- 1Department of Respiratory and Critical Care Medicine, The Second Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Respiratory and Critical Care Medicine, Xiamen Humanity Hospital Fujian Medical University, Xiamen, China
Objective: Chronic obstructive pulmonary disease (COPD) and chronic kidney disease (CKD) are significant global health issues with a well-established association between the two. This study aims to assess the risk of developing CKD in patients with COPD through systematic review and meta-analysis, and to explore the impact of CKD on the prognosis of COPD patients.
Methods: A total of 23 studies were included in the analysis, comprising 11 studies on the risk of CKD in patients with COPD, 6 studies on the impact of CKD on the short-term all-cause mortality risk of patients with acute exacerbation of COPD (AECOPD), and 6 studies on the impact of CKD on the long-term all-cause mortality risk of COPD patients. The meta-analysis showed that the risk of developing CKD in COPD patients was significantly increased (OR 1.54, 95% CI: 1.28–1.84), and CKD significantly increased the short-term all-cause mortality risk in AECOPD patients (OR 1.53, 95% CI: 1.44–1.63) as well as the long-term all-cause mortality risk in COPD patients (OR 1.70, 95% CI: 1.35–2.15).
Results: We searched the PubMed, EMBASE, and Cochrane Library databases in accordance with the PRISMA guidelines, including studies from the inception of the databases through December 31, 2023, to identify research assessing the relationship between COPD and CKD. The quality of the studies was assessed using the Newcastle-Ottawa Scale (NOS). Data were analyzed using either a random effects model or a fixed effects model for the meta-analysis.
Conclusion: This study establishes a significant association between COPD and CKD and reveals the adverse impact of CKD on the prognosis of COPD patients, which may provide important guidance for clinical practice.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/.
1 Introduction
Chronic obstructive pulmonary disease (COPD) is a global public health challenge, with both its incidence and mortality rates on the rise. According to World Health Organization data, in 2019, there were 213.23 million people globally with COPD. This condition resulted in 3.3 million deaths, accounting for 6% of total global deaths. Notably, 90% of these deaths occurred in low-and middle-income countries, placing a significant burden on national healthcare resources. With the intensification of population aging and the extension of life expectancy, the incidence and mortality rates of COPD are expected to continue to increase (1). In addition to progressive lung function impairment, COPD patients often experience systemic complications, including hypertension, diabetes, lung cancer, pulmonary embolism, and pulmonary hypertension (2–5). Among these complications, chronic kidney disease (CKD) has emerged as a significant comorbidity that poses challenges to public health strategies for the older adult population.
CKD is a progressive kidney disease that leads to a decline in renal function, potentially developing into end-stage renal disease (ESRD), which requires dialysis or kidney transplantation (6). Currently, the estimated global prevalence of CKD is about 10%, and this proportion is expected to continue to rise with the aging global population (7). An increasing number of observational studies have shown that the incidence of CKD is closely related to the prevalence of COPD, and research in this area has gradually attracted widespread academic attention.
A German epidemiological study found that the risk of CKD in COPD patients is significantly higher than in the non-COPD population (8). A case–control study from Taiwan also demonstrated that among the middle-aged and older adult population, the prevalence of CKD in COPD patients increased significantly, with an adjusted hazard ratio of 1.61 (p < 0.0001) (9). However, some studies have not observed this phenomenon (10–12).
Previous meta-analyses have discussed the prevalence of CKD in patients with COPD but did not fully consider the impact of confounding factors such as age, gender, comorbidities, and smoking. The prognostic implications of CKD in COPD patients remain unclear (13). This study aims to comprehensively evaluate the association between COPD and CKD, clarify the risk of CKD in patients with COPD, and explore the impact of CKD on the prognosis of these patients through systematic review and meta-analysis. By conducting comprehensive literature searches and strict screenings, we hope to provide more reliable evidence to support clinical practice and a scientific basis for public health strategies in aging societies. We will systematically assess the risk of CKD in patients with COPD, as well as the mortality risks associated with COPD, acute exacerbation of COPD (AECOPD), and CKD, to fully explore the relationship between the two.
2 Method
2.1 Literature retrieval
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (14). A protocol has been registered with PROSPERO under the registration number CRD42024502706.
We conducted a systematic search of literature published in PubMed, EMBASE, and the Cochrane Library from the inception of these databases to December 31, to identify studies assessing the association between COPD and CKD. The search strategy employed keywords such as “chronic obstructive pulmonary disease” and “chronic kidney disease,” optimized with Boolean operators (see Supplementary Table S1 for details). In addition, we manually searched the reference lists of included studies to identify any potentially missed studies.
2.2 Inclusion and exclusion criteria
Inclusion criteria: (1) Studies eligible for inclusion include cohort studies, case–control studies, and cross-sectional studies; (2) Provide relevant data between COPD and CKD, such as COPD-CKD risk, CKD-COPD risk, COPD-CKD mortality risk, and AECOPD-CKD mortality risk; (3) The diagnosis of COPD must be clear and confirmed by pulmonary function testing or COPD diagnosis codes in medical records; (4) CKD diagnosis must be clearly defined and confirmed by renal function tests or CKD diagnostic codes in medical records.
Exclusion criteria: (1) Duplicate publications, non-human studies, non-English literature, case reports, reviews, meta-analyses, and conference papers; (2) If there are multiple publications in the same cohort, select the most recently published data; (3) Full-text literature that cannot be obtained; (4) Observational groups and control groups have other diseases that may lead to poor prognosis (such as hypertension, coronary heart disease, etc.).
2.3 Data extraction and quality assessment
Two researchers (Liu and Ma) independently screened the literature abstracts and conducted comprehensive full-text reviews to verify their eligibility for inclusion. In case of dispute, a third independent author (Ding) made the final decision. The following information was extracted from the included studies: author, publication year, country, sample size, study design type, patient baseline characteristics, disease diagnosis methods, and related risk data (HR, RR, OR). We used the Newcastle-Ottawa Scale (NOS) to assess the quality of the studies, considering studies with NOS scores ≥7 as high quality.
2.4 Data pooling and analysis
HR, RR, and OR were extracted from the included literature and uniformly quantified as OR (15). A meta-analysis was performed using random-effects or fixed-effects models to assess the mutual impact between COPD and CKD. Forest plots were drawn to visually present the combined results of the studies, and the I2 statistic was used to assess the heterogeneity of the included studies. When significant heterogeneity was found (p < 0.1 or I2 > 50%), the random-effects model was used; otherwise, the fixed-effects model was used (16). When significant heterogeneity was present, subgroup analysis was also conducted to explore the sources of heterogeneity. Sensitivity analysis was performed to detect whether the combined results were significantly affected by a single study, and funnel plots and Egger’s test were used to assess publication bias. All analyses were performed using STATA 15.1 statistical software.
3 Results
3.1 Literature retrieval
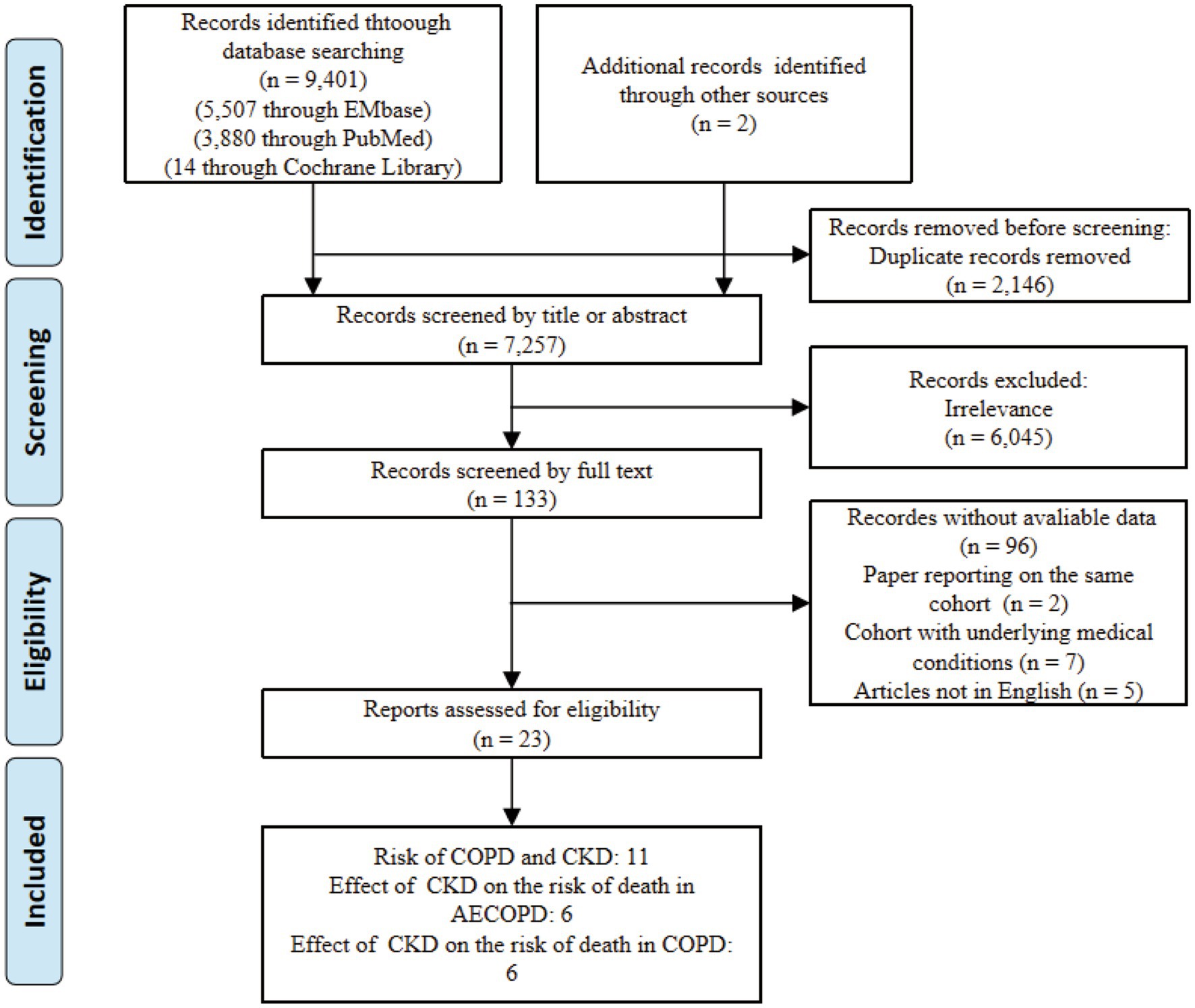
Using the search strategy, we initially identified 9,401 articles. After excluding 2,146 duplicates, we conducted a preliminary screening of the titles and abstracts of the remaining 7,257 articles, eliminating 6,045 irrelevant studies. We then performed a full-text review of the remaining 133 articles and ultimately identified 23 articles that met the inclusion criteria (refer to Figure 1 for the study selection process).
3.2 Basic characteristics of included studies
A total of 23 articles were included, of which 11 examined the risk of CKD in patients with COPD compared to the non-COPD population (8–12, 17–22) (Supplementary Table S2). Six studies assessed the impact of CKD on the short-term all-cause mortality risk in patients with AECOPD (23–28), and another six studies focused on the long-term all-cause mortality risk in COPD patients (Supplementary Table S3) (29–34). These studies encompassed various geographical locations and demographic characteristics, offering a comprehensive perspective. In the 11 studies on the risk of CKD occurrence in COPD, 4 were retrospective cohort studies (8, 9, 19, 20), 2 were case–control studies (10, 21), and 5 were cross-sectional studies (11, 12, 17, 18, 22). Five studies’ data were derived from healthcare databases, with included populations having medical visit records (including outpatient, emergency, and inpatient records) (8–10, 17, 21), four studies’ data were from large-scale population health surveys (11, 12, 18, 22), one study’s data were from a medical center’s health examination population (19), and one study’s data were from outpatients (20). The final results of these studies largely adjusted for risk factors such as age, gender, and comorbidities, but there were still 3 studies that used univariate regression analysis data (8, 10, 20).
Among the studies investigating COPD prognosis, six cohorts reported short-term outcomes for hospitalized patients with AECOPD: four studies assessed in-hospital mortality (23–25, 27), one focused on death within 28 days of admission (26), and one reported mortality within 3 months (28). Furthermore, six cohorts examined the long-term prognosis risk for COPD patients over a duration exceeding 1 year. Among these six cohorts, four studies utilized data from COPD patients enrolled in prospective cohorts established by medical research institutions (29, 31, 32, 34), while the other two studies used data from retrospective cohorts constructed from patient data in medical databases (30, 33).
3.3 Quality assessment
The studies included in the research were assessed using the NOS score, with the majority being high-quality studies (score > 6.0), and only one study considered to be of moderate quality (NOS score of 6 points).
3.4 Data analysis
3.4.1 The impact of COPD on the risk of CKD
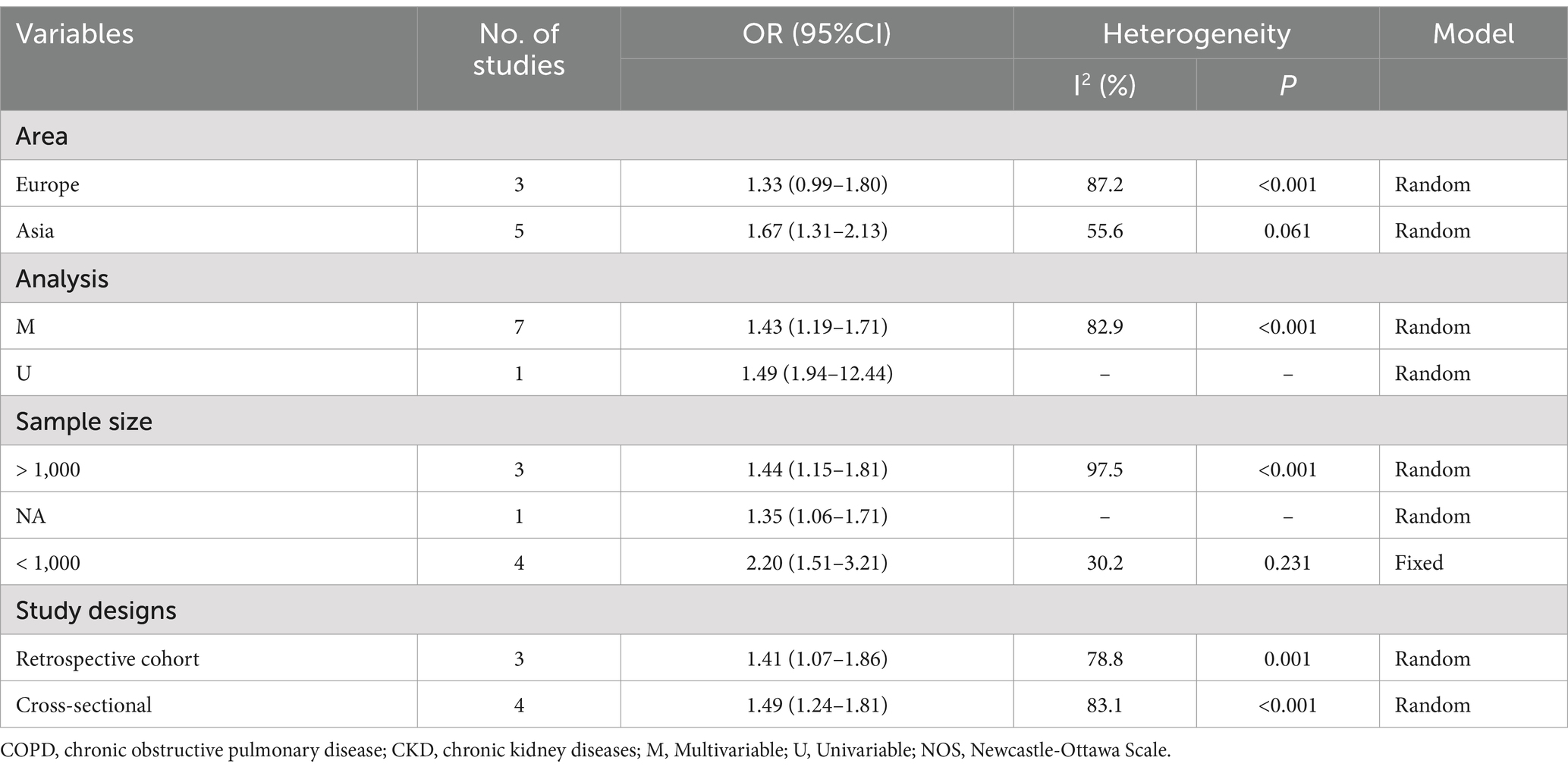
In the 11 studies assessing the risk of CKD in patients with COPD, the odds ratio (OR) for CKD occurrence in COPD patients compared to the normal population was combined using a random effects model: OR 1.54 (95% confidence interval [CI]: 1.28–1.84, I2 = 97.7%, p = 0.000) (see Figure 2A). A sensitivity analysis was conducted by systematically excluding one study at a time and recalculating the combined OR values for the remaining studies. It was found that three studies Greulich et al. (8), Arora et al. (10), and Baty, 2013 (21) significantly influenced the results (see Figure 2B). In these three studies, the first two did not adjust for any confounding factors in the OR value calculation, and the third only adjusted for age and gender, leading to a possible lower precision of the OR value and a smaller range of the corresponding confidence interval. This may have a significant impact on the final combined results when calculated using the inverse variance method. Therefore, after excluding these three studies, the results were recombined as OR 1.49 (95% CI: 1.24–1.81, I2 = 83.1%, p = 0.000) (Figure 2C), which did not qualitatively affect the results and did not reduce the heterogeneity of the combined results. Subgroup analysis was conducted by studying design (cohort or cross-sectional studies), different regions of the study population, sample size, and the analysis methods used in the study results, but no sources of heterogeneity were found (Table 1). A funnel plot was further drawn to observe whether it was symmetrical, and the results did not suggest obvious publication bias (Figure 2D).

Figure 2. Comprehensive analysis of the risk of CKD occurrence in patients with COPD. (A) Forest plot of the risk of CKD occurrence in patients with COPD. (B) Sensitivity analysis of the risk of CKD occurrence in patients with COPD. (C) Forest plot of the risk of CKD occurrence in patients with COPD after excluding influential studies. (D) Funnel plot for publication bias of the risk of CKD occurrence in patients with COPD.
3.4.2 Results of short-term all-cause mortality risk in patients with combined CKD in AECOPD
Six studies assessed the impact of CKD on the short-term all-cause mortality rate in patients with AECOPD. Utilizing a fixed-effects model to combine the short-term all-cause mortality risk data, the results indicated: OR 2.31 (95% CI: 1.80–2.98, I2 = 59.7%, p = 0.03) (see Figure 3). Sensitivity analysis of the included studies revealed that no single study had a significant impact on the results (Figure 4), suggesting that the combined results are relatively robust. Subgroup analysis was conducted based on study design (cohort or cross-sectional studies), different regions of the study population, sample size, and the analysis methods used in the study results. It was found that sample size may be a significant factor contributing to heterogeneity. When the sample size was categorized as >1,000 or < 1,000, the results showed low heterogeneity, but due to the limited number of studies included, it cannot be confidently explained as the source of heterogeneity (Table 2). The funnel plot appeared symmetrical, which may suggest the presence of publication bias (Figure 5). Further Egger’s test was conducted, and the results did not indicate significant publication bias (Egger’s test = 0.954).

Figure 3. Forest plot of the impact of CKD on short-term all-cause mortality risk in patients with AECOPD.
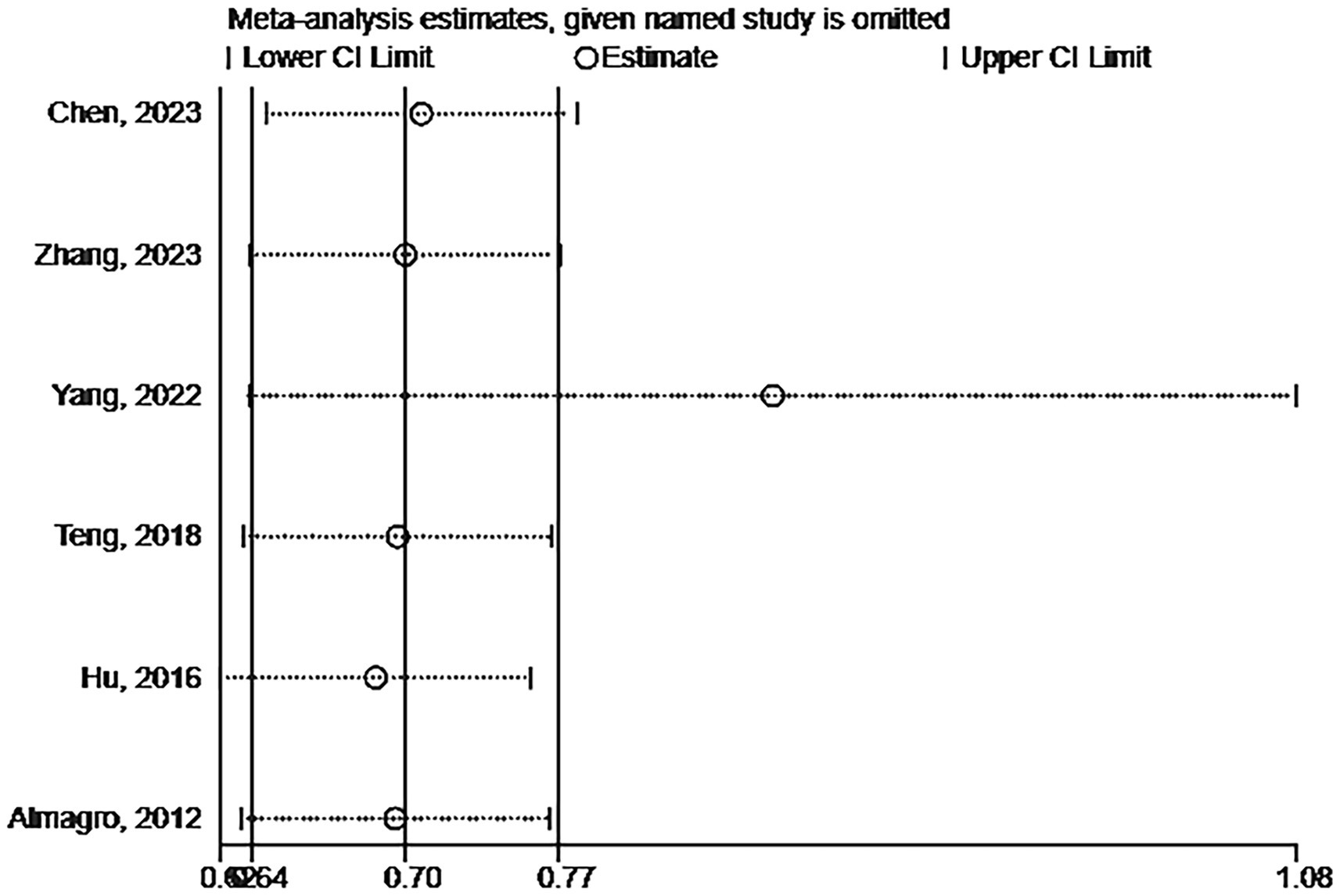
Figure 4. Sensitivity analysis of the impact of CKD on short-term all-cause mortality risk in patients with AECOPD.
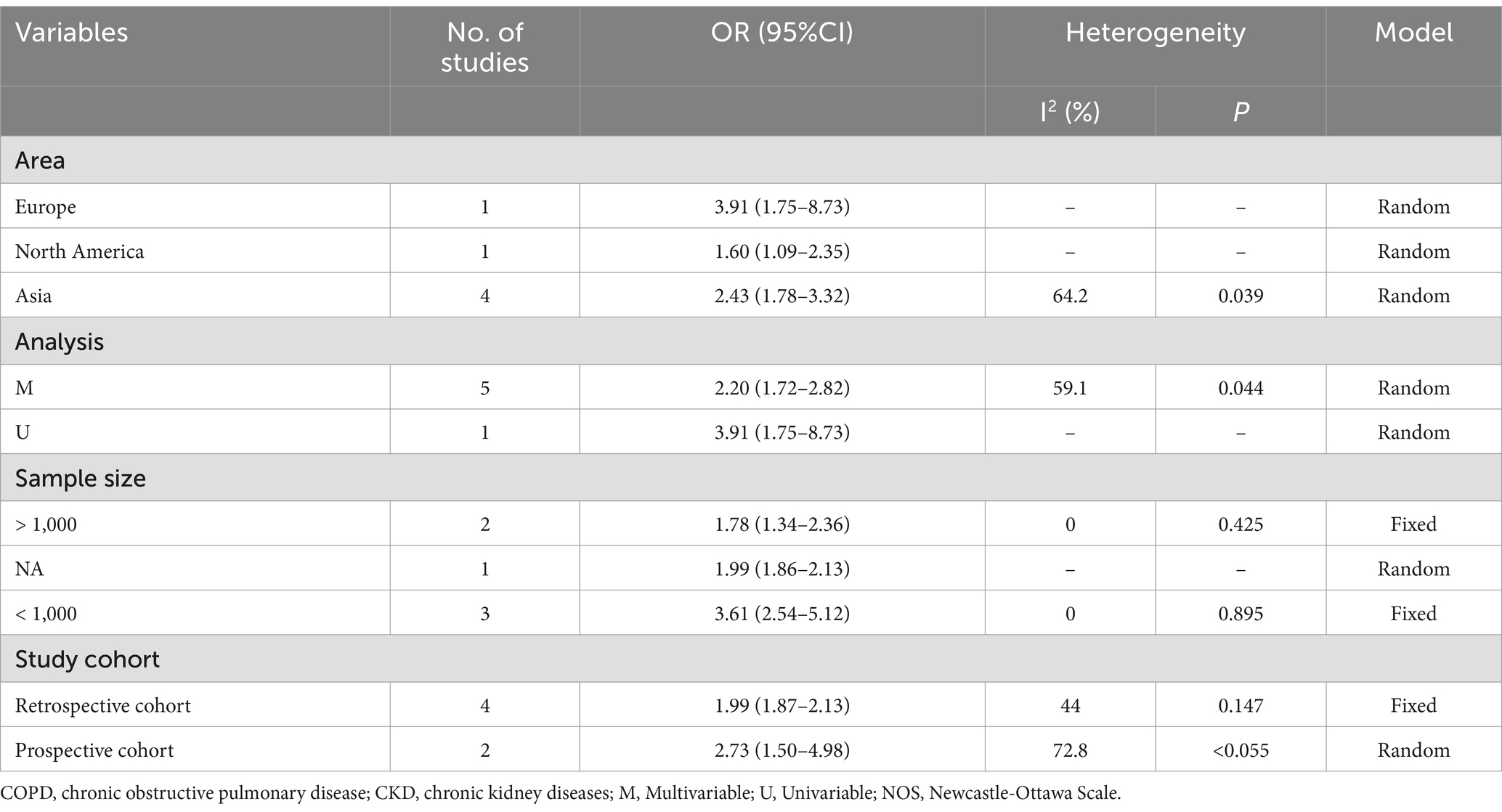
Table 2. The subgroup analysis for studies about Risk of short-term all-cause mortality in AECOPD with CKD.
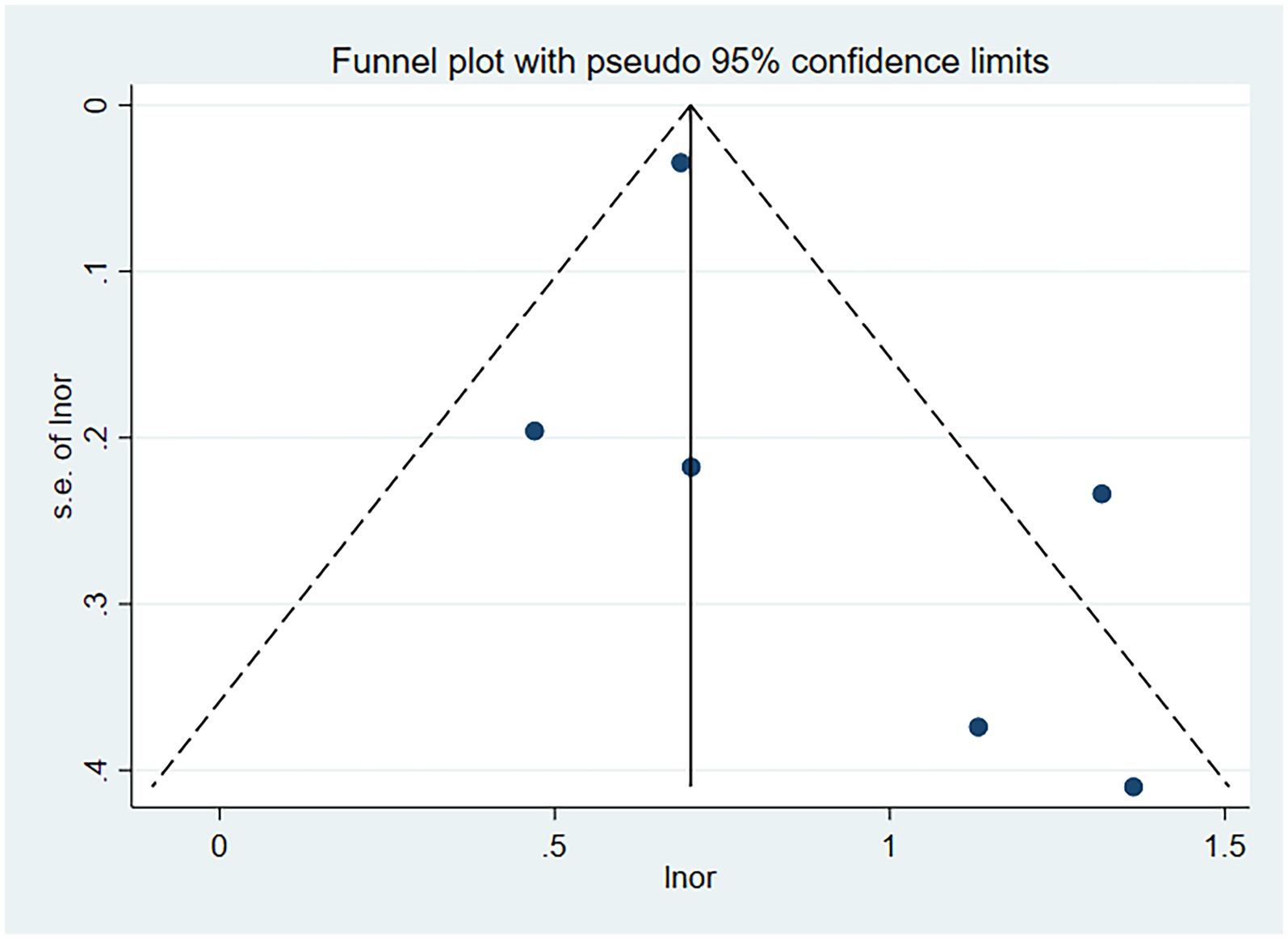
Figure 5. Funnel plot for publication bias of the impact of CKD on short-term all-cause mortality risk in patients with AECOPD.
3.4.3 Results of long-term all-cause mortality risk in COPD patients with CKD
In the six cohort studies analyzing long-term all-cause mortality risk, we employed a random-effects model to combine the relevant mortality risk results, yielding an OR of 1.70 (95% CI: 1.35–2.15, I2 = 61.0%, p = 0.025) (see Figure 6A). Sensitivity analysis showed that Fedeli, 2017 (33) had a significant impact on the combination (Figure 6B). After excluding this study, the results were recombined as OR 1.86 (95% CI: 1.53–2.26, I2 = 0.0%, p = 0.494) (Figure 6C). The funnel plot was basically symmetrical (Figure 6D), and further Egger’s test was performed, with the result being Egger’s test = 0.410.
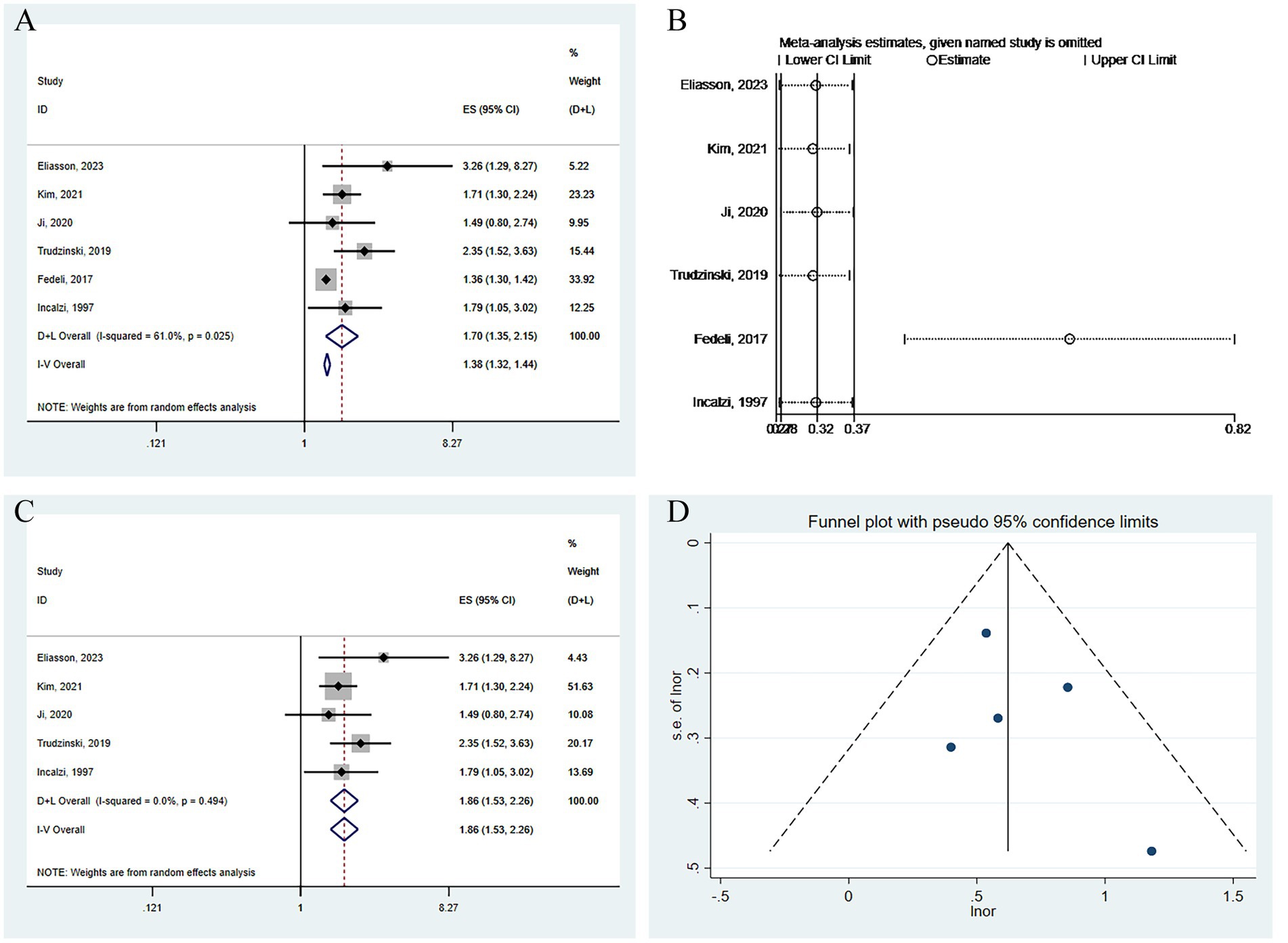
Figure 6. Analysis of the impact of CKD on long-term all-cause mortality risk in patients with COPD. (A) Forest plot of the impact of CKD on long-term all-cause mortality risk in patients with COPD. (B) Sensitivity analysis of the impact of CKD on long-term all-cause mortality risk in patients with COPD. (C) Forest plot of the impact of CKD on long-term all-cause mortality risk in patients with COPD after excluding influential studies. (D) Funnel plot for publication bias of the impact of CKD on long-term all-cause mortality risk in patients with COPD.
4 Discussion
This systematic review and meta-analysis investigated the association between COPD and CKD. Our results indicate that patients with COPD have a significantly increased risk of developing CKD, and the presence of CKD significantly increases the mortality risk of patients with COPD. Specifically, CKD not only increases the short-term all-cause mortality risk of hospitalized patients with AECOPD but also significantly increases the long-term mortality risk of patients with COPD.
Both COPD and CKD are chronic diseases with many common etiological factors, such as advanced age (33), smoking (35, 36), and comorbid diseases.
Firstly, with increasing age, the hospitalization rate, length of hospital stay, and mortality rate of patients with COPD all significantly increase. In the process of repeated acute exacerbations, this can cause chronic kidney damage, leading to an increased incidence of CKD (33), so there is a certain connection between advanced age and the incidence of CKD in patients with COPD. Secondly, smoking is also an important common etiological factor for both COPD and CKD, which plays an indirect pathogenic role by damaging the endothelium of pulmonary blood vessels and alveoli (37, 38). It can also damage the endothelial cells of renal microvessels, possibly through regulating oxidative stress responses and activating the sympathetic nervous system, ultimately increasing the risk of CKD (36). In a meta-analysis of 15 prospective studies including a total of 65,064 cases of COPD, the risk of CKD was significantly higher in people with a history of smoking or current smokers compared to non-smokers (39). Lastly, comorbid diseases also play an important role here. Epidemiological survey studies have shown that CKD caused by diabetes accounts for about 30.7% of the total number of CKD cases (7), so among various comorbid diseases, diabetes has the greatest correlation with CKD. In addition to the kidney, the lung is also considered a target organ for diabetic damage. Some cohort studies have shown that patients with COPD are more likely to have diabetes, and diabetic patients are more likely to have COPD than non-diabetic patients (2, 40), which may be caused by various factors, including microvascular changes in alveolar capillaries and pulmonary arterioles caused by diabetes, chronic pulmonary tissue inflammation, and the impact of diabetes on the elastic recoil force of lung tissue and the autonomic nervous regulation of respiratory muscles (41). In addition to diabetes, the relationship between COPD and CKD is also affected by cardiovascular events. Chronic hypoxia and inflammation in patients with COPD aggravate heart dysfunction through various mechanisms (such as increasing cardiac load, vascular damage, and side effects of beta-agonists and other drugs), thereby affecting renal perfusion (42, 43). The presence of cardiovascular events accelerates the progression of CKD, and the incidence of cardiovascular events in patients with CKD is significantly higher than in the general population (44). Therefore, when patients with COPD also have CKD, comorbidities of the cardiovascular system not only affect the patient’s quality of life but also significantly increase the risk of death.
In the literature we included, many studies have statistically adjusted for these common etiological factors, and the final results still have statistical significance. Therefore, there is reason to believe that there are other potential mechanisms that increase the incidence of CKD in patients with COPD.
Firstly, chronic inflammatory response is one of the important mechanisms between COPD and CKD. The characteristic inflammatory response of COPD triggers the release of pulmonary and systemic inflammatory factors, including tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP). Studies have shown that these factors are not only highly expressed in patients with COPD but are also closely related to the occurrence and progression of CKD (45). For example, Jingli and others have shown that high levels of CRP are positively correlated with the occurrence and progression of CKD, suggesting that chronic inflammation may lead to a decline in renal function by acting on renal tubular cells and glomeruli (46), in addition, inflammatory-mediated apoptosis and fibrotic processes may also play an important role in patients with coexisting COPD and CKD. Relevant studies have shown that TNF-α causes apoptosis of renal tubular epithelial cells and induces renal interstitial fibrosis by activating the nuclear factor-κB (NF-κB) signaling pathway (47).
Secondly, oxidative stress plays a key role in the interaction between COPD and CKD. The lungs of patients with COPD are affected by oxidative stress caused by chronic inflammation, and the body’s redox state is out of balance, leading to a large amount of reactive oxygen species (ROS) being produced (48). Oxidative stress not only directly damages the structural cells of the lungs but also causes microvascular changes by affecting the hemodynamics of the kidneys. For example, oxidative stress can cause apoptosis and dysfunction of renal endothelial cells, thereby reducing kidney perfusion (49). Some studies have pointed out that oxidative stress may cause vasoconstriction and ischemia by enhancing the activity of nitric oxide synthase, further damaging kidney function (50).
In addition, the accumulation of heavy metals in the body of patients with COPD may be a potential mechanism leading to renal tubular damage. A cross-sectional study from South Korea showed that patients with COPD had increased blood cadmium levels, and regardless of smoking status, the FEV1/FVC ratio in patients with COPD was negatively correlated with blood heavy metal (especially cadmium) levels (51), in addition, another study found that the blood cadmium levels in patients with COPD were also negatively correlated with oxygen saturation during the 6-min walk test (6MWT) (52). Long-term hypoxia caused by COPD can simulate iron deficiency, which may promote the overexpression of DMT-1 in the duodenum, thereby increasing the transport of all divalent cations, including toxic heavy metal cadmium. After absorption, cadmium mainly accumulates in the kidneys and liver, especially cadmium in the kidneys, which further causes damage to renal glomeruli and renal tubules (53). Therefore, there is reason to believe that the process of hypoxia/iron deficiency-DMT-1 overexpression-cadmium overload in patients with COPD may be a possibility for the increased incidence of CKD.
This study acknowledges several limitations that should be considered for future research. Firstly, the majority of the studies included are retrospective cohort or cross-sectional studies, which may lead to incomplete or inaccurate case information. Additionally, the adjustment for comorbidities and other confounding factors varied across studies, potentially resulting in inconsistent adjustments and consequently inconsistent outcomes. Secondly, the diagnosis of COPD in patients primarily relied on diagnostic codes within medical databases, which may not adequately reflect the severity of COPD. Concurrently, the GOLD grade of COPD patients, which significantly influences the risk and prognosis of CKD, was not considered in the multifactorial regression models of most included studies, introducing a possible bias in the final outcomes. Furthermore, the treatment of COPD involves a variety of medications, including antimicrobial drugs and long-acting beta2 agonists (LABA), which could increase the risk of kidney damage. However, the vast majority of the studies did not meticulously document the use of COPD-related medications during the follow-up period, and the impact of these drugs was not adjusted for in the statistical analyses. Therefore, more rigorous clinical studies are warranted to refine these findings and address these limitations.
5 Conclusion
In summary, this study, through systematic review and meta-analysis, has established a significant association between COPD and CKD, and has revealed the adverse impact of CKD on both the short-term and long-term prognosis of patients with COPD. Against the backdrop of an aging population, The co-management of COPD and CKD poses significant challenges to public health. Therefore, we recommend that COPD patients undergo regular renal function assessments in clinical management and be managed comprehensively according to the guidelines of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) and Kidney Disease: Improving Global Outcomes (KDIGO) (54) to improve the overall prognosis of patients with COPD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZL: Writing – original draft. ZM: Writing – original draft. CD: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1494291/full#supplementary-material
References
1. Safiri, S, Carson-Chahhoud, K, Noori, M, Nejadghaderi, SA, Sullman, MJM, Ahmadian Heris, J, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the global burden of disease study 2019. BMJ. (2022) 378:e069679. doi: 10.1136/bmj-2021-069679
2. Mannino, DM, Thorn, D, Swensen, A, and Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in Copd. Eur Respir J. (2008) 32:962–9. doi: 10.1183/09031936.00012408
3. Forder, A, Zhuang, R, Souza, VGP, Brockley, LJ, Pewarchuk, ME, Telkar, N, et al. Mechanisms contributing to the comorbidity of Copd and lung Cancer. Int J Mol Sci. (2023) 24:859. doi: 10.3390/ijms24032859
4. Bertoletti, L, Couturaud, F, Sanchez, O, and Jimenez, D. Pulmonary embolism and chronic obstructive pulmonary disease. Semin Thromb Hemost. (2023) 49:809–15. doi: 10.1055/s-0042-1756190
5. Zhang, L, Liu, Y, Zhao, S, Wang, Z, Zhang, M, Zhang, S, et al. The incidence and prevalence of pulmonary hypertension in the Copd population: a systematic review and Meta-analysis. Int J Chron Obstruct Pulmon Dis. (2022) 17:1365–79. doi: 10.2147/copd.S359873
6. Lameire, NH, Levin, A, Kellum, JA, Cheung, M, Jadoul, M, Winkelmayer, WC, et al. Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: improving global outcomes (Kdigo) consensus conference. Kidney Int. (2021) 100:516–26. doi: 10.1016/j.kint.2021.06.028
7. Global, Regional, and National Burden of Chronic Kidney Disease. A systematic analysis for the global burden of disease study. Lancet. (2017) 395:709–33. doi: 10.1016/s0140-6736(20)30045-3
8. Greulich, T, Weist, BJD, Koczulla, AR, Janciauskiene, S, Klemmer, A, Lux, W, et al. Prevalence of comorbidities in Copd patients by disease severity in a German population. Respir Med. (2017) 132:132–8. doi: 10.1016/j.rmed.2017.10.007
9. Chen, CY, and Liao, KM. Chronic obstructive pulmonary disease is associated with risk of chronic kidney disease: a Nationwide case-cohort study. Sci Rep. (2016) 6:25855. doi: 10.1038/srep25855
10. Arora, P, Gupta, A, Golzy, M, Patel, N, Carter, RL, Jalal, K, et al. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol. (2016) 17:112. doi: 10.1186/s12882-016-0325-4
11. Zaigham, S, Christensson, A, Wollmer, P, and Engström, G. Low lung function and the risk of incident chronic kidney disease in the Malmö preventive project cohort. BMC Nephrol. (2020) 21:124. doi: 10.1186/s12882-020-01758-0
12. Joo, H, Park, J, Lee, SD, and Oh, YM. Comorbidities of chronic obstructive pulmonary disease in Koreans: a population-based study. J Korean Med Sci. (2012) 27:901–6. doi: 10.3346/jkms.2012.27.8.901
13. Gaddam, S, Gunukula, SK, Lohr, JW, and Arora, P. Prevalence of chronic kidney disease in patients with chronic obstructive pulmonary disease: a systematic review and Meta-analysis. BMC Pulm Med. (2016) 16:158. doi: 10.1186/s12890-016-0315-0
14. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and Meta-analyses: the Prisma statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
15. Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. (1987) 9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298
16. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a Meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
17. Jurevičienė, E, Burneikaitė, G, Dambrauskas, L, Kasiulevičius, V, Kazėnaitė, E, Navickas, R, et al. Epidemiology of chronic obstructive pulmonary disease (Copd) comorbidities in Lithuanian National Database: a cluster analysis. Int J Environ Res Public Health. (2022) 19:970. doi: 10.3390/ijerph19020970
18. Kim, MY, Boo, S, Yoo, M, Lee, J, and Kang, NR. Impact of chronic kidney disease among Korean adults with chronic obstructive pulmonary disease. Int Urol Nephrol. (2017) 49:1225–32. doi: 10.1007/s11255-017-1572-4
19. Kim, SK, Bae, JC, Baek, JH, Hur, KY, Lee, MK, and Kim, JH. Is decreased lung function associated with chronic kidney disease? A retrospective cohort study in Korea. BMJ Open. (2018) 8:e018928. doi: 10.1136/bmjopen-2017-018928
20. Yoshizawa, T, Okada, K, Furuichi, S, Ishiguro, T, Yoshizawa, A, Akahoshi, T, et al. Prevalence of chronic kidney diseases in patients with chronic obstructive pulmonary disease: assessment based on glomerular filtration rate estimated from creatinine and cystatin C levels. Int J Chron Obstruct Pulmon Dis. (2015) 10:1283–9. doi: 10.2147/copd.S80673
21. Baty, F, Putora, PM, Isenring, B, Blum, T, and Brutsche, M. Comorbidities and burden of Copd: a population based case-control study. PLoS One. (2013) 8:e63285. doi: 10.1371/journal.pone.0063285
22. Nagorni-Obradovic, LM, and Vukovic, DS. The prevalence of Copd co-morbidities in Serbia: results of a National Survey. NPJ Primary Care Respiratory Med. (2014) 24:14008. doi: 10.1038/npjpcrm.2014.8
23. Zhang, J, Qin, Y, Zhou, C, Luo, Y, Wei, H, Ge, H, et al. Elevated bun upon admission as a predictor of in-hospital mortality among patients with acute exacerbation of Copd: a secondary analysis of multicenter cohort study. Int J Chron Obstruct Pulmon Dis. (2023) 18:1445–55. doi: 10.2147/copd.S412106
24. Yang, C, Long, J, Shi, Y, Zhou, Z, Wang, J, Zhao, MH, et al. Healthcare resource utilisation for chronic kidney disease and other major non-communicable chronic diseases in China: a cross-sectional study. BMJ Open. (2022) 12:e051888. doi: 10.1136/bmjopen-2021-051888
25. Chen, S, Shi, Y, Hu, B, and Huang, J. A prediction model for in-hospital mortality of acute exacerbations of chronic obstructive pulmonary disease patients based on red cell distribution width-to-platelet ratio. Int J Chron Obstruct Pulmon Dis. (2023) 18:2079–91. doi: 10.2147/copd.S418162
26. Teng, F, Ye, H, and Xue, T. Predictive value of neutrophil to lymphocyte ratio in patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One. (2018) 13:e0204377. doi: 10.1371/journal.pone.0204377
27. Hu, G, Wu, Y, Zhou, Y, Yu, Y, Liang, W, and Ran, P. Cystatin C as a predictor of in-hospital mortality after exacerbation of Copd. Respir Care. (2016) 61:950–7. doi: 10.4187/respcare.04034
28. Almagro, P, Cabrera, FJ, Diez, J, Boixeda, R, Alonso Ortiz, MB, Murio, C, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of Copd: the Epoc En Servicios De Medicina Interna (Esmi) study. Chest. (2012) 142:1126–33. doi: 10.1378/chest.11-2413
29. Eliasson, G, Janson, C, Johansson, G, Larsson, K, Lindén, A, Löfdahl, CG, et al. Comorbid conditions as predictors of mortality in severe Copd - an eight-year follow-up cohort study. Eur Clin Respiratory J. (2023) 10:2181291. doi: 10.1080/20018525.2023.2181291
30. Kim, Y, Kim, YJ, and Cho, WK. Effect of multiple comorbidities on mortality in chronic obstructive pulmonary disease among Korean population: a Nationwide cohort study. BMC Pulm Med. (2021) 21:56. doi: 10.1186/s12890-021-01424-7
31. Ji, Z, Hernández-Vázquez, J, Domínguez-Zabaleta, IM, Xia, Z, Bellón-Cano, JM, Gallo-González, V, et al. Influence of comorbidities on the survival of Copd patients according to phenotypes. Int J Chron Obstruct Pulmon Dis. (2020) 15:2759–67. doi: 10.2147/copd.S270770
32. Trudzinski, FC, Alqudrah, M, Omlor, A, Zewinger, S, Fliser, D, Speer, T, et al. Consequences of chronic kidney disease in chronic obstructive pulmonary disease. Respir Res. (2019) 20:151. doi: 10.1186/s12931-019-1107-x
33. Fedeli, U, De Giorgi, A, Gennaro, N, Ferroni, E, Gallerani, M, Mikhailidis, DP, et al. Lung and kidney: a dangerous liaison? A population-based cohort study in Copd patients in Italy. Int J Chron Obstruct Pulmon Dis. (2017) 12:443–50. doi: 10.2147/copd.S119390
34. Antonelli Incalzi, R, Fuso, L, De Rosa, M, Forastiere, F, Rapiti, E, Nardecchia, B, et al. Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J. (1997) 10:2794–800. doi: 10.1183/09031936.97.10122794
35. Chandra, D, Stamm, JA, Palevsky, PM, Leader, JK, Fuhrman, CR, Zhang, Y, et al. The relationship between pulmonary emphysema and kidney function in smokers. Chest. (2012) 142:655–62. doi: 10.1378/chest.11-1456
36. Polverino, F, Laucho-Contreras, ME, Petersen, H, Bijol, V, Sholl, LM, Choi, ME, et al. A pilot study linking endothelial injury in lungs and kidneys in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2017) 195:1464–76. doi: 10.1164/rccm.201609-1765OC
37. Wheaton, AG, Liu, Y, Croft, JB, VanFrank, B, Croxton, TL, Punturieri, A, et al. Chronic obstructive pulmonary disease and smoking status - United States, 2017. MMWR Morb Mortal Wkly Rep. (2019) 68:533–8. doi: 10.15585/mmwr.mm6824a1
38. Upadhyay, P, Wu, CW, Pham, A, Zeki, AA, Royer, CM, Kodavanti, UP, et al. Animal models and mechanisms of tobacco smoke-induced chronic obstructive pulmonary disease (Copd). J Toxicol Environ Health B Crit Rev. (2023) 26:275–305. doi: 10.1080/10937404.2023.2208886
39. Xia, J, Wang, L, Ma, Z, Zhong, L, Wang, Y, Gao, Y, et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and Meta-analysis of prospective cohort studies. Nephrol Dialysis Transpl. (2017) 32:475–87. doi: 10.1093/ndt/gfw452
40. Gayle, A, Dickinson, S, Poole, C, Pang, M, Fauconnot, O, and Quint, JK. Incidence of type ii diabetes in chronic obstructive pulmonary disease: a nested case-control study. NPJ Primary Care Respiratory Med. (2019) 29:28. doi: 10.1038/s41533-019-0138-6
41. Zhang, L, Jiang, F, Xie, Y, Mo, Y, Zhang, X, and Liu, C. Diabetic endothelial Microangiopathy and pulmonary dysfunction. Front Endocrinol. (2023) 14:1073878. doi: 10.3389/fendo.2023.1073878
42. Hesse, K, Bourke, S, and Steer, J. Heart failure in patients with Copd exacerbations: looking below the tip of the iceberg. Respir Med. (2022) 196:106800. doi: 10.1016/j.rmed.2022.106800
43. Axson, EL, Ragutheeswaran, K, Sundaram, V, Bloom, CI, Bottle, A, Cowie, MR, et al. Hospitalisation and mortality in patients with comorbid Copd and heart failure: a systematic review and Meta-analysis. Respir Res. (2020) 21:54. doi: 10.1186/s12931-020-1312-7
44. Zoccali, C, Mallamaci, F, Adamczak, M, de Oliveira, RB, Massy, ZA, Sarafidis, P, et al. Cardiovascular complications in chronic kidney disease: a review from the European renal and cardiovascular medicine working Group of the European Renal Association. Cardiovasc Res. (2023) 119:2017–32. doi: 10.1093/cvr/cvad083
45. Guo, P, Li, R, Piao, TH, Wang, CL, Wu, XL, and Cai, HY. Pathological mechanism and targeted drugs of Copd. Int J Chron Obstruct Pulmon Dis. (2022) 17:1565–75. doi: 10.2147/copd.S366126
46. Gao, J, Wang, A, Li, X, Li, J, Zhao, H, Zhang, J, et al. The cumulative exposure to high-sensitivity C-reactive protein predicts the risk of chronic kidney diseases. Kidney Blood Press Res. (2020) 45:84–94. doi: 10.1159/000504251
47. Zhang, JQ, Li, YY, Zhang, XY, Tian, ZH, Liu, C, Wang, ST, et al. Cellular senescence of renal tubular epithelial cells in renal fibrosis. Front Endocrinol. (2023) 14:1085605. doi: 10.3389/fendo.2023.1085605
48. Antus, B, and Kardos, Z. Oxidative stress in Copd: molecular background and clinical monitoring. Curr Med Chem. (2015) 22:627–50. doi: 10.2174/092986732205150112104411
49. Irazabal, MV, and Torres, VE. Reactive oxygen species and redox signaling in chronic kidney disease. Cells. (2020) 9:342. doi: 10.3390/cells9061342
50. Carlström, M. Nitric oxide Signalling in kidney regulation and Cardiometabolic health. Nat Rev Nephrol. (2021) 17:575–90. doi: 10.1038/s41581-021-00429-z
51. Leem, AY, Kim, SK, Chang, J, Kang, YA, Kim, YS, Park, MS, et al. Relationship between blood levels of heavy metals and lung function based on the Korean National Health and nutrition examination survey iv-V. Int J Chron Obstruct Pulmon Dis. (2015) 10:1559–70. doi: 10.2147/copd.S86182
52. Chiu, LC, Hsu, PC, Yen, TH, Kuo, SC, Fang, YF, Lo, YL, et al. Blood cadmium levels and oxygen desaturation during the 6-minute walk test in patients with chronic obstructive pulmonary disease. Medicina. (2021) 57:–160. doi: 10.3390/medicina57111160
53. Cirovic, A, Denic, A, Clarke, BL, Vassallo, R, Cirovic, A, and Landry, GM. A hypoxia-driven occurrence of chronic kidney disease and osteoporosis in Copd individuals: new insights into environmental cadmium exposure. Toxicology. (2022) 482:153355. doi: 10.1016/j.tox.2022.153355
Keywords: chronic obstructive pulmonary disease, chronic kidney disease, systematic review, meta-analysis, comorbidity
Citation: Liu Z, Ma Z and Ding C (2024) Association between COPD and CKD: a systematic review and meta-analysis. Front. Public Health. 12:1494291. doi: 10.3389/fpubh.2024.1494291
Edited by:
Roberto Giovanni Carbone, University of Genoa, Genoa, ItalyReviewed by:
Alessia Verduri, University of Modena and Reggio Emilia, ItalyÖzge Aydın Güçlü, Bursa Uludağ University, Türkiye
Copyright © 2024 Liu, Ma and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaowei Ding, Mzk4OTkyOTg3QHFxLmNvbQ==
 Zexin Liu1
Zexin Liu1 Chaowei Ding
Chaowei Ding