- 1Centre of Excellence in Women and Child Health, Aga Khan University, Nairobi, Kenya
- 2Department of Obstetrics and Gynaecology, Aga Khan University, Nairobi, Kenya
- 3Department of Pathology, Aga Khan University, Nairobi, Kenya
- 4Kilifi County Department of Health and Sanitation Services, Kilifi, Kenya
- 5Department of Women and Children's Health, Kings College London, London, United Kingdom
- 6Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
- 7St. George's University of London, London, United Kingdom
A Corrigendum on
SARS-CoV-2 seroprevalence in pregnant women in Kilifi, Kenya from March 2020 to March 2022
by Koech, A., Omuse, G., Mugo, A. G., Mwaniki, I. G., Mutunga, J. M., Mukhanya, M. W., Wanje, O., Mwashigadi, G. M., Katana, G. G., Craik, R., von Dadelszen, P., Le Doare, K., Temmerman, M., periCOVID-Africa and The PRECISE Network (2023). Front. Public Health. 11:1292932. doi: 10.3389/fpubh.2023.1292932
In the published article, there was an error in Figure 4 as published. The reported antibody seropositivity rates by Wantai and QuantiVac (the maroon line) are incorrect. This error arose from incorrect configuration of cut-off values for determination of qualitative results for the test. The cutoff values configured into the analyzer for the EuroImmun QuantiVac test were for BAU/ml but the results were reported in RU/ml. The corrected Figure 4 and its caption appear below.
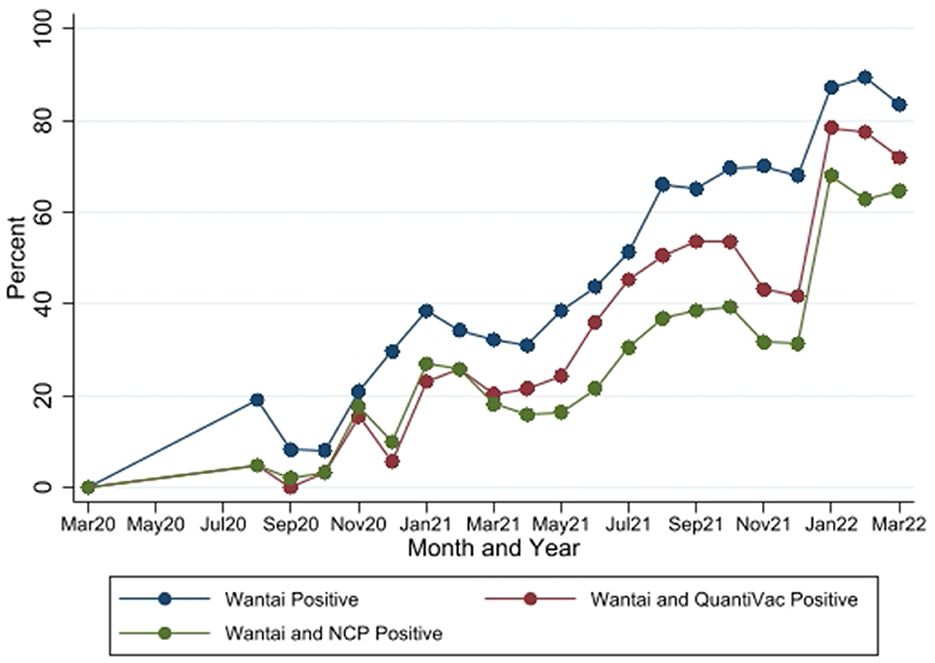
Figure 4. Seropositivity by the three SARS-CoV-2 antibody tests between March 2020 and March 2022. Wantai–SARS-CoV-2 ELISA kit (Wantai). NCP–Euroimmun anti-SARS-CoV-2 NCP ELISA test. QuantiVac–Euroimmun anti-SARS-CoV-2 QuantiVac ELISA test. Samples were tested with NCP and QuantiVac tests only if Wantai test was positive.
In the published article, there was an error. The reported antibody seropositivity for the EuroImmun QuantiVac test was incorrect. This error arose from incorrect configuration of cut-off values for determination of qualitative results for the test. The cutoff values configured into the analyzer for the EuroImmun QuantiVac test were for BAU/ml but the results were reported in RU/ml.
A correction has been made to Abstract, Results, Paragraph 3. This sentence previously stated:
“Of the Wantai test-positive samples, 59.7% [95% CI 57.06–62.34] tested positive by the Euroimmun anti-SARS-CoV-2 NCP test and 37.4% [95% CI 34.83–40.04] tested positive by the Euroimmun anti-SARS-CoV-2 QuantiVac test.”
The corrected sentence appears below:
“Of the Wantai test-positive samples, 59.7% (95% CI 57.06–62.34) tested positive by the Euroimmun anti-SARS-CoV-2 NCP test and 75.9% (95% CI 73.55–78.17) tested positive by the Euroimmun anti-SARS-CoV-2 QuantiVac test.”
In the published article, there was an error. The reported antibody seropositivity for the EuroImmun QuantiVac test was incorrect. This was reported as 37.4% (95% CI 34.8–40.04%). The new corrected result is 75.9% (95% CI 73.55–78.17%).
A correction has been made to Results, Paragraph 2. This sentence previously stated:
“Of all samples analyzed, 1,358 tested positive by Wantai [overall seropositivity of 54.4% (95% CI 52.45–56.39)], of which 811 [59.7%, (95% CI 57.06–62.34)] tested positive by the Euroimmun anti-SARS-CoV-2 NCP ELISA and 508 [37.4%, (95% CI 34.83–40.04)] tested positive by the Euroimmun anti-SARS-CoV-2 QuantiVac ELISA tests respectively; 964 samples [71.0%, (95% CI 68.49–73.39)] were positive by either of the 2 Euroimmun tests.”
The corrected sentence appears below:
“Of all samples analyzed, 1,358 tested positive by Wantai [overall seropositivity of 54.4% (95% CI 52.45–56.39)], of which 811 [59.7%, (95% CI 57.06–62.34)] tested positive by the Euroimmun anti-SARS-CoV-2 NCP ELISA and 1,031 [75.9%, (95% CI 73.55–78.17)] tested positive by the Euroimmun anti-SARS-CoV-2 QuantiVac ELISA tests respectively; 964 samples [71.0%, (95% CI 68.49–73.39)] were positive by either of the 2 Euroimmun tests.”
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: SARS-CoV-2, COVID-19, seroprevalence, pregnancy, Kenya, antibodies
Citation: Koech A, Omuse G, Mugo AG, Mwaniki IG, Mutunga JM, Mukhanya MW, Wanje O, Mwashigadi GM, Katana GG, Craik R, von Dadelszen P, Le Doare K, Temmerman M, periCOVID-Africa and The PRECISE Network (2024) Corrigendum: SARS-CoV-2 seroprevalence in pregnant women in Kilifi, Kenya from March 2020 to March 2022. Front. Public Health 12:1500467. doi: 10.3389/fpubh.2024.1500467
Received: 23 September 2024; Accepted: 27 September 2024;
Published: 14 October 2024.
Edited and reviewed by: Constantinos Tsioutis, European University Cyprus, Cyprus
Copyright © 2024 Koech, Omuse, Mugo, Mwaniki, Mutunga, Mukhanya, Wanje, Mwashigadi, Katana, Craik, von Dadelszen, Le Doare, Temmerman, periCOVID-Africa and The PRECISE Network. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela Koech, YW5nZWxhLmtvZWNoQGFrdS5lZHU=
†These authors have contributed equally to this work and share first authorship
 Angela Koech
Angela Koech Geoffrey Omuse
Geoffrey Omuse Alex G. Mugo
Alex G. Mugo Isaac G. Mwaniki
Isaac G. Mwaniki Joseph M. Mutunga
Joseph M. Mutunga Moses W. Mukhanya
Moses W. Mukhanya Onesmus Wanje
Onesmus Wanje Grace M. Mwashigadi
Grace M. Mwashigadi Geoffrey G. Katana
Geoffrey G. Katana Rachel Craik
Rachel Craik Peter von Dadelszen
Peter von Dadelszen Kirsty Le Doare
Kirsty Le Doare Marleen Temmerman
Marleen Temmerman periCOVID-Africa and The PRECISE Network
periCOVID-Africa and The PRECISE Network