- 1Department of Environmental and Global Health, University of Florida, Gainesville, FL, United States
- 2Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA, United States
- 3Center for African Studies, University of Florida, Gainesville, FL, United States
- 4Department of Statistics, University of Georgia, Athens, GA, United States
- 5College of Agriculture and Environmental Science, Haramaya University, Haramaya, Ethiopia
- 6College of Health and Medical Sciences, Haramaya University, Haramaya, Ethiopia
- 7College of Veterinary Medicine, Haramaya University, Haramaya, Ethiopia
- 8Office of Research Affairs, Haramaya University, Haramaya, Ethiopia
- 9Department of Pediatrics, Washington University, St. Louis, MO, United States
- 10Department of Microbiology and Cell Science, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, FL, United States
- 11Center for Food Animal Health, Department of Animal Sciences, College of Food, Agricultural, and Environmental Sciences, The Ohio State University, Wooster, OH, United States
- 12Department of Animal Sciences, Global Food Systems Institute, and Emerging Pathogens Institute, University of Florida, Gainesville, FL, United States
- 13School of Veterinary Science, Massey University, Palmerston North, New Zealand
- 14Innovation Laboratory for Livestock Systems, University of Florida, Gainesville, FL, United States
Background: Campylobacter is associated with environmental enteric dysfunction (EED) and malnutrition in children. Campylobacter infection could be a linchpin between livestock fecal exposure and health outcomes in low-resource smallholder settings.
Methods: We followed a birth cohort of 106 infants in rural smallholder households in eastern Ethiopia up to 13 months of age. We measured anthropometry, surveyed sociodemographic determinants, and collected stool and urine samples. A short survey was conducted during monthly visits, infant stool samples were collected, and Campylobacter spp. was quantified using genus-specific qPCR. In month 13, we collected stool and urine samples to assay for EED biomarkers. We employed regression analyses to assess the associations of household determinants with Campylobacter colonization, EED, and growth faltering.
Results: The Campylobacter load in infant stools increased with age. The mean length-for-age Z-score (LAZ) decreased from −0.45 at 3–4 months of age to −2.06 at 13 months, while the prevalence of stunting increased from 3 to 51%. The prevalence of EED at 13 months of age was 56%. A higher Campylobacter load was associated with more frequent diarrhea. Prelacteal feeding significantly increased Campylobacter load in the first month of life. Over the whole follow-up period, Campylobacter load was increased by keeping chickens unconfined at home and unsanitary disposal of infant stools while decreased by mothers’ handwashing with soap. Longitudinally, Campylobacter load was positively associated with food insecurity, introduction of complementary foods, and raw milk consumption. There were no significant associations between Campylobacter load, EED, and LAZ.
Conclusion: This study found that most determinants associated with increased Campylobacter infection were related to suboptimal feeding practices and hygiene. The findings related to livestock-associated risks were inconclusive. Although stunting, EED, and Campylobacter prevalence rates all increased to high levels by the end of the first year of life, no significant association between them was identified. While additional research is needed to investigate whether findings from this study are replicable in other populations, community efforts to improve infant and young child feeding practices and food hygiene, and water, sanitation, and hygiene (WaSH) at the household level, could reduce (cross-)contamination at the point of exposure.
Introduction
Stunting [length-for-age (LAZ)/height-for-age Z-score < −2], affects 22% of children under five (CU5) globally and 30% in sub-Saharan Africa (1), is an indicator of chronic undernutrition and is associated with numerous morbidities, all-cause mortality, and reduced lifetime earnings (2–4). Agriculture-to-nutrition pathways, including household income, women’s empowerment, and agricultural production, can be leveraged to reduce undernutrition (5). Particularly, animal-source food (ASF) consumption has been associated with reducing child stunting in low- and middle-income countries (LMICs) (6).
Projected population and economic growth in Africa are expected to increase the demand for ASF fourfold between 2010 and 2050 (7). Ethiopia is home to the largest livestock population on the continent, most of which is produced through extensive smallholder farming systems (i.e., mixed crop-livestock, pastoral) (8). Resources are typically limited in such settings, and WaSH conditions often fail to meet international standards. This reality predisposes CU5 to environmental exposure to human and livestock feces, which are reservoirs of enteric pathogens (9).
Observational studies have associated enteric pathogens with child growth faltering (9, 10), which is hypothesized to be mediated through acute illness (e.g., diarrhea and fever) (11) and environmental enteric dysfunction (EED), a chronic and asymptomatic consequence of both symptomatic and asymptomatic enteric infections (12). EED is well established in the literature as a risk factor for stunting (12–15), and growing evidence points toward EED as an essential mediator between enteric infections and stunting (15). The gold standard for diagnosing EED is intestinal biopsy (16). Still, it is not practical in population-based studies in resource-poor settings, where biomarkers were used to assess different impaired gut functions of EED (17). However, a 2021 study from the Sanitation Hygiene Infant Nutrition Efficacy (SHINE) trial found no consistent association between the examined EED biomarkers and linear growth (18) and a 2022 study in two African countries revealed few correlations between the evaluated EED biomarkers (19). Hence, challenges exist in the use of biomarkers to accurately measure EED.
As landmark randomized controlled trials focusing solely on reducing exposure to human feces through WaSH interventions found no significant effect on linear growth (20), researchers have called for a One Health Approach that considers exposure to human and animal feces (21, 22). A systematic review of benefits and risks of smallholder livestock production found Campylobacter spp. to be the primary zoonotic enteric pathogen associated with increased risks of both EED and undernutrition and proposed a modification to the United Nations Children’s Fund (UNICEF) framework on child undernutrition that incorporates poor gut health as a third immediate determinant of undernutrition (9). These findings suggest that Campylobacter infection could be a critical link between livestock exposure and malnutrition in low-resource settings.
In 2018, the Campylobacter Genomics and Environmental Enteric Dysfunction (CAGED) research team conducted formative research, including a cross-sectional study of smallholder households in rural eastern Ethiopia (23). This study found a high prevalence of infection with diverse Campylobacter spp., EED, and stunting in children aged 12–14 months and identified several associated risk factors (24). These findings supported the design and implementation of a prospective longitudinal birth cohort study (25) to uncover the reservoirs and determinants of Campylobacter spp. colonizing infants, the results of which are presented here. Utilizing the modified UNICEF framework to identify possible putative determinants, this study examines associations in the first year of life between individual- and household-level determinants and Campylobacter infection. Given their importance to Campylobacter, we also explore symptoms of enteric illness (diarrhea and fever) as outcomes associated with Campylobacter. We then examine EED and linear growth faltering as secondary outcomes. Note that the microbiological findings that detail the prevalence and load of Campylobacter from the CAGED longitudinal study have been described in a sister paper (26).
Methods
Study setting
The CAGED longitudinal study was conducted in Haramaya woreda (district), East Hararghe Zone, Oromia Region, Ethiopia. The study site is a rural, agriculture-based area where traditional crop-based agriculture has been replaced in recent years with cash crop production of khat. However, many farmers still cultivate some corn, and most have livestock. Data collection was scheduled to begin in April 2020 but was delayed due to the COVID-19 pandemic. After months of delay, preparation, and online training, data collection was initiated in December 2020, just 1 month after the start of the civil war in the Tigray Region, an armed conflict that continued throughout the data collection period (25).
Study design and field procedures
The study protocol has been described in full elsewhere (25). This study was powered to estimate the prevalence of Campylobacter genus, a sample size of 100 allowed the prevalence estimates to have a precision of 10% at 95% confidence and a power of 80% (25). To account for a 20% attrition rate, we aimed to recruit a total sample of 120 newborn infants (25). Between December 2020 and June 2021, we enrolled six cohorts at 4-week intervals, each consisting of up to 20 randomly selected infants from a birth registry, resulting in a total of 115 infants. Participating families were followed every 4 weeks (hereafter called monthly follow-up) until the infants were approximately 13 months of age (through May 2022). We measured infant’s length and weight at quarterly visits following enrollment using the same procedures described in the formative research (24). Despite extensive efforts to engage with culturally sensitive approaches, some mothers were reluctant to hand over newborn infants to local enumerators for measurement at birth. In addition, birth length measures are error-prone due to infants being bent and curled up (27). For these reasons, we excluded length measures shortly after birth from our analyses.
A Long Survey with mothers and their male partners was conducted at baseline and near the end of the study to collect information including demographics, livelihoods, wealth, animal ownership, management, diseases, WaSH conditions, and child health. During monthly visits, mothers responded to a Short Survey focusing on dynamic indicators, including infant health, diets, food security, contact with animals, and the environment; infant stool samples were collected concurrently. All interview, survey, and anthropometry data were collected on tablets using the REDCap mobile app and were uploaded to a secure REDCap database at the University of Florida1,2. All survey instruments are available in the supplementary material of the study protocol manuscript (25).
At the end of the follow-up period, samples were collected to assess biomarkers of EED as previously reported (24). Briefly, stool samples were collected to measure fecal myeloperoxidase (MPO) and immediately flash-frozen using liquid nitrogen and stored at −0°C until further use. To assess gut permeability, a solution of 1,000-mg lactulose in 10-ml sterile water was administered to the infant for 5 min, and urine was collected over a period of 4 hours. All samples were transported to the United States on dry ice for further analysis. MPO was measured using a commercially available enzyme-linked immunosorbent assay (MPO RUO, Alpco, Salem, NH, United States). Lactulose in urine was assessed using high-performance liquid chromatography (HPLC).
Sampling and laboratory procedures
The collection and preservation of infant stool samples and molecular detection and quantification of Campylobacter by quantitative polymerase chain reaction (qPCR) have been described elsewhere (26). The primers employed detect a large range of species in the Campylobacter genus, including the well-studied species Campylobacter jejuni and Campylobacter coli as well as a wide range of other thermotolerant and non-thermotolerant species such as the recently described Candidatus Campylobacter infans (28). This PCR assay was previously shown to be more sensitive to detect Campylobacter in stool samples from children than culture and enzyme immunoassay. There were no false-positive reactions with DNA from related genera (29). Based on the standard curve of qPCR, Campylobacter load [unit: log10(gene copies per 50 ng of DNA)] was calculated as a linear function of cycle threshold (Ct) values: 10.51–0.24 × Ct (26). The calibration was linear in the full tested range (Ct: 12–42). We chose not to use a Ct-cutoff value to calculate the load of Campylobacter in the following data analyses to avoid left censoring.
Data analyses
Health outcomes
The primary outcome evaluated in this study is the Campylobacter load of infants (including those with and without clinical symptoms). We categorized the ages when the infant stool samples were collected into quartiles to assess temporal variations of the Campylobacter load and its determinants.
Secondary outcomes include EED, linear growth faltering, diarrhea, and fever. EED was categorized using a composite indicator based on the percentage of lactulose excretion (%L) and fecal MPO (24). Cut-off values for %L were 0.2 < %L ≤ 0.45% for moderate gut permeability and %L > 0.45 for severe gut permeability. The thresholds for MPO were 2,000 ng/mL for moderate gut inflammation and the third quartile of the observed data at 3,364 ng/mL for severe gut inflammation. EED was defined as moderate if either %L or MPO was categorized as severe, and as severe if both %L and MPO were in a severe category. A decrease in LAZ characterized the linear growth faltering. We modeled changes in LAZ using two models: one uses the overall decrease between the first and last available LAZ scores as the outcome, referred to as the decrease in LAZ, and is a non-longitudinal model. The other is a longitudinal model, using the LAZ at each visit as the outcome. We use the phrases “linear growth faltering” and “decrease in LAZ” interchangeably. As the anthropometric measurements were conducted on different days than the short surveys and stool sample collection, we employed k-means clustering to categorize the ages at anthropometric measurements into five groups. Neonatal outcomes include the Campylobacter load in the first month of life (day range: 7–39) and the first available LAZ. A case of diarrhea or fever was declared when the mother reported the infant having symptoms on the day of the interview or the day before. The definition of diarrhea provided to the mother was the infant having three or more loose or watery stools in 24 hours.
Identification of putative determinants
We applied the modified UNICEF framework of child undernutrition, as proposed in our systematized review (9), to identify putative determinants of the health outcomes of interest (Campylobacter infection, EED, and linear growth faltering). These included variables from the household surveys that fell into domains of immediate causes (inadequate dietary intake and diseases), underlying causes (household food insecurity, inadequate care and feeding practices, and unhealthy household environment and inadequate health services), and basic causes (social/cultural, economics/livelihood, human capital, basic demographics) from the original UNICEF framework, as well as variables that reflected the domains of benefits (as determined by the agriculture-to-nutrition pathways), risks (of enteric infections from livestock exposure), and control measures (to the risks), as illustrated in the review. For the conceptual overlap between risks and unhealthy household environment underlying Campylobacter infection, we assigned the determinants related to livestock (feces) exposure to risks, given our study’s interest in smallholder farming. Determining eligible variables for analyses involved cross-checking the framework’s domains and the variables in the household surveys, generating a suite of putative determinants for the health outcomes of Campylobacter infection, EED, and linear growth faltering. Some determinants may affect the downstreaming outcomes of poor gut health and undernutrition through other mediators, but may not be directly associated with an increased risk of Campylobacter infection. These determinants include vitamin A and iron supplementation, treatment for malnutrition, use of oral rehydration solution, visiting a health center for diarrhea or fever, vaccination, and receiving prenatal care and its location; thus, we only tested their associations with EED and LAZ to simplify interpretation. We examined the enteric symptoms of diarrhea and fever as possible outcomes of Campylobacter infection. We also considered both enteric symptoms as determinants of growth faltering. While fever was considered a putative determinant for EED, diarrhea was not, as diarrhea is a causative factor for elevated EED biomarkers.
Composite variables
Composite variables were calculated by combining answers from two or more survey questions, and such variables have been validated in other studies. We generated the following composite variables:
1. Minimum Dietary Diversity of Infant and Young Children (MDD-IYC) (30), from which the consumption status of ASF was extracted.
2. Household food insecurity access score (HFIAS) and household food insecurity access (HFIA) category (31).
3. Joint Monitoring Program (JMP) WaSH service ladders for drinking water, sanitation, and hygiene (32).
4. Tropical Livestock Units (TLU), a composite variable that quantifies the holding of all farmed animals in a household by converting the counts of different livestock types into standardized units based on their relative body weight and summing them into a single value (33).
5. Anthropometric Z-scores [i.e., LAZ and weight-for-age Z (WAZ) scores], calculated using the R package anthro (34).
6. Adapted from our formative research (24), we defined a livestock location risk score for each of the four livestock species (cattle, chicken, sheep, or goat). This score was designed to characterize the increasing likelihood of contact between a livestock species and study participants during the day or night. A score of 0 was assigned to any household that did not have the livestock species in question or reported keeping them outside the house at all times; a score of 1 was assigned to any household that reported keeping the livestock species inside the house and confined; and a score of 2 was assigned to any household that reported keeping the livestock species inside the house unconfined.
7. To characterize assets, informed by the literature (35), we employed a latent trait model (using the R package ltm (36)) to calculate a factor score for ownership of sellable household assets for each household.
8. We characterized the age of introducing complementary feeding as the infant’s age at the monthly visit when the mother first reported giving the infant complementary foods. This definition of complementary feeding is based on operational definitions of the WHO (37), which do not consider prelacteal feeding.
9. Complementary feeding refers to an infant receiving any solid, semisolid, or soft foods at any time (note that this differs from appropriate complementary feeding, which should occur after 6 months of age).
10. Prelacteal feeding means feeding any substances other than breastmilk given to the infant during the first 3 days after birth.
We dichotomized some composite variables to facilitate interpretation and minimize the impact of data sparsity. We used accepted cutoffs for meeting MDD as ≥5, an HFIA category of 1 as food secure, and an anthropometric Z-score < −2 as stunted. In the JMP drinking water, sanitation, and hygiene ladders, unimproved, open defecation, and no facility were treated as reference categories, respectively, with all other categories within each ladder merged. Infant stool disposal was binarized as a low-risk category, including infant using toilet or latrine, stool put or rinsed into toilet or latrine or drain or ditch, and a high-risk category, including stool thrown in the garbage, buried, or left in the open. The quartile score for assets was binarized by its median.
Regression analyses
The average Campylobacter load, EED, and the change in LAZ for each infant over the follow-up period were separately regressed on all selected baseline determinants, except for infant feeding practices at birth. As we were interested in the short-term effects of feeding practices at birth (colostrum feeding, early initiation of breastfeeding, and prelacteal feeding) on Campylobacter infection and on linear growth, we assessed their relationship with the Campylobacter load in the first month (age range: 7–39) and the first available LAZ measurement in the regression analysis. EED at endline was regressed on all the selected baseline determinants and the average of longitudinal (time-varying) determinants of the whole follow-up.
To assess the longitudinal associations between the time-varying determinants and health outcomes, we regressed the average Campylobacter load on the average of each time-varying determinant within each age quartile, under the assumption that the effect of each determinant was immediate to the change of Campylobacter load. We used the same approach to regress separately the outcomes of current fever and diarrhea on Campylobacter load. In contrast, the LAZ of each visit was regressed on the average value of each determinant from birth up to that visit, under the assumption that stunting is the result of a chronic process and the effect of each determinant on growth accumulates over time.
We employed linear-mixed models with an individual-level random intercept to account for dependency in repeated measures (using the R package lme4 (38)). For each outcome (except diarrhea and fever), we conducted backward elimination to select a multivariable model, starting with determinants having adjusted p-values <0.2 from a variable screening step where the outcome was regressed on each determinant separately while adjusting for sex and socioeconomic status (SES, binarized household asset score) as fixed confounders. Infant age group was also adjusted as a fixed confounder in the linear-mixed models for Campylobacter load and LAZ, while the age at EED sampling was adjusted as a fixed confounder in the logistic models for EED. These fixed confounders were also constantly kept in the multivariable models, i.e., they are not subject to backward elimination in the multivariable selection. In the models for EED, we categorized variables of proportion of vitamin A supplementation (categories: ≥0.15; >0 and < 0.15; and = 0) and proportion of infants with fever (categories: ≥0.3 and < 0.3) to improve the interpretability of the associated effects and the readability of their confidence intervals. For most variables, there was a higher level of missingness at the baseline than at the endline. For variables that are the same between baseline and endline among >80% of the studied households, and if the baseline value was missing but the endline value was available, we used the endline value to impute the missing baseline value. After this simple ad hoc imputation, we conducted complete case analyses, that is, missing data were dropped under the assumption of missing completely at random. All analyses were conducted in R (version 4.1.0) (39).
Results
Descriptive statistics
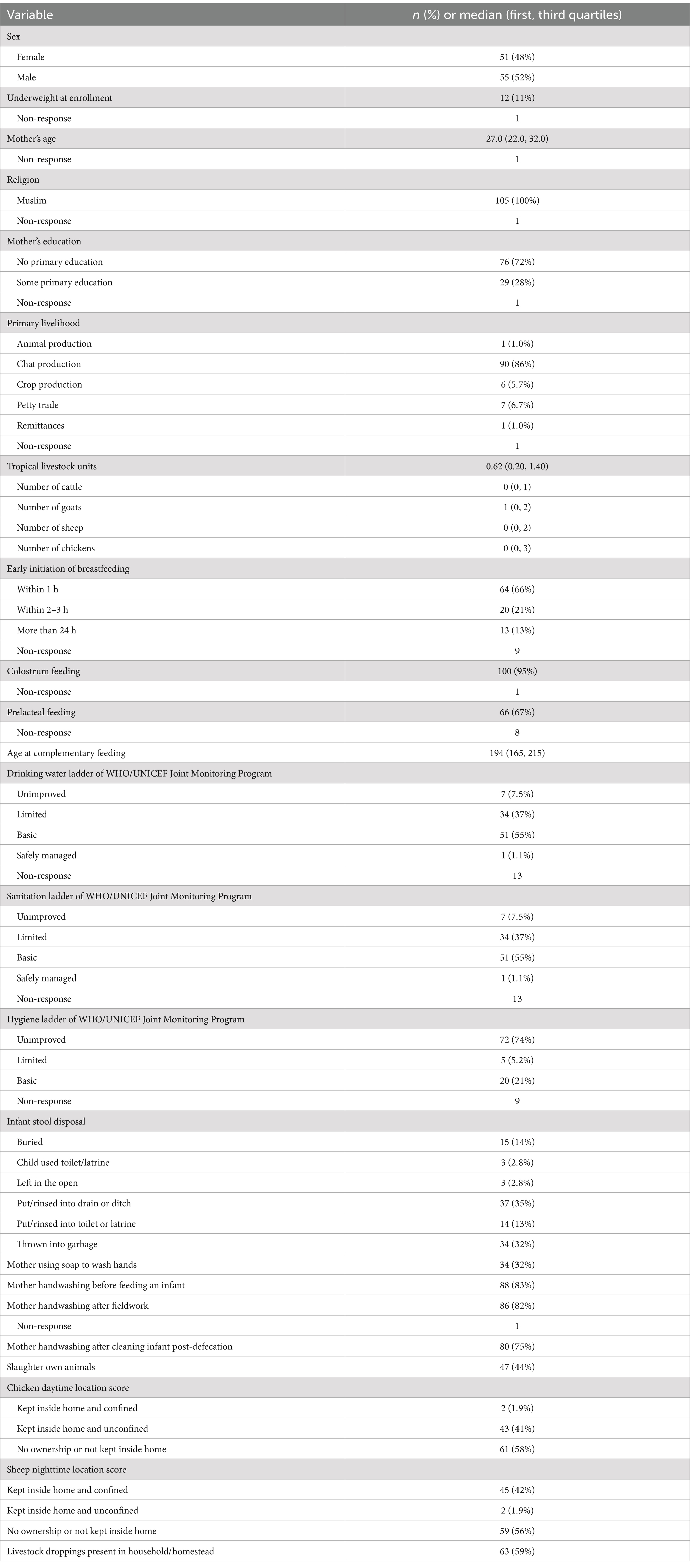
Of the 115 infants enrolled in the study, four households withdrew from the study, three infants died from causes unrelated to the study, and two moved outside of the study area, leaving 106 infants who completed follow-up and were included in the analysis. Of the 1,378 planned household visits, 1,111 (81%) had infant’s stool samples assayed by qPCR, 1,035 (75%) had a monthly short survey, and 980 (71%) had both. Only 13% and 2% of infants had stool samples collected and short surveys completed for all 13 visits, respectively. We present the characteristics of the study population in Table 1.
Forty-eight percent of infants were females, and 11% were underweight at enrollment (age range: 4–27 days). The mothers were, on average, 27 years old, all of whom were Muslim, and 28% had received some formal education. Most households were engaged in a combination of on-farm activities (e.g., animal, crops, and khat production), with khat production dominating as the primary livelihood for 86% of households and an average TLU of 0.62. Almost all infants were fed colostrum, and 66% had early initiation of breastfeeding (within 1 h) after birth; 67% received prelacteal feeding. Few households had access to safely managed drinking water (1.1%) and sanitation facilities (1.1%), and only 21% of households had access to basic hygiene facilities. More than three quarters of mothers reported hand washing before feeding the infant, after fieldwork, and after infant defecation; however, soap use was only reported by one-third of mothers. Most infant stools were reportedly disposed of as garbage or into a drain. During the daytime, all farm animals except chickens were kept outside the household or confined if inside. More farm animals (goats, sheep, cattle, and chickens) were kept inside at night than during the day. The proportion of households confined chickens when kept inside the home (41%) was higher than other livestock and was the same during the day- or nighttime. While 59% of households were observed to have livestock droppings in household/homestead, nearly 90% of households reported collecting animal waste. Forty-four percent of households reported slaughtering their livestock (goat, sheep, or chicken), and all these households reported washing hands before and/or after slaughtering. Further details of household characteristics were reported in Supplementary Table 1.
In the first month of life (day range: 7–39), the average Campylobacter load in infant stools was 1.87 [standard deviation (SD): 1.01, unit: log(gene copies/50 ng of DNA)], whose corresponding prevalence was 32% (95% CI: 24–42%), under a Ct cutoff of 35. The Campylobacter load increased steadily from 1.95 (SD: 0.97) in the first age quartile to 3.83 (SD: 1.14) in the fourth age quartile, see Figure 1A. The average load corresponding to each anthropometry visit also demonstrated a steadily increasing trend, see Figure 1B.
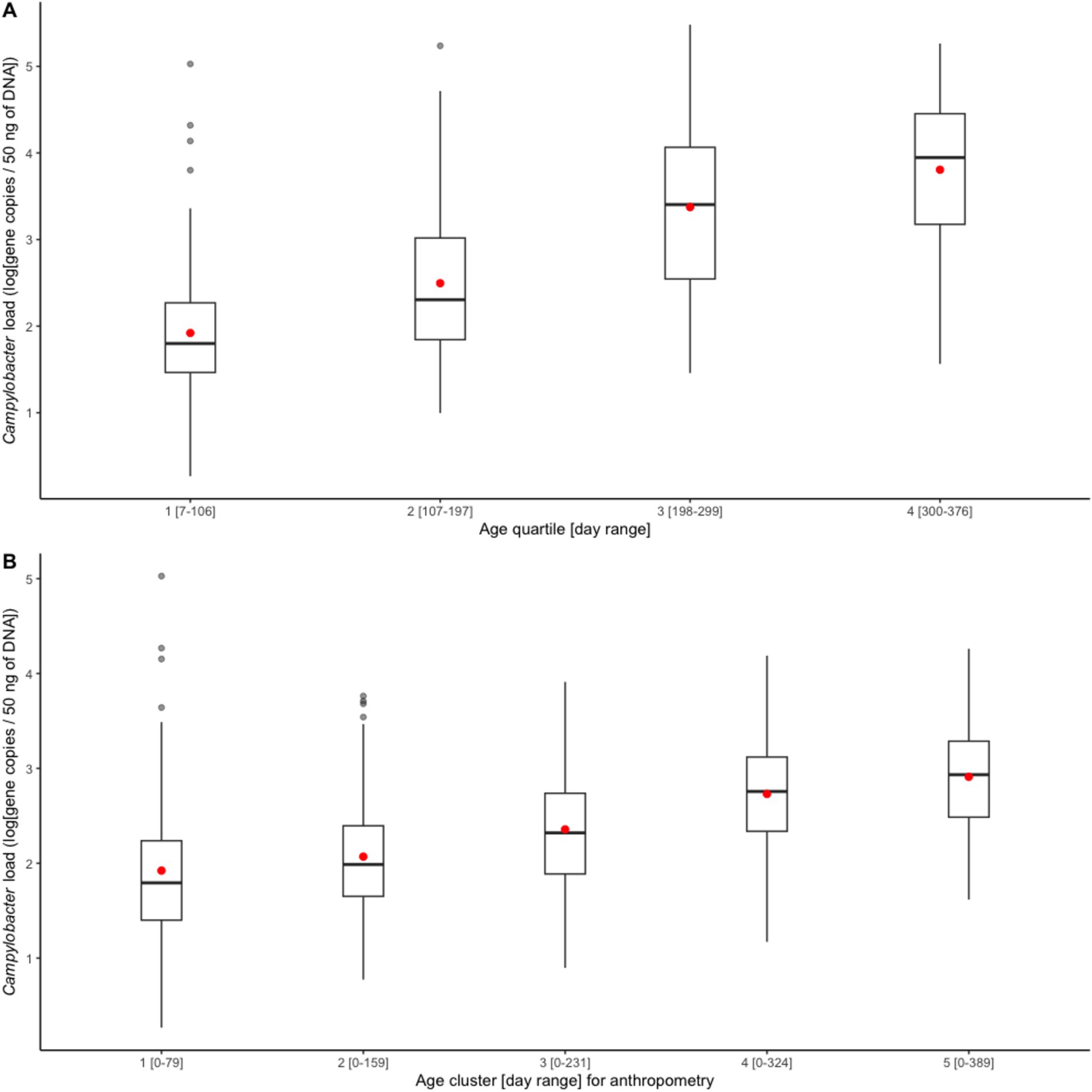
Figure 1. Boxplots of average Campylobacter load (A) by age quartile and (B) from birth to each length-for-age cluster. A red dot indicates the mean of each set.
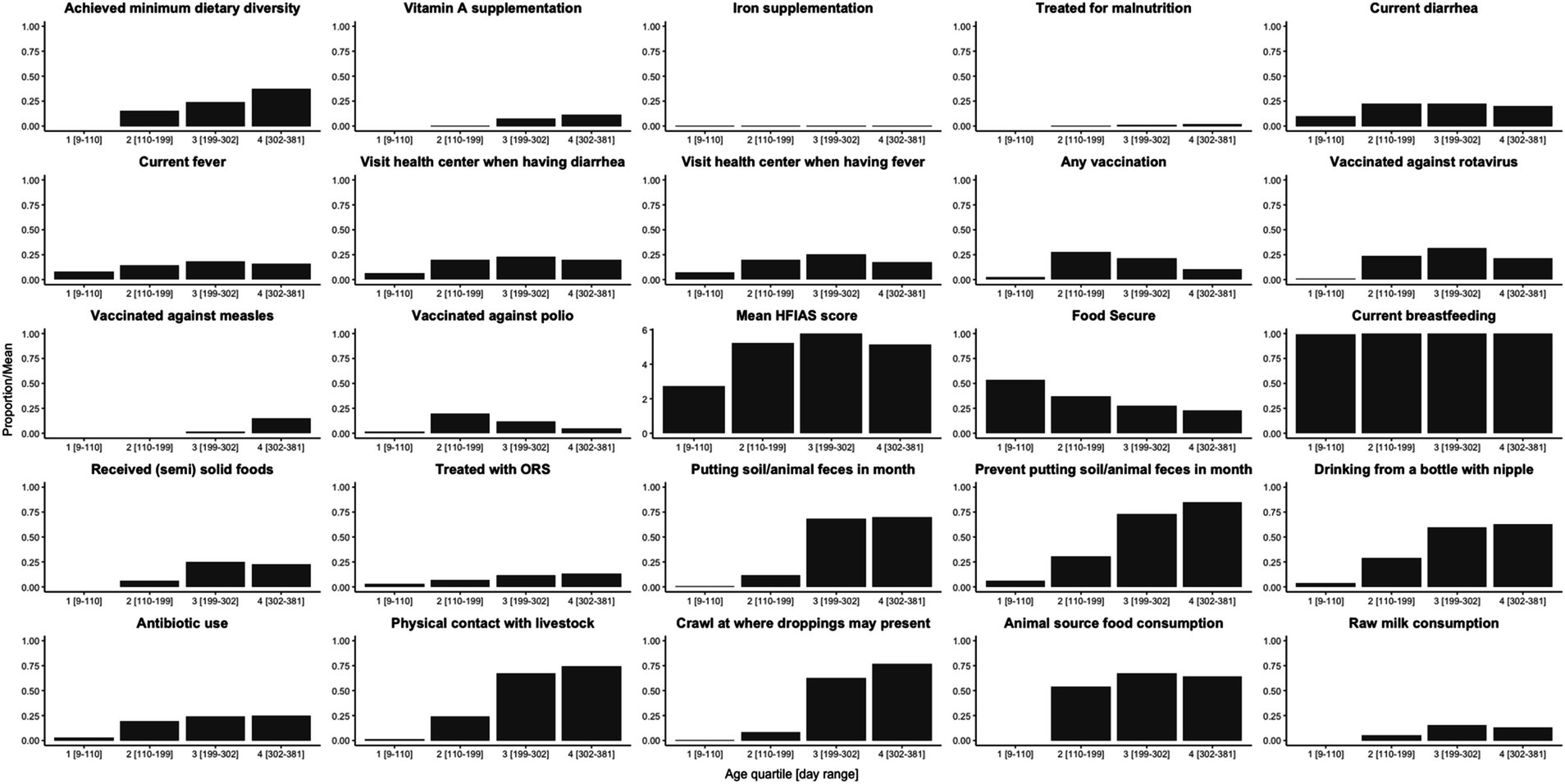
The mean LAZ decreased from −0.45 (SD = 1.08) in age group 1 (mean age = 94 days) to −2.06 (SD = 0.93) in age group 5 (mean age = 428 days), see Figure 2A. Meanwhile, the prevalence of stunting increased steadily from 3% (95% CI: 1–11%) to 51% (95% CI: 40–61%) in the same time frame, see Figure 2B. EED measurements were conducted at an average age of 418 (SD = 35) days, and the prevalence of EED was 56% (95% CI: 46–65%), of which 25% (95% CI: 17–34%) were severe EED. Current diarrhea and fever prevalence rates were higher in the later age quartiles than in the first (Figure 3). Generally, the levels or prevalence of many time-varying determinants increased as the infants aged (Figure 4). However, we observed consistently low proportions of iron supplementation and treatment for malnutrition, a consistently high proportion of breastfeeding, and the highest proportions of vaccination (including polio and rotavirus vaccines) in the second age quartile. The introduction of complementary feeding was reported at a mean age of 187 days (range: 46–319). Based on the HFIAS score, the proportion of the study population that was food secure decreased as infants aged.
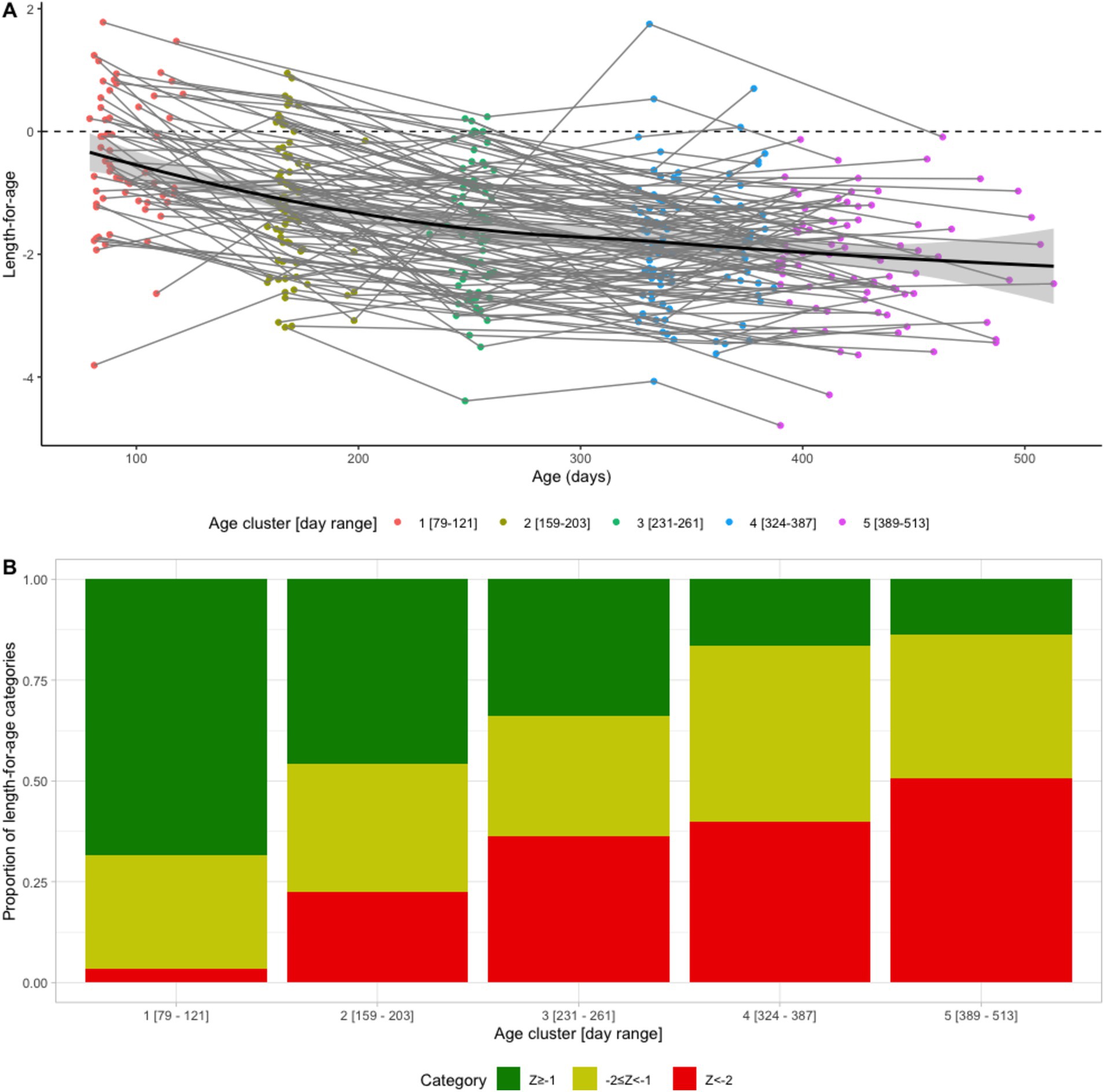
Figure 2. Longitudinal profiles of length-for-age (LAZ) scores (A) LAZ as a function of age for individual infants (thin lines), population average (solid line), and 95% confidence interval (CI) (shaded band). (B) LAZ categories by age cluster. Green: not stunted (LAZ ≥ −1), yellow: at risk of stunting (−2 ≤ LAZ < −1), and red: stunted (LAZ < −2).
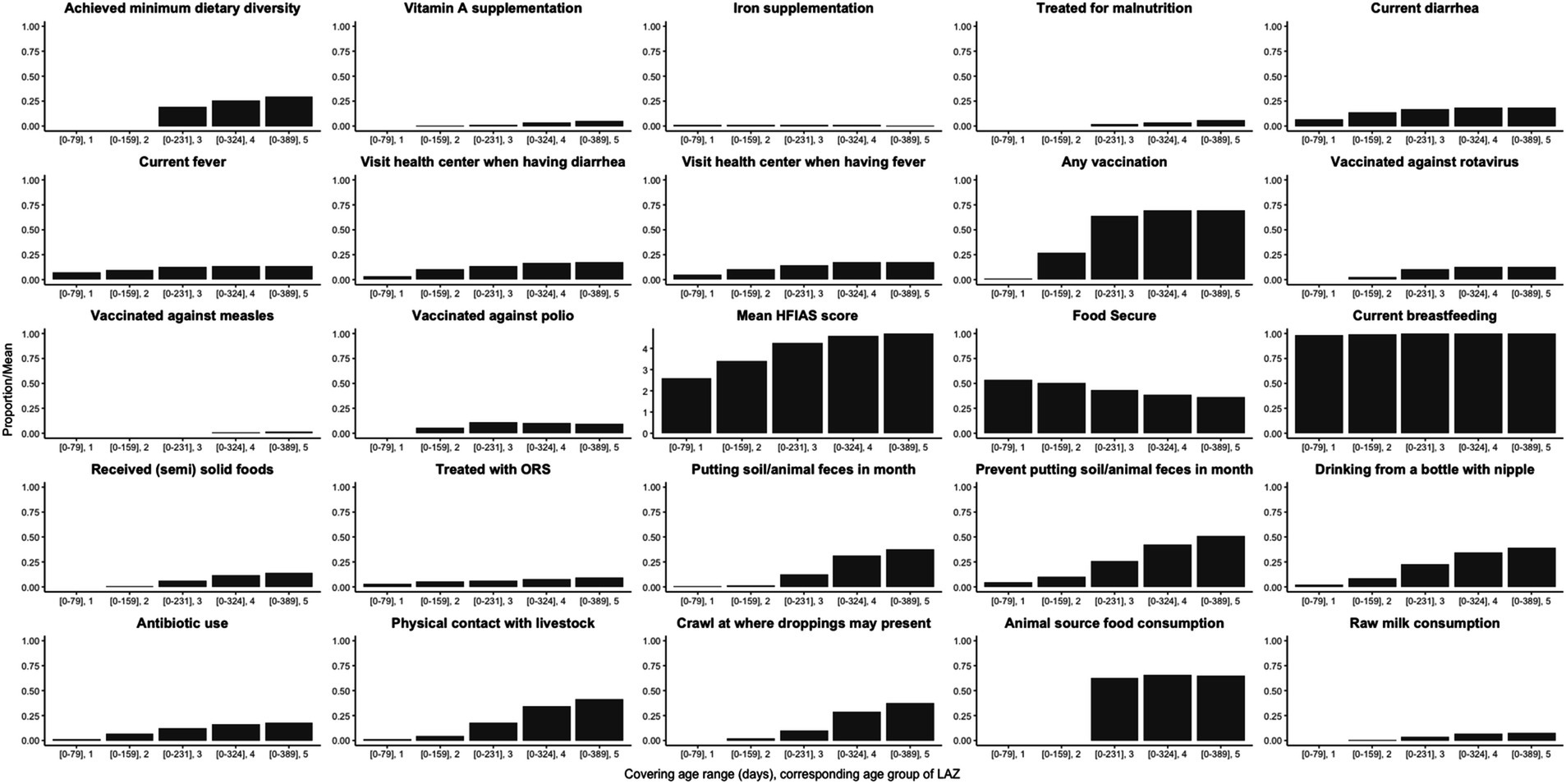
Figure 4. Average proportions/score of putative determinants from birth to each age cluster of visits for length-for-age Z-score (LAZ).
Regression analysis
Campylobacter
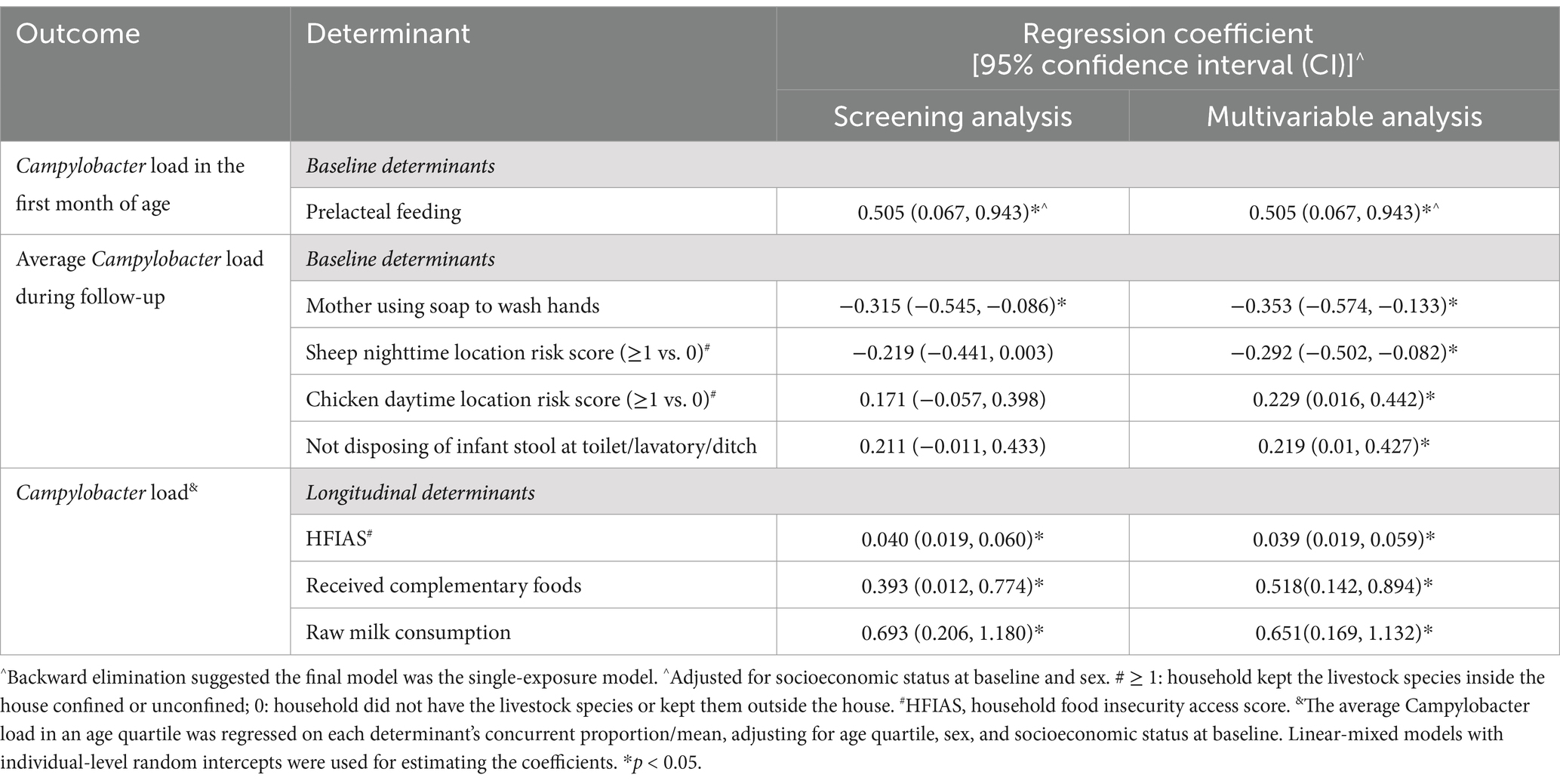
Variable screening results of the individual- and household-level determinants (both with and without adjustment for confounders) are summarized in Supplementary Table S1. Determinants with adjusted p-values <0.2 in the screening analyses were included in multivariable analyses. The final models for determinants of the outcomes of Campylobacter infection at different time scales is presented in Table 2. A lower average Campylobacter load over the follow-up period was significantly associated with mothers washing their hands with soap and a higher sheep nighttime risk score. In contrast, a higher chicken daytime risk score and not disposing of infant stool at a sanitation facility were associated with a higher Campylobacter load. In the first month after birth (day range: 7–39), an increasing Campylobacter load was strongly associated with prelacteal feeding and infant sex did not modify the effect for this association. The longitudinal model over age quartiles found that an increasing Campylobacter load was associated with complementary feeding, consumption of raw milk, and food insecurity.
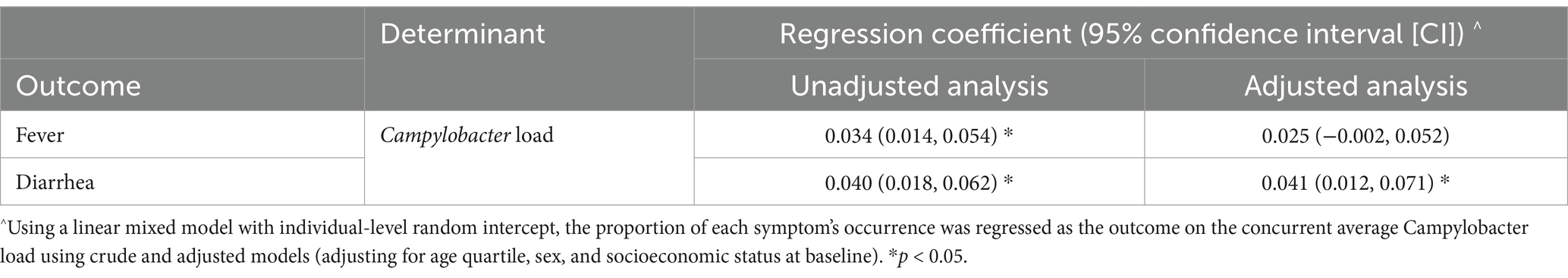
Diarrhea and fever frequency were associated with a higher Campylobacter load, although the latter was not statistically significant after adjustment for confounders (Table 3).
Environmental enteric dysfunction
We found no significant associations between EED or individual indicators of gut function with Campylobacter load (Table 4).
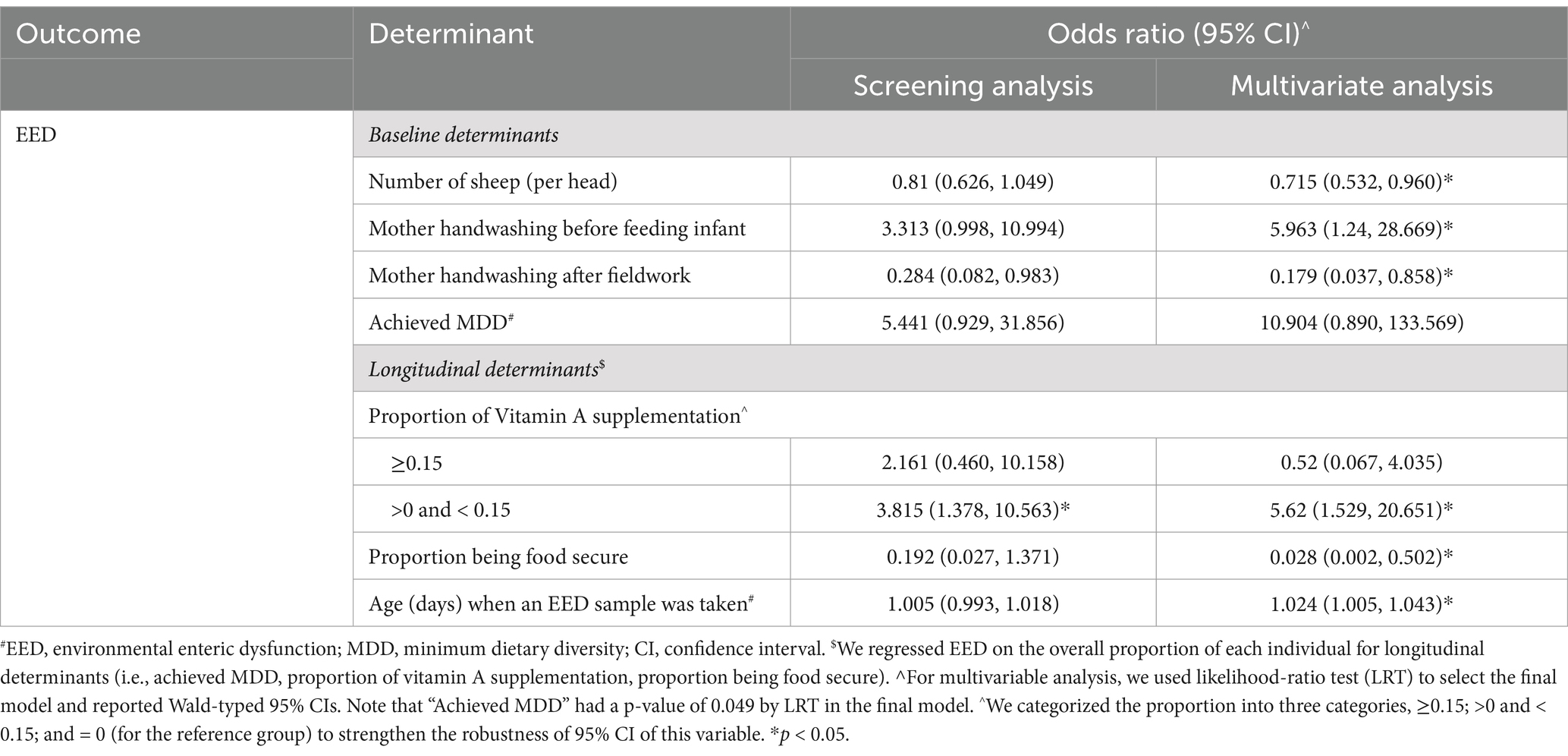
An increased odds of EED was associated with increased infant age at sampling and mother’s handwashing before infant feeding. A lower odds of EED were associated with an increasing number of sheep in the household, the mother washing her hands after the fieldwork, and being food secure (Table 5). A median level (0 < proportion < 0.15) of vitamin A supplementation increased the odds of EED compared to no supplementation at all (proportion = 0).
Length-for-age Z-score
Despite initial findings in a sex- and SES-adjusted linear-mixed model suggesting that the average Campylobacter load had a significant strong effect on increasing growth faltering (regression coefficient: 0.54, p < 0.001), this effect was no longer significant and had a drastic reduction in effect size after adjusting for age (regression coefficient: 0.01, p 0.897; Supplementary Table S1). There were no significant associations between the change in the LAZ score from baseline to endline and indicators of gut function (Table 6).
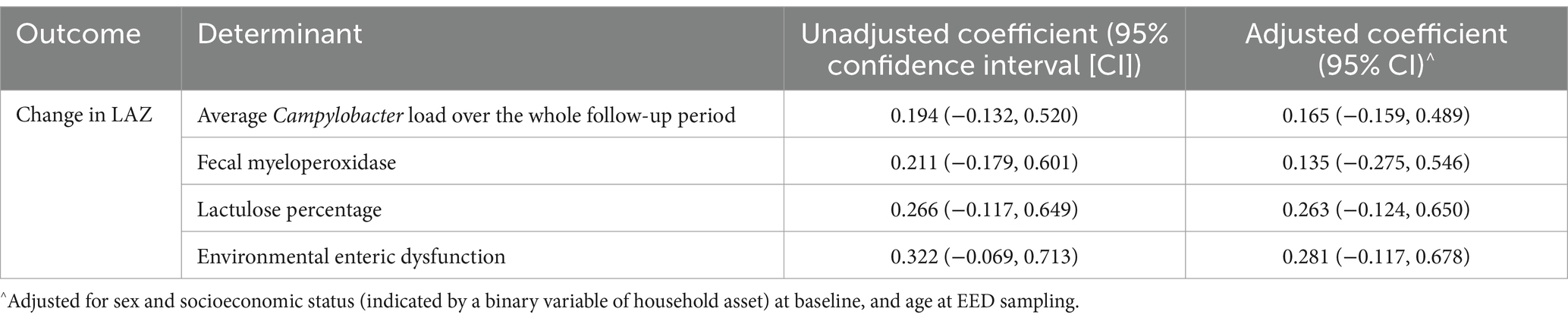
Table 6. Associations between change in LAZ from baseline to endline, Campylobacter load, and gut function indicators.
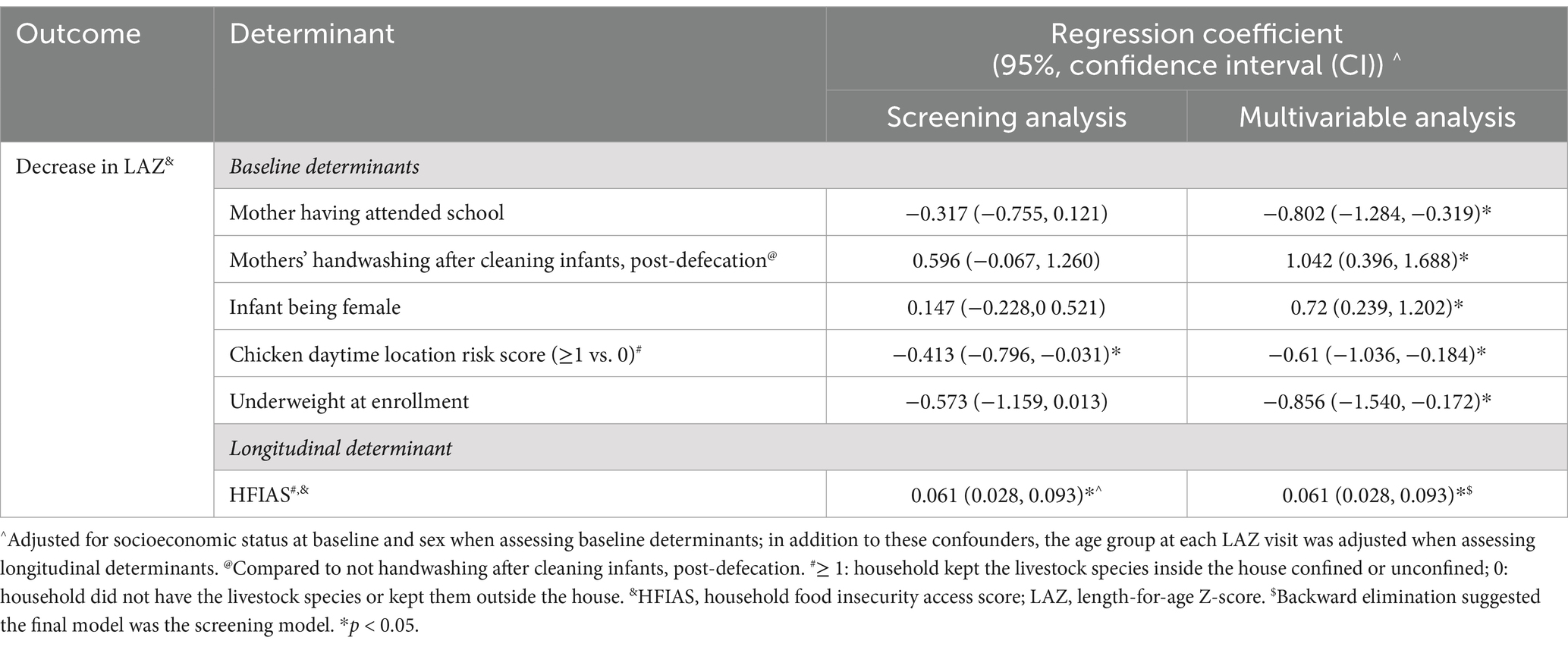
Variable screening results of the individual- and household-level determinants for the change in LAZ from baseline to endline (both with and without adjustment for confounders) are summarized in Supplementary Table S1. Determinants with adjusted p values <0.2 in the screening analyses were included in multivariable analyses. The final model for baseline determinants of LAZ is presented in Table 7. Change in LAZ was calculated as (first available LAZ – last available LAZ). Hence, positive regression coefficients indicate a greater decrease in LAZ and thus are associated with poorer nutrition outcomes. Longitudinally, a greater decrease in LAZ (poorer nutrition outcome) was associated with mothers’ hand washing after cleaning infants’ post-defecation and infants being female. In contrast, mothers having attended school, keeping chickens inside the household during the daytime (i.e., chicken daytime location risk score ≥ 1), and an infant being underweight at enrollment were associated with a smaller decrease in LAZ (better nutrition outcome). Longitudinally, a decrease in LAZ was associated with more household food insecurity (Table 7).
Discussion
In this prospective birth cohort, infants experienced linear growth faltering during the first year of life. More than half of the infants were stunted (LAZ < −2) by endline (day range: 389–513), up from 3% in months 3 and 4 (day range: 79–121). More than half (56%; 95% CI: 46–0.65%) of infants had EED at the end of the longitudinal study, in which 25% (95% CI: 17–34%) had severe EED. More than 30% of infants were colonized with Campylobacter in the first month of life, and the prevalence increased continuously, reaching a plateau of nearly 90% between months 8 and 9 (26). Despite the high occurrence of Campylobacter infection, EED, and stunting in this population, no association was found between Campylobacter load and EED, EED and stunting, or Campylobacter load and stunting.
Several determinants for Campylobacter load, EED, and LAZ were identified. The reported practice of soap use by mothers during handwashing was significantly associated with a lower Campylobacter load and the sheep nighttime location risk score, indicating that increased contact with sheep at night is associated with a lower Campylobacter load. Conversely, a higher chicken daytime location risk score and not disposing of infant stool at a sanitation facility, were both associated with an increased Campylobacter load. Prelacteal feeding contributed to an increase in Campylobacter load in the first month of life. When longitudinal data were analyzed, risk factors associated with increased Campylobacter load included complementary feeding, consumption of raw milk, and food insecurity. Among time-varying determinants, a higher Campylobacter load was associated with more frequent concurrent diarrhea. Determinants for EED and LAZ were also identified. A greater probability of EED was associated with older infant age at the sampling, and mothers reporting handwashing prior to infant feeding. A lower probability of EED was associated with household food security and the mother reporting handwashing after fieldwork. A larger decrease in LAZ of infants, a poorer health outcome, was associated with being female and the mother reporting handwashing after infant cleaning post-defecation. Mother having attended school, the infant being underweight at enrollment (age range: 4–27 days), and keeping chickens inside without confinement during daytime were associated with less decrease of LAZ, a better growth outcome. Among time-varying determinants, only food insecurity was associated with higher risks of linear growth faltering.
Similar to the MAL-ED study (55), we observed a sharp increase in Campylobacter prevalence in the first year of life; however, nearly all infants were colonized with Campylobacter by month 13, which was higher than the prevalence rates observed at that time across MAL-ED sites. Such prevalence increase may be driven by a high force of infection (FOI) of and/or a weakened clearance against Campylobacter (40). The observed difference between studies may also be due to the difference in sensitivity and specificity of assays used for testing Campylobacter. Thus, caution should be taken when attempting to compare pertinent results (e.g., prevalence) between studies. A high FOI is determined by frequent environmental exposures, as observed in our setting. Stunting-associated immunosuppressive effects might weaken clearance capacity. This also implies at least the potential for reverse causality (namely, stunting increases infection) as depicted in the “vicious cycles of diseases of poverty” (41).
Our findings reveal that food insecurity was associated with both higher Campylobacter load and growth faltering which is in line with the role that this determinant plays as an underlying cause in the modified UNICEF framework for undernutrition (9). The significant contribution of raw milk consumption to increasing Campylobacter load is consistent with findings from our formative research, where we found a significant association between animal-sourced foods consumption (primarily consisting of raw milk) and increasing Campylobacter detection in young children (9). Our results indicate that complementary feeding may be a source of enteric infection by Campylobacter. The risk of complementary feeding may be reduced by optimizing or improving existing handwashing behaviors (such as the mother’s handwashing with soap), as handwashing (particularly with soap) has been associated with the reduction of microbial contamination in complementary foods (42).
Being in proximity to sheep at night was found to be associated with lower Campylobacter load, whereas proximity to chicken throughout the day was found to be associated with higher Campylobacter load. These contradictory results for risks associated with animals suggest the relationships between animal ownership and Campylobacter colonization are highly complex and may be confounded by the lack of adjustment for Campylobacter species-level infection. We are undertaking an MLST-based attribution study that aims to tease out the relationships between livestock and Campylobacter infection at the species level.
The practice of prelacteal feeding is common in LMICs despite its potential risks (43). A 2018 meta-analysis suggested the prevalence of this practice in Ethiopia was around 25% (44). A more recent study of children under 2 years conducted near our study area suggested that nearly half of the population received a prelacteal feeding (a cultural practice in the region), which was associated with a lack of knowledge about the risks related to the practice (45). In comparison, the prevalence of prelacteal feeding was even higher in our cohort (67%). Given that the practice is the sole significant feeding practice associated with an increased Campylobacter load in the first month of life, and this was independent of sex, future research may be needed to better understand what drives the practice, the cultural meanings assigned to the practice, and possible interventions to strengthen mothers’ knowledge of its risks.
Comparing the common putative determinants in this study with our cross-sectional study (24), most of them were qualitatively similar. However, overall, we observed fewer livestock holdings in the longitudinal study, which may be related to the timing of our studies. The cross-sectional work was conducted in 2018, while the data presented here were collected after the onsets of both the COVID-19 pandemic and the civil war in Tigray, Ethiopia. Both disruptions and their consequential instability were likely to have caused financial distress, which may have triggered the selling of livestock for income.
The prevalence of child EED in our study population (56%) was similar to findings from the formative research (50, 95% CI: 40–60%), severe EED was higher (25%) but not significantly different from the formative research (17, 95% CI: 11–26%) (24). The lower odds of EED found to be associated with food security and mothers’ handwashing after fieldwork both align and are consistent with broader understandings of EED and its relationship to hygiene and sanitation and other socioeconomic factors (12). The increased odds of EED being associated with mother’s handwashing before feeding the infant and supplementation of vitamin A are additional counterintuitive findings that merit exploration but could also reflect residual confounding.
Despite having a lower prevalence of stunting at the first visit around 3–4 months old, the trend of LAZ in the study population is similar to that which was observed in Haydom, Tanzania (TZH) in the MAL-ED study (46), which was also rural and comprised of smallholder households who relied heavily on corn production as a cash crop (47). Our study population engaged in khat production, another cash crop, as their primary livelihood, the implications of which have been explored elsewhere, with no significant impact on infant nutrition having emerged (48, 49). However, the comparability of the LAZ trends between our site and TZH may reflect a typical trajectory of infant nutrition in rural smallholder households where cash crop agriculture dominates (50).
Previously published literature using enrollment weight (collected ≤17 days of birth) as a surrogate measure for birth weight, found lower weight-for-age at this time increased the odds of being stunted (46). In contrast, being underweight within 27 days of birth in our cohort was associated with less growth faltering. This finding might suggest more attentive care or feeding practices of infants with visible clinical signs of being underweight, resulting in some catch-up growth. Contrary to much literature that finds being male to be a risk factor for stunting (51), female infants had more growth faltering in our setting. Existing literature indicates that any effect of biological sex on linear growth may be modified by its complex interactions with social factors (51). Our study was conducted in a traditional, rural population of Sunni Muslims, where gender norms in society may be favorable for males (52), including infants. Given increasing evidence surrounding the importance of gender inequality as a predictor of child stunting, cultural norms may explain the increased risk of growth faltering among girls (53). A similar finding was observed in Bangladesh, a Muslim-majority country, where female children had lower LAZ (54). The associations between linear growth faltering and the determinants of chicken daytime risk score and the mother reporting handwashing following cleaning an infant after defecation, in opposite directions, are counterintuitive. The following hypothesized scenarios could explain these counterintuitive associations: First, more chickens might indicate higher access to a protein source or more female-controlled revenue, which might outweigh the risk for infant growth. There is increasing evidence that in this study setting women’s empowerment is strongly associated with infant growth outcomes (48, 49). Second, mothers who report handwashing after cleaning infants post-defecation may be more likely to clean their own infants as opposed to having an older child or another person in the house clean the infant; or mother’s hands could be re-contaminated in between the handwashing after cleaning the infant and other infant care practices, thereby exposing the infant to Campylobacter and increased growth faltering. Importantly, a large body of evidence supports the understanding that infant growth, and stunting in particular, is multicausal. Given the small sample size of this analysis, complex models with numerous interacting determinants are not possible. Thus, some of these associations may be spurious. Scenarios like those presented above could not be tested in this study through available instruments. However, additional investigations using parallel observational data collected from structured behavioral observations from the same population (see forthcoming literature from the Exposure Assessment of Campylobacter Infections in Rural Ethiopia [EXCAM] study https://doi.org/10.1101/2024.08.21.24312392) aim to better understand some of the counterintuitive findings.
Built on the formative research, the data from this longitudinal study offers a more thorough understanding of the sociodemographic and exposure landscapes in the Haramaya woreda and similar smallholder settings. The repeated-measures short survey enabled our research team to study the health outcomes at different time scales and allowed a more accurate characterization of the timings related to Infant and Young Child Feeding (IYCF).
This study has limitations. This study was powered for prevalence estimation of the Campylobacter genus (25), limiting our ability to evaluate statistical significance in the regression analyses such as the key association between Campylobacter infection and growth faltering. This was further constrained by an increased level of missing data due at least in part to our fieldwork being disrupted by the global pandemic and civil unrest (25). Despite the adjustment for common confounders (sex, age, and SES) in the regression analyses, as previously mentioned, potential residual confounding, a common problem in survey-based observational studies, could bias the associations of interest. In addition, common survey biases might exist in the survey data. For example, recall bias could exist in the monthly complementary feeding status; and responses to questions about soap use, in our setting where soap use was rare, could be subject to social desirability bias. With a relatively small study population size, the screening of a large set of variables in regression analyses may be subject to type I errors from multiple comparisons. Since the definition of EED was solely based on %L and MPO, it might not suggest the impairments of other gut functions (e.g., translocation, intestinal barrier function).
In summary, this study uncovered an increasing Campylobacter load in the first year of life, a high prevalence of EED by 12–13 months of age, and a concurrent increasing level of growth faltering, none of which were significantly associated with the other, but all three of which were associated with food insecurity. Despite a growing consideration of exposure to livestock feces in both this research and current interventions to improve child growth (22), our findings around livestock risk factors were inconclusive. We found that even though livestock may be the reservoir of Campylobacter infections in infants, these relationships are highly complex and most determinants of the infection in this study were more proximal to the infant, i.e., related to improper IYCF practices and WaSH in our setting. This signifies a need to strengthen both IYCF practices and infant food safety and hygiene to reduce (cross-) contamination at the point of exposure and, ultimately, reduce the risk of infection and improve linear growth. Future research should aim to replicate findings in diverse populations to assess the generalizability of results and identify context-specific factors influencing Campylobacter transmission and health outcomes.
CAGED Research Team
This study is a result of the CAGED Research Team. Beyond those named authors, other CAGED Research Team members include Cyrus Saleem, Efrah Ali Yusuf, Getnet Yimer, Kunuza Adem Umer, Karah Mechlowitz, Mawardi M. Dawid, Mahammad Mahammad Usmail, Wondwossen A. Gebreyes, Yenenesh Demisie Weldesenbet, Zelalem Hailu Mekuria.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the University of Florida Internal Review Board (IRB201903141); the Haramaya University Institutional Health Research Ethics Committee (COHMS/1010/3796/20) and the Ethiopia National Research Ethics Review Committee (SM/14.1/1059/20). Written informed consent was obtained from all participating households (husband and wife), with mothers consenting to infant participation, using forms in the local language (Afan Oromo). The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
DC: Data curation, Formal analysis, Methodology, Project administration, Resources, Writing – original draft. SM: Conceptualization, Funding acquisition, Supervision, Validation, Writing – original draft. YY: Formal analysis, Methodology, Supervision, Validation, Writing – original draft. IU: Writing – review & editing, Investigation, Project administration. IA: Data curation, Project administration, Writing – review & editing. JA: Investigation, Writing – review & editing. AI: Investigation, Resources, Writing – review & editing. AS: Investigation, Writing – review & editing. NuS: Investigation, Writing – review & editing. AO: Conceptualization, Data curation, Project administration, Resources, Writing – review & editing. BH: Conceptualization, Data curation, Writing – review & editing. LD: Conceptualization, Data curation, Project administration, Resources, Writing – review & editing. BA: Investigation, Writing – review & editing. KH: Investigation, Writing – review & editing. MB: Investigation, Writing – review & editing. XL: Formal analysis, Writing – review & editing. NiS: Data curation, Writing – review & editing. KR: Investigation, Writing – review & editing. NF: Methodology, Writing – review & editing. GR: Methodology, Writing – review & editing. MM: Conceptualization, Methodology, Resources, Writing – review & editing. JH: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review & editing. AH: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project is funded by the United States Agency for International Development Bureau for Food Security under Agreement #AID-OAA-L-15-00003 as part of Feed the Future Innovation Lab for Livestock Systems, and by the Bill & Melinda Gates Foundation OPP#1175487. Any opinions, findings, conclusions, or recommendations expressed here are those of the authors alone.
Acknowledgments
This study would not have been possible without the cooperation of study communities and local administration of the study kebeles. We would like to thank the study participants, their households, the Community Advisory Board, and all who supported the study directly or otherwise. We thank Sridevi Devaraj PhD at Texas Children’s Hospital and Baylor College of Medicine, Houston, TX for analysis of lactulose in urine. Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which was partly supported by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427. The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1467462/full#supplementary-material
Footnotes
1. ^http://www.sciencedirect.com/science/article/pii/S1532046408001226
2. ^https://www.sciencedirect.com/science/article/pii/S1532046419301261
References
1. United Nations Children’s Fund, World Health Organization, International bank for reconstruction and development/the World Bank. Levels and trends in child malnutrition: UNICEF/WHO/World Bank Group joint child malnutrition estimates: Key findings of the 2023. New York: UNICEF and WHO (2023). Available at: https://www.who.int/publications-detail-redirect/9789240073791.
2. Olofin, I, McDonald, CM, Ezzati, M, Flaxman, S, Black, RE, Fawzi, WW, et al. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One. (2013) 8:e64636. doi: 10.1371/journal.pone.0064636
3. MAL-ED Network Investigators. Early childhood cognitive development is affected by interactions among illness, diet, enteropathogens and the home environment: findings from the MAL-ED birth cohort study. BMJ Glob Health. (2018) 3:e000752. doi: 10.1136/bmjgh-2018-000752
4. Alderman, H. The economic cost of a poor start to life. J Dev Orig Health Dis. (2010) 1:19–25. doi: 10.1017/S2040174409990158
5. Herthford, A, and Harris, J. Improving nutrition through agriculture. Understanding and applying primary pathways and principles. Arlington, VA: USAID/Strengthening Partnerships, Results, and Innovations in Nutrition Globally (SPRING) Project, p. 16. (2014). Available at: https://www.spring-nutrition.org/sites/default/files/publications/briefs/spring_understandingpathways_brief_1_0.pdf.
6. Headey, D, Hirvonen, K, and Hoddinott, J. Animal sourced foods and child stunting. Am J Agric Econ. (2018) 100:1302–19. doi: 10.1093/ajae/aay053
7. Enahoro, DK, Tran, N, Chan, CY, Komarek, AM, and Rich, KM. The future of animal-source food demand and supply in Africa. SocArXiv. (2021). Available at: https://cgspace.cgiar.org/handle/10568/116954. (Accessed Nov 29, 2023).
8. Management Entity. Ethiopia’s livestock systems: Overview and areas of inquiry. Gainesville. FL, USA: Feed the Future Innovation Lab for Livestock Systems. (2021). Available at: https://livestocklab.ifas.ufl.edu/media/livestocklabifasufledu/pdf-/LSIL_Livestock_Systems_Overview_Ethiopia_2021_08.pdf.
9. Chen, D, Mechlowitz, K, Li, X, Havelaar, AH, and SL, MK. Benefits and risks of smallholder livestock production on child nutrition in low- and middle-income countries. Front Nutr. (2021) 8:836. doi: 10.3389/fnut.2021.751686
10. Rogawski, ET, Liu, J, Platts-Mills, JA, Kabir, F, Lertsethtakarn, P, Siguas, M, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health. (2018) 6:e1319–28. doi: 10.1016/S2214-109X(18)30351-6
11. Weisz, A, Meuli, G, Thakwalakwa, C, Trehan, I, Maleta, K, and Manary, M. The duration of diarrhea and fever is associated with growth faltering in rural Malawian children aged 6-18 months. Nutr J. (2011) 10:25. doi: 10.1186/1475-2891-10-25
12. Budge, S, Parker, AH, Hutchings, PT, and Garbutt, C. Environmental enteric dysfunction and child stunting. Nutr Rev. (2019) 77:240–53. doi: 10.1093/nutrit/nuy068
13. Owino, V, Ahmed, T, Freemark, M, Kelly, P, Loy, A, Manary, M, et al. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics. (2016) 138:e20160641. doi: 10.1542/peds.2016-0641
14. De, MMB, and Da, SGAP. Environmental enteric dysfunction and growth. J Pediatr. (2019) 95:85–94. doi: 10.1016/j.jped.2018.11.004
15. Harper, KM, Mutasa, M, Prendergast, AJ, Humphrey, J, and Manges, AR. Environmental enteric dysfunction pathways and child stunting: a systematic review. PLoS Negl Trop Dis. (2018) 12:e0006205. doi: 10.1371/journal.pntd.0006205
16. Hodges, P, Tembo, M, and Kelly, P. Intestinal biopsies for the evaluation of environmental enteropathy and environmental enteric dysfunction. J Infect Dis. (2021) 224:S856–63. doi: 10.1093/infdis/jiab372
17. Rogawski, ET, and Guerrant, RL. The burden of enteropathy and “subclinical” infections. Pediatr Clin N Am. (2017) 64:815–36. doi: 10.1016/j.pcl.2017.03.003
18. Mutasa, K, Ntozini, R, Mbuya, MN, Rukobo, S, Govha, M, Majo, FD, et al. Biomarkers of environmental enteric dysfunction are not consistently associated with linear growth velocity in rural Zimbabwean infants. Am J Clin Nutr. (2021) 113:1185–98. doi: 10.1093/ajcn/nqaa416
19. Vonaesch, P, Winkel, M, Kapel, N, Nestoret, A, Barbot-Trystram, L, Pontoizeau, C, et al. Putative biomarkers of environmental enteric disease fail to correlate in a cross-sectional study in two study sites in sub-Saharan Africa. Nutrients. (2022) 14:3312. doi: 10.3390/nu14163312
20. Pickering, AJ, Null, C, Winch, PJ, Mangwadu, G, Arnold, BF, Prendergast, AJ, et al. The WASH benefits and SHINE trials: interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Glob Health. (2019) 7:e1139–46. doi: 10.1016/S2214-109X(19)30268-2
21. Prendergast, AJ, Gharpure, R, Mor, S, Viney, M, Dube, K, Lello, J, et al. Putting the “a” into WaSH: a call for integrated management of water, animals, sanitation, and hygiene. Lancet Planetary Health. (2019) 3:e336–7. doi: 10.1016/S2542-5196(19)30129-9
22. Gharpure, R, Mor, SM, Viney, M, Hodobo, T, Lello, J, Siwila, J, et al. A one health approach to child stunting: evidence and research agenda. Am J Trop Med Hyg. (2021) 104:1620–4. doi: 10.4269/ajtmh.20-1129
23. Terefe, Y, Deblais, L, Ghanem, M, Helmy, YA, Mummed, B, Chen, D, et al. Co-occurrence of Campylobacter species in children from eastern Ethiopia, and their association with environmental enteric dysfunction, diarrhea, and host microbiome. Front Public Health. (2020) 8:99. doi: 10.3389/fpubh.2020.00099
24. Chen, D, Mckune, SL, Singh, N, Hassen, JY, Gebreyes, W, Manary, M, et al. Campylobacter colonization, environmental enteric dysfunction, stunting, and associated risk factors among young children in rural Ethiopia: a cross-sectional study from the Campylobacter genomics and environmental enteric dysfunction (CAGED) project. Front Public Health. (2020) 8:1043. doi: 10.3389/fpubh.2020.615793
25. Havelaar, AH, Brhane, M, Ahmed, IA, Kedir, J, Chen, D, Deblais, L, et al. Unravelling the reservoirs for colonisation of infants with Campylobacter spp. in rural Ethiopia: protocol for a longitudinal study during a global pandemic and political tensions. BMJ Open. (2022) 12:e061311. doi: 10.1136/bmjopen-2022-061311
26. Deblais, L, Ojeda, A, Brhane, M, Mummed, B, Hassen, KA, Ahmedo, BU, et al. Prevalence and load of the Campylobacter genus in infants and associated household contacts in rural eastern Ethiopia: a longitudinal study from the Campylobacter genomics and environmental enteric dysfunction (CAGED) project. Appl Environ Microbiol. (2023) 89:e00424–3. doi: 10.1128/aem.00424-23
27. Johnson, TS, and Engstrom, JL. State of the science in measurement of infant size at birth. Newborn Infant Nurs Rev. (2002) 2:150–8. doi: 10.1053/nbin.2002.35122
28. Bian, X, Garber, JM, Cooper, KK, Huynh, S, Jones, J, Mills, MK, et al. Campylobacter abundance in breastfed infants and identification of a new species in the global Enterics multicenter study. mSphere. (2020) 5:e00735–19. doi: 10.1128/mSphere.00735-19
29. Platts-Mills, JA, Liu, J, Gratz, J, Mduma, E, Amour, C, Swai, N, et al. Detection of Campylobacter in stool and determination of significance by culture, enzyme immunoassay, and PCR in developing countries. J Clin Microbiol. (2014) 52:1074–80. doi: 10.1128/JCM.02935-13
30. Data4Diets. INDDEX Project. (2023). Available at: https://inddex.nutrition.tufts.edu/data4diets (Accessed Nov 29, 2023).
31. Coates, J, Swindale, A, and Bilinsky, P. Household food insecurity access scale (HFIAS) for measurement of food access: Indicator guide: Version 3: (576842013–001). (2007).
32. World Health Organization and the United Nations Children’s Fund. WASH in the 2030 agenda - new global indicators for drinking water, sanitation and hygiene. Geneva, Switzeralnd: World Health Organization (2017).
33. Jahnke, HE. Livestock production systems and livestock development in tropical Africa. Kielr Wissenschaftsverlag Vauk, pp. 188–190. (1982) Available at: https://linkinghub.elsevier.com/retrieve/pii/0308521X8390080X.
34. Schumacher, D. Computation of the WHO child growth standards [R package anthro version 1.0.1]. Comprehensive R archive network (CRAN (2023). Available at: https://CRAN.R-project.org/package=anthro (Accessed Nov 29, 2023).
35. Vandemoortele, M. Measuring household wealth with latent trait modelling: an application to Malawian DHS data. Soc Indic Res. (2014) 118:877–91. doi: 10.1007/s11205-013-0447-z
36. Rizopoulos, D. Ltm: an R package for latent variable modeling and item response analysis. J Stat Softw. (2007) 17:1–25. doi: 10.18637/jss.v017.i05
37. World Health Organization. United Nations Children’s fund (UNICEF). Indicators for assessing infant and young child feeding practices: Definitions and measurement methods. Geneva: World Health Organization (2021). Available at: https://www.who.int/publications-detail-redirect/9789240018389.
38. Bates, D, Mächler, M, Bolker, B, and Walker, S. Fitting linear mixed-effects models using lme4. J Stat Softw. (2015) 67:1–48. doi: 10.18637/jss.v067.i01
39. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2023).
40. Chen, D, Havelaar, AH, Platts-Mills, JA, and Yang, Y. Acquisition and clearance dynamics of Campylobacter spp. in children in low- and middle-income countries. Epidemics. (2024) 46:100749. doi: 10.1016/j.epidem.2024.100749
41. Guerrant, RL, DeBoer, MD, Moore, SR, Scharf, RJ, and Lima, AAM. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. (2013) 10:220–9. doi: 10.1038/nrgastro.2012.239
42. Müller-Hauser, AA, Sobhan, S, Huda, TMN, Waid, JL, Wendt, AS, Islam, MA, et al. Key food hygiene behaviors to reduce microbial contamination of complementary foods in rural Bangladesh. Am J Trop Med Hyg. (2022) 107:709–19. doi: 10.4269/ajtmh.21-0269
43. Oakley, L, Benova, L, Macleod, D, Lynch, CA, and Campbell, OMR. Early breastfeeding practices: descriptive analysis of recent demographic and health surveys. Matern Child Nutr. (2018) 14:e12535. doi: 10.1111/mcn.12535
44. Temesgen, H, Negesse, A, Woyraw, W, Getaneh, T, and Yigizaw, M. Prelacteal feeding and associated factors in Ethiopia: systematic review and meta-analysis. Int Breastfeed J. (2018) 13:49. doi: 10.1186/s13006-018-0193-6
45. Adem, A, Assefa, N, Deresa, M, Yuya, M, Ayana, GM, Negash, B, et al. Prelacteal feeding practices and its associated factors among mother of children less than 2 years of age in Kersa District, eastern Ethiopia. Glob Pediatr Health. (2021) 8:2333794X211018321. doi: 10.1177/2333794X211018321
46. MAL-ED Network Investigators. Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: the MAL-ED longitudinal birth cohort study. PLoS Med. (2017) 14:2408. doi: 10.1371/journal.pmed.1002408
47. Mduma, ER, Gratz, J, Patil, C, Matson, K, Dakay, M, Liu, S, et al. The etiology, risk factors, and interactions of enteric infections and malnutrition and the consequences for child health and development study (MAL-ED): description of the Tanzanian site. Clin Infect Dis. (2014) 59:S325–30. doi: 10.1093/cid/ciu439
48. Mechlowitz, K, Singh, N, Li, X, Chen, D, Yang, Y, Rabil, A, et al. Women’s empowerment and child nutrition in a context of shifting livelihoods in eastern Oromia. Ethiopia Front Nutr. (2023) 10:1048532. doi: 10.3389/fnut.2023.1048532
49. Mechlowitz, K, Singh, N, Li, X, Chen, D, Yang, Y, Ahmed, IA, et al. Examining the role of women’s engagement in khat production on child nutritional outcomes using longitudinal data in East Oromia, Ethiopia [internet]. medRxiv. (2024) 2024:5073. doi: 10.1101/2024.04.05.24305073v1
50. Herthford, A, and Harris, J. Improving nutrition through agriculture. Understanding and applying primary pathways and principles. Brief# 1. Arlington, VA: USAID/Strengthening Partnerships, Results, and Innovations in Nutrition Globally (SPRING) Project (2014).
51. Thurstans, S, Opondo, C, Seal, A, Wells, JC, Khara, T, Dolan, C, et al. Understanding sex differences in childhood undernutrition: a narrative review. Nutrients. (2022) 14:948. doi: 10.3390/nu14050948
52. Gouda, M, and Potrafke, N. Gender equality in Muslim-majority countries. Econ Syst. (2016) 40:683–98. doi: 10.1016/j.ecosys.2016.04.002
53. Marphatia, AA, Cole, TJ, Grijalva-Eternod, C, and Wells, JCK. Associations of gender inequality with child malnutrition and mortality across 96 countries. Glob Health Epidemiol Genom. (2016) 1:1–8. doi: 10.1017/gheg.2016.1
54. Abdulla, F, Rahman, A, and Hossain, MM. Prevalence and risk predictors of childhood stunting in Bangladesh. PLoS One. (2023) 18:e0279901. doi: 10.1371/journal.pone.0279901
Keywords: Campylobacter , environmental enteric dysfunction, undernutrition, longitudinal study, prospective birth cohort, Ethiopia, smallholder livestock farming
Citation: Chen D, McKune SL, Yang Y, Usmane IA, Ahmed IA, Amin JK, Ibrahim AM, Seran AJ, Shaik N, Ojeda A, Hassen BM, Deblais L, Ahmedo BU, Hassen KA, Bhrane M, Li X, Singh N, Roba KT, French NP, Rajashekara G, Manary MJ, Hassen JY and Havelaar AH on behalf of the CAGED Research Team (2025) Campylobacter colonization and undernutrition in infants in rural eastern Ethiopia — a longitudinal community-based birth cohort study. Front. Public Health. 12:1467462. doi: 10.3389/fpubh.2024.1467462
Edited by:
Andreas Erich Zautner, University Hospital Magdeburg, GermanyReviewed by:
Hagen Frickmann, Bundeswehr Hospital Hamburg, GermanyNduhiu Gitahi, University of Nairobi, Kenya
Carrie Cowardin, University of Virginia, United States
Copyright © 2025 Chen, McKune, Yang, Usmane, Ahmed, Amin, Ibrahim, Seran, Shaik, Ojeda, Hassen, Deblais, Ahmedo, Hassen, Bhrane, Li, Singh, Roba, French, Rajashekara, Manary, Hassen, Havelaar and on behalf of the CAGED Research Team. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Lindley McKune, c21ja3VuZUB1ZmwuZWR1
†These authors have contributed equally to this work and share first authorship
 Dehao Chen
Dehao Chen Sarah Lindley McKune
Sarah Lindley McKune Yang Yang4†
Yang Yang4† Ibsa Aliyi Usmane
Ibsa Aliyi Usmane Ibsa Abdusemed Ahmed
Ibsa Abdusemed Ahmed Jafer Kedir Amin
Jafer Kedir Amin Abdulmuen Mohammed Ibrahim
Abdulmuen Mohammed Ibrahim Nurmohammad Shaik
Nurmohammad Shaik Bahar Mummed Hassen
Bahar Mummed Hassen Kedir Abdi Hassen
Kedir Abdi Hassen Mussie Bhrane
Mussie Bhrane Xiaolong Li
Xiaolong Li Nitya Singh
Nitya Singh Kedir Teji Roba
Kedir Teji Roba Nigel P. French
Nigel P. French Gireesh Rajashekara
Gireesh Rajashekara Mark J. Manary
Mark J. Manary Jemal Yusuf Hassen
Jemal Yusuf Hassen Arie Hendrik Havelaar
Arie Hendrik Havelaar on behalf of the CAGED Research Team14
on behalf of the CAGED Research Team14