- 1The First People’s Hospital of Lianyungang, Lianyungang, China
- 2The Affiliated Lianyungang Hospital of Xuzhou Medical University and The First People’s Hospital of Lianyungang, Lianyungang, China
- 3The First Affiliated Hospital of Kangda College of Nanjing Medical University and The First People’s Hospital of Lianyungang, Lianyungang, China
- 4Lianyungang Clinical College of Nanjing Medical University and The First People’s Hospital of Lianyungang, Lianyungang, China
Adenoid hypertrophy (AH) is characterized by pathological hyperplasia of the nasopharyngeal tonsils, a component of Waldryer’s ring, which represents the first immune defense of the upper respiratory tract. The pathogenic factors contributing to AH remain to be comprehensively investigated to date. Although some studies suggest that environmental exposure to smoke and allergens, respiratory tract infections, and hormonal influences likely contribute to the development of AH, further research is necessary for fully elucidating the effects of these factors on the onset and progression of AH. AH is the most common cause of airway obstruction in the pediatric population, with a prevalence rate of 49.7%, and is frequently accompanied by various comorbidities. These patients often present with distinctive dental characteristics, including increased overjet, posterior crossbite, a high palatal plane, narrow dental arches, and facial features characterized by disproportionate alterations in facial height, commonly referred to as “adenoid facies.” Individuals with adenoid facies frequently display abnormal breathing patterns, especially mouth breathing. The present review summarizes the findings of research articles sourced from PubMed, IEEE, and Web of Science over the last 20 years up to September 2024. Several high-quality studies screened using the PICOPS framework reported that perioral muscle dysfunction, dental and skeletal malocclusions, and upper airway obstruction caused by AH are interdependent issues and mutually exacerbate one another. The review summarizes the potential associations and mechanisms linking AH, mouth breathing, and the subsequent development of adenoid facies in children.
1 Introduction
Adenoid hypertrophy (AH) is a common condition in pediatric populations that is characterized by a range of respiratory symptoms, including nocturnal snoring, nasal obstruction, mouth breathing, and reduced olfactory sensitivity (1). Apart from these issues, these symptoms of AH contribute to the development of serious secondary complications, including recurrent otitis media (2), obstructive sleep apnea syndrome (3), and sinusitis (4). These complications extend beyond immediate respiratory issues, and can potentially affect normal craniofacial development, neurological functions, and overall health (5). Among these manifestations, mouth breathing is especially predominant, and its prevalence is estimated to range from 11 to 56% in children (6). Despite its high incidence, mouth breathing remains under-recognized by both patients and caregivers, which can potentially delay the administration of appropriate interventions.
Emerging evidence highlights that mouth breathing is a key contributor to the atypical craniofacial development observed in children with AH (7) (8). Although it is traditionally regarded that craniofacial morphology is primarily determined by genetic inheritance, contemporary studies indicate that environmental factors, including oral habits (9), such as pacifier sucking (10), atypical swallowing patterns (11), finger sucking (12), and mouth breathing (7), play a significant role in the etiology of malocclusion (13, 14). Notably, children with AH frequently exhibit distinct dental and facial characteristics, including increased dental overjet, posterior crossbite, high palatal planes, narrow maxillary arches, and adenoid facies, characterized by disproportionate alterations in facial height (15). Malocclusion is especially prevalent in this demographic group, with Class II (16, 17) and Class III (15, 18) malocclusions being more frequently documented.
This review synthesizes the findings of current research on the bidirectional and potentially self-perpetuating relationship between mouth breathing and malocclusion in patients with AH. By integrating the observations of recent studies, the review elucidates the mechanisms by which these conditions reinforce each other in a “vicious cycle” that exacerbates craniofacial anomalies and dental misalignments. The study further aims to provide orthodontists and pediatric dentists with deeper theoretical insights into the mechanisms underlying the development of adenoid facies. The review investigates the factors contributing to this distinct craniofacial presentation to enhance diagnostic precision and ensure the implementation of comprehensive, multidisciplinary, and sequential treatment protocols in clinical practice.
2 Methodology
A comprehensive and systematic review was conducted using a structured search strategy across several databases, including PubMed, IEEE, and Web of Science. Database search was conducted using specific keywords and Medical Subject Headings (MeSH) terms, including “adenoid facies,” “mouth breathing,” “adenoid hypertrophy,” “malocclusion,” and “craniofacial development.”
Inclusion criteria were defined for prioritizing the peer-reviewed studies that examined the relationships among AH, mouth breathing, and malocclusion in pediatric populations. The articles published within the last 20 years were prioritized for capturing the recent advancements in the field. The exclusion criteria encompassed articles not available in English as well as case reports.
The articles that met the selection criteria were reviewed for relevance and quality using the PICOPS framework, and the data were extracted and analyzed according to established guidelines. This approach enables the rigorous synthesis of current evidence, promotes transparency, and ensures the reproducibility of findings for future researchers.
3 Craniofacial anatomy and risk factors of AH
The adenoids, palatine tonsils, and lingual tonsils form Waldeyer’s ring (19), a component of the lymphoid tissue associated with the upper respiratory system, and collectively regulate immune function in the upper respiratory tract (20). Adenoids are encompassed within a specialized lymphoepithelial structure (21) comprising epithelial cells, lymphocytes, macrophages, and dendritic cells. The lymphoepithelium causes the adenoids to be covered in a thick secretion that attracts and binds microorganisms to confer local immunity (Figure 1) (22). The internal structure of adenoids consists of a follicular germinal center and an interfollicular region, which is formed by the aggregation of T lymphocytes. Adenoids secrete large quantities of secretory immunoglobulin A (IgA) antibody that binds to bacteria and inhibits bacterial colonization in the mucosal epithelium (23). Additionally, the effector T lymphocytes within adenoids can generate effective immune responses by secreting cytokines, chemokines, and bactericidal substances. It is worth noting that adenoids are relatively small in infancy, during which their functions are not apparent. They reach their maximum size at 6–10 years of age, at which point they may occupy a substantial portion of the oral-nasal-pharyngeal space in the retro-palatine region, and their immune functions are most pronounced during this period. However, adenoids shrink in size by puberty and their immune functions correspondingly diminish (24).
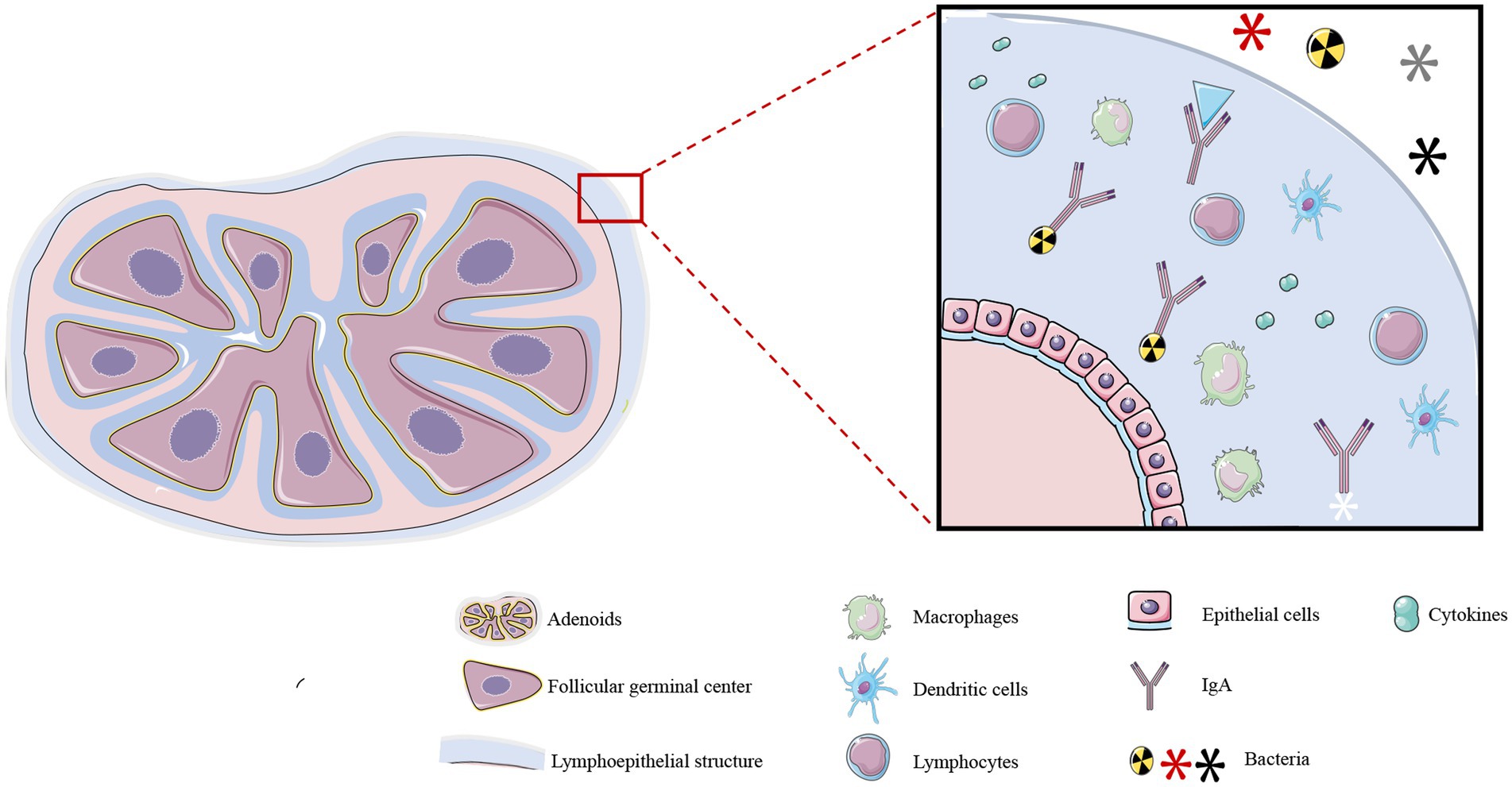
Figure 1. Structure and function of adenoids. Adenoids consist of an inner T lymphocyte-rich follicular germinal center and an interfollicular region, and are covered by a lymphoepithelium. This specialized structure consists of a large number of immune cells, including macrophages, lymphocytes, dendritic cells, and other cell types, and it also secretes cytokines, IgA, and other substances. These structures confer local immunity against microorganisms in the upper respiratory tract. *The illustration was prepared by integrating the findings of existing research. The differences in interpretation may arise due to variations among the conclusions drawn from different research studies.
AH is the most common obstructive upper airway disorder in children and adolescents worldwide, with a prevalence of 49.7% (25), and respiratory tract infections are the major cause of AH. Human adenovirus is the most frequently detected virus in AH, with a detection rate of 47–71% (26). Other viruses with high detection rates include human enterovirus, rhinovirus, bocavirus, respiratory syncytial virus, and others (27). It has been reported that the smoke produced by the burning of tobacco can increase the risk of upper respiratory tract infections, chronic sinusitis, and chronic otitis media in children (28). A previous study demonstrated that passive exposure to tobacco smoke can significantly increase the production of immunoglobulins by adenoid lymphocytes (29). Allergy and sensitivity to various allergens represent another important risk factor for AH (30). The immune system begins to develop between the ages of 1 and 4 years in children, which consequently increases sensitivity to various antigens during this period, and leads to the successive onset of various allergic diseases, including atopic dermatitis, asthma, and allergic rhinitis (31). In a follow-up study in 2015 involving 1,322 children treated for allergies, researchers conducted skin prick tests for the same allergens on all participants, and observed that children with allergic diseases had a higher frequency of AH than control children without allergic diseases (32). In addition, the hypertrophic surface of adenoids is covered by a biofilm that is rich in microorganisms, environmental pollutants, and food antigens, which further increases the risk of asthma and allergic rhinitis (33). The study by Shin et al. recruited 18 atopic subjects sensitized to more than one common allergen and 22 non-atopic subjects who had undergone adenoidectomy. Subsequent immunoassays conducted using adenoid tissue homogenates revealed that the levels of total IgE and allergen-specific antibodies in the adenoid tissues of children with allergic diseases were significantly higher than those of healthy children without allergic diseases (34). It has been demonstrated that local diseases of the upper respiratory tract, including chronic sinusitis, exudative otitis media, and AH, mutually exacerbate one other. Mucociliary clearance is the most important airway defense mechanism, and infections or inflammation of the adenoids can cause localized epithelial metaplasia and loss of ciliary function in the upper respiratory tract, leading to nasal or middle ear diseases (35). Additionally, chronic infections and inflammation of the respiratory epithelium resulting from nasal or middle ear diseases can induce AH and enhance the secretion of inflammatory mediators (22).
4 AH-related mouth breathing promotes dysfunction of perioral muscles
The outer surface of the tooth rests against the labial and buccinator muscles, while the inner surface remains adjacent to the tongue. The opposing forces exerted by these tissues are the primary determinants influencing dental positional stability (36). However, AH-related mouth breathing can lead to atypical tongue positioning, weakening of the orbicularis oris muscle, and overactivity of the buccinator, digastric, mental, and masticatory muscles, leading to malocclusion.
4.1 Atypical tongue positioning
The tongue plays an important role in oral and maxillofacial development, which determines the formation of the dental arch and occlusal relationships (37). The correct positioning of the tongue can be observed in children who breathe through their noses and have proper occlusion (38). The ideal functional position of the tongue is where the lips are lightly closed, the teeth are almost touching, and the tongue is in contact with the palate (39, 40). At rest, the slight force exerted by the tongue is sufficient to move the teeth because the force persists for a prolonged duration (41). There is no absolute balance between the forces exerted by the extraoral and intraoral muscles, which play an important role in the normal positioning of the tongue (42). A cross-sectional study in 2007 compared the effects of mouth opening and closing on lateral cephalometric measurements by fiberoptic nasopharyngoscopy, and the findings revealed that the soft palate moves backward and touches the back wall of the pharynx when breathing through the mouth, thus effectively closing off the nasal cavity. However, during nasal breathing, the base of the tongue moves downwards to reduce the distance between the tongue and the back wall of the pharynx. However, it has been observed that the pressure exerted by the tongue in the pharyngeal region is significantly higher in the supine position than in the upright position when mouth breathing is practiced (Figure 2) (43). Additionally, the position of the tongue is also affected by the posture during mouth breathing. When breathing through the nose, the position and pressure exerted by the tongue remain stable and do not affect breathing irrespective of whether an individual is in an upright or supine position. However, when mouth breathing is performed, the pressure exerted by the tongue is significantly higher in the supine position than in the upright position (44). This is attributed to the weakening of the genioglossus muscle due to mouth breathing (45), which impairs its ability to prevent the tongue from falling back under the action of gravity. This consequently results in the posterior displacement of the tongue, which increases the pressure exerted by the tongue in the pharyngeal region.
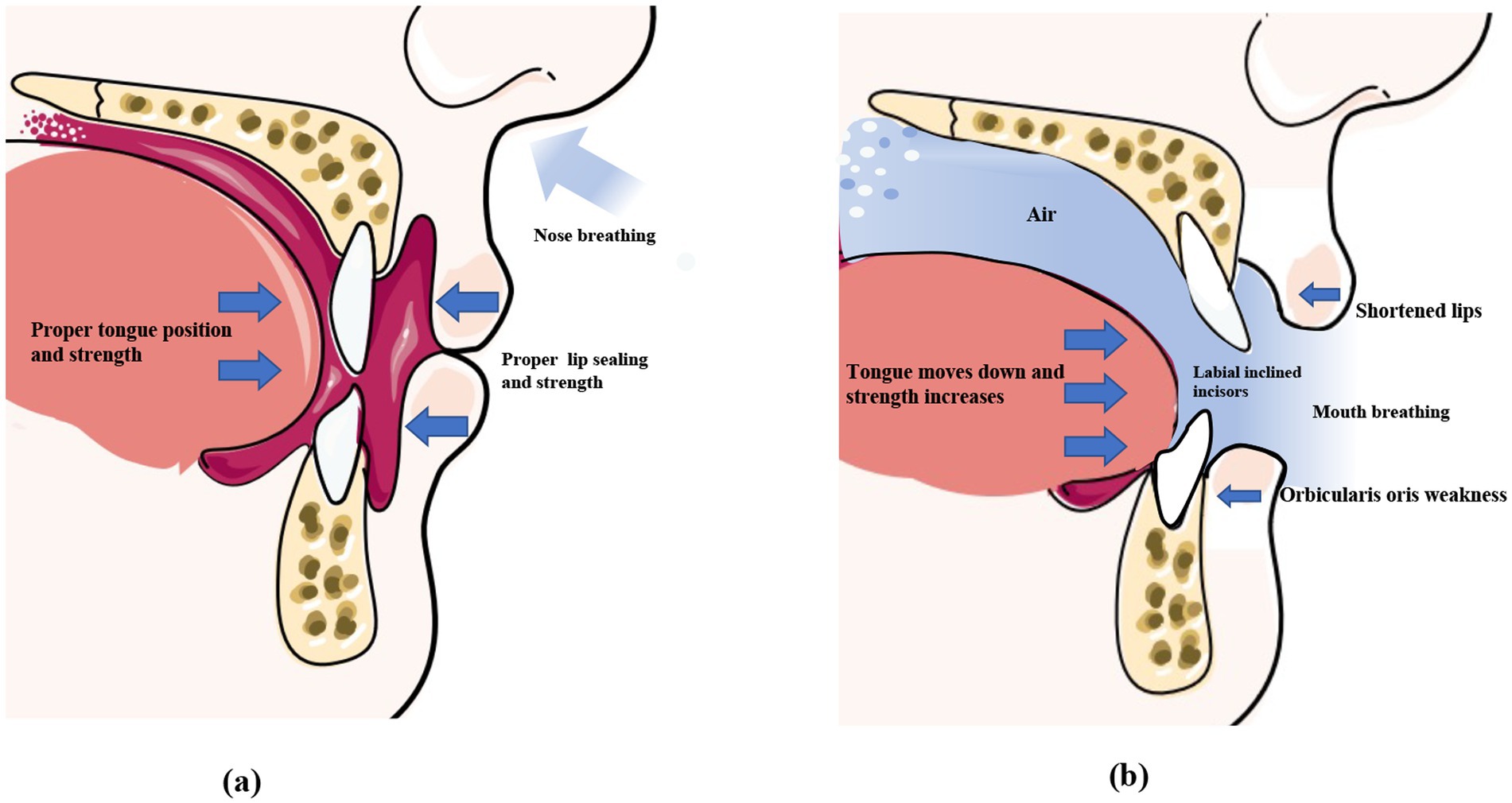
Figure 2. Normal perioral muscle function and the imbalance caused by mouth breathing. Under normal circumstances (left), the lips remain naturally closed at rest, the tongue fills the entire mouth, the forces exerted by the lip and tongue muscles are balanced, and the front teeth remain upright. During mouth breathing (right), the lip seal is insufficient, and the upper lip curls and shortens. The tongue tends to sink, disrupting the balance of perioral muscle strength, which causes the incisors to tilt. *This original illustration depicts the commonly observed clinical patterns. However, individual variations may occur depending on specific cases.
4.2 Weakening of the orbicularis oris muscle
Lip incompetence is a common clinical sign observed in individuals who practice mouth breathing, and affects approximately 30% of children aged 3–12 years (46, 47). Previous studies have demonstrated that the force exerted by the lips greatly affects dental alignment (48, 49). Therefore, mouth breathing due to AH leads to the weakening of the orbicularis oris muscle, which results in an imbalance in the perioral muscles (50). Consequently, patients who practice mouth breathing tend to have shorter, curled, and thick lips (Figure 2) (51). Wagaiyu et al. performed a cross-sectional study involving 201 schoolchildren aged 11–14 years and observed that the mouth breathers tended to have more curled upper lips. Additionally, the area of the upper lip that shortens and separates from the lower lip is reduced, which potentially exposes the surface of the front teeth and thereby increases the risk of gingivitis (52). Additionally, incompetent lip seals, dry lips, and halitosis are some of the common clinical manifestations in individuals who practice mouth breathing (53). By performing electromyographic (EMG) and cephalometric analyses of 20 adolescents, a previous study revealed that mouth breathing weakens the orbicularis oris muscle, especially the inferior orbicularis oris muscle (Figure 2) (54). This manifests as an insufficiency in the strength of the orbicularis oris muscle when pronouncing certain syllables, such as the phonemes /b/ and /m/, and requires higher EMG activity (55). Additionally, individuals who practice mouth breathing are more prone to EMG fatigue during lip muscle training (56).
4.3 Overactivity of the buccinator, digastric, mental, and masticatory muscles
The buccinator muscles, located in the lateral walls of the oral cavity, are responsible for compressing the alveolar bone and increasing the thickness of the cheeks (57). Dysfunction of buccinator muscles can lead to variations in the shape and size of the mandible during growth and development (58). The pressure exerted by the buccal muscles on the alveolar bone increases when the mouth seal is compromised (59), which is likely responsible for the narrow dental arches frequently observed in children who practice mouth breathing (Figure 3). An observational, prospective, multi-center study involving 81 children with a Class II division 1 malocclusion and presenting with one or several functional disorders revealed that the use of muscle function appliances effectively reduces the abnormal tension in the buccal muscles, which can restore the roundness of the dental arches (60).
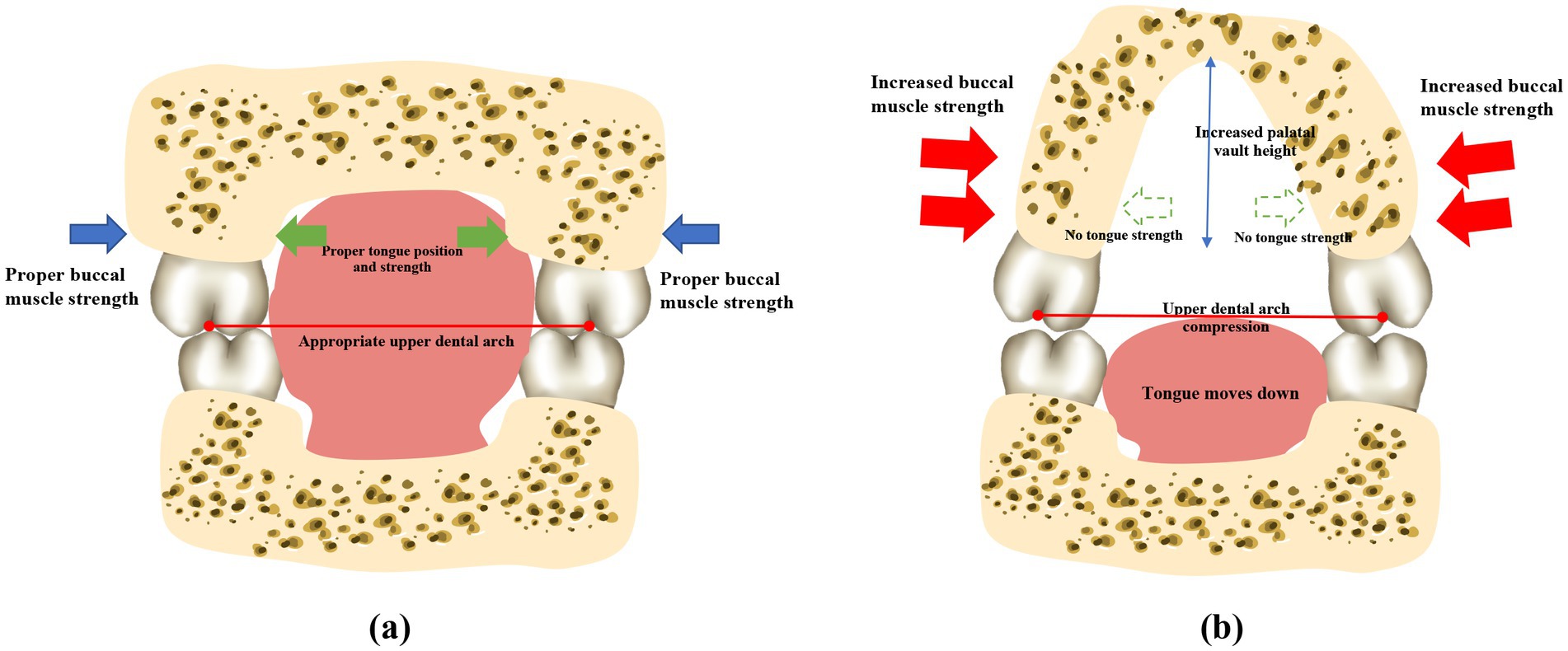
Figure 3. Mouth breathing causes perioral muscle dysfunction and atypical maxillary development. Patients who practice normal nasal respiration generally have balanced perioral muscles, a flat upper palate, and the widths of the upper and lower dental arches are properly coordinated. However, mouth breathing leads to perioral muscle dysfunction, which causes the tongue to sink. The maxilla and the dental arches consequently lose the support provided by the tongue, and the lip strength is weakened due to insufficient lip sealing. The strength of the buccinator muscles is relatively enhanced. The upper dental arch is affected by the imbalanced muscle strength, resulting in a narrow and protruding dental arch, and a higher and sharper palatal fornix. *Note: This original illustration was prepared by integrating the observations of several studies. The variations in the findings may arise due to differences across the various research methodologies and conclusions obtained.
Another study reported that there is a significant relationship between the activity of the masticatory muscles and facial growth patterns (61). Children with Class II division 1 malocclusion often exhibit abnormal overactivity of the mental, anterior temporal, and masseter muscles (62, 63). By blocking the nasal passages of rhesus monkeys using silicone plugs, a previous study demonstrated that mouth breathing is accompanied by rhythmic hyperactivity of the maxillofacial muscles, including the dorsal tongue, digastric, and levator lip muscles (64). The activation of the masseter and submental muscles can be observed during sleep in mouth breathers, and is possibly attributed to the spontaneous stretching of the muscles to expand the upper airway, which is restricted by mouth breathing. Similarly, the use of oral shields has been shown to reduce the activity of the mentalis, buccinator, and digastric muscles (65).
5 AH-related mouth breathing promotes malocclusions
Proper dental alignment and occlusal relationships are essential for oral function, maxillofacial development, and facial esthetics (66, 67). However, the frequency of malocclusions and various occlusal anomalies is significantly higher in children with AH and tonsillar hypertrophy who practice mouth breathing (16, 68, 69).
5.1 Class I malocclusion
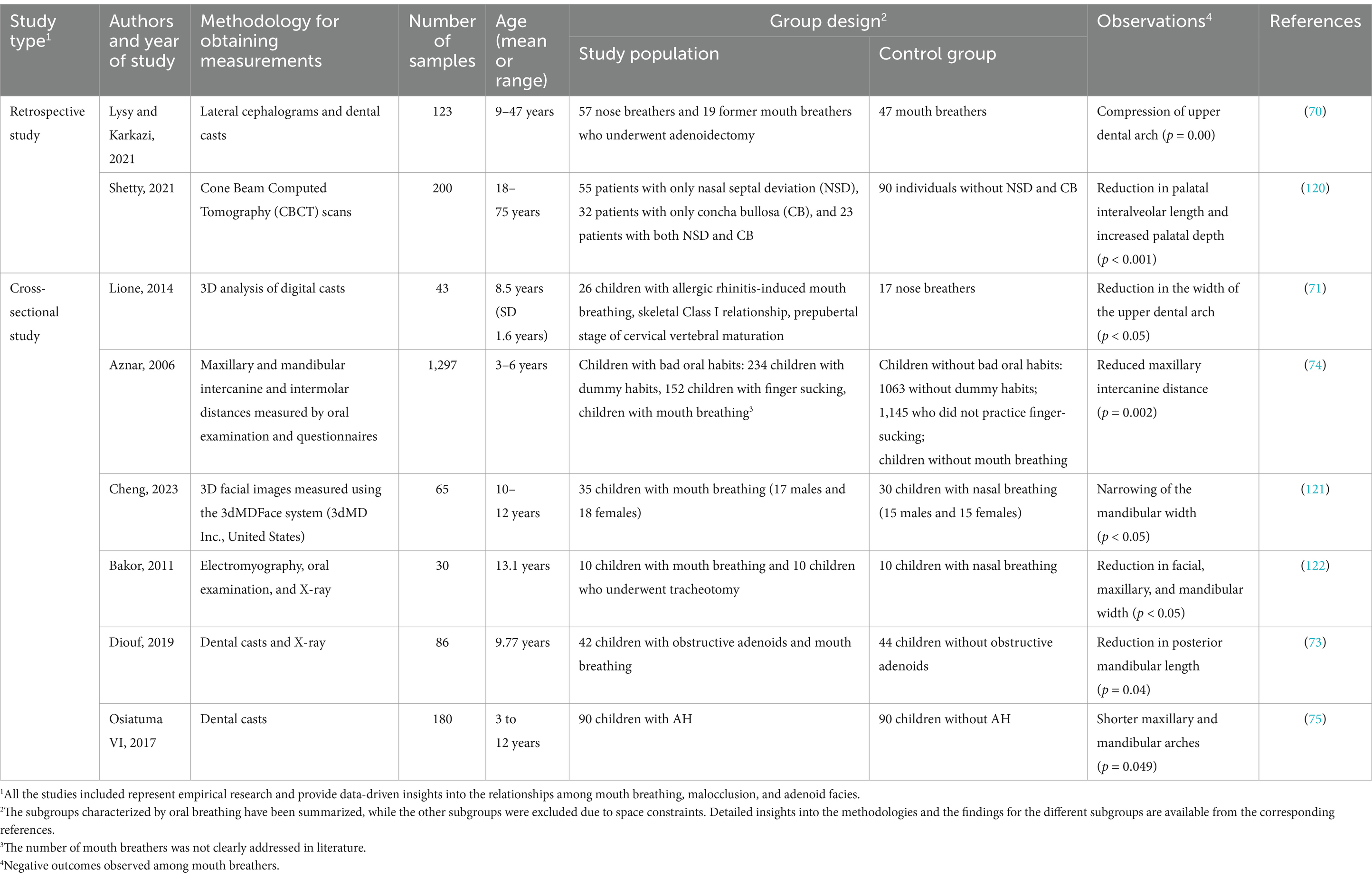
Crowded dentition is an obvious manifestation of Class I malocclusion in children who practice mouth breathing, and is characterized by narrow dental arches (Table 1). The lateral compression of the upper dental arch is a common occlusal deformity caused by mouth breathing (70, 71), which can be observed in children with deciduous dentition at the age of 2.5 years (72). This is often accompanied by an increase in the depth of the dental arch, which results in an open bite (73). In 2017, a cross-sectional study including 90 children aged 3–12 years with AH and 90 children without AH reported that mouth breathing can also reduce the width of the lower dental arch (74). In addition to the changes in width and depth, the lengths of the upper and lower dental arches were also reduced in mouth breathers aged 3–12 years due to AH (75).
5.2 Class II malocclusion
Previous studies have demonstrated that Class II malocclusion is the most common occlusal anomaly in children who practice mouth breathing (7, 76, 77). The occlusal presentation of Class II malocclusion is primarily classified into two types, namely, increased anterior dental overjet (Class II division 1), and a deep anterior overbite (Class II division 2) (78, 79), and the former is frequently accompanied by an increased anterior dental overjet. It has been proposed that children who practice mouth breathing present with narrower dental arches and increased anterior dental overjet (Table 2) (15, 80). A previous study reported that the narrowing of the maxillary dental arch is more severe and the upper incisors are more labially inclined in mouth breathers (81).
In 2021, otolaryngologists and orthodontists conducted a cross-sectional study to evaluate 356 children with AH and tonsillar hypertrophy who practiced mouth breathing. The findings revealed that 81.4% of the mouth breathers presented with Class II malocclusion, with an increased anterior dental overjet being the most prominent feature (16). It has been reported that the long-term use of oral appliances in patients with sleep apnea due to mouth breathing can lead to objective and significant changes in dental malocclusion, including an improvement in dental overjet, independent of the subjective experiences of the patients (82).
5.3 Class III malocclusion
By analyzing the relationship between malocclusion types and respiratory factors in 72 children with and without crossbite during the early mixed dentition phase, a previous study demonstrated that Class III malocclusion is frequently accompanied by ear, nose, and throat (ENT) disorders, which are closely associated with mouth breathing (83). A comparative cephalometric analysis involving 98 children with mouth breathing and 98 children with nasal breathing reported a high prevalence of anterior crossbite and anterior open bite among the mouth breathers (Table 2) (84). Posterior crossbite, including lateral crossbite (85), is also common in children who practice mouth breathing (86, 87). Mechanistically, mouth breathing causes muscular dysfunction, which leads to the forward movement of the tongue, thereby prompting the patient to involuntarily protrude the mandible. This eventually leads to the development of an anterior crossbite, which if not corrected in time, can hinder normal maxillary development, while the mandible may undergo unrestricted overdevelopment and ultimately result in typical skeletal Class III malocclusions. Last but not least, deviations from the intended trajectory of tooth eruption are also frequently observed in children over 3 years of age with mouth breathing (88). Excessive molar eruption has been observed in children with mouth breathing (89), which may lead to a clockwise rotation of the mandible and a disproportional increase in the anterior lower vertical height of the face (90).
6 AH-related mouth breathing promotes the atypical development of the facial skeleton
Mouth breathing, often resulting from AH, is associated with the atypical development of the facial skeleton, and particularly affects the mandible, maxilla, and hyoid bone. However, the effects of mouth breathing on maxillofacial bone structure are less pronounced in adults, which could be attributed to the reduced secretion of growth hormone (somatotropin) observed in children with AH who habitually engage in mouth breathing.
6.1 Maxilla
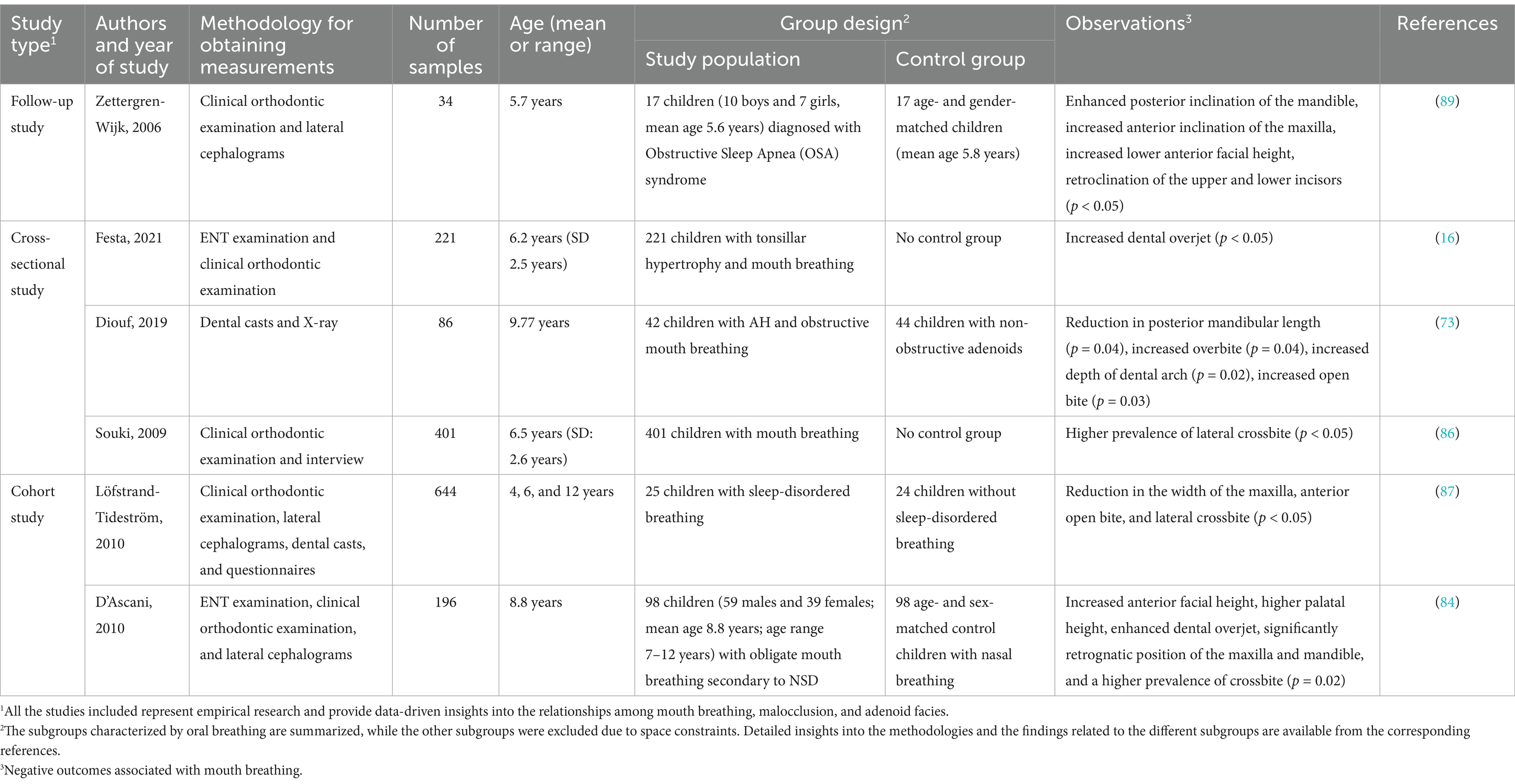
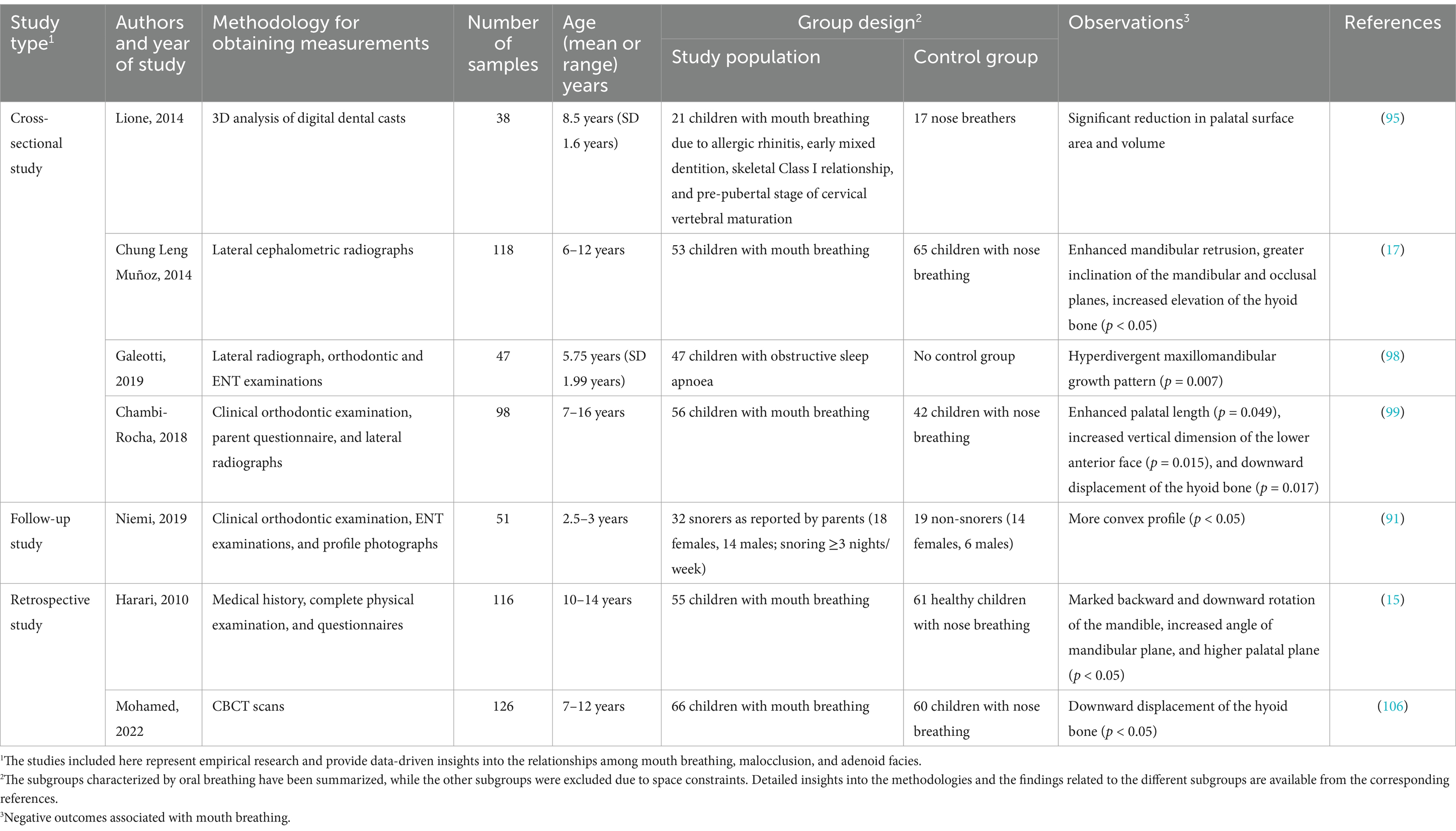
Mouth breathing is associated with maxillofacial growth and development, and affects the development of orofacial structures, including maxillary narrowing (85), enhanced facial convexity (91), mandibular retraction, and increased facial height (92). Mouth breathing leads to physiological changes in the upper respiratory tract that can cause adaptive changes in the maxilla (Table 3) (93, 94). An increase in the height of the palatal vault is the most common manifestation of maxillary dysplasia resulting from mouth breathing (71). Patients with mouth breathing due to AH-induced nasal obstruction tend to have a higher and sharper palatal fornix at the canine, premolar, and molar levels (Figure 3) (75). By performing three-dimensional (3D) analyses of digital dental models, previous studies have demonstrated that mouth breathing markedly reduces the total surface area and volume of the palate (74, 95). Additionally, an observational study conducted over a period of 3 years revealed that maxillary growth is slower in children with mouth breathing (74, 96).
6.2 Mandible
It has been demonstrated that skeletal anomalies, including increased vertical mandibular growth, correlate with the AH-induced narrowing of the upper nasopharyngeal cavity (97, 98). Children who habitually breathe through their mouths often present with a retruded mandible, increased anterior lower facial height, and a steeper inclination of the mandibular and occlusal planes (Table 3) (99) (17). This facial pattern, often referred to as “long face syndrome” or “high-angle profile,” is characterized by vertical discrepancies in the lower face, which can sometimes improve following adenoidectomy (70). Both Class II and Class III malocclusions are frequently observed in children with mouth breathing, as altered breathing patterns can affect craniofacial growth. However, Class II malocclusions, which are marked by mandibular retrusion, are typically more prevalent in this population (100, 101). Although Class III malocclusions have also been observed in children with mouth breathing, they are proportionally less common than Class II malocclusions. Additionally, the mandibular growth pattern in cases with Class II malocclusions is generally neutral to hypo-divergent in the sagittal plane, indicating that vertical growth tendencies are less pronounced in these patients (102). Additionally, a meta-analysis demonstrated that children with mouth breathing tend to exhibit rotational changes in the mandible and maxilla relative to the cranial base, which further affects craniofacial structure (103).
6.3 Hyoid bone
The hyoid bone is a key component of the maxillofacial complex, and its position is also affected by mouth breathing (Table 3) (104). Previous studies have reported varying findings on its position in relation to different breathing modalities. Cephalometric analyses of children aged 7–16 years have shown that the position of the hyoid bone is significantly lower relative to the mandibular plane in mouth breathers compared to nasal breathers (99). Conversely, research on preschool children with airway obstruction suggests that the displacement of the hyoid bone is likely predominantly affected by obstructive conditions instead of breathing habits alone (105). Additionally, cone-beam computed tomography of children aged 10–12 years demonstrated that the hyoid bone adopts a vertically higher and more posterior position in mouth breathers compared to nasal breathers (106). These discrepancies may stem from the differences across the various imaging techniques and developmental factors. However, maintaining the positional stability of the hyoid bone is crucial for ensuring the patency of the nasopharyngeal airway (107). The downward and backward displacement of the hyoid bone associated with mouth breathing may contribute to nasopharyngeal airway stenosis, thereby perpetuating mouth breathing. In summary, the findings obtained from existing literature provide diverse perspectives on the position of the hyoid bone in mouth breathing, which highlights the necessity for further research to elucidate the factors that influence these variations across different populations and methodologies.
6.4 Somatotropin secretion
Mouth breathing is one of the major risk factors that affect normal craniofacial development in children. However, it should be noted that mouth breathing has a relatively minor effect on the maxillofacial bones of adult patients (76), which could be related to the inhibition of somatotropin secretion in children with AH who practice mouth breathing.
It has been observed that the serum levels of insulin-like growth factor-1 (IGF-1) (108), IGF binding protein-3 (IGFBP-3), and plasma ghrelin (GH) (109) are significantly lower in children with AH and tonsillar hypertrophy who practice mouth breathing (62, 110). Furthermore, the reduction in the serum levels of IGF-1, IGFBP-3, and GH is associated with reduced appetite and restricted energy intake (111). This suggests that AH affects maxillofacial growth and development in children, and is also associated with the inhibition of growth hormone secretion. A prospective study examining the growth characteristics of children under 5 years of age following adenoidectomy revealed that the linear growth measures, including height and weight, improved postoperatively and correlated with an improvement in the IGF-1/GH ratio (112).
7 Mutual exacerbation of malocclusion, AH-related mouth breathing, and muscular dysfunction
Interestingly, the structural changes in the teeth, facial muscles, and bones caused by mouth breathing do not occur in an independent manner, but are instead closely interconnected (Figure 4).
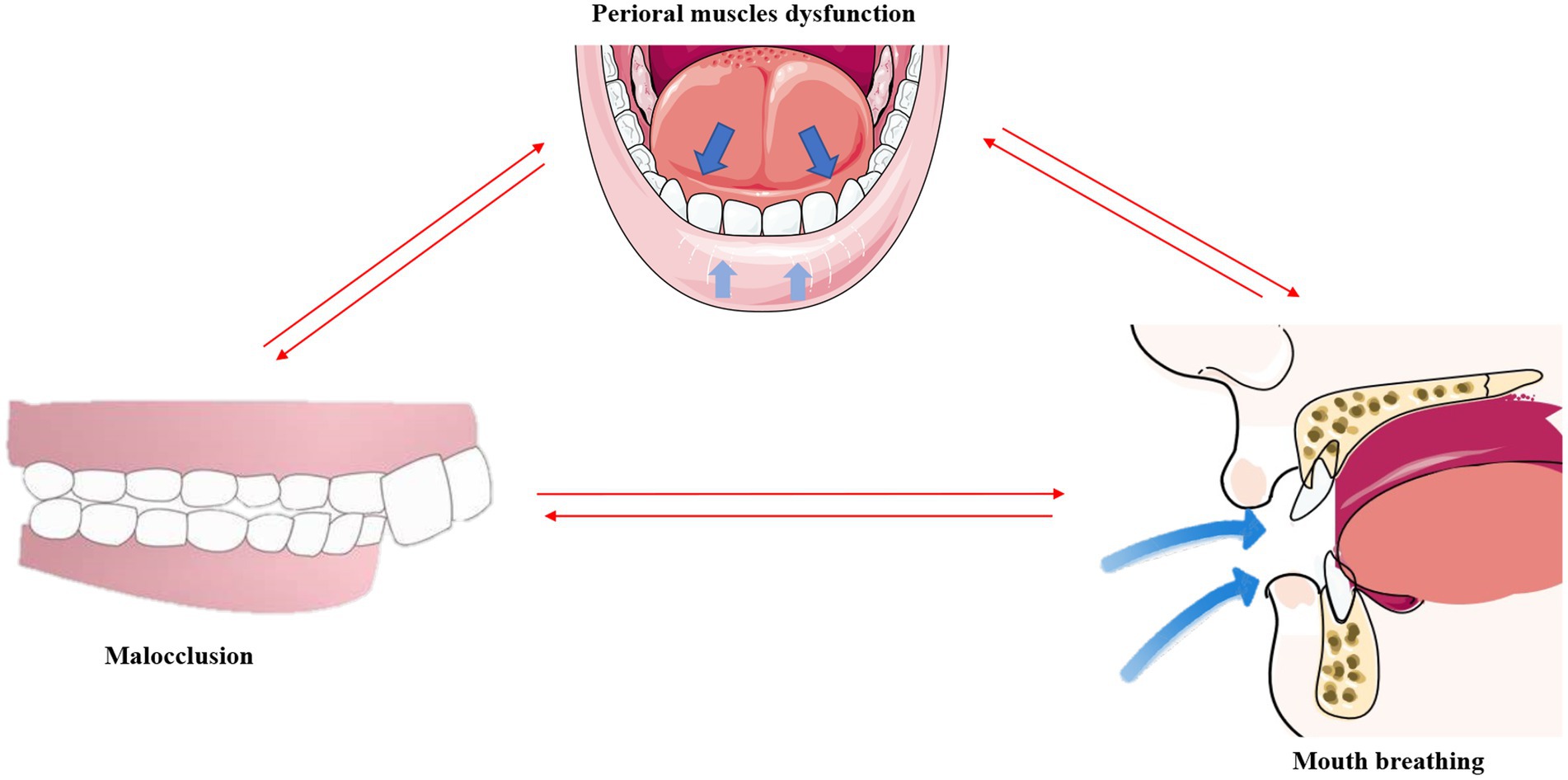
Figure 4. Malocclusion, mouth breathing, and muscular dysfunction mutually exacerbate one another. *This original illustration summarizes the reciprocal influences of these factors; however, future research may present different perspectives and conclusions.
An imbalance in the perioral muscles leads to dental misalignment, and the concurrent onset of malocclusion further exacerbates the muscular imbalance (113). As aforementioned, mouth breathing causes the tongue to drop and shift posteriorly. By employing cone-beam computed tomography (CBCT), a previous study revealed that the tongue adopts a lower position and has a smaller body in Class II malocclusions (114). The tongue also tends to be in a lower position in the mouth in children with a posterior crossbite (115). This indicates that malocclusion further exacerbates muscular dysfunction as proper dental alignment can help balance the strength between the tongue and the labial and buccal muscles.
It has been reported that mouth breathing and muscular dysfunction exacerbate one other. Comparative evaluation of the EMG activities of the orbicularis oris and mentalis muscles in children with mouth breathing revealed that the EMG activity of the mentalis muscle is higher in patients with mouth breathing (55), indicating dysfunction of the perioral muscles in these individuals (116). The overactivity of the submandibular muscles may in turn exacerbate mandibular retraction and mouth breathing, thus forming a negative feedback loop with regards to muscular function.
It is known that mouth breathing and malocclusion exacerbate one other (8). A comparative study of upper and lower pharyngeal airways of 80 subjects with Class I and Class II malocclusions revealed that the width of the nasopharyngeal cavity is narrower in patients with Class I and Class II malocclusions (117), which indicates an increased likelihood of airway obstruction due to malocclusion. Correspondingly, the use of functional appliances for correcting mandibular retrognathism can decrease upper airway resistance and reduce mouth breathing in adolescents (118). A follow-up study involving 49 prepubertal children with severe obstructive mouth breathing revealed a significant increase in transverse maxillary width and a marked improvement in dental crowding after 1 year of adenoidectomy (119).
8 Conclusion
In summary, the occurrence AH in children, caused by the passive inhalation of tobacco smoke, exposure to allergens, or other forms of upper respiratory tract inflammation, can obstruct the nasopharyngeal cavity, leading to mouth breathing. Mouth breathing can cause various functional disorders of the perioral muscles, including the weakening of lip muscles and drooping of the tongue, as well as dental misalignments, such as increased overjet, open bite, crossbite, and narrow dental arches. These factors ultimately contribute to the atypical development of the maxillofacial skeleton, including a higher and sharper palatal fornix, a receding mandible, and a downwardly displaced hyoid bone. AH further inhibits the secretion of somatotropin, which exacerbates maxillofacial skeletal dysplasia in children with mouth breathing. Importantly, perioral muscle dysfunction, malocclusion, and upper airway obstruction caused by hypertrophic adenoids are not independent issues, but are instead closely interconnected and mutually exacerbating, and their combined effects lead to the development of adenoid facies.
Author contributions
JZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YF: Conceptualization, Investigation, Resources, Writing – review & editing. LW: Methodology, Software, Writing – review & editing. GW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors disclose the receipt of the following financial support for research, authorship, and/or publication of this article: Supported in part by the First People’s Hospital of Lianyungang, grant number: QN2414.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang, Y, Jiao, H, Mi, C, Yang, G, and Han, T. Evaluation of adenoid hypertrophy with ultrasonography. Indian J Pediatr. (2020) 87:910–5. doi: 10.1007/s12098-020-03203-4
2. Ciprandi, G, Ameli, F, Asmanov, A, Passali, FM, and Tosca, MA. Risk factors for recurrent acute otitis media: a real-life clinical experience. J Biol Regul Homeost Agents. (2021) 35:33–8. doi: 10.23812/21-1supp2-7
3. Xu, Z, Wu, Y, Tai, J, Feng, G, Ge, W, Zheng, L, et al. Risk factors of obstructive sleep apnea syndrome in children. J Otolaryngol head Neck Surg = Le J d'oto-rhino-laryngologie et de chirurgie cervico-faciale. (2020) 49:11. doi: 10.1186/s40463-020-0404-1
4. Yazıcı, H. Nasal Mucociliary clearance in adenoid hypertrophy and otitis media with effusion. Curr Allergy Asthma Rep. (2015) 15:74. doi: 10.1007/s11882-015-0576-3
5. Gulotta, G, Iannella, G, Vicini, C, Polimeni, A, Greco, A, de Vincentiis, M, et al. Risk factors for obstructive sleep apnea syndrome in children: state of the art. Int J Environ Res Public Health. (2019) 16:3235. doi: 10.3390/ijerph16183235
6. Lin, L, Zhao, T, Qin, D, Hua, F, and He, H. The impact of mouth breathing on dentofacial development: a concise review. Front Public Health. (2022) 10:929165. doi: 10.3389/fpubh.2022.929165
7. Grippaudo, C, Paolantonio, EG, Antonini, G, Saulle, R, La Torre, G, and Deli, R. Association between oral habits, mouth breathing and malocclusion. Acta otorhinolaryngologica Italica: organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. (2016) 36:386–94. doi: 10.14639/0392-100X-770
8. Zicari, AM, Albani, F, Ntrekou, P, Rugiano, A, Duse, M, Mattei, A, et al. Oral breathing and dental malocclusions. Eur J Paediatr Dent. (2009) 10:59–64.
9. Twigg, SR, and Wilkie, AO. New insights into craniofacial malformations. Hum Mol Genet. (2015) 24:R50–9. doi: 10.1093/hmg/ddv228
10. Vázquez-Nava, F, Quezada-Castillo, JA, Oviedo-Treviño, S, Saldivar-González, AH, Sánchez-Nuncio, HR, Beltrán-Guzmán, FJ, et al. Association between allergic rhinitis, bottle feeding, non-nutritive sucking habits, and malocclusion in the primary dentition. Arch Dis Child. (2006) 91:836–40. doi: 10.1136/adc.2005.088484
11. Cenzato, N, Iannotti, L, and Maspero, C. Open bite and atypical swallowing: orthodontic treatment, speech therapy or both? A literature review. Eur J Paediatr Dent. (2021) 22:286–90. doi: 10.23804/ejpd.2021.22.04.5
12. Borrie, FR, Bearn, DR, Innes, NP, and Iheozor-Ejiofor, Z. Interventions for the cessation of non-nutritive sucking habits in children. Cochrane Database Syst Rev. (2015) 2015:CD008694. doi: 10.1002/14651858.CD008694.pub2
13. Kasparaviciene, K, Sidlauskas, A, Zasciurinskiene, E, Vasiliauskas, A, Juodzbalys, G, Sidlauskas, M, et al. The prevalence of malocclusion and oral habits among 5-7-year-old children. Medical Sci Monitor: Int Med J Experiment Clin Res. (2014) 20:2036–42. doi: 10.12659/MSM.890885
14. Rodríguez-Olivos, LHG, Chacón-Uscamaita, PR, Quinto-Argote, AG, Pumahualcca, G, and Pérez-Vargas, LF. Deleterious oral habits related to vertical, transverse and sagittal dental malocclusion in pediatric patients. BMC Oral Health. (2022) 22:88. doi: 10.1186/s12903-022-02122-4
15. Harari, D, Redlich, M, Miri, S, Hamud, T, and Gross, M. The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients. Laryngoscope. (2010) 120:2089–93. doi: 10.1002/lary.20991
16. Festa, P, Mansi, N, Varricchio, AM, Savoia, F, Calì, C, Marraudino, C, et al. Association between upper airway obstruction and malocclusion in mouth-breathing children. Acta otorhinolaryngologica Italica: organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. (2021) 41:436–42. doi: 10.14639/0392-100X-N1225
17. Chung Leng Muñoz, I, and Beltri, OP. Comparison of cephalometric patterns in mouth breathing and nose breathing children. Int J Pediatr Otorhinolaryngol. (2014) 78:1167–72. doi: 10.1016/j.ijporl.2014.04.046
18. Freitas, HV, Alves, CMC, Silva, L, Pereira, ALP, Hugo, FN, and Thomaz, E. Alterations of oral functions and dental malocclusions in adolescents: a cross-sectional population-based study. Ciênc Saúde Colet. (2021) 26:5261–72. doi: 10.1590/1413-812320212611.3.07992020
19. Wright, DH. Lymphomas of Waldeyer's ring. Histopathology. (1994) 24:97–9. doi: 10.1111/j.1365-2559.1994.tb01281.x
20. Min, HJ, and Kim, KS. IL-17C expression and its correlation with pediatric adenoids: a preliminary study. Int J Med Sci. (2020) 17:2603–10. doi: 10.7150/ijms.49244
21. Ogasawara, N, Kojima, T, Go, M, Takano, K, Kamekura, R, Ohkuni, T, et al. Epithelial barrier and antigen uptake in lymphoepithelium of human adenoids. Acta Otolaryngol. (2011) 131:116–23. doi: 10.3109/00016489.2010.520022
22. Masna, K, Zwierz, A, Domagalski, K, and Burduk, P. The impact of the thermal seasons on adenoid size, its mucus coverage and otitis media with effusion: a cohort study. J Clin Med. (2021) 10:5603. doi: 10.3390/jcm10235603
23. Xu, Y, Sun, J, Cui, Y, Yu, S, He, J, Liu, P, et al. Age-related changes in the morphology and the distribution of IgA and IgG in the pharyngeal tonsils of yaks (Bos grunniens). J Morphol. (2019) 280:214–22. doi: 10.1002/jmor.20933
24. Kang, KT, Chou, CH, Weng, WC, Lee, PL, and Hsu, WC. Associations between adenotonsillar hypertrophy, age, and obesity in children with obstructive sleep apnea. PLoS One. (2013) 8:e78666. doi: 10.1371/journal.pone.0078666
25. Pereira, L, Monyror, J, Almeida, FT, Almeida, FR, Guerra, E, Flores-Mir, C, et al. Prevalence of adenoid hypertrophy: a systematic review and meta-analysis. Sleep Med Rev. (2018) 38:101–12. doi: 10.1016/j.smrv.2017.06.001
26. Faden, H, Callanan, V, Pizzuto, M, Nagy, M, Wilby, M, Lamson, D, et al. The ubiquity of asymptomatic respiratory viral infections in the tonsils and adenoids of children and their impact on airway obstruction. Int J Pediatr Otorhinolaryngol. (2016) 90:128–32. doi: 10.1016/j.ijporl.2016.09.006
27. Johnston, JJ, and Douglas, R. Adenotonsillar microbiome: an update. Postgrad Med J. (2018) 94:398–403. doi: 10.1136/postgradmedj-2018-135602
28. Wang, LF, White, DR, Andreoli, SM, Mulligan, RM, Discolo, CM, and Schlosser, RJ. Cigarette smoke inhibits dynamic ciliary beat frequency in pediatric adenoid explants. Otolaryngol Head Neck Surg: Official J American Acad Otolaryngol Head Neck Surg. (2012) 146:659–63. doi: 10.1177/0194599811431414
29. Tagliacarne, SC, Valsecchi, C, Castellazzi, AM, Licari, A, Klersy, C, Montagna, L, et al. Impact of passive smoke and/or atopy on adenoid immunoglobulin production in children. Immunol Lett. (2015) 165:70–7. doi: 10.1016/j.imlet.2015.04.002
30. Sadeghi-Shabestari, M, Jabbari Moghaddam, Y, and Ghaharri, H. Is there any correlation between allergy and adenotonsillar tissue hypertrophy? Int J Pediatr Otorhinolaryngol. (2011) 75:589–91. doi: 10.1016/j.ijporl.2011.01.026
31. Colavita, L, Miraglia Del Giudice, M, Stroscio, G, Visalli, C, Alterio, T, Pidone, C, et al. Allergic rhinitis and adenoid hypertrophy in children: is adenoidectomy always really useful? J Biol Regul Homeost Agents. (2015) 29:58–63.
32. Evcimik, MF, Dogru, M, Cirik, AA, and Nepesov, MI. Adenoid hypertrophy in children with allergic disease and influential factors. Int J Pediatr Otorhinolaryngol. (2015) 79:694–7. doi: 10.1016/j.ijporl.2015.02.017
33. Marazzato, M, Zicari, AM, Aleandri, M, Conte, AL, Longhi, C, Vitanza, L, et al. 16S metagenomics reveals Dysbiosis of nasal Core microbiota in children with chronic nasal inflammation: role of adenoid hypertrophy and allergic rhinitis. Front Cell Infect Microbiol. (2020) 10:458. doi: 10.3389/fcimb.2020.00458
34. Shin, SY, Choi, SJ, Hur, GY, Lee, KH, Kim, SW, Cho, JS, et al. Local production of total IgE and specific antibodies to the house dust mite in adenoid tissue. Pediatric Allergy Immunol: Official Pub European Society of Pediatric Allergy Immunol. (2009) 20:134–41. doi: 10.1111/j.1399-3038.2008.00756.x
35. Andreoli, SM, Schlosser, RJ, Wang, LF, Mulligan, RM, Discolo, CM, and White, DR. Adenoid Ciliostimulation in children with chronic otitis media. Otolaryngol Head Neck Surg: Official J American Acad Otolaryngol Head Neck Surg. (2013) 148:135–9. doi: 10.1177/0194599812462664
36. Proffit, WR. Equilibrium theory revisited: factors influencing position of the teeth. Angle Orthod. (1978) 48:175–86.
37. Hwang, DM, Lee, JY, Choi, YJ, and Hwang, CJ. Evaluations of the tongue and hyoid bone positions and pharyngeal airway dimensions after maxillary protraction treatment. Cranio: J Craniomandibular Prac. (2019) 37:214–22. doi: 10.1080/08869634.2017.1418644
38. Priede, D, Roze, B, Parshutin, S, Arkliņa, D, Pircher, J, Vaska, I, et al. Association between malocclusion and orofacial myofunctional disorders of pre-school children in Latvia. Orthod Craniofacial Res. (2020) 23:277–83. doi: 10.1111/ocr.12367
39. Mew, JR. The postural basis of malocclusion: a philosophical overview. American J Orthodon Dentofacial Orthoped: Official Pub American Association of Orthodontists, its Constituent Societ American Board of Orthodon. (2004) 126:729–38. doi: 10.1016/j.ajodo.2003.12.019
40. Gómez-González, C, González-Mosquera, A, Alkhraisat, MH, and Anitua, E. Mouth breathing and its impact on atypical swallowing: a systematic review and Meta-analysis. Dent J (Basel). (2016) 28:546–550. doi: 10.3390/dj12020021
41. Valentim, AF, Furlan, RM, Perilo, TV, Motta, AR, and Casas, EB. Relationship between perception of tongue position and measures of tongue force on the teeth. CoDAS. (2016) 28:546–50. doi: 10.1590/2317-1782/20162015256
42. Ruan, WH, Chen, MD, Gu, ZY, Lu, Y, Su, JM, and Guo, Q. Muscular forces exerted on the normal deciduous dentition. Angle Orthod. (2005) 75:785–90. doi: 10.1043/0003-3219(2005)75[785:MFEOTN]2.0.CO;2
43. Lee, SH, Choi, JH, Shin, C, Lee, HM, Kwon, SY, and Lee, SH. How does open-mouth breathing influence upper airway anatomy? Laryngoscope. (2007) 117:1102–6. doi: 10.1097/MLG.0b013e318042aef7
44. Takahashi, S, Ono, T, Ishiwata, Y, and Kuroda, T. Effect of changes in the breathing mode and body position on tongue pressure with respiratory-related oscillations. American J Orthodon Dentofacial Orthoped: Official Pub American Association of Orthodontists, its Constituent Societ American Board of Orthodon. (1999) 115:239–46. doi: 10.1016/S0889-5406(99)70324-0
45. Takahashi, S, Ono, T, Ishiwata, Y, and Kuroda, T. Breathing modes, body positions, and suprahyoid muscle activity. J Orthod. (2002) 29:307–13. doi: 10.1093/ortho/29.4.307
46. Wang, L, Wu, Y, Zhu, M, and Zhao, C. Relationship between EMG features and force in orbicularis oris muscle. Technol Health Care: Official J European Society Eng Med. (2023) 31:47–56. doi: 10.3233/THC-213545
47. Masutomi, Y, Goto, T, and Ichikawa, T. Mouth breathing reduces oral function in adolescence. Sci Rep. (2024) 14:3810. doi: 10.1038/s41598-024-54328-x
48. Proffit, WR, McGlone, RE, and Barrett, MJ. Lip and tongue pressures related to dental arch and oral cavity size in Australian aborigines. J Dent Res. (1975) 54:1161–72. doi: 10.1177/00220345750540061101
49. Valentim, AF, Furlan, RM, Perilo, TV, Berbert, MC, Motta, AR, and de Las Casas, EB. Evaluation of the force applied by the tongue and lip on the maxillary central incisor tooth. CoDAS. (2014) 26:235–40. doi: 10.1590/2317-1782/201420130077
50. Nucci, L, Marra, PM, Femiano, L, Isola, G, Flores-Mir, C, Perillo, L, et al. Perioral muscle activity changes after lip bumper treatment. Eur J Paediatr Dent. (2021) 22:129–34. doi: 10.23804/ejpd.2021.22.02.8
51. Li, J, Zhao, Z, Zheng, L, Daraqel, B, Liu, J, and Hu, Y. Effects of mouth breathing on maxillofacial and airway development in children and adolescents with different cervical vertebral maturation stages: a cross-sectional study. BMC Oral Health. (2022) 22:197. doi: 10.1186/s12903-022-02234-x
52. Wagaiyu, EG, and Ashley, FP. Mouthbreathing, lip seal and upper lip coverage and their relationship with gingival inflammation in 11-14 year-old schoolchildren. J Clin Periodontol. (1991) 18:698–702. doi: 10.1111/j.1600-051X.1991.tb00112.x
53. Saitoh, I, Inada, E, Kaihara, Y, Nogami, Y, Murakami, D, Kubota, N, et al. An exploratory study of the factors related to mouth breathing syndrome in primary school children. Arch Oral Biol. (2018) 92:57–61. doi: 10.1016/j.archoralbio.2018.03.012
54. Lowe, AA, Takada, K, and Taylor, LM. Muscle activity during function and its correlation with craniofacial morphology in a sample of subjects with class II, division 1 malocclusions. Am J Orthod. (1983) 84:204–11. doi: 10.1016/0002-9416(83)90127-6
55. Dutra, EH, Maruo, H, and Vianna-Lara, MS. Electromyographic activity evaluation and comparison of the orbicularis oris (lower fascicle) and mentalis muscles in predominantly nose- or mouth-breathing subjects. American J Orthodon Dentofacial Orthoped: Official Pub American Association of Orthodontists, its Constituent Societ American Board of Orthodon. (2006) 129:722.e1–9. doi: 10.1016/j.ajodo.2006.02.027
56. Busanello-Stella, AR, Blanco-Dutra, AP, Corrêa, EC, and Silva, AM. Electromyographic fatigue of orbicular oris muscles during exercises in mouth and nasal breathing children. CoDAS. (2015) 27:80–8. doi: 10.1590/2317-1782/20152014078
57. Dutra, EH, Caria, PH, Rafferty, KL, and Herring, SW. The buccinator during mastication: a functional and anatomical evaluation in minipigs. Arch Oral Biol. (2010) 55:627–38. doi: 10.1016/j.archoralbio.2010.06.004
58. Al Zubaidi, SH, Mha, M, and Hasan, LA. Finite element analysis of the stress released by buccinator muscle in the mandibular dental arch during sucking habits. J Oral Biol Craniofacial Res. (2021) 11:430–4. doi: 10.1016/j.jobcr.2021.05.004
59. Vitti, M, Basmajian, JV, Ouellette, PL, Mitchell, DL, Eastmen, WP, and Seaborn, RD. Electromyographic investigations of the tongue and circumoral muscular sling with fine-wire electrodes. J Dent Res. (1975) 54:844–9. doi: 10.1177/00220345750540042401
60. Alouini, O, and Rollet, D. Morphological and functional peri-oral changes during early treatment of class II division 1 malocclusions using EF line (®) functional education devices. L' Orthodontie francaise. (2018) 89:289–306. doi: 10.1051/orthodfr/2018025
61. Alabdullah, M, Saltaji, H, Abou-Hamed, H, and Youssef, M. Association between facial growth pattern and facial muscle activity: a prospective cross-sectional study. Int Orthod. (2015) 13:181–94. doi: 10.1016/j.ortho.2015.03.011
62. Uysal, T, Yagci, A, Kara, S, and Okkesim, S. Influence of pre-orthodontic trainer treatment on the perioral and masticatory muscles in patients with class II division 1 malocclusion. Eur J Orthod. (2012) 34:96–101. doi: 10.1093/ejo/cjq169
63. Erdem, A, Kilic, N, and Eröz, B. Changes in soft tissue profile and electromyographic activity after activator treatment. Aust Orthod J. (2009) 25:116–22. doi: 10.2478/aoj-2009-0017
64. Miller, AJ, Vargervik, K, and Chierici, G. Experimentally induced neuromuscular changes during and after nasal airway obstruction. Am J Orthod. (1984) 85:385–92. doi: 10.1016/0002-9416(84)90159-3
65. Tallgren, A, Christiansen, RL, Ash, M Jr, and Miller, RL. Effects of a myofunctional appliance on orofacial muscle activity and structures. Angle Orthod. (1998) 68:249–58.
66. Fernandez, CCA, Pereira, C, Luiz, RR, Vieira, AR, and De Castro, CM. Dental anomalies in different growth and skeletal malocclusion patterns. Angle Orthod. (2018) 88:195–201. doi: 10.2319/071917-482.1
67. Sacerdoti, R, and Baccetti, T. Dentoskeletal features associated with unilateral or bilateral palatal displacement of maxillary canines. Angle Orthod. (2004) 74:725–32. doi: 10.1043/0003-3219(2004)074<0725:DFAWUO>2.0.CO;2
68. Galeotti, A, Festa, P, Viarani, V, D'Antò, V, Sitzia, E, Piga, S, et al. Prevalence of malocclusion in children with obstructive sleep apnoea. Orthod Craniofacial Res. (2018) 21:242–7. doi: 10.1111/ocr.12242
69. Granja, GL, Leal, TR, Lima, LCM, Silva, SED, Neves, ÉTB, Ferreira, FM, et al. Predictors associated with malocclusion in children with and without sleep disorders: a cross-sectional study. Braz Oral Res. (2023) 37:e106. doi: 10.1590/1807-3107bor-2023.vol37.0106
70. Lysy, J, Karkazi, F, Stanko, P, and Novak, B. The influence of mouth breathing on skeletal and dental features of splanchnocranium. Bratisl Lek Listy. (2021) 122:196–9. doi: 10.4149/BLL_2021_031
71. Lione, R, Buongiorno, M, Franchi, L, and Cozza, P. Evaluation of maxillary arch dimensions and palatal morphology in mouth-breathing children by using digital dental casts. Int J Pediatr Otorhinolaryngol. (2014) 78:91–5. doi: 10.1016/j.ijporl.2013.09.028
72. Markkanen, S, Niemi, P, Rautiainen, M, Saarenpää-Heikkilä, O, Himanen, SL, Satomaa, AL, et al. Craniofacial and occlusal development in 2.5-year-old children with obstructive sleep apnoea syndrome. Eur J Orthod. (2019) 41:316–21. doi: 10.1093/ejo/cjz009
73. Diouf, JS, Ouédraogo, Y, Souaré, N, Badiane, A, Diop-Bâ, K, Ngom, PI, et al. Comparison of dental arch measurements according to the grade and the obstructive character of adenoids. Int Orthod. (2019) 17:333–41. doi: 10.1016/j.ortho.2019.03.016
74. Aznar, T, Galán, AF, Marín, I, and Domínguez, A. Dental arch diameters and relationships to oral habits. Angle Orthod. (2006) 76:441–5. doi: 10.1043/0003-3219(2006)076[0441:DADART]2.0.CO;2
75. Osiatuma, VI, Otuyemi, OD, Kolawole, KA, Amusa, YB, and Ogunbanjo, BO. Dental arch dimensions of Nigerian children with hypertrophied adenoids. Turkish J Orthodont. (2017) 30:42–9. doi: 10.5152/TurkJOrthod.2017.17019
76. Rossi, RC, Rossi, NJ, Rossi, NJ, Yamashita, HK, and Pignatari, SS. Dentofacial characteristics of oral breathers in different ages: a retrospective case-control study. Prog Orthod. (2015) 16:23. doi: 10.1186/s40510-015-0092-y
77. Dimberg, L, Lennartsson, B, Söderfeldt, B, and Bondemark, L. Malocclusions in children at 3 and 7 years of age: a longitudinal study. Eur J Orthod. (2013) 35:131–7. doi: 10.1093/ejo/cjr110
78. Kallunki, J, Bondemark, L, and Paulsson, L. Early headgear activator treatment of class II malocclusion with excessive overjet: a randomized controlled trial. Eur J Orthod. (2021) 43:639–47. doi: 10.1093/ejo/cjaa073
79. Millett, DT, Cunningham, SJ, O'Brien, KD, Benson, PE, and de Oliveira, CM. Orthodontic treatment for deep bite and retroclined upper front teeth in children. Cochrane Database Syst Rev. (2017) 10:Cd005972. doi: 10.1002/14651858.CD005972.pub3
80. Cabrera Lde, C, Retamoso, LB, Mei, RM, and Tanaka, O. Sagittal and vertical aspects of class II division 1 subjects according to the respiratory pattern. Dental Press J Orthod. (2013) 18:30–5. doi: 10.1590/S2176-94512013000200011
81. Salim, NA, Al-Abdullah, MM, AlHamdan, AS, and Satterthwaite, JD. Prevalence of malocclusion and assessment of orthodontic treatment needs among Syrian refugee children and adolescents: a cross-sectional study. BMC Oral Health. (2021) 21:305. doi: 10.1186/s12903-021-01663-4
82. Marklund, M. Subjective versus objective dental side effects from oral sleep apnea appliances. Sleep Breath = Schlaf & Atmung. (2020) 24:111–7. doi: 10.1007/s11325-019-01852-0
83. Mutlu, E, Parlak, B, Kuru, S, Oztas, E, Pınar-Erdem, A, and Sepet, E. Evaluation of Crossbites in relation with dental arch widths, occlusion type, nutritive and non-nutritive sucking habits and respiratory factors in the early mixed dentition. Oral Health Prev Dent. (2019) 17:447–55. doi: 10.3290/j.ohpd.a42738
84. D'Ascanio, L, Lancione, C, Pompa, G, Rebuffini, E, Mansi, N, and Manzini, M. Craniofacial growth in children with nasal septum deviation: a cephalometric comparative study. Int J Pediatr Otorhinolaryngol. (2010) 74:1180–3. doi: 10.1016/j.ijporl.2010.07.010
85. Löfstrand-Tideström, B, Thilander, B, Ahlqvist-Rastad, J, Jakobsson, O, and Hultcrantz, E. Breathing obstruction in relation to craniofacial and dental arch morphology in 4-year-old children. Eur J Orthod. (1999) 21:323–32. doi: 10.1093/ejo/21.4.323
86. Souki, BQ, Pimenta, GB, Souki, MQ, Franco, LP, Becker, HM, and Pinto, JA. Prevalence of malocclusion among mouth breathing children: do expectations meet reality? Int J Pediatr Otorhinolaryngol. (2009) 73:767–73. doi: 10.1016/j.ijporl.2009.02.006
87. Löfstrand-Tideström, B, and Hultcrantz, E. Development of craniofacial and dental arch morphology in relation to sleep disordered breathing from 4 to 12 years. Effects of adenotonsillar surgery. Int J Pediatr Otorhinolaryngol. (2010) 74:137–43. doi: 10.1016/j.ijporl.2009.10.025
88. Emerich, K, and Wojtaszek-Slominska, A. Clinical practice. Later orthodontic complications caused by risk factors observed in the early years of life. Eur J Pediatr. (2010) 169:651–5. doi: 10.1007/s00431-009-1098-6
89. Zettergren-Wijk, L, Forsberg, CM, and Linder-Aronson, S. Changes in dentofacial morphology after adeno−/tonsillectomy in young children with obstructive sleep apnoea--a 5-year follow-up study. Eur J Orthod. (2006) 28:319–26. doi: 10.1093/ejo/cji119
90. Principato, JJ. Upper airway obstruction and craniofacial morphology. Otolaryngol Head Neck Surg: Official J American Acad Otolaryngol Head Neck Surg. (1991) 104:881–90. doi: 10.1177/019459989110400621
91. Niemi, P, Markkanen, S, Helminen, M, Rautiainen, M, Katila, MK, Saarenpää-Heikkilä, O, et al. Association between snoring and deciduous dental development and soft tissue profile in 3-year-old children. American J Orthodon Dentofacial Orthoped: Official Pub American Association of Orthodontists, its Constituent Societ American Board of Orthodon. (2019) 156:840–5. doi: 10.1016/j.ajodo.2019.02.015
92. Bresolin, D, Shapiro, PA, Shapiro, GG, Chapko, MK, and Dassel, S. Mouth breathing in allergic children: its relationship to dentofacial development. Am J Orthod. (1983) 83:334–40. doi: 10.1016/0002-9416(83)90229-4
93. Kim, DK, Rhee, CS, Yun, PY, and Kim, JW. Adenotonsillar hypertrophy as a risk factor of dentofacial abnormality in Korean children. European Archives Oto-rhino-laryngol: Official J European Federation Oto-Rhino-Laryngological Societies (EUFOS): Aff German Society Oto-Rhino-Laryngol - Head Neck Surg. (2015) 272:3311–6. doi: 10.1007/s00405-014-3407-6
94. Peltomäki, T. The effect of mode of breathing on craniofacial growth--revisited. Eur J Orthod. (2007) 29:426–9. doi: 10.1093/ejo/cjm055
95. Lione, R, Franchi, L, Huanca Ghislanzoni, LT, Primozic, J, Buongiorno, M, and Cozza, P. Palatal surface and volume in mouth-breathing subjects evaluated with three-dimensional analysis of digital dental casts-a controlled study. Eur J Orthod. (2015) 37:101–4. doi: 10.1093/ejo/cju018
96. Gross, AM, Kellum, GD, Franz, D, Michas, K, Walker, M, Foster, M, et al. A longitudinal evaluation of open mouth posture and maxillary arch width in children. Angle Orthod. (1994) 64:419–24.
97. Sousa, JB, Anselmo-Lima, WT, Valera, FC, Gallego, AJ, and Matsumoto, MA. Cephalometric assessment of the mandibular growth pattern in mouth-breathing children. Int J Pediatr Otorhinolaryngol. (2005) 69:311–7. doi: 10.1016/j.ijporl.2004.10.010
98. Galeotti, A, Festa, P, Viarani, V, Pavone, M, Sitzia, E, Piga, S, et al. Correlation between cephalometric variables and obstructive sleep apnoea severity in children. Eur J Paediatr Dent. (2019) 20:43–7. doi: 10.23804/ejpd.2019.20.01.09
99. Chambi-Rocha, A, Cabrera-Domínguez, ME, and Domínguez-Reyes, A. Breathing mode influence on craniofacial development and head posture. J Pediatr. (2018) 94:123–30. doi: 10.1016/j.jped.2017.05.007
100. McNamara, JA Jr. Components of class II malocclusion in children 8-10 years of age. Angle Orthod. (1981) 51:177–202.
101. Cozza, P, Baccetti, T, Franchi, L, De Toffol, L, and McNamara, JA Jr. Mandibular changes produced by functional appliances in class II malocclusion: a systematic review. American J Orthodon Dentofacial Orthoped: Official Pub American Association of Orthodontists, its Constituent Societ American Board of Orthodont. (2006) 129:599, e1–e12. doi: 10.1016/j.ajodo.2005.11.010
102. Siriwat, PP, and Jarabak, JR. Malocclusion and facial morphology is there a relationship? An epidemiologic study. Angle Orthodontist. (1985) 55:127–38.
103. Zhao, Z, Zheng, L, Huang, X, Li, C, Liu, J, and Hu, Y. Effects of mouth breathing on facial skeletal development in children: a systematic review and meta-analysis. BMC Oral Health. (2021) 21:108. doi: 10.1186/s12903-021-01458-7
104. Behlfelt, K, Linder-Aronson, S, and Neander, P. Posture of the head, the hyoid bone, and the tongue in children with and without enlarged tonsils. Eur J Orthod. (1990) 12:458–67. doi: 10.1093/ejo/12.4.458
105. Vieira, BB, Itikawa, CE, de Almeida, LA, Sander, HH, Aragon, DC, Anselmo-Lima, WT, et al. Facial features and hyoid bone position in preschool children with obstructive sleep apnea syndrome. European Archives Oto-rhino-laryngol: Official J European Federation Oto-Rhino-Laryngological Societies (EUFOS): Aff German Society Oto-Rhino-Laryngol - Head Neck Surg. (2014) 271:1305–9. doi: 10.1007/s00405-013-2770-z
106. Mohamed, AS, Habumugisha, J, Cheng, B, Zhao, M, Guo, Y, Zou, R, et al. Three-dimensional evaluation of hyoid bone position in nasal and mouth breathing subjects with skeletal class I, and class II. BMC Oral Health. (2022) 22:228. doi: 10.1186/s12903-022-02257-4
107. Ferraz, MJ, Nouer, DF, Teixeira, JR, and Bérzin, F. Cephalometric assessment of the hyoid bone position in oral breathing children. Braz J Otorhinolaryngol. (2007) 73:45–50. doi: 10.1016/S1808-8694(15)31121-6
108. Keskin, N, and Keskin, S. Association between adenotonsillar hypertrophy and leptin, ghrelin and IGF-1 levels in children. Auris Nasus Larynx. (2021) 48:248–54. doi: 10.1016/j.anl.2020.08.002
109. Jabbari Moghaddam, Y, Golzari, SE, Saboktakin, L, Seyedashrafi, MH, Sabermarouf, B, Gavgani, HA, et al. Does adenotonsillectomy alter IGF-1 and ghrelin serum levels in children with adenotonsillar hypertrophy and failure to thrive? A prospective study. Int J Pediatr Otorhinolaryngol. (2013) 77:1541–4. doi: 10.1016/j.ijporl.2013.06.029
110. Wang, H, Qiao, X, Qi, S, Zhang, X, and Li, S. Effect of adenoid hypertrophy on the upper airway and craniomaxillofacial region. Translational pediatrics. (2021) 10:2563–72. doi: 10.21037/tp-21-437
111. Mazurkiewicz, D, and Bronkowska, M. Circulating insulin and IGF-1 and frequency of food consumption during pregnancy as predictors of birth weight and length. Nutrients. (2021) 13:2344. doi: 10.3390/nu13072344
112. Vontetsianos, HS, Davris, SE, Christopoulos, GD, and Dacou-Voutetakis, C. Improved somatic growth following adenoidectomy and tonsillectomy in young children. Possible pathogenetic mechanisms. Hormones (Athens). (2005) 4:49–54. doi: 10.14310/horm.2002.11143
113. Van Dyck, C, Dekeyser, A, Vantricht, E, Manders, E, Goeleven, A, Fieuws, S, et al. The effect of orofacial myofunctional treatment in children with anterior open bite and tongue dysfunction: a pilot study. Eur J Orthod. (2016) 38:227–34. doi: 10.1093/ejo/cjv044
114. Chen, W, Mou, H, Qian, Y, and Qian, L. Evaluation of the position and morphology of tongue and hyoid bone in skeletal class II malocclusion based on cone beam computed tomography. BMC Oral Health. (2021) 21:475. doi: 10.1186/s12903-021-01839-y
115. Melink, S, Vagner, MV, Hocevar-Boltezar, I, and Ovsenik, M. Posterior crossbite in the deciduous dentition period, its relation with sucking habits, irregular orofacial functions, and otolaryngological findings. American J Orthodon Dentofacial Orthoped: Official Pub American Association of Orthodontists, its Constituent Societ American Board of Orthodont. (2010) 138:32–40. doi: 10.1016/j.ajodo.2008.09.029
116. Ambrosio, AR, Trevilatto, PC, Martins, LP, Santos-Pinto, AD, and Shimizu, RH. Electromyographic evaluation of the upper lip according to the breathing mode: a longitudinal study. Braz Oral Res. (2009) 23:415–23. doi: 10.1590/S1806-83242009000400011
117. de Freitas, MR, Alcazar, NM, Janson, G, de Freitas, KM, and Henriques, JF. Upper and lower pharyngeal airways in subjects with class I and class II malocclusions and different growth patterns. American J Orthodon Dentofacial Orthoped: Official Pub American Association of Orthodontists, its Constituent Societ American Board of Orthodon. (2006) 130:742–5. doi: 10.1016/j.ajodo.2005.01.033
118. Schütz, TC, Dominguez, GC, Hallinan, MP, Cunha, TC, and Tufik, S. Class II correction improves nocturnal breathing in adolescents. Angle Orthod. (2011) 81:222–8. doi: 10.2319/052710-233.1
119. Petraccone Caixeta, AC, and Andrade, I Jr. Bahia Junqueira Pereira T, Franco LP, Becker HM, Souki BQ. Dental arch dimensional changes after adenotonsillectomy in prepubertal children. American J Orthodon Dentofacial Orthoped: Official Pub American Association of Orthodontists, its Constituent Societ American Board of Orthodon. (2014) 145:461–8. doi: 10.1016/j.ajodo.2013.12.018
120. Shetty, SR, Al Bayatti, SW, Al-Rawi, NH, Kamath, V, Reddy, S, Narasimhan, S, et al. The effect of concha bullosa and nasal septal deviation on palatal dimensions: a cone beam computed tomography study. BMC Oral Health. (2021) 21:607. doi: 10.1186/s12903-021-01974-6
121. Cheng, B, Mohamed, AS, Habumugisha, J, Guo, Y, Zou, R, and Wang, F. A study of the facial soft tissue morphology in nasal- and mouth-breathing patients. Int Dent J. (2023) 73:403–9. doi: 10.1016/j.identj.2022.09.002
122. Bakor, SF, Enlow, DH, Pontes, P, and De Biase, NG. Craniofacial growth variations in nasal-breathing, oral-breathing, and tracheotomized children. American J Orthodon Dentofacial Orthoped: Official Pub American Association of Orthodontists, its Constituent Societ American Board of Orthodon. (2011) 140:486–92. doi: 10.1016/j.ajodo.2011.06.017
Keywords: adenoid facies, mouth breathing, adenoid hypertrophy, malocclusion, craniofacial development
Citation: Zhang J, Fu Y, Wang L and Wu G (2024) Adenoid facies: a long-term vicious cycle of mouth breathing, adenoid hypertrophy, and atypical craniofacial development. Front. Public Health. 12:1494517. doi: 10.3389/fpubh.2024.1494517
Edited by:
Kikelomo Adebanke Kolawole, Obafemi Awolowo University, NigeriaReviewed by:
Hong He, Wuhan University, ChinaMaria Cristina Cangussu, Federal University of Bahia (UFBA), Brazil
Copyright © 2024 Zhang, Fu, Wang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geng Wu, NzYwMDIwMjQwMDA2QHh6aG11LmVkdS5jbg==
 Jiaqi Zhang
Jiaqi Zhang Yongwei Fu1,2,3,4
Yongwei Fu1,2,3,4