- 1Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Emergency, Qilu Hospital of Shandong University, Jinan, China
Introduction: Facing Mount Tai in the south and the Yellow River in the north, Zibo District is an important petrochemical base in China. The effect of air pollution on cardiovascular diseases (CVDs) in Zibo was unclear.
Methods: Daily outpatient visits of common CVDs including coronary heart disease (CHD), stroke, and arrhythmia were obtained from 2019 to 2022 in Zibo. Air pollutants contained fine particulate matter (PM2.5), inhalable particulate matter (PM10), nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), and carbon monoxide (CO). Distributed lag non-linear models (DLNM) including single-pollutant model in single-day (lag0-lag7) and cumulative-days (lag01-lag07), concentration-response curve, subgroup analysis, and double-pollutant model were utilized to examine the relationships of daily air pollutants on CHD, stroke, and arrhythmia. Meteorological factors were incorporated to control confounding.
Results: In single-pollutant model, NO2 was positively associated with CHD, stroke and arrhythmia, with the strongest excess risks (ERs) of 4.97% (lag07), 4.71% (lag07) and 2.16% (lag02), respectively. The highest ERs of PM2.5 on CHD, stroke and arrhythmia were 0.85% (lag01), 0.59% (lag0) and 0.84% (lag01), and for PM10, the ERs were 0.37% (lag01), 0.35% (lag0) and 0.39% (lag01). SO2 on CHD was 0.92% (lag6), O3 on stroke was 0.16% (lag6), and CO on CHD, stroke, and arrhythmia were 8.77% (lag07), 5.38% (lag01), 4.30% (lag0). No threshold was found between air pollutants and CVDs. The effects of ambient pollutants on CVDs (NO2&CVDs, PM2.5&stroke, PM10&stroke, CO&stroke, CO&arrhythmia) were greater in cold season than warm season. In double-pollutant model, NO2 was positively associated with CHD and stroke, and CO was also positively related with CHD.
Conclusion: Ambient pollutants, especially NO2 and CO were associated with CVDs in Zibo, China. And there were strong relationships between NO2, PM2.5, PM10, CO and CVDs in cold season.
1 Introduction
The incidence and mortality of cardiovascular diseases (CVDs) are continuously rising, as revealed by the China Cardiovascular Disease Report 2022. There are approximately 330 million CVD patients, including 13 million cases of stroke, 11.4 million cases of coronary heart disease (CHD), and 4.87 million cases of atrial fibrillation in China. CVDs account for over 40% of the total deaths, making it the leading cause of death among the population (1). A staggering 6.67 million deaths worldwide, accounting for 12% of total deaths, were attributed to air pollution. Ambient air pollutants rank as the fourth leading risk factor for the burden of CVDs, as reported by the Global Burden of Disease (GBD) (2). Ambient pollution has become one of the biggest threats of our time (3). Environmental air pollutants are important health hazards (4). Numerous domestic and international studies have indicated that exposure to ambient air pollutants could lead to various cardiovascular health outcomes. Short-term exposure to fine particulate matter (PM2.5), inhalable particulate matter (PM10), and nitrogen oxides (NOx) were associated with increased risks of myocardial infarction and stroke (5). Sulfur dioxide (SO2) and nitrogen dioxide (NO2) significantly increased the daily number of cardiovascular hospitalizations in areas with a low level of air pollution (6). Various studies have shown that exposure to carbon monoxide (CO) was associated with mortality, hospital admissions, and outpatient visits of CVDs (7–12). However, there are discrepancies in the natural environment and population structure across different regions.
Zibo, a modern industrial area and an important petrochemical base, is one of the “2 + 26” cities comprised by of Beijing City, Tianjin City, and 26 surrounding cities-the area with the worst air quality in China. In December 2021, Zibo municipal government declared that various industrial furnaces and kilns in Zibo, accounting for more than one-fifth in Shandong Province, could emit large amounts of pollutants. However, no existing study in the region exploring the risks of ambient air pollutants on CHD, stroke and arrhythmia has been reported. Therefore, this study focused on investigating the effects of ambient air pollutants on common CVDs, and identifying key pollutants that had significant impacts on CHD, stroke and arrhythmia from 2019 to 2022 in Zibo.
2 Materials and methods
2.1 Study area
Zibo district, located in the center of Shandong Province in eastern China (Figure 1), is one of the core cities of the Shandong Peninsula urban agglomeration. It is situated between 35°55′20″ and 37°17′14″ north latitude, 117°32′15″ and 118°31′00″ east longitude, bordered by Mount Tai to the south and the Yellow River to the north. The terrain of Zibo is high in the south and low in the north, with an elevation difference of over 1, 000 meters. Zibo is situated with the warm temperate zone and experiences a semi-humid and semi-arid continental climate. These topographic and climatic conditions restrict the diffusion of ambient air pollutants. The district covered a total area of 5, 965 square kilometers and had a resident population of 4.71 million people as of the end of 2022, as released by the 2023 Statistical Yearbook of Zibo.
2.2 Data
2.2.1 Data on daily outpatient visits
Data on daily outpatient visits of CHD, stroke, and arrhythmia from January 1, 2019 to December 31, 2022 were from all hospitals with hospital information system (HIS) in Zibo. The data were extracted based on the corresponding diagnosis codes from the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10), focusing on CHD (I20-I25), stroke (I60-I61and I63-I64), and arrhythmia (I44-I49). The dataset contained diagnosis codes, outpatient codes, outpatient visit dates, age, gender, and local resident or not. Inclusion criteria: diseases with ICD-10 codes including I20-I25, I44-I50, I60-I61 and I63-I64. Exclusion criteria: (1) patients with residential address outside Zibo; (2) records without complete information.
2.2.2 Data on ambient air pollutants
Data on ambient air pollutants were from the China National Environmental Monitoring Centre (CNEMC), the National Urban Air Quality Real-time Publishing Platform. The data were collected from seven state-controlled monitoring sites in Zibo, which included People’s Park, Shuangshan, Meteorological Station, Putian Park, New District, Sankin Group, Dongfeng Chemical Factory. Pollutants including NO2, PM2.5, PM10, SO2, CO were reported as 24-h average concentrations, while ozone O3 was measured as the daily maximum 8-h concentration.
2.2.3 Data on meteorology factors
Meteorological data were obtained from China Meteorological Administration, including temperature (°C), relative humidity (%), and wind speed (m/s).
2.3 Statistical methods
2.3.1 Descriptive statistical analysis
Continuous variables, including daily outpatient visits, ambient air pollutants, and meteorological factors, were described using mean, standard deviation (SD), and percentiles (1st, 25th, 50th, 75th, and 99th). Categorical data were characterized by frequency (n) and composition ratio (%). Spearman′s correlation coefficient was used to represent the correlation among ambient pollutants and meteorological factors.
2.3.2 Statistical analysis
A distributed lag non-linear model (DLNM) was utilized to examine the associations between air pollutants (NO2, PM2.5, PM10, SO2, O3 and CO) and outpatient visits of CVDs. The main strength of DLNM was its ability to concurrently model exposure-response and lag-response relationships between exposure and outcome, thereby elucidating the relationship between exposure and outcome across both exposure and lag dimensions (13). The basic structure of the model was outlined as follows,
where Yt represented the actual value of outpatient visits on day t, E (Yt) represented the expected value of outpatient visits, α represented the intercept, and cb represented the cross-basis function. Pollutant, Tt, RHt and Windt represented the values of ambient air pollutants, temperature, relative humidity, and wind speed on day t, respectively. Additionally, ttime was incorporated in the form of a natural cubic spline function (ns) to control for long-term and seasonal trends, with the degree of freedom (df) set to 7. In the cross-basis between temperature, relative humidity, wind speed and CVDs, the df was set to 3. VDOW and Vholiday were dummy variables to control the effects of weekday and holiday. Finally, a linear approach was applied to fit the concentration-response relationship (14, 15).
The effect of ambient air pollution on cardiovascular events generally persisted for 3 to 6 days, as shown by previous studies (16–18). To adequately examine the lag effects of ambient pollutants, the maximum lag day in the study was set to 7. Additionally, the effects of air pollutants on CVDs can manifest as both single and cumulative lag effects, therefore, single-pollutant model was used to examine the effects of ambient air pollutants in single-day and cumulative-days on CVDs. Lag0-lag7 was used to denote single-day and lag01-lag07 to denote cumulative-days. Double-pollutant model was developed by adjusting for other air pollutants, including NO2, PM2.5, PM10, SO2, O3 and CO.
2.3.3 Subgroup analysis and sensitivity analysis
To gain further understanding of the associations between air pollutants and outpatient visits of CVDs, subgroup analysis was performed based on gender, age, and season. Gender groups were conducted for males and females. Age groups were stratified into less than 60 years old (<60 years) and those aged 60 years or older (≥60 years). Seasons were categorized as warm season (April to September) and cold season (October to March). Z-tests were used to assess statistical differences between subgroups with the following formula,
where β1 and β2 were the regression coefficients of subgroups, and SE1 and SE2 were the corresponding standard errors (8, 14, 19).
Sensitivity analysis was conducted by adjusting the df of temperature, relative humidity, and wind speed in the cross-basis to 4 and 5, respectively. Additionally, the df of the ns, used to control for long-term and seasonal trends, was modified to 8.
Zero value of daily air pollutant concentration was used as a reference, relative risk (RR) and its 95% confidence interval (95%CI) were used to assess the associations between air pollutants and CVDs. To facilitate the interpretation and comparison of data, RR was converted into excess risk (ER), using the following equation: . ER (95%CI) indicated percentage changes in outpatient visits of CVDs associated with every 10 μg/m3 increase of ambient air pollutants (with CO represented as 1 mg/m3). All analyses were two-sided tests with a significance level of α = 0.05 and performed in R 4.1.3.
3 Results
3.1 Data description
The annual average concentrations of NO2, PM2.5, PM10, SO2, O3, and CO were 36.9 μg/m3, 51.2 μg/m3, 91.3 μg/m3, 16.9 μg/m3, 113.4 μg/m3, and 0.9 mg/m3, respectively (Table 1). A total of 1, 089, 136 outpatient visits were documented. The highest number of outpatient visits was CHD, totaling 809, 792 cases, which accounted for 74.3%, and others included stroke with 142, 625 cases (13.1%), and arrhythmia with 136, 719 cases (12.6%). Statistical differences were found in gender and age subgroups of CHD, stroke and arrhythmia.
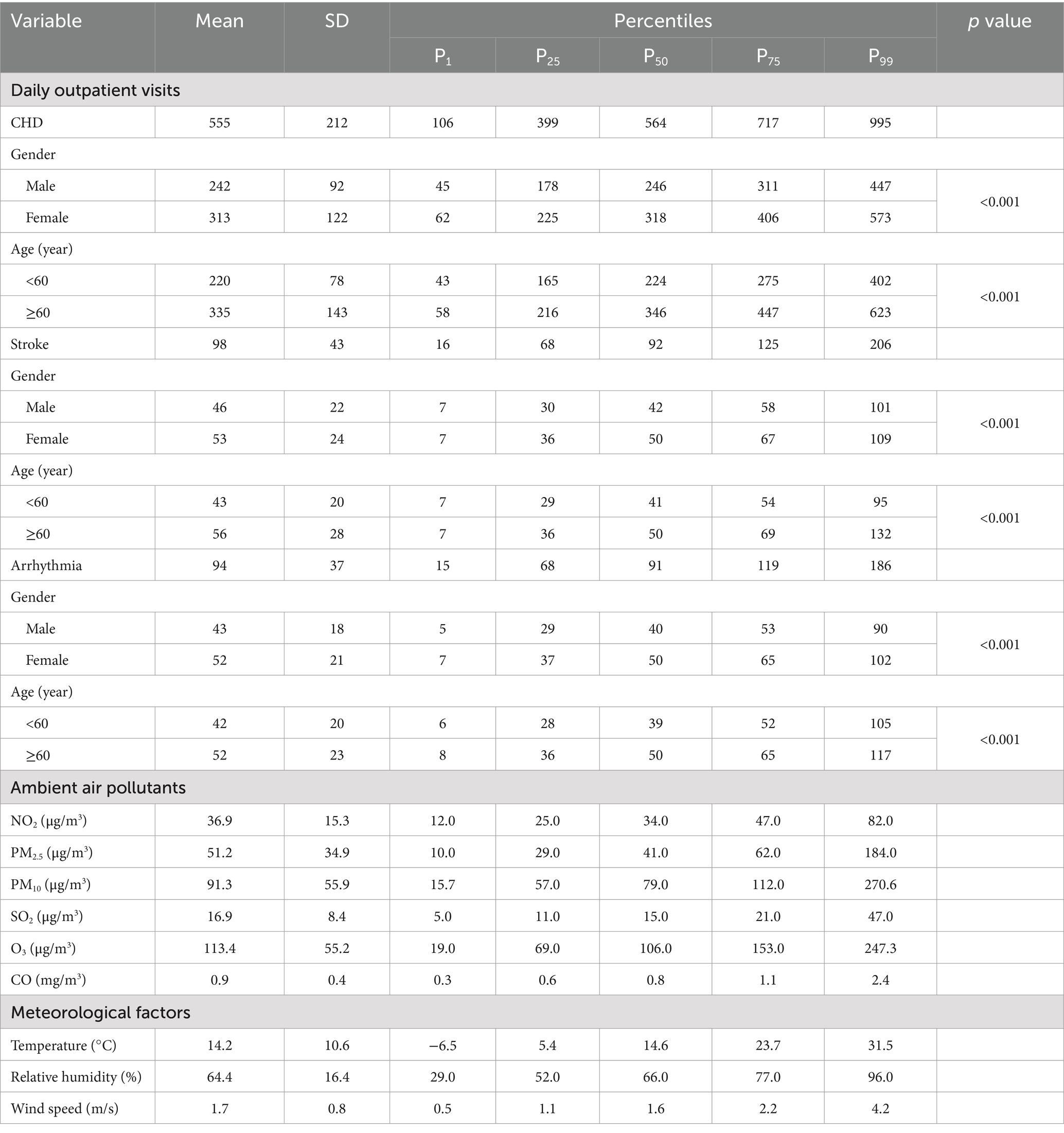
Table 1. Summary statistics of daily outpatient visits of CHD, stroke, arrhythmia, ambient air pollutants, and meteorological factors in Zibo, China, 2019–2022.
There was obvious seasonality in concentrations of air pollutants, the concentrations of NO2, PM2.5, PM10, SO2, and CO were higher in winter and lower in summer, while O3 showed an opposite pattern (Figure 2). In addition, the concentrations of NO2, PM2.5, PM10, SO2 and CO showed a decreasing trend from 2019 to 2022, but the annual change of O3 was less obvious. Compared to PM2.5, the duration of higher NO2 concentration was extended, peaking from January to March and from September to December.
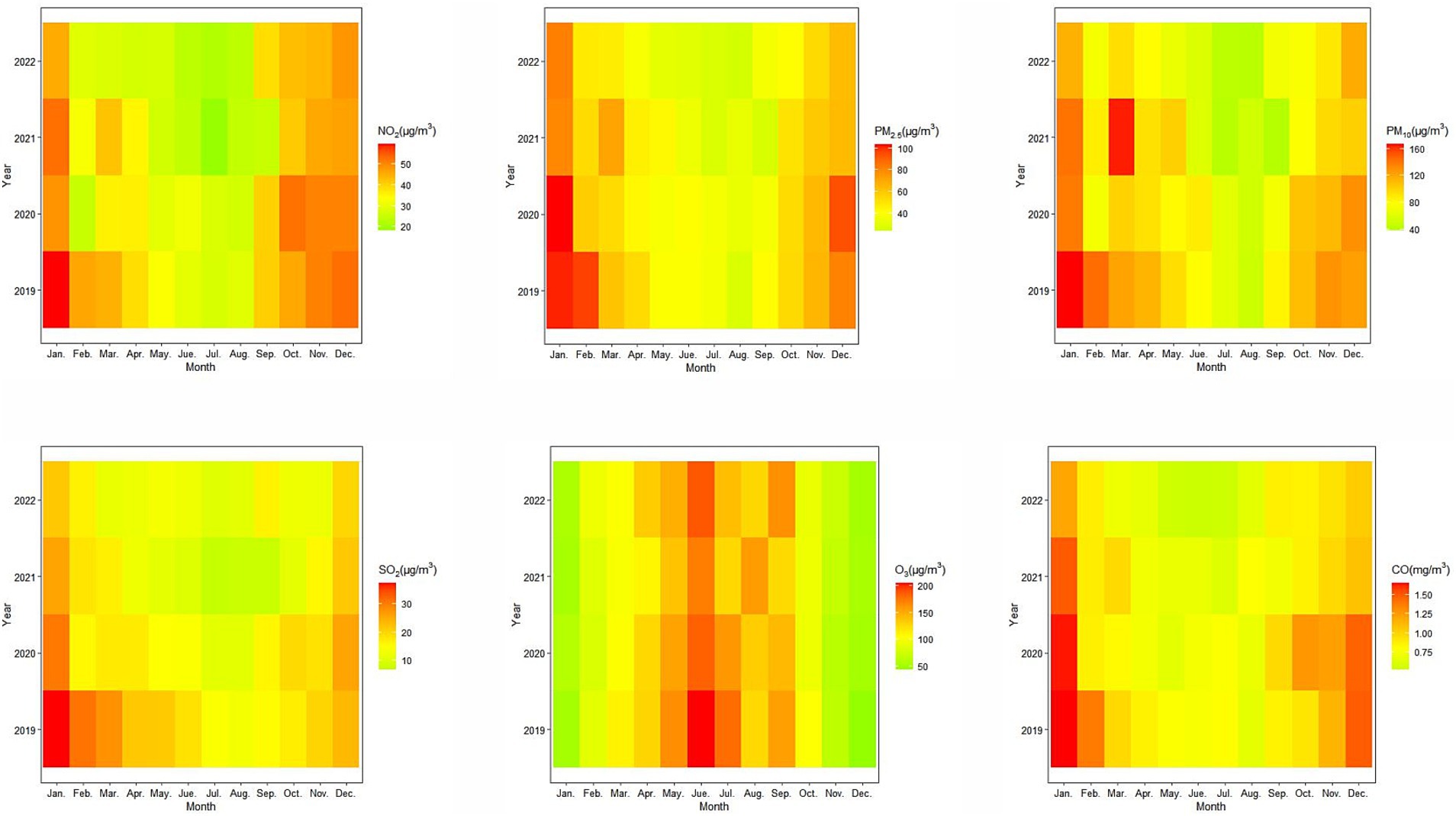
Figure 2. Monthly variations of ambient air pollutants (NO2, PM2.5, PM10, SO2, O3, and CO) in Zibo, China, 2019–2022.
3.2 Regression results
3.2.1 Spearman′s correlation analysis
NO2, PM2.5, PM10, SO2 and CO were positively correlated with each other, while these pollutants mentioned above were negatively correlated with O3, temperature, and wind speed. O3 was positively correlated with temperature and wind speed, but it was negatively correlated with relative humidity. Temperature was positively correlated with relative humidity and wind speed. Additionally, relative humidity was positively correlated with CO, but negatively associated with PM10, SO2, O3, wind speed (see Supplementary Table S1).
3.2.2 Single-pollutant model
The single effects of NO2, PM2.5, PM10 and CO on CHD, stroke and arrhythmia peaked on the current day and then gradually declined, becoming insignificant after approximately 1–2 days (Figure 3). The strongest single ERs of NO2 on CHD, stroke and arrhythmia were 2.14% (95%CI: 1.12, 3.18), 2.02% (95%CI: 0.78, 3.27) and 1.49% (95%CI: 0.05, 2.95) at lag0, respectively. The strongest ERs between PM2.5 and mentioned above diseases were 0.80% (95%CI: 0.43, 1.18), 0.59% (95%CI: 0.13, 1.06) and 0.72% (95%CI: 0.13, 1.06) at lag0, and for PM10, the ERs were 0.34% (95%CI: 0.15, 0.52), 0.35% (95%CI: 0.13, 0.57) and 0.31% (95%CI: 0.03, 0.59) at lag0. CO on CHD, stroke and arrhythmia were 5.26% (95%CI: 2.39, 8.22), 4.74% (95%CI: 0.99, 8.63), and 4.30% (95%CI: 0.14, 8.64) at lag0. SO2 was only associated with CHD, with the strongest ER of 0.92% (95%CI: 0.03, 1.81) at lag6. O3 was solely associated with stroke, with the highest ER of 0.16% (95%CI: 0.01, 0.31) at lag6.
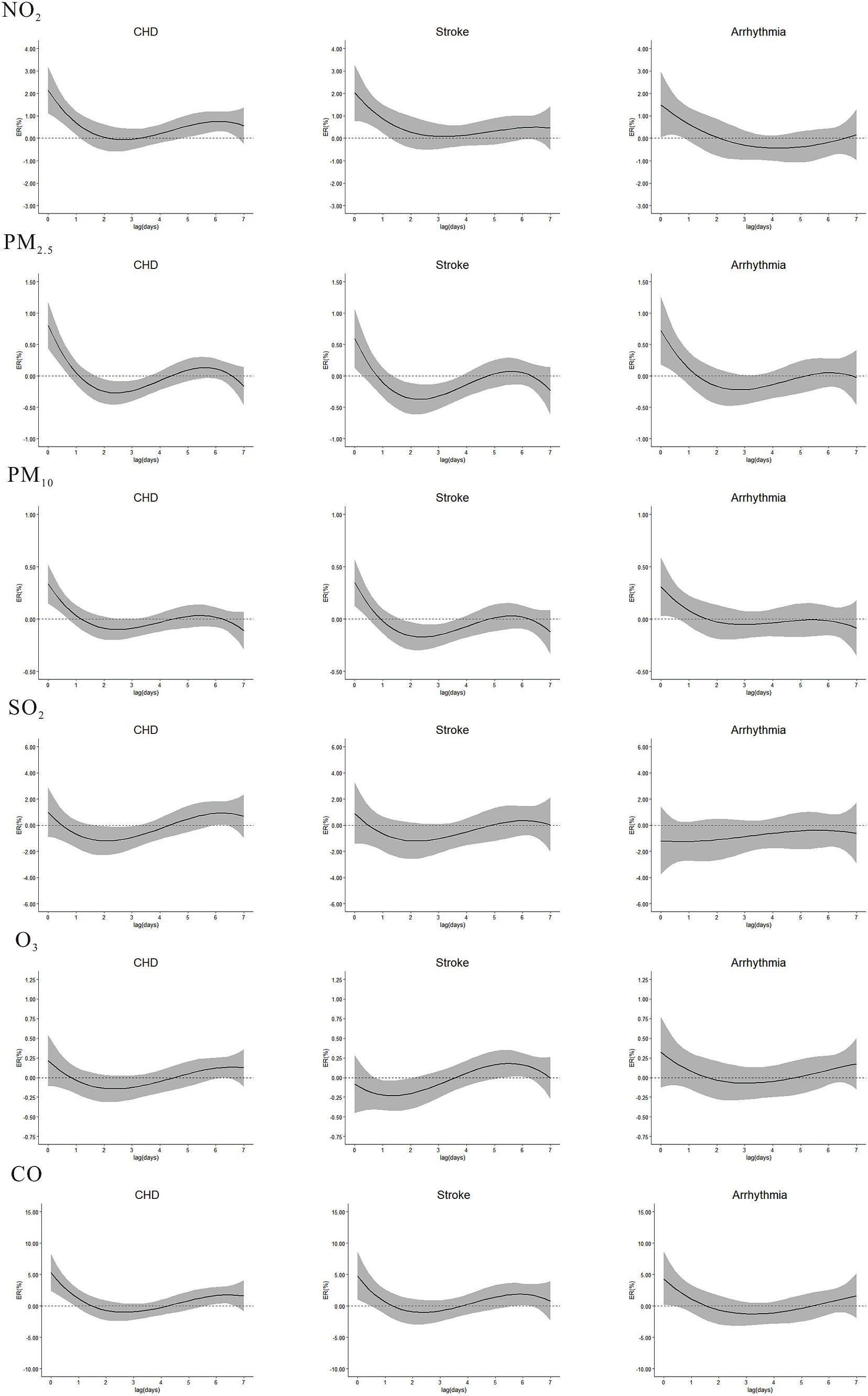
Figure 3. Single excess risks (ER, 95%CI) in outpatient visits of CHD, stroke, and arrhythmia associated with NO2, PM2.5, PM10, SO2, O3, and CO in single-pollutant model, at lag0-lag7.
NO2 was significantly associated with CHD and stroke during the entire cumulative lag period (Figure 4), the highest cumulative effects of NO2 on CHD, stroke and arrhythmia were at lag07 (ER = 4.97, 95%CI: 3.03, 6.94), lag07 (ER = 4.71, 95%CI: 2.37, 7.10), and lag02 (ER = 2.16, 95%CI: 0.12, 4.25). The highest ERs of PM2.5 on CHD and arrhythmia were 0.85% (95%CI: 0.38, 1.33) and 0.84% (95%CI: 0.15, 1.53) at lag01. For PM10, the ERs on CHD, stroke and arrhythmia were 0.37% (95%CI: 0.14, 0.60), 0.33% (95%CI: 0.05, 0.61) and 0.39% (95%CI: 0.04, 0.74) at lag01. The ERs of CO on CHD and stroke were at lag07 (ER = 8.77, 95%CI: 2.06, 15.91) and lag01 (ER = 5.38, 95%CI: 0.45, 10.54). No cumulative effect of PM2.5 on stroke, CO on arrhythmia, SO2 and O3 on CHD, stroke and arrhythmia was observed. ERs of NO2, PM2.5, PM10, SO2, O3 and CO in single-pollutant models of single-day and cumulative-days on outpatient visits of CHD, stroke, and arrhythmia peaked at different lag days (Table 2).
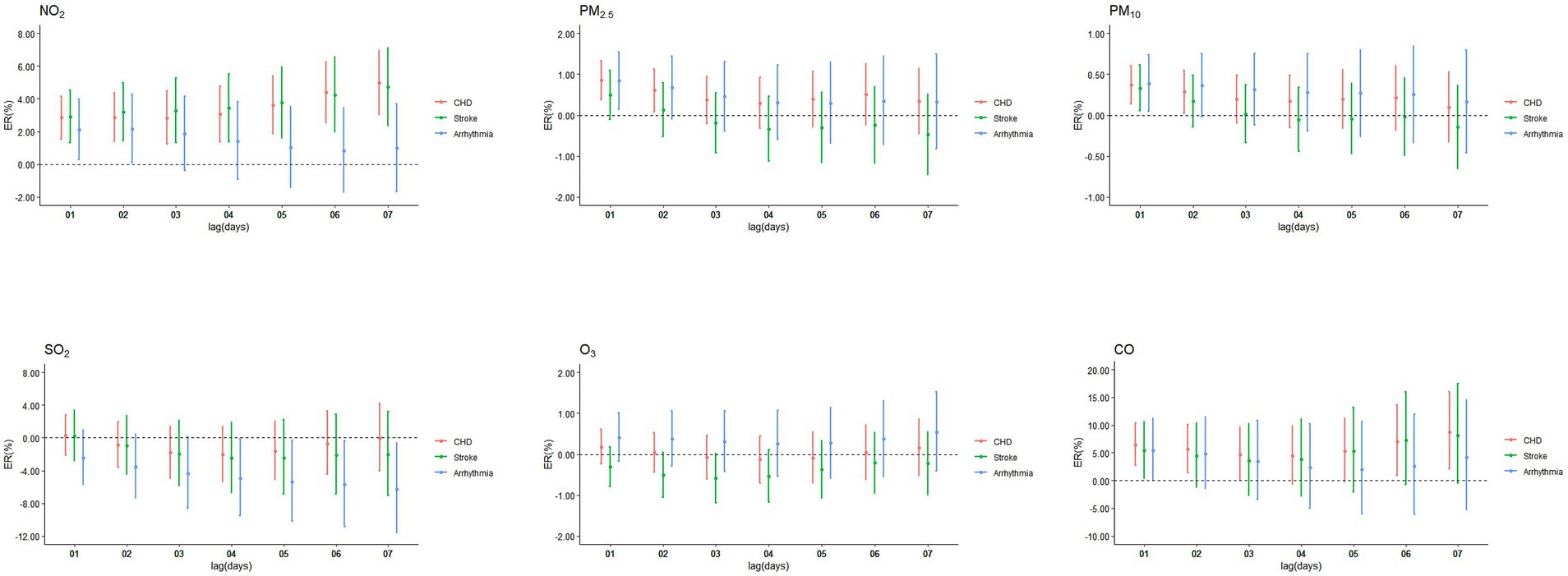
Figure 4. Cumulative excess risks (ER, 95%CI) in outpatient visits of CHD, stroke, and arrhythmia associated with ambient air pollutants in single-pollutant model, at lag01-lag07.
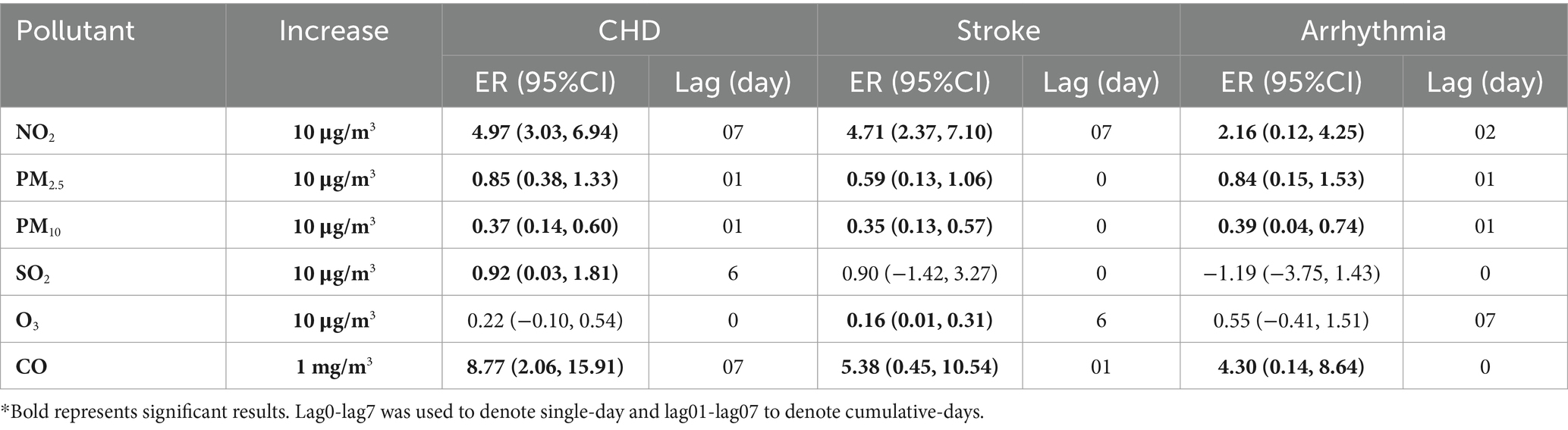
Table 2. The largest excess risks (95%CI) and the lags of NO2, PM2.5, PM10, SO2, O3, and CO on outpatient visits of CHD, stroke, and arrhythmia, in single-pollutant model.
Concentration-response curve was developed based on defining the concentration range of air pollutants from 0 to P99 to mitigate the impact of extreme values. Overall, the curves had no apparent threshold. Specifically, in low-concentration range, the curves were steep, while in high-concentration range, they tended to become less steep (Figure 5). In subgroup analysis, the risks of NO2 on CHD, stroke, and arrhythmia, PM2.5 and PM10 on stroke, and CO on stroke and arrhythmia were stronger during cold season than warm season (Table 3). No difference was found in gender and age subgroups. Sensitivity analyses of NO2, PM2.5, PM10, SO2, O3 and CO on CVDs were robust after changing the df (Supplementary Table S2), except that the relationship between CO and stroke became weakly insignificant (ER = 4.79, 95%CI: −0.11, 9.92) after adjusting the df of ns to 8.
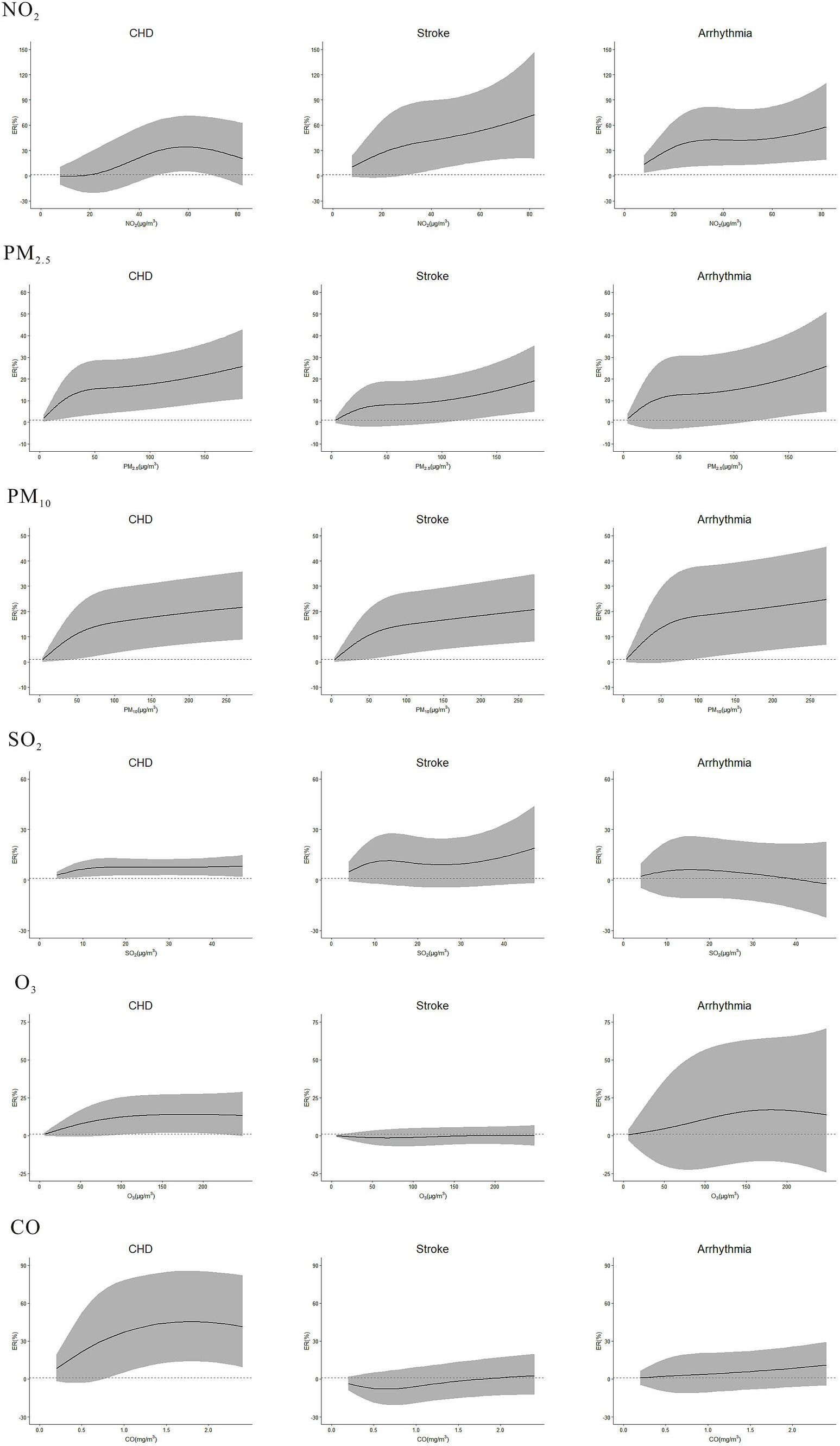
Figure 5. Concentration-response curve between NO2, PM2.5, PM10, SO2, O3, CO and the outpatient visits of CHD, stroke, and arrhythmia in single-pollutant model. The lags of NO2, PM2.5, PM10, SO2, O3, and CO on outpatient visits of CHD, stroke, and arrhythmia: NO2-CHD (lag07), NO2-stroke (lag07), NO2-Arrhythmia (lag02), PM2.5-CHD (lag01), PM2.5-stroke (lag0), PM2.5-Arrhythmia (lag01), PM10-CHD (lag01), PM10-stroke (lag0), PM10-Arrhythmia (lag01), SO2-CHD (lag6), SO2-stroke (lag0), SO2-Arrhythmia (lag0), O3-CHD (lag0), O3-stroke (lag6), O3-Arrhythmia (lag07), CO-CHD (lag07), CO-stroke (lag01), CO-Arrhythmia (lag0).
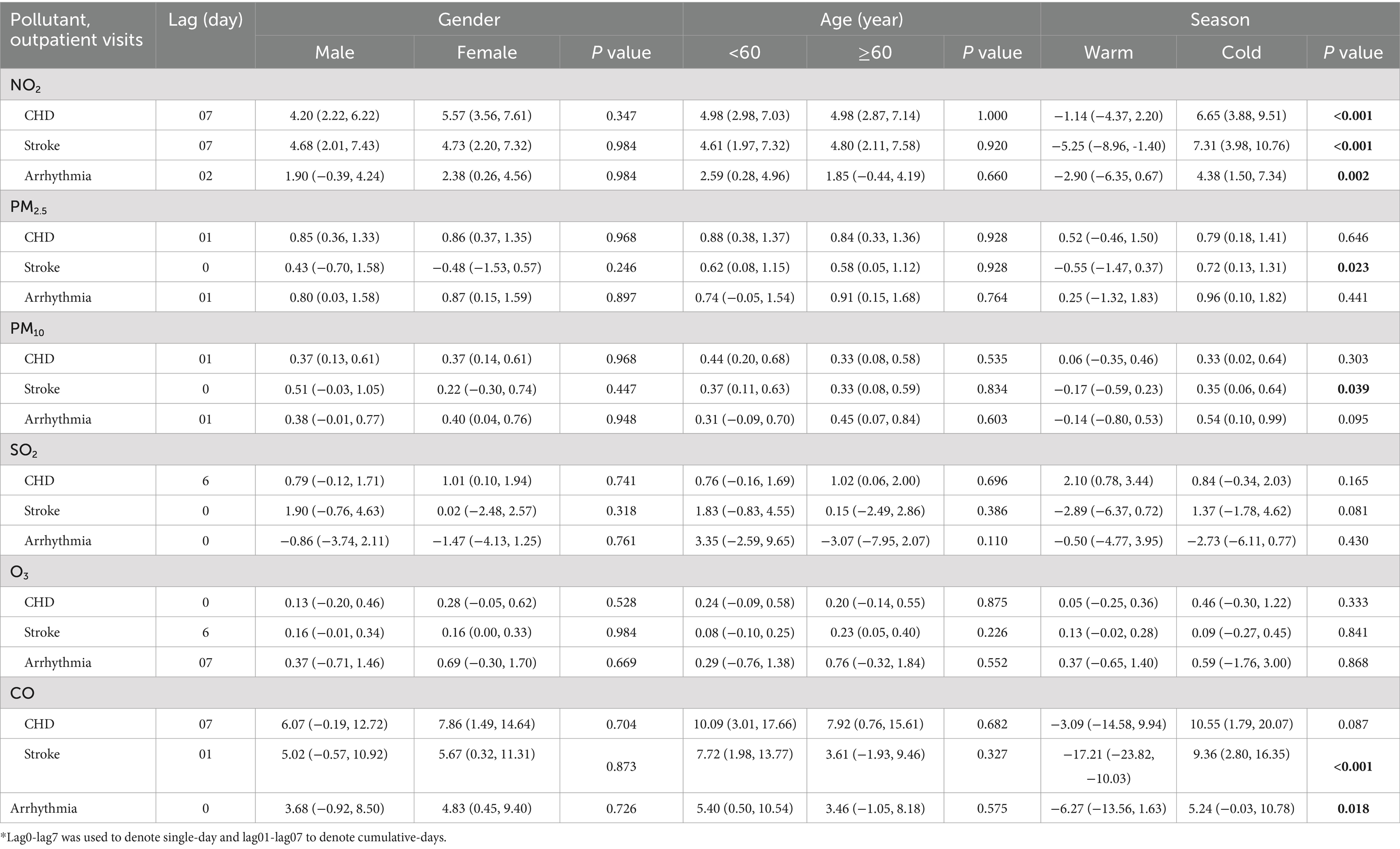
Table 3. Excess risks (95%CI) and the lags in outpatient visits of CHD, stroke, and arrhythmia associated with PM2.5, PM10, NO2, SO2, O3, and CO that stratified by gender, age, and season, in single-pollutant model, respectively.
3.2.3 Double-pollutant model
Considering the strong correlation between PM2.5 and PM10, these two pollutants were not included simultaneously in double-pollutant model. NO2 was positively associated with CHD and stroke when controlling for other air pollutants (Table 4), and CO was also positively related with CHD (except for adjustment of NO2). NO2 and CO were associated with arrhythmia after controlling for SO2. The effects of PM2.5 and PM10 on CVDs remained significant after adjusting for SO2 and O3, but turned out to be insignificant after adjustment of NO2 and CO.
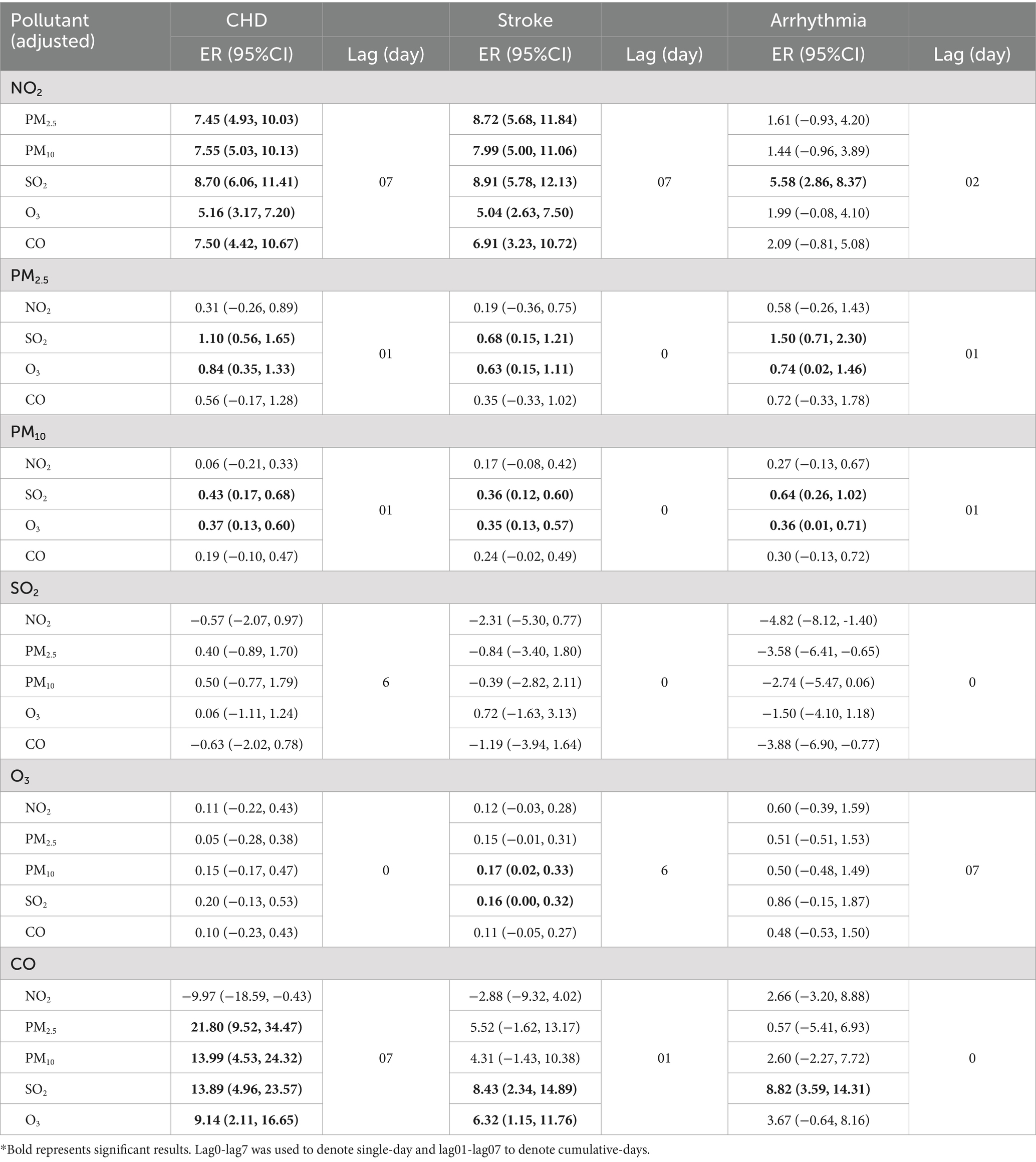
Table 4. Excess risks (95%CI) and the lags of NO2, PM2.5, PM10, SO2, O3, and CO on outpatient visits of CHD, stroke, and arrhythmia with adjustment for other pollutants in double-pollutant model, respectively.
4 Discussion
NO2, PM2.5, PM10, SO2, and CO were positively correlated with each other. In December 2021, Zibo municipal government declared that traditional industries such as chemical materials and products manufacturing industry, non-metallic mineral and products industry accounted for 74% of industrial production in Zibo. Industrial furnaces and kilns in Zibo, accounting for more than one-fifth in Shandong Province, were the main sources of NOx. The industries of foundry, ferroalloy, cement, brick and lime could emit PMs. SO2 and CO were generated during the combustion of energy sources such as coal and fuel oil in industrial manufacturing. Therefore, it should be emphasized that the use of clean energy, improvement of equipment, energy conservation and emission reductions are essential.
The strongest associations between NO2 and CHD, stroke, arrhythmia were at lag07 (ER = 4.97, 95%CI: 3.03, 6.94), lag07 (ER = 4.71, 95%CI: 2.37, 7.10), lag02 (ER = 2.16, 95%CI: 0.12, 4.25), in single-pollutant model, respectively. The findings were consistent with previous studies, which showed significant correlations between NO2 and hospitalizations of CVDs (6), CHD (20) and stroke (16). Additionally, Santos reported an increase in emergency room visits of arrhythmias associated with NO2 (21), and Zhu identified a significant correlation between NO2 and cardiovascular mortality (22). A cohort study in Korea indicated that NO2 increased the risk of atrial fibrillation (23). In contrast, Folino’s research showed no significant association between gaseous pollutants and the occurrence of ventricular tachycardia or ventricular fibrillation (24), which differed from our study. A study indicated a significant association between exposure to NO2 and elevated levels of interleukin-6 (IL-6) among infants (25). Moreover, long-term exposure to NO2 was significantly associated with higher levels of triglycerides and high-sensitivity C-reactive protein (hs-CRP) (26). IL-6 and hs-CRP are representative circulating biomarkers of the inflammatory response in cardiovascular system. There was increasing evidence suggesting that inflammation was associated with an increased risk of CVDs (27, 28).
The highest risks of PM2.5 on CHD, stroke and arrhythmia were at lag01 (ER = 0.85, 95%CI: 0.38, 1.33), lag0 (ER = 0.59, 95%CI: 0.13, 1.06) and lag01 (ER = 0.84, 95%CI: 0.15, 1.53), and for PM10, the strongest ERs were at lag01 (ER = 0.37, 95%CI: 0.14, 0.60), lag0 (ER = 0.35, 95%CI: 0.13, 0.57) and lag01 (ER = 0.39, 95%CI: 0.04, 0.74), in single-pollutant model, respectively. There were studies that concluded significant associations between PMs and cardiovascular mortality (29), myocardial infarction death (30), incidence and daily hospital admissions of stroke (31, 32), incidence and emergency room visits of arrhythmia (21, 23). The associations between PMs and CHD, stroke, arrhythmia can be explained through the following physiological mechanisms. Firstly, PMs can initiate systemic inflammation and vascular endothelial damage, which can ultimately induce atherogenesis (33). Secondly, exposure to PMs may cause systemic inflammation and oxidative stress, promoting vasoconstriction and platelet activation (34). In addition, the health effects of PMs vary according to particle size (35), with smaller diameter particles being more likely to reach and penetrate the bronchial tubes and even cross the air-blood barrier into the circulatory system (36), thereby triggering a series of cardiovascular events. In this study, PM2.5 had stronger effects on CVDs than PM10, which was consistent with the mechanisms.
The strongest risk of SO2 on CHD in single-pollutant model was at lag6 (ER = 0.92, 95%CI: 0.03, 1.81). A study also revealed that exposure to SO2 was significantly associated with CHD (20). Moreover, studies suggested that SO2 increased the risk of cardiovascular emergency department visits and hospital admissions (6, 37). No association was found between SO2 and outpatient visits of stroke and arrhythmia. Therefore, more in-depth studies are needed to confirm the relationship between SO2 and CVDs.
Significant effect was observed between O3 and stroke in single-pollutant model, with the strongest ER at lag6 (ER = 0.16, 95%CI: 0.01, 0.31), but there was no significant association between O3 and CHD and arrhythmia. Exposure to lower concentration of O3 had no effect on mitochondrial DNA (mt-DNA) copy number. Moderate concentration of O3 could damage mitochondrial structure, stimulating the production of endogenous reactive oxygen species (ROS), and releasing the mt-DNA into peripheral blood. Moreover, high concentration of O3 might induce mitochondrial dysfunction and a reduction in mt-DNA levels (38). An animal study revealed that exposure to O3 caused damage of vascular endothelial and atherosclerosis in mice (39). However, a low dose of O3 can modulate the effective antioxidant capacity within the bloodstream and reactivate the antioxidant system (40). Only the association between O3 and stroke was found in this study, thus further investigations are necessary regarding the effects between O3 and CVDs.
CO had the highest associations with CHD, stroke and arrhythmia at lag07 (ER = 8.77, 95%CI: 2.06, 15.91), lag01 (ER = 5.38, 95%CI: 0.45, 10.54), and lag0 (ER = 4.30, 95%CI: 0.14, 8.64), in single-pollutant model. Several studies have demonstrated significant associations between CO and cardiovascular outcomes, including mortality (7, 9), hospitalization (8, 10) and outpatient visits (12). For example, a study in Yichang revealed that an increase of 1 mg/m3 in CO was associated with 39.30% increase in daily outpatient visits of CVDs (12). Franck’s research revealed that both CO and NO2 had adverse effects on cardiovascular admissions (41). An animal experiment discovered that exposure to CO in healthy rats, under experimental conditions simulating urban CO pollution, resulted in the promotion of cardiac remodeling and ventricular arrhythmia (42). CO could rapidly combine with hemoglobin to form carboxyhemoglobin, leading to tissue hypoxia (43). Exposing to CO was associated with CVDs through the following biological mechanisms, including blood coagulation (resulting in elevated fibrinogen levels) (44, 45), inflammatory response (leading to increased IL-6 levels) (46) and oxidative stress (causing an increase in ROS production) (47).
The concentration-response curve of NO2, PM2.5, PM10, SO2, O3 and CO on CVDs, had no apparent threshold. Specifically, in low-concentration range, the curves were steep, while in high-concentration range, they tended to become less steep. A study about hourly air pollutants and acute coronary syndrome onset in 1.29 million patients, also found that there was no obvious threshold between pollutants and acute coronary syndrome (14). It is suggested that controlling the concentration of air pollutants at low levels may achieve better effectiveness.
In subgroup analysis, the risks of NO2 on CHD, stroke and arrhythmia, PM2.5 and PM10 on stroke, CO on stroke and arrhythmia were stronger in cold season than warm season, which was consistent with the study of Ying (37) and Wong (48). A reasonable explanation was that the concentration of NO2, PM2.5, PM10 and CO was higher in cold season compared to warm season due to cold-season heating. Several studies have reported that females and the older adult were more susceptible (16, 18, 49–52), however, no difference was found in subgroup analyses of gender and age in our study, which was consistent with previous findings (8, 53). Therefore, the entire population in Zibo, especially in cold season, protective measures were needed to prevent the effect of ambient pollution. For example, reduce outdoor activities and close windows and doors in cold season when pollution is high, wear a mask when going outside, and wash hands and change clothes when returning home. In addition, further studies are needed on vulnerable populations based on age and gender. Sensitivity analyses of NO2, PM2.5, PM10, SO2, O3 and CO on CVDs were robust after changing the df, except that the relationship between CO and stroke became weakly insignificant (ER = 4,79, 95%CI: -0.11, 9.92) after adjusting the df of ns to 8. Therefore, the results of sensitivity analyses supported the associations between ambient pollutants and CVDs in the study.
In double-pollutant model, NO2 was positively associated with CHD and stroke. The effects of PM2.5 and PM10 on CVDs became insignificant after adjustment of NO2 and CO. The results were consistent with several studies. For example, a study conducted in 272 Chinese cities reported a positive association between NO2 and cardiovascular mortality after controlling for PM2.5 and CO (54). Tian suggested that the associations of NO2 on stroke remained significant after controlling for other air pollutants, but the effects of PM2.5 and CO became insignificant after adjustment of NO2 and SO2 (31). However, there was a study reported that the effect of CO on CHD mortality remained significant after adjustment of PMs, SO2, and NO2 (9). In our study, the risk of CO on CHD became insignificant after adjusting for NO2 and remained significant after adjusting for PMs and SO2. NO2 and CO showed much stronger effects compared with PM2.5, PM10, SO2, and O3, so NO2 and CO were the crucial pollutants affecting CVDs.
There were several strengths in the study. Firstly, data on 1.09 million daily outpatient visits of common CVDs in Zibo were collected over 4 years, which included cases from the entire district with extensive coverage, making the conclusions more persuasive. Secondly, specific diseases such as CHD, stroke and arrhythmia were focused on, rather than CVDs encompassing numerous diseases as a whole, making the interventions more targeted. Additionally, the outcome of this study was outpatient visits of CHD, stroke and arrhythmia, which included lots of milder patients, and therefore, outpatient visits may better reflect the acute effects of diseases, making it more sensitive compared to hospital admissions. However, the study also had limitations. Firstly, as an ecological study, ambient air pollutants were represented by daily average levels measured at monitoring stations instead of individual exposure. The study was conducted at a population level, considering that individuals had different lifestyles and physiological characteristics, which could make it difficult to accurately assess the relationships between air pollutants and CVDs. Secondly, CVD patients’ willingness and frequency of visiting hospital would decrease during the pandemic of COVID-19, which might reduce outpatient visits of CVDs, so the impact of air pollution on CVDs might be underestimated. Finally, the data on outpatient visits of CVDs were from HIS rather than community, but there was no HIS in clinics. It might underestimate the impact of air pollution on CVDs. So, the results of the research could not be extrapolated directly to other realities. Future study should be employed in community to contrast the results.
5 Conclusion
The study adopted single-pollutant model, concentration-response curve, subgroup analysis and double-pollutant model to investigate the association between air pollutants and outpatient visits of CVDs in Zibo. There were positive associations between NO2, PM2.5, PM10, CO and CVDs. While SO2 was only associated with CHD, and O3 was solely associated with stroke. NO2 and CO showed much stronger effects compared with PM2.5, PM10, SO2, and O3. No threshold was found between ambient pollutants and CVDs. The effects of NO2 on CVDs, PM2.5 and PM10 on stroke, and CO on stroke and arrhythmia were stronger in cold season than warm season.
Data availability statement
The datasets presented in this article are not readily available because data on daily outpatient visits of cardiovascular diseases were not publicly available. Requests to access the datasets should be directed to Y2hlbmxpYW5nQHFpbHVob3NwaXRhbC5jb20=.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YW: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. SQ: Methodology, Validation, Writing – review & editing, Writing – original draft. TL: Data curation, Validation, Writing – review & editing. LC: Investigation, Project administration, Resources, Writing – review & editing. LY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1492056/full#supplementary-material
References
1. Wang, Z, Ma, L, Liu, M, Fan, J, and Hu, S. Writing Committee of the Report on Cardiovascular Health and Diseases in China. Summary of the 2022 Report on Cardiovascular Health and Diseases in China. Chin Med J (Engl). (2023) 136:2899–908. doi: 10.1097/CM9.0000000000002927
2. Roth, GA, Mensah, GA, Johnson, CO, Addolorato, G, Ammirati, E, Baddour, LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. Jia, H, Xu, J, Ning, L, Feng, T, Cao, P, Gao, S, et al. Ambient air pollution, temperature and hospital admissions due to respiratory diseases in a cold, industrial city. J Glob Health. (2022) 12:04085. doi: 10.7189/jogh.12.04085
4. Chen, R, Kan, H, Chen, B, Huang, W, Bai, Z, Song, G, et al. Association of Particulate air Pollution with Daily Mortality: the China air pollution and health effects study. Am J Epidemiol. (2012) 175:1173–81. doi: 10.1093/aje/kwr425
5. De Bont, J, Jaganathan, S, Dahlquist, M, Persson, Å, Stafoggia, M, and Ljungman, P. Ambient air pollution and cardiovascular diseases: an umbrella review of systematic reviews and meta-analyses. J Intern Med. (2022) 291:779–800. doi: 10.1111/joim.13467
6. Hasnain, MG, Garcia-Esperon, C, Tomari, YK, Walker, R, Saluja, T, Rahman, MM, et al. Effect of short-term exposure to air pollution on daily cardio- and cerebrovascular hospitalisations in areas with a low level of air pollution. Environ Sci Pollut Res. (2023) 30:102438–45. doi: 10.1007/s11356-023-29544-z
7. Chen, R, Pan, G, Zhang, Y, Xu, Q, Zeng, G, Xu, X, et al. Ambient carbon monoxide and daily mortality in three Chinese cities: the China air pollution and health effects study (CAPES). Sci Total Environ. (2011) 409:4923–8. doi: 10.1016/j.scitotenv.2011.08.029
8. Li, H, Wu, J, Wang, A, Li, X, Chen, S, Wang, T, et al. Effects of ambient carbon monoxide on daily hospitalizations for cardiovascular disease: a time-stratified case-crossover study of 460, 938 cases in Beijing, China from 2013 to 2017. Environ Health. (2018) 17:82. doi: 10.1186/s12940-018-0429-3
9. Liu, C, Yin, P, Chen, R, Meng, X, Wang, L, Niu, Y, et al. Ambient carbon monoxide and cardiovascular mortality: a nationwide time-series analysis in 272 cities in China. Lancet Planet Health. (2018) 2:e12–8. doi: 10.1016/S2542-5196(17)30181-X
10. Liu, H, Tian, Y, Xiang, X, Li, M, Wu, Y, Cao, Y, et al. Association of short-term exposure to ambient carbon monoxide with hospital admissions in China. Sci Rep. (2018) 8:13336. doi: 10.1038/s41598-018-31434-1
11. Tian, L, Ho, KF, Wang, T, Qiu, H, Pun, VC, Chan, CS, et al. Ambient carbon monoxide and the risk of hospitalization due to chronic obstructive pulmonary disease. Am J Epidemiol. (2014) 180:1159–67. doi: 10.1093/aje/kwu248
12. Wang, Y, Yao, C, Xu, C, Zeng, X, Zhou, M, Lin, Y, et al. Carbon monoxide and risk of outpatient visits due to cause-specific diseases: a time-series study in Yichang. China Environ Health. (2019) 18:36. doi: 10.1186/s12940-019-0477-3
13. Gasparrini, A. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Soft. (2011) 43:1–20. doi: 10.18637/jss.v043.i08
14. Chen, R, Jiang, Y, Hu, J, Chen, H, Li, H, Meng, X, et al. Hourly air pollutants and acute coronary syndrome onset in 1.29 million patients. Circulation. (2022) 145:1749–60. doi: 10.1161/CIRCULATIONAHA.121.057179
15. Song, J, Lim, YC, Ko, I, Kim, J-Y, and Kim, D-K. Association between air pollutants and initial hospital admission for ischemic stroke in Korea from 2002 to 2013. J Stroke Cerebrovasc Dis. (2021) 30:106080. doi: 10.1016/j.jstrokecerebrovasdis.2021.106080
16. Czernych, R, Badyda, A, Kozera, G, and Zagożdżon, P. Assessment of low-level air pollution and cardiovascular incidence in Gdansk, Poland: time-series cross-sectional analysis. JCM. (2023) 12:2206. doi: 10.3390/jcm12062206
17. Ma, Y, Zhao, Y, Yang, S, Zhou, J, Xin, J, Wang, S, et al. Short-term effects of ambient air pollution on emergency room admissions due to cardiovascular causes in Beijing, China. Environ. Pollut. (2017) 230:974–80. doi: 10.1016/j.envpol.2017.06.104
18. Zhang, C, Ding, R, Xiao, C, Xu, Y, Cheng, H, Zhu, F, et al. Association between air pollution and cardiovascular mortality in Hefei, China: a time-series analysis. Environ Pollut. (2017) 229:790–7. doi: 10.1016/j.envpol.2017.06.022
19. Altman, DG. Statistics notes: interaction revisited: the difference between two estimates. BMJ. (2003) 326:219–9. doi: 10.1136/bmj.326.7382.219
20. Liu, M, Yu, J, Zhu, A, Ling, J, Chen, R, Zhang, Y, et al. Association between air pollution and coronary heart disease hospitalizations in Lanzhou City, 2013–2020: a time series analysis. J Urban Health. (2023) 100:1246–57. doi: 10.1007/s11524-023-00797-w
21. Santos, UP, Terra-Filho, M, Lin, CA, Pereira, LAA, Vieira, TCB, Saldiva, PHN, et al. Cardiac arrhythmia emergency room visits and environmental air pollution in São Paulo, Brazil. J Epidemiol Comm Health. (2008) 62:267–72. doi: 10.1136/jech.2006.058123
22. Zhu, J, Zhang, X, Zhang, X, Dong, M, Wu, J, Dong, Y, et al. The burden of ambient air pollution on years of life lost in Wuxi, China, 2012–2015: a time-series study using a distributed lag non-linear model. Environ Pollut. (2017) 224:689–97. doi: 10.1016/j.envpol.2017.02.053
23. Kim, I-S, Yang, P-S, Lee, J, Yu, HT, Kim, T-H, Uhm, J-S, et al. Long-term exposure of fine particulate matter air pollution and incident atrial fibrillation in the general population: a nationwide cohort study. Int J Cardiol. (2019) 283:178–83. doi: 10.1016/j.ijcard.2018.12.048
24. Folino, F, Buja, G, Zanotto, G, Marras, E, Allocca, G, Vaccari, D, et al. Association between air pollution and ventricular arrhythmias in high-risk patients (ARIA study): a multicentre longitudinal study. Lancet Planetary Health. (2017) 1:e58–64. doi: 10.1016/S2542-5196(17)30020-7
25. Gruzieva, O, Merid, SK, Gref, A, Gajulapuri, A, Lemonnier, N, Ballereau, S, et al. Exposure to traffic-related air pollution and serum inflammatory cytokines in children. Environ Health Perspect. (2017) 125:067007. doi: 10.1289/EHP460
26. Cai, Y, Hansell, AL, Blangiardo, M, Burton, PR, SHaRE, B, De Hoogh, K, et al. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and lifelines cohorts. Eur Heart J. (2017) 38:2290–6. doi: 10.1093/eurheartj/ehx263
27. Damen, SAJ, Cramer, GE, Dieker, H-J, Gehlmann, H, Aengevaeren, WRM, Oude Ophuis, TJM, et al. A multi-site coronary sampling study on CRP in non-STEMI: novel insights into the inflammatory process in acute coronary syndromes. Atherosclerosis. (2018) 278:117–23. doi: 10.1016/j.atherosclerosis.2018.09.024
28. Wang, A, Liu, J, Li, C, Gao, J, Li, X, Chen, S, et al. Cumulative exposure to high-sensitivity C-reactive protein predicts the risk of cardiovascular disease. JAHA. (2017) 6:e005610. doi: 10.1161/JAHA.117.005610
29. Hayes, RB, Lim, C, Zhang, Y, Cromar, K, Shao, Y, Reynolds, HR, et al. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int J Epidemiol. (2020) 49:25–35. doi: 10.1093/ije/dyz114
30. Mo, S, Hu, J, Yu, C, Bao, J, Shi, Z, Zhou, P, et al. Short-term effects of fine particulate matter constituents on myocardial infarction death. J Environ Sci. (2023) 133:60–9. doi: 10.1016/j.jes.2022.07.019
31. Tian, Y, Liu, H, Zhao, Z, Xiang, X, Li, M, Juan, J, et al. Association between ambient air pollution and daily hospital admissions for ischemic stroke: a nationwide time-series analysis. PLoS Med. (2018) 15:e1002668. doi: 10.1371/journal.pmed.1002668
32. Wu, H, Zhang, B, Wei, J, Lu, Z, Zhao, M, Liu, W, et al. Short-term effects of exposure to ambient PM1, PM2.5, and PM10 on ischemic and hemorrhagic stroke incidence in Shandong Province, China. Environ Res. (2022) 212:113350. doi: 10.1016/j.envres.2022.113350
33. Liang, S, Zhao, T, Xu, Q, Duan, J, and Sun, Z. Evaluation of fine particulate matter on vascular endothelial function in vivo and in vitro. Ecotoxicol Environ Saf. (2021) 222:112485. doi: 10.1016/j.ecoenv.2021.112485
34. Zhu, X, Chen, C, Zhang, B, Ge, Y, Wang, W, Cai, J, et al. Acute effects of personal exposure to fine particulate matter on salivary and urinary biomarkers of inflammation and oxidative stress in healthy adults. Chemosphere. (2021) 272:129906. doi: 10.1016/j.chemosphere.2021.129906
35. Faiz, Y, Siddique, N, He, H, Sun, C, and Waheed, S. Occurrence and profile of organophosphorus compounds in fine and coarse particulate matter from two urban areas of China and Pakistan. Environ Pollut. (2018) 233:26–34. doi: 10.1016/j.envpol.2017.09.091
36. Rossi, S, Buccarello, A, Caffarra Malvezzi, C, Pinelli, S, Alinovi, R, Guerrero Gerboles, A, et al. Exposure to nanoparticles derived from diesel particulate filter equipped engine increases vulnerability to arrhythmia in rat hearts. Environ Pollut. (2021) 284:117163:117163. doi: 10.1016/j.envpol.2021.117163
37. Ying, Z, Gong, WS, Xia, MY, Zheng, SK, Fan, CY, Xu, L, et al. Association between ambient air pollution and hospital emergency admissions for respiratory and cardiovascular diseases in Beijing: a time series study. Biomed Environ Sci. (2015) 28:352–363. doi: 10.3967/bes2015.049
38. Li, Z, Su, Q, Xu, R, Peng, J, Zhu, X, and Wei, Y. Influence of different concentrations of ozone on the alteration of mitochondrial DNA copy numbers in human peripheral blood. Sci Total Environ. (2023) 873:162282. doi: 10.1016/j.scitotenv.2023.162282
39. Chuang, GC, Yang, Z, Westbrook, DG, Pompilius, M, Ballinger, CA, White, CR, et al. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am J Phys Lung Cell Mol Phys. (2009) 297:L209–16. doi: 10.1152/ajplung.00102.2009
40. Bocci, V, Borrelli, E, Travagli, V, and Zanardi, I. The ozone paradox: ozone is a strong oxidant as well as a medical drug. Med Res Rev. (2009) 29:646–82. doi: 10.1002/med.20150
41. Franck, U, Leitte, AM, and Suppan, P. Multiple exposures to airborne pollutants and hospital admissions due to diseases of the circulatory system in Santiago de Chile. Sci Total Environ. (2014) 468-469:746–56. doi: 10.1016/j.scitotenv.2013.08.088
42. Andre, L, Boissière, J, Reboul, C, Perrier, R, Zalvidea, S, Meyer, G, et al. Carbon monoxide pollution promotes cardiac remodeling and ventricular arrhythmia in healthy rats. Am J Respir Crit Care Med. (2010) 181:587–95. doi: 10.1164/rccm.200905-0794OC
43. Prockop, LD, and Chichkova, RI. Carbon monoxide intoxication: an updated review. J Neurol Sci. (2007) 262:122–30. doi: 10.1016/j.jns.2007.06.037
44. Bind, M-A, Baccarelli, A, Zanobetti, A, Tarantini, L, Suh, H, Vokonas, P, et al. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and Epigene-environment interactions in an elderly cohort. Epidemiology. (2012) 23:332–40. doi: 10.1097/EDE.0b013e31824523f0
45. Wu, X, Basu, R, Malig, B, Broadwin, R, Ebisu, K, Gold, EB, et al. Association between gaseous air pollutants and inflammatory, hemostatic and lipid markers in a cohort of midlife women. Environ Int. (2017) 107:131–9. doi: 10.1016/j.envint.2017.07.004
46. Zhang, X, Staimer, N, Gillen, DL, Tjoa, T, Schauer, JJ, Shafer, MM, et al. Associations of oxidative stress and inflammatory biomarkers with chemically-characterized air pollutant exposures in an elderly cohort. Environ Res. (2016) 150:306–19. doi: 10.1016/j.envres.2016.06.019
47. Reboul, C, Boissière, J, André, L, Meyer, G, Bideaux, P, Fouret, G, et al. Carbon monoxide pollution aggravates ischemic heart failure through oxidative stress pathway. Sci Rep. (2017) 7:39715. doi: 10.1038/srep39715
48. Wong, C-M, Atkinson, RW, Anderson, HR, Hedley, AJ, Ma, S, Chau, PY-K, et al. A tale of two cities: effects of air pollution on hospital admissions in Hong Kong and London compared. Environ Health Perspect. (2002) 110:67–77. doi: 10.1289/ehp.0211067
49. Goldberg, MS, Burnett, RT, Stieb, DM, Brophy, JM, Daskalopoulou, SS, Valois, M-F, et al. Associations between ambient air pollution and daily mortality among elderly persons in Montreal, Quebec. Sci. Total Environ. (2013) 463-464:931–42. doi: 10.1016/j.scitotenv.2013.06.095
50. Katsoulis, M, Dimakopoulou, K, Pedeli, X, Trichopoulos, D, Gryparis, A, Trichopoulou, A, et al. Long-term exposure to traffic-related air pollution and cardiovascular health in a Greek cohort study. Sci Total Environ. (2014) 490:934–40. doi: 10.1016/j.scitotenv.2014.05.058
51. Kaufman, JD, Adar, SD, Barr, RG, Budoff, M, Burke, GL, Curl, CL, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the multi-ethnic study of atherosclerosis and air pollution): a longitudinal cohort study. Lancet. (2016) 388:696–704. doi: 10.1016/S0140-6736(16)00378-0
52. Lipsett, MJ, Ostro, BD, Reynolds, P, Goldberg, D, Hertz, A, Jerrett, M, et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. (2011) 184:828–35. doi: 10.1164/rccm.201012-2082OC
53. Liu, H, Tian, Y, Song, J, Cao, Y, Xiang, X, Huang, C, et al. Effect of ambient air pollution on hospitalization for heart failure in 26 of China’s largest cities. Am J Cardiol. (2018) 121:628–33. doi: 10.1016/j.amjcard.2017.11.039
Keywords: ambient air pollutants, coronary heart disease, stroke, arrhythmia, Zibo
Citation: Wang Y, Qu S, Li T, Chen L and Yang L (2025) Association between ambient air pollution and outpatient visits of cardiovascular diseases in Zibo, China: a time series analysis. Front. Public Health. 12:1492056. doi: 10.3389/fpubh.2024.1492056
Edited by:
Arthit Phosri, Mahidol University, ThailandReviewed by:
Worradorn Phairuang, Chiang Mai University, ThailandSitthichok Puangthongthub, Chulalongkorn University, Thailand
Copyright © 2025 Wang, Qu, Li, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Chen, Y2hlbmxpYW5nQHFpbHVob3NwaXRhbC5jb20=; Liping Yang, eWxpcGluZ0BzZHUuZWR1LmNu
†These authors have contributed equally to this work
 Yamei Wang1†
Yamei Wang1† Ting Li
Ting Li Liping Yang
Liping Yang