
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
POLICY AND PRACTICE REVIEWS article
Front. Public Health, 20 March 2025
Sec. Public Health Policy
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1450625
Introduction: The role of public health has evolved from addressing infectious diseases to encompass non-communicable diseases. Individuals with genetic disorders and rare diseases constitute a particularly vulnerable population, requiring tailored public health policies, practical implementation strategies, and a long-term vision to ensure sustainable support. Given the prolonged duration and significant costs often associated with these conditions, comprehensive, patient-centered, and cost-effective approaches are essential to safeguard their physical and mental well-being.
Aims: To summarize definitions and concepts related to health, public health, rare diseases, and to highlight the role of integrating public health interventions into routine care in improving patient outcomes. Hemophilia was selected as an exemplary rare disease due to its significant lifetime treatment costs and the recent approval and pricing of its gene therapy as the world’s most expensive drug, highlighting the critical importance of public health policies in ensuring equitable access to care and treatment.
Methods: A narrative literature review was conducted between July 2023 and December 2024, searching PubMed, Google Scholar, and Google for various topics related to rare diseases, public health, and hemophilia.
Results: Public health can play an important role in improving the health outcomes of people with rare diseases by implementing conceptual and applied models to accomplish a set of objectives. Over the past two decades, legislative and regulatory support in high income countries (HICs) has facilitated the development and approval of diagnostics and treatments for several rare diseases leading to important advancements. In contrast, many low- and middle-income countries (LMICs) face obstacles in enacting legislation, developing regulations, and implementing policies to support rare disease diagnosis and treatment. More investment and innovation in drug discovery and market access pathways are still needed in both LMICs and HICs. Ensuring the translation of public health policies into regulatory measures, and in turn implementing, and regularly evaluating these measures to assess their effectiveness is crucial. In the case of hemophilia, public health can play a pivotal role.
Conclusion: Enhancing public health surveillance, policies, and interventions in hemophilia and other rare diseases can bridge data gaps, support access to equitable treatment, promote evidence-based care, and improve outcomes across the socioeconomic spectrum.
Individuals with genetic disorders and rare diseases constitute a particularly vulnerable population, given the prolonged duration and significant costs often associated with these conditions (1–4). Thus, tailoring public health policies, executing practical implementation strategies, and developing long-term plans to ensure sustainable support can contribute to alleviating the humanistic and economic burden associated with these inherited conditions (5–8). To accomplish that, the definition of health needs to embrace this vulnerable population, whose health is shaped by their unique genetic characteristics, which negatively impact their quality of life and well-being (9, 10). Therefore, providing optimal care for these individuals to address their health problems will enable them to cope with their health condition and to experience and enjoy a sense of health and well-being (11). Hemophilia is the most common inherited bleeding disorder, affecting more than 273,000 people, with an estimated additional 563,000 undiagnosed people worldwide (12, 13). Hemophilia was chosen as an exemplary rare disease due to its substantial lifetime treatment costs and the severe health consequences of inadequate management (14–17). Furthermore, hemophilia stands out as one of the few rare diseases with an approved gene therapy, currently recognized as the most expensive drug in the world (18–20). As such, public health policies play a critical role in ensuring equitable access to care and novel treatments (21–24).
This narrative review aims to summarize and discuss various aspects related to concepts of health, public health, rare diseases in general, and hemophilia in particular, and highlight that the integration of public health interventions into routine care may improve the outcomes for patients affected by rare diseases, including hemophilia.
We embarked on an in-depth narrative literature review to explore and discuss the role of public health in rare diseases in general, using hemophilia disease as an example. First, we conducted a preliminary search of the literature, which yielded a range of heterogenous sources, mostly narrative literature reviews, and highlighted various sub-topics that we believed added value to the main topic. Due to this heterogeneity, a systematic search for primary studies seemed inapplicable, and it was deemed necessary to employ a narrative review methodology because it was more appropriate for our broad topic with its multifaceted aspects (25, 26). Accordingly, we conducted focused and snow-balling searches between July 2023 and December 2024, capitalizing on basic and advanced search techniques on PubMed, Google Scholar, and Google (27, 28). We used several keywords and phrases related to rare diseases, public health, and hemophilia to identify relevant publications in the English language, with no date limits or selective geographical locations. The search terms used were basic definitions, epidemiological aspects, economic burden, psychosocial burden, legislations, regulations, policies, implementation gaps, access to treatment, and other secondary topics. Relevant concepts and themes identified during the search process were then classified and described under headings and sub-headings in this review (25, 26).
Our online searches identified 315 relevant sources of evidence, which were used to synthesize information spanning various topics covered in this review (Supplementary Table 1). After that, we classified the retrieved sources according to (1) relevance (topic-related or methodological references), (2) Publication type (journal articles, textbooks, governmental/official sources, websites, etc.), (3) type of evidence (original research articles, literature/systematic reviews, online books/book chapters, practice guidelines/recommendations, governmental/official reports/papers/guidance, etc.), and (4) evidence category (primary or secondary source of evidence). The topic-related sources were 311 references, of which journal articles represented 76.5% (238) of sources (Figure 1A). Among these 238 journal articles, 179 (75.2%) were narrative reviews and articles, while 36 (15.1%) were original research articles and 23 (9.7%) were systematic and scoping reviews (Figure 1B).
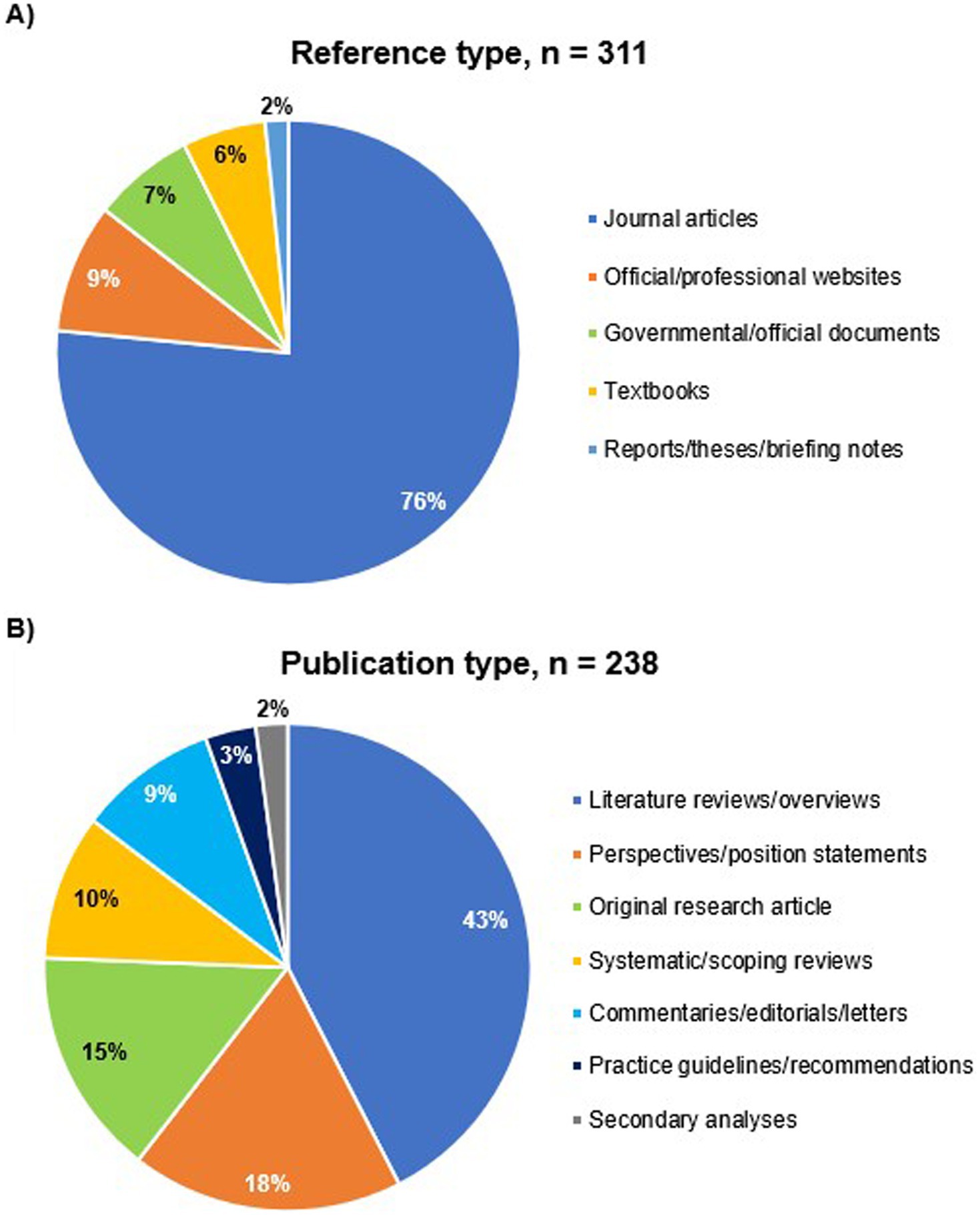
Figure 1. Classification of sources of evidence used to synthesize information in this review. (A) Topic-related references, (B) journal articles.
The original definition of health set by the World Health Organization (WHO) in 1946 necessitated the absence of disease or infirmity to achieve the actual meaning of health (29). However, a contemporary and more dynamic definition of health argues that health and disease or disability may co-exist without prejudicing the value of health (30). Thus, people with chronic diseases and disabilities can enjoy healthy lives if they receive appropriate medical care and are able to cope with their condition (11). As a result, new definitions for health have been proposed, underscoring the dynamic balance among the structural, functional, physical, mental, social, and emotional states of the individual in adapting to life and the environmental conditions to attain an effective state of personal well-being as part of the society (31, 32). Nonetheless, these new definitions may not be suitable for all health conditions due to the complexity of the health notion across various conditions and different stages of life, where both illness and well-being are dynamic and interwoven states. Therefore, health can be attained when a person can cope with these various health states involved in defining the overall health condition (9, 10). Furthermore, the increasing use of technology and the digitalization of healthcare make the adoption of a single definition of health more complex (33).
Public health can be defined as the science and practice of protecting, promoting, and maintaining good health and quality of life, as well as prolonging the lives of all people (34–36). This can be achieved by detecting, preventing, and managing disorders, diseases, illnesses, and injuries through organized public health measures and actions taken by public and private institutions, non-governmental community-based organizations, and individuals (37–39). Therefore, public health constitutes an integral part of the healthcare system (9). While clinical healthcare focuses on treating individuals or subgroups of people in times of sickness, public health focuses on protecting and promoting the health and well-being of the entire population to meet the growing needs and expectations of society (9, 34). This evolving role of public health has led to developing the more recent concept of population health. This term, which can be used as a synonym for public health, emerged to address and improve health outcomes and their distribution among all community members over time, by considering broader factors that influence these outcomes. These factors encompass demographic and socioeconomic variables that contribute to health inequities in the community (40–42).
Figure 2 summarizes the conceptual and applied models of public health practice. The former consists of three overlapping main domains and 11 subdomains. The area of overlap represents the role and activities of public health directories, capitalizing on the use of research methods, information technology, laws, and ethics in public health practice. This conceptual framework establishes a culture of public health assessment that relies on surveillance and monitoring of health hazards and adopts governance and risk management strategies using public health intelligence and information technology (37, 39).
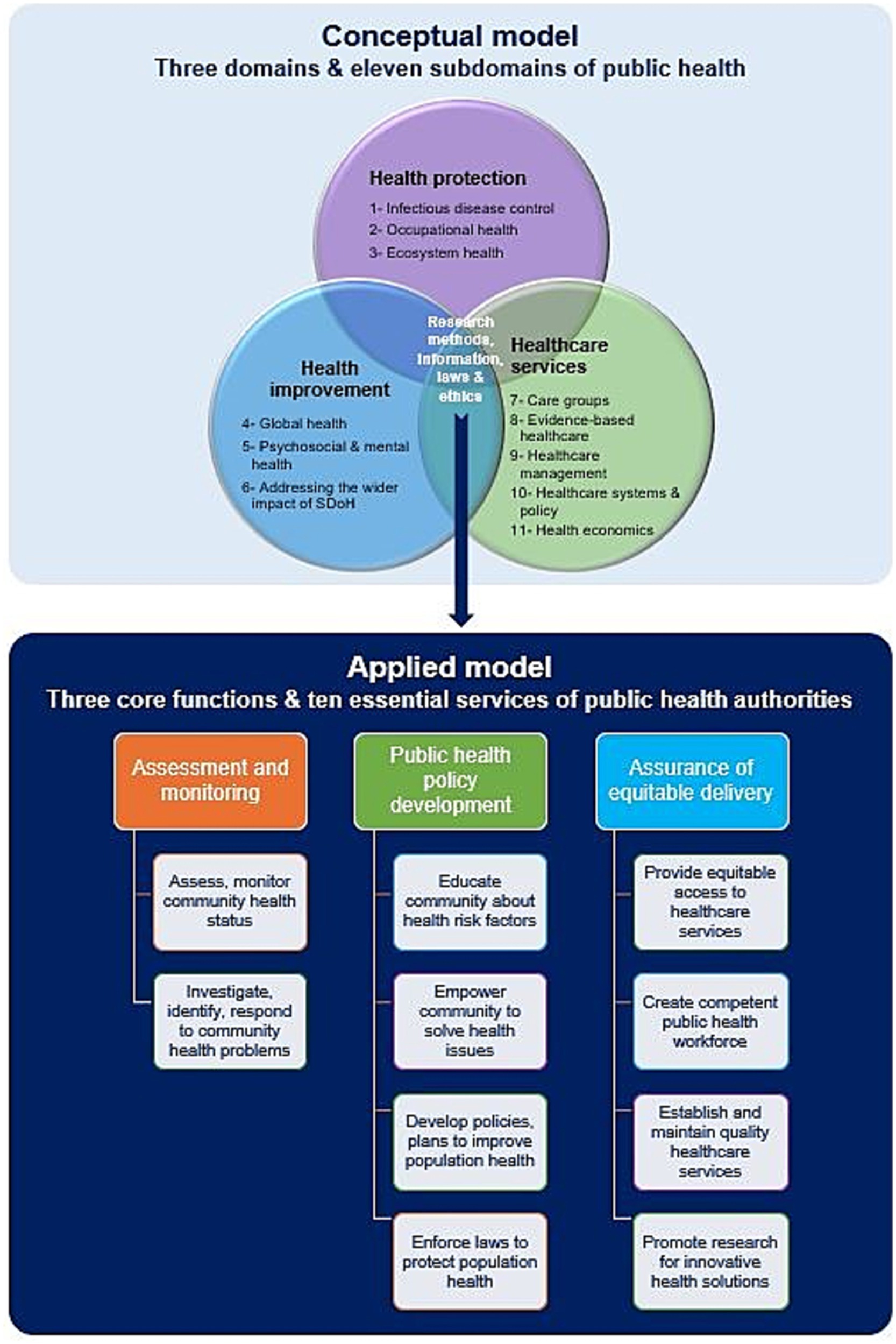
Figure 2. Conceptual and applied models of public health practice. Data modified from references (39, 44, 46). SDoH, Social determinants of health.
The applied model of public health practice is based on the following three core functions of public health authorities and agencies: (1) public health surveillance through assessment and monitoring of health information of populations at risk, (2) development of comprehensive public health policies to tackle the identified health problems and set priorities for their management, and (3) assurance that appropriate and cost-effective public health services are provided to the community equitably. These three core functions can be implemented by public health entities by delivering the 10 essential public health services (9, 43–46).
Determinants of health and health inequities are the social, economic, environmental, and political factors that predict the individual’s health, such as education, income, housing, unemployment, nutritional status, living and working conditions, psychosocial support, and ways of transport, which in turn, are influenced by public policies. Those health-shaping factors mostly exist outside the healthcare system, which itself remains one of the social determinants of health (47–50). We have identified 50 determinants of health in the scientific literature and official websites of main public health authorities, with additional determinants specific to certain diseases and disorders that may be added to the list (Supplementary Table 2) (47, 51–55). These determinants of health are broadly classified into three main categories: the individual’s characteristics and behaviors, the individual’s physical environment, and the individual’s social and economic environment (56, 57). Differences in those factors among society members create avoidable and unjust inequities in health and well-being outcomes (49, 58–61). In addition, biological and genetic characteristics are at the core of several interrelated factors that affect the individual’s health and well-being (55, 62).
Despite the growing international recognition of rare diseases, there is no consensus on a unified global definition for rare diseases (63–73). A systematic search conducted between 2013 and 2014 identified 296 different definitions for rare diseases from 1,109 entities in 32 countries (67). The prevalence threshold for rare diseases ranged from 5 to 76 cases per 100,000 population. Moreover, 21 out of the 32 countries (66%) adopted a prevalence threshold from 40 to 50 cases per 100,000 population. The overall prevalence threshold average was 40–50 cases per 100,000 population (67).
Table 1 summarizes different definitions of rare diseases proposed by various countries and jurisdictions, as an integral part of their public health policies designed for rare diseases, which will be discussed later. The European Union (EU) defines a rare disease as a severely debilitating and life-threatening or seriously progressive and chronic disorder affecting ≤50 per 100,000 people (74) which requires medical attention to reduce its morbidity and mortality, and its impact on the person’s quality of life and social integration (72, 75, 76). In 2019, Health Canada adopted a definition and a prevalence threshold similar to the EU (77, 78).
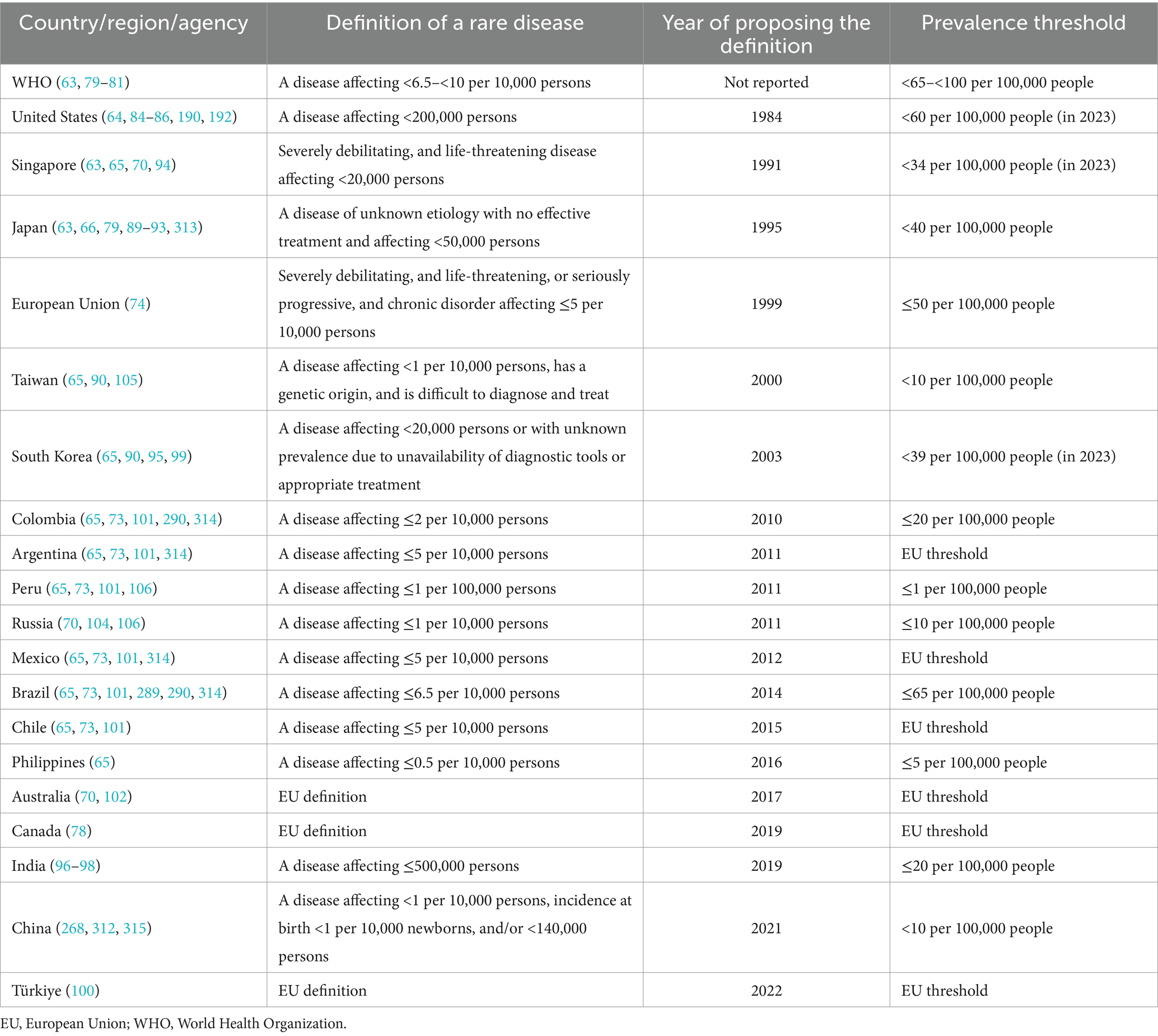
Table 1. Definition and prevalence threshold for rare diseases across several countries, regions, and agencies.
The WHO set a threshold for rare disease prevalence of less than 65 to less than 100 per 100,000 population (63, 79–81). The United States (US) considers a disease rare when it affects <200,000 persons nationally, corresponding to 85 per 100,000 people in 1984 when the Orphan Drug Act was amended. As the US population grew, the prevalence declined over the years until it reached <60 per 100,000 population in 2023 (64, 70, 82–87). The change in the prevalence of rare diseases in the US pertains to the arbitrary absolute threshold of less than 200,000 persons nationally definition, which was derived from previous estimates on narcolepsy, multiple sclerosis, and Tourette syndrome (70, 71, 87, 88). Similarly, Japan adopted an absolute figure approach and identified a prevalence threshold below 50,000 persons in 1993 for each rare disease (63, 66, 79, 89, 90). The prevalence at that time was equal to approximately 40 per 100,000 persons, which was almost the same in 2023 with comparable population size (91–93). Additionally, other countries such as India, Singapore, and South Korea followed a similar approach to that of the US and Japan (63, 65, 70, 90, 94–99).
While some other countries, such as Argentina, Australia, Chile, Mexico, and Türkiye adopted the EU definition and prevalence threshold for rare diseases (65, 70, 73, 78, 100–102), others, such as Colombia, Peru, Philippines, Russia, and Taiwan adopted stricter prevalence thresholds (65, 70, 73, 90, 101, 103–106). In China in 2021, a rare disease definition included a prevalence threshold of <10 per 100,000 persons, incidence at birth of <10 per 100,000 newborns, and/or a total affected population of <140,000 persons nationwide (107).
According to the most widely accepted prevalence thresholds for defining rare diseases worldwide, hemophilia and other inherited bleeding disorders are thus considered rare (79, 108).
Drugs that treat rare diseases are considered orphan medicinal products by the EU, the Food and Drug Administration (FDA), and other regulatory bodies if the available treatment options are unsatisfactory or do not currently exist or may not exist in the future (106).
Disease burden is a term used to estimate the magnitude of a disease or health condition and its impact on a target population by collecting and reporting morbidity and mortality measures (109, 110). Morbidity measures of disease frequency include incidence rates and prevalence proportions (111, 112). The lost healthy life years, calculated as disability-adjusted life years, can be used as a composite outcome measure of the consequences caused by a disease’s morbidity and mortality (113, 114). In addition, estimating the disease burden by considering the economic aspects of disease management and its complications is called cost-of-illness (115, 116). Ideally, it should consider all direct and indirect costs spent by healthcare and non-healthcare sectors in society, as well as productivity loss by patients and caregivers due to the disease (117–119).
Recent estimates show that the number of currently identified rare diseases exceeds 10,390 (120), of which around 80% have a genetic origin and up to 75% have a pediatric onset (72, 79). It was estimated that at least 5.9% of the health conditions affecting humans are caused by 3,585 rare disorders, corresponding to a minimum of 446 million people globally living with rare disorders from 2017 onwards. However, only 4.2% of rare diseases are responsible for up to 80.7% of the rare disease burden. These rare diseases have a prevalence of 1–5 per 100,000 people. On the other hand, 84.5% of rare diseases have a prevalence of <1 per 1,000,000 people (71).
In the year 2000, the number of people living with a rare disease in the 25 countries constituting the EU was approximately 225,000 persons within a population of 450.4 million inhabitants (121). This number remained almost the same in 2023 based on a population of 448.4 million inhabitants in 27 countries (122).
Hemophilia affected more than 273,043 people worldwide in 2023 (13). The prevalence of hemophilia was extrapolated from national patient registries in six high-income countries. Data extrapolations estimate an additional 563,000 undiagnosed people with hemophilia worldwide. Of the total 836,000 diagnosed and undiagnosed patients, approximately 284,000 individuals are expected to be severe cases, based on a world population of 8 billion in 2023 (13, 123). Hemophilia severity is defined according to baseline factor levels (severe <1%, moderate 1–5%, mild >5–<40%) (124, 125) and clinically correlates with the number of bleeding episodes per year (126, 127). Without implementing prophylaxis as a standard of care, severe hemophilia is associated with shorter life expectancy, higher rates of musculoskeletal complications, and reduced quality of life and well-being (128, 129).
Rare diseases significantly reduce health-related quality of life and mental health, leading to negative psychosocial and emotional impacts on both patients and caregivers (130). These negative consequences are caused by stigma in school and workplace, including social exclusion, misappreciation, discrimination, lack of social support or understanding, and bullying from peers and teachers (131).
A recent study found that more than 75% of caregivers of 41 children and adolescents living with rare diseases in Western Australia experienced stigma, and over 46% reported being bullied at school (132). Several factors contribute to these negative behaviors, including a lack of or poor understanding of the rare condition and its complications among non-specialized healthcare professionals and the public, delayed or misdiagnosis of the disease, inadequate medical care—including psychosocial support, and a heavy reliance on caregivers, particularly mothers, lack or unavailability of effective treatment options, loss or reduced productivity, high out-of-pocket expenditure on medical care, and difficulties managing administrative tasks and socio-legal issues to receive the appropriate care they deserve (133–137). These challenges are more prominent in low- and middle-income countries (LMICs), where diagnostic and therapeutic options are limited due to scarce resources allocated for health systems, especially for people with rare diseases (138).
In hemophilia, the psychosocial burden experienced by patients is influenced by musculoskeletal health, which can be affected by the number of target joints, joint disabilities, the duration on episodic treatment or non-optimal prophylaxis, especially during childhood (15, 128, 139).
Rare diseases are associated with a considerable economic burden to both the healthcare sector and society (105, 140–143). In an economic analysis that included 24 rare diseases from five disease categories in the USA, the annual total economic burden per patient was approximately 10 times higher than that of other chronic diseases, such as diabetes and cardiovascular disorders (8). In Hong Kong in 2021, the average annual total cost per person across 106 rare diseases was reported to be 62,084 US$. The total out-of-pocket healthcare expenditure was estimated at 6,646 US$ per patient per year, representing approximately 11% of the total cost. Out-of-pocket expenditure on healthcare exceeded 10% of the total household income in more than 36% of families with a person affected by a rare disease. This catastrophic expenditure on healthcare pushed approximately 9% of families below the poverty line (141). In the US, out-of-pocket expenditure on healthcare for rare diseases was 4% of the total cost (143). Moreover, direct non-medical and indirect costs accounted for 51% and 61% of the total cost of rare diseases in the US and Hong Kong, respectively (141, 143).
Among 83 rare diseases in Sichuan province in China, hemophilia was associated with the highest total cost of care (144). A recent scoping review found that the annual societal cost of severe hemophilia A and B without inhibitor across 14 countries ranged from 479 US$ in India to 700,070 US$ in the US, with clotting factor replacement therapy accounting for 95.1%–99.9% of the total cost. In cases of inhibitor development, the annual cost was five to seven times higher than that for severe patients without inhibitors with a reported range of 1,289,663 US$ to 1,780,903 US$ (14). Other studies found that the annual cost of hemophilia A and B without inhibitors ranged from 201,471 US$ to 621,273 US$ in the USA (145, 146) and from 199,541€ to 246,693€ in Europe (147, 148), with significant out-of-pocket expenditure on hemophilia care in LMICs (149, 150).
Surveillance is an essential function of public health services, defined as the continuous and systematic collection, analysis, interpretation, and dissemination of health data needed for planning, implementing, and evaluating public health activities. These health-related data are collected from various sources, including patient organizations and official healthcare system registers. Without sufficient and systematic data collection, a surveillance system cannot function properly (151–153). It involves the timely dissemination of information to those responsible for disease prevention and control, as well as to those who require the data to take appropriate action (154–158). Additional aspects of the surveillance system include disease management by providing the required diagnostic and clinical services; training and education of the healthcare staff; information management systems that support data collection, data analysis, and reporting of findings; as well as policy formulation and enactment to support the implementation of surveillance (155, 156, 159).
After data collection, the next step is analyzing and interpreting the information using a cross-sectional study design to characterize the target population and to identify potential risk factors, as well as disease and treatment outcomes of interest, which can be further analyzed for predicting and monitoring disease trends over time (160, 161). The final step in public health surveillance is sharing the findings of these analyses and interpretations with those responsible for designing and implementing better health policies, allocating sufficient healthcare resources, and finally improving patient access to available treatment options (154–156, 161).
In hemophilia, surveillance plays a critical role in detecting and monitoring incidence rates, prevalence proportions, and mortality rates (162–164). It is also helpful in collecting and mapping individual and disease characteristics in the affected population, including identifying risk factors for developing subsequent serious and life-threatening disease complications, such as intracranial hemorrhage, inhibitor development, musculoskeletal complications, and other co-morbid conditions, such as blood-borne infections and cardiovascular disease in the adult population (161, 165, 166). Table 2 provides an overview of public health surveillance systems and registries for hemophilia and inherited bleeding disorders (13, 161, 162, 167–174).
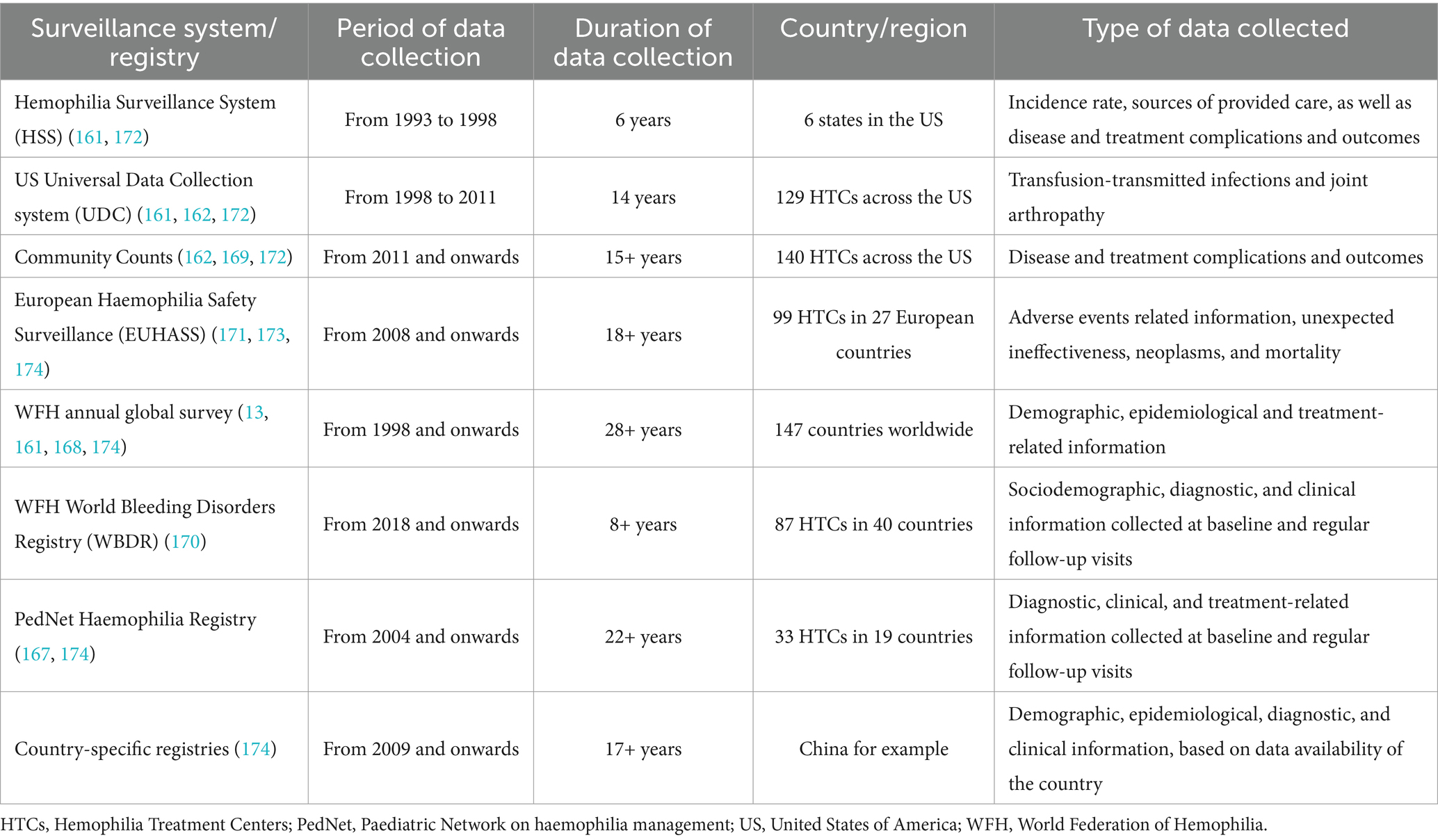
Table 2. Hemophilia and inherited bleeding disorders public health surveillance systems and registries.
Patient registries play a pivotal role in addressing the gaps in epidemiological data for hemophilia and other rare diseases as crucial sources of information for basic and clinical research, as well as for epidemiological and public health purposes (174, 175). It is evident that national and international registries support collecting standardized data, improving data quality, and enhancing our understanding of the disease’s epidemiology for better public health planning (98, 176). These registries provide comprehensive data on patient demographics, disease characteristics, treatment patterns, and outcomes (151, 170, 177). Notably, long-term population registries could improve methods for data collection, enhance the accuracy of estimating epidemiological data, and support informed decision-making in managing hemophilia and rare diseases (68, 175).
Public health policy is a broad term that refers to official laws, regulations, procedures, measures, actions, decisions, plans, and incentives designed by governments, as well as relevant authorities and institutions, to promote the health and well-being of a target population and to ensure achieving specific health goals for that population group (178–180).
In 2015, with the adoption of the 2030 agenda by the United Nations General Assembly (UNGA), one of the targets of sustainable development goal number three (SDG 3) was universal and equitable health coverage for all people without any kind of distinction or financial burden (181). The role of public health has evolved to include individuals living with rare diseases (3). This was accomplished through enacting legislative actions, enforcing regulatory measures, and designing and implementing national plans, frameworks, policies, and strategies (65, 75, 182–185). During the last two decades of the 20th century, various stakeholders, including legislative bodies, regulatory authorities, research institutions, and other governmental and non-governmental entities in several countries started to realize the need for people living with rare diseases to have effective treatments for their lifelong conditions and to support them. This was done by releasing decrees and adopting regulations to incentivize research institutions and the pharmaceutical industry to develop treatment options for various rare diseases (63–66, 68, 70, 75, 76, 79, 182, 184–189).
Figures 3, 4 present the timelines of key legislations, regulations, and national policies related to rare diseases and orphan drugs in the US and globally. A notable example is the US Orphan Drug Act, which was enacted in 1983 and subsequently amended several times, with the latest amendment in 2017 (64, 70, 85, 86, 190–192) (Figure 4).
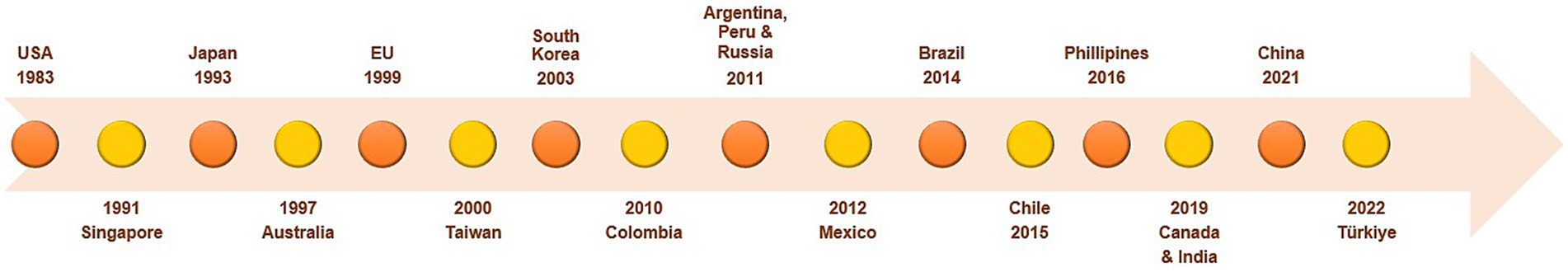
Figure 3. Timeline of legislations, regulations, and national policies for rare diseases and orphan drugs in different countries. Data summarized from references (64–66, 70, 73, 74, 78, 89, 90, 95–97, 99–102, 104, 105, 192, 268, 289, 312). EU, European Union; USA, United States of America.
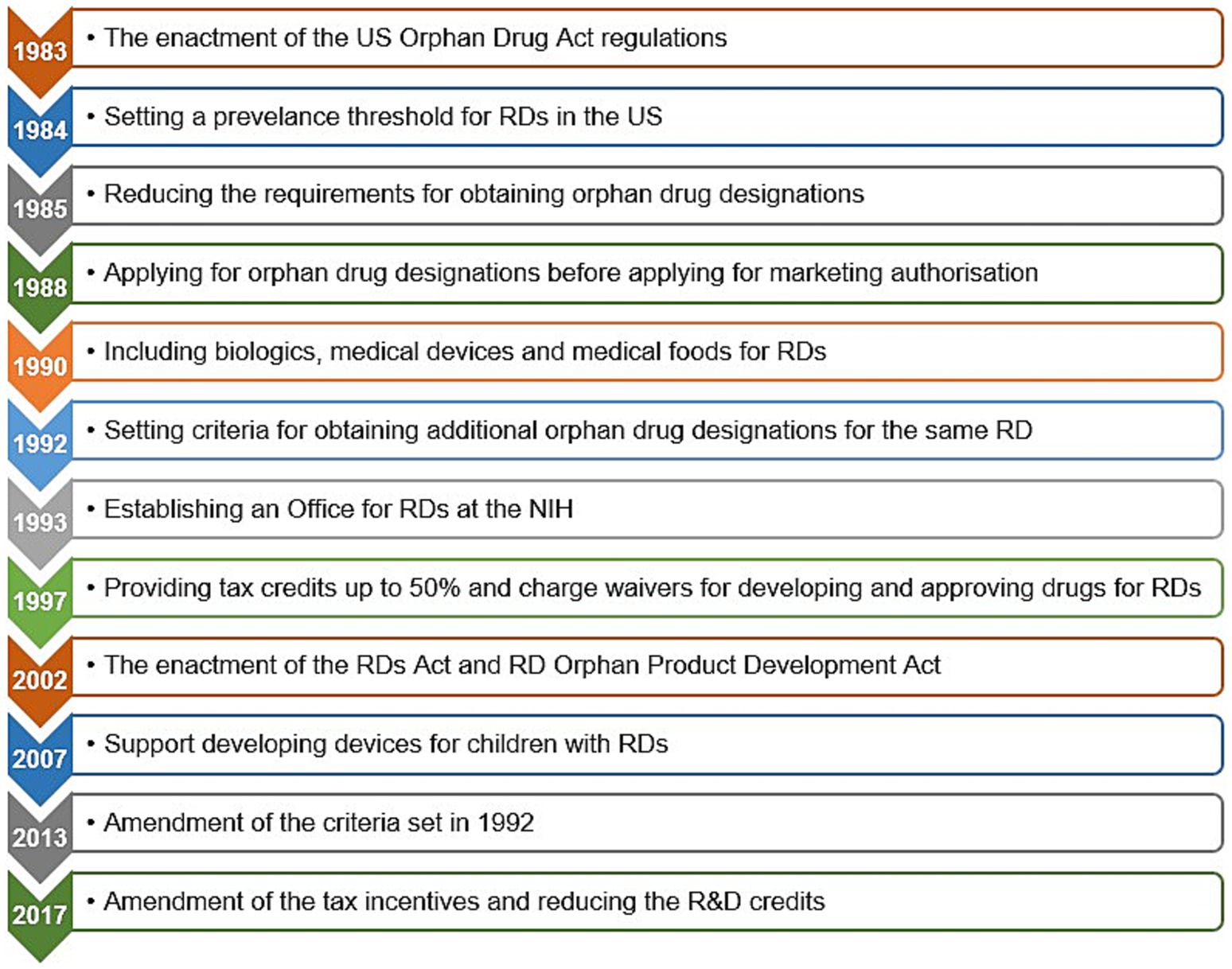
Figure 4. The Orphan Drug Act and subsequent amendments in the United States of America. Data summarized from references (64, 84–86, 190, 192). NIH, National Institutes of Health; RD(s), rare disease(s); R&D, research and development; US, United States.
Since the enactment of these legislative measures and regulatory instruments, rare diseases have been given attention as a global public health priority in health policy, medical research, and regulatory agendas, which in turn reflected positively on orphan drug pipelines (68, 71, 72, 76, 81, 185, 193–195). During the period between the enactment of the Orphan Drug Act by the US Congress in 1983 until the end of 2022, the US FDA granted 6,340 orphan drug designations for 1,079 rare diseases, of which 882 orphan drugs, representing 14% were approved for 392 rare diseases.
Similarly, since the enforcement of the European regulation on orphan medicinal products in 2000, the European Commission designated around 2,000 therapeutic agents as orphan medicinal products and approved 200 of them (74, 196). Furthermore, in 2021 and 2022, 52% and 49% of new drug approvals, respectively, were assigned to rare diseases (197, 198).
Stemming from the overarching principle of providing appropriate healthcare services for all people (199), the UNGA recognized the needs and challenges faced by people living with rare diseases. In December 2021, a complementary resolution was adopted to focus on this specific population and their families (200). The resolution aims to ensure that they can exercise their human rights to achieve the highest level of physical and mental health, as well as to promote their inclusion and participation in society (201).
The International Rare Disease Research Consortium was founded in 2011 with an ambitious goal of discovering diagnostic tools for most rare diseases by 2020 and getting 1,000 new therapies approved for rare diseases by 2027. The first goal was achieved earlier than expected in early 2017 due to the allied global efforts for serving the rare disease community, whereas the second goal is still underway (202–204).
Healthcare systems in several LMICs still face challenges in making orphan drugs available and accessible to people with rare diseases due to unaffordable prices (205, 206) (Figure 5). In a review of value assessment frameworks adopted by health technology assessment (HTA) units in 18 European countries, it was found that 11 (61%) countries still evaluate orphan drugs using conventional cost-effectiveness and cost-utility analyses (207). All approaches presented in the review and their frequencies are summarized in Figure 6.

Figure 5. Challenges of assessing the value of orphan drugs. Data summarized from references (118, 195, 206).
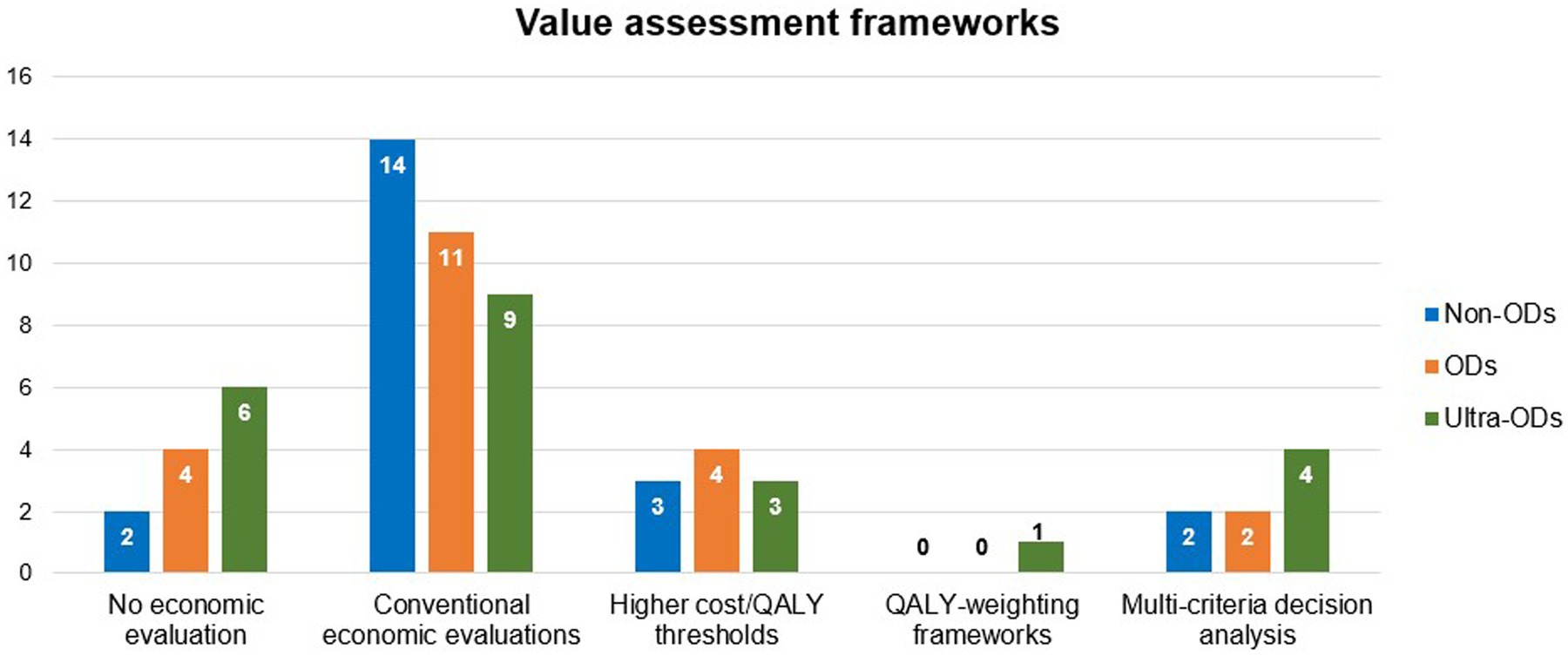
Figure 6. Frequency of using different types of value assessment frameworks in 18 European countries. Data summarized from reference (207). Some countries use more than 1 method for a single drug class. Non-ODs, Non-Orphan Drugs; ODs, Orphan Drugs; QALY, Quality-Adjusted Life Year, Ultra-ODs, Ultra Orphan Drugs; VAFs, Value Assessment Frameworks.
In a comparative analysis of the reimbursement status of 15 orphan drugs in HTA units in four high income countries (HICs) (Australia, Canada, England, and Scotland) from 2017 to 2018, significant heterogeneity in reimbursement assessment criteria and final reimbursement decisions existed among countries (Figure 7). This heterogeneity may be partly explained by variations in the prevalence data of rare diseases used in their respective countries’ assessments (208).
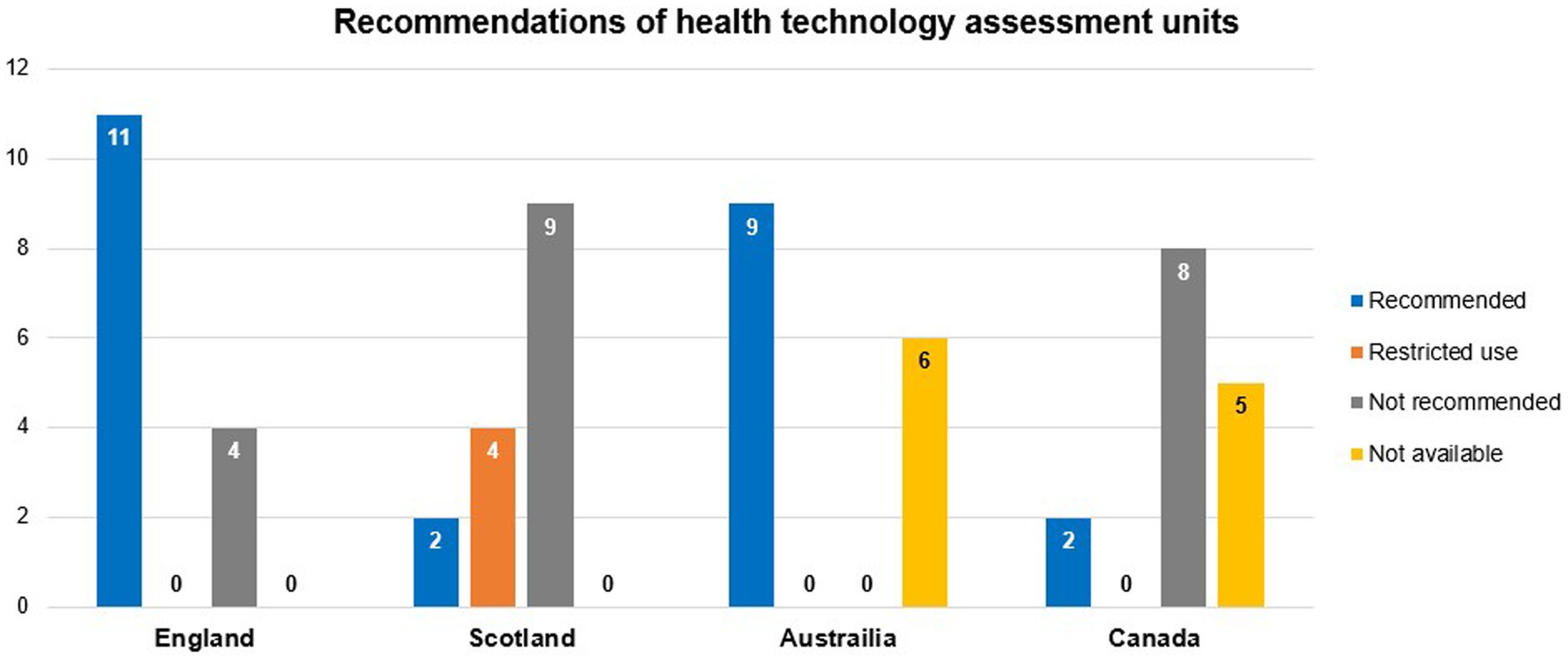
Figure 7. Frequency of HTA units’ recommendations for reimbursing 15 orphan drugs in four HICs from 2017 to 2018. Data summarized from reference (208). HTA, health technology assessment; HICs, high-income countries.
Moreover, a recent study confirmed a similar heterogeneity in reimbursement decisions across 12 European countries. The findings revealed that the recommendations from local HTA agencies do not significantly influence subsequent payer decisions for reimbursing orphan drugs in more than two-thirds of these countries (209). Reimbursement decisions are usually based on market access agreements, known as managed entry agreements between drug manufacturers or their agents and healthcare payers. These agreements are necessary to determine the final price of a drug when its clinical benefits are still uncertain, and its reimbursement poses a significant financial burden (210, 211). Managed entry agreements fall under two main categories, based on their purpose. If the aim is to lower the price of a new intervention or to reduce the budget impact of introducing it for the payer, it will be called a financial agreement. On the other hand, if its aim is to pay for a new intervention based on its performance, it is called an outcome- or performance-based agreement. Both categories can be further divided into two subcategories, depending on whether the agreement focuses on individual patients or a wider target population (212, 213). Each subcategory encompasses various agreement designs and templates, including special price, discount, or rebate agreements, volume- or budget-cap agreements, free initial treatment agreements, agreements for developing real-world evidence, payment-by-results agreements, conditional treatment continuation agreements, price–volume agreements, pay-back agreements, and risk-sharing agreements (210, 211, 213–216).
Equally important, the emergence of innovative funding mechanisms is crucial to ensure sustainable access to costly novel interventions (118, 211, 215–217). A report by IQVIA identified five key archetypes of such programs: (1) Blended Finance: Combines public or non-profit catalytic capital with private sector investments to promote sustainable development. This approach aligns diverse objectives—financial, social, or both—while linking funding to specific outcomes and timelines; (2) Novel Private Insurance: Offers coverage for products or services typically excluded, such as diagnostics, or for underserved patient groups, including those with pre-existing conditions; (3) Government Funding Schemes: Designed and disbursed by governments while involving contributions from multiple sectors; (4) Multi-Source Crowdfunding: Mobilizes funds from diverse stakeholders (individuals, companies, or non-profits), often incentivized through mechanisms like tax benefits; and (5) Financial Services: Provides alternative payment methods, such as credit or savings plans, facilitated by FinTech or traditional banking, enabling patients to manage costs flexibly. These innovative funding models can play a pivotal role in bridging financial gaps and improving access to advanced medical therapies (218).
As an example of rare diseases, equity in global hemophilia care remains a significant challenge. Disparities in access to laboratory and genetic diagnoses, prophylaxis and home treatment, effective treatment options, and comprehensive care still exist across different regions and countries (21–24).
In congenital rare diseases, primary, secondary, tertiary, and quaternary disease prevention strategies have been shown to be beneficial from public health and health economic perspectives (5–8).
In Europe, up to 15% of people living with rare diseases have rare congenital anomalies (219), which can potentially be reduced by the implementation of primary prevention strategies, such as pre-conception or pre-marital carrier genetic screening, preimplantation genetic diagnosis accompanied by in vitro fertilization, healthcare counseling, and educational campaigns, as well as secondary prevention strategies, such as post-conception or prenatal carrier genetic screening and newborn screening (5, 6, 220–222). Therefore, accurate and relevant epidemiological information on the prevalence, morbidity, and mortality of rare diseases is crucial in evaluating and addressing their impact on population health through public health approaches, planning and implementation of health policies, supporting the process of drug development, and the conduct of clinical trials (2, 71, 72, 223, 224).
Public health and precision medicine were initially viewed as competing fields, the former analyzes limited data from large populations with the overarching goal of improving population health, while the latter handles massive sets of data from targeted population cohorts to personalize diagnostic and therapeutic approaches based on these individuals’ needs (225). Precision public health is emerging as a bridge to reconcile these two fields, united by the common goal of achieving equitable provision of health services and reducing disparities in healthcare outcomes, especially in rare diseases (182, 226). Thus, precision public health can play an important role in utilizing big data to re-aggregate small cohorts into large-scale ones, based on biological pathway commonalities, accordingly, enabling novel personalized interventions such as pharmacogenomics, gene editing, and gene therapies to achieve effective and equitable implementation while remaining grounded in the public health values of whole population health improvement and equity (227–229).
Notably, in rare diseases, rarity varies according to demographic and geographical factors, such as rates of consanguinity for congenital disorders, and contextual factors, such as endemicity rates of contagious diseases (120). In rare genetic disorders, phenotypic variability is observed due to the varying disease severities and the different disease subtypes. This non-linear genotype–phenotype relationship is shaped by genetic and environmental disease modifiers that influence the genotype penetrance, expressivity, and pleiotropy of the causative gene of a specific genetic disorder. Understanding this relationship in rare genetic disorders using next-generation sequencing techniques, such as whole-genome sequencing, whole-exome sequencing, and targeted exome sequencing, facilitates accurate, cost-effective, and timely diagnosis (230–232). In addition, the use of novel data generation technologies, including artificial intelligence, will enhance analysis and interpretation of mass biomedical data, helping to close existing gaps in this field and advancing it into new horizons for better diagnosis and treatment of people with rare diseases (233–236).
Advances in genomic and epigenetic analyses have accelerated the research on drugs and biologics that act on disease-specific molecular pathways. Most rare diseases are monogenic disorders (182, 237, 238). Several gene-targeted therapies (GTTs) have shown great promise for rare monogenic disorders. Given the urgent needs of rare disease patients, GTTs are generating interest from the US National Institutes of Health (NIH) to hasten the drug development process for these disorders. This includes using many approaches, platforms, and master protocols to increase the logistical efficiencies for the patients to access these therapeutics (239). Moreover, FDA and the European Medicines Agency (EMA) have issued new scientific guidelines on emerging therapeutic trends including regenerative medicine therapies, gene therapies, and genetically modified cell-based therapies (240, 241).
To reinforce these efforts, the Bespoke Gene Therapy Consortium (BGTC) was recently launched as a bold partnership between the NIH, FDA, 10 pharmaceutical companies, and several non-profit organizations. It aims to optimize the development of gene therapy and fill the gaps and unmet needs of this vulnerable group (242, 243). The NIH and private partners will contribute approximately 76 million US$ over 5 years to support the projects funded by the BGTC. This includes about 39.5 million US$ from the participating NIH institutes and centers, pending the availability of funds. The National Centre for Advancing Translational Sciences (NCATS), the NIH’s lead for BGTC, is expected to contribute approximately 8 million US$ over 5 years (244).
The complexity of health necessitates a unique approach that acknowledges the diverse nature of health conditions and the unique needs of individuals (10). Public health plays a pivotal role in protecting and improving the health and well-being of populations worldwide (9, 37, 39, 40). Over time, the role of public health has evolved from solely focusing on the prevention of infectious diseases to reducing the burden of non-communicable diseases and recognizing the needs of individuals with rare diseases (6, 220–222). Notably, the global landscape of rare diseases presents significant challenges, as there is currently no unified global definition for the prevalence threshold of a rare disease (63–73). With over 10,000 identified rare diseases (120, 245) impacting approximately 450 million people globally (71), it is essential to gather accurate epidemiological information to understand and address their impact on population health effectively.
In this review, we selected hemophilia as our case study because it is one of the most costly rare diseases to manage over the patient’s lifetime (145–148). Moreover, hemophilia is diagnosed shortly after birth and its health outcomes rely heavily on treatment accessibility (124, 125). Without appropriate treatment, people with severe hemophilia will develop long-term and debilitating musculoskeletal complications due to frequent bleeding and their life expectancy will be severely compromised, with early mortality during childhood and adolescence, which is the case in many LMICs (16, 17). In HICs, gene therapy is a viable one-time treatment option for hemophilia, with several vectors approved by the FDA and other regulatory bodies worldwide (18, 20). However, health systems around the globe are still struggling with the pricing, funding, and reimbursement frameworks of gene therapies especially given the expensive upfront payments (19). For these reasons—among others, public health can potentially enhance the access of people with hemophilia to the available treatment options worldwide, through surveillance, advocacy, and planning (246–248).
Public health plays a vital role in managing rare diseases on multiple levels, beginning with the prevention of genetically linked congenital disorders and addressing health inequities that disproportionately impact patients with rare diseases (71, 249). Evidence from HICs has shown that approximately 70% of congenital disorders are preventable or treatable when the appropriate public health measures are implemented (250, 251). Although many congenital diseases that present at birth have a genetic nature (e.g., osteogenesis imperfecta), others develop due to a variety of factors such as environmental risk factors, problems during development, or birth itself (e.g., fetal alcohol syndrome). Thus, congenital and genetic diseases are not identical (251, 252). Yet, the success of public health in controlling congenital disorders with genetic nature should motivate the global community to implement disease prevention strategies to reduce disease prevalence and burden (193). Additionally, to achieve better population health, it is essential to address the determinants of health and health inequities, including the genetic determinants that affect individuals with rare diseases (253, 254).
At the policy making level, legislative and regulatory support has facilitated the development and approval of diagnostic and therapeutic agents for rare diseases, leading to significant advancements in the treatment landscape (255). For example, the cost of developing new treatments for rare diseases may be lowered by approximately 60% when implementing policies and regulations for accelerated drug approval, which also shortens the time to approval to one-third (256). Incentives to drug manufacturers have attracted remarkable investments in developing numerous orphan therapeutic products in recent years (203). Therefore, it is crucial that governmental health directorates realize that investment in research, development, and regulatory reforms for better care for people with rare diseases is highly lucrative from economic and clinical perspectives (257). This process should be ongoing to ensure the sustainability of innovation in the field of rare diseases (258), and aspire to shift the focus of developing orphan drugs from reaching a profitability threshold by marketing those drugs in specific markets, especially HICs, to achieving an equitable environment through attaining comparable health outcomes across various diseases and different income levels (259).
Despite the remarkable progress that has occurred in the legislative and regulatory domains in the current century, greater investment and innovation in drug discovery and market access pathways in LMICs and HICs are still needed (183, 202, 260–262). Patient access to these expensive medications, even in HICs, is not guaranteed unless alternative value-based assessment approaches with complementary elements, as well as innovative pricing and reimbursement schemes, are proposed by relevant HTA units (118, 211, 217). Additionally, payers should adhere to HTA agencies recommendations in line with evidence-based decision-making to facilitate timely patient access to newly discovered orphan drugs (215, 216). By implementing such measures, the principles of health equity can be upheld, ensuring that individuals with rare diseases are fully included as valued members of society (3, 263).
Overall, a key factor to the success of public health policies is developing feasible implementation strategy with a clear and ongoing monitoring and evaluation plan to ensure that these policies are translated into regulatory measures through value assessment frameworks, and support healthcare systems and healthcare professionals while implementing them (65, 81, 176, 185, 211, 258, 264–266). Yet, many countries, especially LMICs face several challenges in enacting legislation, developing regulations, and implementing policies to support the diagnosis and treatment of people with rare diseases (63, 65, 66, 81, 82, 188, 264, 267, 268). These challenges include a lack of awareness about the burden of rare diseases, insufficient financial and human resources, inadequate health systems and infrastructure, absence of national policies and strategies for managing rare diseases and lack of a feasible implementation plan to translate policies into actions. All these limitations compromise patient access to diagnostic and therapeutic tools, which leads to increased morbidity and mortality (68, 81, 182, 226, 261, 269, 270). Efforts are underway to address these challenges through international collaborations, capacity-building initiatives, and raising awareness about the impact of rare diseases on public health (271–273).
Suggested approaches to overcome challenges in LMICs include (1) improving coding for rare diseases in patients’ medical records used in health information systems (68, 182, 274), (2) collecting sufficient information through patient registers (1, 68, 98, 176), (3) establishing precision public health frameworks to enhance genetic and radiological diagnoses (182, 226, 237), (4) facilitating the clinical use of data science and gene sequencing for rare diseases, which improves the quality of epidemiological data and informs public health policy (80, 182, 230, 232), (5) sharing experiences from HICs that have developed and implemented efficient policies and strategies, to support other countries in designing their own (73, 81, 104, 261, 269, 270), (6) raising health literacy, capacity-building, and self-management of the disease and its complications (275, 276), and finally (7) establishing a shared decision-making process for coordinated disease management (68, 277, 278) (Figure 8).
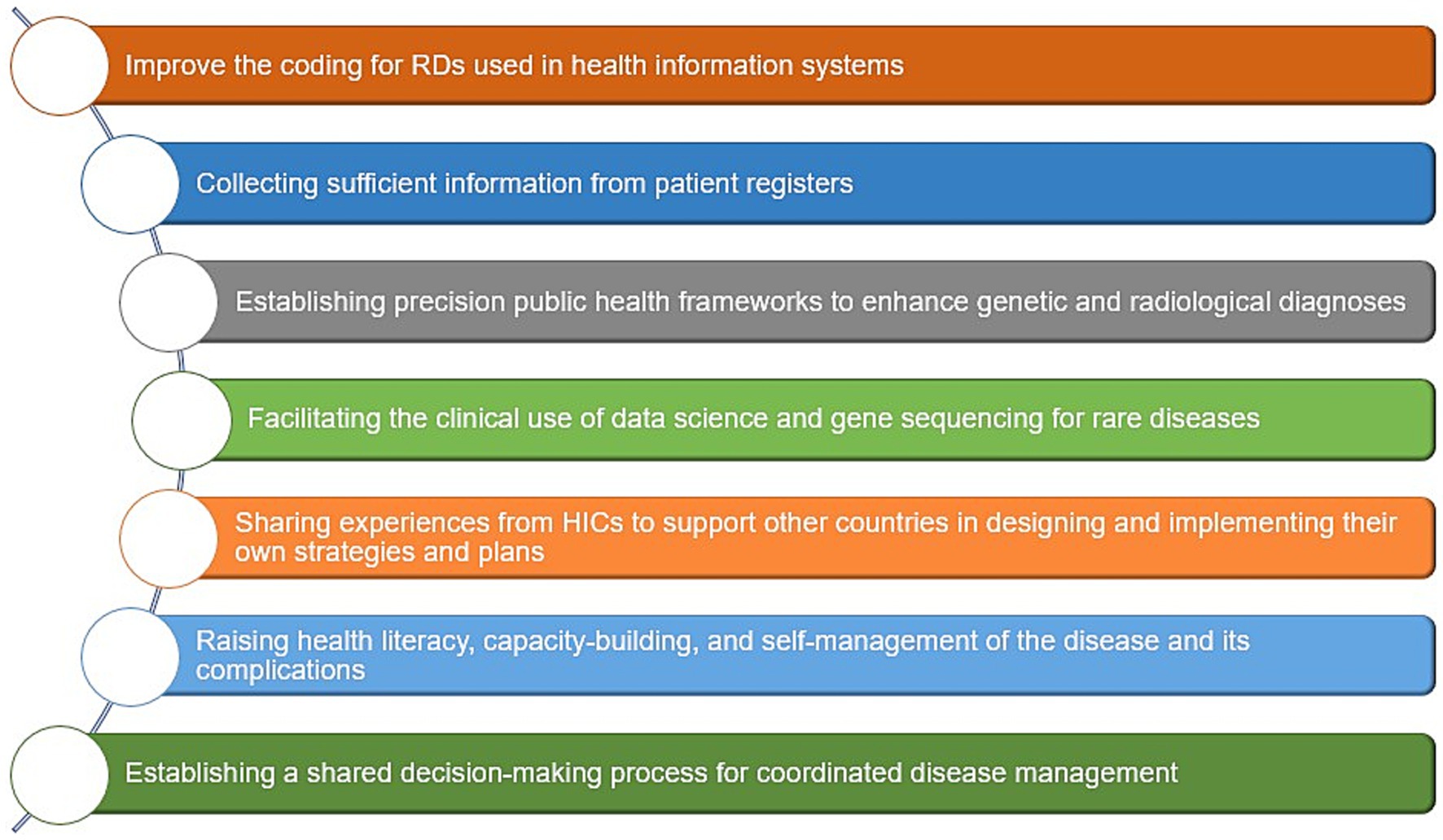
Figure 8. Suggested public health approaches to overcome challenges of RDs in LMICs. Data summarized from references (1, 68, 73, 80, 81, 98, 104, 176, 182, 226, 230, 232, 237, 261, 269, 270, 274–278). HICs, High-income countries; LMICs, low- and middle-income countries; RD(s), rare disease(s).
In the context of hemophilia, public health can play a crucial role in improving hemophilia care by addressing the gaps in the availability and accuracy of epidemiological data, advocating for equitable access to treatment for better hemophilia care (21–24), and implementing evidence-based interventions into routine clinical practice (164, 247, 248). Public health approaches—including health promotion; primordial, primary, secondary, tertiary, and quaternary disease prevention strategies (6, 279); public health surveillance; and policy development and implementation—can improve the overall management and outcomes of hemophilia care (162, 164, 280). Furthermore, public health efforts should focus on overcoming barriers such as inadequate healthcare infrastructure, shortages of trained healthcare professionals, and affordability challenges. Collaborative initiatives involving governments, healthcare systems, professional and patient organizations, and international stakeholders are essential to ensuring equitable access to comprehensive healthcare services for all individuals with hemophilia, regardless of geographic location or socioeconomic status (21–24). As such, by integrating public health principles into hemophilia care, the focus can shift toward more comprehensive, patient-centered, cost-effective approaches that address the broader health needs of individuals with hemophilia and their families to improve their health-related quality of life and achieve sustainable physical and mental health (246, 281).
Thus, to achieve optimal hemophilia care, several priorities need to be met. First, provide laboratory, radiological, and genetic diagnosis, including carrier detection and newborn screening. Second, treat acute bleeds, including serious and life-threatening bleeds. Third, prevent musculoskeletal complications by offering prophylactic treatment to people with hemophilia. Fourth, prevent blood-borne infections by providing safe coagulation factors and other hemostatic therapies. Fifth, delay, reduce, or prevent inhibitor development to avoid putting an additional disease burden on people with hemophilia. Sixth, restore musculoskeletal health by providing the appropriate physiotherapy services and performing the required surgeries. Seventh, offer psychosocial support to patients and families to reduce the humanistic disease burden and improve health-related quality of life (139, 282, 283). And last, create pathways for access to novel therapies through innovative pricing and reimbursement schemes (284, 285).
Brazil stands out as a notable success story among LMICs, demonstrating how targeted policy efforts can improve care for rare diseases (286, 287). Brazil, ranks fourth in the number of people diagnosed with hemophilia worldwide, following China, India, and the USA (13). The Brazilian national health system offers full reimbursement of medical care for people with rare diseases following the establishment of a national policy and treatment guidelines for comprehensive care (81, 288, 289). This policy formulation was complemented by the enactment of legislation and the development of regulations to support the implementation and enforcement of these health policies. Despite this legislative and regulatory support, funding and patient access to treatment remains subject to the availability of appropriate funding (73, 290).
In rare diseases, the availability of clinical practice guidelines facilitates the diagnosis and treatment of these rare conditions, as well as the implementation of preventive public health measures (291, 292). The latest hemophilia management guidelines issued by the World Federation of Hemophilia acknowledge low-dose prophylaxis as a superior treatment option over episodic treatment for people with hemophilia living in low-resource countries with limited access to clotting factor replacement therapies (139).
To achieve equitable and sustainable physical and mental health outcomes for individuals with rare diseases, including hemophilia, it is imperative to adopt innovative, transparent, and evidence-, outcome-, and value-based pricing, reimbursement, and funding strategies for orphan medicinal products to lower the healthcare economic burden and out-of-pocket expenditure on healthcare (183, 210, 214, 216, 293–295). Because the proportion of non-healthcare expenditure on the management of rare diseases is significantly high, it is crucial to use a societal perspective when estimating the economic burden of rare diseases on patients and caregivers (117–119, 296).
The objectives of public health in supporting people with rare diseases should focus on (1) accurately estimating the epidemiological, and economic burden of rare diseases through expanding newborn screening programs, benefiting from genetic diagnostics, strengthening surveillance systems, and assessing costs and cost-effectiveness from a comprehensive societal perspective (115, 116, 140, 142, 249), (2) supporting public health policy formulation and implementation through integrative actions inside and outside health systems (68, 72), (3) boosting basic and clinical research in the rare disease field through international collaborations to accelerate clinical trials and by establishing specialized clinics and centers of excellence (245, 297–300), (4) empowering patients with rare diseases and their caregivers through implementing a comprehensive psychosocial support plan comprising counseling programs, caregiver support networks, and mental health services (64, 202, 248), (5) enhancing patient access to effective treatment through novel pricing and reimbursement schemes (188, 211, 255–257, 269, 284, 285, 301), (6) promoting the efficient use of public health and healthcare services through strengthening health systems and optimizing allocation of resources (3, 302), and finally (7) improving health outcomes of people with rare diseases (119, 265, 303) (Figure 9).
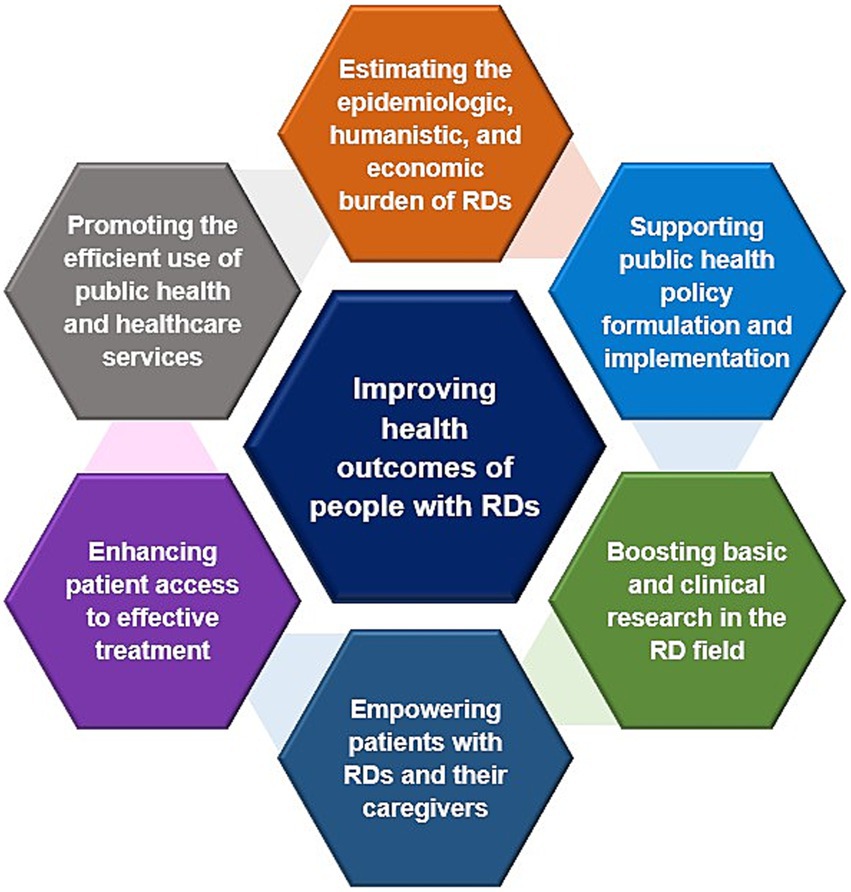
Figure 9. Objectives of public health in supporting the RDs community. Data summarized from references (3, 64, 68, 72, 115, 116, 119, 140, 142, 188, 202, 211, 248, 249, 255–257, 265, 269, 284, 285, 301–303). RD(s), rare disease(s).
The latter and ultimate objective can be strengthened by the invention and availability of innovative therapies, including cell and gene therapies (301, 304–306). In hemophilia, the treatment landscape has dramatically evolved over the past two decades with the licensure of several emerging treatment options, such as extended half-life recombinant factor VIII and FIX products, non-factor replacement subcutaneous agents, and gene therapies (307). These advanced and innovative treatment options have also raised the bar for more ambitious treatment outcomes, making people with hemophilia realize a normal and bleed-free life (308). Despite this scientific and clinical progress in hemophilia care, patient access to these evolving treatment options is still limited to HICs with strong public health systems, sufficient economic resources, and efficient disease awareness and advocacy (22). LMICs with lower capabilities should identify the minimal requirements to provide the best possible care for their people with hemophilia (17). Therefore, a structured plan should be designed, with specific roles and responsibilities for each stakeholder to achieve quality and sustainable care for people with hemophilia (309). Effective collaborative efforts between all concerned stakeholders and strong public health support at best across borders and between LMICs and HICs, can facilitate and overcome these challenges (245, 299, 310, 311).
Public health has evolved to play a vital role in protecting and improving the health and well-being of people globally. Initially focused on preventing infectious diseases, the scope of public health has expanded to address non-communicable diseases and the unique needs of individuals with rare diseases. Addressing the genetic determinants of health and health inequities is essential to providing better care for those with rare diseases. The global landscape of rare diseases presents significant challenges, as there is no universal definition of rarity based on disease prevalence. However, legislative and regulatory support in HICs has facilitated the development and approval of diagnostics and treatments for several rare diseases leading to important advancements. In contrast, many LMICs face obstacles in enacting legislation, developing regulations, and implementing policies to support rare disease diagnosis and treatment. More investment and innovation in drug discovery and market access pathways are still needed in both LMICs and HICs. Ensuring the translation of public health policies into regulatory measures, and in turn implementing and regularly evaluating these measures to assess their effectiveness is important to facilitate the provision of high-quality care for vulnerable populations with rare diseases. Clinical practice guidelines also facilitate diagnosis, treatment, and preventive public health interventions. In the case of hemophilia, public health can play a pivotal role. This includes addressing gaps in epidemiological data, advocating for equitable access to treatment, and implementing evidence-based interventions into routine clinical practice to improve hemophilia care. Overall, public health has a crucial role in ensuring that individuals with rare diseases receive the care and support they need through a multifaceted approach addressing genetic factors, health inequities, legislative frameworks, and evidence-based practices.
AE-S: Conceptualization, Data curation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. UR: Investigation, Validation, Writing – original draft, Writing – review & editing. DH: Writing – original draft, Writing – review & editing. NB: Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The APC of this article was covered by St. Jude Children’s Research Hospital, Memphis, TN, United States. UR and NB are funded in part by the American Lebanese Syrian Associated Charities (ALSAC).
We thank all authors and contributors to the studies included in this review.
AE-S is an employee at Novo Nordisk Egypt. DH is an employee at Phoenix Clinical Research. The conception, design, and conduct of this research project were done completely independently of their employers.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1450625/full#supplementary-material
1. Dale, D, Groft, S, and Harrison, M. Rare disease registries In: RE Gliklich, MB Leavy, and NA Dreyer, editors. Registries for evaluating patient outcomes: a user’s guide, vol. 2. 3rd ed: Agency for Healthcare Research and Quality (US) (2014). 113–34. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/wysiwyg/registries-guide-3rd-edition_research.pdf
2. Epps, C, Bax, R, Croker, A, Green, D, Gropman, A, Klein, AV, et al. Global regulatory and public health initiatives to advance pediatric drug development for rare diseases. Ther Innov Regul Sci. (2022) 56:964–75. doi: 10.1007/s43441-022-00409-w
3. Fantini, B, and Vaccaro, CM. Value based healthcare for rare diseases: efficiency, efficacy, equity. Ann Ist Super Sanita. (2019) 55:251–7. doi: 10.4415/ANN_19_03_10
4. Qamar, JB, Uzair, M, Ahmed, S, Ganny, H, Jafri, L, and Kirmani, S. The role of medical students in advocacy for rare diseases – experience from a Low- and Middle-Income Country (LMIC). Rare. (2023) 1:100004. doi: 10.1016/j.rare.2023.100004
5. Abuhadida, S, Bastaki, L, Bash, B, and Alhindal, B. Return on investment from the prevention of orphan diseases in Kuwait. Ann Public Heal. (2022) 1:637. doi: 10.55085/aph.2022.637
6. Fidan, Ç, Akdur, R, Ünver, ÇN, Şahin, ÖC, Alper, AB, and Ayhan, A. Carrier screening programs for rare diseases in developed countries and the case of Turkey: a systematic review. Intractable Rare Dis Res. (2023) 12:161–9. doi: 10.5582/irdr.2023.01005
7. Tsai, MC, Cheng, CN, Wang, RJ, Chen, KT, Kuo, MC, and Lin, SJ. Cost-effectiveness analysis of carrier and prenatal genetic testing for X-linked hemophilia. J Formos Med Assoc. (2015) 114:722–8. doi: 10.1016/j.jfma.2013.06.017
8. Andreu, P, Karam, J, and Child, C. The burden of rare diseases: an economic evaluation. Chiesi Global Rare Diseases. CHIESI USA, Inc. (2022). Available at: https://chiesirarediseases.com/assets/pdf/chiesiglobalrarediseases.whitepaper-feb.-2022_production-proof.pdf
9. Atrash, HK, and Carpentier, R. The evolving role of public health in the delivery of health care. J Hum Growth Dev. (2012) 22:396–9. doi: 10.7322/jhgd.46349
10. Leonardi, F. The definition of health: towards new perspectives. Int J Heal Serv. (2018) 48:735–48. doi: 10.1177/0020731418782653
11. Huber, M, Knottnerus, JA, Green, L, Van Der Horst, H, Jadad, AR, Kromhout, D, et al. How should we define health? BMJ. (2011) 343:d4163. doi: 10.1136/bmj.d4163
12. Doherty, TM, and Kelley, A. Bleeding disorders. [Updated 2023 Apr 3] In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023)
13. World Federation of Hemophilia. Report on the annual global survey 2023. Montréal. (2024). Available at: https://www1.wfh.org/publications/files/pdf-2525.pdf (Accessed November 19, 2024).
14. El-Sayed, AA, and Bolous, SN. Economic burden of haemophilia from a societal perspective: a scoping review. PharmacoEconomics Open. (2025) 9:179–205. doi: 10.1007/s41669-024-00540-4
15. Fornari, A, Antonazzo, IC, Rocino, A, Preti, D, Fragomeno, A, Cucuzza, F, et al. The psychosocial impact of haemophilia from patients’ and caregivers’ point of view: the results of an Italian survey. Haemophilia. (2024) 30:449–62. doi: 10.1111/hae.14926
16. Ndoumba-Mintya, A, Diallo, YL, Tayou, TC, and Mbanya, DN. Optimizing haemophilia care in resource-limited countries: current challenges and future prospects. J Blood Med. (2023) 14:141–6. doi: 10.2147/JBM.S291536
17. Perolla, A, and Kalaja, B. Improving hemophilia care in low- and middle-income countries: addressing challenges and enhancing quality of life. Cureus. (2024) 16:e62817. doi: 10.7759/cureus.62817
18. Chernyi, N, Gavrilova, D, Saruhanyan, M, Oloruntimehin, ES, Karabelsky, A, Bezsonov, E, et al. Recent advances in gene therapy for hemophilia: projecting the perspectives. Biomol Ther. (2024) 14:854. doi: 10.3390/biom14070854
19. Bolous, NS, Bhatt, N, Bhakta, N, Neufeld, EJ, Davidoff, AM, and Reiss, UM. Gene therapy and hemophilia: where do we go from here? J Blood Med. (2022) 13:559–80. doi: 10.2147/JBM.S371438
20. Kaczmarek, R, Miesbach, W, Ozelo, MC, and Chowdary, P. Current and emerging gene therapies for haemophilia A and B. Haemophilia. (2024) 30:12–20. doi: 10.1111/hae.14984
21. Lane, SJ, Sholapur, NS, Yeung, CHT, Iorio, A, Heddle, NM, Sholzberg, M, et al. Understanding stakeholder important outcomes and perceptions of equity, acceptability and feasibility of a care model for haemophilia management in the US: a qualitative study. Haemophilia. (2016) 22:23–30. doi: 10.1111/hae.13009
22. Pierce, GF, Adediran, M, Diop, S, Dunn, AL, El Ekiaby, M, Kaczmarek, R, et al. Achieving access to haemophilia care in low-income and lower-middle-income countries: expanded Humanitarian Aid Program of the World Federation of Hemophilia after 5 years. Lancet Haematol. (2022) 9:e689–97. doi: 10.1016/S2352-3026(22)00209-5
23. Skinner, MW, Nugent, D, Wilton, P, O’Mahony, B, Dolan, G, O’Hara, J, et al. Achieving the unimaginable: health equity in haemophilia. Haemophilia. (2020) 26:17–24. doi: 10.1111/hae.13862
24. Srivastava, A. The case for equitable haemophilia care. Lancet Haematol. (2021) 8:e626. doi: 10.1016/S2352-3026(21)00132-0
25. Kraus, S, Breier, M, Lim, WM, Dabić, M, Kumar, S, Kanbach, D, et al. Literature reviews as independent studies: guidelines for academic practice. Rev Manag Sci. (2022) 16:2577–95. doi: 10.1007/s11846-022-00588-8
26. Chigbu, U, Atiku, S, and du Plessis, C. The science of literature reviews: searching, identifying, selecting, and synthesising. Publica. (2023) 11:2. doi: 10.3390/publications11010002
27. Saimbert, M. Key principles for searching the literature In: C Holly, S Salmond, and M Saimbert, editors. Comprehensive systematic review for advanced nursing practice. 2nd ed. New York, NY: Springer Publishing Company (2017). 105–38.
28. Thomson, JS, Currier, A, and Gillaspy, M. Basic literature search strategies In: P Kelly, BA Vottero, and CA Christie-McAuliffe, editors. Introduction to quality and safety education for nurses: core competencies. New York, NY: Springer Publishing Company (2014). 309–38.
29. World Health Organization. Constitution of the World Health Organization. Am J Public Health Nations Health. (1946) 36:1315–23. doi: 10.2105/AJPH.36.11.1315
30. Bradley, KL, Goetz, T, and Viswanathan, S. Toward a contemporary definition of health. Mil Med. (2018) 183:204–7. doi: 10.1093/milmed/usy213
31. McCartney, G, Popham, F, McMaster, R, and Cumbers, A. Defining health and health inequalities. Public Health. (2019) 172:22–30. doi: 10.1016/j.puhe.2019.03.023
32. Krahn, GL, Robinson, A, Murray, AJ, Havercamp, SM, Havercamp, S, Andridge, R, et al. It’s time to reconsider how we define health: perspective from disability and chronic condition. Disabil Health J. (2021) 14:101129. doi: 10.1016/j.dhjo.2021.101129
33. Svalastog, AL, Donev, D, Jahren Kristoffersen, N, and Gajović, S. Concepts and definitions of health and health-related values in the knowledge landscapes of the digital society. Croat Med J. (2017) 58:431–5. doi: 10.3325/cmj.2017.58.431
34. American Public Health Association. What is public health? (2023). Available at: https://www.apha.org/what-is-public-health (Accessed July 26, 2023).
35. Capital Area Public Health Network. What is public health? (2023). Available at: https://www.capitalareaphn.org/about/what-is-public-health (Accessed August 29, 2023).
36. CDC. What is public health? (2023). Available at: https://www.cdcfoundation.org/what-public-health#:~:text=Public%20health%20is%20the%20science,and%20responding%20to%20infectious%20diseases (Accessed July 26, 2023).
37. Griffiths, S, Jewell, T, and Donnelly, P. Public health in practice: the three domains of public health. Public Health. (2005) 119:907–13. doi: 10.1016/j.puhe.2005.01.010
38. Thomson, K, Hillier-Brown, F, Todd, A, McNamara, C, Huijts, T, and Bambra, C. The effects of public health policies on health inequalities in high-income countries: an umbrella review. BMC Public Health. (2018) 18:869. doi: 10.1186/s12889-018-5677-1
39. Thorpe, A, Griffiths, S, Jewell, T, and Adshead, F. The three domains of public health: an internationally relevant basis for public health education? Public Health. (2008) 122:201–10. doi: 10.1016/j.puhe.2007.05.013
40. Baba, Z, Belinske, S, and Post, D. Public health, population health, and planning: ideas to improve communities. Delaware J Public Heal. (2018) 4:14–8. doi: 10.32481/djph.2018.03.004
41. Mager, DR. Overview of community, public, and population health In: DR Mager and J Conelius, editors. Population health for nurses: improving community outcomes. New York: Springer Publishing Company (2019). 3–16.
42. Roux, AVD. On the distinction—or lack of distinction—between population health and public health. Am J Public Health. (2016) 106:619–20. doi: 10.2105/AJPH.2016.303097
43. County of Los Angeles. Public health core functions and essential services. Los Angeles, Calif. (2004). Available at: http://publichealth.lacounty.gov/qiap/docs/CoreFunctions.pdf (Accessed August 30, 2023).
44. CDC. The 10 essential public health services. (2020). Available at: https://phaboard.org/wp-content/uploads/EPHS-English.pdf (Accessed July 26, 2023).
45. Hyde, JK, and Shortell, SM. The structure and organization of local and state public health agencies in the U.S.: a systematic review. Am J Prev Med. (2012) 42:S29–41. doi: 10.1016/j.amepre.2012.01.021
46. Karkee, R. Public health education in south asia: a basis for structuring a master degree course. Front Public Health. (2014) 2:88. doi: 10.3389/fpubh.2014.00088
47. World Health Organization. Social determinants of health. (2024). Available at: https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (Accessed September 2, 2023).
48. Wilkinson, RG, and Marmot, M. Social determinants of health: the solid facts. 2nd ed. Copenhagen: World Health Organization. Regional Office for Europe; (2003). 1–32 p.
49. Marmot, M, Friel, S, Bell, R, Houweling, TAJ, and Taylor, S. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. (2008) 372:1661–9. doi: 10.1016/S0140-6736(08)61690-6
50. Braveman, P, and Gottlieb, L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. (2014) 129:19–31. doi: 10.1177/00333549141291S206
51. Healthy People 2030. Social determinants of health. Available at: https://health.gov/healthypeople/priority-areas/social-determinants-health (Accessed April 26, 2024).
52. CDC. Social determinants of health at CDC. (2022). Available at: https://www.cdc.gov/about/sdoh/index.html (Accessed September 2, 2023).
53. Hammond, G, and Joynt Maddox, KE. A theoretical framework for clinical implementation of social determinants of health. JAMA Cardiol. (2019) 4:1189–90. doi: 10.1001/jamacardio.2019.3805
54. Islam, MM. Social determinants of health and related inequalities: confusion and implications. Front Public Health. (2019) 7:11. doi: 10.3389/fpubh.2019.00011
55. Raphael, D, Bryant, T, Mikkonen, J, and Alexander, R. Social determinants of health: the Canadian facts. 2nd ed. Oshawa: Ontario Tech University Faculty of Health Sciences and Toronto: York University School of Health Policy and Management; (2020). 1–93 p.
56. World Health Organization. Determinants of health. (2017). Available at: https://www.who.int/news-room/questions-and-answers/item/determinants-of-health (Accessed September 2, 2023).
57. World Health Organization. Regional Office for the Eastern Mediterranean. Assessment of essential public health functions in countries of the Eastern Mediterranean Region: assessment tool. Cairo PP - Cairo: World Health Organization. Regional Office for the Eastern Mediterranean (2017).
58. Graham, H. Social determinants and their unequal distribution: clarifying policy understandings. Milbank Q. (2004) 82:101–24. doi: 10.1111/j.0887-378X.2004.00303.x
59. Gómez, CA, Kleinman, DV, Pronk, N, Gordon, GLW, Ochiai, E, Blakey, C, et al. Addressing health equity and social determinants of health through healthy people 2030. J Public Heal Manag Pract. (2021) 27:S249–57. doi: 10.1097/PHH.0000000000001297
60. Marmot, M, and Bell, R. Fair society, healthy lives. Public Health. (2012) 126:S4–S10. doi: 10.1016/j.puhe.2012.05.014
61. Solar, O, and Irwin, A. A conceptual framework for action on the Social Determinants of Health Discussion Paper 2 (Policy and Practice). Geneva: World Health Organization (2010).
62. Barton, H, and Grant, M. A health map for the local human habitat. J Roy Soc Promot Health. (2006) 126:252–3. doi: 10.1177/1466424006070466
63. Franco, P. Orphan drugs: the regulatory environment. Drug Discov Today. (2013) 18:163–72. doi: 10.1016/j.drudis.2012.08.009
64. Institute of Medicine In: MJ Field and TF Boat, editors. Rare diseases and orphan products: accelerating research and development. Washington, DC: The National Academies Press (2010). i–xxi). 1–420.
65. Khosla, N, and Valdez, R. A compilation of national plans, policies and government actions for rare diseases in 23 countries. Intractable Rare Dis Res. (2018) 7:213–22. doi: 10.5582/irdr.2018.01085
66. O’Connor, DJ. Orphan drug designation–Europe, the USA and Japan. Expert Opin Orphan Drugs. (2013) 1:255–9. doi: 10.1517/21678707.2013.769876
67. Richter, T, Nestler-Parr, S, Babela, R, Khan, ZM, Tesoro, T, Molsen, E, et al. Rare disease terminology and definitions-a systematic global review: report of the ISPOR rare disease special interest group. Value Heal J Int Soc Pharmacoeconomics Outcomes Res. (2015) 18:906–14. doi: 10.1016/j.jval.2015.05.008
68. Rodwell, C, and Aymé, S. Rare disease policies to improve care for patients in Europe. Biochim Biophys Acta Mol Basis Dis. (2015) 1852:2329–35.
69. Canadian Agency for Drugs and Technologies in Health. Drugs for rare diseases: evolving trends in regulatory and health technology assessment perspectives. Ottawa (ON). (2016). Available at: https://www.cadth.ca/drugs-rare-diseases-evolving-trends-regulatory-and-health-technology-assessment-perspectives (Accessed October 19, 2023).
70. Birkelund, CH. Rare disease thresholds-An analysis of different definitions, laws and arguments. University of Oslo. (2019). Available at: https://www.duo.uio.no/bitstream/handle/10852/72584/ferdig-oppgave------.pdf?sequence=1&isAllowed=y (Accessed October 23, 2023).
71. Nguengang Wakap, S, Lambert, DM, Olry, A, Rodwell, C, Gueydan, C, Lanneau, V, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. (2020) 28:165–73. doi: 10.1038/s41431-019-0508-0
72. Chung, CCY, Chu, ATW, and Chung, BHY. Rare disease emerging as a global public health priority. Front Public Health. (2022) 10:10. doi: 10.3389/fpubh.2022.1028545
73. Wainstock, D, and Katz, A. Advancing rare disease policy in Latin America: a call to action. Lancet Reg Heal Am. (2023) 18:100434.
74. European Union. Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products. OJ L 18. (2000) 15:1–5.
75. Valdez, R, Ouyang, L, and Bolen, J. Public health and rare diseases: oxymoron no more. Prev Chronic Dis. (2016) 13:E05. doi: 10.5888/pcd13.150491
76. Moliner, AM, and Waligora, J. The European Union policy in the field of rare diseases In: M Posada de la Paz, D Taruscio, and SC Groft, editors. Rare diseases epidemiology: update and overview. 2nd ed. Cham: Springer International Publishing (2017). 561–87.
77. House of Commons of Canada. Canadians affected by rare diseases and disorders: improving access to treatment - report of the Standing Committee on Health. Ottawa (ON). (2019). Available at: https://www.ourcommons.ca/Content/Committee/421/HESA/Reports/RP10349306/hesarp22/hesarp22-e.pdf (Accessed October 23, 2023).
78. Health Canada. Building a national strategy for high-cost drugs for rare diseases: a discussion paper for engaging Canadians. Ottawa (ON). (2021). Available at: https://www.canada.ca/content/dam/hc-sc/documents/services/health-related-consultation/National-Strategy-High-Cost-Drugs-eng.pdf (Accessed October 23, 2023).
79. Lavandeira, A. Orphan drugs: legal aspects, current situation. Haemophilia. (2002) 8:194–8. doi: 10.1046/j.1365-2516.2002.00643.x
80. Derayeh, S, Kazemi, A, Rabiei, R, Hosseini, A, and Moghaddasi, H. National information system for rare diseases with an approach to data architecture: a systematic review. Intractable Rare Dis Res. (2018) 7:156–63. doi: 10.5582/irdr.2018.01065
81. Lopes-Júnior, LC, Ferraz, VEF, Lima, RAG, Schuab, SIPC, Pessanha, RM, Luz, GS, et al. Health policies for rare disease patients: a scoping review. Int J Environ Res Public Health. (2022) 19:15174. doi: 10.3390/ijerph192215174
82. Budhwar, V, Singh, AK, and Choudhary, M. Regulations of orphan drugs in USA, EU and India-a comparative study. Int J Drug Regul Aff. (2016) 4:30–7. doi: 10.22270/ijdra.v4i3.187
83. Congressional Budget Office. The demographic outlook: 2023 to 2053. (2023). Available at: https://www.cbo.gov/publication/58612 (Accessed December 19, 2023).
84. Herder, M. What is the purpose of the orphan drug act? PLoS Med. (2017) 14:e1002191. doi: 10.1371/journal.pmed.1002191
85. Kim, S. The orphan drug act: how the FDA unlawfully usurped market exclusivity. Nw J Tech Intell Prop. (2013) 11:541.
86. Orphanet. Orphan drugs in the United States of America. (2023). Available at: https://www.orpha.net/consor/cgi-bin/Education_AboutOrphanDrugs.php?lng=EN&stapage=ST_EDUCATION_EDUCATION_ABOUTORPHANDRUGS_USA (Accessed October 19, 2023).
87. Partin, C. Rarely mentioned: how we arrived at the quantitative definition of a rare disease. Proc (Bayl Univ Med Cent). (2022) 35:498–504. doi: 10.1080/08998280.2022.2048613
88. Mikami, K. Orphans in the market: the history of orphan drug policy. Soc Hist Med. (2019) 32:609–30. doi: 10.1093/shm/hkx098
89. Song, P, Tang, W, and Kokudo, N. Rare diseases and orphan drugs in Japan: developing multiple strategies of regulation and research. Expert Opin Orphan Drugs. (2013) 1:681–3. doi: 10.1517/21678707.2013.832201
90. Song, P, Gao, J, Inagaki, Y, Kokudo, N, and Tang, W. Rare diseases, orphan drugs, and their regulation in Asia: current status and future perspectives. Intractable Rare Dis Res. (2012) 1:3–9. doi: 10.5582/irdr.2012.v1.1.3
91. countryeconomy.com. Japan - population. Available at: https://countryeconomy.com/demography/population/japan?year=1993 (Accessed October 25, 2023).
92. Orphanet. Orphan drugs in Japan. (2023). Available at: https://www.orpha.net/consor/cgi-bin/Education_AboutOrphanDrugs.php?lng=EN&stapage=ST_EDUCATION_EDUCATION_ABOUTORPHANDRUGS_JAP (Accessed October 26, 2023).
93. Worldometer. Japan population. Available at: https://www.worldometers.info/world-population/japan-population/ (Accessed October 25, 2023).
94. Australian Government - Department of Health - Therapeutic Goods Administration. Orphan drugs program discussion paper. Canberra. (2015). Available at: https://www.tga.gov.au/sites/default/files/consultation-orphan-drugs-program.pdf (Accessed May 03, 2024).
95. Lim, SS, Lee, W, Kim, YK, Kim, J, Park, JH, Park, BR, et al. The cumulative incidence and trends of rare diseases in South Korea: a nationwide study of the administrative data from the National Health Insurance Service database from 2011-2015. Orphanet J Rare Dis. (2019) 14:49. doi: 10.1186/s13023-019-1032-6
96. Bajaj, A, Mathew, S, Vellarikkal, SK, Sivadas, A, Bhoyar, RC, Joshi, K, et al. Genomics of rare genetic diseases—experiences from India. Hum Genomics. (2019) 13:52. doi: 10.1186/s40246-019-0215-5
97. Rajueni, K, and Chakraborty, CM. Assessment of the availability of repurposed orphan drugs in India. PLOS Glob Public Heal. (2023) 3:e0001498. doi: 10.1371/journal.pgph.0001498
98. Sharma, M, Jain, N, Singh, V, Singla, S, and Aftab, I. Ophthalmic registries for rare eye diseases. Indian J Ophthalmol. (2022) 70:2225–30. doi: 10.4103/ijo.IJO_302_22
99. Song, Y, Kwon, B, Al-Abdulwahhab, AH, Nam, YK, Ahn, Y, Jeong, SY, et al. Rare neurovascular diseases in Korea: classification and related genetic variants. Korean J Radiol. (2021) 22:1379–96. doi: 10.3348/kjr.2020.1171
100. Ertok, Ö, Akbil, Ş, and Cebeci, Z. Rare diseases in Turkey Istanbul. (2023). Available at: https://www.aifd.org.tr/nadirhastaliklarraporu/en.pdf (Accessed May 03, 2024).
101. Mayrides, M, Ruiz de Castilla, EM, and Szelepski, S. A civil society view of rare disease public policy in six Latin American countries. Orphanet J Rare Dis. (2020) 15:60. doi: 10.1186/s13023-020-1314-z
102. Parliament of the Commonwealth of Australia - House of Representatives Standing Committee on Health; Aged Care and Sport. The New Frontier - Delivering better health for all Australians - Inquiry into approval processes for new drugs and novel medical technologies in Australia. Canberra. (2021). Available at: https://parlinfo.aph.gov.au/parlInfo/download/committees/reportrep/024755/toc_pdf/TheNewFrontier-DeliveringbetterhealthforallAustralians.pdf;fileType=application%2Fpdf (Accessed October 23, 2023).
103. Czech, M, Baran-Kooiker, A, Holownia-Voloskova, M, Kooiker, C, and Sykut-Cegielska, J. Bridging East with West of Europe – A comparison of orphan drug policies in Poland, Russia and the Netherlands. Acta Pol Pharm Drug Res. (2019) 75:1409–22. doi: 10.32383/appdr/90995
104. Czech, M, Baran-Kooiker, A, Atikeler, K, Demirtshyan, M, Gaitova, K, Holownia-Voloskova, M, et al. A review of rare disease policies and orphan drug reimbursement systems in 12 Eurasian countries. Front Public Health. (2019) 7:416. doi: 10.3389/fpubh.2019.00416
105. Hsu, JC, Wu, HC, Feng, WC, Chou, CH, Lai, ECC, and Lu, CY. Disease and economic burden for rare diseases in Taiwan: a longitudinal study using Taiwan’s National Health Insurance Research Database. PLoS One. (2018) 13:e0204206. doi: 10.1371/journal.pone.0204206
106. Schouten, A. Selected government definitions of orphan or rare diseases - KEI briefing note 2020:4. (2020). Available at: https://www.keionline.org/wp-content/uploads/KEI-Briefing-Note-2020-4-Defining-Rare-Diseases.pdf (Accessed May 03, 2024).
107. Lu, Y, and Han, J. The definition of rare disease in China and its prospects. Intractable Rare Dis Res. (2022) 11:29–30. doi: 10.5582/irdr.2022.01034
108. Miller, CH, Soucie, JM, Byams, VR, Payne, AB, Abe, K, Lewandowska, M, et al. Occurrence rates of inherited bleeding disorders other than haemophilia and von Willebrand disease among people receiving care in specialized treatment centres in the United States. Haemophilia. (2022) 28:e75–8. doi: 10.1111/hae.14529
109. Edelstein, BL, and Yoder, KM. The child in context of the family, community, and society In: JA Dean, editor. McDonald and Avery’s dentistry for the child and adolescent. 10th ed. St. Louis: Mosby (2016). 645–52.
110. Udompap, P, Kim, D, and Kim, WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol. (2015) 13:2031–41. doi: 10.1016/j.cgh.2015.08.015
111. Spronk, I, Korevaar, JC, Poos, R, Davids, R, Hilderink, H, Schellevis, FG, et al. Calculating incidence rates and prevalence proportions: not as simple as it seems. BMC Public Health. (2019) 19:512. doi: 10.1186/s12889-019-6820-3
112. Ward, MM. Estimating disease prevalence and incidence using administrative data: some assembly required. J Rheumatol. (2013) 40:1241–3. doi: 10.3899/jrheum.130675
113. Devleesschauwer, B, Havelaar, AH, Maertens de Noordhout, C, Haagsma, JA, Praet, N, Dorny, P, et al. Calculating disability-adjusted life years to quantify burden of disease. Int J Public Health. (2014) 59:565–9. doi: 10.1007/s00038-014-0552-z
114. Nolte, MT, Nasser, JS, and Chung, KC. A systematic review of orthopedic global outreach efforts based on WHO-CHOICE thresholds. Hand Clin. (2019) 35:487–97. doi: 10.1016/j.hcl.2019.07.015
115. García-Pérez, L, Linertová, R, Valcárcel-Nazco, C, Posada, M, Gorostiza, I, and Serrano-Aguilar, P. Cost-of-illness studies in rare diseases: a scoping review. Orphanet J Rare Dis. (2021) 16:178. doi: 10.1186/s13023-021-01815-3
116. Delaye, J, Cacciatore, P, and Kole, A. Valuing the “burden” and impact of rare diseases: a scoping review. Front Pharmacol. (2022) 13:914338. doi: 10.3389/fphar.2022.914338
117. Armeni, P, Cavazza, M, Xoxi, E, Taruscio, D, and Kodra, Y. Reflections on the importance of cost of illness analysis in rare diseases: a proposal. Int J Environ Res Public Health. (2021) 18:1101. doi: 10.3390/ijerph18031101
118. Postma, MJ, Noone, D, Rozenbaum, MH, Carter, JA, Botteman, MF, Fenwick, E, et al. Assessing the value of orphan drugs using conventional cost-effectiveness analysis: is it fit for purpose? Orphanet J Rare Dis. (2022) 17:157. doi: 10.1186/s13023-022-02283-z
119. Zanello, G, Chan, CH, and Pearce, DA Group IrdW. Recommendations from the IRDiRC Working Group on methodologies to assess the impact of diagnoses and therapies on rare disease patients. Orphanet J Rare Dis. (2022) 17:181. doi: 10.1186/s13023-022-02337-2
120. Haendel, M, Vasilevsky, N, Unni, D, Bologa, C, Harris, N, Rehm, H, et al. How many rare diseases are there? Nat Rev Drug Discov. (2020) 19:77–8. doi: 10.1038/d41573-019-00180-y
121. Eurostat. Population statistics. Luxembourg: Office for Official Publications of the European Communities (2006).
122. Eurostat. EU population increases again after two years decrease. (2023). Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/edn-20230711-1 (Accessed October 25, 2023).
123. Iorio, A, Stonebraker, JS, Chambost, H, Makris, M, Coffin, D, Herr, C, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta-analytic approach using national registries. Ann Intern Med. (2019) 171:540–6. doi: 10.7326/M19-1208
124. Berntorp, E, Fischer, K, Hart, DP, Mancuso, ME, Stephensen, D, Shapiro, AD, et al. Haemophilia. Nat Rev Dis Prim. (2021) 7:45. doi: 10.1038/s41572-021-00278-x
125. Mehta, P, and Reddivari, AKR. Hemophilia In: StatPearls. Treasure Island (FL): StatPearls Publishing (2024)
126. Rejtő, J, Kraemmer, D, Grilz, E, Königsbrügge, O, Gabler, C, Schuster, G, et al. Bleeding phenotype in nonsevere hemophilia by International Society on Thrombosis and Haemostasis bleeding assessment tool, bleeding frequency, and the joint status. Res Pract Thromb Haemost. (2023) 7:100047. doi: 10.1016/j.rpth.2023.100047
127. Kloosterman, FR, Zwagemaker, AF, Bagot, CN, Beckers, EAM, Castaman, G, Cnossen, MH, et al. The bleeding phenotype in people with nonsevere hemophilia. Blood Adv. (2022) 6:4256–65. doi: 10.1182/bloodadvances.2022007620
128. Malec, L, and Matino, D. Targeting higher factor VIII levels for prophylaxis in haemophilia A: a narrative review. Haemophilia. (2023) 29:1419–29. doi: 10.1111/hae.14866
129. Coppola, A, Franchini, M, Pappagallo, G, Borchiellini, A, De Cristofaro, R, Molinari, AC, et al. Current choices and management of treatment in persons with severe hemophilia A without inhibitors: a mini-Delphi consensus. J Clin Med. (2022) 11:801. doi: 10.3390/jcm11030801
130. Atkins, JC, and Padgett, CR. Living with a rare disease: psychosocial impacts for parents and family members – a systematic review. J Child Fam Stud. (2024) 33:617–36. doi: 10.1007/s10826-024-02790-6
131. Belzer, LT, Wright, SM, Goodwin, EJ, Singh, MN, and Carter, BS. Psychosocial considerations for the child with rare disease: a review with recommendations and calls to action. Children. (2022) 9:933. doi: 10.3390/children9070933
132. Adama, EA, Arabiat, D, Foster, MJ, Afrifa-Yamoah, E, Runions, K, Vithiatharan, R, et al. The psychosocial impact of rare diseases among children and adolescents attending mainstream schools in Western Australia. Int J Incl Educ. (2023) 27:1273–86. doi: 10.1080/13603116.2021.1888323
133. Witt, S, Schuett, K, Wiegand-Grefe, S, Boettcher, J, and Quitmann, J. Living with a rare disease - experiences and needs in pediatric patients and their parents. Orphanet J Rare Dis. (2023) 18:242. doi: 10.1186/s13023-023-02837-9
134. Uhlenbusch, N, Löwe, B, and Depping, MK. Perceived burden in dealing with different rare diseases: a qualitative focus group study. BMJ Open. (2019) 9:e033353. doi: 10.1136/bmjopen-2019-033353
135. Rihm, L, Dreier, M, Rezvani, F, Wiegand-Grefe, S, and Dirmaier, J. The psychosocial situation of families caring for children with rare diseases during the COVID-19 pandemic: results of a cross-sectional online survey. Orphanet J Rare Dis. (2022) 17:449. doi: 10.1186/s13023-022-02595-0
136. Richardson, T, Rice, M, Lyon, ME, Kobernick, M, and Brackbill, L. Impact of mental health in persons living with rare disease: findings from the AMCP Market Insights Program. J Manag Care Spec Pharm. (2024) 30:S1–S11. doi: 10.18553/jmcp.2024.30.7-b.s1
137. Kenny, T, and Stone, J. Psychological support at diagnosis of a rare disease. A Rev Lit Rare Dis Res Partners. (2022) 1:1–25. doi: 10.20517/rdodj.2022.04
138. Palagyi, A, Sengupta, A, Moorthy, M, Malik, C, Barratt, J, Devuyst, O, et al. Systematic scoping review of socioeconomic burden and associated psychosocial impact in patients with rare kidney diseases and their caregivers. Kidney Int Reports. (2024). Available at: https://www.sciencedirect.com/science/article/pii/S2468024924034041 (Accessed December 21, 2024).
139. Srivastava, A, Santagostino, E, Dougall, A, Kitchen, S, Sutherland, M, Pipe, SW, et al. WFH guidelines for the management of hemophilia. Haemophilia. (2020) 26:1–158. doi: 10.1111/hae.14046
140. Angelis, A, Tordrup, D, and Kanavos, P. Socio-economic burden of rare diseases: a systematic review of cost of illness evidence. Health Policy. (2015) 119:964–79. doi: 10.1016/j.healthpol.2014.12.016
141. Chung, CCY, Ng, NYT, Ng, YNC, Lui, ACY, Fung, JLF, Chan, MCY, et al. Socio-economic costs of rare diseases and the risk of financial hardship: a cross-sectional study. Lancet Reg Heal Pacific. (2023) 34:100711. doi: 10.1016/j.lanwpc.2023.100711
142. Makarova, EV, Krysanov, IS, Valilyeva, TP, Vasiliev, MD, and Zinchenko, RA. Evaluation of orphan diseases global burden. Eur J Transl Myol. (2021) 31:9610. doi: 10.4081/ejtm.2021.9610
143. Yang, G, Cintina, I, Pariser, A, Oehrlein, E, Sullivan, J, and Kennedy, A. The national economic burden of rare disease in the United States in 2019. Orphanet J Rare Dis. (2022) 17:163. doi: 10.1186/s13023-022-02299-5
144. Li, J, Yang, L, Zhang, Y, Liao, H, Ma, Y, and Sun, Q. Rare disease curative care expenditure-financing scheme-health provider–beneficiary group analysis: an empirical study in Sichuan Province, China. Orphanet J Rare Dis. (2022) 17:373. doi: 10.1186/s13023-022-02524-1
145. Burke, T, Asghar, S, O’Hara, J, Sawyer, EK, and Li, N. Clinical, humanistic, and economic burden of severe hemophilia B in the United States: Results from the CHESS US and CHESS US+ population surveys. Orphanet J Rare Dis. (2021) 16:143. doi: 10.1186/s13023-021-01774-9
146. Zhou, ZY, Koerper, MA, Johnson, KA, Riske, B, Baker, JR, Ullman, M, et al. Burden of illness: direct and indirect costs among persons with hemophilia A in the United States. J Med Econ. (2015) 18:457–65. doi: 10.3111/13696998.2015.1016228
147. O’Hara, J, Hughes, D, Camp, C, Burke, T, Carroll, L, and Diego, DAG. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. (2017) 12:1–8. doi: 10.1186/s13023-017-0660-y
148. Burke, T, Asghar, S, O’Hara, J, Chuang, M, Sawyer, EK, and Li, N. Clinical, humanistic, and economic burden of severe haemophilia B in adults receiving factor IX prophylaxis: findings from the CHESS II real-world burden of illness study in Europe. Orphanet J Rare Dis. (2021) 16:521. doi: 10.1186/s13023-021-02152-1
149. Malhan, S, Öksüz, E, Antmen, B, Ar, MC, Balkan, C, and Kavaklı, K. Cost of hemophilia A in Turkey: an economic disease burden analysis. J Med Econ. (2021) 24:1052–9. doi: 10.1080/13696998.2021.1965388
150. Dharmarajan, S, Phadnis, S, Gund, P, and Kar, A. Out-of-pocket and catastrophic expenditure on treatment of haemophilia by Indian families. Haemophilia. (2014) 20:382–7. doi: 10.1111/hae.12324
151. Mazzucato, M, Visonà Dalla Pozza, L, Manea, S, Minichiello, C, and Facchin, P. A population-based registry as a source of health indicators for rare diseases: the ten-year experience of the Veneto Region’s rare diseases registry. Orphanet J Rare Dis. (2014) 9:37. doi: 10.1186/1750-1172-9-37
152. Institute of Medicine (US) Committee on a National Surveillance System for Cardiovascular and Select Chronic Diseases. Existing surveillance data sources and systems In: A nationwide framework for surveillance of cardiovascular and chronic lung diseases. Washington (DC): National Academies Press (US) (2011). 65–89.
153. Dang, A. Real-world evidence: a primer. Pharmaceut Med. (2023) 37:25–36. doi: 10.1007/s40290-022-00456-6
154. Choi, BCK. The past, present, and future of public health surveillance. Scientifica (Cairo). (2012) 2012:875253:1–26. doi: 10.6064/2012/875253
155. Groseclose, SL, and Buckeridge, DL. Public health surveillance systems: recent advances in their use and evaluation. Annu Rev Public Health. (2017) 38:57–79. doi: 10.1146/annurev-publhealth-031816-044348
156. Nsubuga, P, White, ME, Thacker, SB, Anderson, MA, Blount, SB, Broome, CV, et al. Public health surveillance: a tool for targeting and monitoring interventions In: DT Jamison, JG Breman, and AR Measham, et al., editors. Disease Control Priorities in Developing Countries. 2nd ed. Washington (DC): The International Bank for Reconstruction and Development/The World Bank (2006). 997–1015.
157. World Health Organization. Regional Office for Africa. Overview (Public health surveillance). (2023). Available at: https://www.afro.who.int/node/5685 (Accessed November 19, 2023).
158. CDC. Introduction to public health surveillance. (2018). Available at: https://www.cdc.gov/training/publichealth101/surveillance.html (Accessed November 19, 2023).
159. Hamilton, JJ, and Hopkins, RS. Using technologies for data collection and management In: SA Rasmussen and RA Goodman, editors. The CDC field epidemiology manual, Part I. 4th ed. New York, NY: Oxford University Press (2019). 71–103.
160. Capili, B. Cross-sectional studies. Am J Nurs. (2021) 121:59–62. doi: 10.1097/01.NAJ.0000794280.73744.fe
161. Soucie, JM. Public health surveillance and data collection: general principles and impact on hemophilia care. Hematology. (2012) 17:s144–6. doi: 10.1179/102453312X13336169156537
162. Schieve, LA, Byams, VR, Dupervil, B, Oakley, MA, Miller, CH, Soucie, JM, et al. Evaluation of CDC’s hemophilia surveillance program - universal data collection (1998-2011) and community counts (2011-2019), United States. MMWR Surveill Summ. (2020) 69:1–18. doi: 10.15585/mmwr.ss6905a1
163. Okolo, AI, Soucie, JM, Grosse, SD, Roberson, C, Janson, IA, Allen, M, et al. Population-based surveillance of haemophilia and patient outcomes in Indiana using multiple data sources. Haemophilia. (2019) 25:456–62. doi: 10.1111/hae.13734
164. Parker, CS, Tsai, J, Siddiqi, AA, Atrash, HK, and Richardson, LC. Meeting the emerging public health needs of persons with blood disorders. Am J Prev Med. (2014) 47:658–63. doi: 10.1016/j.amepre.2014.07.008
165. Soucie, JM, Miller, CH, Kelly, FM, Aschman, D, DiMichele, D, Konkle, BA, et al. National surveillance for hemophilia inhibitors in the United States: summary report of an expert meeting. Am J Hematol. (2014) 89:621–5. doi: 10.1002/ajh.23704
166. Soucie, JM, Miller, CH, Kelly, FM, Payne, AB, Creary, M, Bockenstedt, PL, et al. A study of prospective surveillance for inhibitors among persons with haemophilia in the United States. Haemophilia. (2014) 20:230–7. doi: 10.1111/hae.12302
167. Fischer, K, Ljung, R, Platokouki, H, Liesner, R, Claeyssens, S, Smink, E, et al. Prospective observational cohort studies for studying rare diseases: the European PedNet Haemophilia Registry. Haemophilia. (2014) 20:e280–6. doi: 10.1111/hae.12448
168. Stonebraker, JS, Bolton-Maggs, PHB, Brooker, M, Evatt, B, Iorio, A, Makris, M, et al. The world federation of hemophilia annual global survey 1999-2018. Haemophilia. (2020) 26:591–600. doi: 10.1111/hae.14012
169. American Thrombosis and Hemostasis Network. Community counts: CDC public health surveillance project for bleeding disorders. (2023). Available at: https://athn.org/what-we-do/national-projects/community-counts.html (Accessed November 19, 2023).
170. Coffin, D, Gouider, E, Konkle, B, Hermans, C, Lambert, C, Diop, S, et al. The world federation of hemophilia world bleeding disorders registry: insights from the first 10,000 patients. Res Pract Thromb Haemost. (2023) 7:102264. doi: 10.1016/j.rpth.2023.102264
171. Makris, M, Calizzani, G, Fischer, K, Gilman, EA, Hay, CRM, Lassila, R, et al. EUHASS: the European haemophilia safety surveillance system. Thromb Res. (2011) 127:S22–5. doi: 10.1016/S0049-3848(10)70150-X
172. Manco-Johnson, MJ, Byams, VR, Recht, M, Dudley, B, Dupervil, B, Aschman, DJ, et al. Community counts: Evolution of a national surveillance system for bleeding disorders. Am J Hematol. (2018) 93:E137–40. doi: 10.1002/ajh.25076
173. van Vulpen, LFD, Saccullo, G, Iorio, A, and Makris, M. The current state of adverse event reporting in hemophilia. Expert Rev Hematol. (2017) 10:161–8. doi: 10.1080/17474086.2017.1272410
174. Tran, H, Yang, R, Fischer, K, Makris, M, and Konkle, BA. The importance and evolution of bleeding disorder registries. Haemophilia. (2024) 30:21–8. doi: 10.1111/hae.14993
175. Dolan, G, Makris, M, Bolton-Maggs, PHB, and Rowell, JA. Enhancing haemophilia care through registries. Haemophilia. (2014) 20:121–9. doi: 10.1111/hae.12406
176. Ali, SR, Bryce, J, Kodra, Y, Taruscio, D, Persani, L, and Ahmed, SF. The quality evaluation of rare disease registries—an assessment of the essential features of a disease registry. Int J Environ Res Public Health. (2021) 18:11968. doi: 10.3390/ijerph182211968
177. Ninomiya, K, and Okura, M. Nationwide comprehensive epidemiological study of rare diseases in Japan using a health insurance claims database. Orphanet J Rare Dis. (2022) 17:140. doi: 10.1186/s13023-022-02290-0
178. Columbia University Mailman School of Public Health. Public health policy: definition, examples, and more. (2021). Available at: https://www.publichealth.columbia.edu/news/public-health-policy-definition-examples-more (Accessed July 22, 2024).
179. CDC. Definition of policy. (2015). Available at: https://www.cdc.gov/policy/paeo/process/definition.html (Accessed July 22, 2024).
180. Moutselos, K, and Maglogiannis, I. Evidence-based public health policy models development and evaluation using big data analytics and web technologies. Med Arch (Sarajevo, Bosnia Herzegovina). (2020) 74:47–53. doi: 10.5455/medarh.2020.74.47-53
181. United Nations. Transforming our world: the 2030 Agenda for Sustainable Development: resolution 70/1/adopted by the General Assembly on 25 September 2015. New York, NY; Report No.: A/RES/70/1. (2015). Available at: https://digitallibrary.un.org/record/809145?ln=en (Accessed October 16, 2023).
182. Baynam, G, Bowman, F, Lister, K, Walker, CE, Pachter, N, Goldblatt, J, et al. Improved diagnosis and care for rare diseases through implementation of precision public health framework In: M Posada de la Paz, D Taruscio, and SC Groft, editors. Rare diseases epidemiology: update and overview. 2nd ed. Cham: Springer (2017). 55–94.
183. Yates, N, and Hinkel, J. The economics of moonshots: value in rare disease drug development. Clin Transl Sci. (2022) 15:809–12. doi: 10.1111/cts.13270
184. European Parliament. Public health - fact sheets on the European Union. (2023). Available at: https://www.europarl.europa.eu/factsheets/en/sheet/49/public-health (Accessed October 28, 2023).
185. Valdez, R, Grosse, SD, and Khoury, MJ. The need for a next-generation public health response to rare diseases. Genet Med. (2017) 19:489–90. doi: 10.1038/gim.2016.166
186. Shah, RR. Regulatory framework for the treatment of orphan diseases In: A Mehta and MSPG Beck, editors. Fabry disease: perspectives from 5 years of FOS. Oxford: Oxford PharmaGenesis (2006)
187. Hall, AK, and Carlson, MR. The current status of orphan drug development in Europe and the US. Intractable Rare Dis Res. (2014) 3:1–7. doi: 10.5582/irdr.3.1
188. Gammie, T, Lu, CY, and Babar, ZUD. Access to orphan drugs: a comprehensive review of legislations, regulations and policies in 35 countries. PLoS One. (2015) 10:e0140002. doi: 10.1371/journal.pone.0140002
189. Takemura, S, and Sone, T. Research and development on intractable & rare diseases in Japan: Contribution of the National Institute of Public Health to research program management. J Natl Inst Public Heal. (2019) 68:45–54.
190. Knowledge Ecology International. Orphan drug timeline. (2023). Available at: https://www.keionline.org/orphan-drugs/orphan-drug-timeline (Accessed October 19, 2023).
191. U.S. Congress. Orphan Drug Act. USA. (1983) p. 96 Stat. 2049-2066. Available at: https://www.fda.gov/media/99546/download (Accessed October 19, 2023).
192. U.S. Department of Health and Human Services. The orphan drug act: implementation and impact. (2001). Available at: https://www.govinfo.gov/app/details/GOVPUB-HE-PURL-gpo75081 (Accessed October 19, 2023).
193. European Economic and Social Committee. Opinion of the European Economic and Social Committee on Ensuring strong European solidarity for rare disease patients. OJ C 75. (2023) 10:67–74.
194. Mu, Y, Song, K, and Song, Y. A cross-sectional study of price and affordability of drugs for rare diseases in Shandong Province, China. Int J Environ Res Public Health. (2022) 19:13319. doi: 10.3390/ijerph192013319
195. Pearson, C, Schapiro, L, and Pearson, SD. The next generation of rare disease drug policy: ensuring both innovation and affordability. J Comp Eff Res. (2022) 11:999–1010. doi: 10.2217/cer-2022-0120
196. European Commission. Orphan medicinal products. (2023). Available at: https://health.ec.europa.eu/medicinal-products/orphan-medicinal-products_en (Accessed October 20, 2023).
197. U.S. Food and Drug Administration. CDER continues to make rare diseases a priority with drug approvals and programming to speed therapeutic development. (2022). Available at: https://www.fda.gov/news-events/fda-voices/cder-continues-make-rare-diseases-priority-drug-approvals-and-programming-speed-therapeutic (Accessed October 20, 2023).
198. Fermaglich, LJ, and Miller, KL. A comprehensive study of the rare diseases and conditions targeted by orphan drug designations and approvals over the forty years of the Orphan Drug Act. Orphanet J Rare Dis. (2023) 18:163. doi: 10.1186/s13023-023-02790-7
199. United Nations. Political Declaration of the High-Level Plenary Meeting on Universal Health Coverage: resolution 74/2/adopted by the General Assembly on 10 October 2019. New York, NY: United Nations (2019).
200. United Nations. Addressing the challenges of persons living with a rare disease and their families: resolution 76/132/adopted by the General Assembly on 16 December 2021. New York, NY: United Nations (2021).
201. Taruscio, D. The long journey of people with rare diseases: from darkness to the UN Resolution 2021. Ann Ist Super Sanita. (2022) 58:79–80. doi: 10.4415/ANN_22_02_01
202. Monaco, L, Zanello, G, Baynam, G, Jonker, AH, Julkowska, D, Hartman, AL, et al. Research on rare diseases: ten years of progress and challenges at IRDiRC. Nat Rev Drug Discov. (2022) 21:319–20. doi: 10.1038/d41573-022-00019-z
203. Austin, CP, Cutillo, CM, Lau, LPL, Jonker, AH, Rath, A, Julkowska, D, et al. Future of rare diseases research 2017-2027: an IRDiRC perspective. Clin Transl Sci. (2018) 11:21–7. doi: 10.1111/cts.12500
204. Hivert, V, Jonker, AH, O’Connor, D, and Ardigo, D. IRDiRC: 1000 new rare diseases treatments by 2027, identifying and bringing forward strategic actions. Rare Dis Orphan Drugs J. (2022) 1:3. doi: 10.20517/rdodj.2021.02
205. Pearson, I, Rothwell, B, Olaye, A, and Knight, C. Economic modeling considerations for rare diseases. Value Heal J Int Soc Pharmacoeconomics Outcomes Res. (2018) 21:515–24. doi: 10.1016/j.jval.2018.02.008
206. Ollendorf, DA, Chapman, RH, and Pearson, SD. Evaluating and valuing drugs for rare conditions: no easy answers. Value Heal. (2018) 21:547–52. doi: 10.1016/j.jval.2018.01.008
207. Blonda, A, Denier, Y, Huys, I, and Simoens, S. How to value orphan drugs? A review of european value assessment frameworks. Front Pharmacol. (2021) 12:12. doi: 10.3389/fphar.2021.631527
208. Zhou, N, Ji, H, Li, Z, Hu, J, Xie, JH, Feng, YH, et al. Influencing factors of health technology assessment to orphan drugs: empirical evidence in England, Scotland, Canada, and Australia. Front Public Health. (2022) 10:861067. doi: 10.3389/fpubh.2022.861067
209. Jakubowski, S, Holko, P, Nowak, R, Warmuth, M, Dooms, M, Salminen, O, et al. Clinical and non-clinical aspects of reimbursement policy for orphan drugs in selected European countries. Front Pharmacol. (2024) 15:15. doi: 10.3389/fphar.2024.1498386
210. European Commission. Innovative payment models for high-cost innovative medicines. Report of the Expert Panel on effective ways of investing in Health (EXPH). Luxembourg: Publications Office of the European Union (2018).
211. Chan, AYL, Chan, VKY, Olsson, S, Fan, M, Jit, M, Gong, M, et al. Access and unmet needs of orphan drugs in 194 countries and 6 areas: a global policy review with content analysis. Value Health. (2020) 23:1580–91. doi: 10.1016/j.jval.2020.06.020
212. Jommi, C, Addis, A, Martini, N, Nicod, E, Pani, M, Scopinaro, A, et al. Price and reimbursement for orphan medicines and managed entry agreements: does Italy need a framework? Glob Reg Heal Technol Assess. (2021) 8:114–9. doi: 10.33393/grhta.2021.2278
213. Wenzl, M, and Chapman, S. Performance-based managed entry agreements for new medicines in OECD countries and EU member states: how they work and possible improvements going forward. Paris: OECD (2019).
214. Eichler, HG, Kossmeier, M, Zeitlinger, M, and Schwarzer-Daum, B. Orphan drugs’ clinical uncertainty and prices: addressing allocative and technical inefficiencies in orphan drug reimbursement. Front Pharmacol. (2023) 14:14. doi: 10.3389/fphar.2023.1074512
215. Decker, B, Mlcoch, T, Pustovalova, A, and Dolezal, T. Novel approach to decision making for orphan drugs. Int J Technol Assess Health Care. (2023) 39:e10. doi: 10.1017/S0266462323000053
216. Mincarone, P, Leo, CG, Sabina, S, Sarriá-Santamera, A, Taruscio, D, Serrano-Aguilar, PG, et al. Reimbursed price of orphan drugs: current strategies and potential improvements. Public Health Genomics. (2017) 20:1–8. doi: 10.1159/000464100
217. Rubin, JL, Lopez, A, Booth, J, Gunther, P, and Jena, AB. Limitations of standard cost-effectiveness methods for health technology assessment of treatments for rare, chronic diseases: a case study of treatment for cystic fibrosis. J Med Econ. (2022) 25:783–91. doi: 10.1080/13696998.2022.2077550
218. IQVIA. Innovative funding models for treatment of rare diseases. Singapore. (2021). Available at: https://www.iqvia.com/-/media/iqvia/pdfs/asia-pacific/white-papers/innovative-funding-models-for-treatment-of-rare-diseases.pdf (Accessed December 31, 2024).
219. EUROCAT Central Registry. EUROCAT special report: congenital anomalies are a major group of mainly rare diseases. Jordanstown. (2012). Available at: https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/eurocat-pub-docs/Special-Report-Major-Group-of-Mainly-Rare-Diseases.pdf (Accessed July 29, 2023).
220. Cornel, MC, Rigter, T, Jansen, ME, and Henneman, L. Neonatal and carrier screening for rare diseases: how innovation challenges screening criteria worldwide. J Community Genet. (2021) 12:257–65. doi: 10.1007/s12687-020-00488-y
221. Loeber, JG, Platis, D, Zetterström, RH, Almashanu, S, Boemer, F, Bonham, JR, et al. Neonatal screening in europe revisited: an ISNS perspective on the current state and developments since 2010. Int J Neonatal Screen. (2021) 7:15. doi: 10.3390/ijns7010015
222. Taruscio, D, Arriola, L, Baldi, F, Barisic, I, Bermejo-Sánchez, E, Bianchi, F, et al. European recommendations for primary prevention of congenital anomalies. Public Health Genomics. (2014) 17:115–23. doi: 10.1159/000360602
223. Bruckner-Tuderman, L. Epidemiology of rare diseases is important. J Eur Acad Dermatol Venereol. (2021) 35:783–4. doi: 10.1111/jdv.17165
224. Chang, X, Zhang, J, Jiang, Y, Shang, M, and Wu, Y. A survey of registered pharmacological clinical trials on rare neurological diseases in children in 2010-2020. Front Pediatr. (2022) 10:963601. doi: 10.3389/fped.2022.963601
225. Velmovitsky, PE, Bevilacqua, T, Alencar, P, Cowan, D, and Morita, PP. Convergence of precision medicine and public health into precision public health: toward a big data perspective. Front Public Health. (2021) 9:561873. doi: 10.3389/fpubh.2021.561873
226. Bilkey, GA, Burns, BL, Coles, EP, Mahede, T, Baynam, G, and Nowak, KJ. Optimizing precision medicine for public health. Front Public Health. (2019) 7:42. doi: 10.3389/fpubh.2019.00042
227. Dolley, S. Big data’s role in precision public health. Front Public Health. (2018) 6:68. doi: 10.3389/fpubh.2018.00068
228. Naumova, EN. Precision public health: is it all about the data? J Public Health Policy England. (2022) 43:481–6. doi: 10.1057/s41271-022-00367-5
229. Yang, X, Huang, K, Yang, D, Zhao, W, and Zhou, X. Biomedical big data technologies, applications, and challenges for precision medicine: a review. Glob Challenges. (2024) 8:2300163. doi: 10.1002/gch2.202300163
230. Incerti, D, Xu, XM, Chou, JW, Gonzaludo, N, Belmont, JW, and Schroeder, BE. Cost-effectiveness of genome sequencing for diagnosing patients with undiagnosed rare genetic diseases. Genet Med. (2022) 24:109–18. doi: 10.1016/j.gim.2021.08.015
231. Kingdom, R, and Wright, CF. Incomplete penetrance and variable expressivity: from clinical studies to population cohorts. Front Genet. (2022) 13:920390. doi: 10.3389/fgene.2022.920390
232. Rahit, KMTH, and Tarailo-Graovac, M. Genetic modifiers and rare mendelian disease. Genes (Basel). (2020) 11:239. doi: 10.3390/genes11030239
233. Choon, YW, Choon, YF, Nasarudin, NA, Al Jasmi, F, Remli, MA, Alkayali, MH, et al. Artificial intelligence and database for NGS-based diagnosis in rare disease. Front Genet. (2024) 14:14. doi: 10.3389/fgene.2023.1258083
234. Adams, DR, van Karnebeek, CDM, Agulló, SB, Faùndes, V, Jamuar, SS, Lynch, SA, et al. Addressing diagnostic gaps and priorities of the global rare diseases community: recommendations from the IRDiRC diagnostics scientific committee. Eur J Med Genet. (2024) 70:104951. doi: 10.1016/j.ejmg.2024.104951
235. Ou, P, Wen, R, Shi, L, Wang, J, and Liu, C. Artificial intelligence empowering rare diseases: a bibliometric perspective over the last two decades. Orphanet J Rare Dis. (2024) 19:345. doi: 10.1186/s13023-024-03352-1
236. van Karnebeek, CDM, O’Donnell-Luria, A, Baynam, G, Baudot, A, Groza, T, Jans, JJM, et al. Leaving no patient behind! Expert recommendation in the use of innovative technologies for diagnosing rare diseases. Orphanet J Rare Dis. (2024) 19:357. doi: 10.1186/s13023-024-03361-0
237. Villalón-García, I, Álvarez-Córdoba, M, Suárez-Rivero, JM, Povea-Cabello, S, Talaverón-Rey, M, Suárez-Carrillo, A, et al. Precision medicine in rare diseases. Dis (Basel, Switzerland). (2020) 8:42. doi: 10.3390/diseases8040042
238. Dugger, SA, Platt, A, and Goldstein, DB. Drug development in the era of precision medicine. Nat Rev Drug Discov. (2018) 17:183–96. doi: 10.1038/nrd.2017.226
239. Park, JJH, Siden, E, Zoratti, MJ, Dron, L, Harari, O, Singer, J, et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. (2019) 20:572. doi: 10.1186/s13063-019-3664-1
240. Committee for Advanced Therapies (CAT). Guideline on quality, non-clinical and clinical aspects of medicinal products containing genetically modified cells - Revision 1. Amsterdam. (2021). Available at: https://www.ema.europa.eu/en/quality-non-clinical-and-clinical-aspects-medicinal-products-containing-genetically-modified-cells-scientific-guideline (Accessed December 31, 2024).
241. U.S. Department of Health and Human Services. Expedited programs for regenerative medicine therapies for serious conditions - guidance for industry. Rockville, MD. (2019). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expedited-programs-regenerative-medicine-therapies-serious-conditions (Accessed December 31, 2024).
242. Brooks, PJ, Miller, TM, Revah, F, Suh, J, Garrison, BR, Starke, LC, et al. The Bespoke Gene Therapy Consortium: facilitating development of AAV gene therapies for rare diseases. Nat Rev Drug Discov England. (2024) 23:157–8. doi: 10.1038/d41573-024-00020-8
243. National Institutes of Health. NIH, FDA and 15 private organizations join forces to increase effective gene therapies for rare diseases. (2021). Available at: https://www.nih.gov/news-events/news-releases/nih-fda-15-private-organizations-join-forces-increase-effective-gene-therapies-rare-diseases (Accessed January 23, 2024).
244. National Centre for Advancing Translational Sciences. Bespoke Gene Therapy Consortium (BGTC). (2024). Available at: https://ncats.nih.gov/research/research-activities/BGTC (Accessed January 23, 2024).
245. Nabbout, R, Zanello, G, Baker, D, Black, L, Brambilla, I, Buske, OJ, et al. Towards the international interoperability of clinical research networks for rare diseases: recommendations from the IRDiRC Task Force. Orphanet J Rare Dis. (2023) 18:109. doi: 10.1186/s13023-023-02650-4
246. Valentino, LA, Baker, JR, Butler, R, Escobar, M, Frick, N, Karp, S, et al. Integrated hemophilia patient care via a national network of care centers in the United States: a model for rare coagulation disorders. J Blood Med. (2021) 12:897–911. doi: 10.2147/JBM.S325031
247. Tran, DQ, Benson, CC, Boice, JA, Chitlur, M, Dunn, AL, Escobar, MA, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research priorities to transform the care of people with hemophilia. Expert Rev Hematol. (2023) 16:19–37. doi: 10.1080/17474086.2023.2171981
248. Byams, VR, Baker, JR, Bailey, C, Connell, NT, Creary, MS, Curtis, RG, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research priorities in health services; diversity, equity, and inclusion; and implementation science. Expert Rev Hematol. (2023) 16:87–106. doi: 10.1080/17474086.2023.2183836
249. Tisdale, A, Cutillo, CM, Nathan, R, Russo, P, Laraway, B, Haendel, M, et al. The IDeaS initiative: pilot study to assess the impact of rare diseases on patients and healthcare systems. Orphanet J Rare Dis. (2021) 16:429. doi: 10.1186/s13023-021-02061-3
250. Nacul, LC, Stewart, A, Alberg, C, Chowdhury, S, Darlison, MW, Grollman, C, et al. A Toolkit to assess health needs for congenital disorders in low- and middle-income countries: an instrument for public health action. J Public Health (Bangkok). (2014) 36:243–50. doi: 10.1093/pubmed/fdt048
251. Modell, B, Darlison, MW, Malherbe, H, Moorthie, S, Blencowe, H, Mahaini, R, et al. Congenital disorders: epidemiological methods for answering calls for action. J Community Genet. (2018) 9:335–40. doi: 10.1007/s12687-018-0390-4
252. World Health Organization. Congenital disorders. (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/birth-defects (Accessed July 25, 2024).
253. Lorey, F. Genetic determinants of disease and genetics in public health In: RB Wallace, editor. Maxcy-Rosenau-last public health and preventive medicine. 15th ed. New York, NY: McGraw-Hill Medical (2017). 63–74.
254. Patwardhan, B, Mutalik, G, and Tillu, G. Concepts of health and disease. In: PR Sarsinadi and T Mariateresa, editors. Integrative approaches for health: biomedical research, ayurveda, and yoga. Boston: Academic Press; (2015). p. 53–78.
255. Rollet, P, Lemoine, A, and Dunoyer, M. Sustainable rare diseases business and drug access: no time for misconceptions. Orphanet J Rare Dis. (2013) 8:109. doi: 10.1186/1750-1172-8-109
256. Miyamoto, BE, and Kakkis, ED. The potential investment impact of improved access to accelerated approval on the development of treatments for low prevalence rare diseases. Orphanet J Rare Dis. (2011) 6:49. doi: 10.1186/1750-1172-6-49
257. Adachi, T, El-Hattab, AW, Jain, R, Nogales Crespo, KA, Quirland Lazo, CI, Scarpa, M, et al. Enhancing equitable access to rare disease diagnosis and treatment around the world: a review of evidence, policies, and challenges. Int J Environ Res Public Health. (2023) 20:4732. doi: 10.3390/ijerph20064732
258. Annemans, L, Aymé, S, Le Cam, Y, Facey, K, Gunther, P, Nicod, E, et al. Recommendations from the European working group for value assessment and funding processes in rare diseases (ORPH-VAL). Orphanet J Rare Dis. (2017) 12:50. doi: 10.1186/s13023-017-0601-9
259. Magalhaes, M. Should rare diseases get special treatment? J Med Ethics. (2022) 48:86–92. doi: 10.1136/medethics-2021-107691
260. David, A, Alimohamed, MZ, Modern, G, Mbarak, S, Ahmad, H, Ozcan, B, et al. the plight of rare diseases in southern Africa: health and social services policy recommendations. Qeios. (2023). doi: 10.32388/LGHFL8
261. Baynam, GS, Groft, S, van der Westhuizen, FH, Gassman, SD, du Plessis, K, Coles, EP, et al. A call for global action for rare diseases in Africa. Nat Genet. (2020) 52:21–6. doi: 10.1038/s41588-019-0552-2
262. Bouwman, ML, Sousa, JJS, and Pina, MET. Regulatory issues for orphan medicines: a review. Heal Policy Technol. (2020) 9:115–21. doi: 10.1016/j.hlpt.2019.11.008
263. Gahl, WA, Wong-Rieger, D, Hivert, V, Yang, R, Zanello, G, and Groft, S. Essential list of medicinal products for rare diseases: recommendations from the IRDiRC Rare Disease Treatment Access Working Group. Orphanet J Rare Dis. (2021) 16:308. doi: 10.1186/s13023-021-01923-0
264. Dharssi, S, Wong-Rieger, D, Harold, M, and Terry, S. Review of 11 national policies for rare diseases in the context of key patient needs. Orphanet J Rare Dis. (2017) 12:63. doi: 10.1186/s13023-017-0618-0
265. Ferrelli, RM, Gentile, AE, De Santis, M, and Taruscio, D. Sustainable public health systems for rare diseases. Ann Ist Super Sanita. (2017) 53:170–5. doi: 10.4415/ANN_17_02_16
266. Ince, Ö, and MDP, GÜRE. Evaluation of rare diseases policy performance of oecd countries using mcdm methods. Heal Policy Technol. (2021) 10:100537. doi: 10.1016/j.hlpt.2021.100537
267. Cox, TM, Tylki-Szymańska, A, Aymé, S, and Dooms, M. Editorial: Prevention, diagnosis and treatment of rare disorders. Front Pharmacol. (2022) 13:1026064. doi: 10.3389/fphar.2022.1026064
268. Li, X, Wu, L, Yu, L, He, Y, Wang, M, and Mu, Y. Policy analysis in the field of rare diseases in China: a combined study of content analysis and Bibliometrics analysis. Front Med. (2023) 10:10. doi: 10.3389/fmed.2023.1180550
269. Edu, E, Okesanya, OJ, and Lucero-Prisno, DE. Burden of rare diseases in Africa: recommendations for improving access to medications and healthcare. J Med Surgery Public Heal. (2024) 2:100032. doi: 10.1016/j.glmedi.2023.100032
270. Kaywanga, F, Alimohamed, MZ, David, AB, Maeda, D, Mbarak, S, Mavura, T, et al. Rare diseases in Tanzania: a National Call for Action to address policy and urgent needs of individuals with rare diseases. Orphanet J Rare Dis. (2022) 17:343. doi: 10.1186/s13023-022-02498-0
271. European Commission. Draft proposal for a European Partnership under Horizon Europe Rare Diseases. (2022). Available at: https://research-and-innovation.ec.europa.eu/system/files/2022-02/ec_rtd_he-partnerships-rare-diseases.pdf (Accessed October 28, 2023).
272. Mukherjee, K. Care for rare: spotlight on rare diseases. Trends Pharmacol Sci. (2019) 40:227–8. doi: 10.1016/j.tips.2019.02.008
273. Verma, IC, El-Beshlawy, A, Tylki-Szymańska, A, Martins, A, Duan, YL, Collin-Histed, T, et al. Transformative effect of a Humanitarian Program for individuals affected by rare diseases: building support systems and creating local expertise. Orphanet J Rare Dis. (2022) 17:87. doi: 10.1186/s13023-022-02192-1
274. Angin, C, Mazzucato, M, Weber, S, Kirch, K, Abdel Khalek, W, Ali, H, et al. Coding undiagnosed rare disease patients in health information systems: recommendations from the RD-CODE project. Orphanet J Rare Dis. (2024) 19:28. doi: 10.1186/s13023-024-03030-2
275. De Santis, M, Hervas, C, Weinman, A, Bosi, G, and Bottarelli, V. Patient empowerment of people living with rare diseases. Its contribution to sustainable and resilient healthcare systems. Ann Ist Super Sanita. (2019) 55:283–91. doi: 10.4415/ANN_19_03_15
276. Marinello, D, Del Bianco, A, Manzo, A, Mosca, M, and Talarico, R. Empowering rare disease patients through patient education: the new BehçeTalk programme. BMC Rheumatol. (2022) 6:17. doi: 10.1186/s41927-022-00247-1
277. Aymé, S, Kole, A, and Groft, S. Empowerment of patients: lessons from the rare diseases community. Lancet. (2008) 371:2048–51. doi: 10.1016/S0140-6736(08)60875-2
278. Nori, M, Fisher-Vance, D, Wuerth, L, Colenso, R, and Donovan, DJ. The global role of patients, advocates and caregivers in rare diseases. Futur Rare Dis. (2023) 2:FRD22. doi: 10.2217/frd-2022-0003
279. Kisling, LA, and Das, JM. Prevention strategies In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023)
280. Pai, M, Key, NS, Skinner, M, Curtis, R, Feinstein, M, Kessler, C, et al. NHF-McMaster guideline on care models for haemophilia management. Haemophilia. (2016) 22:6–16. doi: 10.1111/hae.13008
281. van Balen, EC, O’Mahony, B, Cnossen, MH, Dolan, G, Blanchette, VS, Fischer, K, et al. Patient-relevant health outcomes for hemophilia care: development of an international standard outcomes set. Res Pract Thromb Haemost. (2021) 5:e12488. doi: 10.1002/rth2.12488
282. Peyvandi, F, Berger, K, Seitz, R, Hilger, A, Hecquet, ML, Wierer, M, et al. Kreuth V initiative: European consensus proposals for treatment of hemophilia using standard products, extended half-life coagulation factor concentrates and non-replacement therapies. Haematologica. (2020) 105:2038–43. doi: 10.3324/haematol.2019.242735
283. Soucie, JM, Miller, CH, Kelly, FM, Oakley, M, Brown, DL, and Kucab, P. A public health approach to the prevention of inhibitors in hemophilia. Am J Prev Med. (2014) 47:669–73. doi: 10.1016/j.amepre.2014.07.007
284. Carvalho, M, Sepodes, B, and Martins, AP. Patient access to gene therapy medicinal products: a comprehensive review. BMJ Innov. (2021) 7:123–34. doi: 10.1136/bmjinnov-2020-000425
285. Salzman, R, Cook, F, Hunt, T, Malech, HL, Reilly, P, Foss-Campbell, B, et al. Addressing the value of gene therapy and enhancing patient access to transformative treatments. Mol Ther. (2018) 26:2717–26. doi: 10.1016/j.ymthe.2018.10.017
286. Giugliani, R, Vairo, FP, Riegel, M, de Souza, CFM, Schwartz, IVD, and Pena, SDJ. Rare disease landscape in Brazil: report of a successful experience in inborn errors of metabolism. Orphanet J Rare Dis. (2016) 11:76. doi: 10.1186/s13023-016-0458-3
287. de Oliveira, BM, Bernardi, FA, Baiochi, JF, Neiva, MB, Artifon, M, Vergara, AA, et al. Epidemiological characterization of rare diseases in Brazil: a retrospective study of the Brazilian Rare Diseases Network. Orphanet J Rare Dis. (2024) 19:405. doi: 10.1186/s13023-024-03392-7
288. Arnold, RJG, Bighash, L, Bryón Nieto, A, Branco, T, de Araújo, G, Gay-Molina, JG, et al. The role of globalization in drug development and access to orphan drugs: orphan drug legislation in the US/EU and in Latin America. F1000Research. (2015) 4:1–9. doi: 10.12688/f1000research.4268.1
289. Cunico, C, Vicente, G, and Leite, SN. Initiatives to promote access to medicines after publication of the Brazilian Policy on the Comprehensive Care of People with Rare Diseases. Orphanet J Rare Dis. (2023) 18:259. doi: 10.1186/s13023-023-02881-5
290. Dias, AG, Daher, A, Barrera Ortiz, L, Carreño-Moreno, S, Hafez, HSR, Jansen, AM, et al. Rarecare: a policy perspective on the burden of rare diseases on caregivers in Latin America. Front Public Health. (2023) 11:11. doi: 10.3389/fpubh.2023.1127713
291. Gittus, M, Chong, J, Sutton, A, Ong, ACM, and Fotheringham, J. Barriers and facilitators to the implementation of guidelines in rare diseases: a systematic review. Orphanet J Rare Dis. (2023) 18:140. doi: 10.1186/s13023-023-02667-9
292. Pavan, S, Rommel, K, Mateo Marquina, ME, Höhn, S, Lanneau, V, and Rath, A. Clinical practice guidelines for rare diseases: the orphanet database. PLoS One. (2017) 12:e0170365. doi: 10.1371/journal.pone.0170365
293. Simoens, S. Pricing and reimbursement of orphan drugs: the need for more transparency. Orphanet J Rare Dis. (2011) 6:42. doi: 10.1186/1750-1172-6-42
294. Lee, JH. Pricing and reimbursement pathways of new orphan drugs in South Korea: a longitudinal comparison. Healthc (Basel, Switzerland). (2021) 9:296. doi: 10.3390/healthcare9030296
295. Henrard, S, and Arickx, F. Negotiating prices of drugs for rare diseases. Bull World Health Organ. (2016) 94:779–81. doi: 10.2471/BLT.15.163519
296. López-Bastida, J, Oliva-Moreno, J, Linertová, R, and Serrano-Aguilar, P. Social/economic costs and health-related quality of life in patients with rare diseases in Europe. Eur J Heal Econ. (2016) 17:1–5. doi: 10.1007/s10198-016-0780-7
297. Bhattacharya, K, Millis, N, Jaffe, A, and Zurynski, Y. Rare diseases research and policy in Australia: on the journey to equitable care. J Paediatr Child Health. (2021) 57:778–81. doi: 10.1111/jpc.15507
298. Marolt Presen, D, Lainšček, D, Kinghorn, J, Sebestyen, Z, Kuball, J, Amini, L, et al. CTGCT, Centre of Excellence for the Technologies of Gene and Cell Therapy: collaborative translation of scientific discoveries into advanced treatments for neurological rare genetic diseases and cancer. Comput Struct Biotechnol J. (2025) 27:10–6. doi: 10.1016/j.csbj.2024.11.051
299. Sheng, B. Connecting each other in rare diseases: a call for cross-regional collaboration BT In: BYF Fong and WCW Wong, editors. Gaps and actions in health improvement from hong kong and beyond: all for health. Singapore: Springer Nature Singapore (2023). 391–400.
300. Tumienė, B, Juozapavičiūtė, A, and Andriukaitis, V. Rare diseases: still on the fringes of universal health coverage in Europe. Lancet Reg Heal Eur. (2024) 37:100783. doi: 10.1016/j.lanepe.2023.100783
301. Fox, TA, and Booth, C. Improving access to gene therapy for rare diseases. Dis Model Mech. (2024) 17:dmm050623. doi: 10.1242/dmm.050623
302. Halley, MC, Smith, HS, Ashley, EA, Goldenberg, AJ, and Tabor, HK. A call for an integrated approach to improve efficiency, equity and sustainability in rare disease research in the United States. Nat Genet. (2022) 54:219–22. doi: 10.1038/s41588-022-01027-w
303. Czerska, I, and Skweres-Kuchta, M. Integrative medicine as a new treatment model and the future of health care systems in the world in the context of rare diseases. Eur Res Stud J. (2021) XXIV:800–9. doi: 10.35808/ersj/2265
304. Henderson, ML, Zieba, JK, Li, X, Campbell, DB, Williams, MR, Vogt, DL, et al. Gene therapy for genetic syndromes: understanding the current state to guide future care. Biotech. (2024) 13:1. doi: 10.3390/biotech13010001
305. De Luca, M, and Cossu, G. Cost and availability of novel cell and gene therapies. EMBO Rep. (2023) 24:e56661. doi: 10.15252/embr.202256661
306. Kohn, DB, Chen, YY, and Spencer, MJ. Successes and challenges in clinical gene therapy. Gene Ther. (2023) 30:738–46. doi: 10.1038/s41434-023-00390-5
307. Gogia, P, Tarantino, M, Schramm, W, and Aledort, L. New directions to develop therapies for people with hemophilia. Expert Rev Hematol. (2023) 16:417–33. doi: 10.1080/17474086.2023.2184341
308. Holme, PA, Blatný, J, Chowdary, P, Lassila, R, O’Connell, N, Hermans, C, et al. Moving towards normalization of haemostasis and health equity: evolving treatment goals for haemophilia A. Haemophilia. (2024) 30:1109–14. doi: 10.1111/hae.15031
309. Mahlangu, J, Diop, S, and Lavin, M. Diagnosis and treatment challenges in lower resource countries: state-of-the-art. Haemophilia. (2024) 30:78–85. doi: 10.1111/hae.14956
310. Yoon, S, Lee, M, Jung, HI, Khan, MM, Kim, SY, Kim, H, et al. Prioritization of research engaged with rare disease stakeholders: a systematic review and thematic analysis. Orphanet J Rare Dis. (2023) 18:363. doi: 10.1186/s13023-023-02892-2
311. Hedley, V, Bolz-Johnson, M, Hernando, I, Kenward, R, Nabbout, R, Romero, C, et al. Together4RD position statement on collaboration between European reference networks and industry. Orphanet J Rare Dis. (2023) 18:272. doi: 10.1186/s13023-023-02853-9
312. Liu, J, Yu, Y, Zhong, M, Ma, C, and Shao, R. Long way to go: progress of orphan drug accessibility in China from 2017 to 2022. Front Pharmacol. (2023) 14:1138996. doi: 10.3389/fphar.2023.1138996
313. Sakushima, K, Takeda, H, and Aoi, Y. Orphan drug designation and development in Japan: 25 years of experience and assessment. Nat Rev Drug Discov. (2021) 20:893–4. doi: 10.1038/d41573-021-00045-3
314. Encina, G, Castillo-Laborde, C, Lecaros, JA, Dubois-Camacho, K, Calderón, JF, Aguilera, X, et al. Rare diseases in Chile: challenges and recommendations in universal health coverage context. Orphanet J Rare Dis. (2019) 14:289. doi: 10.1186/s13023-019-1261-8
Keywords: public health, rare diseases, hemophilia, orphan drugs, health inequities, public health policy, public health surveillance, disease burden
Citation: El-Sayed AA, Reiss UM, Hanna D and Bolous NS (2025) The role of public health in rare diseases: hemophilia as an example. Front. Public Health. 13:1450625. doi: 10.3389/fpubh.2025.1450625
Received: 17 June 2024; Accepted: 10 February 2025;
Published: 20 March 2025.
Edited by:
Segundo Mariz, European Medicines Agency, NetherlandsReviewed by:
Armando Magrelli, National Institute of Health (ISS), ItalyCopyright © 2025 El-Sayed, Reiss, Hanna and Bolous. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amr A. El-Sayed, YWFzeUBub3Zvbm9yZGlzay5jb20=; ZHIuYW1yLmVsc2F5ZWQuMTk4M0BnbWFpbC5jb20=
†ORCID: Amr A. El-Sayed, http://orcid.org/0009-0008-3713-237X
Ulrike M. Reiss, http://orcid.org/0000-0002-2258-3687
Diana Hanna, http://orcid.org/0000-0002-8967-273X
Nancy S. Bolous, http://orcid.org/0000-0001-6619-5291
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.