- 1Department of Stomatology, Xi’an Central Hospital, Xi’an, Shaanxi, China
- 2Department of Epidemiology and Health Statistics, School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an, Shaanxi, China
Objective: This study aimed to examine the association between maternal pesticide exposure during the periconceptional period and birth defects in their offspring.
Methods: A survey was conducted among 29,204 women with infants born between 2010 and 2013 in Shaanxi Province, Northwest China. All cases of birth defects were diagnosed using the International Classification of Diseases, Tenth Revision (ICD-10). Given the multistage sampling design, the generalized estimating equation (GEE) binomial regression models with log link and exchangeable correlation structures were used to analyze the association between maternal pesticide exposures and birth defects.
Results: Among the 29,204 subjects, 562 mothers had children with birth defects, resulting in an incidence rate of 192.44 per 10,000 live births. The incidence of birth defects was higher in the pesticide-exposed group compared to the control group (737.46/10,000 vs. 186.04/10,000). After adjusting for baseline demographic characteristics, fertility status, nutritional factors, and environmental factors in the GEE model, the results indicated that the risk of birth defects and cardiovascular system defects in mothers exposed to pesticides during the periconceptional period was 2.39 times (95% CI: 1.84–3.10) and 3.14 times (95% CI: 1.73–5.71) higher, respectively, compared to the control group.
Conclusion: This study demonstrated that maternal exposure to pesticides during the periconceptional period was associated with an increased risk of birth defects, particularly cardiovascular system defects in offspring. Consequently, it would be beneficial to avoid pesticide exposure from three months before pregnancy through the first trimester to lower birth defects in infants.
1 Introduction
Congenital abnormalities, commonly known as birth defects, include a wide range of anatomical and functional irregularities that develop during embryonic or fetal growth. The International Classification of Diseases, Tenth Revision (ICD-10), identifies approximately 10 systems in which birth defects can occur. Over 20 significant birth defects are frequently encountered in clinical practice, including congenital heart disease, neural tube defects, limb shortening, cleft lip and palate, and trisomy 21 syndrome. In China, the prevalence of birth defects was 123 per 10,000 perinatal births during 2000–2021 (1). These defects contribute significantly to early abortion, stillbirth, perinatal death, infant mortality, and congenital disabilities (2).
A growing body of evidence suggests that the risk of birth defects is influenced by a complex interaction of genetic and environmental factors. The widespread use of pesticides, especially in agriculture, has been shown through experimental research to produce endocrine disruptors, neurodevelopmental toxicants, immunotoxicants, and carcinogens (3, 4). Numerous studies, both domestic and international, indicate that prenatal exposure to pesticides may increase the risk of adverse birth outcomes, including miscarriage, premature delivery, stillbirth, and birth defects (5–9). These exposures may also impact the long-term cognitive and physical development of offspring (10–13). A case–control study in Northern Netherlands identified maternal occupational exposure to pesticides and dust as potential risk factors for oral clefts in offspring (14). Additionally, exposure to personal, household, and agricultural pesticides during pregnancy may raise the risk of holoprosencephaly (15). An animal study provides new insights into the harmful effects of pesticide and environmental chemical exposure on craniofacial skeletal development in zebrafish embryos (16).
However, some studies have produced conflicting results regarding the correlation between prenatal pesticide exposure and birth defects. For instance, a case–control study in California found no evidence of a correlation between maternal residential exposure to agricultural pesticides and specific congenital heart defects in children (17). Moreover, most pesticides analyzed did not show a statistically significant relationship with an increased risk of hypospadias in male infants (18). These conflicts might result from differences in pesticide exposure assessment methods and study population heterogeneity (19). Different agricultural practices, pesticide types, genetic backgrounds in various regions, as well as small sample sizes in some studies and possible protective genetic factors in certain areas with low pesticide usage, can make the exposure birth defect association less obvious and reduce statistical power (20, 21).
The period extending from 3 months prior to conception through the first trimester of pregnancy is critical for both conception and fetal development. Notably, during the first trimester, pregnant women are particularly susceptible to the effects of pesticides due to physiological changes and the immature state of the placenta (22). In many agricultural regions of low- and middle-income countries, pesticide use coincides with the periconceptional stage, increasing exposure risk (23). Pesticide exposure in the early stage of life can also lead to long term issues like developmental delays, autism spectrum disorder and learning disabilities (24). Given the current understanding of the link between maternal pesticide exposure during early pregnancy and birth defects in offspring, further research is needed.
Therefore, this study used data from a large population-based survey of birth defects conducted in Shaanxi Province, Northwest China, to explore the potential connection between maternal pesticide exposure during the periconceptional period and the occurrence of birth defects in offspring. The findings of this study could help inform strategies for the prevention and management of birth defects.
2 Methods
2.1 Study design and participants
This study used data from a population-based cross-sectional survey on birth defects conducted in Shaanxi Province, Northwest China, between July and November 2013. Mothers with documented pregnancy outcomes and their infants born between 2010 and 2013 were selected using a stratified multistage sampling approach. The detailed sampling methodology has been previously published (25). The study was approved by the Human Research Ethics Committee of Xi’an Jiaotong University (approval number: 2012008). Written informed consent was obtained from all participants. After excluding 823 subjects due to unclear pregnancy outcomes or incomplete questionnaires, the final analysis included 29,204 women.
2.2 Definition and diagnosis of birth defects
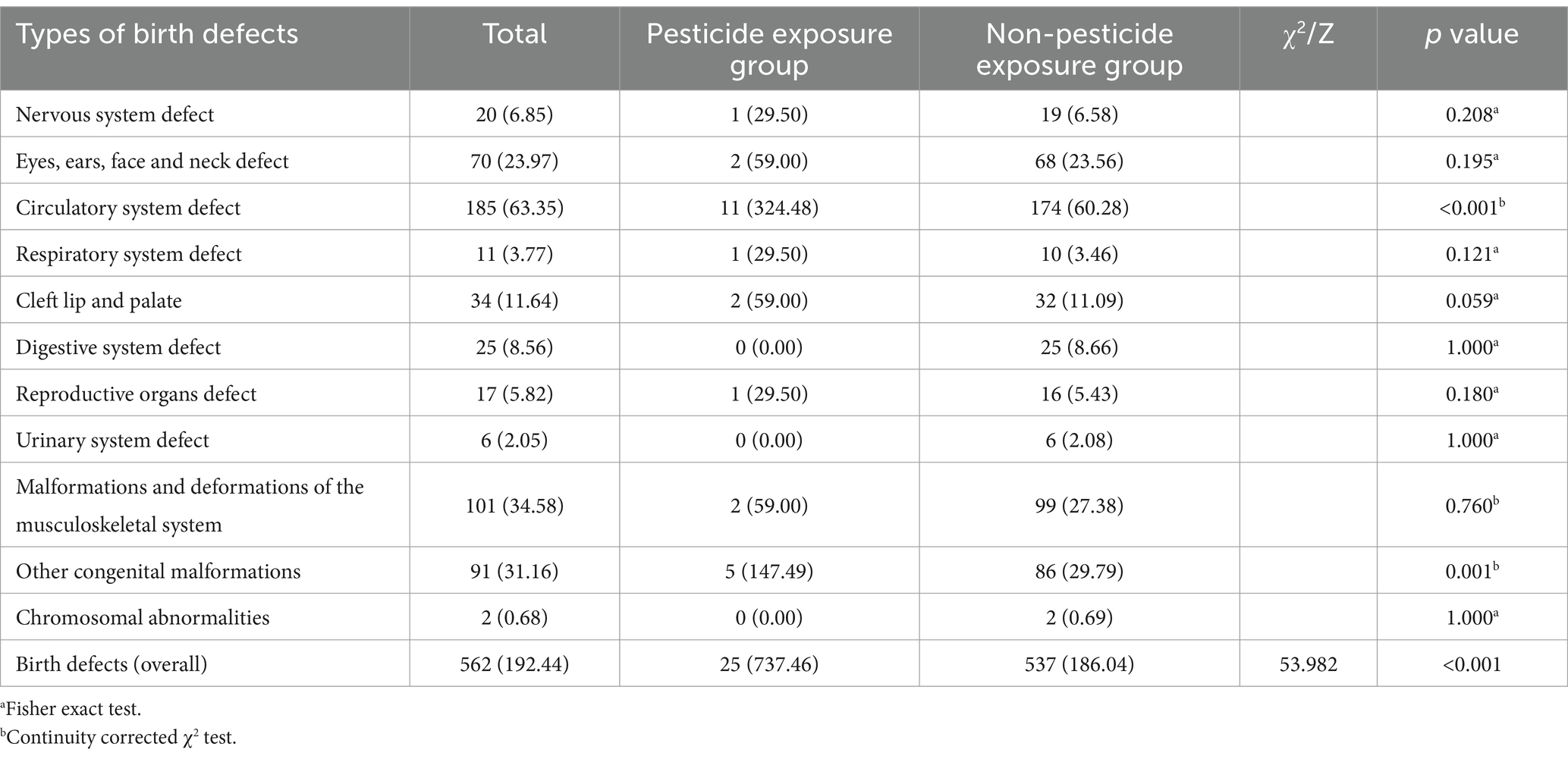
Birth defects were classified according to ICD-10 codes, covering malformations of various body systems, including defects of the nervous system; eyes, ears, face, and neck; circulatory system; respiratory system; cleft lip and palate; digestive system; reproductive organs; urinary system; musculoskeletal system; other congenital malformations; and chromosomal abnormalities not classified elsewhere.
An expert team, including senior medical technicians from the departments of congenital heart surgery, ultrasound, obstetrics, and gynecology at the First Affiliated Hospital of Xi’an Jiaotong University, was assembled for the diagnosis of birth defects. The diagnosis of congenital anomalies was determined by this team using the ICD-10. For pediatric patients with external anomalies, medical records were reviewed, and malformations were documented with photographic evidence to aid in diagnosis. For congenital internal malformations, including cardiovascular anomalies, medical records were reviewed, and supplementary ultrasound examinations were conducted at the First Affiliated Hospital of Xi’an Jiaotong University to establish a definitive diagnosis.
2.3 Definition of pesticide exposure and the periconceptional period
Pesticides included various chemical classes such as pyrethroids, carbamates, organophosphorus compounds, organofluorides, organochlorines, avermectin, carbendazim, dafenazine, sofril, rodenticides, and herbicides, among others. The periconceptional period was defined as the span from 3 months before pregnancy to the first 3 months of pregnancy.
2.4 Definitions of covariates
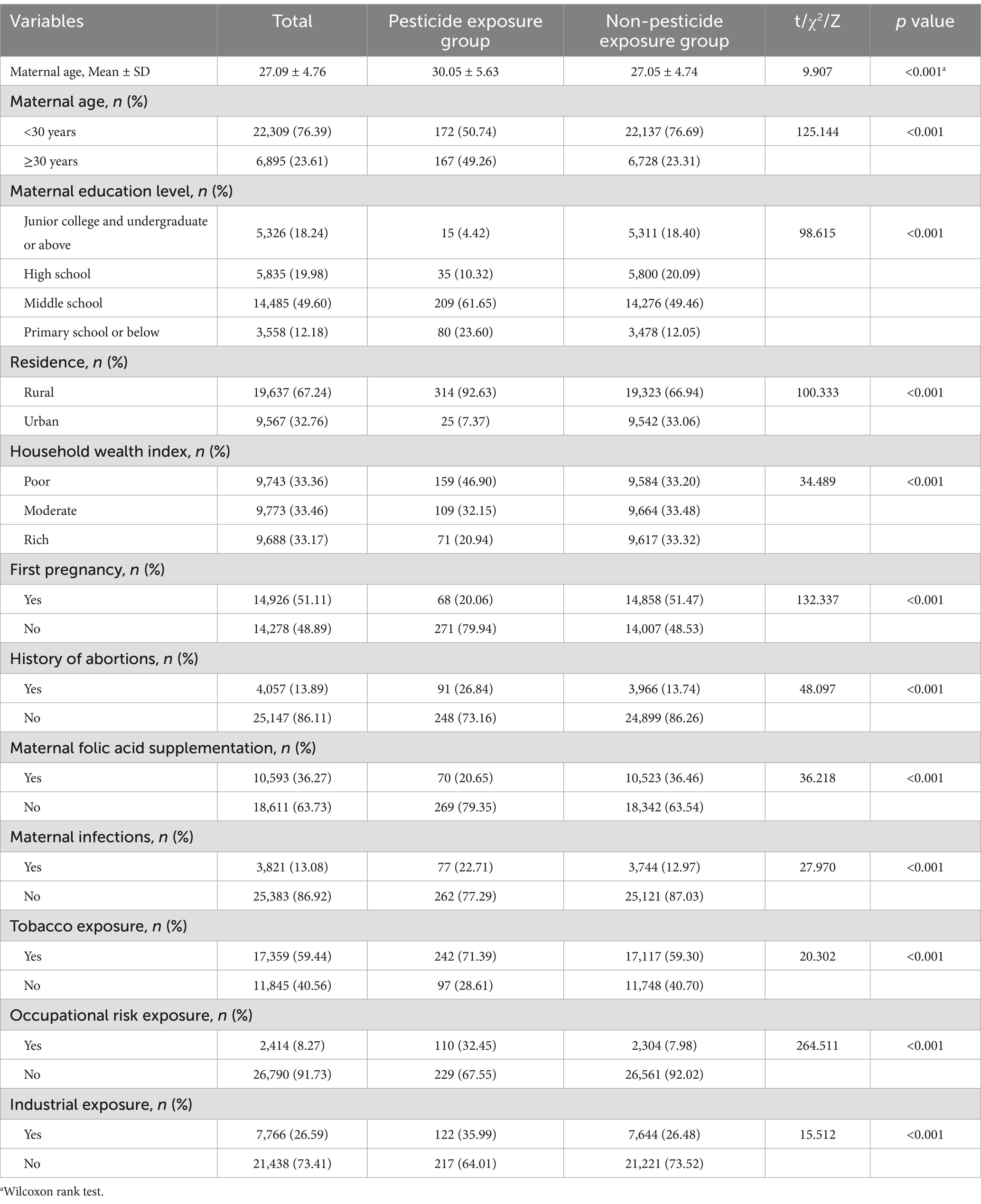
Previous research (26–28) has identified several factors correlated with birth defects, including maternal socio-demographic characteristics, fertility status, nutritional factors, and environmental factors. Specifically, these variables included maternal age (<30 and ≥ 30 years), education level (college degree and above, high school or technical secondary school, junior high school, primary school and below), ethnicity (Han, other), residence (rural, urban), household wealth index (poor, moderate, rich), first pregnancy (yes, no), history of abortions (yes, no), type of pregnancy (singleton, multiple), family history of birth defects (yes, no), folic acid supplementation (yes, no), infections (yes, no), tobacco exposure (yes, no), occupational risk exposure (yes, no), and industrial exposure (yes, no).
The folic acid supplementation regimen was defined as the consistent intake of at least 400 μg of folic acid per day during the periconceptional period, sustained for more than three consecutive months. Infection was characterized by febrile episodes (temperature exceeding 38°C) and symptoms related with influenza or the common cold, including influenza-like symptoms and seasonal influenza during pregnancy. Tobacco exposure during pregnancy was categorized into two groups: active smoking, defined as the consumption of at least one cigarette per week for 3 consecutive months, and passive smoking, defined as inhaling secondhand smoke for a minimum of 15 min per day over one consecutive month. Occupational exposure was defined by the presence of biological, physical, chemical, or psychosocial risk factors in the workplace. Industrial exposure was defined as residing within a one-kilometer radius of mines, cement factories, power plants, fertilizer factories, or major traffic thoroughfares during pregnancy.
2.5 Data collection
A standardized questionnaire was developed by the Department of Epidemiology and Health Statistics at the School of Public Health, Xi’an Jiaotong University Health Science Center. This questionnaire gathered information on maternal socio-demographic characteristics, fertility status, nutritional factors, and environmental factors during pregnancy, and child birth outcomes as reported by the mothers. Trained field staff conducted face-to-face interviews to administer the questionnaire. Additionally, diagnostic information regarding birth outcomes, including the timing of diagnosis and types of birth defects, was collected from local hospitals. Data on perinatal pesticide exposure were self-reported by mothers, detailing contact with pesticides via skin or respiratory routes during this period.
2.6 Statistical analysis
Categorical variables were summarized as frequencies and percentages, and intergroup comparisons were performed using the chi-squared test, corrected chi-squared test, or Fisher’s exact test, as appropriate. Continuous variables were reported as mean and standard deviation, with group comparisons conducted using the t-test for normally distributed variables or the Wilcoxon rank-sum test for non-normally distributed variables. The generalized estimating equation (GEE) extends the generalized linear model, specifically addressing the analysis of non-independent data. The GEE is adept at fitting suitable statistical models to dependent variables that conform to a variety of distributions, such as normal, binomial, and Poisson. This statistical method effectively mitigates the issue of correlation among dependent variables in longitudinal datasets, thereby enhancing the reliability of the estimates obtained. Given the multistage sampling design, the GEE was used to analyze the association between maternal pesticide exposures during the periconceptional period and the incidence of birth defects, aiming to mitigate intra-group correlations. Additionally, to explore the potential heterogeneity in the effects of pesticide exposures on birth defects, effects were estimated within subgroups defined by various maternal characteristics.
The data entry process was conducted in duplicate with error checking using EpiData 3.1 software. Statistical analysis was performed using SAS 9.4 software (Statistics Analysis System, Inc., Cary, North Carolina, United States), with statistical significance set at a two-tailed p-value of less than 0.05.
3 Results
3.1 Participants’ characteristics
Table 1 and Supplementary Table S1 presents the baseline characteristics of the 29,204 pregnant women included in the study. The mean age of the participants was 27.09 ± 4.76 years. Most participants had a junior high school education level (49.60%), were of Han ethnicity (99.36%), and resided in rural areas (67.24%). Among the participants, 339 women were in the pesticide exposure group, while 28,865 were in the non-exposure group. Compared to the non-exposure group, women in the pesticide exposure group were more likely to be older, live in rural areas, have a history of abortion and infection, be exposed to tobacco, and encounter occupational and environmental risk factors. They also tended to have lower education levels and household wealth index and were less likely to take folic acid supplements. No significant differences were observed between the two groups in terms of maternal ethnicity, type of pregnancy and family history of birth defects.
3.2 Prevalence of birth defects
Table 2 shows the prevalence of birth defects among the 29,204 participants. A total of 562 women gave birth to children with birth defects, resulting in an overall incidence rate of 192.44 per 10,000 live births. The prevalence of birth defects (737.46/10,000 vs. 186.04/10,000), congenital malformations of the circulatory system (324.48/10,000 vs. 60.28/10,000), and other congenital malformations (147.49/10,000 vs. 29.79/10,000) was significantly higher in the pesticide-exposed group compared to the unexposed group.
3.3 Pesticide exposure and birth defects
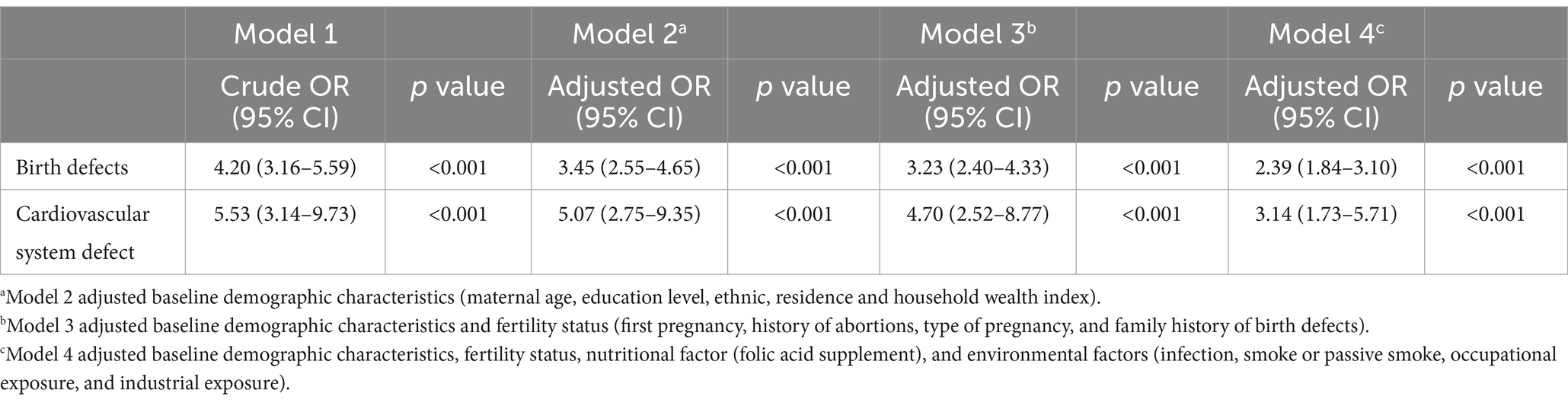
Due to the hierarchical structure of the data, with aggregation at the county level, a GEE model was used to account for the county (district) as a random effect in the multifactor analysis of pesticide exposure and birth defects (Table 3). In the unadjusted model (model 1), the risks of birth defects and cardiovascular system defects in the offspring of mothers exposed to pesticides were 4.20 times (95% CI: 3.16–5.59) and 5.53 times (95% CI: 3.14–9.73) higher, respectively, compared to the control group. After adjusting for baseline demographic characteristics (model 2), these risks decreased to 3.45 times (95% CI: 2.55–4.65) and 5.07 times (95% CI: 2.75–9.35) higher, respectively. Further adjustment for fertility status (model 3) reduced the risks to 3.23 times (95% CI: 2.40–4.33) and 4.70 times (95% CI: 2.52–8.77) higher, respectively. Finally, after adjusting for baseline demographic characteristics, fertility status, nutritional factors, and environmental factors (model 4), the risks of birth defects and cardiovascular system defects were 2.39 times (95% CI: 1.84–3.10) and 3.14 times (95% CI: 1.73–5.71) higher, respectively, compared to the control group.
3.4 Sensitivity analyses
The relationship between pesticide exposure and birth defects was further examined across various demographic subgroups. Pesticide exposure was consistently associated with an increased risk of birth defects in all subgroups. This association was statistically significant for women under 30 years of age, women 30 years or older, those with a high school education or above, those with a middle school education or below, those living in rural areas, and those experiencing a non-first pregnancy. No significant interaction was observed between pesticide exposure and any covariates for birth defects (Supplementary Table S2).
4 Discussion
Drawing on a comprehensive population-based survey conducted in Shaanxi Province, Northwest China, this study investigated the relationship between pesticide exposure during the periconceptional period and the risk of birth defects. Using a GEE model, we adjusted for baseline demographic characteristics, fertility status, nutritional factors, and environmental factors. Our findings indicated that maternal pesticide exposure during the periconceptional period was significantly associated with an increased risk of birth defects, particularly congenital circulatory malformations, in offspring.
This study identified a correlation between maternal pesticide exposure during the periconceptional period and birth defects based on data from a cross-sectional survey conducted across 20 counties and 10 districts in Shaanxi Province, China. Our results were aligned with international findings in previous reports. For instance, a systematic review found that maternal exposure to pesticides during pregnancy is associated with a significantly increased risk of birth defects, with an odds ratio of 4.44 (95% CI: 2.61–7.57) compared to unexposed pregnant women (29). Evidence from a nested case–control study also suggested that pregnant women in southwest China were ubiquitously exposed to low-level pyrethroid pesticide (PYRs), and maternal household pesticides use was related to congenital anomalies (30). Similarly, a previous case control study conducted in a rural area of Northern China suggested that prenatal exposure to Organochlorine pesticides (OCPs) was associated with increased risk for neural tube defects (NTDs), the OCPs also had a joint effect on NTD as its risk increased almost linearly with concentrations of the 16 OCPs as a mixture (31). Another birth cohort study also revealed that exposure to pesticides in maternal residences increased the likelihood of congenital heart disease, reproductive system malformations, and skeletal muscle malformations in offspring (32). Unlike previous studies, this research specifically focused on pesticide exposure during the 3 months preceding pregnancy and the first trimester—a critical period for fetal development. The odds ratios of similar international studies were basically like those of this study, with case—control studies as the main research methods and maternal self-reporting often used for exposure assessment. Therefore, it is of great necessity to conduct large sample prospective studies and quantitatively measure the level of maternal pesticide exposure in the future to reveal the causal relationship between pesticides and birth defects.
In addition to the acute toxic effects of cholinergic toxicity, organophosphorus pesticides have been shown to cause genetic toxicity, including DNA damage, gene mutations, chromosome aberrations, and the induction of cell carcinogenesis. Moreover, evidence suggests that these pesticides disrupt embryogenesis and development, have long-term impacts on the structural and functional integrity of the nervous system, and may increase the risk of neurodegenerative diseases (33, 34). Organochlorine pesticides, which are commonly used in agriculture, can accumulate in the maternal body and be transmitted to the fetus through the placental barrier, potentially leading to birth defects (35).
This study identified a correlation between maternal pesticide exposure during the periconceptional period and cardiovascular system defects, consistent with prior research. A birth cohort study conducted in North Carolina found a significant association between maternal exposure to pendimethalin and an increased risk of atrial septal defect in offspring (OR = 2.09, 95% CI = 1.63–2.68), as well as an elevated risk of patent ductus arteriosus (OR = 1.64, 95% CI = 1.29–2.08) (36). Similarly, results from a nested case–control study suggested a trend toward a higher risk of septal defects (ORs ranged from 1.80 to 2.36) with greater serum neonicotinoid pesticides (NEOs), especially nitro-containing NEOs represented by imidacloprid (IMI) (37). Most animal studies have used nitrofen to induce congenital heart malformations in mouse embryos (38, 39), with the primary mechanism involving the impact of toxic substances on the proliferation or apoptosis of cells involved in heart development. Due to poor perceptions of biological control and lack of capacity, many low- and middle-income countries are reluctant to pursue biological control, increasing a dependency on pesticides, and increases the risk of pesticide exposure for mothers during pregnancy (40). In addition, since biopesticides can promote the increase of crop production without compromising human health, they have already received widespread attention (41). However, there are very few studies on the relationship between exposure to new pesticides during pregnancy and cardiovascular system defects, further research is needed to confirm its safety to mothers and children.
This research represented a large-scale, population-based cross-sectional survey conducted in Northwest China, focusing on the correlation between maternal pesticide exposure during the periconceptional period and the incidence of birth defects. However, several limitations should be acknowledged. Firstly, pesticide exposure was assessed retrospectively through self-reporting, which might introduce recall bias due to the time elapsed between pregnancy and the survey. To mitigate this bias, we established a rigorous investigative protocol and selected clear indices pertinent to pesticide exposure, thereby enhancing participants’ ability to accurately recall long-term exposure histories. For example, the indices encompassed commonly used local pesticides and their corresponding application seasons. Secondly, the low incidence of birth defects and the limited number of cases of malformations outside the cardiovascular system prevented an analysis of other types of birth defects. Thirdly, the study did not differentiate between specific types of pesticide exposures, limiting our ability to perform sensitivity analyses. Finally, despite adjusting for potential confounders using multivariable regression, unmeasured or latent confounders, such as pregestational diabetes mellitus, genetics, environmental toxins and other environmental risk factors, might still influence the results.
5 Conclusion
Our findings demonstrated that maternal exposure to pesticides during the periconceptional period was associated with an increased risk of birth defects, particularly those affecting the cardiovascular system in offspring. This association is particularly pronounced among individuals residing in rural areas and those undergoing subsequent pregnancies. Therefore, in order to prevent birth defects in infants, it may be beneficial to pesticide exposure from 3 months before conception through the first trimester of pregnancy. These findings carry significant policy implications for maternal health interventions in China.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Human Research Ethics Committee of Xi’an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
FL: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. XL: Data curation, Formal analysis, Writing – original draft. JC: Data curation, Formal analysis, Writing – original draft. YH: Data curation, Formal analysis, Writing – original draft. SD: Conceptualization, Funding acquisition, Investigation, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Project of Birth Defect Control and Prevention in Shaanxi (No. Sxwsjswzfcght2016-013).
Acknowledgments
We thank all the participants and investigators in this study. We also thank the staff from Xi’an Jiaotong University for their assistance with the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1489365/full#supplementary-material
References
1. Kang, L, Guo, Z, Shang, W, Cao, G, Zhang, Y, Wang, Q, et al. Perinatal prevalence of birth defects in the mainland of China, 2000-2021: a systematic review and meta-analysis. World J Pediatr. (2024) 20:669–81. doi: 10.1007/s12519-023-00786-8
2. Zhao, L, Chen, L, Yang, T, Wang, T, Zhang, S, Chen, L, et al. Birth prevalence of congenital heart disease in China, 1980-2019: a systematic review and meta-analysis of 617 studies. Eur J Epidemiol. (2020) 35:631–42. doi: 10.1007/s10654-020-00653-0
3. Lerro, CC, Hofmann, JN, Andreotti, G, Koutros, S, Parks, CG, Blair, A, et al. Dicamba use and cancer incidence in the agricultural health study: an updated analysis. Int J Epidemiol. (2020) 49:1326–37. doi: 10.1093/ije/dyaa066
4. Bahadar, H, Abdollahi, M, Maqbool, F, Baeeri, M, and Niaz, K. Mechanistic overview of immune modulatory effects of environmental toxicants. Inflamm Allergy Drug Targets. (2015) 13:382–6. doi: 10.2174/1871528114666150529103003
5. Kalliora, C, Mamoulakis, C, Vasilopoulos, E, Stamatiades, GA, Kalafati, L, Barouni, R, et al. Association of pesticide exposure with human congenital abnormalities. Toxicol Appl Pharmacol. (2018) 346:58–75. doi: 10.1016/j.taap.2018.03.025
6. Jaacks, LM, Diao, N, Calafat, AM, Ospina, M, Mazumdar, M, Ibne Hasan, MOS, et al. Association of prenatal pesticide exposures with adverse pregnancy outcomes and stunting in rural Bangladesh. Environ Int. (2019) 133:105243. doi: 10.1016/j.envint.2019.105243
7. Ferguson, KK, van den Dries, MA, Gaillard, R, Pronk, A, Spaan, S, Tiemeier, H, et al. Organophosphate pesticide exposure in pregnancy in association with ultrasound and delivery measures of fetal growth. Environ Health Perspect. (2019) 127:87005. doi: 10.1289/ehp4858
8. Lin, S, Li, J, Yan, X, Pei, L, and Shang, X. Maternal pesticide exposure and risk of preterm birth: a systematic review and meta-analysis. Environ Int. (2023) 178:108043. doi: 10.1016/j.envint.2023.108043
9. Sharma, RK, Singh, P, Setia, A, and Sharma, AK. Insecticides and ovarian functions. Environ Mol Mutagen. (2020) 61:369–92. doi: 10.1002/em.22355
10. Jusko, TA, van den Dries, MA, Pronk, A, Shaw, PA, Guxens, M, Spaan, S, et al. Organophosphate pesticide metabolite concentrations in urine during pregnancy and offspring nonverbal IQ at age 6 years. Environ Health Perspect. (2019) 127:17007. doi: 10.1289/ehp3024
11. Vidart d'Egurbide Bagazgoïtia, N, Bailey, HD, Orsi, L, Lacour, B, Guerrini-Rousseau, L, Bertozzi, AI, et al. Maternal residential pesticide use during pregnancy and risk of malignant childhood brain tumors: a pooled analysis of the ESCALE and ESTELLE studies (SFCE). Int J Cancer. (2018) 142:489–97. doi: 10.1002/ijc.31073
12. Rowe, C, Gunier, R, Bradman, A, Harley, KG, Kogut, K, Parra, K, et al. Residential proximity to organophosphate and carbamate pesticide use during pregnancy, poverty during childhood, and cognitive functioning in 10-year-old children. Environ Res. (2016) 150:128–37. doi: 10.1016/j.envres.2016.05.048
13. Midya, V, Colicino, E, Conti, DV, Berhane, K, Garcia, E, Stratakis, N, et al. Association of Prenatal Exposure to endocrine-disrupting chemicals with liver injury in children. JAMA Netw Open. (2022) 5:e2220176. doi: 10.1001/jamanetworkopen.2022.20176
14. Spinder, N, Bergman, JEH, Boezen, HM, Vermeulen, RCH, Kromhout, H, and de Walle, HEK. Maternal occupational exposure and oral clefts in offspring. Environ Health. (2017) 16:83. doi: 10.1186/s12940-017-0294-5
15. Addissie, YA, Kruszka, P, Troia, A, Wong, ZC, Everson, JL, Kozel, BA, et al. Prenatal exposure to pesticides and risk for holoprosencephaly: a case-control study. Environ Health. (2020) 19:65. doi: 10.1186/s12940-020-00611-z
16. Huang, W, Wu, T, Au, WW, and Wu, K. Impact of environmental chemicals on craniofacial skeletal development: insights from investigations using zebrafish embryos. Environ Pollut. (2021) 286:117541. doi: 10.1016/j.envpol.2021.117541
17. Carmichael, SL, Yang, W, Roberts, E, Kegley, SE, Padula, AM, English, PB, et al. Residential agricultural pesticide exposures and risk of selected congenital heart defects among offspring in the San Joaquin Valley of California. Environ Res. (2014) 135:133–8. doi: 10.1016/j.envres.2014.08.030
18. Carmichael, SL, Yang, W, Roberts, EM, Kegley, SE, Wolff, C, Guo, L, et al. Hypospadias and residential proximity to pesticide applications. Pediatrics. (2013) 132:e1216–26. doi: 10.1542/peds.2013-1429
19. Ohlander, J, Fuhrimann, S, Basinas, I, Cherrie, JW, Galea, KS, Povey, AC, et al. Impact of occupational pesticide exposure assessment method on risk estimates for prostate cancer, non-Hodgkin's lymphoma and Parkinson's disease: results of three meta-analyses. Occup Environ Med. (2022) 79:566–74. doi: 10.1136/oemed-2021-108046
20. Kosnik, MB, Antczak, P, and Fantke, P. Data-driven characterization of genetic variability in disease pathways and pesticide-induced nervous system disease in the United States population. Environ Health Perspect. (2024) 132:57003. doi: 10.1289/ehp14108
21. Felisbino, K, Milhorini, SDS, Kirsten, N, Bernert, K, Schiessl, R, and Guiloski, IC. Exposure to pesticides during pregnancy and the risk of neural tube defects: a systematic review. Sci Total Environ. (2024) 913:169317. doi: 10.1016/j.scitotenv.2023.169317
22. Varshavsky, JR, Rayasam, SDG, Sass, JB, Axelrad, DA, Cranor, CF, Hattis, D, et al. Current practice and recommendations for advancing how human variability and susceptibility are considered in chemical risk assessment. Environ Health. (2023) 21:133. doi: 10.1186/s12940-022-00940-1
23. Bliznashka, L, Roy, A, Christiani, DC, Calafat, AM, Ospina, M, Diao, N, et al. Pregnancy pesticide exposure and child development in low- and middle-income countries: a prospective analysis of a birth cohort in rural Bangladesh and meta-analysis. PLoS One. (2023) 18:e0287089. doi: 10.1371/journal.pone.0287089
24. von Ehrenstein, OS, Ling, C, Cui, X, Cockburn, M, Park, AS, Yu, F, et al. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study. BMJ. (2019) 364:l962. doi: 10.1136/bmj.l962
25. Qu, P, Zhao, D, Yan, M, Liu, D, Pei, L, Zeng, L, et al. Risk assessment for birth defects in offspring of Chinese pregnant women. Int J Environ Res Public Health. (2022) 19:8584. doi: 10.3390/ijerph19148584
26. Perry, MF, Mulcahy, H, and DeFranco, EA. Influence of periconception smoking behavior on birth defect risk. Am J Obstet Gynecol. (2019) 220:588.e1–7. doi: 10.1016/j.ajog.2019.02.029
27. Lipinski, RJ, and Krauss, RS. Gene-environment interactions in birth defect etiology: challenges and opportunities. Curr Top Dev Biol. (2023) 152:1–30. doi: 10.1016/bs.ctdb.2022.10.001
28. Howley, MM, Papadopoulos, EA, Van Bennekom, CM, Van Zutphen, AR, Carmichael, SL, Munsie, JW, et al. Asthma medication use and risk of birth defects: National Birth Defects Prevention Study, 1997-2011. J Allergy Clin Immunol Pract. (2020) 8:3490–3499.e9. doi: 10.1016/j.jaip.2020.07.033
29. Demelash Enyew, H, Bogale, BG, Hailu, AB, and Mereta, ST. Environmental exposures and adverse pregnancy outcomes in Ethiopia: a systematic review and meta-analysis. PLoS One. (2023) 18:e0288240. doi: 10.1371/journal.pone.0288240
30. Xu, Q, Zhu, B, Dong, X, Li, S, Song, X, Xiao, X, et al. Pyrethroid pesticide exposure during early pregnancy and birth outcomes in Southwest China: a birth cohort study. J Toxicol Sci. (2020) 45:281–91. doi: 10.2131/jts.45.281
31. Yin, S, Sun, Y, Yu, J, Su, Z, Tong, M, Zhang, Y, et al. Prenatal exposure to organochlorine pesticides is associated with increased risk for neural tube defects. Sci Total Environ. (2021) 770:145284. doi: 10.1016/j.scitotenv.2021.145284
32. Rappazzo, KM, Warren, JL, Meyer, RE, Herring, AH, Sanders, AP, Brownstein, NC, et al. Maternal residential exposure to agricultural pesticides and birth defects in a 2003 to 2005 North Carolina birth cohort. Birth Defects Res A Clin Mol Teratol. (2016) 106:240–9. doi: 10.1002/bdra.23479
33. Thistle, JE, Ramos, A, Roell, KR, Choi, G, Manley, CK, Hall, AM, et al. Prenatal organophosphorus pesticide exposure and executive function in preschool-aged children in the Norwegian mother, father and child cohort study (MoBa). Environ Res. (2022) 212:113555. doi: 10.1016/j.envres.2022.113555
34. Hawkey, AB, Unal, D, Holloway, ZR, and Levin, ED. Developmental exposure of zebrafish to neonicotinoid pesticides: long-term effects on neurobehavioral function. Neurotoxicology. (2023) 96:240–53. doi: 10.1016/j.neuro.2023.05.003
35. Qi, S, Xu, X, Ma, W, Deng, S, Lian, Z, and Yu, K. Effects of organochlorine pesticide residues in maternal body on infants. Front Endocrinol. (2022) 13:890307. doi: 10.3389/fendo.2022.890307
36. Rappazzo, KM, Warren, JL, Davalos, AD, Meyer, RE, Sanders, AP, Brownstein, NC, et al. Maternal residential exposure to specific agricultural pesticide active ingredients and birth defects in a 2003-2005 North Carolina birth cohort. Birth Defects Res. (2019) 111:312–23. doi: 10.1002/bdr2.1448
37. Qu, Y, Li, AJ, Liu, X, Lin, S, Bloom, MS, Wang, X, et al. Maternal serum neonicotinoids during early-mid pregnancy and congenital heart diseases in offspring: an exploratory study. Environ Pollut. (2024) 342:123046. doi: 10.1016/j.envpol.2023.123046
38. Zhaorigetu, S, Bair, H, Lu, J, Jin, D, Olson, SD, and Harting, MT. Perturbations in endothelial dysfunction-associated pathways in the Nitrofen-induced congenital diaphragmatic hernia model. J Vasc Res. (2018) 55:26–34. doi: 10.1159/000484087
39. Takahashi, T, Friedmacher, F, Zimmer, J, and Puri, P. Fibrillin-1 expression is decreased in the diaphragmatic muscle connective tissue of Nitrofen-induced congenital diaphragmatic hernia. Eur J Pediatr Surg. (2017) 27:026–31. doi: 10.1055/s-0036-1587586
40. Day, M, Witt, A, and Winston, R. Weed biological control in low- and middle-income countries. Curr Opin Insect Sci. (2020) 38:92–8. doi: 10.1016/j.cois.2020.02.004
Keywords: pesticides, birth defects, perinatal pregnancy, cardiovascular system defects, crosssectional survey
Citation: Liu F, Li X, Chen J, Huang Y and Dang S (2024) Maternal pesticide exposure and risk of birth defects: a population-based cross-sectional study in China. Front. Public Health. 12:1489365. doi: 10.3389/fpubh.2024.1489365
Edited by:
Paolo Lauriola, International Society Doctors for the Environment (ISDE), ItalyReviewed by:
Sotirios Maipas, National and Kapodistrian University of Athens, GreeceMuzafar Riyaz, St. Xavier’s College, Palayamkottai, India
Copyright © 2024 Liu, Li, Chen, Huang and Dang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaonong Dang, dGpkc2huQG1haWwueGp0dS5lZHUuY24=
 Fangfang Liu
Fangfang Liu Xiayang Li2
Xiayang Li2 Shaonong Dang
Shaonong Dang