- 1Department of Clinical Laboratory, The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, Zhejiang, China
- 2Department of Hospital Management, The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, Zhejiang, China
- 3Department of Osteoporosis Care and Control, The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, Zhejiang, China
Background: Mercury exposure poses significant health risks, yet its effects on bone mineral density (BMD) and appendicular lean mass index (ALMI) in middle-aged women remain poorly understood. This study aimed to investigate the associations between blood mercury levels (BML) and these key indicators of skeletal health and body composition, with special attention to the potential modifying effect of menopausal status.
Methods: We analyzed data from 1,648 women aged 40–59 years (782 premenopausal, 866 postmenopausal) using the National Health and Nutrition Examination Survey (NHANES) 2011–2018. Multiple linear regression models were employed to assess the relationships between LnBML and both lumbar BMD and ALMI, adjusting for relevant covariates.
Results: There was complex, non-linear associations between LnBML and skeletal health parameters. Notably, the relationship between LnBML and lumbar BMD differed significantly based on menopausal status (P for interaction <0.001). In premenopausal women, LnBML was negatively associated with BMD (β = −0.018, 95% CI: −0.029, −0.007), while in postmenopausal women, a positive association was observed (β = 0.025, 95% CI: 0.014, 0.036). Conversely, LnBML demonstrated a significant positive association with ALMI (β = 0.054, 95% CI: 0.025, 0.083, p < 0.001) in the fully adjusted model.
Conclusion: Our findings revealed intricate, menopause-dependent relationships between BML and skeletal health parameters in middle-aged women. These results underscore the complex interplay between environmental exposures and women’s health across the menopausal transition, highlighting the need for further research to elucidate underlying mechanisms and inform targeted interventions.
Introduction
Mercury, a pervasive and highly toxic heavy metal, ranks among the top three priority substances of global public health concern due to its profound impacts on human health and ecosystems (1). In the general population, exposure to mercury predominantly transpires via three principal avenues: ingestion of dietary sources, notably the consumption of fish and seafood contaminated with methylmercury; occupational hazards, encompassing dental amalgams and industrial activities; and environmental contamination, such as polluted air and tainted water (2, 3). Moreover, the speciation of mercury—elemental, inorganic, or organic—profoundly affects its toxicokinetic properties and the health outcomes associated with exposure (4). These findings carry particularly alarming implications for vulnerable populations, underscoring the urgent need for stringent global policies to mitigate mercury pollution (5).
Concurrently, bone mineral density (BMD) serves as a critical indicator of skeletal integrity and a primary predictor of osteoporosis risk (6). The perimenopausal transition in women is characterized by accelerated bone loss, significantly increasing their susceptibility to fractures and osteoporosis (7). This life stage is further marked by notable changes in body composition, particularly in appendicular lean mass (8). The appendicular lean mass index (ALMI), a valuable measure of skeletal muscle mass, has been closely associated with sarcopenia and functional capacity (9, 10).
Emerging evidence suggests that environmental factors, including exposure to heavy metals, may exert substantial influence on bone metabolism and body composition (11–13). However, the specific relationship between blood mercury levels (BML) and skeletal health parameters remains understudied, representing a critical gap in our understanding of environmental toxicology and women’s health. Therefore, this study aimed to elucidate the association between BML, BMD and ALMI in middle-aged women using large-scale population data, with particular focus on examining whether these relationships differ by menopausal status. Our findings may have important implications for public health policies, environmental regulations, and clinical practices related to women’s health and aging.
Methods
Study design and population
This study analyzed data from the National Health and Nutrition Examination Survey (NHANES), a comprehensive, ongoing cross-sectional survey designed to assess the health and nutritional status of adults and children in the United States. The NHANES protocol was approved by the ethics review board of the National Center for Health Statistics, with all participants providing written informed consent.
We extracted data from the NHANES database spanning 2011 to 2018, focusing on women aged 40–59 years (n = 3,908). After excluding participants with missing data for BML (n = 1,240), lumbar BMD (n = 645), ALMI (n = 243), and those with indeterminate menstrual status (n = 132), the final cohort comprised 1,648 participants (782 premenopausal and 866 postmenopausal women).
Study variables
The primary exposure variable was BML, measured using inductively coupled plasma mass spectrometry (ICP-MS) with quadrupole technology. BML data underwent natural log-transformation (Ln) to approximate a normal distribution. Outcome variables included lumbar BMD and ALMI, both measured via dual-energy X-ray absorptiometry. ALMI was calculated as appendicular lean mass [g] divided by height squared [m2]. Covariates encompassed both categorical variables (race, educational level, menstrual status, history of hypertension, diabetes, cancer or malignancy, and moderate recreational activities) and continuous variables (age, body mass index [BMI], total protein, blood urea nitrogen, serum uric acid, and serum calcium). Detailed methodologies for BML, BMD, and ALMI measurements are available at wwwn.cdc.gov/nchs/nhanes/.
Statistical analyses
All analyses utilized weighted NHANES samples to ensure national representativeness. To examine the independent associations of BML with BMD and ALMI, we employed weighted multiple linear regression models. Following the statement of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (14), we constructed four models: Model 1 (unadjusted), Model 2 (adjusted for age and race), Model 3 (adjusted for age, race, and BMI), and Model 4 (fully adjusted model including all screened covariates). Smooth curve fittings and generalized additive models were applied to explore potential non-linear relationships.
Continuous variables were expressed as mean ± standard deviation, while categorical variables were presented as weighted percentages. Statistical significance was set at p < 0.05. All analyses were performed using Empowerstats1 and R software (version 3.4.3). Statistical significance was set at p < 0.05.
Results
Participant characteristics
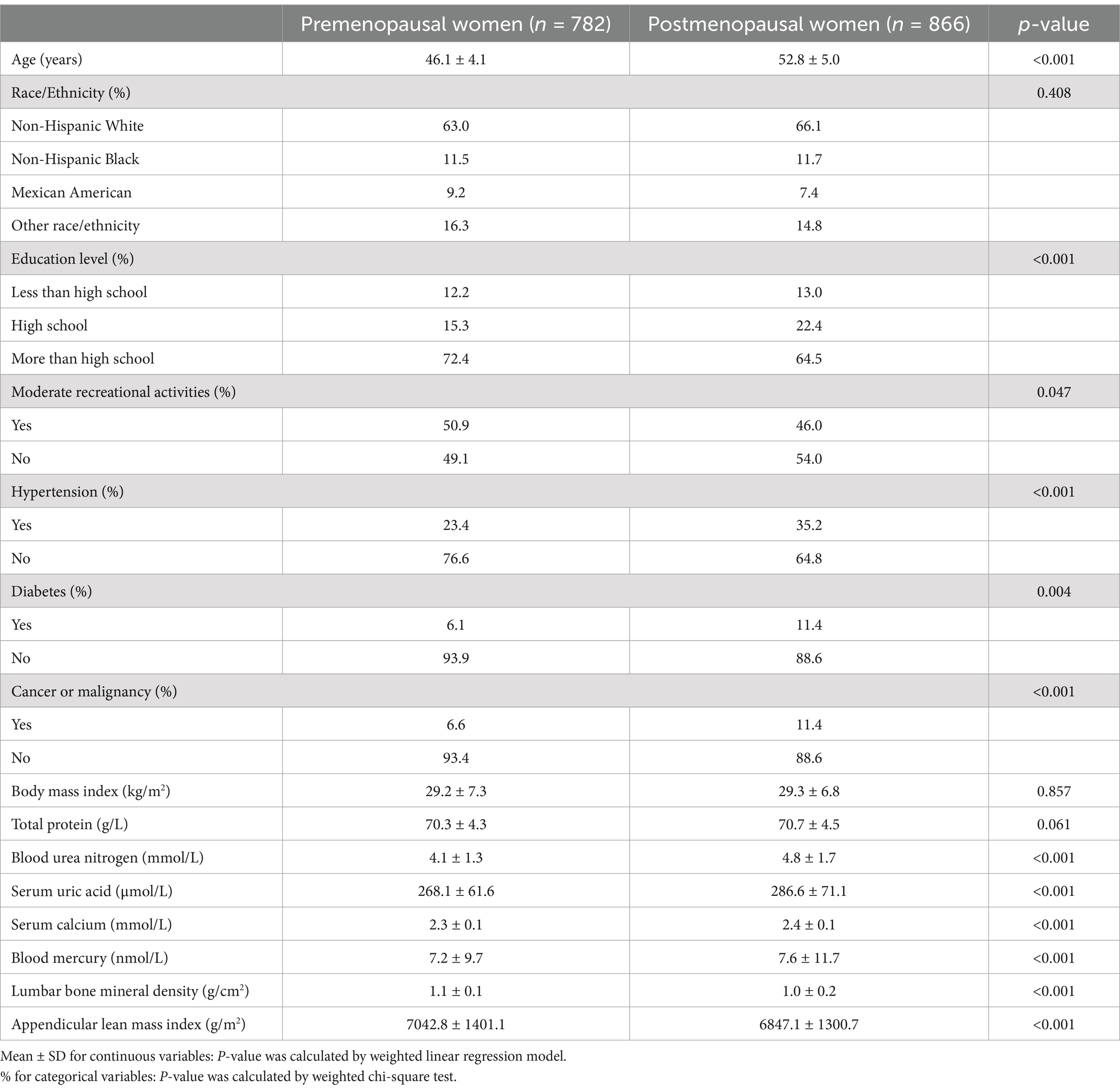
Table 1 presents participant characteristics stratified by menopausal status. Postmenopausal women exhibited higher prevalence of hypertension, diabetes, and cancer, alongside elevated levels of blood urea nitrogen, serum uric acid, and serum calcium. Notably, postmenopausal women demonstrated significantly lower lumbar BMD and ALMI compared to their premenopausal counterparts.
Associations between BML, BMD, and ALMI
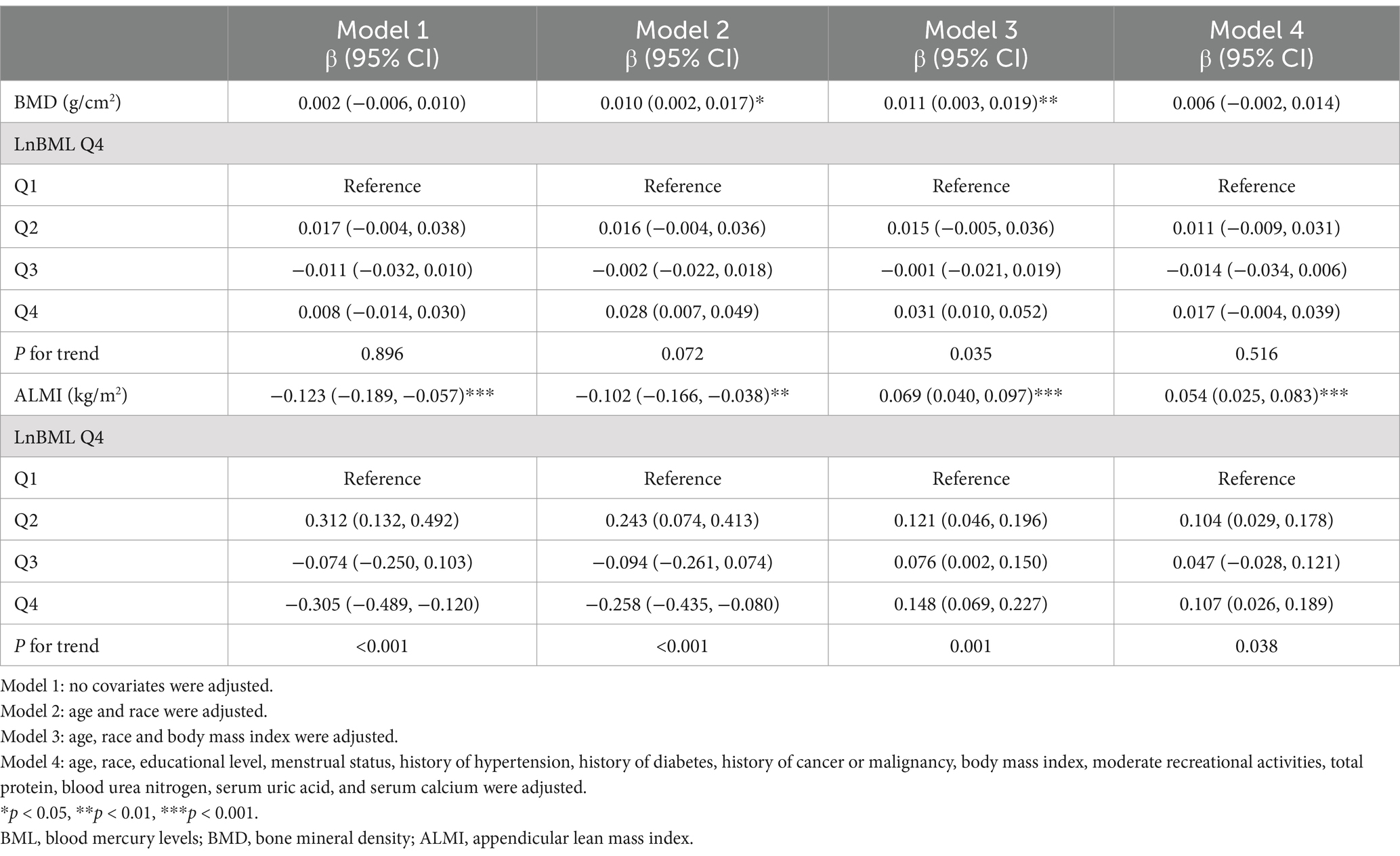
LnBML exhibited complex associations with BMD and ALMI (Table 2). In fully adjusted models (Model 4), LnBML showed no significant association with BMD (β = 0.006, 95% CI: −0.002, 0.014). However, LnBML quartiles revealed a non-linear relationship with BMD, albeit without statistical significance (p for trend = 0.516). Conversely, LnBML demonstrated a significant positive association with ALMI (β = 0.054, 95% CI: 0.025, 0.083, p < 0.001). This relationship was corroborated by a significant trend across LnBML quartiles (p for trend = 0.038), with the highest quartile showing the strongest association. These non-linear and positive relationships were further substantiated by smooth curve fittings (Figures 1, 2).
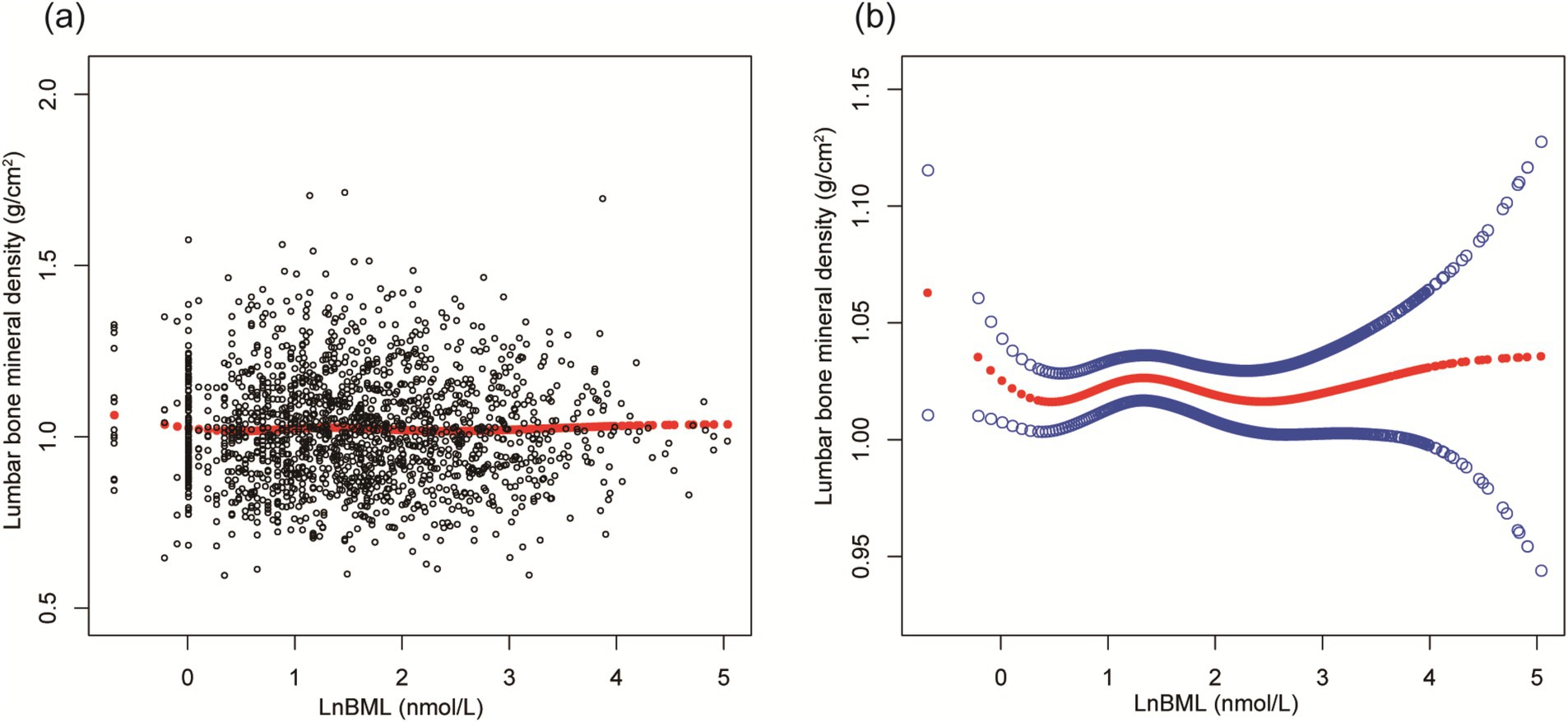
Figure 1. The association between LnBML and lumbar BMD (g/cm2). (A) Each black point represents a sample. (B) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. Age, race, educational level, menstrual status, history of hypertension, history of diabetes, history of cancer or malignancy, body mass index, moderate recreational activities, total protein, blood urea nitrogen, serum uric acid, and serum calcium were adjusted. BML, blood mercury levels; BMD, bone mineral density.
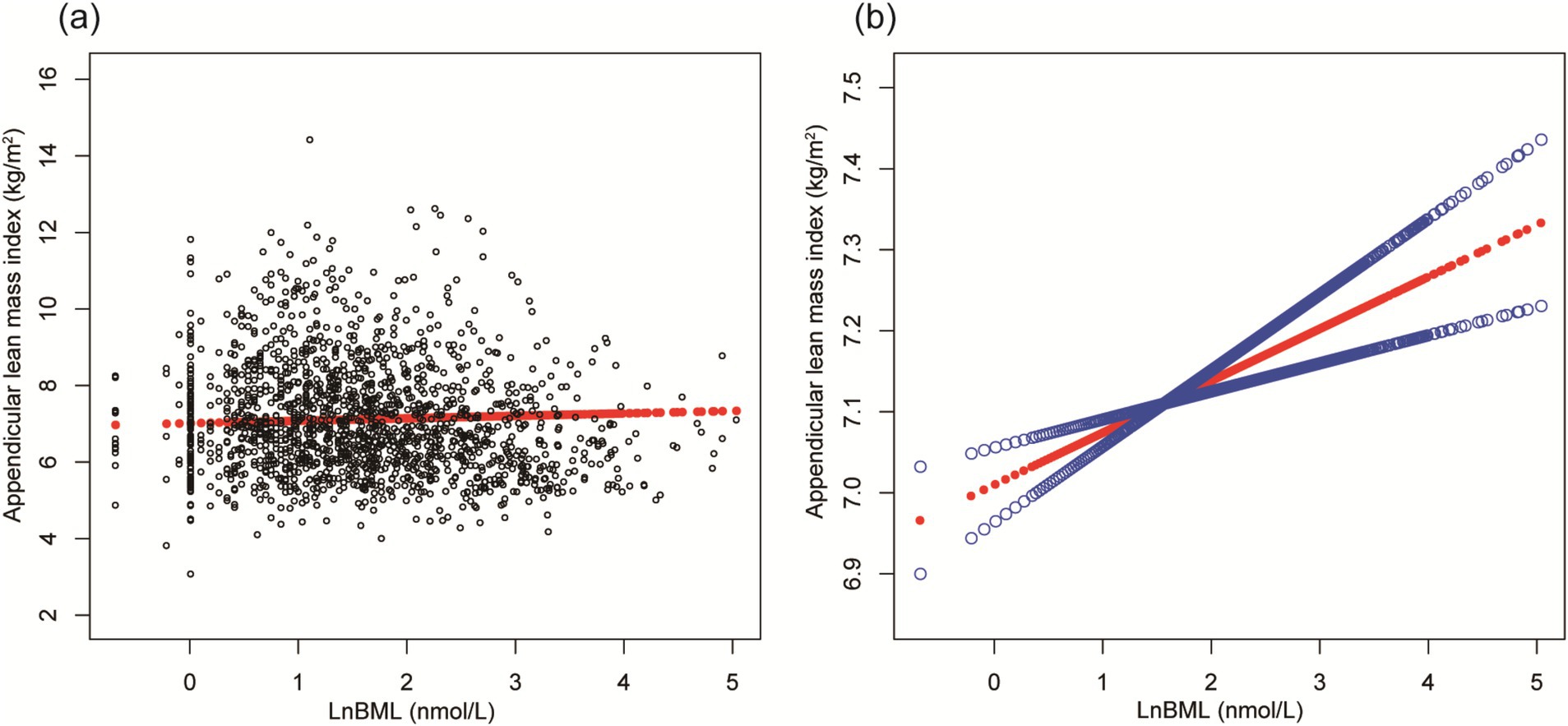
Figure 2. The association between LnBML and ALMI (g/m2). (A) Each black point represents a sample. (B) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. Age, race, educational level, menstrual status, history of hypertension, history of diabetes, history of cancer or malignancy, body mass index, moderate recreational activities, total protein, blood urea nitrogen, serum uric acid, and serum calcium were adjusted. BML, blood mercury levels; ALMI, appendicular lean mass index.
Subgroup analyses
Figures 3, 4 illustrate subgroup analyses of LnBML correlations with lumbar BMD and ALMI across various demographic and health-related strata. Notably, the relationship between LnBML and BMD exhibited a significant dichotomy based on menopausal status (p for interaction <0.001). A negative correlation was observed in premenopausal women (β = −0.018, 95% CI: −0.029, −0.007), contrasting with a positive correlation in postmenopausal women (β = 0.025, 95% CI: 0.014, 0.036). The association between LnBML and ALMI demonstrated a positive trend, reaching statistical significance in postmenopausal women (β = 0.075, 95% CI: 0.035, 0.115).
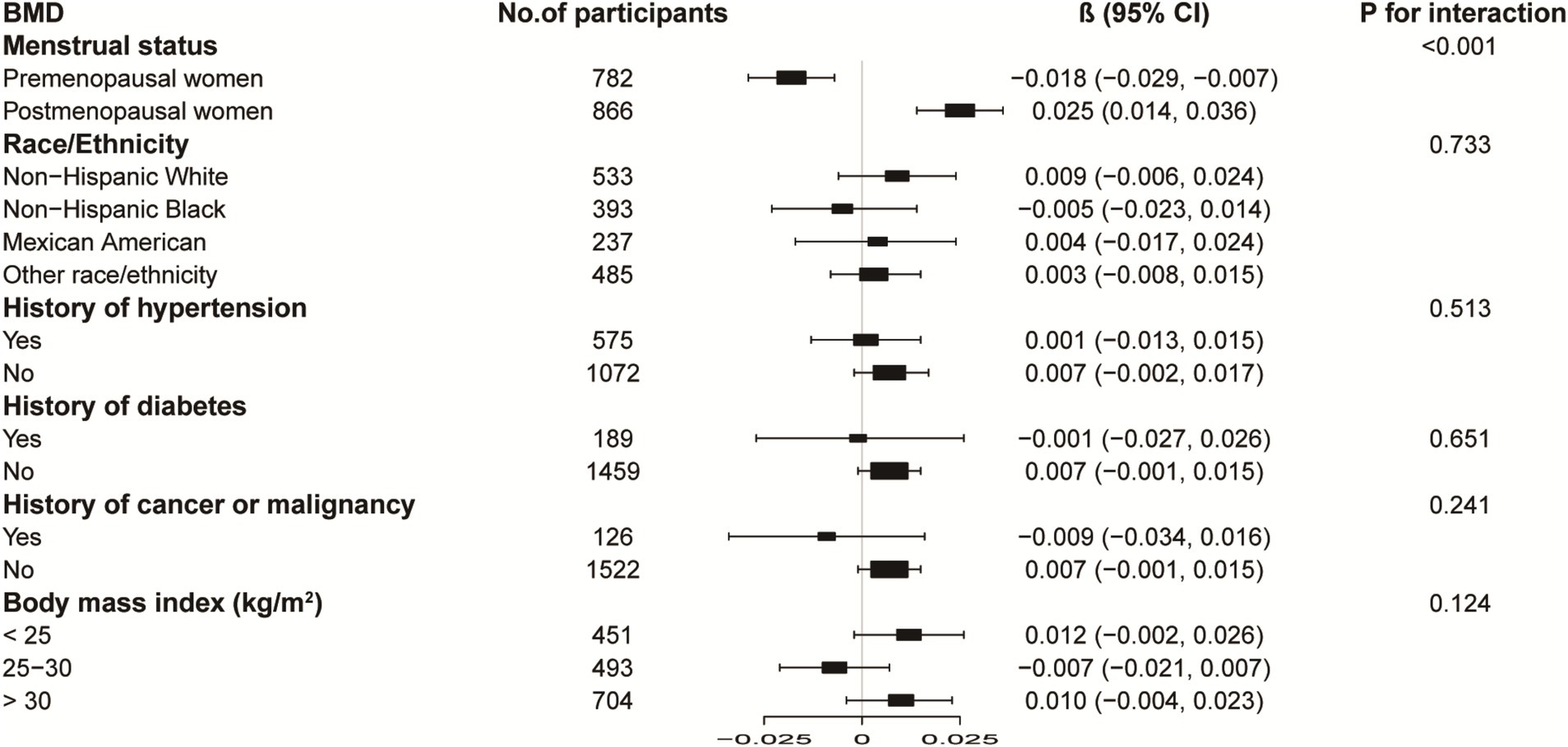
Figure 3. Subgroup analysis of the association between LnBML and lumbar BMD (g/cm2). Age, race, educational level, menstrual status, history of hypertension, history of diabetes, history of cancer or malignancy, body mass index, moderate recreational activities, total protein, blood urea nitrogen, serum uric acid, and serum calcium were adjusted. In the subgroup analysis, the model is not adjusted for the stratification variable itself. BML, blood mercury levels; BMD, bone mineral density.
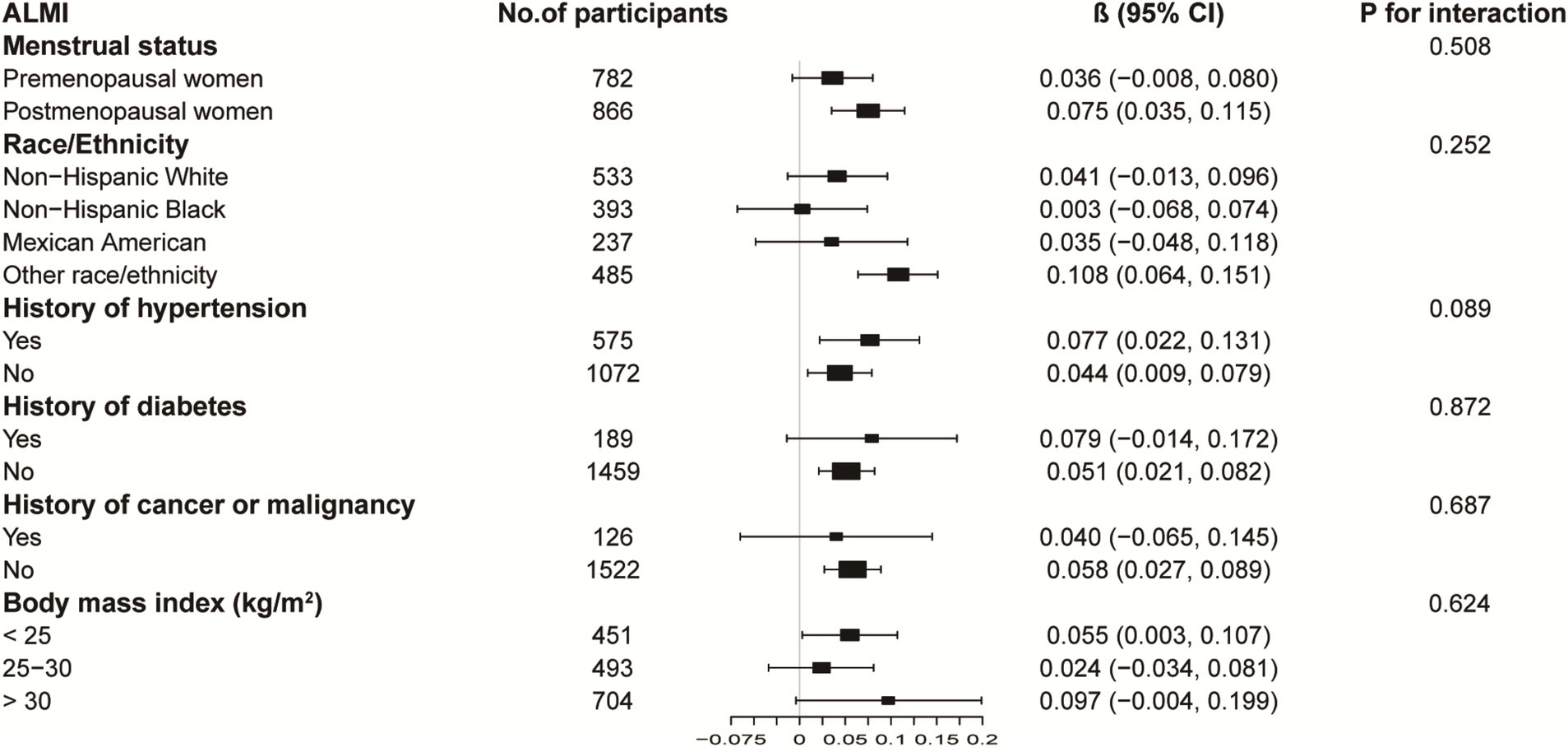
Figure 4. Subgroup analysis of the association between LnBML and ALMI (g/m2). Age, race, educational level, menstrual status, history of hypertension, history of diabetes, history of cancer or malignancy, body mass index, moderate recreational activities, total protein, blood urea nitrogen, serum uric acid, and serum calcium were adjusted. In the subgroup analysis, the model is not adjusted for the stratification variable itself. BML, blood mercury levels; ALMI, appendicular lean mass index.
Figure 5 further confirms these menstrual status-stratified associations between LnBML and both lumbar BMD and ALMI through smooth curve fittings and generalized additive models.
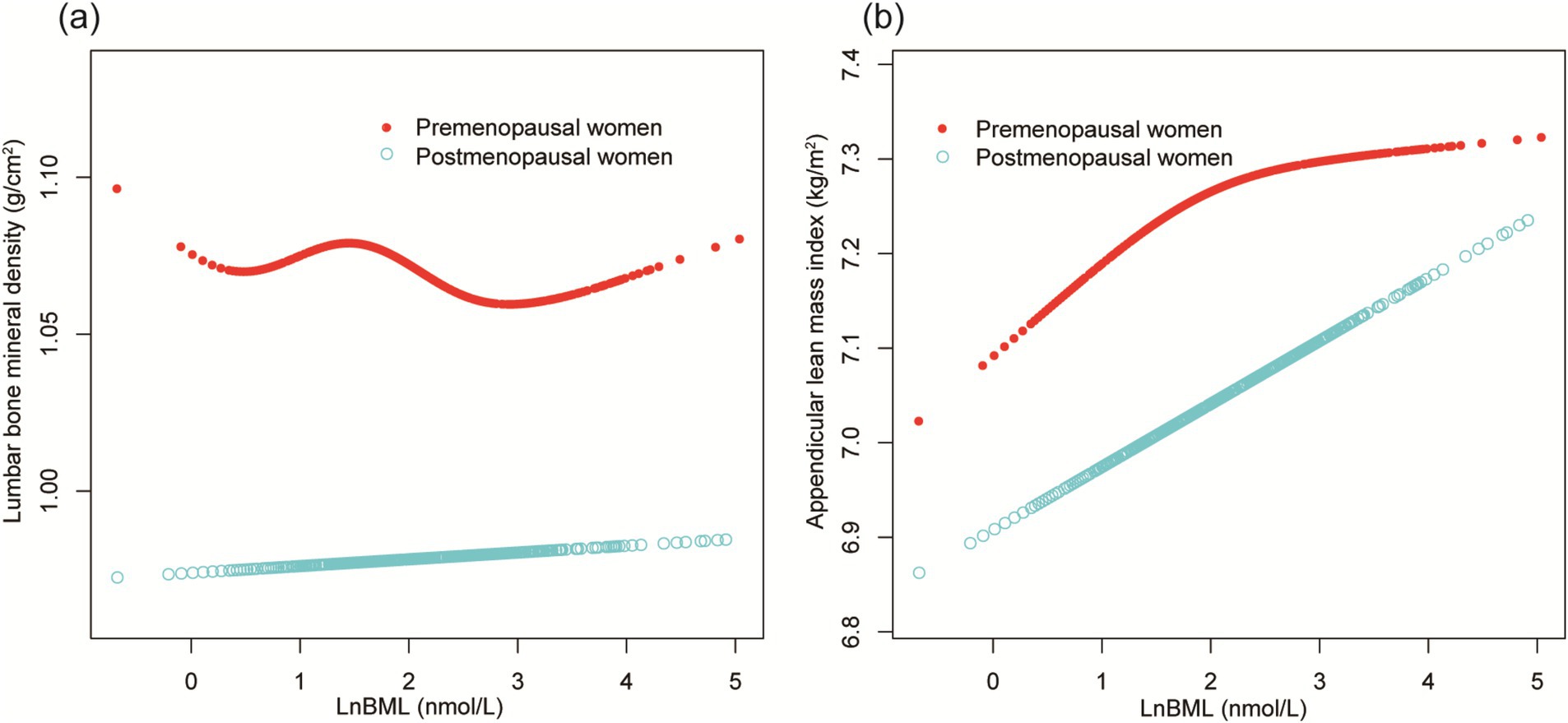
Figure 5. The association of LnBML with lumbar BMD (g/cm2) and ALMI (g/m2), stratified by menstrual status. (A) The association between LnBML and lumbar BMD. (B) The association between LnBML and ALMI. Age, race, educational level, history of hypertension, history of diabetes, history of cancer or malignancy, body mass index, moderate recreational activities, total protein, blood urea nitrogen, serum uric acid, and serum calcium were adjusted. BML, blood mercury levels; BMD, bone mineral density; ALMI, appendicular lean mass index.
Discussion
Our findings revealed complex, non-linear relationships between BML, lumbar BMD, and ALMI in middle-aged women, with notable differences based on menopausal status. The significant interaction between BML and menopausal status for BMD emerged as a key finding, demonstrating that the impact of environmental mercury exposure on musculoskeletal health fundamentally differs across the menopausal transition.
Evidence suggests that there is a complex relationship between mercury exposure and osteoporosis. A groundbreaking study in Korea (15) revealed a counterintuitive protective effect of elevated BML against osteoporosis in postmenopausal women, suggesting a nuanced interplay between mercury exposure and bone health. Contrastingly, research in Spain (16) found no significant correlations between dietary mercury intake and bone health parameters in premenopausal women, highlighting the potential importance of exposure route and physiological state. Our findings align with and extend these observations, revealing a striking interaction between BML and menopausal status that suggests a fundamental shift in mercury-bone interactions across the menopausal transition.
Further complicating this picture, a recent analysis of NHANES data (17) demonstrated a negative association between BML and total BMD in female adolescents, underscoring the age-dependent nature of mercury’s impact on bone metabolism. Our findings align with and extend these observations, revealing a striking interaction between BML and menopausal status. We observed diametrically opposed correlations in premenopausal and postmenopausal women, a discovery that not only corroborates previous studies but also suggests a fundamental shift in mercury-bone interactions across the menopausal transition.
The mechanistic underpinnings of mercury’s effects on bone metabolism remain elusive and controversial. Short-term mercury exposure studies have yielded conflicting results: one investigation (18) reported enhanced osteoclast activity and increased blood calcium levels in fish scales, while a subsequent experiment (19) observed reduced osteoclast activity and potential osteoblast protection. These contradictory findings underscore the complexity of mercury’s biological effects and the need for further research to elucidate the precise molecular pathways involved.
The well-established link between estrogen deficiency and increased osteoporosis risk (20, 21) provides a potential framework for understanding our observations. Intriguingly, previous research has demonstrated that mercuric chloride can exert estrogen-like effects on osteoblast activity (22). This phenomenon may offer a plausible explanation for the differential effects of BML on BMD observed in our menstrual status-stratified analysis.
The relationship between mercury exposure and its effects on lean mass and sarcopenia remains largely unexplored, representing a significant gap in our understanding of environmental toxicants and musculoskeletal health. While direct evidence is limited, the established similarities in pathophysiology and risk factors between osteoporosis and sarcopenia (23–25) provide a compelling rationale for investigating potential associations between chronic mercury exposure and muscle-related outcomes.
Our study presents novel findings that contribute to this emerging field of inquiry. Initially, our unadjusted models (Models 1 and 2) aligned with previous research from the Korea National Health and Nutritional Examination Surveys, which reported an increased prevalence of sarcopenia in older adult populations with elevated BML (26). However, upon adjusting for BMI in Models 3 and 4, we observed a striking reversal in this relationship. This unexpected shift underscores the complex interplay between mercury exposure and muscle mass, highlighting the critical importance of considering confounding factors in environmental health research. Future studies should aim to elucidate the underlying biological mechanisms, explore dose–response relationships, and investigate potential interactions with other environmental toxicants and lifestyle factors.
Our study, while illuminating the intricate relationships between BML, BMD, and ALMI in middle-aged women, is constrained by several methodological limitations. First, the cross-sectional nature of our NHANES-based analysis precludes causal inferences, necessitating longitudinal investigations to establish temporal relationships. Second, we relied solely on total blood mercury measurements without detailed information about exposure sources or mercury species. Future studies would benefit from collecting comprehensive exposure data, including dietary patterns, occupational history, and environmental exposure assessments, to better understand the relationship between specific mercury exposure pathways and skeletal health outcomes. Third, despite rigorous covariate adjustment, residual confounding from unmeasured variables such as occupational exposures, genetic polymorphisms, and hormonal factors cannot be ruled out. Fourth, the research was confined to middle-aged women aged 40–59 years, which may limit the generalizability of our findings. Future studies should explore mercury’s effects across broader age ranges and include male populations to provide a more comprehensive understanding of environmental mercury exposure’s impact on skeletal health. Lastly, while we identify significant associations between BML and musculoskeletal parameters, the underlying biological mechanisms remain elusive. The observed differential effects based on menopausal status underscore the necessity for mechanistic studies to elucidate the molecular pathways mediating mercury’s impact on skeletal health.
Conclusion
Our study unveils complex, menopause-dependent relationships between BML and skeletal health parameters in middle-aged women. The striking dichotomy in BML-BMD correlations between pre- and post-menopausal women highlights the critical role of menopausal status in modulating environmental mercury’s effects on bone health. These findings emphasize the need for targeted interventions and further research to understand the underlying mechanisms of these relationships across the menopausal transition.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
FX: Data curation, Writing – original draft. YW: Data curation, Writing – original draft. JF: Formal analysis, Writing – original draft. ZZ: Conceptualization, Data curation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors appreciate the time and effort given by participants during the data collection phase of the NHANES project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Crespo-Lopez, ME, Barthelemy, JL, Lopes-Araújo, A, Santos-Sacramento, L, Leal-Nazaré, CG, Soares-Silva, I, et al. Revisiting genetic influence on mercury exposure and intoxication in humans: a scoping review. Toxics. (2023) 11:967. doi: 10.3390/toxics11120967
2. Clarkson, TW. The toxicology of mercury. Crit Rev Clin Lab Sci. (1997) 34:369–403. doi: 10.3109/10408369708998098
3. Lv, D, Wu, Q, Ouyang, D, Wen, M, Zhang, G, Wang, S, et al. Differentiated emission control strategy based on comprehensive evaluation of multi-media pollution: case of mercury emission control. J Environ Sci. (2023) 123:222–34. doi: 10.1016/j.jes.2022.03.028
4. Crespo-Lopez, ME, Lopes-Araújo, A, Basta, PC, Soares-Silva, I, de Souza, CBA, Leal-Nazaré, CG, et al. Environmental pollution challenges public health surveillance: the case of mercury exposure and intoxication in Brazil. Lancet Reg Health Am. (2024) 39:100880. doi: 10.1016/j.lana.2024.100880
5. Basu, N, Bastiansz, A, Dórea, JG, Fujimura, M, Horvat, M, Shroff, E, et al. Our evolved understanding of the human health risks of mercury. Ambio. (2023) 52:877–96. doi: 10.1007/s13280-023-01831-6
6. Pinheiro, MB, and Naganathan, V. Appraisal of clinical practice guideline: clinical practice guideline for management of osteoporosis and fracture prevention in Canada: 2023 update. J Physiother. (2024) 70:241. doi: 10.1016/j.jphys.2024.05.002
7. Ip, TP, Lee, CA, Lui, TD, Wong, RMY, Cheung, C, Chiu, K, et al. 2024 OSHK guideline for clinical Management of Postmenopausal Osteoporosis in Hong Kong. Hong Kong Med J. (2024) 30:1–44.
8. Divaris, E, Anagnostis, P, Gkekas, NK, Kouidi, E, and Goulis, DG. Early menopause and premature ovarian insufficiency may increase the risk of sarcopenia: a systematic review and meta-analysis. Maturitas. (2023) 175:107782. doi: 10.1016/j.maturitas.2023.05.006
9. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
10. Gandham, A, Gregori, G, Johansson, L, Johansson, H, Harvey, NC, Vandenput, L, et al. Sarcopenia definitions and their association with fracture risk in older Swedish women. J Bone Miner Res. (2024) 39:453–61. doi: 10.1093/jbmr/zjae026
11. Li, H, Li, G, Yi, M, Zhou, J, Deng, Y, Huang, Y, et al. Sex-specific associations of urinary mixed-metal concentrations with femoral bone mineral density among older people: an NHANES (2017-2020) analysis. Front Public Health. (2024) 12:1363362. doi: 10.3389/fpubh.2024.1363362
12. Snega Priya, P, Pratiksha Nandhini, P, and Arockiaraj, J. A comprehensive review on environmental pollutants and osteoporosis: insights into molecular pathways. Environ Res. (2023) 237:117103. doi: 10.1016/j.envres.2023.117103
13. Huang, Q, Wan, J, Nan, W, Li, S, He, B, and Peng, Z. Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in US adults from NHANES 2011-2018. J Hazard Mater. (2024) 464:133005. doi: 10.1016/j.jhazmat.2023.133005
14. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
15. Cho, GJ, Park, HT, Shin, JH, Hur, JY, Kim, SH, Lee, KW, et al. The relationship between blood mercury level and osteoporosis in postmenopausal women. Menopause. (2012) 19:576–81. doi: 10.1097/gme.0b013e3182377294
16. Lavado-García, JM, Puerto-Parejo, LM, Roncero-Martín, R, Moran, J, Pedrera-Zamorano, J, Aliaga, I, et al. Dietary intake of cadmium, Lead and mercury and its association with bone health in healthy premenopausal women. Int J Environ Res Public Health. (2017) 14:1437. doi: 10.3390/ijerph14121437
17. Xu, K, Gao, B, Liu, T, Li, J, Xiang, Y, Fu, Y, et al. Association of blood mercury levels with bone mineral density in adolescents aged 12-19. Environ Sci Pollut Res Int. (2023) 30:46933–9. doi: 10.1007/s11356-023-25701-6
18. Suzuki, N, Yamamoto, M, Watanabe, K, Kambegawa, A, and Hattori, A. Both mercury and cadmium directly influence calcium homeostasis resulting from the suppression of scale bone cells: the scale is a good model for the evaluation of heavy metals in bone metabolism. J Bone Miner Metab. (2004) 22:439–46. doi: 10.1007/s00774-004-0505-3
19. Yachiguchi, K, Sekiguchi, T, Nakano, M, Hattori, A, Yamamoto, M, Kitamura, KI, et al. Effects of inorganic mercury and methylmercury on osteoclasts and osteoblasts in the scales of the marine teleost as a model system of bone. Zool Sci. (2014) 31:330–7. doi: 10.2108/zs130265
20. Yao, Y, Cai, X, Chen, Y, Zhang, M, and Zheng, C. Estrogen deficiency-mediated osteoimmunity in postmenopausal osteoporosis. Med Res Rev. (2024). doi: 10.1002/med.22081
21. Cheng, CH, Chen, LR, and Chen, KH. Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int J Mol Sci. (2022) 23:1376. doi: 10.3390/ijms23031376
22. Jin, GB, Inoue, S, Urano, T, Cho, S, Ouchi, Y, and Cyong, JC. Induction of anti-metallothionein antibody and mercury treatment decreases bone mineral density in mice. Toxicol Appl Pharmacol. (2002) 185:98–110. doi: 10.1006/taap.2002.9531
23. Olmos Martínez, JM, Hernández Martínez, P, and González, MJ. Frailty, sarcopenia and osteoporosis. Med Clin. (2024) 163:e17–23. doi: 10.1016/j.medcli.2024.03.004
24. Lu, L, and Tian, L. Postmenopausal osteoporosis coexisting with sarcopenia: the role and mechanisms of estrogen. J Endocrinol. (2023) 259:e230116. doi: 10.1530/JOE-23-0116
25. Clynes, MA, Gregson, CL, Bruyère, O, Cooper, C, and Dennison, EM. Osteosarcopenia: where osteoporosis and sarcopenia collide. Rheumatology. (2021) 60:529–37. doi: 10.1093/rheumatology/keaa755
Keywords: blood mercury levels, bone mineral density, appendicular lean mass index, menopausal status, environmental exposure
Citation: Xu F, Wang Y, Fang J and Zhu Z (2025) Menopause-dependent correlations of blood mercury levels with bone mineral density and appendicular lean mass index in middle-aged women. Front. Public Health. 12:1501162. doi: 10.3389/fpubh.2024.1501162
Edited by:
Mohiuddin Md. Taimur Khan, Washington State University Tri-Cities, United StatesReviewed by:
Keith Dana Thomsen, Washington River Protection Solutions, United StatesNan Wang, Jiangnan Hospital Affiliated to Zhejiang University of Traditional Chinese Medicine, China
Copyright © 2025 Xu, Wang, Fang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongxin Zhu, b3J0aG96enhAMTYzLmNvbQ==
 Feng Xu1
Feng Xu1 Zhongxin Zhu
Zhongxin Zhu