- 1College of Physical Education, Minzu University of China, Beijing, China
- 2Sports Nutrition Center, National Institute of Sports Medicine, Beijing, China
- 3Rehabilitation Medicine Center, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 4Department of Rehabilitation, Nanjing Qixia District Hospital, Nanjing, China
Background: Knee osteoarthritis (KOA) presents a significant public health challenge due to its hazards and increasingly severe trends. Addressing this challenge requires targeted investigation into the prevalence and identification of risk factors for KOA across different regions, especially in populous and vast China. Therefore, a cross-sectional survey was conducted in Nanjing, China, with the aim of investigating the prevalence and risk factors of KOA among individuals aged 50 and above.
Method: A total of 1,045 subjects were selected using the stratified random sampling method and diagnosed with KOA based on the diagnostic criteria established by the Chinese Medical Association. Data on 14 potential risk factors were collected through a self-designed questionnaire and standardized on-site tests. The association between KOA and these risk factors was explored using t-tests, Chi-square tests, and logistic regression analysis.
Results: The prevalence of KOA among the subjects was 23.64%. Multiple logistic regression models indicated that the risk of KOA was significantly higher among women (OR: 5.34, 95% CI: 3.13–9.11), subjects aged 60–69 (OR: 1.83, 95% CI: 1.25–2.69) and over 70 (OR: 2.87, 95% CI: 1.80–4.59), individuals with high school education and above (OR:2.22, 95% CI: 1.37–3.60), those with flatfoot (OR: 1.74, 95% CI: 1.10–2.74), and subjects classified as overweight (OR: 1.91, 95% CI: 1.21–3.04) and obese (OR: 4.63, 95% CI: 2.18–9.85) based on their BMI status. Additionally, the models identified weight (OR: 1.04, 95% CI: 1.01–1.08), 30-s chair stand performance (OR: 0.94, 95% CI: 0.91–0.97), and single-leg stand performance (OR: 0.96, 95% CI: 0.93–0.99) as independent risk factors for KOA.
Conclusion: The prevalence of KOA is remarkable in Nanjing city. The risk factors for KOA include women, older age, higher education, flatfoot, increased weight and BMI, as well as poor performance in 30-s chair stand and single-leg stand tests.
1 Introduction
Knee osteoarthritis (KOA) is a chronic and irreversible joint disease accompanied by pain and limited joint activity (1, 2). It may pose difficulties for patients in their daily activities such as bathing, toileting, dressing, walking, and household chores (3). It may also lead to psychological problems such as insomnia, anxiety, and depression due to long-term pain and inconvenience (1). In severe cases, KOA may even cause patients to lose their ability of live independently and experience disabilities (4, 5). Evidence suggests that KOA is the fourth leading cause of disability for women and the eighth leading cause for men (6). Its disability rate ranks high among all disabling diseases worldwide (5, 7). Meanwhile, almost all epidemiological surveys indicate a continued increase in the prevalence of KOA in recent decades (7–9). According to the 2019 Global Burden of Disease Study, approximately 364.58 million people were suffering from KOA, with an age-standardized prevalence of 4.38%, and the estimated annual percentage growth was 0.32% from 1990 to 2019 (7). Given these hazards and prevalence trends, KOA has become a major public health challenge.
Investigating the prevalence and risk factors of KOA is a prerequisite for addressing this challenge, but its findings cannot be easily generalized. Studies have indicated that the prevalence of KOA among individuals aged 60 and above in the United States was 37.4%, whereas in Japan and Germany, it stood at 26.1 and 12.3%, respectively (10–12). A systematic review indicated that the prevalence of KOA in China was 21.5% (13), while the China Health and Retirement Longitudinal Study suggested a lower rate of 8.1% (14). These discrepancies in KOA prevalence may be attributed to various social risk factors, including population dynamics, economic development, and geographical location (9, 11). For example, the prevalence in rural areas of the United States was significantly higher than that in urban areas due to the impact of economic development (10), and the prevalence in France decreased gradually from northeast mountainous areas to southwest coastal plain due to the impact of geographical location (15). Moreover, evidences also suggest that personal characteristics and lifestyle factors such as gender, education, obesity, aging, smoking, drinking, and prolonged sitting may all be associated with KOA prevalence, but the effects of these factors vary accross different studies (1, 16–19). For instance, Ji Shuqing et al. reported that the risk of KOA was 1.51 and 2.24 times higher in overweight and obese individuals, respectively, compared to normal-weight individuals (20), while Ren Yan et al. found no significant differences of KOA risk among overweight, obese and normal-weight individuals (16). These discrepancies highlight the diversity and uncertainty of KOA risk factors, emphasizing the necessity of targeted identification, especially in a vast and populous country like China.
This study was carried out in Nanjing, a city in southern China. China has conducted fewer cross-sectional surveys on KOA compared to developed countries (14). Simultaneously, Nanjing has barely reported a KOA survey in the past 10 years, according to our systematic search on Web of Science and PubMed databases. In order to enrich the epidemiological data on KOA, investigate the current prevalence and risk factors of KOA, and develop targeted strategies for KOA prevention and control in Nanjing, we designed and conducted this study.
2 Subjects and methods
This study obtained ethical approval from Nanjing Qixia District Hospital (No. 2022QX0901). It was conducted by standardized trained general practitioners and nursing staff at two community hospitals in Qixia District from September to October 2022.
2.1 Subjects
The random stratified sampling method was used to select individuals aged 50 and above as study subjects in an urban district of Nanjing, Jiangsu Province, East China. Firstly, 10 communities from two subdistricts (township-level regions) were randomly selected in Qixia District, Nanjing. Subsequently, residents were selected based on gender and age stratification, with an equal proportion of men and women, and a ratio of 2:2:1 for age groups 50–59, 60–69 and ≥ 70 years old. Eligible subjects were required to have resided in the local community for 5 years or more, voluntarily participate in this study, and be able to complete tests and answer questions independently. Individuals with stroke, dementia, and severe mental illness were excluded. A total of 1,114 subjects were selected for this study, and 1,045 subjects were finally included after processing outliers and missing data. All subjects provided informed consent.
2.2 KOA diagnosis
The diagnostic criteria for KOA released by the Chinese Medical Association in 2018 were used (21), including the following parameters: (1) Recurrent knee pain experienced within the past month; (2) X-ray examination (conducted in a standing or weight-bearing position) revealing joint space narrowing, subchondral bone sclerosis and/or cystic degeneration, as well as the presence of osteophytes at the joint edge; (3) Age ≥ 50 years old; (4) Morning stiffness lasting ≤30 min; (5) The presence of bone friction sound or sensation during activities. KOA diagnosis is established when both the first condition and any other two conditions are simultaneously met. Throughout this investigation, general practitioners first diagnosed KOA based on symptomatic manifestations such as pain, morning stiffness, and bone friction sound or sensation. In cases where a diagnosis could not be solely confirmed by symptoms, bilateral or unilateral knee X-ray examinations were assist in the diagnosis.
2.3 Risk factors
In this study, we screened 14 potential risk factors associated with KOA based on literature reports and clinician recommendations. Specifically, gender, age, weight, and body mass index (BMI) have been extensively validated as associated with KOA (22, 23) and were included to provide new data. Thigh circumference (TC) and calf circumference (CC) were selected based on clinical observations by local doctors, despite limited evidence linking them to KOA. The remaining eight factors are commonly reported in KOA epidemiological surveys, but no consensus has been reached regarding their associations with KOA (22–25); these were chosen to supplement the evidence in Nanjing. The 14 factors were categorized into three types. The first type refers to personal characteristics and lifestyle factors, including gender, age, marriage, education, smoking, and drinking. The second type comprises factors related to obesity and lower limb morphology, such as weight, BMI, TC, CC and flatfoot. The third type involves factors associated with lower limb strength and balance, including 30-s chair stand (30-s CS), single-leg stand (SLS), and timed up-and-go (TUG) tests.
2.4 Data collection
Data on these factors were collected through a self-designed questionnaire and standardized on-site tests. Some of the testing details were as follows. BMI data were calculated using the formula weight (kg) / height (m)2. According to the Chinese BMI evaluation criteria, BMI < 24 indicated that the subject was not overweight or obese, 24 ≤ BMI < 28 indicated overweight, and BMI ≥ 28 indicated obesity (26). For the TC and CC tests, we measured the thickest part of the thighs and calves separately, and calculated the averages of both legs. For the 30-s CS test, we recorded the number of repetitions of the subjects standing up and sitting down within 30 s. For the SLS test, subjects were asked to stand on one leg with their eyes closed, and we recorded the time until subjects open their eyes or move their supporting feet. For the TUG test, subjects were asked to stand up from an armchair, walk 3 meters forward, turn around at a marked line, walk back to the chair, turn around again, and sit down. Then we recorded the time taken to complete this sequence. Both the SLS and TUG tests were conducted twice, and the optimal values were used as the final data.
2.5 Statistical analysis
Epidata 3.1 software was used for data entry, while SPSS 21.0 software was utilized for single-factor or multiple-factor analysis. The dependent variable was the presence or absence of KOA, denoted by “yes” or “no.” The independent variables consisted of 14 potential KOA risk factors divided into 3 types. The normality of continuous variables was confirmed using the Kolmogorov-Smirnova test. Continuous data were presented as means ± standard deviations, and intergroup differences were assessed through independent-samples t-tests. Discrete data were presented as frequencies and composition ratios, with intergroup differences analyzed using Chi-square (χ2) tests. The significance level was set at 0.05. Subsequently, multiple logistic regression was used to establish risk factor models for KOA. Three models were developed by sequentially incorporating three types of risk factors using the stepwise entry method. Mode 1 was established based on personal characteristics and lifestyle factors. Model 2 expanded on Mode 1 by including obesity and lower limb morphology factors. Model 3 further incorporated factors related to lower limb strength and balance on the basis of Model 2. The fitting degree of these models was evaluated using the Hosmer-Lemeshow tests. Odds Ratios (ORs) and 95% Confidence Intervals (CIs) were used to quantitatively describe the association between risk factors and KOA.
3 Results
3.1 Descriptive characteristics and single-factor analysis results
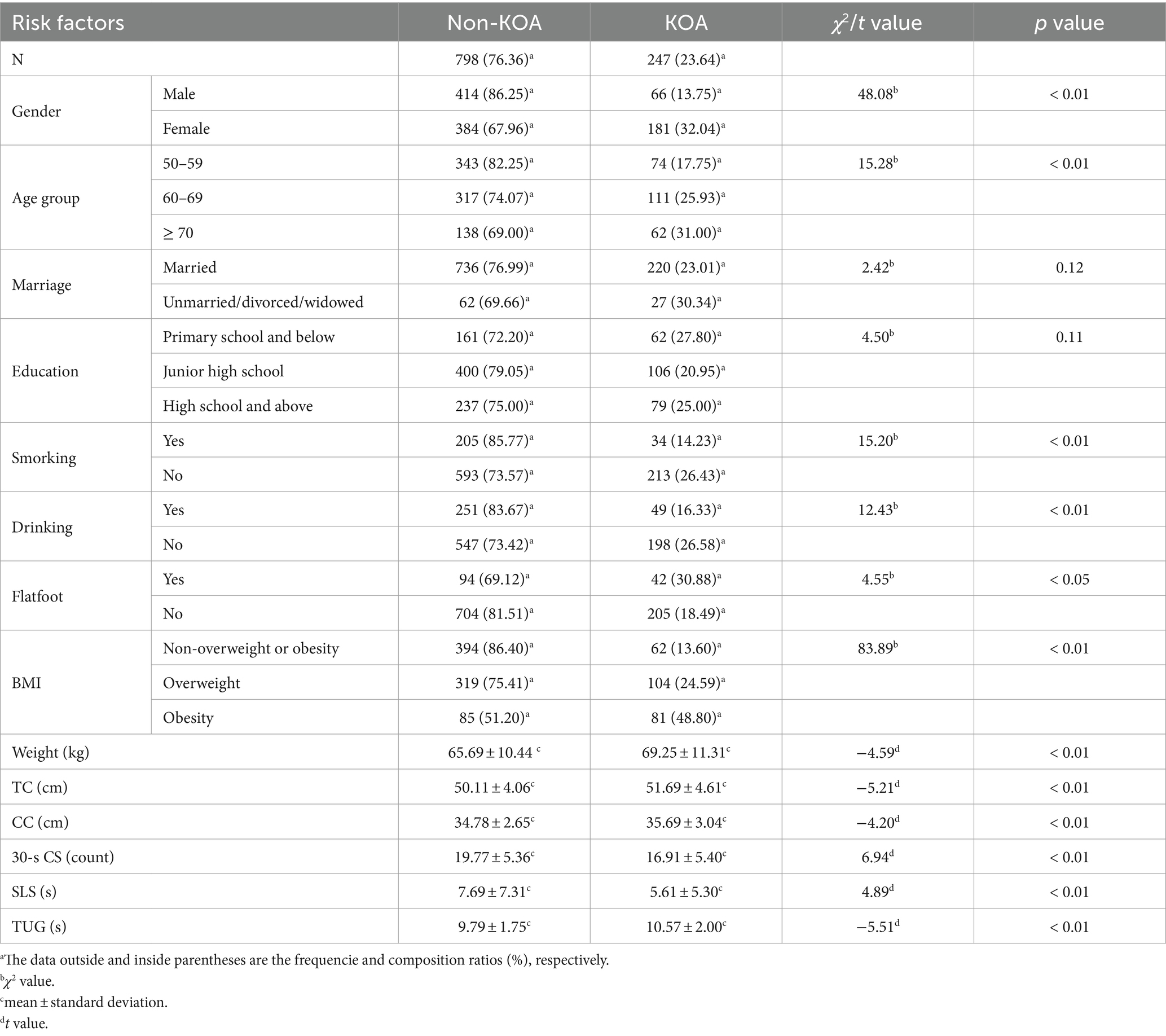
This study included 1,045 subjects, among whom 247 suffered from KOA, resulting in a prevalence of 23.64%. The Kolmogorov-Smirnova test indicated that all continuous data followed a normal distribution. Single-factor analysis revealed that gender, age, smoking, drinking, weight, BMI, TC, CC, flatfoot, 30-s CS, SLS, and TUG were all associated with KOA. Specifically, the prevalence of KOA was 32.04% in women and 13.75% in men, with women exhibiting a 2.33 times higher prevalence than men. Among subjects aged 50–59, 60–60 and ≥ 70, the prevalence rates were 17.75, 25.93 and 31.00%, respectively, indicating an increasing trend with age groups. Smokers had a lower prevalence rate (14.23%) compared to non-smokers (26.43%), as did drinkers (16.33%) compared to non-drinkers (26.58%). Subjects with flatfoot exhibited a higher prevalence rate (30.88%) than those without flatfoot (18.49%). Notably, the prevalence of KOA among obese subjects was as high as 48.80%, which was 1.98 times higher than that among overweight subjects (24.59%) and 3.59 times higher than that among non-overweight or obese subjects (13.60%). Meanwhile, the mean weight, TC, CC, and TUG performance were higher among KOA subjects compared to non-KOA subjects, while the mean 30-s CS and SLS performance were lower among KOA subjects compared to non-KOA subjects. All of these differences were statistically significant (p < 0.05, Table 1). Furthermore, there were no significant differences in marriage and education between KOA subjects and non-KOA subjects (p > 0.05, Table 1).
3.2 Multiple-factor analysis results
We established three logistic regression models. The p-values of the Hosmer-Lemeshow tests for Model 1, Model 2, and Model 3 were 0.87, 0.57, and 0.10, respectively, indicating good calibration of the models. In this study, both Model 1 and Model 2 identified gender and age group as significant independent risk factors for KOA (p < 0.05, Tables 2, 3), with Model 2 showing higher effect sizes. The results of Model 2 indicated that women (OR: 5.34, 95% CI: 3.13 to 9.11) were more likely to suffer from KOA compared to men, and subjects aged 60–69 (OR: 1.83, 95% CI: 1.25 to 2.69) and those over 70 (OR: 2.87, 95% CI: 1.80 to 4.59) had a higher likelihood of experiencing KOA compared to those aged 50–59. Moreover, both Model 2 and Model 3 identified education, flatfoot, and BMI as independent risk factors for KOA (p < 0.05, Tables 3, 4), with Model 3 demonstrating higher effect sizes. The results of Model 3 suggested that subjects with high school education and above (OR: 2.22, 95% CI: 1.37 to 3.60) were more likely to suffer from KOA compared to those with primary school education and below, and subjects with flatfoot (OR: 1.74, 95% CI: 1.10 to 2.74) were more predisposed to KOA compared to those without flatfoot. Similarly, overweight subjects (OR: 1.91, 95% CI: 1.21 to 3.04) and obese subjects (OR: 4.63, 95% CI: 2.18 to 9.85) were at an increased risk of KOA compared to those who were not overweight or obese based on their BMI status. Additionally, Model 2 also considered weight as an independent risk factor for KOA (p < 0.05, Table 3), indicating that the risk of KOA increased by 4% for every 1 kg increase in weight (OR: 1.04, 95% CI: 1.01 to 1.08). Model 3 revealed that 30-s CS and SLS were independent risk factors for KOA (p < 0.05, Table 4). The risk of KOA decreased by 6% for every 1 repetition increase in 30-s CS (OR: 0.94, 95% CI: 0.91 to 0.97) and by 4% for every 1 s increase in SLS (OR: 0.96, 95% CI: 0.93 to 0.99).
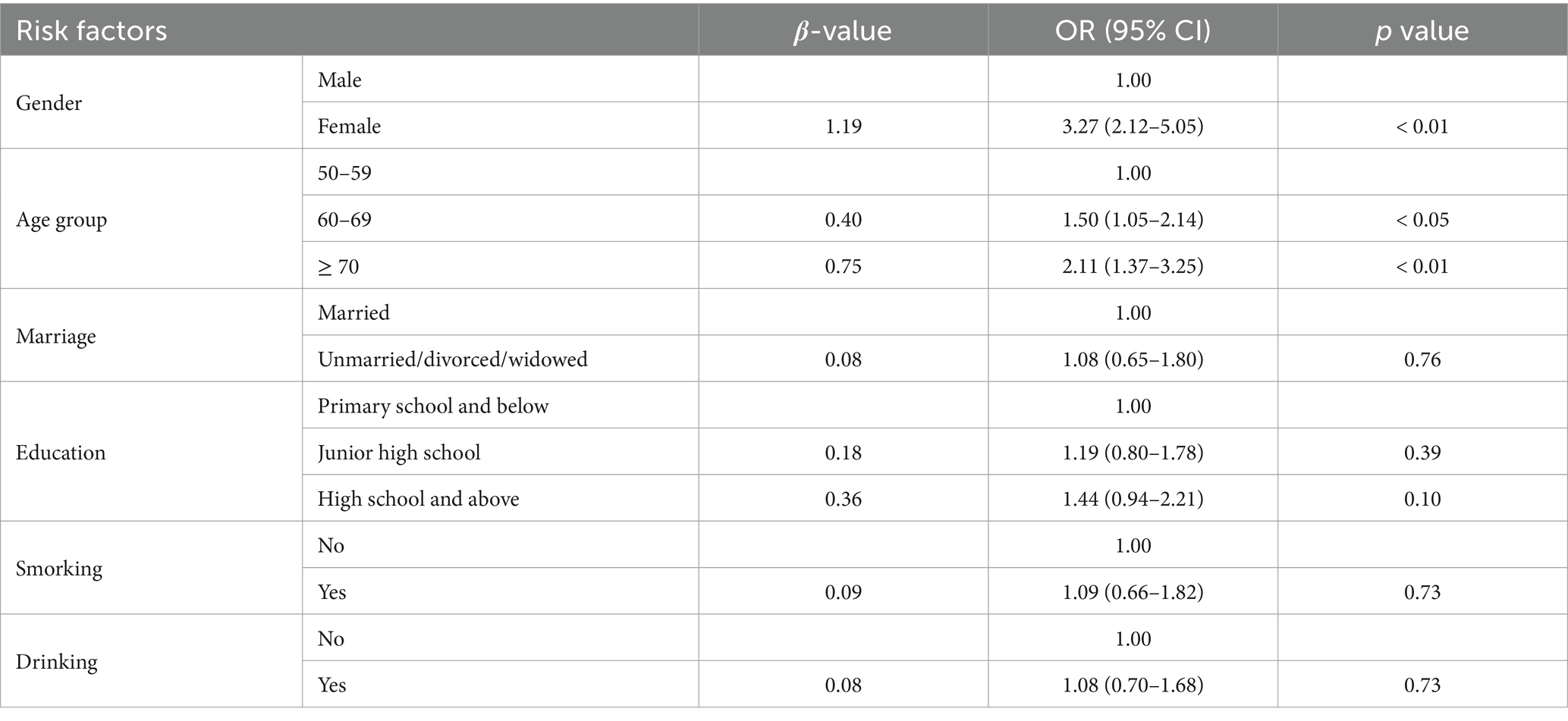
Table 2. Binary logistic regression model of personal characteristics and lifestyle factors related to KOA (Model 1).
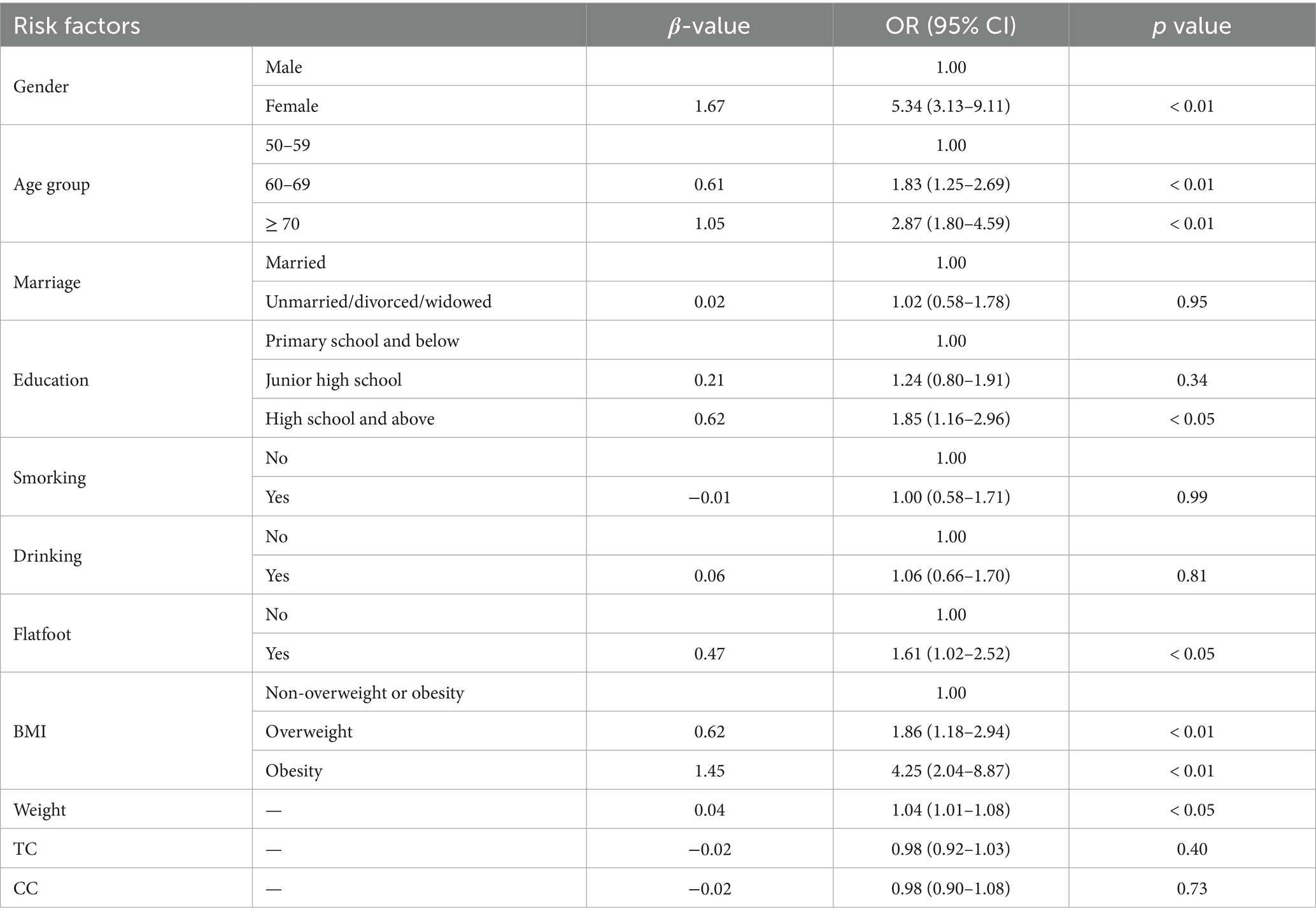
Table 3. Binary logistic regression model of personal characteristics, lifestyle, obesity, and lower limb morphology factors related to KOA (Model 2).
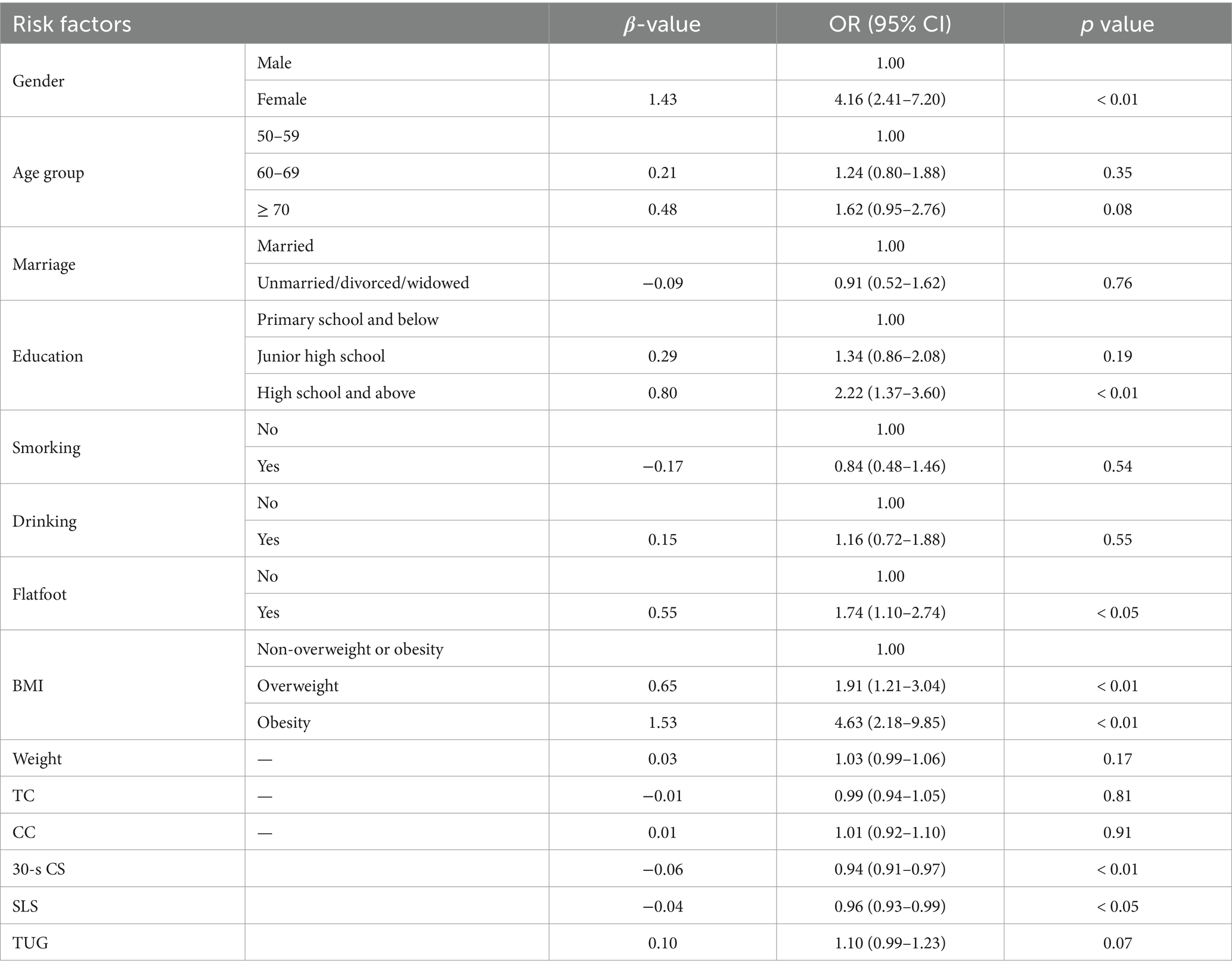
Table 4. Binary logistic regression model of the 14 potential risk factors related to KOA (Model 3).
4 Discussion
In this cross-sectional survey targeting middle-aged and older adults in Nanjing, China, we observed a KOA prevalence rate of 23.64%. This figure is lower than the prevalence rates in the United States (37.4%) and Japan (26.1%) among those aged 60 and older (10, 11), but higher than the rates found in England (17.4% for those over 50), Bangladesh (14.8% for those over 58), and Germany (12.3% for those over 60) (12, 27, 28). However, considering the influence of factors such as diagnostic methods for KOA, geographical location, ethnicity, and age range, the comparability of these figures requires further validation. Nevertheless, the high prevalence of KOA globally is undisputed. Furthermore, in China, the 23.64% prevalence is slightly higher than the estimate of 21.51% reported by Sun et al. after summarizing findings from 21 studies and significantly surpasses the estimate of 8.1% reported by Tang et al. (13, 14). Upon further analysis, we noted that both our study and the aforementioned 21 studies collected data through direct measurements, while the study of Tang et al. collected data through face-to-face household interviews and identified KOA patients by inquiring whether subjects had received a doctor’s diagnosis of KOA. Consequently, the estimate of 8.1% appears relatively low, and the prevalence rates of 23.64 and 21.51% may better reflect the actual prevalence, suggesting a serious challenge posed by KOA among the middle-aged and older adult population in Nanjing.
Subsequently, we investigated the risk factors for KOA and identified gender, age, education, flatfoot, weight, BMI, 30-s CS, and SLS as independent contributors. Gender and age emerged as the primary risk factors. Numerous studies, including ours, have consistently reported a higher prevalence of KOA among women compared to men (8, 10–12, 15, 29). This gender difference may be attributed to declining estrogen levels in perimenopausal women, which can diminish the metabolic capacity of joint cartilage and contribute to the onset of KOA. Additionally, women’s daily activities, such as squatting for defecation and participating in household chores, often entail repetitive stress on their knees, potentially accelerating knee joint wear and the onset of KOA. Furthermore, studies have consistently indicated an increasing prevalence of KOA with advancing age (13, 14, 29). This age-related trend was also observed in the univariate analysis, Model 1, and Model 2 of our study but not in Model 3. In contrast to Model 2, Model 3 incorporated three factors reflecting lower limb strength and balance function: 30-s CS, SLS, and TUG. We speculate that these three factors serve as intermediate variables linking age and KOA. With increasing age, the prevalence of KOA may rise due to declining muscle strength and deteriorating balance associated with aging, which may lead to knee joint wear and the development of KOA (30, 31).
After adjusting for other factors, education emerged as an independent risk factor for KOA. In both Model 2 and Model 3 of our study, we observed that the prevalence of KOA was 1.85 times and 2.22 times higher, respectively, in individuals with a high school education and above, compared to those with primary school education and below. However, some studies have reported contrasting results, suggesting a higher prevalence of KOA in individuals with lower education (20, 32). This discrepancy might be attributed to the association between KOA and occupation. Studies by Hulshof et al. and Zhou et al. suggested that individuals with lower education were more likely to engage in repetitive tasks such as kneeling, squatting, carrying heavy objects, and climbing stairs, which increased the risk of KOA (33, 34). Unfortunately, our study did not collect data related to occupational factors or other confounding factors, making it difficult to explain why opposite results were observed.
Flatfoot is recognized as an independent risk factor for KOA. Studies by Gross et al. and Lijima et al. have indicated that flatfoot is correlated with knee pain, knee cartilage damage, and can exacerbate disability in KOA patients (35, 36). Additionally, both Model 2 and Model 3 of our study reported that individuls with flatfoot had a 1.61 and 1.74 times greater risk of developing KOA, respectively, compared to those without flatfoot. The association between flatfoot and KOA can be explained through mechanical stress. During weight-bearing activities, the posture and movement of the feet and knees form a closed kinematic chain, working together to support weight and absorb impact. Flatfoot is characterized by weak arch support and limited ability to absorb impacts, inevitably leading to increased mechanical stress on the knee (37, 38). This stress may cause damage to the cartilage and soft tissue of the knee joint, thereby increasing the risk of KOA.
As is well-known, weight and BMI serve as significant risk factors for KOA (39, 40). This study revealed a notable association that with each additional 1 kg of weight, there was a 4% increase in the prevalence of KOA. Moreover, the study indicated that the prevalence of KOA among overweight individuals was 1.91 times higher compared to non-overweight and obese individuals, and the prevalence of KOA among obese individuals was 4.63 times higher in comparison. The mechanism by which weight and BMI impact KOA can be explained from two perspectives. Firstly, from a mechanical load perspective, the knee joint bears the greatest weight in the human body. As weight and BMI increase, the load on the knee joint escalates, heightening the risk of cartilage degradation. Secondly, from a fat metabolism perspective, higher weight and BMI are associated with increased body fat content. Fat-related factors can trigger inflammatory reactions in the joints, activate proteinases, and accelerate the degeneration of joint cartilage. Simultaneously, fat metabolism may interfere with cholesterol reverse transcription in joint cartilage, leading to cholesterol accumulation, hypertrophy of cartilage cells, cartilage ossification, and other factors that can trigger or exacerbate KOA (41–43).
The 30s-CS and SLS tests are not only widely used to objectively evaluate physical function in KOA patients, but are also considered as risk factors for KOA. This study reported that for every additional repetition in 30s-CS test and one-second increase in SLS test, the risk of KOA decreased by 6 and 4%, respectively. The 30s-CS reflects lower limb muscle strength, while SLS indicates lower limb static balance ability, and their association with KOA can be well explained. Specifically, insufficient muscle strength may lead to knee joint instability, causing it to swing during activities, thereby accelerating joint degeneration and contributing to KOA. Moreover, the correlation between muscle strength and KOA is influenced by gender, with women having less strength being more likely to suffer from KOA (44). The possible reason is that women have lower strength capacity and are closer to the risk threshold of KOA (45).
In this study, marriage, smoking, drinking, TC, CC, and TUG were not considered as independent risk factors for KOA. Regarding marriage, evidence suggests that it may be a risk factor for KOA, as the prevalence of KOA in married, divorced and widowed individuals was significantly higher than that in unmarried individuals (32, 46). However, our study did not find a significant correlation between marriage and KOA, which may be attributed to the relatively small sample size of unmarried, divorced, and widowed individuals, accounting for only 8.52% of the total. This sample size might not be sufficient to yield statistically significant results. Regarding smoking and drinking, the univariate analysis of our study suggested a notably lower prevalence of KOA among smokers or drinkers compared to non-smokers or non-drinkers, respectively, implying that smoking and drinking might act as protective factors for KOA. However, after adjusting for gender, age group, marriage, and education, no significant association was found between smoking or drinking and KOA, indicating the initial conclusion drawn from the univariate analysis was inaccurate. To investigate the reasons for this discrepancy, we further compared the differences in smoking and drinking among subjects of different genders, age groups, marriages, and educational levels using Chi-square tests. The results revealed substantial gender disparities in smoking (χ2 = 315.70, p < 0.01) and drinking (χ2 = 304.62, p < 0.01), with males exhibiting significantly higher rates than females. The erroneous conclusion likely stemmed from the interference of the gender factor. Regarding TC and CC, they to some extent reflect lower limb muscle mass and cross-sectional area, both of which have been demonstrated to be related to KOA (47, 48). Consequently, we attempted to explore the correlation between TC or CC and KOA, but unfortunately, we did not obtain statistically significant results. As for the TUG test, it has been widely utilized to evaluate the dynamic balance ability of KOA patients (49). The univariate analysis of our study also reported a significant association between TUG performance and KOA. However, the multivariate analysis did not confirm this association, as evidenced by a p-value of 0.07. We speculate that expanding the geographical and age distribution of the samples might influence this outcome, necessitating further validation.
5 Limitations
This study has several limitations. Firstly, all subjects were sourced from urban communities rather than rural areas, selected for the convenience of receiving KOA diagnosis in community hospitals, potentially introducing selection bias. It is recommended to broaden the sample coverage in future studies to ensure greater representativeness. Secondly, our study was a cross-sectional survey, which may reveal correlations between indicators and KOA, but cannot assess direct causality. Further cohort studies in Nanjing are necessary to address this limitation and provide more robust evidence.
6 Conclusion
The prevalence of KOA is remarkable in Nanjing city, indicating the urgency of developing and implementing targeted measures. This study demonstrates that factors such as women, older age, higher education, flatfoot, increased weight, higher BMI, as well as poor performance in 30s-CS and SLS tests, all contribute to the risk of KOA. These findings help identify vulnerable groups for KOA and are instrumental in the development of prevention and control measures for the condition.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: Figshare Dryad Digital Repository (https://doi.org/10.6084/m9.figshare.25679568).
Ethics statement
The studies involving humans were approved by the Ethics Committee of Qixia District Hospital in Nanjing (No. 2022QX0901). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. HH: Conceptualization, Formal analysis, Methodology, Writing – review & editing. QH: Formal analysis, Writing – review & editing. KC: Funding acquisition, Investigation, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the Major Sports Research Project of the Jiangsu Sports Bureau in China (grant number ST222102).
Acknowledgments
We would like to express our appreciation to Maigaoqiao, Yanziji, Baguazhou, Longtan, Xiaohang,Daishan, Qixia, and Xigang community hospitals for providing necessary facilities and resources to conduct our experiments. We are grateful for the collaboration and assistance provided by Baoyi Chen, Lan Wang, Qihua Zheng, Yasheng Li, Song Qin, Zhengguo Yin, and Junpeng Wu in disease diagnosis and data collection. Lastly, we extend our gratitude to all the participants who devoted their time and efforts to contribute to this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
KOA, knee osteoarthritis; BMI, body mass index; TC, thigh circumference; CC, calf circumference; 30-s CS, 30-s chair stand; SLS, single-leg stand; TUG, timed up-and-go.
References
1. Sharma, L . Osteoarthritis of the knee. N Engl J Med. (2021) 384:51–9. doi: 10.1056/NEJMcp1903768
2. Bijlsma, JW, Berenbaum, F, and Lafeber, FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. (2011) 377:2115–26. doi: 10.1016/S0140-6736(11)60243-2
3. Baird, CL . Living with hurting and difficulty doing: older women with osteoarthritis. Clin Excell Nurse Pract. (2000) 4:231–7.
4. Brooks, PM . Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. Curr Opin Rheumatol. (2002) 14:573–7. doi: 10.1097/00002281-200209000-00017
5. Cross, M, Smith, E, Hoy, D, Nolte, S, Ackerman, I, Fransen, M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. (2014) 73:1323–30. doi: 10.1136/annrheumdis-2013-204763
6. Jordan, KM, Arden, NK, Doherty, M, Bannwarth, B, Bijlsma, JW, Dieppe, P, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. (2003) 62:1145–55. doi: 10.1136/ard.2003.011742
7. Long, H, Liu, Q, Yin, H, Wang, K, Diao, N, Zhang, Y, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. Arthritis Rheumatol. (2022) 74:1172–83. doi: 10.1002/art.42089
8. Nguyen, U-SDT, Zhang, Y, Zhu, Y, Niu, J, Zhang, B, Felson, DT, et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. (2011) 155:725–32. doi: 10.7326/0003-4819-155-11-201112060-00004
9. Safiri, S, Kolahi, A-A, Smith, E, Hill, C, Bettampadi, D, Mansournia, MA, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis. (2020) 79:819–28. doi: 10.1136/annrheumdis-2019-216515
10. Lawrence, RC, Felson, DT, Helmick, CG, Arnold, LM, Choi, H, Deyo, RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheumatol. (2008) 58:26–35. doi: 10.1002/art.23176
11. Muraki, S, Oka, H, Akune, T, Mabuchi, A, En-yo, Y, Yoshida, M, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthr Cartil. (2009) 17:1137–43. doi: 10.1016/j.joca.2009.04.005
12. Postler, A, Ramos, AL, Goronzy, J, Günthe, KP, Lange, T, Schmitt, J, et al. Prevalence and treatment of hip and knee osteoarthritis in people aged 60 years or older in Germany: an analysis based on health insurance claims data. Clin Interv Aging. (2018) 13:2339–49. doi: 10.2147/CIA.S174741
13. Sun, X, Zhen, X, Hu, X, Li, Y, Gu, S, Gu, Y, et al. Osteoarthritis in the middle-aged and elderly in China: prevalence and influencing factors. Int J Environ Res Public Health. (2019) 16:4701. doi: 10.3390/ijerph16234701
14. Tang, X, Wang, S, Zhan, S, Niu, J, Tao, K, Zhang, Y, et al. The prevalence of symptomatic knee osteoarthritis in China: results from the China health and retirement longitudinal study. Arthritis Rheumatol. (2016) 68:648–53. doi: 10.1002/art.39465
15. Guillemin, F, Rat, AC, Mazieres, B, Pouchot, J, Fautrel, B, Euller-Ziegler, L, et al. Prevalence of symptomatic hip and knee osteoarthritis: a two-phase population-based survey. Osteoarthr Cartil. (2011) 19:1314–22. doi: 10.1016/j.joca.2011.08.004
16. Ren, Y, Hu, J, Tan, J, Tang, X, Li, Q, Yang, H, et al. Incidence and risk factors of symptomatic knee osteoarthritis among the Chinese population: analysis from a nationwide longitudinal study. BMC Public Health. (2020) 20:1491. doi: 10.1186/s12889-020-09611-7
17. Michael, JWP, Schlüter-Brust, KU, and Eysel, P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. (2010) 107:152–62. doi: 10.3238/arztebl.2010.0152
18. Chang, AH, Lee, JJ, Chimiel, JS, Almagor, O, Song, J, and Sharma, L. Association of Long-term Strenuous Physical Activity and Extensive Sitting with Incident Radiographic Knee Osteoarthritis. JAMA Netw Open. (2020) 3:e204049. doi: 10.1001/jamanetworkopen.2020.4049
19. Rethorn, ZD, Rethorn, TJ, Cook, CE, Sharpe, JA, Hastings, SN, and Allen, KD. Association of burden and prevalence of arthritis with disparities in social risk factors, findings from 17 US states. Prev Chronic Dis. (2022) 19:E08. doi: 10.5888/pcd19.210277
20. Ji, S, Liu, L, Li, J, Zhao, G, Cai, Y, Dong, Y, et al. Prevalence and factors associated with knee osteoarthritis among middle-aged and elderly individuals in rural Tianjin: a population-based cross-sectional study. J Orthop Surg Res. (2023) 18:266. doi: 10.1186/s13018-023-03742-4
21. Zhang, Z, Huang, C, Jiang, Q, Zheng, Y, Liu, Y, Liu, S, et al. Guidelines for the diagnosis and treatment of osteoarthritis in China (2019 edition). Ann Transl Med. (2020) 8:1213. doi: 10.21037/atm-20-4665
22. Blagojevic, M, Jinks, C, Jeffery, A, and Jordan, KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthr Cartil. (2010) 18:24–33. doi: 10.1016/j.joca.2009.08.010
23. Silverwood, V, Blagojevic-Bucknall, M, Jinks, C, Jordan, JL, Protheroe, J, and Jordan, KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr Cartil. (2015) 23:507–15. doi: 10.1016/j.joca.2014.11.019
24. Lee, JY, Han, K, Park, YG, and Park, SH. Effects of education, income, and occupation on prevalence and symptoms of knee osteoarthritis. Sci Rep. (2021) 11:13983. doi: 10.1038/s41598-021-93394-3
25. Dekker, J, van Dijk, GM, and Veenhof, C. Risk factors for functional decline in osteoarthritis of the hip or knee. Curr Opin Rheumatol. (2009) 21:520–4. doi: 10.1097/BOR.0b013e32832e6eaa
26. Luo, H, Li, J, Zhang, Q, Cao, P, Ren, X, Fang, A, et al. Obesity and the onset of depressive symptoms among middle-aged and older adults in China: evidence from the CHARLS. BMC Public Health. (2018) 18:909. doi: 10.1186/s12889-018-5834-6
27. Peat, G, Rathod-Mistry, T, Paskins, Z, Marshall, M, Thomas, MJ, Menz, HB, et al. Relative prevalence and distribution of knee, hand and foot symptomatic osteoarthritis subtypes in an English population. Musculoskeletal Care. (2020) 18:219–24. doi: 10.1002/msc.1457
28. Haider, MZ, Bhuiyan, R, Ahmed, S, Zahid-Al-Quadir, A, Choudhury, MR, Haq, SA, et al. Risk factors of knee osteoarthritis in Bangladeshi adults: a national survey. BMC Musculoskelet Disord. (2022) 23:333. doi: 10.1186/s12891-022-05253-5
29. Yoshimura, N, Muraki, S, Oka, H, Mabuchi, A, En-Yo, Y, Yoshida, M, et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab. (2009) 27:620–8. doi: 10.1007/s00774-009-0080-8
30. Øiestad, BE, Juhl, CB, Culvenor, AG, Berg, B, and Thorlund, JB. Knee extensor muscle weakness is a risk factor for the development of knee osteoarthritis: an updated systematic review and meta-analysis including 46 819 men and women. Br J Sports Med. (2022) 56:349–55. doi: 10.1136/bjsports-2021-104861
31. Mat, S, Tan, MP, Kamaruzzaman, SB, and Ng, CT. Physical therapies for improving balance and reducing falls risk in osteoarthritis of the knee: a systematic review. Age Ageing. (2015) 44:16–24. doi: 10.1093/ageing/afu112
32. Jørgensen, KT, Pedersen, BV, Nielsen, NM, Hansen, AV, Jacobsen, S, and Frisch, M. Socio-demographic factors, reproductive history and risk of osteoarthritis in a cohort of 4.6 million Danish women and men. Osteoarthr Cartil. (2011) 19:1176–82. doi: 10.1016/j.joca.2011.07.009
33. Hulshof, CTJ, Pega, F, Neupane, S, van der Molen, HF, Colosio, C, Daams, JG, et al. The prevalence of occupational exposure to ergonomic risk factors: a systematic review and meta-analysis from the WHO/ILO joint estimates of the work-related burden of disease and injury. Environ Int. (2021) 146:106157. doi: 10.1016/j.envint.2020.106157
34. Zhou, G, Zhao, M, Wang, X, Geng, X, and Tian, H. Demographic and radiographic factors for knee symptoms and range of motion in patients with knee osteoarthritis: a cross-sectional study in Beijing, China. BMC Musculoskelet Disord. (2023) 24:24. doi: 10.1186/s12891-023-06432-8
35. Gross, KD, Felson, DT, Niu, J, Hunter, DJ, Guermazi, A, Roemer, FW, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res. (2011) 63:937–44. doi: 10.1002/acr.20431
36. Iijima, H, Ohi, H, Isho, T, Aoyama, T, Fukutani, N, Kaneda, E, et al. Association of bilateral flat feet with knee pain and disability in patients with knee osteoarthritis: a cross-sectional study. J Orthop Res. (2017) 35:2490–8. doi: 10.1002/jor.23565
37. Moudy, SC, Tillin, NA, Sibley, AR, and Strike, S. Foot strike alters ground reaction force and knee load when stepping down during ongoing walking. Gait Posture. (2020) 76:327–33. doi: 10.1016/j.gaitpost.2019.12.019
38. Nakazato, K, Taniguchi, M, Yagi, M, Motomura, Y, Fukumoto, Y, Saeki, J, et al. Assessment of fore-, mid-, and rear-foot alignment and their association with knee symptoms and function in patients with knee osteoarthritis. Clin Rheumatol. (2023) 42:511–7. doi: 10.1007/s10067-022-06421-7
39. Martel-Pelletier, J, Barr, AJ, Cicuttini, FM, Conaghan, PG, Cooper, C, Goldring, MB, et al. Osteoarthritis. Nat Rev Dis Primers. (2016) 2:16072. doi: 10.1038/nrdp.2016.72
40. Grotle, M, Hagen, KB, Natvig, B, Dahl, FA, and Kvien, TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. (2008) 9:132. doi: 10.1186/1471-2474-9-132
41. Ding, C, Stannus, O, Cicuttini, F, Antony, B, and Jones, G. Body fat is associated with increased and lean mass with decreased knee cartilage loss in older adults: a prospective cohort study. Int J Obes. (2013) 37:822–7. doi: 10.1038/ijo.2012.136
42. Zhou, M, Guo, Y, Wang, D, Shi, D, Li, W, Liu, Y, et al. The cross-sectional and longitudinal effect of hyperlipidemia on knee osteoarthritis: results from the Dongfeng-Tongji cohort in China. Sci Rep. (2017) 7:9739. doi: 10.1038/s41598-017-10158-8
43. Ertürk, C, Altay, MA, Bilge, A, and Çelik, H. Is there a relationship between serum ox-LDL, oxidative stress, and PON1 in knee osteoarthritis? Clin Rheumatol. (2017) 36:2775–80. doi: 10.1007/s10067-017-3732-4
44. Eckstein, F, Hitzl, W, Duryea, J, Kent Kwoh, C, and Wirth, W. Baseline and longitudinal change in isometric muscle strength prior to radiographic progression in osteoarthritic and pre-osteoarthritic knees – data from the osteoarthritis initiative. Osteoarthr Cartil. (2013) 21:682–90. doi: 10.1016/j.joca.2013.02.658
45. Culvenor, AG, Wirth, W, Ruhdorfer, A, and Eckstein, F. Thigh muscle strength predicts knee replacement risk independent of radiographic disease and pain in women: data from the osteoarthritis initiative. Arthritis Rheumatol. (2016) 68:1145–55. doi: 10.1002/art.39540
46. Vennu, V, Abdulrahman, TA, Alenazi, AM, and Bindawas, SM. Associations between social determinants and the presence of chronic diseases: data from the osteoarthritis initiative. BMC Public Health. (2020) 20:1323. doi: 10.1186/s12889-020-09451-5
47. Wada, O, Kurita, N, Kamitani, T, and Mizuno, K. Implications of evaluating leg muscle mass and fat mass separately for quadriceps strength in knee osteoarthritis: the SPSS-OK study. Clin Rheumatol. (2020) 39:1655–61. doi: 10.1007/s10067-019-04879-6
48. Yamauchi, K, Suzuki, S, Kato, C, and Kato, T. Atrophy of individual thigh muscles measured by MRI in older adults with knee osteoarthritis: a cross-sectional study. Ann Phys Rehabil Med. (2020) 63:38–45. doi: 10.1016/j.rehab.2019.06.018
Keywords: knee osteoarthritis, prevalence, risk factors, middle aged and older adult, China
Citation: Shao W, Hou H, Han Q and Cai K (2024) Prevalence and risk factors of knee osteoarthritis: a cross-sectional survey in Nanjing, China. Front. Public Health. 12:1441408. doi: 10.3389/fpubh.2024.1441408
Edited by:
Mario Salazar-Paramo, University of Guadalajara, MexicoReviewed by:
Fuzhou Hua, Second Affiliated Hospital of Nanchang University, ChinaJinyi Zhou, Jiangsu Provincial Center for Disease Control and Prevention, China
Copyright © 2024 Shao, Hou, Han and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keshu Cai, Y2Fpa2VzaHVAc2luYS5jb20=; Huisheng Hou, aGhzaGVuZzc2MDJAMTI2LmNvbQ==
 Wenjuan Shao
Wenjuan Shao Huisheng Hou
Huisheng Hou Qi Han
Qi Han Keshu Cai3,4*
Keshu Cai3,4*