
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 17 February 2025
Sec. Environmental Health and Exposome
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1512603
 Qi Jiang1†‡
Qi Jiang1†‡ Liu Junjun2†
Liu Junjun2† Xiaochuan Wang3‡
Xiaochuan Wang3‡ Li Luo3‡
Li Luo3‡ Gaoyan He3‡
Gaoyan He3‡ Xiaojuan Wu3‡
Xiaojuan Wu3‡ Qian Min3‡
Qian Min3‡ Ying Long3‡
Ying Long3‡ Wang Wenjun2*
Wang Wenjun2* Tao Zhu3*‡
Tao Zhu3*‡ Yu Yao3*‡
Yu Yao3*‡Background: It is well-known that sex and age play critical roles in smoking-related diseases and mortality. However, quantification of the extent of smoking requires self-reports in these studies, which may yield only partially accurate results. This study investigated sex-and age-related differences in the association between smoking and all-cause, cardiovascular disease, and cancer mortality by measuring serum cotinine levels.
Methods: Participants aged 20–85 years from the US National Health and Nutrition Examination Survey (1999–2018) were included. All-cause and disease-specific mortality data were obtained from publicly available user-linked mortality files. Multivariate Cox regression was performed to identify serum cotinine as an independent risk factor of mortality. Subgroup and interaction analyses were performed to investigate these sex and age differences. Smooth curve fitting was conducted to discover potential nonlinear relationships and threshold saturation effects.
Results: Sex was significantly associated with all-cause and cancer mortality. Threshold saturation effects were observed in all-cause mortality among both males and females, cancer mortality among females, and cardiovascular disease mortality among males. Age markedly associated with all-cause and cardiovascular disease mortality. Threshold saturation effects were found in cardiovascular disease mortality among younger adults and cancer mortality among the all-age population.
Conclusion: These findings suggest that there are threshold saturation effects between smoking and mortality, and sex and age differences in smoking-related mortality are inconsistent in different diseases.
Despite annualized rates of decline in smoking prevalence, smoking remains the second leading risk factor for early death and disability worldwide, contributing to 11.5% of global deaths (6.4 million), of which more than half occur in four countries (1).
Evidence from several extensive contemporary studies illustrates sex and age differences in the health outcomes of tobacco exposure, suggesting that the comprehensive risk of smoking is considerably more significant in women than in men, and in young adults than in older adults (2–8). For example, in 2014, a cohort study with an extended follow-up indicated that the full hazards of light smoking (1–14 cigarettes per day) at baseline were more significant for women than for men, while the effect decreased with increasing extent of smoking, suggesting a dose–response relationship (9). The risks of mortality and health outcomes vary according to the age at smoking initiation. A prospective cohort study including a contemporary US population showed that delayed smoking initiation reduced the mortality risk of cancer (7).
A limitation in these studies is that the quantification of the extent of smoking was subjective and self-reported, including the pack-year index and daily cigarette consumption. Self-reports are frequently affected by memory and emotion-expression biases (10). Meanwhile, the adverse health impact of actual exposure to smoke toxicants is determined by multiple factors, including the number of smoked cigarettes, smoking years, cigarette brands, cigarette emissions, and even smoking topography (11–14). Therefore, cigarette consumption may not fully reflect the extent of the harmful effects of smoking. Moreover, it is complicated to quantify the extent of passive smoking by the frequency of smoking and the number of cigarettes consumed. The methods of tobacco exposure quantification may have resulted in inaccuracies in these previous studies regarding the sex and age differences in smoking. Hence, an objectively quantifiable indicator is required to evaluate these differences in smoking.
Cotinine is the most predominant metabolite of nicotine, which is similarly a tobacco-specific biomarker (15–17). Serum cotinine levels measured at a single time point are positively associated with the degree of tobacco smoke exposure, considered one of the most reliable indicators of both smoking intensity and exposure to environmental tobacco smoke, which includes first-hand, second-hand, and third-hand smoke exposure (18, 19). Meanwhile, cotinine-assessed smoking status is generally more accurate than self-reported smoking status (20). Smoking is associated with mortality from various diseases, including cardiovascular diseases and cancer (1). A National Health and Nutrition Examination Survey (NHANES) of 20,175 self-reported non-smokers aged ≥20 years revealed that serum cotinine levels were significantly associated with death from lung cancer, all cancers, and heart diseases (21).
These findings support the assertion that cotinine is an effective indicator to evaluate smoking-related mortality risk. Another related question arises from these results, namely, whether there are thresholds and differences among different diseases in sex-and age-related differences in smoking. In particular, there is a need for long-term prospective studies to completely confirm these differences by measuring serum cotinine with standard statistical methods. Therefore, this study aimed to investigate sex-and age-related differences in smoking-related mortality by measuring serum cotinine levels, emphasizing subgroup analyses and possible nonlinear relationships and threshold saturation effects.
This prospective cohort study utilized data from 10 cycles (1999 and 2018) of the US NHANES (RRID:SCR_013201). The NHANES is a series of ongoing cross-sectional surveys with a nationally representative sample of non-institutionalized individuals conducted by the National Center for Health Statistics (NCHS) at the US Center for Disease Control. It includes data from health survey interviews, physical examinations, questionnaires, laboratory, and mortality. All-cause and disease-specific mortality data from 1999 to 2018 were obtained from publicly available-user-linked mortality files, linked to the National Death Index. All participants provided written consent, and the NCHS Ethics Review Board approved this study (Protocols #98–12, #2005–06, #2011–17, and #2018–01).
Participants aged 20–85 years in the NHANES (1999–2018) with serum cotinine and follow-up data were included in this study. The exclusion criteria were (1) missing data; (2) positive test results for tuberculosis (TB); (3) human immunodeficiency virus (HIV) antibody; (4) hepatitis C antibody; (5) hepatitis B surface antibody; (6) ever had chronic obstructive pulmonary disease; (7) ever had and still have a liver condition; (8) ever had a stroke, heart attack, angina/angina pectoris, coronary heart disease, congestive heart failure; (9) ever received blood transfusions; (10) ever had attention deficit disorder; (11) received treatment for anemia in/the past three months.
Serum cotinine concentration was measured using an isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometric method.
In the NHANES 2005–2007, 2008–2010/2015–2016, and 2017–2018, routine biochemical profiles and CBC were analyzed using Beckman Synchron LX20 Beckman DxC800 (Beckman Coulter, Fullerton, CA, US), and Roche Cobas 6,000 (c501 module) (Cobas 6,000; Roche Diagnostics USA), respectively were used. Further details of this analysis are in the Supplementary material.
BMI, smoking status, family income to poverty ratio, blood pressure (BP), glycohemoglobin, high-density lipoprotein cholesterol (HDL-C), and total nutrient intake.
Obesity was defined as BMI ≥30. Smoking status was defined as SHS (not actively smoking but exposed to tobacco smoke in the workplace or at home in the past 7 days); non-smoking (smoking <100 cigarettes during the lifetime, not smoking currently, and not exposed to environmental tobacco smoke); and active smoking (smoked >100 cigarettes during the lifetime and currently smoking cigarettes). The family income to poverty ratio was calculated by comparing family (or individual) income to the poverty guidelines specific to the survey year.
BP was measured by Shared Care Research and Education Consulting trained investigators, certified to measure the BP.
HDL-C and glycohemoglobin were measured using Cobas 6,000 Chemistry Analyzer and Tosoh G8 Glycohemoglobin Analyzer, respectively.
Total nutrient intakes were used to estimate the types and amounts of foods and beverages (including all types of water) consumed during the 24-h period prior to the interview (midnight to midnight) conducted at mobile examination centers, and to estimate the intake of energy, nutrients, and other food components from those foods and beverages.
Normally distributed data was presented by mean ± standard deviation (SD). Categorical variables were analyzed by Chi-square test. Continuous variables with normal distribution were analyzed by Student t test. Ordinal variables and continuous variables without normal distribution were analyzed by Mann–Whitney U-test. Variance inflation factor (VIF) was performed to analyze data collinearity. Least absolute shrinkage and selection operator (LASSO) was used to select potential variables associated with all-cause, cardiovascular disease, and cancer mortality. Subsequently, based on the variables selected by LASSO regression, three multivariate Cox regression models were established to identify the independent risk factors for mortality. Prior to applying the Cox proportional hazards model, a time-dependent model was employed to verify the proportionality of risk for the aforementioned factors, all of which satisfied the equal proportionality hypothesis. The potential effect modifications of sex (male vs. female) and age (<65 years vs. ≥65 years) were evaluated using subgroup and interaction analyses. Smooth curve fitting was performed to examine whether serum cotinine levels were partitioned into intervals, and segmented regression. Log-likelihood ratio tests were performed to determine whether a threshold existed in the association between serum cotinine levels and mortality. These analyses were conducted using both logarithmic transformed and untransformed data. The log (relative risk) was converted to a relative risk by taking the antilog. Nomograms and receiver operating characteristic (ROC) curves were used to visualize and verify the three multivariate Cox regression models. The R project (http://www.Rproject.Org/; RRID:SCR_001905) and Empower Stats (http://www.Empowerstats.com) were used for all statistical analyses. We utilized the MEC subsample weights, specifically WTMEC4YR for the years 1999–2002 and WTMEC2YR for 2003–2018, along with the masked variance unit Pseudo-PSU variable (SDMVPSU) and the masked variance unit Pseudo-Stratum variable (SDMVSTRA) for variance estimation in this analysis. These sampling weights were recalculated to account for the combination of 10 NHANES cycles. Based on the NCHS edited analytical guidelines. Statistical significance was set at p < 0.05.
In total, 16,393 participants (male 52.94%; Figure 1) were included in the study. The baseline characteristics of the study participants are presented in Table 1 and Supplementary Table S1. In both groups, all baseline characteristics differed significantly. Of these, the males had significantly higher serum cotinine levels and higher all-cause, cardiovascular disease, and cancer mortality than the females.
LASSO was used to reduce the dimensions of the data and select the potential variables of all-cause, cardiovascular disease, and cancer mortality (Supplementary Figure S1). We identified 22, 15, and 8 variables, respectively (Table 2).
Three multivariate Cox regression analysis models, including the variables selected using LASSO, were established (Table 3). After adjusting for Albumin, ALP, BUN, globulin, serum glucose, GGT, LDH, potassium, uric acid, SBP, neutrophils number, MCV, RDW, gender, age, race, the ratio of family income to poverty, glycohemoglobin, and cancer or malignancy history, after adjusting for Albumin, ALP, BUN, globulin, LDH, uric acid, SBP, monocyte number, RDW, gender, age, the ratio of family income to poverty, and glycohemoglobin, after adjusting for Albumin, ALP, neutrophils number, RDW, gender, age, and cancer or malignancy history, serum cotinine levels were found to be positively associated with all-cause, cardiovascular disease, and cancer mortality, respectively.
The subgroup and interaction analyses of the association between serum cotinine levels and mortality stratified by various causes are shown in Table 4 after adjusting the above covariates. The positive role of serum cotinine level in increasing mortality was significant in most subgroups. However, the positive associations of all-cause and cardiovascular disease mortality with serum cotinine levels were not significant in older adults. The positive association between cardiovascular disease mortality and serum cotinine levels remained insignificant in the participants with obesity. In the interaction analyses, the hazard ratio (HR) among females was greater than that for males for all mortality outcomes, suggesting that serum cotinine levels have a higher mortality risk in females. Moreover, we found that sex interacted with serum cotinine levels for all-cause and cancer mortality, and age (<65 years vs. ≥65 years) interacted with serum cotinine levels for all-cause and cardiovascular disease mortality.
Additive Cox proportional models (Figure 2) were used to visually assess the functional relationship between serum cotinine and all mortality outcomes after adjusting the above covariates. Our data showed that the relationships between serum cotinine levels and mortality outcomes were nonlinear and showed threshold saturation effects (Supplementary Figures S2A1–A3). As shown in Table 5, the cutoffs of the associations of serum cotinine levels with all-cause, cardiovascular disease, and cancer mortality were 11.6, 32.1, and 307 ng/mL, respectively. On the left side of the cutoff values, the risks of these mortality outcomes increased with increasing serum cotinine levels. On the right side of the cutoffs, their risks did not significantly increase with the serum cotinine levels.
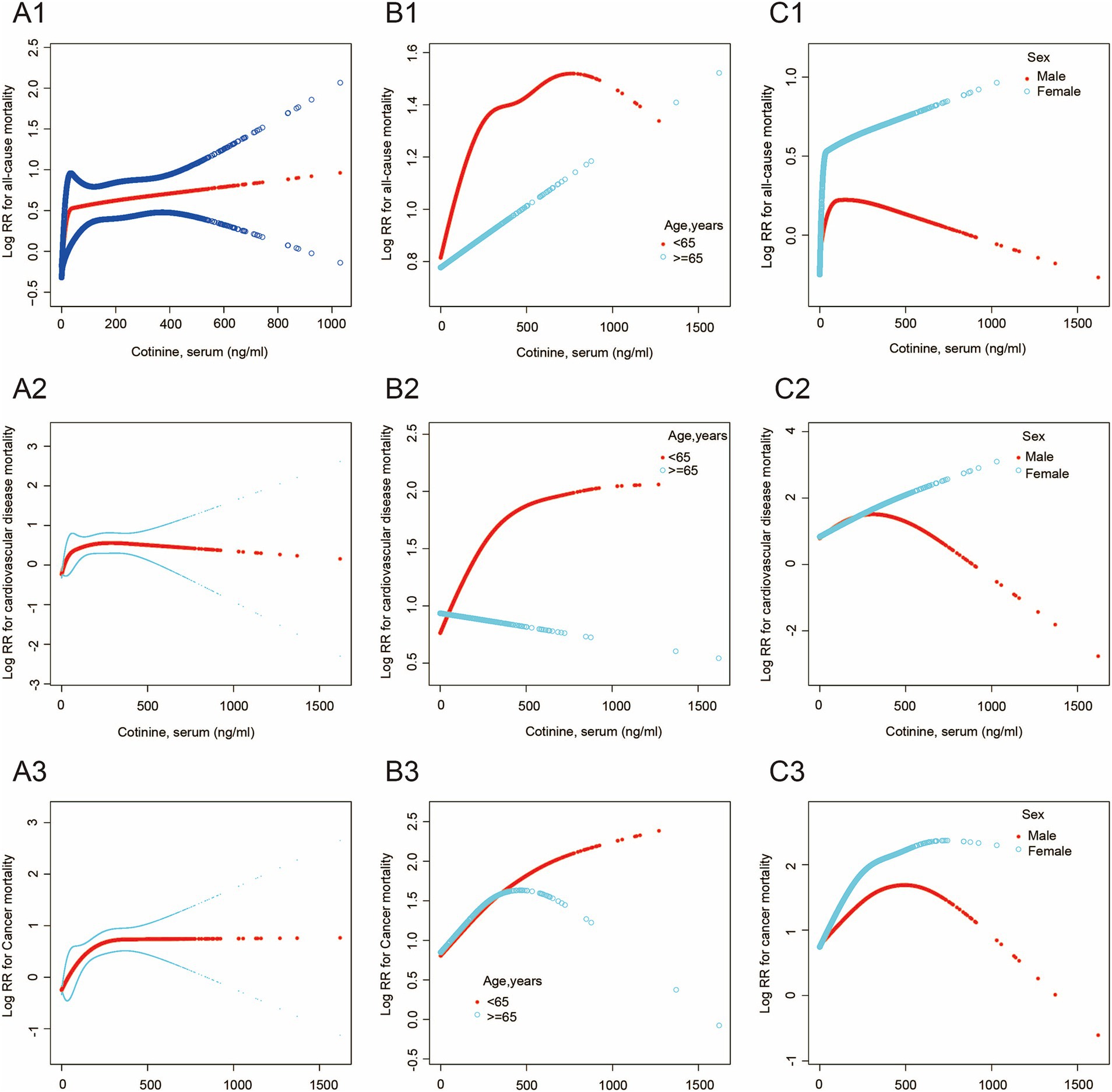
Figure 2. The association between serum cotinine level and mortality. The blue and black lines indicate 95% CI, and the red lines indicate a smooth curve fitting line. (A1–A3) The smooth curve of the relationship between serum cotinine level and all-cause, cardiovascular disease, and cancer mortality is shown. (B1–B3) The smooth curve of the relationship between serum cotinine level and the above mortality in different age groups is shown. (C1–C3) The smooth curve of the relationship between serum cotinine level and the above mortality in different sex groups is shown.
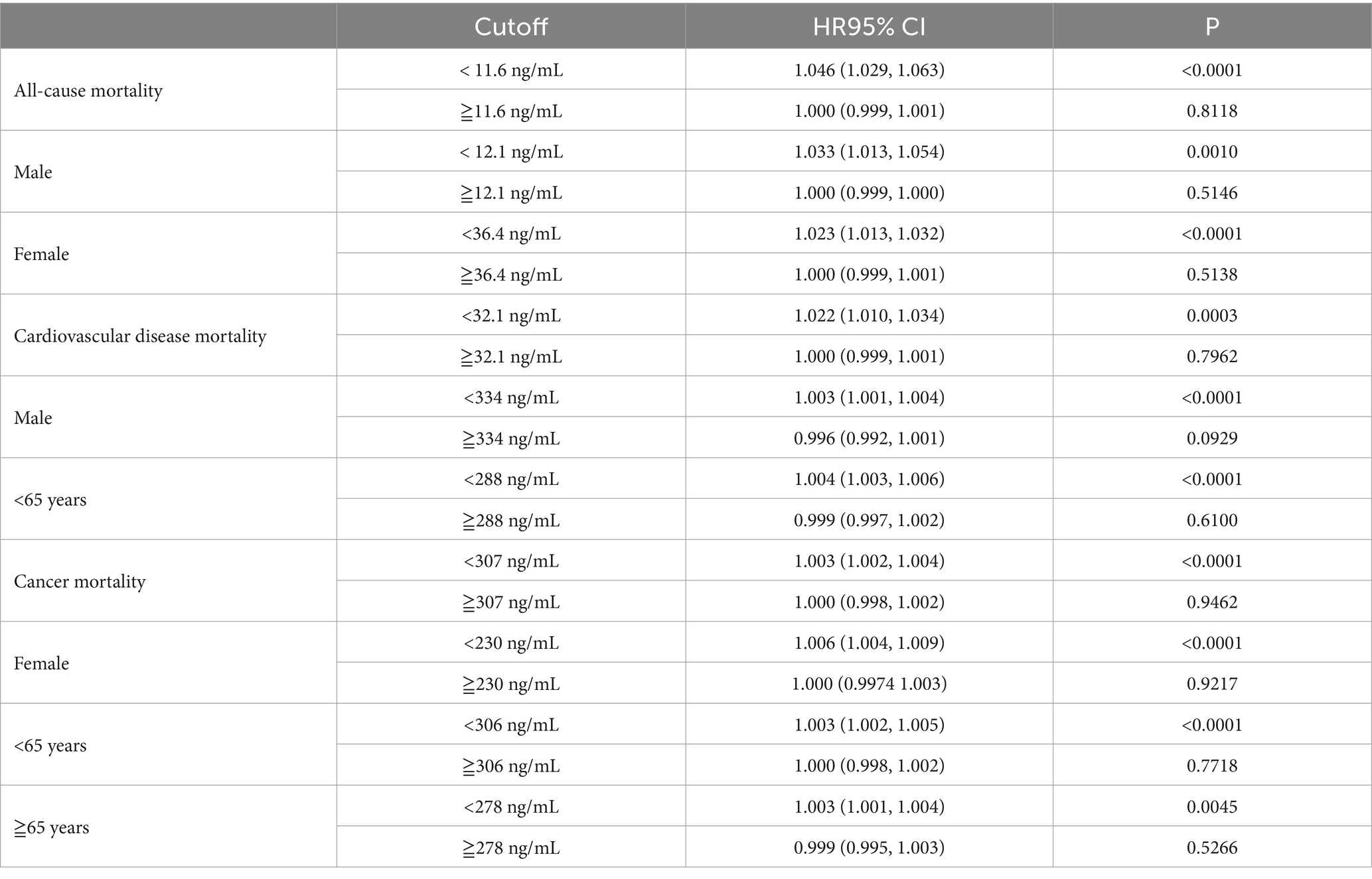
Table 5. Association between serum cotinine and mortality by segmented regression and log-likelihood ratio test.
Meanwhile, threshold saturation effects were observed in the relationships between serum cotinine levels and either all-cause mortality in males (cutoff = 12.1 ng/mL) and females (cutoff = 36.4 ng/mL), cardiovascular disease mortality in males (cutoff = 334 ng/mL), cancer mortality in females (cutoff = 230 ng/mL), cardiovascular disease mortality in younger adults (age < 65 years; cutoff = 288 ng/mL), and cancer mortality in younger (age < 65 years; cutoff = 306 ng/mL), and older adults (age ≥ 65 years cutoff = 278 ng/mL) (Supplementary Figures S2C1–C3, S2B2,B3). Moreover, Serum cotinine levels were associated with a greater risk of all mortality outcomes in females than in males and in the younger adults compared to older adults.
Nomograms were constructed based on the three multivariate Cox regression models. The total points calculated as the sum of the individual points of each of the variables included in the nomogram, and the months of follow-up were 116.5, 115, and 111 months for all-cause, cardiovascular disease, and cancer, respectively (Supplementary Figures S2A1–A3). The AUC of the ROC curve were 0.821 [95% confidence interval (CI) 0.699–0.813], 0.867 (95% CI 0.699–0.813), and 0.833 (95% CI 0.699–0.813) for all-cause, cardiovascular disease, and cancer mortality, respectively (Supplementary Figures S2B1–B3).
This study explored the influence of sex and age on the impact of cigarette exposure on all-cause, cardiovascular disease, and cancer mortality. To the best of our knowledge, no previous study has explored sex-and age-related differences in the association between cigarette exposure and mortality by measuring serum cotinine levels. In summary, our significant findings were that females and younger adults had higher risks of all-cause cardiovascular disease and cancer mortality associated with cigarette exposure. Sex differences were significant only in all-cause and cancer mortality. Threshold saturation effects were observed in all-cause mortality among both males and females, cancer mortality among females, and cardiovascular disease mortality among males. Age differences were significant only in all-cause and cardiovascular disease mortality. Threshold saturation effects were found in cardiovascular disease mortality among younger adults and cancer mortality among all-age population. Furthermore, the nomogram and ROC curves demonstrated the accuracy and reliability of the established models.
Inconsistent with the decreasing trend in overall smoking prevalence worldwide, smoking prevalence in more places changed minimally or increased among women (22). A major finding of this study was that younger adults demonstrated a higher proportion of all-cause, cardiovascular disease, and cancer mortality associated with cigarette exposure than older adults. These age differences are consistent with those reported in previous studies. Hiroyasu et al. demonstrated that the relative risk of smoking-induced cardiovascular disease mortality in Japanese women was greater in persons aged 40–64 years than in older persons (23). Furthermore, many studies have found that early smoking-onset age indicates a high risk of smoking-related mortality. A retrospective study that included 4,499 current or former smokers in a median 7.02 years of follow-up suggested that each year of delay in early smoking-onset age was inversely associated with cardiovascular mortality in the group aged ≤12 years and 4% lower risk of all-cause mortality in all groups (8, 24). However, our study revealed more comprehensive findings that the relationship between serum cotinine and cardiovascular disease in younger adults, and cancer mortality in all-age population showed threshold saturation effects. Additionally, the positive correlation was only significant in cancer mortality among older adults. For cardiovascular diseases mortality, the risk in younger adults was increased with increasing serum cotinine, while when serum cotinine increased to 306 ng/mL, this dose–response trend was no longer significant. There were, however, no dose–response trends in older adults. For cancer mortality, the risk was increased with increasing serum cotinine in all ages and the increased risk was early weakened in older adults after serum cotinine increased beyond the threshold, compared to younger adults. This implies that there is a dose-effect relationship in smoking-related carcinogenesis among all ages, while smoking-related atherosclerosis does not present a dose-effect relationship in older adults. Our results suggested that a delay in early smoking-onset age reduced the risk of smoking-induced cardiovascular disease mortality but did not significantly decrease the risk of smoking-induced cancer mortality.
Females have greater health consequences associated with tobacco exposure. For example, earlier large-scale cohort studies in the Asia-Pacific region reported that women presented a two-fold higher risk of lung cancer mortality associated with current smoking in Australia and New Zealand than men (2). Similarly, increased all-cause mortality in females from myocardial infarction was previously reported (5). Hurley found that for women, the all-cause mortality risk induced by light smoking may be approximately three times that for men, whereas no such relationship was observed for medium/heavy smoking (9), which was consistent with our study. We found that females had a higher risk of all-cause and cancer mortality than males before serum cotinine increased to the threshold. However, for cardiovascular diseases mortality, the risk was increased simultaneously with increasing serum cotinine in males and females and the increased risk was early weakened in males after serum cotinine rose beyond the threshold. This indicates that mild and moderate tobacco exposure resulted in a higher risk of all-cause and cancer mortality in females and heavy tobacco exposure brought little risk of mortality in males and females. Interestingly, the harm of cardiovascular diseases from mild and moderate tobacco exposure in females and males is approximately equal.
One of the underlying mechanisms of the sex difference may be that due to the anti-estrogenic effect of smoking (25). Women smokers suffer from the twice “struck” phenomena: smoking directly increases the risk of ischemic heart disease by inducing atherosclerosis in both women and men, and in women, smoking may substantially attenuate the protective role of estrogen in atherosclerosis, and gastric and colon cancers (26–28). Another underlying mechanism for their higher risk of all-cause mortality is because women are more vulnerable to chronic obstructive pulmonary disease (29). Additionally, smoking cessation is more difficult in females than males (30, 31). Females experience more weight gain related to smoking cessation worries and suffer severe side effects from smoking cessation medications and craving, leading to fewer antismoking medications (32–35). Cepeda-Benito et al. reported that nicotine replacement therapy is less effective in females than males (35). Difficulty in quitting smoking increases the lasting detrimental effects of smoking in women who attempt to quit.
The age difference effect is relatively easy to understand. A prospective study reported that younger smokers have a greater mortality risk from cancer than those who start later (7). Similar results have been reported for coronary heart disease (36). The initiation of premature smoking is typically associated with more difficult cessation. This study suggests that younger adults are at a higher risk of smoking-related mortality. To interpreting this result, possibility of a cumulative effect need be considered; younger adults with the same serum cotinine level as the older adults may have a longer duration of smoking exposure (37).
In the present study, dose–response relationships and saturation effects of tobacco exposure showed sex and age differences, while sex differences in cardiovascular disease and age differences in cancer mortality were not significant; these results were not found in the previous study. These results suggest that and sex and age differences and corresponding saturation effects in smoking-related mortality are inconsistent in different diseases. Finally, this study included several laboratory parameters and demographic data. Most underlying diseases from the more representative and multiracial samples of the US population were excluded, which improved the reliability and accuracy of the study. The major study limitations were the long-term prognostic outcome, and a single point detection of serum cotinine concentration may not completely reflect the degree of smoking exposure during the follow-up period.
Taken together, our study demonstrated sex and age differences in smoking risk by measuring serum cotinine levels. The findings of dose–response relationships and threshold saturation effects provide evidence that mild and moderate tobacco exposure brought a higher risk of all-cause and cancer mortality in females. It has been noted that sex differences in cardiovascular disease and age differences in cancer mortality were insignificant, indicating inconsistency in different diseases. Cotinine could be an effective indicator to reveal novel findings for sex and age differences in smoking risk.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the NCHS Ethics Review Board (Protocol #2005–06, Protocol #2011–17 and Protocol #2018–01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
QJ: Formal analysis, Investigation, Writing – original draft. LJ: Methodology, Writing – original draft. XWa: Investigation, Writing – original draft. LL: Investigation, Data curation, Methodology, Software, Writing – review & editing. GH: Investigation, Formal analysis, Validation, Visualization, Writing – review & editing. XWu: Investigation, Methodology, Conceptualization, Software, Writing – review & editing. QM: Investigation, Methodology, Writing – review & editing. YL: Writing – review & editing, Investigation, Methodology, Software, Supervision. WW: Writing – review & editing. TZ: Writing – review & editing. YY: Software, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Sciences Foundation of Sichuan [23NSFSC0667]; Health Commission Technology Project of Sichuan [23LCYJ008]; and China International Medical Foundation of Youth Practical Research Project for respiratory disease [Z-2017-24-2301].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1512603/full#supplementary-material
ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; BMI, Body mass index; BUN, Blood urea nitrogen; CBC, Complete blood count; GGT, Gamma-glutamyl transferase; ISE, Ion selective electrode; LASSO, Least absolute shrinkage and selection operator; LDH, Lactate dehydrogenase; NCHS, National Center for Health Statistics; NHANES, National Health and Nutrition Examination Survey; ROC, Receiver operating characteristic; SHS, Second-hand smoke; VCS, Volume, conductivity, and scatter; VIF, Variance inflation factor; WBC, White blood cell.
1. Reitsma, MB, Fullman, N, Ng, M, Salama, JS, Abajobir, A, Abate, KH, et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. (2017) 389:1885–906. doi: 10.1016/s0140-6736(17)30819-x
2. Huxley, R, Jamrozik, K, Lam, T, Barzi, F, Ansary-Moghaddam, A, Jiang, C, et al. Impact of smoking and smoking cessation on lung cancer mortality in the Asia-Pacific region. Am J Epidemiol. (2007) 165:1280–6. doi: 10.1093/aje/kwm002
3. Huxley, RR, and Woodward, MJTL. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. (2011) 378:1297–305. doi: 10.1016/S0140-6736(11)60781-2
4. Peters, SA, Huxley, RR, and Woodward, M. Smoking as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 81 cohorts, including 3, 980, 359 individuals and 42, 401 strokes. Stroke. (2013) 44:2821–8. doi: 10.1161/strokeaha.113.002342
5. Prescott, E, Hippe, M, Schnohr, P, Hein, HO, and Vestbo, J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. (1998) 316:1043–7. doi: 10.1136/bmj.316.7137.1043
6. Prescott, E, Bjerg, AM, Andersen, PK, Lange, P, and Vestbo, J. Gender difference in smoking effects on lung function and risk of hospitalization for COPD: results from a Danish longitudinal population study. Eur Respir J. (1997) 10:822–7. doi: 10.1183/09031936.97.10040822
7. Thomson, B, Emberson, J, Lacey, B, Lewington, S, Peto, R, and Islami, F. Association of Smoking Initiation and Cessation across the life course and Cancer mortality: prospective study of 410 000 US adults. JAMA Oncol. (2021) 7:1901–3. doi: 10.1001/jamaoncol.2021.4949
8. Choi, SH, Stommel, M, Broman, C, and Raheb-Rauckis, C. Age of smoking initiation in relation to multiple health risk factors among US adult smokers: National Health Interview Survey (NHIS) data (2006-2018). Behav Med. (2023) 49:312–9. doi: 10.1080/08964289.2022.2060930
9. Hurley, MA. Light smoking at base-line predicts a higher mortality risk to women than to men; evidence from a cohort with long follow-up. BMC Public Health. (2014) 14:95. doi: 10.1186/1471-2458-14-95
10. Akbar, F, Mark, G, Pavlidis, I, and Gutierrez-Osuna, R. An empirical study comparing unobtrusive physiological sensors for stress detection in computer work. Sensors. (2019) 19. doi: 10.3390/s19173766
11. Hammond, D, Wiebel, F, Kozlowski, LT, Borland, R, Cummings, KM, O'Connor, RJ, et al. Revising the machine smoking regime for cigarette emissions: implications for tobacco control policy. Tob Control. (2007) 16:8–14. doi: 10.1136/tc.2005.015297
12. Marian, C, O'Connor, RJ, Djordjevic, MV, Rees, VW, Hatsukami, DK, and Shields, PG. Reconciling human smoking behavior and machine smoking patterns: implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol Biomarkers Prev. (2009) 18:3305–20. doi: 10.1158/1055-9965.Epi-09-1014
13. Reilly, SM, Goel, R, Bitzer, Z, Elias, RJ, Foulds, J, Muscat, J, et al. Effects of topography-related puff parameters on carbonyl delivery in mainstream cigarette smoke. Chem Res Toxicol. (2017) 30:1463–9. doi: 10.1021/acs.chemrestox.7b00104
14. Talhout, R, Richter, PA, Stepanov, I, Watson, CV, and Watson, CH. Cigarette design features: effects on emission levels, user perception, and behavior. Tob Regul Sci. (2018) 4:592–604. doi: 10.18001/trs.4.1.6
15. Benowitz, N, Jacob, P, Fong, I, and Gupta, S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. (1994) 268:296–303. doi: 10.1016/S0022-3565(25)38479-X
16. Benowitz, N, and Jacob, PJ. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. (1994) 56:483–93. doi: 10.1038/clpt.1994.169
17. Spaiuc, D, Spac, AF, Agoroaei, L, Dorneanu, V, and Butnaru, E. Development and validation of a GC-MS method for determination of nicotine in tobacco. Rev Med Chir Soc Med Nat Iasi. (2012) 116:611–6.
18. Ana, F, Roberta, F, Tom, E, Peter, S, Offie, S, and Gideon, K. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit. (2009) 31:14-30. doi: 10.1097/FTD.0b013e3181957a3b
19. Suzaynn, F, Benjamin, C, Peyton, R, Najat, A, and John, T. Biomarkers of exposure to new and emerging tobacco delivery products. Am J Physiol Lung Cell Mol Physiol. (2017) 313:L425–l452. doi: 10.1152/ajplung.00343.2016
20. Sarah, C, Sean, S, Jill, H, Geneviève, L, and Mark, T. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. (2009) 11:12–24. doi: 10.1093/ntr/ntn010
21. Flores, R, Liu, B, and Taioli, EJC. Association of serum cotinine levels and lung cancer mortality in non-smokers. Carcinogenesis. (2016) 37:1062–9. doi: 10.1093/carcin/bgw094
22. GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the global burden of disease study 2015. Lancet. (2017) 389:1885–906. doi: 10.1016/S0140-6736(17)30819-X
23. Iso, H, Date, C, Yamamoto, A, Toyoshima, H, Watanabe, Y, Kikuchi, S, et al. Smoking cessation and mortality from cardiovascular disease among Japanese men and women: the JACC study. Am J Epidemiol. (2005) 161:170–9. doi: 10.1093/aje/kwi027
24. Fa-Binefa, M, Clará, A, Pérez-Fernández, S, Grau, M, Dégano, IR, Marti-Lluch, R, et al. Early smoking-onset age and risk of cardiovascular disease and mortality. Prev Med. (2019) 124:17–22. doi: 10.1016/j.ypmed.2019.04.022
25. Baron, JA, La Vecchia, C, and Levi, F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol. (1990) 162:502–14. doi: 10.1016/0002-9378(90)90420-c
26. Meng, Q, Li, Y, Ji, T, Chao, Y, Li, J, Fu, Y, et al. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor α-mediated autophagy. J Adv Res. (2021) 28:149–64. doi: 10.1016/j.jare.2020.08.010
27. Fu, Z, Wang, X, Wang, Z, and Liu, L. Estrogen receptor-α36-mediated rapid estrogen signaling regulates 78 kDa glucose-regulated protein expression in gastric carcinoma cells. Oncol Lett. (2018) 15:10031–6. doi: 10.3892/ol.2018.8542
28. Kuo, CH, Kuo, CC, Wu, HY, Wu, DC, and Yang, CY. Higher parity and earlier age at first birth are associated with lower risk of death from colon cancer. Cancer Sci. (2012) 103:1553–7. doi: 10.1111/j.1349-7006.2012.02336.x
29. Zhang, Y, Wang, L, Mutlu, GM, and Cai, H. More to explore: further definition of risk factors for COPD-differential gender difference, modest elevation in PM (2).(5), and e-cigarette use. Front Physiol. (2021) 12:669152. doi: 10.3389/fphys.2021.669152
30. Zvolensky, MJ, Jardin, C, Wall, MM, Gbedemah, M, Hasin, D, Shankman, SA, et al. Psychological distress among smokers in the United States: 2008-2014. Nicotine Tob Res. (2018) 20:707–13. doi: 10.1093/ntr/ntx099
31. Smith, PH, Bessette, AJ, Weinberger, AH, Sheffer, CE, and McKee, SA. Sex/gender differences in smoking cessation: a review. Prev Med. (2016) 92:135–40. doi: 10.1016/j.ypmed.2016.07.013
32. Halperin, AC, McAfee, TA, Jack, LM, Catz, SL, McClure, JB, Deprey, TM, et al. Impact of symptoms experienced by varenicline users on tobacco treatment in a real world setting. J Subst Abus Treat. (2009) 36:428–34. doi: 10.1016/j.jsat.2008.09.001
33. Jarvis, MJ, Cohen, JE, Delnevo, CD, and Giovino, GA. Dispelling myths about gender differences in smoking cessation: population data from the USA, Canada and Britain. Tob Control. (2013) 22:356–60. doi: 10.1136/tobaccocontrol-2011-050279
34. Lombardi, EM, Prado, GF, Santos Ude, P, and Fernandes, FL. Women and smoking: risks, impacts, and challenges. J Bras Pneumol. (2011) 37:118–28. doi: 10.1590/s1806-37132011000100017
35. Cepeda-Benito, A, Reynoso, JT, and Erath, S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol. (2004) 72:712–22. doi: 10.1037/0022-006x.72.4.712
36. Fielding, JE. Smoking: health effects and control (1). N Engl J Med. (1985) 313:491–8. doi: 10.1056/nejm198508223130807
Keywords: cotinine, cigarette exposure, all-cause mortality, cardiovascular disease, cancer mortality, threshold saturation effects transferase, ion selective electrode, least absolute shrinkage and selection operator
Citation: Jiang Q, Junjun L, Wang X, Luo L, He G, Wu X, Min Q, Long Y, Wenjun W, Zhu T and Yao Y (2025) Beyond self-reports: serum cotinine reveals sex-and age-related differences of smoking on all-cause and disease-specific mortality. Front. Public Health. 13:1512603. doi: 10.3389/fpubh.2025.1512603
Received: 17 October 2024; Accepted: 29 January 2025;
Published: 17 February 2025.
Edited by:
Chuanwei Ma, Guangdong Medical University, ChinaCopyright © 2025 Jiang, Junjun, Wang, Luo, He, Wu, Min, Long, Wenjun, Zhu and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Yao, eXV5YW8wODI1QDE2My5jb20=; Tao Zhu, emh1dGFvMDYzMDIwQDE2My5jb20=; Wang Wenjun, d2FuZ3dlbmp1bjIwMDVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡ORCID: Qi Jiang, orcid.org/0000-0001-6883-769X
Xiaochuan Wang, orcid.org/0000-0002-5452-3400
Li Luo, orcid.org/0000-0002-4660-6361
Gaoyan He, orcid.org/0000-0001-5241-262X
Xiaojuan Wu, orcid.org/0000-0001-8606-5347
Qian Min, orcid.org/0009-0000-7618-2224
Ying Long, orcid.org/0000-0002-4292-7029
Tao Zhu, orcid.org/0000-0001-9622-2721
Yu Yao, orcid.org/0000-0001-8649-9372
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.