- 1Department of Otorhinolaryngology, Organization Lishui People’s Hospital, Lishui, Zhejiang, China
- 2Medical College, Organization Zhejiang University, Hangzhou, Zhejiang, China
- 3Department of Nursing, Organization Lishui People’s Hospital, Lishui, Zhejiang, China
- 4Department of Radiation Oncology, Organization Lishui People’s Hospital, Lishui, Zhejiang, China
Background: Multimorbidity is a major aging and public health problem that has a significant burden on a global scale. The number of risk prediction models for mortality in patients with multimorbidity is increasing; however, the quality and applicability of these prediction models in clinical practice and future research remain uncertain.
Objective: To systematically review published studies on risk prediction models for mortality in patients with multimorbidity.
Methods: The Wanfang, China National Knowledge Infrastructure, China Science and Technology Journal (VIP), PubMed, SinoMed, Cochrane Library, Web of Science, Embase, and Cumulative Index to Nursing and Allied Health Literature databases were searched from inception until May 30, 2024. Two independent reviewers performed study selection, data extraction, and quality assessment. The Prediction Model Risk of Bias Assessment Tool (PROBAST) checklist was utilized to assess the risk of bias and applicability.
Results: Overall, 18 studies with 21 prediction models were included in this review. Logistic regression was used for model development in 12 studies, Cox regression in four, a parametric Weibull regression in one, and machine learning in one study. The incidence of mortality in patients with multimorbidity ranged from 7.6–50.0%. The most frequently used predictors were age and body mass index. The reported area under the receiver operating characteristic curve (AUC) and C-index values ranged from 0.700–0.907. Three studies were rated as having a low risk of bias, 11 as high, and four as unclear, primarily owing to poor reporting of the analysis domain. The pooled AUC value of the seven validated models was 0.81, with a 95% confidence interval ranging from 0.77–0.86, signifying a fair level of discrimination.
Conclusion: The included studies revealed a degree of discriminatory ability in predicting mortality in patients with multimorbidity; however, they all demonstrated significant risks of bias based on the PROBAST checklist assessment. Future researchers should prioritize the development of new models that incorporate rigorous study designs and multicenter external validation, which may improve the precision of risk predictions and help the development of global strategies for this significant public health problem.
Registration: The study protocol was registered in PROSPERO (registration number: CRD42024543170).
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/recorddashboard, PROSPERO CRD42024543170.
1 Introduction
Multimorbidity is defined as the coexistence of two or more chronic conditions in the same individual and is frequently observed in medical and health research (1). The concept of comorbidity is related to that of multimorbidity (2) as comorbidity refers to any ailment that coexists with an index disease (3). Multimorbidity is estimated to affect a large proportion of the world’s population and demonstrates an S-shaped relationship with aging (4). The overall incidence of multimorbidity is 37.2%, and its highest incidence occurred in South America (45.7%), followed by North America (43.1%) and Asia (35.0%). More than half of the global adult population older than 60 years (51.0%) are suffering from multimorbidity. Multimorbidity has become increasingly prevalent in the last two decades, although the global incidence appears to have remained stable among adults in the recent decade (5). Multimorbidity has been associated with functional dependence, reduced productivity, poor quality of life, and increased mortality, threatening the sustainability of healthcare systems (6, 7). This represents a persistent and significant burden on individuals, families, healthcare systems, and societies. However, to the best of our knowledge, numerous evidence gaps exist regarding the relationship between multimorbidity and mortality. For instance, cancer and vascular disease are the two leading causes of mortality; however, the impact of multimorbidity on these disease outcomes remains unexplored (8). Multimorbidity is linked to a high mortality rate (9), and previous research has indicated that individuals with multimorbidity have an all-cause mortality risk that is approximately 2–3 times higher than that of those without multimorbidity (10–12). Nevertheless, it is potentially preventable (4) and easily preventable among individuals without chronic illnesses. For individuals diagnosed with a chronic illness, additional diagnoses are likely, and these linkages have been theorized to be mediated via sleep disturbance and circadian rhythm dysfunction (13). Multiple guidelines suggest tailoring the preventive care of individuals with multimorbidity based on life expectancy and consequently, their mortality risk (14, 15). Indeed, patients with a high short-term mortality risk, such as those with multimorbidity, may not benefit from preventive care interventions. Considering the rapid increase in the incidence of multimorbidity and the fact that the specific roles of various long-term conditions and their interactions in predicting mortality risk remain unclear, establishing a valid index for predicting mortality in patients with multimorbidity is necessary. The accurate prediction of mortality risk in such patients is crucial, as it can assist medical decision-making and enable health professionals to provide individualized treatments based on patients’ condition, orientation, and prognosis (16), and it is essential for early identification and intervention.
Prognostic models are mathematical equations incorporating multiple variables that can be used to estimate the probability that an individual in a specific health state will experience a particular health outcome (17). In this context, modeling the mortality risk for patients with multimorbidity to accurately estimate their individual risk has garnered increasing interest. Specifically, establishing risk of mortality models for patients with multimorbidity have been considered as the solution to many research problems. However, studies have found that the risk of mortality is differentially distributed among different comorbidity types (18, 19). Prediction models can assist healthcare providers in optimizing decisions, including accurately and rapidly identifying patients who require extensive education and preventive measures (e.g., weight control and regular monitoring); moreover, these models inform patients with multimorbidity and their family members on the mortality risk, enhancing their awareness and facilitating compliance with prevention (20, 21).
In the past decade, an increasing number of studies have been conducted to develop and validate risk prediction models for mortality in patients with multimorbidity (22, 23). However, a recognized and authoritative prediction model recommended for use by guidelines has not been established. Moreover, these models have been developed using small cohorts, lack external validation, and have rarely been applied in clinical practice (24), and the methodological quality has rarely been thoroughly and critically assessed. New models have been published; however, this problem remains unsolved. Hence, this systematic review aimed to screen and systematically review published studies on existing risk prediction models (developed or validated) for mortality in patients with multimorbidity.
2 Methods
2.1 Study design
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. The study protocol was registered in PROSPERO (registration number: CRD42024543170).
2.2 Search strategy
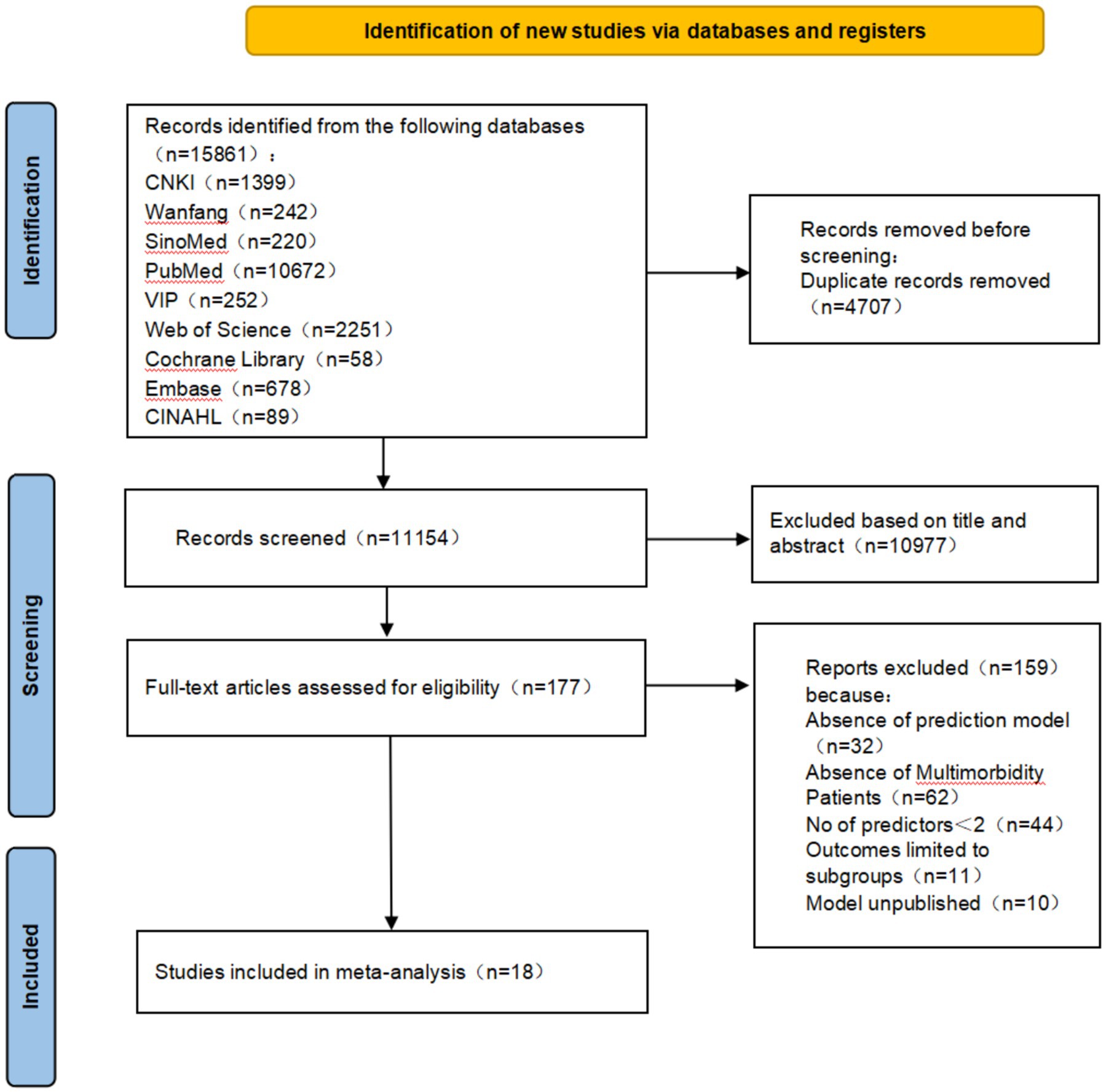
Chinese and English databases were targeted to conduct a comprehensive search, considering the large population size and language universality. The Wanfang, China National Knowledge Infrastructure (CNKI), China Science and Technology Journal (VIP), PubMed, SinoMed, Cochrane Library, Web of Science, Embase and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases were searched from inception until May 30, 2024, using the following keywords: “multimorbidity,” “comorbidity,” “polymorbidity,” “mortality,” “death,” “case fatality rate,” “Risk prediction model,” “Risk factor,” “Predictor,” “Model,” and “Risk Score.” The retrieval method, using PubMed as an example, is shown in Figure 1. Additional relevant studies were identified by reviewing the reference lists of the retrieved studies and review articles.
We used the PICOTS system for the systematic review, which allows framing of the review’s aim, search strategy, and study inclusion and exclusion criteria, as described below.
P (Population): Patients with multimorbidity.
I (Intervention model): Risk prediction models for mortality in patients with multimorbidity that were developed and published (prediction score ≥ 2).
C (Comparator): No competing model.
O (Outcome): The outcome focused on mortality, rather than its subgroups.
T (Timing): The outcome was predicted after evaluating basic information upon admission, clinical scoring scale results, and laboratory indicators.
S (Setting): The intended use of the risk prediction models was to individualize the prediction of mortality risk in patients with multimorbidity, thereby facilitating the implementation of preventive measures for adverse events.
2.3 Inclusion and exclusion criteria
The inclusion criteria for studies were as follows: (1) inclusion of patients with multimorbidity, (2) use of observational study design, (3) report of a prediction model, and (4) death as the outcome of interest.
The exclusion criteria were as follows: (1) no development of a prediction model, (2) outcomes limited to death subgroups, (3) not written in English or Chinese, and (4) full text not retrievable despite contacting authors via email.
2.4 Study selection and screening
Two authors (Chen Yuanyuan and Shang Xuli) independently conducted the screening process. Initially, duplicate studies were removed, and the remaining studies were assessed based on their titles and abstracts to determine eligibility. Following the application of the inclusion and exclusion criteria, the full texts were reviewed, and the reference lists of all eligible studies were examined to identify any potentially relevant studies. In cases of disagreements regarding study selection, a discussion involving three authors (Chen Yuanyuan, Shang Xuli, and Dong Luga) was held to reach a consensus.
2.5 Data extraction
Two reviewers independently screened the search results. The eligibility of the full-text reports was assessed, and any discrepancies were resolved through discussion or by a third reviewer. Data from the articles selected for final inclusion were extracted using the Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modeling Studies (CHARMS) checklist. The information extracted from the selected studies was categorized into two groups: (1) Basic information, including details on the authors, year of publication, research design, participants, outcome indicator, observation time, and mortality; and (2) Model information, including information related to the prediction model, such as model development method, model validation type, variable selection method, predictive factors, model performance, calibration method, and model presentation.
2.6 Quality assessment
The risk of bias and the applicability of the included studies were assessed using the Prediction Model Risk of Bias Assessment Tool (PROBAST) checklist. Two authors (Chen Yuanyuan and Shang Xuli) independently evaluated the presence of bias and concerns regarding the applicability of the studies. The PROBAST checklist is utilized for the critical appraisal of studies on developing, validating, or updating prediction models for individualized predictions. It comprises 20 signaling questions categorized into four domains: participants, predictors, outcomes, and analysis. Each signaling question can be answered as “yes,” “probably yes,” “no,” “probably no,” or “no information.” If at least one signaling question in a domain is answered as “no” or “probably no,” that domain would be considered at high risk of bias. The overall risk of bias is considered low only when all domains are judged to have a low risk of bias.
2.7 Data analysis
Review Manager, MedCalc16.4.3 and R Studio were used for data analysis. Heterogeneity was assessed using the I2 index and Cochrane’s Q test. The I2 index is a measure of heterogeneity, with values of 25, 50, and 75% indicating low, moderate, and high levels of heterogeneity, respectively (25). Random- or fixed-effects models were used based on the heterogeneity of the analysis results. Additionally, Egger’s test was used to identify publication bias, with p-values <0.05 indicating a high likelihood of publication bias (26). A sensitivity analysis was performed using the leave-one-out method.
3 Results
3.1 Study selection
The PRISMA 2020 guideline flowchart is shown in Figure 2. The initial database search yielded 15,861 references. After removing duplicates, 11,154 records remained across all databases. In total, 177 titles and abstracts were screened. Overall, 159 studies were excluded based on the following eligibility criteria: prediction models not established in 32 studies, inconsistent study population in 62, fewer than two predictors in 44, outcomes limited to mortality subgroups in 11, and prediction models not published in 10 studies. Ultimately, 18 studies were included in the analysis.
3.2 Study characteristics
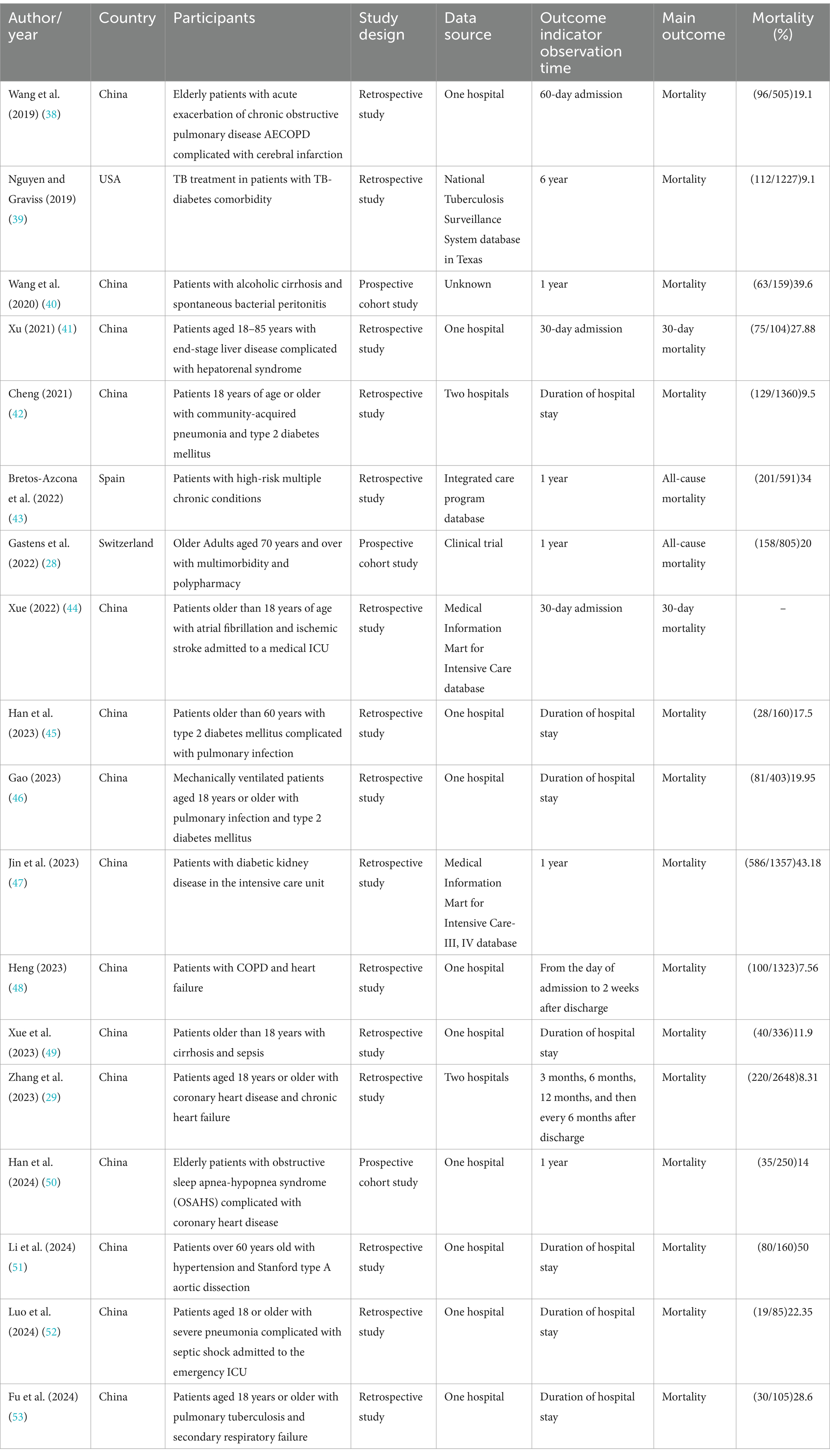
Table 1 shows the characteristics of the 18 included studies. The studies were published between 2019 and 2024. Fifteen studies were conducted in China, one in the United States, one in Spain, and one in Switzerland. In addition, 15 studies were retrospective, and three were prospective. Each study was conducted in a single center. Regarding the study population, two studies focused on multimorbidity, two focused on pulmonary infection and type 2 diabetes mellitus, and the others focused on acute exacerbation of chronic obstructive pulmonary disease complicated with cerebral infarction; tuberculosis diabetes comorbidity; alcoholic cirrhosis with spontaneous bacterial peritonitis; end-stage liver disease complicated with hepatorenal syndrome; community-acquired pneumonia and type 2 diabetes mellitus; atrial fibrillation and ischemic stroke; diabetic kidney disease; chronic obstructive pulmonary disease with heart failure; cirrhosis and sepsis; coronary heart disease and chronic heart failure; obstructive sleep apnea-hypopnea syndrome with coronary heart disease; hypertension and Stanford type A aortic dissection; pulmonary tuberculosis; and secondary respiratory failure. The sample sizes ranged from 85 to 2,648 participants across the studies.
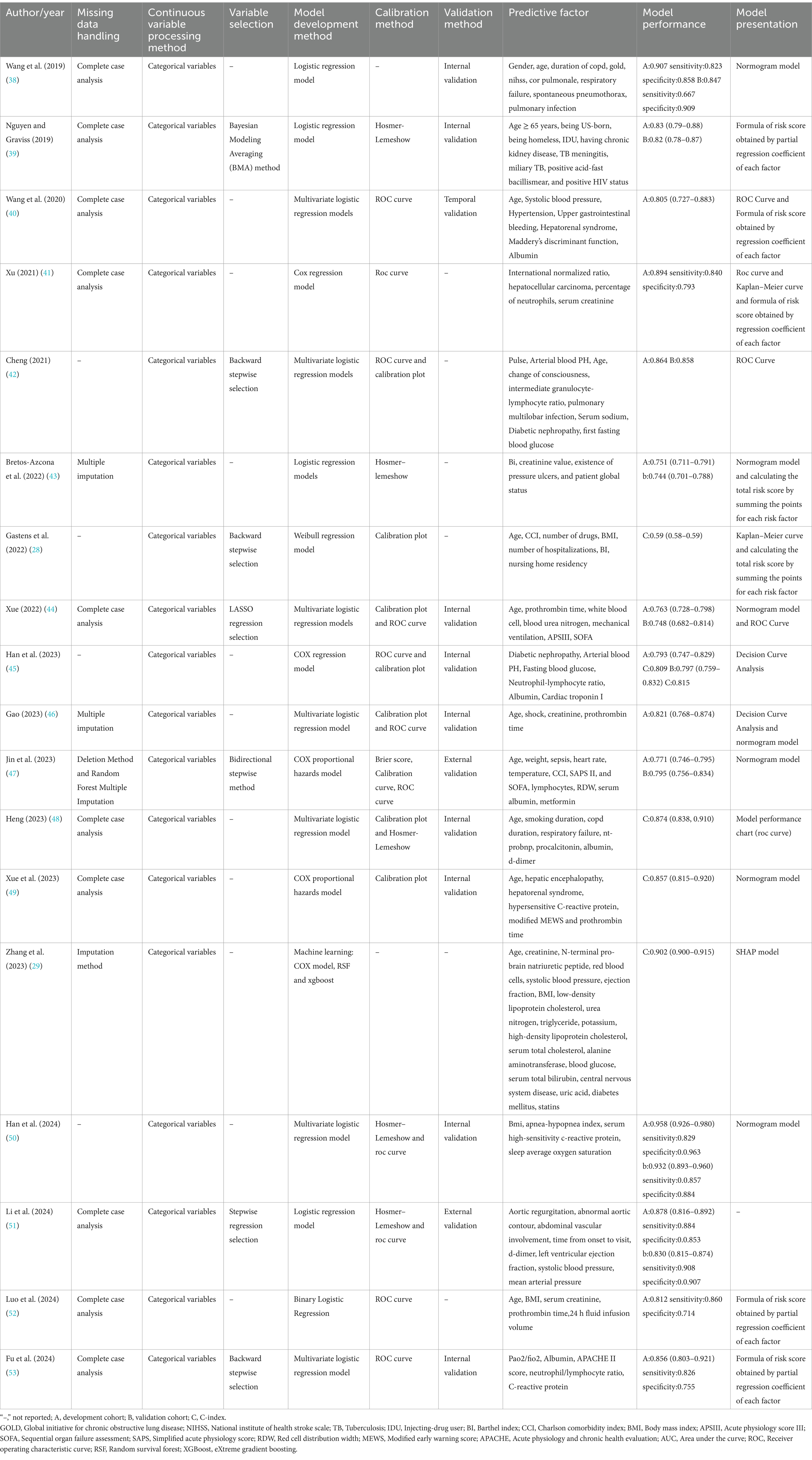
Table 2 presents detailed information on the utilized models in the 18 studies. Logistic regression was used for model development in 12 studies, Cox regression in four, a parametric Weibull regression in one, and machine learning, such as random survival forests and extreme gradient boosting, in one study. The most commonly used predictor was age, utilized in 14 studies. Other frequently used predictors included body mass index (BMI) in five studies and sequential organ failure assessment (SOFA) in three studies. The Charlson Comorbidity Index and Barthel Index were individually used in two studies.
Model performance was reported in all studies. The area under the receiver operating characteristic curve (AUC) or C-statistic values were the most frequently used indices for evaluating discrimination performance, ranging between 0.700 and 0.907. Calibration was performed in nine models, with the Hosmer–Lemeshow test being the most frequently used method.
3.3 Model validation
Most models in the 18 studies were internally validated, 13 of which reported 15 internal validations. Machine learning was used to develop and validate the prediction models.
3.4 Results of quality assessment
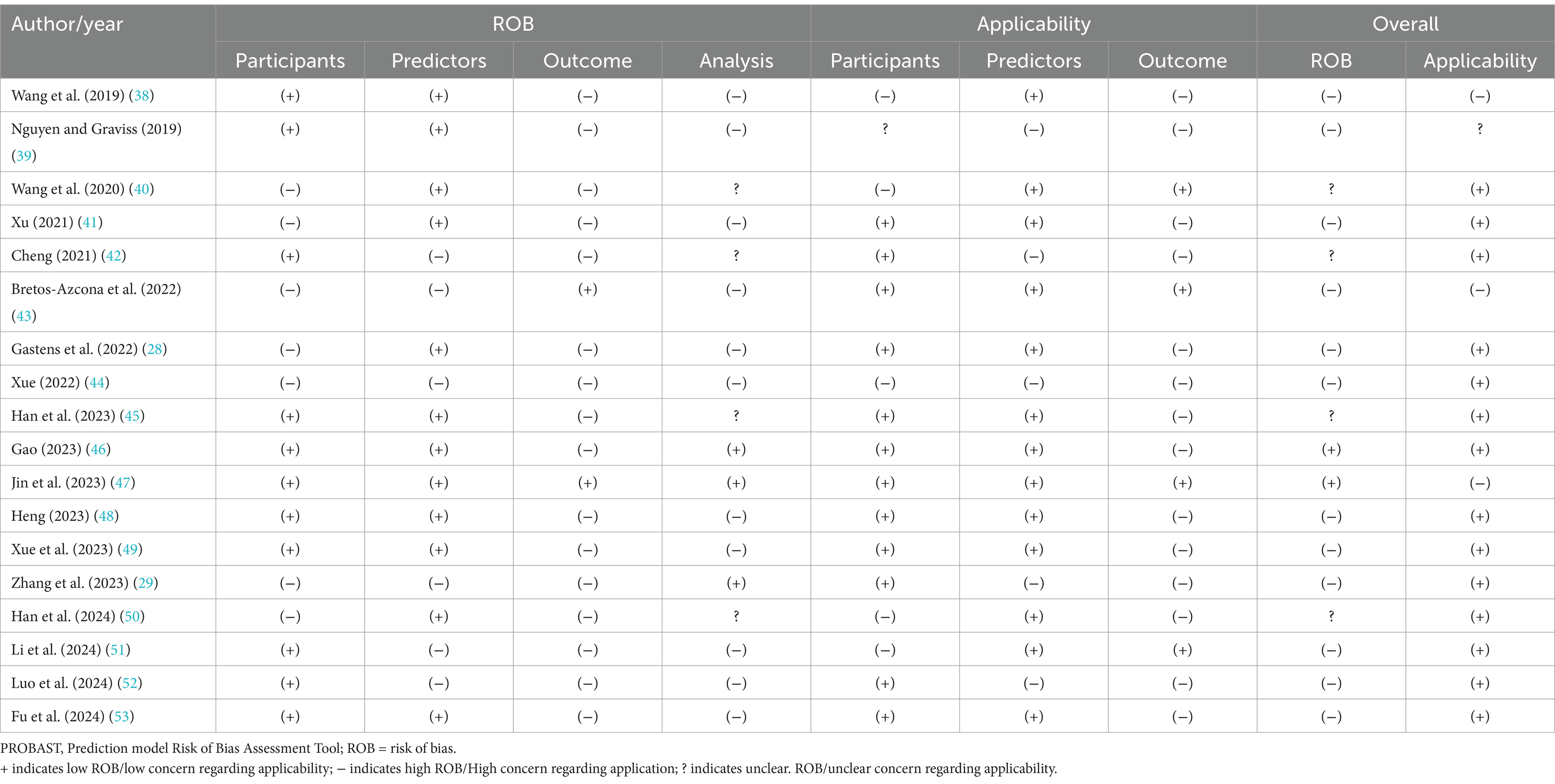
All the studies were evaluated as having a high overall risk of bias according to the PROBAST checklist. The assessments of the risk of bias and applicability for all the studies are summarized in Table 3. Eleven studies were judged as having a high risk of bias, indicating methodological concerns in the models’ development or validation processes.
Six studies primarily had a high risk of bias in the participant domain, mainly attributed to the use of inappropriate data sources. Seven studies were rated as having a high risk of bias in the predictor domain because the outcome data were mainly known before assessments, and the predictors were not similarly defined or assessed for all participants.
Sixteen studies had a high risk of bias in the outcome domain, primarily owing to the lack of a pre-specified or standard outcome, blind determination between the outcome and predictors, and inappropriate time intervals between predictor assessment and outcome determination.
Eleven studies were considered to have a high risk of bias and four studies an unclear risk of bias in the analysis domain. Thirteen studies had insufficient sample sizes, with the participant numbers being <20 events per variable. Ten studies involved partial conversion of continuous variables into categorical variables. Eleven studies did not include all enrolled participants in the analysis, and missing data were inappropriately handled. Sixteen studies did not avoid the selection of predictors based on univariate analysis. Complexities in the data were not accounted for in 13 studies, and this was not reported in four. The predictive performance of the models was not reported in seven studies. Five studies did not comprehensively consider the overfitting, underfitting, and optimism performances of the models, and this was not reported in only one study. Correspondence between the predictors and their assigned weights in the final model and multivariate analysis results were not reported in four studies.
Regarding the assessment of applicability concerns, two studies were rated as having low concerns, 15 as high, and one as unclear. In the participant domain, five studies had high concerns regarding the inclusion of participants aged ≥60 years, and one study had unclear concerns regarding participant inclusion. In the predictor domain, three studies involved predictor assessments based on knowledge of the outcome data. Fourteen studies had high concerns regarding the time interval between predictor assessment and outcome determination.
3.5 Meta-analysis of validation models
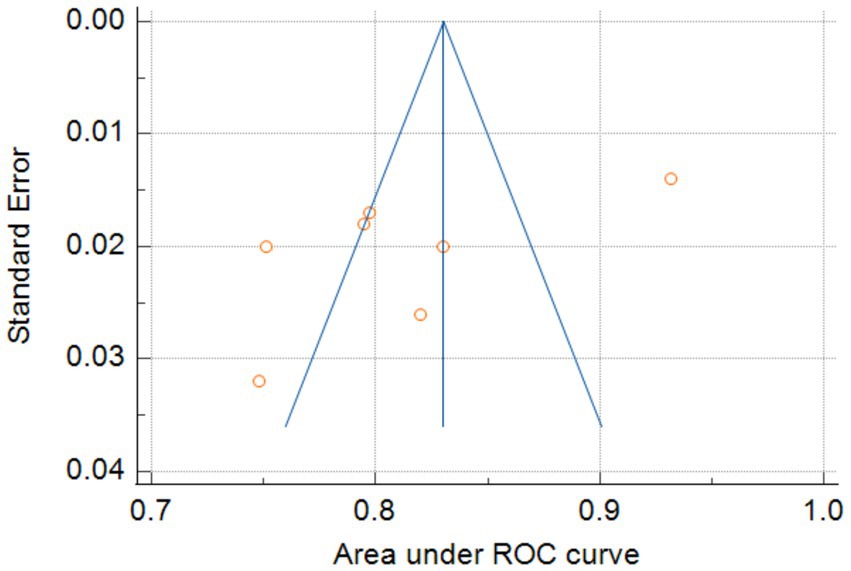
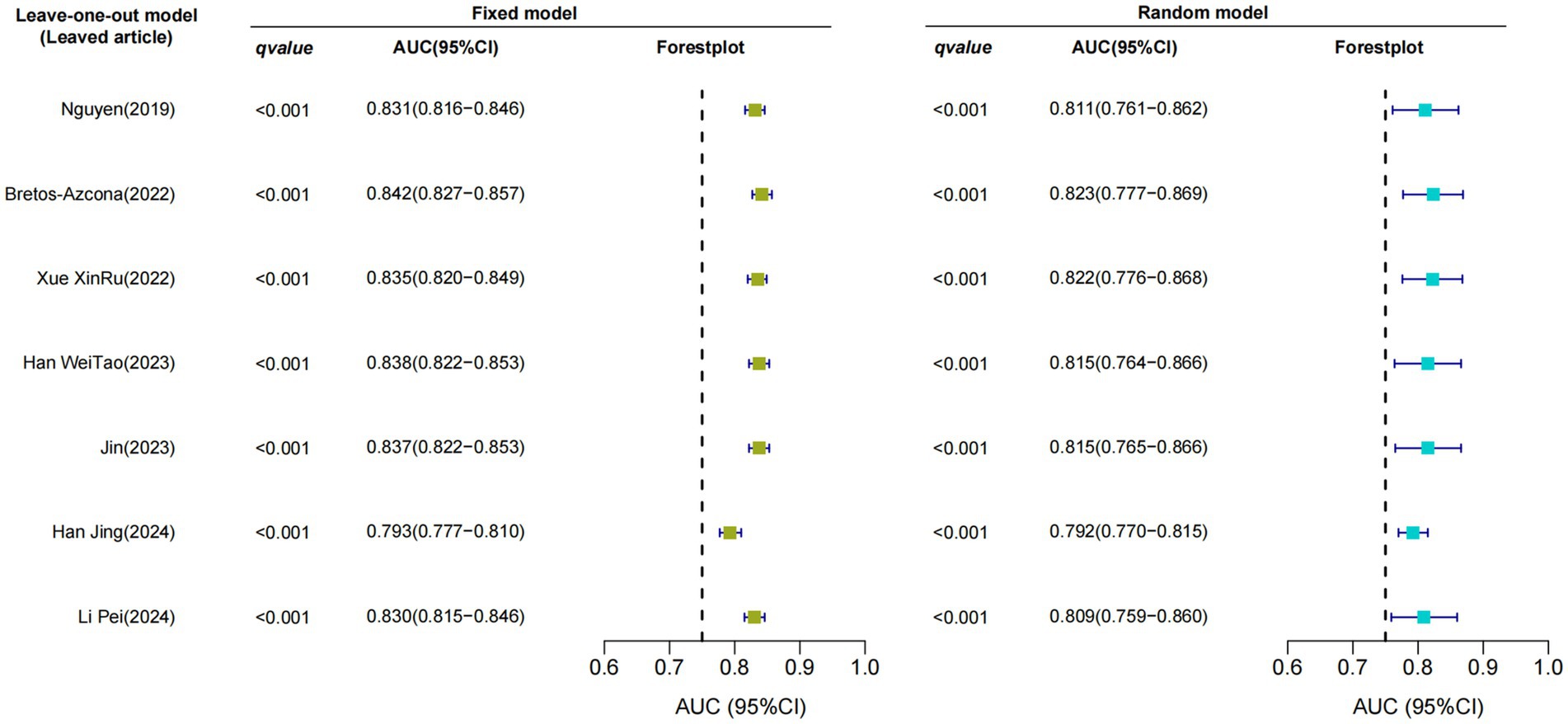
Only seven studies were eligible for synthesis owing to insufficient details on the models. A random-effects model was used to calculate the pooled AUC of seven studies, which resulted in a value of 0.81 (95% confidence interval, 0.77–0.86) (Figure 3). The I2 value was 92.77% (p < 0.001), indicating a high degree of heterogeneity. For models that used mortality, the pooled AUC was 0.81 (95% confidence interval, 0.79–0.83; Figure 4). The I2 value for the models was 0.0% (p = 0.501), indicating a low degree of heterogeneity. The Egger’s test of seven studies showed no significant publication bias, with a value of −9.39 (p = 0.122; Figure 5). The leave-one-out analysis showed relatively stable overall results when each study was sequentially excluded and the meta-analysis results were reconstructed. The results demonstrated good diagnostic value (AUC > 0.75; Figure 6).
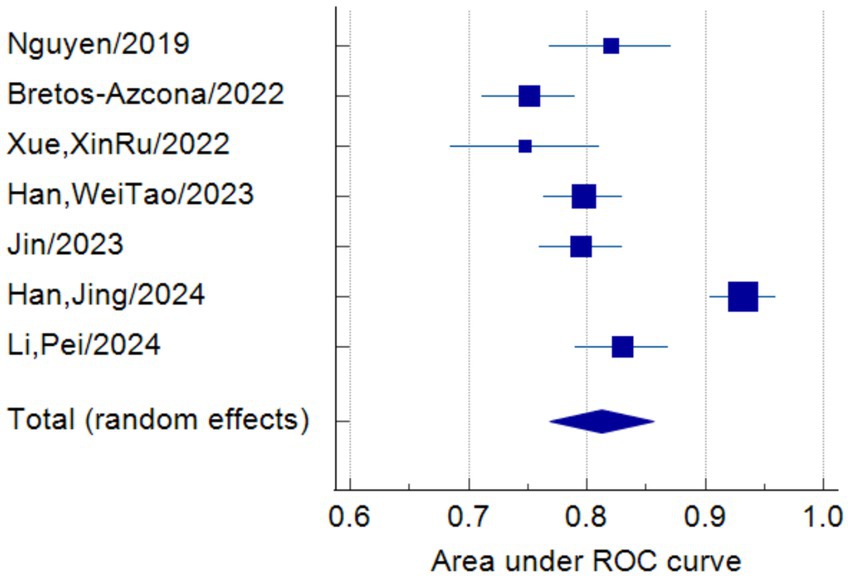
Figure 3. Forest plot of the random effects meta-analysis of pooled AUC estimates for seven validation models.
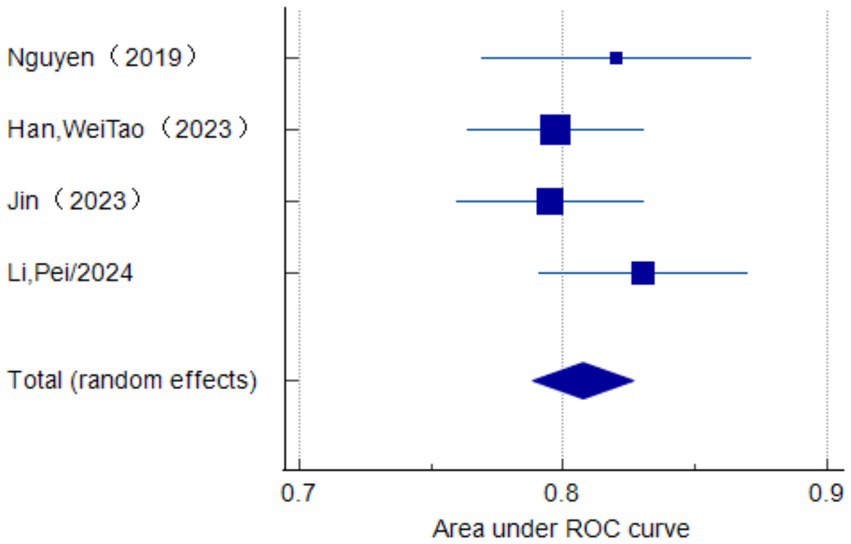
Figure 4. Forest plot of the random effects meta-analysis of pooled AUC estimates for the mortality outcome of four validation models.
4 Discussion
This systematic review revealed an increasing number of risk prediction models for mortality in patients with multimorbidity; however, most models used Chinese patient data. We evaluated the internal research or external validation of models from 18 studies, which demonstrated moderate-to-good predictive performance, with AUC values ranging from 0.70–0.91. However, the risk of bias was judged as high in 11 studies and unknown in four according to the PROBAST checklist, limiting the practical utility of the models. The meta-analysis revealed a pooled AUC value of the seven validated models of 0.81 (95% confidence interval: 0.77–0.86). However, heterogeneity was high in these models, which may be attributed to differences in predictors, population, and methodology. In addition, we observed inadequate reporting in adherence to the Transparent Reporting of a Multivariate Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement in some articles (27) during model evaluation. This opacity introduces doubts and possible prejudice hazards into the models. Future researchers should prioritize the development of novel models that incorporate larger sample sizes, adhere to rigorous research designs, undergo multicenter external validation, and exhibit enhanced reporting transparency.
Valuable insights can be obtained from the development processes of the included models. For example, Gastens et al. (28) used a prospective cohort study design with a large sample size; however, they did not appropriately handle missing data and continuous and categorical predictors, which have often been neglected by other studies. In contrast, Zhang et al. (29) used a large sample size but a retrospective design, resulting in a high risk of bias. The data were sourced from the Electronic Medical Record System, with a degree of bias risk in the participant, predictor, and outcome PROBAST domains. However, their study excelled in the analysis domain. The multiple imputation method was employed to handle missing data, and comprehensive evaluations of model calibration and discrimination were conducted, which were frequently overlooked in other models. Zhang et al. (29) used a machine-learning method during model development. Machine learning methods tend to yield higher accuracy than logistic regression (30). Most included studies had issues with continuous variable treatment, sample size, and predictor selection. Some of these challenges can be addressed by incorporating machine learning into model development. However, a drawback of machine learning lies in the current lack of suitable presentation tools. Therefore, researchers must select a suitable model development method that considers the current circumstances. Generally, all models exhibited moderate or good performance; however, the risk of bias remained high. Improvements regarding the number of events, data source (case–control or cohort study), continuous variable treatment, data complexity, predictor selection method, and model calibration and fitting are required.
The existing predictor models reported in this review had some clinical implications. Initially, predictors with high frequency were demonstrated to possess a certain reference significance for future research. Furthermore, age was consistently identified as an important risk factor for mortality and multimorbidity (31, 32). The incidence of mortality increases for individuals aged ≥80 years, accounting for approximately 82% of mortality cases (33). Therefore, nurses should remain alert regarding mortality risk in patients aged ≥80 years with multimorbidity. BMI was well documented as a predictor in the models (34); however, some studies revealed certain limitations regarding using BMI for diseases such as acute heart failure in hospitalized patients or patients with coronary heart disease after a meal (35, 36). Additionally, if BMI is to be clinically utilized for assessing mortality in patients with multimorbidity, the corresponding disease-adjusted BMI cutoff value requires further exploration. In addition, SOFA is widely used in clinical practice as a disease assessment scale, demonstrating excellent predictive performance in many diseases; however, it does not directly evaluate mortality risk in patients with multimorbidity. Disease severity assessed using SOFA is associated with mortality in patients with multimorbidity (37).
4.1 Limitations
This review had some limitations. First, most included models were from studies conducted in mainland China, which may limit the generalizability of the findings to Western countries. Adjustments may be required when applying the models to different populations. Future researchers should develop risk prediction models for mortality in patients with multimorbidity from diverse populations. Second, a risk-of-bias tool for machine learning models has not yet been published, and the PROBAST tool may be unsuitable for assessing all included models. Third, only studies published in English and Chinese were included, and issues related to language barriers or gray literature may have been omitted. Fourth, model impact studies were not considered. Determining whether a model can be implemented into clinical practice is important and should be investigated in future research. Fourth, only seven validated models from seven studies were included in our study owing to differences in reporting methodology and transparency. This may have resulted in problems related to the sources of study heterogeneity, which could not be discussed further. Sixth, the publication bias test exhibited low statistical power. However, these problems did not impact the evaluation of the models and partly mirrored the identified methodological and reporting problems. Therefore, more transparent and rigorous methodologies are required. Moreover, the comorbid diseases and primary outcomes of the model in each article were not entirely identical, which could potentially affect the results. However, we conducted in-depth sensitivity analyses, and the findings demonstrate that our conclusions are reliable.
4.2 Conclusion
This systematic review revealed a pooled AUC value of seven validated models of 0.81 (95% confidence interval: 0.77–0.86), suggesting a certain level of discrimination. However, according to the PROBAST checklist, 11 included studies had a high risk of bias, and 15 studies raised concerns about their applicability in clinical practice. Most current prediction models for mortality in patients with multimorbidity do not meet the PROBAST standards. Researchers must familiarize themselves with the PROBAST checklist and strictly adhere to the TRIPOD statement to improve the quality of future research. Moreover, they should prioritize the development of new models that incorporate rigorous study designs and multicenter external validation. Our findings provide valuable insights on prediction models for clinical practice and future research, which may contribute to the development of global strategies to address this significant public health challenge.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YC: Writing – original draft. MJ: Writing – review & editing. LJ: Conceptualization, Writing – review & editing. LD: Methodology, Writing – review & editing. MC: Conceptualization, Writing – review & editing. XS: Data curation, Writing – review & editing. XL: Data curation, Writing – review & editing. YH: Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research project was supported by the Lishui City public welfare project (grant number: 2022GYX64).
Acknowledgments
We thank Jiaxing Li and Yuanliang Gu for their valuable assistance with the statistical analysis during the revision of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ho, ISS, Azcoaga-Lorenzo, A, Akbari, A, Black, C, Davies, J, Hodgins, P, et al. Examining variation in the measurement of multimorbidity in research: a systematic review of 566 studies. Lancet Public Health. (2021) 6:e587–97. doi: 10.1016/S2468-2667(21)00107-9
2. Zöller, B, and Connors, JM. Multimorbidity, comorbidity, frailty, and venous thromboembolism. Haematologica. (2024) 109:3852–9. doi: 10.3324/haematol.2023.284579
3. Harrison, C, Fortin, M, van den Akker, M, Mair, F, Calderon-Larranaga, A, Boland, F, et al. Comorbidity versus multimorbidity: why it matters. UK: London: SAGE Publications Sage (2021).
4. Xu, X, Mishra, GD, and Jones, M. Evidence on multimorbidity from definition to intervention: an overview of systematic reviews. Ageing Res Rev. (2017) 37:53–68. doi: 10.1016/j.arr.2017.05.003
5. Chowdhury, SR, Chandra Das, D, Sunna, TC, Beyene, J, and Hossain, A. Global and regional prevalence of multimorbidity in the adult population in community settings: a systematic review and meta-analysis. EClinicalMedicine. (2023) 57:101860. doi: 10.1016/j.eclinm.2023.101860
6. Marengoni, A, Angleman, S, Melis, R, Mangialasche, F, Karp, A, Garmen, A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. (2011) 10:430–9. doi: 10.1016/j.arr.2011.03.003
7. Zhou, Y, Dai, X, Ni, Y, Zeng, Q, Cheng, Y, Carrillo-Larco, RM, et al. Interventions and management on multimorbidity: an overview of systematic reviews. Ageing Res Rev. (2023) 87:101901. doi: 10.1016/j.arr.2023.101901
8. Department of Health UK (2015). Available online at: https://www.gov.uk/government/publications/living-well-for-longer-progress-1-year-on (accessed March 18, 2019)
9. Menotti, A, Mulder, I, Nissinen, A, Giampaoli, S, Feskens, EJ, and Kromhout, D. Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: the FINE study (Finland, Italy, Netherlands, elderly). J Clin Epidemiol. (2001) 54:680–6. doi: 10.1016/S0895-4356(00)00368-1
10. Jani, BD, Hanlon, P, Nicholl, BI, McQueenie, R, Gallacher, KI, Lee, D, et al. Relationship between multimorbidity, demographic factors and mortality: findings from the UK biobank cohort. BMC Med. (2019) 17:74. doi: 10.1186/s12916-019-1305-x
11. Ryan, BL, Allen, B, Zwarenstein, M, Stewart, M, Glazier, RH, Fortin, M, et al. Multimorbidity and mortality in Ontario, Canada: a population-based retrospective cohort study. J Comorb. (2020) 10:2235042X20950598. doi: 10.1177/2235042X20950598
12. Nunes, BP, Flores, TR, Mielke, GI, Thumé, E, and Facchini, LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. (2016) 67:130–8. doi: 10.1016/j.archger.2016.07.008
13. Brown, R, and Thorsteinsson, E. Comorbidity: what is it and why is it important? In: R Brown and E Thorsteinsson, editors. Comorbidity. Cham: Palgrave Macmillan (2020)
14. Takeuchi, S, Kohno, T, Goda, A, Shiraishi, Y, Kawana, M, Saji, M, et al. Multimorbidity, guideline-directed medical therapies, and associated outcomes among hospitalized heart failure patients. ESC Heart Fail. (2022) 9:2500–10. doi: 10.1002/ehf2.13954
15. Muth, C, and Glasziou, PP. Guideline recommended treatments in complex patients with multimorbidity. BMJ. (2015) 351:h5145. doi: 10.1136/bmj.h5145
16. Schneider, C, Aubert, CE, Del Giovane, C, Donzé, JD, Gastens, V, Bauer, DC, et al. Comparison of 6 mortality risk scores for prediction of 1-year mortality risk in older adults with multimorbidity. JAMA Netw Open. (2022) 5:e2223911. doi: 10.1001/jamanetworkopen.2022.23911
17. Damen, JAA, Moons, KGM, van Smeden, M, and Hooft, L. How to conduct a systematic review and meta-analysis of prognostic model studies. Clin Microbiol Infect. (2023) 29:434–40. doi: 10.1016/j.cmi.2022.07.019
18. Xu, Z, Zhang, J, Zhang, Q, Xuan, Q, and Yip, P. A comorbidity knowledge-aware model for disease prognostic prediction. IEEE Trans Cybern. (2021) 52:9809–19. doi: 10.1109/TCYB.2021.3070227
19. Bao, Y, Lu, P, Wang, M, Zhang, X, Song, A, Gu, X, et al. Exploring multimorbidity profiles in middle-aged inpatients: a network-based comparative study of China and the United Kingdom. BMC Med. (2023) 21:495. doi: 10.1186/s12916-023-03204-y
20. Fan, J, Sun, Z, Yu, C, Guo, Y, Pei, P, Yang, L, et al. Multimorbidity patterns and association with mortality in 0.5 million Chinese adults. Chin Med J. (2022) 135:648–57. doi: 10.1097/CM9.0000000000001985
21. Li, FR, Wang, S, Li, X, Cheng, ZY, Jin, C, Mo, CB, et al. Multimorbidity and mortality among older patients with coronary heart disease in Shenzhen. China J Geriatr Cardiol. (2024) 21:81–9. doi: 10.26599/1671-5411.2024.01.005
22. Singh, M, Gulati, R, Lewis, BR, Zhou, Z, Alkhouli, M, Friedman, P, et al. Multimorbidity and mortality models to predict complications following percutaneous coronary interventions. Circ Cardiovasc Interv. (2022) 15:e011540. doi: 10.1161/CIRCINTERVENTIONS.121.011540
23. Kato, D, Kawachi, I, Saito, J, and Kondo, N. Complex multimorbidity and mortality in Japan: a prospective propensity-matched cohort study. BMJ Open. (2021) 11:e046749. doi: 10.1136/bmjopen-2020-046749
24. Anthonimuthu, DJ, Hejlesen, O, Zwisler, AO, and Udsen, FW. Application of machine learning in multimorbidity research: protocol for a scoping review. JMIR Res Protoc. (2024) 13:e53761. doi: 10.2196/53761
25. Higgins, JPT, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
26. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
27. Collins, GS, Reitsma, JB, Altman, DG, and Moons, KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. (2015) 350:g7594. doi: 10.1136/bmj.g7594
28. Gastens, V, Chiolero, A, Anker, D, Schneider, C, Feller, M, Bauer, DC, et al. Development and validation of a new prognostic index for mortality risk in multimorbid adults. PLoS One. (2022) 17:e0271923. doi: 10.1371/journal.pone.0271923
29. Zhang, WC, Tian, J, Yang, H, Han, QH, and Zhang, YB. 5-year all-cause mortality survival analysis and interpretable study in patients with coronary artery disease combined with chronic heart failure. Chin J Dis Control Prev. (2023) 27:391. doi: 10.16462/j.cnki.zhjbkz.2023.04.001
30. Churpek, MM, Yuen, TC, Winslow, C, Meltzer, DO, Kattan, MW, and Edelson, DP. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. (2016) 44:368–74. doi: 10.1097/CCM.0000000000001571
31. Svensson, T, Saito, E, Svensson, AK, Melander, O, Orho-Melander, M, Mimura, M, et al. Association of sleep duration with all- and major-cause mortality among adults in Japan, China, Singapore, and Korea. JAMA Netw Open. (2021) 4:e2122837. doi: 10.1001/jamanetworkopen.2021.22837
32. Hanlon, P, Nicholl, BI, Jani, BD, Lee, D, McQueenie, R, and Mair, FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK biobank participants. Lancet Public Health. (2018) 3:e323–32. doi: 10.1016/S2468-2667(18)30091-4
33. Su, Z, Huang, L, Zhu, J, and Cui, S. Effects of multimorbidity coexistence on the risk of mortality in the older adult population in China. Front Public Health. (2023) 11:1110876. doi: 10.3389/fpubh.2023.1110876
34. Hussin, NM, Shahar, S, Din, NC, Singh, DKA, Chin, AV, Razali, R, et al. Incidence and predictors of multimorbidity among a multiethnic population in Malaysia: a community-based longitudinal study. Aging Clin Exp Res. (2019) 31:215–24. doi: 10.1007/s40520-018-1007-9
35. Yoshihisa, A, Sato, T, Kajimoto, K, Sato, N, and Takeishi, Y. Acute decompensated heart failure syndromes (ATTEND) investigators. Heterogeneous impact of body mass index on in-hospital mortality in acute heart failure syndromes: an analysis from the ATTEND registry. Eur Heart J Acute Cardiovasc Care. (2019) 8:589–98. doi: 10.1177/2048872617703061
36. Zhang, YN, Pang, YX, Chen, TL, Gu, GQ, Xie, RQ, and Cui, W. The clinical observation of postprandial hypotension in patients with obesity or overweight and coronary heart disease. J Hebei Med Univ. (2019) 40:144–8. doi: 10.3969/j.issn.1007-3205.2019.02.006
37. Qin, W, Zhang, X, Yang, L, Li, Y, Yang, S, Li, X, et al. Predictive value of the sequential organ failure assessment (SOFA) score for prognosis in patients with severe acute ischemic stroke: a retrospective study. J Int Med Res. (2020) 48:300060520950103. doi: 10.1177/0300060520950103
38. Wang, WJ, Huang, ZD, Li, GY, and Luo, YJ. Establishment of a nomogram model for short-term mortality risk in elderly patients with acute exacerbation of COPD complicated by cerebral infarction. J Pract Med. (2019) 35:2128–32. doi: 10.3969/j.issn.1006-5725.2019.13.022
39. Nguyen, DT, and Graviss, EA. Development and validation of a risk score to predict mortality during TB treatment in patients with TB-diabetes comorbidity. BMC Infect Dis. (2019) 19:10. doi: 10.1186/s12879-018-3632-5
40. Wang, RR, Jiang, YY, Wang, XB, and Yang, JX. A prognostic model for long-term survival in alcoholic liver cirrhosis patients complicated with spontaneous bacterial peritonitis. Hepatoday. (2020) 25:1042. doi: 10.3969/j.issn.1008-1704.2020.10.007
41. Xu, T. Establishment of death risk model for patients with hepatorenal syndrome in end-stage liver disease and relationship between TCM syndromes and laboratory indexes. Chengdu, China: Chengdu Univ TCM (2021).
42. Cheng, SJ. Risk factors analysis and prediction models establishment of in-hospital mortality among community-acquired pneumonia patients with type 2 diabetes mellitus. Liaoning, China: China Med. University (2021).
43. Bretos-Azcona, PE, Ibarrola Guillén, C, Sánchez-Iriso, E, Cabasés Hita, JM, Gorricho, J, and Librero, LJ. Multisystem chronic illness prognostication in non-oncologic integrated care. BMJ Support Palliat Care. (2022) 12:e112–9. doi: 10.1136/bmjspcare-2019-002055
44. Xue, XR. Development of a 30-day mortality risk prediction model in ischemic stroke patients with atrial fibrillation. Chongqing, China: Chongqing Med. University (2022).
45. Han, WT, Feng, M, Yang, QM, Hu, H, and Xu, RH. Prognostic risk factors for elderly patients with T2DM and pulmonary infection. P Prev Med. (2023) 30:1373–7. doi: 10.3969/j.issn.1006-3110.2023.11.023
46. Gao, JX. Risk factors and prediction models for in-hospital death in patients with pulmonary infections and type 2 diabetes requiring mechanical ventilation. Beijing, China: Peking Union Med. College (2023).
47. Jin, W, Jin, H, Su, X, Che, M, Wang, Q, Gu, L, et al. Development and validation of the prediction model for mortality in patients with diabetic kidney disease in intensive care unit: a study based on medical information Mart for intensive care. Ren Fail. (2023) 45:2257808. doi: 10.1080/0886022X.2023.2257808
48. Heng, X. Nomogram modeling of short-term mortality risk in patients with COPD and heart failure comorbidity. Luzhou, China: Southwest Med Universidad (2023).
49. Xue, LL, Li, BH, Liu, CY, Li, WK, Chang, LX, and Liu, L. Establishment and evaluation of a multivariate cox proportional hazards prediction model for mortality during short-term hospitalization in patients with liver cirrhosis and sepsis. J Clin Hepatol. (2023) 39:1089–97. doi: 10.3969/j.issn.1001-5256.2023.05.014
50. Han, J, Zhu, W, and Jiang, F. Construction of nomogram prediction model for poor prognosis of elderly patients with obstructive sleep apnea hypopnea syndrome and coronary heart disease based on multicenter. J Clin Exp Med. (2024) 23:462–5. doi: 10.3969/j.issn.1671-4695.2024.05.004
51. Li, P, Xue, WT, and Zhao, P. Relationship between postoperative blood pressure control level and in hospital death in elderly patients with hypertension complicated with Stanford type a aortic dissection. Chin Heart J. (2024) 36:294–301. doi: 10.12125/j.chj.202308002
52. Luo, SP, Liu, DX, Wei, ZJ, Dong, WX, Liu, SL, Qu, HR, et al. Multivariate analysis and risk model of ICU death in patients with severe pneumonia and septic shock. J Clin Intern Med. (2024) 41:104–6. doi: 10.3969/j.issn.1001-9057.2024.02.009
Keywords: mortality, risk prediction model, systematic review, meta-analysis, multimorbidity
Citation: Chen YY, Ji MF, Jin LH, Dong LG, Chen MH, Shang XL, Lan X and He YY (2025) Risk prediction models for mortality in patients with multimorbidity: a systematic review and meta-analysis. Front. Public Health. 13:1505541. doi: 10.3389/fpubh.2025.1505541
Edited by:
Priyank Shukla, Ulster University, United KingdomReviewed by:
Rhonda Frances Brown, Australian National University, AustraliaBodhayan Prasad, University of Glasgow, United Kingdom
Copyright © 2025 Chen, Ji, Jin, Dong, Chen, Shang, Lan and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-hong Jin, NjI0ODczMjEyQHFxLmNvbQ==
†These authors share first authorship
 Yuan-yuan Chen
Yuan-yuan Chen Mei-fen Ji2†
Mei-fen Ji2† Yuan-yuan He
Yuan-yuan He