- 1Department of Neurology, Shanghai Putuo People’s Hospital, Tongji University, Shanghai, China
- 2Department of Thoracic Surgery, Changhai Hospital, Naval Medical University, Shanghai, China
Background: The Magnesium depletion score (MDS) serves as a novel metric for quantifying magnesium deficiency in the human body, comprehensively assessing four indicators: diuretic use, proton pump inhibitor use, estimated glomerular filtration rate, and alcohol abuse. However, there have been no studies examining the potential association between MDS and depression.
Methods: The study population for this cross-sectional study comprised adults from the National Health and Nutrition Examination Survey database from 2009 to 2018. Participants with a score of 10 or above on the Patient Health Questionnaire-9 were defined as having depression. We employed multivariable logistic regression models to investigate the association between MDS and depression. Furthermore, subgroup analyses were conducted to assess potential differences in this association among populations with diverse characteristics.
Results: A total of 13,197 participants were included in this study. After adjusting for all covariates, a significant positive correlation was observed between MDS and depression. Specifically, for every unit increase in MDS, the likelihood of developing depression increased by 13% (OR = 1.13, 95% CI: 1.04–1.22, p = 0.0025). This positive correlation was consistent across MDS groups, with a 19% increase in depression likelihood in the medium group (OR = 1.19, 95% CI: 1.01–1.41, p = 0.0404) and a 58% increase in the high group (OR = 1.58, 95% CI: 1.21–2.07, p = 0.0007), using the low subgroup as a reference. Subgroup analyses revealed significant differences in the relationship between MDS and depression across races, marital statuses, and hypertension status.
Conclusion: Our study has uncovered a significant positive association between MDS and depression. Reducing MDS in individuals may play a positive role in both the prevention and treatment of depression.
1 Introduction
Depression is a common mental disorder that has become one of the most important public problems worldwide. People with depression often exhibit negative self-esteem, low energy, loss of self-confidence, and even a high risk of suicide (1). The negative effects of depression are particularly severe in adults (2). According to the World Health Organization (3), 5 percent of adults globally suffer from depression. In 2020, depression adversely affected 17.2 percent of young adults aged 18 to 25 years in the United States, and there is a trend of continued growth in prevalence (4). This suggests that prevention and intervention for depression in adults is critical. However, there are still numerous challenges in the current treatment methods for depression, such as drug side effects and poor treatment adherence. Therefore, it is particularly important to search for safe and effective non-pharmacological treatment methods. In recent years, the role of micronutrients in depression has attracted attention. Numerous studies have demonstrated that some micronutrients affect depression through biological mechanisms, such as magnesium, zinc, and selenium (5–7).
Magnesium is one of the essential micronutrients that play a crucial role in human health and disease prevention (8). Its role in stress coping, neurotransmitter regulation, and other functions is irreplaceable (9). Previous studies have demonstrated that magnesium enhances neurocognitive functions and exhibits significant potential in the treatment of depression, suicidal behavior, and anxiety (10–12). Magnesium, as a safe and easily accessible nutrient, may emerge as a novel and promising treatment for depression. In research related to magnesium and depression, investigators frequently focus on magnesium levels or magnesium acquisition, encompassing serum magnesium levels and dietary magnesium intake (13, 14). However, it has been observed that serum magnesium levels and dietary magnesium intake do not accurately reflect the body’s magnesium content or its deficiency (15, 16). Therefore, there is an urgent need for an accurate, simple, and convenient tool that can be widely used to assess magnesium bioavailability.
In 2021, Fan et al. developed the Magnesium Depletion Score (MDS) (17) to assess the magnesium depletion status of the human body. The MDS takes into account four risk factors affecting the efficiency of renal magnesium absorption and is effective in identifying the degree of whole-body magnesium deficiency in humans. The MDS is more accurate and reliable than other magnesium-related clinical indicators (18). Since the MDS was proposed, many studies have confirmed its significant association with a variety of public health problems, such as hypertension, abdominal aortic calcification, and metabolic syndrome (19–21). However, to the best of our knowledge, no study has yet demonstrated whether there is an association between MDS and depression. To fill the gap in this research area, we provide new scientific evidence for the role of magnesium in the prevention and treatment of depression by deeply exploring the association between MDS and depression. Therefore, we used a cross-sectional study to verify whether MDS is associated with depression in US adults, utilizing data from the 2009–2018 National Health and Nutrition Examination Survey (NHANES).
2 Materials and methods
2.1 Inclusion of the population
In this study, all participants were selected from the NHANES database, a nationally representative survey administered by the National Center for Health Statistics (NCHS), a division of the Centers for Disease Control and Prevention. Before data collection, the NHANES database underwent a stringent ethical review and obtained approval from the NCHS Ethics Review Board. Moreover, all individuals involved in the study provided written informed consent. Interested parties can visit the official website for comprehensive details and updated information on the NHANES database.
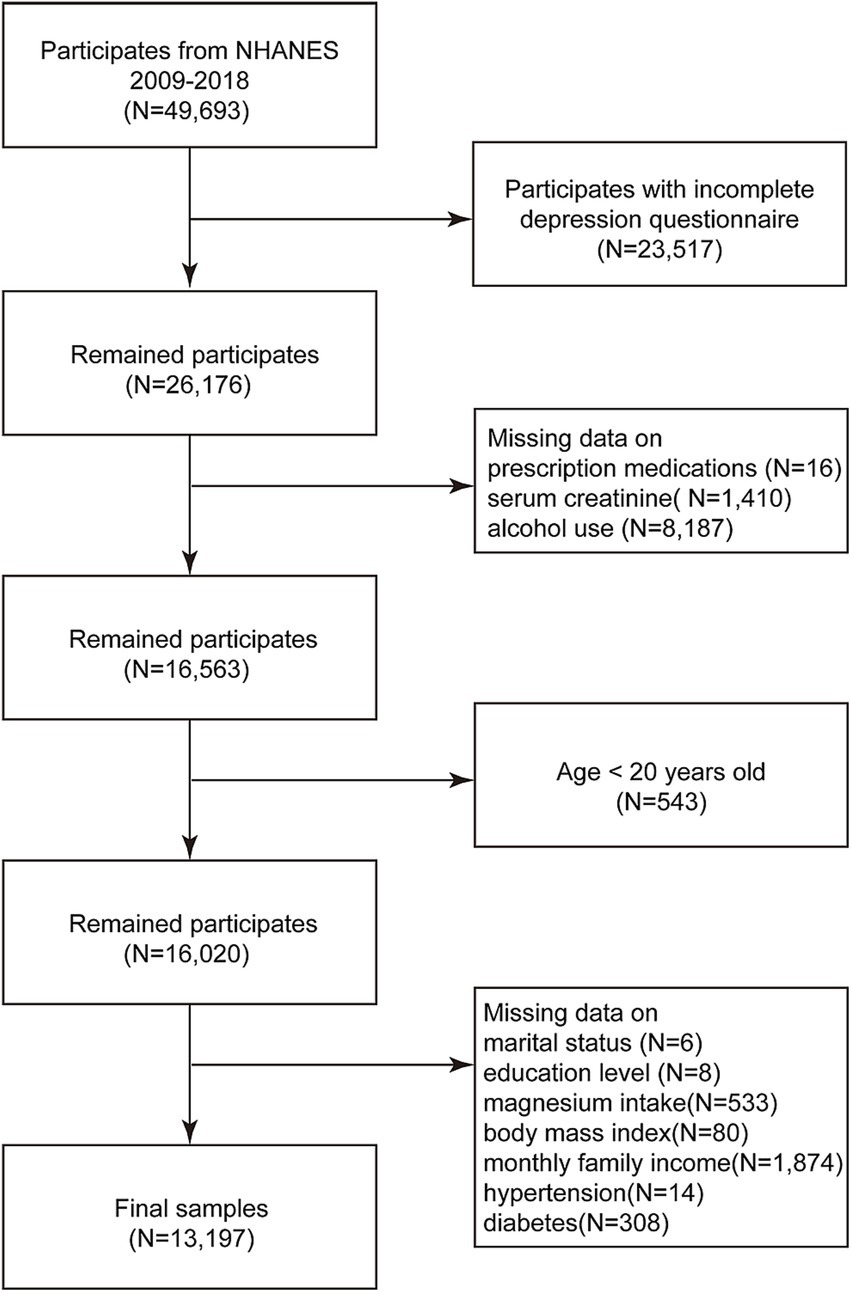
We incorporated the data of all participants from 5 cycles of the NHANES database spanning 2009 to 2018, comprising a total of 49,693 individuals. After rigorous screening, we excluded participants with missing depression status (N = 23,517), ambiguous prescription drug use status (N = 16), absent blood creatinine data (N = 1,410), unclear alcohol consumption status (N = 8,187), those younger than 20 years (N = 543), and those with missing covariates (N = 2,823). Ultimately, our analyses encompassed a final count of 13,197 participants, as depicted in Figure 1.
2.2 Calculation of MDS
The MDS was determined by quantifying four obtained criteria. Firstly, diuretic use was assessed, awarding one point for current use and zero points otherwise. Secondly, proton pump inhibitor use was considered, with one point awarded for current use and zero points for non-use. Thirdly, the estimated glomerular filtration rate (eGFR) was calculated using blood creatinine levels (22). Two points were given if the eGFR value was below 60, one point if it ranged from 60 to 90, and zero points otherwise. Lastly, alcohol abuse was evaluated, awarding one point if women consumed more than one drink per day or men drank more than two drinks per day, and zero points in other cases.
These calculations were based on the questionnaires administered to NHANES participants and their relevant physiological indicators. To facilitate in-depth analyses, apart from computing continuous MDS scores, we also categorized MDS into three groups as categorical variables: Low (0 points), Medium (1–2 points), and High (3 or more points).
2.3 Depression judgments
To assess the presence of depression among participants, we employed the Patient Health Questionnaire (PHQ-9) (23), a well-established and reliable tool for gauging the severity of depressive symptoms. This questionnaire comprises nine specific inquiries, each designed to evaluate the frequency of depressive symptoms, with scores ranging from 0 to 3. Specifically, answers indicating a lower frequency of symptoms receive a score of 0, while those reflecting a higher frequency are assigned a score of 3. By aggregating these individual scores, we determined the total score for each participant. Participants who attained a cumulative score of 10 or above across all nine questions were categorized as experiencing depression. The PHQ-9 scores were derived directly from the questionnaire administered to participants in the National Health and Nutrition Examination Survey.
2.4 Covariates
Drawing upon previous research (15, 24), we identified 10 potential confounding variables that could potentially influence the outcomes. These included factors such as gender, age, race, marital status, education level, monthly income, body mass index (BMI), hypertension status, diabetes status, and magnesium intake. These covariates encompassed four key domains: demographics, biochemistry, dietary factors, and physical measurements.
2.5 Statistical methods
We utilized R software (version 4.4.1) for the comprehensive data processing and statistical evaluation. In this study, categorical variables were presented as percentages and subjected to chi-square analysis for comparison. Meanwhile, continuous variables were expressed as the mean accompanied by the standard deviation (SD). Statistical significance was set at a two-sided p-value below 0.05.
To delve deeper into the correlation between MDS and depression, we employed multivariate logistic regression analysis, establishing three distinct models with varying degrees of covariate adjustments. Specifically, model 1 served as the baseline without any covariate adjustments, while model 2 accounted for gender, age, and ethnicity. Furthermore, model 3 built upon model 2 by incorporating additional factors such as marital status, education level, monthly income, BMI, hypertension, diabetes mellitus, and magnesium intake. Additionally, we conducted a subgroup analysis by grouping all the covariates and utilizing them as stratification factors. This approach aimed to capture the variability in the MDS-depression relationship across different population subsets.
3 Results
3.1 Comparison of baseline characteristics based on depression status
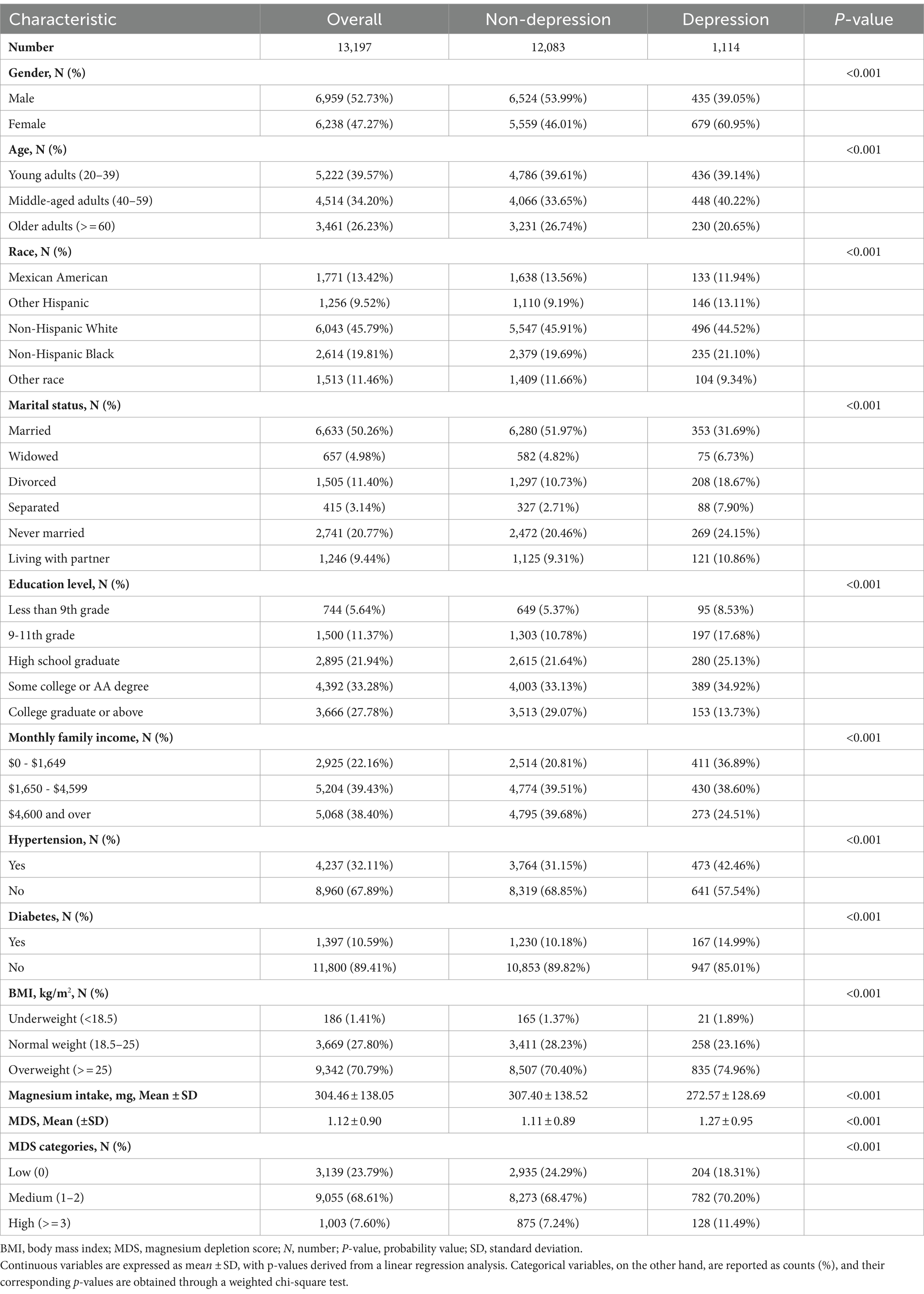
Overall, out of the 13,197 subjects participating in this study, 52.73% (6,959) were males. Among these participants, a substantial portion comprising 39.57% (5,222) were young adults aged 20–39 years. MDS across all subjects averaged 1.12 ± 0.90 (Mean ± SD), and notably, 1,114 individuals (8.44%) exhibited depressive symptoms.
In a comparative analysis of depressed individuals versus those without depression, it was observed that the depressed group tended to have a higher proportion of females, were aged between 40 and 59, belonged to the Other Hispanic ethnic group, were divorced, had completed 9th-11th grade education, had a lower monthly income, were hypertensive, diabetic, overweight, consumed less magnesium, and had a higher MDS score. Table 1 offers a detailed breakdown of the baseline characteristics of the study participants, segmented based on the presence or absence of depressive symptoms.
3.2 The association between MDS and depression
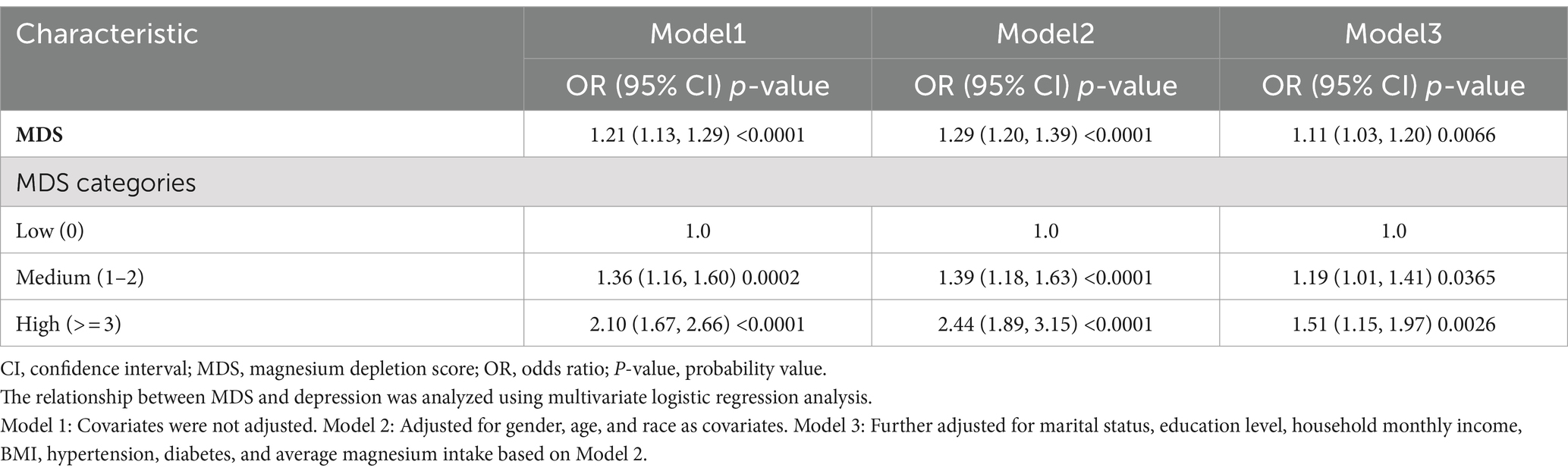
Table 2 displays the relationship between MDS and depression via a weighted multivariate logistic regression analysis. Across all three models, a statistically significant positive correlation was observed between MDS and depression (p < 0.05). Following the adjustment for all relevant variables, every incremental point in MDS corresponded to an 11% increase in the risk of developing depression (OR = 1.11, 95% CI 1.03–1.20). This positive relationship persisted when MDS was categorized into three distinct groups based on its score. In all three models, using the low-score subgroup as a benchmark, both the medium and high-score subgroups exhibited a greater likelihood of depression. In the comprehensive adjusted model, the medium-score subgroup showed a significantly increased likelihood of depression by 19%, when compared to the low-score subgroup (OR = 1.19, 95% CI 1.01–1.41). Similarly, the high-score subgroup exhibited a remarkably higher likelihood of depression, standing at 51% above the baseline (OR = 1.51, 95%CI 1.15–1.97).
3.3 Subgroup analyses
To delve deeper into the varying associations between MDS and depression across various populations, we conducted subgroup analysis interaction tests. As Figure 2 illustrates, our findings reveal inconsistencies in these associations. Specifically, we found remarkable disparities in the linkage between MDS and depression when considering race, marital status, and hypertension, all with statistical significance (p < 0.05). The positive correlation between MDS and depression stood out among Mexican Americans, individuals of other races, married individuals, those who remain unmarried, and individuals free from hypertension. Nonetheless, it is noteworthy that a negative correlation emerged between MDS and depression in the widowed population (OR = 0.70, 95% CI 0.55–0.90). Interestingly, the consumption of magnesium did not seem to alter this relationship between MDS and depression.
4 Discussion
This cross-sectional study examined the relationship between MDS and depression, utilizing cohort data comprising 13,197 adult participants from the NHANES database spanning the years 2009 to 2018. Notably, our in-depth analysis uncovered a pronounced and statistically significant positive correlation between MDS and depression, consistent across the three models evaluated. Furthermore, this correlation exhibited noteworthy variations among subgroups stratified by marital status, race, and hypertension.
The relationship between magnesium and depression has garnered significant attention for quite some time. Magnesium plays a pivotal role in brain biochemistry, influencing numerous neurotransmission pathways linked to depression’s development (25). The research indicates that magnesium deficiency contributes to the pathophysiology of mood disorders (26). This may be because magnesium deficiency can affect glutamatergic transmission in the limbic system and cerebral cortex, brain regions that play crucial roles in the pathogenesis of depression (27). Furthermore, magnesium deficiency may alter the composition and signaling of N-methyl-D-aspartate receptors, leading to enhanced depressive-like behaviors (28). Additionally, magnesium exhibits certain immunoregulatory effects, which potentially interact with depression, particularly as magnesium deficiency may be associated with elevated levels of inflammatory markers such as serum C-reactive protein (29).
It is well-established in the literature that balancing magnesium levels in depressed patients has a beneficial impact on antidepressant effectiveness (30). Numerous studies have highlighted a substantial correlation between magnesium deficiency and depression (31, 32). Dietary magnesium intake may be associated with depression through its protective effects on the nervous system (33). A cross-sectional survey among Iranian postgraduate students noted a negative association between dietary magnesium intake and depression (34). Similarly, a cross-sectional study conducted in the Polish region among postmenopausal women revealed a negative correlation between serum magnesium levels and the severity of depressive symptoms (35).
In our current study, we employed MDS to assess the magnesium status of workers. MDS comprehensively accounts for the key factors that influence magnesium reabsorption in the human body, encompassing prescription drug use, renal function, and lifestyle habits. Regarding prescription drug use, studies have validated that prolonged use of diuretics and proton pump inhibitors can cause a decrease in magnesium levels in the body due to the interplay of various pathogenic mechanisms, potentially resulting in hypomagnesemia (36, 37). As for renal function, eGFR serves as a reliable indicator (38), and individuals with lower eGFR tend to have lower serum magnesium levels compared to those with normal renal function (39). Additionally, alcohol abuse disrupts intestinal magnesium absorption, a frequent cause of hypomagnesemia (40). In summary, the utilization of MDS offers a comprehensive assessment of magnesium status in humans.
Although the current study lacks a definitive biological mechanism to explain the interaction between MDS and depression, there are possible explanations for the underlying causes and mechanisms of this interaction. Notably, in subgroup analyses, the significant positive correlation between MDS and depression persisted among Mexican Americans (OR = 1.55, 95% CI 1.25–1.92) and individuals of Other Races (OR = 1.28, 95% CI 1.01–1.61). This suggests that the positive association between MDS and depression may not be uniform across different racial groups. Varied accessibility to mental health care (41) and disparities in renal magnesium handling (42) among different races could potentially influence the relationship between MDS and depression. Furthermore, alcohol consumption, a crucial factor in the calculation of MDS, exhibits distinct patterns across ethnic groups. For instance, Mexican-American adults have been identified as having high alcohol consumption rates within the Hispanic community (43), indicating that drinking habits may vary significantly between ethnicities.
In subgroup analyses, marital status emerged as a significant factor modulating the association between MDS and depression. Notably, the positive correlation between MDS and depression remained prominent in married (OR = 1.25, 95% CI 1.11–1.40) and never-married individuals (OR = 1.21, 95% CI 1.02–1.44). Conversely, among widowed individuals, this relationship shifted to a significant negative association (OR = 0.70, 95% CI 0.55–0.90). Previous research has highlighted the negative implications of marital dysfunction on health, particularly through depression (44). A study found a substantial link between the severity of depression and a lack of marital intimacy (45), indicating that such intimacy deficits can adversely affect individuals’ financial stability, behavior, and emotional well-being. A cross-sectional investigation among older adults in the United States revealed variations in energy expenditure among different marital statuses. Widowed individuals exhibited the lowest energy expenditure, rendering them susceptible to malnutrition (46). Furthermore, a robust association was observed between nutritional status, body magnesium levels, and depression. Moreover, numerous studies have documented that widows are more vulnerable to a range of conditions, including stroke, inflammation, and psychiatric disorders. This group also faces a significantly heightened risk of cardiovascular and all-cause mortality (47–49). These findings collectively suggest that such conditions may contribute to the observed association between MDS and depression.
In our subgroup analyses, we observed that hypertension modified the positive association between MDS and depression. Specifically, this association remained significant in the non-hypertensive population (OR = 1.27, 95% CI 1.12–1.43), but not among hypertensive patients (p < 0.05). The significance of magnesium in hypertension has been extensively researched. A meta-analysis revealed that magnesium intake exhibited the most beneficial effect on blood pressure reduction compared to other micronutrients (50). A study involving patients with essential hypertension highlighted a significant difference in magnesium excretion between hypertensive and non-hypertensive individuals (51), potentially explaining the variance in body magnesium levels between these two groups. Furthermore, several studies have demonstrated a high prevalence of comorbidities between hypertension and psychiatric disorders (52, 53). The physiological impacts of depression on cardiovascular health include hypothalamic–pituitary–adrenal axis and sympathoadrenal activation, rhythm disturbances, inflammation, and hypercoagulability. These factors could potentially contribute to the observed differences in the association between MDS and depression among hypertensive and non-hypertensive individuals.
In subgroup analysis, we observed an intriguing phenomenon: magnesium intake had no significant impact on the positive correlation between MDS and depression (p for interaction >0.05). This may be due to the complex absorption and consumption process of dietary magnesium within the human body, which could result in differences in functional magnesium status even among individuals with the same dietary magnesium intake (9). Furthermore, not all magnesium components are equally absorbed into the bloodstream (54).
To our knowledge, this marks the inaugural study to explore the correlation between MDS and depression. From a clinical perspective, our study provides evidence that magnesium deficiency may be a potential risk factor for depression, not only supporting previous research on the crucial role of magnesium in the nervous system and mental health but also offering new insights into the potential value of magnesium supplements as a preventive or adjuvant therapeutic means for depression. Among its strengths, this study boasts a sizable, nationally representative participant pool. We have accounted for confounding variables to bolster the reliability of our findings. Furthermore, through subgroup analyses, we have dissected the relationship between MDS and depression across diverse populations, thereby enhancing statistical rigor. However, our study does have its limitations. Firstly, the cross-sectional design precludes us from establishing a causal link between MDS and depression. Secondly, the extensive use of questionnaires in our data collection may have introduced deviations from the participants’ actual circumstances. Lastly, despite adjusting for a broad spectrum of 10 variables, we acknowledge that not all potential confounding factors have been accounted for.
5 Conclusion
In summary, there exists a notable positive correlation between MDS and depression. This finding underscores the importance of heightened vigilance regarding the risk of depression among individuals with elevated MDS levels. Reducing individual MDS scores could potentially play a pivotal role in mitigating the incidence of depression. To further validate our observations, additional prospective studies are warranted. In future research, we will continue to include covariates that may potentially influence the relationship between the two, and track changes in individual MDS to observe its impact on the incidence or severity of depressive symptoms, to establish causality.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WZ: Data curation, Methodology, Software, Writing – original draft. HJ: Data curation, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thanks to all participants in the NHANES database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Park, LT, and Zarate, CA Jr. Depression in the primary care setting. N Engl J Med. (2019) 380:559–68. doi: 10.1056/NEJMcp1712493
2. Marwaha, S, Palmer, E, Suppes, T, Cons, E, Young, AH, and Upthegrove, R. Novel and emerging treatments for major depression. Lancet. (2023) 401:141–53. doi: 10.1016/S0140-6736(22)02080-3
3. WHO. Depressive disorder (depression). (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/depression (Accessed June 1, 2024).
4. Goodwin, RD, Dierker, LC, Wu, M, Galea, S, Hoven, CW, and Weinberger, AH. Trends in U.S. depression prevalence from 2015 to 2020: the widening treatment gap. Am J Prev Med. (2022) 63:726–33. doi: 10.1016/j.amepre.2022.05.014
5. Wang, J, Um, P, Dickerman, BA, and Zinc, LJ. Magnesium, selenium and depression: a review of the evidence, potential mechanisms and implications. Nutrients. (2018) 10:584. doi: 10.3390/nu10050584
6. Quan, Z, Li, H, Quan, Z, and Qing, H. Appropriate macronutrients or mineral elements are beneficial to improve depression and reduce the risk of depression. Int J Mol Sci. (2023) 24:7098. doi: 10.3390/ijms24087098
7. Li, Z, Li, B, Song, X, and Zhang, D. Dietary zinc and iron intake and risk of depression: a meta-analysis. Psychiatry Res. (2017) 251:41–7. doi: 10.1016/j.psychres.2017.02.006
8. Volpe, SL. Magnesium in disease prevention and overall health. Adv Nutr. (2013) 4:378s–83s. doi: 10.3945/an.112.003483
9. Chou, MH, Yang, YK, Wang, JD, Lin, CY, and Lin, SH. The Association of Serum and Dietary Magnesium with depressive symptoms. Nutrients. (2023) 15:774. doi: 10.3390/nu15030774
10. Du, J, Zhu, M, Bao, H, Li, B, Dong, Y, Xiao, C, et al. The role of nutrients in protecting mitochondrial function and neurotransmitter signaling: implications for the treatment of depression, PTSD, and suicidal behaviors. Crit Rev Food Sci Nutr. (2016) 56:2560–78. doi: 10.1080/10408398.2013.876960
11. Botturi, A, Ciappolino, V, Delvecchio, G, Boscutti, A, Viscardi, B, and Brambilla, P. The role and the effect of Magnesium in mental disorders: a systematic review. Nutrients. (2020) 12:1661. doi: 10.3390/nu12061661
12. Noah, L, Dye, L, Bois De Fer, B, Mazur, A, Pickering, G, and Pouteau, E. Effect of magnesium and vitamin B6 supplementation on mental health and quality of life in stressed healthy adults: post-hoc analysis of a randomised controlled trial. Stress Health. (2021) 37:1000–9. doi: 10.1002/smi.3051
13. Hajhashemy, Z, Shirani, F, and Askari, G. Dietary Magnesium intake in relation to depression in adults: a GRADE-assessed systematic review and dose-response Meta-analysis of epidemiologic studies. Nutr Rev. (2024). doi: 10.1093/nutrit/nuae056
14. Abdelmoneam, AH, Khafagy, GM, Elbeh, KA, and Hasan, MDA. Impact of Magnesium and ferritin deficiency on depression among adolescent students. J Prim Care Community Health. (2024) 15:21501319241252570. doi: 10.1177/21501319241252570
15. Luo, X, Tang, M, Wei, X, and Peng, Y. Association between magnesium deficiency score and sleep quality in adults: a population-based cross-sectional study. J Affect Disord. (2024) 358:105–12. doi: 10.1016/j.jad.2024.05.002
16. Reinhart, RA. Magnesium metabolism. A review with special reference to the relationship between intracellular content and serum levels. Arch Intern Med. (1988) 148:2415–20. doi: 10.1001/archinte.1988.00380110065013
17. Fan, L, Zhu, X, Rosanoff, A, Costello, RB, Yu, C, Ness, R, et al. Magnesium depletion score (MDS) predicts risk of systemic inflammation and cardiovascular mortality among US adults. J Nutr. (2021) 151:2226–35. doi: 10.1093/jn/nxab138
18. Tian, Z, Qu, S, Chen, Y, Fang, J, Song, X, He, K, et al. Associations of the magnesium depletion score and magnesium intake with diabetes among US adults: an analysis of the National Health and nutrition examination survey 2011-2018. Epidemiol Health. (2024) 46:e2024020. doi: 10.4178/epih.e2024020
19. Wang, X, Zeng, Z, Wang, X, Zhao, P, Xiong, L, Liao, T, et al. Magnesium depletion score and metabolic syndrome in US adults: analysis of NHANES 2003-2018. J Clin Endocrinol Metab. (2024). doi: 10.1210/clinem/dgae075
20. Lu, J, Li, H, and Wang, S. The kidney reabsorption-related magnesium depletion score is associated with increased likelihood of abdominal aortic calcification among US adults. Nephrol Dial Transplant. (2023) 38:1421–9. doi: 10.1093/ndt/gfac218
21. Song, J, Zhang, Y, Lin, Z, Tang, J, Yang, X, and Liu, F. Higher Magnesium depletion score increases the risk of all-cause and cardiovascular mortality in hypertension participants. Biol Trace Elem Res. (2024). doi: 10.1007/s12011-024-04254-w
22. Levey, AS, Stevens, LA, Schmid, CH, Zhang, YL, Castro, AF 3rd, Feldman, HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
23. Kroenke, K, Spitzer, RL, and Williams, JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
24. Huang, J, Shi, P, Zhao, Y, Zhang, H, Gao, T, and Wang, X. Associations between smoking, sex steroid hormones, trouble sleeping, and depression among U.S. adults: a cross-sectional study from NHANES (2013-2016). BMC Public Health. (2024) 24:1541. doi: 10.1186/s12889-024-19045-0
25. Serefko, A, Szopa, A, and Poleszak, E. Magnesium and depression. Magnes Res. (2016) 29:112–9. doi: 10.1684/mrh.2016.0407
26. Moabedi, M, Aliakbari, M, Erfanian, S, and Milajerdi, A. Magnesium supplementation beneficially affects depression in adults with depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Front Psych. (2023) 14:1333261. doi: 10.3389/fpsyt.2023.1333261
27. Mlyniec, K. Zinc in the glutamatergic theory of depression. Curr Neuropharmacol. (2015) 13:505–13. doi: 10.2174/1570159X13666150115220617
28. Ghafari, M, Whittle, N, Miklósi, AG, Kotlowski, C, Schmuckermair, C, Berger, J, et al. Dietary magnesium restriction reduces amygdala-hypothalamic GluN1 receptor complex levels in mice. Brain Struct Funct. (2015) 220:2209–21. doi: 10.1007/s00429-014-0779-8
29. Dibaba, DT, Xun, P, and He, K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: meta-analysis and systematic review. Eur J Clin Nutr. (2014) 68:510–6. doi: 10.1038/ejcn.2014.7
30. Eby, GA, and Eby, KL. Rapid recovery from major depression using magnesium treatment. Med Hypotheses. (2006) 67:362–70. doi: 10.1016/j.mehy.2006.01.047
31. Rajizadeh, A, Mozaffari-Khosravi, H, Yassini-Ardakani, M, and Dehghani, A. Serum Magnesium status in patients subjects with depression in the City of Yazd in Iran 2013-2014. Biol Trace Elem Res. (2016) 171:275–82. doi: 10.1007/s12011-015-0542-x
32. Rajizadeh, A, Mozaffari-Khosravi, H, Yassini-Ardakani, M, and Dehghani, A. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: a randomized, double-blind, placebo-controlled trial. Nutrition. (2017) 35:56–60. doi: 10.1016/j.nut.2016.10.014
33. Rajasekar, R, VanderMolen, J, Barnhart, K, and Anguilim, N. Dietary intake with supplementation of vitamin D, vitamin B6, and magnesium on depressive symptoms: a public health perspective. Front Public Health. (2024) 12:1369666. doi: 10.3389/fpubh.2024.1369666
34. Yary, T, Aazami, S, and Soleimannejad, K. Dietary intake of magnesium may modulate depression. Biol Trace Elem Res. (2013) 151:324–9. doi: 10.1007/s12011-012-9568-5
35. Stanisławska, M, Szkup-Jabłońska, M, Jurczak, A, Wieder-Huszla, S, Samochowiec, A, Jasiewicz, A, et al. The severity of depressive symptoms vs. serum mg and Zn levels in postmenopausal women. Biol Trace Elem Res. (2014) 157:30–5. doi: 10.1007/s12011-013-9866-6
36. Katopodis, P, Karteris, E, and Katopodis, KP. Pathophysiology of drug-induced hypomagnesaemia. Drug Saf. (2020) 43:867–80. doi: 10.1007/s40264-020-00947-y
37. Bosman, W, Hoenderop, JGJ, and de Baaij, JHF. Genetic and drug-induced hypomagnesemia: different cause, same mechanism. Proc Nutr Soc. (2021) 80:327–38. doi: 10.1017/S0029665121000926
38. Helal, I, Fick-Brosnahan, GM, Reed-Gitomer, B, and Schrier, RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. (2012) 8:293–300. doi: 10.1038/nrneph.2012.19
39. Hernández-Rubio, A, Sanvisens, A, Barbier-Torres, L, Blanes, R, Miquel, L, Torrens, M, et al. Associations of hypomagnesemia in patients seeking a first treatment of alcohol use disorder. Drug Alcohol Depend. (2023) 245:109822. doi: 10.1016/j.drugalcdep.2023.109822
40. Figueres, L, Bruneau, S, Prot-Bertoye, C, Brideau, G, Néel, M, Griveau, C, et al. Hypomagnesemia, hypocalcemia, and Tubulointerstitial nephropathy caused by Claudin-16 autoantibodies. J Am Soc Nephrol. (2022) 33:1402–10. doi: 10.1681/ASN.2022010060
41. Hardaway, CR, and McLoyd, VC. Escaping poverty and securing middle class status: how race and socioeconomic status shape mobility prospects for African Americans during the transition to adulthood. J Youth Adolesc. (2009) 38:242–56. doi: 10.1007/s10964-008-9354-z
42. Palacios, C, Wigertz, K, Braun, M, Martin, BR, McCabe, GP, McCabe, L, et al. Magnesium retention from metabolic-balance studies in female adolescents: impact of race, dietary salt, and calcium. Am J Clin Nutr. (2013) 97:1014–9. doi: 10.3945/ajcn.112.039867
43. Tam, CC, Li, L, Kosai, S, Duhart Clarke, SE, Ehlers, CL, and Karriker-Jaffe, KJ. Protective effects of ethnic enclaves: testing pathways to alcohol use and use disorders in Mexican American young adults. Am J Community Psychol. (2024). doi: 10.1002/ajcp.12756
44. Kiecolt-Glaser, JK, and Newton, TL. Marriage and health: his and hers. Psychol Bull. (2001) 127:472–503. doi: 10.1037/0033-2909.127.4.472
45. Waring, EM, and Patton, D. Marital intimacy and depression. Br J Psychiatry. (1984) 145:641–4. doi: 10.1192/bjp.145.6.641
46. Heuberger, R, and Wong, H. The association between depression and widowhood and nutritional status in older adults. Geriatr Nurs. (2014) 35:428–33. doi: 10.1016/j.gerinurse.2014.06.011
47. Wong, CW, Kwok, CS, Narain, A, Gulati, M, Mihalidou, AS, Wu, P, et al. Marital status and risk of cardiovascular diseases: a systematic review and meta-analysis. Heart. (2018) 104:1937–48. doi: 10.1136/heartjnl-2018-313005
48. Ennis, J, and Majid, U. "death from a broken heart": a systematic review of the relationship between spousal bereavement and physical and physiological health outcomes. Death Stud. (2021) 45:538–51. doi: 10.1080/07481187.2019.1661884
49. Elwert, F, and Christakis, NA. The effect of widowhood on mortality by the causes of death of both spouses. Am J Public Health. (2008) 98:2092–8. doi: 10.2105/AJPH.2007.114348
50. Iqbal, S, Klammer, N, and Ekmekcioglu, C. The effect of electrolytes on blood pressure: a brief summary of Meta-analyses. Nutrients. (2019) 11:1362. doi: 10.3390/nu11061362
51. Tillman, DM, and Semple, PF. Calcium and magnesium in essential hypertension. Clin Sci (Lond). (1988) 75:395–402. doi: 10.1042/cs0750395
52. Grimsrud, A, Stein, DJ, Seedat, S, Williams, D, and Myer, L. The association between hypertension and depression and anxiety disorders: results from a nationally-representative sample of south African adults. PLoS One. (2009) 4:e5552. doi: 10.1371/journal.pone.0005552
53. Joynt, KE, Whellan, DJ, and O'Connor, CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. (2003) 54:248–61. doi: 10.1016/S0006-3223(03)00568-7
Keywords: depression, magnesium depletion score, micronutrients, magnesium, NHANES
Citation: Zhao W and Jin H (2024) Magnesium depletion score and depression: a positive correlation among US adults. Front. Public Health. 12:1486434. doi: 10.3389/fpubh.2024.1486434
Edited by:
Joanna Rog, Warsaw University of Life Sciences, PolandReviewed by:
Richa Tripathi, All India Institute of Medical Sciences Gorakhpur, IndiaShima Erfanian, Isfahan University of Medical Sciences, Iran
Copyright © 2024 Zhao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Jin, cHJvamluaGFpQDE2My5jb20=
 Wei Zhao1
Wei Zhao1 Hai Jin
Hai Jin