- 1School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON, Canada
- 2Children’s Hospital of Eastern Ontario, Ottawa, ON, Canada
- 3Division of Clinical Epidemiology, McGill University, Montreal, QC, Canada
- 4Foundation for Innovative New Diagnostics (FIND), Geneva, Switzerland
Introduction: HIV self-testing (HIVST) is an innovative strategy that has been shown to increase uptake of HIV testing compared to conventional facility-based testing. HIVST implementation with digital-based supports may help facilitate testing accessibility and linkage to care after a reactive self-test. Economic evidence around community-based implementation of HIVST is growing; however, economic evidence around digital-based HIVST approaches remains limited.
Methods: We used previously published cost and efficacy data from HIVST interventions, with the specific intervention model differing between scenarios. Digital-based interventions included text messaging campaigns and online websites that promoted uptake and linkage to HIVST care. Community-based interventions included door-to-door distribution, peer-incentivized distribution, and mobile testing units. Using data obtained from the literature, we parameterized a combined Markov and decision analytic model to evaluate the cost-utility of digital-based HIVST implementation across Malawi, South Africa, and Brazil compared to both community-based HIVST and facility-based testing.
Results: We found that HIVST was cost-effective compared to facility-based testing in all settings investigated. Our scenarios predicted that digital-based HIVST was associated with an incremental cost in the range of $769–$17,839/DALY (disability-adjusted life year) averted compared to facility-based testing across Malawi, South Africa, and Brazil. Digital-based HIVST cost savings had an incremental cost of $7,300/DALY averted compared to community-based HIVST. The main drivers of cost-utility included HIV test and treatment costs, HIV test-positivity, rates of linkage to care, and antiretroviral therapy (ART) initiation rates. Digital-based supports were associated with an increased cost compared to facility-based testing, but they also had increased utility, which led to favorable cost-utility estimates.
Discussion: HIVST with digital supports has the potential to be a highly cost-effective approach, with the potential to make HIV testing more available and accessible, thereby increasing overall uptake and coverage of HIV testing. Digital supports can also support linkage to care, which we have identified as a major driver of cost-utility. Strategies to improve cost-utility include reducing testing costs, targeting key populations with increased rates of HIV test-positivity, and ensuring strong support for linkage to care.
Introduction
The United Nations General Assembly has established ambitious goals for HIV diagnosis and care to be met by the end of 2030 (1). Although global progress has been made, many countries have yet to attain critical targets (2–4). There have been significant advances in diagnostic technologies and in novel treatment and supportive care options for HIV (5).
An innovative approach to promote HIV screening and diagnosis is HIV self-testing (HIVST), where individuals can perform their own HIV screening. HIVST provides a private and anonymous method for testing that is perceived by users as more convenient than accessing testing through conventional health clinics (6, 7). Self-testing is a highly promising strategy with the potential to reduce the global HIV burden by bringing the currently undiagnosed to care (8). Previous literature strongly suggests that HIVST is preferred by clients with higher rates of uptake compared to conventional testing. However, there are many factors that affect testing outcomes (9–11). A recent systematic review found that HIVST had a variable uptake rate of 20–92% depending on the implementation strategy and specific subpopulation being tested (6).
There are many advantages to HIVST; however, concern has been concern raised regarding the linkage to care, with some studies suggesting that linkage to care may be lower with HIVST (particularly with home-based approaches) (12–14). Varoious strategies have been used to implement HIVST and support and improve uptake and linkage to care, including pairing HIVST with community-based and digital-based supports. HIVST with community-based support involves utilizing resources from within the community, such as existing health infrastructure, volunteers, or peer support groups, to support uptake and linkage to care (15). HIVST with digital-based support typically includes the use of digital interventions such as SMS messaging, websites, downloadable applications, or social media to improve uptake, help with user experience and ease of testing, provide counseling and help assist with clinical care and follow-up. As access to the internet improves globally (16), HIVST with digital support offers improved accessibility and confidentiality for HIVST, especially among hard-to-reach populations (17) or stigmatized groups (7).
Population-level screening programs can be resource-intense, and economic evidence is critical in providing evidence to guide implementation and scaling up of such programs. Previous studies have strongly supported that HIVST is cost-effective among a broad range of contexts, primarily using community-based distribution approaches. Matsimela et al. (18) conducted a micro-costing and economic evaluation of eleven community-based HIVST distribution strategies in South Africa. They observed that HIVST distribution strategies across South Africa varied significantly by volume distributed, cost per kit, underlying test positivity of HIV in the population tested, and rates of linkage to care. These factors contributed to the wide range of cost-effectiveness estimates between distribution strategies ($5–19 USD/person tested, $61–1,277 USD/person diagnosed).
HIVST has two published interventions using digital-based supports that include both costing and outcome measures. Both studies reported cost-effectiveness; however, cost-utility measures were not evaluated. Kelvin et al. used SMS-based messaging to target truck drivers and female sex workers for their HIVST campaign (19, 20). They found that HIVST was preferable compared to facility-based testing (FBT) and that it improved overall rates of HIV testing. HIVST was associated with a cost of $10.13/person tested, compared to $5.01 for FBT (20). Because they did not report diagnostic outcomes, costs per diagnosis were unavailable. DeBoni et al. (21) evaluated an HIVST intervention using website-based supports in Brazil. They found that digital-based HIVST was associated with a cost of $176/person tested or $15,717/person diagnosed (21, 22).
To our knowledge, this is the first model evaluating the cost-utility of a digital-based implementation of HIVST. Cost-utility implies that costs are reported per utility measure, which includes both duration and quality of life impacted, compared to cost-effectiveness, which reports cost per outcome (e.g., cost per quality-adjusted life year versus cost per person diagnosed). The aim of this study is to evaluate the cost-utility of HIVST using digital-based (DB) supports compared to HIVST using community-based (CB) supports or facility-based testing (FBT) alone.
Methodology
Design and setting
We evaluated the cost-utility of implementing HIVST using digital-based modalities compared to two comparator arms, namely HIVST using a community-based approach or FBT alone. We considered FBT to be the current standard of care and therefore modeled HIVST so that it was implemented in addition to the standard of care rather than replacing FBT.
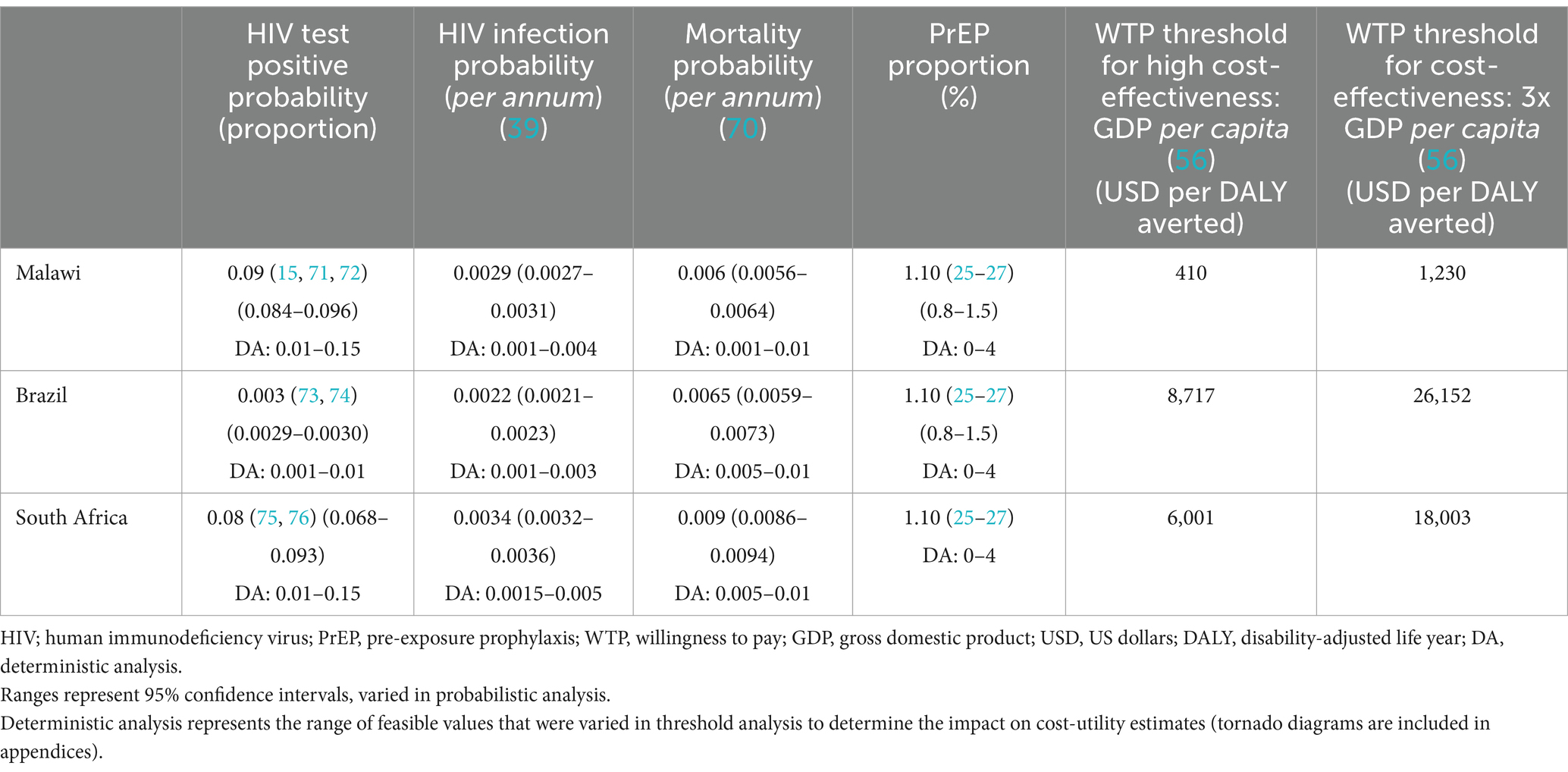
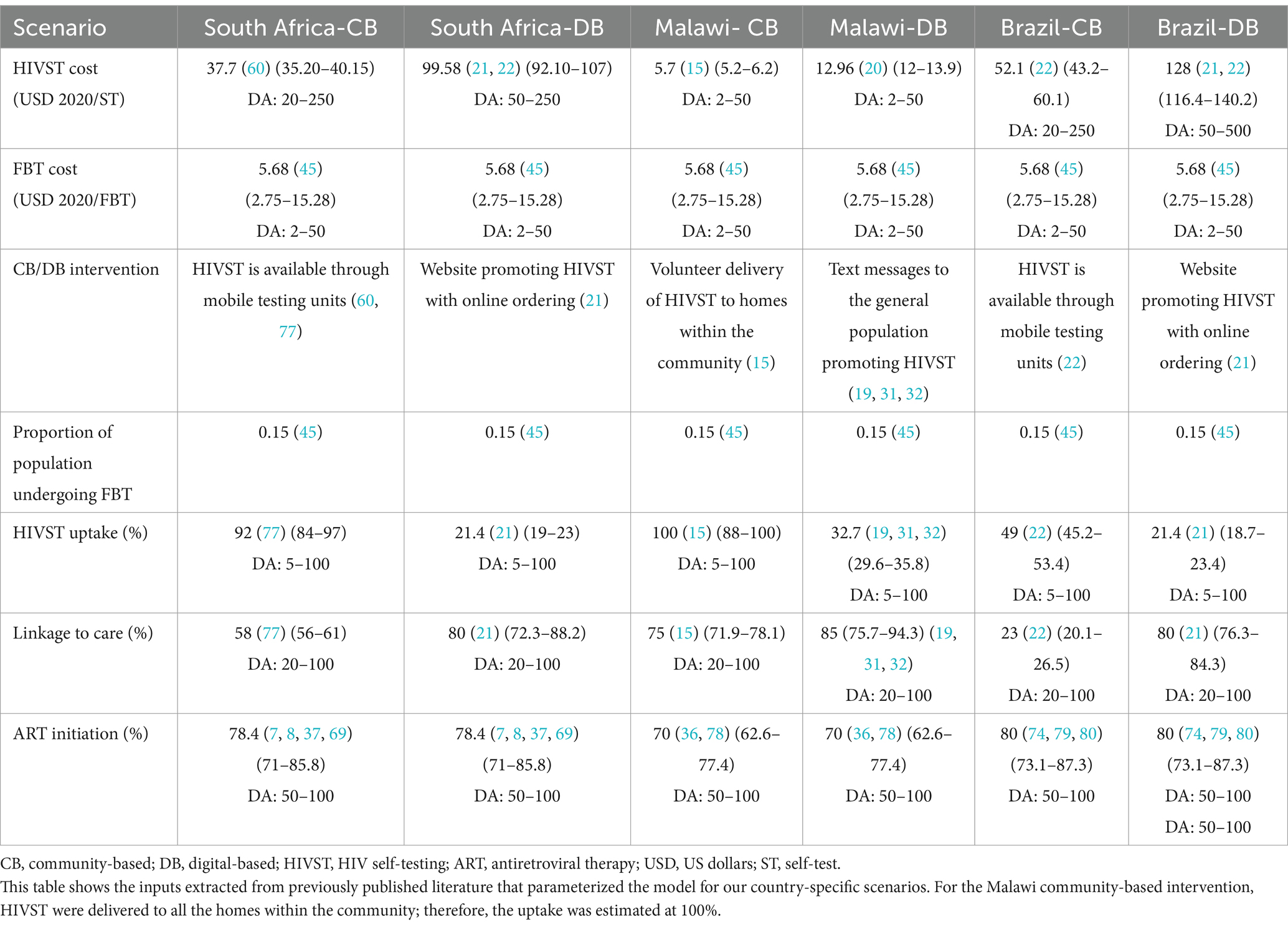
We developed an embedded decision tree within a Markov model structure using TreeAge Pro 2021 (23). We used a decision tree, with mutually exclusive branches, to capture the diagnostic testing process, confirmatory testing, and linkage to care (Figures 1A,B) and a Markov model for the chronic health states of HIV positive (on and off antiretroviral therapy), HIV negative, and death (Figure 2). The Markov cycle duration was one year and the time horizon was 30 years, which was varied from 5 to 50 years in sensitivity analysis. This implies that the intervention modeled incurred costs and benefits for a period of thirty years. The time horizon will impact cost (which accumulates yearly), but it will also impact the ability to detect benefits of screening programs on disease-related morbidity and mortality, especially in infections like HIV, which have a chronic course. We modeled the cost-utility of HIVST across three countries—Malawi, South Africa, and Brazil. These countries were selected to represent a variety of income levels and endemic HIV rates.
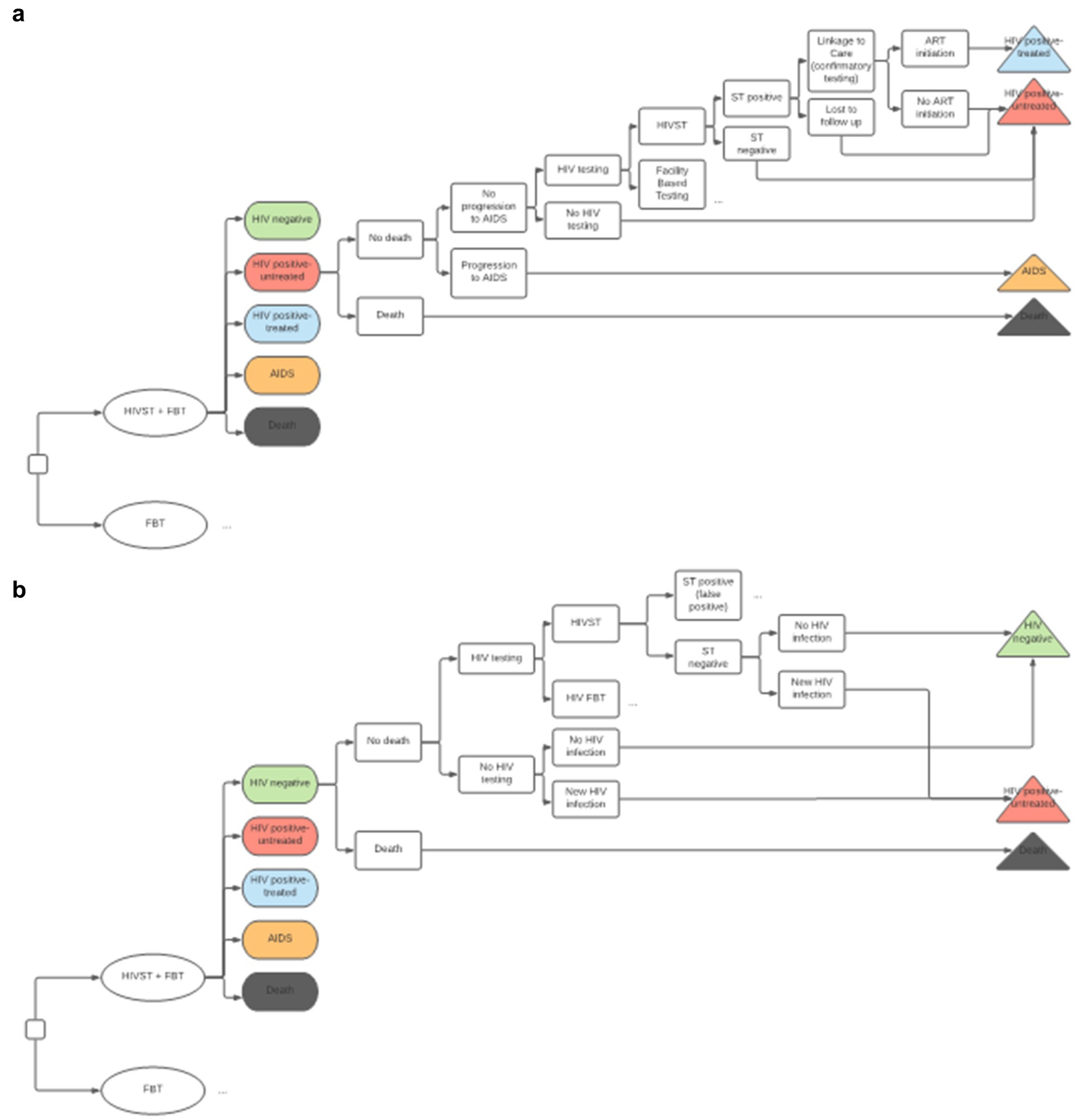
Figure 1. (A) Decision Tree for HIV positive Markov state. HIVST, HIV self-testing; FBT, facility-based testing; AIDS, acquired immunodeficiency syndrome; ST, self-testing; ART, antiretroviral therapy. Description: This diagram depicts the combined decision tree and Markov structure of the economic model. We have compared HIVST and FBT (the standard of care) in addition to FBT alone. The color-coded ovals in this diagram represent Markov states. A cohort progresses through the decision tree structure with every Markov cycle, and a proportion of the cohort may progress to a different Markov state, as illustrated by the terminal triangles. This diagram shows how PLHIV who are untreated may get transitioned to starting ART. (B) Decision tree for HIV negative Markov state. HIVST, HIV self-testing; FBT, facility-based testing; AIDS, acquired immunodeficiency syndrome; ST, self-testing; ART, antiretroviral therapy. Description: this diagram illustrates how individuals can transform from the HIV negative stage to the HIV positive stage. Depending on the specific test-positivity, a percentage of the population entered the model in the HIV positive-untreated state. This reflects the HIV test positive rate of the cohort. The other individuals entered the model with HIV negative state. With every cycle, there is a small probability of new HIV infection, where individuals who are HIV negative can move to the HIV positive-untreated state.
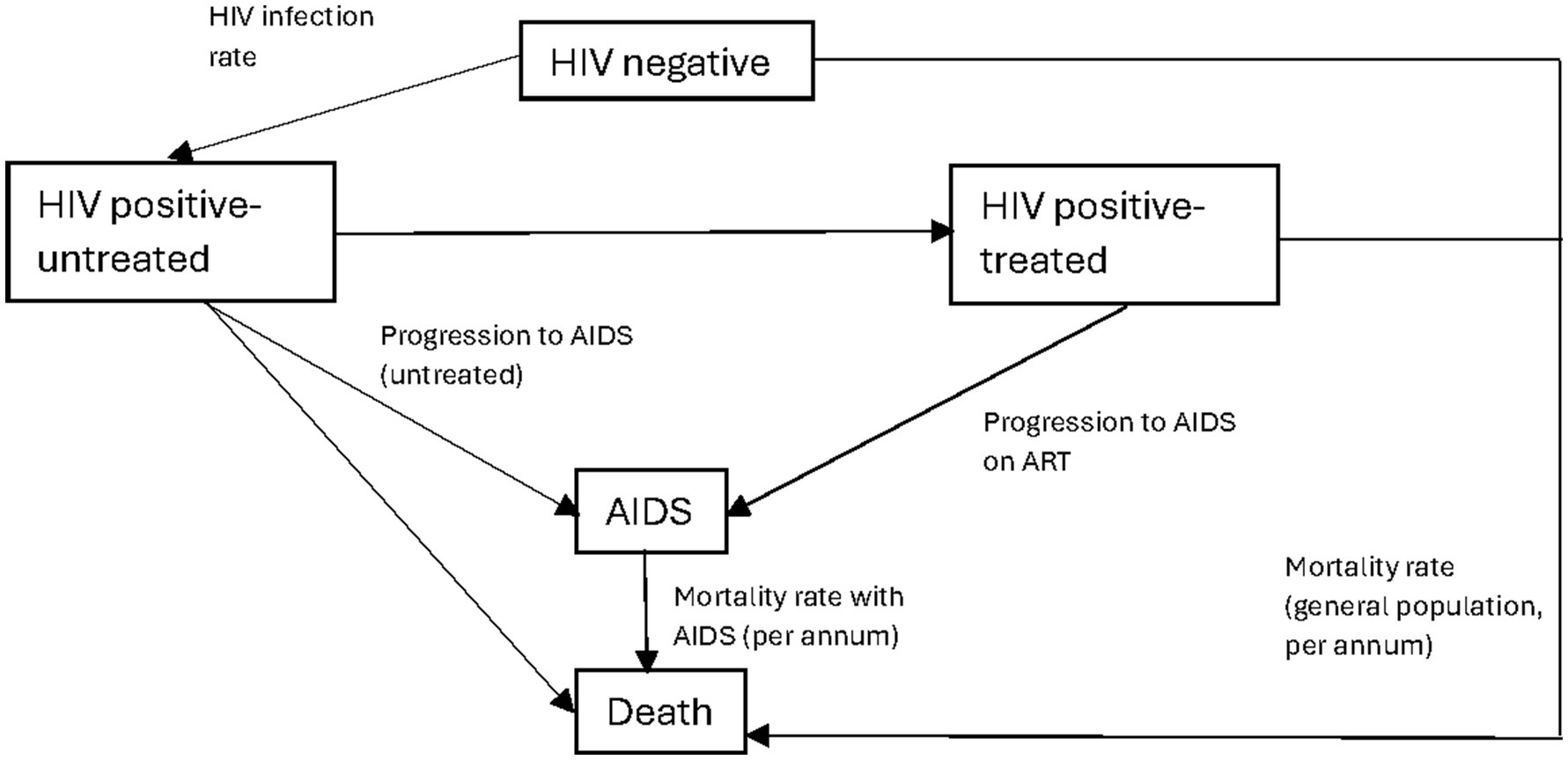
Figure 2. Markov model schematic. AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; ART, antiretroviral therapy. Description: this figure shows the general schema of the Markov model and how the cohort moves between different Markov states. The transition probabilities between states are depicted on the diagram. The corresponding quantitative values are included in Table 2. Individuals who enter the model are either the HIV positive (undiagnosed) or HIV negative. With each Markov cycle, there exists a transitional probability to undergo HIV testing. For individuals who are diagnosed, they can either be treated or choose not to start ART. With each cycle, there is a transitional probability of a new HIV infection or death. The probability of death is dependent on the Markov state, with those within the AIDS Markov state having a higher probability of death.
We reported our findings based on the CHEERs (Consolidated Health Economic Evaluation Reporting Standards) checklist for model-based economic evaluations (24).
Patient population
We modeled the cost-utility of HIVST assuming a general population (ages 15–65 years) who had not previously tested positive for HIV. We also included scenario analyses where HIVST was restricted to key populations, such as men who have sex with men (MSM), with higher underlying rates of test positivity for HIV. PrEP was included in our model, with the percentage of the population on PrEP based on existing literature (25–27). Individuals on PrEP were eligible for HIVST and FBT annually and were subject to incurred regular testing and treatment costs as per country-specific guidelines. Individuals on PrEP incurred additional treatment costs but had a greatly decreased risk of becoming HIV positive (28–30).
Interventions of interest and comparator
Our primary intervention of interest was HIVST using a digital-based approach, which was implemented in addition to the current standard of care, which consisted of HIV testing offered at a clinic (FBT). We used pre-existing literature evaluating digital-based HIVST, community-based HIVST, and facility-based HIV testing to inform cost and effectiveness parameters within the model (19–22, 31, 32). Those with a reactive self-test required follow-up (linkage to care) with confirmatory testing and post-test counseling provided at a health care facility. The costs associated with confirmatory FBT from true and false positive HIVST screens were attributed to the intervention arm. Linkage to care rates were varied depending on the HIVST implementation strategy. FBT required an individual to present to the health care facility for screening and confirmatory testing as per the current standard of care based on country-specific HIV confirmatory testing guidelines (33–35). After confirmatory testing (in both the HIVST and FBT scenarios), individuals could be initiated on antiretroviral therapy (ART). Rates of ART acceptance rates were country-specific, based on published literature (6, 7, 36–38).
Model parameters
Key epidemiological parameters such as HIV test-positivity, HIV incidence, and population mortality were sourced from the national databases (Table 1). Our model accounted for the rate of new HIV infections (HIV incidence) by using country-specific infection rates (39). Individuals who were HIV negative experienced an annual rate of transition to become HIV positive based on the HIV infection rate. The underlying HIV test-positivity rates were sourced from the UNAIDS (Joint United Nations Programme on HIV and AIDS) data bank (4) and were country and age specific.
The specific HIV self-test model was saliva-based OraQuick™, given that it is the HIV self-test that has been most commonly implemented on a global basis and for which there is existing economic literature in the context of HIVST (40–44).
To model digital and community-based supports, we used data from previously published interventional studies to parametrize each country-specific model (Table 2). In order to identify all relevant literature, a comprehensive literature review focusing on economic studies for HIVST (both community and digital-based) was conducted (45). For our Malawi digital-based (DB) model, parameters relevant to the DB intervention, including cost, uptake, and linkage to care, were sourced from a randomized control trial conducted in Kenya, using SMS-based messaging to target truck drivers and female sex workers (FSWs) for HIVST (19, 20). In Malawi, there are no current publications on the cost-effectiveness of DB HIVST, which is why a Kenyan study was chosen.
We parameterized our model with effectiveness data from a published study that used a digital application to promote HIVST in South Africa along with counseling and linkage services (46). We extrapolated costing data from Brazil to estimate the cost-utility of DB HIVST in South Africa, as there is no published cost-effectiveness data for DB HIVST in South Africa. For both Malawi and South Africa, where there were no country-specific costing data available for DB HIVST, a corrective ratio was applied based on GDP per capita.
The DB intervention from Brazil is based on a study conducted by DeBoni et al. (21), using a website targeted at the MSM population to facilitate HIVST. For further details of the CB or DB HIVST intervention used in each scenario, we have included detailed summaries of the studies used to parameterize our model in our appendices.
Economic approach
The cost per HIV self-test (including unit test and implementation cost) was sourced from the relevant published literature for both digital and community-based HIVST (Table 2). For the comparator arm (facility-based testing), the average cost per HIVST ($5.68/test) was used based on a literature heterogeneity of all available published economic data for HIVST. Unweighted mean costs were employed and sourced from the 22 relevant studies identified by the literature review (45). We assumed individuals who were HIV positive and had initiated ART would have incurred annual ART and monitoring costs that were sourced from country-specific literature (47–49).
We have adopted a health care system perspective since there was inadequate data to inform patient incurred costs around HIVST. A mid-cycle correction was applied to the costs and utilities. Both costs and utilities are discounted by 3% per annum. All costs were converted back to their original currency (50), adjusted for inflation (51, 52) and then converted into USD 2020.
Outcomes
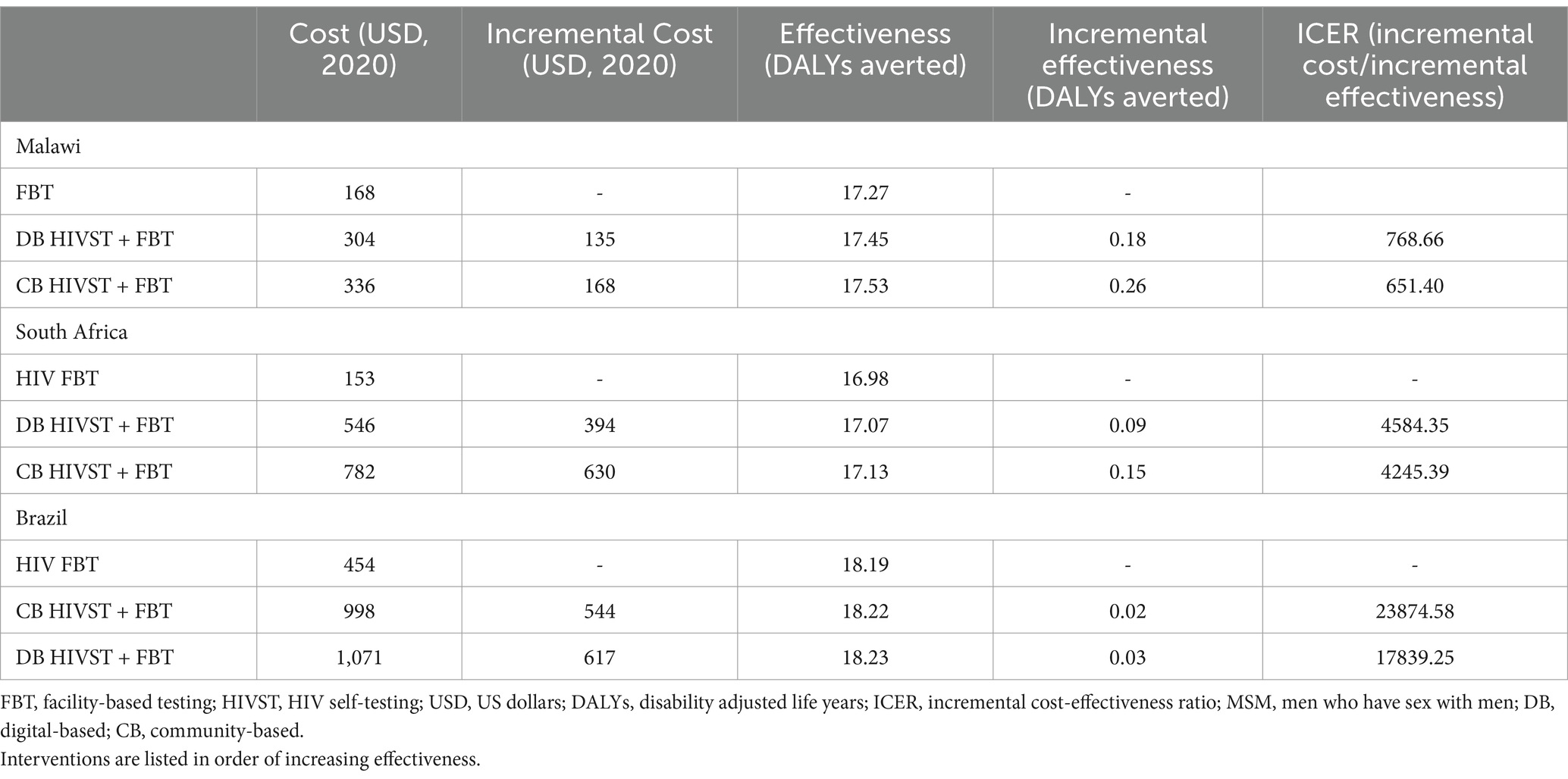
The primary outcome evaluated was the incremental cost-effectiveness ratio (ICER), defined as the incremental cost in USD per disability-adjusted life year (DALY) averted (53). Values for disability weights were sourced from the WHO Global Burden of Disease study (54). ICER calculations were conducted with digital-based testing compared to both community-based HIVST and FBT only. ICER per DALY averted was used as a primary outcome because it is a standardized measure that allows comparison between studies and accounts for quality and quantity of life.
The ICER estimates were compared against a willingness to pay threshold (WTP) established a priori to determine whether the intervention should be considered cost-effective. As per the WHO Choosing Interventions that are Cost-Effective (CHOICE) guidelines (55), the intervention could be considered cost-effective if it is less than three times the national gross domestic product (GDP) per capita per DALY averted and highly cost-effective if it is less than the national GDP per capita (56).
Sensitivity analyses
Uncertainty around parameter values was explored through one- and two-way deterministic sensitivity analyses to understand the impact of key parameters varied along plausible ranges on model results and to identify key drivers of cost-effectiveness. Key parameters identified comprised of test positivity, cost of HIVST, HIVST uptake, linkage to care, and ART initiation rates (57–60). We varied the horizon from 5 to 50 years to understand the impact of intervention duration on cost-utility estimates.
A probabilistic sensitivity analysis was conducted using 10,000 Monte Carlo repetitions to generate 95% uncertainty ranges around model point estimates. Costs are represented by gamma distributions, transitional probabilities are modeled by beta distributions, and utility values are represented by triangular distributions.
As the HIVST unit costs employed in the model included costs associated with program implementation costs as well as individual kit costs, we sought to explore the further benefits associated with economies of scale wherein implementation costs could be spread over different target population sizes. To address this, a scenario analysis was performed where the programmatic portion of HIVST is divided by an increasing number of people to evaluate how increased uptake leading to reduced unit test costs might affect model estimates. The scale-up factor for this sensitivity analysis was varied from 0.5 to 10x.
Malawi
Results
In the Malawian scenario, digital-based HIVST was more cost-effective compared to FBT, but it was associated with a lower ICER than CB HIVST. DB HIVST was associated with an ICER of $769/DALY averted compared to facility-based testing. We have discovered that DB HIVST was less effective but cost-saving compared to community-based testing. Community-based testing was associated with an additional cost of $400/DALY averted compared to digital-based HIVST. Using a WTP threshold of 3x Malawian GDP per capita, the digital-based HIVST was cost-effective in 88% of the probabilistic scenarios (Figure 3). HIVST with DB supports was associated with a total cost of $10.13/person tested per annum, which includes testing costs, programmatic costs, and treatment for the additional people diagnosed with HIV. If digital-based HIVST was implemented on a national level in Malawi (among a population of approximately 19.9 million), it would be associated with an additional 318,400 individuals initiating ART and 278,600 deaths averted compared to FBT. Compared to FBT, digital-based HIVST with FBT was associated with a 32% increase in ART initiation among people living with HIV (PLHIV).

Figure 3. Probabilistic analysis for Malawi digital-based HIVST compared to FBT. HIVST, HIV self-testing; GDP, gross domestic product; WTP, willingness to pay; USD, US Dollars; DALY, disability-adjusted life year; CB, community-based. Description: WTP = 3x Malawian GDP per capita.
The drivers of cost-utility for digital-based HIVST in Malawi were the cost of the HIVST, HIV test-positivity rates, linkage to care, and annual ART costs (Appendix Figure 1). In threshold analysis, the minimum linkage to care required for DB HIVST to be considered cost-effective was 35%.
In order to examine the impact of targeting the intervention to key subgroups with increased rates of HIV test positivity, we modeled the cost-utility of a DB intervention limited to the MSM population (4, 61, 62). We found that DB HIVST was more cost-effective within this subgroup, with a decreased ICER of $511/DALY averted. This would be considered cost-effective (costing less than 3x the Malawian GDP per capita).
South Africa
In the South African scenario, DB HIVST was considered highly cost-effective compared to FBT, with an ICER of $4,584/DALY averted (Table 3). However, it was associated with a lower ICER than CB HIVST. Probabilistic analysis showed that the chance that HIVST would be highly cost-effective when implemented through a digital-based approach was 55% (Figure 4). If we used the less conservative WTP threshold of 3x GDP per capita, DB HIVST would have >99% chance of being cost-effective compared to FBT. Our model predicts that DB HIVST would be less effective but more economical compared to CB HIVST. CB HIVST was associated with an additional cost of $3,933/DALY averted compared to DB HIVST. HIVST with DB supports was associated with a total cost of $152.81/person tested per annum. If DB HIVST was enacted at a national level in South Africa (among a population of approximately 59.4 million), it would be associated with an additional 356,340 individuals starting on ART and 297,000 deaths averted. Compared to FBT, digital-based HIVST with FBT was associated with a 20% increase in ART initiation among PLHIV.
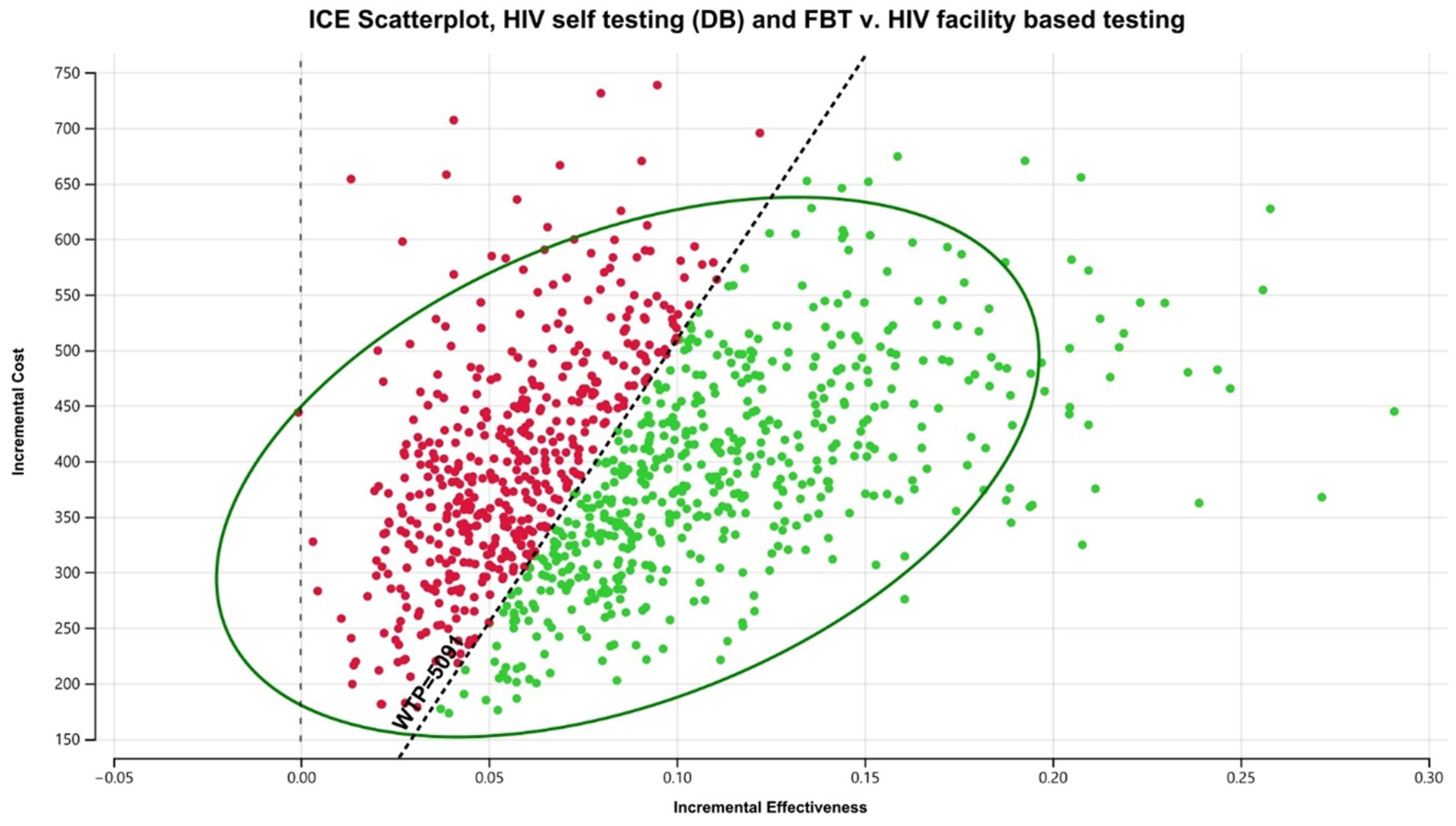
Figure 4. Probabilistic analysis for South Africa digital-based HIVST compared to FBT. HIVST, HIV self-testing; GDP, gross domestic product; WTP, willingness to pay; USD, US dollars; CB, community-based. Description: WTP = 1 x South African GDP per capita.
In sensitivity analyses, the major driver of cost-utility was the rate of HIV test positivity (Appendix Figure 2). In threshold analysis, DB HIVST continued to be cost-effective throughout a widely varied linkage to care rate (20–100%).
When DB HIVST was targeted toward MSM, we found that HIVST with a DB support was more cost-effective with a decreased ICER of $2,871/DALY averted. This would be considered highly cost-effective (costing less than the South African GDP per capita).
Brazil
Within Brazil, DB HIVST would be considered cost-effective with an average ICER of $17,839/DALY averted compared to FBT. Digital-based HIVST was more cost-effective than CB HIVST. A probability analysis indicated that DB HIVST would have an 82% probability of being cost-effective (Figure 5). Within this context, DB HIVST was more expensive and more effective than CB HIVST with an ICER of $7,300/DALY averted. HIVST with DB supports was associated with a total cost of $594.64/person tested per annum.
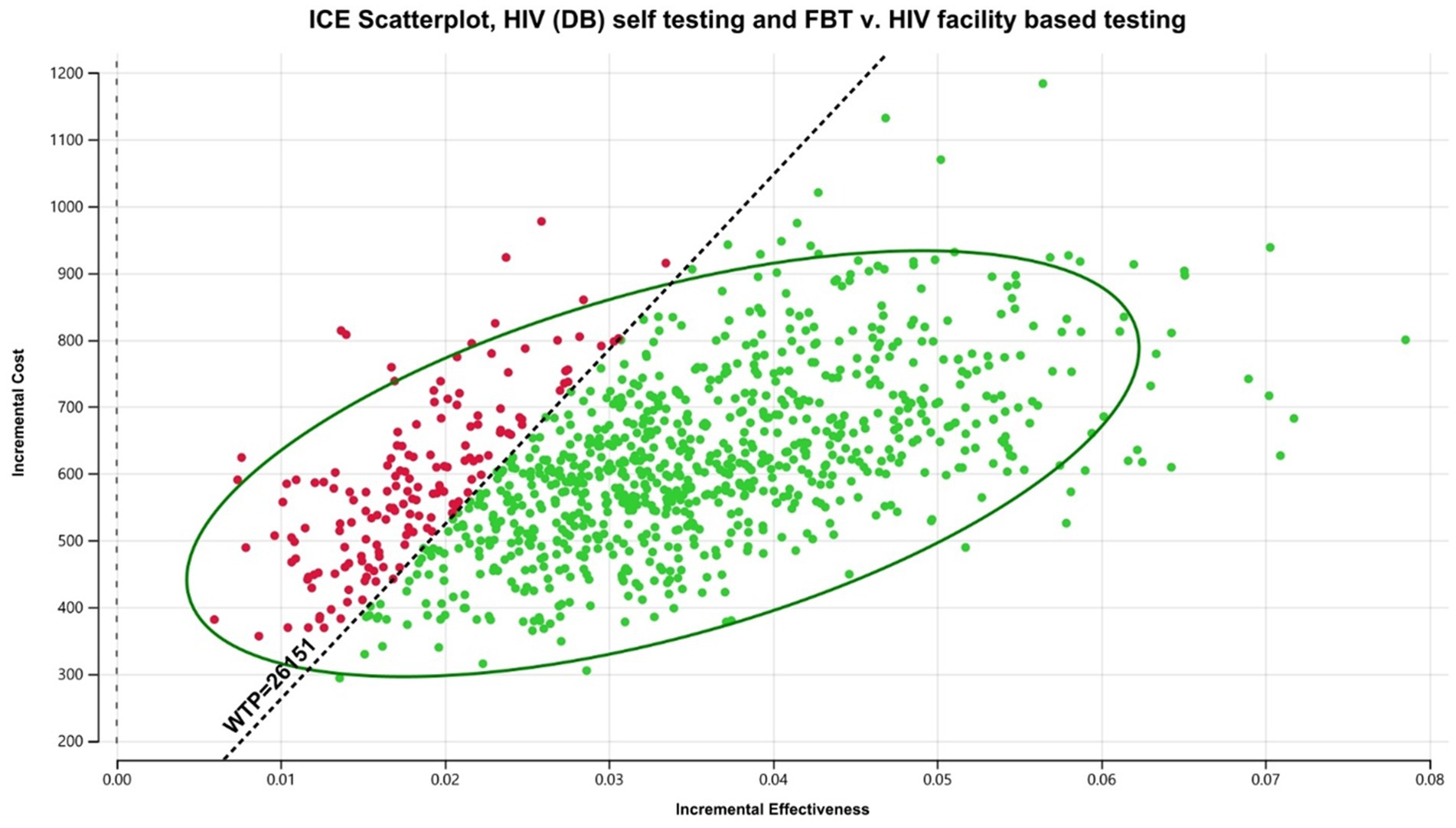
Figure 5. Probabilistic analysis for Brazil digital-based HIVST compared to FBT. HIVST, HIV self-testing; GDP, gross domestic product; WTP, willingness to pay; USD, US dollars; DB, digital-based. Description: WTP = 3 x Brazilian GDP per capita.
If digital-based HIVST was enacted on a national level in Brazil (among a population of approximately 214.3 million), it would be associated with an additional 1.5 million individuals starting on ART and 857,200 deaths averted. When compared to FBT, digital-based HIVST with FBT was associated with a 33% increase in ART initiation among PLHIV.
The key determinants of cost-utility within this scenario were the cost of HIVST and linkage to care (Appendix Figure 3). In threshold analysis, the minimum linkage to care for DB HIVST to be considered cost-effective was 48%.
When DB HIVST was targeted toward MSM, we noticed that HIVST with a DB support was more cost-effective, resulting in a decreased ICER of $5,703/DALY averted. It would be considered highly cost-effective (costing less than the Brazilian GDP per capita). Increasing uptake of PrEP among this population reduce the cost-effectiveness of HIVST by decreasing HIV infection rates and underlying seropositivity of the group.
Scenario analysis: horizontal duration
We found that the cost-utility of HIVST improved with an increased horizon (Table 4). The cost-utility of HIVST improved with decreased horizon despite decreased intervention costs because the long-term benefits in DALYs averted were properly captured. HIVST continued to be increasingly cost-effective up to a 50-year horizon; however, the rate of improved cost-utility plateaued between 30 and 50 years.
Scenario analysis: HIVST uptake
To evaluate the potential impact of economies of scale on unit cost per person tested and cost-effectiveness, we performed a sensitivity analysis in which a proportion of the HIVST cost, representing the implementation or “one-time programmatic” costs, was divided among an increasing number of participants. This analysis showed that as uptake increased, the cost per person tested decreased, and HIVST became increasingly cost-effective (Figures 6A–C).
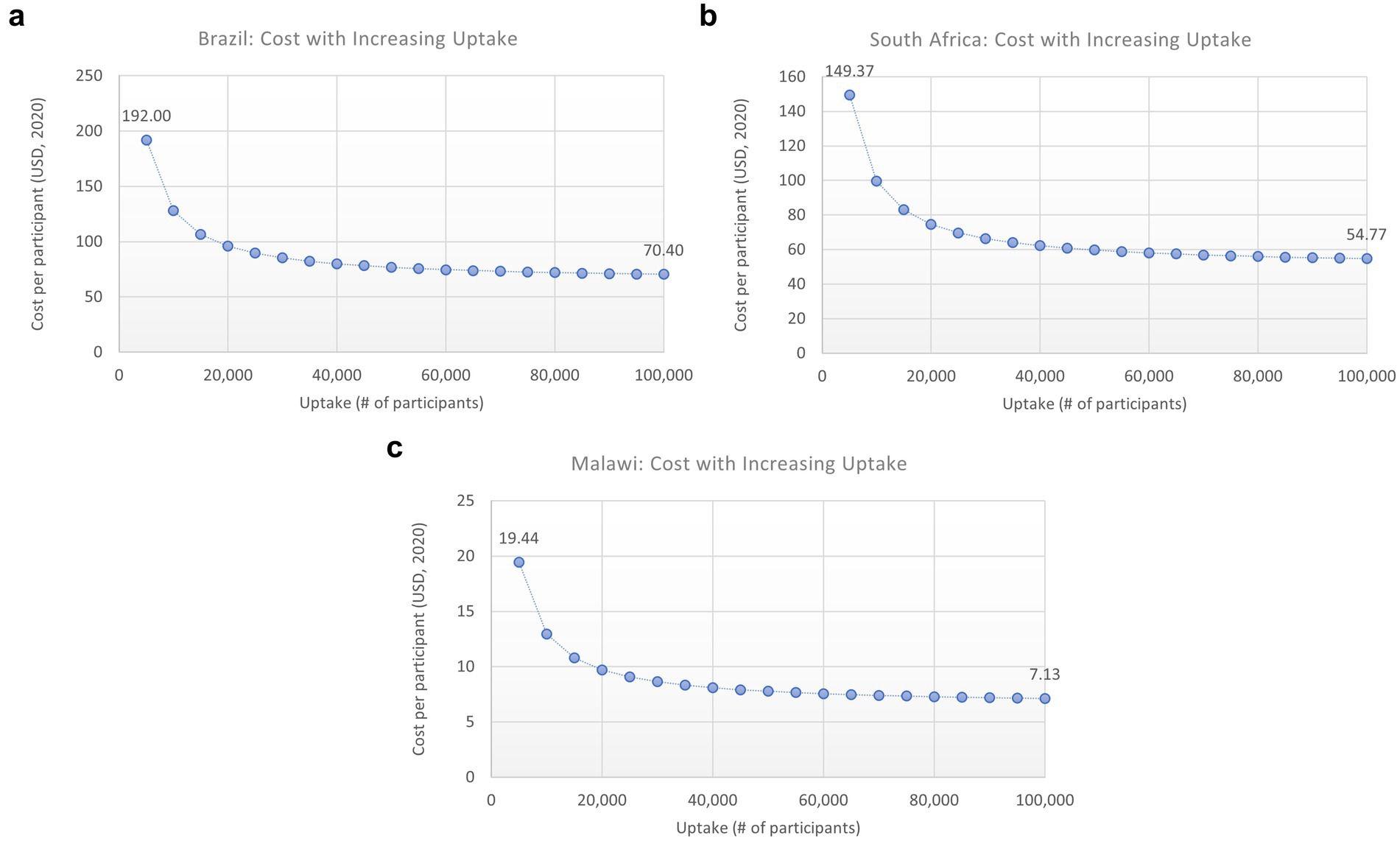
Figure 6. (A–C) Impact of increased uptake on cost/person. HIVST, HIV self-testing; ICER, incremental cost-effectiveness ratio; USD, US Dollars. Description: this graph shows how the ICER would change for each scenario with increasing uptake (number of participants), which would decrease the overhead cost per person tested.
Discussion
Our model suggests that digital-based HIVST in addition to FBT resulted in more new diagnoses than FBT alone, but that it was associated with an additional cost that varied by implementation approach. In all the scenarios evaluated, digital-based HIVST was considered cost-effective compared to FBT. Compared to community-based HIVST, digital-based HIVST ranged from cost-saving to $7,300/DALY averted. The cost-utility of digital-based HIVST varied between scenarios, with an average ICER/DALY averted ranging from $769 to $17,839 compared to facility-based testing. The upper estimate for this range comes from HIVST in Brazil, where testing and treatment costs were significantly higher compared to Malawi and South Africa.
Compared to facility-based testing, CB HIVST was more cost-effective in Malawi and South Africa than DB HIVST but less cost-effective in Brazil. In Brazil, costs for DB supports were much lower than CB supports, which drove the superior cost-utility profile of DB HIVST in the Brazilian setting. In addition, linkage to care for the CB HIVST was lower than DB HIVST (23% vs. 80%) within the Brazilian setting, which was also a major determinant of cost-effectiveness. Rates of linkage to care are highly variable within the published literature for HIVST. When we compared linkage to care rates from 20 to 100% through deterministic analysis, the DB HIVST and CB HIVST cost-utility estimates overlapped (Appendix Figures 1–3).
The major drivers of cost-utility in our model were largely consistent across different scenarios investigated and highlighted the importance of linkage to confirmatory testing and care, underlying HIV test-positivity, acceptability of ART, and cost of the HIVST test. By modeling previously published DB HIVST interventions on a national scale, using country-specific epidemiological inputs, we were able to enhance the conclusions from prior studies and increase the generalizability of results.
A recent systematic review suggested that HIVST, especially when implemented without support for follow-up, may be associated with lower linkage to care compared to FBT (63). By modeling different rates of linkage to care, we were able to determine the proportion of individuals who would need to link to care to meet cost-utility thresholds in different settings. In the scenarios evaluated, the minimum linkage to care to meet cost-utility thresholds was 20–48%. This is a critical element for consideration by programs when implementing or scaling up self-test approaches.
Although our model suggests that digital-based supports for HIVST are likely to be cost-effective, they require the user to possess device and/or internet access. In January 2023, it was estimated that 64% of the global population had access to the internet, and this has been increasing rapidly (56). Despite improvements, the lack of uniform devices and internet access could lead to gaps in coverage and impact uptake of DB HIVST. We anticipate that HIVST using digital-based supports serves as an adjunct rather than a replacement for facility-based diagnosis. This is why the interventional arm of our Markov model included both digital-based and facility-based testing, compared to only facility-based testing alone. If resources were re-allocated to the DB HIVST from the current standard of care (FBT), then it would pose a health equity concern for individuals without access to devices or the internet.
This is the initial model to estimate cost-utility outcomes [in either DALYs averted or quality-adjusted life years (QALYs)] for digital-based programs. For our CB HIVST results, our estimates were similar to previously published literature once results were adjusted for inflation. Cambiano et al. estimated that CB HIVST was associated with a cost-utility of $23-418/DALY averted when implemented among high-risk populations in Malawi (58). Our estimate for the general population (with a lower test-positivity rate) was $651/DALY averted compared to FBT. Maheswaran et al. also modeled the cost-utility of CB HIVST but they used QALYs as their outcome measures (64). The lack of generalizability across studies suggests a need for reporting standardization among economic modeling studies.
To allow for generalization, we have combined a variety of interventions into the category of digital-based HIVST. It is important to recognize that different programs may employ different digital strategies employed and have a different cost-utility profile. There was limited costing data around digital-based HIVST, with only two relevant studies providing cost data for DB interventions. All current studies have focused on key populations with high underlying rates of HIV test positivity. This would be an important area for further research moving forward, given the increasing digitalization of health care on a global level. Our findings highlight the emerging opportunity to use digital-based support in conjunction with HIVST to help testing become more accessible to communities and support linkage to care, especially among those who face barriers to accessing health care through conventional means. This could serve as an effective means to improving the accessibility of HIVST among currently “hard to reach” populations.
Limitations
Although we did account for new HIV infections over time within the Markov modeling structure, our cohort model did not include dynamic transmission or account for the potential impact of HIVST on transmission and the underlying community prevalence of HIV over time. An earlier HIV diagnosis has the potential to increase pre-symptomatic treatment and decrease transmissibility (65). Without including dynamic transmission modeling, the cost-utility of HIVST might be underestimated. If HIVST was capable of reducing the underlying community prevalence of HIV, there may be a gradual decrease in new HIV infections and therefore a reduction in ART costs and increased DALYs averted. Alternatively, with a decrease in the test-positivity of HIV (which we found was a driver of cost-effectiveness), the cost-utility of the HIVST intervention could decrease. There is a lack of literature to inform how HIVST might impact long-term community incidence of HIV; thus, it is challenging to predict how changing incidence would impact cost-utility.
We have followed the WHO’s CHOICE economic reporting guidelines (55) and used a WTP threshold based on GDP per capita. This does have health equity implications, as an intervention that was cost-effective in South Africa has a much lower chance of being cost-effective in Malawi despite similar efficacy. Recently, various alternatives have been introduced to determine WTP, including using an opportunity cost approach, reporting costs as a percentage of GDP per capita, or requiring participants to assign a value to outcome measures using a standard gamble and time trade-off (66, 67). These have been primarily used for QALYs, and DALYs averted continue to be the most common utility outcome used within a global health context. Therefore, to allow generalizability across studies, we have continued to use cost per DALY averted as our primary outcome. The ICERs, costs, and efficacy are presented in addition to conclusions around cost-effectiveness which allows alternate WTP thresholds to be applied.
There are many prevention strategies for HIV transmission (beyond PrEP), that are not specifically included in our model. Since we have reported incremental cost-utility ratios (ICERs), our cost-utility estimates would not be affected by these unless there was a differential uptake of the preventative strategies between the digital-based HIVST cohort and the FBT cohort.
We have used the relevant existing economic data to inform our model, but unfortunately there is a lack of literature around the costs of digital-based HIVST, which has limited the strength of our inputs. Since there were no country-specific studies for either Malawi or South Africa; therefore, we had to generalize from other countries using a ratio of GDP per capita. This highlights the need for further cost-effectiveness data from digital-based HIVST initiatives. There was a lack of literature around the effectiveness of DB HIVST that required us to generalize across contexts.
Key policy implications
As internet access becomes increasingly available across nations, digital-based approaches offer a promising avenue for promoting HIVST while maintaining anonymity. Digital-based HIVST can be offered digitally with websites and applications that focus on key populations, such as MSM dating applications (21). A recent systematic review found that digital-based strategies were associated with a 1.5 times increased rate of HIVST among the MSM population and were perceived as more accessible and convenient (68).
Based on our model, ST (both CB and DB) was cost-effective compared to FBT, but depended on key drivers including underlying HIV test positivity, linkage to care, ART acceptability, and HIVST cost. In areas with low endemic rates of HIV, DB HIVST is most likely to be cost-effective when implemented among key populations with high rates of undiagnosed HIV. Strategies to improve cost-utility include ensuring adequate linkage to confirmatory testing and treatment, negotiating reduced unit test cost, and reducing costs associated with implementation. HIVST implemented with digital-based supports is a key strategy that can improve cost-utility (despite increasing overall costs). It increases accessibility and support linkage to care.
Confidentiality is a major concern with both facility-based and self-testing for HIV. Concerns about recognized in a health care facility while testing for HIV have been reported as perceived benefits of self-testing, which can be done in the privacy of your home (69). Privacy and security in the context of digital-based health care is an increasingly relevant issue as electronic medical records become common. It would need to be considered when implementing a DB HIVST program.
Conclusion
Self-testing is a promising new strategy that may improve access to diagnosis of infectious disease among hard-to-reach populations. Our model found that the cost-utility of HIVST using DB interventions varied between $769-17839/DALY averted.
As HIV diagnosis and treatment evolve, it becomes increasingly crucial to ensure that marginalized patient populations can also derive benefit from advancements in HIV care. The improved accessibility of digital-based HIVST makes it an appealing strategy for reaching individuals who face barriers to conventional testing. Our model suggests that DB HIVST is cost-effective in a variety of different contexts. Both digital and community-based interventions can increase accessibility to HIVST and support improving the linkage to care and rates of ART initiation (which are key drivers of cost-effectiveness).
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: available via prior publications (referenced).
Ethics statement
This study was submitted to our institutional REB but because it relies on previously published parameters to inform the model, was deemed not to require ethics approval as it did not involve any new data collection.
Author contributions
BE: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. AK: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. MF-S: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. SC: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. NP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is funded from FIND – RIMUHC agreement no 8647 PI award to Dr. NP.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material of this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1440104/full#supplementary-material
Appendix Figure 1 | Deterministic Analysis of Digital-Based HIVST in Malawi. This tornado diagram shows that the major drivers of cost-effectiveness of DB HIVST in Malawi were cost of the HIV self-test, underlying HIV test-positivity, linkage to care and the cost of ART. The willingness to pay threshold used for this analysis was 3xGDP per capita ($1230/DALY averted). HIVST, HIV self-testing; DB, digital-based; ART, antiretroviral therapy; WTP, willingness to pay; EV, expected value.
Appendix Figure 2 | Deterministic Analysis of Digital-Based HIVST in South Africa. This tornado diagram shows that the major driver of cost-effectiveness of DB HIVST in South Africa was underlying HIV test-positivity. The willingness to pay threshold used for this analysis was 3xGDP per capita ($18003/DALY averted). HIVST, HIV self-testing; DB, digital-based; ART, antiretroviral therapy; WTP, willingness to pay; EV, expected value.
Appendix Figure 3 | Deterministic Analysis of Digital-Based HIVST in Brazil. This tornado diagram shows that the major driver of cost-effectiveness of DB HIVST in South Africa was cost of the HIV self-test and linkage to care. The willingness to pay threshold used for this analysis was 3xGDP per capita ($26152/DALY averted). HIVST, HIV self-testing; DB, digital-based; ART, antiretroviral therapy; WTP, willingness to pay; EV, expected value.
References
1. Van Hout, MC, Stover, H, Benamara, K, Bauer, P, and Salah, E. 90-90-90: catalysing the response to HIV by enhancing prison visibility in the joint United Nations Programme on HIV and AIDS (UNAIDS) strategy beyond 2021. Public Health. (2021) 190:e5–6. doi: 10.1016/j.puhe.2020.10.016
2. Fuente-Soro, L, Lopez-Varela, E, Augusto, O, Sacoor, C, Nhacolo, A, Honwana, N, et al. Monitoring progress towards the first UNAIDS target: understanding the impact of people living with HIV who re-test during HIV-testing campaigns in rural Mozambique. J Int AIDS Soc. (2018) 21:e25095. doi: 10.1002/jia2.25095
3. Ssekalembe, G, Isfandiari, MA, and Suprianto, H. Current status towards 90-90-90 UNAIDS target and factors associated with HIV viral load suppression in Kediri City, Indonesia. HIV AIDS. (2020) 12:47–57. doi: 10.2147/HIV.S231173
5. Frost, LJ, and Reich, MR. Creating access to health technologies in poor countries. Health Aff. (2009) 28:962–73. doi: 10.1377/hlthaff.28.4.962
6. Hamilton, A, Thompson, N, Choko, AT, Hlongwa, M, Jolly, P, Korte, JE, et al. HIV self-testing uptake and intervention strategies among men in sub-Saharan Africa: a systematic review. Front Public Health. (2021) 9:594298. doi: 10.3389/fpubh.2021.594298
7. McGuire, M, de Waal, A, Karellis, A, Janssen, R, Engel, N, Sampath, R, et al. HIV self-testing with digital supports as the new paradigm: a systematic review of global evidence (2010-2021). EClinicalMedicine. (2021) 39:101059. doi: 10.1016/j.eclinm.2021.101059
8. Johnson, CC, Kennedy, C, Fonner, V, Siegfried, N, Figueroa, C, Dalal, S, et al. Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis. J Int AIDS Soc. (2017) 20:21594. doi: 10.7448/IAS.20.1.21594
9. Aluisio, AR, Bergam, SJ, Sugut, J, Kinuthia, J, Bosire, R, Ochola, E, et al. HIV self-testing acceptability among injured persons seeking emergency care in Nairobi, Kenya. Glob Health Action. (2023) 16:2157540. doi: 10.1080/16549716.2022.2157540
10. Boisvert Moreau, M, Kintin, FD, Atchekpe, S, Batona, G, Béhanzin, L, Guédou, FA, et al. HIV self-testing implementation, distribution and use among female sex workers in Cotonou, Benin: a qualitative evaluation of acceptability and feasibility. BMC Public Health. (2022) 22:589. doi: 10.1186/s12889-022-12917-3
11. Salvadori, N, Achalapong, J, Boontan, C, Piriya, C, Arunothong, S, Nangola, S, et al. Uptake, acceptability and interpretability of 3-in-1 rapid blood self-testing for HIV, hepatitis B and hepatitis C. J Int AIDS Soc. (2022) 25:e26053. doi: 10.1002/jia2.26053
12. Wesolowski, L, Chavez, P, Sullivan, P, Freeman, A, Sharma, A, Mustanski, B, et al. Distribution of HIV self-tests by HIV-positive men who have sex with men to social and sexual contacts. AIDS Behav. (2019) 23:893–9. doi: 10.1007/s10461-018-2277-0
13. Chanda, MM, Ortblad, KF, Mwale, M, Chongo, S, Kanchele, C, Kamungoma, N, et al. HIV self-testing among female sex workers in Zambia: a cluster randomized controlled trial. PLoS Med. (2017) 14:e1002442. doi: 10.1371/journal.pmed.1002442
14. Jamil, MS, Guy, RJ, Bavinton, BR, Fairley, CK, Grulich, AE, Holt, M, et al. HIV testing self-efficacy is associated with higher HIV testing frequency and perceived likelihood to self-test among gay and bisexual men. Sex Health. (2017) 14:170–8. doi: 10.1071/SH16100
15. Indravudh, PP, Fielding, K, Kumwenda, MK, Nzawa, R, Chilongosi, R, Desmond, N, et al. Effect of community-led delivery of HIV self-testing on HIV testing and antiretroviral therapy initiation in Malawi: a cluster-randomised trial. PLoS Med. (2021) 18:e1003608. doi: 10.1371/journal.pmed.1003608
16. Reddick, CG, Enriquez, R, Harris, RJ, and Sharma, B. Determinants of broadband access and affordability: an analysis of a community survey on the digital divide. Cities. (2020) 106:102904. doi: 10.1016/j.cities.2020.102904
17. Qin, Y, Han, L, Babbitt, A, Walker, JS, Liu, F, Thirumurthy, H, et al. Experiences using and organizing HIV self-testing. AIDS. (2018) 32:371–81. doi: 10.1097/QAD.0000000000001705
18. Matsimela, K, Sande, LA, Mostert, C, Majam, M, Phiri, J, Zishiri, V, et al. The cost and intermediary cost-effectiveness of oral HIV self-test kit distribution across 11 distribution models in South Africa. BMJ Glob Health. (2021) 6:e005019. doi: 10.1136/bmjgh-2021-005019
19. Kelvin, EA, George, G, Kinyanjui, S, Mwai, E, Romo, ML, Oruko, F, et al. Announcing the availability of oral HIV self-test kits via text message to increase HIV testing among hard-to-reach truckers in Kenya: a randomized controlled trial. BMC Public Health. (2019) 19:7. doi: 10.1186/s12889-018-6345-1
20. George, G, Chetty, T, Strauss, M, Inoti, S, Kinyanjui, S, Mwai, E, et al. Costing analysis of an SMS-based intervention to promote HIV self-testing amongst truckers and sex workers in Kenya. PLoS One. (2018) 13:e0197305. doi: 10.1371/journal.pone.0197305
21. De Boni, RB, Veloso, VG, Fernandes, NM, Lessa, F, Corrêa, RG, De Souza Lima, R, et al. An internet-based HIV self-testing program to increase HIV testing uptake among men who have sex with men in Brazil: descriptive cross-sectional analysis. J Med Internet Res. (2019) 21:e14145. doi: 10.2196/14145
22. da Cruz, MM, Cota, VL, Lentini, N, Bingham, T, Parent, G, Kanso, S, et al. Comprehensive approach to HIV/AIDS testing and linkage to treatment among men who have sex with men in Curitiba, Brazil. PLoS One. (2021) 16:e0249877. doi: 10.1371/journal.pone.0249877
24. Husereau, D, Drummond, M, Augustovski, F, de Bekker-Grob, E, Briggs, AH, Carswell, C, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Clin Ther. (2022) 44:158–68. doi: 10.1016/j.clinthera.2022.01.011
25. Stelzle, D, Godfrey-Faussett, P, Jia, C, Amiesimaka, O, Mahy, M, Castor, D, et al. Estimating HIV pre-exposure prophylaxis need and impact in Malawi, Mozambique and Zambia: a geospatial and risk-based analysis. PLoS Med. (2021) 18:e1003482. doi: 10.1371/journal.pmed.1003482
26. Luz, PM, Benzaken, A, Alencar, TM, Pimenta, C, Veloso, VG, and Grinsztejn, B. PrEP adopted by the Brazilian national health system: what is the size of the demand? Medicine. (2018) 97:S75–7. doi: 10.1097/MD.0000000000010602
27. Wyatt, MA, Pisarski, EE, Kriel, Y, Smith, PM, Mathenjwa, M, Jaggernath, M, et al. Influences on PrEP uptake and adherence among south African women during Periconception and pregnancy: a qualitative analysis. AIDS Behav. (2023) 27:208–17. doi: 10.1007/s10461-022-03757-8
28. Zhang, L, Peng, P, Wu, Y, Ma, X, Soe, NN, Huang, X, et al. Modelling the epidemiological impact and cost-effectiveness of PrEP for HIV transmission in MSM in China. AIDS Behav. (2019) 23:523–33. doi: 10.1007/s10461-018-2205-3
29. Sanchez Conde, M, Vivancos Gallego, MJ, and Moreno, GS. Pre-exposure prophylaxis (PrEP) against HIV: efficacy, safety and uncertainties. Farm Hosp. (2017) 41:630–7. Profilaxis preexposicion (PrEP) frente al VIH: eficacia, seguridad e incertidumbres. doi: 10.7399/fh.10821
30. Sun, S, Yang, C, Zaller, N, Zhang, Z, Zhang, H, and Operario, D. PrEP willingness and adherence self-efficacy among men who have sex with men with recent Condomless anal sex in urban China. AIDS Behav. (2021) 25:3482–93. doi: 10.1007/s10461-021-03274-0
31. Kelvin, EA, George, G, Mwai, E, Kinyanjui, S, Romo, ML, Odhiambo, JO, et al. A randomized controlled trial to increase HIV testing demand among female sex Workers in Kenya through Announcing the availability of HIV self-testing via text message. AIDS Behav. (2019) 23:116–25. doi: 10.1007/s10461-018-2248-5
32. Kelvin, EA, George, G, Mwai, E, Nyaga, EN, Mantell, JE, Romo, ML, et al. Offering self-administered Oral HIV testing as a choice to truck drivers in Kenya: predictors of uptake and need for guidance while self-testing. AIDS Behav. (2018) 22:580–92. doi: 10.1007/s10461-017-1783-9
33. Ministry of Health and Population. (2018). Malawi guidelines for clinical management of HIV in children and adults, 4th edition. Available at: https://www.differentiatedservicedelivery.org/Portals/0/adam/Content/yb4xSSLvE0SW98_z7wTm_w/File/Malawi%20Clinical%20HIV%20Guidelines%202018%20(1).pdf (Accessed September 24, 2018).
34. Department of Health SA. (2015). National Consolidated Guidelines: for the prevention of mother-to-child transmission of HIV and the management of HIV in children, adolescents and adults. Available at: https://sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf (Accessed September 24, 2022).
35. Department of Health B. (2018). Guidelines for the diagnosis of HIV Available at: http://www.aids.gov.br (Accessed September 24, 2021).
36. Rivera, AS, Hernandez, R, Mag-usara, R, Sy, KN, Ulitin, AR, O’Dwyer, LC, et al. Implementation outcomes of HIV self-testing in low- and middle- income countries: a scoping review. PLoS One. (2021) 16:e0250434. doi: 10.1371/journal.pone.0250434
37. Garrett, N, Norman, E, Leask, K, Naicker, N, Asari, V, Majola, N, et al. Acceptability of early antiretroviral therapy among south African women. AIDS Behav. (2018) 22:1018–24. doi: 10.1007/s10461-017-1729-2
38. Stevens, DR, Vrana, CJ, Dlin, RE, and Korte, JE. A global review of HIV self-testing: themes and implications. AIDS Behav. (2018) 22:497–512. doi: 10.1007/s10461-017-1707-8
39. UNAIDS. (2021). New HIV infections. Available at: https://aidsinfo.unaids.org. (Accessed July 4, 2021).
40. Zelin, J, Garrett, N, Saunders, J, Warburton, F, Anderson, J, Moir, K, et al. An evaluation of the performance of OraQuick® ADVANCE rapid HIV-1/2 test in a high-risk population attending genitourinary medicine clinics in East London, UK. Int J STD AIDS. (2008) 19:665–7. doi: 10.1258/ijsa.2008.008132
41. Technologies. O. OraQuick HIV self tests. (2021). Available at: (https://www.orasure.com/products-infectious/OraQuick-Self-Test.html).
42. Holguin, A, Gutierrez, M, Portocarrero, N, Rivas, P, and Baquero, M. Performance of OraQuick Advance rapid HIV-1/2 antibody test for detection of antibodies in oral fluid and serum/plasma in HIV-1+ subjects carrying different HIV-1 subtypes and recombinant variants. J Clin Virol. (2009) 45:150–2. doi: 10.1016/j.jcv.2009.04.002
43. O'Connell, RJ, Merritt, TM, Malia, JA, VanCott, TC, Dolan, MJ, Zahwa, H, et al. Performance of the OraQuick rapid antibody test for diagnosis of human immunodeficiency virus type 1 infection in patients with various levels of exposure to highly active antiretroviral therapy. J Clin Microbiol. (2003) 41:2153–5. doi: 10.1128/JCM.41.5.2153-2155.2003
44. Watson, V, Dacombe, RJ, Williams, C, Edwards, T, Adams, ER, Johnson, CC, et al. Re-reading of OraQuick HIV-1/2 rapid antibody test results: quality assurance implications for HIV self-testing programmes. J Int AIDS Soc. (2019) 22:e25234. doi: 10.1002/jia2.25234
45. Empringham, B, Karellis, A, Kashkary, A, D’Silva, O, Carmona, S, Suarez, MF, et al. How much does HIV self-testing cost in low and middle income countries? A systematic review of evidence from economic studies. Front Public Health. (2023) 11:1135425. doi: 10.3389/fpubh.2023.1135425
46. Pai, N, Esmail, A, Saha Chaudhuri, P, Oelofse, S, Pretorius, M, Marathe, G, et al. Impact of a personalised, digital, HIV self-testing app-based program on linkages and new infections in the township populations of South Africa. Health. (2021) 6, 1–12. doi: 10.1136/bmjgh-2021-006032
47. Orlando, S, Diamond, S, Palombi, L, Sundaram, M, Shear Zimmer, L, Marazzi, MC, et al. Cost-effectiveness and quality of Care of a Comprehensive ART program in Malawi. Medicine. (2016) 95:e3610. doi: 10.1097/MD.0000000000003610
48. Tagar, E, Sundaram, M, Condliffe, K, Matatiyo, B, Chimbwandira, F, Chilima, B, et al. Multi-country analysis of treatment costs for HIV/AIDS (MATCH): facility-level ART unit cost analysis in Ethiopia, Malawi, Rwanda, South Africa and Zambia. PLoS One. (2014) 9:e108304. doi: 10.1371/journal.pone.0108304
49. Fatti, G, Ngorima-Mabhena, N, Chirowa, F, Chirwa, B, Takarinda, K, Tafuma, TA, et al. The effectiveness and cost-effectiveness of 3- vs. 6-monthly dispensing of antiretroviral treatment (ART) for stable HIV patients in community ART-refill groups in Zimbabwe: study protocol for a pragmatic, cluster-randomized trial. Trials. (2018) 19:79. doi: 10.1186/s13063-018-2469-y
50. World Bank. (2021). Official exchange rate. Available at: (Accessed August 1, 2021https://data.worldbank.org/indicator/PA.NUS.FCRF).
51. World Bank. (2021). Inflation, consumer prices (%). Available at: (Accessed August 13, 2021https://data.worldbank.org/indicator/FP.CPI.TOTL.ZG).
52. Bank of Canada. (2021). Inflation calculator. Available at: (Accessed August 31, 2021https://www.bankofcanada.ca/rates/related/inflation-calculator/).
53. Diseases, GBD, and Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
54. Wu, J, Lai, T, Han, H, Liu, J, Wang, S, and Lyu, J. Global, regional and national disability-adjusted life years due to HIV from 1990 to 2019: findings from the global burden of disease study 2019. Trop Med Int Health. (2021) 26:610–20. doi: 10.1111/tmi.13565
55. Bertram, MY, Lauer, JA, Stenberg, K, and Edejer, TTT. Methods for the economic evaluation of health care interventions for priority setting in the health system: an update from WHO CHOICE. Int J Health Policy Manag. (2021). doi: 10.34172/ijhpm.2020.244
56. World Bank (2020). GDP per capita: current USD. Retrieved July 11, 2021 from https://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
57. Cambiano, V, Ford, D, Mabugu, T, Napierala Mavedzenge, S, Miners, A, Mugurungi, O, et al. Assessment of the potential impact and cost-effectiveness of self-testing for HIV in low-income countries. J Infect Dis. (2015) 212:570–7. doi: 10.1093/infdis/jiv040
58. Cambiano, V, Johnson, CC, Hatzold, K, Terris-Prestholt, F, Maheswaran, H, Thirumurthy, H, et al. The impact and cost-effectiveness of community-based HIV self-testing in sub-Saharan Africa: a health economic and modelling analysis. J Int AIDS Soc. (2019) 22:e25243. doi: 10.1002/jia2.25243
59. Bulterys, MA, Mujugira, A, Nakyanzi, A, Nampala, M, Taasi, G, Celum, C, et al. Costs of providing HIV self-test kits to pregnant women living with HIV for secondary distribution to male Partners in Uganda. Diagnostics. (2020) 10, 75–85. doi: 10.3390/diagnostics10050318
60. d’Elbée, M, Makhetha, MC, Jubilee, M, Taole, M, Nkomo, C, Machinda, A, et al. Using HIV self-testing to increase the affordability of community-based HIV testing services. AIDS. (2020) 34:2115–23. doi: 10.1097/QAD.0000000000002664
61. Kerr, L, Kendall, C, Guimarães, MDC, Salani Mota, R, Veras, MA, Dourado, I, et al. HIV prevalence among men who have sex with men in Brazil: results of the 2nd national survey using respondent-driven sampling. Medicine (Baltimore). (2018) 97:S9–S15. doi: 10.1097/MD.0000000000010573
62. Teixeira, SLM, Jalil, CM, Jalil, EM, Nazer, SC, Silva, SCC, Veloso, VG, et al. Evidence of an untamed HIV epidemic among MSM and TGW in Rio de Janeiro, Brazil: a 2018 to 2020 cross-sectional study using recent infection testing. J Int AIDS Soc. (2021) 24:e25743. doi: 10.1002/jia2.25743
63. Njau, B, Damian, DJ, Abdullahi, L, Boulle, A, and Mathews, C. The effects of HIV self-testing on the uptake of HIV testing, linkage to antiretroviral treatment and social harms among adults in Africa: a systematic review and meta-analysis. PLoS One. (2021) 16:e0245498. doi: 10.1371/journal.pone.0245498
64. Maheswaran, H, Petrou, S, MacPherson, P, Choko, AT, Kumwenda, F, Lalloo, DG, et al. Cost and quality of life analysis of HIV self-testing and facility-based HIV testing and counselling in Blantyre, Malawi. Medicine. (2016) 14:34. doi: 10.1186/s12916-016-0577-7
65. Sobrino-Vegas, P, Miguel, LGS, Caro- Murillo, AM, Miro, JM, Viciana, P, Tural, C, et al. Delayed diagnosis of HIV infection in a multicenter cohort: prevalence, risk factors, response to HAART and impact on mortality. Curr HIV Res. (2009) 7:224–30. doi: 10.2174/157016209787581535
66. Iino, H, Hashiguchi, M, and Hori, S. Estimating the range of incremental cost-effectiveness thresholds for healthcare based on willingness to pay and GDP per capita: a systematic review. PLoS One. (2022) 17:e0266934. doi: 10.1371/journal.pone.0266934
67. McDougall, JA, Furnback, WE, Wang, BCM, and Mahlich, J. Understanding the global measurement of willingness to pay in health. J. Mark Access Health Policy. (2020) 8:1717030. doi: 10.1080/20016689.2020.1717030
68. Veronese, V, Ryan, KE, Hughes, C, Lim, MS, Pedrana, A, and Stoove, M. Using digital communication technology to increase HIV testing among men who have sex with men and transgender women: systematic review and Meta-analysis. J Med Internet Res. (2020) 22:e14230. doi: 10.2196/14230
69. Krause, J, Subklew-Sehume, F, Kenyon, C, and Colebunders, R. Acceptability of HIV self-testing: a systematic literature review. BMC Public Health. (2013) 13:735. doi: 10.1186/1471-2458-13-735
70. World Bank Data Group: Data Gender Portal. Death rate, crude. (2022) Available at: https://genderdata.worldbank.org/en/indicator/sp-dyn-cdrt-in
71. Mwenge, L, Sande, L, Mangenah, C, Ahmed, N, Kanema, S, d’Elbée, M, et al. Costs of facility-based HIV testing in Malawi, Zambia and Zimbabwe. PLoS One. (2017) 12:e0185740. doi: 10.1371/journal.pone.0185740
72. Burke, RM, Henrion, MYR, Mallewa, J, Masamba, L, Kalua, T, Khundi, ME, et al. Incidence of HIV-positive admission and inpatient mortality in Malawi [2012-2019]: a population cohort study. AIDS. (2021) 35:2191–9. doi: 10.1097/QAD.0000000000003006
73. Grinberg, G, Giron, LB, Knoll, RK, Galinskas, J, Camargo, M, Arif, MS, et al. High prevalence and incidence of HIV-1 in a counseling and testing center in the city of Itajaí, Brazil. Brazil. J. Infect. Dis. (2015) 19:631–5. doi: 10.1016/j.bjid.2015.08.001
74. Melo, MC, Almeida, VC, and Donalisio, MR. Trend incidence of HIV-AIDS according to different diagnostic criteria in Campinas-SP, Brazil from 1980 to 2016. Ciênc Saúde Colet. (2021) 26:297–307. Tendencia da incidencia de HIV-aids segundo diferentes criterios diagnosticos em Campinas-SP, Brasil de 1980 a 2016. doi: 10.1590/1413-81232020261.08652019
75. Akullian, A, Vandormael, A, Miller, JC, Bershteyn, A, Wenger, E, Cuadros, D, et al. Large age shifts in HIV-1 incidence patterns in KwaZulu-Natal, South Africa. Proc Natl Acad Sci USA. (2021) 118, 126–134. doi: 10.1073/pnas.2013164118
76. Bärnighausen, T, Wallrauch, C, Welte, A, McWalter, TA, Mbizana, N, Viljoen, J, et al. HIV incidence in rural South Africa: comparison of estimates from longitudinal surveillance and cross-sectional cBED assay testing. PLoS One. (2008) 3:e3640. doi: 10.1371/journal.pone.0003640
77. Pettifor, A, Lippman, SA, Kimaru, L, Haber, N, Mayakayaka, Z, Selin, A, et al. HIV self-testing among young women in rural South Africa: a randomized controlled trial comparing clinic-based HIV testing to the choice of either clinic testing or HIV self-testing with secondary distribution to peers and partners. EClinicalMedicine. (2020) 21:100327. doi: 10.1016/j.eclinm.2020.100327
78. Hatzold, K, Gudukeya, S, Mutseta, MN, Chilongosi, R, Nalubamba, M, Nkhoma, C, et al. HIV self-testing: breaking the barriers to uptake of testing among men and adolescents in sub-Saharan Africa, experiences from STAR demonstration projects in Malawi, Zambia and Zimbabwe. J Int AIDS Soc. (2019) 22:e25244. doi: 10.1002/jia2.25244
79. Ministerio de saude Brazil. Relatorio de moniotramento clinico do HIV. Relatorio de moniotramento clinico do HIV. (2017). Available at: https://antigo.aids.gov.br/pt-br/pub/2017/relatorio-de-monitoramento-clinico-do-hiv
Keywords: HIV, Markov model, self-testing, health economics, digital-based intervention
Citation: Empringham B, Karellis A, Fernandez-Suarez M, Carmona S, Pai NP and Zwerling A (2025) Understanding the cost-utility of implementing HIV self-testing with digital-based supports. Front. Public Health. 12:1440104. doi: 10.3389/fpubh.2024.1440104
Edited by:
Kyriakos Souliotis, University of Peloponnese, GreeceReviewed by:
Theresa Hoke, Family Health International 360, United StatesSamson Malwa Haumba, Georgetown University Medical Center, United States
Copyright © 2025 Empringham, Karellis, Fernandez-Suarez, Carmona, Pai and Zwerling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brianna Empringham, YmVtcHJpbmdoYW1AY2hlby5vbi5jYQ==
 Brianna Empringham
Brianna Empringham Angela Karellis
Angela Karellis Marta Fernandez-Suarez4
Marta Fernandez-Suarez4 Nitika Pant Pai
Nitika Pant Pai