- 1Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 2Department of Urology, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 3Department of Urology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 4Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 5Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 6Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 7Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 8Research Center for Precision Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
Background: Active smokers are known to be at an increased risk of both gastroesophageal reflux disease (GERD) and peptic ulcer disease (PUD), however the role of passive smoking remains unclear. In this study, we aimed to examine whether secondhand smoke (SHS) is associated with PUD and GERD.
Methods: In this population-based study, we conducted a large-scale analysis with 88,297 never-smokers (male: 18,595; female: 69,702; mean age 50.1 ± 11.0 years) from the Taiwan Biobank. The exposure group was comprised of those who had been exposed to SHS, and the no exposure group as those without SHS exposure. According to the frequency of exposure, we further divided the participants into “no exposure,” “<1 h per week,” and “≥1 h per week” groups. A cutoff point of 1 h per week was chosen according to the median exposure time in our participants. Associations between SHS and SHS frequency with PUD and GERD were assessed.
Results: Of the 88,297 enrolled participants, 11,909 (13.5%) had PUD and 76,388 (86.5%) did not. In addition, 11,758 (13.3%) had GERD and 76,539 (86.7%) did not. Multivariable analysis showed a significant association between SHS with PUD (odds ratio [OR] = 1.166; 95% confidence interval [CI] = 1.084–1.254; p < 0.001), and GERD (OR = 1.131; 95% CI = 1.053–1.216; p = 0.001). Furthermore, those exposed to SHS ≥ 1 h per week (vs. no exposure) were associated with higher risks of PUD (OR = 1.232; 95% CI = 1.121–1.355; p < 0.001) and GERD (OR = 1.200; 95% CI = 1.093–1.319; p < 0.001).
Conclusion: SHS was significantly associated with PUD and GERD. Furthermore, exposure to SHS ≥ 1 h per week (vs. no exposure) was associated with a 1.23-fold higher risk of PUD and 1.20-fold higher risk of GERD. This study represents the largest population-based investigation to explore the association between SHS with PUD and GERD in Taiwanese never-smokers.
Introduction
Peptic ulcer disease (PUD) is a gastrointestinal mucosal defect, characterized by asymptomatic or symptomatic abdominal pain, bloating, and abdominal fullness (1). The Global Burden of Disease, Injuries and Risk Factors Study reported that approximately 8 million people had PUD in 2019 worldwide (2). In addition, in Taiwan, a prospective study of 6,457 individuals undergoing health examinations reported that of those diagnosed with PUD in esophagogastroduodenoscopy, two-thirds were asymptomatic (3). Proposed risk factors for PUD include increased body mass index (BMI), smoking tobacco, malignancy, stress, radiation therapy, non-steroidal anti-inflammatory drugs, chemotherapy, and helicobacter pylori infection (3–5). Complications include ulcer bleeding, gastric outlet obstruction, bowel penetration and fistulation, and even perforation.
The 2006 Montreal Definition and Classification defined gastroesophageal reflux disease (GERD) as troublesome symptoms and/or complications caused by reflux of stomach contents (6). The estimated incidence of GERD is 5.0 per 1,000 person-years globally, with a lower prevalence in Asia than in Western countries (7). In addition, the reported weekly prevalence of gastroesophageal reflux symptoms is highest in Southeast Europe and South Asia (>25%) followed by Central America (19.6%), and lowest in Southeast Asia (7.4%) (7–9). A recent meta-analysis concluded that increased BMI, tobacco smoking, heredity, and Helicobacter pylori infection were the major risk factors for GERD (9, 10). Other risk factors including alcohol consumption and dietary factors (10) have also been proposed. Established complications include esophageal adenocarcinoma, Barrett’s esophagus, reflux esophagitis, and reflux stricture, while proposed complications include idiopathic pulmonary fibrosis and recurrent otitis media (6). Due to the growing incidence of PUD and GERD and their complications (2, 8), more research is needed to elucidate the associated risk factors.
Smoking is associated with increased prevalence and mortality of many diseases, including atherosclerotic cardiovascular disease, malignancy, chronic obstructive pulmonary disease, infection, osteoporosis, hip fracture, reproductive disorders, PUD, periodontal disease and ophthalmologic disorders (11–17). Notably, many studies have also reported the deleterious effects of secondhand smoke (SHS). According to the 2012 International Agency for Research on Cancer published report, SHS mainly consisted of two parts. The first part compromised burning end of a cigarette (or other burned tobacco compounds), which called diluted sidestream smoke. The second part includes smoke emitted during puffing and gasses diffused during smoking through the cigarette paper, which also called exhaled mainstream smoke (18). SHS was also names as passive smoking, which signified its involuntariness (18). A 2011 retrospective study (19) with data from 192 countries identified associations between SHS exposure with asthma, premature death, lower respiratory infection, and ischemic heart disease. SHS has also been associated with an elevated risk of lung cancer (20), cardiovascular disease (21–23), stroke (11, 24), type 2 diabetes mellitus (DM) (25), gestational DM (26, 27), and female sexual dysfunction (28). Regarding gastrointestinal diseases, a 2021 Japanese case–control study disclosed that people exposed to passive smoking at home had a higher risk of developing ulcerative colitis (29). In addition, a study (30) of people who had never used tobacco reported that higher SHS exposure was correlated with an increased risk of esophageal squamous cell carcinoma. Moreover, a case–control study (31) of children with pathologically confirmed esophagitis reported a significantly higher risk of esophagitis in those whose parents smoked, and a retrospective study (32) of 34 infants reported that those exposed to environmental tobacco smoke (ETS) had elevated pH parameters and higher reflux index. Furthermore, the authors emphasized that ETS was a strong risk factor for infantile gastroesophageal reflux (32).
Despite these findings, few studies have investigated the associations between SHS with PUD and GERD. Therefore, the aims of this population-based study was to examine the association between SHS with PUD and GERD in a large cohort of never-smokers from the Taiwan Biobank (TWB), and also to determine the relative risk of SHS exposure frequency with PUD and GERD.
Materials and methods
TWB
The TWB is a pioneering project initiated in 2008 with the aim of advancing healthcare in Taiwan by recording health information including genetic and lifestyle factors and storing biological samples from Taiwanese participants (33, 34). Ethical approval for the TWB was granted by the Institutional Review Board on Biomedical Science Research, Academia Sinica, Taiwan and the Ethics and Governance Council of the TWB.
Ethics statement
All enrollees in the TWB are requested to sign written informed consent forms. The current study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20210058), and was conducted following the Helsinki Declaration.
Study participants
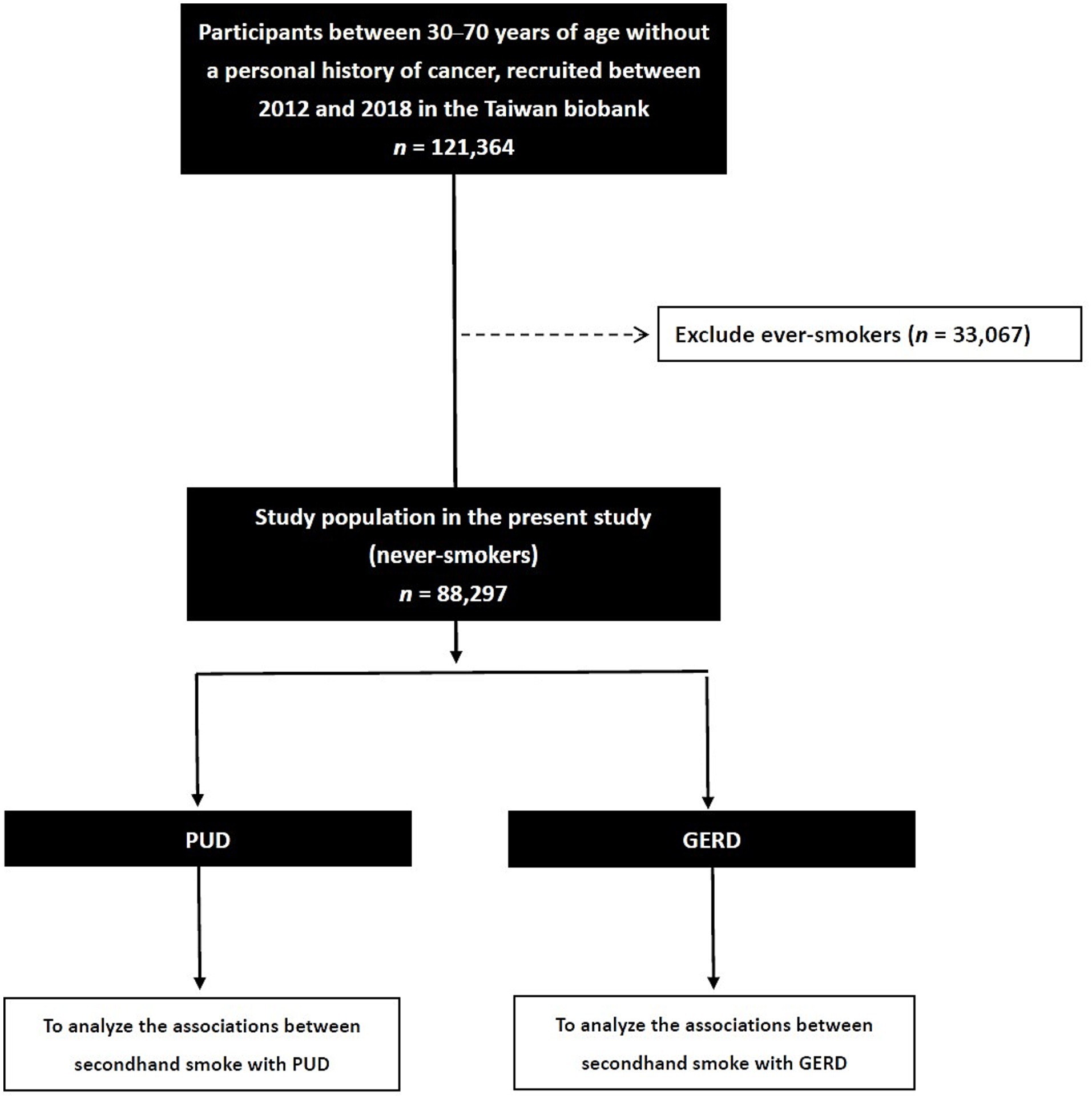
Of the 121,364 subjects enrolled in the TWB, those with a history of smoking (n = 33,067) were excluded from this study, and the remaining 88,297 were included (male: 18,595; female: 69,702; mean age 50.1 ± 11.0 years) for analysis (Figure 1).
Collection of study variables
We recorded body height and weight, and systolic and diastolic blood pressures (BP). The average of three BP measurements taken after a 1–2-min break using an electronic BP device after refraining from smoking, exercise and caffeine intake for at least 30 min was used in the analysis. In addition, information on the presence of hypertension and DM, smoking status, age and sex were also obtained, and the participants were also asked whether they had a history of PUD or GERD. Those who reported a history were then classified into the PUD or GERD group accordingly.
Other variables of interest included estimated glomerular filtration rate [eGFR; calculated as reported previously (35)], triglycerides, total cholesterol, high- and low-density lipoprotein cholesterol (HDL-C/LDL-C), hemoglobin, uric acid and fasting glucose.
Smoking and SHS assessments
We first grouped the participants as “never-smokers,” “ex-smokers,” or “active smokers” according to questionnaires which they were asked to complete. The never-smokers were then asked whether they had ever been exposed to SHS. Those who replied that they had were classified into the exposure group, and those without exposure to SHS were classified into the no exposure group. The participants who had been exposed to SHS were then asked, “How many hours per week have you been exposed to SHS?” According to their answer, they were further classified into “no exposure,” “<1 h per week,” and “≥1 h per week” groups. The cutoff point of 1 h per week was chosen according to the median time of exposure in our participants.
Statistical analysis
The study variables are presented in percentage or mean ± SD as appropriate. Independent t-tests were used for comparing differences in continuous variables, and chi-square tests were used for categorical variables. Factors associated with PUD and/or GERD were identified by multivariable logistic regression. All tests were two-tailed, and a statistically significant association was considered at a p-value <0.05. SPSS was used for the analysis (v19, IBM Inc., Armonk, NY, United States).
Results
The 88,297 participants were classified into those with PUD (n = 11,909; 13.5%) or without PUD (n = 76,388; 86.5%), and with GERD (n = 11,758; 13.3%) or without GERD (n = 76,539; 86.7%).
Clinical characteristics of the PUD groups
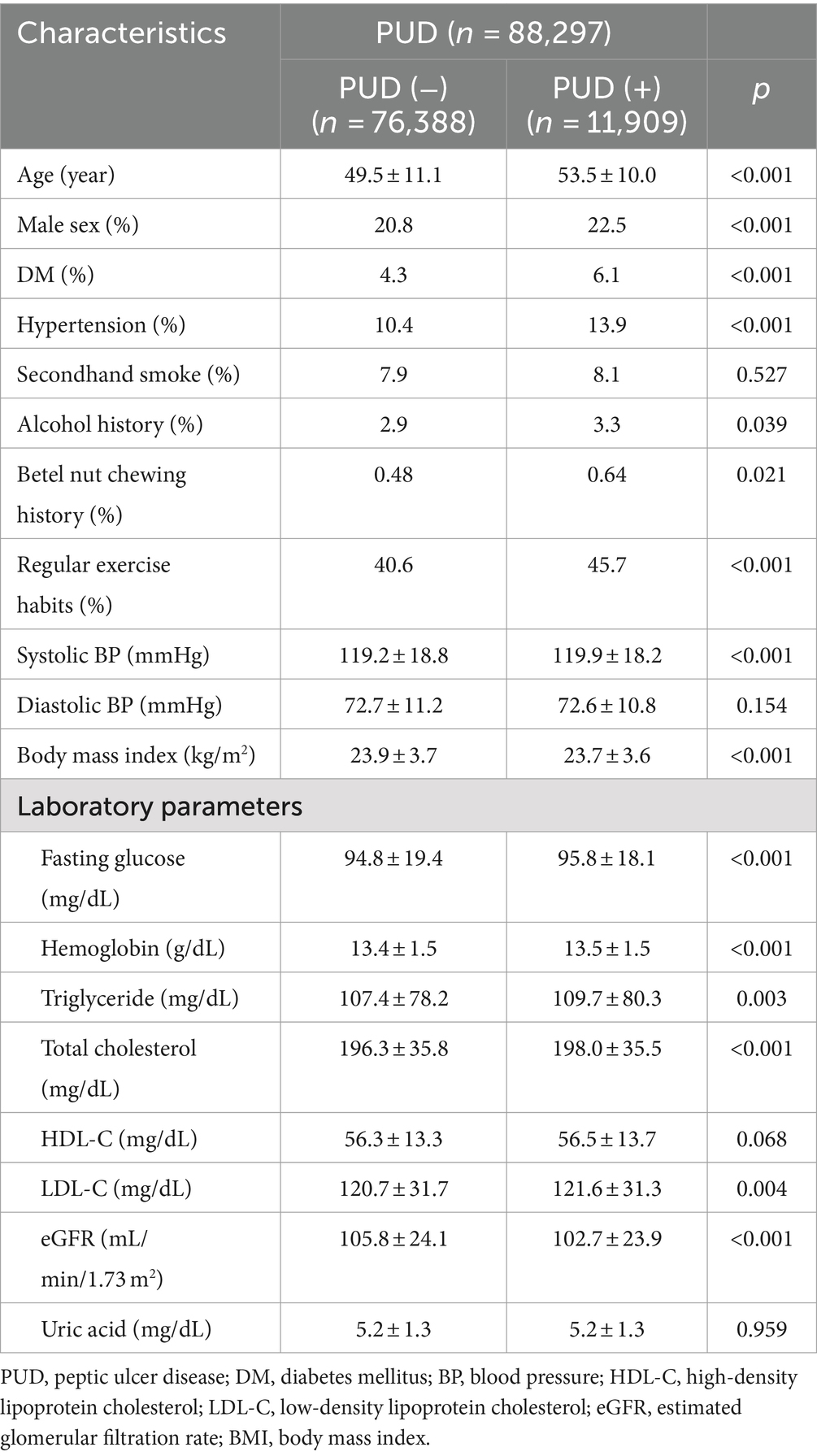
The clinical characteristics of the participants with and without PUD are shown in Table 1. Compared to the participants without PUD, those with PUD were older, predominantly male, had higher prevalence rates of DM and hypertension, higher prevalence rates of alcohol and betel nut chewing history, higher prevalence of regular exercise habit, higher systolic BP, higher fasting glucose, higher hemoglobin, higher triglyceride, higher total cholesterol, higher LDL-C and lower eGFR.
Association of SHS with PUD
Multivariable analysis adjusting for age, sex, hypertension, DM, SHS, alcohol intake, betel quid chewing, regular exercise, systolic BP, BMI, hemoglobin, fasting glucose, triglycerides, total cholesterol, LDL-C and eGFR, showed significant associations between SHS (odds ratio [OR] = 1.166; 95% confidence interval [CI] = 1.084–1.254), old age, male sex, hypertension, DM, low systolic BP, low BMI (all p < 0.001), no regular exercise and low fasting glucose (both p = 0.003), and high hemoglobin (p = 0.014) with PUD (Table 2).

Table 2. Association of secondhand smoke with PUD using multivariable logistic regression analysis in never smokers (n = 88,297).
Clinical characteristics of the GERD groups
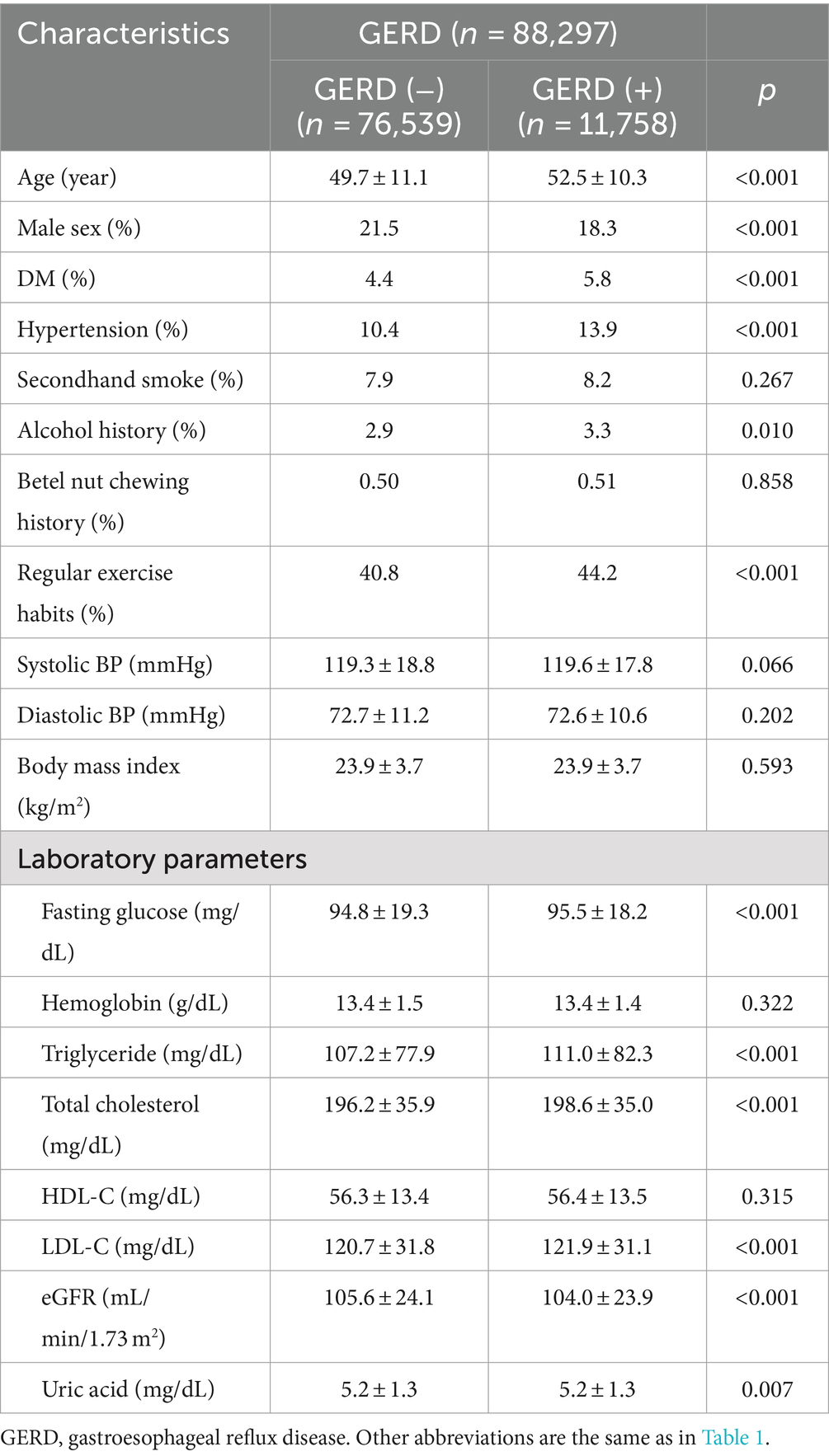
The clinical characteristics of the participants with and without GERD are shown in Table 3. Compared to the participants without GERD, those with GERD were older, predominantly female, had higher prevalence rates of DM and hypertension, higher prevalence rates of alcohol history, higher prevalence of regular exercise habit, higher fasting glucose, higher triglyceride, higher total cholesterol, higher LDL-C, lower eGFR and lower uric acid.
Association of SHS with GERD
Multivariable analysis adjusting for age, sex, hypertension, DM, SHS, alcohol intake, regular exercise, uric acid, fasting glucose, triglycerides, total cholesterol, eGFR and LDL-cholesterol showed significant associations between SHS (OR = 1.131; 95% CI = 1.053–1.216), alcohol history, low fasting glucose (all p = 0.001), old age, female sex, low uric acid, hypertension (all p < 0.001), DM (p = 0.003), and high triglycerides (p = 0.005) with GERD (Table 4).
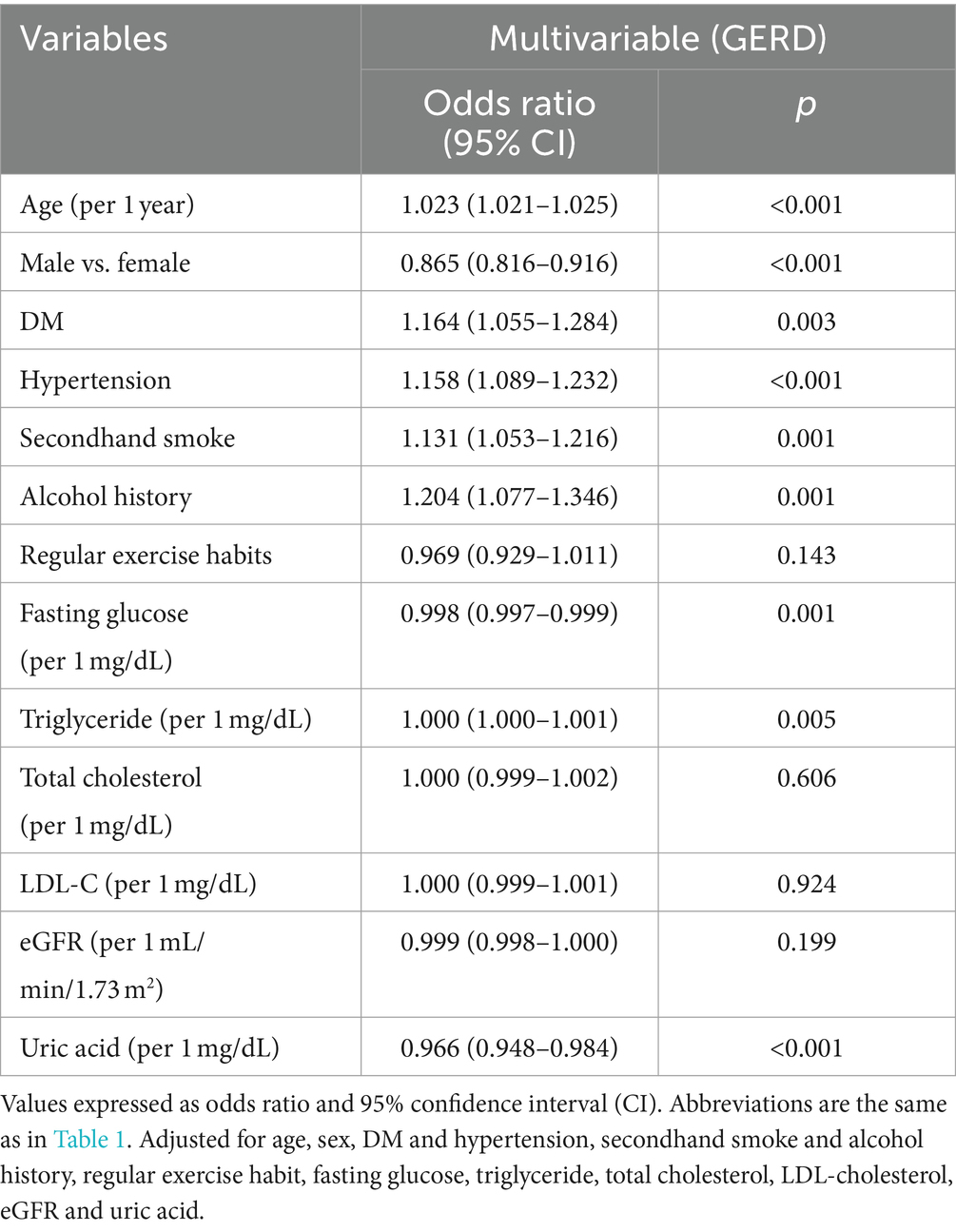
Table 4. Association of secondhand smoke with GERD using multivariable logistic regression analysis in never smokers (n = 88,297).
Association between SHS frequency with PUD and GERD
After adjusting for confounders, the participants who were exposed to SHS ≥ 1 h per week (vs. no exposure; OR = 1.232; 95% CI = 1.121–1.355; p < 0.001) were associated with a higher risk of PUD. In addition, those who were exposed to SHS ≥ 1 h per week (vs. no exposure; OR = 1.200; 95% CI = 1.093–1.319; p < 0.001) were associated with a higher risk of GERD (Table 5).
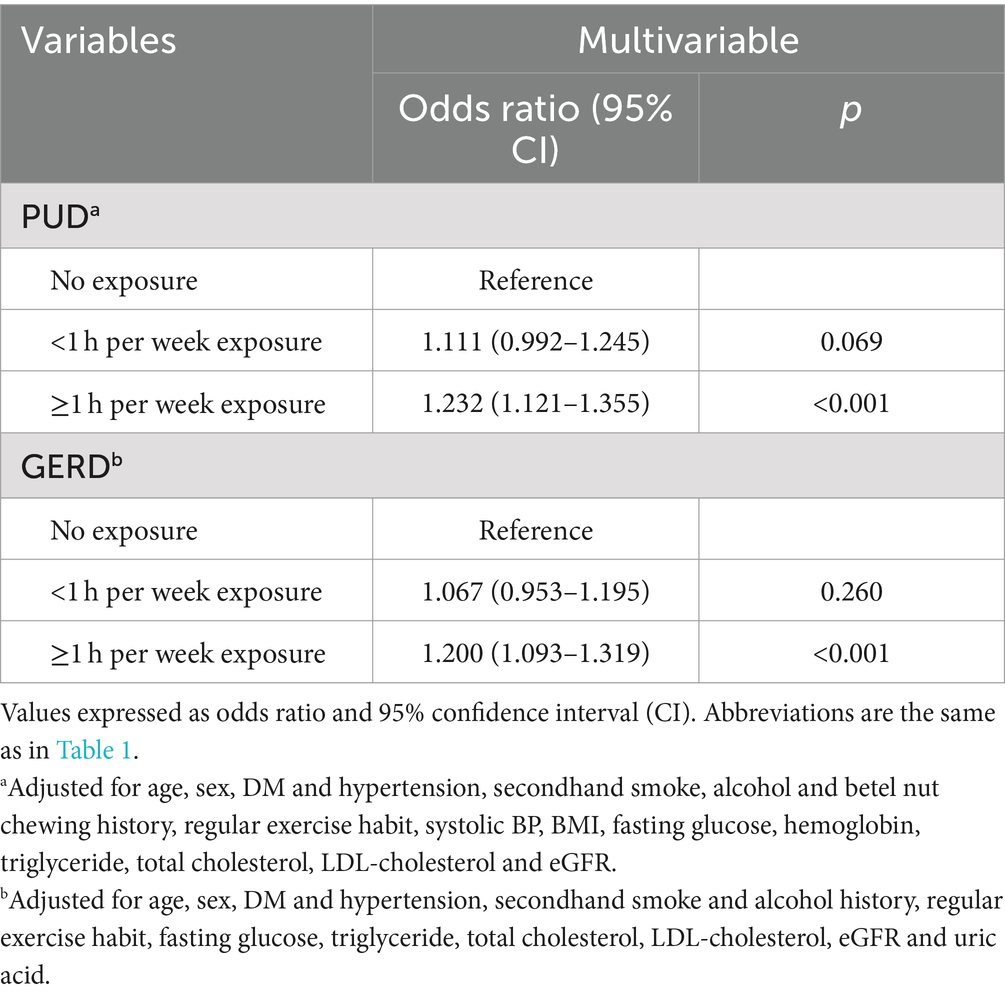
Table 5. Relative risk for PUD and GERD according to frequency of secondhand smoke in never smokers (n = 88,297).
Association of smoke status with PUD using multivariable logistic regression analysis
Supplementary Table S1 showed association of smoke status with PUD using multivariable logistic regression analysis in all participants (n = 121,364). Multivariable analysis showed that never smokers, SHS (+) [vs. never smokers, SHS (−); OR = 1.163; 95% CI = 1.082–1.249; p < 0.001], and ex-, or active smokers [vs. never smokers, SHS (−); OR = 1.274; 95% CI = 1.218–1.331; p < 0.001] were significantly associated with PUD.
Association of smoke status with GERD using multivariable logistic regression analysis
Supplementary Table S2 showed association of smoke status with GERD using multivariable logistic regression analysis in all participants (n = 121,364). Multivariable analysis showed that never smokers, SHS (+) [vs. never smokers, SHS (−); OR = 1.124; 95% CI = 1.046–1.207; p < 0.001], and ex-, or active smokers [vs. never smokers, SHS (−); OR = 1.264; 95% CI = 1.209–1.322; p < 0.001] were significantly associated with GERD.
Discussion
The results of this population-based study of 88,297 participants showed that the individuals in the SHS exposure group had increased risks of PUD and GERD. Furthermore, compared with no exposure, the participants exposed to SHS for ≥1 h per week were associated with a 1.23-fold higher risk of PUD and 1.20-fold higher risk of GERD. Furthermore, ex-, or active smokers also had increased risks of PUD and GERD.
The first important finding of this study is the association between SHS and PUD. A previous rat study (36) designed a 20-L smoke chamber with smoke/air mixture continuously delivered at a flow rate of 250 mL/h for a 1-h experimental period, and then applied an oral dose of 70% ethanol in a volume of 10 mL/kg 15 min later. The results showed that a higher smoke concentration resulted in larger ethanol-induced gastric mucosal lesions. However, there were no significant differences in possible stress factors including serum pH, partial pressure of carbon dioxide, partial pressure of oxygen, bicarbonate, systemic BP or heart rate before and after tobacco and alcohol exposure. Active smoking was simulated using nicotine administered via an oral route, but this study is the first and to date the only paper to demonstrate that passive smoking with nicotine absorbed through the respiratory tract also worsened ethanol-induced gastric ulcers in a rat model. Although very few studies have elucidated the pathogenesis between SHS and PUD, an increasing amount of research has been conducted to explain the relationship between active smoking and PUD. Ulcer healing (37) involves ulcer margin epithelial cell proliferation, migration, angiogenesis, gastric gland reconstruction and migration. Previous studies (38–40) have demonstrated the positive effects of nitric oxide (NO), prostaglandin E2, and vascular endothelial growth factor on vasodilation, increased mucosal blood flow, and angiogenesis. In another experimental rat model (41) of acetic acid-induced ulcers, rats were exposed to 0, 2% or 4% concentrations of tobacco smoke for 1 h daily for 6 days. The ulcers healed spontaneously after 10 days in the control group, but delayed ulcer healing was noted in the smoke-exposed group (p < 0.05 in the 2% group and p < 0.01 in the 4% group). In subgroup analysis (41), exposure to tobacco smoke at 4% concentration was associated with a marked reduction in ulcer base constitutive NO synthase activity and ulcer margin micro-vessels. In addition, caspase-3 was found to be activated during apoptosis during the process of programmed cell death. In another rodent study (42), the smoke-exposed group showed higher levels of activated caspase-3 in immunohistochemistry compared with the air-exposed group. Epidermal growth factor (EGF) plays a vital role in gastrointestinal ulcer cell reconstruction and proliferation (43), and it is mainly formed by the salivary glands, Brunner’s glands in the duodenum, and pancreas (44). Konturek et al. (44) recruited 36 healthy male volunteers and asked them to smoke one cigarette every 30 min. EGF concentrations were then measured and found to be significantly decreased in the saliva and duodenum. Ma et al. (45) also reported decreased serum and gastric mucosal EGF concentrations as well as salivary gland EGF messenger ribonucleic acid expression in the smoke-exposed group of acetic acid-induced ulcer rats. Surprisingly, the intravenous administration of EGF reduced ulcer size, angiogenesis, and muscular cell proliferation in the smoke-exposed group (45). In summary, active smoking affects ulcer site healing by increasing apoptosis, inhibiting angiogenesis, and decreasing vasodilation and reconstruction based on current evidence. The aforementioned mechanisms may partially explain the association between SHS and PUD.
Our results also showed an association between SHS and GERD. One case–control study (31) of 278 children with esophageal biopsy-confirmed esophagitis identified a 6-fold higher risk of esophagitis if at least one parent smoked (p < 0.001; relative risk 6.1, 95% CI 3.2 to 11.3). Proposed pathogeneses include a combination of increased free radical producing activity and a lower antioxidant level (31, 46). Monajemzadeh et al. (47) investigated the association between nicotinine, a nicotine metabolite, and esophagitis in children with GERD, and found an increased risk of developing esophagitis in children exposed to ETS. However, there is currently little evidence to explain the association between SHS exposure and GERD in adults. Several studies have investigated the connection between active smoking and GERD. One study (48) using 24-h ambulatory esophageal pH monitoring showed that chronic smokers had more reflux episodes and increased duration of esophageal acid exposure. Many studies (48–50) have found that decreased gastroesophageal sphincter pressure contributes to GERD in smokers. Dennish and Castell (49) enrolled six normal men who were chronic smokers and studied the relationship between smoking and lower esophageal sphincter pressure using a triple-lumen polyvinyl tube. They found that the mean lower esophageal sphincter pressure fell to 11.4 mmHg from a baseline value of 19.6 mmHg 2 to 3 min after smoking, and the difference was significant (p < 0.001). Moreover, they found that the gastroesophageal sphincter pressure returned to baseline level 5 min after the men stopped smoking. Stanciu and Bennett (48) also found a significant change in end-expiratory gastroesophageal sphincter pressure before and after smoking (from a mean 10.8 cmH2O to 6.4 cmH2O, p < 0.01). Stanciu and Bennett (48) hypothesized that this decrease may be due to the cholinergic system being blocked by nicotine, as a previous in-vitro study found that nicotine could cause relaxation of lower esophagus circular muscle fibers. Kahrilas and Gupta (51) further confirmed these findings, as they showed that smokers with heartburn and endoscopically confirmed esophagitis had a lower esophagus sphincter pressure compared with asymptomatic smokers. Smoking has also been shown to inhibit acid-clearing capacity as assessed using a modified acid-clearing test (52). Another study Koelz et al. (53) also found that smoking was positively correlated with ranitidine treatment failure in patients with peptic esophagitis during a 6-week treatment period. Taken together, these studies shed light on the possible pathophysiology of active smoking and GERD, including more reflux episodes, increased duration of exposure to esophageal acid, reduced acid-clearing ability, and decreased lower gastroesophageal sphincter pressure.
In the study, multivariable analysis (Table 2) showed that high hemoglobin was associated with a high risk of PUD, and regular exercise habit was associated with a low risk of PUD. Stress-induced hemoconcentration (54) may be the reasons of high hemoglobin in PUD group. Hemoconcentration attributes to the conditions when the ratio of serum cellular components to the plasma volume increases, especially red blood cells (55). Stress-hemoconcentration specified the condition that an acute elevation of blood pressure and a net efflux of plasma into third spacing which results in increased colloid osmotic pressure and raised plasma protein concentration after stressors (55). Evidence (56) has shown that the presence of gastro-intestinal ulcer after burns is directly connected to the occurrence of hemoconcentration. As for the association between regular exercise habit and PUD, there is growing evidence that the circulation cells of the innate immune system and the anti-inflammatory and antioxidant effect increase after exercise (57). In one review article, there was evidence that increased physical activity enhance the ability to deal with physical distress and anxiety (58). In the experimental study, peak gastric acid level had fallen to about 60% after exercise with statistically significance (59). Study had shown reduced risk of duodenal ulcer formation in physical active individuals (60). Decrease gastric acid secretion, rebust innate immune system, increase anti-inflammatory effect may explain regular exercise habit with low risk of PUD.
Various social, psychological, and biological factors also play a role in the development of PUD. In some population-based studies, stress, depressed mood (61), suicidal thoughts (61), panic disorders (62), and childhood abuse (63) showed positively correlation with PUD. However, TWB did not collect psychological status of the involved volunteers and thus may underestimated their effect on occurrence of PUD.
The results of this study are enhanced by the inclusion of a large cohort. The limitations of this cross-sectional study include that the durations of PUD and GERD were not evaluated, so that causal relationships between SHS with PUD and GERD could also not be evaluated. Longitudinal studies are needed to investigate this issue. Another limitation is that the occurrence of PUD and GERD was ascertained through the participants’ responses to questionnaires without endoscopic verification, and thus their type and severity could not be ascertained, which may lead to incorrect information due to recall bias. However, a previous study (64) from Taiwan noted moderate agreement between claims records and diseases identified through questionnaires. In addition, we did not conduct subgroup analyses on the brand of cigarette, the extent and amount of SHS, and the place and distance where the non-smoker was exposed. Fourth, the TWB collects health-related data on healthy volunteers across Taiwan, and women may be more willing or able to participate in research studies compared with men due to greater health awareness. Thus, our findings may not be generalizable to the general population. Finally, the generalizability of our findings may be limited by the ethnicity of our participants, all of whom were of Chinese ethnicity.
In conclusion, we found significant associations between SHS with PUD and GERD. Furthermore, exposure to SHS for ≥1 h per week (vs. no exposure) was associated with 1.23- and 1.20-fold higher risks of PUD and GERD, respectively. This study represents the largest population-based investigation to date to explore the association between SHS with PUD and GERD in Taiwanese never-smokers.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20210058). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
P-CY: Writing – original draft. J-HG: Writing – review & editing, Methodology, Data curation, Conceptualization. P-YW: Writing – review & editing, Data curation, Conceptualization. J-CH: Writing – review & editing, Data curation, Conceptualization. H-MH: Writing – review & editing, Supervision, Conceptualization. C-HK: Writing – review & editing, Supervision, Data curation, Conceptualization. S-CC: Writing – original draft, Writing – review & editing, Supervision, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Ministry of Education (MOE) in Taiwan and by Kaohsiung Medical University Research Center Grant (KMU-TC113A01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1450481/full#supplementary-material
References
1. Lanas, A, and Chan, FKL. Peptic ulcer disease. Lancet. (2017) 390:613–24. doi: 10.1016/S0140-6736(16)32404-7
2. Xie, X, Ren, K, Zhou, Z, Dang, C, and Zhang, H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study. BMC Gastroenterol. (2022) 22:58. doi: 10.1186/s12876-022-02130-2
3. Lu, CL, Chang, SS, Wang, SS, Chang, FY, and Lee, SD. Silent peptic ulcer disease: frequency, factors leading to “silence,” and implications regarding the pathogenesis of visceral symptoms. Gastrointest Endosc. (2004) 60:34–8. doi: 10.1016/S0016-5107(04)01311-2
4. Kavitt, RT, Lipowska, AM, Anyane-Yeboa, A, and Gralnek, IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. (2019) 132:447–56. doi: 10.1016/j.amjmed.2018.12.009
5. Chan, FK, and Leung, WK. Peptic-ulcer disease. Lancet. (2002) 360:933–41. doi: 10.1016/S0140-6736(02)11030-0
6. Vakil, N, van Zanten, SV, Kahrilas, P, Dent, J, and Jones, R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. (2006) 101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x
7. Richter, JE, and Rubenstein, JH. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology. (2018) 154:267–76. doi: 10.1053/j.gastro.2017.07.045
8. Eusebi, LH, Ratnakumaran, R, Yuan, Y, Solaymani-Dodaran, M, Bazzoli, F, and Ford, AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. (2018) 67:430–40. doi: 10.1136/gutjnl-2016-313589
9. Dent, J, El-Serag, HB, Wallander, MA, and Johansson, S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. (2005) 54:710–7. doi: 10.1136/gut.2004.051821
10. Maret-Ouda, J, Markar, SR, and Lagergren, JJJ. Gastroesophageal reflux disease: a review. JAMA. (2020) 324:2536–47. doi: 10.1001/jama.2020.21360
11. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of Progress: A report of the surgeon general. Atlanta (GA): Centers for Disease Control and Prevention (US) (2014).
12. Ezzati, M, Henley, SJ, Thun, MJ, and Lopez, AD. Role of smoking in global and regional cardiovascular mortality. Circulation. (2005) 112:489–97. doi: 10.1161/CIRCULATIONAHA.104.521708
13. United States Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking cessation: a report of the surgeon general. Washington (DC): US Department of Health and Human Services (2020).
14. Christenson, SA, Smith, BM, Bafadhel, M, and Putcha, N. Chronic obstructive pulmonary disease. Lancet. (2022) 399:2227–42. doi: 10.1016/S0140-6736(22)00470-6
15. Kanis, JA, Johnell, O, Oden, A, Johansson, H, De Laet, C, Eisman, JA, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. (2005) 16:155–62. doi: 10.1007/s00198-004-1640-3
16. Parasher, G, and Eastwood, GL. Smoking and peptic ulcer in the Helicobacter pylori era. Eur J Gastroenterol Hepatol. (2000) 12:843–53. doi: 10.1097/00042737-200012080-00003
17. Zee, KY. Smoking and periodontal disease. Aust Dent J. (2009) 54:S44–50. doi: 10.1111/j.1834-7819.2009.01142.x
18. National Research Council (US) Committee on Passive Smoking. Environmental tobacco smoke: Measuring exposures and assessing health effects. Washington (DC): National Academies Press (US) (1986).
19. Oberg, M, Jaakkola, MS, Woodward, A, Peruga, A, and Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. (2011) 377:139–46. doi: 10.1016/S0140-6736(10)61388-8
20. Taylor, R, Najafi, F, and Dobson, A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol. (2007) 36:1048–59. doi: 10.1093/ije/dym158
21. Jones, MR, Magid, HS, Al-Rifai, M, McEvoy, JW, Kaufman, JD, Hinckley Stukovsky, KD, et al. Secondhand smoke exposure and subclinical cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. (2016) 5:2965. doi: 10.1161/JAHA.115.002965
22. Steenland, K, Thun, M, Lally, C, and Heath, C. Environmental tobacco smoke and coronary heart disease in the American Cancer Society CPS-II cohort. Circulation. (1996) 94:622–8. doi: 10.1161/01.CIR.94.4.622
23. Wells, AJ. Passive smoking as a cause of heart disease. J Am Coll Cardiol. (1994) 24:546–54. doi: 10.1016/0735-1097(94)90315-8
24. Zhang, X, Shu, XO, Yang, G, Li, HL, Xiang, YB, Gao, YT, et al. Association of passive smoking by husbands with prevalence of stroke among Chinese women non-smokers. Am J Epidemiol. (2005) 161:213–8. doi: 10.1093/aje/kwi028
25. Pan, A, Wang, Y, Talaei, M, Hu, FB, and Wu, T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2015) 3:958–67. doi: 10.1016/S2213-8587(15)00316-2
26. Zhang, H, Zhou, X, Tian, L, Huang, J, Meng, E, and Yin, J. Passive smoking and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Tob Induc Dis. (2023) 21:115. doi: 10.18332/tid/169722
27. Na, J, Chen, H, An, H, Ren, M, Jia, X, Wang, B, et al. Passive smoking and risk of gestational diabetes mellitus among non-smoking women: a prospective cohort study in China. Int J Environ Res Public Health. (2022) 19:4712. doi: 10.3390/ijerph19084712
28. Ju, R, Ruan, X, Xu, X, Yang, Y, Cheng, J, Zhang, L, et al. Importance of active and passive smoking as one of the risk factors for female sexual dysfunction in Chinese women. Gynecol. Endocrinol. (2021) 37:541–5. doi: 10.1080/09513590.2021.1913115
29. Nishikawa, A, Tanaka, K, Miyake, Y, Nagata, C, Furukawa, S, Andoh, A, et al. Active and passive smoking and risk of ulcerative colitis: a case-control study in Japan. J Gastroenterol Hepatol. (2022) 37:653–9. doi: 10.1111/jgh.15745
30. Rafiq, R, Shah, IA, Bhat, GA, Lone, MM, Islami, F, Boffetta, P, et al. Secondhand smoking and the risk of esophageal squamous cell carcinoma in a high incidence region, Kashmir, India: a case-control-observational study. Medicine. (2016) 95:e2340. doi: 10.1097/MD.0000000000002340
31. Shabib, SM, Cutz, E, and Sherman, PM. Passive smoking is a risk factor for esophagitis in children. J Pediatr. (1995) 127:435–7. doi: 10.1016/S0022-3476(95)70078-1
32. Alaswad, B, Toubas, PL, and Grunow, JE. Environmental tobacco smoke exposure and gastroesophageal reflux in infants with apparent life-threatening events. J Okla State Med Assoc. (1996) 89:233–7.
33. Chen, CH, Yang, JH, Chiang, CWK, Hsiung, CN, Wu, PE, Chang, LC, et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan biobank project. Hum Mol Genet. (2016) 25:5321–31. doi: 10.1093/hmg/ddw346
34. Fan, CT, Hung, TH, and Yeh, CK. Taiwan regulation of biobanks. J Law Med Ethics. (2015) 43:816–26. doi: 10.1111/jlme.12322
35. Levey, AS, Bosch, JP, Lewis, JB, Greene, T, Rogers, N, and Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. (1999) 130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002
36. Chow, JY, Ma, L, and Cho, CH. An experimental model for studying passive cigarette smoking effects on gastric ulceration. Life Sci. (1996) 58:2415–22. doi: 10.1016/0024-3205(96)00245-7
37. Tarnawski, A, and Halter, F. Cellular mechanisms, interactions, and dynamics of gastric ulcer healing. J Clin Gastroenterol. (1995) 21:S93–7.
38. Ziche, M, Morbidelli, L, Masini, E, Amerini, S, Granger, HJ, Maggi, CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. (1994) 94:2036–44. doi: 10.1172/JCI117557
39. Leung, DW, Cachianes, G, Kuang, WJ, Goeddel, DV, and Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. (1989) 246:1306–9. doi: 10.1126/science.2479986
40. Form, DM, and Auerbach, R. PGE2 and Angiogenesis. Proc Soc Exp Biol Med. (1983) 172:214–8. doi: 10.3181/00379727-172-41548
41. Ma, L, Chow, JY, and Cho, CH. Cigarette smoking delays ulcer healing: role of constitutive nitric oxide synthase in rat stomach. Am J Phys. (1999) 276:G238–48. doi: 10.1152/ajpgi.1999.276.1.G238
42. Verschuere, S, Bracke, KR, Demoor, T, Plantinga, M, Verbrugghe, P, Ferdinande, L, et al. Cigarette smoking alters epithelial apoptosis and immune composition in murine GALT. Lab Invest. (2011) 91:1056–67. doi: 10.1038/labinvest.2011.74
43. Wright, NA, Pike, C, and Elia, G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. (1990) 343:82–5. doi: 10.1038/343082a0
44. Konturek, JW, Bielanski, W, Konturek, SJ, Bogdal, J, and Oleksy, J. Distribution and release of epidermal growth factor in man. Gut. (1989) 30:1194–200. doi: 10.1136/gut.30.9.1194
45. Ma, L, Wang, WP, Chow, JY, Yuen, ST, and Cho, CH. Reduction of EGF is associated with the delay of ulcer healing by cigarette smoking. Am J Physiol Gastrointest Liver Physiol. (2000) 278:G10–7. doi: 10.1152/ajpgi.2000.278.1.G10
46. Kalra, J, Chaudhary, AK, and Prasad, K. Increased production of oxygen free radicals in cigarette smokers. Int J Exp Pathol. (1991) 72:1–7.
47. Monajemzadeh, M, Haghi-Ashtiani, MT, Soleymani, R, Shams, S, Taleb, S, Motamed, F, et al. Is there any association between passive smoking and esophagitis in pediatrics? Iran J Pediatr. (2013) 23:194–8.
48. Stanciu, C, and Bennett, JR. Smoking and gastro-oesophageal reflux. Br Med J. (1972) 3:793–5. doi: 10.1136/bmj.3.5830.793
49. Dennish, GW, and Castell, DO. Inhibitory effect of smoking on the lower esophageal sphincter. N Engl J Med. (1971) 284:1136–7.
50. Chattopadhyay, DK, Greaney, MG, and Irvin, TT. Effect of cigarette smoking on the lower oesophageal sphincter. Gut. (1977) 18:833–5. doi: 10.1136/gut.18.10.833
51. Kahrilas, PJ, and Gupta, RR. Mechanisms of acid reflux associated with cigarette smoking. Gut. (1990) 31:4–10. doi: 10.1136/gut.31.1.4
52. Kjellén, G, and Tibbling, L. Influence of body position, dry and water swallows, smoking, and alcohol on esophageal acid clearing. Scand J Gastroenterol. (1978) 13:283–8. doi: 10.3109/00365527809179821
53. Koelz, HR, Birchler, R, Bretholz, A, Bron, B, Capitaine, Y, Delmore, G, et al. Healing and relapse of reflux esophagitis during treatment with ranitidine. Gastroenterology. (1986) 91:1198–205. doi: 10.1016/S0016-5085(86)80017-8
54. Allen, MT, and Patterson, SM. Hemoconcentration and stress: a review of physiological mechanisms and relevance for cardiovascular disease risk. Biol Psychol. (1995) 41:1–27. doi: 10.1016/0301-0511(95)05123-R
55. Austin, AW, Patterson, SM, and von Känel, R. Hemoconcentration and hemostasis during acute stress: interacting and independent effects. Ann Behav Med. (2011) 42:153–73. doi: 10.1007/s12160-011-9274-0
56. Friesen, SR, and Wangensteen, OH. Role of hemoconcentration in production of gastric and duodenal ulcer following experimental burns. Proc Soc Exp Biol Med. (1947) 64:81–5.
57. Nieman, DC, and Wentz, LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. (2019) 8:201–17. doi: 10.1016/j.jshs.2018.09.009
58. Scully, D, Kremer, J, Meade, MM, Graham, R, and Dudgeon, K. Physical exercise and psychological well being: a critical review. Br J Sports Med. (1998) 32:111–20. doi: 10.1136/bjsm.32.2.111
59. Ramsbottom, N, and Hunt, JN. Effect of exercise on gastric emptying and gastric secretion. Digestion. (1974) 10:1–8. doi: 10.1159/000197517
60. Cheng, Y, Macera, CA, Davis, DR, and Blair, SN. Does physical activity reduce the risk of developing peptic ulcers? Br J Sports Med. (2000) 34:116–21. doi: 10.1136/bjsm.34.2.116
61. Lee, YB, Yu, J, Choi, HH, Jeon, BS, Kim, HK, Kim, SW, et al. The association between peptic ulcer diseases and mental health problems: a population-based study: a STROBE compliant article. Medicine. (2017) 96:e7828. doi: 10.1097/MD.0000000000007828
62. Goodwin, RD, Talley, NJ, Hotopf, M, Cowles, RA, Galea, S, and Jacobi, F. A link between physician-diagnosed ulcer and anxiety disorders among adults. Ann Epidemiol. (2013) 23:189–92. doi: 10.1016/j.annepidem.2013.01.003
63. Fuller-Thomson, E, Bottoms, J, Brennenstuhl, S, and Hurd, M. Is childhood physical abuse associated with peptic ulcer disease? Findings from a population-based study. J Interpers Violence. (2011) 26:3225–47. doi: 10.1177/0886260510393007
Keywords: secondhand smoke, peptic ulcer disease, gastroesophageal reflux disease, Taiwan Biobank, risk factors
Citation: Yen P-C, Geng J-H, Wu P-Y, Huang J-C, Hu H-M, Kuo C-H and Chen S-C (2024) Secondhand smoke is associated with peptic ulcer disease and gastroesophageal reflux disease in non-smokers in a large Taiwanese population study. Front. Public Health. 12:1450481. doi: 10.3389/fpubh.2024.1450481
Edited by:
Bhawna Gupta, Torrens University Australia, AustraliaReviewed by:
Fu-Chen Kuo, I-Shou University, TaiwanLi-Nien Chien, National Yang Ming Chiao Tung University, Taiwan
Copyright © 2024 Yen, Geng, Wu, Huang, Hu, Kuo and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao-Hung Kuo, a2poODhrbXVAZ21haWwuY29t; Szu-Chia Chen, c2NhcmNoZW5vbmVAeWFob28uY29tLnR3
 Pei-Chi Yen
Pei-Chi Yen Jiun-Hung Geng
Jiun-Hung Geng Pei-Yu Wu
Pei-Yu Wu Jiun-Chi Huang
Jiun-Chi Huang Huang-Ming Hu
Huang-Ming Hu Chao-Hung Kuo
Chao-Hung Kuo Szu-Chia Chen
Szu-Chia Chen