- 1Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan-Aman, Ethiopia
- 2Southwest Ethiopian People Regional State Health Bureau, Dawro Zone, Ethiopia
- 3School of Pharmacy, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan-Aman, Ethiopia
Background: Intestinal parasitic infections continue to pose a major threat to human health globally, with a particularly high prevalence in developing countries. Soil-borne helminthiasis and schistosomiasis are notably widespread.
Objective: The objective of the study was to determine the prevalence and contributing factors of intestinal parasites infection among participants aged 7–14 years.
Methods: Community-wide prevalence study was undertaken from 30 August to 30 September 2021 in Mizan Aman Town. Socio-demographic information was collected using questionnaires. Three of the five kebels were randomly chosen. Households with children aged 7–14 were gathered from the chosen kebels and health post to recruit one eligible subject. Allocation of study subjects to each of the chosen kebels was computed proportionally. Two thick smear of Kato Katz technique was applied to examine stool samples. Data were entered and analyzed using SPSS version 20. To investigate the association between the dependent and independent variables, a logistic regression analysis was conducted. Statistics were considered significant for p-values under 0.05.
Results: The overall prevalence of intestinal parasites was 64.6% (215/333). Of these, 51.05% (170/333) were infected with STHs, while 13.5% (45/333) had S. mansoni. T. trichiura was the most prevalent helminth. Infection intensity ranged from light to moderate was observed. Prior information about STHs (aORr:2.022 = CI:1.222–3.340), poor knowledge about STHs (aOR:1.677 = CI:1.057–2.660), unaware of deworming as prevention method of S. mansoni (aOR:2.620:CI:1267–5.418), swimming (aOR:0.448:CI:0.176–0.992) and contact with water (aOR:0.402:CI:0.169–0.957) were significantly associated with the S. mansoni infection.
Conclusion and recommendation: The prevalence of intestinal parasite was high. Heavy infection was not recorded. Beyond mass deworming, the report emphasizes the necessity of ongoing public health interventions to address the high prevalence of these intestinal helminths.
Introduction
Infection caused by intestinal parasites remains a significant global health issue, particularly affecting regions with high poverty rates, limited access to clean water, inadequate sanitation, and unsanitary living environments. In tropical regions where helminth infections are prevalent, it is not uncommon for individuals to acquire multiple types of gastrointestinal parasites, such as schistosomiasis and soil-transmitted helminthiasis, due to the high incidence of worm diseases (1, 2). The duration of infection, parasitic load, and type of species involved a role in determining the impact of intestinal worms. High levels of intestinal helminths and schistosomes in school-aged children can hinder their development, reduce their overall physical health, and impair their cognitive abilities (3).
The negative health effects mentioned above have a cumulative impact on the academic performance of children, leading to decreased school attendance (4). Additionally, it has been observed that hookworm infection (and potentially other diseases caused by parasitic worms) can hinder future earning potential (5). Moreover, hookworm and schistosomiasis are significant diseases that can affect pregnant women, resulting in premature births, lower birth weights, and increased health risks for mothers (6).
Globally, soil-transmitted helminths infection and schistosomiasis affect more than 1.5 billion individuals (24%) and 240 million individuals, respectively (7). Worldwide, there are over 267 million preschool-age children and 568 million school-age children residing in regions where STHs are highly prevalent and require treatment and preventive measures (8). In sub-Saharan Africa, approximately 198 million, 192 million, 173 million, and 162 million people are infected with hookworm, Schistosoma spp., Ascaris lumbricoides, and Trichuris trichiura, respectively (9, 10).
In Ethiopia, approximately 79 million individuals residing in areas where STHs are prevalent. This includes 9.1 million pre-school age children, 25.3 million school-age children, and 44.6 million adults above 18 years old. Moreover, an estimated 37.3 million people live in areas endemic to schistosomiasis, including 3.4 million pre-school children, 12.3 million school-aged children, and 21.6 million adults. According to estimates from 2013, the number of schistosomiasis cases in Ethiopia was approximately 35,775,100 (11). The overall pooled estimate of STHs in Ethiopia regions was reported to be 33%. The Southern Nations, Nationalities, and Peoples’ Region, Amhara Region, Oromia Region, and Tigray Region accounted for 44, 34, 31, and 10%, respectively (12). Ten years ago, a survey conducted in Mizan-Aman revealed a significant occurrence of intestinal parasites, with a prevalence rate of 76.7%. However, the research conducted at the institutional level failed to consider the environmental factors (13).
The government has been closely monitoring the prevention and control of these parasites, particularly in regions with high prevalence. In (14), the Federal Ministry of Health (FMOH) intensified its efforts to address schistosomiasis and STHs in regions where these diseases are prevalent, leading to the successful treatment of over 19 million people (15). Bench Sheko Health Department implemented school-based deworming activities by June 2023. Despite all efforts, there is often a lack of recent, localized data on the prevalence and distribution of these infections, making it difficult to design effective public health interventions. The purpose of this study was, therefore, to fill this gap by providing current data on the prevalence of intestinal parasite helminths, intensity and associated factors among this particular age group at the community level following deworming. This assessment can offer valuable insights for the development of a focused and effective strategy for prevention and control.
Methods and materials
Study setting, period and design
A community-based cross-sectional study was carried out in Mizan Aman City from August 30 to September 30, 2021. Mizan-Aman city is situated in Bench Sheko Zone of the Southwest Ethiopian People’s Regional State. Twelve ethnic groups make up this region. The region consists of six Zones: Keffa, Sheka, Bench Sheko, Dawro, West Omo, and Konta (In the context of Ethiopia, a zone functions as a second-tier administrative division, situated below regions and above woredas, which are also known as districts). Bonga, Tercha, Mizan Aman, and Tepi are the four capital cities in the region. Although ethnic groups speak twelve different languages, Amharic is the working language in the region (16). Mizan-Aman city is located at an elevation of 1,451 meters above sea level and has latitudes between 6°55′0″N and 7°2′30″N and longitudes between 35°31′30″E and-35°37′30″E (Figure 1). The city is located 561 kilometers from Addis Ababa, the capital of Ethiopia, 50 kilometers from Tepi and 230 kilometers from Jimma. The city comprises five administrative kebels. The average yearly temperature in the area is 19.6°C. The annual rainfall is 2000 mm. The city is home to three higher education institutions—Mizan Tepi University, Aman Health Science College and Mizan ATVET College. In the city, clinical services are provided by the Mizan Aman Teaching Hospital, two health centers, and several private pharmacies and clinics (68).
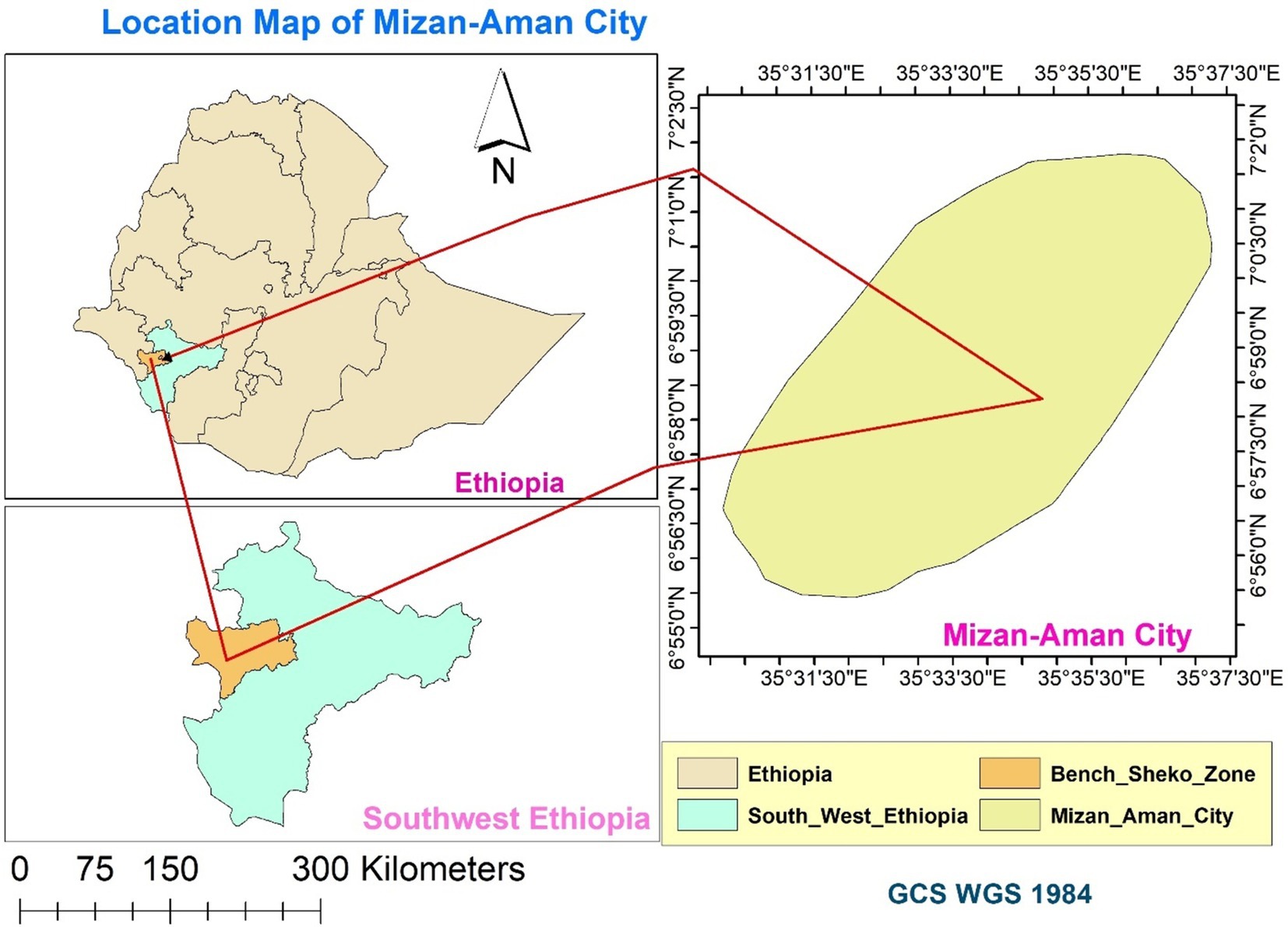
Figure 1. Map of the Study Area: Mizan-Aman City, Bench Sheko Zone, Southwest Ethiopian People’s Regional State.
Population
The source population consisted of all individuals aged 7–14 residing in Mizan Aman city. The study population comprised children within the same age range, who were randomly selected from the designated Kebeles (the smallest and final level of administrative units in Ethiopia) based on specific inclusion criteria. These criteria required that the children had lived in these Kebles for at least 6 months and had not received any anthelmintic treatments (Albendazole, Mebendazole, Levamisole, Pyrantel pamoate and Praziquantel) for a duration of 2 weeks before the data collection commenced.
Sample size determination
The sample size was calculated using methods suitable for a cross-sectional survey (18), based on a previously reported intestinal parasite prevalence of 73.9% (13). The calculation assumed a 15% non-response rate, a 5% margin of error, and a 95% confidence level. The initial sample size calculation resulted in 296 participants. After accounting for the 15% non-response rate (45 individuals), the final sample size was adjusted to 341 children.
Sampling technique
Three kebeles (the smallest administrative units within a woreda or districts, which is the third level of administrative divisions next to Zone in Ethiopia) were selected at random from a pool of five kebels in the town. The households that were chosen had children between the ages of 7 and 14, and this selection was based on the registration records and family folders that were accessible at the health posts. In these three Kebels, there was a combined total of 2053 children. Specifically, there were 691 children in Hibert Kebel, 609 children in Kometa Kebel, and 753 children in Addis Ketema Kebel. The selection process involved allocating and sampling children in a proportional manner. Specifically, 112 children from Hibert Kebel, 99 children from Kometa Kebel, and 122 children from Addis Ketema Kebel were chosen. In cases where a household had only one child between the ages of 7 and 14, that child was selected. However, if there were multiple children within this age range, one child was randomly chosen through a lottery method.
Questionnaire survey
The questionnaire was written primarily in English and translated into Amharic for ease of understanding. A comparison was made regarding the consistency of the two versions. Three skilled data collectors conducted face-to-face interviews to gather the data. A preliminary study was conducted using 5% of the study subjects who were not from the study area but possessed comparable attributes in order to facilitate the required adjustments. All ambiguous inquiries and duplicated concepts were rectified. Sociodemographic factors and risk elements linked to STH and S. mansoni were gathered through a questionnaire. A survey was conducted to evaluate the type and condition of latrines, the existence of waste disposal sites, the occurrence of open defecation near the residence, the presence of animals, and the hygiene of the child’s fingernails.
Parasitological examination
Approximately 5 grams of fresh fecal specimens were gathered utilizing labeled containers. The specimens were then stored in an ice box and transported to Mizan-Tepi University Parasitology Laboratory for processing and examination. The protocol of Kato-Katz was as follows: Fresh fecal sample was filtered through a mesh sieve to remove larger particles. A total of 41.7 mg of the strained stool was transferred on a microscope slide using 41.7 mg template. One pieces of cellophane soaked in a glycerol-malachite green solution for 24 h was placed on a slide. In order to produce a smear, the cellophane was pressed down with another microscope slide. Two thick smears were prepared from a single stool sample. The slides were then examined under a microscope within a time frame of 40–60 min after preparation to prevent the loss of hookworm parasites. Eggs in each prepared slide were counted and multiplied by a factor 24 to express the eggs per gram (EPG) of feces (19). Species-specific infection intensity categories had been classified as mild, moderate, and severe according to thresholds set by the World Health Organization (20).
Quality assurance
The data collectors and the laboratory technologist underwent training in collecting questionnaire data and utilizing Kato-Katz techniques. The data collectors were supervised during the data collection process. The Principal Investigator confirmed the questionnaire’s completeness and the overall activities in the laboratory. Reagent and smear preparation for microscopic examination were reviewed. The egg quantification of 10% of post-analyzed slides was re-evaluated by an experienced laboratory technician and the Principal Investigator.
Date processing and analysis
The data analysis underwent a process of data validation, data cleaning, editing, coding, inputting, and analyzing using SPSS version 20. The prevalence of soil-transmitted helminthiasis and schistosomiasis infection was reported in percentage. Logistic regression analysis was conducted to examine the potential significant associations. The potential candidates for multivariate analysis were determined by selecting the independent variables with a p-value ≤0.2 in bivariate analysis. In the multivariate analysis, the adjusted odds ratios (aOR) with a 95% confidence interval (CI) and a p-value <0.05 were considered to be statistically significant.
Operational definition
In our research context, an operational definition refers to how a variable defined for the purposes of the study.
Prior Information (heard) about Helminths: we are specifically referring to whether an individual had been previously informed or not about helminth infections before evaluating their knowledge based on the questions, we had set based on the criteria listed below:
Previously informed (Prior information)
If participants who can correctly answer at least one or more questions about STHs, such as:
• Can they identify at least one type of helminth?
• Do they know at least one method of preventing STHs?
• Have they heard of deworming treatments and their importance?
• Have they ever been exposed to information about helminths through health education programs, schools, media, or community health outreach?
Not previously informed (no prior information)
If participants who were unaware of key aspects of helminths and their prevention, such as:
• Unable to identify any specific helminth species or transmission routes.
• Not familiar with basic prevention methods like handwashing or the use of latrines.
• Not previously exposed to educational messages about helminths.
Good knowledge: refers to those who answered and scored greater than the mean value of all the question we had set to assess the participants knowledge.
Poor knowledge: refers to those who answered and scored less than the mean value of all the question we had set to assess the participants knowledge.
Woredas (Districts): are the third level of administrative divisions in Ethiopia, following zones and regional states.
Keble: The smallest and final level of administrative units in Ethiopia.
Animals: refers to animals play vital roles in Ethiopian households, particularly in rural areas, where they contribute to the family’s livelihood, transportation, and food security.
Results
Socio-demographic characteristics of the study subjects
The household survey revealed that 333 school children participated, with a response rate of 97.6%. Of these, 187 (56.2%) were male, and the majority, 186 (55.9%) fell within the age range of 7–10 years old. The average age was 10.4 ± 1.2 years old. Furthermore, 184 (55.3%) children were in school grades 1–4. The educational status indicated that 253 fathers (76%) and 211 mothers (63.4%) were able to read and write (Table 1).
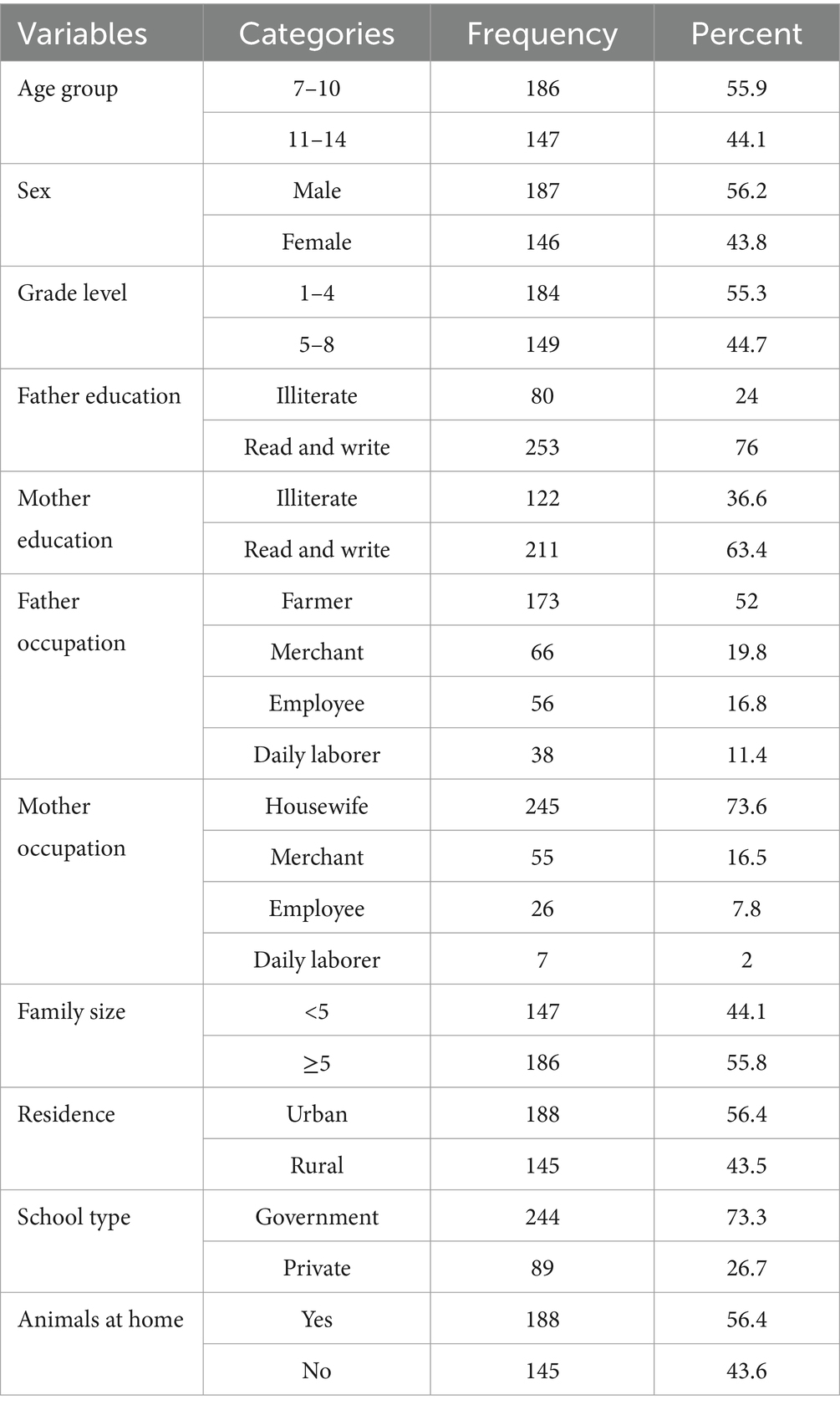
Table 1. Socio-demographic characteristics of study participants in Mizan-Aman City, Bench Sheko Zone, Southwest Ethiopia, 2021.
Soil transmitted helminths and Schistosoma infection related to sanitation
Among the study participants, 308 individuals (92.5%) possessed a toilet in their homes, while 249 individuals (74.8%) consistently utilized their home toilets. It was observed that 107 children (32.4%) had a habit of defecating in open fields. Additionally, 233 children (69.9%) engaged in swimming activities. As for hygiene practices, 247 children (74.2%) occasionally used soap and water after using the toilet (Table 2).
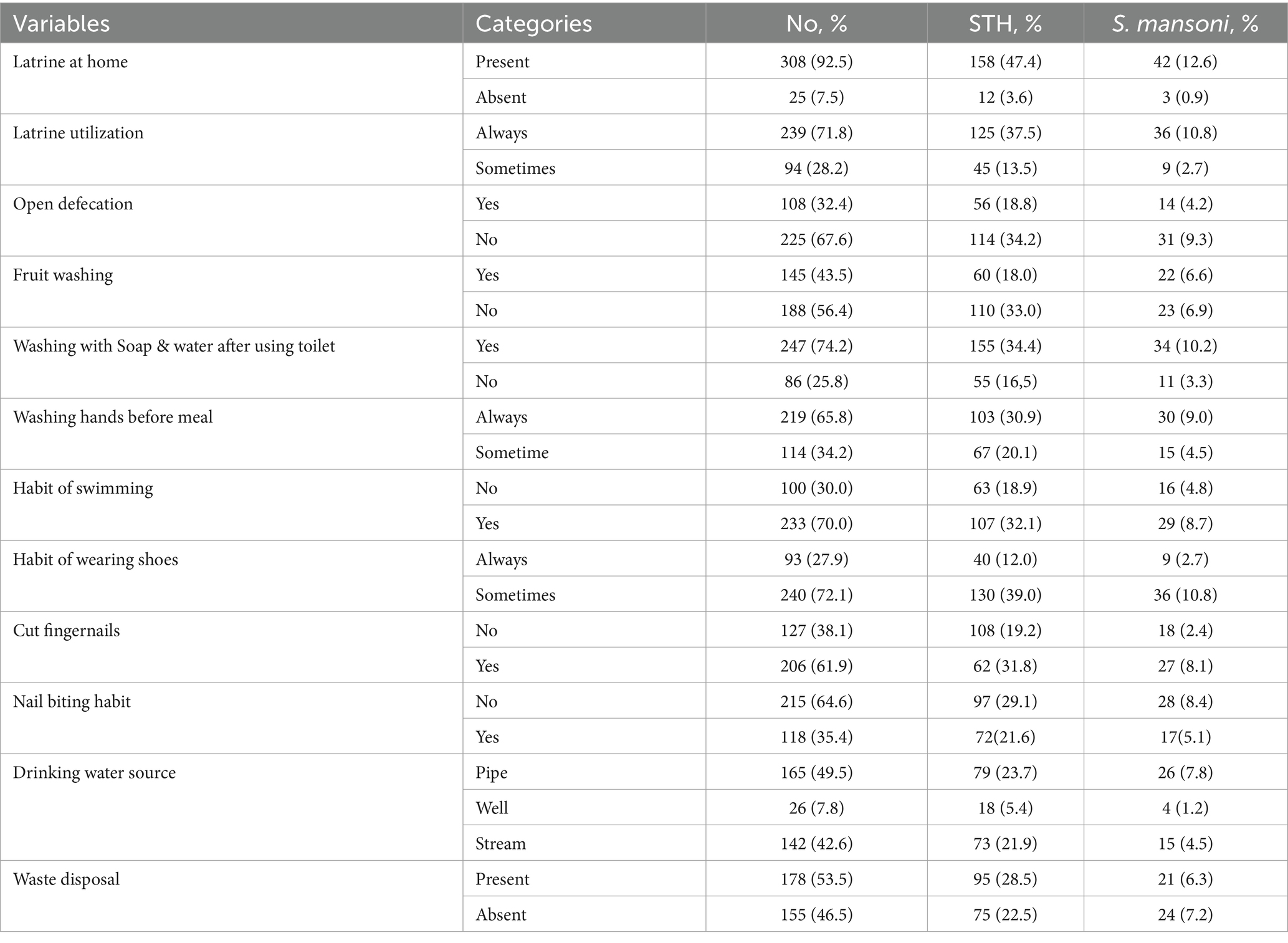
Table 2. The distribution of soil transmitted helminths and S. mansoni infection in related to respondent sanitation practices, Mizan-Aman City. Bench Sheko Zone, Southwest Ethiopia, 2021.
Prevalence of soil transmitted helminths and Schistosoma mansoni
The combined prevalence of soil-transmitted helminths (STHs) and S. mansoni was 215 (64.6%). Specifically, 170 individuals (51.05%) were infected with STHs, while 45 individuals (13.5%) were infected with S. mansoni. A total of five different types including S. mansoni were identified during the study. T. trichiura was the most common parasite, infecting 97 individuals (29.1%), followed by Ascaris lumbricoides with 64 case (19.2%), and S. mansoni with 45 cases (13.5%). The age group most affected with both groups by the consisted mainly of children aged 7–10 years old. Among the 333 students who took part in the study, 165 (49.5%) had a single infection, while 50 (15.0%) were co-infected with more than one helminth (Table 3).
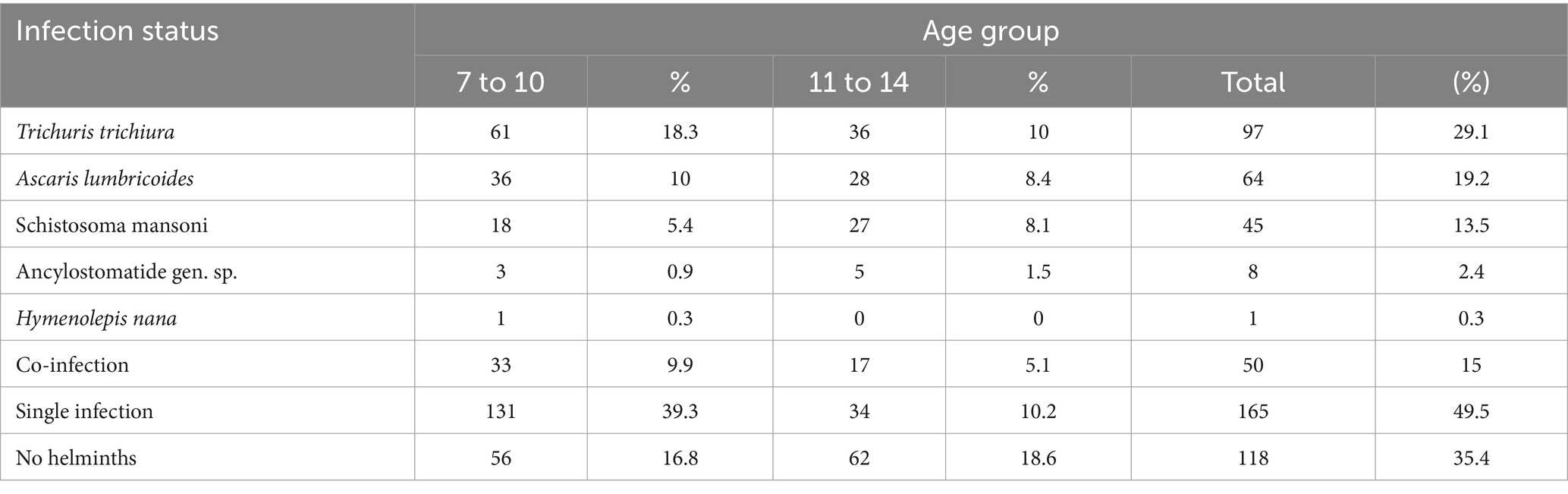
Table 3. Infection status of respondents by age distribution in Mizan-Aman City, Bench Sheko Zone, Southwest Ethiopia, 2021.
Co-infection with intestinal helminths
This study also found that multiple intestinal helminths can coexist in the same individuals. Among the 50 study participants, the most common co-infection was A. lumbricoides and T. trichiura, which occurred in 56% (28/50) of respondents. Other significant co-infections included A. lumbricoides and S. mansoni (16%), T. trichiura and S. mansoni (14%), A. lumbricoides, T. trichiura and S. mansoni co-infection (8%), and Hookworm and T. trichiura (6%).
Helminth infection intensity and distribution
The study found that the majority of participants who tested positive for STH and S. mansoni had low-level infections. Specifically, the Kato-Katz detection method 253 showed that out of 170 individuals, 85.3% (145) had mild infections and 14.1% (24/170) 254 had moderate infections of soil-transmitted helminths. As for S. mansoni, 82.2% 255 (37/45) had light infections and 17.8% (8/45) had moderate infections (Table 4).
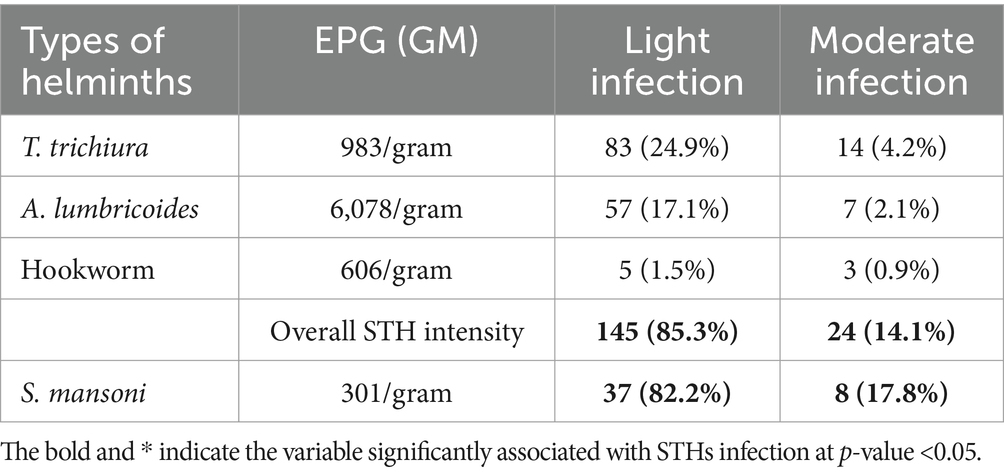
Table 4. Intensity of STHs and S. mansoni infection among study participants in Mizan-Aman City, Bench Sheko Zone, Southwest Ethiopia, 2021.
Factors associated with soil transmitted helminths
Using the information from the table, the parameters linked to the existence of soil-transmitted helminth (STH) infections were analyzed. Crude odds ratios (COR), adjusted odds ratios (aOR), and p-values for the relationships between different independent variables and infection status were all included in the analysis. To choose the candidate, the COR was examined. The parameters linked to the STHs infection were revealed by the adjusted odd ratio with p < 0.05. Notably, children who had not never heard of STHs before (p = 0.006) and children with poor knowledge about STHs (p = 0.028) were significantly associated with increased infection (Table 5).
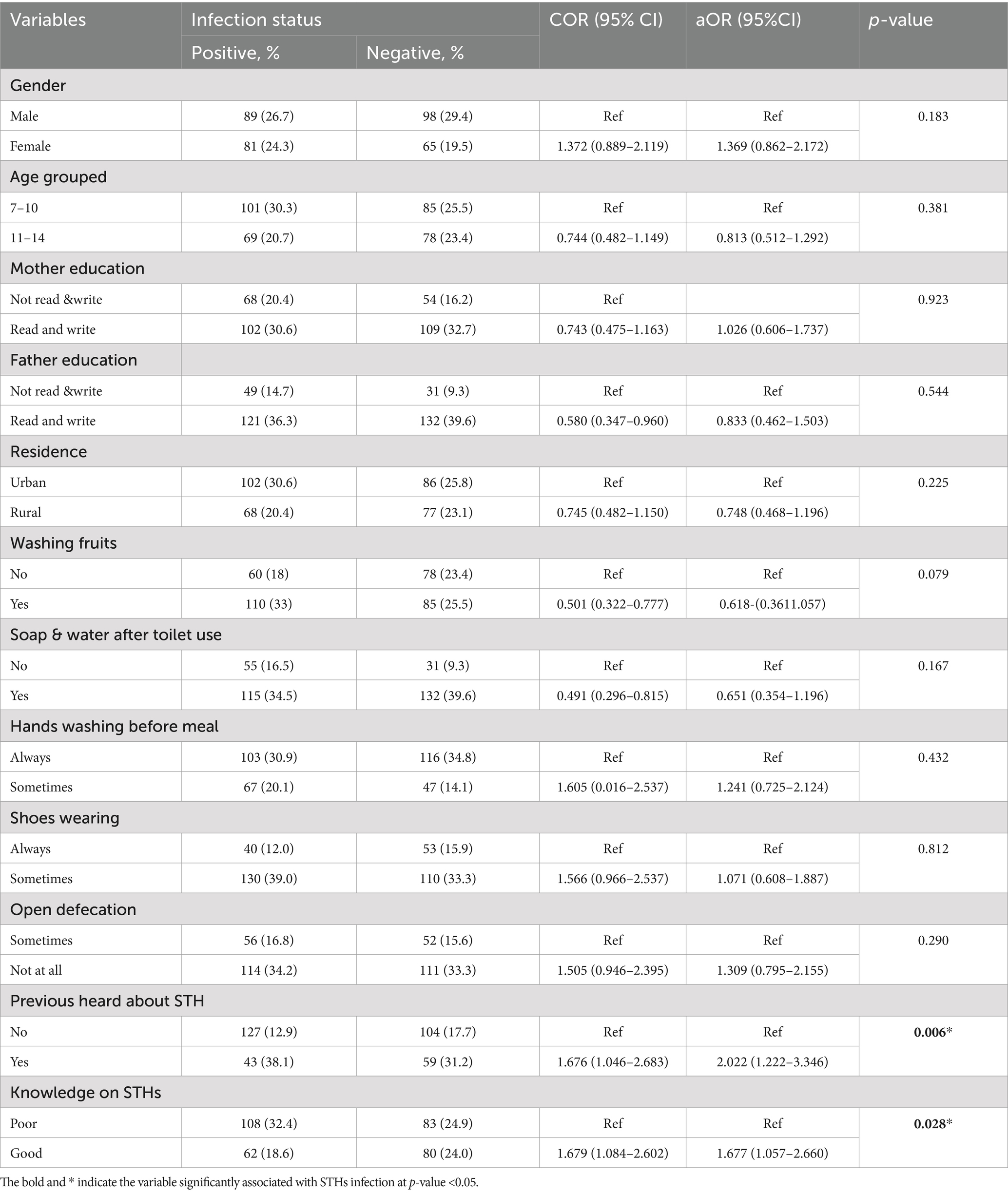
Table 5. Bivariate and multivariate analysis of STHs infection among children of Mizan- Aman City, Bench Sheko Zone, Southwest Ethiopia, 2021.
Factors associated with Schistosoma mansoni
It was evident in our research that participants who were unaware of deworming as a preventative and control presented an odds of being infected 2.6 times higher than children who understood that deworming was a means of control and prevention. Children who engaged in swimming had a 99.5% higher chance of contracting S. mansoni. Children who knew that S. mansoni is transmitted through contact with fecally contaminated water were 99.6% less likely to be infected with S. mansoni (Table 6).
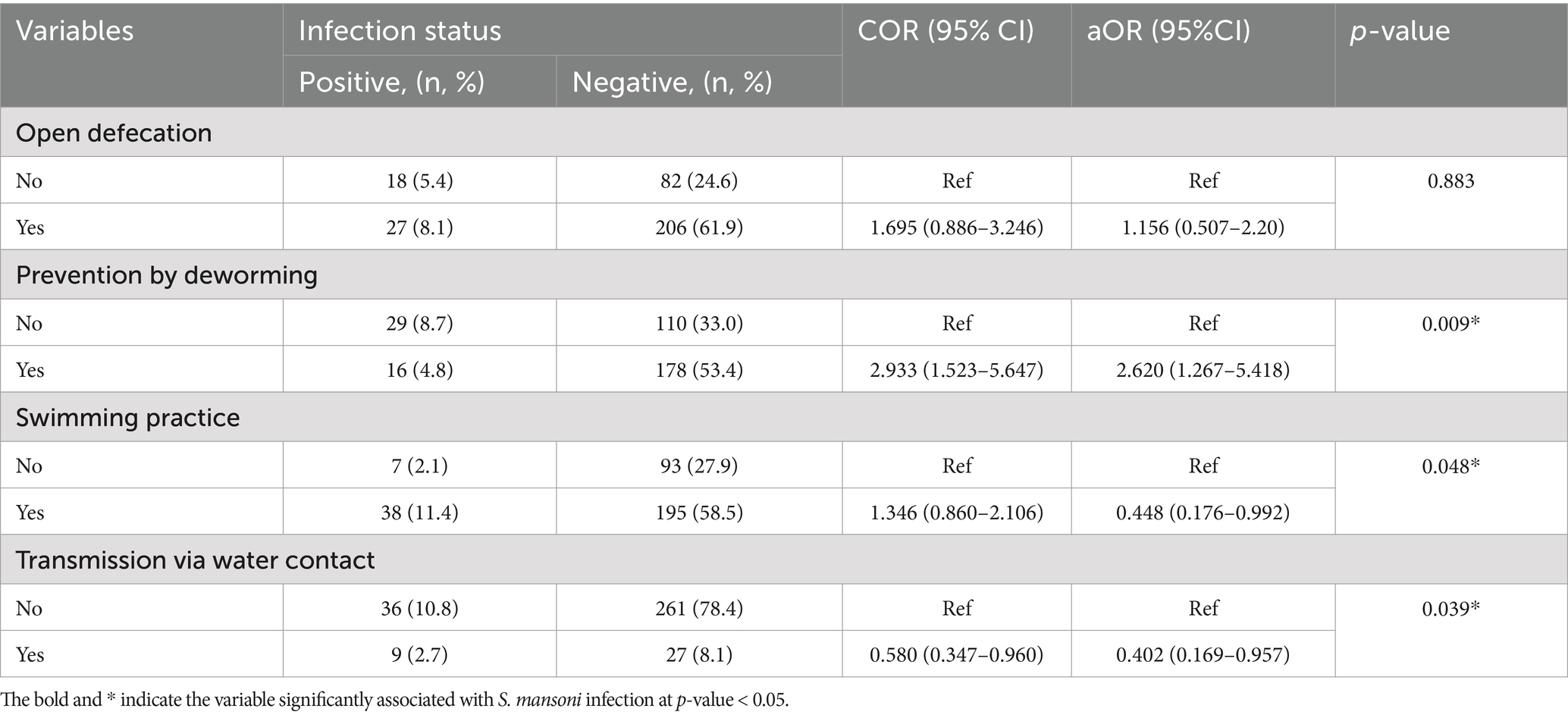
Table 6. Bivariate and multivariate analysis and associated factors of S. mansoni infection among children of Mizan-Aman City, Bench Sheko Zone, Southwest Ethiopia, 2021.
Discussion
There is still a public health concern regarding soil-transmitted helminthiasis and schistosomiases, although there has been a significant difference in their distribution. The transmission of the infection occurs through water and soil contaminated with human waste. This research aimed to showcase the prevalence and intensity of infection of STHs and S. mansoni, individually. The combined prevalence of STHs and S. mansoni was 64.9%. Meanwhile, STHs make up a total prevalence of 170 (51.1%). It is believed that the local factors identified in the study area contributed to creating favorable conditions for this prevalence. Our study’s results also showed that T. trichiura was the most common helminth, with light to moderate infection intensity. A. lumbricoides and hookworms were next, with comparable infection intensity levels. The findings of a previous study conducted in the same area also emphasized this concern, reporting infection intensity ranging from light to heavy. However, our study found a lower prevalence, with infection intensity primarily categorized as light to moderate (13). The reduction in overall prevalence and the difference in intensity range might be attributed to the government’s focus on implementing the neglected tropical disease prevention and control program (69). However, the continued prevalence reflects the issue and underscores the need for public health intervention in these resource-limited settings. The documented results indicated that, based on the World Health Organization’s risk classification, the study subjects were categorized as high-risk, with an infection rate of 50% or higher (21).
In this study, the prevalence of T. trichiura and A. lumbricoides decreased with age; however, age was not identified as a significant associated factor, which is consistent with the findings of a previous study (22). Similarly, another study (23) also observed that age was associated with a reduction in both the likelihood of infection and the severity of any soil-transmitted helminth (STH) infection. However, as people aged, the prevalence of S. mansoni and Hookworm infections rose. Spending more time in high-risk environment, participation in high-risk activities like playing barefoot or swimming in tainted water might be contributing factors. The relationship between age and the risk of acquiring these infections is complex and can vary based on factors like immune response, behavior, environmental exposure, and access to public health interventions. Intestinal helminths infections are typically associated with poor sanitation, contaminated water, and soil, but their patterns of transmission and the age groups most at risk can differ.
The prevalence in our study is lower than that reported in Indian studies, where it stands at 75.6%, with A. lumbricoides being the most STHs and the majority of infections being of low intensity (24). The higher prevalence in India might be attributed to several factors, including the large sample size, risk variables similar to those in our study, and the absence of a deworming program in India prior to (14) during the time of the investigation. In Runda, the prevalence of STH infections (77%) is higher than in our study, with T. trichiura being the most commonly observed species. The study also found moderate to high-intensity infections of T. trichiura and A. lumbricoides (25). Various factors, including altitude, soil types, temperature, the level of awareness among participants, differences in sample sizes, the season during which the study was conducted, study designs might be contributed to these observed variations.
The prevalences in Bahir Dar City, Mettu Town, Yachi, and Zarima were higher than in our study, with rates of 85.9% (26), 84.4% (27), 70.7% (28), and 82.4% (29), respectively. In those areas, hookworm, A. lumbricoides, S. mansoni, and A. lumbricoides were the most commonly identified species, in that order. The differences in prevalence might be attributed to factors such as variations in sample sizes, lack of awareness and the presence water bodies used by residents for irrigation and household activities. However, comparable results were observed in Gonder, 66.7% (30), Hawassa Tula Sub-City, 67.7% (31), Adwa (Tigray), 58.8% (32), and West Hararghe, 61.5% (33). The figures implicated that intensity of soil-transmitted helminths and prevalence are clearly influenced by ecological conditions, which could explain the differences observed (34).
Although T. trichiura and A. lumbricoides were the most prevalent species in Indonesia, the Indonesian data showed that the prevalence of STHs was lower and that the severity of STH infection ranged from mild to severe. The results variation might be linked to personal, environmental aspects (35), exposure and small number of study participants involved. In Afghanistan, a polyparasitic infection involving soil-transmitted helminths (STHs) and a low prevalence of intestinal parasites (39.8%) was reported. In contrast to our findings, A. lumbricoides was the most common causative agent. One potential contributing factor identified was living in mud homes, which increased the risk of poor clearance of parasite eggs and facilitated the spread of infection. Additionally, the study’s large sample size likely contributed to the higher number of infected children compared to our study, even though the overall prevalence was low (17). Studies from other countries had reported varying lower prevalences of soil-transmitted helminthiasis, 9.3% in China (36), 37% in Malaysia (37), 30% in Nigeria (14), 12.95% in Kenya (38), and 32% in Tajikistan (39), all with low infection intensity. These lower prevalences and intensities may be linked to sociodemographic factors as well as the implementation of deworming programs as a control measure.
Other studies had shown that STH infections can lead to significant health issues. In Gonder, 32.3% of patients were reported to have STHs, with Ascaris lumbricoides being the predominant species, ranging in intensity from mild to high. In contrast, other helminths were associated with mild to moderate infections (40). In The prevalence in Hawassa City, Ethiopia, was 49.7% (67), and the lower rate in schools may be attributed to intervention programs. Most study participants in Jimma exhibited light infection intensity, with a prevalence of 45.6% (42). The differences in detection sensitivity between the McMaster and Kato-Katz methods, which are based on fecal egg counts, might explain the study’s lower magnitude and infection intensity. Numerous studies conducted in our country had reported a variety of dominant species, with prevalences ranging from 9.5 to 36.2%, typically with light infections, though some cases show very low to moderate heavy intensity infections (40, 43–47).
In the tropics and subtropics, especially in sub-Saharan African nations like Ethiopia, schistosomiasis is a prevalent helminthic infection. S. mansoni infection is a serious public health issue in these counties despite multiple rounds of preventative treatment, there is a chance of reinfection and recurrent illness. Age, water body contact habits, and gender all affect the frequency of S. mansoni in Ethiopia. By 2030, the nation aimed to stop the spread of several neglected tropical diseases, such as schistosomiasis. To meet the goals set by the nation, an epidemiological survey with updated findings is therefore necessary. In this investigation, the prevalence of S. mansoni was 13.5%, and the infection severity ranged from mild to moderate. The finding suggested that schistosomiasis remains a significant public health concern.
Studies conducted in various regions of Ethiopia had reported prevalence rates ranging from 20 to 89.9%, with infection intensity varying from low to heavy (29, 41, 48–53). In other countries such as Tanzania, Kenya, and Madagascar, prevalence ranged from 41.3 to 73.2%, with infections ranging from light to heavy, although no cases of heavy infection were reported in Tanzania (38, 54, 55). These variations in prevalence and intensity may be attributed to several factors, including altitude, temperature (which favors the multiplication and survival of the intermediate host), water contact behaviors, snail density, proximity of schools and homes to rivers (which may increase contact), sample size, occupation (e.g., fishing and irrigated farming), and the level of local awareness.
Our finding is comparable to those reported from other endemic areas, such as Guangua District (12.6%) (56), Wondo District (11.4%) (53), the Niger River basin (12.2%) (57), and Dembia (15.4%) (58). This similarity may be attributed to common recreational practices and the endemic nature of these areas. In contrast, lower prevalences were observed in Sudan (5.9%) (59) and Bamako, Mali (1.5%) (60). These could be due to less contamination of water bodies with feces, a lower distribution of the intermediate host, and the use of single egg counts in parasite examination. Our study, which used two slides per child, likely provided a more accurate estimate of both the prevalence and intensity of S. mansoni.
The participants in this study were found to have limited knowledge about the prevention, transmission, and control of soil-transmitted helminths (STHs). This lack of knowledge was associated with a higher likelihood of STH infection. It is consistent with a previous study conducted in the Lake Tana region, which also observed higher infection rates among individuals with limited awareness of prevention and control measures (26). Other study had also shown that children with inadequate knowledge are at a reduced risk of soil-transmitted helminth (STH) infections (61). In Nigeria, children who did not have prior knowledge about soil transmitted helminths infections had high likelihood of infection (62). Our findings align with this observation. This phenomenon may be attributed to a lack of awareness among parents about how to prevent, transmit, and control STH infections. As a result, this knowledge gap leaves children poorly informed, leading to higher infection rates, as also observed in study from Bangladesh (63). The analysis of multiple variables showed that engaging in swimming, having insufficient knowledge about preventing and controlling S. mansoni through deworming, and the transmission of the disease through contact with water contaminated with feces were all linked to S. mansoni infection. Studies conducted in Mekelle City (64), Tanzania (55), Wolaita (53), Zarima (29), Gorgora (65), Jimma (42) and Sanja, students’ behaviors related to water activities were identified as risk factors for S. mansoni (50). However, in contrast, study in the Gambia indicated that swimming and bathing in bodies of water presented a lower risk, suggesting that children’s actual water interaction behavior may not be fully captured (66).
Strength
The strength of a cross-sectional study lies in its ability to rapidly assess the prevalence and distribution of these infections, identify risk factors, and provide essential baseline data for public health planning. It is a powerful tool for guiding both immediate interventions and long-term strategies to combat these neglected tropical diseases in vulnerable populations. There are various benefits to using the double Kato-Katz procedure, which entails extracting two distinct stool smears from the same sample and running the test twice. The accuracy and dependability of the study’s findings are increased by these benefits, which include increased sensitivity, accuracy, and overall efficacy in identifying and measuring helminth infections, especially in regions with low-intensity infections, multiple species, and when eggs are unevenly distributed in the stool sample (Reduce false negative). This is especially crucial when assessing the efficacy of public health initiatives, such deworming campaigns, when accurate prevalence and severity data are essential. Additionally, the study underscored its dedication to participant welfare by promptly referring individuals diagnosed with intestinal parasitic infections to local health facilities for suitable treatment.
Limitation
Cross-sectional studies have many advantages, but it’s crucial to remember that they are correlational rather than causative. Since the data is gathered all at once, the study is unable to monitor the beginning or progression of infections over time, making it impossible to determine cause-and-effect links.
Conclusion and recommendation
The results of this community-based cross-sectional study revealed a high prevalence of STHs (≥50 WHO threshold) and a moderate risk of morbidity caused by S. mansoni, with a prevalence greater than 10% but less than 50%, according to the WHO threshold. Both infections were found to be associated with factors such as Lack of prior information about STHs and poor knowledge with regard to the transmission, prevention, and control methods. Regarding S. mansoni, the identified associated factors included lack of knowledge about its transmission through contact with fecally contaminated water and unawareness of deworming as a preventive measure and swimming habit. The study emphasizes the importance of ongoing public health interventions to address the issue. Despite current deworming programs, the continued presence of these infections highlights the need for comprehensive strategies that not only focus on treatment but also address the environmental and behavioral factors contributing to transmission.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The Mizan-Tepi University Teaching Hospital, College of Medicine and Health Sciences granted ethical permission under the approval number CHS/00971/21. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
ET: Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. DN: Writing – original draft, Writing – review & editing. TD: Writing – original draft, Writing – review & editing. TT: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors sincerely appreciate the cooperation of the families and participants in participating and providing the necessary data and stool samples. The authors are also grateful to the technologist for collecting and examining the stool samples. The authors are grateful to the Mizan-Tepi University, College of Medicine and Health Sciences for providing approval letter to conduct the research. We would also like to express our deepest gratitude to Bench Sheko Zone Health Department and Municipality workers for their assistance in writing a letter to the kebeles (the lowest administrative level of the country) prior to undertaking the investigation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
aOR, Adjusted odds ratio; COR, Crude odds ratio; SNNPR, Southern Nation Nationality People Region; STHs, Soil transmitted helminths; SPSS, Statistical package for social sciences; WHO, World Health Organization; EPG, Eggs per gram of stool; GM, Geometric mean; ATVET, Agriculture Technical Vocational Education and Training.
References
1. World Health Organization. Schistosomiasis and soil transmitted helminth infections – preliminary estimates of the number of children treated with albendazole or mebendazole. Weekly epidemiological record. Geneva: World Health Organization (2006).
2. Tchuem Tchuenté, LA, Behnke, JM, Gilbert, FS, Southgate, VR, and Vercruysse, J. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Trop Med Int Health. (2003) 8:975–86. doi: 10.1046/j.1360-2276.2003.01120.x
3. Crompton, DW, and Nesheim, MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. (2002) 22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539
4. Miguel, E, and Kremer, M. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica. (2004) 72:159–217. doi: 10.1111/j.1468-0262.2004.00481.x
5. Bleakley, H. Disease and development: evidence from hookworm eradication in the American South. Q J Econ. (2007) 122:73–117. doi: 10.1162/qjec.121.1.73
6. Christian, P, Khatry, SK, and West, KP Jr. Antenatal anthelmintic treatment, birthweight, and infant survival in rural Nepal. Lancet. (2004) 364:981–3. doi: 10.1016/S0140-6736(04)17023-2
7. Hotez, PJ, Asojo, OA, and Adesina, AM. Nigeria: “ground zero” for the high prevalence neglected tropical diseases. PLoS Negl Trop Dis. (2012) 6:e1600. doi: 10.1371/journal.pntd.0001600
8. World Health Organization. Soil-transmitted helminth infections. (2019). Available at: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections
9. Hotez, PJ, and Kamath, A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. (2009) 3:e412. doi: 10.1371/journal.pntd.0000412
10. Steinmann, P, Keiser, J, Bos, R, Tanner, M, and Utzinger, J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. (2006) 6:411–25. doi: 10.1016/S1473-3099(06)70521-7
11. Federal Democratic Republic of Ethiopia Ministry of Health. Second edition of Ethiopia National Master Plan for neglected tropical diseases. Addis Ababa, Ethiopia: Federal Ministry of Health (2016).
12. Chelkeba, L, Mekonnen, Z, Emana, D, Jimma, W, and Melaku, T. Prevalence of soil-transmitted helminths infections among preschool and school-age children in Ethiopia: a systematic review and meta-analysis. Glob Health Res Policy. (2022) 7:9. doi: 10.1186/s41256-022-00239-1
13. Ayalew, J, Endalew, Z, Yayehirad, A, and Zemenu, M. High prevalence of Schistosoma mansoni and other intestinal parasites among elementary school children in Southwest Ethiopia: a cross-sectional study. BMC Public Health. (2015) 15:600–7. doi: 10.1186/s12889-015-1952-6
14. Odinaka, KK, Nwolisa, EC, Mbanefo, F, Iheakaram, AC, and Okolo, S. Prevalence and pattern of soil-transmitted helminthic infection among primary school children in a rural community in Imo State, Nigeria. J Trop Med. (2015) 2015:349439. doi: 10.1155/2015/349439
15. Nebiyu, N, Birhan, M, Biruck, K, Kebede,, Ephrem, E, Gemechu, T, et al. Ethiopia schistosomiasis and soil-transmitted helminthes control programme: progress and prospects. Ethiop Med J. (2017) 1:75–80. doi: 10.33140/AIDT/01/01/00010
16. South West Ethiopia Peoples Region. Regional Profile. Available at: https://epo.acleddata.com/south-west-ethiopia-peoples-region-regional/
17. Rahimi, BA, Mahboobi, BA, Wafa, MH, Sahrai, MS, Stanikzai, MH, and Taylor, WR. Prevalence and associated risk factors of soil-transmitted helminth infections in Kandahar, Afghanistan. BMC Infect Dis. (2022) 22:361. doi: 10.1186/s12879-022-07336-z
18. Charan, J, and Biswas, T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. (2013) 35:121–6. doi: 10.4103/0253-7176.116232
19. Ash, LR, Orihel, TC, and Savioli, L. Bench aids for the diagnosis of intestinal parasites 2nd ed. Geneve: World Health Organization (2019) Licence: CC BY-NC-SA 3.0 IGO.
20. World Health Organization. Prevention and control of intestinal parasitic infections, World Health Organization Technical Report series 749. Geneva. (1987). Available at: https://www.who.int/publications/i/item/WHO-TRS-749
21. World Health Organization. Soil-transmitted helminthiasis: eliminating soil-transmitted helminthiasis as a public health problem in children: progress report 2001–2010 and strategic plan 2011–2020. Geneva, Switzerland: World Health Organization (2012).
22. Stothard, JR, Imison, E, French, MD, Sousa-Figueiredo, JC, Khamis, IS, and Rollinson, D. Soil-transmitted helminthiasis among mothers and their pre-school children on Unguja Island, Zanzibar with emphasis upon ascariasis. Parasitology. (2008) 135:1447–55. doi: 10.1017/S0031182008004836
23. Nute, AW, Edenshaw, T, Stewart, AEP, Sata, E, Bayissasse, B, Zerihun, M, et al. Prevalence of soil-transmitted helminths and Schistosoma mansoni among a population-based sample of school-age children in Amhara region, Ethiopia. Parasites Vectors. (2018) 11:431. doi: 10.1186/s13071-018-3008-0
24. Ganguly, S, Barkataki, S, Karmakar, S, Sanga, P, Boopathi, K, Kanagasabai, K, et al. High prevalence of soil-transmitted helminth infections among primary school children, Uttar Pradesh, India, 2015. Infect Dis Poverty. (2017) 6:139. doi: 10.1186/s40249-017-0354-7
25. Kabatende, J, Mugisha, M, Ntirenganya, L, Barry, A, Ruberanziza, E, Mbonigaba, JB, et al. Prevalence, intensity, and correlates of soil-transmitted helminth infections among school children after a decade of preventive chemotherapy in Western Rwanda. Pathogens. (2020) 9:1076. doi: 10.3390/pathogens9121076
26. Afework Bitew, A, Abera, B, Seyoum, W, Endale, B, Kiber, T, Goshu, G, et al. Soil-transmitted helminths and Schistosoma mansoni infections in Ethiopian orthodox church students around Lake Tana, Northwest Ethiopia. PLoS One. (2016) 11:e0155915. doi: 10.1371/journal.pone.0155915
27. Solomon, Y, Teshome, B, Zemenay, T, Melaku, T, Embiet, A, and Habtamu, D. Soil-transmitted helminthiasis and undernutrition among schoolchildren in Mettu town, Southwest Ethiopia. Sci Rep. (2022) 12:3614. doi: 10.1038/s41598-022-07669-4
28. Bekana, T, Hu, W, Liang, S, and Erko, B. Transmission of Schistosoma mansoni in Yachi areas, southwestern Ethiopia: new foci. Infect Dis Poverty. (2019) 8:1–8. doi: 10.1186/s40249-018-0513-5
29. Alemu, A, Atnafu, A, Shiferaw, Y, Teklu, T, Mathewos, B, Addis, Z, et al. Soil transmitted helminths and Schistosoma mansoni infections among school children in Zarima town, Northwest Ethiopia. BMC Infect Dis. (2011) 11:189. doi: 10.1186/1471-2334-11-189
30. Mathewos, B, Alemu, A, Woldeyohannes, D, Alemu, A, Addis, Z, Tiruneh, M, et al. Current status of soil transmitted helminths and Schistosoma mansoni infection among children in two primary schools in North Gondar, Northwest Ethiopia. BMC Res Notes. (2014) 7:88. doi: 10.1186/1756-0500-7-88
31. Eyamo, T, Girma, M, Alemayehu, T, and Bedewi, Z. Soil-transmitted helminths and other intestinal parasites among schoolchildren in Southern Ethiopia. Res Rep Trop Med. (2019) 10:137–43. doi: 10.2147/RRTM.S210200
32. Legese, L, Erko, B, and Hailu, A. Current status of intestinal schistosomiasis and soil transmitted helminthiasis among primary school children in Adwa town, Northern Ethiopia. Ethiop J Health Dev. (2010) 24:91–197. doi: 10.4314/ejhd.v24i3.68384
33. Wolde, AM. Prevalence of intestinal and soil transmitted helminths among primary school children of Badessa District, West Hararghe Zone, Oromia region, Eastern Ethiopia. Gastro Digestive Syst. (2023) 7:1–7. doi: 10.33140/JGDS.07.01.01
34. Oyewole, OE, and Simon-Oke, IA. Ecological risk factors of soil-transmitted helminths infections in Ifedore district, Southwest Nigeria. Bull Natl Res Cent. (2022) 46:13. doi: 10.1186/s42269-022-00700-8
35. Brahmantya, IBY, Iqra, HHP, Hartawan, IGNBM, Anjani, IAW, Sudarmaja, IM, and Ryalino, C. Risk factors and prevalence of soil-transmitted helminth infections. Open Access Maced J Med Sci. (2020) 8:521–4. doi: 10.3889/oamjms.2020.4440
36. Balen, J, Raso, G, Li, YS, Zhao, ZY, Yuan, LP, Williams, GM, et al. Risk factors for helminth infections in a rural and a peri-urban setting of the Dongting Lake area, People’s Republic of China. Int J Parasitol. (2011) 41:1165–73. doi: 10.1016/j.ijpara.2011.07.006
37. Huat, LB, Mitra, AK, Jamil, NI, Dam, PC, Mohamed, HJ, and Muda, WA. Prevalence and risk factors of intestinal helminth infection among rural malay children. J Glob Infect Dis. (2012) 4:10–4. doi: 10.4103/0974-777X.93753
38. Okoyo, C, Campbell, SJ, Williams, K, Simiyu, E, Owaga, C, and Mwandawiro, C. Prevalence, intensity and associated risk factors of soil-transmitted helminth and schistosome infections in Kenya: impact assessment after five rounds of mass drug administration in Kenya. PLoS Negl Trop Dis. (2020) 14:e0008604. doi: 10.1371/journal.pntd.0008604
39. Matthys, B, Bobieva, M, Karimova, G, Mengliboeva, Z, Jean-Richard, V, Hoimnazarova, M, et al. Prevalence and risk factors of helminths and intestinal protozoa infections among children from primary schools in western Tajikistan. Parasit Vectors. (2011) 4:1–13. doi: 10.1186/1756-3305-4-195
40. Eyayu, T, Yimer, G, Workineh, L, Tiruneh, T, Sema, M, Legese, B, et al. Prevalence, intensity of infection and associated risk factors of soil-transmitted helminth infections among school children at Tachgayint woreda, Northcentral Ethiopia. PLoS One. (2022) 17:e0266333. doi: 10.1371/journal.pone.0266333
41. Workineh, L, Yimer, M, Gelaye, W, and Desalegn, M. The magnitude of Schistosoma mansoni and its associated risk factors among Sebatamit primary school children, rural Bahir Dar, Northwest Ethiopia. Res Notes. (2019) 12:447. doi: 10.1186/s13104-019-4498-3
42. Ephrem, T, Tariku, B, Sileshi, K, Zelek, M, Ahmed, Z, and Belachew, T. Prevalence and intensity of soil transmitted helminths among school children of Mendera elementary school, Jimma, Southwest Ethiopia. Pan Afr Med J. (2017) 27:88. doi: 10.11604/pamj.2017.27.88.8817
43. Addisu, T, and Mebrate, D. The prevalence of soil-transmitted helminths and associated risk factors among school children at Sekela primary school, Western Ethiopia. J Parasitol Res. (2020) 2020:1–7. doi: 10.1155/2020/8885734
44. Workineh, L, Kiros, T, Damtie, S, Andualem, T, and Dessie, B. Prevalence of soil-transmitted helminth and Schistosoma mansoni infection and their associated factors among Hiruy Abaregawi primary school children, rural Debre Tabor, North West Ethiopia: a cross-sectional study. J Parasitol Res. (2020) 2020:2521750. doi: 10.1155/2020/2521750
45. Zerdo, Z, Bastiaens, H, Anthierens, S, Massebo, F, Masne, M, Biresaw, G, et al. Prevalence, intensity and endemicity of intestinal schistosomiasis and soil-transmitted helminthiasis and its associated factors among school-aged children in southern Ethiopia. Sci Rep. (2022) 12:4586. doi: 10.1038/s41598-022-08333-7
46. Ayalew, J, Adane, D, Abebe, G, John, SG, and Tegegne, E. Prevalence, infection intensity and associated factors of soil-transmitted helminthiasis among school-aged children from selected districts in Northwest Ethiopia. Res Rep Trop Med. (2021) 12:15–23. doi: 10.2147/RRTM.S289895
47. Weldesenbet, H, and Worku, A. Shumbej prevalence, infection intensity and associated factors of soil transmitted helminths among primary school children in Gurage zone, South Central Ethiopia: a cross-sectional study design. BMC Res Notes. (2019) 12:231. doi: 10.1186/s13104-019-4254-8
48. Tiruneh, A, Zemene, E, Mizana, A, Girma, H, Dereje, E, Sharew, B, et al. Schistosoma mansoni infections and morbidities among school children in hotspot areas of Jimma town, Southwest Ethiopia: a cross-sectional study. Environ Health Insights. (2023) 17:1–8. doi: 10.1177/11786302231161047
49. Weldegebreal, HH. Prevalence and re-infection of Schistosoma mansoni among school children in Mekele town, North Ethiopia. Int J Infect Dis. (2016) 45:249. doi: 10.1016/j.ijid.2016.02.557
50. Worku, L, Damte, D, Endris, M, Tesfa, H, and Aemero, M. Schistosoma mansoni infection and associated determinant factors among school children in Sanja town, Northwest Ethiopia. J Parasitol Res. (2014) 2014:1–7. doi: 10.1155/2014/792536
51. Feleke, DG, Arega, S, Tekleweini, M, Kindie, K, and Gedefie, A. Schistosoma mansoni and other helminthes infections at Haike primary school children, north-east, Ethiopia. BMC Res Notes. (2017) 10:609. doi: 10.1186/s13104-017-2942-9
52. Mekonnen, Z, Meka, S, Zeynudin, A, and Suleman, S. Schistosoma mansoni infection and undernutrition among school age children in Fincha’a sugar estate, rural part of West Ethiopia. BMC Res Notes. (2014) 7:763. doi: 10.1186/1756-0500-7-763
53. Alemayehu, B, Tomass, Z, Wadilo, F, Leja, D, Liang, S, and Erko, B. Epidemiology of intestinal helminthiasis among school children with emphasis on Schistosoma mansoni infection in Wolaita zone, Southern Ethiopia. BMC Public Health. (2017) 17:587. doi: 10.1186/s12889-017-4499-x
54. Stephen, SA, James, M, Penney, SJ, Russell, HJ, Howe, AP, and Linder, C. High burden of Schistosoma mansoni infection in school-aged children in Marolambo District, Madagascar. Parasites Vectors. (2017) 10:1–8. doi: 10.1186/s13071-017-2249-7
55. Ogweno, G, Mushi, V, Silvestri, V, Bonaventura, W, Justine, NC, Noah, M, et al. Burden and risk factors for Schistosoma mansoni infection among primary school children: a quantitative school-based cross-sectional survey in Busega district, northern Tanzania. PLoS One. (2023) 18:e0280180. doi: 10.1371/journal.pone.0280180
56. Tazebew, B, Temesgen, D, Alehegn, M, Salew, D, and Tarekegn, M. Prevalence of S. mansoni infection and associated risk factors among school children in Guangua District, Northwest Ethiopia. J Parasitol Res. (2022) 2022:1–9. doi: 10.1155/2022/1005637
57. Sacko, M, Magnussen, P, Keita, AD, Traoré, MS, Landouré, A, Doucouré, A, et al. Impact of Schistosoma haematobium infection on urinary tract pathology, nutritional status and anaemia in school-aged children in two different endemic areas of the Niger River Basin, Mali. Acta Trop. (2011) 120:S142–50. doi: 10.1016/j.actatropica.2010.12.009
58. Kahisay, M, Birhanie, M, and Derso, A. Prevalence and intensity of Schistosoma mansoni infection and its associated risk factors among patients with and without HIV at Chuahit Health Center, Dembia District, Northwest Ethiopia. Res Rep Trop Med. (2021) 12:25–32. doi: 10.2147/RRTM.S292899
59. Ismail, HA, Hong, ST, Babiker, AT, Hassan, RM, Sulaiman, MA, Jeong, HG, et al. Prevalence, risk factors, and clinical manifestations of schistosomiasis among school children in the White Nile River basin, Sudan. Parasit Vectors. (2014) 7:478. doi: 10.1186/s13071-014-0478-6
60. Dabo, A, Diarra, AZ, Machault, V, Touré, O, Niambélé, DS, Kanté, A, et al. Urban schistosomiasis and associated determinant factors among school children in Bamako, Mali, West Africa. Infect Dis Poverty. (2015) 4:4. doi: 10.1186/2049-9957-4-4
61. Alemu, G, Nibret, E, Amor, A, Munshea, A, and Anegagrie, M. Knowledge, attitude and practice towards intestinal schistosomiasis among school-aged children and adults in Amhara regional state, Northwest Ethiopia a cross-sectional study. Trop Med Health. (2024) 52:23. doi: 10.1186/s41182-024-00584-6
62. Imalele, EE, Braide, EI, Emanghe, UE, Effanga, EO, and Usang, AU. Soil-transmitted helminth infection among school-age children in Ogoja, Nigeria: implication for control. Parasitol Res. (2023) 122:1015–26. doi: 10.1007/s00436-023-07809-3
63. Sujan, MSH, Islam, MS, Naher, S, Banik, R, and Gozal, D. Predictors associated with knowledge and practice of helminthic infection prevention among rural school-aged children’s parents in Bangladesh. Front Public Health. (2020) 8:484. doi: 10.3389/fpubh.2020.00484
64. Assefa, A, Tadesse Dejenie, T, and Zewdneh, TZ. Infection prevalence of Schistosoma mansoni and associated risk factors among schoolchildren in suburbs of Mekelle city, Tigray, Northern Ethiopia. Momona Ethiopian J Sci. (2013) 5:174–88. doi: 10.4314/mejs.v5i1.85339
65. Tekeste, Z, Belyhun, Y, Gebrehiwot, A, Moges, B, Workineh, M, Ayalew, G, et al. Epidemiology of intestinal schistosomiasis and soil transmitted helminthiasis among primary school children in Gorgora, Northwest Ethiopia. Asian Pac J Trop Dis. (2013) 3:61–4. doi: 10.1016/S2222-1808(13)60013-4
66. Joof, E, Sanyang, AM, Camara, Y, Sey, AP, Baldeh, I, Jah, SL, et al. Prevalence and risk factors of schistosomiasis among primary school children in four selected regions of the Gambia. PLoS Negl Trop Dis. (2021) 15:e0009380. doi: 10.1371/journal.pntd.0009380
67. Bokicho, B, Hailu, D, Eshetu, B, Matie, M, and Tadele, T. Soil-transmitted helminthiases among school-age children and their association with water, sanitation, and hygiene, Hawassa City, Southern Ethiopia. PLoS Negl Trop Dis. (2023) 17:e0011484. doi: 10.1371/journal.pntd.0011484
68. Yemaneh, Y, Sahile, E, Niguse, W, Menberu, M, Asmare, M, Gosa Mekaleya, G, et al. Assessment of attitudes towards induced abortion among adults residing in Mizan-Aman town bench-Maji zone, SNNPR, south West Ethiopia. J Gynecol Obstetrics. (2017) 5:50–5. doi: 10.11648/j.jgo.20170504.12
Keywords: Intestinal parasitic infection, children aged 7-14 years, Mizan-Aman City, Southwest Ethiopia, community-based study
Citation: Tekalign E, Sebeta A, Nureye D, Duguma T and Tesfaye T (2024) Intestinal parasitic infections among children aged 7–14 years in Mizan-Aman city, Southwest Ethiopia: a community-based cross-sectional study. Front. Public Health. 12:1478293. doi: 10.3389/fpubh.2024.1478293
Edited by:
Carlos Landaeta-Aqueveque, University of Concepcion, ChileReviewed by:
Viviana Randazzo, National University of the South, ArgentinaPablo Oyarzún-Ruiz, University of Concepcion, Chile
Copyright © 2024 Tekalign, Sebeta, Nureye, Duguma and Tesfaye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eyob Tekalign, ZXlvYnRhc2Zhd0BnbWFpbC5jb20=
†Present address: Asresash Sebeta, Family Health International 360. Alive and Thrive. Regional Multisectoral Food System and Nutrition Advisor, Bonga, Ethiopia
 Eyob Tekalign
Eyob Tekalign Asresash Sebeta2†
Asresash Sebeta2† Dejen Nureye
Dejen Nureye Tadesse Duguma
Tadesse Duguma