling bacteria from bird droppings or secretions through close contact. Individuals who interact with birds as part of their leisure or professional activities, including pet bird enthusiasts and breeders, employees in pet shops, zoo workers, poultry industry workers, veterinarians, and wildlife keepers, were at the highest risk (6).
Although psittacosis is a rare disease, fewer than 10 cases were reported annually in the United States from 2006 to 2012 (7, 8). Sporadic outbreaks of psittacosis have also been documented in certain locations (9). Due to restricted testing and deficiencies in historical diagnostic methods, the reported figures may not accurately reflect the true incidence of human cases. In China, most human psittacosis cases were sporadic. However, a small number of clusters have been reported in recent years (10). Nevertheless, experts believe that there is significant underreporting and potential misdiagnosis of human psittacosis, which may be attributed to insufficient awareness of the disease and restricted testing capabilities.
As metagenomic next-generation sequencing (mNGS) technology has been increasingly utilized in recent years, an increasing number of human psittacosis cases have been identified and reported. Unlike conventional diagnostic methods, mNGS is less affected by antibiotic use and is able to detect rare, novel, and unforeseen pathogens without preconceived biases (11).
Hangzhou, a central city in the Yangtze River Delta region, is situated in eastern China, surrounded by mountains and lush greenery. Although human psittacosis is not considered a notifiable infectious disease in China, cases have been reported in Hangzhou since 2021. The case data were collected and epidemiological investigations were conducted. In this study, we undertook a comprehensive analysis to understand the epidemiological features and risk factors associated with human psittacosis in Hangzhou from 2021 to 2024.
2 Materials and methods
2.1 Data collection
Data were collected on laboratory-verified cases of human psittacosis from the China information system for diseases control and prevention infectious disease surveillance system from January 1, 2021, to June 30, 2024.
2.2 Study design, case, and control definition
The study was a community-based 1:3 matched case-control investigation involving 80 cases and 239 controls. The criteria for the diagnosis of human psittacosis case were as follows: (1) Individuals who meet the diagnostic criteria for community-acquired pneumonia (CAP) according to the Chinese Guidelines for the Diagnosis and Treatment of Community-acquired Pneumonia in Adults (12); (2) The presence of specific gene fragments of C. psittaci in samples detected by real-time reverse transcription polymerase chain reaction (RT-PCR) or metagenomic next-generation sequencing (mNGS). The controls were neighbors who had lived in the same community or village as the cases for over 6 months and were no more than 5 years older than the cases. The controls had no respiratory symptoms, such as fever, cough, or chest tightness, in the previous 3 months.
2.3 Methodology and content of the survey
The “Questionnaire for human psittacosis case” was independently developed and administered by trained professionals acting as investigators. Face-to-face interviews were conducted with the cases and their families, and their responses were accurately recorded. The control survey was conducted simultaneously by the same researchers. The investigation encompassed general information, details regarding medical consultations and treatments, clinical manifestations, laboratory test results, medical history, family circumstances, and exposure to live birds or poultry.
2.4 Sample collection and testing
The samples of alveolar lavage fluid, sputum, and peripheral blood from the patient were collected and transported in a refrigerated container (4–8°C) to the laboratory for testing. Real-time reverse transcription polymerase chain reaction (RT-PCR) and metagenomic next-generation sequencing (mNGS) were employed to detect specific gene fragments of C. psittaci in the samples.
2.5 Statistical analysis
Data entry was conducted using EpiData 3.1, and database consistency checks were performed. Data cleaning and organization were carried out using WPS Office software (Kingsoft Corporation Limited). The Chi-square test was employed as a univariable regression analysis. Multivariable logistic regression was performed using SPSS version 25.0 (Statistical Product and Service Solutions, Chicago, IL). The interquartile range (IQR), odds ratios (OR), and 95% confidence intervals (CI) were calculated.
3 Results
3.1 Demographic characteristics of the study population
A total of 137 laboratory-confirmed cases of human psittacosis were reported during the study period, of which 24 (17.52%) were classified as critical cases. 136 patients improved clinically and were discharged home; however, one patient died. Among the cases, 135 (98.54%) were diagnosed using mNGS, while 2 cases (1.46%) were diagnosed by RT-PCR. Female-confirmed psittacosis cases numbered 67 (48.91%), resulting in a male-to-female ratio of 1.04:1. Notification rates for those aged < 60 years were higher among females, while rates for those aged ≥60 years were higher in males. The median age was 63 years [interquartile range (IQR) = 53–70 years). Eighty eight cases (64.23%) were mainly in the age group ≥60 years and the notification rate was 4.06 per 100,000, which was higher than those aged < 60 years (P < 0.05). Most confirmed cases were homemakers, accounting for 46.72% (64 of 137), followed by farmers at 24.82% (34 of 137). Some cases also had a history of chronic medical conditions, including cardiovascular disease (15.33%, 21/137), diabetes (10.22%, 14/137) and others (2.92%, 4/137), as shown in Table 1 and Figure 1.
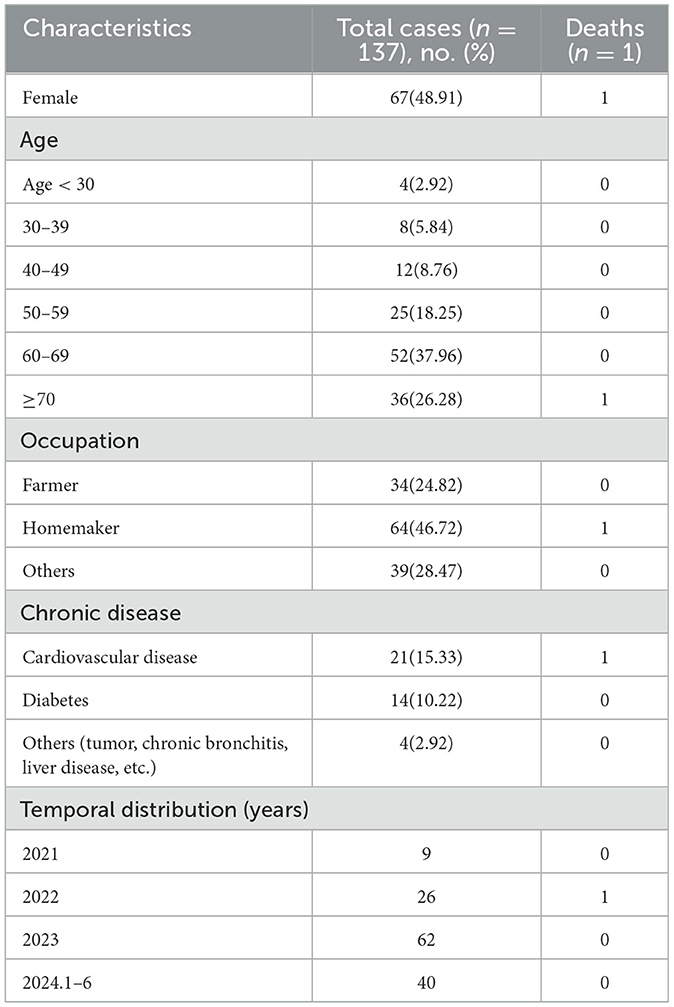
Table 1. Summary of epidemiological characteristics of psittacosis in Hangzhou City from 2021 to 2024.
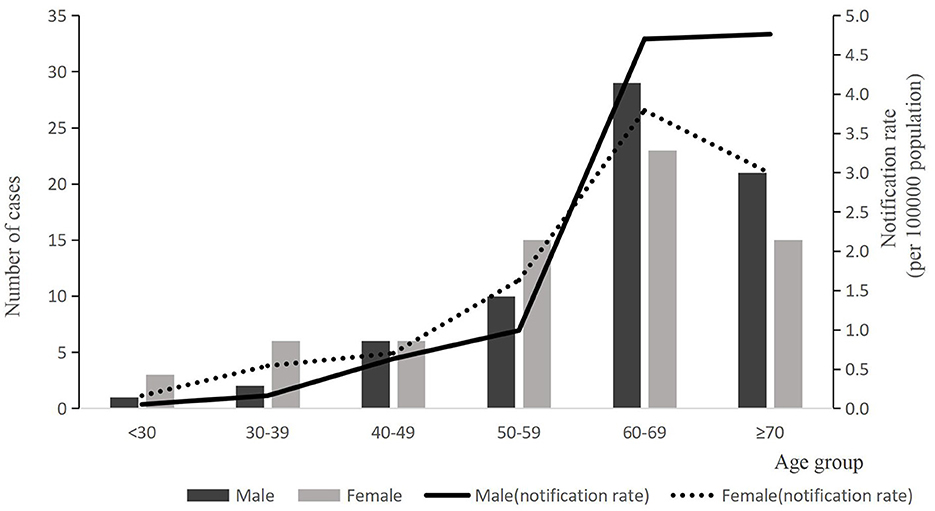
Figure 1. The number of reported case and notification rate of psittacosis by age group of gender, 2021–2024(n = 137). Bars express number of cases by age group. Black bar indicates male cases and gray shows female cases. Notification rate per 100,000 population is indicated by lines (solid line indicates male and dotted line indicates female).
3.2 Temporal trends
The number of confirmed human psittacosis cases from January 1, 2021, to June 30, 2024, was as follows: 9, 26, 62, and 40 cases per year. Confirmed cases were reported monthly. A rapid increase in the number of cases was observed in January, followed by a slowdown in May, as shown in Figure 2. One outbreak was identified in June 2022, involving two female cases in one family that kept two parrots at home.
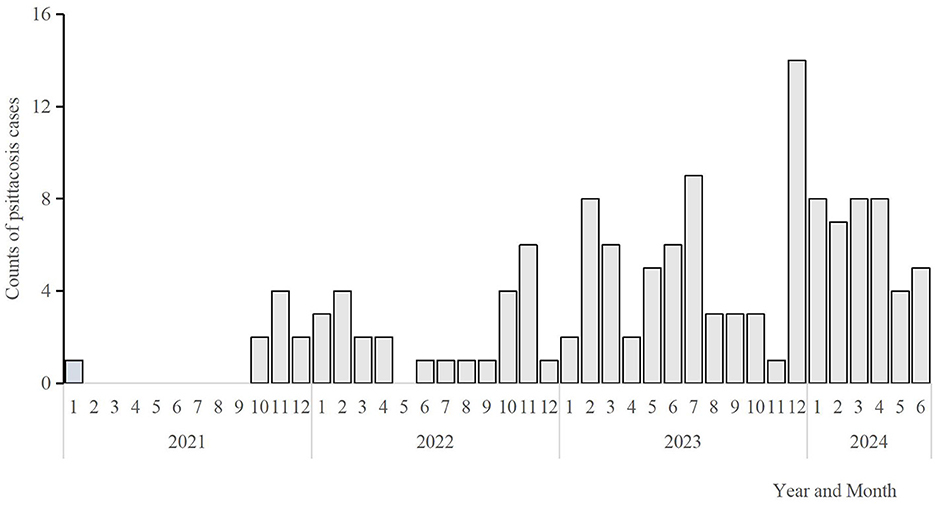
Figure 2. Monthly distribution of Pisstoci cases in Hangzhou City, 2021–2024(n = 137). The bars express the number of cases on a monthly basis from January 2021 to June 2024.
3.3 Symptoms and treatment
The most common clinical symptoms included fever (100%, 137/137), cough (56.20%, 77/137), expectoration (40.15%, 55/137), chest tightness (27.74%, 38/137), fatigue (59.12%, 81/137), limb fatigue (27.74%, 38/137), and headache (23.36%, 32/137). Twenty-four critical patients required endotracheal intubation and mechanical ventilation. Most cases exhibited flake or strip-shaped high-density shadows, and some patients had pleural effusion. We studied 83 cases with results from computed tomography (CT) of the lungs. Among these, 23 cases (27.71%, 23/83) presented with bilateral lesions. The lesions were unilateral in 58 cases (69.88%, 58/83), including 41 cases (49.40%, 41/83) on the left side and 17 cases (20.48%, 17/83) on the right side. Pleural effusion was observed in 13 cases. All patients recovered after receiving antibiotics, with the exception of one fatality.
3.4 Death case
A total of one death was reported, which occurred in November 2022. The deceased was an 81-year-old housewife with a 20-year history of hypertension. On October 11, she purchased a parrot and began caring for it at home, feeding and playing with it daily. On October 22, the patient developed symptoms such as a cough and sore throat but did not seek medical treatment at that time. She was admitted to the hospital for treatment on October 29 after her symptoms worsened on October 27. A chest CT scan revealed an infection in the right lung and a small amount of fluid in both pleural cavities. Sputum samples indicated a C. psittaci infection through mNGS testing. On November 5, she died due to psittacosis, which was accompanied by organ failure.
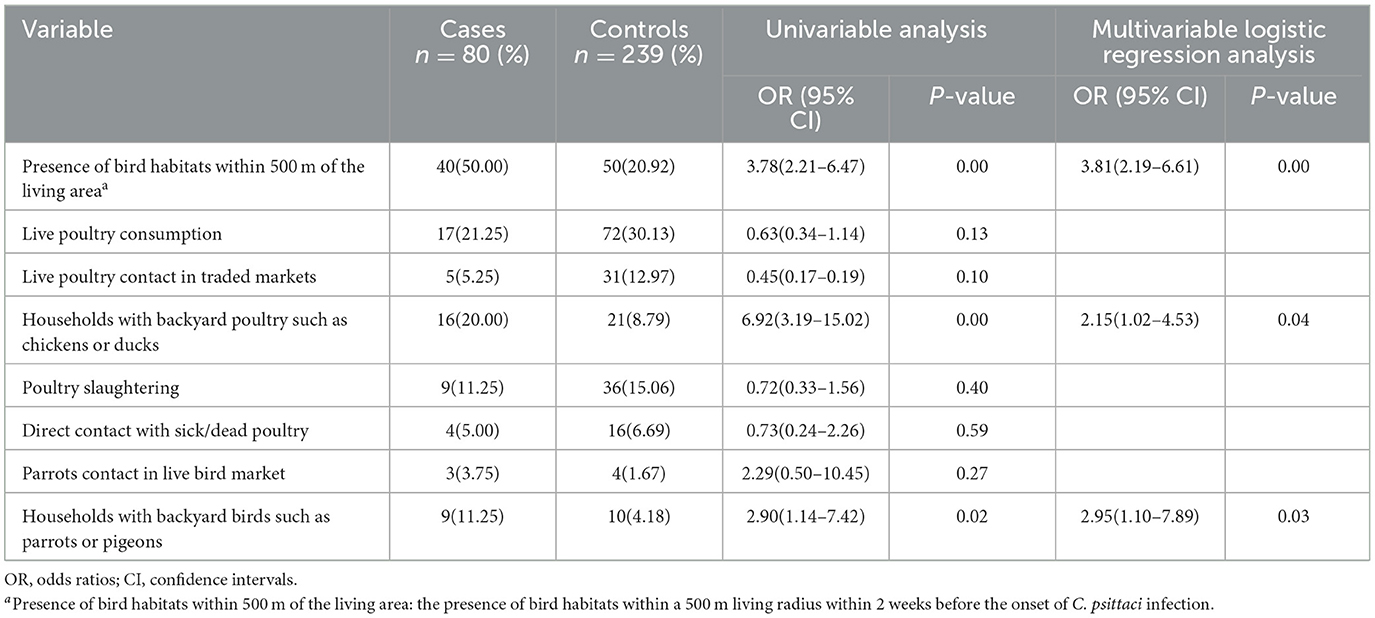
3.5 Risk factors associated with C. psittaci infection
The study included a total of 319 participants, comprising 80 cases and 239 matched controls. The ratio of cases to controls was ~1:3. All cases exhibited positive results on the mNGS test. No significant demographic differences (such as sex, age, and occupation) were observed between the cases and controls, as shown in Table 2.

Table 2. Demographic characteristics of psittacosis cases and controls by sex, age, and occupation in this study.
As shown in the univariable analysis, potential risk factors for infection included the presence of bird habitats within 500 m of the living area, households with backyard birds such as parrots or pigeons, and households with backyard poultry such as chickens or ducks. Other potential risk factors, including poultry consumption, contact with poultry in trade markets, contact with poultry during slaughter, raising pigeons, and interaction with parrots in live bird markets, were not significantly more prevalent among cases than among controls.
Multivariable logistic regression analysis indicated that the presence of bird habitats within 500 m of the living area(P < 0.05, OR = 3.81, 95%CI = 2.19–6.61), households with backyard birds such as parrots or pigeons (P < 0.05, OR = 2.95, 95%CI = 1.10–7.89) and households with backyard poultry such as chickens or ducks (P < 0.05, OR = 2.15, 95% CI = 1.02–4.53) remained significantly associated with risk of disease, as shown in Table 3.
4 Discussion
The range of clinical symptoms associated with human psittacosis is extensive and varies significantly, encompassing asymptomatic cases or mild influenza-like illnesses to severe instances of atypical pneumonia, which can occasionally result in fatalities (13). The clinical manifestations of psittacosis resemble those associated with other pathogens (e.g., SARS-CoV-2, seasonal influenza, etc.) (14). The primary symptoms include fever, cough, dyspnea, and chest tightness, with most cases exhibiting unilateral or bilateral pneumonia, similar to findings in other studies (15). In our research, we also observed that the initial symptoms primarily included fever, malaise, and other non-respiratory symptoms, indicating that respiratory symptoms were not the predominant initial presentations. One fatal case had a history of hypertension and diabetes. The cause of death was multi-organ failure attributed to advanced age, highlighting the severe complications associated with psittacosis.
In recent years, the number of reported cases of human psittacosis has increased significantly worldwide (16). This study indicates that the incidence of human psittacosis cases in Hangzhou has risen annually since 2021, with the majority being laboratory-confirmed cases. Furthermore, the median age of individuals affected by psittacosis was 63 years. A substantial proportion of cases involved homemakers, accounting for 46.72% of the total. Additionally, one death was recorded in 2022. A family outbreak was also reported, with two family members infected after exposure to parrots. Austria, Denmark, Germany, Sweden, and the Netherlands have observed a rise in psittacosis cases since November-December 2023, continuing into early 2024 (17). Sweden has suggested that the overall increase in human psittacosis cases may be attributed to the increased use of more sensitive polymerase chain reaction (PCR) panels (18).
There was no significant difference in sex distribution, which differed from the Japanese and Australian studies (19). Although the total number of cases is roughly the same for men and women overall, the distribution was age dependent. Specifically, there were more female cases among individuals aged < 60 years, while male cases predominated among those aged ≥60 years. Similar trends were observed in Japan's national surveillance data, which reported 115 cases of psittacosis from 2007 to 2016, indicating a higher number of female cases among individuals aged < 50 years (20). In contrast, studies conducted in England and Italy revealed an overall male predominance, with female cases being older (21). While these differences between countries may reflect varying exposures to birds, it is important to note that these studies were conducted in the 1980s using complement fixation (CF) tests, and comparisons should be made with caution.
Our current community-based case-control study identified three risk factors for human psittacosis infection: the presence of bird habitats within 500 m of the living area, and the raising of poultry, including chickens, ducks, and parrots. The study also indicates that exposure to parrots and backyard poultry, such as chickens and ducks, were the primary source of psittacosis infection. Previous research has shown that human psittacosis cases are associated with lovebirds and pet birds, such as parrots and cockatoos, which harbor C. psittaci. The disease can be contracted by buyers, sales center personnel, and workers at hatcheries (22). Poultry-associated cases have been reported on a poultry farm in France and in rural areas of China, linked to infected chickens, ducks, and geese (23). Direct contact with poultry, particularly during slaughter and processing, increases the risk of infection. Several outbreaks of severe community-acquired pneumonia of C. psittaci have also been reported in various countries (24). In this study, the occupations of homemaker and farmer accounted for the majority of cases. Individuals in both occupational categories were more likely to be exposed to and interact with parrots and poultry at home. Some studies have found that social isolation and restrictions on recreational activities due to COVID-19 since 2020 may have led to an increased demand for pets, especially parrots (25). People spent a lot of time at home with their pets, which can elevate the risk of psittacosis. In addition, some studies suggest that human-to-human transmission of C. psittaci is an emerging public health concern, particularly among healthcare workers and their close contacts (26). Furthermore, 14 patients in this study denied any history of close contact with live birds or poultry, suggesting that they may have been infected by inhaling C. psittaci organisms present in the environment. This environment may have been contaminated by the feces of live birds or poultry infected with C. psittaci. But human-to-human transmission cannot be completely ruled out.
It is essential to strengthen surveillance systems to facilitate early detection and rapid response. Although psittacosis is not classified as a notifiable infectious disease in China, some countries require reporting to authorities within 48 h (27). Since 2021, human psittacosis has been designated as a notifiable disease in Hangzhou City, with cases reported to the China Information System for Disease Control and Prevention Infectious Disease Surveillance System. Epidemiological investigations have been conducted to identify potential exposures and outbreaks. Additionally, external environmental surveillance systems have been established, which include laboratory examinations of poultry specimens submitted for avian influenza testing. This analysis aims to monitor the prevalence of C. psittaci in poultry on a monthly basis and to provide early warnings of human psittacosis.
Several measures should be proposed to prevent and control psittacosis and to mitigate the effects of the increasing number of patients affected by this disease. Firstly, we need to encourage clinicians to test suspected psittacosis cases for diagnosis using RT-PCR, mNGS or specific antibodies. However, the cost of mNGS is relatively high in clinic, so it cannot completely replace current conventional identification methods, especially in outbreaks. Secondly, we should conduct continuous surveillance of C. psittaci in the environments of poultry and wild birds. Thirdly, we should advise bird owners to keep cages clean, ensure that cages are positioned to prevent the transfer of feces between them, and avoid overcrowding (28). Fourthly, owners of caged or domesticated birds and poultry should maintain good personal hygiene and ensure proper ventilation when handling birds, bird droppings, and the surrounding environment. They should take precautions by wearing masks and always wash their hands after handling. Fifthly, ensure that purchase channels are legitimate and avoid bringing home birds from unknown sources. Avoid direct contact with sick birds or poultry.
However, our study has several limitations. First, we only included confirmed cases of human psittacosis because, due to the high cost of testing, physicians only performed mNGS testing on those cases with relatively severe symptoms. In addition, the controls refused to provide throat swabs or blood samples, making it difficult to exclude the possibility of subclinical infection among them. This introduced a selective bias as some cases with atypical symptoms were undetected. Second, our study was unable to establish a dose-response relationship between the frequency of exposure to birds and poultry. Third, there were many older adult participants in our study who could not accurately recall the exact information, such as the date of onset, clinical manifestations, exposure to birds or poultry, which contributed to recall bias.
5 Conclusions
In conclusion, we have observed an increasing number of reported human psittacosis cases in Hangzhou City in recent years. Our study shows that C. psittaci can be found in poultry such as chickens, ducks and parrots. The transmission of the pathogen from poultry and birds to humans is common and human-to-human transmission is rare. Urgent actions to contain psittacosis cases and mitigate the impact of outbreaks. These actions should include strengthening surveillance, raising public awareness and promoting collaboration between the agricultural and health sectors. In addition, health education on personal hygiene should be provided to the public, especially those who keep poultry and birds.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The investigation was reviewed and approved by the Medical Ethics Committee of the Hangzhou Municipal Center for Disease Control and Prevention (Approval number:2024-09). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
ZS: Writing – original draft, Conceptualization, Data curation, Investigation, Methodology, Software. KX: Investigation, Supervision, Writing – review & editing. LH: Methodology, Writing – review & editing. XZ: Investigation, Writing – review & editing. YW: Investigation, Writing – review & editing. YG: Formal analysis, Investigation, Software, Validation, Writing – review & editing. BC: Project administration, Resources, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by Zhejiang Medical and Health Technology General Project (grant numbers 2020KY236 and 2020KY777) and Medical Key Discipline of Hangzhou (Infectious Diseases).
Acknowledgments
We thank the staff of Xihu District Center for Disease Control and Prevention of Hangzhou for their assistance with the epidemiological survey; we also thank all the participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hogerwerf L, DE Gier B, Baan B, VAN DER Hoek W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. (2017) 145:3096–105. doi: 10.1017/S0950268817002060
2. Rybarczyk J, Versteele C, Lernout T, Vanrompay D. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg. (2020) 75:42–8. doi: 10.1080/17843286.2019.1590889
3. Heddema ER, van Hannen EJ, Bongaerts M, Dijkstra F, Ten Hove RJ, de Wever B, et al. Typing of Chlamydia psittaci to monitor epidemiology of psittacosis and aid disease control in the Netherlands, 2008 to 2013. Euro Surveill. (2015) 20:21026. doi: 10.2807/1560-7917.ES2015.20.5.21026
4. Stewardson AJ, Grayson ML. Psittacosis. Infect Dis Clin North Am. (2010) 24:7–25. doi: 10.1016/j.idc.2009.10.003
5. Harkinezhad T, Geens T, Vanrompay D. Chlamydophila psittaci infections in birds: a review with emphasis on zoonotic consequences. Vet Microbiol. (2009) 135:68–77. doi: 10.1016/j.vetmic.2008.09.046
6. Balsamo G, Maxted AM, Midla JW, Murphy JM, Wohrle R, Edling TM, et al. Compendium of measures to control Chlamydia psittaci infection among humans (Psittacosis) and pet birds (Avian Chlamydiosis), 2017. J Avian Med Surg. (2017) 31:262–82. doi: 10.1647/217-265
7. Shi Y, Chen J, Shi X, Hu J, Li H, Li X, et al. A case of chlamydia psittaci caused severe pneumonia and meningitis diagnosed by metagenome next-generation sequencing and clinical analysis: a case report and literature review. BMC Infect Dis. (2021) 21:621. doi: 10.1186/s12879-021-06205-5
8. Centers for Disease Control and Prevention (CDC). Surveillance and Reporting (2022). Available from: https://www.cdc.gov/pneumonia/atypical/psittacosis/surveillance-reporting/index.html (accessed June 12, 2024).
9. Nieuwenhuizen AA, Dijkstra F, Notermans DW, van der Hoek W. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect Dis. (2018) 18:442. doi: 10.1186/s12879-018-3317-0
10. Qin XC, Huang J, Yang Z, Sun X, Wang W, Gong E, et al. Severe community-acquired pneumonia caused by Chlamydia psittaci genotype E/B strain circulating among geese in Lishui city, Zhejiang province, China. Emerg Microbes Infect. (2022) 11:2715–23. doi: 10.1080/22221751.2022.2140606
11. Liu K, Wu L, Chen G, Zeng D, Zhong Q, Luo L, et al. Clinical characteristics of Chlamydia psittaci infection diagnosed by metagenomic next-generation sequencing: a retrospective multi-center study in Fujian, China. Infect Drug Resist. (2024) 17:697–708. doi: 10.2147/IDR.S443953
12. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. (2007) 44:S27–72. doi: 10.1086/511159
13. Gaede W, Reckling KF, Dresenkamp B, Kenklies S, Schubert E, Noack U, et al. Chlamydophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses Public Health. (2008) 55:184–8. doi: 10.1111/j.1863-2378.2008.01108.x
14. Chaber AL, Jelocnik M, Woolford L. Undiagnosed cases of human pneumonia following exposure to Chlamydia psittaci from an infected rosella parrot. Pathogens. (2021) 10:968. doi: 10.3390/pathogens10080968
15. Huang Y, Zheng W, Gan W, Zhang T. Chlamydia psittaci pneumonia: a clinical analysis of 12 patients. Ann Transl Med. (2023) 11:144. doi: 10.21037/atm-22-6624
16. Olatunji G, Kokori E, Ajibola F, Akpovona O, Daniel BO, Iyore O, et al. Addressing the new wake of psittacosis outbreak in Europe. New Microbes New Infect. (2024) 60–61:101436. doi: 10.1016/j.nmni.2024.101436
17. World Health Organization. Psittacosis—European region. Retrieved from: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON509 (accessed June 12, 2024).
18. Chereau F, Rehn M, Pini A, Kühlmann-Berenzon S, Ydring E, Ringberg H, et al. Wild and domestic bird faeces likely source of psittacosis transmission-A case-control study in Sweden, 2014-2016. Zoonoses Public Health. (2018) 65:790–7. doi: 10.1111/zph.12492
19. Kozuki E, Arima Y, Matsui T, Sanada Y, Ando S, Sunagawa T, et al. Human psittacosis in Japan: notification trends and differences in infection source and age distribution by gender, 2007 to 2016. Ann Epidemiol. (2020) 44:60–63. doi: 10.1016/j.annepidem.2020.03.001
20. Coutts II, Mackenzie S, White RJ. Clinical and radiographic features of psittacosis infection. Thorax. (1985) 40:530–2. doi: 10.1136/thx.40.7.530
21. Maffei C, Marracino A, Di Stanislao F, Pauri P, Clementi M, Varaldo PE. Psittacosis in a highly endemic area in Italy. Epidemiol Infect. (1987) 99:413–9. doi: 10.1017/S095026880006790X
22. De Boeck C, Dehollogne C, Dumont A, Spierenburg M, Heijne M, Gyssens I, et al. Managing a cluster outbreak of psittacosis in Belgium linked to a pet shop visit in The Netherlands. Epidemiol Infect. (2016) 144:1710–6. doi: 10.1017/S0950268815003106
23. Laroucau K, Aaziz R, Meurice L, Servas V, Chossat I, Royer H, et al. Outbreak of psittacosis in a group of women exposed to Chlamydia psittaci-infected chickens. Euro Surveill. (2015) 20:21155. doi: 10.2807/1560-7917.ES2015.20.24.21155
24. Liu S, Yang Y, Pang Z, Liu Y, Li H, Cai J, et al. A cluster of two psittacosis cases among women farmers exposed to Chlamydia psittaci-infected domestic poultry in Zhejiang Province, China. Zoonoses Public Health. (2023) 70:93–102. doi: 10.1111/zph.13004
25. Li N, Li S, Tan W, Wang H, Xu H, Wang D. Metagenomic next-generation sequencing in the family outbreak of psittacosis: the first reported family outbreak of psittacosis in China under COVID-19. Emerg Microbes Infect. (2021) 10:1418–28. doi: 10.1080/22221751.2021.1948358
26. Zhang Z, Zhou H, Cao H, Ji J, Zhang R, Li W, et al. Human-to-human transmission of Chlamydia psittaci in China, 2020: an epidemiological and aetiological investigation. Lancet Microbe. (2022) 3:e512–20. doi: 10.1016/S2666-5247(22)00064-7
27. Liu S, Cui Z, Carr MJ, Meng L, Shi W, Zhang Z. Chlamydia psittaci should be a notifiable infectious disease everywhere. Lancet Microbe. (2023) 4:e62–3. doi: 10.1016/S2666-5247(22)00306-8
Keywords: epidemiological, human psittacosis, Chlamydia psittaci, transmission, risk factors
Citation: Sun Z, Xu K, Huo L, Zhang X, Wang Y, Gong Y and Chen B (2025) Epidemiological features and risk factors of human psittacosis in Hangzhou City, eastern China. Front. Public Health 13:1512841. doi: 10.3389/fpubh.2025.1512841
Received: 17 October 2024; Accepted: 17 January 2025;
Published: 06 February 2025.
Edited by:
Senthil Kumaran Satyanarayanan, Hong Kong Institute of Innovation and Technology, Hong Kong SAR, ChinaReviewed by:
Jie Chen, Shanghai Jiao Tong University, ChinaBrian Crook, Health and Safety Laboratory (HSL), United Kingdom
Copyright © 2025 Sun, Xu, Huo, Zhang, Wang, Gong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingbing Chen, aHpjYmJAZm94bWFpbC5jb20=
 Zhou Sun
Zhou Sun Ke Xu
Ke Xu Liangliang Huo1
Liangliang Huo1 Yi Wang
Yi Wang Bingbing Chen
Bingbing Chen