
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Public Health , 07 January 2025
Sec. Public Health Education and Promotion
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1455623
This article is part of the Research Topic Exploring the Interaction between Health-promoting and Health Risk Behaviors in Health, Volume II View all 23 articles
Objective: This study aimed to update baseline data on monkeypox (mpox)-related knowledge and vaccination willingness among human immunodeficiency virus (HIV) diagnosed and suspected males.
Methods: The cross-sectional survey was conducted in Changsha, a provincial capital in China, during 5 JULY to 5 SEPTEMBER 2023. Among the three study groups, the participants in the “previously diagnosed” group were recruited from a cohort of HIV-infected patients. The “newly diagnosed” and the “suspected” groups were recruited from the outpatients and grouped according to their confirmatory test results. The the exploratory factor analysis was firstly applied to capture the latent structure of participants’ response to the questionnaire about monkeypox. The component and factor scores were compared between groups using the Kruskal-Wallis H tests. The chi-square test was then used to assess the difference of mpox vaccination willingness between MSM and non-MSM in each group. Finally, multivariate logistic regression analysis was performed to identify the determinants of vaccination willingness.
Results: A total of 481 males were included in the final analysis. The results revealed that there was a gap in knowledge about monkeypox between the three participant groups. The vaccination willingness rate of HIV-infected participants was above 90%, while the rate in the HIV-suspected group was 72.60%. Multivariate logistic regression analysis revealed that the previously diagnosed group (adjusted odds ratio [aOR] = 0.314, 95% confidence interval [CI]: 0.105–0.940) and the suspected group (aOR = 0.111, 95% CI: 0.034–0.363) had a lower level of vaccination willingness and they were referred to the newly diagnosed group. Participants in the age groups ranging 25–34 (aOR = 0.287, 95% CI: 0.086–0.959) and 35–44 (aOR = 0.136, 95% CI: 0.039–0.478) years showed a lower level of vaccination willingness, referred to the 15–24 year age group. A better knowledge about monkeypox was associated with a higher level of vaccination willingness (aOR = 1.701, 95% CI: 1.165–2.483). Additionally, a considerable percentage of heterosexual individuals in each group indicated their acceptance of monkeypox vaccines.
Conclusion: An overall high level of vaccination willingness was observed among HIV-infected and-suspected male individuals with disparities noted among those with different HIV infection status, knowledge levels of monkeypox, and age. Addressing the existing knowledge gap and engaging people with persistent risks—regardless of their sexual orientation—for a timely HIV diagnosis may facilitate vaccine-based mitigation measures against monkeypox.
Monkeypox (mpox) is caused by an orthopoxvirus which is similar to smallpox. The virus is symptomatic in the majority of the cases and is characterized by a vesicular rash (1, 2). Prior to 2022, the documented mpox cases outside of Africa had a history of either traveling to the endemic region or having contact with infected animals (2, 3), with no or very limited subsequent human-to-human transmission. However, an unexpected mpox pandemic occurred in 2022 and then spread worldwide rapidly within a year. During the 2022–2023 mpox pandemic, men under the category ‘men who have sex with men’ (MSM) were disproportionately affected, whereas male-to-male sexual contacts were not seen as a dominant transmission route of mpox prior to 2022 (2).
Worldwide, MSM are also at high risk for human immunodeficiency virus (HIV) infection, with acquisition risk 26 times higher than the general population (4). Literature from multiple countries revealed that approximately 40% of mpox cases tested positive for HIV in the recent wave of mpox pandemic (5), and the proportion that HIV-positive males considered in the reported mpox cases was higher than the prevalence of HIV in MSM, implying that people living with HIV (PLHIV) were overrepresented among mpox cases (6–8). Apart from the overlapping at-risk population and a high prevalence of HIV co-infection, mpox also intersected with HIV in clinic treatment (5). For individuals with uncontrolled HIV viral loads, mpox could present a more severe or chronic illness (5, 9). Furthermore, PLHIV coinfected with mpox are more likely subject to dual stigma and higher levels of stress, which may worsen their mental health and clinical outcomes, and impede them from accessing mpox testing, treatment, and vaccination (10–13).
Unfortunately, the current public reporting on mpox has reinforced the stereotypes of “homosexual infection” and exacerbated information barriers (10, 11, 14). Lessons should be learned from HIV/AIDS which is also labeled as a “gay infection” in the early stages. More recently, the epidemiology of HIV infection has changed, and sexual transmission through heterosexuality has begun to predominate in some parts of China. The latest reports on the 2024 outbreak of mpox have shown a similar sign: a broader demographic was affected via heterosexual intimate or sexual contact (15). Therefore, it is to be emphasized that the risk of contracting mpox is not limited to MSM, and any person with multiple or new sexual partners is also at risk (16). Without efficient prevention and control measures, there is a possibility of a sustained spillover of the mpox epidemic to the general population, affecting vulnerable groups, such as untreated PLHIV, older adults, immunocompromised individuals, young children, and pregnant women (17, 18).
Considering the aforementioned information, PLHIV and key populations at increased risk of HIV/sexually transmitted infections (STIs) should be valued in preventing mpox virus spillovers, and opportunities should be identified to mitigate the unfavorable impacts of the mpox epidemic on their health (19). First and foremost, promoting an unbiased understanding of mpox infection among the stakeholders can reduce panic and motivate them to adopt preventive behaviors during the epidemic (20). More importantly, pre-exposure prophylactic vaccination should be encouraged for eligible populations to curtail the further spread of mpox (21). Although, currently, there is no mpox-specific vaccine, various generations of smallpox vaccines have been used to protect individuals against mpox due to their cross-immunity to mpox (22). With two doses on a 4-week schedule, the third generation of Modified Vaccinia Ankara live-attenuated vaccine, Bavarian Nordic A/S, Denmark is predicted to provide 71.8% protection against mpox after 2 years (22). MVA-BN shows promising safety and efficacy for PLHIV and is recommended for mpox prevention in many countries, including the UK, Europe, and the USA (18, 23, 56–58). Currently, mpox vaccines are not available in China. Still, it is worth studying vaccination willingness and its determinants among potential target populations to better prepare for the vaccine roll-out in the future. In China, the majority of the existing studies have focused on the vaccination willingness of MSM (with and/or without HIV) on a self-reported basis (24–28); information is limited about people infected with and/or suspected of HIV regardless of gender or sexual orientation. In the context of highly interconnected sexual networks shared by the transmission of HIV/STIs and mpox, louder voices are evident that mpox vaccination should be accessible for anyone who can benefit from it, with a diversity of gender and sexual identity, to achieve a substantial vaccination coverage for cost-effectively decreasing the spread of mpox (18, 19, 29–31). This primary study extended the scope of discussion to HIV-diagnosed and-suspected males without restrictions on self-reported sexual orientation, which brings out two significant tasks: investigating the knowledge gap of mpox and exploring the potential influencing factors of vaccination willingness against mpox among the populations of interest. We foresee this study can update the baseline for addressing the potential disparities in mpox-related health education and the upcoming roll-out of mpox vaccines in China.
This study was conducted in Changsha, a provincial city in China with a population of 1,042 million. It started on 5 July 2023 (briefly after the official announcement of the first mpox case in Changsha) and ended on 5 September 2023. As shown in Figure 1A, we adopted the convenience sampling method combining online and field recruitment. Any confirmed and probable cases of mpox were excluded from the recruitment. The first source was obtained through online interviews from a cohort of PLHIV who had received the HIV case management jointly initiated by the local Centers for Disease Control and Prevention (CDC) and antiretroviral therapy (ART) clinics. The CDC interviewers ensured the eligibility criteria by checking digital health records in the recent follow-up visits, including males of age 15 years and above, CD4 cell counts greater than 350/μl, and not inpatients. Finally, 294 people submitted the survey and were labelled a previously diagnosed group. The remainder of the participants were recruited from the outpatient clinic in the municipal centre of the CDC (where a designated HIV confirmatory laboratory is located, offering free confirmatory antibody tests to people with possible HIV exposure). During the data collection period, all male visitors (of age 15 years and above) who consulted about an HIV antibody test were invited to participate. A total of 187 males consented to fill out the survey before taking a test. According to Chinese Guidelines for the Diagnosis and Treatment of HIV/AIDS, the reactive samples in screen tests (test 1 [T1]) are not reported as positive and should be subjected to the following: retesting (test 2 [T2]) and further supplementary testing (test 3 [T3]) before being diagnosed as HIV positive (32). The test takers were further divided into the newly diagnosed (114 people, HIV positive in T3) and the suspected (73 people, HIV negative in T1/T2/T3 or inconclusive in T3 or declined to test finally) groups according to their test results.
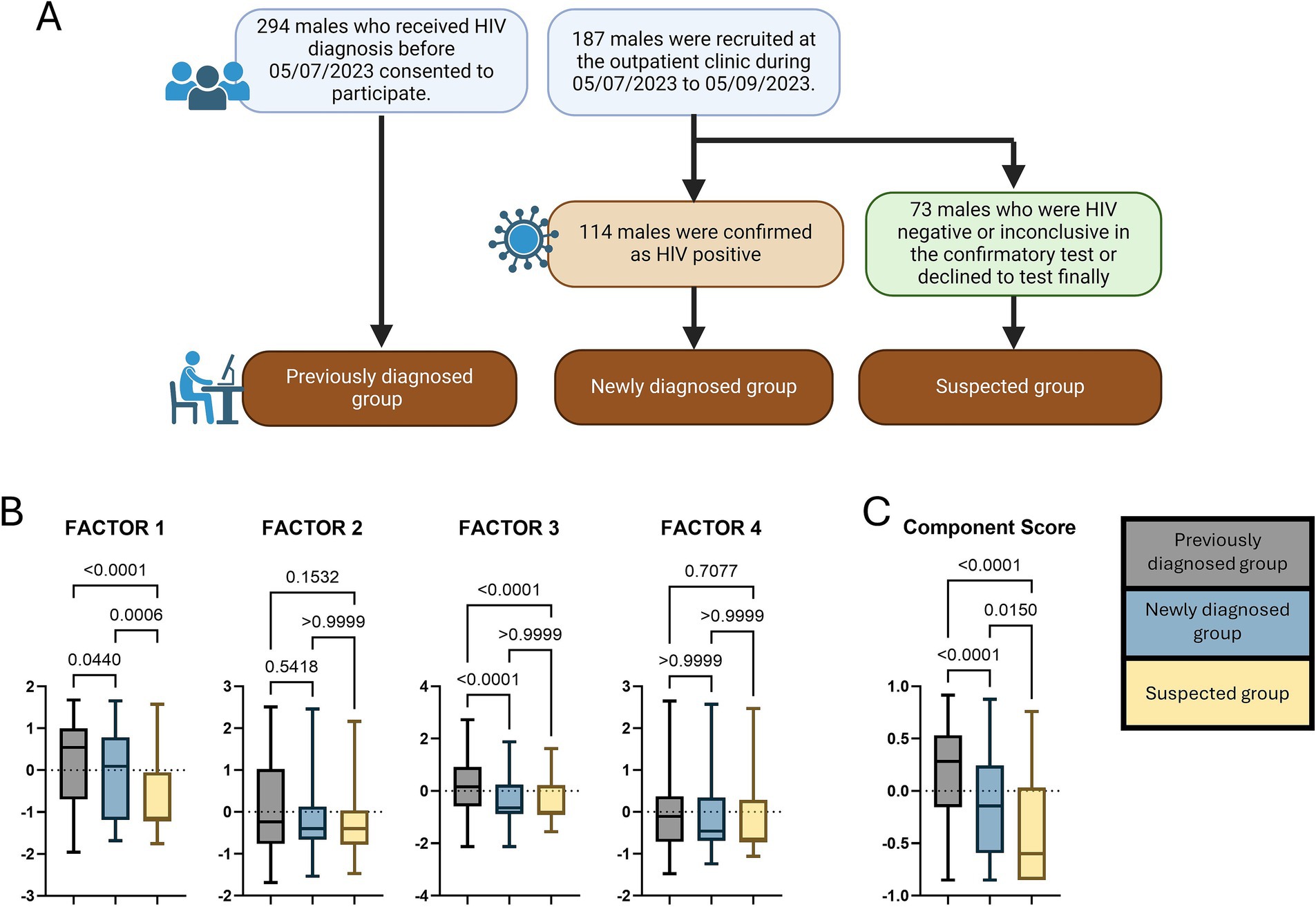
Figure 1. (A) The workflow of the participant recruitment; (B,C) The comparisons of the component score and the factor scores between participant groups. The p-values were acquired by the Kruskal–Wallis H tests.
The study protocol was approved by the Ethics Committee of the Municipal Changsha Center for Disease Control and Prevention (CSCDC). Before the survey, the interviewers explained to the participants about the study’s aims, contents, the potential benefits and dangers of participating in the study, and the participants’ rights. This information was prompted at the beginning of the digitalised questionnaire to ensure that potential participants were sufficiently informed, and the survey submission was regarded as implied consent to participate. The submitted questionnaires, which could be exclusively accessed by the data analyst, were anonymised and saved in the digital storage device secured by a password.
The participants used smartphones to access the digitalised survey comprised of three parts: sociodemographics; mpox awareness and related knowledge; sexualities; and mpox vaccination willingness. The instrument for evaluating mpox-related knowledge is a 15-item questionnaire (for details, see Table 1). For each item, the response of “yes” was scored as 1, and the reactions of “no” and “I do not know” were scored as 0. The item scores were added to give a sum ranging from 0 to 15. A training dataset on 100 respondents indicated the feasibility of the factor analysis and an overall Cronbach’s α of 0.845 (33). In the formal survey, the exploratory factor analysis (EFA) helped us gather valuable information about the interrelationships of the instrument items (34). Kaiser’s criterion and scree plot were examined to determine the number of factors to be extracted. The varimax orthogonal factor rotation was used to minimize the number of variables with high loadings on each factor. The Kruskal–Wallis H tests were applied to compare the component and factor scores between different participant groups. The chi-square test was used to assess the difference in mpox vaccination willingness between MSM and non-MSM in each group. The variance inflation factor (VIF) was introduced as a multicollinearity diagnosis for the variables to be studied, and we adopted a cutoff value of VIF < 2.5 for selecting variables to enter the logistic regression analysis (35). Finally, multivariate logistic regression was performed to identify determinants of vaccination willingness. All p-values were set at <0.05 for statistical significance. SPSS Version 29.0, IBM Corporation, USA. GraphPad Prism Version 9.5.1, GraphPad Software, LLC, USA.
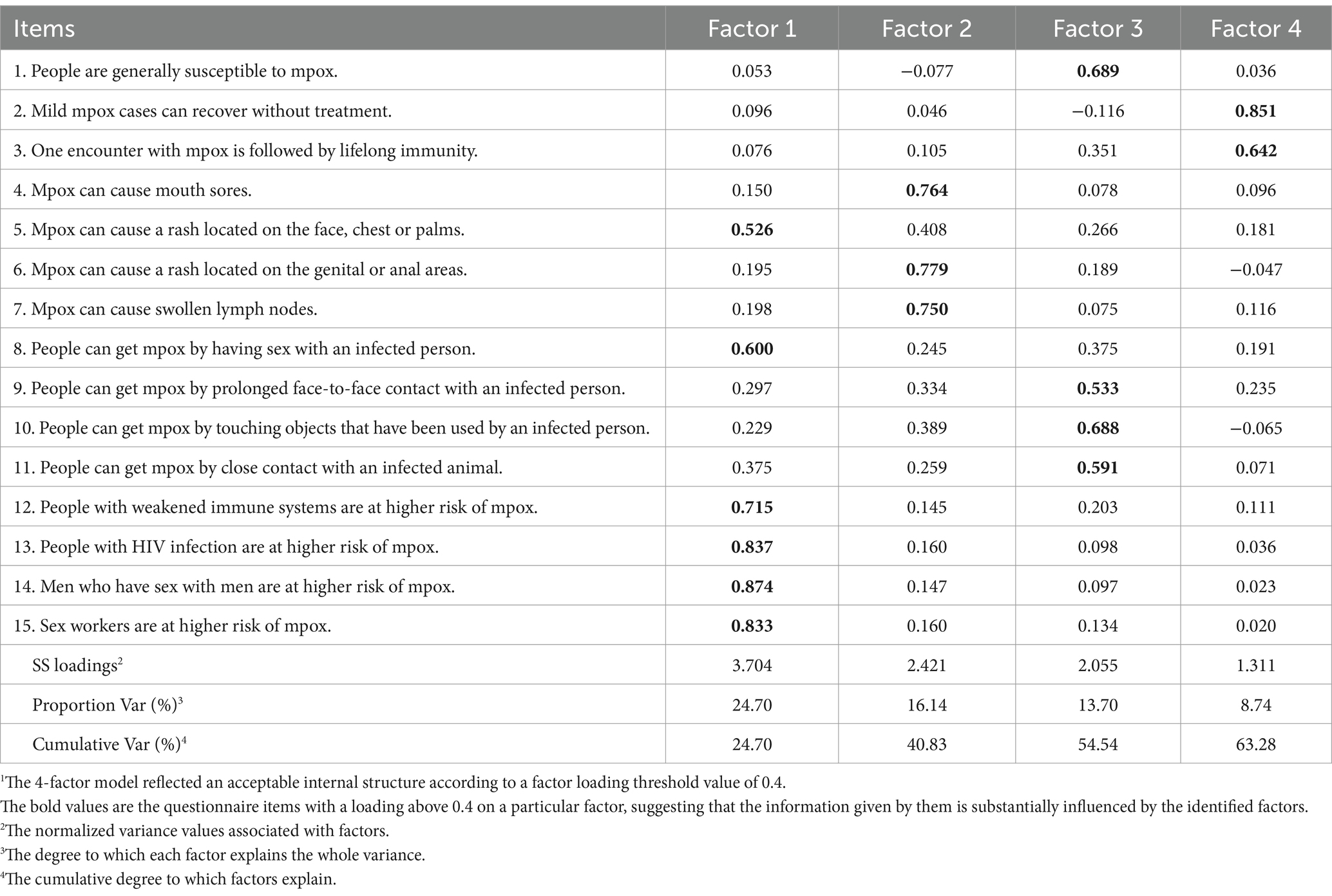
Table 1. The matrix of factor loadings, proportion variance and cumulative variance after orthogonal rotation1.
Sociodemographic information is listed in Table 2. The majority of participants in each group were those aged below 35 years, held a college degree or higher, were employed full-time, and were not married. The answers to five sexuality questions are listed in Table 2. MSM (those whose sexual orientation was reported as gay and bisexual) were responsible for a larger part either in the previously diagnosed (84.70%) or newly diagnosed (73.68%) groups, contrary to the suspected group where heterosexual men predominated (69.86%). Males who had ever used condoms inconsistently in the preceding year made up 78.07% of the newly diagnosed group, much higher than the percentages of the previously diagnosed (44.90%) and the suspected (34.25%) groups. Over one-third of the previously diagnosed and newly diagnosed groups had experienced chemsex (including both legal and illegal use of sexual stimulants, such as Viagra, Cialis, rush poppers, and amyl nitrite) in the past year, and more than 20% of them had same-sex behavior in the past 1 month. Meanwhile, recent casual sex (including commercial sex and group sex) saw the highest level among the suspected ones (21.92%).
People with awareness of the mpox epidemic in China had the largest share in the previously diagnosed group, at 84.01%, followed by the newly diagnosed group, at 67.54%, and the suspected group, at 38.36%. People who considered their risk of contracting mpox to be moderate and higher accounted for 46.59 and 42.11% of the previously diagnosed and newly diagnosed HIV-infected persons, respectively. Meanwhile, 23.19% of the participants of the suspected group assessed their infection risk as moderate or higher. Over 90% of HIV-infected persons expressed their vaccination willingness, displaying a higher level than the suspected people, 72.60% of whom opted “yes” to this question. MSM had a stronger vaccination willingness than non-MSM in the newly diagnosed group (98.82% vs. 86.67%, p = 0.005). In comparison, no statistical difference was in the previously diagnosed group (91.97% vs. 88.89%, p = 0.496) and the suspected group (77.27% vs. 70.60%, p-value: 0.557).
Table 1 demonstrates that four main factors were recommended by the scree plot for EFA and explained 63.277% of the variance on instrument items. Factor 1 was responsible for 24.70% of the total variance, containing 6 items, namely, a rash on the face, chest, and palms; the mode of sexual transmission; and the at-risk populations (MSM, PLHIV, sex workers, and immunocompromised people) had higher loadings. Three items on other common symptoms had higher loadings on Factor 2, which explained 16.14% of the total variance. Four items investigating the general susceptibility and the rest of the documented transmission modes contributed to 13.170% of the total variance on Factor 3. Finally, 8.74% of the total variance was seen in Factor 4 where 2 items about the prognosis had the higher loadings.
The component score and the factor scores between participant groups are compared as shown in Figures 1B,C. Statistical significance of the Kruskal–Wallis H tests was found in the scores of the component, Factor 1, and Factor 3 (p < 0.001). The median of the component score was highest in the previously diagnosed group, followed by the newly diagnosed and the suspected groups. The three groups had the same ranks in comparing the median of Factor 1 score. In terms of the Factor 3 score, the median of the previously diagnosed group was higher than that of the newly diagnosed and the suspected groups. At the same time, there was no statistical significance between the newly diagnosed and the suspected groups’ participants.
A total of 15 variables, including infected group, age group, education level, marital status, employment status, sexual orientation, condom use, chemsex, recent same-sex behavior, casual sex, self-assessed risk, and Factors 1–4 scores, were examined using the VIF, with the results ranging from 1.041 to 2.298, indicating a tolerant multicollinearity consideration. As such, they all entered multivariate logistic regression. As listed in Table 3, three variables had statistical significance (p < 0.05) in the final model: participant group, age category, and mpox knowledge. Referring to the newly diagnosed group, the previously diagnosed group (aOR = 0.314, 95% CI: 0.105–0.940) and the suspected group (aOR = 0.111, 95% CI: 0.034–0.363) had lower odds of vaccination willingness. The participants aged between 25 and 34 (aOR = 0.287, 95% CI: 0.086–0.959) and 35–44 (aOR = 0.136, 95% CI: 0.039–0.478) years had lower odds of vaccination willingness in comparison with the participants in 15–24 age category. A higher score in Factor 1 predicted higher odds of vaccination willingness (aOR = 1.701, 95% CI: 1.165–2.483).
This study provided preliminary insights into the knowledge gap of mpox and the possible determinates of vaccine willingness among males living with or at increased risk of HIV during the 2022–2023 mpox pandemic in Changsha. Significant findings are as follows: (1) over 90% of HIV-infected participants were willing to vaccinate for mpox, with a higher level in the newly diagnosed cases; (2) the vaccination willingness level in the HIV-suspected males was above 70%, lower than that in the newly diagnosed patients; (3) a better grasp of key points concerning mpox knowledge (Factor 1) predicted a higher level of vaccination willingness; (4) participants aged between 25 and 44 years were less likely to accept mpox vaccines than the youngest ones; (5) a considerate percentage of heterosexual persons in each group also expressed their acceptance for mpox vaccines; (6) a disparity of mpox knowledge level existed between the three participant groups.
We found a very high level of willingness to vaccinate against mpox in HIV-infected males regardless of sexual orientation. Our findings are on the upper end of homogeneous studies where the levels of mpox vaccine uptake or vaccination willingness were reported in a range of 56.8 to 91.7% among at-risk populations in China (24–27, 36). In a publication issued by China CDC Weekly, 78.9% of the under-treated HIV-infected persons with diverse gender and sexual identity were willing to vaccinate in Beijing (36). In our study, the higher level of willingness rates can be partly ascribed to the male-focused design with recruitment of newly confirmed HIV cases who had not been referred to ART treatment and healthcare. These new cases displayed a stronger vaccination willingness than the previously diagnosed patients in our study. A similar finding from a survey of PLHIV in Washington, DC, USA showed that respondents with a recent HIV diagnosis were more likely to be vaccinated for mpox (29).
We also found that newly diagnosed cases were more likely to be vaccinated for mpox than the suspected group’s participants. A possible reason is that these new HIV-infected persons, compared to the suspected ones, had a higher level of previous engagement in high-risk sexuality (e.g., same-sex behavior, inconsistent condom use, and chemsex), and had been more likely to perceive their seroconversion, making them more worried about their vulnerabilities in the mpox epidemic (12, 29). Similarly, Yuwei Li et al. found that MSM with self-reported HIV infection were more prone to vaccine uptake (27). It was noteworthy that, in China, approximately 30% of HIV-infected people were not knowing their status in China (37). For the HIV-suspected people, some were not entirely excluded from HIV infection considering the antibody-negative “window period” or the depletion of antibodies caused by severe immunosuppression (HIV nucleic acid amplification testing can be recommended in these cases) (38, 39). More crucially, frequent HIV tests may indicate a persistent risk for HIV/STIs as well as mpox infection (30, 40). Moreover, STIs and mpox can afflict users of postexposure prophylaxis (PEP) and pre-exposure prophylaxis (PrEP) despite HIV negativity (41). Healthcare providers should engage at-risk populations to adopt risk reduction behaviors and adhere to HIV and STIs (including mpox) testing for a timely medical care. They should also provide quality information about mpox and vaccination to encourage vaccine acceptance in the future vaccination promotion campaigns.
In addition, our results demonstrated that an increased knowledge of mpox predicted a raised level of vaccination willingness in participants, consistent with previous studies (24, 26, 42, 43). However, the statistical significance was only seen with an increase in Factor 1. The first subdomain had the highest correlations with the updated features of epidemiology in the 2022–2023 mpox pandemic. Since mpox has intersected with HIV, according to multiple scientific research reports; public health guidance and messaging; and social media (5, 6, 8, 10, 14, 40), MSM, PLHIV, sex workers, and immunocompromised people are recommended to vaccinate against mpox in multiple regions (13). It is not surprising that an improved knowledge of the intersection between HIV and mpox can make people living with or at risk of HIV better understand the importance of vaccinating for mpox.
Furthermore, we found that willingness to get vaccinated was related to age category, which aligns with several publications (26, 29, 42). However, our analysis did not support a statistical significance in terms of educational levels, sexual orientation/identities, high-risk sexual practices, and perceived risks of mpox infection, which had been reported as the determinants of mpox vaccine uptake or vaccination willingness among at-risk populations in other homogenous studies (25, 29–31, 44). Nevertheless, these studies mainly targeted MSM with a self-reported nature and the results varied across regions and survey periods. We surveyed HIV-infected and-suspected males without restrictions on self-reported sexual orientations and found that a considerable number of heterosexual men in each participant group also expressed their willingness to get inoculated. There was a possibility that the intersection of mpox and HIV raised a health concern among heterosexual persons who had multiple sex partners, making them feel a need to receive mpox vaccines. Meanwhile, we should also consider the high occurrence of MSM’s concealing same-sex behaviors to healthcare providers in China (45, 46). Existing evidence suggested that concealers were more likely to be MSM who were less experienced in HIV testing, had lower self-perceived risk of HIV infection, and had not received HIV-related medical care (46–48). Notably, closeting about sexual orientation can undermine healthcare service utilization, such as STI screening, vaccine uptake, and preventive information seeking (11, 45). Therefore, we believe that the expanded vaccine eligibility for both HIV-infected and-suspected persons inclusive of diverse sexual orientation should be considered and carefully assessed in the development of the vaccination and immunization guidelines for fostering high efficacy of mitigation measures against mpox (29).
The previously diagnosed HIV-infected persons had a better understanding of mpox, including the highest awareness rate of the domestic mpox pandemic and a similar result was reported in an MSM-targeted study (49). It may be primarily contributed to HIV-related healthcare which offered regular health education on coinfection prevention to the diagnosed patients (49). Moreover, a previous HIV diagnosis may have raised their awareness of HIV-related illness (50, 51) and motivated them to use patient peer networks and search engines to gain mpox knowledge (49). A Baidu-index-based study in mainland China indicated that provinces with higher HIV/AIDS incidence had more online search activity related to mpox (52).
Interestingly, in the EFA of mpox knowledge, a skin rash on the face, chest, and palms was most correlated to the items about the updated epidemiological intersection of HIV and mpox. The correlation may reflect a lag in updating public health guidance and messaging on the changes in clinical manifestations. The most prominent symptom of mpox is the typical vesicular rash, based on which a test for mpox virus is recommended (2). In the records before 2022, mpox rash usually started in the face, hands, or feet before spreading to other parts of the body (1), whereas, in the 2022–2023 mpox pandemic, skin lesions predominantly appear on the genital or perianal area (suggesting direct inoculation in sexual contacts), sometimes without preceding prodrome symptoms, and less typical at the beginning, behaving like an STI (2, 18). Failure to understand that mpox can mimic a common STI (e.g., herpes and syphilis) can pose challenges to timely diagnosis because of the diminished suspicion in self-monitoring (53). Moreover, worries over the visibility of symptoms may increase as skin eruptions are mistakenly believed to appear more often in the exposed parts of the body (11, 12). We believe that filling the gap in clinical presentation in public health guidance and messaging has significant implications for guiding at-risk populations in self-monitoring and reducing worries.
There were several limitations to this study. Firstly, our design used a convenient sampling and failed to survey the previously diagnosed HIV-infected persons who had dropped out from the follow-up visits and individuals who refused to participate. Also, the survey was based on self-reports, so there may be recall bias and underpotted involvement in unsafe sexual practices. In addition, the high-risk behaviors were not assessed by frequency, degree, and subcategory, so we were unable to grade the risk of exposure further. Another limitation involves that the newly diagnosed and the suspected group’s participants were recruited from a single center, which may raise a concern about the generalizability of the results. Nevertheless, the municipal centre of the CDC was one of two health institutions authorised to conduct HIV confirmatory antibody tests in Changsha (the only test which was available free of charge for the participants). Additionally, this survey is observational in nature, so there was no causality established. The survey time was carried out when mpox pandemic hit China; these results may change as the pandemic is controlled. Finally, the self-reported data on other STIs were not included in the design. We made this choice mainly due to two reasons. First, HIV/AIDS is distinct from other STIs in terms of etiology, treatments and management, and long-term effects. In this study, we prioritized the intersection of HIV/AIDS and mpox. Second, HIV/AIDS is a chronic infection without a cure and can be confirmed by laboratory tests; in contrast, the diagnosis of an ongoing STI should consider the clinical manifestations. The municipal centre of the CDC is not qualified to make the diagnosis of other STIs, and we were concerned about the incomparability of the self-reported data on STIs with the laboratory-confirmed HIV infection status.
This study is an attempt to identify the knowledge gap of mpox and determine the potential factors influencing vaccination willingness against mpox among males living with or at increased risk of HIV amid the 2022–23 mpox pandemic in Changsha. We found an overall high degree of vaccination willingness among HIV-infected and-suspected males. Notwithstanding, the willingness levels varied with respect to their HIV infection status, understanding of the intersection of HIV and mpox, and age. Healthcare providers should engage the people who have unsafe sex for a timely HIV and STI (including mpox) diagnosis and treatment, raise their awareness of behavioral modification, and encourage mpox vaccination acceptance of those with persistent exposure, regardless of sexual orientation. Our findings also highlighted the importance of eliminating the potential knowledge gap of mpox for better guiding the at-risk populations in self-monitoring and reducing worries in the mpox epidemic.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Municipal Center for Disease Control and Prevention of Changsha. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YZ: Writing – original draft, Writing – review & editing. JW: Investigation, Writing – review & editing. ZX: Investigation, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors want to acknowledge all the participants in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rabaan, AA, Alasiri, NA, Aljeldah, M, Alshukairiis, AN, AlMusa, Z, Alfouzan, WA, et al. An updated review on monkeypox viral disease: emphasis on genomic diversity. Biomedicines. (2023) 11:1832. doi: 10.3390/biomedicines11071832
2. Okoli, GN, Van Caeseele, P, Askin, N, and Abou-Setta, AM. Comparative evaluation of the clinical presentation and epidemiology of the 2022 and previous Mpox outbreaks: a rapid review and meta-analysis. Infect Dis (Lond). (2023) 55:490–508. doi: 10.1080/23744235.2023.2214609
3. Borges, V, Duque, MP, Martins, JV, Vasconcelos, P, Ferreira, R, Sobral, D, et al. Viral genetic clustering and transmission dynamics of the 2022 mpox outbreak in Portugal. Nat Med. (2023) 29:2509–17. doi: 10.1038/s41591-023-02542-x
4. Global HIV, Hepatitis and STIs programmes: Men who have sex with men. The Joint United Nations Programme on HIV/AIDS (UNAIDS) (2023).
5. Saldana, CS, Kelley, CF, Aldred, BM, and Cantos, VD. Mpox and HIV: a narrative review. Curr HIV/AIDS Rep. (2023) 20:261–9. doi: 10.1007/s11904-023-00661-1
6. Ortiz-Saavedra, B, Montes-Madariaga, ES, Cabanillas-Ramirez, C, Alva, N, Ricardo-Martinez, A, Leon-Figueroa, DA, et al. Epidemiologic situation of HIV and monkeypox coinfection: A systematic review. Vaccines (Basel). (2023) 11:246. doi: 10.3390/vaccines11020246
7. Pilkington, V, Quinn, K, Campbell, L, Payne, L, Brady, M, and Post, FA. Clinical presentation of monkeypox in people with and without HIV in the United Kingdom during the 2022 global outbreak. AIDS Res Hum Retrovir. (2023) 39:581–6. doi: 10.1089/aid.2023.0014
8. Girometti, N, Ogoina, D, Tan, DHS, Pozniak, A, and Klein, MB. Intersecting HIV and mpox epidemics: more questions than answers. J Int AIDS Soc. (2022) 25:e26043. doi: 10.1002/jia2.26043
9. Mitja, O, Alemany, A, Marks, M, Lezama Mora, JI, Rodriguez-Aldama, JC, Torres Silva, MS, et al. Mpox in people with advanced HIV infection: a global case series. Lancet. (2023) 401:939–49. doi: 10.1016/S0140-6736(23)00273-8
10. Hong, C. Mpox on reddit: a thematic analysis of online posts on Mpox on a social media platform among key populations. J Urban Health. (2023) 100:1264–73. doi: 10.1007/s11524-023-00773-4
11. May, T, Towler, L, Smith, LE, Horwood, J, Denford, S, Rubin, GJ, et al. Mpox knowledge, behaviours and barriers to public health measures among gay, bisexual and other men who have sex with men in the UK: a qualitative study to inform public health guidance and messaging. BMC Public Health. (2023) 23:2265. doi: 10.1186/s12889-023-17196-0
12. Jiao, K, Xu, Y, Huang, S, Zhang, Y, Zhou, J, Li, Y, et al. Mpox risk perception and associated factors among Chinese young men who have sex with men: results from a large cross-sectional survey. J Med Virol. (2023) 95:e29057. doi: 10.1002/jmv.29057
13. Acharya, A, Kumar, N, Singh, K, and Byrareddy, SN. Mpox in MSM: tackling stigma, minimizing risk factors, exploring pathogenesis, and treatment approaches. Biom J. (2024) 100746:100746. doi: 10.1016/j.bj.2024.100746
14. Logie, CH. What can we learn from HIV, COVID-19 and mpox stigma to guide stigma-informed pandemic preparedness? J Int AIDS Soc. (2022) 25:e26042. doi: 10.1002/jia2.26042
15. Ratevosian, J, Heisler, M, Carpino, T, McHale, T, Mushekuru, J, and Beyrer, C. Addressing transnational exploitation and armed conflict in the response to mpox. Lancet. (2024) 404:2137–40. doi: 10.1016/S0140-6736(24)02418-8
16. World Health Organization. Mpox fact sheet (2024). Available at: https://www.who.int/news-room/fact-sheets/detail/mpox.
17. Amer, F, Khalil, HES, Elahmady, M, ElBadawy, NE, Zahran, WA, Abdelnasser, M, et al. Mpox: risks and approaches to prevention. J Infect Public Health. (2023) 16:901–10. doi: 10.1016/j.jiph.2023.04.001
18. Nakamura, H, and Yamamoto, K. Mpox in people with HIV: A narrative review. HIV Med. (2024) 25:910–8. doi: 10.1111/hiv.13661
19. Curran, KG, Eberly, K, Russell, OO, Snyder, RE, Phillips, EK, Tang, EC, et al. HIV and sexually transmitted infections among persons with monkeypox-eight U.S. jurisdictions, May 17-July 22, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1141–7. doi: 10.15585/mmwr.mm7136a1
20. World Health Organization. Public health advice on understanding, preventing and addressing stigma and discrimination related to mpox (2024). Available at: https://www.who.int/publications/m/item/public-health-advice-on-understanding-preventing-and-addressing-stigma-and-discrimination-related-to-mpox.
21. Mohapatra, RK, Singh, PK, Branda, F, Mishra, S, Kutikuppala, LVS, Suvvari, TK, et al. Transmission dynamics, complications and mitigation strategies of the current mpox outbreak: A comprehensive review with bibliometric study. Rev Med Virol. (2024) 34:e2541. doi: 10.1002/rmv.2541
22. Berry, MT, Khan, SR, Schlub, TE, Notaras, A, Kunasekaran, M, Grulich, AE, et al. Predicting vaccine effectiveness for mpox. Nat Commun. (2024) 15:3856. doi: 10.1038/s41467-024-48180-w
23. NHS. Find a mpox vaccination site. Available online: https://www.nhs.uk/conditions/mpox/find-a-mpox-vaccination-site/ (Accessed December 28, 2024).
24. Zheng, M, Qin, C, Qian, X, Yao, Y, Liu, J, Yuan, Z, et al. Knowledge and vaccination acceptance toward the human monkeypox among men who have sex with men in China. Front Public Health. (2022) 10:997637. doi: 10.3389/fpubh.2022.997637
25. Fu, L, Sun, Y, Li, Y, Wang, B, Yang, L, Tian, T, et al. Perception of and vaccine readiness towards Mpox among men who have sex with men living with HIV in China: A cross-sectional study. Vaccines. (2023) 11:528. doi: 10.3390/vaccines11030528
26. Chen, Y, Li, Y, Fu, L, Zhou, X, Wu, X, Wang, B, et al. Knowledge of human Mpox (monkeypox) and attitude towards Mpox vaccination among male sex Workers in China: A cross-sectional study. Vaccines. (2023) 11:285. doi: 10.3390/vaccines11020285
27. Li, Y, Peng, X, Fu, L, Wang, B, Sun, Y, Chen, Y, et al. Monkeypox awareness and low vaccination hesitancy among men who have sex with men in China. J Med Virol. (2023) 95:e28567. doi: 10.1002/jmv.28567
28. Wong, NS, Wong, BC, Lee, MP, Tsang, OT, Cheung, DKF, Sit, AY, et al. Mpox vaccination for men who have sex with men and their differential risk of exposure and infection. Hum Vaccin Immunother. (2023) 19:2252263. doi: 10.1080/21645515.2023.2252263
29. Andersen, EW, Kulie, P, Castel, AD, Lucar, J, Benator, D, Greenberg, AE, et al. Mpox awareness, risk reduction, and vaccine acceptance among people with HIV in, vol. 13. Washington, DC. Pathogens: (2024).
30. Svartstein, AW, Knudsen, AD, Heidari, SL, Heftdal, LD, Gelpi, M, Benfield, T, et al. Mpox incidence and vaccine uptake in men who have sex with men and are living with HIV in Denmark. Vaccines. (2023) 11:1761. doi: 10.3390/vaccines11071167
31. Del Duca, G, Tavelli, A, Mastrorosa, I, Aguglia, C, Lanini, S, Brita, AC, et al. Risk awareness as a key determinant of early vaccine uptake in the Mpox vaccination campaign in an Italian region: A cross-sectional analysis. Vaccines (Basel). (2023) 11:1761. doi: 10.3390/vaccines11121761
32. Zhao, W. Chinese guidelines for the diagnosis and treatment of HIV/AIDS (2021 edition). Infect Dis Immun. (2022) 2:145–67. doi: 10.1097/ID9.0000000000000044
33. Taber, KS. The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res Sci Educ. (2017) 48:1273–96. doi: 10.1007/s11165-016-9602-2
34. Shrestha, N. Factor analysis as a tool for survey analysis. Am J Appl Math Stat. (2021) 9:4–11. doi: 10.12691/ajams-9-1-2
35. TMJ, AC, and NAMR, S. Diagnosing multicollinearity of logistic regression model. Asian J Prob Statis. (2019) 5:1–9. doi: 10.9734/ajpas/2019/v5i230132
36. Gu, Y, Ren, R, Han, J, Bai, W, Zhang, Y, Liu, H, et al. Knowledge, attitude, and practice towards Mpox and associated factors among HIV-infected individuals-Beijing municipality, China, 2023. China CDC Wkly. (2024) 6:109–17. doi: 10.46234/ccdcw2024.024
37. Yan, X, Su, H, Zhang, B, Li, Y, Zhang, L, and Jia, Z. Adherence of HIV self-testing among men who have sex with men in China: longitudinal study. J Med Internet Res. (2020) 22:e19627. doi: 10.2196/19627
38. Stekler, JD, Swenson, PD, Coombs, RW, Dragavon, J, Thomas, KK, Brennan, CA, et al. HIV testing in a high-incidence population: is antibody testing alone good enough? Clin Infect Dis. (2009) 49:444–53. doi: 10.1086/600043
39. Guan, M. Frequency, causes, and new challenges of indeterminate results in Western blot confirmatory testing for antibodies to human immunodeficiency virus. Clin Vaccine Immunol. (2007) 14:649–59. doi: 10.1128/CVI.00393-06
40. Torres, TS, Silva, MST, Coutinho, C, Hoagland, B, Jalil, EM, Cardoso, SW, et al. Evaluation of Mpox knowledge, stigma, and willingness to vaccinate for Mpox: cross-sectional web-based survey among sexual and gender minorities. JMIR Public Health Surveill. (2023) 9:e46489. doi: 10.2196/46489
41. Pestel, J, Matthews, H, Schmiedel, S, Hufner, A, Jordan, S, Scheiter, RL, et al. Screening for monkeypox infection in asymptomatic high-risk-behaviour men having sex with men (MSM). Infect Dis Rep. (2022) 14:794–7. doi: 10.3390/idr14050081
42. Chow, EPF, Samra, RS, Bradshaw, CS, Chen, MY, Williamson, DA, Towns, JM, et al. Mpox knowledge, vaccination and intention to reduce sexual risk practices among men who have sex with men and transgender people in response to the 2022 mpox outbreak: a cross-sectional study in Victoria, Australia. Sex Health. (2023) 20:390–402. doi: 10.1071/SH23075
43. Dong, C, Yu, Z, Zhao, Y, and Ma, X. Knowledge and vaccination intention of monkeypox in China's general population: A cross-sectional online survey. Travel Med Infect Dis. (2023) 52:102533. doi: 10.1016/j.tmaid.2022.102533
44. Dukers-Muijrers, N, Evers, Y, Widdershoven, V, Davidovich, U, Adam, PCG, Op de Coul, ELM, et al. Mpox vaccination willingness, determinants, and communication needs in gay, bisexual, and other men who have sex with men, in the context of limited vaccine availability in the Netherlands (Dutch Mpox-survey). Front. Public Health. (2022) 10:1058807. doi: 10.3389/fpubh.2022.1058807
45. Cao, W, You, X, Li, J, Peng, L, Gu, J, Hao, C, et al. Same-sex behavior disclosure to health care providers associated with greater awareness of pre-exposure prophylaxis. BMC Public Health. (2021) 21:2243. doi: 10.1186/s12889-021-12317-z
46. Qiao, S, Zhou, G, and Li, X. Disclosure of same-sex Behaviors to health-care providers and uptake of HIV testing for men who have sex with men: A systematic review. Am J Mens Health. (2018) 12:1197–214. doi: 10.1177/1557988318784149
47. Zhuoma, L, Zhang, Y, Yan, T, Kang, F, Hou, X, Chen, J, et al. Non-disclosed men who have sex with men within local MSM HIV-1 genetic transmission networks in Guangyuan, China. Front Public Health. (2022) 10:956217. doi: 10.3389/fpubh.2022.956217
48. Sun, CJ, Tobin, K, Spikes, P, and Latkin, C. Correlates of same-sex behavior disclosure to health care providers among black MSM in the United States: implications for HIV prevention. AIDS Care. (2019) 31:1011–8. doi: 10.1080/09540121.2018.1548753
49. Zheng, M, Chen, W, Qian, X, Tao, R, Ma, L, Zhou, F, et al. Awareness of mpox-related knowledge among men who have sex with men in China. BMC Public Health. (2023) 23:600. doi: 10.1186/s12889-023-15503-3
50. Collins, RL, Kanouse, DE, Gifford, AL, Senterfitt, JW, Schuster, MA, McCaffrey, DF, et al. Changes in health-promoting behavior following diagnosis with HIV: prevalence and correlates in a national probability sample. Health Psychol. (2001) 20:351–60. doi: 10.1037/0278-6133.20.5.351
51. Arias-Colmenero, T, Perez-Morente, MA, Ramos-Morcillo, AJ, Capilla-Diaz, C, Ruzafa-Martinez, M, and Hueso-Montoro, C. Experiences and attitudes of people with HIV/AIDS: A systematic review of qualitative studies. Int J Environ Res Public Health. (2020) 17:639. doi: 10.3390/ijerph17020639
52. Du, M, Yan, W, Zhu, L, Liang, W, Liu, M, and Liu, J. Trends in the Baidu index in search activity related to Mpox at geographical and economic levels and associated factors in China: National Longitudinal Analysis. JMIR Form Res. (2023) 7:e44031. doi: 10.2196/44031
53. Minhaj, FS, Singh, V, Cohen, SE, Townsend, MB, Scott, H, Szumowski, J, et al. Prevalence of undiagnosed monkeypox virus infections during global Mpox outbreak, United States, June-September 2022. Emerg Infect Dis. (2023) 29:2307–14. doi: 10.3201/eid2911.230940
54. Fu, L, Wang, B, Wu, K, Yang, L, Hong, Z, Wang, Z, et al. Epidemiological characteristics, clinical manifestations, and mental health status of human mpox cases: A multicenter cross-sectional study in China. J Med Virol. (2023) 95:e29198. doi: 10.1002/jmv.29198
55. Islam, MR, Nowshin, DT, Khan, MR, Shahriar, M, and Bhuiyan, MA. Monkeypox and sex: sexual orientations and encounters are key factors to consider. Health Sci Rep. (2023) 6:e1069. doi: 10.1002/hsr2.1069
56. Centers for Disease Control and Prevention (CDC). Mpox vaccines and vaccine recommendations. Available online: https://www.cdc.gov/poxvirus/mpox/vaccines/vaccine-recommendations.html (Accessed December 28, 2024).
57. Australian Government Department of Health. Mpox (monkeypox) vaccines. Available online: https://www.health.gov.au/diseases/mpox-monkeypox/vaccines (Accessed December 28, 2024).
58. New York State Department of Health. Mpox vaccine information. Available online: https://www.health.ny.gov/diseases/communicable/zoonoses/mpox/vaccine.htm (Accessed December 28, 2024).
Keywords: monkeypox, HIV, men who have sex with men (MSM), sexually transmitted infections (STIs), vaccination, health education
Citation: Zhou Y, Wang J and Xie Z (2025) Monkeypox-related knowledge and vaccination willingness among HIV-diagnosed and -suspected males: a cross-sectional survey in Changsha. Front. Public Health. 12:1455623. doi: 10.3389/fpubh.2024.1455623
Received: 27 June 2024; Accepted: 16 December 2024;
Published: 07 January 2025.
Edited by:
Huixuan Zhou, Beijing Sport University, ChinaReviewed by:
Mehrdad Mohammadi, Shiraz University of Medical Sciences, IranCopyright © 2025 Zhou, Wang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Xie, emhpeGllX2NzY2RjQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.