
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 25 March 2025
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1502036
Introduction: Acute respiratory tract infections impose a considerable burden on the health services. The development of improved prevention and treatment measures requires a better understanding of the mechanisms of infection. Since sex has been shown to be an important biological variable in the immune response to infections, we aimed to assess sex differences in the incidence rates of respiratory infections.
Materials and methods: We obtained data on cases hospitalized with diagnosed respiratory tract infections by sex and age group over a period of 11 years (2012–2022) from the Sheba Medical Center (SMC), the largest tertiary care medical center in Israel. Nasopharyngeal samples collected from the patients with symptoms of a respiratory tract infection were examined for adenovirus, influenza, rhinovirus, parainfluenza-3, human metapneumovirus (hMPV) and respiratory syncitial virus (RSV) in the Central Virology Laboratory and Viral RNA/DNA was extracted and tested using a real-time reverse transcription-PCR (rRT-PCR) assay. We calculated annual male to female incidence rate ratios (IRRs) which were combined over the period of the study using meta-analysis methodology.
Results: There was a male excess in infection rates for all viruses, particularly in the youngest age groups of <0 and 1–4 years. Our analyses revealed that the influenza incidence rates were 42 and 28% higher in males in infants and toddlers. The male dominance was similar for adenovirus with 33 and 38% in infancy and age group 1–4. For RSV, the male to female IRR was higher at ages <1 and 1–4 (22 and 21% respectively). Males were more likely to be positive for rhinovirus in infancy and toddlers, by 40 and 25%, respectively.
Conclusion: There is evidence of an excess incidence of respiratory diseases in males. The mechanism is unclear. Other than behavioral factors, there is a need to study the role of sex hormones and genetic factors.
Acute respiratory tract infections (ARTIs) are among the most common causes of morbidity worldwide (1, 2). These infections are caused by a variety of viruses (3, 4) and are responsible for a large proportion of physician visits (5, 6). The most common respiratory viruses that infect humans include adenoviruses, coronaviruses, human metapneumoviruses (hMPVs), rhinoviruses (RVs), influenza viruses, enteroviruses, parainfluenza virus (PIV), and respiratory syncytial virus (RSV) and COVID-19 (7).
There are varying clinical manifestations for each virus, occasionally with complications severe enough to require hospitalization (8, 9). Each of these viruses can be associated with wide range of symptoms, from influenza-like symptoms to acute respiratory distress syndrome that can lead to hospitalization, comorbidities and death (7). In the 20th century, influenza and pneumonia constituted the highest proportion of infectious disease deaths in the United States with a higher age-adjusted mortality rates in males than females (10). Rhinovirus is the factor that causes more than 50% of upper respiratory tract infections worldwide and together with RSV, RV is one of the leading causes of viral infections in infants (11). Influenza A viruses are responsible for seasonal epidemics and occasional outbreaks, and sporadic pandemics (12). The COVID-19 pandemic began with an outbreak of pandemic, caused the public health emergency of international concern with 7,079,129 confirmed deaths (13). Sex differences in selected infections including COVID-19 have been described (14).
There are reports indicating that males are overrepresented in incidence of infectious diseases (15–19) although this varies by disease and age. In a retrospective cohort Dutch study, the researchers found that female patients had a significantly higher incidence of respiratory symptoms compared with males (20). In another study on sex differences in the incidence of respiratory tract infections, males develop otitis media, croup and respiratory tract infections more frequently than females (21).
Many funding agencies in Europe and North America support and encourage researchers to consider sex as a biological variable in medical research (22). Sex is a biological variable that influences both the innate and adaptive immune systems. The presence of sex hormone receptors across multiple cell tissues and the pattern of expression of X-linked genes means that sex is a biological factor that influence the function of many physiological systems, including the immune system (23). The understanding of the ways in which sex impact on the immune response to respiratory virus infections is limited by the relative lack of data on this variable.
The objective of this study was to explore possible sex differences in the incidence of infections caused by selected respiratory viruses by age group, among patients hospitalized in a largest tertiary care hospital in Israel, between years 2012–2022.
This study includes the results of laboratory tests of all nasopharyngeal samples collected from patients hospitalized at Sheba Medical Center due to respiratory illness and influenza-like symptoms between January 2012 and June 2022. Sheba is the largest tertiary care medical center encompassing six hospitals and centers, treating over 1.2 million people each year (24) which provides medical treatment and service to the population in the center of Israel. The source population (about 1.200.000 population) was estimated on the basis of the number of patients treated relative to the total number of patients receiving health care in Israel.
The samples were analyzed by the Central Virology Laboratory and tested for respiratory viruses including adenovirus, influenza, rhinovirus, parainfluenza-3, human metapneumovirus (hMPV) and respiratory syncytial virus (RSV).
If a patient had a culture sent simultaneously from the nose or pharynx or mouth or both the nose and throat and the same pathogen was detected in all tests, then the duplicates were removed and just one result was included. If a patient was diagnosed with infections due to different pathogens, each infection was recorded separately. If the patient was diagnosed with multiple infections during different time periods, all infections were included.
All the samples were collected before treatment using Ʃ-Virocult Swab and Virus Transport Medium (MWE Medical Wire, England) and transported to the laboratory within 8 h from collection. Samples were stored at 4°C pending molecular analysis within 24 h of sample collection. Until January 2020, viral RNA/DNA was extracted using MagNA PURE 96 (Roch, Manheim, Germany). After January 2020, viral RNA/DNA extraction was performed using the STARMag Viral DNA/RNA 200C kit (Seegene Inc., South Korea).
Viral RNA/DNA of influenza, RSV, hMPV, parainfluenza, and adenoviruses were tested using real-time reverse transcription-PCR (rRT-PCR) assay (25, 26). The test master mix was prepared with Ambion Ag-Path master mix (Life technologies, United States) in multiplex reactions. Two different multiplex tests were performed: one reaction that included primers for influenza A (H3N2), influenza A (H1N1pdm), influenza B, RSV and the other containing primers for hMPV, parainfluenza and adenovirus. The RT-PCR assay was performed on an ABI 7500 instrument (Thermo Fisher Scientific, United Kingdom). From January 2020, rRT-PCR assays were also performed using the AllplexTM RV essential assay (Seegene Inc., South Korea) in a CFX Real-time PCR system (Bio-Rad, United States) (27, 28).
The annual male to female incidence rate ratios (IRRs) were calculated for each year and combined over the period of the study using meta-analytic methods. The grouping by age was as follows: infants <1, toddlers 1–4, middle childhood (5–9), puberty (10–14), reproductive age (15–44), middle adulthood (45–64) years, senior adulthood (65–79) and older adulths (80+). The annual disease incidence rate (IR) for each disease/age group was calculated by dividing the number of test positive patients by the size of the relevant total population for each year according to sex and age group expressed per 100,000 of population. The combined male to female incidence rate ratios over the years of the study were calculated using both the Mantel–Haenszel estimator for the fixed effects model and the Desmonian-Laird estimator for the random effects model. The results for both the fixed and random effects models are presented. The statistical significance level was set at 0.05 for both the fixed and random effects models (29). Analyses were carried out using WinPepi (Version 11.65, Aug, 2016).
All data collected from the Central Virology Laboratory were anonymous. The SMC institutional review board approved the research (Helsinki Number 0801-23-SMC).
The summary of isolates by age and sex for acute respiratory tract infections (for influenza, adenovirus, RSV, parainfluenza-3, rhinovirus and hMPV) between years 2012–2022 and midpoint population size is presented in Table 1. In absolute numbers, influenza was more common in older ages of 15–44, 45–64, 65–79, and 80+, while RSV was more common at ages of <1, 1–4, 15–44, 45–64, 65–80+. Adenovirus was more common at ages <1 and 1–4. Low incidence of rhinovirus, parainfluenza- 3 and hMPV cases was observed at ages 5–9 and 10–14 (Supplementary data on Table 3 shows data on the source population estimation for each calendar year by age and sex).
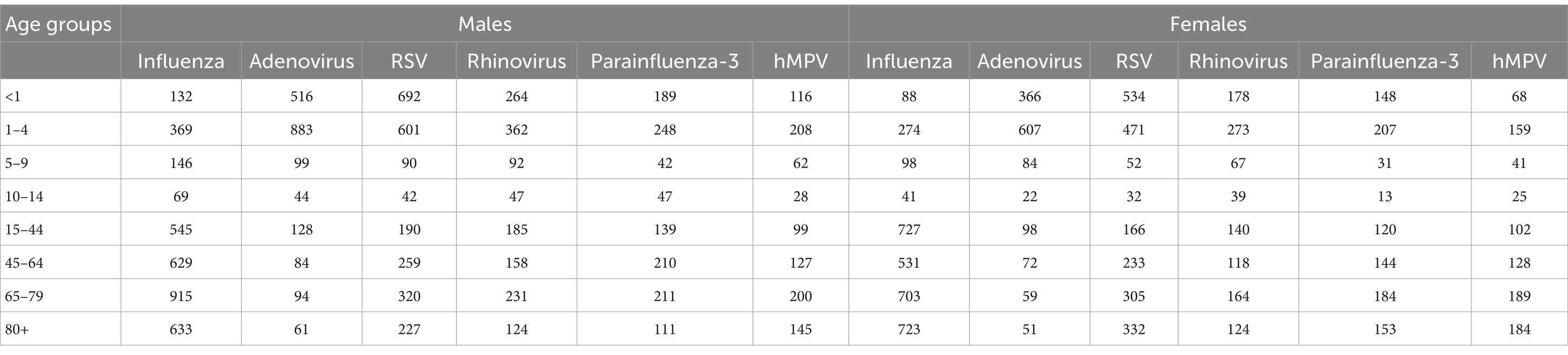
Table 1. Summary of the absolute numbers of isolates for influenza, adenovirus, RSV, rhinovirus, parainfluenza-3, and hMPV viruses at Sheba Medical Center in Israel, by age and sex, between the years 2012–2022.
The data on the annual male to female annual incidence rates with 95% confidence intervals and p-values are presented in Table 2. At young ages between infancy and puberty (<1, 1–4, 5–9, 10–14) and at later ages (45–64, 65–79 and 80+), significantly higher incidence rates of influenza were observed in males than in females (IRR = 1.42, IRR = 1.28, IRR = 1.42, IRR = 1.60 and IRR = 1.25, IRR = 1.53 and IRR = 1.33 respectively). A similar trend was revealed for adenovirus. Male adenovirus incidence rates were higher in all ages with significant differences at ages <1, 1–4, 10–14, 65–79 and 80+ (IRR = 1.33, IRR = 1.38, IRR = 1.90, IRR = 1.88 and IRR = 1.82 respectively). Higher incidence rates of RSV in males were observed at all ages with the significant differences between sexes at ages <1 (IRR = 1.22),1–4 (IRR = 1.21), 5–9 (IRR = 1.65) and 65–79 (IRR = 1.24).

Table 2. Male to female incidence rate ratios (IRR) with 95% confidence intervals, for influenza, adenovirus, RSV, rhinovirus, parainfluenza-3, and hMPV viruses, by age groups, at the Sheba Medical Center, between the years 2012–2022.
The incidence rates of parainfluenza-3 in males were higher in all age groups. Significant differences between males and females were demonstrated at ages 10–14, 45–64 and 65–79 (IRR = 3.35, IRR = 1.54 and 1.35 respectively). Higher male incidence rates in rhinovirus were observed at all ages with significant differences between sexes at ages <1, 1–4, 15–44, 45–64, 65–79, and 80+ (IRR = 1.40, IRR = 1.25, IRR = 1.30, IRR = 1.40, IRR = 1.65 and IRR = 1.50 for age groups respectively). The excess male hMPV incidence rates were significantly higher in infancy and senior adulthood (IRR = 1.61 and IRR = 1.25).
Data on respiratory tract viruses Isolation rates per 100.000 population, by age and sex for years 2012–2022 are presented in Figures 1A,B. For all viruses, isolation rates per 100.000 population in young ages were higher in males than in females of the same age. For adenovirus and RSV isolation rates per 100.000 population at ages <1, 1–4 were as follows: 427 vs. 320, 185 vs. 134 (for adenovirus) and 573 vs. 442, 126 vs. 99 (for RSV) respectively by age groups. The same trend was observed for influenza, rhinovirus, parainfluenza-3 and hMPV. For young ages of <1 and 1–4 the isolation rates per 100.000 population at ages were as follows 109 vs. 77, 77 vs. 61 for influenza, 219 vs. 156,76 vs. 60 for rhinovirus, 157 vs. 123, 52 vs. 43 for parainfluenza-3 and 96 vs. 60, 44 vs. 35 for hMPV.
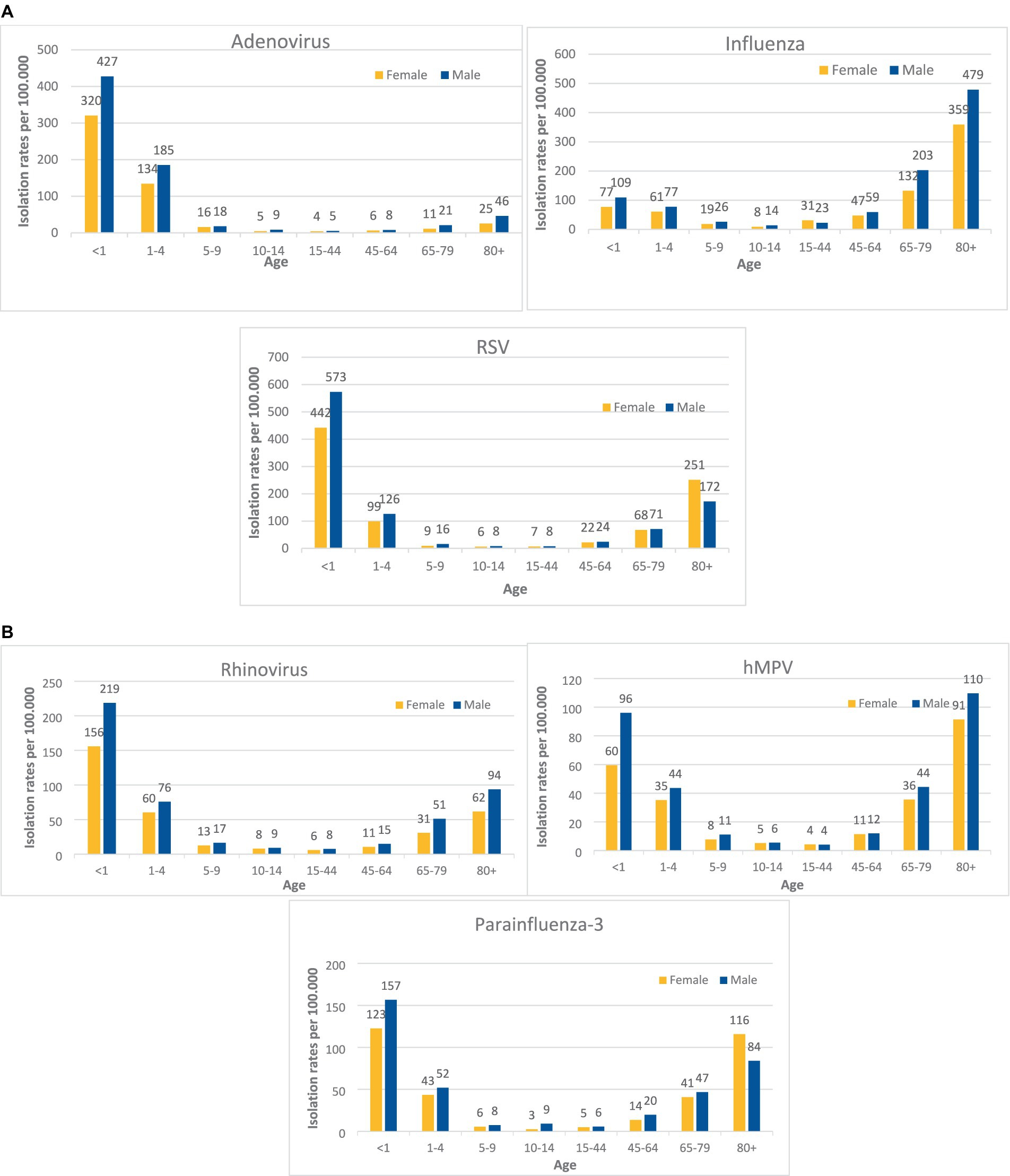
Figure 1. (A) Virus isolation rates per 100.000 population, at the Sheba Medical Center, by age and sex, 2012–2022. (B) Virus isolation rates per 100.000 population, at the Sheba Medical Center, by age and sex, 2012–2022.
For all viruses, isolation rates per 100.000 population at ages 5–9, 10–14, 15–44, and 45–64 were low and the differences between sexes were negligible. Influenza virus isolation rate showed a male dominance for older age groups (except 15–44), 203 vs. 132 and 479 vs. 359 for ages 65–79 and 80+ respectively. Similar to the influenza virus, other pathogens isolation rates per 100.000 population at older ages (65–79, 80+) were higher in males in compare to females:44 vs. 36 and 110 vs. 91 for hMPV, 51 vs. 31 and 94 vs. 62 for rhinovirus, and 46 vs. 25 for adenovirus at age 80+. Parainfluenza 3 and RSV viruses isolation rates per 100.000 population were higher in females aged 80+ then in males of same age (116 vs. 84 and 251 vs. 172 respectively).
We observed seasonal variations in most of the viruses with the highest incidence during the winter months from November through March. hMPV and Adenovirus demonstrated a distinct seasonal variation with peaks in December to April and with decreased cases in September through November (Figure 2). Substantial levels of influenza virus and RSV activity and well-defined seasonality were demonstrated from December to March.
In this study of respiratory tract viruses isolated from patients from the largest hospital in Israel, we found that there was generally an excess of males in the incidence rates. Our analyses revealed that the influenza incidence rates were 42, 28, 42 and 60% higher in males in infants, toddlers, middle childhood and puberty, respectively. The trends of male dominance were similar for adenovirus with 33, 38, and 90% excess incidence in infancy, age group 1–4 and 10–14, respectively. For RSV male to female IRR was significantly higher at ages <1, 1–4, and 5–9 (22, 21 and 65% respectively). For parainfluenza-3 significant differences were evident in puberty and at ages 45–64, 65–79 with male preponderance of incidence of the disease (235, 54 and 35% respectively). Males were more likely to be positive for rhinovirus in infancy (40%), toddler age (25%) and in in older ages. Significant sex difference was demonstrated in hMPV incidence with 61% excess in males in infancy. In older ages (65+), the male: female IRR for almost all respiratory viruses was high and significant, except the RSV, parainfluenza-3 and hMPV at ages 80 + .
In a study that provides an overview of RSV prevalence in Iran from 1996 to 2013 the male–female ratio of RSV-positive patients was 1.5:1 (30). In the study on 16,018 patients with acute lower respiratory tract infection from a study in Buenos Aires, adenovirus infection was associated with age ≥ 12 months and male sex (31). A retrospective cohort study in Israel, on 1,227 children aged 0–23 months, showed that being a male was significantly associated with an increased incidence of RSV (male:female ratio of 1.35) (32).
There are contradictory data regarding the excess male incidence rates for acute respiratory tract infections. In Victoria, Australia, females were more likely to be positive for influenza and hMPV. There was no significant difference in sex distribution for the other viruses as RSV and parainfluenza (33). In study on hospitalized children<5 years old from Naples, Southern Italy, respiratory infection viruses demonstrated no substantial differences in sex and age (34).
In a large epidemiologic study in China, the overall viral detection rates were not significantly different between male and female patients, although female patients displayed higher rates for influenza virus, hMPV, and adenovirus (35). In a prospective cohort study among >1,000 children aged 0–35 months in Quebec City, Canada, with acute respiratory infection female sex was a risk factors for severe hMPV disease (36). In the study on children and adult patients n Córdoba, Argentina, of the 795 clinical specimens analyzed, the incidence of hMPV was higher in female patients in compared to male patients with ratio of 0.8 (37). In the prospective study of rhinovirus infections in adults (median age 50) recruited from 16 primary care networks in 11 European countries, the male to female ratio was 1:1.5 (38).
In this study we cannot provide mechanisms that may explain the sex differences in incidence of acute respiratory tract viral infections. Nevertheless, we can hypothesize that sex hormones and genetic differences are involved. We do not believe that in infancy and young children, behavioral differences between the sexes could affect the incidence rates of respiratory tract viral infections. We suggest that particularly in these ages, sex hormones and genetic factors could contribute a significant role in the sex differences observed. The mini-puberty period in infancy is associated with the transient sex-specific activation of the hypothalamic–pituitary-gonadal axis during the first 6 months of life in boys and during the first 2 years in girls. This phenomenon leads to a rise of sex hormones including estradiol, and testosterone and essential for physiological, including immune system body functions (39). Epigenetic modifications that affect X-linked gene expression could affect the immune response to infections in all ages (40).
At later ages, the differences between the sexes in incidence of respiratory tract infections may partly be explained by behavioral and social differences. Females may be more exposed to viruses when caring for children and in the workplace such as schools and kindergartens. Asymptomatic persons, especially children can be an important source of infection in their environment and could explain the increase of female disease incidence rate at childbearing ages of 15–44 and the older ages. In a study of Johnston SL et al. the viruses were isolated from asymptomatic subjects in 12% of children for rhinovirus (41). In Netherlands, the frequencies of subclinical infection and of asymptomatic subjects was high, 68%, for children at ages 0–15 (42). Occupations that involve considerable interaction with infected children can explain at least in part the differences in respiratory tract infection between ages15–44 and older.
In older ages, for males and females with the same chronological age, males prone to be biologically older (43). This also fits the established longer lifespan of females compared to males (44) which could be cause by biological factors, including genetic differences, sex hormones, age-related decline in testosterone (45, 46).
Some extrinsic factors such as preexisting immunity and microbiota could give at least partial explanation for sex differences in acute respiratory tract infections (47). The airway microbiome is involved in interactions between sex hormones, and immune systems, and it is highly influence on disease susceptibility. The number of shared microbiome species between the sexes was significantly lower than expected in the case in the respiratory tract (48).
Sex hormones affect the functioning of immune cells. Estrogen influence and enhance humoral responses to viral infection (49), Interferons (IFNs) and IRF5 express estrogen response elements (50, 51), are critical for protection from viral infection and cause the increased cytokine and chemokine production (52–56). Estradiol activity shows profound dose- and context-dependent effects on innate immune signaling pathways and promotes the production of interferons (57). Testosterone can decrease NK cell, neutrophil, and macrophage activity and reduce the production of pro-inflammatory cytokines, such as TNF-α (58). Testosterone diminishes pulmonary tissue inflammation, including virus-specific CD8+ T cells after the virus has been cleared (59). The pregnancy-related sex hormone concentrations and profound hormonal changes underlie many of the important immunological processes (60).
Genetic factors also could be implicated. There are many important genes, part of immune regulatory pathways, on the X chromosome, which escape the X inactivation event (61). Damaged genes on the X chromosome in males of significant immunological importance in males than in females, introducing sex-based bias and influencing the sex differences in immune response to infection (62, 63).
Existing clinical data show that the population, including children, young and aged individuals, males and females differ in vaccine-induced immune responses and protection. The study of Flanagan et al. (23) revealed that males are more likely to get vaccines, but following vaccination, females prone to develop higher antibody levels than do males. In Israel, male sex and older age were found to be associated with a higher willingness to be get vaccination (64).
For all the viruses studied, the information about seasonality is important in vaccination and health services planning, especially when viruses co-circulate and putting pressure on hospitals. In Israel, influenza epidemics occur a little later than respiratory syncytial virus (December–April vs. November–March). Compared to other reports, in Israel, parainfluenza virus epidemics were found mostly in period between November to April (65). hMPV epidemics occurred in winter and spring. There is no demonstrated seasonality in adenovirus and rhinovirus epidemics, but there are peaks in the incidence of the disease in months November–March and November–December, respectively.
In Israel, during the COVID-19 pandemic a dramatic reduction in RSV and influenza virus activity was found. The circulation of both the parainfluenza and adenoviruses were mildly affected during the COVID-19 pandemic. For RSV, there was a seasonality shift with a delayed disease outbreak and greater weekly incidence rates (66, 67).
We collected the molecular data from the analyses of respiratory samples over 11 years (2012 to 2022), which allowed us to obtain robust estimations of the incidence of these viruses in hospitalized patients in Israel. Our study population is a good representation of most of the population in Israel as Sheba Hospital, is the largest tertiary care medical center in Israel and in the entire Middle East (24) which provides medical treatment and service to the population in the center of Israel, treats over 1.200.000 patients annually.
The population seeks care in other primary or secondary hospitals and incidence estimates may be underestimated. There is no reason to believe that it will have a different effect on males and females and on sex ratios. In addition, this is a single-center study, thus, the generalizability of the results may be limited.
Underreporting is a known limitation, but it is unlikely to influence the findings since the objective was to study IRR and not absolute differences. The structure of the health system and society in Israel minimizes sex-related differences in access to medical service. We did not have information on possible exposure differences. In addition, the study was a single-center, thus, the generalizability of the results may be limited. In addition, cases with two or more pathogens were included more than once. This would affect the incidence rates, but the sex ratios should not be affected. It has been reported that the respiratory infection patterns and pathogen distributions changed significantly during the pandemic (68). Comparing age and sex differences in SARS-CoV-2 hospitalization and mortality with MERS-CoV, seasonal coronaviruses, influenza and other health outcomes revealed that age-specific sex differences in hospitalizations are largely similar across endemic and emerging infections (69). There is no reason to consider sex related differences in behavior or social distancing measures in children that would affect the infections transmission during COVID-19 pandemic (70).
The results of this study add to the evidence of about a male dominance in the incidence rates of respiratory viruses, especially in young ages. Sex differences in the incidence of respiratory virus infections can contribute to a better understanding of the mechanisms of disease, and the development of improved prevention and treatment.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
All data collected from the Central Virology Laboratory were anonymous. The SMC institutional review board approved the research (Helsinki Number 0801-23-SMC). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was not provided by the participants’ legal guardians/next of kin because all data collected from the Central Virology Laboratory were anonymous. Written informed consent was not obtained from the individual(s), nor the minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article because all data collected from the Central Virology Laboratory were anonymous. There is no potentially identifiable information.
VP: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Visualization, Writing – original draft, Writing – review & editing. MM: Conceptualization, Data curation, Project administration, Resources, Writing – review & editing. MJ: Data curation, Resources, Writing – review & editing. MG: Conceptualization, Data curation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1502036/full#supplementary-material
1. Li, Y, Wang, X, Blau, DM, Caballero, MT, Feikin, DR, Gill, CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. (2022) 399:2047–64. doi: 10.1016/S0140-6736(22)00478-0
2. Shi, T, Denouel, A, Tietjen, AK, Campbell, I, Moran, E, Li, X, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. (2020) 222:S577–83. doi: 10.1093/infdis/jiz059
3. Peltola, V, Waris, M, Osterback, R, Susi, P, Ruuskanen, O, and Hyypia, T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. (2008) 197:382–9. doi: 10.1086/525542
4. Zaas, AK, Chen, M, Varkey, J, Veldman, T, Hero, AO 3rd, Lucas, J, et al. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell Host Microbe. (2009) 6:207–17. doi: 10.1016/j.chom.2009.07.006
5. Troy, NM, and Bosco, A. Respiratory viral infections and host responses; insights from genomics. Respir Res. (2016) 17:156. doi: 10.1186/s12931-016-0474-9
6. Gannon, CJ, Pasquale, M, Tracy, JK, McCarter, RJ, and Napolitano, LM. Male gender is associated with increased risk for postinjury pneumonia. Shock. (2004) 21:410–4. doi: 10.1097/00024382-200405000-00003
7. Ursin, RL, and Klein, SL. Sex differences in respiratory viral pathogenesis and treatments. Annu Rev Virol. (2021) 8:393–414. doi: 10.1146/annurev-virology-091919-092720
8. Nguyen-Van-Tam, JS, O'Leary, M, Martin, ET, Heijnen, E, Callendret, B, Fleischhackl, R, et al. Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur Respir Rev. (2022) 31:220105. doi: 10.1183/16000617.0105-2022
9. Caini, S, Kroneman, M, Wiegers, T, El Guerche-Séblain, C, and Paget, J. Clinical characteristics and severity of influenza infections by virus type, subtype, and lineage: a systematic literature review. Influenza Other Respir Viruses. (2018) 12:780–92. doi: 10.1111/irv.12575
10. Ashraf, H, Ashfaq, H, Ahmed, S, and Ashraf, A. Two decades of influenza and pneumonia mortality trends: demographics, regional shifts and disparities in the United States: 1999 to 2020. Am J Infect Control. (2024) 52:00495-4, 1152–1159. doi: 10.1016/j.ajic.2024.05.003
11. Vandini, S, Biagi, C, Fischer, M, and Lanari, M. Impact of rhinovirus infections in children. Viruses. (2019) 11:521. doi: 10.3390/v11060521
12. Krammer, F, Smith, GJD, Fouchier, RAM, Peiris, M, Kedzierska, K, Doherty, PC, et al. Influenza. Nat Rev Dis Primers. (2018) 4:3. doi: 10.1038/s41572-018-0002-y
13. Mathieu, E, Ritchie, H, Rodés-Guirao, L, Appel, C, Giattino, C, Hasell, J, et al. “Coronavirus pandemic (COVID-19) ” (2020–2024). Our World in Data. https://ourworldindata.org/coronavirus (Accessed December 30, 2024).
14. Haitao, T, Vermunt, JV, Abeykoon, J, Ghamrawi, R, Gunaratne, M, Jayachandran, M, et al. COVID-19 and sex differences: mechanisms and biomarkers. Mayo Clin Proc. (2020) 95:2189–203. doi: 10.1016/j.mayocp.2020.07.024
15. Peer, V, Schwartz, N, and Green, MS. A pooled analysis of sex differences in rotaviral enteritis incidence rates in three countries over different time periods. Womens Health Rep. (2022) 3:228–37. doi: 10.1089/whr.2021.0096
16. Peer, V, Schwartz, N, and Green, MS. Consistent, excess viral meningitis incidence rates in young males: a multi-country, multi-year, meta-analysis of national data. The importance of sex as a biological variable. EClinicalMedicine. (2019) 15:62–71. doi: 10.1016/j.eclinm.2019.08.006
17. Green, MS, Schwartz, N, and Peer, V. Sex differences in campylobacteriosis incidence rates at different ages – a seven country, multi-year, meta-analysis. A potential mechanism for the infection. BMC Infect Dis. (2020) 20:625. doi: 10.1186/s12879-020-05351-6
18. Green, MS, Schwartz, N, and Peer, V. Sex differences in hepatitis a incidence rates-a multi-year pooled-analysis based on national data from nine high-income countries. PLoS One. (2023) 18:e0287008. doi: 10.1371/journal.pone.0287008
19. Walter, F, Ott, JJ, Claus, H, and Krause, G. Sex- and age patterns in incidence of infectious diseases in Germany: analyses of surveillance records over a 13-year period (2001-2013). Epidemiol Infect. (2018) 146:372–8. doi: 10.1017/S0950268817002771
20. Groeneveld, JM, Ballering, AV, van Boven, K, Akkermans, RP, Olde Hartman, TC, and Uijen, AA. Sex differences in incidence of respiratory symptoms and management by general practitioners. Fam Pract. (2020) 37:631–6. doi: 10.1093/fampra/cmaa040
21. Falagas, ME, Mourtzoukou, EG, and Vardakas, KZ. Sex differences in the incidence and severity of respiratory tract infections. Respir Med. (2007) 101:1845–63. doi: 10.1016/j.rmed.2007.04.011
22. Clayton, JA, and Collins, FS. Policy: NIH to balance sex in cell and animal studies. Nature. (2014) 509:282–3. doi: 10.1038/509282a
23. Flanagan, KL, Fink, AL, Plebanski, M, and Klein, SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. (2017) 33:577–99. doi: 10.1146/annurev-cellbio-100616-060718
24. Available online at: https://www.shebaonline.org/ (accessed January 2, 2025).
25. Heim, A, Ebnet, C, Harste, G, and Pring-Akerblom, P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol. (2003) 70:228–39. doi: 10.1002/jmv.10382
26. Meningher, T, Hindiyeh, M, Regev, L, Sherbany, H, Mendelson, E, and Mandelboim, M. Relationships between a(H1N1)pdm09 influenza infection and infections with other respiratory viruses. Influenza Other Respir Viruses. (2014) 8:422–30. doi: 10.1111/irv.12249
27. Folgueira, L, Moral, N, Pascual, C, and Delgado, R. Comparison of the panther fusion and allplex assays for the detection of respiratory viruses in clinical samples. PLoS One. (2019) 14:e0226403. doi: 10.1371/journal.pone.0226403
28. Fratty, IS, Reznik-Balter, S, Nemet, I, Atari, N, Kliker, L, Sherbany, H, et al. Outbreak of influenza and other respiratory viruses in hospitalized patients alongside the SARS-CoV-2 pandemic. Front Microbiol. (2022) 13:902476. doi: 10.3389/fmicb.2022.902476
29. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
30. Salimi, V, Tavakoli-Yaraki, M, Yavarian, J, Bont, L, and Mokhtari-Azad, T. Prevalence of human respiratory syncytial virus circulating in Iran. J Infect Public Health. (2016) 9:125–35. doi: 10.1016/j.jiph.2015.05.005
31. Bakir, J, Juárez, MDV, Lución, MF, Areso, MS, Viegas, M, Mistchenko, AS, et al. Clinical and epidemiological study of acute lower respiratory tract infections caused by adenovirus in hospitalized children. Nineteen years of active epidemiological surveillance. Arch Argent Pediatr. (2020) 118:193–201. doi: 10.5546/aap.2020.eng.193
32. Na'amnih, W, Kassem, E, Tannous, S, Kagan, V, Jbali, A, Hanukayev, E, et al. Incidence and risk factors of hospitalisations for respiratory syncytial virus among children aged less than 2 years. Epidemiol Infect. (2022) 150:e45. doi: 10.1017/S0950268822000152
33. Price, OH, Sullivan, SG, Sutterby, C, Druce, J, and Carville, KS. Using routine testing data to understand circulation patterns of influenza a, respiratory syncytial virus and other respiratory viruses in Victoria, Australia. Epidemiol Infect. (2019) 147:e221. doi: 10.1017/S0950268819001055
34. Botti, C, Micillo, A, Ricci, G, Russo, A, Denisco, A, Cantile, M, et al. Characterization of respiratory infection viruses in hospitalized children from Naples province in southern Italy. Exp Ther Med. (2018) 15:4805–9. doi: 10.3892/etm.2018.6061
35. Liao, X, Hu, Z, Liu, W, Lu, Y, Chen, D, Chen, M, et al. New epidemiological and clinical signatures of 18 pathogens from respiratory tract infections based on a 5-year study. PLoS One. (2015) 10:e0138684. doi: 10.1371/journal.pone.0138684
36. Papenburg, J, Hamelin, MÈ, Ouhoummane, N, Carbonneau, J, Ouakki, M, Raymond, F, et al. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis. (2012) 206:178–89. doi: 10.1093/infdis/jis333
37. Rodriguez, PE, Frutos, MC, Adamo, MP, Cuffini, C, Cámara, JA, Paglini, MG, et al. Human Metapneumovirus: epidemiology and genotype diversity in children and adult patients with respiratory infection in Cordoba, Argentina. PLoS One. (2020) 15:e0244093. doi: 10.1371/journal.pone.0244093
38. Zlateva, KT, van Rijn, AL, Simmonds, P, Coenjaerts, FEJ, van Loon, AM, Verheij, TJM, et al. Molecular epidemiology and clinical impact of rhinovirus infections in adults during three epidemic seasons in 11 European countries (2007-2010). Thorax. (2020) 75:882–90. doi: 10.1136/thoraxjnl-2019-214317
39. Becker, M, and Hesse, V. Minipuberty: why does it happen? Horm Res Paediatr. (2020) 93:76–84. doi: 10.1159/000508329
40. Chlamydas, S, Markouli, M, Strepkos, D, and Piperi, C. Epigenetic mechanisms regulate sex-specific bias in disease manifestations. J Mol Med (Berl). (2022) 100:1111–23. doi: 10.1007/s00109-022-02227-x
41. Johnston, SL, Sanderson, G, Pattemore, PK, Smith, S, Bardin, PG, Bruce, CB, et al. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. (1993) 31:111–7. doi: 10.1128/jcm.31.1.111-117.1993
42. van Gageldonk-Lafeber, A, Heijnen, M, Bartelds, A, Peters, M, van der Plas, S, and Wilbrink, B. A case-control study of acute respiratory tract infection in general practice patients in the Netherlands. Clin Infect Dis. (2005) 41:490–7. doi: 10.1086/431982
43. Horvath, S, Gurven, M, Levine, ME, Trumble, BC, Kaplan, H, Allayee, H, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. (2016) 17:171. doi: 10.1186/s13059-016-1030-0
44. Austad, SN. Why women live longer than men: sex differences in longevity. Gend Med. (2006) 3:79–92. doi: 10.1016/s1550-8579(06)80198-1
45. Mauvais-Jarvis, F, Bairey Merz, N, Barnes, PJ, Brinton, RD, Carrero, JJ, DeMeo, DL, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. (2020) 396:565–82. doi: 10.1016/S0140-6736(20)31561-0
46. Phyo, AZZ, Fransquet, PD, Wrigglesworth, J, Woods, RL, Espinoza, SE, and Ryan, J. Sex differences in biological aging and the association with clinical measures in older adults. Geroscience. (2024) 46:1775–88. doi: 10.1007/s11357-023-00941-z
47. Zimmermann, P, and Curtis, N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. (2019) 32:e00084–18. doi: 10.1128/CMR.00084-18
48. Ma, ZS, and Li, W. How and why men and women differ in their microbiomes: medical ecology and network analyses of the microgenderome. Adv Sci (Weinh). (2019) 6:1902054. doi: 10.1002/advs.201902054
49. Potluri, T, Fink, AL, Sylvia, KE, Dhakal, S, Vermillion, MS, Vom Steeg, L, et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines. (2019) 4:29. doi: 10.1038/s41541-019-0124-6
50. Phiel, KL, Henderson, RA, Adelman, SJ, and Elloso, MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. (2005) 97:107–13. doi: 10.1016/j.imlet.2004.10.007
51. Fox, HS, Bond, BL, and Parslow, TG. Estrogen regulates the IFN-gamma promoter. J Immunol. (1991) 146:4362–7. doi: 10.4049/jimmunol.146.12.4362
52. Klein, SL, and Roberts, CW. Sex hormones and immunity to infection. Berlin, Heidelberg: Springer-Verlag (2010).
53. Qi, F, Wang, D, Liu, J, Zeng, S, Xu, L, Hu, H, et al. Respiratory macrophages and dendritic cells mediate respiratory syncytial virus-induced IL-33 production in TLR3- or TLR7-dependent manner. Int Immunopharmacol. (2015) 29:408–15. doi: 10.1016/j.intimp.2015.10.022
54. Crotta, S, Davidson, S, Mahlakoiv, T, Desmet, CJ, Buckwalter, MR, Albert, ML, et al. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog. (2013) 9:e1003773. doi: 10.1371/journal.ppat.1003773
55. Stanifer, ML, Guo, C, Doldan, P, and Boulant, S. Importance of type I and III interferons at respiratory and intestinal barrier surfaces. Front Immunol. (2020) 11:608645. doi: 10.3389/fimmu.2020.608645
56. Bartlett, NW, Slater, L, Glanville, N, Haas, JJ, Caramori, G, Casolari, P, et al. Defining critical roles for NF-kappa B p 65 and type I interferon in innate immunity to rhinovirus. EMBO Mol Med. (2012) 4:1244–60. doi: 10.1002/emmm.201201650
57. Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. (2015) 294:63–9. doi: 10.1016/j.cellimm.2015.01.018
58. Hou, J, and Zheng, WF. Effect of sex hormones on NK and ADCC activity of mice. Int J Immunopharmacol. (1988) 10:15–22. doi: 10.1016/0192-0561(88)90145-2
59. Vom Steeg, LG, Dhakal, S, Woldetsadik, YA, Park, HS, Mulka, KR, Reilly, EC, et al. Androgen receptor signaling in the lungs mitigates inflammation and improves the outcome of influenza in mice. PLoS Pathog. (2020) 16:e1008506. doi: 10.1371/journal.ppat.1008506
60. Veenstra van Nieuwenhoven, AL, Bouman, A, Moes, H, Heineman, MJ, de Leij, LF, Santema, J, et al. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil Steril. (2002) 77:1032–7. doi: 10.1016/s0015-0282(02)02976-x
61. Bhattacharya, S, Sadhukhan, D, and Saraswathy, R. Role of sex in immune response and epigenetic mechanisms. Epigenetics Chromatin. (2024) 17:1. doi: 10.1186/s13072-024-00525-x
62. Abramowitz, LK, Olivier-Van Stichelen, S, and Hanover, JA. Chromosome imbalance as a driver of sex disparity in disease. J Genomics. (2014) 2:77–88. doi: 10.7150/jgen.8123
63. Schurz, H, Salie, M, Tromp, G, Hoal, EG, Kinnear, CJ, and Möller, M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics. (2019) 13:2. doi: 10.1186/s40246-018-0185-z
64. Green, MS, Abdullah, R, Vered, S, and Nitzan, D. A study of ethnic, gender and educational differences in attitudes toward COVID-19 vaccines in Israel – implications for vaccination implementation policies. Isr J Health Policy Res. (2021) 10:26. doi: 10.1186/s13584-021-00458-w
65. Li, Y, Reeves, RM, Wang, X, Bassat, Q, Brooks, WA, Cohen, C, et al. RSV global epidemiology network; RESCEU investigators. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health. (2019) 7:e1031–45. doi: 10.1016/S2214-109X(19)30264-5
66. Oster, Y, Abu Ahmad, W, Michael-Gayego, A, Rivkin, M, Levinzon, L, Wolf, D, et al. Viral and bacterial respiratory pathogens during the COVID-19 pandemic in Israel. Microorganisms. (2023) 11:166. doi: 10.3390/microorganisms11010166
67. Weinberger Opek, M, Yeshayahu, Y, Glatman-Freedman, A, Kaufman, Z, Sorek, N, and Brosh-Nissimov, T. Delayed respiratory syncytial virus epidemic in children after relaxation of COVID-19 physical distancing measures, Ashdod, Israel, 2021. Euro Surveill. (2021) 26:2100706. doi: 10.2807/1560-7917.ES.2021.26.29.2100706
68. Yang, MC, Su, YT, Chen, PH, Tsai, CC, Lin, TI, and Wu, JR. Changing patterns of infectious diseases in children during the COVID-19 pandemic. Front Cell Infect Microbiol. (2023) 13:1200617. doi: 10.3389/fcimb.2023.1200617
69. Metcalf, CJE, Paireau, J, O'Driscoll, M, Pivette, M, Hubert, B, Pontais, I, et al. Comparing the age and sex trajectories of SARS-CoV-2 morbidity and mortality with other respiratory pathogens. R Soc Open Sci. (2022) 9:211498. doi: 10.1098/rsos.211498
Keywords: sex differences, acute respiratory tract infections, adenovirus, influenza, rhinovirus, parainfluenza-3, human metapneumovirus, respiratory syncytial virus
Citation: Peer V, Mandelboim M, Jurkowicz M and Green MS (2025) Sex differences in acute respiratory tract infections—multi-year analysis based on data from a large tertiary care medical center in Israel. Front. Public Health. 13:1502036. doi: 10.3389/fpubh.2025.1502036
Received: 26 September 2024; Accepted: 03 March 2025;
Published: 25 March 2025.
Edited by:
Laura Petrarca, Sapienza University of Rome, ItalyCopyright © 2025 Peer, Mandelboim, Jurkowicz and Green. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victoria Peer, dmljdG9yaWFwYXBpQHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.