- 1The First Clinical Medical School, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2Department of Cardiology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 3Institute of Child and Adolescent Health, School of Public Health, Peking University, Beijing, China
- 4School of Laboratory Animal & Shandong Laboratory Animal Center, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, Shandong, China
Background: Previous studies have found that poor sleep quality promotes the occurrence of cognitive impairment (CI), but this relationship has been rarely reported in older adults hypertensive patients. The purpose of this study was to investigate the relationship between sleep quality and CI in older adults hypertensive patients and the mediating effect of sleep quality between physical activity (PA) and CI.
Methods: A total of 2072 older adults hypertensive patients were included in this case–control study. Five hundred and eighteen older adults hypertensive patients with CI were matched 1:3 by age and sex to 1,554 older adults hypertensive patients with normal cognitive function. The International Physical Activity Questionnaire-Long Form, Pittsburgh Sleep Quality Index, and Mini-Mental State Examination were used to evaluate PA intensity, sleep quality, and cognitive function in older adults hypertensive patients. Multivariate logistic regression and the mediation package in R Language were used to analyze the relationship between sleep quality and CI and the mediating effect of sleep quality between PA intensity and CI in older adults hypertensive patients.
Results: After adjusting for all confounding factors, sleep quality was positively correlated with CI in older adults hypertensive patients (OR = 2.565, 95%CI: 1.958–3.360, p < 0.001), and this relationship also existed in the older adults hypertensive patients with education levels of primary school and below and junior high school and above (OR = 2.468, 95%CI: 1.754–3.473, p < 0.001; OR = 2.385, 95%CI: 1.367–4.161, p = 0.002). In addition, sleep quality mediated part of the mediating effect between PA intensity and CI in older adults hypertensive patients (Za*Zb: - 17.19339; 95%CI: −0.37312, −0.04194).
Conclusion: Poor sleep quality was associated with the occurrence of CI in older adults hypertensive patients, and this relationship also existed in older adults hypertensive patients with education levels of primary school and below and junior high school and above.
1 Introduction
High systolic blood pressure has become a major risk factor for the global burden of disease by 2017 (1). By 2019, approximately 49% of men and 59% of women globally have been hypertensive patients (2). Hypertension is not only associated with an increased risk of cardiovascular disease, chronic kidney disease, stroke, and other diseases (3), but also increases the risk of cognitive impairment (CI) (4). Studies have found that the prevalence of hypertension with mild cognitive impairment (MCI) is 30% (5), and the prevalence of CI in hypertensive patients in China is 37.6% (6). CI has aggravated the economic burden of patients and increased the rehospitalization rate of patients (7, 8), which has become a public health problem plaguing the world. Unfortunately, there is no specific drug for the treatment of CI (9). Therefore, it is very important to prevent and delay the occurrence of CI, especially for hypertensive patients.
Sleep plays a variety of basic biological functions in the human body, such as the organism’s maintenance, repair, and construction (10). Studies have found that sleep has many benefits for human health. It is beneficial to the formation and consolidation of human brain memory (11), the regulation of emotion (12), the balance of energy metabolism (13), the synthesis of biological macromolecules (14), and the clearance of metabolites (15). Poor sleep quality is an important symptom of sleep disorders and reflects the nature and severity of sleep deprivation (16, 17). Recent studies have found that poor sleep quality can promote cognitive decline and there was also a synergistic effect between poor sleep quality and hypertension. Two meta-analyses found that poor sleep quality was associated with MCI (18); the sleep quality of older adults patients with MCI was lower than that of healthy older adults people (19). In addition, a meta-analysis study and a cross-sectional study found that poor sleep quality was associated with hypertension, and hypertension promoted poor sleep quality (20, 21). However, there are few studies on the association between sleep quality and CI in older adults hypertensive patients. Physical activity (PA) is closely related to human health. Studies have found that PA is associated with improvements in sleep quality and cognitive function (22–24), but the relationship among them is rarely reported.
This study aimed to analyze the relationship between sleep quality and CI in older adults hypertensive patients, and whether sleep quality mediates the association between PA and CI.
2 Materials and methods
2.1 Study design and population
2.1.1 Source of study cases
The data of this case–control study came from the hypertension electronic community follow-up system of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine. The system collected hypertension patients from outpatients or wards of 9 hospitals which were the Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan Hospital of Traditional Chinese Medicine, Jinan Minzu Hospital, Qingzhou People’s Hospital, Penglai Hospital of Traditional Chinese Medicine, Tai ‘an First People’s Hospital, Guangrao County Hospital of Traditional Chinese Medicine, Qufu Hospital of Traditional Chinese Medicine, and Weifang Hospital of Traditional Chinese Medicine. The matched case–control sample size was calculated using the following formula:
Among them, uα was 1.96, uβ was 1.28, OR was 2.16, p0 was 0.15, p1 was 0.28, q0 was 0.85, and q1 was 0.72 (25, 26). Since 1:3 matching was performed in this study, it was calculated that the case group was 269 (1.2 × M) at least required, and the control group was 807 at least required. In this study, the hypertensive cases of the system from May 2022 to February 2024 were exported and summarized into an Excel table, with a total of 5,118 cases. After excluding 1714 hypertensive cases with severe data loss and age < 60 years, the total number of included cases was 3,404. The diagnostic criteria for hypertension meet one of the following criteria: (1) office systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg on 3 times on different days without using antihypertensive drugs.; (2) SBP ≥ 135 mmHg and/or DBP ≥ 85 mmHg measured at home 3 times on different days without using antihypertensive drugs; (3) 24 h ambulatory average blood pressure ≥ 130/80 mmHg; daytime ≥135/85 mmHg; nighttime ≥120/70 mmHg. (4) The patient has a history of hypertension and is currently taking antihypertensive drugs. Although the blood pressure is <140/90 mmHg, it should still be diagnosed as hypertension (27). Cognitive function was assessed by Mini-mental State Examination (MMSE). MMSE score between 27 and 30 was defined as normal cognitive function, and MMSE score less than 27 was defined as CI (28).
2.1.2 Inclusion criteria
The inclusion criteria were as follows: (1) age ≥ 60 years old; (2) patients with essential hypertension; (3) signing informed consent; (4) voluntarily completing the collection of hypertension-related information.
2.1.3 Exclusion criteria
The exclusion criteria were as follows: (1) patients with various types of secondary hypertension; (2) vascular dementia or cognitive decline due to vascular factors; (3) new drug clinical trials in the past 3 months; (4) pregnant women, pre-pregnant women, and lactating women; (5) comorbid psychosis and/or psychotropic substance use disorder or dependence; (6) malignant tumor and liver, kidney, and heart function are seriously damaged.
2.1.4 Matching of cases and study indicators
The older adults hypertensive patients with CI and normal cognitive function were matched 1:3 by age and gender, and a total of 2072 older adults hypertensive patients were enrolled, including 518 hypertensive patients with CI and 1,554 hypertensive patients with normal cognitive function. In this study, the baseline data of older adults hypertensive patients were collected, including basic information: age, gender (male/female), education level (primary school and below/junior high school and above), type of work (physical work mainly, mental work mainly, and both), smoking (no/yes), alcohol drinking (no/yes), course of hypertension, hypertension classification (grade 1, 2, and 3), systolic pressure at ordinary times, diastolic pressure at ordinary times, waist-to-hip ratio (WHR): Waist circumference/hip circumference, body mass index (BMI): weight/height^2, hypertension medication (no/yes): calcium channel blocker (CCB), diuretic, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (ACEI or ARB), beta-blockers, sympathetic nerve inhibitor; auxiliary examination: serum metabolic indicators (fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), serum creatinine (Scr)), left ventricular end-diastolic diameter (LVEDD), right ventricular end-diastolic diameter (RVEDD), left atrial diameter (LAD), and subjective evaluation scale: International Physical Activity Questionnaire-Long Form (IPAQ-L), Pittsburgh Sleep Quality Index (PSQI), MMSE.
2.1.5 Ethics statement
This study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine ((2023) Review No. (109) -KY). Hypertensive patients in the electronic community follow-up system of hypertension signed written informed consent.
2.2 Assessment of cognitive function
The Chinese version of MMSE can effectively and reliably assess the cognitive function of the Chinese population (29). The MMSE contains seven simple task domains of time orientation, place orientation, immediate memory, attention and calculation, delayed memory, language ability, and visuospatial ability and is divided into five sections: orientation (10 points), memory (3 points), attention and numeracy (5 points), recall (3 points), and language (9 points). Scores range from 0 to 30, with higher scores indicating better cognitive function (30).
2.3 Assessment of sleep quality
PSQI has good reliability and validity in evaluating sleep quality in the Chinese population. The scale consists of 19 items, including 7 elements: (1) subjective sleep quality; (2) sleep latency; (3) sleep duration; (4) sleep efficiency; (5) sleep disorders; (6) sleeping pills; and (7) daytime dysfunction. Each component is scored on a scale of 0 to 3, and the sum of the scores from the seven components produces a subjective sleep-quality score (which ranges from 0 to 21 points). The PSQI total score > 5 was considered poor sleep quality (31).
2.4 Assessment of physical activity
IPAQ-L can effectively and reliably assess PA intensity in the Chinese population (32, 33). The IPAQ-L is used to assess daily work, daily living, daily transportation, sports, and recreational activities in the past 7 days. IPAQ-L data are converted into metabolic equivalent task scores (METs) for each dimension or intensity of PA. PA per week (MET-min/week) is calculated by multiplying the total number of minutes per week of each activity by the specific METs for that activity and then summing the total metabolic equivalent task (MET) for each activity. The METs are 3.3 for walking, 4 for moderate activity, 6 for cycling, and 8 for vigorous activity. PA is classified into three categories: (1) Low intensity (category 1): this is the lowest level of PA. Patients do not meet grade 2 or 3 criteria. (2) Moderate intensity (category 2): one of the following three criteria: ① vigorous activity for at least 20 min per day for more than 3 days; ② do at least 30 min of moderate intensity activity or walking daily for more than 5 days; ③ 5 or more days of any combination of walking, moderate or vigorous intensity activity to achieve a minimum of at least 600 MET-min per week. (3) High intensity (category 3): one of the following two criteria: ① vigorous activity for at least 3 days, accumulating at least 1,500 MET-min per week; ② walking at least 3,000 MET-min per week for 7 consecutive days at moderate or vigorous intensity (34).
2.5 Measurement of cardiac structure
By a professional cardiac color Doppler technician in the hospital using the American GE color Doppler ultrasound instrument, LVEDD and RVEDD were measured at the level of the mitral tendon cable in parasternal left ventricular long-axis view at end-diastole and LAD which was also the anteroposterior diameter of the left ventricle was measured by taking a vertical line from the posterior wall of the distal aorta to the posterior wall of the left atrium from the parasternal long axis view of the left ventricle at the end of ventricular systole (35).
2.6 Measurement of serum metabolic indexes
Medically trained nurses collected blood samples from patients who had fasted for more than 12 h at each hospital and blood samples of patients were centrifuged until patient serum was obtained. The levels of FBG, TG, TC, HDL-C, and LDL-C were determined by enzymatic and homogeneous methods on an automatic biochemical analyzer (Roche Cobas 8,000) and matching kits.
2.7 Statistical analysis
All statistical analyses were performed using SPSS software (version 26.0; SPSS Inc., Chicago, IL, USA), GraphPad Prism software (version 9.0.0; GraphPad Software, San Diego, CA, USA), and R Language software (version 4.3.3; R Language Software, Auckland, NI, NZ). Quantitative data were expressed as mean ± standard deviation or median and interquartile range, and a comparison between groups was performed using the t-test or Mann–Whitney U test. Qualitative data were expressed as percentages, and a comparison between groups was performed using the Chi-square test. Spearman correlation coefficient was used to analyze the correlation between sleep quality and the total score of MMSE components. Multivariate logistic regression was used to analyze the relationship between sleep quality and cognitive function. The Mediation package in R Language was used to analyze the mediating effect of sleep quality between PA intensity and CI in older adults hypertensive patients. Two-sided p-values of less than 0.05 were considered to indicate statistical significance.
3 Results
3.1 Baseline differences between the CI group and the normal cognitive function group in older adults hypertensive patients
Table 1 shows the differences between the CI group and the normal cognitive function group in the basic information, auxiliary examinations, hypertension medication use, and subjective evaluation scales in older adults hypertensive patients. The results showed that compared with the normal cognitive function group, the CI group had a higher proportion of education level of primary school and below, work type with physical work mainly, no alcohol drinking, and no CCB use, lower WHR, serum TC, LDL-C levels, and PA intensity, and worse sleep quality. However, there was no statistically significant difference in hypertension classification, systolic pressure at ordinary times, diastolic pressure at ordinary times, and course of hypertension between the CI group and the normal cognitive function group in older adults hypertensive patients (p < 0.05).
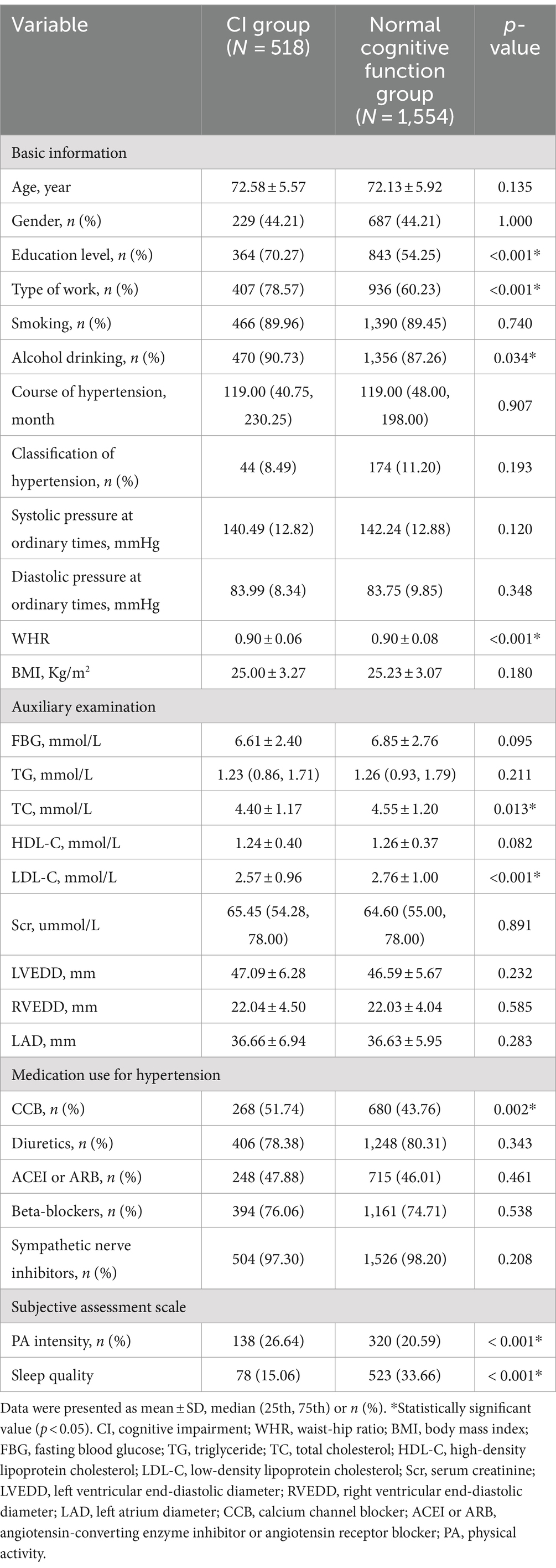
Table 1. Baseline differences between older adults hypertensive patients with CI and normal cognitive function.
Table 2 compares differences in each component of PSQI between the CI group and the normal cognitive function group in older adults hypertensive patients. The results showed that the PSQI total score, subjective sleep quality score, sleep latency score, sleep duration score, sleep efficiency score, sleep disorders score, sleeping pills score, and daytime dysfunction score of the CI group were higher than that of normal cognitive function group in older adults hypertensive patients.
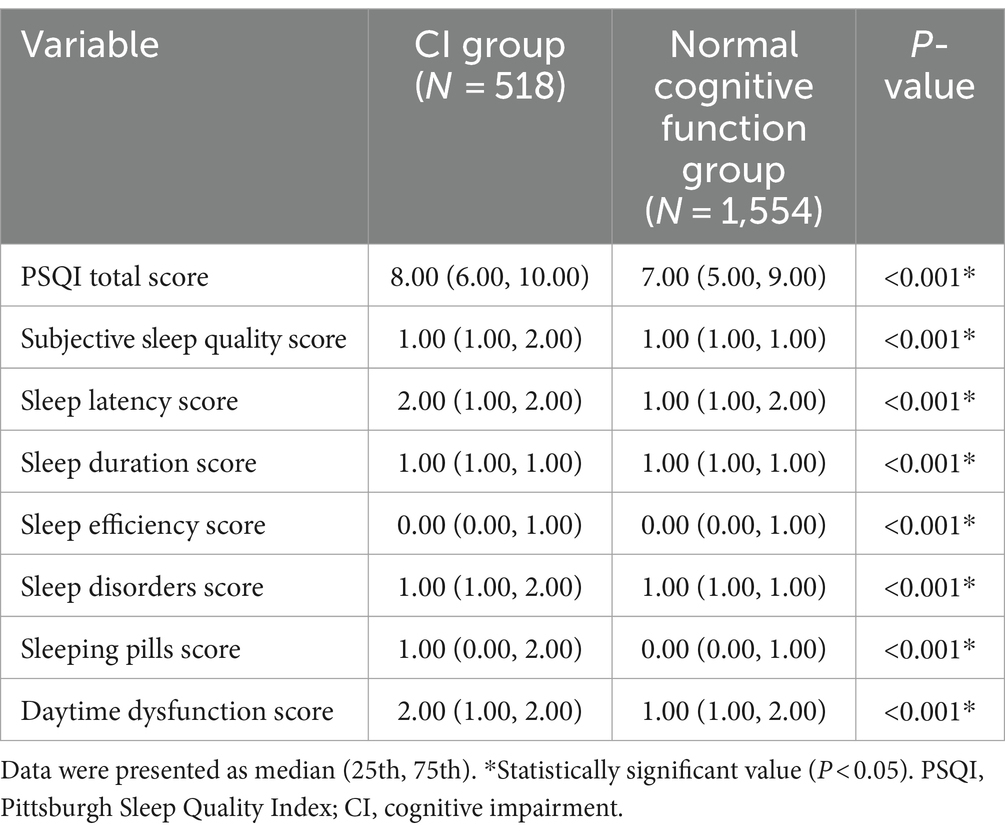
Table 2. Differences in each component of PSQI between the CI group and the normal cognitive function group in older adults hypertensive patients.
Figure 1 analyzes of differences in cognitive function among older adults hypertensive patients with different educational levels using the Chi-square test. The proportion of CI in older adults hypertensive patients with primary school education and below was higher than that in older adults hypertensive patients with junior high school education and above (30.16% vs. 17.80%, p < 0.001).
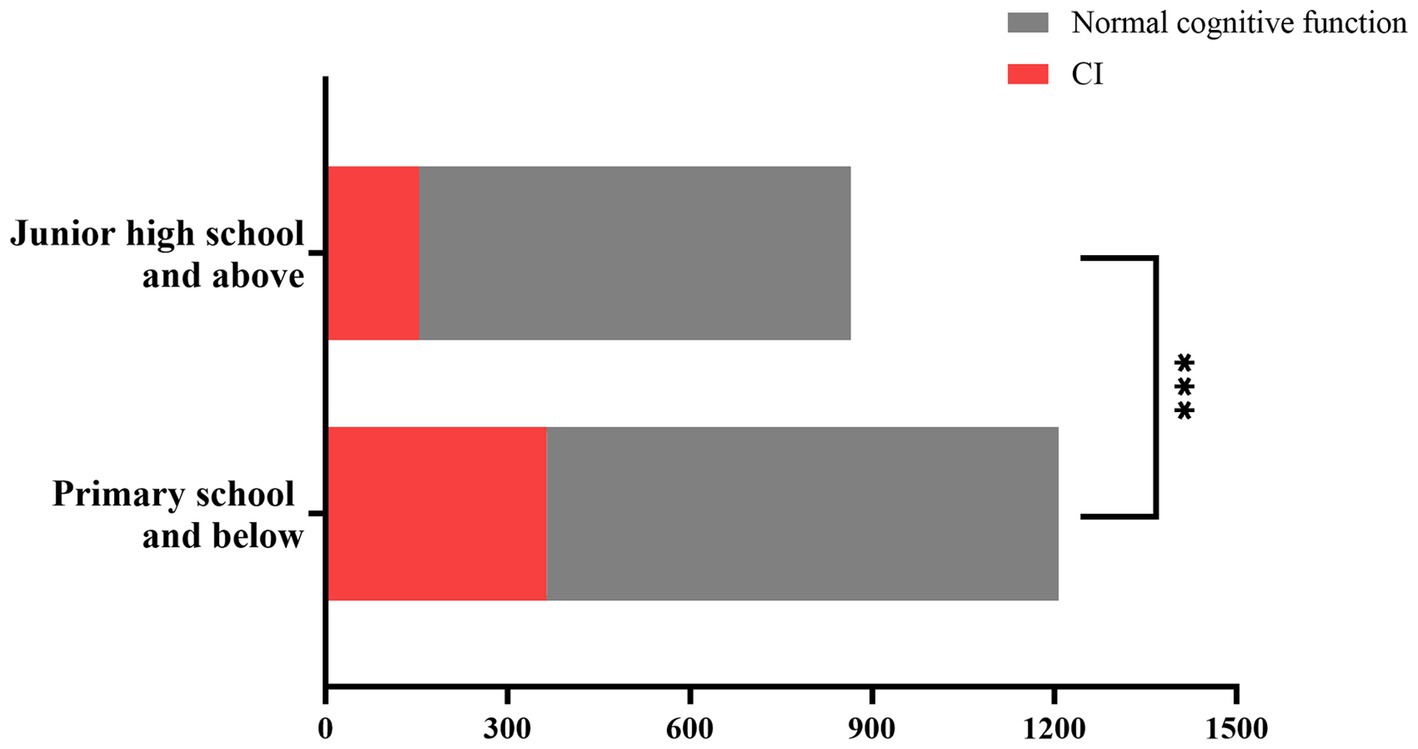
Figure 1. Differences in cognitive function among older adults hypertensive patients with different levels of education. Older adults hypertensive patients with primary school and below had a higher proportion of CI compared with those with junior high school education and above (30.16% vs 17.80%, P < 0.001). ***Statistically significant value (P < 0.001). CI, cognitive impairment.
3.2 The relationship between sleep quality and cognitive function in older adults hypertensive patients
Table 3 uses logistic regression to analyze the relationship between sleep quality and cognitive function in older adults hypertensive patients. Without adjusting for any factors, sleep quality was positively correlated with CI in older adults hypertensive patients (OR = 2.862, 95%CI: 2.200–3.722, p < 0.001), and this correlation still existed in the older adults hypertensive patients with education levels of primary school and below and junior high school and above (OR = 2.633, 95%CI: 1.887–3.673, p < 0.001; OR = 2.697, 95%CI: 1.585–4.592, p < 0.001). After adjusting for type of work, alcohol drinking, systolic pressure at ordinary times, serum TC and LDL-C levels, LAD, CCB, and PA intensity, sleep quality was also positively correlated with CI in older adults hypertensive patients (OR = 2.565, 95%CI: 1.958–3.360, p < 0.001). This relationship still existed in the older adults hypertensive patients with education levels of primary school and below and junior high school and above (OR = 2.468, 95%CI: 1.754–3.473, p < 0.001; OR = 2.385, 95%CI: 1.367–4.161, p = 0.002).
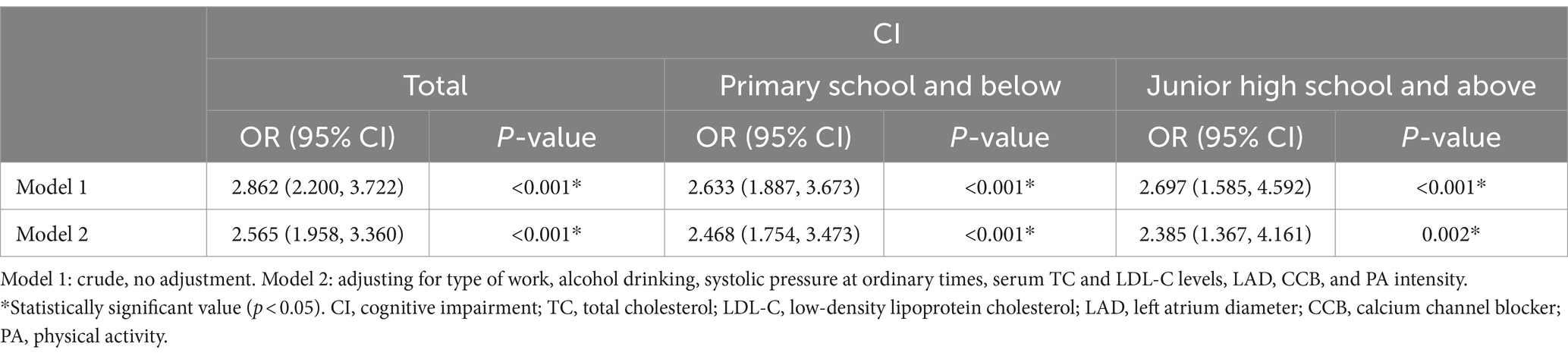
Table 3. The relationship between sleep quality and cognitive function in older adults hypertensive patients.
3.3 Relationship between each component of PSQI and CI in older adults hypertensive patients
Figure 2 uses multivariate logistic regression analysis to analyze the relationship between each component of PSQI and CI in older adults hypertensive patients. After adjusting for education level, type of work, alcohol drinking, systolic pressure at ordinary times, serum TC and LDL-C levels, LAD, CCB, and PA intensity, the scores of subjective sleep quality (OR = 1.685, 95%CI: 1.438–1.975, p < 0.001), sleep latency (OR = 1.529, 95%CI: 1.351–1.730, p < 0.001), sleep duration (OR = 1.425, 95%CI: 1.178–1.723, p < 0.001), sleep disorders (OR = 1.715, 95%CI: 1.485–1.981, p < 0.001), sleeping pills (OR = 1.206, 95%CI: 1.083–1.342, p = 0.001), and daytime dysfunction (OR = 1.488, 95%CI: 1.317–1.680, p < 0.001) were positively correlated with CI, and the score of sleep efficiency was negatively correlated with CI in older adults hypertensive patients (OR = 0.815, 95%CI: 0.728–0.913, p < 0.001).
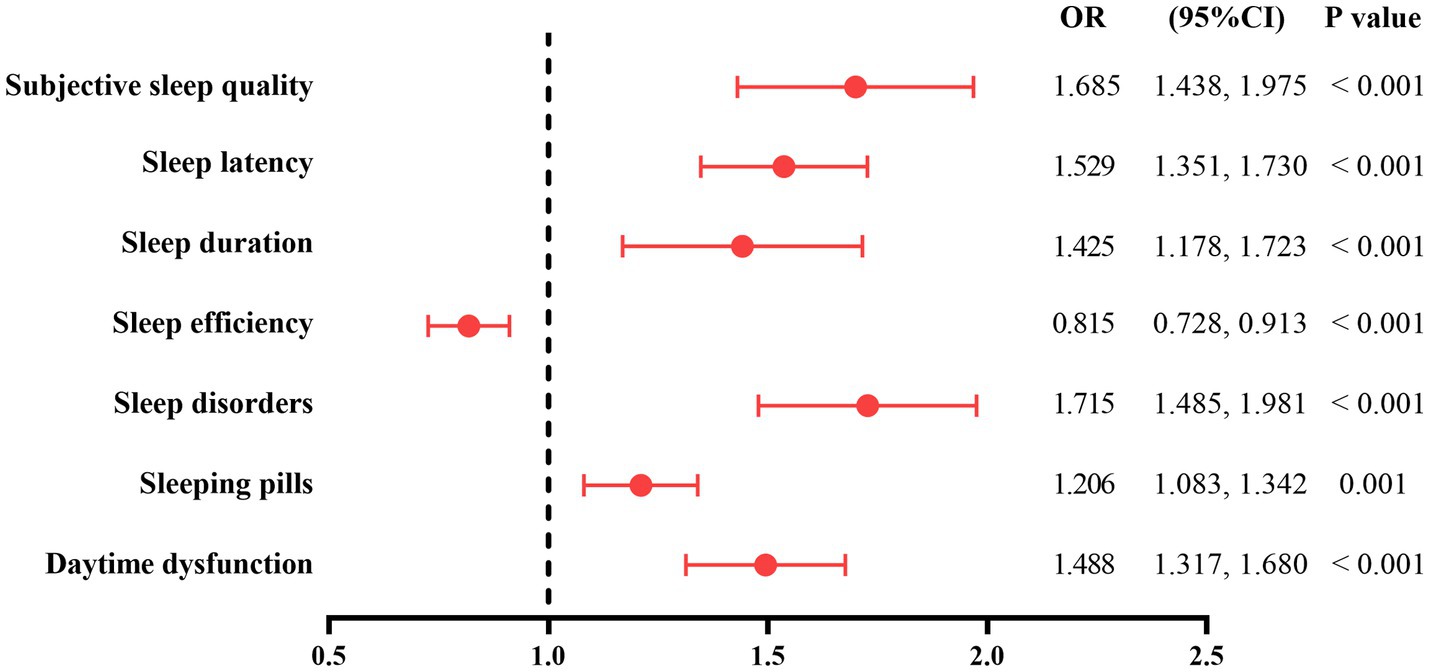
Figure 2. The relationship between each component of PSQI and CI in older adults hypertensive patients. The model was adjusted for education level, type of work, alcohol drinking, systolic pressure at ordinary times, serum TC and LDL-C levels, LAD, CCB, and PA intensity. PSQI, Pittsburgh Sleep Quality Index; CI, cognitive impairment; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; LAD, left atrium diameter; CCB, calcium channel blocker; PA, physical activity.
3.4 The correlation between sleep quality and each part of the MMSE score in older adults hypertensive patients
Table 4 shows that the Spearman correlation coefficient is used to study the correlation between sleep quality and each part of the MMSE score in older adults hypertensive patients. The results showed that sleep quality was negatively associated with orientation (r = −0.139, p < 0.001), memory (r = −0.085, p < 0.001), attention and numeracy (r = −0.232, p < 0.001), recall ability (r = −0.131, p < 0.001), and language ability (r = −0.188, p < 0.001) in older adults hypertensive patients.
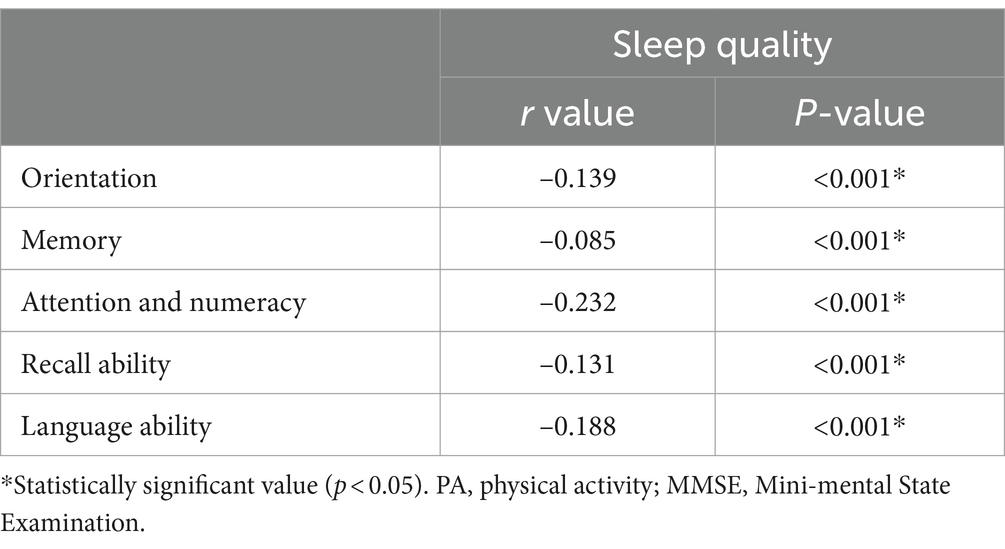
Table 4. Correlation of sleep quality and each part of MMSE scores in older adults hypertensive patients.
3.5 The mediating effect of sleep quality between PA intensity and CI in older adults hypertensive patients
Table 5 uses the Mediation package in R Language to analyze the mediating effect of sleep quality between PA intensity and CI in older adults hypertensive patients. PA intensity was used as the independent variable, sleep quality was the mediator variable, and CI was the outcome variable. In addition, type of work, alcohol drinking, systolic pressure at ordinary times, serum TC and LDL-C levels, LAD, and CCB were included in the model as adjusted variables. The results showed that sleep quality had a partial mediating effect between PA intensity and CI in older adults hypertensive patients (Za * Zb: –17.19339; 95%CI: –0.37312, –0.04194).
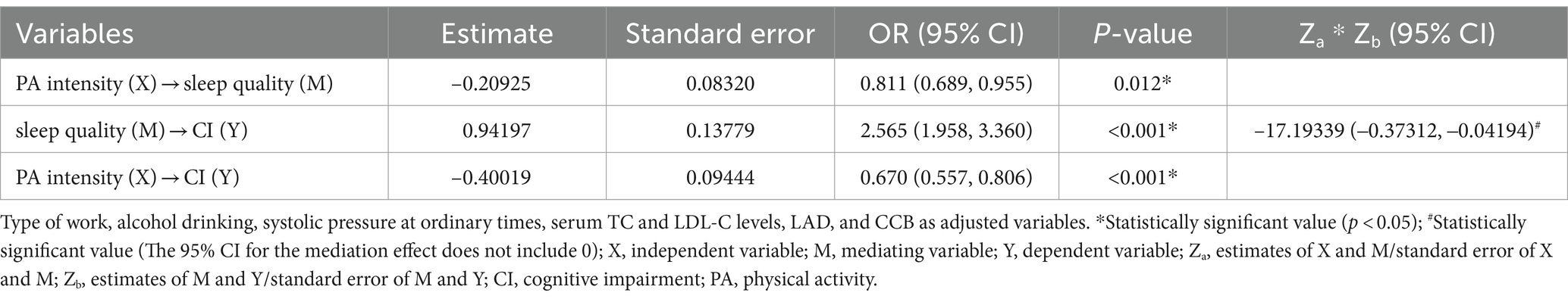
Table 5. Mediating effect of sleep quality between PA intensity and CI in older adults hypertensive patients.
4 Discussion
The purpose of this study was to investigate the relationship between sleep quality and CI in older adults hypertensive patients. The results of this study showed that poor sleep quality was associated with CI in older adults hypertensive patients, and this correlation still existed in older adults hypertensive patients with education levels of primary school and below and junior high school and above. In each part of the PSQI, the scores of subjective sleep quality, sleep latency, sleep duration, sleep disorders, sleeping pills, and daytime dysfunction were positively correlated with CI, and the score of sleep efficiency was negatively correlated with CI. Sleep quality in older adults hypertensive patients was negatively correlated with orientation, memory, attention and numeracy, recall ability, and language ability in MMSE. In addition, sleep quality partly mediated the association between PA intensity and CI in older adults hypertensive patients.
In this study, the baseline data characteristics of older adults hypertensive patients with CI and those with normal cognitive function were analyzed. The results showed that compared with the normal cognitive function group, the CI group had a higher proportion of no CCB use and work type with physical work mainly. A cross-sectional study found that the total score of MMSE of the Chinese older adults with work type of mental work was significantly higher than that of the Chinese older adults with work type of physical work (36), which might be because work type of mental work could improve the cognitive reserve (CR) of the older adults and thus help them maintain better cognitive function (37). A prospective study found that older hypertensive patients who used CCB had better memory (38). In an epidemiological investigation, alcohol drinking less than 40 g per day in women and less than 80 g per day in men reduced the occurrence of CI (39). This study did not calculate the alcohol consumption of older adults hypertensive patients, but the results of this study showed that older adults hypertensive patients with CI had a higher proportion of no alcohol drinking than those with normal cognitive function. This study also found that the older adults hypertensive patients with CI had lower WHR, serum TC and LDL-C levels, and PA intensity than those with normal cognitive function, which were similar to the results of some studies (28, 40–42).
Previous studies have found that sleep quality affects the cognitive function of patients. Five cross-sectional studies found that poor sleep quality was negatively correlated with MMSE total score in older adults (21); sleep disorders (PSQI total score > 5) were associated with MCI in Chinese community population (43); poor sleep quality was associated with CI in Chinese older adults and centenarians (44, 45); poor sleep quality was associated with CI in older adults hypertensive patients (46); PSQI total score was positively correlated with CI in older adults hypertensive patients (47). In a cross-sectional study using a multistage cluster random sampling method, Insomnia status (Athens Insomnia Scale >6) was associated with a lower total MMSE score in rural older adults (48). A longitudinal survey found that poor sleep quality was associated with CI in Chinese older adults (49). A prospective cohort study found that a higher PSQI total score was associated with CI in older adults men (50). A retrospective cohort study found that poor sleep quality was associated with the development of MCI in Germans (51). Similarly, this study also found that poor sleep quality was associated with CI in older adults hypertensive patients, and this relationship existed in hypertensive patients with education levels of primary school and below and junior high school and above. In each part of the PSQI, the results of this study showed that the scores of subjective sleep quality, sleep latency, sleep duration, sleep disorders, sleeping pills, and daytime dysfunction had a positive correlation with CI, which were similar to the results of previous studies. Two cross-sectional studies have found that poor subjective sleep quality and daytime dysfunction contribute to the occurrence of CI in Chinese older adults (44, 52). A prospective cohort study found that longer sleep latency in Korean older adults with normal cognitive function or MCI was associated with a decline in cognitive function 4 years later (53). Two cross-sectional studies found that sleep duration of 6–7 h, < 6 h, 8–9 h, and ≧ 9 h compared with sleep duration of 7–8 h promoted the occurrence of CI in older adults Chinese (44); the older adults with sleep duration <6 h and > 8 h were more likely to have CI compared with the older adults with 6–8 h of sleep (54). Another prospective study found that older women who slept ≥8 and ≤ 6 h/night had a higher risk for MCI or dementia compared with 7 h/night (55). Both long and short sleep duration contributed to circadian dysfunction and thus contributed to CI (56). This study showed that the score of sleep efficiency had a negative correlation with CI, which was in contrast to the results of previous studies (43, 52). In addition, the results of the present study also found that sleep quality was negatively correlated with orientation, memory, attention and numeracy, recall ability, and language ability in older adults hypertensive patients, which were similar to the results of the following studies. A cross-sectional study using multiple linear regression analysis showed that insomnia (AIS > 6) was negatively correlated with attention and numeracy, memory, and language ability in older adults (48). A community epidemiology study found that PSQI total score was negatively correlated with memory in non-demented older adults (57). In conclusion, poor sleep quality promoted the occurrence of CI and decreased MMSE scores of orientation, memory, attention and numeracy, recall ability, and language ability in older adults hypertensive patients.
The mechanism of the relationship between poor sleep quality and CI is still unclear, and it may be related to the deposition of brain amyloid β-protein (Aβ), the decrease of brain-derived neurotrophic factor (BDNF), and cortical atrophy. Aβ caused the decline in cognitive function by causing erroneous neural network activity and impairing synapses between neurons that form and maintain microcircuits that support learning, memory, and other cognitive functions (58). A cross-sectional study found that poor sleep quality was associated with increased brain Aβ in community-dwelling older adults (59). Two cohort studies have shown that poor sleep efficiency and reduced low-frequency < 1 Hz slow waves during non-rapid eye movement sleep promote the accumulation rate of brain Aβ in older adults people with normal cognitive function (60); poor sleep quality in middle-aged and older Koreans increases the deposition of pathological Aβ in the brain (61). BDNF is a neuroprotective factor that promotes long-term memory storage by long-term potentiation of hippocampal glutamatergic neurons and enhances dendritic spine growth and reorganization in response to altered neural activity (62–64). Low levels of BDNF, both in peripheral blood and in cerebrospinal fluid, were associated with cognitive decline (65, 66). A systematic review and meta-analysis found that peripheral BDNF was lower in insomnia patients than in controls (67). A case–control study found that higher insomnia scores were associated with lower serum BDNF levels (68). Animal experimental studies have shown that the mRNA and protein levels of BDNF in the hippocampus of sleep-deprived rats are lower than those of caged control rats (69). Cortical atrophy was related to cognitive function (70), especially posterior cortical atrophy (71). A cohort study found that poor sleep quality was associated with cortical atrophy in community adults (72). A cross-sectional study found that the cortical and subcortical volumes of older adults with poor sleep were smaller than those of older adults with normal sleep (73). Hypertension was a risk factor for CI in the older adults (28), and poor sleep quality was related to the occurrence of CI in older adults patients with hypertension. Therefore, older adults hypertensive patients should maintain good sleep quality.
The results of the present study also showed that the prevalence of CI in older adults hypertensive patients with primary school education and below was significantly higher than that in older adults hypertensive patients with junior high school education and above, which was similar to the results of some studies. Two systematic reviews and meta-analyses found that Chinese hypertensive patients with primary school education and below promoted the occurrence of CI compared with those with junior high school education and above (6); community residents aged 50 and above with education level > 6 years had a lower prevalence of MCI than community residents aged 50 and above with education level ≤ 6 years (74). Two cross-sectional studies have found that Chinese hypertensive patients with education level of junior high school and below have an increased risk of MCI compared with those with education level of junior high school and above (75); Chinese older adults with education levels of junior high school and above and primary school have a lower incidence of CI than illiterate Chinese older adults (76). The mechanism by which higher education level reduces the prevalence of CI in older adults hypertensive patients may be related to better CR. Studies have found that higher levels of education are associated with better CR (77). CR could increase the functional connectivity of the human brain’s large network and promote the formation of better organization of human brain network topology, which improved the ability of the human brain to process information (78), thereby reducing the occurrence of CI (77).
There were few studies on the relationship between PA, sleep quality, and CI in older adults hypertensive patients, but there were some studies in other populations. Two cross-sectional studies have found that sleep quality has a partial mediating effect between physical exercise and CI in older adults with type 2 diabetes (79); sleep quality mediates the relationship between PA and cognitive function in adults aged 50 years or above to a certain extent (80). A randomized controlled study showed that PA in older adults could improve the cognitive ability of older adults by improving sleep quality (81). The present study found that sleep quality mediated part of the mediating effect between PA intensity and CI in older adults hypertensive patients. Therefore, higher PA intensity in older adults hypertensive patients reduced the occurrence of CI in part by improving sleep quality.
Admittedly, this study has several limitations. Firstly, this study was a case–control study and could not determine the causal relationship between sleep quality and CI in older adults hypertensive patients. Therefore, cohort studies are needed to determine this relationship. Second, although a multivariate logistic regression model was used to adjust for confounders in this study, the possibility of residual bias due to other relevant confounders not included cannot be excluded. Third, PSQI is a subjective evaluation scale, which may lead to recall bias due to the older age and CI of the patients. Finally, the cases included in this study were limited to older adults hypertensive patients attending outpatient clinics or wards of hospitals in Shandong Province, China, and future studies with large samples and multiple regions are needed. However, this study found that poor sleep quality can promote the occurrence of CI in older adults hypertensive patients, and this relationship still existed in older adults hypertensive patients with education levels of primary school and below and junior high school and above. In addition, sleep quality had a partial mediating effect between PA intensity and CI in older adults hypertensive patients. Therefore, it is suggested that older adults hypertensive patients should maintain good sleep quality.
5 Conclusion
Poor sleep quality promoted the occurrence of CI in older adults hypertensive patients, and this relationship also existed in older adults hypertensive patients with education levels of primary school and below and junior high school and above. The mechanism may be related to the deposition of Aβ in the brain, decreased BDNF, and cortical atrophy. In addition, sleep quality partly mediated the mediating effect between PA intensity and CI in older adults hypertensive patients. Therefore, this study suggests that older adults hypertensive patients should improve sleep quality by improving subjective sleep quality, shortening sleep latency, maintaining sleep duration for about 7 h, and so on to reduce the occurrence of CI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SL: Conceptualization, Data curation, Investigation, Writing – original draft. HJ: Funding acquisition, Supervision, Writing – review & editing. XZ: Writing – review & editing. YQ: Data curation, Writing – original draft. MZ: Data curation, Writing – original draft. RW: Data curation, Writing – original draft. DL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Key R&D Program of Shandong Province, China (Major Scientific and Technological Innovation Project) (NO.2021SFGC0503).
Acknowledgments
I thank the participants of this study, teachers for their guidance, and partners for their help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CI, cognitive impairment; MCI, mild cognitive impairment; PA, physical activity; SBP, systolic blood pressure; DBP, diastolic blood pressure; MMSE, Mini-mental State Examination; WHR, waist-to-hip ratio; BMI, body mass index; CCB, calcium channel blocker; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; Scr, serum creatinine; LVEDD, left ventricular end-diastolic diameter; RVEDD, right ventricular end-diastolic diameter; LAD, left atrial diameter; IPAQ-L, International Physical Activity Questionnaire-Long Form; PSQI, Pittsburgh Sleep Quality Index; METs, metabolic equivalent task scores; CR, cognitive reserve; Aβ, amyloid β-protein; BDNF, brain-derived neurotrophic factor.
References
1. GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017 [published correction appears in lancet. 2019;393(10167):132]. Lancet. (2018) 392:1923–94. doi: 10.1016/S0140-6736(18)32225-6
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398:957–80. doi: 10.1016/S0140-6736(21)01330-1
3. Mills, KT, Stefanescu, A, and He, J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16:223–37. doi: 10.1038/s41581-019-0244-2
4. Ou, YN, Tan, CC, Shen, XN, Xu, W, Hou, XH, Dong, Q, et al. Blood pressure and risks of cognitive impairment and dementia: a systematic review and Meta-analysis of 209 prospective studies. Hypertension. (2020) 76:217–25. doi: 10.1161/HYPERTENSIONAHA.120.14993
5. Qin, J, He, Z, Wu, L, Wang, W, Lin, Q, Lin, Y, et al. Prevalence of mild cognitive impairment in patients with hypertension: a systematic review and meta-analysis. Hypertens Res. (2021) 44:1251–60. doi: 10.1038/s41440-021-00704-3
6. Xie, C, Zhong, D, Zhang, Y, Liu, X, Zhang, L, Luo, X, et al. Prevalence and risk factors of cognitive impairment in Chinese patients with hypertension: a systematic review and meta-analysis. Front Neurol. (2024) 14:1271437. doi: 10.3389/fneur.2023.1271437
7. Guan, D, Lewis, MO, Li, P, Zhang, Y, Zhang, P, Tang, S, et al. Incremental burden on health-related quality of life, health service utilization and direct medical expenditures associated with cognitive impairment among non-institutionalized people with diabetes aged 65 years and older. Diabetes Obes Metab. (2024) 26:275–82. doi: 10.1111/dom.15313
8. Fogg, C, Meredith, P, Culliford, D, Bridges, J, Spice, C, and Griffiths, P. Cognitive impairment is independently associated with mortality, extended hospital stays and early readmission of older people with emergency hospital admissions: a retrospective cohort study. Int J Nurs Stud. (2019) 96:1–8. doi: 10.1016/j.ijnurstu.2019.02.005
9. McCollum, L, and Karlawish, J. Cognitive impairment evaluation and management. Med Clin North Am. (2020) 104:807–25. doi: 10.1016/j.mcna.2020.06.007
10. Baranwal, N, Yu, PK, and Siegel, NS. Sleep physiology, pathophysiology, and sleep hygiene. Prog Cardiovasc Dis. (2023) 77:59–69. doi: 10.1016/j.pcad.2023.02.005
11. Diekelmann, S, and Born, J. The memory function of sleep. Nat Rev Neurosci. (2010) 11:114–26. doi: 10.1038/nrn2762
12. Gruber, R, and Cassoff, J. The interplay between sleep and emotion regulation: conceptual framework empirical evidence and future directions. Curr Psychiatry Rep. (2014) 16:500. doi: 10.1007/s11920-014-0500-x
13. Poggiogalle, E, Jamshed, H, and Peterson, CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. (2018) 84:11–27. doi: 10.1016/j.metabol.2017.11.017
14. Mackiewicz, M, Shockley, KR, Romer, MA, Galante, RJ, Zimmerman, JE, Naidoo, N, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. (2007) 31:441–57. doi: 10.1152/physiolgenomics.00275.2006
15. Xie, L, Kang, H, Xu, Q, Chen, MJ, Liao, Y, Thiyagarajan, M, et al. Sleep drives metabolite clearance from the adult brain. Science. (2013) 342:373–7. doi: 10.1126/science.1241224
16. Fabbri, M, Beracci, A, Martoni, M, Meneo, D, Tonetti, L, and Natale, V. Measuring subjective sleep quality: a review. Int J Environ Res Public Health. (2021) 18:1082. doi: 10.3390/ijerph18031082
17. Liu, R, Liu, X, Zee, PC, Hou, L, Zheng, Z, Wei, Y, et al. Association between sleep quality and C-reactive protein: results from national health and nutrition examination survey, 2005-2008. PLoS One. (2014) 9:e92607. doi: 10.1371/journal.pone.0092607
18. Smith, L, Shin, JI, Jacob, L, Carmichael, C, López Sánchez, GF, Oh, H, et al. Sleep problems and mild cognitive impairment among adults aged ≥50 years from low- and middle-income countries. Exp Gerontol. (2021) 154:111513. doi: 10.1016/j.exger.2021.111513
19. Casagrande, M, Forte, G, Favieri, F, and Corbo, I. Sleep quality and aging: a systematic review on healthy older people, mild cognitive impairment and Alzheimer's disease. Int J Environ Res Public Health. (2022) 19:8457. doi: 10.3390/ijerph19148457
20. Lo, K, Woo, B, Wong, M, and Tam, W. Subjective sleep quality, blood pressure, and hypertension: a meta-analysis. J Clin Hypertens (Greenwich). (2018) 20:592–605. doi: 10.1111/jch.13220
21. Chen, J, Chen, X, Mao, R, Fu, Y, Chen, Q, Zhang, C, et al. Hypertension, sleep quality, depression, and cognitive function in elderly: a cross-sectional study. Front Aging Neurosci. (2023) 15:1051298. doi: 10.3389/fnagi.2023.1051298
22. Vanderlinden, J, Boen, F, and van Uffelen, JGZ. Effects of physical activity programs on sleep outcomes in older adults: a systematic review. Int J Behav Nutr Phys Act. (2020) 17:11. doi: 10.1186/s12966-020-0913-3
23. De Nys, L, Anderson, K, Ofosu, EF, Ryde, GC, Connelly, J, and Whittaker, AC. The effects of physical activity on cortisol and sleep: a systematic review and meta-analysis. Psychoneuroendocrinology. (2022) 143:105843. doi: 10.1016/j.psyneuen.2022.105843
24. Iso-Markku, P, Aaltonen, S, Kujala, UM, Halme, HL, Phipps, D, Knittle, K, et al. Physical activity and cognitive decline among older adults: a systematic review and Meta-analysis. JAMA Netw Open. (2024) 7:e2354285. doi: 10.1001/jamanetworkopen.2023.54285
25. Wang, R, Zhao, H, and Li, B. Analytical clinical medicine research: key points of case-control study design. Shanghai Med. (2023) 44:23–5. doi: 10.3969/j.issn.1006-1533.2023.21.005
26. Cui, G, Li, S, Yin, Y, Chen, L, Liu, X, Yu, P, et al. Effects of sedentary behavior and sleep quality on cognitive function in community elderly. Modern Prevent Med. (2020) 47:3339–42.
27. Revision committee of Guidelines for the Prevention and Treatment of Hypertension in China. Chinese guidelines for the prevention and treatment of hypertension 2018 revision. Cardio-Cerebrovascular Disease Prevent Treat. (2019) 19:1–44. doi: 10.3969/j.issn.1009-816X.2019.01.001
28. Han, F, Luo, C, Lv, D, Tian, L, and Qu, C. Risk factors affecting cognitive impairment of the elderly aged 65 and over: a cross-sectional study. Front Aging Neurosci. (2022) 14:903794. doi: 10.3389/fnagi.2022.903794
29. Jia, X, Wang, Z, Huang, F, Su, C, du, W, Jiang, H, et al. A comparison of the Mini-mental state examination (MMSE) with the Montreal cognitive assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: a cross-sectional study. BMC Psychiatry. (2021) 21:485. doi: 10.1186/s12888-021-03495-6
30. Folstein, MF, Folstein, SE, and McHugh, PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
31. Tsai, PS, Wang, SY, Wang, MY, Su, CT, Yang, TT, Huang, CJ, et al. Psychometric evaluation of the Chinese version of the Pittsburgh sleep quality index (CPSQI) in primary insomnia and control subjects. Qual Life Res. (2005) 14:1943–52. doi: 10.1007/s11136-005-4346-x
32. Ren, YJ, Su, M, Liu, QM, Tan, YY, du, YK, Li, LM, et al. Validation of the simplified Chinese-character version of the international physical activity questionnaire-long form in Urban Community-dwelling adults: a cross-sectional study in Hangzhou, China. Biomed Environ Sci. (2017) 30:255–63. doi: 10.3967/bes2017.035
33. Wang, X. Reliability and validity of the international physical activity length questionnaire in Chinese elderly population. Chin J Gerontol. (2015) 35:5912–4. doi: 10.3969/j.issn.1005-9202.2015.20.115
34. Fan, M, Lyu, J, and He, P. Chinese guidelines for data processing and analysis concerning the international physical activity questionnaire. Zhonghua Liu Xing Bing Xue Za Zhi. (2014) 35:961–4.
35. Echocardiography Group of the Ultrasound Society of the Chinese Medical Association. Echocardiographic measurement guidelines for Chinese adults. Chin J Ultrasound Imaging. (2016) 25:645–66. doi: 10.3760/cma.j.issn.1004-4477.2016.08.001
36. Zhu, J, Xing, X, Tian, X, and Li, A. Analysis of the differences between job type and cognitive ability of the elderly based on propensity score matching. Chinese Manag Sci. (2016) 24:183–8.
37. Ko, K, Yi, D, Byun, MS, Lee, JH, Jeon, SY, Kim, WJ, et al. Cognitive reserve proxies, Alzheimer pathologies, and cognition. Neurobiol Aging. (2022) 110:88–95. doi: 10.1016/j.neurobiolaging.2021.10.005
38. Watfa, G, Rossignol, P, Kearney-Schwartz, A, Fay, R, Bracard, S, Felblinger, J, et al. Use of calcium channel blockers is associated with better cognitive performance in older hypertensive patients with subjective memory complaints. J Hypertens. (2010) 28:2485–93. doi: 10.1097/HJH.0b013e32833e4108
39. Zuccalà, G, onder, G, Pedone, C, Cesari, M, Landi, F, Bernabei, R, et al. Dose-related impact of alcohol consumption on cognitive function in advanced age: results of a multicenter survey. Alcohol Clin Exp Res. (2001) 25:1743–8. doi: 10.1111/j.1530-0277.2001.tb02185.x
40. Cen, Y, Dou, M, Kui, Q, and Lu, X. The relationship between obesity and brain volume and cognitive function in the elderly. Pract Gerontol. (2023) 37:178–82. doi: 10.3969/j.issn.1003-9198.2023.02.017
41. Lv, YB, Yin, ZX, Chei, CL, Brasher, MS, Zhang, J, Kraus, VB, et al. Serum cholesterol levels within the high Normal range are associated with better cognitive performance among Chinese elderly. J Nutr Health Aging. (2016) 20:280–7. doi: 10.1007/s12603-016-0701-6
42. Wang, X, Zhang, J, Chen, C, Lu, Z, Zhang, D, and Li, S. The association between physical activity and cognitive function in the elderly in rural areas of northern China. Front Aging Neurosci. (2023) 15:1168892. doi: 10.3389/fnagi.2023.1168892
43. Gao, F, Wei, S, Dang, L, Gao, Y, Gao, L, Shang, S, et al. Sleep disturbance is associated with mild cognitive impairment: a community population-based cross-sectional study. BMC Public Health. (2022) 22:2000. doi: 10.1186/s12889-022-14391-3
44. Liu, X, Xu, P, Wei, R, Cheng, B, Sun, L, Yang, L, et al. Gender-and age-specific associations of sleep duration and quality with cognitive impairment in community-dwelling older adults in Anhui Province, China. Front Public Health. (2024) 11:1047025. doi: 10.3389/fpubh.2023.1047025
45. Yang, S, Wang, S, Liu, G, Li, R, Li, X, Chen, S, et al. Association of Sleep Status with Cognitive Functions in centenarians: evidence from Hainan centenarian cohort. J Gerontol B Psychol Sci Soc Sci. (2023) 79:gbad185. doi: 10.1093/geronb/gbad185
46. Chen, H. Correlation between sleep quality and cognitive function in elderly patients with hypertension and analysis of influencing factors of cognitive function. Shenyang Medical College (2023).
47. He, Z. The effect of sleep quality on cognitive dysfunction in elderly patients with hypertension. Anhui Medical University (2021). doi: 10.26921/d.cnki.ganyu.2020.000169
48. Xiong, Y, Yang, J, Zhou, Q, Qu, F, Chen, M, Yang, X, et al. The relationship between sleep quality and cognitive function and the mediating effect of depression in the rural elderly in Guizhou province. Chin J Disease Control. (2023) 27:645–649+661. doi: 10.16462/j.cnki.zhjbkz.2023.06.005
49. Xie, Y, Bai, C, Feng, Q, and Gu, D. Serum vitamin D3 concentration, sleep, and cognitive impairment among older adults in China. Nutrients. (2023) 15:4192. doi: 10.3390/nu15194192
50. Potvin, O, Lorrain, D, Forget, H, Dubé, M, Grenier, S, Préville, M, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. (2012) 35:491–9. doi: 10.5665/sleep.1732
51. Brachem, C, Winkler, A, Tebrügge, S, Weimar, C, Erbel, R, Jöckel, KH, et al. Associations between self-reported sleep characteristics and incident mild cognitive impairment: the Heinz Nixdorf recall cohort study. Sci Rep. (2020) 10:6542. doi: 10.1038/s41598-020-63511-9
52. Ma, XQ, Jiang, CQ, Xu, L, Zhang, WS, Zhu, F, Jin, YL, et al. Sleep quality and cognitive impairment in older Chinese: Guangzhou biobank cohort study. Age Ageing. (2019) 49:119–24. doi: 10.1093/ageing/afz120
53. Suh, SW, Han, JW, Lee, JR, Byun, S, Kwon, SJ, Oh, SH, et al. Sleep and cognitive decline: a prospective nondemented elderly cohort study. Ann Neurol. (2018) 83:472–82. doi: 10.1002/ana.25166
54. Chen, WC, and Wang, XY. Longitudinal associations between sleep duration and cognitive impairment in Chinese elderly. Front Aging Neurosci. (2022) 14:1037650. doi: 10.3389/fnagi.2022.1037650
55. Chen, JC, Espeland, MA, Brunner, RL, Lovato, LC, Wallace, RB, Leng, X, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement. (2016) 12:21–33. doi: 10.1016/j.jalz.2015.03.004
56. Yaffe, K, Falvey, CM, and Hoang, T. Connections between sleep and cognition in older adults. Lancet Neurol. (2014) 13:1017–28. doi: 10.1016/S1474-4422(14)70172-3
57. Guan, Q, Hu, X, Ma, N, He, H, Duan, F, Li, X, et al. Sleep quality, depression, and cognitive function in non-demented older adults. J Alzheimers Dis. (2020) 76:1637–50. doi: 10.3233/JAD-190990
59. Spira, AP, Gamaldo, AA, An, Y, Wu, MN, Simonsick, EM, Bilgel, M, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. (2013) 70:1537–43. doi: 10.1001/jamaneurol.2013.4258
60. Winer, JR, Mander, BA, Kumar, S, Reed, M, Baker, SL, Jagust, WJ, et al. Sleep disturbance forecasts β-amyloid accumulation across subsequent years. Curr Biol. (2020) 30:4291–4298.e3. doi: 10.1016/j.cub.2020.08.017
61. For the KBASE Research GroupChoe, YM, Byun, MS, Yi, D, Lee, JH, Jeon, SY, et al. Sleep experiences during different lifetime periods and in vivo Alzheimer pathologies. Alzheimers Res Ther. (2019) 11:79. doi: 10.1186/s13195-019-0536-6
62. Huang, EJ, and Reichardt, LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. (2001) 24:677–736. doi: 10.1146/annurev.neuro.24.1.677
63. Malenka, RC, and Bear, MF. LTP and LTD: an embarrassment of riches. Neuron. (2004) 44:5–21. doi: 10.1016/j.neuron.2004.09.012
64. McAllister, AK, Katz, LC, and Lo, DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. (1999) 22:295–318. doi: 10.1146/annurev.neuro.22.1.295
65. Qin, XY, Cao, C, Cawley, NX, Liu, TT, Yuan, J, Loh, YP, et al. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer's disease: a meta-analysis study (N=7277). Mol Psychiatry. (2017) 22:312–20. doi: 10.1038/mp.2016.62
66. Li, G, Peskind, ER, Millard, SP, Chi, P, Sokal, I, Yu, CE, et al. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS One. (2009) 4:e5424. doi: 10.1371/journal.pone.0005424
67. Ballesio, A, Zagaria, A, Curti, DG, Moran, R, Goadsby, PJ, Rosenzweig, I, et al. Peripheral brain-derived neurotrophic factor (BDNF) in insomnia: a systematic review and meta-analysis. Sleep Med Rev. (2023) 67:101738. doi: 10.1016/j.smrv.2022.101738
68. Mikoteit, T, Brand, S, Eckert, A, Holsboer-Trachsler, E, and Beck, J. Brain-derived neurotrophic factor is a biomarker for subjective insomnia but not objectively assessable poor sleep continuity. J Psychiatr Res. (2019) 110:103–9. doi: 10.1016/j.jpsychires.2018.12.020
69. Guzman-Marin, R, Ying, Z, Suntsova, N, Methippara, M, Bashir, T, Szymusiak, R, et al. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. (2006) 575:807–19. doi: 10.1113/jphysiol.2006.115287
70. Wang, R, Guo, Q, Yuan, X, Zhao, J, Zheng, B, Liu, J, et al. Study on the correlation between cognitive impairment and CT measurement of brain structure in the elderly. J Pract Radiol. (2007) 3:289–92. doi: 10.3969/j.issn.1002-1671.2007.03.001
71. Wang, L, Chu, L, and Liu, F. Changes of cognitive function and neuropsychological characteristics in patients with anterior and posterior cerebral cortical atrophy. Chin J Gerontol. (2018) 38:2578–80. doi: 10.3969/j.issn.1005-9202.2018.11.007
72. Sexton, CE, Storsve, AB, Walhovd, KB, Johansen-Berg, H, and Fjell, AM. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. (2014) 83:967–73. doi: 10.1212/WNL.0000000000000774
73. Alperin, N, Wiltshire, J, Lee, SH, Ramos, AR, Hernandez-Cardenache, R, Rundek, T, et al. Effect of sleep quality on amnestic mild cognitive impairment vulnerable brain regions in cognitively normal elderly individuals. Sleep. (2019) 42:zsy254 doi: 10.1093/sleep/zsy254
74. Bai, W, Chen, P, Cai, H, Zhang, Q, Su, Z, Cheung, T, et al. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a meta-analysis and systematic review of epidemiology studies. Age Ageing. (2022) 51:afac173 doi: 10.1093/ageing/afac173
75. Wang, B, Shen, T, Mao, L, Xie, L, Fang, QL, and Wang, X. Establishment of a risk prediction model for mild cognitive impairment among elderly Chinese. J Nutr Health Aging. (2020) 24:255–61. doi: 10.1007/s12603-020-1335-2
76. Xu, T, Bu, G, Yuan, L, Zhou, L, Yang, Q, Zhu, Y, et al. The prevalence and risk factors study of cognitive impairment: analysis of the elderly population of Han nationality in Hunan province, China. CNS Neurosci Ther. (2024) 30:e14478. doi: 10.1111/cns.14478
77. Wöbbeking-Sánchez, M, Bonete-López, B, Cabaco, AS, Urchaga-Litago, JD, and Afonso, RM. Relationship between cognitive reserve and cognitive impairment in autonomous and institutionalized older adults. Int J Environ Res Public Health. (2020) 17:5777. doi: 10.3390/ijerph17165777
78. Marques, P, Moreira, P, Magalhães, R, Costa, P, Santos, N, Zihl, J, et al. The functional connectome of cognitive reserve. Hum Brain Mapp. (2016) 37:3310–22. doi: 10.1002/hbm.23242
79. Zhang, H, Zhang, Y, Sheng, S, Xing, Y, Mou, Z, Zhang, Y, et al. Relationship between physical exercise and cognitive impairment among older adults with type 2 diabetes: chain mediating roles of sleep quality and depression. Psychol Res Behav Manag. (2023) 16:817–28. doi: 10.2147/PRBM.S403788
80. Cheval, B, Maltagliati, S, Sieber, S, Cullati, S, Zou, L, Ihle, A, et al. Better subjective sleep quality partly explains the association between self-reported physical activity and better cognitive function. J Alzheimers Dis. (2022) 87:919–31. doi: 10.3233/JAD-215484
Keywords: older adults hypertension, sleep quality, cognitive impairment, physical activity, mediation analysis
Citation: Lv S, Jiao H, Zhong X, Qu Y, Zhang M, Wang R and Liu D (2024) Association between sleep quality and cognitive impairment in older adults hypertensive patients in China: a case–control study. Front. Public Health. 12:1446781. doi: 10.3389/fpubh.2024.1446781
Edited by:
Chenjuan Gu, GlaxoSmithKline, ChinaReviewed by:
Lenise Jihe Kim, George Washington University, United StatesChen Jiajie, Huazhong University of Science and Technology, China
Copyright © 2024 Lv, Jiao, Zhong, Qu, Zhang, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huachen Jiao, TGl5aXh1YW4wNTMxQDE2My5jb20=; Donghai Liu, TGl1ZG9uZ2hhaUBzZGZtdS5lZHUuY24=
 Shunxin Lv
Shunxin Lv Huachen Jiao
Huachen Jiao Xia Zhong
Xia Zhong Ying Qu1
Ying Qu1 Mengdi Zhang
Mengdi Zhang