- 1Department of Internal Medicine, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 2Department of Pediatrics and Child Health Nursing, School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 3Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 4Department of Health Informatics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Introduction: Anemia is a critical global public health issue, especially among women of reproductive age (15–49 years) in low- and middle-income countries. Mozambique has the highest prevalence of anemia in women of reproductive age in Sub Saharan Africa in 2019. This study aims to assess the spatial variation and predictors of anemia among women of reproductive age in Mozambique.
Methods: Individual record and spatial coordinates data from the Mozambique Demographic and Health Survey (DHS 2022/23) were used. A stratified two-stage cluster sampling method was applied. Global autocorrelation analysis was performed to determine clustering of anemia. A weighted sample of 5,907 women of reproductive age was analyzed using ArcGIS 10.7. Multiscale geographically weighted regression was employed to identify predictors of anemia.
Results: The national prevalence of anemia among women of reproductive age in Mozambique was 51.89% (95% CI: 50.66, 53.12%). Higher rates were observed in Nampula, Zambezia, and Sofala. Unimproved drinking water, pregnancy, and being underweight were positively correlated with anemia, while contraceptive use and obesity were negatively correlated. Geographic variability in these associations was evident (Global Moran’s I = 0.0.444359 and p < 0.001). Anemia was the highest in Tete and Manica due to unimproved drinking water the effect of pregnancy on anemia was significant in Manica and Sofala. Being underweight was strongly related to anemia in Niassa. Conversely, contraceptive use and obesity had a protective effect in Nampula, Zambezia, Niassa, and Cabo Delgado.
Conclusion: Anemia remains a critical public health issue among women of reproductive age in Mozambique, with marked regional disparities. Hotspot clusters were identified in Nampula, Zambezia, Tete, and Sofala. Factors such as unimproved drinking water, pregnancy, and being underweight were associated with higher anemia rates in certain regions, while obesity and contraceptive use indicated a protective effect in specific provinces. To effectively combat anemia, policymakers should focus on improving access to clean water and maternal health services, and enhancing nutritional support through the USAID Advancing Nutrition Project, the Global Alliance for Improved Nutrition, Integrated Community Case Management, and Supervised Weekly Iron and Folic Acid Supplementation.
Introduction
Anemia, a condition characterized by a reduced concentration of hemoglobin in the blood (hemoglobin level < 12 g/dL and < 11 g/dL for non-pregnant and pregnant women respectively), is a global public health concern, particularly among women of reproductive age (WRA) in low- and middle-income countries (LMICs) (1). Anemia in WRA is associated with increased risks of maternal mortality, preterm birth, low birth weight, and impaired cognitive development in children (2, 3). According to the Ministry of Health (MISAU), improving nutritional support and addressing anemia among women is a priority in the National Health Policy and Strategic Plan 2022–2026 (4).
Worldwide, the prevalence of anemia among WRA is estimated to be around 30%, with the highest burden observed in Africa and Asia (1). In Mozambique, a country in Southeast Africa, anemia is a major nutritional deficiency problem, with an estimated prevalence of 55% among WRA as of 2019 (5). The burden of anemia is influenced by a variety of factors, with iron deficiency being the main cause (6). However, other micronutrient deficiencies (such as vitamin A, vitamin B12, and folate), chronic bleeding, acute or chronic infections, and parasitic infections (like hookworm and malaria) can also contribute to the development of anemia (7–9).
Estimates suggest that about half of anemia cases in low- and middle-income countries are attributable to iron deficiency, while the remaining cases may be due to diseases like parasitic infections, malaria, and HIV (2). A systematic review revealed that the proportion of anemia caused by iron deficiency was below 50% in LMICs, with regional variations, poor sanitary conditions, and increased occurrence of infections also contributing to the burden of anemia (10).
Spatial variation in anemia prevalence among women of reproductive age was observed in studies conducted in Ethiopia (11), Rwanda (12), and Nigeria (13). Understanding the spatial distribution and determinants of anemia in Mozambique is essential for developing targeted intervention strategies and allocating resources effectively. Previous studies have identified various factors associated with anemia in the country, including socioeconomic status, education level, access to healthcare, nutritional intake, and the burden of infectious diseases (14, 15). However, the geospatial patterns and clustering of anemia in WRA have not been comprehensively examined.
The Sustainable Development Goals (SDGs), specifically Goal 2: Zero Hunger, aim to end all forms of malnutrition, including anemia, by 2030 (16). The target is to reduce anemia among women of reproductive age by 50% by 2030 (16).
This study aimed to investigate the spatial distribution of anemia prevalence and identify the key determinant factors among WRA in Mozambique. By exploring the geographic distribution and analyzing the associated risk factors, the findings from this research can inform the development and implementation of tailored public health strategies to address anemia in this vulnerable population.
Methods
Study setting
Mozambique is situated on the eastern coast of Southern Africa, facing Madagascar. It boasts approximately 2,800 km of coastline and covers an area of around 800,000 km2. Moving southward to Beira, the country consists of a large plain that lies 200–500 meters above sea level. To the west, the high plateaus of Zimbabwe, Swaziland, Natal, and Transvaal dominate the landscape. The central region features a high plateau nestled between Zambia and Zimbabwe along the Zambezi River. As we move eastward, Mozambique gradually descends toward the Indian Ocean, situated to the east of Lake Malawi. The country is divided into 3 regions (North, Center and South), 11 provinces and 148 districts (as shown in Figure 1). Maputo, the capital, is located in the southernmost part of the country. With a population estimated at slightly over 20 million, Mozambique experiences an annual growth rate of 2.5 percent. Agriculture plays a pivotal role in the country’s economy, employing more than 80 percent of the labor force and providing livelihoods for the majority of the population (17). Mozambique has a population density of 44 people per square kilometer (114 people per square mile). The current population of Mozambique is 35,063,881. The country’s total land area is 786,380 square kilometers (303,623 square miles). Approximately 41% of the population lives in urban areas (18). The north-central provinces of Nampula and Zambezia are the most populous regions in Mozambique. These provinces account for 45% of the total population. The largest city and capital, Maputo, has a population of around 1.2 million people (19).
Figure 1. Graphical description of the study setting; Shape file source: https://data.humdata.org/dataset/5e8d83a5-1210-49be-b7d9-cf286dbc15df.
Study design and sample size
We conducted a cross-sectional study using data from the 2022/23 Mozambique Demographic Health Survey (DHS). The standard sampling technique used by DHS including the Mozambique 2022/23 involves a stratified two-stage cluster sampling approach to guarantee nationally representative data. First, a country is categorized into various strata based on relevant characteristics, such as urban or rural location and geographic regions. Within each stratum, enumeration areas (clusters) are randomly chosen as the primary sampling units, with these clusters typically containing several households. In the second stage, a sample of households is drawn from each selected enumeration area using either a systematic or random sampling method. This study focused on anemia among reproductive-age women within the 5 years preceding the survey. A total weighted sample of 5,907 reproductive age women. To analyze the data, we utilized individual recode (IR) data extracted from the Mozambique DHS 2022/23 data set (20). This can be accessed through: https://dhsprogram.com/data/dataset_admin/index.cfm.
Variables of the study
The dependent variable of interest was anemia among women (15–49 years) in Mozambique. Anemia status was determined based on hemoglobin measurements collected as part of the 2022–23 Demographic and Health Survey (DHS) in Mozambique. As per the guide to DHS Statistics-8, individual non-pregnant women with a hemoglobin level of below 12 g/dL and pregnant women with hemoglobin level of less than 11 g/dL were classified as anemic and coded as “1,” otherwise non-anemic and coded as “0.”
Drawing from an extensive literature review, we examined several factors that may influence anemia in this population (21–27). These factors included maternal education, place of residence, maternal body mass index (BMI), maternal age, paternal education, marital status, maternal employment, use of antenatal care services, wealth status, family size, distance to health facilities, use of mosquito bed nets during sleep, media exposure, smoking status, type of toilet facility, source of drinking water, pregnancy status, use of contraceptive methods, breastfeeding status, sex of the household head, and parity (Table 1).
Data management
The data were processed and cleaned using STATA version 17. This involved editing, verifying, and recoding the raw data as necessary (28). To account for the complex sampling design of the Mozambique Demographic and Health Survey (DHS), we generated a weighting variable. All subsequent analyses were conducted using the weighted data (29). This allowed us to restore the representativeness of the survey sample and obtain reliable statistical estimates. To explore the distribution of the dependent variable (anemia) and the independent variables, we generated cross-tabulations with the cluster variable (v001) and saved the results as comma-separated values (CSV) file in Excel. Then, we imported the data from Excel into ArcGIS 10.7 and fitted an ordinary least squares (OLS) regression model to further examine the spatial patterns and associations. The use of appropriate weighting and accounting for the complex survey design ensures the validity and generalizability of the study findings.
Management of missing values
Women who were not tested, as well as those whose values were not recorded, were excluded from both the denominators and numerators.
Statistical analysis
Spatial autocorrelation
The software Arc GIS version 10.7 was utilized to map model parameters between local models and look for spatial variance. Mozambique’s anemic distribution was determined by calculating the global spatial autocorrelation, also known as global Moran’s I (30, 31). A spatial statistic called Global Moran’s I uses the complete dataset to generate a single output value between −1 and +1. This allows for the measurement of spatial autocorrelation. A closer distance from −1 to Moran’s output suggests that the event of interest is scattered, whereas a closer distance from +1 suggests clustering, and a closer distance from 0 suggests a random pattern. The distribution of anemia among women of reproductive age is nonrandom, either clustered or scattered, according to a statistically significant Moran’s I (p < 0.05) (32).
Spatial interpolation
Based on measured values from the neighborhood, the standard Kriging method of spatial interpolation was used to forecast the percentage of anemia in un-sampled places. The Kriging method was preferred above other interpolation methods because an ideal interpolator that provides a minimum mean error (ME) and root mean square error (RMSE) is kriging interpolation (33).
Spatial scan statistical analysis
Bernoulli and merely spatial statistical analysis of Kulldorff’s scan were applied. Using SaTScanTM version 10.1.3 software, only regions with a high risk of prevalence were used to identify the geographic locations of statistically significant clusters of anemia. Because the data is binary (anemic or non-anemic), the Bernoulli model was applied (34). Anemia was classified as case (1) and its absence as non-case (0). To locate the important clusters, the coordinate (latitude and longitude) file, case file (1), and non-case file (0) were imported into the SaTScanTM program. A scale was used to determine the maximum scanning window size based on the proportion of the entire population at danger. The maximum size of a geographic cluster was set at 50% of the population at risk in order to account for both very tiny and very big clusters. Clusters that were most likely (primary) were found by means of the likelihood ratio and p-value tests. The most likely cluster is the one with the highest likelihood ratio (35).
Factor analysis
The Ordinary Least Squares Model (OLS) is a global regression model that assumes the homogeneity of each variable’s coefficients throughout the study region and estimates the connection between the dependent and independent variables using a single equation (36). The initial step in selecting the suitable predictor variables for the geographical variation of anemia is to use the OLS model (37). Verification that the anemia does not have a stationary percentage is required before fitting the global and local regression models. Global spatial autocorrelation was used to determine the spatial no stationary. Global geographic regression modeling was then calibrated to find variables related to the percentage of anemia.
Before moving on to the local model, the six assumptions of the OLS model (that is, that the explanatory variables should have the relationship that we expected, that each explanatory variable is significant, that the residuals are random, that the Jarque-Bera statistics have no statistical significance, the VIF value, and the strength of R-square) were verified (38). The Variance Inflation Factor (VIF) values were used to evaluate the multicollinearity. Predictors with VIF values higher than 7.5, or the cut point to indicate that multicollinearity is present, were not seen in this set of data (28).
After verifying the assumptions of the OLS model, we employed the local model, Geographically Weighted Regression (GWR), to analyze spatially varying relationships at 95% confidence interval. GWR assumes that the relationships between variables change across space (39). We utilized the latest version of GWR, known as Multiscale Geographically Weighted Regression (MGWR) version 2.2.1 software. Unlike classic GWR, which assumes that all processes occur on the same spatial scale, MGWR allows for different processes to operate at varying scales (40). This advanced version avoids the single bandwidth assumption for all covariates, instead permitting covariate-specific bandwidths (40). Additionally, between local regression (MGWR) and global regression (OLS), corrected Akaike Information Criteria (AICc) and Adjusted R2 were used as model selection criteria. As the best fitted model, the one with the lowest AICc and the highest Adjusted R2 was chosen (37).
Ethical consideration
Registration was completed and a permission letter was obtained in order to access the Mozambique 2022/23 DHS Dataset and Global Positioning System (GPS) data through the DHS measure website, http://www.dhsprogram.com/. As a result, all required information was obtained from the website of the Demographic and Health Surveys (DHS) Program. However, participant permission was not necessary since the authors used a supplementary dataset from the Mozambique DHS for 202/23.
Results
Characteristics of the study participants
In our study, a total weighted sample of 5,907 reproductive-age women with measured hemoglobin levels was included. Anemia was observed in 54.4% of women aged 15–19 years and 52.2% of women aged 35–49 years. Additionally, 54.5% of women residing in rural areas had anemia. The majority of women who were pregnant (60.6%), underweight (59.1%), poor (57.8%), or using unimproved sources of drinking water (59.6%) experienced anemia. Slightly more than half of the women with unimproved toilet facilities also had anemia (Table 2).
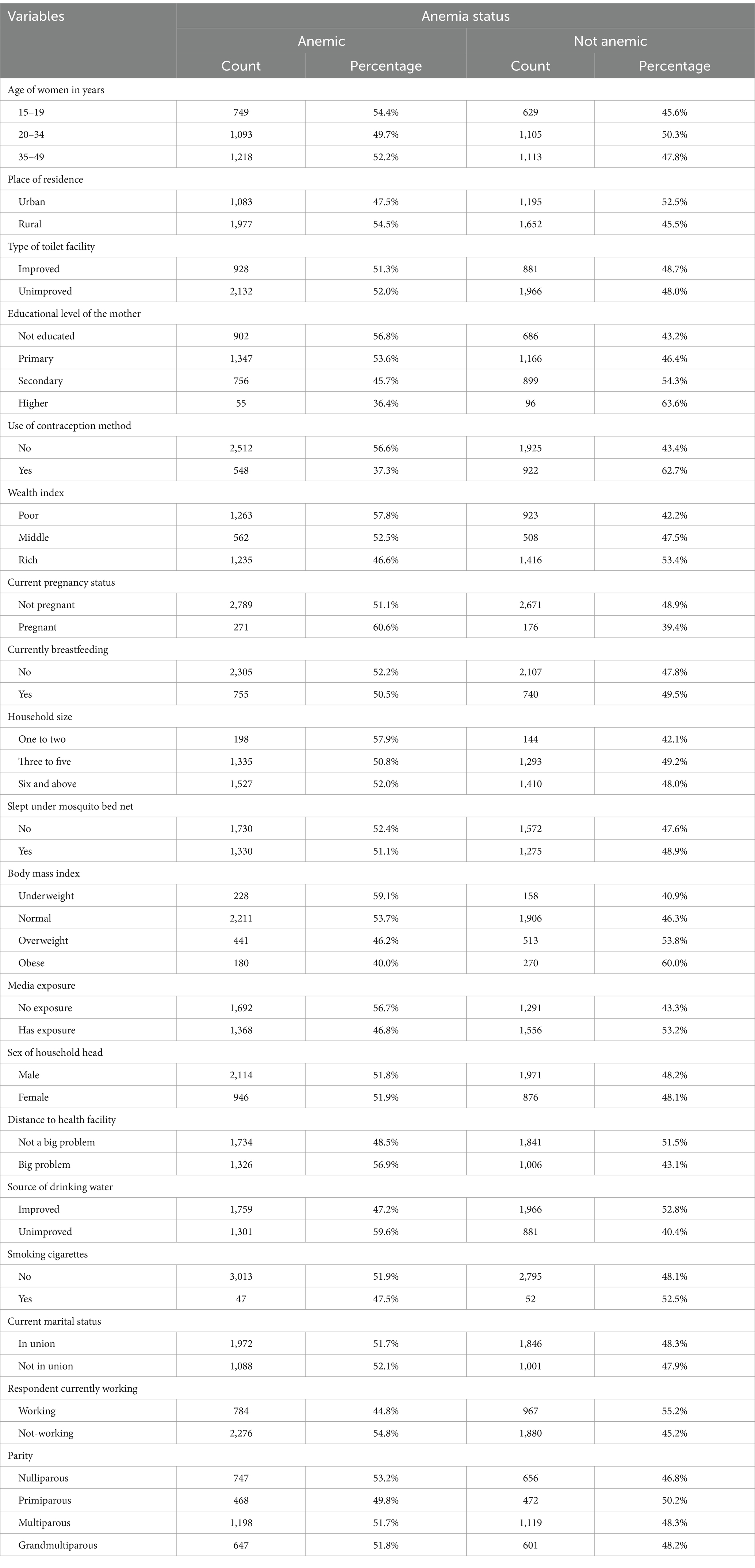
Table 2. Descriptive characteristics of the study participants (n = 5,907) in Mozambique DHS, 2022/23.
Prevalence of anemia among reproductive age women in Mozambique
The national pooled prevalence of anemia among women of reproductive age in Mozambique was 51.89% (95% CI: 50.66, 53.12%). Significant regional variations in anemia prevalence were observed. The highest prevalence was in Zambezia at 70.28% (95% CI: 67.37, 73.19%), while the lowest prevalence was in Niassa at 27.47% (95% CI: 22.98, 31.96%) (Figure 2).
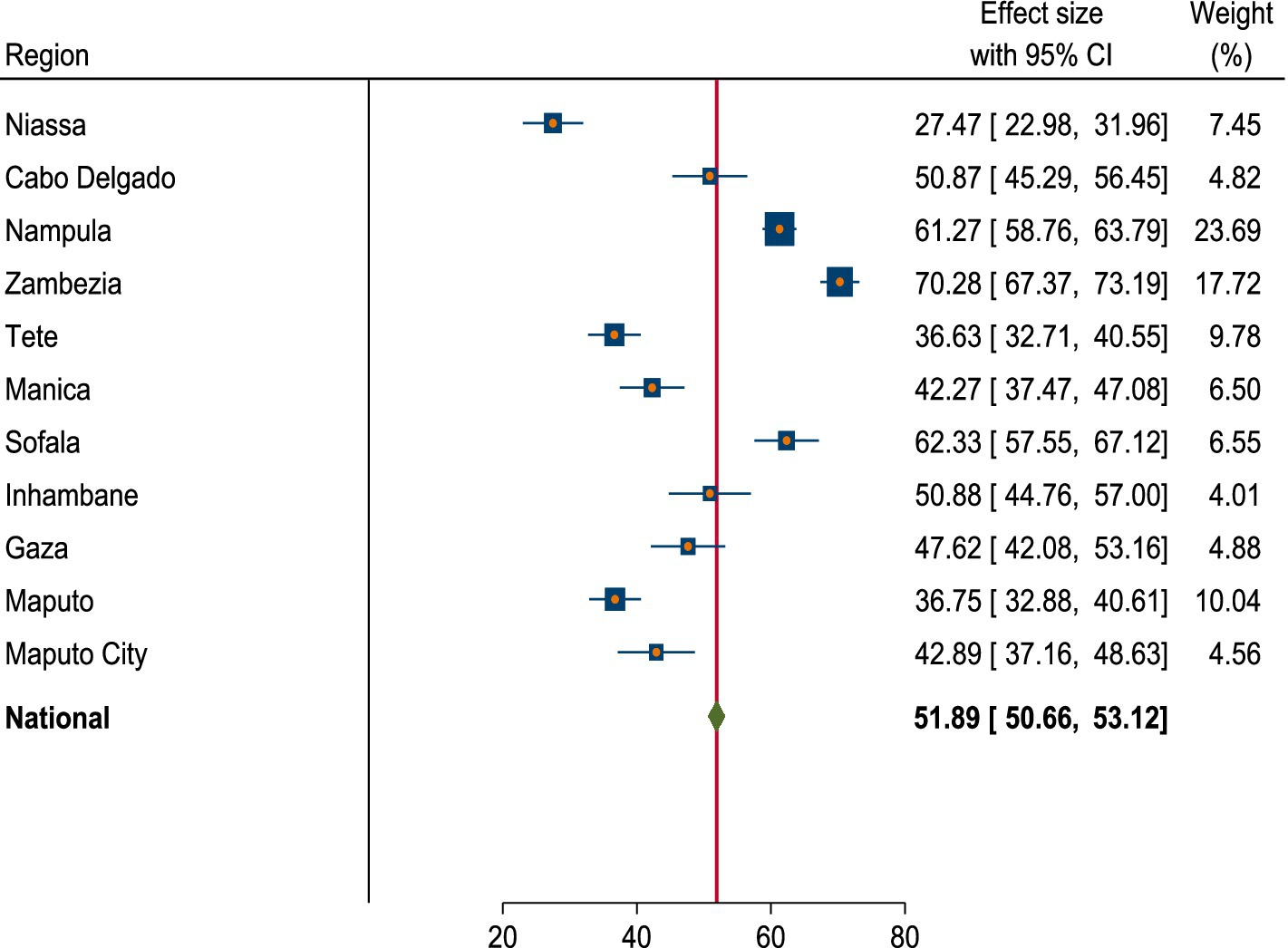
Figure 2. Forest plot of prevalence of anemia among women of reproductive age in Mozambique DHS, 2022/23.
Spatial distribution of anemia among reproductive age women in Mozambique
The spatial autocorrelation model found that the spatial distribution of anemia among reproductive-age women was non-random, with a global Moran’s I value of 0.444359 and a p-value of less than 0.001. This high confidence level indicates that the probability of the observed spatial pattern being due to randomness is less than 1%. The positive Moran’s I value signifies the clustering of anemia cases in Mozambique (Figure 3).
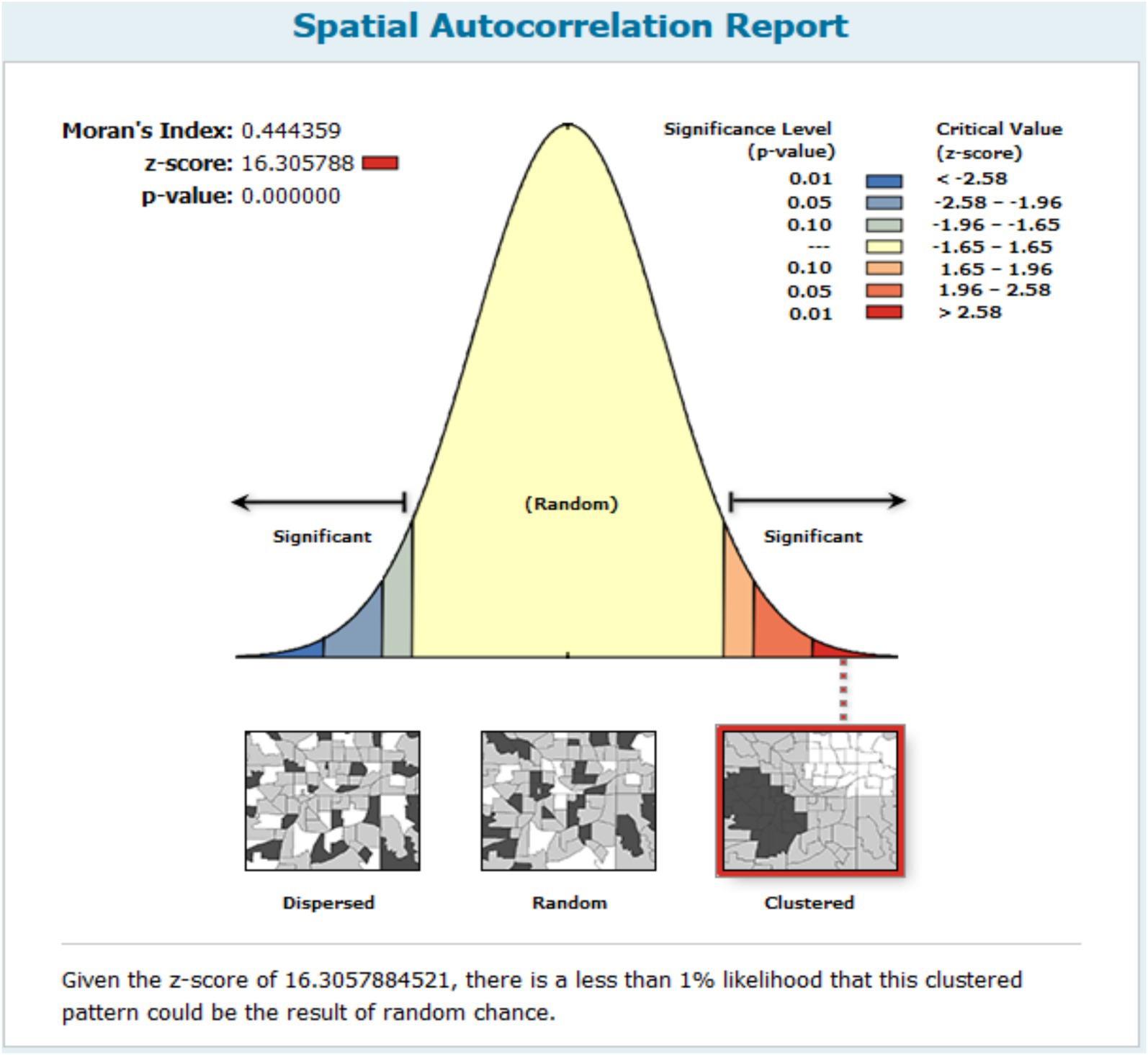
Figure 3. Global spatial autocorrelation result of anemia among reproductive age women in Mozambique DHS, 2022/23.
Cluster and outlier analysis
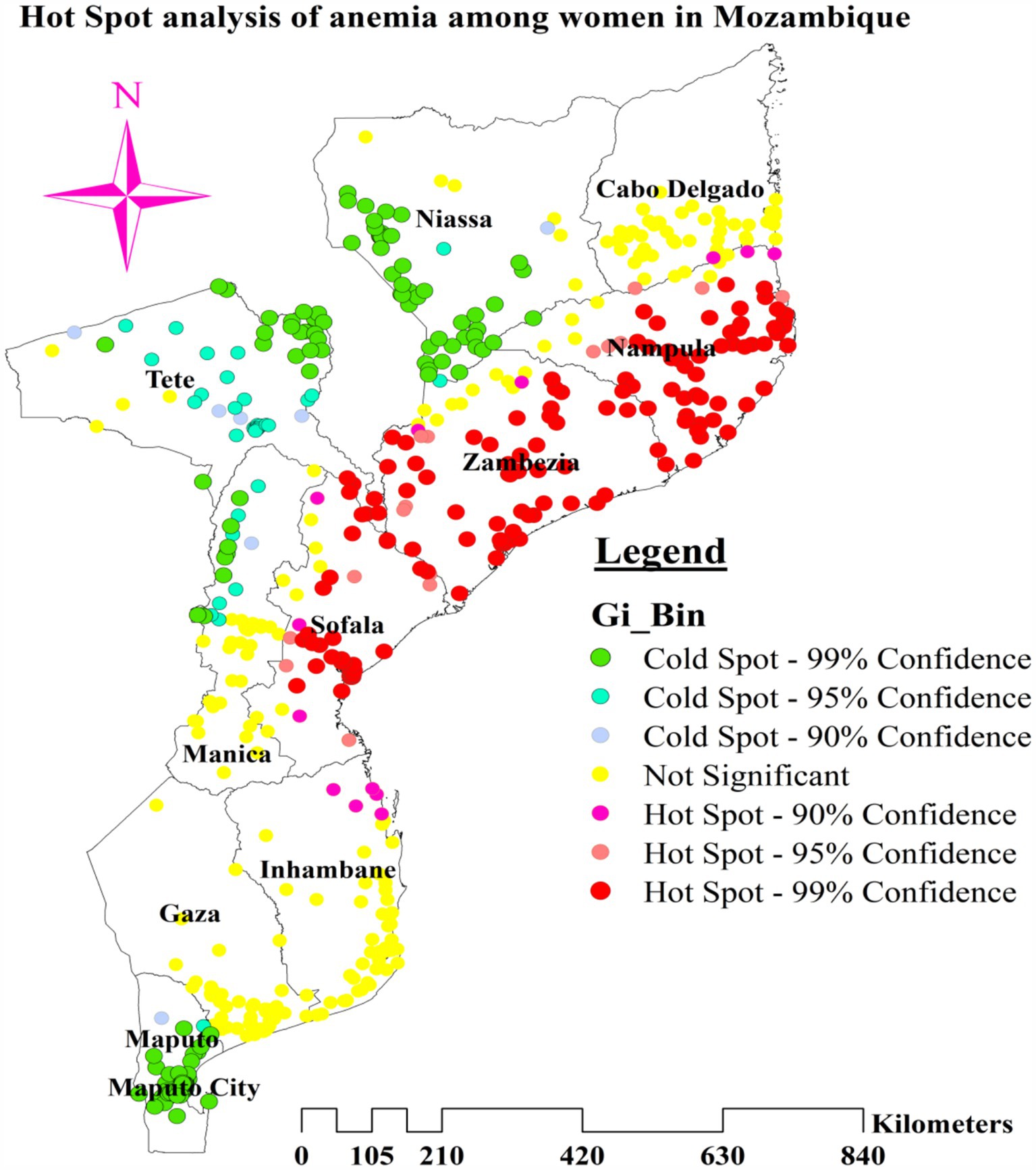
We used the Anselin Local Moran’s I cluster and outlier analysis to illustrate the geographic distribution of anemia across the nation. This analysis identified specific clusters and their surrounding regions, as shown in Figure 4. The red tint in the panels indicates high-high clustering of anemia, signifying regions with a higher than average incidence of similar cases. These regions include most of Nampula, all of Zambezia, the northern and central areas of Sofala, and the eastern tip of Tete. Conversely, the northern half of Tete, the western and northwest boundaries of Manica, most of Maputo, and the northern section of Maputo City are low-low clustering zones, represented by green hues (Figure 5).
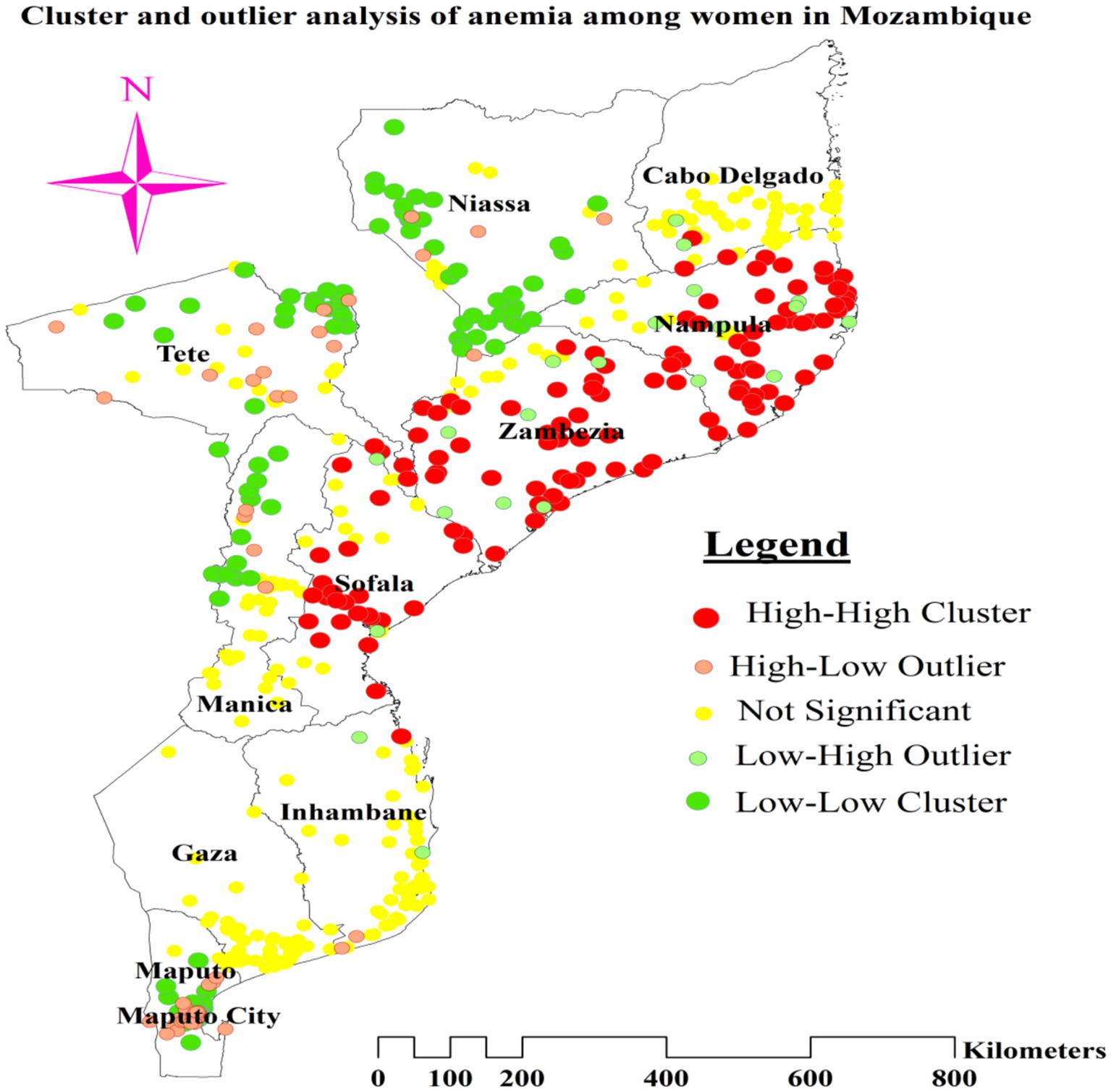
Figure 4. Cluster and outlier analysis of anemia among reproductive age women in Mozambique DHS, 2022/23.
Hotspot analysis of anemia among reproductive age women
The greater percentage of anemia (red dots) was centered in the majority of Nampula and Zambezia, the eastern tip of Tete, and the central and northern regions of Sofala. On the other hand, a lower proportion (represented by green dots) was found in the southern and southwest areas of Niassa, the northeastern part of Tete, a greater portion of Maputo, the northern section of Maputo City, and the Northwestern border of Manica (Figure 4).
Spatial scan statistics of anemia among reproductive age women
Kulldorff’s spatial scan statistics identified three statistically significant clusters of anemia among reproductive-age women, using 50% of the total population as the maximum spatial circular window size. The most likely cluster had a total enumeration area of 101, a population size of 1,515, a number of cases of 1,047, and a LLR of 124.33. The primary cluster, centered at 17.269259 S and 37.822882 E, has a radius of 214.40 km, a relative risk of 1.51, and a p-value of less than 0.001. This reveals that women within this spatial window had a 51% elevated risk of anemia compared to women outside the window, and this risk was unlikely to have occurred by chance. The secondary cluster 1 (LLR = 105.69, p-value<0.001, and RR = 1.50) incorporated 79 enumeration areas, a population of 1,148, and 813 anemia cases among reproductive-age women. This first secondary cluster is centered at 17.269259 S, 37.822882 E, with a radius of 141.53 km. The population size and anemia cases in the scanning window of secondary cluster 2 were 841 and 540, respectively. It was located at 14.559803 S, 40.689502 E, with a 164.35 km radius. Its RR, LLR, and p-value were 1.29, 30.45, and <0.001, respectively. The third secondary cluster (total population = 74, anemia cases = 60, RR = 1.58, LLR = 13.91, and p-value <0.001) was centered at 19.339681 S, 34.339890 E, with a 51.93 km radius. This cluster with 12 enumeration areas is located in the central part of Sofala (Table 3).
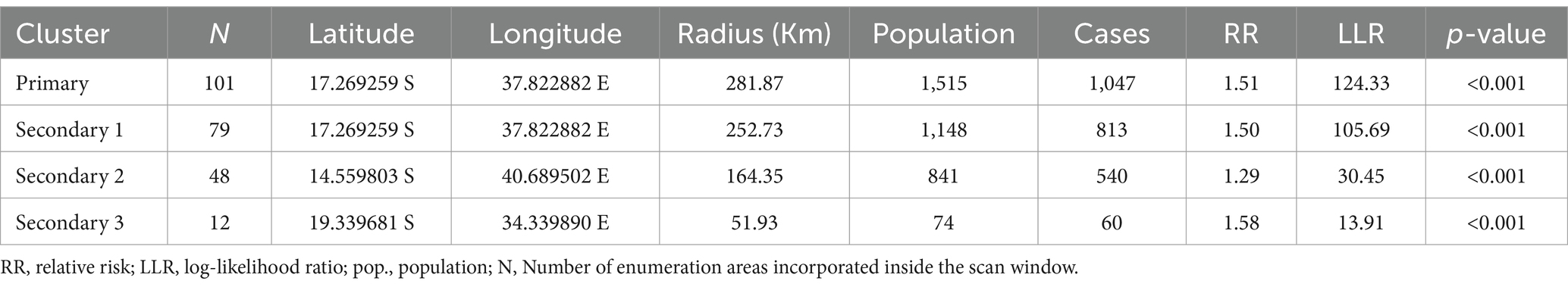
Table 3. Cluster detection analysis results for anemia among reproductive age women in Mozambique DHS, 2022/23.
In addition to the most likely cluster, three statistically significant secondary clusters were found in the western and northwestern parts of Mozambique, in the Nampula, Zambezia, and Sofala regions. The most likely cluster was concentrated in the whole of Zambezia, southern and southwestern Nampula, and northeastern Sofala. The secondary cluster 1 was situated in most parts of Zambezia, the northeastern border of Sofala, and southern and southwestern Nampula. The scanning window of secondary cluster 2 was situated in the eastern half of Nampula and the southeastern part of Cabo Delgado. The RR of 1.29 suggests that reproductive-age women within the spatial window had a 29% higher risk of anemia than women outside the window (Figure 6).
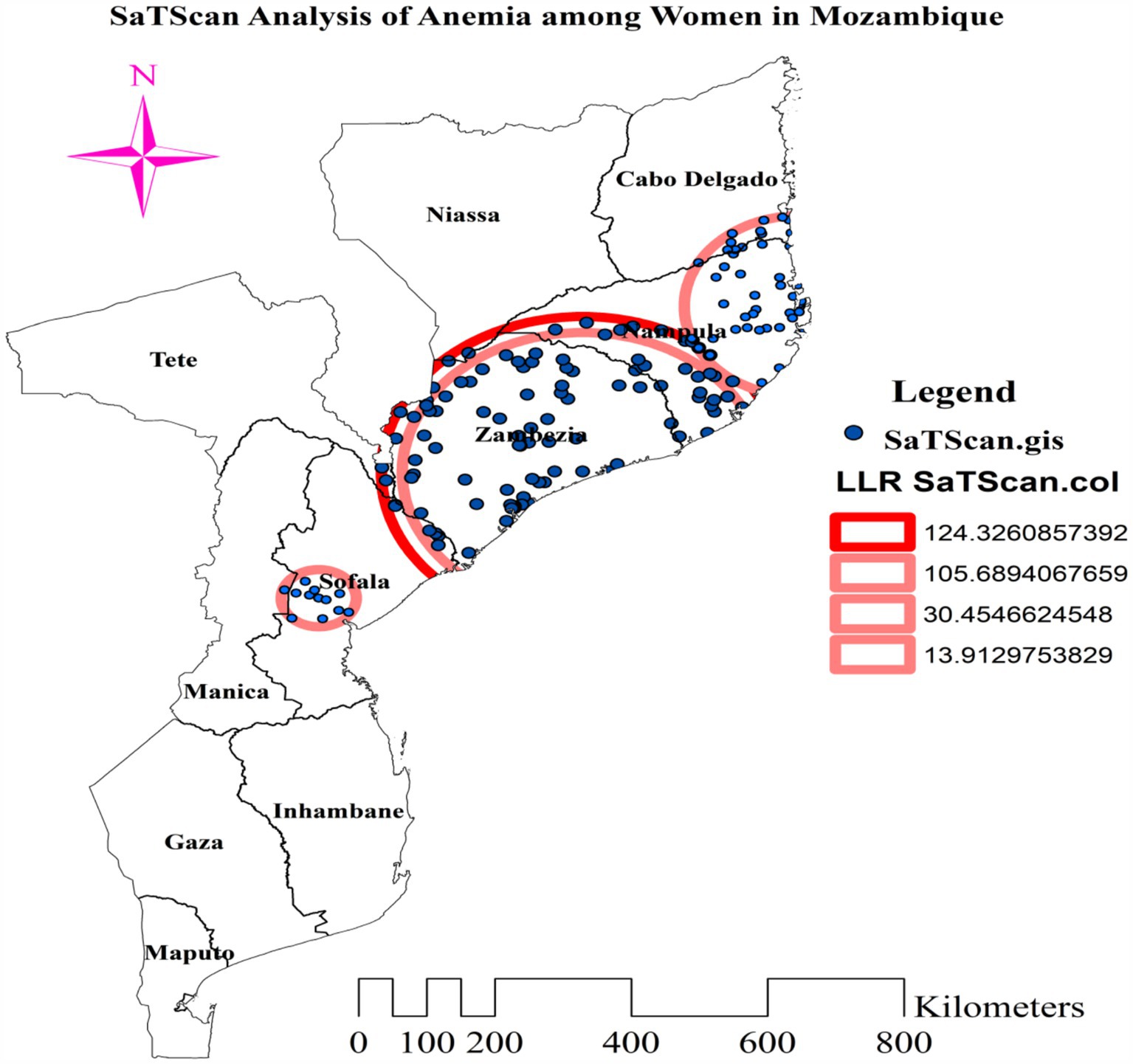
Figure 6. Most likely and secondary clusters with high rate of anemia among reproductive age women in Mozambique DHS, 2022/23.
Spatial interpolation of anemia among reproductive age women
We mapped the prevalence of anemia among women of reproductive age across various regions in Mozambique using ordinary Kriging (OK). Higher proportions of predicted anemia (indicated by red shaded areas) were observed in nearly all of Nampula and Zambezia, the western, central, and eastern parts of Sofala, the southern portion of Cabo Delgado, the eastern tip of Tete, and a small area in central Niassa. Conversely, areas with green shading, which indicate expected low anemia prevalence, included most of Niassa, Tete, Manica, Cabo Delgado, and Gaza; the southern half of Maputo; and the southern and western borders of Inhambane (Figure 7).
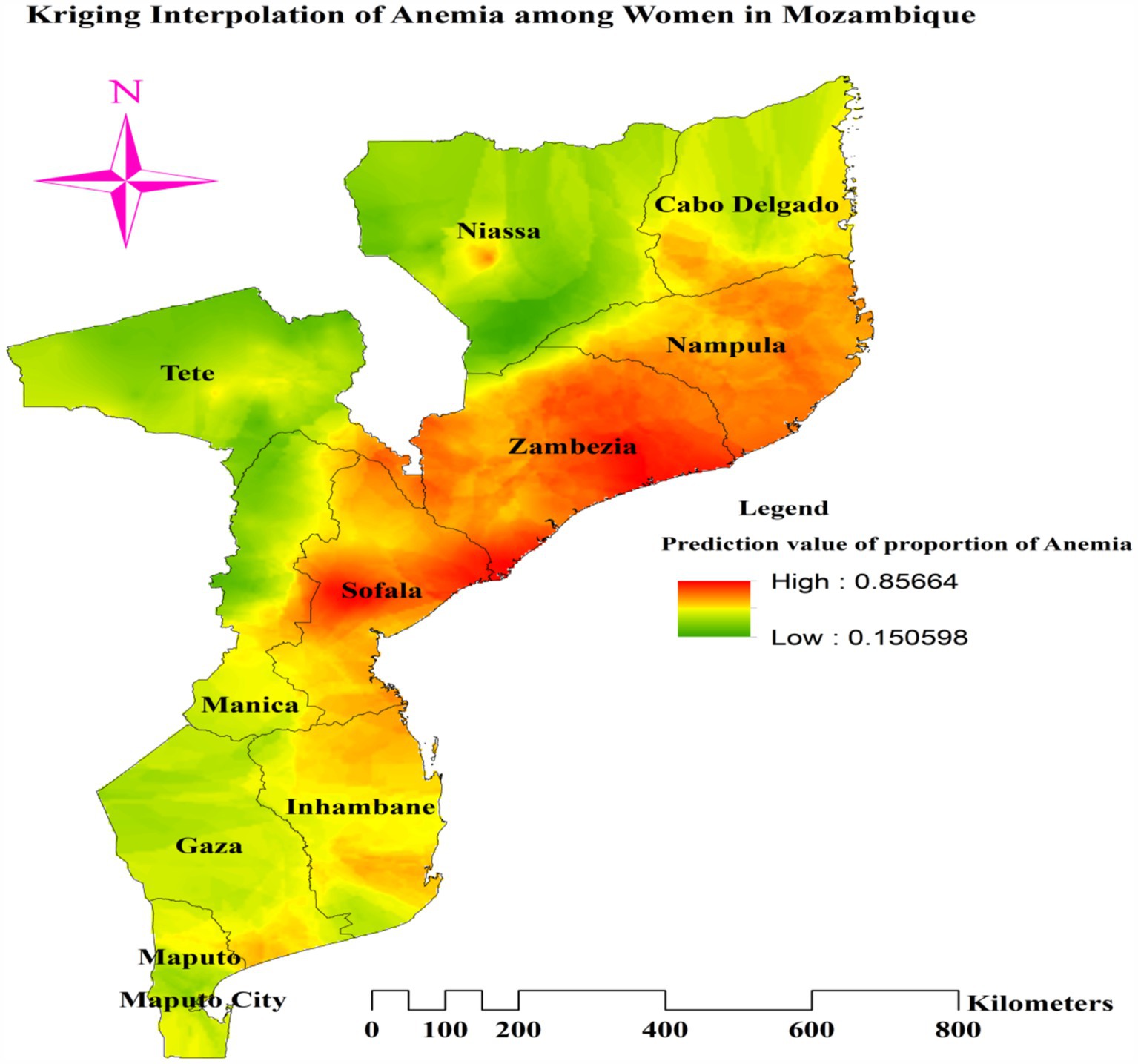
Figure 7. Ordinary Kriging of interpolation of proportion of anemia among reproductive age women in Mozambique DHS, 2022/23.
Spatial regression of the predictors of anemia
Ordinary least squares model results
We employed the OLS model to identify factors associated with spatial variation in anemia among reproductive-age women. Anemia was positively associated with using an unimproved drinking water source, being pregnant, and being underweight. Conversely, using contraceptive methods and being obese were negatively associated with anemia in this population. The minimum, maximum, and mean variance inflation factor (VIF) values were 1.61, 2.72, and 2.036, respectively, indicating the absence of multicollinearity among the independent variables used in the OLS model, as these values were below the cut-off threshold for multicollinearity diagnostics (Table 4).
The adjusted R2 value of 0.1116 from the OLS model indicates that 11.165% of the variation in anemia is explained by the five explanatory variables (Table 4). This study demonstrates a statistically significant linear relationship between the dependent variable and the explanatory variables, as evidenced by significant joint Wald and F-statistics. The p-value of 0.099909 for the Jarque-Bera statistic suggests that the residuals are normally distributed, indicating that the OLS model predictions are unbiased. However, the statistically significant Koenker statistic indicates that the regression model is inconsistent across the study area, suggesting that the relationship between variables varies with geographic location. Consequently, the GWR model was deemed more appropriate and was used to estimate the model parameters (Table 4).
Multiscale geographically weighted regression analysis
Comparing the OLS model and the Multiscale Geographically Weighted Regression (MGWR) model using diagnostic parameters (AICc and R2), the AICc value decreased from 1712.38 (OLS model) to 1538.93 (MGWR model). The adjusted R2 value increased from 0.116 (11.16%) in the OLS model to 0.442 (44.2%) in the MGWR model. These diagnostic parameters indicate that the MGWR model is superior to the OLS model, suggesting that the Multiscale Geographically Weighted Regression (local) model provides a better fit than the OLS (global) model (Table 5).
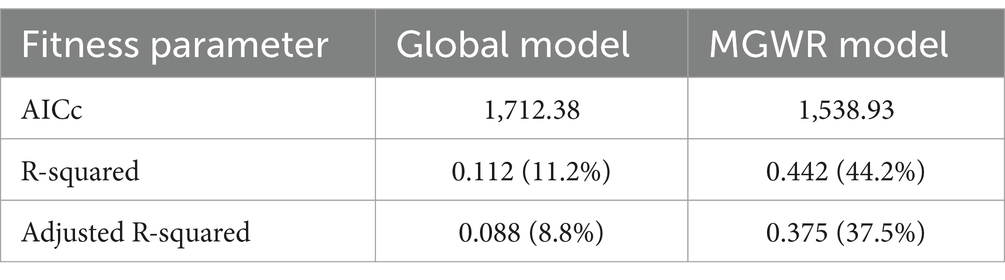
Table 5. Model comparison of OLS and MGWR model fit/performance of anemia among reproductive age women in Mozambique DHS, 2022/23.
The mean and median beta coefficients for using an unimproved water source, being pregnant, and being underweight were positive, indicating a positive association between these factors and the spatial variation of anemia. Conversely, the mean and median beta coefficients for using contraceptive methods and being obese were negative, suggesting a negative association between these factors and the spatial variation of anemia (Table 6).
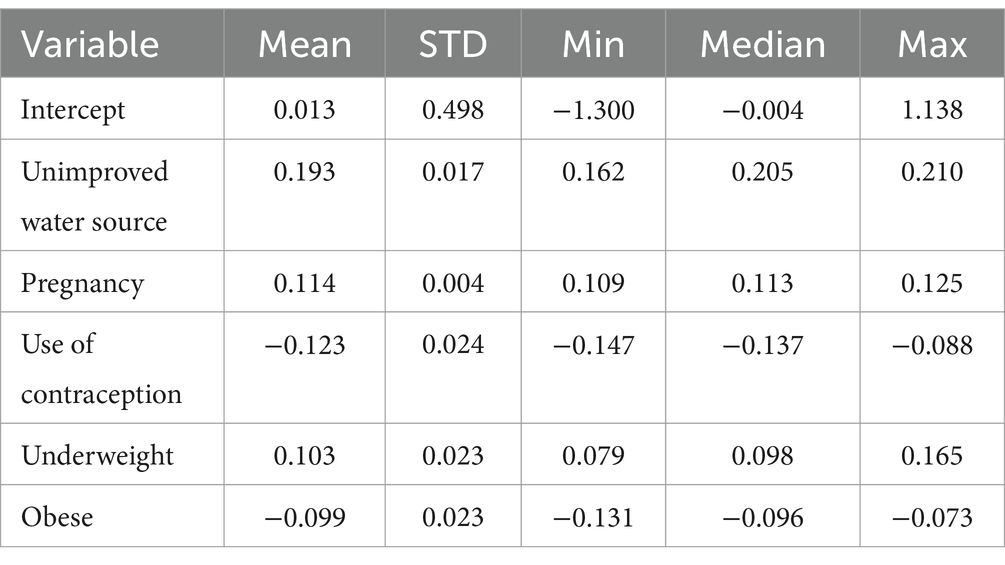
Table 6. Summary statistics for MGWR parameter estimates describing the spatially varying relationships between anemia among reproductive age and predictors in Mozambique DHS, 2022/23.
Figure 8A illustrates the model’s performance (local R-squared) across the study area. The model performed well, with R-squared values ranging from 46.28 to 55.61% in the entire areas of Zambezia, southern, southeastern, and southwestern Niassa, and the northern border of Nampula. However, the model showed a poor fit for data from provinces in the south, southwest, and southeast of Maputo, the north, northeast, and northwest of Maputo City, the south of Gaza, and the east, southeast, and northeast of Inhambane, with adjusted R-squared values ranging from 21.22 to 28.31% (Figure 8A).
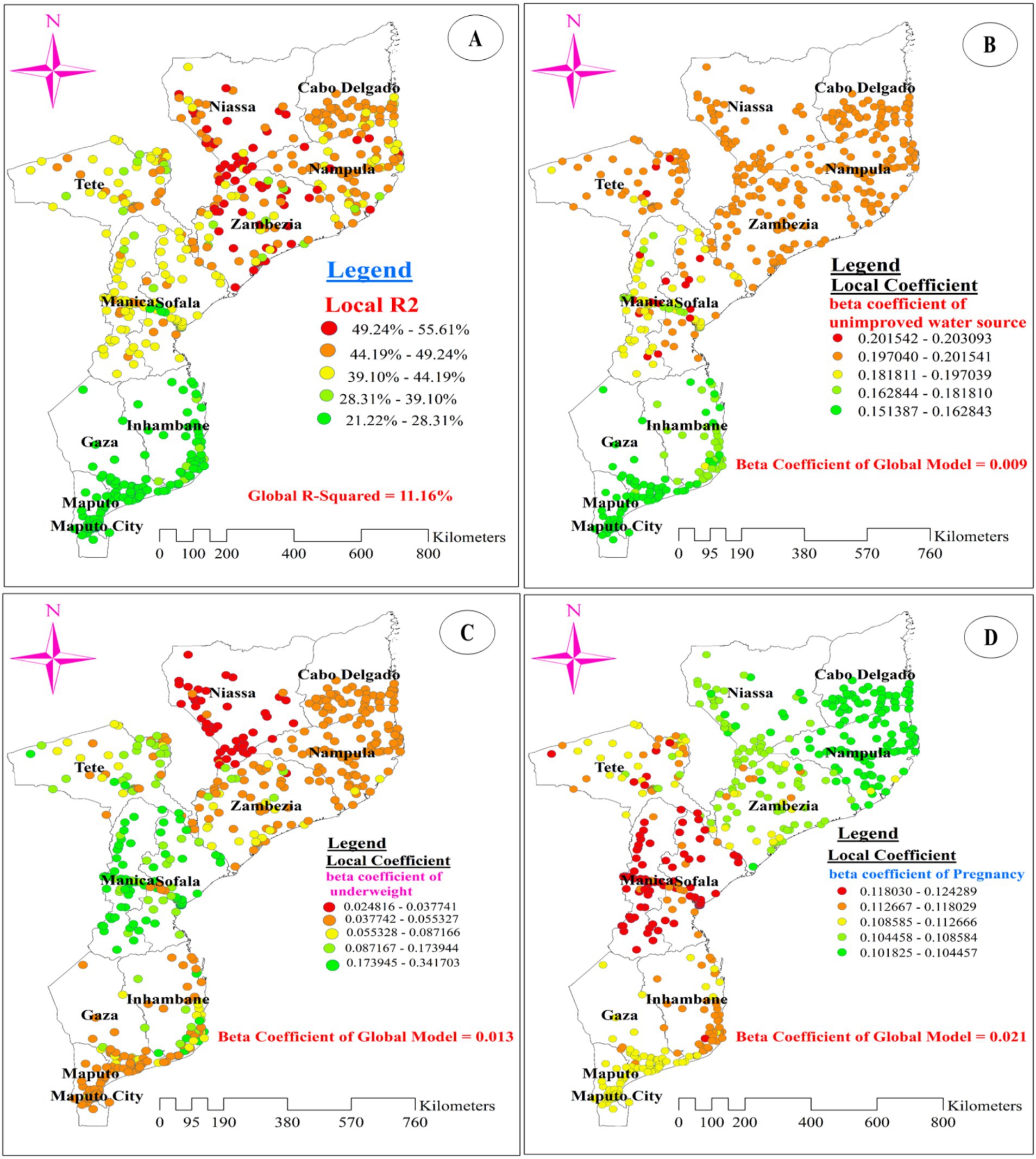
Figure 8. The spatial mapping of local R-squared (A) and local regression coefficients of unimproved drinking water source (B), underweight (C), and pregnancy (D) in Mozambique DHS, 2022/23.
Figures 8B–D, 9A,B display the spatial distribution of the beta coefficients for the five predictor variables. The red dotted areas indicate a strong positive influence (high beta coefficient) of three explanatory variables (using an unimproved drinking water source, being pregnant, and being underweight) on anemia among reproductive-age women (Figures 8B–D). Conversely, the green dotted areas show a strong negative influence (high protective effect) of two explanatory variables (using contraceptive methods and being obese) on anemia among reproductive-age women (Figures 9A,B).
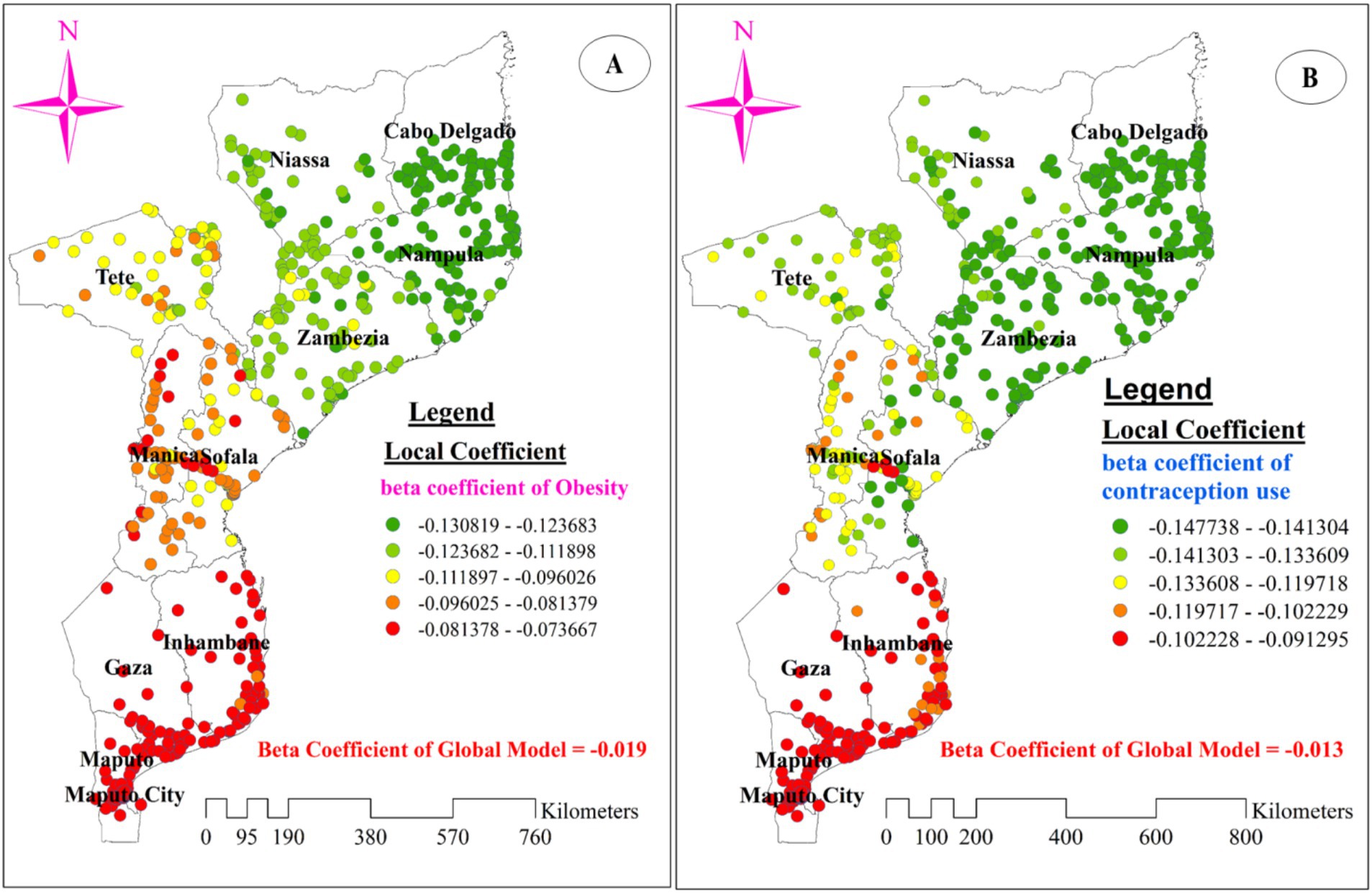
Figure 9. The spatial mapping of local regression coefficients of obesity (A), and use of contraception methods (B) in Mozambique DHS, 2022/23.
The beta coefficient for using an unimproved drinking water source shows substantial variation across the study area, indicating an inconsistent relationship between this variable and the proportion of anemia. A strong positive influence of using an unimproved drinking water source on anemia was observed in the northeast of Tete, central and southeast Manica, and the west and central parts of Sofala. Conversely, the lowest coefficients for women using an unimproved drinking water source were predominantly found in southern Gaza, southern Maputo, and northern Maputo City. Additionally, low coefficients were scattered across the central part of Gaza and the northern, eastern, and southern borders of Inhambane (Figure 8B).
Regarding the beta coefficient for pregnant women, a strong positive influence (red dotted areas on the map) was concentrated in most parts of Manica and Sofala. Although scattered, it was also observed in the southeast and northeast parts of Tete, with beta coefficient values ranging from 0.118 to 0.124 in these areas. Conversely, the lowest coefficients (green dotted areas on the map) for pregnant women were predominantly found in the entire area of Nampula and the southern part of Cabo Delgado (Figure 8D). Additionally, being underweight was associated with spatial variation in anemia. A strong positive relationship (red dotted areas) between being underweight and anemia was identified in most parts of Niassa, particularly in the southern and western regions of the province. The beta coefficients in these areas ranged from 0.025 to 0.038. Conversely, low beta coefficients were notably observed in most parts of Manica and Sofala (Figure 8C).
Interestingly, the negative beta coefficients for using contraceptive methods and being obese imply a protective effect of these explanatory variables on anemia among reproductive-age women. A strong protective effect, indicated by green-dotted areas on the map, was concentrated in all parts of Nampula and the southern part of Cabo Delgado. This effect was also observed in Zambezia (especially for using contraceptive methods) and the eastern and western portions of Niassa (Figures 9A,B).
Discussion
Mozambique, one of the countries with the highest prevalence of anemia in sub-Saharan Africa, was the focus of this study, which identified the prevalence, predictors, and spatial variation of anemia among women of reproductive age (5).
The national pooled prevalence of anemia among women of reproductive age in this study was 51.89% (95% CI: 50.66, 53.12%). This finding aligns with the prevalence reported in the National Family Health Survey (NFHS)-4 in India, which stands at 53% (41).
However, it is higher than the prevalence reported in studies conducted in Rwanda (19.2%) (24), Sudan (35.6%) (42), and China (18.9%) (43). These significant differences in anemia prevalence can be attributed to variations in healthcare access, nutrition, public health interventions, cultural beliefs, food insecurity and socio-economic status. In Mozambique, frequent natural disasters such as cyclones, floods, and droughts disrupt livelihoods and food production, exacerbating the challenges faced by vulnerable populations and thereby increasing the prevalence of anemia among women of reproductive age (44). Despite ongoing interventions in Mozambique such as the USAID Advancing Nutrition Project (45), the Global Alliance for Improved Nutrition (GAIN) (46), the prevalence of anemia in women of reproductive age is still higher. In China, the low prevalence of anemia can be attributed to a more intensive and geographically extensive multifaceted approach that includes Iron and Folic Acid Supplementation, Dietary Diversification, and Nutrition Counseling and Education (47, 48). In Rwanda, socio-economic indicators such as literacy rates and poverty levels are generally better than those in Mozambique (49, 50), contributing to the lower prevalence of anemia. In Sudan, the relatively better Malaria Control, Healthcare Access, Nutritional Interventions, and Public Health Programs compared to in Mozambique contribute the lower prevalence of anemia (51).
Conversely, the prevalence of anemia in this study is slightly lower than the national prevalence in Yemen, which stands at 57.4% (27). The slightly lower prevalence compared to Yemen may be due to differences in healthcare access, nutritional programs, and public health interventions. In Yemen, the higher prevalence of anemia can be attributed to ongoing conflict, food insecurity, and restricted access to healthcare facilities (52, 53). These factors increase the risk of anemia, especially for women of reproductive age who require more iron because of menstruation, pregnancy, and lactation (54, 55).
The results from the spatial global Moran’s analysis in our study illustrate that the proportion of anemia among reproductive age women in Mozambique demonstrate significant geographical variation and clustering. This finding is supported by similar studies conducted in Democratic republic of Congo (DRC), Ethiopia, Timore-Leste, India, and Bangladesh which showed considerable clustering and high prevalence of anemia in particular areas of the countries (25, 41, 56–58).
Considering the explanatory variables, the spatial clustering of anemia can be attributed to various underlying factors that are geographically concentrated. These factors include poverty, illiteracy, poor sanitation, malnutrition, insufficient regional health services, inadequate dietary practices like low dietary diversity and poor meal frequency, limited nutritional supplementation like insufficient coverage of supplementation programs and challenges in implementation, limited healthcare access (including antenatal care), recent childbirth, and the incidence of malaria (25, 41, 57). Additionally, maternal age, pregnancy status, body mass index, and HIV status were significant predictors for the geographical variation of anemia among reproductive age women (56, 58).
This study’s spatial analysis revealed distinct geographical patterns in the prevalence of anemia among women of reproductive age in Mozambique. The hotspot analysis identified several regions with significantly higher proportions of anemia, particularly in Nampula, Zambezia, Tete, and Sofala. These hotspots can be attributed to a combination of factors, including nutritional deficiencies, socioeconomic conditions, infectious diseases, and natural disasters (notably in Nampula and Sofala). Additionally, reproductive health factors such as access to reproductive health services, HIV/AIDS, and sexually transmitted infections (STIs) contribute to the high prevalence of anemia in these provinces. Firstly, the availability of a balanced diet high in iron, folate, and vitamin B12—all necessary for the synthesis of healthy red blood cells—is restricted for many women in these areas (59). Secondly, the high prevalence of parasitic infections such as malaria and hookworm resulting in hemolysis and chronic blood loss, respectively, is another significant factor, leading to anemia (60, 61). Thirdly, many women cannot afford or access the essential treatments and preventative measures for anemia due to high levels of poverty and restricted access to healthcare facilities (62). Finally, Short time spans between pregnancies and repeated pregnancies can deplete a woman’s iron levels, enhancing the risk of anemia (63).
On the other hand, a lower proportion was found in Niassa, Tete, Maputo, Maputo City, and Manica. The relatively lower proportion of maternal anemia in these specific areas of Mozambique might be attributed to several factors like healthcare access and quality, malaria control, nutritional programs, higher socioeconomic status, and higher levels of education and awareness about maternal health. For example, compared to more remote areas, places like Maputo and Maputo City have better access to medical services and a stronger healthcare infrastructure. Better prenatal care is one aspect of this, since it can aid in the early diagnosis and treatment of anemia (64).
The GWR analysis revealed substantial spatial variation in the relationship between using an unimproved drinking water source and the proportion of anemia among reproductive women across Mozambique. This suggests that the impact of water quality on anemia prevalence is highly context-dependent and cannot be adequately captured by a single, global model (65, 66). Specifically, the GWR results showed a strong positive influence of using an unimproved water source on anemia levels in specific regions of the country, namely the northeast of Tete, central and southeast Manica, and the west and central parts of Sofala. In these areas, women who rely on unimproved water sources, such as surface water, unprotected wells, or unprotected springs, are at a significantly higher risk of being anemic compared to those with access to improved water sources. This aligns with finding from previous study in low and middle income countries in 2011 (2). This spatial variation in the water source-anemia relationship suggests that there are likely other contextual factors, such as local environmental conditions, sociocultural practices, or access to health services that modify the impact of water quality on anemia prevalence (67, 68). These factors may differ across the regions of Mozambique, leading to the observed heterogeneity in the GWR model results (65, 66).
Additionally, being pregnant was significantly related to anemia, with its stronger positive influence concentrated in most parts of Manica, Sofala, southeast and northeast parts of Tete. This finding aligns with evidence from previous studies (69–73). The observed spatial variation in the pregnancy and anemia relationship can be attributed to a combination of physiological, access to care, socioeconomic, and environmental factors. Physiological factors such as maternal nutritional status and underlying health conditions can contribute to the risk of anemia during pregnancy (69, 70). Access to quality antenatal care, including regular checkups and appropriate supplementation, has also been shown to play a crucial role in mitigating anemia prevalence (71, 72). Socioeconomic factors, such as household wealth and education levels, significantly influence the spatial distribution of anemia among pregnant women. Women from lower socioeconomic backgrounds often have limited access to nutritious food, healthcare services, and health education, which can lead to higher rates of anemia. Additionally, cultural determinants, such as traditional dietary practices and beliefs about health and illness, also play a crucial role. For example, certain cultural practices may restrict the consumption of iron-rich foods, contributing to higher anemia prevalence (73, 74). Additionally, environmental exposures, such as the availability of iron-rich foods and the presence of infectious diseases, can also contribute to the observed spatial variation in the pregnancy-anemia relationship (75, 76).
Moreover, aligning with existing evidence (71, 72, 75, 76), being underweight was associated with spatial variation in anemia. A stronger positive relationship between being underweight and anemia was identified in Niassa. This finding suggests that maternal nutritional status, as indicated by underweight, is an important determinant of the spatial distribution of anemia among reproductive age pregnant women. Underweight women are more likely to have inadequate nutrient intake, which can directly contribute to the development of anemia during pregnancy (70, 77). Additionally, underlying health conditions related to underweight, such as chronic infections or malabsorption disorders, can further exacerbate the risk of anemia (71, 72). The spatial clustering of the underweight-anemia relationship in Niassa may be influenced by socioeconomic factors, such as poverty and food insecurity, which can limit access to a diverse and nutrient-rich diet (73, 74). The availability of iron-rich foods and the presence of infectious diseases, may also contribute to the observed spatial patterns (75, 76). Addressing the underlying causes of underweight and improving maternal nutritional status through Integrated Community Case Management (iCCM) (78) and Supervised Weekly Iron and Folic Acid Supplementation (WIFS) (79) could be crucial interventions for reducing the spatial disparities in anemia prevalence among pregnant women in the region. Furthermore, our analysis revealed that using contraceptive methods was inversely related with anemia among women in Mozambique, with a stronger protective effect concentrated in Nampula, Zambezia, Cabo Delgado and Niassa provinces. This finding suggests that access to and utilization of contraceptive services may play a crucial role in mitigating the burden of anemia among women of reproductive age in these regions. The observed spatial variation in the contraceptive use-anemia relationship can be attributed to several factors. First, the availability and quality of family planning services, including the provision of a diverse range of contraceptive methods, may be more robust in the identified provinces compared to other areas of the country. Improved access to contraception can empower women to plan their pregnancies, which is particularly important given the strong association between pregnancy and anemia risk (75, 76). Additionally, the use of certain contraceptive methods, such as intrauterine devices or implants, can help prevent frequent or closely spaced pregnancies, reducing the physiological strain that repetitive pregnancies can have on a woman’s iron stores and overall nutritional status (80, 81).
The GWR analysis also revealed an intriguing finding - being obese was inversely related to anemia among women in Mozambique, with a stronger protective effect in Nampula, Cabo Delgado, and Niassa provinces. This inverse association between obesity and anemia prevalence within these regions merits further study. However, this finding may be explained by the notion that these regions of Mozambique may have stronger health infrastructure and services, including more effective anemia screening, diagnosis, and management programs that target both overweight/obese and underweight individuals (68, 82, 83). Furthermore, improved roads on access to health care, addressing health care disruption in rural Mozambique could be the potential explanation (84, 85). Additionally, the interplay between obesity, inflammation, and anemia should be considered. Obesity is often associated with chronic low-grade inflammation, which can lead to the development of functional iron deficiency and anemia of inflammation (86, 87). However, in certain contexts, the inflammatory response may be less pronounced or better managed, allowing obese individuals to maintain adequate iron status and hemoglobin levels. Further research is needed to unpack the complex mechanisms underlying the spatial patterns of the obesity-anemia relationship in Mozambique.
Conclusion
Anemia remained a critical public health issue among women of reproductive age in Mozambique, with marked regional disparities. Hotspot clusters of anemia were found in Nampula, Zambezia, Tete, and Sofala, while cold spot clusters were located in Niassa, Tete, Maputo, Maputo City, and Manica. Factors such as unimproved drinking water, pregnancy, and being underweight were associated with higher anemia rates in certain regions, whereas obesity and contraceptive use indicated a protective effect in specific provinces. Targeted interventions addressing these factors are essential for reducing anemia and improving health outcomes for women across Mozambique; therefore, policymakers should focus on improving access to clean water, increasing the availability of maternal health services and enhancing nutritional support through the USAID Advancing Nutrition Project, the Global Alliance for Improved Nutrition (GAIN), Integrated Community Case Management (iCCM), and Supervised Weekly Iron and Folic Acid Supplementation (WIFS), particularly in the identified hotspot regions.
Limitation
The study’s conclusions should be interpreted with the following limitations in mind: Firstly, due to its cross-sectional design, the study cannot establish the chronological order of events, which restricts our ability to make causal inferences about anemia predictors. Secondly, the study relied on secondary data from Mozambique’s Demographic and Health Survey (DHS), which did not account for genetic causes of anemia such as iron-refractory iron-deficiency anemia, sickle cell anemia, thalassemia, and hereditary spherocytosis. These genetic factors could potentially influence anemia risk. Additionally, the study’s narrow focus on Mozambique limits the generalizability of findings to other regions with varying healthcare access, nutrition programs, public health interventions, socio-economic status, and cultural practices. Lastly, the lack of information on anemia type—due to the absence of peripheral morphology assessment and iron panel studies (including ferritin, serum iron, transferrin saturation, and total iron binding capacity), serum vitamin B12 levels, and serum folate levels—poses challenges in characterizing the specific anemia subtypes.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.dhsprogram.com/data/available-datasets.cfm.
Author contributions
DG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. TT: Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. GM: Conceptualization, Investigation, Software, Visualization, Writing – review & editing. YK: Formal analysis, Software, Visualization, Writing – review & editing. DT: Data curation, Software, Visualization, Writing – review & editing. SN: Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AN: Conceptualization, Data curation, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AICc, corrected Akaike Information Criterion; BMI, Body Mass Index; CSV, Comma-Separated Values; DHS, Demographic and Health Survey; DRC, Democratic Republic of Congo; GPS, Global Positioning System; GWR, Geographically Weighted Regression; HIV, Human Immunodeficiency Virus; IR, Individual Record; LLR, Log Likelihood Ratio; LMICs, Low and Middle Income Countries; ME, Minimum Mean Error; MGWR, Multiscale Geographically Weighted Regression; NFHS, National Family Health Survey; OLS, Ordinary Least Square; RMSE, Root Mean Square Error; RR, Relative Risk; WRA, Women of Reproductive Age.
References
2. Balarajan, Y, Ramakrishnan, U, Özaltin, E, Shankar, AH, and Subramanian, S. Anaemia in low-income and middle-income countries. Lancet. (2011) 378:2123–35. doi: 10.1016/S0140-6736(10)62304-5
3. Haider, BA, Olofin, I, Wang, M, Spiegelman, D, Ezzati, M, Fawzi, WW, et al. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. (2013) 346:346. doi: 10.1136/bmj.f3443
4. Report GN. Ministry of Health (MISAU). (2024). Available at: https://globalnutritionreport.org/resources/naf/tracker/org/ministry-of-health-misau/.
5. Chicanequisso, EM, Sabonete, A, Sacarlal, J, Rossetto, EV, and Baltazar, CS. Factors associated with high HIV infection among adolescents and young adults in Mozambique: Sub-Analysis of Immunization, Malaria and HIV/AIDS Indicators Survey in Mozambique-IMASIDA 2015. Research Square (2023).
6. Pokropek, C, Sobolewski, P, Getachew, R, Paul, A, Boura, J, and Ogunyemi, D. 621: Dietary intake patterns and insulin resistance in women with a history of gestational diabetes in the National Health and Nutrition Examination Survey (NHANES) 2000-2010. Am J Obstetrics Gynecol. (2015) 212:S308. doi: 10.1016/j.ajog.2014.10.827
7. Asres, Y, Yemane, T, and Gedefaw, L. Determinant factors of anemia among nonpregnant women of childbearing age in southwest Ethiopia: a community based study. Int Scholarly Res Notices. (2014) 2014:391580:1–8. doi: 10.1155/2014/391580
8. Brooker, S, Hotez, PJ, and Bundy, DA. Hookworm-related anaemia among pregnant women: a systematic review. PLoS Negl Trop Dis. (2008) 2:e291:e291. doi: 10.1371/journal.pntd.0000291
9. World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in populations. Geneva, Switzerland: World Health Organization (2020).
10. Petry, N, Olofin, I, Hurrell, RF, Boy, E, Wirth, JP, Moursi, M, et al. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients. (2016) 8:693. doi: 10.3390/nu8110693
11. Liyew, AM, Kebede, SA, Agegnehu, CD, Teshale, AB, Alem, AZ, Yeshaw, Y, et al. Spatiotemporal patterns of anemia among lactating mothers in Ethiopia using data from Ethiopian Demographic and Health Surveys (2005, 2011 and 2016). PLoS One. (2020) 15:e0237147. doi: 10.1371/journal.pone.0237147
12. Habyarimana, F, Zewotir, T, and Ramroop, S. Spatial distribution and analysis of risk factors associated with Anemia among women of reproductive age: case of 2014 Rwanda demographic and health survey data. Open Public Health J. (2018) 11:425–37. doi: 10.2174/1874944501811010425
13. Ogunsakin, RE, Akinyemi, O, Babalola, BT, and Adetoro, G. Spatial pattern and determinants of anemia among women of childbearing age in Nigeria. Spatial Spatiotemporal Epidemiol. (2021) 36:100396. doi: 10.1016/j.sste.2020.100396
14. Stevens, GA, Paciorek, CJ, Flores-Urrutia, MC, Borghi, E, Namaste, S, Wirth, JP, et al. National, regional, and global estimates of anaemia by severity in women and children for 2000–19: a pooled analysis of population-representative data. Lancet Glob Health. (2022) 10:e627–39. doi: 10.1016/S2214-109X(22)00084-5
15. Sacarlal, J, Nhacolo, AQ, Sigaúque, B, Nhalungo, DA, Abacassamo, F, Sacoor, CN, et al. A 10 year study of the cause of death in children under 15 years in manhiça, mozambique. BMC Int Health Hum Rights. (2009) 9:1–10. doi: 10.1186/1471-2458-9-67
16. Nations U. Transforming our world: the 2030 agenda for sustainable development. New York: United Nations, Department of Economic and Social Affairs (2015).
17. Pavelic, P, Giordano, M, Keraita, BN, Ramesh, V, and Rao, T. Groundwater availability and use in Sub-Saharan Africa: a review of 15 countries. Colombo, Sri Lanka: International Water Management Institute. (2012).
18. Worldometer. Mozambique population. (2024). Available at: https://www.worldometers.info/world-population/mozambique-population/.
19. Review WP. Mozambique population. (2024). Available at: https://worldpopulationreview.com/countries/mozambique.
20. Program D. Mozambique: standard DHS, 2022–23. (2023). Available at: https://dhsprogram.com/methodology/survey/survey-display-564.cfm.
21. Tirore, LL, Areba, AS, Habte, A, Desalegn, M, and Kebede, AS. Prevalence and associated factors of severity levels of anemia among women of reproductive age in sub-Saharan Africa: a multilevel ordinal logistic regression analysis. Front Public Health. (2024) 11:1349174. doi: 10.3389/fpubh.2023.1349174
22. Alem, AZ, Efendi, F, McKenna, L, Felipe-Dimog, EB, Chilot, D, Tonapa, SI, et al. Prevalence and factors associated with anemia in women of reproductive age across low- and middle-income countries based on national data. Sci Rep. (2023) 13:20335. doi: 10.1038/s41598-023-46739-z
23. Teshale, AB, Tesema, GA, Worku, MG, Yeshaw, Y, and Tessema, ZT. Anemia and its associated factors among women of reproductive age in eastern Africa: a multilevel mixed-effects generalized linear model. PLoS One. (2020) 15:e0238957. doi: 10.1371/journal.pone.0238957
24. Hakizimana, D, Nisingizwe, MP, Logan, J, and Wong, R. Identifying risk factors of anemia among women of reproductive age in Rwanda–a cross-sectional study using secondary data from the Rwanda demographic and health survey 2014/2015. BMC Public Health. (2019) 19:1–11. doi: 10.1186/s12889-019-8019-z
25. Rahman, MA, Rahman, MS, Aziz Rahman, M, Szymlek-Gay, EA, Uddin, R, and Islam, SMS. Prevalence of and factors associated with anaemia in women of reproductive age in Bangladesh, Maldives and Nepal: Evidence from nationally-representative survey data. PLoS One. (2021) 16:e0245335. doi: 10.1371/journal.pone.0245335
26. Worku, MG, Tesema, GA, and Teshale, AB. Prevalence and determinants of anemia among young (15–24 years) women in Ethiopia: A multilevel analysis of the 2016 Ethiopian demographic and health survey data. PLoS One. (2020) 15:e0241342. doi: 10.1371/journal.pone.0241342
27. Kinyoki, D, Osgood-Zimmerman, AE, Bhattacharjee, NV, Local Burden of Disease Anaemia CollaboratorsKassebaum, NJ, and Hay, SI. Anemia prevalence in women of reproductive age in low-and middle-income countries between 2000 and 2018. Nat Med. (2021) 27:1761–82. doi: 10.1038/s41591-021-01498-0
28. Montgomery, DC, Peck, EA, and Vining, GG. Introduction to linear regression analysis. Geneva, Switzerland: John Wiley & Sons (2021).
29. Lumley, T. Analysis of complex survey samples. J Stat Softw. (2004) 9:1–19. doi: 10.18637/jss.v009.i08
30. Tsai, P-J, Lin, M-L, Chu, C-M, and Perng, C-H. Spatial autocorrelation analysis of health care hotspots in Taiwan in 2006. BMC Public Health. (2009) 9:464. doi: 10.1186/1471-2458-9-464
31. Tesema, GA, Tessema, ZT, Angaw, DA, Tamirat, KS, and Teshale, AB. Geographic weighted regression analysis of hot spots of anemia and its associated factors among children aged 6–59 months in Ethiopia: a geographic weighted regression analysis and multilevel robust Poisson regression analysis. PLoS One. (2021) 16:e0259147. doi: 10.1371/journal.pone.0259147
32. Chen, Y. New approaches for calculating Moran’s index of spatial autocorrelation. PLoS One. (2013) 8:e68336. doi: 10.1371/journal.pone.0068336
33. Siska, PP, and Hung, I-K, eds. Assessment of kriging accuracy in the GIS environment. 21st Annual ESRI International Conference, San Diego, CA. (2001).
34. Kulldorff, M. A spatial scan statistic. Commun. Statistics Theory Methods. (1997) 26:1481–96. doi: 10.1080/03610929708831995
35. Martin, K. SaTScanTM user guide. Boston: The National Cancer Institute, and the New York City Department of Health and Mental Hygiene (2006).
36. Shrestha, PM. Comparison of ordinary least square regression, spatial autoregression, and geographically weighted regression for modeling forest structural attributes using a Geographical Information System (GIS). Remote Sensing (RS) Approach Thesis Canada: University of Calgary. (2006). Available at: https://theijes.com/papers/v3-i11/Version-2/A0311020109.pdf (Accessed October 30, 2012).
37. Nazeer, M, and Bilal, M. Evaluation of ordinary least square (OLS) and geographically weighted regression (GWR) for water quality monitoring: a case study for the estimation of salinity. J Ocean Univ China. (2018) 17:305–10. doi: 10.1007/s11802-018-3380-6
38. Kleinbaum, DG, Kupper, LL, Muller, KE, and Nizam, A. Applied regression analysis and other multivariable methods. Belmont, CA: Duxbury Press (1988).
39. Charlton, M, Fotheringham, S, and Brunsdon, C. Geographically weighted regression. Maynooth, Ireland: White paper National Centre for Geocomputation National University of Ireland Maynooth (2009). 2 p.
40. Fotheringham, AS, Yang, W, and Kang, W. Multiscale geographically weighted regression (MGWR). Ann Am Assoc Geogr. (2017) 107:1247–65. doi: 10.1080/24694452.2017.1352480
41. Let, S, Tiwari, S, Singh, A, and Chakrabarty, M. Prevalence and determinants of anaemia among women of reproductive age in Aspirational Districts of India: an analysis of NFHS 4 and NFHS 5 data. BMC Public Health. (2024) 24:437. doi: 10.1186/s12889-024-17789-3
42. Elmardi, KA, Adam, I, Malik, EM, Abdelrahim, TA, Elhag, MS, Ibrahim, AA, et al. Prevalence and determinants of anaemia in women of reproductive age in Sudan: analysis of a cross-sectional household survey. BMC Public Health. (2020) 20:1–12. doi: 10.1186/s12889-020-09252-w
43. Wu, Y, Ye, H, Liu, J, Ma, Q, Yuan, Y, Pang, Q, et al. Prevalence of anemia and sociodemographic characteristics among pregnant and non-pregnant women in southwest China: a longitudinal observational study. BMC Pregnancy Childbirth. (2020) 20:1–10. doi: 10.1186/s12884-020-03222-1
44. Mugabe, VA, Gudo, ES, Inlamea, O, Kitron, U, and Ribeiro, GS. Natural disasters, population displacement and health emergencies: multiple public health threats in Mozambique. BMJ Glob Health. (2021) 6:e006778:e006778. doi: 10.1136/bmjgh-2021-006778
45. USAID. USAID advancing nutrition Mozambique; (2024). Available at: https://pdf.usaid.gov/pdf_docs/PA00ZZG6.pdf. Final Report. Fiscal Years 2019–2023.
46. Nutrition gGAfI. Mozambique. (2021). Available at: https://copilot.microsoft.com/chats/e39MrMV7vN9Ge2GSAKKhc.
47. Zhao, J, Zhu, X, Dai, Q, Hong, X, Zhang, H, Huang, K, et al. The prevalence and influencing factors of anaemia among pre-pregnant women in mainland China: A large population-based, cross-sectional study. Br J Nutr. (2022) 127:439–50. doi: 10.1017/S0007114521001148
48. Zhou, Y, Lyu, Y, Ye, W, Shi, H, Peng, Y, Wen, Z, et al. The prevalence of anemia among pregnant women in china: a systematic review and meta-analysis. Nutrients. (2024) 16:1854. doi: 10.3390/nu16121854
49. Bizoza, A, Jäger, P, and Simons, A. Understanding poverty dynamics in Rwanda. Essen, Germany: Ruhr Economic Papers (2018).
50. World Bank. Literacy rate, adult total (% of people ages 15 and above). Washington, D.C., USA. (2020).
51. Elmardi, KA, Adam, I, Malik, EM, Kafy, HT, Abdin, MS, Kleinschmidt, I, et al. Impact of malaria control interventions on malaria infection and anaemia in areas with irrigated schemes: a cross-sectional population-based study in Sudan. BMC Infect Dis. (2021) 21:1–11. doi: 10.1186/s12879-021-06929-4
52. World Health Organization. Regional Overview of Food Security and Nutrition in the Near East and North Africa 2019: Rethinking food systems for healthy diets and improved nutrition. Cairo, Egypt: FAO (2020).
53. Maxwell, D, Haan, N, Bilukha, O, Hailey, P, Seal, A, and Lopez, J. Famine review of the IPC acute food insecurity and acute malnutrition analyses. Conclusions and Recommendations for Five Areas in Yemen (Abs, Haradh and Midi In Hajjah Governorate and Al Hali and Al Hawak in Al Hudaydah Governorate). University College London (UCL). (2022).
54. Lu, J, Cai, J, Ren, T, Wu, J, Mao, D, Li, W, et al. Physiological requirements for iron in women of reproductive age assessed by the stable isotope tracer technique. Nutr Metabolism. (2019) 16:1–8. doi: 10.1186/s12986-019-0384-1
55. Houghton, L, and O’Connor, D. Pregnancy and lactation. Optimizing Women’s Health Through Nutr. (2008) 1:65–92. doi: 10.1201/9781420043013.ch5
56. Soda, MA, Hamuli, EK, Batina, SA, and Kandala, N-B. Determinants and spatial factors of anemia in women of reproductive age in Democratic Republic of Congo (drc): a Bayesian multilevel ordinal logistic regression model approach. BMC Public Health. (2024) 24:202. doi: 10.1186/s12889-023-17554-y
57. Lover, AA, Hartman, M, Chia, KS, and Heymann, DL. Demographic and spatial predictors of anemia in women of reproductive age in Timor-Leste: implications for health program prioritization. PLoS One. (2014) 9:e91252. doi: 10.1371/journal.pone.0091252
58. Ejigu, BA, Wencheko, E, and Berhane, K. Spatial pattern and determinants of anaemia in Ethiopia. PLoS One. (2018) 13:e0197171. doi: 10.1371/journal.pone.0197171
59. Report GN. Mozambique nutrition profile. (2022). Available at: https://globalnutritionreport.org/resources/nutrition-profiles/africa/eastern-africa/mozambique/.
60. Afai, G, Rossetto, EV, Baltazar, CS, Candrinho, B, Saifodine, A, and Zulliger, R. Factors associated with knowledge about malaria prevention among women of reproductive age, Tete Province, Mozambique, 2019–2020. Malar J. (2022) 21:76. doi: 10.1186/s12936-022-04090-0
61. de Sousa, PL, Arroz, JA, Martins, MRO, Hartz, Z, Negrao, N, Muchanga, V, et al. Malaria prevention knowledge, attitudes, and practices in Zambezia Province, Mozambique. Malar J. (2021) 20:293–303. doi: 10.1186/s12936-021-03825-9
62. Llop-Gironés, A, Julià, M, Chicumbe, S, Dulá, J, Odallah, AAP, Alvarez, F, et al. Inequalities in the access to and quality of healthcare in Mozambique: evidence from the household budget survey. Int J Qual Health Care. (2019) 31:577–82. doi: 10.1093/intqhc/mzy218
63. Gonçalves, SD, and Moultrie, TA. Short preceding birth intervals and child mortality in Mozambique. Afr J Reprod Health. (2012) 16:29–42. doi: 10.2307/23485773
64. Muhajarine, N, Adeyinka, DA, Matandalasse, M, and Chicumbe, S. Inequities in childhood anaemia at provincial borders in Mozambique: cross-sectional study results from multilevel Bayesian analysis of 2018 National Malaria Indicator Survey. BMJ Open. (2021) 11:e051395. doi: 10.1136/bmjopen-2021-051395
65. Fotheringham, AS, Brunsdon, C, and Charlton, M. Geographically weighted regression. Sage Handbook Spatial Analysis. (2009) 1:243–54. doi: 10.4135/9780857020130.n13
66. Páez, A, Uchida, T, and Miyamoto, K. A general framework for estimation and inference of geographically weighted regression models: 1. Location-specific kernel bandwidths and a test for locational heterogeneity. Environ Plan A. (2002) 34:733–54. doi: 10.1068/a34110
67. Victor, C, Vega Ocasio, D, Cumbe, ZA, Garn, JV, Hubbard, S, Mangamela, M, et al. Spatial heterogeneity of neighborhood-level water and sanitation access in informal urban settlements: a cross-sectional case study in Beira, Mozambique. PLoS Water. (2022) 1:e0000022. doi: 10.1371/journal.pwat.0000022
68. Maulide Cane, R, Chidassicua, JB, Varandas, L, and Craveiro, I. Anemia in pregnant women and children aged 6 to 59 months living in Mozambique and Portugal: an overview of systematic reviews. Int J Environ Res Public Health. (2022) 19:4685. doi: 10.3390/ijerph19084685
69. Onoh, R, Lawani, O, Ezeonu, P, Nkwo, P, Onoh, T, and Ajah, L. Predictors of anemia in pregnancy among pregnant women accessing antenatal care in a poor resource setting in South Eastern Nigeria. Sahel Med J. (2015) 18:182–7. doi: 10.4103/1118-8561.176588
70. Toteja, G, Singh, P, Dhillon, B, Saxena, B, Ahmed, F, Singh, R, et al. Prevalence of anemia among pregnant women and adolescent girls in 16 districts of India. Food Nutr Bull. (2006) 27:311–5. doi: 10.1177/156482650602700405
71. Gebre, A, and Mulugeta, A. Prevalence of anemia and associated factors among pregnant women in North Western Zone of Tigray, Northern Ethiopia: a cross-sectional study. J Nutr Metabolism. (2015) 2015:165430:1–7. doi: 10.1155/2015/165430
72. Nair, M, Choudhury, MK, Choudhury, SS, Kakoty, SD, Sarma, UC, Webster, P, et al. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Glob Health. (2016) 1:e000026. doi: 10.1136/bmjgh-2015-000026
73. Assefa, S, Mossie, A, and Hamza, L. Prevalence and severity of anemia among school children in Jimma Town, Southwest Ethiopia. BMC Hematol. (2014) 14:3. doi: 10.1186/2052-1839-14-3
74. Ngnie-Teta, I, Kuate-Defo, B, and Receveur, O. Multilevel modelling of sociodemographic predictors of various levels of anaemia among women in Mali. Public Health Nutr. (2009) 12:1462–9. doi: 10.1017/S1368980008004400
75. Banhidy, F, Acs, N, Puho, EH, and Czeizel, AE. Iron deficiency anemia: pregnancy outcomes with or without iron supplementation. Nutrition. (2011) 27:65–72. doi: 10.1016/j.nut.2009.12.005
76. Yakoob, MY, and Bhutta, ZA. Effect of routine iron supplementation with or without folic acid on anemia during pregnancy. BMC Public Health. (2011) 11:1–10. doi: 10.1186/1471-2458-11-S3-S21
77. Dim, CC, and Onah, HE. The prevalence of anemia among pregnant women at booking in Enugu, South Eastern Nigeria. Medscape Gen Med. (2007) 9:11.
78. Story, WT, Pritchard, S, Hejna, E, Olivas, E, and Sarriot, E. The role of integrated community case management projects in strengthening health systems: case study analysis in Ethiopia, Malawi and Mozambique. Health Policy Plan. (2021) 36:900–12. doi: 10.1093/heapol/czaa177
79. World Health Organization. Weekly Iron-Folic Acid Supplementation (WIFS) in women of reproductive age: its role in promoting optimal maternal and child health. Manila, Philippines: World Health Organization (2009).
80. Cleland, J, Bernstein, S, Ezeh, A, Faundes, A, Glasier, A, and Innis, J. Family planning: the unfinished agenda. Lancet. (2006) 368:1810–27. doi: 10.1016/S0140-6736(06)69480-4
81. Vogel, JP, Pileggi-Castro, C, Chandra-Mouli, V, Pileggi, VN, Souza, JP, Chou, D, et al. Millennium Development Goal 5 and adolescents: looking back, moving forward. Arch Dis Child. (2015) 100:S43–7. doi: 10.1136/archdischild-2013-305514
82. Mamabolo, R, Alberts, M, Levitt, N, Delemarre-Van De Waal, H, and Steyn, N. Prevalence of gestational diabetes mellitus and the effect of weight on measures of insulin secretion and insulin resistance in third-trimester pregnant rural women residing in the Central Region of Limpopo Province, South Africa. Diabet Med. (2007) 24:233–9. doi: 10.1111/j.1464-5491.2006.02073.x
83. Muthayya, S, Rah, JH, Sugimoto, JD, Roos, FF, Kraemer, K, and Black, RE. The global hidden hunger indices and maps: an advocacy tool for action. PLoS One. (2013) 8:e67860. doi: 10.1371/journal.pone.0067860
84. Iimi, A. Estimating the impact of improved roads on access to health care: evidence from Mozambique. Washington, D.C., USA: The World Bank (2021).
85. Rossi, B, Formenti, B, Cerini, C, Tique, N, da Celma, CR, Boniotti, F, et al. Addressing health care disruption in rural Mozambique due to extreme climate events: mobile units tackling cyclones, vaccine-preventable diseases, and beyond. Front Trop Dis. (2024) 5:1328926. doi: 10.3389/fitd.2024.1328926
86. Longo, DL, and Camaschella, C. Iron-deficiency anemia. N Engl J Med. (2015) 372:1832–43. doi: 10.1056/NEJMra1401038
Keywords: anemia, predictors, women, geographically weighted, Mozambique normal
Citation: Gebrehana DA, Tamir TT, Molla GE, Kebede Y, Tegegne D, Nigatu SG and Nigatu AM (2025) Spatial variation and predictors of anemia among women of reproductive age in Mozambique, 2022/23: a multiscale geographically weighted regression. Front. Public Health. 13:1502177. doi: 10.3389/fpubh.2025.1502177
Edited by:
Wei Wang, Capital Medical University, ChinaReviewed by:
Guangda He, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaRu Zhang, Peking Union Medical College Hospital (CAMS), China
Copyright © 2025 Gebrehana, Tamir, Molla, Kebede, Tegegne, Nigatu and Nigatu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deresse Abebe Gebrehana, ZGVyZXNzZWFiZWJlNEBnbWFpbC5jb20=
 Deresse Abebe Gebrehana
Deresse Abebe Gebrehana Tadesse Tarik Tamir
Tadesse Tarik Tamir Gebretsadik Endeshaw Molla3
Gebretsadik Endeshaw Molla3 Solomon Gedlu Nigatu
Solomon Gedlu Nigatu