- 1Tianjin Centers for Disease Control and Prevention, Tianjin, China
- 2Tianjin Key Laboratory of Pathogenic Microbiology of Infectious Disease, Tianjin Centers for Disease Control and Prevention, Tianjin, China
- 3School of Public Health, Tianjin Medical University, Tianjin, China
Background: Men who have sex with men (MSM) are vulnerable to HPV infection. This study aims to explore the HPV infection status at different sites among HIV-positive MSM, HIV-negative MSM, and men who have sex with women (MSW), and to investigate their willingness to receive HPV vaccination.
Methods: From September 2023 to April 2024, three groups were recruited in Tianjin, China. Participants completed an electronic self-administered questionnaire, which included demographic information, knowledge related to sexually transmitted diseases, behavioral information, and willingness to receive the HPV vaccine. Samples were collected from the anal region, genitals, and oral cavity for HPV typing.
Results: A total of 1,559 participants were recruited, including 300 HIV-positive MSM, 600 HIV-negative MSM, and 659 MSW. The HPV infection prevalence for any site were 62.0, 53.7 and 8.3%, respectively (p < 0.001). The infection prevalence for HPV genes covered by the 9-valent vaccine were 47.0, 36.8, and 3.5%, respectively (p < 0.001). Co-infection prevalence at anal and genital were 20.3, 14.2, 0.6%, respectively. Co-infection prevalence at anal and genital and oral were 1.3, 0.3%, 0, respectively. A total of 77.0% HIV-positive MSM and 75.3% HIV-negative MSM expressed willingness to receive the HPV vaccine, whereas 58.9% of MSW were unwilling (p < 0.001). Being HIV-positive (aOR, 3.119; 95% CI, 2.213–4.395), being over 46 years old (aOR, 1.994; 95% CI, 1.266–3.142), with an occupation classified as “white collar workers” (aOR, 1.620; 95% CI, 1.111–2.362) and “freelancing” (aOR, 2.025; 95% CI, 1.371–2.993) and a history of homosexual behavior in the past 6 months (aOR, 5.338; 95% CI, 3.802–7.495) were risk factors for HPV infection among men in Tianjin. Consistently using condoms in the past 6 months (aOR, 0.667; 95% CI, 0.513–0.867) were protective factors.
Conclusion: The HPV infection prevalence among MSM in Tianjin is significantly higher than among MSW, with higher prevalence in the anal region compared to the genital and oral region. HPV infection is associated with HIV infection, older age, and homosexual behavior. Most MSM showed a positive willingness to receive the HPV vaccine, indicating the necessity to implement targeted HPV vaccination programs for MSM and to enhance necessary preventive knowledge and behavioral interventions.
1 Introduction
Human papillomavirus (HPV) infection is one of the most common sexually transmitted infections (STIs) (1). Previous studies indicated that HPV infection is a significant contributing factor in the development of cervical cancer (2), anal cancer (3), and genital warts (4).
Men who have sex with men (MSM) are at high risk for HPV infection and transmission due to their unique sexual behavior (5) and multiple and casual sexual partnerships. A meta-analysis published in 2019 revealed that the prevalence of any anal HPV genotype infection was significantly higher among MSM compared to men who have sex with women (MSW), with rates of 79 vs. 43% in HIV-positive men and 47 vs. 12% in HIV-negative men (6). A meta-analysis published in 2021 indicated that the prevalence of anal HPV infection among MSM in China ranged from 53.60 to 85.10%, with a higher prevalence of high-risk HPV types in northern China (7).
HPV vaccination is an effective method for preventing HPV-related diseases in both men and women. An observed vaccine efficacy rate of 60.2% in men (8), and efficacy of the qHPV vaccine against anal intraepithelial neoplasia was 77.5% in the per-protocol efficacy population (9). In mainland China, the 2-valent, 4-valent, and 9-valent HPV vaccines were approved for use (in women) in 2016, 2017, and 2018, respectively (10). However, there has been no specific HPV vaccination program for men. It is of great importance to comprehend the willingness of men to receive the HPV vaccine if future HPV vaccination efforts are to be feasible. Any concerns that men may have about receiving the vaccine need to be addressed. Despite several studies reporting the prevalence of HPV among Chinese MSM, the majority of those studies have focused on the prevalence of HPV infection in anal region (11–13). Few studies investigated the prevalence of multi-site HPV infections among different types of men in a single city. It is estimated that there will be significant differences in HPV infection rates among individuals with different sexual behaviors, so this study examines the prevalence and distribution of HPV genotype at anal, genital, and oral sites among HIV-positive and HIV-negative MSM, and MSW in Tianjin. Additionally, the study examines the willingness of those individuals to receive the HPV vaccine, providing a reference for the development of comprehensive HPV prevention strategies and for the evaluation of the clinical application value of the HPV vaccine in different male populations.
2 Materials and methods
2.1 Ethical approval
The study was approved by the Ethics Committee of the Tianjin Center for Disease Control and Prevention (Approval no. TJCDC-R-2023-013).
2.2 Study population
The calculation of sample size is based on the sample size formula N = 400 × (Q/P) of the current situation survey, where P is the HPV infection rate and Q = 1-P. According to previous literature reports (7), the HPV infection rate of HIV positive men who have sex with men is calculated according to p = 0.60, and the HPV infection rate of HIV negative men who have sex with men is calculated according to p = 0.42. Considering possible factors such as questionnaire or sample failure, the sample size of the HIV positive men who have sex with men group is 300, the sample size of the HIV negative men who have sex with men group is 600, and the MSW group and the HIV negative MSM group form a control in a 1:1 ratio, with a sample size of 600.
From September 2023 to April 2024, 300 HIV-positive MSM, 600 HIV-negative MSM and 600 MSW were recruited in Tianjin, China. The study participants were recruited in a stratified manner by age, with a ratio of 2:3:1 for the 18–26, 27–45, and ≥ 46 years, respectively. The questionnaire was completed by the participants in a private space after the purpose of the study was explained to them and their informed consent was obtained. The questionnaire included demographic information, knowledge related to sexually transmitted diseases, behavioral information, and willingness to receive the HPV vaccine. Individuals who had received the HPV vaccine were excluded. Education is not a limiting condition.
2.2.1 Inclusion criteria for HIV-positive MSM
(1) Males aged 18 or older. (2) History of anal or oral sex with men in the past year. (3) HIV-infected individuals or AIDS patients. Recruitment of HIV-positive MSM was conducted at designated antiretroviral therapy hospital. On a voluntary basis, those who met the inclusion criteria were consecutive recruited.
2.2.2 Inclusion criteria for HIV-negative MSM
(1) Males aged 18 or older. (2) History of anal or oral sex with men in the past year. (3) HIV antibody-negative. Recruitment of HIV-negative MSM was conducted in collaboration with non-governmental organizations (NGOs) in Tianjin, with strategies involving online recruitment, venue-based recruitment, and snowball sampling.
2.2.3 Inclusion criteria of MSW
(1) Males aged 18 or older. (2) History of vaginal or oral sex with women in the past year. Recruitment of MSW was conducted at community health check-up institutions. On a voluntary basis, those who met the inclusion criteria were consecutive recruited.
2.3 Sample collection
Samples of exfoliated cells were collected from three sites for each participant: an anal swab, a genital swab, and an oral swab. Participants were instructed not to have sexual intercourse for 2 days before sampling and not to wash their genital and anal areas on the day of sampling. Three sterile cotton swabs moistened with saline were used to collect exfoliated cells from the anal, genital (glans, foreskin, urethral opening), and oral mucosa. Samples were placed in a specialized preservation solution and stored at 4°C for testing within 1 week. Additionally, a 5 mL sample of venous blood was collected for the purpose of HIV antibody testing.
2.4 Laboratory testing
2.4.1 HPV DNA testing and genotyping
HPV DNA and genotyping were performed using the HPV23 nucleic acid typing detection kit (Kedean, Hangzhou, China), based on fluorescence PCR melting curve analysis. DNA extraction was conducted using the EB1000 nucleic acid extractor (Kedean, Hangzhou, China). High-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) and low-risk genotypes (6, 11, 42, 70, 81, 83) were classified according to their carcinogenicity.
2.4.2 HIV serology testing
For HIV-negative MSM recruited through NGOs, initial screening was conducted using the HIV Ag/Ab colloidal gold method test kit (Zhongsheng Kejv, Tianjin, China). Samples reactive in the initial screening were confirmed with the Western blot method (MP, Singapore) and excluded from the study if positive.
2.5 Statistical analysis
Descriptive statistics were used for categorical variables (e.g., HPV genotype positivity, willingness to vaccinate), expressed as frequencies and percentages. Chi-square tests compared HPV genotype positivity rates and willingness to vaccinate among the three groups. Multivariate logistic regression identified factors associated with HPV infection. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) were reported. Data were analyzed using SPSS 24.0 (SPSS, Inc., Chicago, IL, USA), with significance set at p < 0.05.
3 Results
3.1 Demographic characteristics of the participants
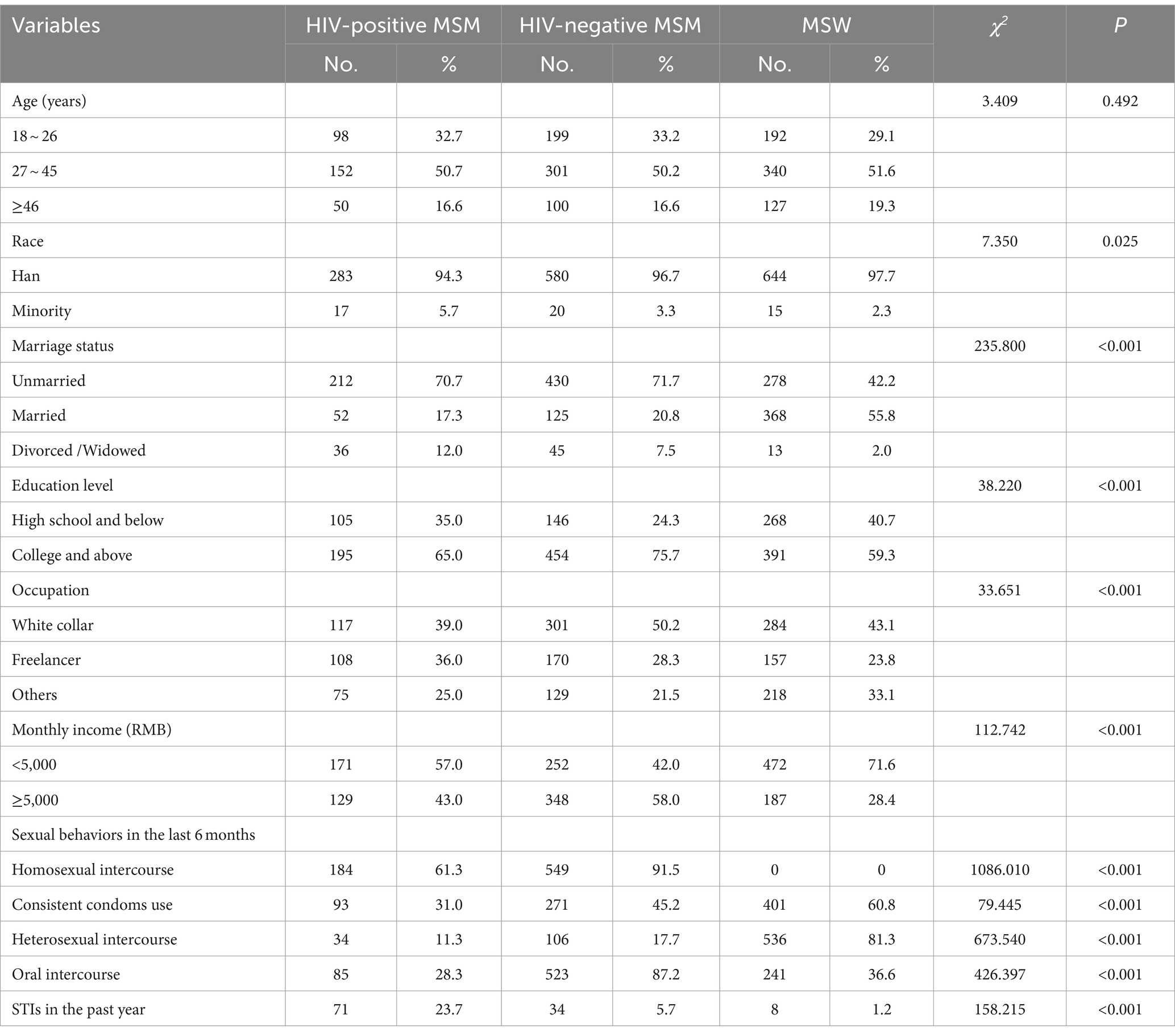
A total of 300 HIV-positive MSM, 600 HIV-negative MSM and 659 MSW were recruited in this study. The age distributions among the three groups were similar, with a median age of 32 (IQR, 25–40) for HIV-positive MSM, 32 (IQR, 25–39) for HIV-negative MSM, and 35 (IQR, 24–43) for MSW. With regard to marital status, the majority of HIV-positive MSM and HIV-negative MSM were unmarried (70.7 and 71.7%, respectively), while the majority of MSW were married (55.8%). In terms of education level, all three groups exhibited a high level of education, with the majority having completed college or above. With regard to occupational, all three groups were predominantly engaged in white-collar work. Socio-demographic characteristics and sexual behaviors of participants are shown in Table 1.
3.2 HPV infection status
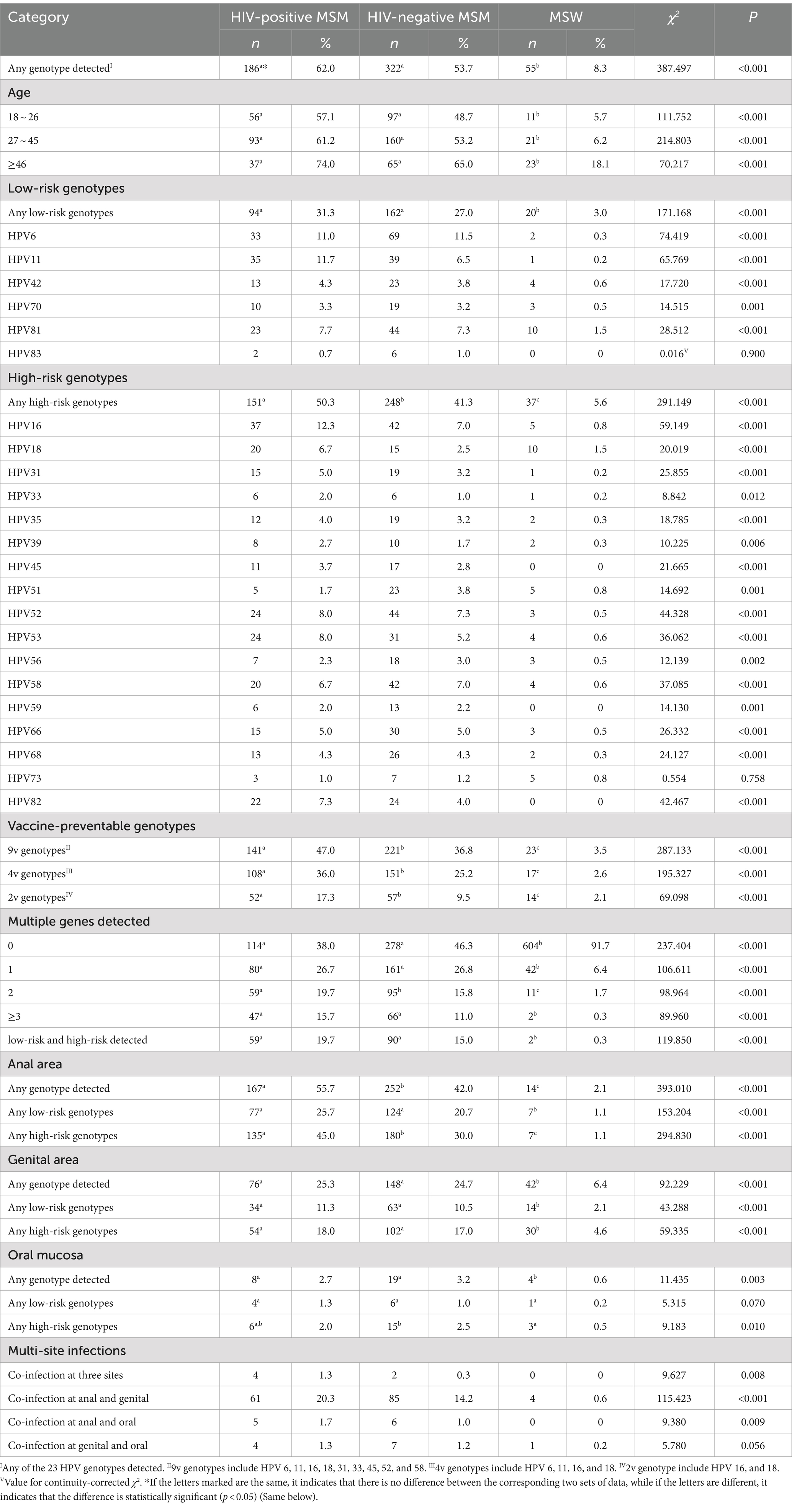
The overall HPV infection prevalence was 62.0% for HIV-positive MSM, 53.7% for HIV-negative MSM, and 8.3% for MSW (p < 0.001). The prevalence for the genotypes covered by the 9-valent, 4-valent, and 2-valent vaccines were 47.0, 36.8, and 17.3% among HIV-positive MSM, 36.8, 25.2, and 9.5% among HIV-negative MSM, and 3.5, 2.6, and 2.1% among MSW, respectively (p < 0.001). The anal HPV infection prevalence for HIV-positive MSM, HIV-negative MSM, and MSW were 55.7, 42.0, and 2.1%, respectively. The genital HPV infection prevalence were 25.3, 24.7, and 6.4%, respectively. The oral HPV infection prevalence were 2.7, 3.2, and 0.6%, respectively. Co-infection prevalence at anal and genital were 20.3, 14.2, 0.6%, respectively. Co-infection prevalence at anal and genital and oral were 1.3, 0.3%, 0, respectively. Prevalence of HPV genotype and multiple HPV genotypes are shown in Table 2.
3.3 Willingness to receive the HPV vaccine
Among HIV-positive MSM and HIV-negative MSM, 77.0 and 75.3%, respectively, expressed willingness to receive the HPV vaccine if it was available for men, whereas 58.9% of MSW were unwilling (p < 0.001). The most common reasons for unwillingness were concerns about side effects and safety, high cost, and a lack of knowledge about where to get vaccinated. The relevant issues regarding willingness to receive HPV vaccine are shown in Figure 1.
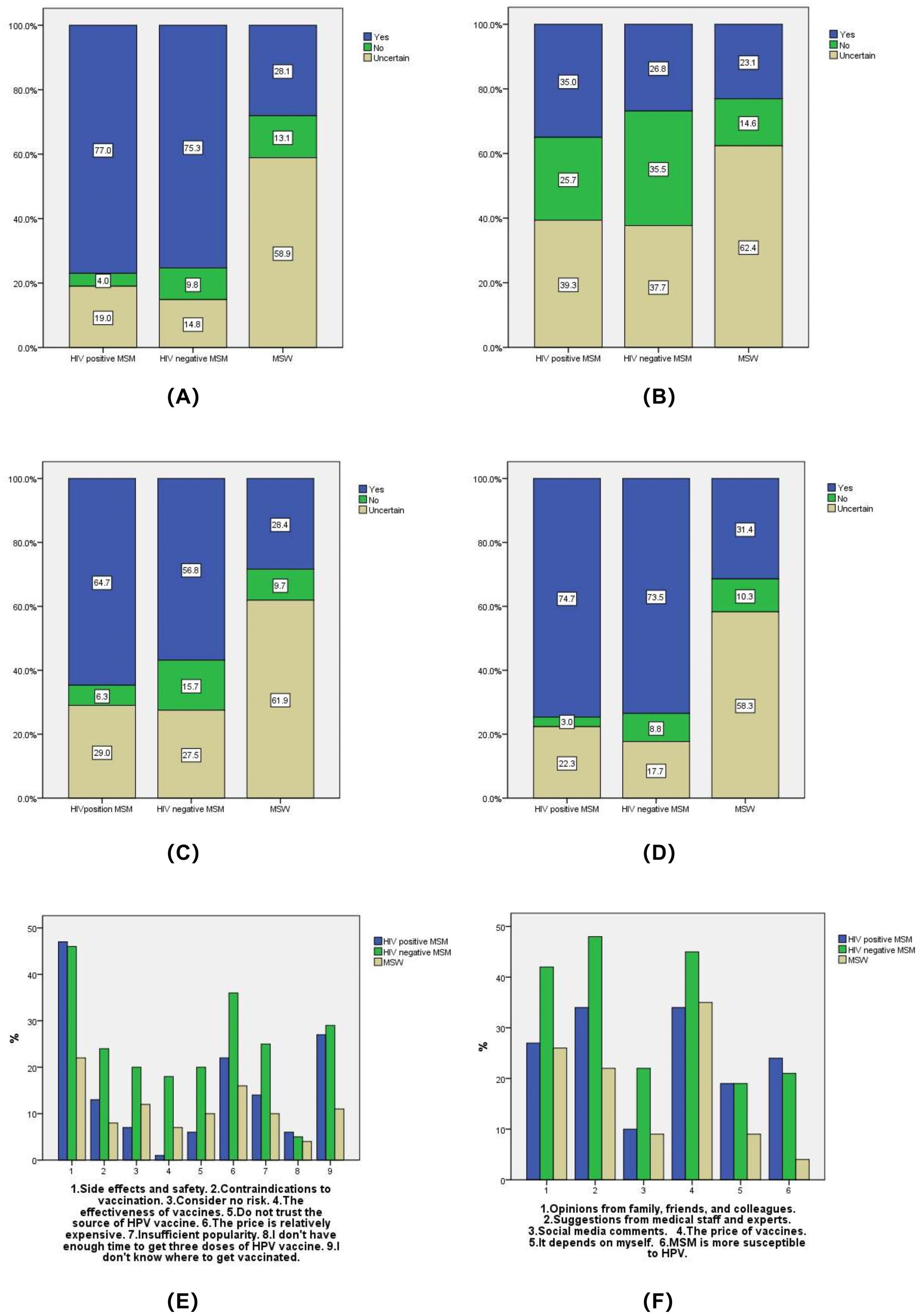
Figure 1. Willingness to receive HPV vaccine. (A) If the HPV vaccine does not restrict gender, are you willing to receive the HPV vaccine? (B) Do you have any family or friends who have already been received the HPV vaccine? (C) Would you be willing to recommend your family and friends to receive the HPV vaccine? (D) If the HPV test result is positive, are you willing to receive the HPV vaccine? (E) What is the main reason why you are unwilling to receive the HPV vaccine? (F) What factors will affect your willingness to receive the HPV vaccine?
3.4 Factors influencing HPV infection
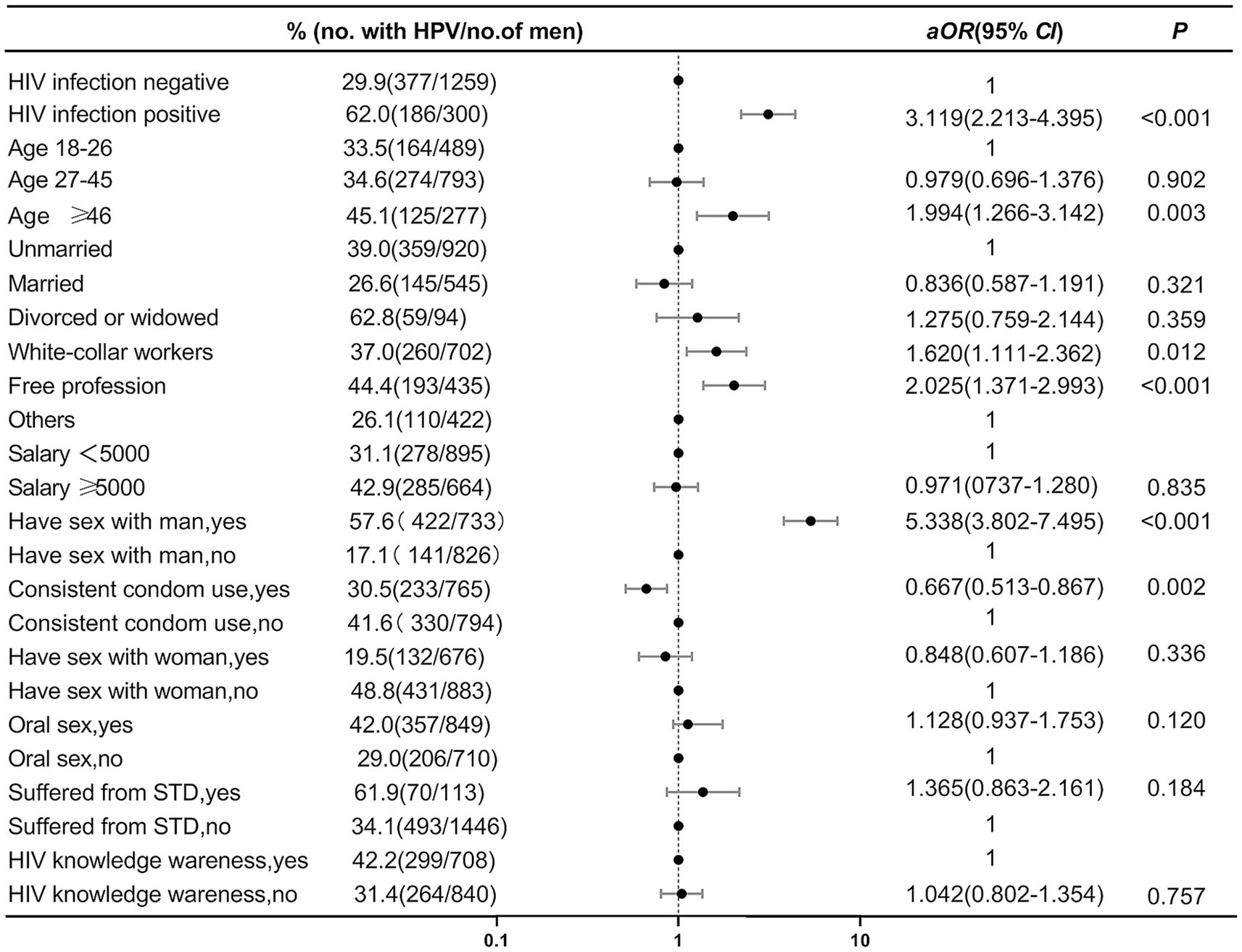
In the multivariable analysis, HIV-positive (aOR, 3.119; 95% CI, 2.213–4.395), over 46 years old (aOR, 1.994; 95% CI, 1.266–3.142), with an occupation of “white collar” (aOR, 1.620; 95% CI, 1.111–2.362) and “freelancer” (aOR, 2.025; 95% CI, 1.371–2.993) and having a history of homosexual behavior in the past 6 months (aOR, 5.338; 95% CI, 3.802–7.495) were risk factors for HPV infection among men in Tianjin. Consistent condoms use in the past 6 months (aOR, 0.667; 95% CI, 0.513–0.867) was found to be a protective factor for HPV infection. The results of the multivariable logistic regression analysis were presented in Figure 2.
4 Discussion
This study shows that the HPV infection prevalence among MSM (HIV-positive 62.0%, HIV-negative 53.7%) in Tianjin is significantly higher than among MSW (8.3%), with higher prevalence in the anal region compared to the genital and oral region. Being HIV-positive, being over 46 years old, with an occupation classified as “white collar workers” and “freelancing” and a history of homosexual behavior in the past 6 months were risk factors for HPV infection among men in Tianjin. Most MSM showed a positive willingness to receive the HPV vaccine.
The anal region is the most severely affected site for HPV infection among MSM populations. This study demonstrates that the anal HPV infection prevalence among HIV-positive MSM in Tianjin is 55.7%, which is lower than previous reports from China [65.6% (12) and 77.9% (13)], Rome (14) (83.5%), Peru (15) (77.1%), and Italy (16) (60.0%). Anal HPV infection prevalence among HIV-negative MSM was found to be 42.0%, which is also lower than previous reports from China (47.1–58.0%) (7, 11), South Africa (50.8%) (17), Rome (68.3%) (14), and Thailand (18) (59.0%), but higher than the report from Italy 15 (37.8%) (16).The reason for the difference may be that the participants in different studies have different demographic characteristics, behavioral characteristics and the course of AIDS. Other studies can be conducted in the future to verify this hypothesis.
Compared to the anal region, the genital region has a relatively lower HPV infection prevalence. This study indicates that the genital HPV infection prevalence among HIV-positive MSM is 25.3%, which is higher than that reported in Italy (18.5%). The genital HPV infection prevalence among HIV-negative MSM is 24.7%, which is higher than that reported in Taiwan (18.0%) (19), lower than that reported in Beijing (36.7%) (20), and comparable to the report from Italy (23.5%) (15). Among the three surveyed sites, the oral region has the lowest HPV infection prevalence. The oral HPV infection prevalence among HIV-positive and HIV-negative MSM in Tianjin are 2.7 and 3.2%, respectively, which is similar to the report from southern China (2.7%) (7). The data from Tianjin, situated in northern China, combined with data from southern China, suggests that the oral region is currently not a high-risk site for HPV infection among MSM.
There is a significant difference in the prevalence of HPV infection between MSM and MSW. The overall HPV infection prevalence among MSW is 8.3%, which is similar to the prevalence reported in Guangxi (21) (10.5%) and Jiangsu (22) (11.31%) in China. The genital HPV infection prevalence among MSW is higher than that in the anal region (6.4 vs. 2.1%), a trend also reported in Guangxi (10.8 vs. 3.8%) (21). However, international reports indicate that HPV infection prevalence among MSW was considerably higher, with a global overall prevalence of 50.8% (23) and a study from the United States reporting a genital HPV infection prevalence of 45.2% (95% CI, 41.3–49.3%) among men aged 18–59 (24), indicating significant regional differences worldwide.
The HPV infection prevalence for the genotypes covered by the 9-valent, 4-valent, and 2-valent vaccines are higher in HIV-positive MSM compared to HIV-negative MSM and MSW. The HPV infection prevalence for the genotypes covered by the 9-valent and 2-valent vaccines in HIV-positive MSM is lower than that reported from South Africa (47.0 vs. 75.0%, 17.3 vs. 20.0%), while the 4-valent rate is higher (36.0 vs. 35.0%) (17). In HIV-negative MSM, the rates for the 9-valent and 4-valent genotypes are higher than in South Africa (36.8 vs. 28.0%, 25.2 vs. 13.0%), but the 2-valent rate is lower (9.5 vs. 10.0%) (17). The HPV infection prevalence for the genotypes covered by the 9-valent, 4-valent, and 2-valent vaccines in MSW are lower than the rates reported in a global meta-analysis (3.5 vs. 16.0%, 2.6 vs. 11.0%, 2.1 vs. 7.0%) (25).
HPV vaccination can effectively reduce the prevalence and mortality of related diseases among MSM in China, particularly anal-genital warts with the 4-valent vaccine and anal cancer with the 9-valent vaccine (26). Our study found that over two-thirds of MSM would be willing to get vaccinated if the HPV vaccine were available to men, which is slightly lower than that reported in other studies from China (87.6%) (27). However, most MSW are reluctance to get vaccinated, mainly due to concerns about vaccine side effects and safety, vaccine cost, and lack of knowledge about vaccination locations. Studies conducted in Quebec, France, and other regions have also found that safety concerns are a significant barrier to HPV vaccine acceptance (28, 29). Factors influencing HPV vaccine uptake among men in China include vaccine cost, recommendations from healthcare providers and experts, and opinions from family and friends. Other studies have also shown a positive correlation between the advice of significant others and the HPV vaccination rate (30). To further enhance vaccination willingness and improve cost-effectiveness, it is necessary to adjust vaccine prices appropriately or reduce the economic burden of vaccination through other means. Research has demonstrated that individuals who receive HPV vaccine recommendations from healthcare providers are more likely to be vaccinated (31). To enhance vaccination rates, it is essential to implement strategies that strengthen publicity and education through various channels such as hospitals, communities, and online platforms. Additionally, government regulation of vaccine prices, enhanced training for healthcare workers, and tailored interventions based on different population needs, can also be employed to enhance vaccination rates.
Our multivariate analysis indicate that being HIV-positive, aged 46 or above, and a history of same-sex behavior in the past 6 months are risk factors for HPV infection among men in Tianjin. The specific sexual behaviors of MSM, particularly anal intercourse, increase the risk of HPV infection due to mucosal damage. It has been demonstrated that HIV infection can induce immunosuppression, thereby increasing the risk of HPV infection and prolonging the persistence and reactivation of latent HPV. Consequently, HIV-positive MSM are a priority group for anal cancer screening research (32). Our study observed a higher risk of HPV infection among older MSM, which contrasts with some previous studies indicating that the prevalence of anal HPV infection among MSM does not vary with age (33, 34). It is important to note that the infection of HPV in middle-aged and older men, as older MSM may have higher cumulative HPV infection, warranting increased HPV screening for middle-aged and older MSM. Since there is no specific HPV vaccination plan for men in Chinese Mainland at present, and the vaccines are approval in other countries up to 45 years old, so vaccination is not relevant for the oldest group in the study, and cancer screening is the only practical recommendation. However, our findings also indicate that nearly half of MSM younger than 27 years old were already infected with HPV. This highlights the importance of vaccinating before sexual debut for better prevention, which is also a long-term solution for preventing anal cancer in MSM (35).
Interestingly, we found that white-collar workers and freelancers are risk factors of HPV infection compared to other professions. It is worth noting that the “other” category in this study includes some university students who have been in society for a shorter time and have a higher level of education, which may contribute to their better awareness of disease transmission and safe sexual practices, possibly explaining the lower HPV infection rate in this group.
This study strictly followed the inclusion criteria for subjects, recruiting participants through a stratified snowball sampling method both online and offline, which yield a good representativeness sample. However, this study is subject to certain limitations. Firstly, data collection is based on self-reporting, which may be subject to recall bias and potentially incomplete, truthful responses due to the personal nature of sexual behavior. Secondly, this study was conducted in Tianjin, China, and the HPV infection prevalence assessment may not represent the national level, therefore caution is needed when extrapolating the results. Thirdly, this study is a cross-sectional survey and cannot confirm the causal relationship between HPV infection and its influencing factors.
Overall, the findings of this study indicate that the prevalence of HPV infection among MSM in Tianjin is significantly higher than among MSW, with the anal region having a higher infection rate than the genital region. HPV infection among men is associated with HIV infection, age 46 or above, and same-sex sexual behavior. There is a need to promote HPV prevention and intervention measures for this population, particularly focusing on older MSM, and to include anal cancer screening in the follow-up and treatment of HIV-positive individuals. The high prevalence of HPV genotypes covered by vaccines among MSM underscores the necessity for HPV vaccination programs tailored to this group. Further research and technical preparation for such vaccination initiatives are essential to address this public health concern effectively.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Tianjin Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JB: Formal analysis, Conceptualization, Writing – original draft. XD: Writing – original draft, Project administration, Investigation. TN: Writing – review & editing, Supervision, Methodology. JZ: Writing – review & editing, Methodology, Investigation. ZW: Writing – original draft, Validation, Software. HL: Writing – original draft, Investigation, Data curation. MY: Writing – review & editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Tianjin Medical Key Disciplines (TJYXZDXK-050A) and National Bureau of Disease Control and Prevention Public Health Talent Training Support Project.
Acknowledgments
The authors appreciate MSD China Holding Co., Ltd. for supported in part by a research grant from Investigator Sponsored Non-interventional Research Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sethi, S, Ju, X, Antonsson, A, Canfell, K, Smith, MA, Garvey, G, et al. Oral HPV infection among indigenous Australians; incidence, persistence and clearance at 12-months follow-up. Cancer Epidemiol Biomarkers Prev. (2022) 31:604–13. doi: 10.1158/1055-9965.EPI-21-1056
2. Walboomers, JM, Jacobs, MV, Manos, MM, Bosch, FX, Kummer, JA, Shah, KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. (1999) 189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F
3. Welton, ML, Sharkey, FE, and Kahlenberg, MS. The etiology and epidemiology of anal cancer. Surg Oncol Clin N Am. (2004) 13:263–75. doi: 10.1016/j.soc.2003.12.005
4. Brown, DR, Schroeder, JM, Bryan, JT, Stoler, MH, and Fife, KH. Detection of multiple human papillomavirus types in condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. (1999) 37:3316–22. doi: 10.1128/JCM.37.10.3316-3322.1999
5. Teng, Q, Niu, M, Liu, Y, and Ren, S. Harm of HPV infection in men and protection of men with HPV vaccination. Chin J Immunol. (2022) 38:507–14. doi: 10.3969/j.issn.1000-484X.2022.04.023
6. Marra, E, Lin, C, and Clifford, GM. Type-specific anal human papillomavirus prevalence among men, according to sexual preference and HIV status: a systematic literature review and meta-analysis. J Infect Dis. (2019) 219:590–8. doi: 10.1093/infdis/jiy556
7. Zhou, Y, Lin, Y, Gao, L, Dai, J, Luo, G, Li, L, et al. Human papillomavirus prevalence among men who have sex with men in China: a systematic review and meta-analysis. Eur J Clin Microbiol. (2021) 40:1357–67. doi: 10.1007/s10096-021-04229-y
8. Giuliano, AR, Palefsky, JM, Goldstone, S, Moreira, ED Jr, Penny, ME, Aranda, C, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. (2011) 364:401–11. doi: 10.1056/NEJMoa0909537
9. Palefsky, JM, Giuliano, AR, Goldstone, S, Moreira, ED Jr, Aranda, C, Jessen, H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. (2011) 365:1576–85. doi: 10.1056/NEJMoa1010971
10. Lin, W, Wang, Y, Liu, Z, Chen, B, Yuan, S, Wu, B, et al. Awareness and attitude towards human papillomavirus and its vaccine among females with and without daughter(s) who participated in cervical cancer screening in Shenzhen. China. Trop Med Int Health. (2019) 24:1054–63. doi: 10.1111/tmi.13283
11. Li, H, Yu, M, Bai, J, Dong, X, and Zhang, Y. Research progress on male HPV infection in China. Chin J AIDS STD. (2024) 30:330–4. doi: 10.13419/j.cnki.aids.2024.03.21
12. Jiang, J, Zhang, W, Guo, Z, Yang, J, and Pan, X. Meta-analysis of human papillomavirus infection and associated factors among HIV high-risk population in China. Chin J AIDS STD. (2014) 20:818–22. doi: 10.13419/j.cnki.aids.2014.11.008
13. Gao, M, Li, Y, Li, L, Deng, X, Nie, Y, Li, F, et al. Prevalence of anal HPV infection among HIV positive men who have sex with men. J Trop Med. (2019) 19:785–8. doi: 10.3969/j.issn.1672-3619.2019.06.029
14. Fracella, M, Oliveto, G, Roberto, P, Cinti, L, Gentile, M, Coratti, E, et al. The epidemiology of anal human papillomavirus (HPV) in HIV-positive and HIV-negative women and men: a ten-year retrospective observational study in Rome (Italy). Pathogens. (2024) 13:163. doi: 10.3390/pathogens13020163
15. del Pino, M, Vorsters, A, Joura, AE, Doorbar, J, Haniszewski, M, Gudina, IA, et al. Risk factors for human papillomavirus infection and disease: a targeted literature summary. J Med Virol. (2024) 96:e29420. doi: 10.1002/jmv.29420
16. Ucciferri, C, Tamburro, M, Falasca, K, Sammarco, ML, Ripabelli, G, and Vecchiet, J. Prevalence of anal, oral, penile and urethral human papillomavirus in HIV infected and HIV uninfected men who have sex with men. J Med Virol. (2018) 90:358–66. doi: 10.1002/jmv.24943
17. Mbulawa, ZZ, Coetzee, D, and Williamson, AL. Human papillomavirus prevalence in south African women and men according to age and human immunodeficiency virus status. BMC Infect Dis. (2015) 15:459. doi: 10.1186/s12879-015-1181-8
18. Supindham, T, Chariyalertsak, S, Utaipat, U, Miura, T, Ruanpeng, D, Chotirosniramit, N, et al. High prevalence and genotype diversity of anal HPV infection among MSM in northern Thailand. PLoS One. (2015) 10:e0124499. doi: 10.1371/journal.pone.0124499
19. Cheng, S-H, Chu, F-Y, Lin, Y-S, and Hsueh, Y-M. Influence of age and CD4+ T cell counts on the prevalence of genital human papillomavirus infection among HIV-seropositive men who have sex with men in Taiwan. J Med Virol. (2012) 84:1876–83. doi: 10.1002/jmv.23413
20. Qian, HZ, Hu, Y, Carlucci, JG, Yin, L, Li, X, Giuliano, AR, et al. Human immunodeficiency virus status differentially associated with genital and anal human papillomavirus infection among Chinese men who have sex with men: a cross-sectional survey. Sex Transm Dis. (2017) 44:656–62. doi: 10.1097/OLQ.0000000000000672
21. Wei, FX. Observational cohort study on the natural history of human papillomavirus infection in natural populations in Liuzhou, Guangxi. Xiamen: Xiamen University (2021).
22. Zhang, R, Jiang, Z, Lin, N, Shi, H, Wang, LJ, Chen, W, et al. Prevalence of human papilloma virus infection and its risk factors in some rural areas of Jiangsu, China. Natl J Androl. (2018) 24:795–801. doi: 10.13263/j.cnki.nja.2018.09.005
23. Gamboa-Hoil, SI. Human papillomavirus in men. Rev Int Androl. (2023) 21:100325. doi: 10.1016/j.androl.2021.09.001
24. Han, JJ, Beltran, TH, Song, JW, Klaric, J, and Choi, YS. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and nutrition examination survey (NHANES) 2013-2014. JAMA Oncol. (2017) 3:810–6. doi: 10.1001/jamaoncol.2016.6192
25. Bruni, L, Albero, G, Rowley, J, Alemany, L, Arbyn, M, Giuliano, AR, et al. Global and regional estimates of genital human papillomavirus prevalence among men: a systematic review and meta-analysis. Lancet Glob Health. (2023) 11:e1345–62. doi: 10.1016/S2214-109X(23)00305-4
26. Li, Y, Lin, YF, Wu, X, Zhou, X, Tian, T, Guo, Z, et al. Effectiveness and cost-effectiveness of human papillomavirus vaccination strategies among men who have sex with men in China: a modeling study. Front Immunol. (2023) 14:1197191. doi: 10.3389/fimmu.2023.1197191
27. Cao, Z, Jiang, H, He, W, Pan, H, Zhang, C, and Zhong, X. The potential risk compensation after receiving HPV vaccination among men who have sex with men in Southwest China: a HAPA-based analysis. Vaccine. (2023) 11:1429. doi: 10.3390/vaccines11091429
28. Cable, CE, Watson, KE, and Tsuyuki, RT. Case-finding for HPV vaccination eligibility within a dental office with concurrent development of a dialogue tool. Vaccine X. (2024) 18:100492. doi: 10.1016/j.jvacx.2024.100492
29. Bruel, S, Rakotomampionona, Z, Gignon, M, Agrinier, N, Ndiaye, NC, Lasset, C, et al. The intentions of French health university students to recommend and to receive the HPV vaccine are mainly influenced by vaccine knowledge, confidence in vaccines and personal HPV vaccination. Vaccine. (2024) 42:1934–40. doi: 10.1016/j.vaccine.2024.02.033
30. Zian, L, Siyu, C, Lixian, S, He, C, Hongbiao, C, Yuan, F, et al. Associations of mothers’ decisional conflicts and satisfaction with governmental health promotion materials with their daughters’ HPV vaccination uptake in China: a cross-sectional survey. Vaccine X. (2024) 19:100529. doi: 10.1016/j.jvacx.2024.100529
31. Chido-Amajuoyi, OG, Osaghae, I, Onyeaka, HK, and Shete, S. Barriers to the assessment and recommendation of HPV vaccination among healthcare providers in Texas. Vaccine X. (2024) 18:100471. doi: 10.1016/j.jvacx.2024.100471
32. Wei, F, Gaisa, MM, D'Souza, G, Xia, N, Giuliano, AR, Hawes, SE, et al. Epidemiology of anal human papillomavirus infection and high-grade squamous intraepithelial lesions in 29 900 men according to HIV status, sexuality, and age: a collaborative pooled analysis of 64 studies. Lancet HIV. (2021) 8:e531–43. doi: 10.1016/S2352-3018(21)00108-9
33. Donà, MG, Latini, A, Benevolo, M, Moretto, D, Cristaudo, A, and Giuliani, M. Anal human papillomavirus infection prevalence in men who have sex with men is age independent: a role for recent sexual behavior? Future Microbiol. (2014) 9:837–44. doi: 10.2217/fmb.14.44
34. Torres, M, González, C, del Romero, J, Viciana, P, Ocampo, A, Rodríguez-Fortúnez, P, et al. Anal human papillomavirus genotype distribution in HIV-infected men who have sex with men by geographical origin, age, and cytological status in a Spanish cohort. J Clin Microbiol. (2013) 51:3512–20. doi: 10.1128/JCM.01405-13
35. Chow, EPF, Tabrizi, SN, Fairley, CK, Wigan, R, Machalek, DA, Garland, SM, et al. Fall in human papillomavirus in young men who have sex with men after the implementation of gender-neutral HPV vaccination: a repeated cross-sectional study. Lancet Infect Dis. (2021) 21:1448–57. doi: 10.1016/S1473-3099(20)30687-3
Keywords: human papillomavirus (HPV), HPV vaccines, human immunodeficiency virus (HIV), men who have sex with men (MSM), influencing factors, vaccine willingness
Citation: Bai J, Dong X, Ning T, Zhu J, Wu Z, Li H and Yu M (2024) Analysis of multi-site HPV infection and vaccination willingness among men who have sex with men in Tianjin, China. Front. Public Health. 12:1453024. doi: 10.3389/fpubh.2024.1453024
Edited by:
Junjie Xu, Key Laboratory of AIDS Immunology of National Health and Family Planning Commission, ChinaReviewed by:
Andrew Pavelyev, Merck, United StatesZixin Wang, The Chinese University of Hong Kong, China
Copyright © 2024 Bai, Dong, Ning, Zhu, Wu, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maohe Yu, eXVtYW9oZUB0ai5nb3YuY24=
†These authors share first authorship
 Jianyun Bai
Jianyun Bai Xiaoyue Dong1†
Xiaoyue Dong1†