- 1West China School of Medicine, Sichuan University, Chengdu, China
- 2Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, China
- 3Chengdu Medical College, Chengdu, China
- 4Department of Outpatient, West China Hospital, Sichuan University, Chengdu, China
- 5Mental Health Center, West China Hospital, Sichuan University, Chengdu, China
Background: Ethylene oxide (EO) is a volatile compound positively correlated with respiratory and cardiovascular diseases. Currently, evidence suggests that environmental exposure may contribute to depressive symptoms. This study evaluated the correlation between EO exposure and depressive symptoms and investigated whether inflammatory indicators had a mediation effect on this correlation.
Methods: Patients were enrolled from the National Health and Nutrition Examination Survey during 2013–2016, and 2,764 (49.67% male and 50.33% female) participants were ultimately included. EO exposure was determined by measuring hemoglobin-EO adduct (Hb-EO) concentration due to its long half-life, which was log2-transformed. Depressive symptoms were assessed using the Patient Health Questionnaire-9. Multivariable logistic regression analysis was performed to identify any correlations before and after covariate adjustment. Sensitivity analysis, subgroup analyses, and interaction tests were performed to further evaluate identified correlations. Mediation analysis was conducted to reveal whether specific inflammatory indicators mediated the correlation.
Results: A high prevalence of depressive symptoms was observed in quartiles with increased levels of EO exposure, and male individuals exhibiting higher Hb-EO levels than female individuals. A positive correlation was observed between EO exposure and depressive symptoms (odds ratio [OR]: 1.439, 95% confidence interval [CI]: 1.310, 1.581), which remained stable even after covariate adjustment (OR: 1.332, 95% CI: 1.148, 1.545). Interaction tests showed significant effects of sex (p < 0.001) and thyroid diseases (p = 0.048) on this correlation. In the mediation analysis, white blood cell (p = 0.010) and neutrophil counts (p = 0.010) exerted a mediating effect, accounting for 13.6 and 11.9%, respectively.
Conclusion: Increased exposure to EO is associated with an elevated risk of depressive symptoms, where white blood cell and neutrophil counts exert a significant mediating effect. Further prospective studies are required to investigate the potential link among EO, other environmental pollutants, and human mental health.
1 Introduction
Depression is a common psychiatric disease characterized by constant sadness and loss of interest (1). According to cross-sectional epidemiological studies, the prevalence of depressive disorders has reached 6.8% in China and 6.38% in Europe (2, 3). Depression is also one of the leading contributors to the Global Burden of Disease, accounting for the largest proportion (37.3%) of disability-adjusted life years among all mental disorders (4). Although the pathogenesis of depression remains under investigation, previous studies have indicated that environmental exposure may play a role in its development and progression (5–7). A retrospective study on 535 older participants revealed that the concentrations of particular phthalate metabolites were positively correlated with depression-related symptoms (5). Further, a systematic review of agricultural workers indicated a link between pesticide exposure and depression (6). Supporting these findings, a cross-sectional study on 10 volatile organic compounds showed that blood levels of benzene, 2,5-dimethylfuran, and furan were positively correlated with depression (7). Therefore, it is essential to explore the impact of environmental exposure on the pathogenesis of depression.
Ethylene oxide (EO), a reactive epoxide derived from ethylene, has been widely applied in chemical compound manufacturing and sterilization of medical equipment (8). Individuals may be exposed to EO through both endogenous and exogenous pathways. Endogenously, the human gut microbiota can convert endogenous ethylene into EO. Exogenously, additional sources of EO exposure include fruit consumption, cigarette smoking, occupational exposure, and environmental pollution (8, 9). EO can combine with DNA or proteins to form adducts, leading to cellular damage and genetic mutations (10). Furthermore, EO is known to bind to hemoglobin, forming hemoglobin-EO adducts that serve as critical biomarkers for assessing the extent of EO exposure (11, 12). Evidence suggests that chronic exposure to EO may increase the risk to a range of neurological symptoms, including memory loss, anxiety and depression (13). EO exposure is also associated with various chronic diseases such as chronic obstructive pulmonary disease (14), asthma (15), and hypertension (16). However, the relationship between exposure to EOs and depression-related symptomology remains unclear.
Inflammatory indicators, including the white blood cell (WBC) count, neutrophil count, lymphocyte count, and neutrophil-to-lymphocyte ratio (NLR), may become essential biomarkers of EO exposure. A cross-sectional study showed that various inflammatory indicators, including the total WBC, neutrophil, and lymphocyte counts, were positively correlated with EO exposure (17). Another population-based study revealed that WBC, neutrophil, and lymphocyte counts exert a mediating effect on the correlation observed between EO and asthma. As a correlation between inflammatory response and depressive symptoms has been widely recognized (18, 19), it is hypothesized that specific inflammatory indicators may play a mediating role between EO exposure and depressive symptoms.
Therefore, we conducted a cross-sectional study and a mediation analysis based on the National Health and Nutrition Examination Survey (NHANES) database. We aimed (1) to evaluate the correlation between EO exposure and depressive symptoms and (2) to investigate whether inflammatory indicators had a mediating effect on this correlation.
2 Methods
2.1 Data source and exclusion criteria
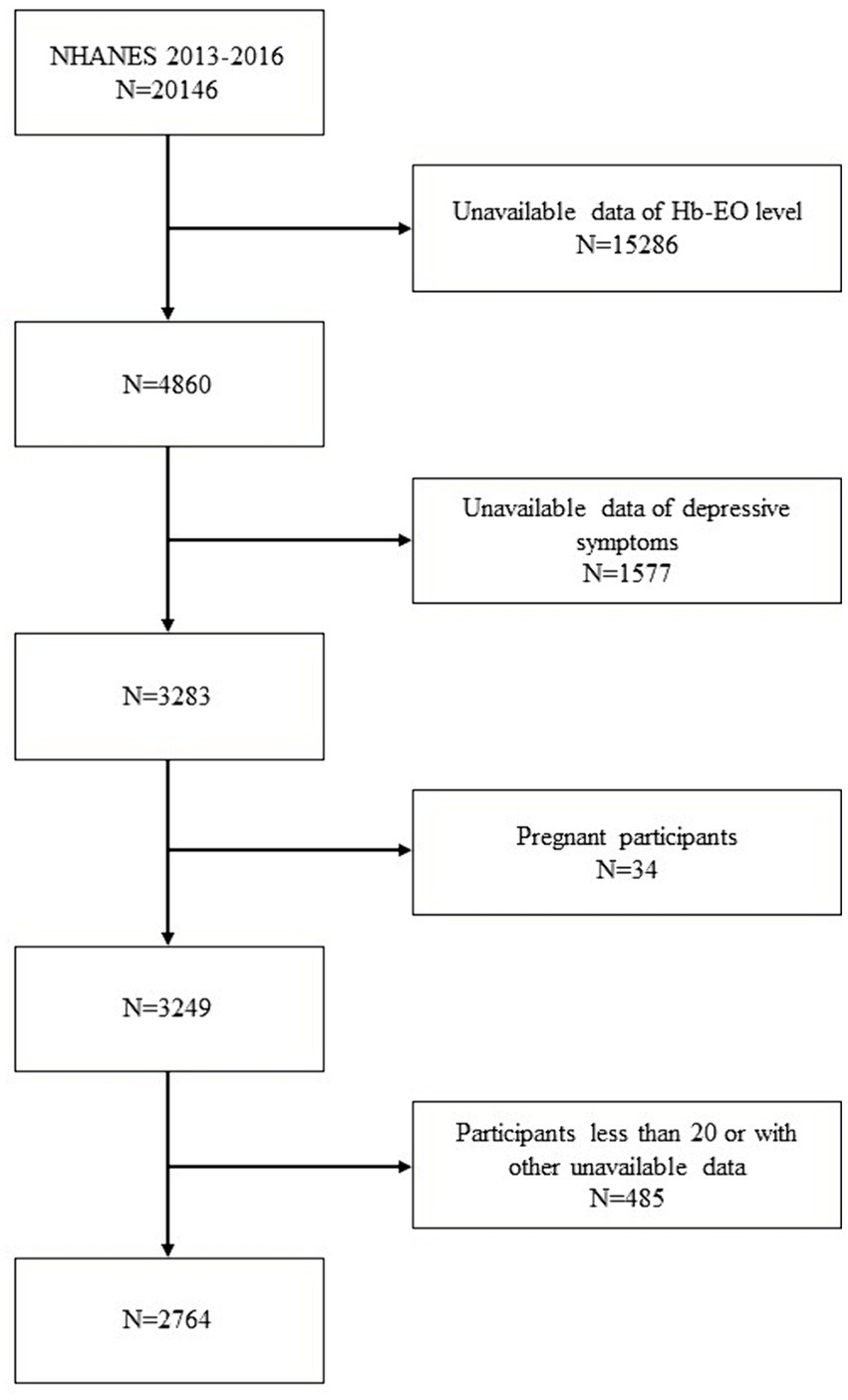
The NHANES is a nationally representative cross-sectional survey conducted by the National Center for Health Statistics (NCHS). A complex multistage probability sampling design was applied to select individuals who could represent a non-institutionalized American population (20). Data from the NHANES comprised demographic information, dietary data, physical examination findings, laboratory test results, and healthcare questionnaire responses. In this cross-sectional study, participants from NHANES 2013–2016 were selected based on available data regarding both EO exposure and depressive symptoms (14). The exclusion criteria were as follows: (1) unavailable EO exposure data, depressive symptom assessments, or other covariates; (2) pregnancy, and (3) participants aged <20 years. The selection process for enrolled participants is shown in Figure 1. The NHANES program was approved by the NCHS Ethics Review Board (Protocol number: Protocol #2011-17). All information concerning ethnic approval of this study can be viewed at NHANES - NCHS Research Ethics Review Board Approval (cdc.gov).
2.2 Measurements of EO exposure, inflammatory indicators, and depressive symptoms
All examinations were performed in the mobile examination centers during NHANES cycles. EO adducts with hemoglobin to form a hemoglobin-EO adduct (Hb-EO), which has been reported to have a longer half-life than EO in vivo, can serve as a sensitive indicator for EO exposure assessments (11, 12, 14). In NHANES 2013–2016, Hb-EO level was measured according to the modified Edman reaction, which forms Edman products under alkaline or neutral conditions with the effect of N-alkylated amino acids. The Hb-EO assessment process consisted of four parts: (1) Specimen preparation: At the NHANES mobile examination center, blood was collected, processed, and aliquoted into vials, then stored at −30°C in preparation for Hb-EO level measurements; (2) Assessment of total hemoglobin in the sample solution: The total Hb levels were determined via photometric measurements accompanied by rigorous quality assessments; (3) Modified Edman reaction and Edman product isolation: This reaction was used to identify the Hb-EO adducts and prepare them for analysis; and (4) Edman product analysis and result processing via high-performance liquid chromatography and tandem mass spectrometry. All measurements were conducted based on a well-established procedure in clinical chemistry [ETHOX_H (cdc.gov); the standardized methods can be found at 2016_MEC_Laboratory_Procedures_Manual.pdf (cdc.gov)].
The blood samples for inflammatory indicators, including total WBC, neutrophils, lymphocytes, and NLR, were also collected in the mobile examination center and analyzed via a complete blood count with 5-Part differential and counted using the UniCel DxH 800 Analyzer [Complete Blood Count (cdc.gov)] (21). NLR was calculated by dividing the neutrophil count by the lymphocyte count.
Depressive symptoms were evaluated using the Patient Health Questionnaire-9 (PHQ-9), a 9-item questionnaire that determines the frequency of depressive symptoms experienced during the most recent 2 weeks (11, 22). The response for each question included “not at all,” “several days,” “more than half the days,” and “nearly every day,” which was scored 0, 1, 2, and 3, respectively. A total score of ≥10 was defined as the presence of depressive symptoms.
2.3 Covariates
Covariates were selected based on prior research and available NHANES data (11, 14). Demographic factors, including sex, age, race, education status, marital status, and economic status (represented by the poverty-to-income ratio [PIR]), are essential characteristics to include. Moreover, given the demonstrated impact of body composition on mental health, body mass index (BMI) was included. Comorbidities such as hypertension, diabetes (including HbA1c levels), cancers, and thyroid diseases were also considered. Lifestyle factors, including measured nicotine levels to assess smoke exposure and annual alcohol consumption (more/less than 12 drinks/year), were included to evaluate drinking status.
2.4 Ethics approval
This population-based study included human participants, which was approved by the Ethics Review Board of NCHS [NHANES - NCHS Research Ethics Review Board Approval (cdc.gov)]. Written informed consent were obtained from all individuals in this survey.
2.5 Statistical analyses
All statistical analyses were conducted according to the primary sample units and strata of the NHANES. An appropriate subsample weight was also applied according to the NHANES recommendation [ETHOX_H (cdc.gov)]. Continuous variables are presented as mean with standard estimate (SE) and were analyzed using the t-test, and categorical variables are presented as percentages with SE and were analyzed using the chi-square test.
Due to the skewed distribution of Hb-EO levels, log2Hb-EO was applied to reach a normal distribution. Multivariate logistic regression analysis was performed using the three models to explore the correlation between EO exposure and depressive symptoms. In Model 1, no covariates were adjusted for. Model 2 was adjusted for sex, age, and race only. Model 3 was adjusted for all covariates mentioned in Section 2.3. Sensitivity analysis was conducted by dividing log2Hb-EO into four quartiles to test the stability and trend of this correlation. Subgroup analyses with interaction tests was also performed for all categorical variables to further investigate correlations. Multivariate logistic regression was applied again to reveal the role of inflammatory indicators in the relationship between EO exposure and depressive symptoms. Mediation analysis, including multiple regression analysis, path analysis, mediation model construction, and the bootstrap method, was performed to explore whether inflammatory indicators were involved in EO’s effect on depressive symptoms (23). The results of mediation analysis were presented as total effect, direct effect, mediation effect, and mediation proportion. All statistical analyses were performed using the R and Empower software (EmpowerStats | Data Analysis for Biostatistics & Epidemiology), and a p-value of < 0.05 was considered statistically significant.
3 Results
3.1 Summary of the key findings
Participants with higher levels of EO exposure exhibited a higher prevalence of depressive symptoms. Multivariate logistic regression suggested that EO exposure increased the risk of depressive symptoms, even after adjusting for covariates. Interaction tests revealed that sex and a history of thyroid diseases had remarkable interacting effects on this association. Additionally, mediation analysis showed that both WBC and neutrophil counts mediated the association between EO exposure and depressive symptoms.
3.2 Baseline characteristics of the enrolled participants
A total of 2,764 (49.67% male and 50.33% female) participants were selected for enrollment from NHANES 2013–2016. Table 1 shows the baseline characteristics of the eligible participants in the four log2Hb-EO quartiles; there were significant differences among the quartiles, except for hypertension (p = 0.9360) and cancer prevalence (p = 0.5253). The log2Hb-EO ranges for quartiles 1, 2, 3, and 4 were 2.54–3.91, 3.91–4.39, 4.40–5.41, and 5.42–10.35, respectively. The prevalence rates of depressive symptoms in quartiles 1, 2, 3, and 4 were 4.08, 4.54, 5.39, and 15.95%, respectively (p < 0.001). Moreover, male individuals exhibited higher levels of log2Hb-EO than female individuals (p = 0.003) (Supplementary Table S1).
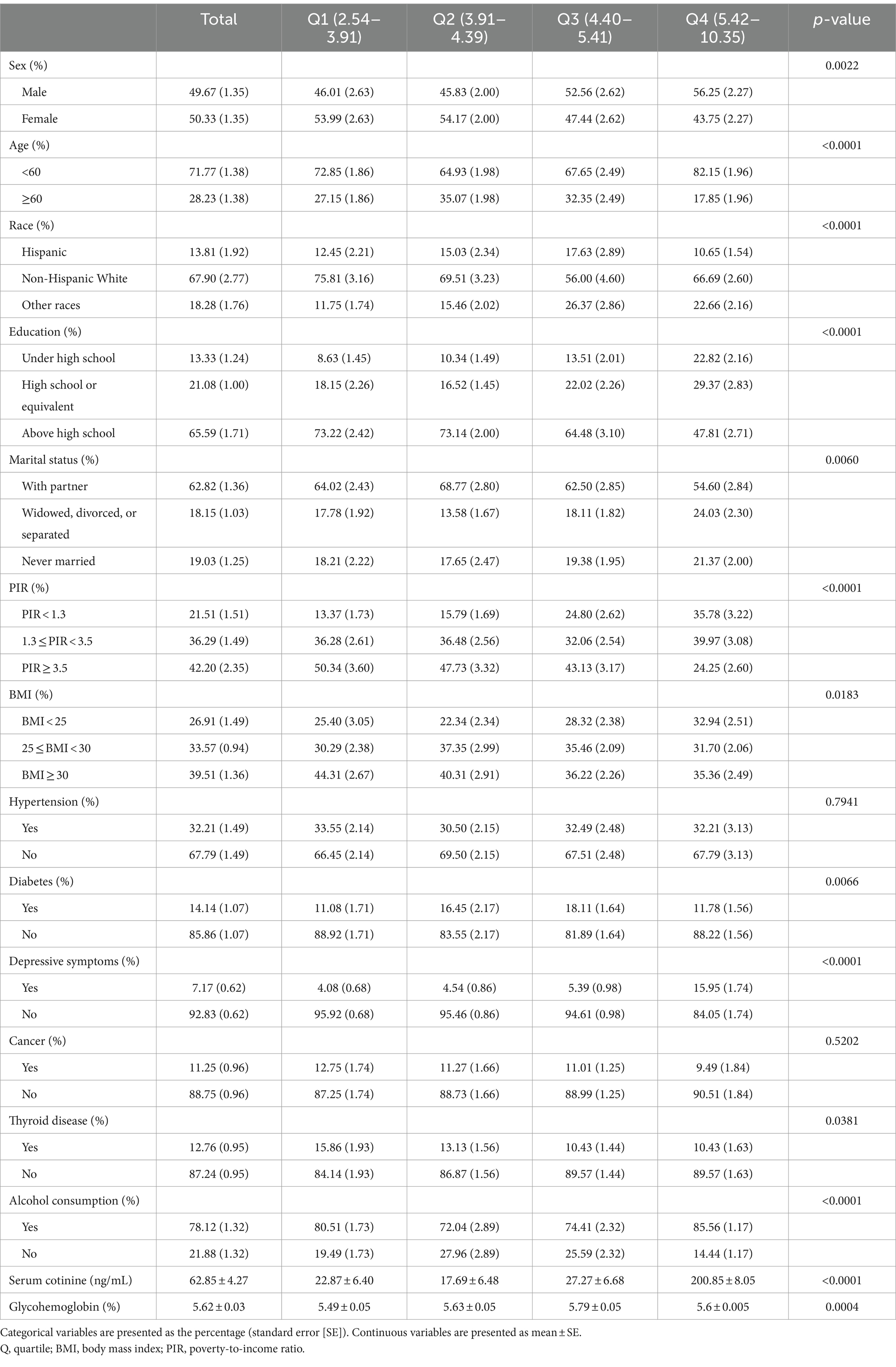
Table 1. Weighted baseline characteristics of eligible participants enrolled in this study (divided by different quartiles of EO exposure).
3.3 Association between EO exposure and depressive symptoms
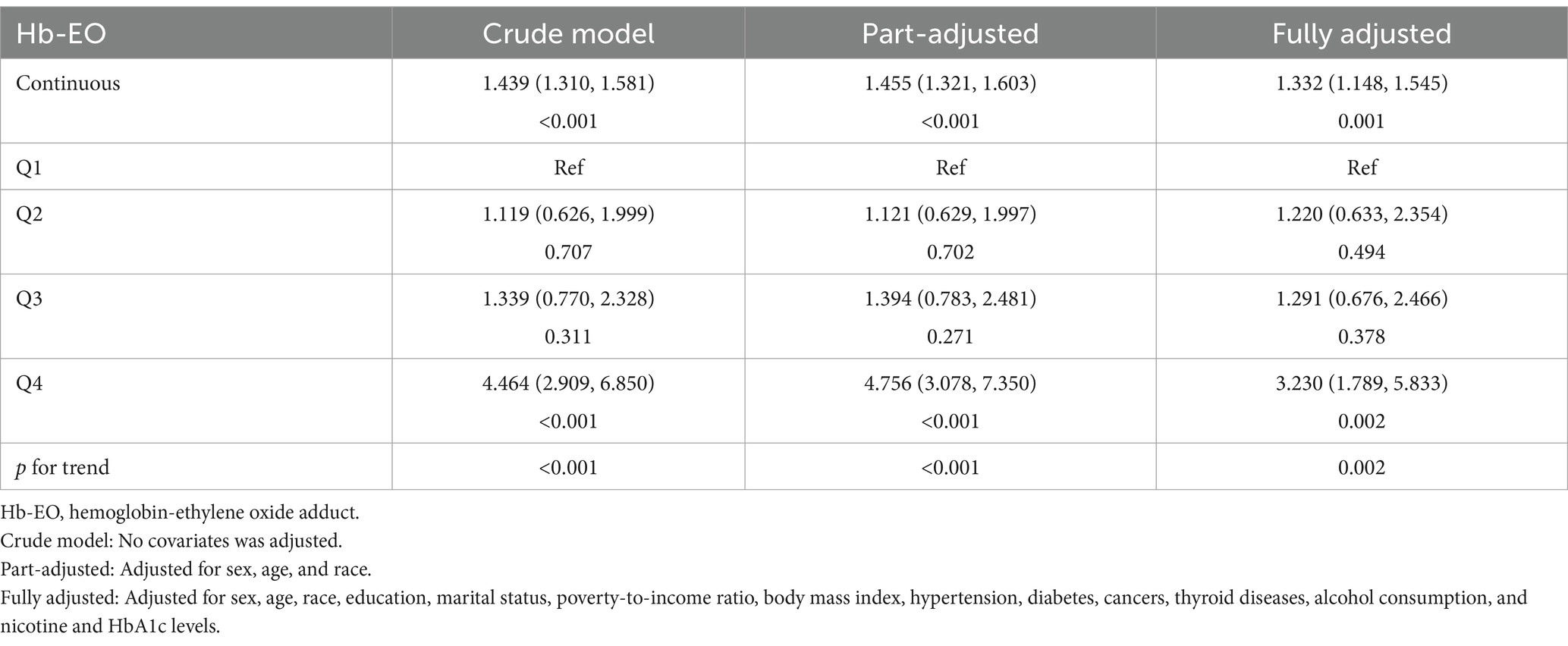
Table 2 shows the results of multivariate logistic regression analysis of the correlation between EO exposure and depressive symptoms. Positive correlations were observed among the crude (odds ratio [OR]: 1.439, 95% confidence interval [CI]: 1.310, 1.581), part-adjusted (OR: 1.455, 95% CI: 1.321, 1.603) and fully adjusted models (OR: 1.332, 95% CI: 1.148, 1.545). Compared with quartile 1 (although quartiles 2 and 3 in the three models did not reach significance), quartile 4 showed a significantly higher risk of depressive symptoms in the three models. The p-values for the trends in the three models were all <0.05.
3.4 Subgroup analyses and interaction tests
The results of the subgroup analyses are shown in Figure 2. The significant correlation between EO exposure and depressive symptoms was not significant in certain subgroups, including male sex, age ≥ 60 years, race (except non-Hispanic White), education status (under high school education), never married, PIR (<1.3 or ≥3.5), BMI <25 kg/m2, or history of hypertension or diabetes. The interaction tests showed that sex (p < 0.001) and thyroid diseases (p = 0.048) had a significant impact on the correlation between EO and depressive symptoms.
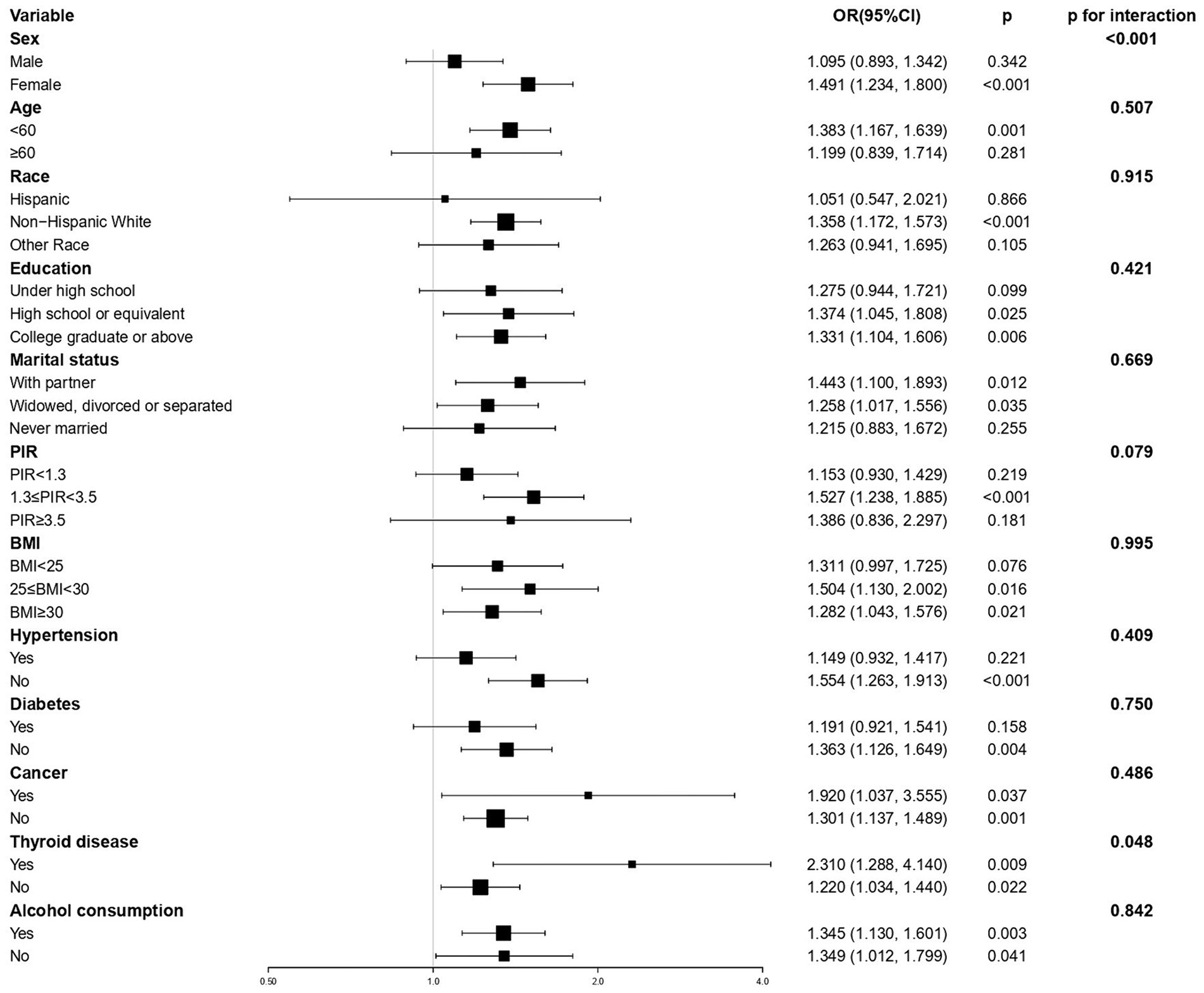
Figure 2. Subgroup analyses of the association between ethylene oxide exposure and depressive symptoms. All covariates (sex, age, race, education, marital status, PIR, BMI, hypertension, diabetes, cancer thyroid disease, alcohol consumption, and level of nicotine and HbA1c) were adjusted in subgroup analyses. PIR, poverty-to-income ratio; BMI, body mass index.
3.5 Mediation analysis of inflammatory indicators
Table 3 shows the correlations between Hb-EO levels and different inflammatory indicators using multivariate logistic regression analysis. Hb-EO level was positively correlated with WBC (β = 0.374, 95% CI: 0.266, 0.482), neutrophil (β = 0.257, 95% CI: 0.175, 0.339), and lymphocyte (β = 0.089, 95% CI: 0.055, 0.123) counts. However, no significant association was observed between Hb-EO and NLR (β = 0.043, 95% CI: −0.005, 0.091) after covariate adjustment. Mediation analysis was then applied to explore whether inflammatory indicators played a mediating role in the association between EO exposure and depressive symptoms. As shown in Figure 3, WBC (p = 0.010) and neutrophil (p = 0.010) counts significantly mediated this correlation, accounting for 13.6 and 11.9%, respectively. However, the mediating effects of lymphocyte count (p = 0.548) and NLR (p = 0.210) were not statistically significant.

Table 3. Association between ethylene oxide exposure and inflammatory indicators after covariate adjustment.
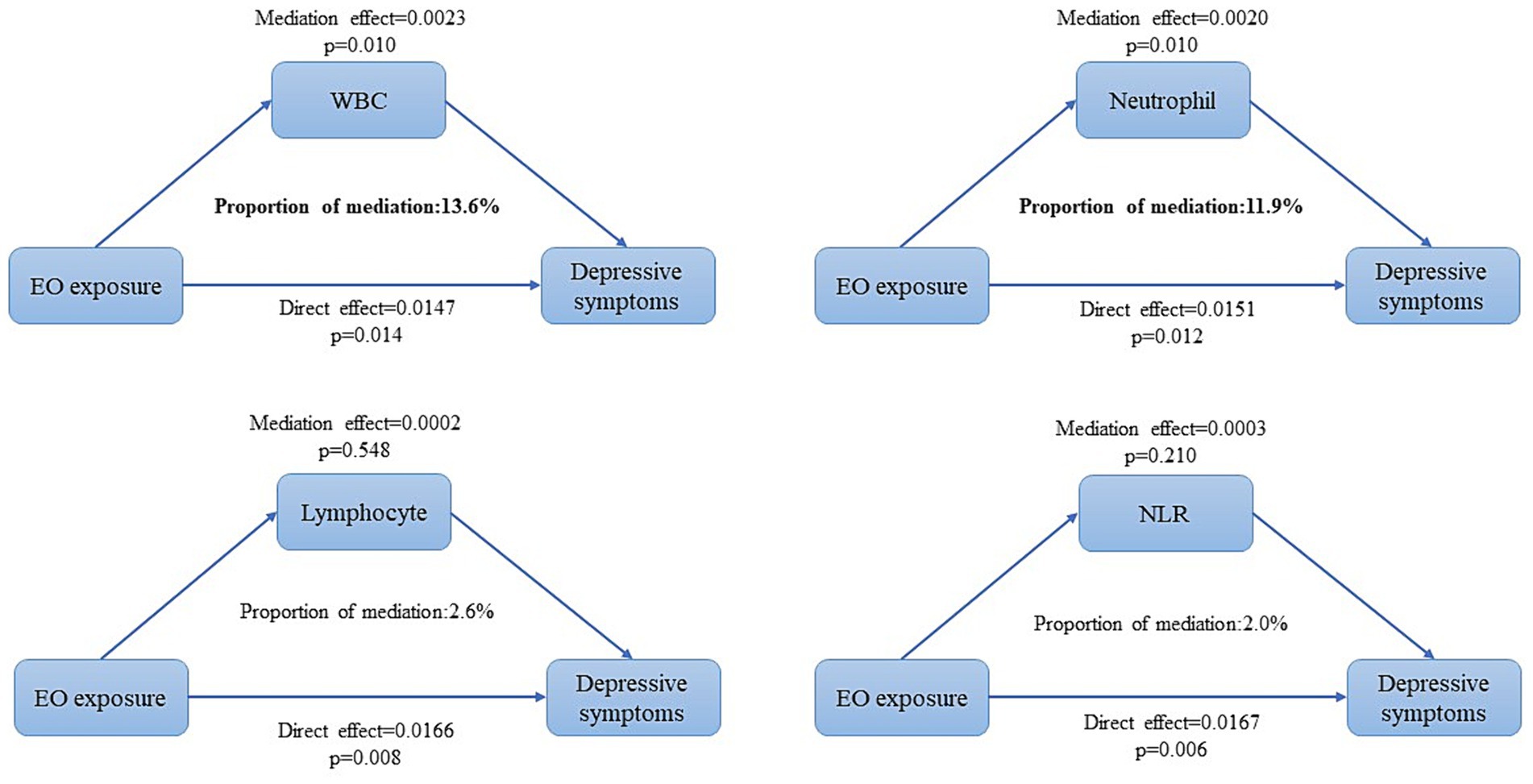
Figure 3. Mediation analysis of WBC, neutrophil, and lymphocyte counts and NLR over the association between EO exposure and depressive symptoms. EO, ethylene oxide; WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio. All covariates (sex, age, race, education, marital status, poverty-to-income ratio, body mass index, hypertension, diabetes, cancer thyroid disease, alcohol consumption, and levels of nicotine and HbA1c) were adjusted in mediation analyses.
4 Discussion
This cross-sectional, population-based study provides evidence that EO exposure is positively correlated with depressive symptoms. Moreover, it is identified that some inflammatory indicators, including WBC and neutrophil counts, exerted mediating effects on this correlation.
4.1 Potential sources of EO exposure
Humans are exposed to EO through a variety of unavoidable endogenous and exogenous sources within the general population (8). Previous evidence has suggested that ethylene may act as a potential precursor to EO (8). The consumption of fruit may increase EO exposure via metabolizing dietary ethylene into ethylene oxide (24, 25). A preliminary study suggested that fruit store workers exhibited a 23 pmol/g increase in Hb-EO levels (26). Moreover, some ethylene-producing bacteria have been detected in the human gastrointestinal tract, suggesting that the gut microbiota may also result in EO formation (8). Smoking can also increase the chance of EO exposure due to the incomplete combustion of cigarette smoke (8). Other evidence suggests that EO exposure may result from occupational sources because EO is applicable in medical equipment sterilization (8). As EO may be produced after the burning of vegetation or degeneration of agricultural waste, environmental pollution may become another source of EO exposure (9). However, although the aforementioned sources have been identified as potential contributors to EO exposure, further quantitative details are necessary to determine the amount of exposure from each potential source.
As a biomarker for quantifying EO exposure, Hb-EO has shown promising reliability. Walker et al. reported that after 4 weeks of EO exposure, Hb-EO formation was linear at concentrations ranging from 3 to 33 ppm and increased in slope at concentrations above 33 ppm in mice (27). Another human-based study suggested that Hb-EO levels were significantly elevated in smokers, aligning quantitatively with the exposure source (28). These findings highlight the reliability of Hb-EO in reflecting EO exposure accurately.
4.2 Hypothesis of how EO exposure affects the pathogenesis of depression
The possible mechanisms underlying the impact of EO on depressive symptoms are summarized in Figure 4. Mediation analysis showed that WBC count accounted for 13.6% of the mediation proportion, whereas the neutrophil count accounted for 11.9%, indicating that neutrophils may play an essential role on this correlation. Previous evidence suggested that neutrophils accelerate the formation of neutrophil extracellular traps in response to various extrinsic or intrinsic stimuli (29). Another study revealed that the formation of neutrophil extracellular traps aggravates pro-inflammatory cytokine release and promotes neuroinflammation in mice, which subsequently cause further depressive symptoms (30). Therefore, exposure to EO may promote neutrophil activation and neutrophil extracellular trap formation, subsequently leading to neuroinflammation and depressive symptoms. However, this hypothesis warrants further investigations as other potential inflammation-related factors, such as infection, metabolic diseases, and immunological diseases, may also affect the pathogenesis of depression through inflammatory responses. Moreover, these inflammatory indicators may only reflect a certain aspect of inflammation.
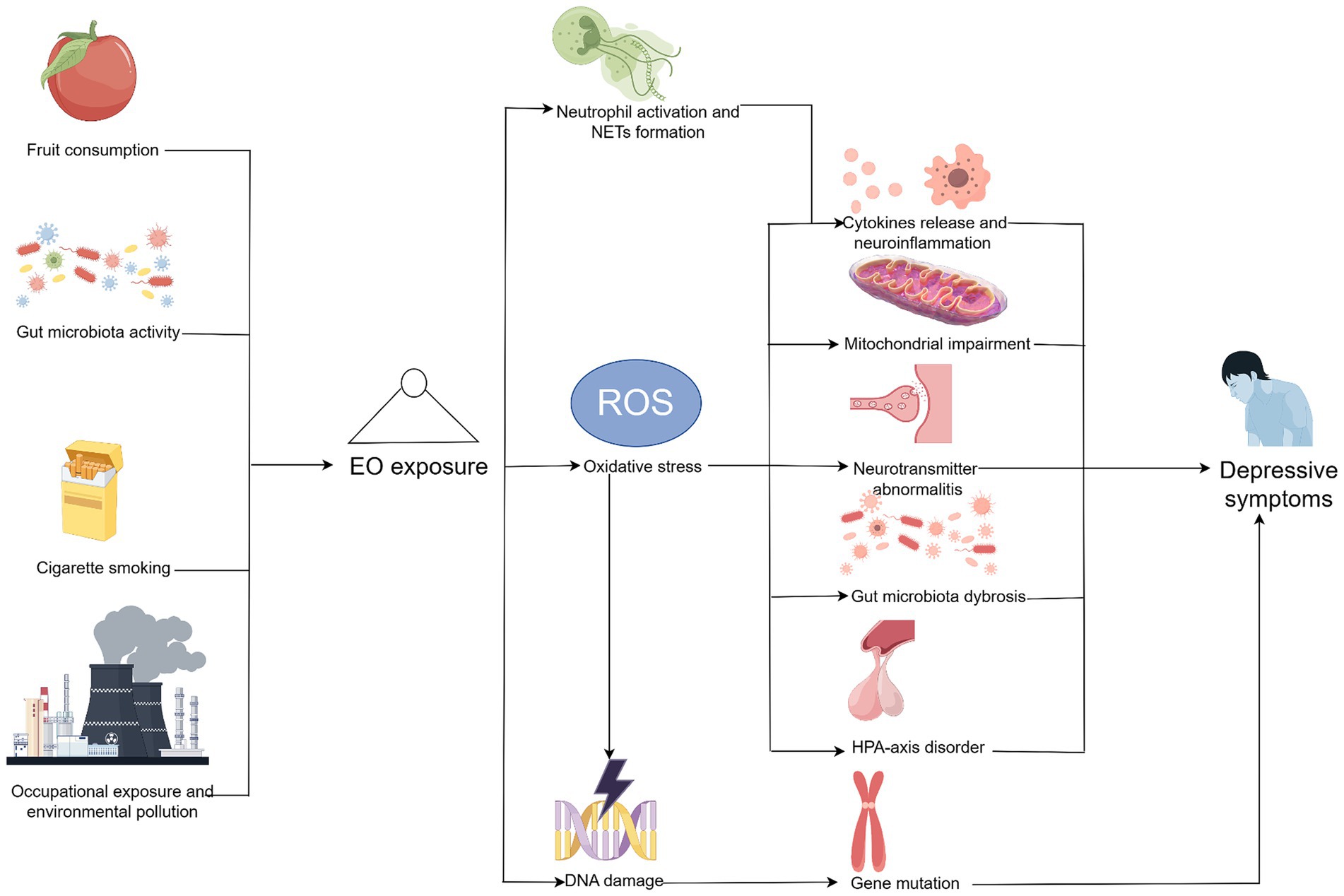
Figure 4. Potential mechanisms underlying the impact of ethylene oxide on depressive symptoms. Created by Figdraw. Further studies are needed to verify these pathways.
Moreover, oxidative stress may serve as an essential contributor to depressive symptoms as a consequence of EO exposure. Compared with controlled rats, rats exposed to EO showed elevated levels of malondialdehyde and advanced oxidation protein products and lower levels of superoxide dismutase, glutathione, and catalase, suggesting the existence of oxidative stress under EO exposure (9). Oxidative stress may contribute to depressive symptoms through multiple pathways, including neuroinflammation, mitochondrial impairment, neurotransmitter abnormalities, gut microbiota dysbiosis, and hypothalamic–pituitary–adrenal disorders (31). Oxidative stress promoted pro-inflammatory cytokine (such as interleukin-6 and tumor necrosis factor-α) release, leading to dysregulation of the inflammatory response and cell damage (32). Consistent with this, Liu et al. reported decreased levels of malondialdehyde, which is regarded as a biomarker of oxidative stress, in stress-exposed mice after muscone (an active ingredients from the musk) treatment (33). Meanwhile, the exposed mice showed decreased inflammatory levels and more adaptive behavioral responses compared with those that were not treated (33). Oxidative stress was also associated with overproduction of reactive oxygen species (ROS) in mitochondria, causing enzyme inactivation and mitochondrial dysfunction, which may promote depression-related symptomology (34, 35). Salvi et al. also suggested that air pollutants can induce oxidative stress and mitochondrial impairments, thereby disrupting neural circuitry and leading to depressive-like behaviors (36). Moreover, oxidative stress is closely associated with alterations in neurotransmitter function. For example, accumulation of excessive ROS may inhibit glutamate transporter 1 expression in astrocytes and affect glutamine synthetase levels, leading to glutamate excitotoxicity and subsequent depressive symptoms (31). Oxidative stress may also produce neurotoxic substances via the oxidation of tryptophan, which is a precursor of serotonin and is associated with depression pathogenesis (32). Furthermore, dysbiosis of the gut microbiota may be a consequence of excessive oxidative stress. Current evidence suggests that gut-brain axis disturbance may serve as a vital downstream pathway of oxidative stress, affecting the abundance and function of the microbiota (37). Since the gut microbiota is also responsible for regulating oxidative stress, its dysfunction may further promote oxidative stress, contributing to the pathogenesis of depression (38). Furthermore, oxidative stress may disturb activity of the hypothalamic–pituitary–adrenal axis. A clinical trial in adolescent children with depression suggested that morning cortisol concentration was positively correlated with lipoperoxide expression, while the aldosterone concentration was positively correlated with 8-isoprostane (p = 0.019), suggesting that stress hormone secretion is positively correlated with oxidative stress in adolescents with depression (39). Another study reported that corticosterone administration promoted deficits in the forced swim test in rodents and contributed to the development of depressive-like behaviors, such as anhedonia and learned helplessness (40).
Additionally, the increased incidence of genetic mutations caused by exposure to EOs may promote depressive symptoms. EO-DNA adducts, including N3-hydroxyehyladenine and O6-hydroxyethylguanine, are regarded as the initial steps in the development of genetic mutations (8, 10). N3-hydroxyethyladenine may adduct with the minor groove of the DNA helix, whereas O6-hydroxyethylguanine directly affects the base pairing of nucleotides (10). Evidence suggests that genetic mutations may also contribute to depressive symptoms. A study on patients with Alzheimer’s disease suggested that a carboxypeptidase E/neurotrophic factor-α1 gene mutation in humans leads to impaired memory acquisition and depressive-like symptoms in transgenic mice (41). Another study on patients with primary aldosteronism uncovered that KCNJ5 mutations were associated with higher baseline PHQ scores and a better response to treatment with adrenalectomy, indicating that KCNJ5 mutations may participate in the formation of depressive symptoms (42). Moreover, subsequent effects of EO exposure, including oxidative stress, promote genetic mutations. A comparative study showed that patients with major depressive disorder had greater mitochondrial oxidative damage (p < 0.001) and lower leukocyte mtDNA copy numbers (p = 0.037) (43). Therefore, genetic mutations may also contribute to depressive symptoms resulting from EO exposure.
4.3 Interpretation of interaction test results
The results of this cross-sectional study also showed that sex (p < 0.001) and history of thyroid diseases (p = 0.048) significantly affected the association between EO exposure and depressive symptoms. We also observed that the significant association disappeared in male participants after subgroup analysis. This may be attributed to more EO exposure and less depressive symptoms in male individuals than in female individuals. Current evidence suggests that higher concentrations of Hb-EO adducts are detected in males than in females, which may be explained by a higher smoking prevalence in male individuals (8). By contrast, the prevalence of depressive disorders in female individuals is significantly higher than that in male individuals (44). Regarding the interactive effect of thyroid diseases, a history of thyroid diseases may make patients more vulnerable to EO exposure and more susceptible to depressive symptoms (45). However, the specific role of thyroid diseases in the association between EO exposure and depressive symptoms warrants further investigations.
4.4 Strengths and limitations of this study
This study has some strengths that should be discussed. By investigating the correlation between EO exposure and depressive symptoms and the mediating role of inflammatory indicators, this study suggested that reduction of EO exposure may become a feasible way of preventing depressive disorders. Moreover, as EO exposure has been linked to an increased risk of chronic respiratory diseases (14), cardiovascular diseases (16), and metabolic diseases (46), this study may also draw additional attention to reveal impact of environmental factors on various psychiatry diseases. Additionally, due to the public health implications of EO exposure, this study may prompt ethical considerations that could influence public health policies. However, there are still some limitations that are worth mentioning. First, owing to the cross-sectional design, we could not determine a causal relationship between EO exposure and depressive symptoms (47). Second, although we conducted covariate adjustments in the part-adjusted and fully adjusted models, potential confounders (such as those unavailable in the NHANES database) may still affect the study results. Third, the diagnosis of depressive symptoms was based on self-reported PHQ-9 scores, which could introduce bias. Fourth, since the NHANES program focused on the non-institutionalized American population, the generalizability of the aforementioned findings warrants further investigations. Fifth, further details of EO exposure (such as specific amounts and durations that lead to increased levels of Hb-EO) remain limited and warrant further investigations. Sixth, although both laboratory tests and the PHQ-9 questionnaires were completed at the mobile examination centers, the precise intervals between these tests for each individual were not available, which may also lead to potential bias.
5 Conclusion
This cross-sectional study demonstrated that EO exposure (measured by Hb-EO adduct) positively correlated with depressive symptoms. Inflammatory indicators, including WBC and neutrophil counts, exerted a mediating effect on this correlation, while the mediating effects of lymphocytes and the NLR remained insignificant. Further research should investigate the underlying mechanisms linking environmental pollutants to mental disorders. Such studies may offer new insights into the prevention of mental diseases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Review Board of NCHS [NHANES - NCHS Research Ethics Review Board Approval (cdc.gov)]. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DD: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. YY: Data curation, Investigation, Methodology, Writing – original draft. XG: Data curation, Investigation, Methodology, Writing – original draft. QX: Data curation, Investigation, Methodology, Writing – original draft. ZD: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China [Grant No. 81801350] and the Key R&D Projects of the Science and Technology Department of Sichuan Province [Grant Nos. 2023YFS0292 and 2022YFS0350]. These funding agencies were not involved in study design, data analysis or journal selection.
Acknowledgments
We would like to thank Editage for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1445257/full#supplementary-material
Abbreviations
AI, Artificial intelligence; BMI, Body mass index; EO, Ethylene oxide; NCHS, National Center for Health Statistics; NHANES, National Health and Nutrition Examination Survey; NLR, Neutrophil-to-lymphocyte ratio; PIR, Poverty-to-income ratio; ROS, Reactive oxygen species; SE, Standard estimate; WBC, White blood cell.
References
1. McCarron, RM, Shapiro, B, Rawles, J, and Luo, J. Depression. Ann Intern Med. (2021) 174:ITC65–80. doi: 10.7326/AITC202105180
2. Huang, Y, Wang, Y, Wang, H, Liu, Z, Yu, X, Yan, J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2019) 6:211–24. doi: 10.1016/S2215-0366(18)30511-X
3. Arias-de la Torre, J, Vilagut, G, Ronaldson, A, Serrano-Blanco, A, Martín, V, Peters, M, et al. Prevalence and variability of current depressive disorder in 27 European countries: a population-based study. Lancet Public Health. (2021) 6:e729–38. doi: 10.1016/S2468-2667(21)00047-5
4. Drevets, WC, Wittenberg, GM, Bullmore, ET, and Manji, HK. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Discov. (2022) 21:224–44. doi: 10.1038/s41573-021-00368-1
5. Lee, KS, Lim, YH, Kim, KN, Choi, YH, Hong, YC, and Lee, N. Urinary phthalate metabolites concentrations and symptoms of depression in an elderly population. Sci Total Environ. (2018) 625:1191–7. doi: 10.1016/j.scitotenv.2017.12.219
6. Cancino, J, Soto, K, Tapia, J, Muñoz-Quezada, MT, Lucero, B, Contreras, C, et al. Occupational exposure to pesticides and symptoms of depression in agricultural workers. A systematic review. Environ. Res. (2023) 231:116190. doi: 10.1016/j.envres.2023.116190
7. Zhu, Y, Ju, Y, Wang, M, Yang, Y, and Wu, R. Association of volatile organic compounds exposure with the risk of depression in U.S. adults: a cross-sectional study from NHANES 2013-2016. Int Arch Occup Environ Health. (2023) 96:1101–11. doi: 10.1007/s00420-023-01993-6
8. Kirman, CR, Li, AA, Sheehan, PJ, Bus, JS, Lewis, RC, and Hays, SM. Ethylene oxide review: characterization of total exposure via endogenous and exogenous pathways and their implications to risk assessment and risk management. J Toxicol Environ Health B Crit Rev. (2021) 24:1–29. doi: 10.1080/10937404.2020.1852988
9. Rasool, M, Malik, A, Abdul Basit Ashraf, M, Mubbin, R, Ayyaz, U, Waquar, S, et al. Phytochemical analysis and protective effects of Vaccinium macrocarpon (cranberry) in rats (Rattus norvegicus) following ethylene oxide-induced oxidative insult. Bioengineered. (2021) 12:4593–604. doi: 10.1080/21655979.2021.1955528
10. Lynch, HN, Kozal, JS, Russell, AJ, Thompson, WJ, Divis, HR, Freid, RD, et al. Systematic review of the scientific evidence on ethylene oxide as a human carcinogen. Chem Biol Interact. (2022) 364:110031. doi: 10.1016/j.cbi.2022.110031
11. Cao, S, Meng, L, Bai, H, Yang, W, Hu, X, and Li, X. The association between ethylene oxide and testosterone in the United States population: a cross-sectional study based on the National Health and nutrition examination survey (NHANES) 2013-2016. Endocrine. (2024). doi: 10.1007/s12020-024-03979-x
12. Ogawa, M, Oyama, T, Isse, T, Yamaguchi, T, Murakami, T, Endo, Y, et al. Hemoglobin adducts as a marker of exposure to chemical substances, especially PRTR class I designated chemical substances. J Occup Health. (2006) 48:314–28. doi: 10.1539/joh.48.314
13. O'Kelley, L, Swanson, B, and Bishop-Royse, JC. Integrative literature review: ethylene oxide exposure signs and symptoms. Public Health Nurs (Boston, MA). (2023) 40:790–809. doi: 10.1111/phn.13216
14. Huang, Q, Li, S, Wan, J, Nan, W, and He, B. Association between ethylene oxide exposure and prevalence of COPD: evidence from NHANES 2013-2016. Sci Total Environ. (2023) 885:163871. doi: 10.1016/j.scitotenv.2023.163871
15. Li, Z, Shi, P, Chen, Z, Zhang, W, Lin, S, Zheng, T, et al. The association between ethylene oxide exposure and asthma risk: a population-based study. Environ Sci Pollut Res Int. (2023) 30:24154–67. doi: 10.1007/s11356-022-23782-3
16. Wu, N, Cao, W, Wang, Y, and Liu, X. Association between blood ethylene oxide levels and the prevalence of hypertension. Environ Sci Pollut Res Int. (2022) 29:76937–43. doi: 10.1007/s11356-022-21130-z
17. Zhu, X, Kong, X, Chen, M, Shi, S, Cheang, I, Zhu, Q, et al. Blood ethylene oxide, systemic inflammation, and serum lipid profiles: results from NHANES 2013-2016. Chemosphere. (2022) 299:134336. doi: 10.1016/j.chemosphere.2022.134336
18. Cao, P, Chen, C, Liu, A, Shan, Q, Zhu, X, Jia, C, et al. Early-life inflammation promotes depressive symptoms in adolescence via microglial engulfment of dendritic spines. Neuron. (2021) 109:2573–89.e9. doi: 10.1016/j.neuron.2021.06.012
19. Beurel, E, Toups, M, and Nemeroff, CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
20. You, Y, Chen, Y, Wei, M, Tang, M, Lu, Y, Zhang, Q, et al. Mediation role of recreational physical activity in the relationship between the dietary intake of live microbes and the systemic immune-inflammation index: a real-world cross-sectional study. Nutrients. (2024) 16:777. doi: 10.3390/nu16060777
21. Du, D, Zhang, G, Xu, D, Xu, D, Liu, L, Hu, X, et al. Association between systemic inflammatory markers and chronic obstructive pulmonary disease: a population-based study. Heliyon. (2024) 10:e31524. doi: 10.1016/j.heliyon.2024.e31524
22. Kroenke, K, Spitzer, RL, and Williams, JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Imai, K, Keele, L, and Tingley, D. A general approach to causal mediation analysis. Psychol Methods. (2010) 15:309–34. doi: 10.1037/a0020761
24. Xu, J, Liu, S, Cai, L, Wang, L, Dong, Y, Qi, Z, et al. SPINDLY interacts with EIN2 to facilitate ethylene signalling-mediated fruit ripening in tomato. Plant Biotechnol J. (2023) 21:219–31. doi: 10.1111/pbi.13939
25. Walker, VE, Wu, KY, Upton, PB, Ranasinghe, A, Scheller, N, Cho, MH, et al. Biomarkers of exposure and effect as indicators of potential carcinogenic risk arising from in vivo metabolism of ethylene to ethylene oxide. Carcinogenesis. (2000) 21:1661–9. doi: 10.1093/carcin/21.9.1661
26. Törnqvist, MA, Almberg, JG, Bergmark, EN, Nilsson, S, and Osterman-Golkar, SM. Ethylene oxide doses in ethene-exposed fruit store workers. Scand J Work Environ Health. (1989) 15:436–8. doi: 10.5271/sjweh.1829
27. Walker, VE, Fennell, TR, Upton, PB, MacNeela, JP, and Swenberg, JA. Molecular dosimetry of DNA and hemoglobin adducts in mice and rats exposed to ethylene oxide. Environ Health Perspect. (1993) 99:11–7. doi: 10.1289/ehp.939911
28. Törnqvist, M, Osterman-Golkar, S, Kautiainen, A, Jensen, S, Farmer, PB, and Ehrenberg, L. Tissue doses of ethylene oxide in cigarette smokers determined from adduct levels in hemoglobin. Carcinogenesis. (1986) 7:1519–21. doi: 10.1093/carcin/7.9.1519
29. Döring, Y, Libby, P, and Soehnlein, O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ Res. (2020) 126:1228–41. doi: 10.1161/CIRCRESAHA.120.315931
30. Kong, Y, He, G, Zhang, X, and Li, J. The role of neutrophil extracellular traps in lipopolysaccharide-induced depression-like behaviors in mice. Brain Sci. (2021) 11:1514. doi: 10.3390/brainsci11111514
31. Ji, N, Lei, M, Chen, Y, Tian, S, Li, C, and Zhang, B. How oxidative stress induces depression? ASN Neuro. (2023) 15:17590914231181037. doi: 10.1177/17590914231181037
32. Correia, AS, Cardoso, A, and Vale, N. Oxidative stress in depression: the link with the stress response, Neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants (Basel, Switzerland). (2023) 12:470. doi: 10.3390/antiox12020470
33. Meng, D, Yang, M, Hu, L, Liu, T, Zhang, H, Sun, X, et al. Rifaximin protects against circadian rhythm disruption-induced cognitive impairment through preventing gut barrier damage and neuroinflammation. J Neurochem. (2022) 163:406–18. doi: 10.1111/jnc.15701
34. Shao, A, Lin, D, Wang, L, Tu, S, Lenahan, C, and Zhang, J. Oxidative stress at the crossroads of aging, stroke and depression. Aging Dis. (2020) 11:1537–66. doi: 10.14336/AD.2020.0225
35. Casaril, AM, Dantzer, R, and Bas-Orth, C. Neuronal mitochondrial dysfunction and bioenergetic failure in inflammation-associated depression. Front Neurosci. (2021) 15:725547. doi: 10.3389/fnins.2021.725547
36. Salvi, A, Liu, H, and Salim, S. Involvement of oxidative stress and mitochondrial mechanisms in air pollution-related neurobiological impairments. Neurobiol Stress. (2020) 12:100205. doi: 10.1016/j.ynstr.2019.100205
37. Xie, XD, Zhou, Y, Sun, YB, Yi, SL, Zhao, Y, Chen, Q, et al. RNA-Seq and 16S rRNA reveals that Tian-Dong-Tang-Gan powder alleviates environmental stress-induced decline in immune and antioxidant function and gut microbiota Dysbiosis in Litopenaeus vannami. Antioxidants (Basel, Switzerland). (2023) 12:1262. doi: 10.3390/antiox12061262
38. Hao, WZ, Li, XJ, Zhang, PW, and Chen, JX. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. (2020) 284:112691. doi: 10.1016/j.psychres.2019.112691
39. Oravcova, H, Katrencikova, B, Garaiova, I, Durackova, Z, Trebaticka, J, and Jezova, D. Stress hormones cortisol and aldosterone, and selected markers of oxidative stress in response to long-term supplementation with Omega-3 fatty acids in adolescent children with depression. Antioxidants (Basel, Switzerland). (2022) 11:1546. doi: 10.3390/antiox11081546
40. Mikulska, J, Juszczyk, G, Gawrońska-Grzywacz, M, and Herbet, M. HPA Axis in the Pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci. (2021) 11:1298. doi: 10.3390/brainsci11101298
41. Cheng, Y, Cawley, NX, Yanik, T, Murthy, SRK, Liu, C, Kasikci, F, et al. A human carboxypeptidase E/NF-α1 gene mutation in an Alzheimer's disease patient leads to dementia and depression in mice. Transl Psychiatry. (2016) 6:e973. doi: 10.1038/tp.2016.237
42. Adolf, C, Murck, H, Sarkis, AL, Schneider, H, Heinrich, DA, Williams, TA, et al. Differential central regulatory mineralocorticoidreceptor systems for anxiety and depression - could KCNJ5 be an interesting target for further investigations in major depression? J Psychiatr Res. (2022) 156:69–77. doi: 10.1016/j.jpsychires.2022.09.008
43. Chang, CC, Jou, SH, Lin, TT, Lai, TJ, and Liu, CS. Mitochondria DNA change and oxidative damage in clinically stable patients with major depressive disorder. PLoS One. (2015) 10:e0125855. doi: 10.1371/journal.pone.0125855
44. Lu, J, Xu, X, Huang, Y, Li, T, Ma, C, Xu, G, et al. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2021) 8:981–90. doi: 10.1016/S2215-0366(21)00251-0
45. Song, Y, Wang, X, Ma, W, Yang, Y, Yan, S, Sun, J, et al. Graves' disease as a driver of depression: a mechanistic insight. Front Endocrinol. (2023) 14:1162445. doi: 10.3389/fendo.2023.1162445
46. Zhou, C, Wang, S, Ju, L, Zhang, R, Yang, Y, and Liu, Y. Positive association between blood ethylene oxide levels and metabolic syndrome: NHANES 2013-2020. Front Endocrinol. (2024) 15:1365658. doi: 10.3389/fendo.2024.1365658
Keywords: depressive symptoms, ethylene oxide, inflammatory marker, lymphocytes, neutrophils, white blood cells
Citation: Du D, Yuan Y, Guan X, Xie Q and Dong Z (2024) Ethylene oxide exposure, inflammatory indicators, and depressive symptoms: a cross-sectional study and mediation analysis based on a non-institutionalized American population. Front. Public Health. 12:1445257. doi: 10.3389/fpubh.2024.1445257
Edited by:
Liana Fattore, CNR Neuroscience Institute (IN), ItalyReviewed by:
Louise Bennett, Monash University, AustraliaYuquan Chen, Monash University, Australia
Zhongxia Shen, Huzhou Third Municipal Hospital, The Affiliated Hospital of Huzhou University, China
Copyright © 2024 Du, Yuan, Guan, Xie and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zaiquan Dong, emFpcXVhbmRvbmdAd2Noc2N1LmNu
 Dongru Du1
Dongru Du1 Zaiquan Dong
Zaiquan Dong