- 1Department of Pediatrics, the First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, Fujian, China
- 2Department of Pediatrics, Pediatric Key Laboratory of Xiamen, the First Affiliated Hospital of Xiamen University, Xiamen, Fujian, China
- 3Institute for Clinical Medical Research, the First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, Fujian, China
Background: To counteract the COVID-19 pandemic, nonpharmaceutical interventions (NPIs) were implemented globally, exerting a profound influence on a wide spectrum of infectious diseases, encompassing respiratory tract infections (RTIs). Subsequent to the easing of NPIs, China experienced a significant outbreak of Mycoplasma pneumoniae (MP).
Methods: Over a decade from 2015 to 2024, our study scrutinized 12 common infectious diseases among pediatric children. Etiologically diagnostic data and clinical outcome metrics of children with RTIs, tested for 13 pathogens, were analyzed to evaluate changes during and after the pandemic compared to pre-pandemic periods, with a notable emphasis on age profile and coinfection patterns of MP.
Results: Among 57,471 hospitalized children, 23,178 were diagnosed with infectious diseases. Under NPIs, most respiratory infections declined compared to pre-pandemic levels, rebounding by 69.64% in 2023. While the infection rate of common respiratory pathogens decreased, cases of respiratory syncytial virus increased during the period of extensive NPI implementation. In 2023, pediatric intensive care unit durations for these pathogens increased, suggesting greater severity of illness compared to 2019. MP exhibited the highest infection rate (31.38% average), with a notable outbreak post-pandemic due to severity increase in <3 year olds and rise among older children. NPIs reduced MP coinfections and mitigated their severity, while exerting a significant influence on bacterial coinfections with MP over the span of 5 years, in contrast to their impact on viral pathogens.
Conclusion: NPIs effectively curb transmission of respiratory infections by most pathogens, resulting in increased average age of MP infections and altered patterns of coinfection post-pandemic.
Introduction
In early 2020, China implemented a wide range of public health and social measures (such as movement restrictions, mask mandates, social and physical distancing, environmental cleaning and disinfection) in response to the COVID-19 pandemic, which is also known as nonpharmaceutical interventions (NPIs). These measures not only successfully contained the spread of syndrome coronavirus 2 (SARS-CoV-2) but also significantly decreased the incidence of various community-acquired infectious diseases, including respiratory tract infection (RTI) which is primarily transmitted through aerial droplets, aerosols, and direct contacts (1–3).
RTIs may induce uncontrolled systemic inflammatory responses, leading to severe complications such as septic shock, acute respiratory distress syndrome, pneumonia, encephalitis, and multiple organ failure. They represent one of the primary causes of hospitalization and mortality in children. According to research statistics, in the year 2000 alone, approximately 1.9 million children globally died from RTIs, thereby imposing a significant burden on both families and society (4–6). Respiratory pathogens causing RTI primarily include respiratory syncytial virus (RSV), human adenovirus (HAdV), human rhinoviruses (HRV), influenza virus (IFV), Bordetella pertussis (causative agent of pertussis), Haemophilus influenzae (HI), and Streptococcus pneumoniae (SP). After NPI implementation during the COVID-19 pandemic, respiratory infections significantly declined, but their resurgence in children post-NPI withdrawal exceeded historical levels (7).
Among these pathogens, there has been also a resurgence of Mycoplasma pneumoniae (MP) in multiple countries occurring thereafter, especially China, triggering large-scale non-seasonal outbreaks (8–11). It serves as one of the most prevalent causative agents of community-acquired pneumonia in children, particularly infants and young children because of the lack of cross-protective antibodies generated from previous infectious diseases and high resistance to macrolide antibiotics (12, 13). Previous research has confirmed that MP can coinfect with other pathogens and the emergence of COVID-19 highlighted the severity and complexity of diseases caused by viral-bacterial coinfections again (14–16). However, existing studies have primarily focused on single-pathogen detection, with relatively few investigations into the mixed infection patterns dominated by MP in RTIs. Additionally, there is a lack of focus regarding the age-specific characteristics of MP infections in children before and after the implementation of NPIs.
Against the backdrop of a large-scale outbreak of MP in Chinese children post the COVID-19 pandemic, this work aims at investigating the overall infection situation among children (≤ 14 years old) hospitalized due to community-acquired infections in Xiamen, Fujian Province, China. We examined the epidemiologic characteristics of infectious diseases, especially RTIs from January 2015 to January 2024, and profile the changes of MP infection as a whole as well as by age group. Besides, we characterized its coinfection patterns with other respiratory pathogens. These findings are informative about the broad impact of NPIs post COVID-19 pandemic, especially on MP infection patterns, guiding the control of respiratory pathogens among children.
Methods
Study design and patient enrollment
A retrospective cohort study was conducted using data from the First Affiliated Hospital of Xiamen University. The First Affiliated Hospital of Xiamen University, the largest tertiary Grade-A comprehensive hospital in southwestern Fujian Province, serves the majority of children residing in the region.
This study enrolled all pediatric inpatients with infectious disease admitted from January 2015 to January 2024. To analyze trends in traditional respiratory pathogens, children infected with SARS-CoV-2 were excluded from the study. Diagnostics were recorded by each pediatrician using the International Classification of Disease 10th edition, which were detailed in Supplementary Table 1. Patients were divided into three groups according to their ages: 0–3 years, 3–6 years, and 6–14 years. This study followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.
Study design and patient enrollment
Data were collected through review of medical records, including demographic information, timeline of symptom onset, hospitalization and pediatric intensive care unit (PICU) admission (if applicable), clinical presentations, laboratory test results, microbiological findings, and clinical outcomes. Trained clinical physicians inputted the standardized database.
To ensure the reliability of pathogen detection, all samples were meticulously collected by specialized nurses within 48 h of patient admission, followed by testing within 4–6 h of collection, while being stored in a controlled environment at 4°C throughout the process. Blood and sputum bacterial culture tests were used to detect SP, HI, Staphylococcus aureus (SA), Klebsiella pneumoniae (KLP), Pseudomonas aeruginosa (PA), Legionella pneumophila (LP), Branhamella Catarrhalis, Pertussis, and Acinetobacter baumannii. Urine can be utilized for the detection of bacteria such as Escherichia coli, KLP, PA, as well as the antigen of SP. Additionally, oral pharyngeal swabs were collected for viral pathogen detection, with total nucleic acids (both DNA and RNA) being extracted from these specimens. Subsequently, RT-PCR assays were conducted to identify IFV, RSV, human parainfluenza virus (HPIV), human metapneumovirus (HMPV), seasonal human coronavirus (HCoV), and HRV, while PCR assays were used to detect HAdV, human bocavirus (HBoV), MP, and Chlamydia. All tests followed the standard operating procedures established by the Chinese Center for Disease Control and Prevention, utilizing commercially available, mature pathogen detection kits (Shengxiang Biotechnology, Changsha, China) tailored for specific pathogens. Nucleic acid extraction and PCR/RT-PCR testing were performed in parallel with both positive and negative controls, and rigorous quality control measures were implemented. Each positive result was reviewed by at least one infectious disease physician. If testing positive for more than one pathogen, mixed infection is determined. We referred to cases that test positive for more than one pathogen as mixed infection.
Infection rate
To better characterize the infection condition of various pathogens, we defined the infection rate (IR) as the number of cases infected by a specific pathogen divided by the total number of respiratory infections in that year.
Regional interventions in Xiamen
Since January 26, 2020, Xiamen region initiated the promotion of NPIs to reduce the transmission of SARS-CoV-2. In June 2020, enhanced medical observation was implemented for incoming individuals to Xiamen. Subsequently, high-traffic areas such as entertainment venues, parks, and tourist attractions were gradually closed to curb the spread. Areas with confirmed COVID-19 cases were placed under lockdown. Notably, starting from March 21, 2020, school offline teaching activities were intermittently suspended based on the epidemic trend. Stringent epidemic containment policies were enforced from September 18, 2021, to August 25, 2022. Xiamen region lifted the epidemic containment policies on August 26, 2022.
Data management and statistical analysis
All data are extracted from electronic medical records from January 2015 to January 2024, and the number of participants is counted bi-weekly. Descriptive statistics include the frequency (proportion) of categorical variables and the interquartile range (IQR) and median of continuous variables. To evaluate the impact of NPIs, we calculated the frequency and percentage of infectious diseases and pathogen diagnoses from January 2019 to January 2024. Using the 2019 data as the baseline, we quantified the changes from 2020 to January 2024 using a binary logistic regression model to analyze the trends. Statistical analysis was performed using STATA 15 (StataCorp 2015, College Station, TX) and R 4.3.2, with the significance level for assessing the relationships between variables set at p value <0.05.
Results
2015–2024 changes of pediatric infectious diseases
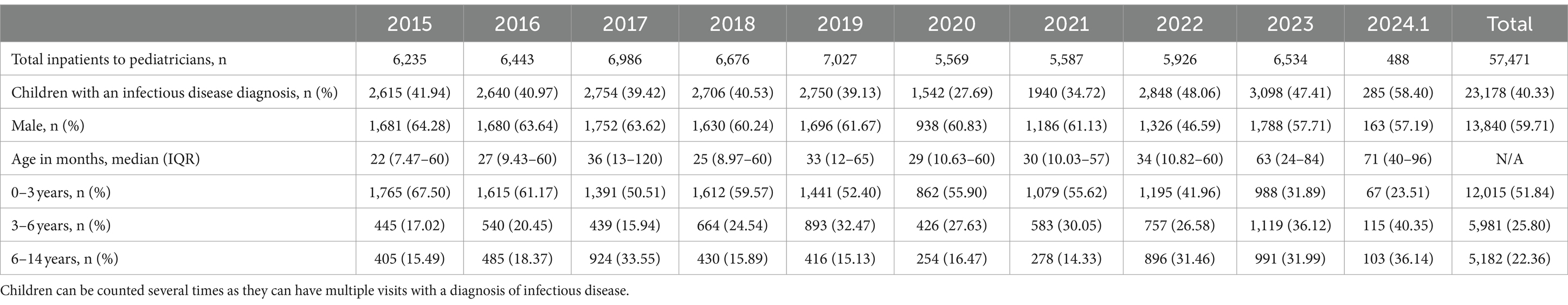
This retrospective cohort study, conducted at the First Affiliated Hospital of Xiamen University, encompassed pediatric inpatients (≤ 14 years old) admitted from January 2015 to January 2024, excluding cases of COVID-19 infection (Table 1). The majority of patients hailed from southwestern Fujian Province, where the hospital predominantly serves. Out of 57,471 children, there were 23,178 diagnosed cases of infection, with males accounting for 13,840 cases (59.71%).
Compared to 2015–2019, the number of annual infectious patients reported in 2020–2022 decreased by 583 (21.65%). Particularly, in 2020, the proportion of children diagnosed as community-acquired infections dropped sharply to no more than 30% of all those admitted. Notably, the number of cases crept up with the easing of the NPIs. Besides, a noticeable shift in the age distribution of affected children has been observed after the outbreak of COVID-19. The age group with the highest number of cases transitioned from 0 to 3 years old before the pandemic to 3–6 years old afterward (Table 1).
Table 2 presents that in 2020, almost all RTIs monitored, except for non-annual epidemic infections such as influenza-like-illness and bronchitis, showed a downward trend compared to 2015–2019 (OR = 0.62, p < 0.0001). As the largest proportion of respiratory infections, the number of pneumonia case declined from 54.29% for 2015 to 2019 to 51.44% immediately after the implementation of NPIs in the end of January 2020 (OR = 0.892, p = 0.019), then surged sharply toward the end of 2022 after the cessation of NPIs, with the number of patients markedly exceeding those in previous years, reaching 69.64% in 2023 (OR = 1.913, p < 0.0001; Table 2; Figures 1A,B). In addition, the incidence rates have increased for certain non-RTIs such as viral enteritis and urinary tract infections (Table 2, OR from 1.77 to 3.02, p < 0.0001). However, overall, there is not a substantial difference in the number of non-respiratory infections before and after the lockdown (Figure 1C). Over the past decade, the seasonal pattern of enterovirus infections has remained consistent, but during the pandemic and post-pandemic period, there has been a reduction in the peak number of cases during the summer months, with the autumn peak appearing later than usual (Figure 1D). Bronchitis and urinary tract infections have shown high incidence rates, with occasional peaks (Supplementary Figure 1), but overall, there is not a substantial seasonal difference during the pandemic and post-pandemic period compared to the years 2015–2019.
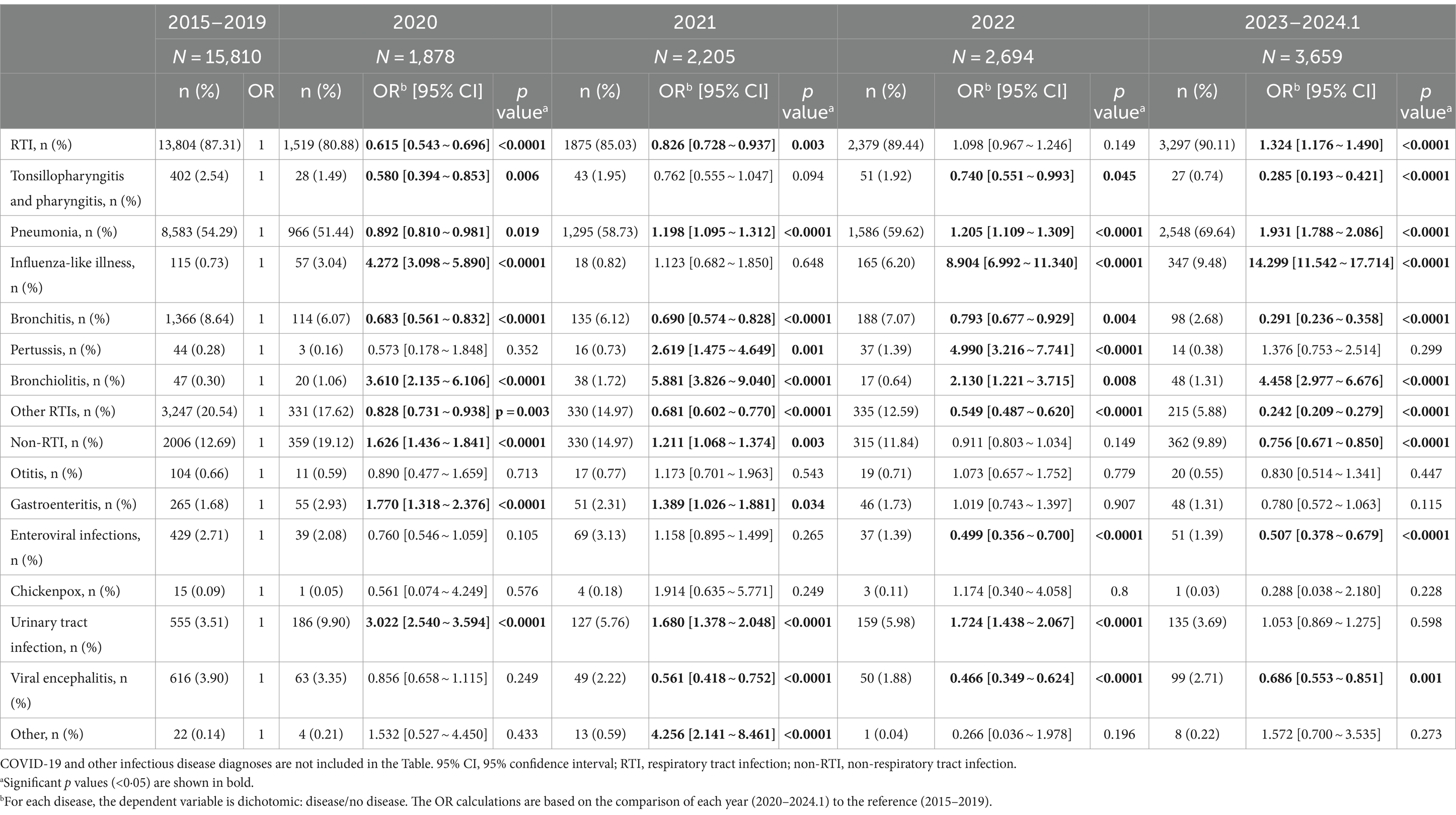
Table 2. The annual frequencies and percentages of infectious disease from 2020 to January 2024 compared to the 2015–2019 period.
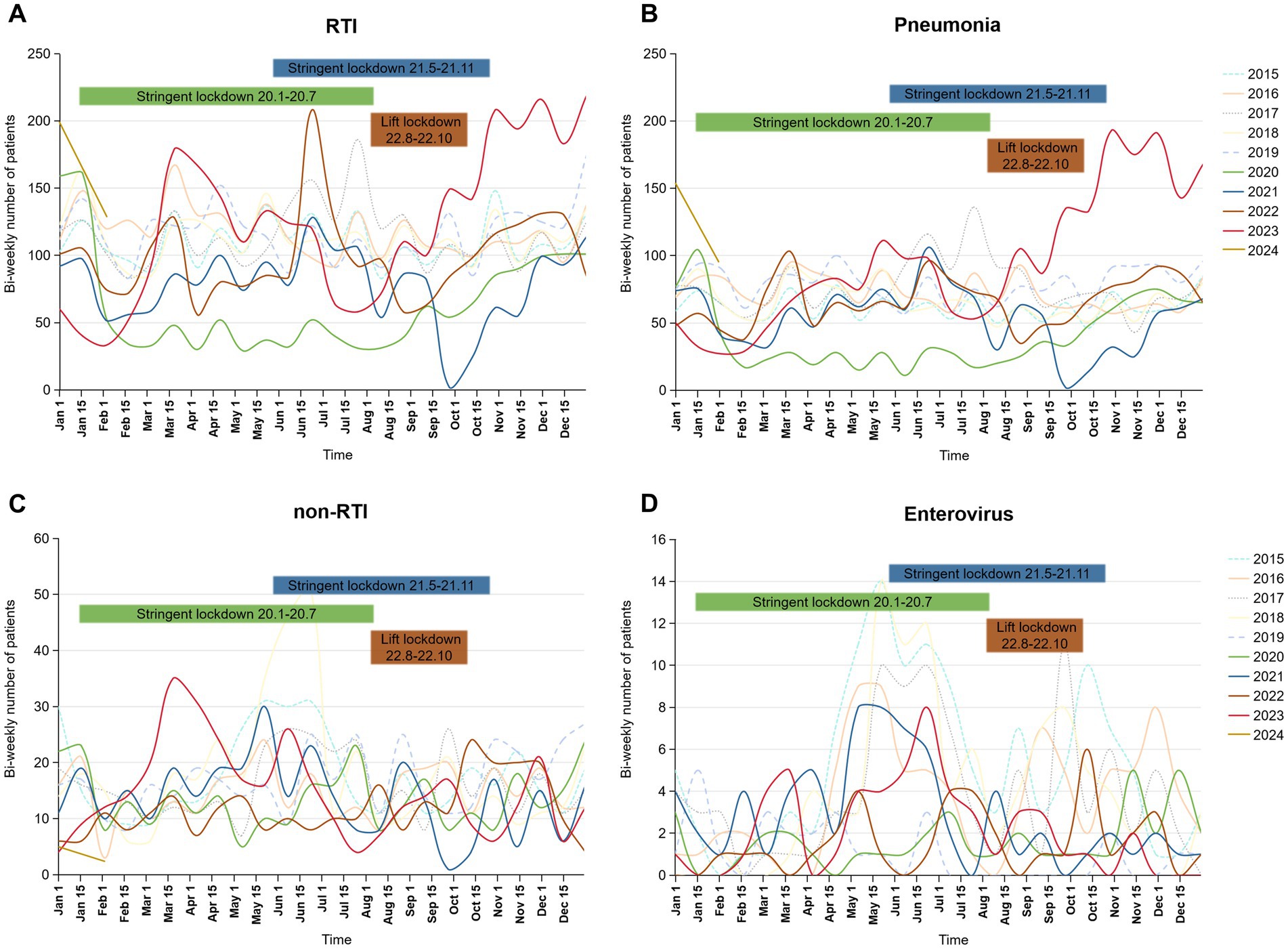
Figure 1. Bi-weekly number of RTI, pneumonia, non-RTI and enteroviral infections during January 2015 to January 2024 in the pediatric inpatient case database. (A) RTI, respiratory infection; (B) pneumonia; (C) non-RTI, non-respiratory tract infection; (D) enteroviral infections. The bars labeled “Stringent Lockdown” correspond to the years indicated by their colors: green for 2020 and blue for 2021. The bars labeled “Lift Lockdown” are represented in brown for 2022.
Our research findings indicate that the frequency and proportion of respiratory infections among hospitalized children has been weakened by the implementation of NPIs. Following the withdrawal, there was a significant rebound in the number and proportion of respiratory infections, with pneumonia as the representative condition, compared to pre-pandemic levels. Consequently, we conducted further analysis of respiratory infections.
Trends of respiratory pathogens
In 2019, the hospital saw 1944 patients with respiratory pathogen infections. During the 2020 pandemic, only 934 cases were reported, rising to 2031 post-NPI lifting in 2023. Compared to 2019, significant epidemiological variations in the prevalence of common respiratory pathogens were observed during and after the COVID-19 pandemic. Notably, MP, a major pathogen, constituted about one-third of cases yearly, peaking at 35.79% in 2019 (Supplementary Table 2). While bacterial pathogens had low and stable detection rates, SP and HI saw notable outbreaks post-pandemic (p < 0.0001). Viral pathogen fluctuation was observed, with a decrease during the pandemic and a recovery afterward, with RSV cases surging post-pandemic.
Six pathogens were selected with the highest number of IRs. During the pandemic, NPIs reduced the IR of these pathogens, rebounding post-NPI lifting, surpassing pre-pandemic levels. MP had the highest IR at the average of 31.38%, with SP and HI seeing significant increases post-NPI measures in 2023, reaching 138 and 246% of the annual averages, respectively (Figure 2A). The prevalence of MP infections exhibited multiple peaks annually, with stable monthly cases around 140 in the latter half of 2019 (Figure 2B). In 2023, there was a significant surge in infections, reaching as high as 285 cases in November. Similar seasonal patterns were observed for SP, HI, and HAdV (Figures 2B,C), characterized by low IR before and during the initial stages of the pandemic, followed by a rapid increase post-lifting of NPIs. RSV transmission displayed clear winter peaks, persisting until the following March–April period. Notably, the peak of RSV infections in 2020 significantly declined, but following the peaks in 2023 advanced to the summer months, while HRV transmission persisted year-round, with prolonged period during the later stages of the pandemic (Figure 2C).
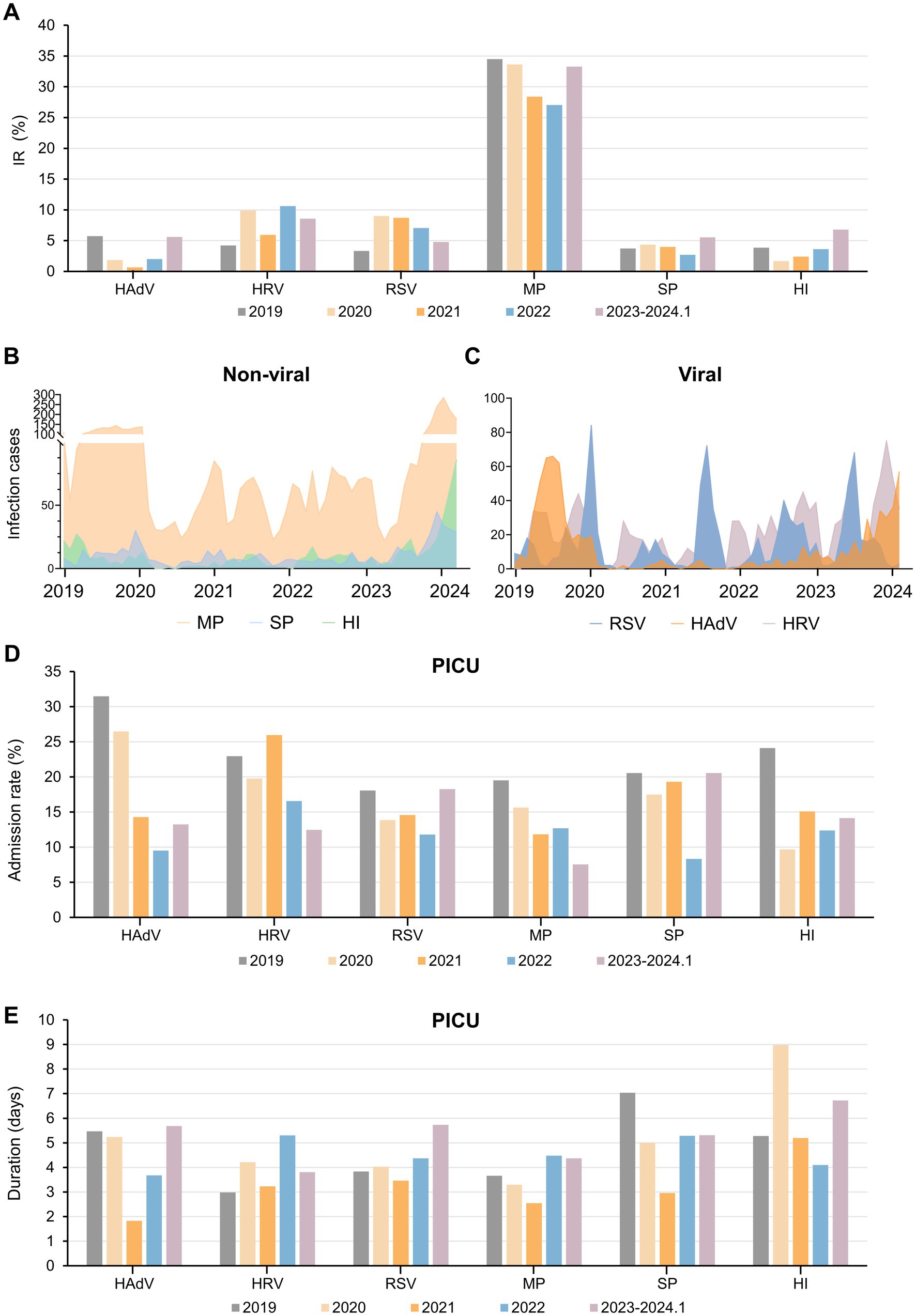
Figure 2. Overview of the IR, infection cases and PICU admissions of six respiratory pathogens during the past 5 years. (A) IR of six important respiratory pathogens pre-, during and post-epidemic. (B,C) Infection cases of several non-viral (B) and viral (C) respiratory pathogens before and after the epidemic. Admission rate to the PICU (D) and duration of PICU (E) for each type of pathogen-infected patients. Abbreviations: IR, infection rate; PICU, Pediatric Intensive Care Unit; HAdV, human adenovirus; HRV, human rhinoviruses; RSV, respiratory syncytial virus; MP, Mycoplasma pneumoniae; SP, Streptococcus pneumoniae; HI, Haemophilus influenzae.
Consistent with the significant reduction in the number and IR of various pathogens, the pandemic led to reduced PICU admission rates among infected children (Figure 2D). From 2019 to 2024, the duration of PICU duration for pediatric patients exhibited an overall decreasing trend, followed by an increase, except for HI (Figure 2E). Despite MP’s highest IR, PICU admission rates were initially low, with short total hospital stay compares to other pathogens (Figure 2D; Supplementary Figure 2). However, post-pandemic, the duration of PICU stay for MP patients increased, indicating the occurrence of more severe respiratory conditions (Figure 2E).
Age profiles of Mycoplasma pneumoniae infection
Our study revealed the age distribution of MP infections among children (Figure 3). The age distribution of MP infections remained stable till the withdrawal of NPIs. Preschool children (aged 1 month to 6 years) were at higher risk of respiratory diseases caused by MP. Following NPIs relaxation, there was a notable decrease in infections among infants aged 1 month to 3 years (from 35 to 15%), accompanied by a significant increase among children aged 6 to 14 years (from 20 to 40%; Figure 3A).
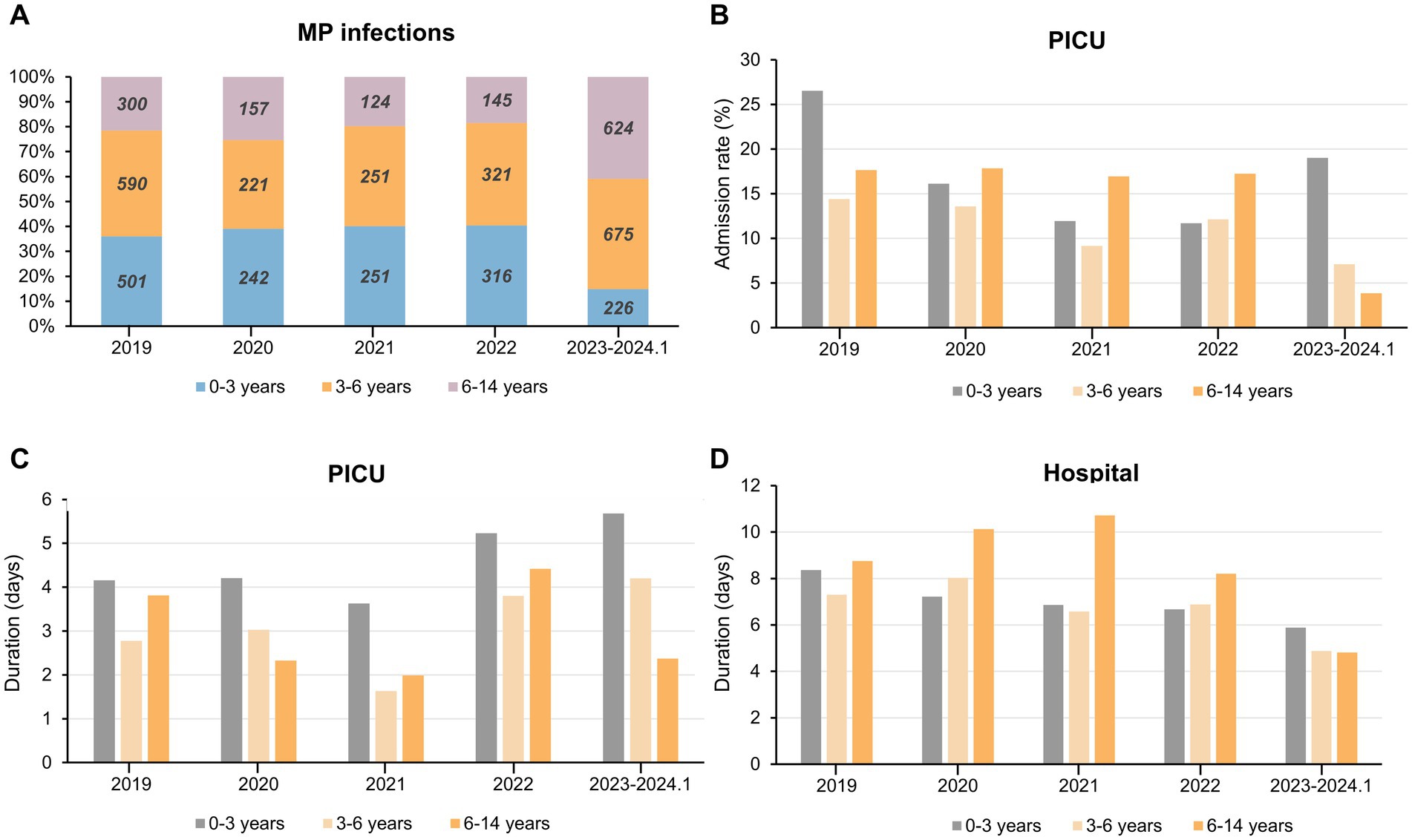
Figure 3. Overview of MP infection and PICU admission among children in different age groups during the past 5 years. (A) Yearly proportion and cases of children infected with MP in different age groups. (B–D) The admission rate to PICU (B) duration of PICU (C) and duration of total hospital stay (D) for children of different age groups of MP infection. Abbreviations: MP, Mycoplasma pneumoniae; PICU, Pediatric Intensive Care Unit.
Infants aged 1 month to 3 years exhibited the highest PICU admission rate and longest PICU duration both pre- and post-pandemic compared to other age groups (Figures 3B,C). For children aged 3 to 6 years, these two metrics along with total duration of hospitalization gradually declined during NPIs. Interestingly, for patients aged 6 years and older, there was a swift decline in these three metrics following the cessation of NPIs in 2023 (Figures 3B–D). In children afflicted with respiratory illnesses stemming from MP infection, the PICU duration showed a nadir in 2021 and peaked in 2023. Among children aged 6 and below, there was an increment of approximately 1.3 days compared to 2019 and 2.1 days compared to 2021 (Figure 3C). During the period of NPIs, the average duration of hospitalization for children aged 6–14 years increased by approximately 2 day and experienced a rapid decline in 2023 (Figure 3D). In summary, after the pandemic, children under 3 experienced higher severity of MP infections following the withdrawal of NPIs, evident in longer PICU duration as well as MP infections being more prevalent among older children.
Changes of coinfection pattern with Mycoplasma pneumoniae
Significant rates of coinfection involving MP with other bacteria and viruses have been reported, leading to severe illnesses (17). Therefore, we conducted an investigation into cases of coinfection involving MP and other pathogens (Figure 4). From January 2019 to January 2024, the number and proportion of mixed infections involving MP showed a decreasing trend followed by an increase. In 2021, there were only 176 cases of mixed infections, accounting for 28.11% of all cases. However, from 2023 to January 2024, the number of mixed infection cases peaked at 719, accounting for 43.6%, resembling the levels observed in 2019 (Figure 4A). In 2019 and 2023, the predominant coinfecting pathogens with MP were HRV and HAdV. Conversely, during the pandemic, RSV coinfection was most prevalent. Notably, in 2019, HRV and SP were the most common coinfecting agents; in 2020 and 2021, RSV and SP prevailed; in 2022, RSV and IFV were prominent; and in 2023, IFV and HAdV were predominant (Figures 4B–F).
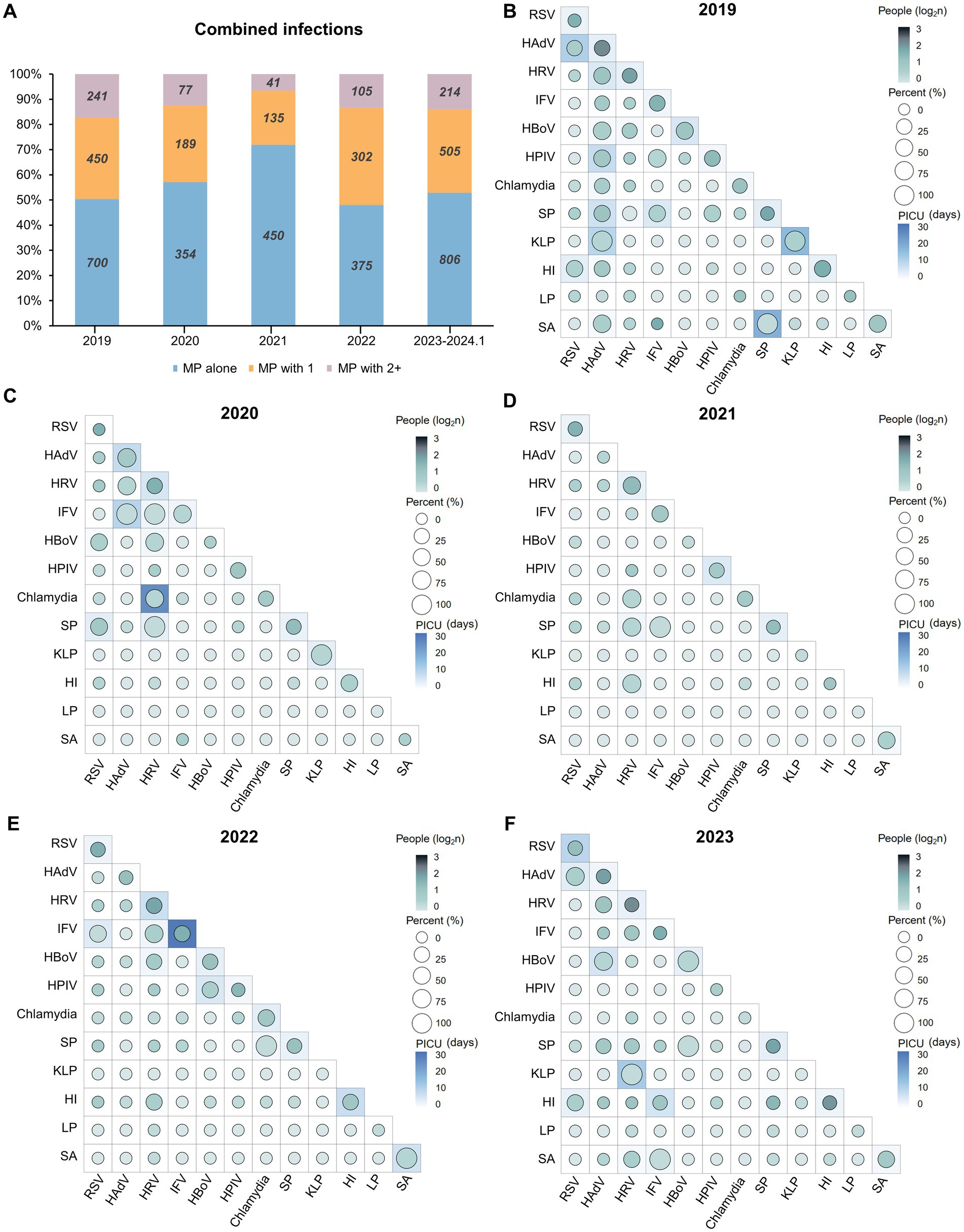
Figure 4. Landscape of mixed infections involving MP. (A) The proportion and cases of combined infections involving MP and multiple pathogens. (B–F) The association between the types of mixed infections involving MP and the PICU admission rate and duration of PICU from 2019 to January 2024 (Data from 2023 and January 2024 were consolidated). Each square represents the presence of additional pathogens in patients infected with MP; for example, “RSV alone (top left)” indicates mixed infection with MP and RSV. The circle color denotes the count of individuals coinfected with MP, with the number undergoing a log2 transformation to enhance differentiation. The size of the circles and the background color of the squares indicates the PICU admission rate (%) and the PICU duration (days) resulting from coinfections of one or both pathogens with MP, respectively. Abbreviations: MP, Mycoplasma pneumoniae; PICU, Pediatric Intensive Care Unit; RSV, respiratory syncytial virus; HAdV, human adenovirus; HRV, human rhinoviruses; IFV, influenza virus; HBoV, human bocavirus; SP, Streptococcus pneumoniae; KLP, Klebsiella pneumoniae; HI, Haemophilus influenzae; LP, Legionella pneumophila; SA, Staphylococcus aureus.
Before the pandemic, more severe cases of mixed infections (longer PICU duration) often occurred due to coinfection of MP alongside one or two other bacterial pathogens, such as SP or KLP, resulting in a PICU hospital stay of up to 17.65 days, associated with a PICU admission rate exceeding 50% (Figure 4B). The year 2022 was particularly notable, as the outbreak of influenza led to cases of IFV coinfection, which were more severe compared to previous years, with some cases even requiring a PICU stay of up to 24.04 days (totaling 52 cases) (Figure 4E). Aligned with the notable surge in the IRs of SP and HI, in 2023 and January 2024 (Figure 2), the count of patients coinfected with MP & HI surpassed 100. Likewise, the number of patients with concurrent infections of MP & SP & HI reached 30. However, their PICU hospital stays ranged merely from 1 to 3 days (Figure 4F). The analysis suggests that the implementation of NPIs has not only effectively reduced the number of cases of MP coinfections, but also alleviated their severity, despite the occurrence of special pathogen outbreaks each year. Bacterial coinfections with MP, compared to viral pathogens, were markedly affected by NPIs during 5 years.
Discussion
Our study stands as the sole investigation focusing on the trends of pediatric infectious diseases in the southeastern part of Fujian Province over a span of 10 years (2015–2024). This research not only delved into the impact of NPIs on the transmission patterns of 13 common respiratory pathogens but also placed emphasis on MP. We analyzed its age distribution characteristics as well as both dual and triple coinfections revolving around MP.
China’s implementation of NPIs resulted in a significant decrease in pediatric RTIs, including pneumonia, bronchitis, and tonsillitis, in Xiamen City since January 2020. However, the lifting of these measures led to a rapid surge in RTIs, particularly pneumonia. Conversely, the incidence of non-RTIs remained relatively stable throughout the pandemic, underscoring the effectiveness of NPI policies in mitigating respiratory diseases transmitted via droplets and reducing disease burden (18–20). Besides severe restrictions on public movement and limited access to hospitals, NPIs, such as mask-wearing, home isolation, and enhanced surface cleaning and disinfection were instrumental in curbing disease transmission by interrupting transmission chains and reducing the pathogen load in the environment, thereby achieving effective disease control.
Over the 10-year period, with technological advancements, hospitals progressively optimized pathogen detection methods. Our observation of the overall trends in respiratory pathogens revealed that despite the presence of similar seasonal distribution patterns before and after the COVID-19 pandemic, the IR of common bacteria and most viruses saw a significant increase post-pandemic, surpassing pre-pandemic levels. Of note, although RSV admission rate to the PICU decreased during the pandemic period, the IR increased. This could be attributed to the relatively reduced IR of other pathogens or suggest that RSV derived limited benefit from NPIs. Conclusions from researches on the epidemiological characteristics of RSV before and after the COVID-19 pandemic differ between China and other countries. While most foreign studies (21–23) indicate that NPI implementation effectively curbs RSV transmission, the majority of Chinese researchers (24–26) believe RSV continues to be highly transmissible during the pandemic. This discrepancy may be attributed to the prolonged survival of RSV in hosts and its strong capacity for rapid transmission, or alterations in RSV spectrum due to preventive measures against COVID-19. In addition, due to the special climate of Xiamen, RSV outbreaks were concentrated in summer, which was inconsistent with the period when the NPIs were most strictly implemented in 2020, this may also explain the differences in the transmission.
MP, as a prokaryotic organism without cell walls and unable to self-replicate, is one of the clinically significant smallest pathogens in children and a major causative agent of community-acquired pneumonia (27, 28). Our work indicates that MP had the highest IR both before and after the pandemic, with an average of 43.08%. Although its overall numbers of infection decreased significantly during the pandemic, along with reduced PICU admission rates and durations, it remains the most prevalent of all respiratory tract pathogens. Moreover, it re-emerged and led to another outbreak after the pandemic. And as a result, the PICU duration soared, indicating a more severe respiratory diseases. These findings are consistent with previous studies (29–32), reaffirming the effectiveness of NPI implementation in preventing respiratory tract infections. Additionally, we observed a higher IR among preschool children in previous studies, whereas after the end of the pandemic, IR increased among school-aged children, possibly due to susceptible individuals being protected from infection by NPIs, leading to a higher likelihood of infection after their cessation, resulting in an overall increase in the average age of patients.
However, it is worth noting that although there was a resurgence of MP infections following the end of the pandemic, the study results showed a decrease in both the PICU admission rate and the average length of hospital stay for children aged 3 and above. This could be attributed to the severity of MP infections in 2023, which were characterized by extensive pulmonary involvement, resulting in a higher number of hospital admissions being required. Due to limited pediatric ward capacity, efforts were made to promptly treat more patients by increasing hospital bed capacity, providing early treatment guidance to all patients during outpatient visits, aiming to shorten the length of hospital stay, increase bed turnover rate, and facilitate early discharge with outpatient follow-up visits. In contrast, children under 3 years old often exhibit severe symptoms such as dyspnea, respiratory distress, and septicemia, necessitating admission to the PICU for advanced respiratory support and treatment.
Furthermore, we found a high likelihood of MP mixed infections, primarily with viruses, which significantly decreased in 2021, followed by a peak in cases in 2023. Studies (17) suggest that apart from MP itself being one of the most important potential coinfecting pathogens, the surge in macrolide resistance contributes to increased susceptibility to mixed infections. Evidence indicates that macrolide resistance in Asian countries, especially China and Japan, can exceed 90%, primarily due to inappropriate or excessive antibiotic use (33–35). Additionally, mixed infections increase the incidence of severe MP pneumonia, leading to prolonged duration of PICU. Despite the rise in MP mixed bacterial infections post-2023, PICU admission and hospital stay decreased, possibly due to enhanced bed turnover rates and increased utilization of tetracyclines and fluoroquinolones to counteract rising macrolide resistance, thereby partially alleviating the severity of bacterial infections.
Our study also revealed that post-pandemic, the age of patients suffering from respiratory infections increased, with the age group most affected shifting from 0 to 3 years before the COVID-19 pandemic to 3–6 years after the pandemic. Delayed immunity may be one reason for this transition, as the immune system of children, after being “compensating for lost time” upon exposure to certain pathogens post-NPIs, could lead to the resurgence of infectious diseases (36). Furthermore, implementation of NPIs has led to prolonged low exposure to specific pathogens in young children during the COVID-19 pandemic, resulting in a higher susceptibility to infections, leading to an “immunity debt” and subsequent outbreaks of infectious diseases in many countries post-COVID-19, along with an increase in the median age of onset (37–40). However, the mechanism of immunity debt remains controversial, with some scholars suggesting that the outbreak of specific pathogens may be related to the comprehensive and long-term damage to the immune system after SARS-CoV-2 infection, leading to a decrease in the population’s immune barrier (36). Further in-depth research is warranted on the mechanism of immunity debt.
Several limitations exist in our study. Firstly, being a single-center study, its findings may not be generalizable to other regions despite the large sample size. Secondly, focusing solely on hospitalized patients limits the understanding of the overall situation among children in the region. Many children with infections who do not require hospitalization are not represented in our data. Therefore, our findings may not fully capture the spectrum of disease severity or the true incidence and prevalence of infections in the broader community. Lastly, while our results provide population-level insights, they may not apply to individual cases due to differences in host factors (such as age, immune status, or genetic predispositions) and environmental influences. The interaction between these factors may lead to variability in how different individuals respond to the same pathogen or combination of pathogens.
In conclusion, our study affirms the effectiveness of NPIs in preventing respiratory infections. However, further mechanistic research is needed on delayed immunity and immunity debt post-pandemic. MP, as a special pathogen, continues to exhibit cyclical outbreaks globally, with its increasing resistance and the risks associated with resistance warranting attention.
Brief summary
Most common infectious diseases and respiratory pathogens declined under NPIs but rebounded notably in 2023. The MP outbreak was characterized by a higher severity of illness in children under 3 years of age and a rise in the number of cases among older children. NPIs reduced MP coinfections primarily by decreasing the MP coinfections with other bacteria.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University (XMYY-2023KY128-01). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Written informed consent for participation was not required from the participants or the participants’ parents and/or legal guardians in accordance with the institutional requirements.
Author contributions
QC: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Resources, Writing – original draft, Writing – review & editing. RX: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YG: Data curation, Formal analysis, Investigation, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JP: Data curation, Formal analysis, Investigation, Project administration, Validation, Writing – original draft, Writing – review & editing. CM: Data curation, Formal analysis, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. DS: Data curation, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SL: Software, Validation, Visualization, Writing – original draft, Writing – review & editing. DG: Conceptualization, Formal analysis, Supervision, Writing – review & editing. YY: Conceptualization, Data curation, Investigation, Project administration, Resources, Supervision, Writing – review & editing. WN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R & D Program of China [2021ZD0201300 and 2022YFC2704300]. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments
The authors would like to thank all the patients who participated in this trial as well as their families. We thank other team members for assisting with manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1437508/full#supplementary-material
References
1. Kim, JH, Roh, YH, Ahn, JG, Kim, MY, Huh, K, Jung, J, et al. Respiratory syncytial virus and influenza epidemics disappearance in Korea during the 2020-2021 season of COVID-19. Int J Infect Dis. (2021) 110:29–35. doi: 10.1016/j.ijid.2021.07.005
2. Ren, L, Lin, L, Zhang, H, Wang, Q, Cheng, Y, Liu, Q, et al. Epidemiological and clinical characteristics of respiratory syncytial virus and influenza infections in hospitalized children before and during the COVID-19 pandemic in Central China. Influenza Other Respir Viruses. (2023) 17:e13103. doi: 10.1111/irv.13103
3. Ning, W, Lei, S, Yang, J, Cao, Y, Jiang, P, Yang, Q, et al. Open resource of clinical data from patients with pneumonia for the prediction of COVID-19 outcomes via deep learning. Nat Biomed Eng. (2020) 4:1197–207. doi: 10.1038/s41551-020-00633-5
4. Li, Y, Wang, X, Blau, DM, Caballero, MT, Feikin, DR, Gill, CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. (2022) 399:2047–64. doi: 10.1016/S0140-6736(22)00478-0
5. Zhu, G, Xu, D, Zhang, Y, Wang, T, Zhang, L, Gu, W, et al. Epidemiological characteristics of four common respiratory viral infections in children. Virol J. (2021) 18:10. doi: 10.1186/s12985-020-01475-y
6. Williams, BG, Gouws, E, Boschi-Pinto, C, Bryce, J, and Dye, C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. (2002) 2:25–32. doi: 10.1016/S1473-3099(01)00170-0
7. Li, ZJ, Yu, LJ, Zhang, HY, Shan, CX, Lu, QB, Zhang, XA, et al. Broad impacts of coronavirus disease 2019 (COVID-19) pandemic on acute respiratory infections in China: an observational study. Clin Infect Dis. (2022) 75:e1054–62. doi: 10.1093/cid/ciab942
8. Gao, LW, Yin, J, Hu, YH, Liu, XY, Feng, XL, He, JX, et al. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol Infect. (2019) 147:e192. doi: 10.1017/S0950268819000839
9. Meyer Sauteur, PM, and Beeton, ML. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) study Group for Mycoplasma and Chlamydia Infections (ESGMAC), and the ESGMAC Mycoplasma pneumoniae surveillance (MAPS) study group. Mycoplasma pneumoniae: Delayed re-emergence after COVID-19 pandemic restrictions. Lancet Microbe. (2024) 5:e100–1. doi: 10.1016/S2666-5247(23)00344-0
10. Wang, X, Li, M, Luo, M, Luo, Q, Kang, L, Xie, H, et al. Mycoplasma pneumoniae triggers pneumonia epidemic in autumn and winter in Beijing: a multicentre, population-based epidemiological study between 2015 and 2020. Emerg Microbes Infect. (2022) 11:1508–17. doi: 10.1080/22221751.2022.2078228
11. Zhang, XB, He, W, Gui, YH, Lu, Q, Yin, Y, Zhang, JH, et al. Current Mycoplasma pneumoniae epidemic among children in Shanghai: unusual pneumonia caused by usual pathogen. World J Pediatr. (2024) 20:5–10. doi: 10.1007/s12519-023-00793-9
12. Edens, C, Clopper, BR, DeVies, J, Benitez, A, McKeever, ER, Johns, D, et al. Notes from the field: reemergence of Mycoplasma pneumoniae infections in children and adolescents after the COVID-19 pandemic, United States, 2018-2024. MMWR Morb Mortal Wkly Rep. (2024) 73:149–51. doi: 10.15585/mmwr.mm7307a3
13. Yan, C, Xue, GH, Zhao, HQ, Feng, YL, Cui, JH, and Yuan, J. Current status of Mycoplasma pneumoniae infection in China. World J Pediatr. (2024) 20:1–4. doi: 10.1007/s12519-023-00783-x
14. De Francesco, MA, Poiesi, C, and Gargiulo, F. Coinfection of chlamydia pneumoniae and mycoplasma pneumoniae with SARS-CoV-2 is associated with more severe features. J Inf Secur. (2021) 82:e4–7. doi: 10.1016/j.jinf.2021.01.009
15. Wu, Q, Xing, Y, Shi, L, Li, W, Gao, Y, Pan, S, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. (2020) 146:e20200961. doi: 10.1542/peds.2020-0961
16. Chen, Q, Lin, L, Zhang, N, and Yang, Y. Adenovirus and Mycoplasma pneumoniae coinfection as a risk factor for severe community-acquired pneumonia in children. Front Pediatr. (2024) 12:1337786. doi: 10.3389/fped.2024.1337786
17. Shin, S, Koo, S, Yang, YJ, and Lim, HJ. Characteristics of the Mycoplasma pneumoniae epidemic from 2019 to 2020 in Korea: macrolide resistance and coinfection trends. Antibiotics (Basel). (2023) 12:1623. doi: 10.3390/antibiotics12111623
18. Yoon, Y, Lee, HS, Yang, J, Gwack, J, Kim, BI, Cha, JO, et al. Impact of nonpharmacological interventions on severe acute respiratory infections in children: from the National Surveillance Database. J Korean Med Sci. (2023) 38:e311. doi: 10.3346/jkms.2023.38.e311
19. Olsen, SJ, Winn, AK, Budd, AP, Prill, MM, Steel, J, Midgley, CM, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic - United States, 2020-2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1013–9. doi: 10.15585/mmwr.mm7029a1
20. Chiapinotto, S, Sarria, EE, Mocelin, HT, Lima, JAB, Mattiello, R, and Fischer, GB. Impact of non-pharmacological initiatives for COVID-19 on hospital admissions due to pediatric acute respiratory illnesses. Paediatr Respir Rev. (2021) 39:3–8. doi: 10.1016/j.prrv.2021.04.003
21. Bardsley, M, Morbey, RA, Hughes, HE, Beck, CR, Watson, CH, Zhao, H, et al. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID-19 pandemic, measured by laboratory, clinical, and syndromic surveillance: a retrospective observational study. Lancet Infect Dis. (2023) 23:56–66. doi: 10.1016/S1473-3099(22)00525-4
22. di Mattia, G, Nenna, R, Mancino, E, Rizzo, V, Pierangeli, A, Villani, A, et al. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol. (2021) 56:3106–9. doi: 10.1002/ppul.25582
23. Stamm, P, Sagoschen, I, Weise, K, Plachter, B, Münzel, T, Gori, T, et al. Influenza and RSV incidence during COVID-19 pandemic-an observational study from in-hospital point-of-care testing. Med Microbiol Immunol. (2021) 210:277–82. doi: 10.1007/s00430-021-00720-7
24. Qiu, W, Zheng, C, Huang, S, Zhang, Y, and Chen, Z. Epidemiological trend of RSV infection before and during COVID-19 pandemic: a three-year consecutive study in China. Infect Drug Resist. (2022) 15:6829–37. doi: 10.2147/IDR.S388231
25. Wan, L, Li, L, Zhang, H, Liu, C, Li, R, Wu, X, et al. The changing pattern of common respiratory viruses among children from 2018 to 2021 in Wuhan, China. Arch Virol. (2023) 168:291. doi: 10.1007/s00705-023-05891-7
26. Jiang, W, Chen, S, Lv, M, Zhang, Z, Wang, Z, Shao, X, et al. Are we ready to face the next wave of RSV surge after the COVID-19 omicron pandemic in China? Front Cell Infect Microbiol. (2023) 13:1216536. doi: 10.3389/fcimb.2023.1216536
27. Hu, J, Ye, Y, Chen, X, Xiong, L, Xie, W, and Liu, P. Insight into the pathogenic mechanism of Mycoplasma pneumoniae. Curr Microbiol. (2022) 80:14. doi: 10.1007/s00284-022-03103-0
28. Kumar, S, and Kumar, S. Mycoplasma pneumoniae: among the smallest bacterial pathogens with great clinical significance in children. Indian J Med Microbiol. (2023) 46:100480. doi: 10.1016/j.ijmmb.2023.100480
29. Sauteur, PMM, Chalker, VJ, Berger, C, Nir-Paz, R, Beeton, ML, Pereyre, S, et al. ESGMAC and the ESGMAC–MyCOVID study group. Mycoplasma pneumoniae Beyond the COVID-19 pandemic: where is it? Lancet Microbe. (2022) 3:e897. doi: 10.1016/S2666-5247(22)00190-2
30. Li, X, Li, T, Chen, N, Kang, P, and Yang, J. Changes of Mycoplasma pneumoniae prevalence in children before and after COVID-19 pandemic in Henan, China. J Inf Secur. (2023) 86:256–308. doi: 10.1016/j.jinf.2022.12.030
31. Cheng, Y, Cheng, Y, Dai, S, Hou, D, Ge, M, Zhang, Y, et al. The prevalence of Mycoplasma Pneumoniae among children in Beijing before and during the COVID-19 pandemic. Front Cell Infect Microbiol. (2022) 12:854505. doi: 10.3389/fcimb.2022.854505
32. Meyer Sauteur, PM, and Beeton, ML. Mycoplasma pneumoniae: delayed re-emergence after COVID-19 pandemic restrictions. Pneumonia outbreaks due to re-emergence of Mycoplasma pneumoniae. Lancet Microbe. (2024) S2666-5247:00406–8.
33. Kim, K, Jung, S, Kim, M, Park, S, Yang, HJ, and Lee, E. Global trends in the proportion of macrolide-resistant Mycoplasma pneumoniae infections: a systematic review and Meta-analysis. JAMA Netw Open. (2022) 5:e2220949. doi: 10.1001/jamanetworkopen.2022.20949
34. Loconsole, D, de Robertis, AL, Sallustio, A, Centrone, F, Morcavallo, C, Campanella, S, et al. Update on the epidemiology of macrolide-resistant Mycoplasma pneumoniae in Europe: a systematic review. Infect Dis Rep. (2021) 13:811–20. doi: 10.3390/idr13030073
35. Oishi, T, and Ouchi, K. Recent trends in the epidemiology, diagnosis, and treatment of macrolide-resistant Mycoplasma pneumoniae. J Clin Med. (2022) 11:1782. doi: 10.3390/jcm11071782
36. Cohen, R, Levy, C, Rybak, A, Angoulvant, F, Ouldali, N, and Grimprel, E. Immune debt: recrudescence of disease and confirmation of a contested concept. Infect Dis Now. (2023) 53:104638. doi: 10.1016/j.idnow.2022.12.003
37. Leung, C, Konya, L, and Su, L. Postpandemic immunity debt of influenza in the USA and England: an interrupted time series study. Public Health. (2024) 227:239–42. doi: 10.1016/j.puhe.2023.12.009
38. Li, T, Chu, C, Wei, B, and Lu, H. Immunity debt: hospitals need to be prepared in advance for multiple respiratory diseases that tend to co-occur. Biosci Trends. (2024) 17:499–502. doi: 10.5582/bst.2023.01303
39. Wang, C, Li, X, Ning, W, Gong, S, Yang, F, Fang, C, et al. Multi-omic profiling of plasma reveals molecular alterations in children with COVID-19. Theranostics. (2021) 11:8008–26. doi: 10.7150/thno.61832
40. Cohen, PR, Rybak, A, Werner, A, Béchet, S, Desandes, R, Hassid, F, et al. Trends in pediatric ambulatory community acquired infections before and during COVID-19 pandemic: a prospective multicentric surveillance study in France. Lancet Reg Health Eur. (2022) 22:100497. doi: 10.1016/j.lanepe.2022.100497
Keywords: COVID-19 pandemic, nonpharmaceutical interventions, respiratory pathogens, Mycoplasma pneumoniae, children
Citation: Chen Q, Xu R, Gu Y, Peng J, Ma C, Su D, Liu S, Ge D, Yang Y and Ning W (2024) Respiratory pathogen analysis in pediatric inpatients unraveled the infection pattern of Mycoplasma pneumoniae post the COVID-19 pandemic. Front. Public Health. 12:1437508. doi: 10.3389/fpubh.2024.1437508
Edited by:
Vitali Sintchenko, The University of Sydney, AustraliaReviewed by:
Mark Aaron Poritz, Faro Molecular, United StatesMarcelo Trindade Dos Santos, National Laboratory for Scientific Computing (LNCC), Brazil
Copyright © 2024 Chen, Xu, Gu, Peng, Ma, Su, Liu, Ge, Yang and Ning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yungang Yang, eG15eWdAc2luYS5jb20=; Wanshan Ning, bmluZ3dhbnNoYW5AMTI2LmNvbQ==
†These authors have contributed equally to this work
 Qihong Chen
Qihong Chen Ruizhi Xu
Ruizhi Xu Ying Gu
Ying Gu Jie Peng1,2†
Jie Peng1,2† Chiyuan Ma
Chiyuan Ma Dubin Su
Dubin Su Shuai Liu
Shuai Liu Dandan Ge
Dandan Ge Yungang Yang
Yungang Yang Wanshan Ning
Wanshan Ning