- 1Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 2School of Pharmacy, Curtin Medical School, Faculty of Health Sciences, Curtin University, Bentley, WA, Australia
- 3College of Medicine and Health Sciences, Woldia University, Woldia, Ethiopia
- 4School of Population Health, Curtin University, Bentley, WA, Australia
- 5Departement of Pharmacognosy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 6Department of Pharmaceutical Chemistry, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 7Department of Health Informatics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Background: Human papillomavirus (HPV) vaccinations protect against HPV infections. The infection might lead to vaginal cancer, vulvar cancer, genital warts, cervical intraepithelial neoplasia lesions, and cervical cancer. This study assessed hesitancy of HPV vaccination, associated factors, and barriers to vaccination among youth girls in Ethiopia.
Methods: An institutional-based cross-sectional study was conducted among female undergraduate students at the University of Gondar, College of Medicine and Health Sciences, between July and August 2022. The data was collected using a self-administered questionnaire. A simple random sampling method was used to recruit participants. The data were entered and analyzed with SPSS version 26. Descriptive statistics were used to describe the participants’ demographic characteristics. Logistic regression was performed to identify the significant factors associated with acceptance of the HPV vaccine. A p-value <0.05 was considered statistically significant.
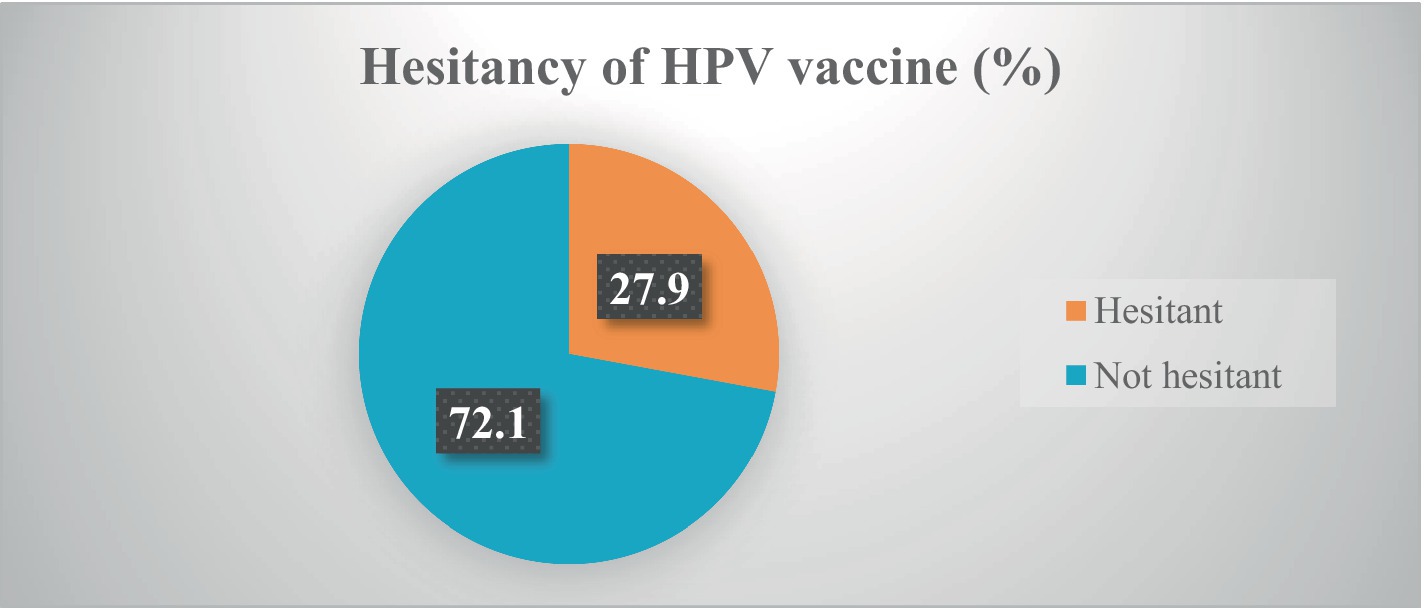
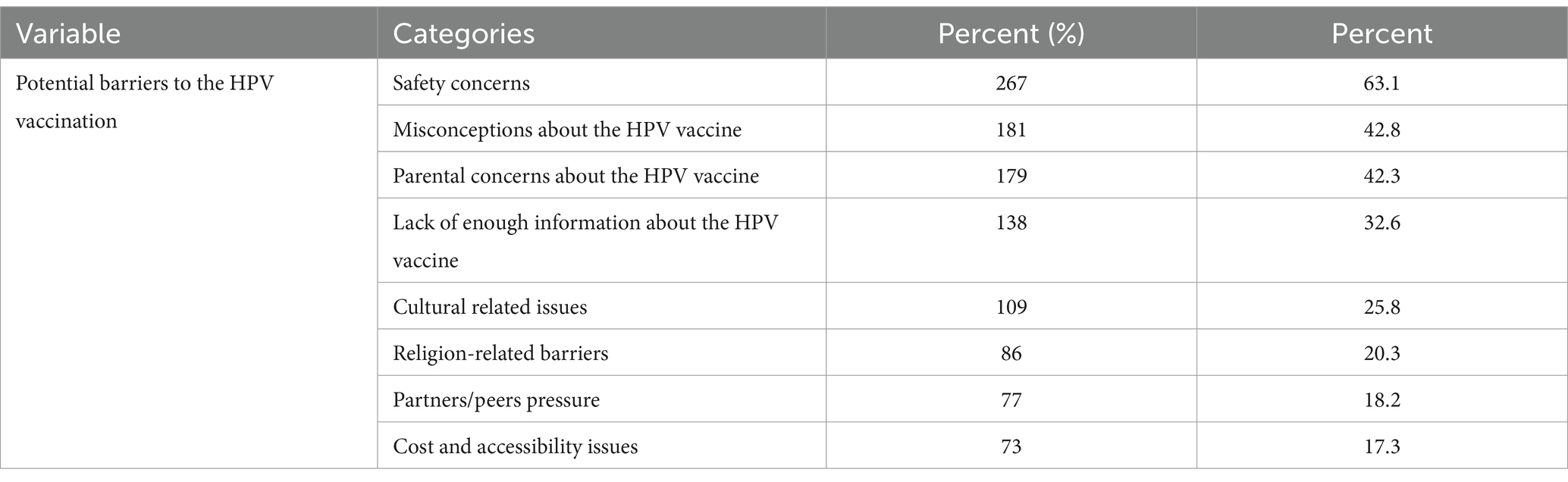
Results: The study included 423 participants with a mean age of 22.5 ± 6.7 years. Only more than one-third (35.2, 95% CI: 27.2–44.1) received the HPV vaccine. Currently, more than one-fourth (27.9, 95% Cl: 21.4–33.8) of participants are hesitant to receive the HPV vaccine. Higher monthly income (AOR = 1.52, 95% CI: 1.08–6.34), good knowledge of the HPV vaccine (AOR = 2.12, 95% CI: 1.12–4.87), and a positive attitude towards the vaccine (AOR = 3.03, 95% CI: 1.63–9.56) were significantly associated with acceptance of HPV vaccination. Safety concerns (63.1%), misinformation (42.8%), and parental concerns (42.3%) about the HPV vaccine were among the top perceived reported barriers to receiving the HPV vaccine.
Conclusion: This result showed that more than a quarter number of youth girls are still hesitant to receive HPV vaccinations. To increase vaccination acceptance, interventions should focus on awareness-raising programs about HPV infection and vaccines and addressing safety and parental concerns.
Introduction
Human papillomavirus (HPV) is a common sexually transmitted infection (STI) that can cause a variety of health problems, including cervical cancer, genital warts, and oropharyngeal cancer (1). The World Health Organization (WHO) estimates that approximately 630,000 women worldwide develop cervical cancer each year, resulting in 317,000 deaths. Most of these deaths occur in low- and middle-income countries, including Ethiopia. More than 85% of cases are diagnosed in developing countries (2). In Ethiopia, cervical cancer is the second most common cause of cancer death in women, with an age-specific incidence and mortality rate of 21.5 and 16 deaths per 100,000 females, respectively (3, 4).
The development of HPV vaccines has been a breakthrough in preventing HPV-related diseases (5–7). These vaccines are highly effective in preventing HPV infections and the subsequent development of cancer. The WHO recommends that all girls and young women aged 9–14 years receive two doses of the HPV vaccine as part of routine immunization programs (8). It is also recommended for girls and women aged 13 through 26 who have not yet been vaccinated or completed the vaccine series (9). Older people could be vaccinated, although this strategy is thought to be less effective given that this age group is more likely to have previously been exposed to HPV (10).
However, despite the availability and effectiveness of HPV vaccines, global vaccination rates remain suboptimal, and vaccination rates vary widely across different regions and countries (11). In some countries, vaccination rates are high, while in others they are low. Several factors contribute to vaccination hesitancy, including a lack of awareness about HPV and its consequences, concerns about vaccine safety, and cultural and religious barriers (12–15).
In Ethiopia, the HPV vaccination program is a relatively recent introduction and was introduced in December 2019 as part of the national immunization schedule for all 14-year-old girls through a school-based approach and in health centers (16). However, the uptake of the vaccine has been slow, with many girls and young women remaining hesitant to receive it (17–22). This is due to a variety of factors, including limited knowledge about HPV and its consequences (20), and concerns about vaccine safety (19), and cultural and religious beliefs that may discourage vaccination (13, 18).
Despite evidence showing barriers to HPV vaccination acceptance of youth girls in Ethiopia (17, 18, 20, 22, 23), most of the studies focused on knowledge and attitude-related factors and failed to comprehensively address other important potential barriers that influence vaccination acceptance in youth girls such as safety concerns and misconceptions about the HPV vaccine, parental and peer-related concerns, and cultural and religious factors. This study aims to assess hesitancy and associated factors and potential perceived barriers to HPV vaccination. By identifying the barriers to HPV vaccination in Ethiopia, policymakers and healthcare providers can develop targeted interventions to address these challenges and increase HPV vaccination acceptance.
Methods
Study design, setting, and samples
An institutional-based cross-sectional study was employed among female medical and health science students at the University of Gondar (UoG) from 17 July 2022 to 30 August 2022. The UoG has five campuses, such as Atse Tewodros, Atse Fasil, the College of Medicine and Health Sciences (CMHS), Maraki, and Teda campuses. At present, with nine academic offices, it offers about 87 undergraduate and 167 postgraduate programs in regular, distance, extension, and summer programs.
All selected regular undergraduate female students in the College of Medicine and Health Sciences were used as a study population. Participants who were interested in completing the self-administered questionnaire were included in the study and those unable to take part in data collection because of illness or other problems were excluded.
Sample size determination and sampling technique
The sample size was calculated using the single population proportion formula: n = (Z α/2)2*P *(1-P)/D2. This is where 1.96 was used for Z α/2, 50% for P, and 5% for D because there is no previous study in the study area. Where: D is the margin of error; P is the proportion of an outcome variable; and Z α/2 is the 95% confidence interval.
After considering a 10% non-response rate, the final sample size was 424. Study participants were included in the study using a simple random sampling technique.
Study variables
Dependent variable
HPV vaccine hesitancy is the main outcome variable in this study, which is categorized as hesitant and not hesitant.
Independent variables
Independent variables include sociodemographic variables (age, religion, residence, marital status, sex experience, department of current study, year of study, academic performance, economic status, mother’s education status, father’s education status, family income monthly, family can afford the vaccine, family history of the HPV vaccine), knowledge, and attitudes of HPV infection and vaccinations.
Data collection instruments and procedure
Data were collected using a self-administered structured questionnaire adopted after reviewing previous literature on the topic (18, 22–25). Two data collectors with previous experience in data collection were recruited. The questionnaire consisted of sociodemographic characteristics and questions that were used to assess participants’ hesitancy of HPV vaccinations; knowledge; attitudes toward cervical cancer, HPV infection, and HPV vaccination, and potential perceived barriers to HPV vaccination (Supplementary file 1).
Hesitancy to receive the HPV vaccine
Vaccine hesitancy is a delay or refusal to accept vaccines, even when they are readily available. The hesitancy of the HPV vaccination was determined directly from the respondents’ responses, which asked for their willingness to accept the HPV vaccine (18). This item consisted of three questions related to previous vaccination status, current acceptance behavior, and their recommendation for families to receive HPV vaccines with a response of yes and no. The item’s internal reliability was assessed using a Kuder–Richardson Formula 20 (KR-20) and found to be in an acceptable range of 0.74.
Knowledge of HPV and HPV vaccine
The knowledge of participants was assessed using a total of 15 questions of knowledge-related questions, specifically related to cervical cancer, HPV infection, and HPV vaccine. Participants are expected to respond Yes (score = 1) and No (score = 0) with a maximum possible score of 15. The were five questions for assessing knowledge of cervical cancer, five questions for knowledge of HPV infection, and five questions for the knowledge of HPV vaccine. The total score was calculated and those with mean and below mean scores were considered as having poor overall knowledge, while those with the above mean score were displayed as having good overall knowledge (23, 24). A KR-20 score of the items was found to be 0.71 and showed good internal reliability of the constructed items.
Attitude of HPV and HPV vaccine
Attitude towards HPV vaccination was measured using seven items that assessed attitudes of participants related to cervical cancer, HPV infection, and HPV vaccines on a five-point Likert scale: one for strongly disagreeing to five for strongly agree. Attitude scores for each participant then were calculated and attitudes towards HPV ranged from 1 to 7. Participants’ attitude was categorized as follows: negative if they obtained a score of less than the mean score and positive for those who obtained more than the mean score of the total attitude score (23). The internal reliability of the constructed items found an acceptable score of a Cronbach alpha value of 0.81.
Data quality control
The quality of the data collection instrument was maintained. The instrument was assessed for content and face validity with expertise. It was also pretested using a 5% sample to ensure the accuracy of the responses, the clarity of the questionnaire, and applicability. The data collectors received one-day training about the purposes of the study and the ethical aspects of data collection. The collected data was checked for clarity, consistency, and cleanliness throughout the collection period.
Data entry and statistical analysis
The data were coded and entered using EpiData version 4.6 and analyzed with SPSS version 26 statistical packages. Shapiro-Willick test (p > 0.05), histogram, and Q-Q plots were used to assess the normality of continuous variables. Descriptive data statistics were used to describe the demographic characteristics of the participants, and the results were presented through text, tables, figures, and graphs. Multivariable logistic regression was performed to identify associations between other sociodemographic variables and knowledge and HPV vaccination acceptance among study participants.
Results
Sociodemographic characteristics of study participants
A total of 423 participants were included in the current study with a 99.9% response. The mean age of the participants was 22.5 ± 6.7 years. Most students reported to have academic performance (60.8%) and economic status (60.3%). Approximately three-fourths (74%) of the participants reported a personal history of sexual intercourse. Around one-third (30%) of the participants’ families were vaccinated with the HPV vaccine (Table 1).
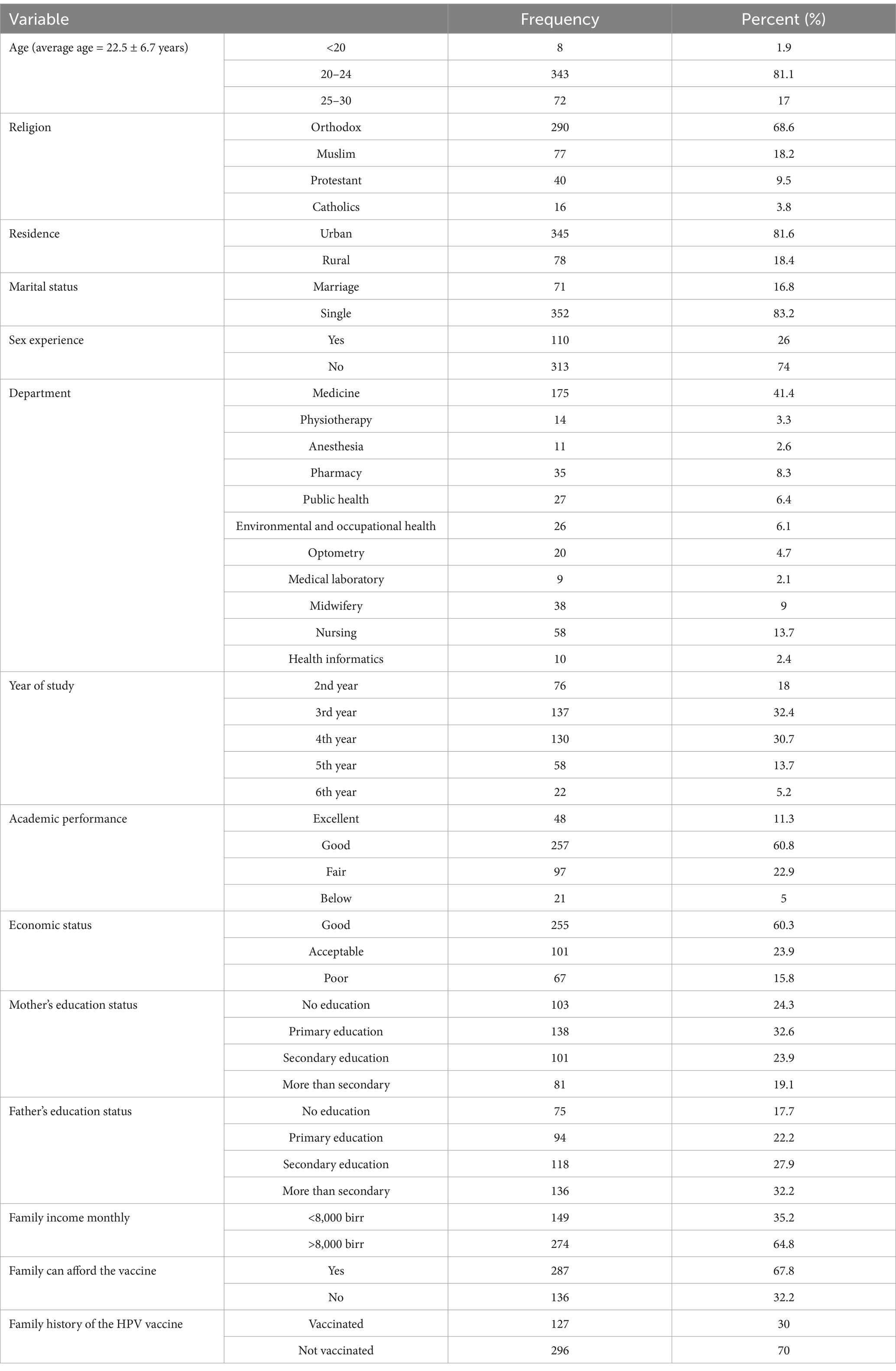
Table 1. Sociodemographic characteristics of study participants at the University of Gondar, 2022 (N = 423).
Participants’ level of hesitancy of the HPV vaccine
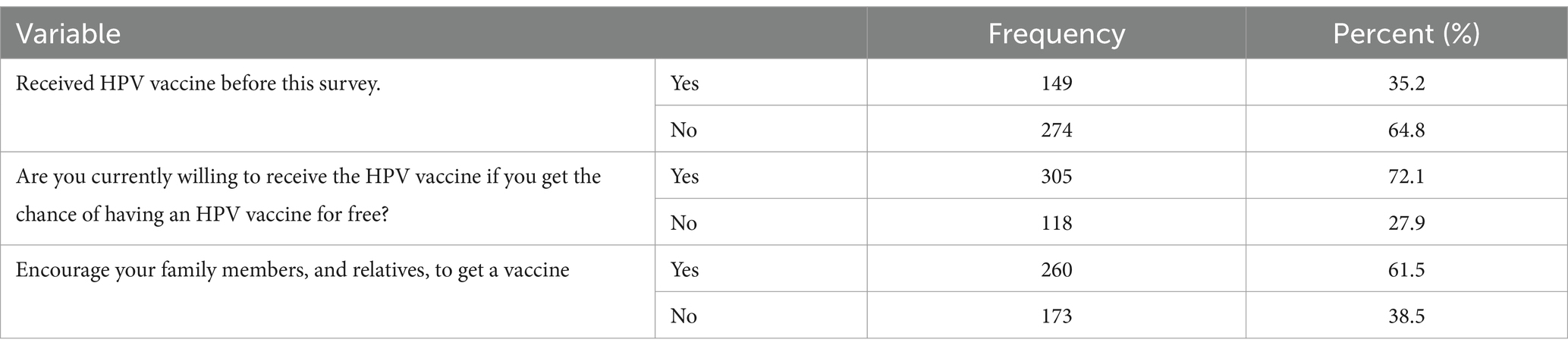
Of the 423 participants, only 149 (35.2, 95% CI: 27.2–44.1) received the HPV vaccine before the survey. Currently, more than one-fourth (27.9, 95% Cl: 21.4–33.8) of participants were hesitant to receive the HPV vaccine. About two-fifths (38.5, 95% CI: 31.4–44.9) of respondents were not confidently informing and recommending their family members and relatives to get the vaccines (Table 2) and (Figure 1).
Associated factors of HPV vaccination acceptance
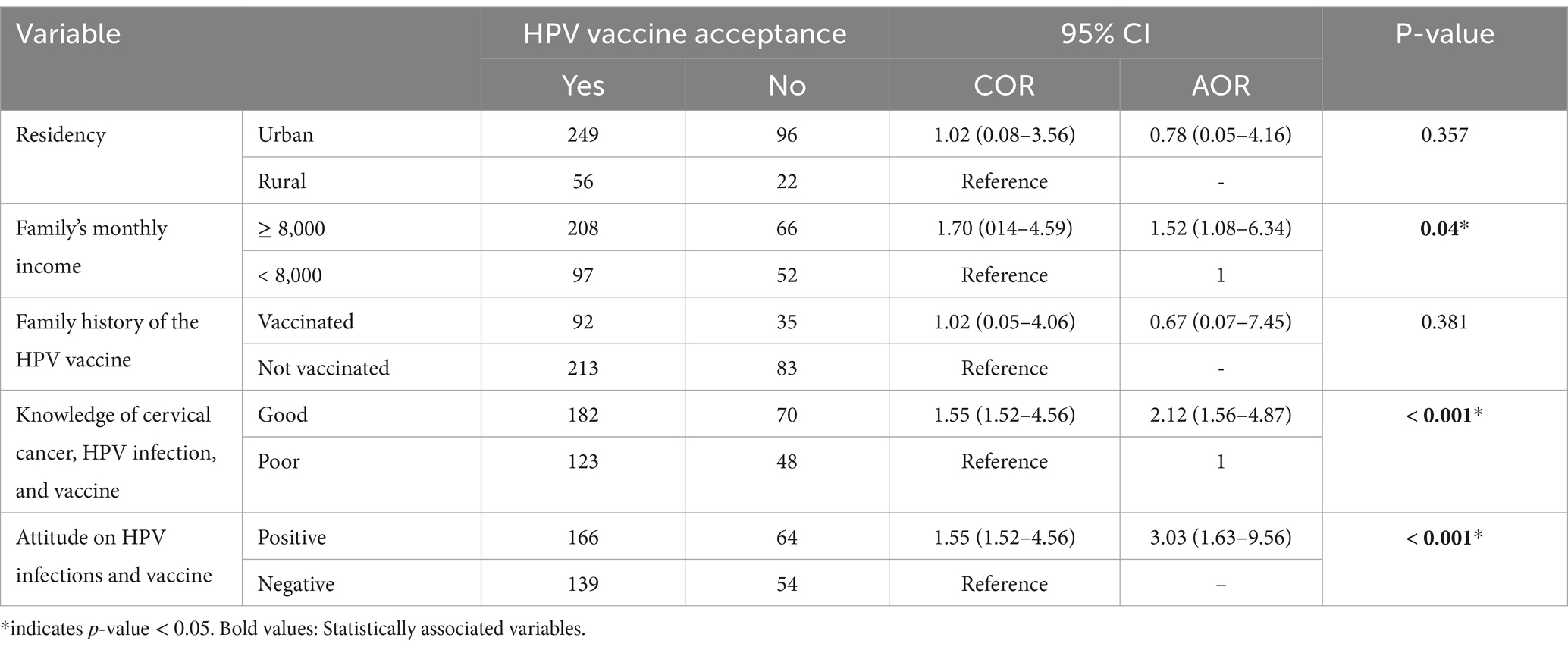
Multivariable logistic regression analysis showed that vaccine acceptability was associated with the participants’ monthly income, attitude, and knowledge of the HPV vaccine. HPV vaccine acceptance was significantly associated with higher monthly income (AOR = 1.52, 95% CI: 1.08–6.34), good knowledge of participants about cervical cancer, HPV infection, and HPV vaccine (AOR = 2.12, 95% CI: 1.12–4.87), and a positive attitude towards HPV vaccine (AOR = 3.03, 95% CI: 1.63–9.56) (Table 3).
Perceived barriers to the HPV vaccination
Less than two-thirds of the participants (63.1%) revealed that safety concern was the main barrier to receiving the HPV vaccine, followed by misconceptions about the HPV vaccine (42.8%) and parental concerns about the HPV vaccine (42.3%) (Table 4).
Participants’ level of knowledge
Most participants heard about cervical cancer (92.9%), HPV infection (93.4%), and HPV vaccine (78%). However, most of them did not know about the risk factors of cervical cancer (87.9), risk factors of HPV infections (62.6%), and the method of prevention of HPV infection (83.9%) (Supplementary Table S1). Overall, 252 (59.6%) had good knowledge of cervical cancer, HPV infections, and vaccines (Figure 2).
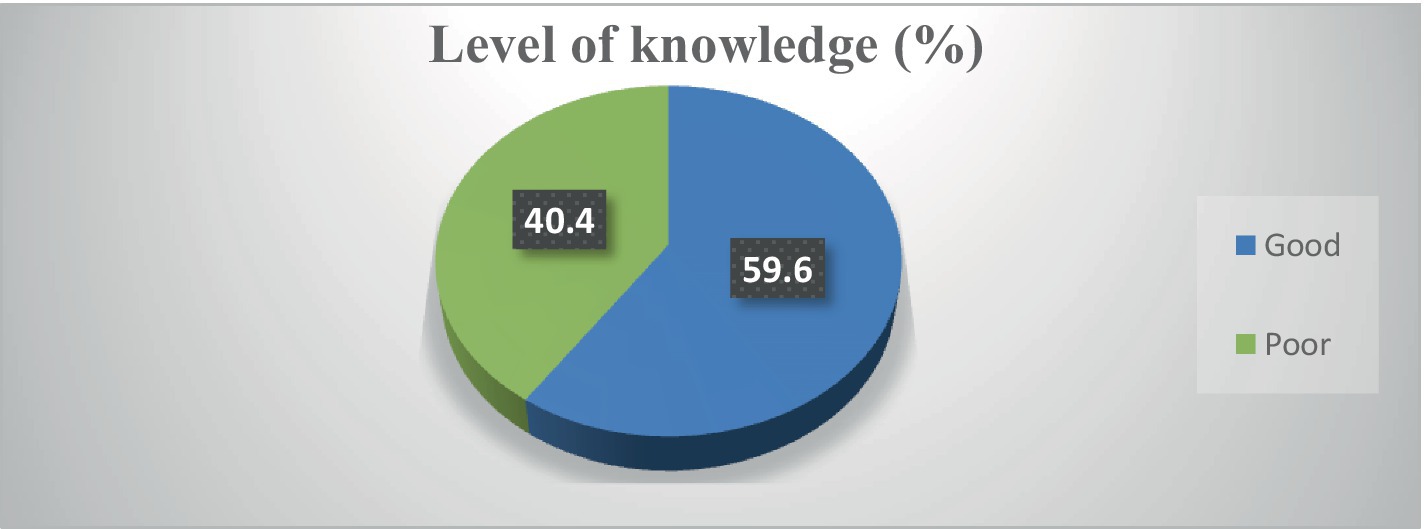
Figure 2. Participants’ level of knowledge about cervical cancer, HPV infection, and vaccine (N = 423).
Participants’ level of attitudes
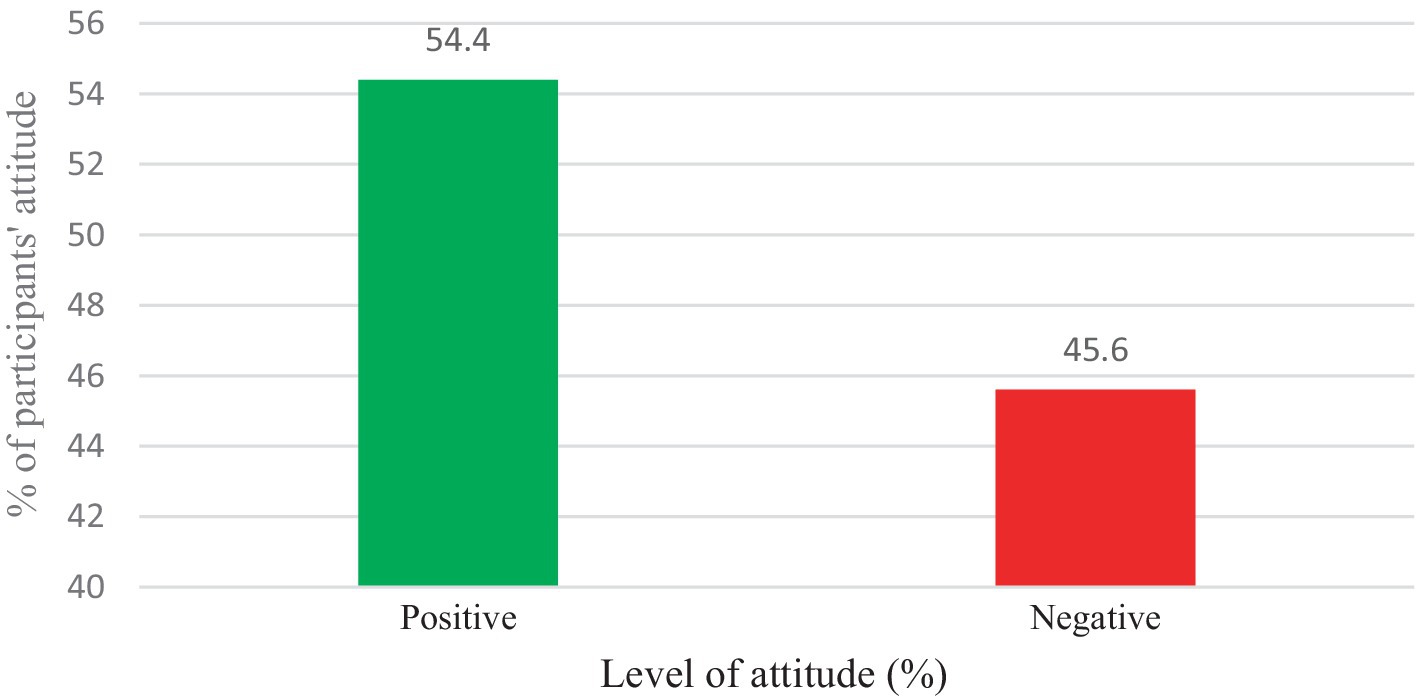
In the current study, more than one-third of the participants (33.5%) agreed or strongly agreed that cervical cancer is a deadly disease. About 97.2% also strongly agreed or agreed that vaccination helps to prevent HPV infection. Moreover, about 93.2% also agreed or strongly agreed that the vaccination was beginning to minimize cervical cancer (Supplementary Table S2). Overall, most (54.4%) respondents had a positive attitude towards the HPV vaccine (Figure 3).
Discussion
Vaccination hesitancy has continued to be a significant barrier to youths’ HPV vaccination and this study also revealed that more than one-quarter of youth girls were hesitant to receive HPV vaccines. HPV vaccination acceptance was significantly associated with the family’s monthly income, knowledge, and attitude about cervical cancer, HPV infection, and HP vaccine. This study also reported that safety concerns, misconceptions about the HPV vaccine, parental concerns about the HPV vaccine, lack of enough information about the HPV vaccine, and cultural and religious beliefs were among the top barriers to HPV vaccination of youth girls.
The findings of the current study showed that more than one-fourth of young girls are hesitant to receive the HPV vaccine currently. This figure is much lower than systematic reviews conducted on HPV vaccine uptake among adolescents in Ethiopia (17, 19, 26). A study conducted in South Africa (27), China (28), and other primary studies in Ethiopia (18, 20–22) also reported higher hesitancy levels than this study. This discrepancy could be due to differences in sociodemographic characteristics and health literacy. The study participants in the current study were health science students who were close to medical information, and they might be more aware of the importance of the HPV vaccine. The participants in China’s study were also medical students, however, the methods of hesitancy scoring are different in that this study directly asked respondents about their willingness to vaccine acceptance, while the previous study calculated from composite scores of three components such as complacency, convenience, and confidence (28). However, the current finding is much higher as compared to other studies reported in China which ranged from 10.3 to 15.5% (29, 30). This could be because of differences in socioeconomic status and access to healthcare and information, which can have an impact or contribute to vaccine acceptance or hesitancy. This finding suggests that vaccine hesitancy continues to be a public challenge and seeking tailored interventions considering contributing factors and potential barriers to vaccination uptake.
Consistent with earlier studies, parental monthly income was associated with acceptance of the HPV vaccine. Those participants with lower monthly incomes were less willing to vaccinate against the HPV vaccine compared to those with higher incomes (31). This justifies that the HPV vaccine can be expensive, especially when multiple doses are required. Lower-income families may struggle to afford this out-of-pocket expense, even if insurance partially covers it. However, in an Ethiopian context, this vaccination service is among the exempted healthcare services aiming to promote health service access. Therefore, public awareness creation could be important regarding the cost of the services.
This study also revealed that the knowledge and attitude of youth girls about cervical cancer, HPV infection, and HPV vaccines were determinant factors for HPV vaccine acceptance. These findings are consistent with other studies conducted in Ethiopia (17, 19, 20, 32) and Jordan (33). Participants with a positive attitude towards the HPV vaccine increased their chances of vaccine uptake by three times. This is consistent with a study conducted in Ethiopia (31) and Thailand (34). This might be because individuals who have a positive attitude towards the vaccine may have a higher intention of receiving the vaccine. Participants who had good knowledge of the HPV vaccine increased their vaccination uptake by about two-fold. This is a consistent study conducted in Nigeria (35) and Uganda (36). The findings highlight the importance of public awareness creation to enhance HPV vaccine acceptance in hesitant youth girls.
In the current study, safety concerns, misconceptions, and lack of enough information about the HPV vaccine were reported as the main barriers to HPV vaccination. This finding is consistent with earlier evidence (14, 15, 37, 38). This could suggest that healthcare providers should address specific concerns about vaccine safety and provide reassurance based on scientific evidence. Moreover, awareness creation campaigns may help dispel myths and provide evidence-based information about HPV, its risks, and the benefits of vaccination. For instance, awareness creation campaigns focusing on cancer prevention and HPV vaccine knowledge have resulted in reduced misinformation and increased vaccination uptake (39, 40). In addition, vaccination promotion campaigns implemented in schools and integrating HPV vaccination as school routine vaccination increased vaccination acceptance (39, 41). Furthermore, effective eHealth communication messages and delivery methods could have a positive impact on increasing the uptake of HPV vaccinations in adolescents (42).
Parental concerns about the HPV vaccine were among the major barriers to receiving HPV vaccines among youth girls. Pooled evidence meta-analysis study also revealed that parental willingness to vaccinate their children was lower (19, 43–45). This finding highlights the importance of engaging parents and caregivers in the decision-making process to build trust and address their concerns. Clear and effective communication with parents or caregivers can play a crucial role in supporting them as they decide about vaccinating their children (46).
Cultural and religious beliefs were also among the reported potential barriers to HPV vaccination of youth girls. This is in line with previous studies (13, 47, 48). The finding underscores the need for vaccination campaign interventions to address cultural and religious beliefs, emphasizing the safety and effectiveness of the vaccines (49). Furthermore, a detailed exploration of the impacts of cultural and religious beliefs could be conducted using qualitative data.
Strengths and limitations of the study
This study presents a comprehensive finding regarding current vaccination status and their willingness to receive HPV vaccination among university students using a relatively larger sample. However, it has some limitations. Firstly, the study was conducted among only health science students, which is difficult to conclude for all young girls in Ethiopia. Secondly, this study focused only on HPV cancers. Additionally, despite HPV being recommended for males globally the current study accounts only for young girls. Furthermore, the nature of data collection, a self-administered survey, causes a social disability bias, and the outcome might be overestimated. Finally, the causal relationship might be difficult to estimate using a cross-sectional study. As a result, the authors recommend a prospective follow-up study that incorporates qualitative exploration to understand potential barriers to the vaccination of youths.
Implication of the study
This study has implication highlights for important parties in this area such as healthcare providers, policymakers, and researchers.
Healthcare providers
Healthcare providers should be equipped with accurate and up-to-date information about HPV, its risks, and the benefits of vaccination. They should be able to effectively communicate this information to young girls and their parents, addressing common concerns and misconceptions. Providers should offer personalized counseling to address individual concerns and provide tailored information based on the patient’s needs and cultural background. Establishing a trusting relationship with patients is essential. Providers should be empathetic, respectful, and patient in addressing concerns and building trust. Healthcare providers can also play a crucial role in advocating for HPV vaccination policies and programs, both at the local and national levels.
Policymakers
Policymakers should allocate sufficient funding for HPV vaccination programs, including education campaigns, vaccine procurement, and healthcare provider training. While mandating vaccination may be controversial, it can be a powerful tool for increasing coverage. Policymakers should carefully consider the potential benefits and drawbacks of mandatory vaccination. Implementing school-based vaccination programs can make it easier for young girls to receive the vaccine and improve access in underserved communities. Policymakers should also ensure that HPV vaccination programs are equitable and accessible to all young girls, regardless of their socioeconomic status, race, or ethnicity.
Researchers
Further research is needed to better understand the factors contributing to HPV vaccination hesitancy, particularly in diverse populations. This research can inform the development of more effective interventions. Longitudinal studies can track changes in vaccine hesitancy over time and evaluate the effectiveness of different interventions. Qualitative research can provide valuable insights into the perspectives and experiences of young girls, parent concerns, and their decisions regarding HPV vaccination and the potential impacts of cultural and religious beliefs. Researchers should also ensure that their studies are culturally competent and consider the diverse cultural and religious beliefs of different populations. Since HPV also causes other cancers and genital warts, future studies should consider including questions about additional HPV-related diseases. Furthermore, as HPV vaccination is administered to males globally, it would be valuable to include males in such studies to better inform and target future vaccination efforts.
Conclusion
The current study revealed that more than one-quarter of young girls are hesitant to receive the HPV vaccine, indicating that it remains a persistent challenge in today’s context. Interventional programs aiming to increase HPV vaccination in youth girls should focus on promoting awareness among youths and the public about the impact of HPV infection and the safety and importance of the HPV vaccine. Tailored interventions addressing parental concerns, and religious and cultural beliefs are also crucial to enhancing HPV vaccination in youth girls.
Data availability statement
The data supporting the conclusions of this article will be made available by the correspondence author upon reasonable request, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethical review committee of the School of Pharmacy at the University of Gondar with a reference number of SOP/257/2022. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BBA: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. BAA: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. AT: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. KM: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. MA: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. YT: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. AD: Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing, Data curation. MM: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. GB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank data collectors and study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1507832/full#supplementary-material
Abbreviations
CDC, Communicable Disease Control; HPV, Human papillomavirus; WHO, World Health Organization.
References
1. WHO. Human papillomavirus and cancer. Available: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer#:~:text=Persistent%20HPV%20infection%20with%20highcases%20in%20men% (Accessed September 25, 2024).
2. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Singh, D, Vignat, J, Lorenzoni, V, Eslahi, M, Ginsburg, O, Lauby-Secretan, B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO global cervical Cancer elimination initiative. Lancet Glob Health. (2023) 11:e197–206. doi: 10.1016/S2214-109X(22)00501-0
4. Bruni, LB, Albero, G, Serrano, B, Mena, M, Collado, JJ, Gómez, D, et al. ICO/IARC information Centre on HPV and cancer (HPV information Centre). Human papillomavirus and related diseases in the world. Summary Rep. (2023).
5. Kim, YT, Serrano, B, Lee, JK, Lee, H, Lee, SW, Freeman, C, et al. Burden of human papillomavirus (HPV)-related disease and potential impact of HPV vaccines in the Republic of Korea. Papillomavirus Res. (2019) 7:26–42. doi: 10.1016/j.pvr.2018.12.002
6. Patel, C, Brotherton, JM, Pillsbury, A, Jayasinghe, S, Donovan, B, Macartney, K, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Euro Surveill. (2018) 23:1700737. doi: 10.2807/1560-7917.ES.2018.23.41.1700737
7. Charde, SH, and Warbhe, RA. Human papillomavirus prevention by vaccination: a review article. Cureus. (2022) 14:e30037. doi: 10.7759/cureus.30037
8. Centers for Disease Control and Prevention, (2021). HPV Vaccination Recommendations. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html.
9. Adjei Boakye, E, Lew, D, Muthukrishnan, M, Tobo, BB, Rohde, RL, Varvares, MA, et al. Correlates of human papillomavirus (HPV) vaccination initiation and completion among 18-26 year olds in the United States. Hum Vaccin Immunother. (2018) 14:2016–24. doi: 10.1080/21645515.2018.1467203
10. Garbuglia, AR, Lapa, D, Sias, C, Capobianchi, MR, and Del Porto, P. The use of both therapeutic and prophylactic vaccines in the therapy of papillomavirus disease. Front Immunol. (2020) 11:188. doi: 10.3389/fimmu.2020.00188
11. WHO. Immunization coverage. Available at: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (Accessed August 25, 2024.
12. Agana-Norman, DFG, Martinez Martinez, M, Shanmugasundaram, M, and Berenson, AB. Understanding barriers to human papillomavirus vaccination among parents of 9-10-year-old adolescents: a qualitative analysis. Vaccines. (2024) 12:245. doi: 10.3390/vaccines12030245
13. Netfa, F, King, C, Davies, C, Rashid, H, Tashani, M, Booy, R, et al. Perceived facilitators and barriers to the uptake of the human papillomavirus (HPV) vaccine among adolescents of Arabic-speaking mothers in NSW, Australia: a qualitative study. Vaccine X. (2023) 14:100335. doi: 10.1016/j.jvacx.2023.100335
14. Kutz, JM, Rausche, P, Gheit, T, Puradiredja, DI, and Fusco, D. Barriers and facilitators of HPV vaccination in sub-saharan Africa: a systematic review. BMC Public Health. (2023) 23:974. doi: 10.1186/s12889-023-15842-1
15. Zheng, L, Wu, J, and Zheng, M. Barriers to and facilitators of human papillomavirus vaccination among people aged 9 to 26 years: a systematic review. Sex Transm Dis. (2021) 48:e255–62. doi: 10.1097/OLQ.0000000000001407
16. WHO. Ethiopia. Ethiopia launches Human Papillomavirus Vaccine for 14 year old girls. Available at: https://www.afro.who.int/news/ethiopia-launches-human-papillomavirus-vaccine-14-year-old-girls (Accessed August 25, 2023).
17. Zewdie, A, Kasahun, AW, Habtie, A, Gashaw, A, and Ayele, M. Human papillomavirus vaccine acceptance among adolescent girls in Ethiopia: a systematic review and meta-analysis. BMC Public Health. (2023) 23:1369. doi: 10.1186/s12889-023-16305-3
18. Dera, M, Wondimagegnehu, A, and Asfaw, ZG. Determinants for hesitancy in human papillomavirus (HPV) vaccine uptake among school girls in Jimma town, Ethiopia. A mixed approach: quantitative and qualitative. Reprod Health. (2023) 20:175. doi: 10.1186/s12978-023-01711-y
19. Derbie, A, Mekonnen, D, Misgan, E, Maier, M, Woldeamanuel, Y, and Abebe, T. Acceptance of human papillomavirus vaccination and parents' willingness to vaccinate their adolescents in Ethiopia: a systematic review and meta-analysis. Infect Agent Cancer. (2023) 18:59. doi: 10.1186/s13027-023-00535-6
20. Kassie, N, Sema, A, Amsalu, B, Sintayehu, Y, Abie, A, Mengist, B, et al. Willingness to take human papillomavirus vaccine and its associated factors among school adolescent girls: a case of school-based dose one human papillomavirus vaccine campaign in Dire Dawa. Ethiopia SAGE Open Med. (2024) 12:20503121231225333. doi: 10.1177/20503121231225333
21. Hailu, G, Wirtu, D, Tesfaye, T, and Getachew, M. Human papillomavirus vaccine uptake and associated factors among adolescent girls in high schools of Nekemte city, Western Ethiopia, 2020. BMC Womens Health. (2023) 23:560. doi: 10.1186/s12905-023-02702-8
22. Beyen, MW, Bulto, GA, Chaka, EE, Debelo, BT, Roga, EY, Wakgari, N, et al. Human papillomavirus vaccination uptake and its associated factors among adolescent school girls in ambo town, Oromia region, Ethiopia, 2020. PLoS One. (2022) 17:e0271237. doi: 10.1371/journal.pone.0271237
23. Yohannes, E, Beyen, MW, Bulto, GA, Chaka, EE, Debelo, BT, Erena, MM, et al. Knowledge and attitude toward human papillomavirus vaccination and associated factors among adolescent school girls in ambo town, Ethiopia, 2021: a multicenter cross-sectional study. Health Sci Rep. (2023) 6:e1305. doi: 10.1002/hsr2.1305
24. Lee, K-N, Chang, KHJ, Cho, SS, Park, SH, and Park, ST. Attitudes regarding HPV vaccinations of children among mothers with adolescent daughters in Korea. J Korean Med Sci. (2017) 32:130–4. doi: 10.3346/jkms.2017.32.1.130
25. Lakneh, EA, Mersha, EA, Asresie, MB, and Belay, HG. Knowledge, attitude, and uptake of human papilloma virus vaccine and associated factors among female preparatory school students in Bahir Dar City, Amhara region, Ethiopia. PLoS One. (2022) 17:e0276465. doi: 10.1371/journal.pone.0276465
26. Addisu, D, Gebeyehu, NA, and Belachew, YY. Knowledge, attitude, and uptake of human papillomavirus vaccine among adolescent schoolgirls in Ethiopia: a systematic review and meta-analysis. BMC Womens Health. (2023) 23:279. doi: 10.1186/s12905-023-02412-1
27. Khosa, LA, Meyer, JC, Motshwane, FMM, Dochez, C, and Burnett, RJ. Vaccine hesitancy drives low human papillomavirus vaccination coverage in girls attending public schools in South Africa. Front Public Health. (2022) 10:10. doi: 10.3389/fpubh.2022.860809
28. Zhou, L, Wang, J, Cheng, P, Li, Y, Liu, G, and Zhang, X. HPV vaccine hesitancy among medical students in China: a multicenter survey. Front Public Health. (2022) 10:774767. doi: 10.3389/fpubh.2022.774767
29. Huang, Y, Chen, C, Wang, L, Wu, H, Chen, T, and Zhang, L. HPV vaccine hesitancy and influencing factors among university students in China: a cross-sectional survey based on the 3Cs model. Int J Environ Res Public Health. (2022) 19:14025. doi: 10.3390/ijerph192114025
30. Chen, C, Chen, T, Huang, M, Huang, Y, Zhang, L, and Li, P. Factors associated with HPV vaccine hesitancy among college students: a cross-sectional survey based on 3Cs and structural equation model in China. Hum Vaccin Immunother. (2024) 20:2309731. doi: 10.1080/21645515.2024.2309731
31. Dereje, N, Ashenafi, A, Abera, A, Melaku, E, Yirgashewa, K, Yitna, M, et al. Knowledge and acceptance of HPV vaccination and its associated factors among parents of daughters in Addis Ababa, Ethiopia: a community-based cross-sectional study. Infect Agents Cancer. (2021) 16:1–7. doi: 10.1186/s13027-021-00399-8
32. Woldehawaryat, EG, Geremew, AB, and Asmamaw, DB. Uptake of human papillomavirus vaccination and its associated factors among adolescents in Gambella town, southwest, Ethiopia: a community-based cross-sectional study. BMJ Open. (2023) 13:e068441. doi: 10.1136/bmjopen-2022-068441
33. Sallam, M, Al-Mahzoum, K, Eid, H, Assaf, AM, Abdaljaleel, M, Al-Abbadi, M, et al. Attitude towards HPV vaccination and the intention to get vaccinated among female university students in health schools in Jordan. Vaccine. (2021) 9:1432. doi: 10.3390/vaccines9121432
34. Chanprasertpinyo, W, and Rerkswattavorn, C. Human papillomavirus (HPV) vaccine status and knowledge of students at a university in rural Thailand. Heliyon. (2020) 6:e04625. doi: 10.1016/j.heliyon.2020.e04625
35. Fagbule OFKanmodi, KK, Aliemeke, EO, Ogunniyi, KE, Ogbeide, M, Victor, SO, et al. Knowledge of HPV and HPV vaccine among senior secondary school students in Nigeria: implications on cancer prevention strategies, the CHANCE study. Popul Med. (2020) 2:1–10. doi: 10.18332/popmed/127237
36. Nabirye, J, Okwi, LA, Nuwematsiko, R, Kiwanuka, G, Muneza, F, Kamya, C, et al. Health system factors influencing uptake of human papilloma virus (HPV) vaccine among adolescent girls 9-15 years in Mbale District, Uganda. BMC Public Health. (2020) 20:8302. doi: 10.1186/s12889-020-8302-z
37. Giede, C, McFadden, LL, Komonoski, P, Agrawal, A, Stauffer, A, and Pierson, R. The acceptability of HPV vaccination among women attending the University of Saskatchewan Student Health services. J Obstet Gynaecol Can. (2010) 32:679–86. doi: 10.1016/S1701-2163(16)34572-8
38. Muthukrishnan, M, Loux, T, Shacham, E, Tiro, JA, and Arnold, LD. Barriers to human papillomavirus (HPV) vaccination among young adults, aged 18-35. Prev Med Rep. (2022) 29:101942. doi: 10.1016/j.pmedr.2022.101942
39. Xu, MA, Choi, J, Capasso, A, and DiClemente, RJ. Improving HPV vaccination uptake among adolescents in low resource settings: sociocultural and socioeconomic barriers and facilitators. Adolesc Health Med Ther. (2024) 15:73–82. doi: 10.2147/AHMT.S394119
40. Fu, LY, Bonhomme, LA, Cooper, SC, Joseph, JG, and Zimet, GD. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. (2014) 32:1901–20. doi: 10.1016/j.vaccine.2014.01.091
41. Rane, MS, Page, LC, McVeigh, E, Miller, K, Baure, D, Elizabeth Halloran, M, et al. Improving adolescent human papillomavirus (HPV) immunization uptake in school-based health centers through awareness campaigns. Vaccine. (2021) 39:1765–72. doi: 10.1016/j.vaccine.2021.02.006
42. Kim, Y, Lee, H, Park, J, Kim, YC, Kim, DH, and Lee, YM. eHealth communication intervention to promote human papillomavirus vaccination among middle-school girls: development and usability study. JMIR Form Res. (2024) 8:e59087. doi: 10.2196/59087
43. Bittew, SM, Masresha, SA, Mulaw, GF, Yimam, MA, Zimamu, AA, Abriham, AA, et al. Parental willingness to vaccinate their daughters against human papilloma virus and its associated factors in Woldia town. Northeast Ethiopia Front Glob Womens Health. (2024) 5:1243280. doi: 10.3389/fgwh.2024.1243280
44. Wassie, M, Zegeye, AF, Worku, W, Sisay, T, Eyob, T, and Gebeyehu, DA. Willingness to accept human papilloma virus vaccination and its associated factors among parents with eligible daughters in Addis Zemen town, Northwest Ethiopia. Infect Agent Cancer. (2023) 18:84. doi: 10.1186/s13027-023-00551-6
45. Aragaw, GM, Anteneh, TA, Abiy, SA, Bewota, MA, and Aynalem, GL. Parents' willingness to vaccinate their daughters with human papillomavirus vaccine and associated factors in Debretabor town, Northwest Ethiopia: a community-based cross-sectional study. Hum Vaccin Immunother. (2023) 19:2176082. doi: 10.1080/21645515.2023.2176082
46. Karafillakis, E, Peretti-Watel, P, Verger, P, Chantler, T, and Larson, HJ. ‘I trust them because my mum trusts them’: exploring the role of trust in HPV vaccination decision-making among adolescent girls and their mothers in France. Vaccine. (2022) 40:1090–7. doi: 10.1016/j.vaccine.2022.01.028
47. Hittson, H, McAleer, L, Saucedo, L, Mahler, L, Andino, G, Zorba, A, et al. Association between religious beliefs and HPV vaccination attitudes among college students. Vaccines. (2023) 11:1623. doi: 10.3390/vaccines11101623
48. Marlow, LA, Wardle, J, Forster, AS, and Waller, J. Ethnic differences in human papillomavirus awareness and vaccine acceptability. J Epidemiol Community Health. (2009) 63:1010–5. doi: 10.1136/jech.2008.085886
Keywords: Human papillomavirus, HPV vaccine, vaccine hesitancy, vaccination acceptance, knowledge, attitude, young girls, Ethiopia
Citation: Sendekie AK, Abate BB, Adamu BA, Tefera AM, Mekonnen KT, Ashagrie MA, Tadesse YB, Dagnaw AD, Melaku MS and Bizuneh GK (2025) Human papillomavirus vaccination hesitancy among young girls in Ethiopia: factors and barriers to uptake. Front. Public Health. 13:1507832. doi: 10.3389/fpubh.2025.1507832
Edited by:
Marina Pekmezovic, Independent Researcher, Berlin, GermanyReviewed by:
Andrew Pavelyev, Merck, United StatesJuliana Sesma, Hospital Provincial de Rosario, Argentina
Copyright © 2025 Sendekie, Abate, Adamu, Tefera, Mekonnen, Ashagrie, Tadesse, Dagnaw, Melaku and Bizuneh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashenafi Kibret Sendekie, YXNodWtpYjAyQHlhaG9vLmNvbQ==; QXNoZW5hZmkua2licmV0QHVvZy5lZHUuZXQ=
 Ashenafi Kibret Sendekie
Ashenafi Kibret Sendekie Biruk Beletew Abate
Biruk Beletew Abate Betelhem Anteneh Adamu5
Betelhem Anteneh Adamu5 Yabibal Berie Tadesse
Yabibal Berie Tadesse Mequannent Sharew Melaku
Mequannent Sharew Melaku Gizachew Kassahun Bizuneh
Gizachew Kassahun Bizuneh