- 1Vanke School of Public Health, Tsinghua University, Beijing, China
- 2Department of Pharmacy, Peking University People's Hospital, Beijing, China
- 3School of Pharmaceutical Sciences, Tsinghua University, Beijing, China
- 4Key Laboratory of Innovative Drug Research and Evaluation, National Medical Products Administration, Beijing, China
- 5School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, China
- 6School of Pharmacy, Massachusetts College of Pharmacy and Health Sciences University, Boston, MA, United States
- 7BCPMdata Pharma Technology (Chengdu) Co., Ltd, Chengdu, China
Background: As China is one of the countries with the highest recorded cases of Immune-Mediated Inflammatory Diseases (IMIDs), these diseases have also emerged as a serious public health concern. Biosimilars, potentially lower-cost versions of biologics, may improve access to more affordable yet comparably effective treatments. Encouragingly, China launched its abbreviated biosimilar pathway in 2015, and since then, a large number of biosimilars have been approved. However, systematic studies on the therapeutic efficacy and economic impact of IMIDs biosimilars are lacking in China. This study aims to assess the clinical benefits (including efficacy/effectiveness, safety, and immunogenicity), cost and uptake of adalimumab biosimilars, tocilizumab biosimilars, and infliximab biosimilars compared with their reference biologics in patients with IMIDs in China.
Methods: IMIDs biosimilars and their reference drugs approved in China between 2015 and 2024 were identified. Head-to-head randomized clinical trials (RCTs) and real-world cohort studies on adalimumab, tocilizumab and infliximab and their biosimilars for the treatment of IMIDs were assessed. PubMed, Embase, Cochrane Library,
Findings: A total of 12 RCTs involving 5,717 patients with IMIDs were analyzed, including 12 approved biosimilars of adalimumab, infliximab, and tocilizumab. The primary endpoints of adalimumab (7 RCTs with 3,174 patients; RR, 1.02; 95% CrI, 0.99–1.06, p = 0.33), infliximab (3 RCTs with 1,291 patients; RR, 1.02; 95% CrI, 0.94–1.11, p = 0.98), tocilizumab (2 RCTs with 1,252 patients; RR, 1.01, 95% CrI, 0.94–1.08) met equivalence with reference biologics. Additionally, there was no significant difference between biosimilars and their reference biologics in the secondary endpoints. Overall, biosimilars demonstrated comparable safety (TEAEs: RR, 0.99; 95% CrI, 0.95–1.02, p = 0.44) (SAEs: RR, 0.80; 95% CrI, 0.42–1.54, p = 0.50) and immunogenicity (ADA: RR, 1.00; 95% CrI, 0.95–1.04, p = 0.85) (Nabs: RR, 0.93; 95% CrI, 0.82–1.05, p = 0.25) profiles to reference biologics. These findings were consistent with the cohort studies. In 2024, IMIDs biosimilars are available at 63 to 82% of the price per unit of the reference drugs, with uptake rates of 16.5 to 72.1% in China. Patients with IMIDs using these biosimilars could save between $874 and $2,184 per month in treatment costs, equivalent to 1.8 to 7.0 times the per capita monthly disposable income in China in 2024. Simulation showed that with 100% biosimilar substitution, savings would increase to $22.98 M, $33.83 M, and $3.82 M for adalimumab, infliximab, and tocilizumab, respectively. This would enable treatment for an additional 6,700, 9,863, and 4,373 patients, respectively.
Interpretation: Our study revealed that IMID biosimilars in China provide clinical benefits comparable to their reference biologics evidenced by high-quality RCTs and cohort studies with offer significant cost savings in China. Encouraging China’s national volume-based procurement and multi-stakeholder collaboration may help accelerate the substitution of IMIDs biosimilars.
1 Introduction
Immune-mediated inflammatory diseases (IMIDs) are characterized by excessive and uncontrolled inflammation and are highly prevalent across a group of conditions. These conditions are often accompanied by severe cardiovascular, metabolic disorders, cognitive impairment, and other complications, which severely impact the quality of life of patients (1). Common IMIDs include rheumatoid arthritis (RA), ankylosing spondylitis (AS), plaque psoriasis, Crohn’s disease and uveitis (2). Over the past two decades, there have been landmark achievements in the treatment of IMIDs, especially with the development of monoclonal antibody-based biologics, which have significantly improved patient symptoms (3). For example, tumor necrosis factor-α inhibitors, such as infliximab, adalimumab, and tocilizumab are biologic disease-modifying antirheumatic drugs used worldwide to treat IMIDs. The latest annual sales figures forecast that adalimumab ranks fourth globally, with expected sales of $13.6 billion (4). However, it should be noted that the high cost of IMIDs drugs remains a serious concern for patients, prescribers, and payors even in high-income countries (5).
Biosimilars, drugs that are highly similar in structure, efficacy and safety to the approved reference drugs, are often significantly less expensive than the reference drugs, promising to alleviate the burden on patients (6). Hence, to address the high expense of novel biologics, abbreviated approval pathway for biosimilars were established in the EU (2006), Japan (2009), and the US (2010), to improve affordability for patients (7). As of November 1, 2024, the European Medicines Agency (EMA) (8), the US Food and Drug Administration (FDA) (9), and the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) (10), have approved more than 97, 50, and 41 biosimilars, respectively. Among them, the number of biosimilar approvals for adalimumab, infliximab and tocilizumab for the treatment of IMIDs reaches up to 37 (Supplementary Table S1). Previous studies have reported that biosimilars offer potentially cost-effective treatment options for autoimmune diseases (11, 12). However, as with other biosimilars, the use of biosimilars for IMIDs remains confronted with many challenges, including uncertainty about clinical benefits, patent and data exclusivity, substitution and interchangeability policies, and healthcare payments (13). These issues have contributed to the suboptimal use of biosimilars (14, 15).
In China, the prevalence of IMIDs is among the highest in the world, making these diseases a critical public health concern (16). The prevalence of RA in China was reported to be 0.42%, with a total affected population of about 5 million, making it the second leading cause of disability in in the country (17). The annual total cost of RA in China was about ¥12.67 billion RMB, among which direct medical costs accounting for 33.3%, with direct non-medical costs account for 29.8% and indirect costs account for 37.0% (18). In 2015, the China Food and Drug Administration (now known as the National Medical Products Administration, NMPA) established a streamlined biosimilar approval pathway and issued a series of policies in the field of biosimilars aimed at clarifying the definition and regulatory principles of biosimilars while encouraging their development (13, 19, 20). More importantly, the NMPA has developed specific guidelines for adalimumab biosimilars and infliximab biosimilars. These incentives have indeed led to the development of local biosimilars. As of November 1, 2024, China has approved 15 biosimilars for the treatment of IMIDs, with another 25 biosimilars in the pipeline (Supplementary Tables S1–S3). However, evidence of the clinical benefits, cost, and uptake of these biosimilars for the treatment of IMIDs is rarely reported in China.
To address the issues mentioned above, this study aims to compare the evidence for IMIDs biosimilars with their reference drugs by including RCTs and real-world cohort studies assessing in efficacy/effectiveness, safety, and immunogenicity. Furthermore, it evaluates the cost and uptake of these biosimilars versus their reference drugs after the biosimilars’ entry into the market. This evidence may facilitate a better understanding of the value of biosimilars and promote their adoption and utilization in China.
2 Methods
2.1 Data sources
Head-to-head RCTs and cohort studies on biosimilars of adalimumab, infliximab and tocilizumab and their reference biologics for IMIDs were assessed. PubMed, Embase, Cochrane library, Clinicaltrials.gov and Listed Drug Database of Center for Drug Evaluation, NMPA (21) were searched from their inception to November 1, 2024. Price and uptake data from the launch date to July 1, 2024 were retrieved from the Pharnexcloud database (22).
2.2 Data extraction
2.2.1 Identification of RCTs and cohort studies
This study included RCTs and cohort studies that involved patients with IMIDs. The intervention groups consisted of patients treated with any biosimilars of adalimumab, infliximab, or tocilizumab, while control groups consisted of patients who received respective reference drugs. No restrictions were applied to the dosage, treatment, regimen, or patient number. Non-comparative studies (e.g., reviews, expert commentary, editorials, and clinical guidelines) were excluded. A detailed description of the eligibility and exclusion criteria can be found in Appendix 1. Two investigators (X.D., X.L.) extracted the data of IMIDs biosimilars from RCTs and cohort studies, including patient numbers, whether the study was sponsored by a manufacturer, study design, duration of study, therapy lines, endpoints for efficacy/effectiveness, safety, and immunogenicity. The primary endpoints of efficacy included American College of Rheumatology 20% improvement criteria (ACR20), Psoriasis Area and Severity Index (PASI), and Assessment of Spondylo Arthritis international Society 20% improvement criteria (ASAS20). The safety outcomes included Treatment Emergent Adverse Event (TEAE), Serious Adverse Event (SAE), Drug-related TEAE hypersensitivity, drug interruption and drug discontinuations. In addition, immunogenicity outcomes included the incidence of neutralizing antibodies (Nabs) and anti-drug antibodies (ADA).
2.2.2 Price, cost and uptake data extraction
2.2.2.1 Price and cost
China has a three-tier healthcare delivery system, with healthcare institutions and providers operating at different levels: county, township, and village levels in rural areas, and at the municipal, district, and community levels in urban areas (23, 24). We extracted the winning bid price from each province to calculate the annual weighted average price of biosimilars per milligram. Meanwhile, we adjusted the price of biosimilars to reflect 2024 values, accounting for inflation. Finally, we converted these values into US dollars based on the 2024 exchange rate between the Chinese Yuan (RMB) and the US Dollar (USD) in 2024, which was 1 USD to 7.1 RMB (25).
In addition, we calculated the monthly treatment costs for both reference biologics and biosimilars individually, using information from the drug labels. The annual treatment cost was determined by multiplying the dose administered to a patient over 1 year (52 weeks) by the unit price. Subsequently, we calculated the average monthly treatment cost over 12 months.
2.2.2.2 Uptake
In China, healthcare institutions are categorized into primary healthcare institutions, secondary comprehensive hospitals and tertiary comprehensive hospitals. Normally, higher-level institutions provide more sophisticated treatments. In this study, we utilized the national hospital sales volume data from the Pharnexcloud database module, which covered the sales volumes of cancer biosimilars in secondary and tertiary hospitals nationwide, as there was no public database available for drug sales volumes (26). We separately extracted the sales volume data of biosimilars and reference drugs in China for each quarter. The uptake of biosimilar was defined as the ratio of biosimilar sales volume to the total sales volume (combining sales volumes of both biosimilars and reference drugs). The dataset spans from the introduction of biosimilars to the market until October 1, 2023. A detailed description of price, cost and uptake of biosimilars and reference drugs in China can be found in Appendix 2.
2.2.2.3 Simulation
To better evaluate the effect of biosimilar substitution, we designed two scenarios for simulation, including biosimilar substitution in 2023 and 100% of biosimilar substitution. Since the data for 2024 was still incomplete, the simulation took the annual treatment cost, sales volume and revenue of the reference biologics and biosimilars in 2023 as the baseline. Given the prices of different biosimilars vary, the annual treatment cost of biosimilars is weighted according to their market share. The specific calculations can be found in Supplementary Table S12.
2.3 Assessment of risk of bias
Two investigators (X.D., X.L.) assessed the risk of bias in the RCTs and cohort studies in accordance with the Cochrane Collaboration’s tool (CCT) and Newcastle-Ottawa Risk of Bias Assessment Tool (NOS). The assessment of CCT included selection bias (sequence generation and allocation concealment), performance bias (blinding of participants, personnel, and outcome assessors), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other potential biases (27). There are three main dimensions involved in NOS, including selection, comparability and outcome (28).
2.4 Statistical analysis
Medians (IQRs) were used for continuous variables. Counts and percentages were used for categorical variables. Similar to the previous study, we calculated the median weighted average price (WAP) separately when there were multiple biosimilars approved (13). For biosimilars or reference drugs with different dose strengths, price were standardized to price per milligram (eg, adalimumab is available in 40 mg per vial or 20 mg per vial in China) to facilitate comparison. The uptake rate of biosimilars was defined as the ratio of quarterly (3-month) sales volume of the biosimilar to combined quarterly sales volume of the biosimilar and its reference drugs as the sales volume was obtained quarterly.
We pooled relative estimates of adalimumab biosimilars, infliximab biosimilars and tocilizumab biosimilars compared to their respective reference drugs, considering the significant differences in indications and mechanisms of the tested drugs. This study also analyzed subgroups of these biosimilars for primary and secondary endpoints (efficacy/effectiveness, safety and immunogenicity) and indications. Considering the possible heterogeneity of different biosimilars in terms of design, patient population, and efficacy endpoints, restricted maximum likelihood random-effects meta-analysis was used for pooling efficacy, safety and immunogenicity outcomes of IMIDs biosimilars, following methodologies from a previous study (24).
Statistical analyses were performed and graphical representations were generated using IBM SPSS, (version 20 IBM Corp) and R (version 4.1.0 R Project for Statistical Computing). The R packages used in the analysis included meta (version 5.2.0), forestplot (version 1.10.1), and ggplot2 (version 3.4.0). Two-sided tests were conducted with a significance threshold of p < 0.05.
3 Results
3.1 RCT and cohort study characteristics
Table 1 summarizes the characteristics of the 12 RCTs, involving 5,717 patients with IMIDs, with a median (IQR) sample size of 482 (370 to 601) patients. Of those, 9 RCTs were published in journals (26, 29–36), and 3 were reported in NMPA reviews (37–39). All identified studies were funded by various sponsors, encompassing 12 biosimilars marketed across 3 disease settings in China: plaque psoriasis (PP), ankylosing spondylitis (AS) and rheumatoid arthritis (RA). Among the 12 RCTs, 7 studied adalimumab biosimilars (26, 29–33, 37), 3 studied infliximab biosimilars (34, 35, 38), and 2 studied tocilizumab biosimilars (36, 39).
The majority of the RCTs were designed as equivalence designs, while only Infliximab-CMAB008 was prespecified as a noninferiority design. The median (IQR) proportion of females was 14% (13 to 22%) for AS, 23% (19 to 27%) for PP, and 84.5% (84 to 85%) for RA. The median (IQR) study duration for assessing the primary efficacy endpoints was 10.12 (5.62–18.20) months for AS, 21.93 months for PP, and 29.4 (16.57–31.50) months for RA, respectively. The risk of bias assessment in RCTs, as detailed in Supplementary Table S5, indicated that 8 RCTs (66.7%) were of low risk and 4 were at uncertain risk.
Four cohort studies with 3,918 comparing biosimilars with their reference drugs were found (40–43). All cohort studies were conducted out of China and were rated as low risk (Supplementary Tables S4, S6) summarize the characteristics of the 4 cohort studies with a total of 3,918 patients. Of the 4 cohort studies, 2 (50.0%) focus on a adalimumab biosimilar (40, 41), 2 (50.0%) were of infliximab biosimilars (42, 43).
3.2 Clinical benefits
3.2.1 Adalimumab biosimilars vs. adalimumab
Seven RCTs compared adalimumab biosimilars with the originator (26, 29–33, 37), four studies including patients with AS (30–33), two studies including patients with PP (26, 29), and one study including patients with RA (37). The primary endpoints were ASAS20, PASI and ACR20, respectively, (Table 1).
The overall pooled results showed no significant differences between adalimumab biosimilars and the originators in the primary endpoints (Figure 1A). Subgroup analysis showed that no significant differences in the primary endpoint of PASI rate (RR, 1.01, 95% CI, 0.95–1.08; p = 0.86), ACR20 rate (RR, 1.10; 95% CI, 0.99–1.22) or ASAS20 rate (RR, 1.02; 95% CI, 0.97–1.07; p = 0.24) between adalimumab biosimilars and the reference drugs were observed among patients with RA and AS, respectively. For secondary endpoints, the assessments of ASAS40 and PASI75 were consistent with the primary endpoints. Furthermore, no significant differences were found in safety outcomes (TEAE, Drug-related TEAE, SAE, hypersensitivity, drug interruption, and drug discontinuations), immunogenicity (ADA and Nabs) outcomes or disease subgroups between the Adalimumab biosimilars and their reference drugs (Supplementary Tables S4, S7, S8; Supplementary Figures S2–S6).
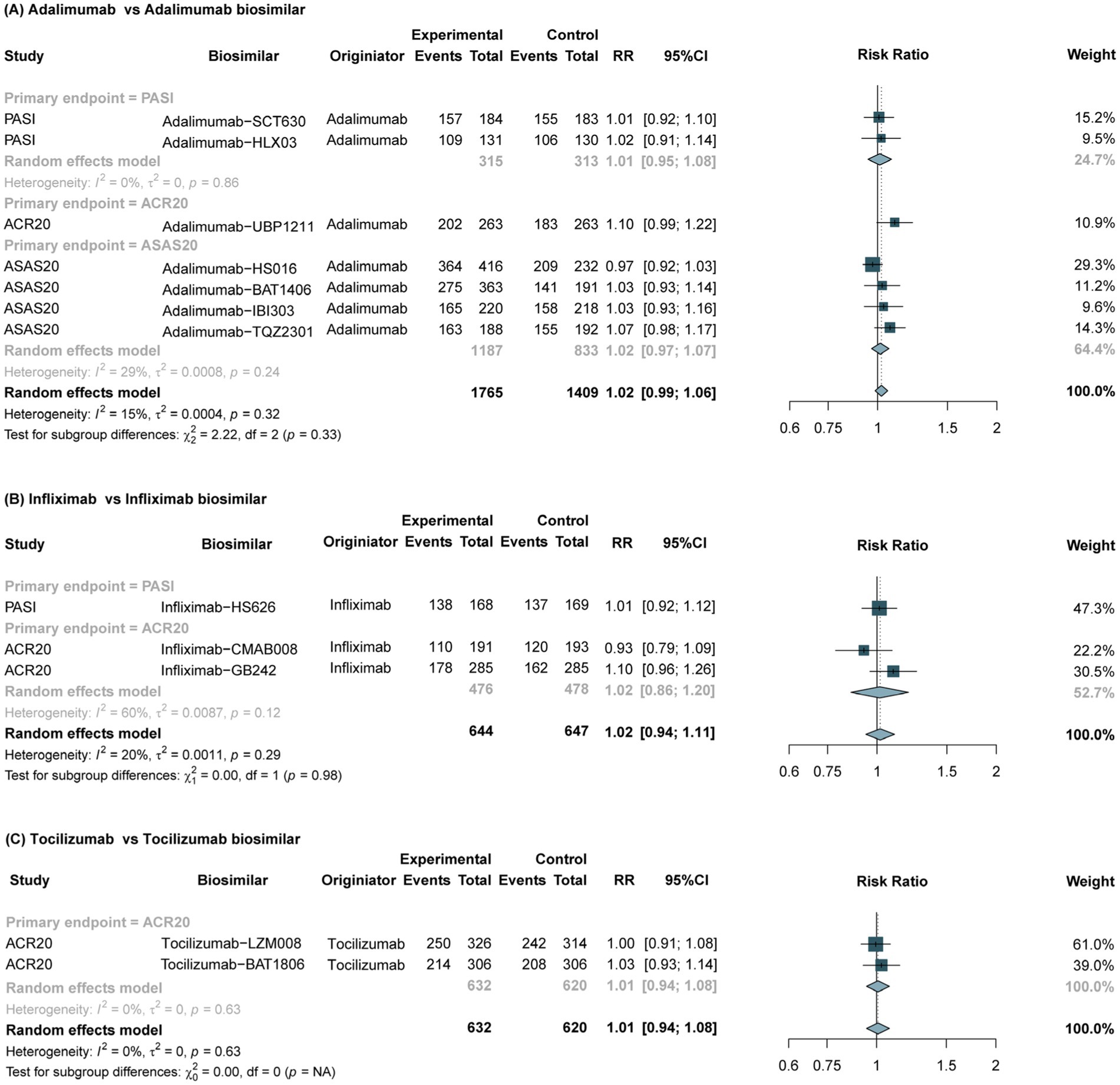
Figure 1. Forest plots of the primary endpoints between biosimilars and reference drugs for IMIDs. IMIDs, Immune-Mediated Inflammatory Diseases; RR, risk ratio; CI, confidence interval; ACR20, American College of Rheumatology 20% improvement criteria; PASI, Psoriasis Area and Severity Index; ASAS20, Assessment of Spondylo Arthritis international Society 20% improvement criteria.
Two cohort studies (40, 41) with low risk of adalimumab biosimilars were for Rheumatoid arthritis and Inflammatory Bowel Diseases, respectively (Supplementary Table S6). The results showed that no differences in clinical benefit between adalimumab biosimilars and reference drugs were observed among real-world studies.
3.2.2 Infliximab biosimilars vs. infliximab
Three RCTs compared the Infliximab originator with biosimilars: two studies involved patients with RA (35, 38), and one study involved patients with PP (34). The primary endpoints were ACR20 and PASI, respectively (Table 1).
The meta-analysis of 3 RCTs showed that the primary endpoint of Infliximab biosimilars was comparable to that of the reference drugs in the treatment of IMIDs (RR, 1.02; 95% CI, 0.94–1.11; p = 0.98) (Figure 1B). No significant differences in the primary endpoint of ACR20 rate (RR, 1.02; 95% CI, 0.86–1.20; p = 0.12) or PASI rate (RR, 1.01; 95% CI, 0.92–1.12) between infliximab biosimilars and the reference drugs were observed among patients with RA and AS, respectively. The assessments of secondary endpoints (ACR50 and PASI 75) were consistent with the primary endpoint. Furthermore, no significant differences were found in safety (TEAE, SAE and other outcomes), immunogenicity endpoints (ADA and Nabs) and and disease subgroups between the Infliximab biosimilars and their reference drugs (Supplementary Tables S4, S7, S8; Supplementary Figures S2–S6).
There were two cohort studies (42, 43) of infliximab biosimilars for inflammatory bowel disease, and both of two cohort studies were low risk (Supplementary Table S6). The results showed no significant differences in efficacy and safety between the Infliximab biosimilar and the reference drug.
3.2.3 Tocilizumab biosimilars vs. tocilizumab
The meta-analysis of 2 RCTs (36, 39) revealed no significant difference in the primary efficacy endpoint (ACR20) between tocilizumab biosimilars and the reference drug in the treatment of RA, (RR, 1.01; 95% CI, 0.94–1.08; p = 0.63) (Figure 1C). The assessments of secondary efficacy were consistent with the primary endpoints. Furthermore, no significant differences were found in safety and immunogenicity outcomes between the tocilizumab biosimilars and the reference drugs (Supplementary Tables S7, S8).
3.3 Cost and uptake of Biosimilars vs. reference drugs
The median weighted average price (WAP) of IMIDs biosimilars and their reference drugs from 2015 to 2024 is shown in Figure 2; Supplementary Tables S9, S10. Adalimumab and tocilizumab were listed on China’s National Reimbursement Drug List (NRDL) in 2019, since then their prices have dropped significantly. Infliximab was included in the NRDL in 2020. As of November 1, 2024, NMPA has approved 7, 5, and 3 biosimilars for adalimumab, infliximab, and tocilizumab, respectively. The estimated median WAP was 82% of the reference drugs for adalimumab biosimilars, 63% for infliximab biosimilars, and 79% for tocilizumab biosimilars.
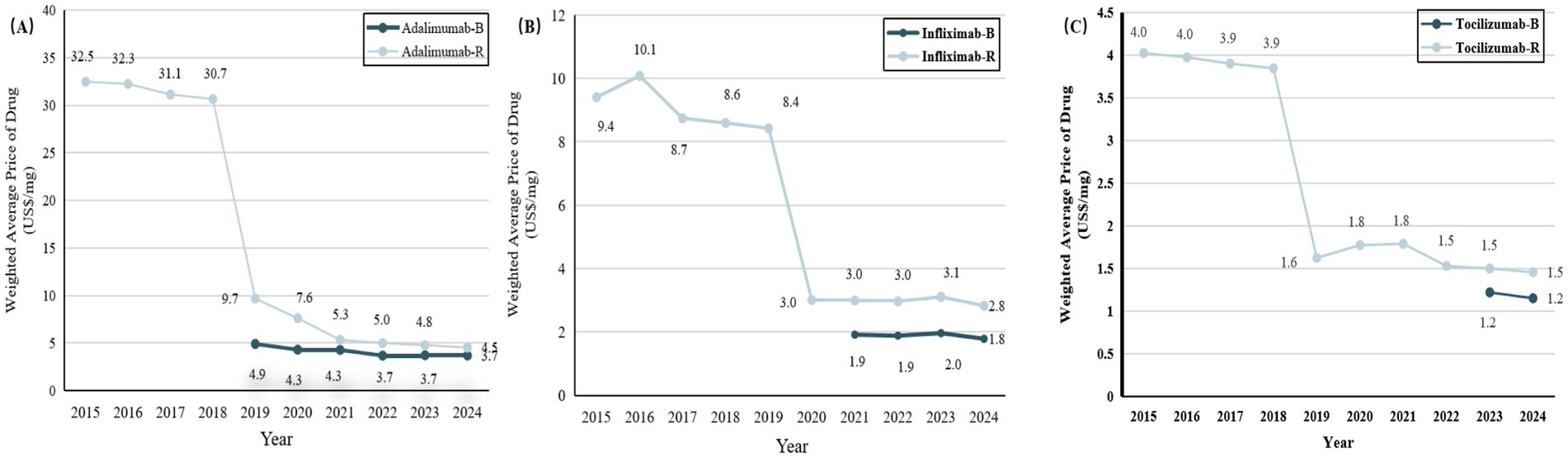
Figure 2. Trends in weighted average price (WAP) per unit for biosimilars and reference drugs for IMIDs between 2015 and 2024. (A) Adalimumab versus adalimumab biosimilars. (B) Infliximab versus infliximab biosimilars. (C) Tocilizumab versus tocilizumab biosimilars. IMIDs, Immune-Mediated Inflammatory Diseases; B, biosimilars; R, reference drugs.
In terms of monthly treatment costs, IMIDs biosimilars showed varying degrees of cost savings across different indications (Figure 3; Supplementary Table S6). Taking RA as an example, infliximab biosimilars had the lowest monthly treatment cost of $3,430, representing a monthly savings of $1,993 compared to the originator. Tocilizumab was the last to enter the market, with the biosimilar priced at $8,736, resulting in a savings of $2,184, compared to the originator (Figure 3).
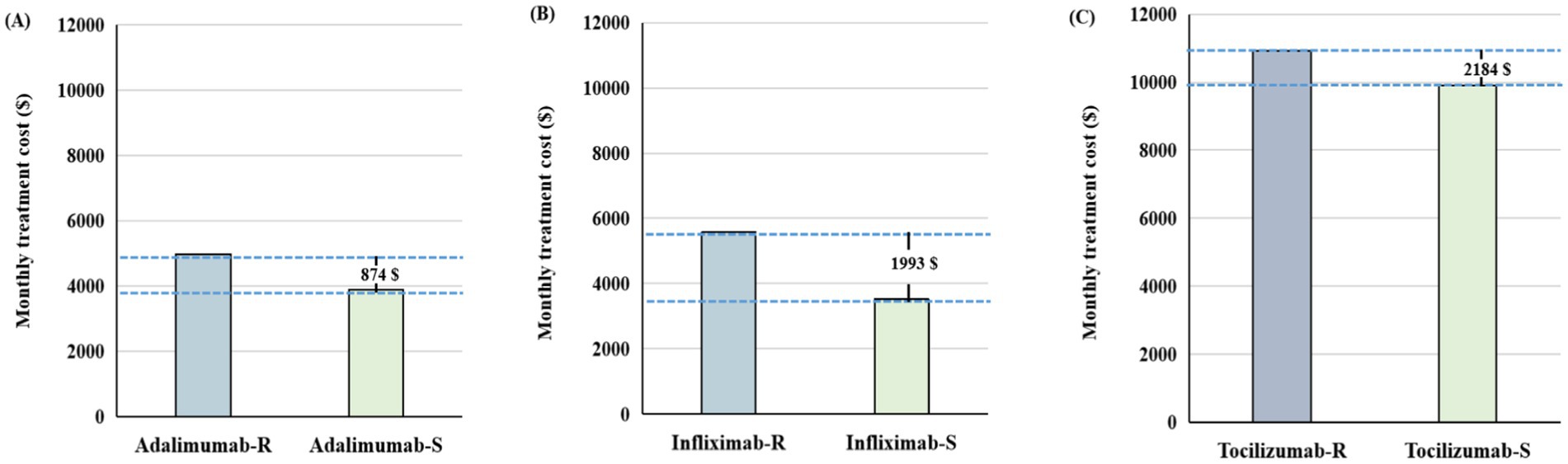
Figure 3. The difference in monthly treatment costs between biosimilars and reference drugs for patients with rheumatoid arthritis in 2024. (A) Adalimumab versus adalimumab biosimilars. (B) Infliximab versus infliximab biosimilars. (C) Tocilizumab versus tocilizumab biosimilars. IMIDs, Immune-Mediated Inflammatory Diseases; B, biosimilars; R, reference biologics.
IMIDs biosimilars have shown steady uptake growth since the first biosimilar entered the market, despite varying growth rates compared to the originator (Figure 4; Supplementary Table S11). In China, the uptake was highest for adalimumab (36%) and lowest for infliximab (2%) 1 year after market entry. The latest data for 2024 showed that biosimilars of adalimumab, infliximab, and tocilizumab had a market share of 72.1, 16.5, and 53.3%, respectively.
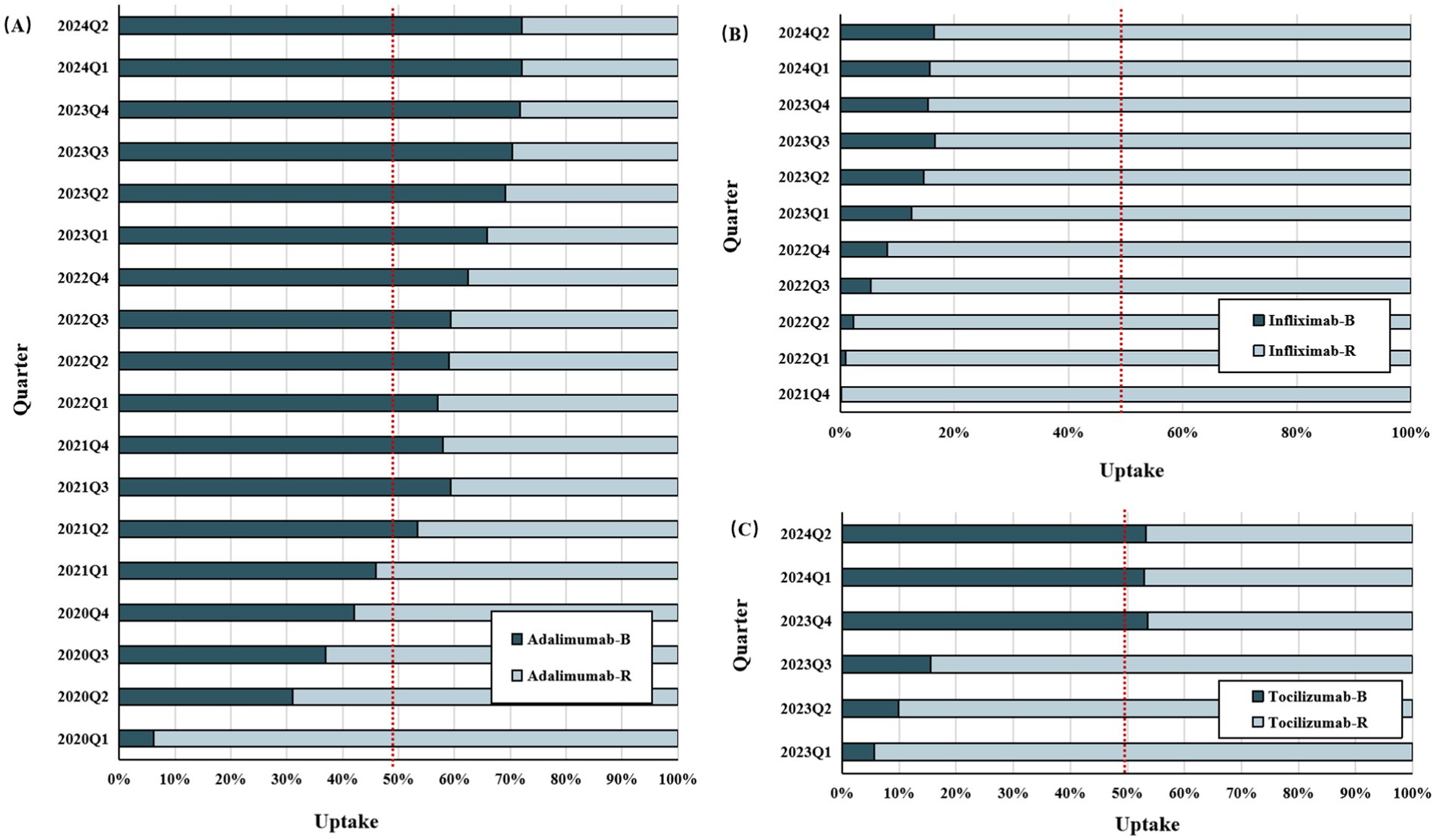
Figure 4. The uptake rate of biosimilars versus reference drugs since biosimilars’ entry into the Chinese market (As of July 2024). (A) Adalimumab versus adalimumab biosimilars. (B) Infliximab versus infliximab biosimilars. (C) Tocilizumab versus tocilizumab biosimilars. IMIDs, Immune-Mediated Inflammatory Diseases; B, biosimilars; R, reference biologics.
3.4 Substitution scenarios of biosimilars
For the first scenario, the results indicated that, in 2023, the use of biosimilars could save $15.9 M for adalimumab, $5.2 M for infliximab, and $0.9 M for tocilizumab. Moreover, substituting biosimilars for reference biologics would allow to support an additional 4,643, 1,527, and 1,071 patients annually for adalimumab, infliximab, and tocilizumab, respectively. Additionally, for the second scenario, with 100% biosimilar substitution, savings would increase to $22.98 M for adalimumab, $33.83 M for infliximab, and $3.82 M for tocilizumab, enabling treatment for 6,700, 9,863, and 4,373 more patients, respectively(Supplementary Table S12).
4 Discussion
This study was the first systematic analysis evaluating the relationship between the clinical benefits (efficacy/effectiveness, safety and immunogenicity) and the costs of biosimilars for autoimmune diseases in China. Additionally, the uptake of these biosimilars and their impact on treatment costs were also evaluated. The outcomes of the study showed that all RCTs of IMIDs biosimilars were conducted using double-blinded design. Among these RCTs, 10 (83.3%) were classified as low risks, while 2 (17.0%) had uncertain risk. Most of the biosimilars followed an equivalence trial design, except for Infliximab-GB242, which employed a non-inferiority design despite meeting equivalence thresholds and showing similar safety and immunogenicity. Non-inferiority trials are smaller than equivalence trials but cannot rule out the possibility that a biosimilar may have increased activity, potentially leading to more adverse events or suggesting it could be considered a biobetter (44). Given the risks of non-inferiority design, the FDA, EMA, and NMPA all recommend using an equivalence design in general, while non-inferiority designs should be chosen with caution (45).
This study identified 12 head-to-head RCTs involving 5,717 patients with IMIDs that compared the effects of biosimilars of adalimumab, infliximab, and tocilizumab to their reference drugs. Summary estimates met the prespecified criteria for equivalence based on 12 trials for primary endpoints (including ACR20, ASAS20, and PASI). In addition, our analysis of secondary outcomes revealed no significant differences between biosimilars and originators in PASI75, ASAS40 and ACR50. Safety outcomes, including TEAE, drug-related TEAE, SAE, hypersensitivity reactions, drug interruptions and discontinuations were also evaluated. Biosimilars demonstrated a comparable level of safety to the originators. In terms of immune response, biosimilars did not show a higher incidence of immunogenicity, which was consistent with previous studies (13, 46). This evidence supports the approval of these biosimilars in China with similar clinical benefits to the reference drugs. However, when we conducted further subgroup analyses of diseases, it was found that in the treatment of RA, IMIDs biosimilar may have higher immunogenicity than originators (Nabs, p = 0.006) (Supplementary Table S8). This also suggests that post-market immunogenicity monitoring should be strengthened in the future.
While RCTs are valuable, they may have limitations due to their short study duration and strict patient inclusion and exclusion criteria. Real-world studies provide important additional evidence by assessing patient clinical benefit through the data from real clinical patient encounters. In other countries such as the US, EU and Japan, real-world studies are widely used as supplementary evidence to evaluate the clinical benefits of biosimilars in various fields, including oncology, rheumatoid arthritis (28, 40–45, 47–52). Our study included 4 real-world studies of IMIDs biosimilars conducted worldwide, all of which showed that biosimilars have similar clinical benefits to originators (Supplementary Table S4). Real-world studies on oncology biosimilars are currently conducted in China. Previous studies have shown that three retrospective real-world studies conducted in China on bevacizumab were instrumental in supporting the approval for use in combination with platinum-based chemotherapy regimens as a first-line treatment for advanced non-squamous non-small cell lung cancer (53, 54). Given the high malignancy of cancer and the potential risks associated with treatment efficacy differences leading to disease progression, the CDE has emphasized the need for post-market immunogenicity monitoring in its guidelines (51–53). Strengthening real-world evidence for IMID biosimilars regulatory will be essential moving forward.
Indication extrapolation of biosimilars from the reference drugs may contribute to improving patient accessibility and accelerating the uptake of biosimilars. The US, the EU, Japan and other countries have established extrapolation policies, while adopting patent and data exclusivity systems to maintain the balance between innovation and imitation (54, 55). China has also issued the Technical Guidelines for Similarity Evaluation and Indication Extrapolation of Biosimilars in 2020 to allow for the extrapolation of biosimilars (56). Our study has shown that biosimilars of adalimumab, infliximab, and tocilizumab have been approved for the majority of originator indications through extrapolation (Supplementary Tables S13, S14). Additionally, our previous study has demonstrated that China’s patent term extension and data exclusivity protection systems are also being actively explored (52). Therefore, it can be expected that China will establish a mechanism to enhance the balance between biosimilars and originators.
The affordability of biologic treatments poses a significant barrier to patient access, highlighting the importance of IMIDs biosimilars in reducing treatment costs (57). A recent study indicated that biosimilars typically ranged from 55 to 90% of the list prices of their reference drugs in the US, Germany, and Switzerland (58). The utilization of adalimumab biosimilars in the US alone could yield healthcare savings of approximately $2.19 billion between 2016 and 2019 (5). In our investigation, we found that IMIDs biosimilars were priced at 63 to 82% of their originators, consistent with the previous studies (58). It should be noted that drug price negotiations in China have been instrumental in reducing the prices of IMIDs biosimilars and their reference drugs (13, 59). According to the China National Health Security Administration, these negotiations have led to an average price reduction of 61.7% for novel drugs (60). Specifically, the prices of reference drugs such as adalimumab, infliximab, and tocilizumab have decreased by 86.0, 69.9, and 63.7%, respectively, following their inclusion in the Chinese health insurance schemes. In this study, biosimilars of adalimumab, infliximab, and tocilizumab were found to offer monthly savings of $874 to $3,322 compared to their originators, which were equivalent to 1.8 to 7.0 times the per capita disposable income ($473.6) in China for 2023. This suggests that the use of IMIDs biosimilars could be critical to reducing costs and alleviating the financial burden on patients. Besides, our simulations align with previous studies, showing that biosimilar substitution helps reduce healthcare costs, allowing limited resources to benefit more patients (61).
The substitution effect of biosimilars is influenced by multiple factors. Within the same regulatory region, different uptake rates of IMIDs biosimilars may be related to market competition, cost savings, and extrapolated indications (adalimumab biosimilars have the highest number of approvals and indications, while tocilizumab biosimilars offer the greatest monthly treatment cost savings). The uptake of biosimilars varies significantly across countries, influenced by a variety of factors, such as healthcare negotiations, utilization policies (7, 62, 63). A previous study showed that, within the first year of launch, adalimumab biosimilars achieved market shares of 45% in the US, 55% in Germany and 17.5% in Switzerland. In comparison, Denmark showcased a remarkably higher uptake rate, with adalimumab biosimilar reaching a 95.1% market share just 3 months after launch, significantly surpassing other countries. Our study found that the uptake rate of adalimumab biosimilars in China was 42% in the first year after launch, closely aligning with the US but considerably lower than Denmark. The high uptake rate of the Danish model can be mainly attributed to the national tendering and procurement strategies, alternative treatment recommendations issued by the Danish Medicines Agency, and effective multi-stakeholder cooperation. These strategies were similar to China’s national volume-based procurement (NVBP), which has also yielded favorable outcomes in insulin. Therefore, accelerating the uptake of IMIDs biosimilars through China’s national volume-based procurement (NVBP) and multistakeholder consensus should be considered in China. Additionally, the uptake rate of biosimilars can be influenced by the preferences of both patients and clinicians. Further efforts to enhance the understanding of biosimilars among physicians and patients are needed in China.
5 Limitation
This study has certain limitations. Firstly, while the study makes thorough use of RCT data, the absence of RWE weakens its generalizability. Real-world studies are crucial to validate RCT findings, particularly in understanding long-term safety and effectiveness, especially for biologics and biosimilars. Although RWE from other countries has been included, the lack of data from China highlights a critical gap, underscoring the need for future research to address this context-specific evidence deficit. Secondly, our study was limited to the clinical benefits, costs and uptake of IMIDs biosimilars approved in China, making our findings not necessarily applicable to other countries. Third, this study sought to incorporate, to the extent possible, publicly available data from published articles and review reports on IMIDs biosimilars. Despite these efforts, it is possible that some relevant unpublished studies may not have been included. To ensure more comprehensive data inclusion, future studies should expand their search to include gray literature, such as dissertations, clinical trial registries, and preprints, capturing unpublished or ongoing studies.
6 Conclusion
Our study revealed that IMID biosimilars in China provide clinical benefits comparable to their reference biologics evidenced by high-quality RCTs and cohort studies with offer significant cost savings in China. In the future, encouraging China’s national volume-based procurement and multi-stakeholder collaboration may help accelerate the substitution of IMIDs biosimilars. Additionally, research on doctors, physicians, and patient behaviors, along with education, may also help ensure rational use and improve accessibility.
Data availability statement
The original contributions presented in the study are included in the article and supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval and informed consent were not considered necessary in accordance with the requirements of the Biomedical Ethics Committee of Tsinghua University because this research was based on publicly available data.
Author contributions
XD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. QG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XJ: Data curation, Formal analysis, Methodology, Project administration, Writing – review & editing. ZS: Data curation, Formal analysis, Methodology, Project administration, Writing – review & editing. WZ: Data curation, Formal analysis, Methodology, Validation, Writing – review & editing. ZW: Resources, Software, Writing – review & editing. JL: Data curation, Investigation, Software, Writing – review & editing. YY: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. YZ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Research Fund, Vanke School of Public Health, Tsinghua University and the National Natural Science Foundation of China (reference 72304168).
Conflict of interest
ZW was employed by BCPMdata Pharma Technology (Chengdu) Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1476213/full#supplementary-material
References
1. McInnes, IB, and Gravallese, EM. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. (2021) 21:680–6. doi: 10.1038/s41577-021-00603-1
2. Scherlinger, M, Richez, C, Tsokos, GC, Boilard, E, and Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat Rev Immunol. (2023) 23:495–510. doi: 10.1038/s41577-023-00834-4
3. McInnes, IB, and Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. (2017) 389:2328–37. doi: 10.1016/S0140-6736(17)31472-1
4. Girton, M, Tomsig, J, and Bazydlo, L. Triazole antifungal quantification for therapeutic drug monitoring in serum by liquid chromatography-tandem mass spectrometry (LC-MS/MS): Posaconazole, Voriconazole, Itraconazole, and Hydroxyitraconazole. Methods Mol Biol. (2024) 2737:55–65. doi: 10.1007/978-1-0716-3541-4_6
5. Lee, CC, Najafzadeh, M, Kesselheim, AS, and Sarpatwari, A. Cost to Medicare of delayed adalimumab biosimilar availability. Clin Pharmacol Ther. (2021) 110:1050–6. doi: 10.1002/cpt.2322
6. Sarpatwari, A, Barenie, R, Curfman, G, Darrow, JJ, and Kesselheim, AS. The US biosimilar market: stunted growth and possible reforms. Clin Pharmacol Ther. (2019) 105:92–100. doi: 10.1002/cpt.1285
7. Bennett, CL, Schoen, MW, Hoque, S, Witherspoon, BJ, Aboulafia, DM, Hwang, CS, et al. Improving oncology biosimilar launches in the EU, the USA, and Japan: an updated policy review from the southern network on adverse reactions. Lancet Oncol. (2020) 21:e575–88. doi: 10.1016/S1470-2045(20)30485-X
8. European Medicines Agency. European public assessment reports. (2024). Available at: https://www.ema.europa.eu/en/medicines/download-medicine-data#european-publicassessment-reports-(epar)-section (Accessed November 01, 2024).
9. US Food and Drug Administration. Biosimilars. (2024). Available at: https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars (Accessed November 01, 2024).
10. Pharmaceuticals and Medical Devices Agency. Review reports: drugs. (2024). Available at: https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0001.html (Accessed November 01, 2024).
11. Kvien, TK, Patel, K, and Strand, V. The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems. Semin Arthritis Rheum. (2022) 52:151939. doi: 10.1016/j.semarthrit.2021.11.009
12. Kay, J, Schoels, MM, Dörner, T, Emery, P, Kvien, TK, Smolen, JS, et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis. (2018) 77:165–74. doi: 10.1136/annrheumdis-2017-211937
13. Luo, X, Du, X, Li, Z, Liu, J, Lv, X, Li, H, et al. Clinical benefit, Price, and uptake for Cancer Biosimilars vs reference drugs in China: a systematic review and Meta-analysis. JAMA Netw Open. (2023) 6:e2337348. doi: 10.1001/jamanetworkopen.2023.37348
14. Gellad, WF, and Good, CB. Adalimumab and the challenges for Biosimilars. JAMA. (2019) 322:2171–2. doi: 10.1001/jama.2019.16275
15. Yazdany, J, Dudley, RA, Lin, GA, Chen, R, and Tseng, CW. Out-of-pocket costs for infliximab and its biosimilar for rheumatoid arthritis under Medicare part D. JAMA. (2018) 320:931–3. doi: 10.1001/jama.2018.7316
16. Wu, D, Jin, Y, Xing, Y, Abate, MD, Abbasian, M, Abbasi-Kangevari, M, et al. Global, regional, and national incidence of six major immune-mediated inflammatory diseases: findings from the global burden of disease study 2019. eClinicalMedicine. (2023) 64:102193. doi: 10.1016/j.eclinm.2023.102193
17. Xie, W, Yang, X, and Zhang, Z. The plight and light of treating rheumatoid arthritis in China. Lancet Rheumatol. (2019) 1:e81–2. doi: 10.1016/S2665-9913(19)30039-6
18. Yuan, N, Chen, C, Xing, C, Zhang, Y, Liu, A, Wang, J, et al. PSY3 disease burden of rheumatoid arthritis in China. Value Health. (2019) 22:S901. doi: 10.1016/j.jval.2019.09.2631
19. Luo, X, Du, X, Huang, L, Guo, Q, Tan, R, Zhou, Y, et al. The price, efficacy, and safety of within-class targeted anticancer medicines between domestic and imported drugs in China: a comparative analysis. Lancet. (2023) 32:100670. doi: 10.1016/j.lanwpc.2022.100670
20. Luo, X, Du, X, Huang, L, Guo, Q, Lv, X, Wang, C, et al. Evidence of pre-approval clinical trial supporting the granted conditional approval for novel cancer drugs in China between 2015 and 2022. eClinicalMedicine. (2023) 63:102177. doi: 10.1016/j.eclinm.2023.102177
21. The database of listed drugs. China National Medical Products Administration. (2024). Available at: https://www.cde.org.cn/main/xxgk/listpage/b40868b5e21c038a6aa8b4319d21b07d (Accessed November 01, 2024).
22. Pharnexcloud. National hospital sales volume. Available at: https://pharma (Accessed November 01, 2024).
23. Meng, Q, Mills, A, Wang, L, and Han, Q. What can we learn from China’s health system reform? BMJ. (2019) 365:l2349. doi: 10.1136/bmj.l2349
24. Rui, M, and Li, H. Cost-effectiveness of Osimertinib vs docetaxel-bevacizumab in third-line treatment in EGFR T790M resistance mutation advanced non-small cell lung Cancer in China. Clin Ther. (2020) 42:2159–2170.e6. doi: 10.1016/j.clinthera.2020.08.018
25. State Administration of Foreign Exchange. Renminbi Exchange Rate Intermediate Price. Available at: https://www.safe.gov.cn/safe/rmbhlzjj/index.html (Accessed November 01, 2024).
26. Yu, C, Zhang, F, Ding, Y, Li, Y, Zhao, Y, Gu, J, et al. A randomized, double-blind phase III study to demonstrate the clinical similarity of biosimilar SCT630 to reference adalimumab in Chinese patients with moderate to severe plaque psoriasis. Int Immunopharmacol. (2022) 112:109248. doi: 10.1016/j.intimp.2022.109248
27. Higgins, JP, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
28. Deng, W, Yang, S, Yang, C, Chen, H, Huang, G, Wu, H, et al. Rituximab biosimilar HLX01 versus reference rituximab in the treatment of diffuse large B-cell lymphoma: real-world clinical experience. J Oncol Pharm Pract. (2022) 10781552221110470. doi: 10.1177/10781552221110470
29. Cai, L, Li, L, Cheng, H, Ding, Y, Biao, Z, Zhang, S, et al. Efficacy and safety of HLX03, an adalimumab biosimilar, in patients with moderate-to-severe plaque psoriasis: a randomized, double-blind, phase III study. Adv Ther. (2022) 39:583–97. doi: 10.1007/s12325-021-01899-0
30. Huang, F, Sun, F, Wan, W, Wu, LJ, Dong, LL, Zhang, X, et al. Fri0414 Secukinumab provides rapid and significant improvement in the signs and symptoms of ankylosing spondylitis: Primary (16-week) results from a phase 3 China-centric study, measure 5. Chin Med J (Engl). (2019) 133:2521–31.
31. Li, J, Xue, Z, Wu, Z, Bi, L, Liu, H, Wu, L, et al. Comparison of the efficacy and safety of the adalimumab biosimilar TQ-Z2301 and adalimumab for the treatment of Chinese patients with active ankylosing spondylitis: a multi-center, randomized, double-blind, phase III clinical trial. Clin Rheumatol. (2022) 41:3005–16. doi: 10.1007/s10067-022-06199-8
32. Su, J, Li, M, He, L, Zhao, D, Wan, W, Liu, Y, et al. Comparison of the efficacy and safety of adalimumab (Humira) and the adalimumab biosimilar candidate (HS016) in Chinese patients with active ankylosing spondylitis: a multicenter, randomized, double-blind, parallel, phase III clinical trial. BioDrugs. (2020) 34:381–93. doi: 10.1007/s40259-020-00408-z
33. Xu, H, Li, Z, Wu, J, Xing, Q, Shi, G, Li, J, et al. IBI303, a biosimilar to adalimumab, for the treatment of patients with ankylosing spondylitis in China: a randomised, double-blind, phase 3 equivalence trial. Lancet Rheumatol. (2019) 1:e35–43. doi: 10.1016/S2665-9913(19)30013-X
34. Liu, Y, Liu, S, Liu, L, Gong, X, Liu, J, Sun, L, et al. Fine comparison of the efficacy and safety between GB242 and infliximab in patients with rheumatoid arthritis: a phase III study. Rheumatol Ther. (2022) 9:175–89. doi: 10.1007/s40744-021-00396-8
35. Ye, H, Liu, S, Xu, J, Chai, K, He, D, Fang, Y, et al. Efficacy and safety of CMAB008 compared with innovator infliximab in patients with moderate-to-severe rheumatoid arthritis receiving concomitant methotrexate: a randomized, double-blind, multi-center, phase III non-inferiority study. Rheumatol Ther. (2023) 10:757–73. doi: 10.1007/s40744-023-00544-2
36. Meng, C, Rajesh, D, Jannat-Khah, D, Bruce, O, Jivanelli, B, and Bykerk, V. Pos0286 can patients with controlled Ra receiving any class of targeted therapy with methotrexate (Mtx) sustain disease control after tapering Mtx? A systematic review and Meta-analysis. Ann Rheum Dis. (2022) 81:387–8. doi: 10.1136/annrheumdis-2022-eular.211
37. China National Medical Products Administration. Review reports of Adalimumab biosimilar. (2024). Available at: https://www.cde.org.cn/main/xxgk/postmarketpage?acceptidCODE=4e535af6c6b64d430b967bda35039e1f (Accessed November 01, 2024).
38. China National Medical Products Administration.review reports of Infliximab biosimilar. Available at: https://www.cde.org.cn/main/xxgk/postmarketpage?acceptidCODE=a3ab02b753af80706f30073b45a7d140 (Accessed November 1, 2024).
39. China National Medical Products Administration.review reports of Tocilizumab biosimilar. Available at: https://www.cde.org.cn/main/xxgk/postmarketpage?acceptidCODE=7d426010254d5e4dceddabf06c0ce702 (Accessed November 1, 2024).
40. Bae, SC, and Lee, YH. Comparative efficacy and safety of biosimilar adalimumab and originator adalimumab in combination with methotrexate in patients with active rheumatoid arthritis: a Bayesian network meta-analysis of randomized controlled trials. Clin Rheumatol. (2018) 37:1199–205. doi: 10.1007/s10067-018-4002-9
41. Mocci, G, Bodini, G, Allegretta, L, Cazzato, AI, Chiri, S, Aragona, G, et al. Adalimumab biosimilar GP2017 versus adalimumab originator in treating patients with inflammatory bowel diseases: a real-life, multicenter, observational study. Biomedicines. (2022) 10:1799. doi: 10.3390/biomedicines10081799
42. Kumar, P, Vuyyuru, SK, Kante, B, Kedia, S, Sahu, P, Ranjan, MK, et al. Efficacy and safety of biosimilar versus originator infliximab in patients with inflammatory bowel disease: a real-world cohort analysis. Indian J Gastroenterol. (2022) 41:446–55. doi: 10.1007/s12664-022-01252-5
43. Smith, JT, Velayos, FS, Niu, F, Liu, V, Delate, T, Pola, S, et al. Retrospective cohort study comparing infliximab-dyyb and infliximab in biologic-naive patients with inflammatory bowel disease in the United States. Crohns Colitis. (2021) 3:otab051. doi: 10.1093/crocol/otab051
44. The Center for Drug Evaluation of NMPA. Guidelines for the Research and Evaluation of Biosimilars. Accessed November 1, 2024. Available at:https://www.nmpa.gov.cn/xxgk/ggtg/ypggtg/ypqtggtg/20150228155701114.html.
45. Wells, G, Shea, B, O’Connell, D, Peterson, J, Welch, V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed November 01, 2024).
46. Ascef, BO, Almeida, MO, Medeiros-Ribeiro, AC, Oliveira de Andrade, DC, Oliveira Junior, HA, and de Soarez, PC. Therapeutic equivalence of biosimilar and reference biologic drugs in rheumatoid arthritis: a systematic review and Meta-analysis. JAMA Netw Open. (2023) 6:e2315872. doi: 10.1001/jamanetworkopen.2023.15872
47. Lai, Z, and La Noce, A. Key design considerations on comparative clinical efficacy studies for biosimilars: adalimumab as an example. RMD Open. (2016) 2:e000154. doi: 10.1136/rmdopen-2015-000154
48. Bankar, A, Korula, A, Abraham, A, Viswabandya, A, George, B, Srivastava, A, et al. Comparison of the efficacy of innovator rituximab and its Biosimilars in diffuse large B cell lymphoma patients: a retrospective analysis. Indian J Hematol Blood Transfus. (2020) 36:71–7. doi: 10.1007/s12288-019-01167-w
49. Yang, C, Khwaja, R, Tang, P, Nixon, N, King, K, and Lupichuk, S. A review of Trastuzumab Biosimilars in early breast Cancer and real world outcomes of neoadjuvant MYL-1401O versus reference Trastuzumab. Curr Oncol. (2022) 29:4224–34. doi: 10.3390/curroncol29060337
50. Tang, N, and Wang, Z. Comparison of bevacizumab plus chemotherapy with chemotherapy alone in advanced non-small-lung cancer patients. Onco Targets Ther. (2016) 9:4671–9. doi: 10.2147/OTT.S110339
51. Xing, P, Mu, Y, Wang, Y, Hao, X, Zhu, Y, Hu, X, et al. Real world study of regimen containing bevacizumab as first-line therapy in Chinese patients with advanced non-small cell lung cancer. Thorac Cancer. (2018) 9:805–13. doi: 10.1111/1759-7714.12650
52. Luo, X, Yang, L, Du, X, Yang, J, Qian, F, and Yang, Y. Analysis of patent and regulatory exclusivity for novel agents in China and the United States: a cohort study of drugs approved between 2018 and 2021. Clin Pharmacol Ther. (2022) 112:335–43. doi: 10.1002/cpt.2625
53. The Center for Drug Evaluation of NMPA. Guidelines for Clinical Trials of Trastuzumab Biosimilars for Injection. Available at: https://www.nmpa.gov.cn/directory/web/nmpa/images/16LJ5NPDxrN19bptaWucn6zuA4MvG0qnB2bSyytTR6da4tbzUrdTyLnBkZg==.pdf (Accessed November 1, 2024).
54. Yamanaka, T, and Kano, S. Patent term extension systems differentiate Japanese and US drug lifecycle management. Drug Discov Today. (2016) 21:111–7. doi: 10.1016/j.drudis.2015.09.005
55. Van de Wiele, VL, Kesselheim, AS, Nagar, S, and Tu, SS. The prevalence of drug patent term extensions in the United States, 2000–2018. Nat Biotechnol. (2023) 41:903–6. doi: 10.1038/s41587-023-01847-z
56. China Center for drug evaluation, National Medical Products Administration. Circular on the Publication of Technical Guidelines on Similarity Evaluation and Extrapolation of Indications for Biosimilars. Available at: https://www.cde.org.cn/main/news/viewInfoCommon/d92c6507a57bee9ccfc5baa1ee87fda9 (Accessed November 1, 2024).
57. Alten, R, and Weinbrecht-Mischkewitz, M. Maximizing the success of biosimilar implementation. Nat Rev Rheumatol. (2023) 19:757–8. doi: 10.1038/s41584-023-01048-7
58. Carl, DL, Laube, Y, Serra-Burriel, M, Naci, H, Ludwig, W-D, and Vokinger, KN. Comparison of uptake and prices of Biosimilars in the US, Germany, and Switzerland. JAMA Netw Open. (2022) 5:e2244670. doi: 10.1001/jamanetworkopen.2022.44670
59. Dickson, SR, and Kent, T. Association of Generic Competition with Price Decreases in physician-administered drugs and estimated Price decreases for biosimilar competition. JAMA Netw Open. (2021) 4:e2133451. doi: 10.1001/jamanetworkopen.2021.33451
60. National Healthcare Security Administration. (2023) National Health Insurance Drug List. Available at:http://www.nhsa.gov.cn/art/2023/12/13/art_14_11671.html (Accessed November 1, 2024).
61. Clarke, K, Ainslie-Garcia, M, Ferko, N, and Shastri, K. Modelling the opportunity for cost-savings or patient access with biosimilar adalimumab and tocilizumab: a European perspective. J Med Econ. (2024) 27:952–62. doi: 10.1080/13696998.2024.2379212
62. Sosulski, N. A brief overview of biosimilars and factors limiting their uptake. Can Pharm J (Ott). (2019) 152:364–6. doi: 10.1177/1715163519879411
Keywords: immune-mediated inflammatory diseases, biosimilars, clinical benefit, cost, uptake
Citation: Du X, Luo X, Guo Q, Jiang X, Su Z, Zhou W, Wang Z, Li J, Yang Y and Zhang Y (2024) Assessment of clinical benefit, cost and uptake of biosimilars versus reference biologics in immune-mediated inflammatory diseases in China. Front. Public Health. 12:1476213. doi: 10.3389/fpubh.2024.1476213
Edited by:
Irma Convertino, University of Pisa, ItalyReviewed by:
Mihaela-Simona Naidin, University of Medicine and Pharmacy of Craiova, RomaniaMarcel Henrique Marcondes Sari, Federal University of Paraná, Brazil
Copyright © 2024 Du, Luo, Guo, Jiang, Su, Zhou, Wang, Li, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Yang, eWFuZ2hhcHB5QHRzaW5naHVhLmVkdS5jbg==; Yi Zhang, eWlfemhhbmdAbWFpbC50c2luZ2h1YS5lZHUuY24=
†These authors share first authorship
 Xin Du
Xin Du Xingxian Luo2†
Xingxian Luo2† Qixiang Guo
Qixiang Guo Xiaomeng Jiang
Xiaomeng Jiang Ziling Su
Ziling Su Weiting Zhou
Weiting Zhou Zhongjian Wang
Zhongjian Wang Jiarun Li
Jiarun Li Yue Yang
Yue Yang Yi Zhang
Yi Zhang