- 1The First School of Clinical Medicine, Gansu University of Chinese Medicine, Lanzhou, Gansu, China
- 2Gansu Provincial Hospital, Lanzhou, Gansu, China
- 3Institute of Modern Physics, Chinese Academy of Sciences, Lanzhou, China
- 4Gansu Provincial Hospital of TCM, Lanzhou, Gansu, China
- 5Evidence-based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu, China
Objective: The use of proton beam therapy (PBT) for treating ocular conjunctival malignancies is on the rise across numerous medical centers. This study conducts a systematic review and meta-analysis to assess the effectiveness and safety of PBT in treating malignant conjunctival tumors.
Methods: We searched for studies on PBT for ocular conjunctival malignancies in PubMed, Embase, Cochrane Library, and Web of Science (WoS) databases up to November 25, 2023. Studies were selected and data were extracted by two independent reviewers based on pre-established inclusion and exclusion criteria. The quality of evidence was assessed using the GRADE method. Meta-analysis was performed using STATA version 16.0.
Results: An initial search yielded 586 articles, from which six retrospective case series studies were selected involving 291 patients with ocular conjunctival malignancies, including 240 cases of conjunctival melanoma and 51 cases of conjunctival squamous cell carcinoma (SCC). Meta-analysis with a random-effects model showed that PBT is effective and relatively safe, with 2-, 4-, and 5-year overall survival (OS) rates of 98% (95% CI 95–102%), 87% (95% CI 69–104%), and 78% (95% CI 70–87%) respectively. Reported toxicity rates included 19% for cataracts, 10% for glaucoma, 5% for lacrimal stenosis, 52% for sicca symptoms, and 11% for limbal stem cell deficiency. The GRADE assessment yielded a low certainty of evidence.
Conclusions: Proton therapy offers a viable alternative treatment for patients with conjunctival malignancies, with acceptable treatment-related toxicity rates.
1 Introduction
Ocular surface malignancies, including conjunctival melanoma (CM) and SCC, affect primarily middle-aged and older adults and pose significant ocular and systemic threats (1, 2). Despite their low prevalence, these tumors are notoriously aggressive, leading to potential blindness or tumor-related mortality, and require intensive treatment (3). Managing unresectable diffuse conjunctival malignancies is challenging; treatments risk damaging essential ocular functions and may lead to vision loss or, in extreme cases, enucleation. Therapeutic efforts to preserve ocular function cover various modalities, such as surgical resection, cryotherapy, immunotherapy, chemotherapy, and radiotherapy (4). In cases of conjunctival SCC, tumors classified as T2–T3 show higher recurrence rates post-surgery (5). Treatments with local chemotherapy and immunotherapy are widely used and effective (MMC 74%; IFN 72–85%; 5 FU 57%). However, issues of secondary complications and recurrence remain significant clinical challenges (6–9).
In recent years, advances in radiation therapy such as PBT have introduced significant innovations. The development of more compact and cost-effective single-chamber proton beam devices has facilitated the widespread adoption of this technology (10). PBT utilizes an external beam where radiation dosage is precisely controlled. Unlike conventional radiotherapy, PBT delivers a lower incident dose and concentrates the majority of radiation directly on the tumor, reducing exposure to surrounding healthy tissues (11). Produced via a linear accelerator or cyclotron, the proton beam reaches the tumor with high energy and forms a narrow, intense Bragg peak. Beyond this peak, the energy falls off sharply, allowing precise energy deposition at the target. This dosimetric property provides a clear advantage over conventional radiation therapy, enhancing treatment effectiveness while protecting nearby normal tissue. During planning, the physician carefully defines the tumor target at the Bragg peak's apex to optimize tumor destruction and minimize damage to adjacent structures (12). Studies suggest that proton radiotherapy's radiobiological effects may offer a stronger tumoricidal effect than photon radiotherapy, potentially improving treatment outcomes (13, 14). Thus, PBT represents a promising approach for treating conjunctival tumors. Although clinical studies of PBT have been limited to small samples and case reports, the efficacy and safety are not fully established. Therefore, this study aims to systematically review and analyze the evidence on PBT's effectiveness in treating conjunctival tumors to support clinical treatment, guideline development, and policy implementation for PBT.
2 Materials and methods
This systematic review and meta-analysis was registered with PROSPERO (registration number CRD42023486574) and adhered to the PRISMA guidelines (15) and additional methodological standards (16–18).
2.1 Search strategy
A comprehensive search was conducted in the Cochrane Library, Embase, PubMed, and WoS databases for articles published through November 25, 2023. Only English publications were included. The search terms used were: ((((((conjunctival lymphoma) OR (conjunctival melanoma)) OR (Conjunctiva)) OR (squamous cell conjunctival cancer)) OR (conjunctival cancer)) OR (conjunctival neoplasms)) AND (((((proton radiotherapy) OR (proton radiation therapy)) OR (proton radiation)) OR (proton irradiation)) OR (proton therapy)). Additionally, references from relevant reviews and primary studies were examined to identify further pertinent articles.
2.2 Inclusion and exclusion criteria
Inclusion criteria:
(a) Population: Patients diagnosed with conjunctival malignancy;
(b) Intervention: PBT alone or in combination with other therapies;
(c) Comparison: No restrictions;
(d) Outcomes: OS, toxicity;
(e) Study type: No restrictions.
Exclusion criteria:
(a) Use of radiotherapy modalities other than PBT;
(b) Duplicate study reports;
(c) Case reports, reviews, meta-analyses, abstracts, letters, commentaries, and consensus statements;
(d) Studies lacking sufficient data;
(e) Studies on irrelevant topics.
2.3 Study selection
All articles were processed in EndNote 20 to eliminate duplicates. Titles and abstracts were independently screened by two reviewers to exclude irrelevant studies, and the full texts of potentially relevant studies were assessed for eligibility. Discrepancies were resolved through discussion or by consulting a third reviewer. Data extraction included:
(a) First author, year, research institution, country, study design, and period;
(b) Patient demographics, tumor specifics, total treatment dose, and follow-up;
(c) Outcomes focusing on survival rates and toxicity;
(d) Risk of bias assessment items.
2.4 Risk of bias assessments
To assess the quality of the case series studies, a scale developed by the Canadian Institute of Health Economics (IHE) was used (19). This method comprises 20 items distributed over seven areas, each assessed as “yes”, “no”, or “unclear”. A study was considered to have acceptable quality and moderate risk of bias if more than 14 items received a “yes” rating. Disagreements were resolved through discussion among researchers or consultation with a third party.
2.5 Statistical analysis
Descriptive statistics were used to summarize basic characteristics and toxicity incidence. Dichotomous data were presented as frequencies and percentages, and continuous data were summarized as means or medians with their respective ranges. Meta-analysis employed a random-effects model in STATA 16.0, calculating effect sizes as proportions with 95% confidence intervals (CI). The I2 statistic assessed heterogeneity, with I2 ≤ 50% and P ≥ 0.05 indicating homogeneity; values outside this range suggested heterogeneity. Publication bias was evaluated using a funnel plot for outcomes from at least ten studies.
3 Results
3.1 Study selection and characteristics
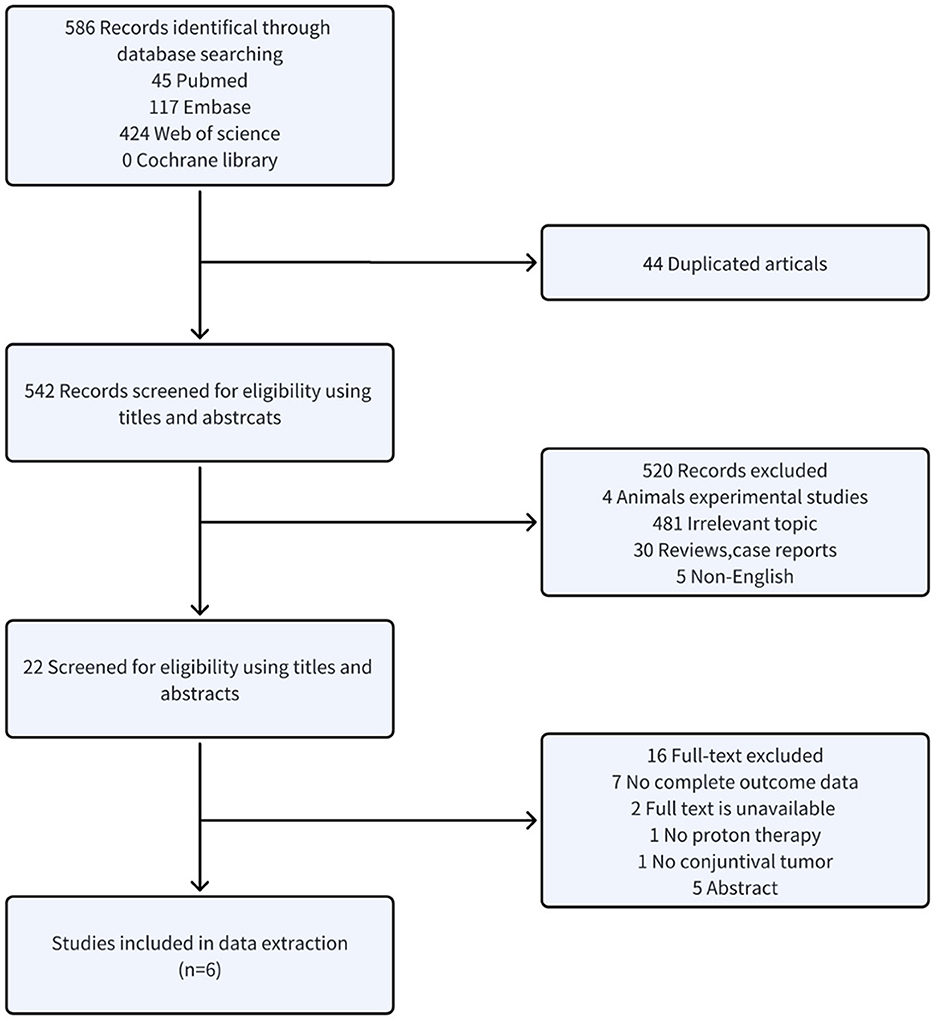
As illustrated in Figure 1, the search yielded 586 studies. After removing 44 duplicates, 542 studies remained for title and abstract screening. Of these, 522 were excluded as unsuitable. The full texts of the remaining 22 studies were examined; 16 were excluded for reasons including seven with incomplete data, two lacking full text, one missing proton beam irradiation, one lacking conjunctival malignancies, and five that were abstracts. Ultimately, six retrospective studies involving 291 patients were included.
3.2 Basic characteristics
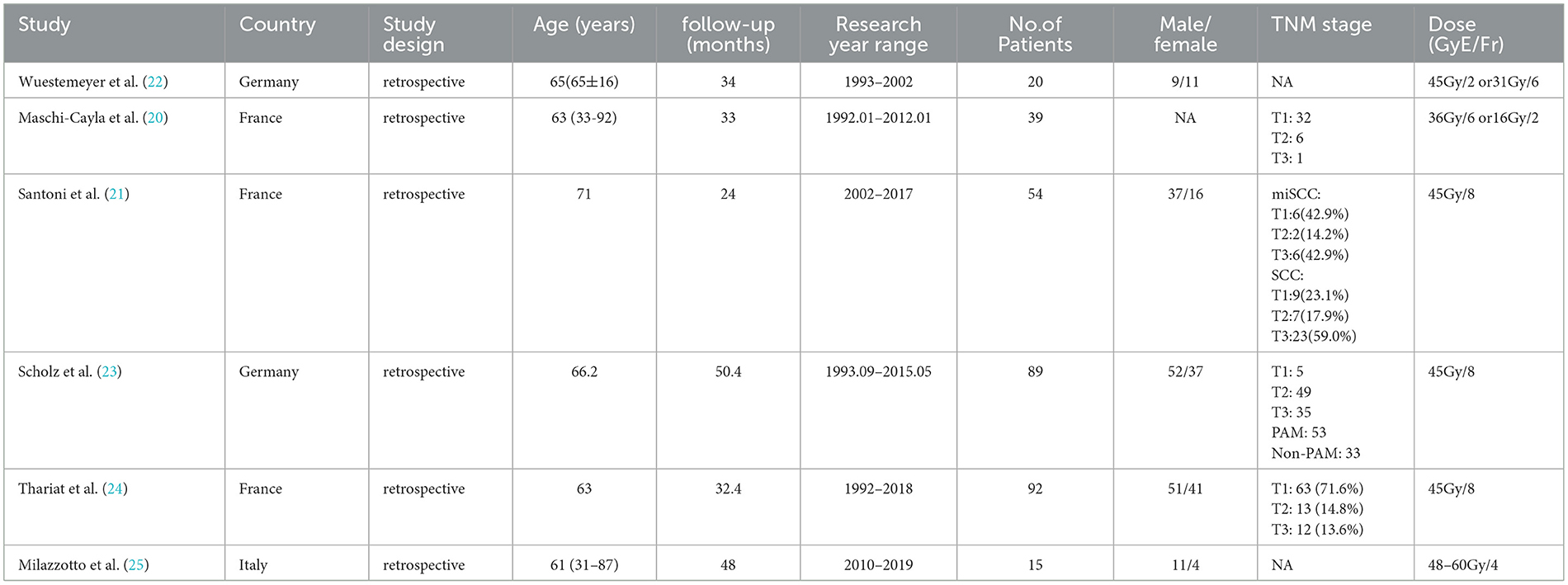
Six retrospective case series studies were included, involving 291 patients with conjunctival tumors treated with proton therapy (20–25). Of these, four studies focused on CM (n = 240) (20, 22–24), and two addressed conjunctival SCC (n = 51) (21, 25). The studies originated from Germany (published in 2006 and 2019) (22, 23), France (published in 2013 and 2019) (20, 21, 24), and Italy (published in 2022) (25). The sample size ranged from 15 to 92 patients, with follow-up periods spanning 24 to 50.4 months (Table 1).
3.3 Outcomes
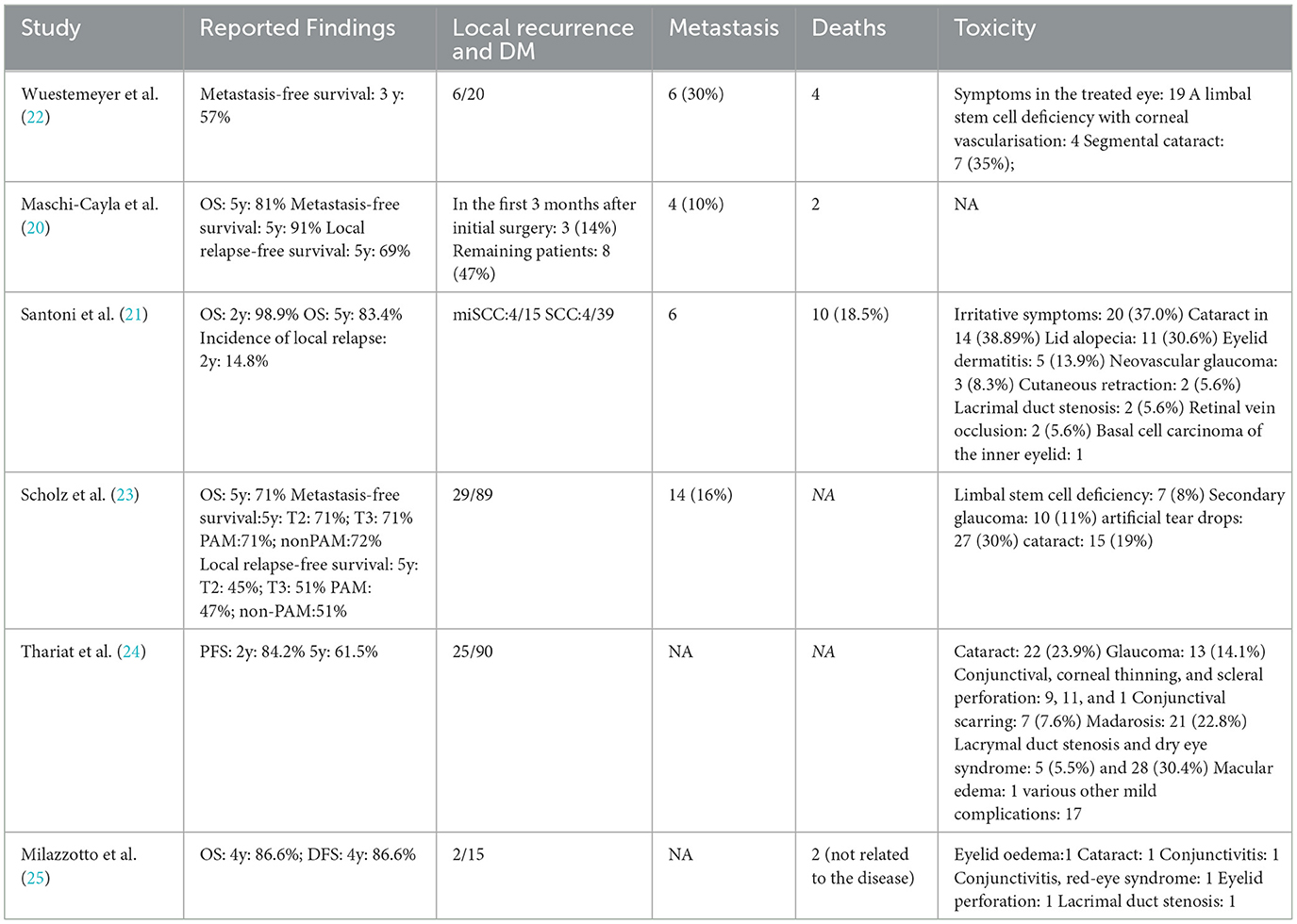
The articles covered conjunctival malignant melanoma and squamous cell tumors. Three studies reported on metastasis-free survival (20, 22, 23), four on OS (20, 21, 23, 25), and two on local relapse-free survival (20, 23). Additionally, one study reported on PFS (24), and another on DFS (25) (Table 2).
3.4 Risk of bias and certainty of evidence assessment results
Figure 2 depicts the overall suboptimal quality of the included case series, primarily due to their retrospective nature and the lack of blinding among outcome assessors. Three studies were conducted at a single center (21, 23, 24); three used consecutive participant recruitment (21, 22, 24); none measured outcomes before and after intervention; two reported patient loss to follow-up (20, 22); two provided estimates of random variables in data analyses of relevant outcomes (20, 21). GRADE profiler was used to assess the quality of evidence for proton radiotherapy's effects over 2, 4, and 5 years and its impact on cataracts, glaucoma, lacrymal duct stenosis, limbal stem cell deficiency, and sicca symptoms. The evidence for OS and the ocular conditions is considered LOW certainty, while the evidence linking to sicca symptoms is rated VERY LOW (Table 3).
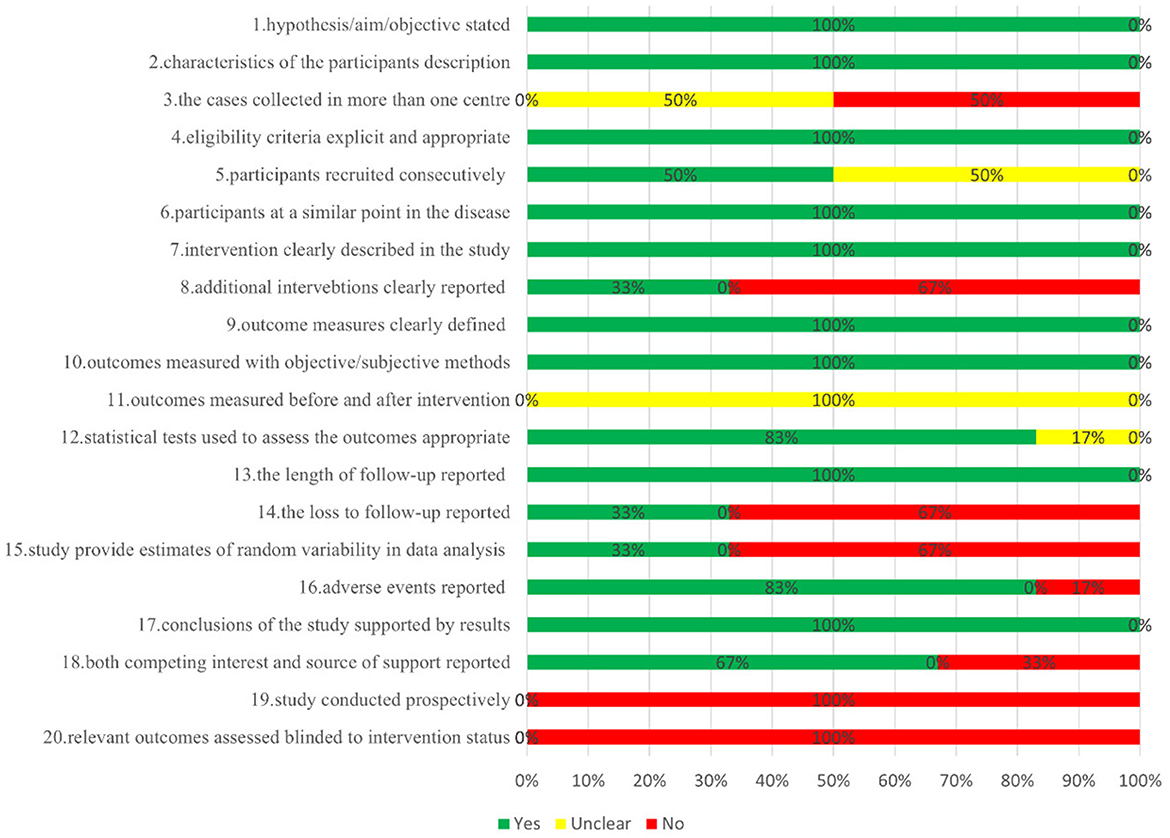
Figure 2. Risk of bias assessment of included case series study of proton therapy for ocular conjunctival malignancies.
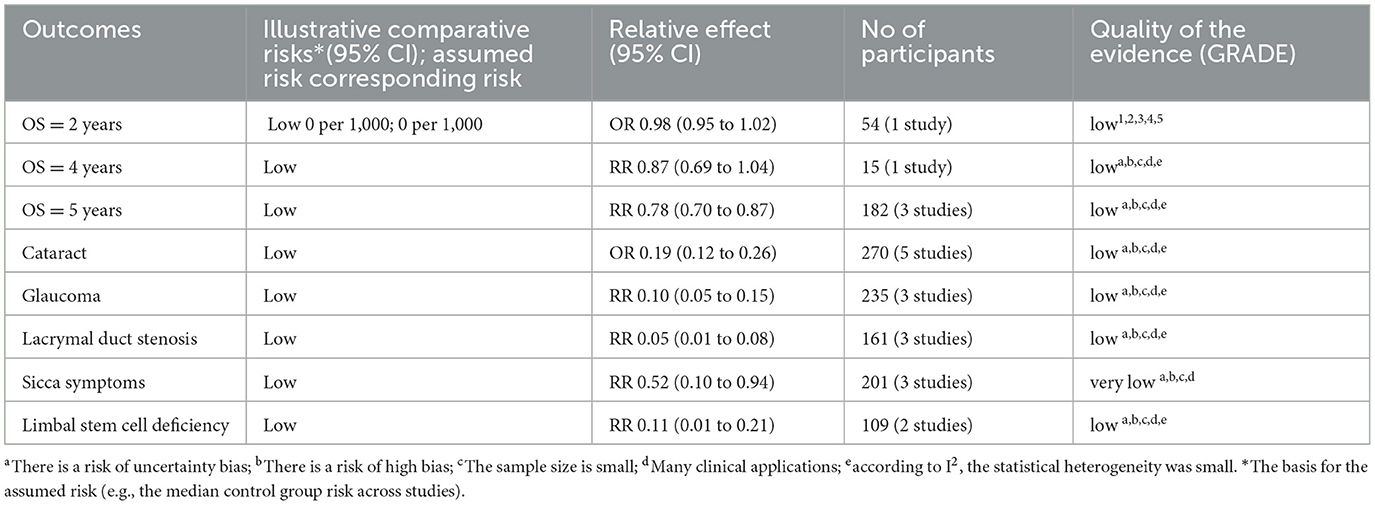
Table 3. Grading of the quality of evidence in case series studies of proton therapy for conjunctival malignancies.
3.5 OS
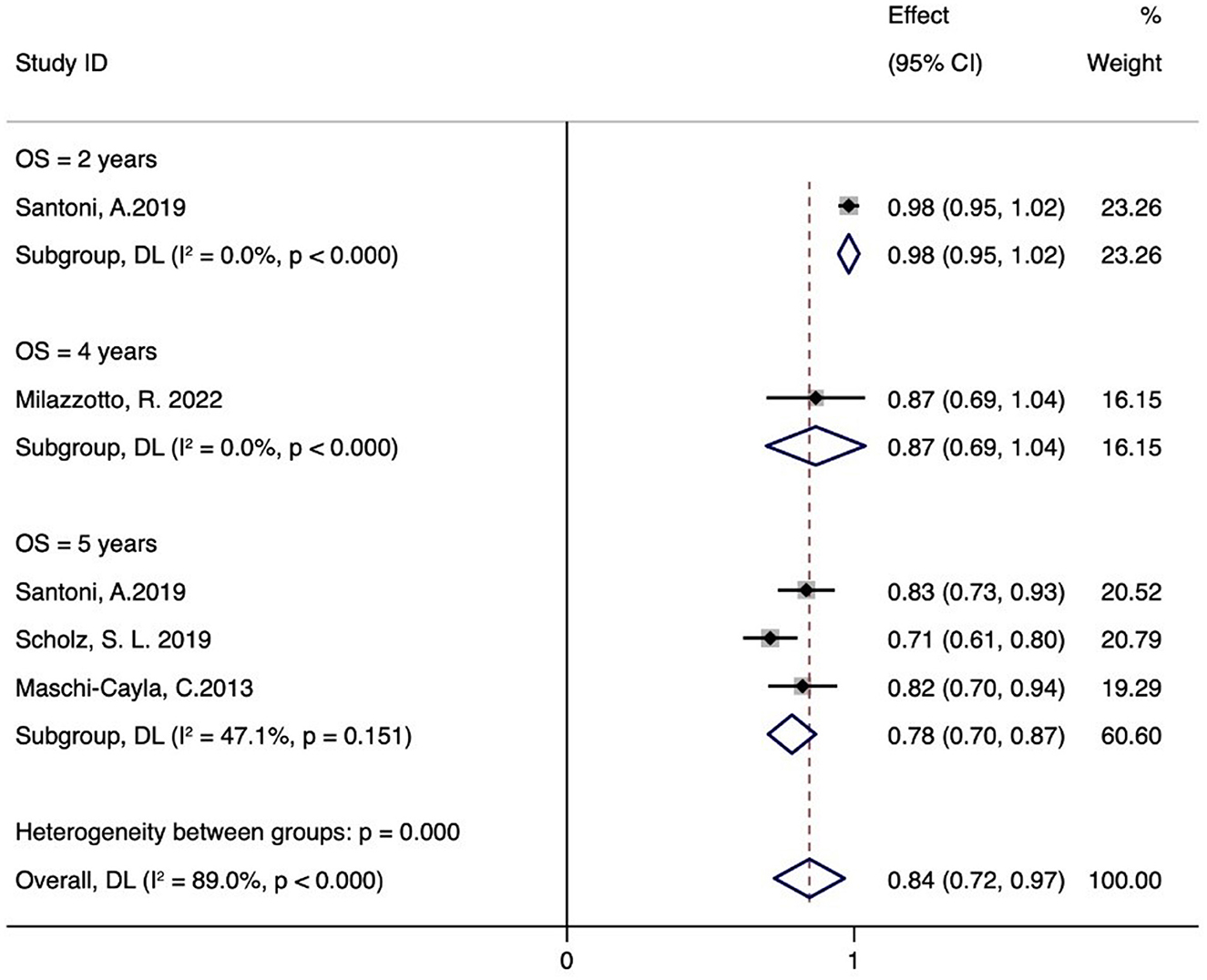
Figure 3 illustrates six case series studies on PBT for ocular conjunctival malignancies. These include reports on 2-year OS (21), 4-year OS (25), and 5-year OS (20, 21, 23); one study did not include OS data (22). Five studies analyzed the OS associated with PBT (20, 21, 23, 25). A random-effects meta-analysis showed the 2-, 4-, and 5-year OS rates to be 98% (95% CI 95-102%), 87% (95% CI 69-104%), and 78% (95% CI 70–87%), respectively.
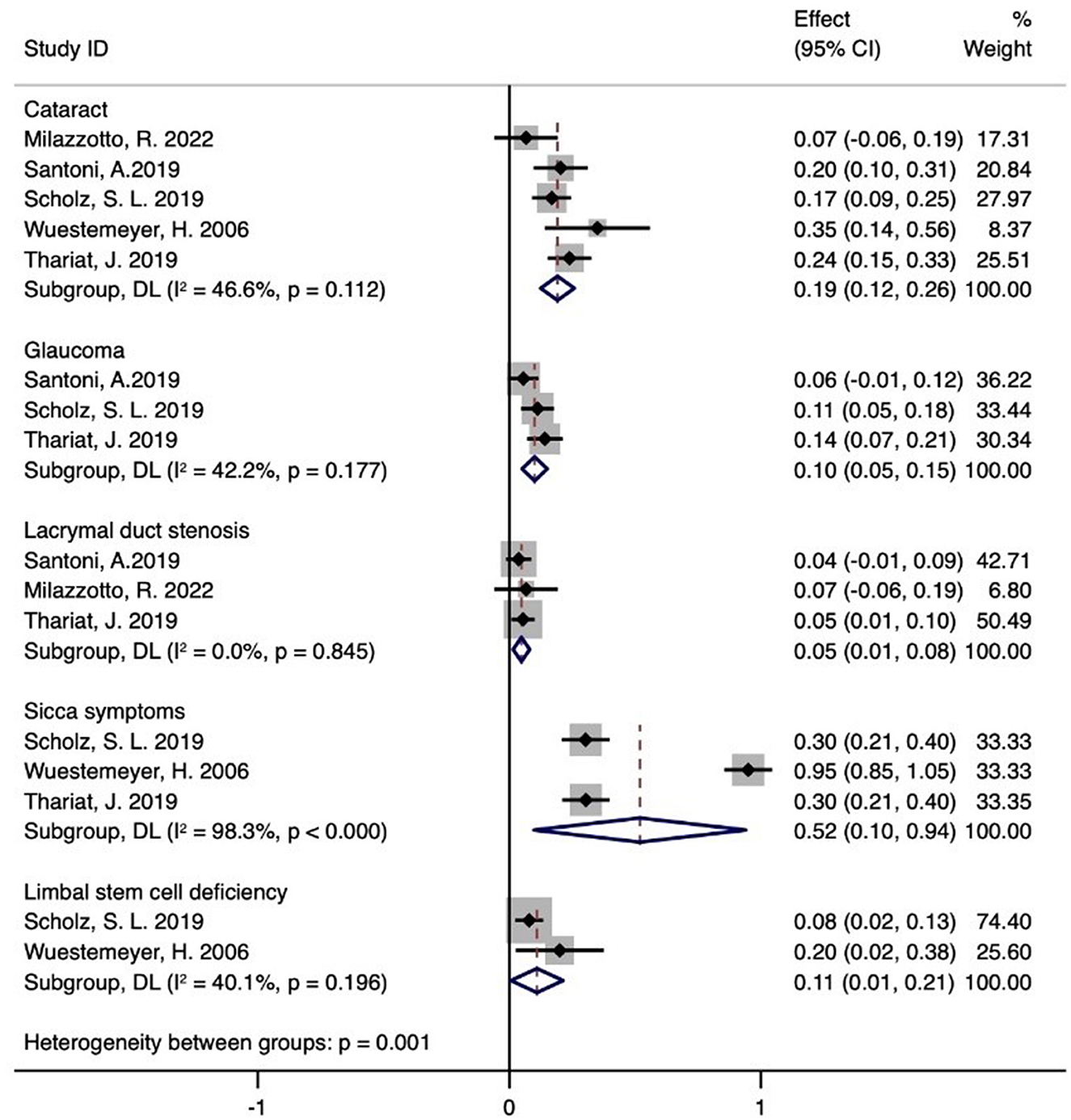
3.6 Toxicity
Figure 4 shows that five studies documented adverse effects (21–25), with five reporting cataracts (n = 59) (21–25), three detailing glaucoma (n = 26) (21, 23, 24), and three describing lacrimal duct stenosis (n = 8) (21, 24, 25). Three studies reported sicca symptoms (n = 51) (22–24), and two noted limbal stem cell defects (n = 11) (22, 23). The meta-analysis indicated the following incidence rates for proton therapy: 19% for cataracts (95% CI 12–26%), 10% for glaucoma (95% CI 5–15%), 5% for lacrimal duct stenosis (95% CI 1–8%), 52% for sicca symptoms (95% CI 10–94%), and 11% for limbal stem cell defects (95% CI 1–21%). Additional adverse reactions included eyelid alopecia (30.6%, n = 11), eyelid dermatitis (13.9%, n = 5), and skin degeneration (5.6%, n = 2) reported by Santoni et al. (21). Retinal vein occlusion (5.6%, n = 2) and inner eyelid basal cell carcinoma (n = 1) were also reported. Thariat et al. noted conjunctival thinning, corneal thinning, scleral perforation (n = 9, 11, and 1, respectively), conjunctival scarring (n = 7), osteoporosis (n = 21), macular edema (n = 1), and other minor complications (n = 17) (24). Milazzotto et al. reported eyelid edema, cataract, conjunctivitis, red-eye syndrome, eyelid perforation, and lacrimal duct stenosis (all n = 1) (25).
4 Discussion
We analyzed six peer-reviewed studies involving 291 patients from Germany, France, and Italy. The results suggest that PBT for conjunctival malignancies offers high survival rates and an acceptable level of treatment-related adverse effects. The quality of the methodologies in the studies was considered modest, and the confidence in the evidence ranged from very low to low.
Conjunctival malignancies, though rare, pose significant challenges in ophthalmology. These tumors often display aggressive behavior, requiring intensive treatments that may lead to severe ocular complications, including blindness, and potentially result in tumor-related mortality. The primary conjunctival malignancies include SCC, malignant melanoma, and malignant lymphoma, with surgical treatment being predominant for SCC and malignant melanoma. Often, adjuvant treatments such as local chemotherapy or radiotherapy are necessary (3). A comprehensive therapeutic approach is adopted, involving surgical excision, brachytherapy, cryotherapy, chemotherapy, systemic immunotherapy, photon radiation therapy, and PBT, tailored to the tumor's characteristics and the patient's health.
An independent study of 31 patients with CM reported a survival rate of 75.8% at 2 years and 51.5% at 5 years (26). Another study reported a five-year disease-free survival (DFS) rate of 67.5% for patients with conjunctival lymphoma treated with brachytherapy (27). Surgical interventions have shown a five-year recurrence rate of 36% to 45%, highlighting the variability and challenges in long-term effectiveness (28–33). Our meta-analysis revealed that PBT achieved a 98% two-year and a 78% five-year OS rate. These findings suggest that the five-year survival rate with proton therapy may exceed those of surgical methods. Additionally, a study found an 86.6% four-year DFS rate after PBT for conjunctival SCC (25), indicating potential advantages over brachytherapy. Our analysis also showed a five-year recurrence rate of 31% in patients treated with PBT for malignant melanoma (20), demonstrating its potential superiority over surgical methods (34). Scholz et al. (23) noted a 16% incidence of metastasis after PBT, highlighting its effectiveness in controlling metastasis compared to cryotherapy. However, the limited empirical evidence and the retrospective nature of the studies may affect the reliability of these findings.
PBT has emerged as a promising and revolutionary treatment modality within radiation oncology. Its precision targeting, enhanced by the Bragg peak phenomenon, significantly improves therapeutic efficacy. This advanced approach not only aims to reduce the adverse effects associated with conventional radiotherapy but also expands the range of treatable conditions, providing new management options for various oncological diseases. In managing conjunctival malignancies, adverse reactions are an inevitable challenge. It is notable that many patients develop ocular complications post-treatment, underscoring the need for effective management and mitigation of these side effects. One study involving 51 participants who underwent adjuvant electron beam radiation therapy (EBRT) post-surgical excision of CM found that a majority, 96% (25/26), suffered from keratoconjunctivitis sicca, while 27% (7/26) developed localized cataracts, and 15% (4/26) experienced limbal stem cell insufficiency (22, 35, 36). Another study with 24 subjects undergoing postoperative radiotherapy reported a 62% incidence of cataracts (37). Systemic immunotherapy can cause a spectrum of side effects, including fatigue, diarrhea, colitis, hepatitis, pneumonia, and endocrinopathies, along with less common complications. For conjunctival malignancies, chemotherapy is typically used as an ancillary treatment in conjunction with surgery or radiotherapy to enhance efficacy. Although effective in destroying cancer cells, chemotherapy also harms healthy cells, leading to various side effects.
Our meta-analysis found sicca symptoms in 52% of cases, cataracts in 19%, limbal stem cell deficiency in 11%, glaucoma in 10%, and lacrimal duct stenosis in 5%. Due to the high radiation doses required for treating malignant melanoma and SCC, proton therapy offers precise dosing that covers the entire conjunctival region while minimizing exposure to other ocular structures (38). Milazzotto and colleaguesand (21) reported a superior OS rate, possibly due to higher radiation doses. This suggests that proton therapy, either alone or in combination with complex surgical techniques, could be a viable and safe treatment option for this cancer type. The minimal toxicity of proton therapy, due to its dosimetric advantage where radiation dose distribution is more uniform, helps preserve more ocular tissue by avoiding electron scatter. Consequently, the incidence of harmful reactions such as xerophthalmia, cataractogenesis, and limbal stem cell insufficiency is significantly reduced in patients treated with proton therapy for conjunctival malignancies. Our findings show that proton therapy maintains a commendably benign toxicity profile for treating conjunctival malignancies. Managing and controlling adverse reactions after therapeutic interventions in patients with conjunctival malignant tumors is a critical area that requires further research and academic focus.
The quality assessment of the included studies identifies several areas for enhancement in future clinical trials: First, it is crucial to involve multiple centers in patient recruitment to improve the generalizability of the results. Second, comprehensive reporting of any additional interventions is essential. Third, it is imperative to ensure the mandatory disclosure of conflicts of interest and funding sources (16, 39, 40). Finally, the importance of blinding patients in clinical trials must be emphasized (41, 42). Addressing these aspects can significantly reduce the risk of bias in study outcomes (43). The literature analyzed shows a variable risk of bias, often uncertain or high, and is characterized by small sample sizes, necessitating a downgrade in the evidence grading. However, the presence of numerous clinical applications and minimal statistical heterogeneity warrants an upgrade and careful evaluation. The findings suggest that the evidence level for OS at 2, 4, and 5 years, as well as for Cataract, Glaucoma, Lacrymal duct stenosis, and Limbal stem cell deficiency, is generally rated as LOW. The evidence level for Sicca symptoms is rated as VERY LOW, which calls into question the reliability of the current results. The overall level of evidence is deemed average, highlighting the need for future studies to include multi-center, high-quality randomized controlled trials for further investigation.
Although systematic reviews and meta-analyses suggest that PBT is an effective treatment for conjunctival malignancies, these findings should be cautiously interpreted and applied due to notable limitations. The variability in clinical efficacy observed among patients could stem from the lower quality and small number of studies in this meta-analysis, highlighting the need for additional research to explore this observation. Moreover, despite a thorough literature search and stringent inclusion criteria, only six studies met the inclusion standards, potentially impacting the robustness of the findings. Furthermore, the predominance of retrospective studies in the analysis curtails the scope for more exhaustive comparative studies. While PBT is emerging as a promising clinical treatment option, its growing acceptance underscores the imperative for more extensive prospective studies to validate its efficacy.
5 Conclusions
Proton therapy holds promise as a viable alternative treatment for patients with conjunctival malignancies, with acceptable levels of treatment-related toxicity. However, the confirmation of PBT's superiority over other radiotherapy modalities necessitates further high-quality prospective randomized controlled trials.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
TZ: Resources, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Software. DW: Conceptualization, Formal analysis, Methodology, Writing – original draft. YM: Conceptualization, Data curation, Software, Writing – review & editing. MD: Data curation, Methodology, Resources, Writing – original draft. QL: Data curation, Methodology, Writing – original draft. QZ: Software, Supervision, Validation, Writing – review & editing. HB: Conceptualization, Data curation, Methodology, Writing – original draft. HL: Conceptualization, Data curation, Methodology, Writing – original draft. ML: Software, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Science and Technology Plan Project of Gansu Province (23JRRA1291), the National Key Research and Development Program of China (No. 2022YFC2401500), Talent Innovation and Venture Project of Lanzhou City (2020-RC-113), the Central Guidance Local Science and Technology Development Program (Grant No. 24ZYQA029), the Science and Technology Project of Lanzhou City (Grant No. 2023-1-9), Gansu Province Project of Science and Technologies (Grant No. 20JR10RA680, 22CX8JA149), and the Lanzhou Heavy Ion Accelerator High-End User Project (HIR20GY007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shields JA, Shields CL, De Potter P. Surgical management of conjunctival tumors. The 1994 Lynn B. McMahan lecture. Arch Ophthalmol. (1997) 115:808–15. doi: 10.1001/archopht.1997.01100150810025
2. Chalasani R, Giblin M, Conway RM. Role of topical chemotherapy for primary acquired melanosis and malignant melanoma of the conjunctiva and cornea: review of the evidence and recommendations for treatment. Clin Exp Ophthalmol. (2006) 34:708–14. doi: 10.1111/j.1442-9071.2006.01356.x
3. Auw-Hädrich C, Reinhard T. [Conjunctival malignancies]. Ophthalmologe. (2019) 116:989–1004. doi: 10.1007/s00347-019-00978-6
4. Marr BP, Abramson DH, Cohen GN, Williamson MJ, McCormick B, Barker CA. Intraoperative high-dose rate of radioactive phosphorus 32 brachytherapy for diffuse recalcitrant conjunctival neoplasms: a retrospective case series and report of toxicity. JAMA Ophthalmol. (2015) 133:283–9. doi: 10.1001/jamaophthalmol.2014.5079
5. Galor A, Karp CL, Oellers P, Kao AA, Abdelaziz A, Feuer W, et al. Predictors of ocular surface squamous neoplasia recurrence after excisional surgery. Ophthalmology. (2012) 119:1974–81. doi: 10.1016/j.ophtha.2012.04.022
6. Russell H C, Chadha V, Lockington D, Kemp EG. Topical mitomycin C chemotherapy in the management of ocular surface neoplasia: a 10-year review of treatment outcomes and complications. Br J Ophthalmol. (2010) 94:1316–21. doi: 10.1136/bjo.2009.176099
7. Yeatts RP, Engelbrecht NE, Curry CD, Ford JG, Walter KA. 5-Fluorouracil for the treatment of intraepithelial neoplasia of the conjunctiva and cornea. Ophthalmology. (2000) 107:2190–5. doi: 10.1016/s0161-6420(00)00389-4
8. Kim HJ, Shields CL, Shah S U, Kaliki S, Lally SE. Giant ocular surface squamous neoplasia managed with interferon alpha-2b as immunotherapy or immunoreduction. Ophthalmology. (2012) 119:938–44. doi: 10.1016/j.ophtha.2011.11.035
9. Boehm MD, Huang AJ. Treatment of recurrent corneal and conjunctival intraepithelial neoplasia with topical interferon alfa 2b. Ophthalmology. (2004) 111:1755–61. doi: 10.1016/j.ophtha.2004.01.034
10. LaRiviere MJ, Santos PMG, Hill-Kayser CE, Metz JM. Proton Therapy. Hematol Oncol Clin North Am. (2019) 33:989–1009. doi: 10.1016/j.hoc.2019.08.006
11. Vitti ET, Parsons JL. The radiobiological effects of proton beam therapy: impact on DNA damage and repair. Cancers. (2019) 11:946. doi: 10.3390/cancers11070946
12. Ge YL, Tu WZ, Liu Y. Research progress in proton in oncology radiobiology. Chin J Radiat Oncol. (2018) 27:5. doi: 10.3760/cma.j.issn.1004-4221.2018.08.015
13. Alan Mitteer R, Wang Y, Shah J, Gordon S, Fager M, Butter PP, et al. Proton beam radiation induces DNA damage and cell apoptosis in glioma stem cells through reactive oxygen species. Sci Rep. (2015) 5:13961. doi: 10.1038/srep13961
14. Narang H, Kumar A, Bhat N, Pandey B N, Ghosh A. Effect of proton and gamma irradiation on human lung carcinoma cells: Gene expression, cell cycle, cell death, epithelial-mesenchymal transition and cancer-stem cell trait as biological end points. Mutat Res. (2015) 780:35–46. doi: 10.1016/j.mrfmmm.2015.07.006
15. Moher D, Liberati A, Tetzlaff J, Altman D G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
16. Li Y, Cao L, Zhang Z, Hou L, Qin Y, Hui X, et al. Reporting and methodological quality of COVID-19 systematic reviews needs to be improved: an evidence mapping. J Clin Epidemiol. (2021) 135:17–28. doi: 10.1016/j.jclinepi.2021.02.021
17. Li X, Li Y, Guo K, Chen N, Cui X, Bai Z, et al. Evidence Based Social Science in China Paper 3: The quality of social science RCTs published from 2000-2020. J Clin Epidemiol. (2022) 141:64–73. doi: 10.1016/j.jclinepi.2021.09.014
18. Wang Y, Chen N, Guo K, Li Y, Fenfen E, Yang C, et al. Reporting and methodological quality of acupuncture network meta-analyses could be improved: an evidence mapping. J Clin Epidemiol. (2023) 153:1–12. doi: 10.1016/j.jclinepi.2022.11.004
19. Wang XQ, Chen YL, Qu QY, Li Y, Yang C, Shang X, et al. Reporting and methodological quality of acupuncturenetwork meta-analyses could be improved: an evidence mapping. J Clin Epidemiol. (2023) 153:1–12. doi: 10.1016/j.jclinepi.2022.11.004
20. Maschi-Cayla C, Doyen J, Gastaud P, Caujolle JP. Conjunctival melanomas and proton beam therapy. Acta Ophthalmol. (2013) 91:e647. doi: 10.1111/aos.12202
21. Santoni A, Thariat J, Maschi C, Herault J, Baillif S, Lassalle S, et al. Management of invasive squamous cell carcinomas of the conjunctiva. Am J Ophthalmol. (2019) 200:1–9. doi: 10.1016/j.ajo.2018.11.024
22. Wuestemeyer H, Sauerwein W, Meller D, Chauvel P, Schueler A, Steuhl KP, et al. Proton radiotherapy as an alternative to exenteration in the management of extended conjunctival melanoma. Graefes Arch Clin Exp Ophthalmol. (2006) 244:438–46. doi: 10.1007/s00417-005-0093-5
23. Scholz SL, Hérault J, Stang A, Griewank KG, Meller D, Thariat J, et al. Proton radiotherapy in advanced malignant melanoma of the conjunctiva. Graefes Arch Clin Exp Ophthalmol. (2019) 257:1309–18. doi: 10.1007/s00417-019-04286-2
24. Thariat J, Salleron J, Maschi C, Fevrier E, Lassalle S, Gastaud L, et al. Oncologic and visual outcomes after postoperative proton therapy of localized conjunctival melanomas. Radiat Oncol. (2019) 14:239. doi: 10.1186/s13014-019-1426-6
25. Milazzotto R, Liardo RLE, Privitera G, Raffaele L, Salamone V, Arena F, et al. Proton beam radiotherapy of locally advanced or recurrent conjunctival squamous cell carcinoma: experience of the CATANA Centre. J Radiother Pract. (2022) 21:97–104. doi: 10.1017/S1460396920000953
26. Ma R, Ren H, Zhou X, Gan L, Xu B, Guo J, et al. Orbital exenteration for conjunctival melanoma: comparison of long-term outcome between individualised and conventional techniques. Eye (Lond). (2021) 35:3410–8. doi: 10.1038/s41433-021-01454-9
27. Regueiro C A, Valcárcel F J, Romero J, de la Torre A. Treatment of conjunctival lymphomas by beta-ray brachytherapy using a strontium-90-yttrium-90 applicator. Clin Oncol. (2002) 14:459–63. doi: 10.1053/clon.2002.0148
28. Tuomaala S, Eskelin S, Tarkkanen A, Kivelä T. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. (2002) 43:3399–408.
29. Missotten GS, Keijser S, De Keizer RJ, De Wolff-Rouendaal D. Conjunctival melanoma in the Netherlands: a nationwide study. Invest Ophthalmol Vis Sci. (2005) 46:75–82. doi: 10.1167/iovs.04-0344
30. Shields CL, Markowitz JS, Belinsky I, Schwartzstein H, George N S, Lally SE, et al. Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases. Ophthalmology. (2011) 118:389–95.e1-2. doi: 10.1016/j.ophtha.2010.06.021
31. Esmaeli B, Wang X, Youssef A, Gershenwald JE. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. (2001) 108:2101–5. doi: 10.1016/s0161-6420(01)00782-5
32. Werschnik C, Lommatzsch PK. Long-term follow-up of patients with conjunctival melanoma. Am J Clin Oncol. (2002) 25:248–55. doi: 10.1097/00000421-200206000-00009
33. Shields CL, Kaliki S, Al-Dahmash SA, Lally SE, Shields JA. American Joint Committee on Cancer (AJCC) clinical classification predicts conjunctival melanoma outcomes. Ophthalmic Plast Reconstr Surg. (2012) 28:313–23. doi: 10.1097/IOP.0b013e3182611670
34. Koç I, Kiratli H. Current Management of Conjunctival Melanoma Part 2: Treatment and Future Directions. Turk J Ophthalmol. (2020) 50:362–70. doi: 10.4274/tjo.galenos.2020.22567
35. Hsu A, Frank SJ, Ballo MT, Garden AS, Morrison WH, Rosenthal DI, et al. Postoperative adjuvant external-beam radiation therapy for cancers of the eyelid and conjunctiva. Ophthalmic Plast Reconstr Surg. (2008) 24:444–9. doi: 10.1097/IOP.0b013e31818be098
36. Norregaard JC, Gerner N, Jensen OA, Prause JU. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. (1996) 234:569–72. doi: 10.1007/bf00448801
37. Belad A, Nasr C, Jmour O, Cherif A, Benna F. Post- operative radiotherapy of conjunctival malignancies: a series of 24 cases. Asian Pacific J Cancer Biol. (2017) 2:3–8. doi: 10.31557/apjcb.2017.2.1.3-8
38. Stannard C, Sauerwein W, Maree G, Lecuona K. Radiotherapy for ocular tumours. Eye (Lond). (2013) 27:119–27. doi: 10.1038/eye.2012.241
39. Xiu-xia L, Ya Z, Yao-long C, Ke-hu Y, Zong-jiu Z. The reporting characteristics and methodological quality of Cochrane reviews about health policy research. Health Policy. (2015) 119:503–10. doi: 10.1016/j.healthpol.2014.09.002
40. Yao L, Sun R, Chen Y L, Wang Q, Wei D, Wang X, et al. The quality of evidence in Chinese meta-analyses needs to be improved. J Clin Epidemiol. (2016) 74:73–9. doi: 10.1016/j.jclinepi.2016.01.003
41. Tian J, Zhang J, Ge L, Yang K, Song F. The methodological and reporting quality of systematic reviews from China and the USA are similar. J Clin Epidemiol. (2017) 85:50–8. doi: 10.1016/j.jclinepi.2016.12.004
42. Yan P, Yao L, Li H, Zhang M, Xun Y, Li M, et al. The methodological quality of robotic surgical meta-analyses needed to be improved: a cross-sectional study. J Clin Epidemiol. (2019) 109:20–9. doi: 10.1016/j.jclinepi.2018.12.013
Keywords: proton beam therapy, ocular conjunctival malignancies, systematic review, meta-analysis, effectiveness and safety
Citation: Zheng T, Wang D, Miao Y, Dong M, Liu Q, Zhang Q, Bai H, Luo H and Li M (2025) Clinical efficacy and safety of proton radiotherapy for ocular conjunctival malignancies: a systematic review and meta-analysis. Front. Public Health 13:1486988. doi: 10.3389/fpubh.2025.1486988
Received: 27 August 2024; Accepted: 20 January 2025;
Published: 11 February 2025.
Edited by:
Weihua Yang, Jinan University, ChinaReviewed by:
Maurizio Tomasi, New Mexico State University, United StatesLiqiu Ma, National Institutes for Quantum Science and Technology, Japan
Pengsen Wu, Sun Yat-sen University, China
Copyright © 2025 Zheng, Wang, Miao, Dong, Liu, Zhang, Bai, Luo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiling Bai, MTUyOTMxMTIxMjdAMTYzLmNvbQ==; Hongtao Luo, aG9uZ3Rhb2x1b0AxMjYuY29t
 Tingwei Zheng
Tingwei Zheng Dandan Wang3
Dandan Wang3 Yuxin Miao
Yuxin Miao Qin Liu
Qin Liu Qiuning Zhang
Qiuning Zhang Hongtao Luo
Hongtao Luo