- 1Research Group Experimental Pharmacology, Department of Pharmaceutical Chemistry, Drug Analysis and Drug Information, Center for Neurosciences, Vrije Universiteit Brussel, Jette, Belgium
- 2Centre for Environmental Sciences, Hasselt University, Diepenbeek, Belgium
- 3Department of Analytical Chemistry, Applied Chemometrics and Molecular Modelling, Vrije Universiteit Brussel, Jette, Belgium
Attention Deficit/Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder that has long been considered a concern only in the pediatric population. However, symptoms often sustain into adulthood and may require medication. For women with ADHD, this also means dealing with the disorder during the reproductive period. Medication safety during pregnancy and breastfeeding is a critical concern, and the potential transfer of ADHD medication to infants remains a topic of scientific interest. The quantification of ADHD medications in both maternal blood and breast milk are vital for understanding their pharmacokinetics and potential exposure risks for (nursing) infants. This review aims (1) to compile and critically assess existing research on the transfer of ADHD medications into breast milk and the potential implications for nursing infants and (2) to provide a comprehensive overview and discussion of the literature regarding the quantification of methylphenidate, amphetamine, atomoxetine, viloxazine, guanfacine, clonidine and bupropion in the blood, urine, oral fluid, and breast milk with liquid chromatography. A literature search was conducted using PubMed, Scopus, and Web of Science, to identify relevant articles published from January 2014 up to December 2023. We illustrate the lack of methods to simultaneously monitor multiple ADHD medications as well as the lack of developed methods for breast milk. Finally, we highlight the need for continued research to refine our understanding of medication transfer into breast milk and potential risks, and to develop clinical guidelines to support mothers with ADHD in making informed choices regarding medication use during pregnancy and lactation.
1 Introduction
Attention Deficit/Hyperactivity Disorder (ADHD), a neurodevelopmental disorder characterized by inattention, hyperactivity and/or impulsivity (1), has long been primarily considered a concern only in the pediatric population (2, 3). However, in about 50% of children with ADHD, symptoms persist into adulthood (4), exhibiting a global prevalence of adult ADHD of 2.5–2.8% (5). These adults might at some point require treatment, including medication. In 2023, the first longitudinal multinational study on ADHD medication consumption was published, with an overall increase in use of 9.7% per year from 2015 to 2019, mostly in high-income countries (6). Methylphenidate (MPH), (dex)amphetamine [(d)AMP] and its prodrug lisdexamphetamine (LDX) were consumed the most (6) and are also considered the first line drugs (3). Other options consumed less (6) are atomoxetine (ATX), a selective norepinephrine reuptake inhibitor (3) or a new extended-release form of viloxazine, another norepinephrine reuptake inhibitor (7). Guanfacine (GUA) and clonidine (CLO), which are selective alpha-A2 adrenergic receptor agonists, are also frequently used, as well as bupropion (BUP), a norepinephrine/dopamine-reuptake inhibitor (3).
A subgroup within the adult ADHD population is pregnant and breastfeeding women. In 2022, around 3.88 million (8) and 3.67 million (9) children were born in Europe and the United States, respectively. Using a conservative prevalence of 2.5% (5), this translates into about 97,000 mothers in Europe and 91,750 mothers in the United States with ADHD. In recent years, the treatment of this subgroup has become more prevalent (10–14), raising questions about potential risks for both mother and child. Interestingly, ADHD without pharmacotherapy has been linked to various adverse pregnancy outcomes (15), as the combination of (untreated) ADHD symptoms with the physical and psychosocial effects of pregnancy worsens mental health, academic challenges, and socioeconomic difficulties (16). In general, reviews regarding the safety of ADHD medication during pregnancy conclude that physicians should carefully weigh the risks of medication exposure against the risks associated with untreated ADHD (17–20). Despite some research interest in the use of ADHD medication during pregnancy, research regarding the breastfeeding period is lacking (18, 20–26). In this stage there seems to be a reluctance by both clinicians and women to support and maintain breastfeeding when the mother is taking ADHD medication (27), notwithstanding it being associated with benefits for the mother and her child (28). This is mainly due to concerns about the transfer of the medication into the milk and possible adverse health effects on the infant (27). This leads to temporary, though potentially unnecessary interruption of treatment (2) and reluctance to resume medication post-childbirth (29). Indeed, only about 35% of women restarts their ADHD medication in the 6 months postpartum (12). Data regarding breast milk levels of ADHD medication are therefore limited, as presented in Table 1. However, discontinuing ADHD medication may render risks (12), as ADHD comes with specific challenges toward breastfeeding, such as sensory overload and distractibility. This increases the likelihood of early breastfeeding cessation and missed follow-up healthcare appointments for both mother and child (16, 30).
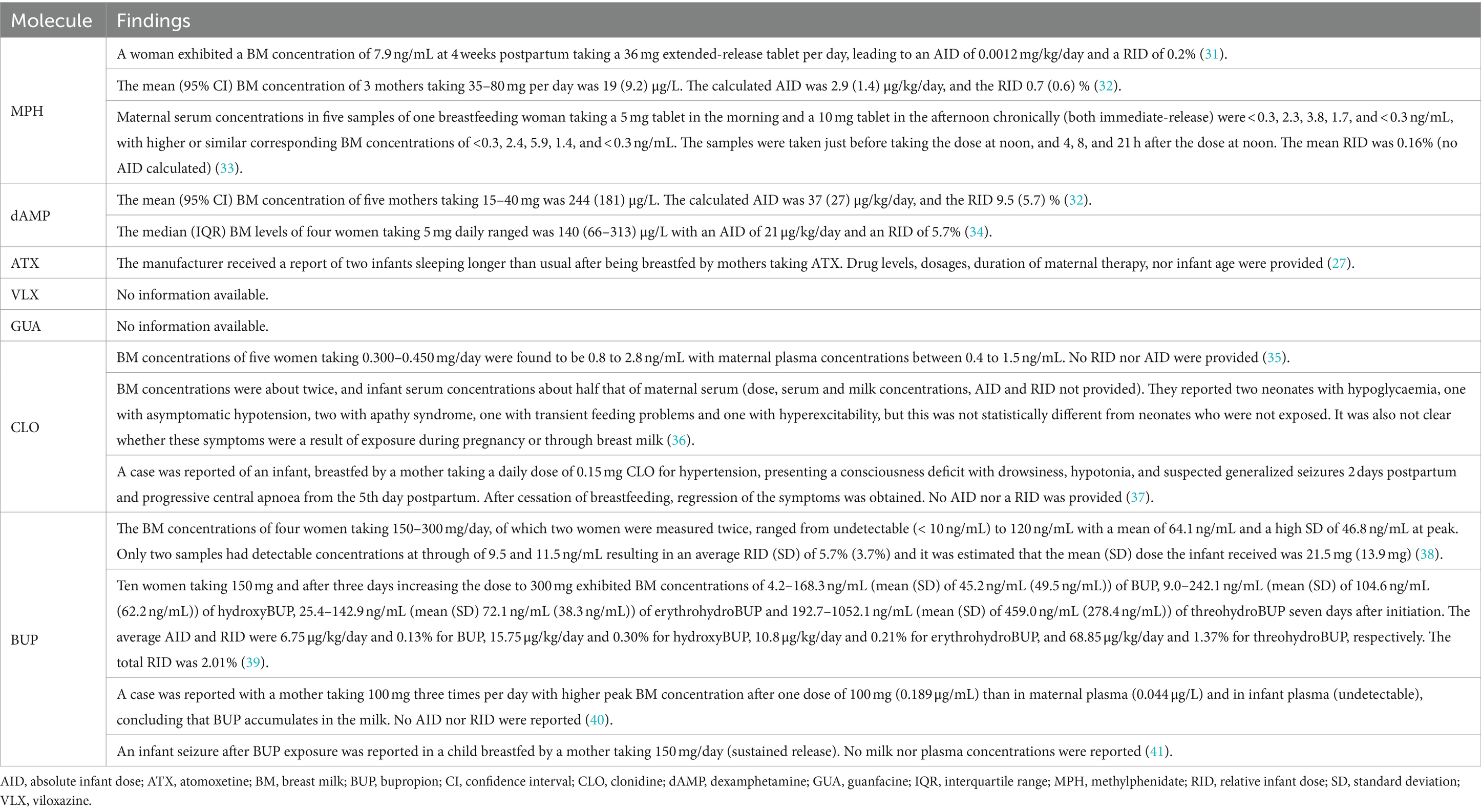
Table 1. Drug levels and clinical outcomes of infants after being breastfed by a mother taking ADHD medication.
To acquire information about the pharmacokinetics of ADHD medication throughout pregnancy and lactation, monitoring the concentration levels of the mother and assessing the correlation with infant blood levels is required. Therefore, the current review aims to provide a comprehensive overview of the recent literature (published between January 2014 and December 2023) from PubMed, Scopus, and Web of Science regarding the quantification of the most frequently used ADHD medications (i.e., MPH, (d)AMP, LDX, ATX, VLX, GUA, CLO and BUP) and their metabolites with liquid chromatography in biological human fluids, namely blood, plasma, serum, urine, and oral fluid, and we put a specific emphasis on breast milk. Only methods with the aim to quantify these medications in human matrices for ADHD treatment were included to assess their applicability for therapeutic drug monitoring (TDM) of ADHD medication. The results are summarized in Table 2 and the most important findings per (group of) molecule(s) are described here below.
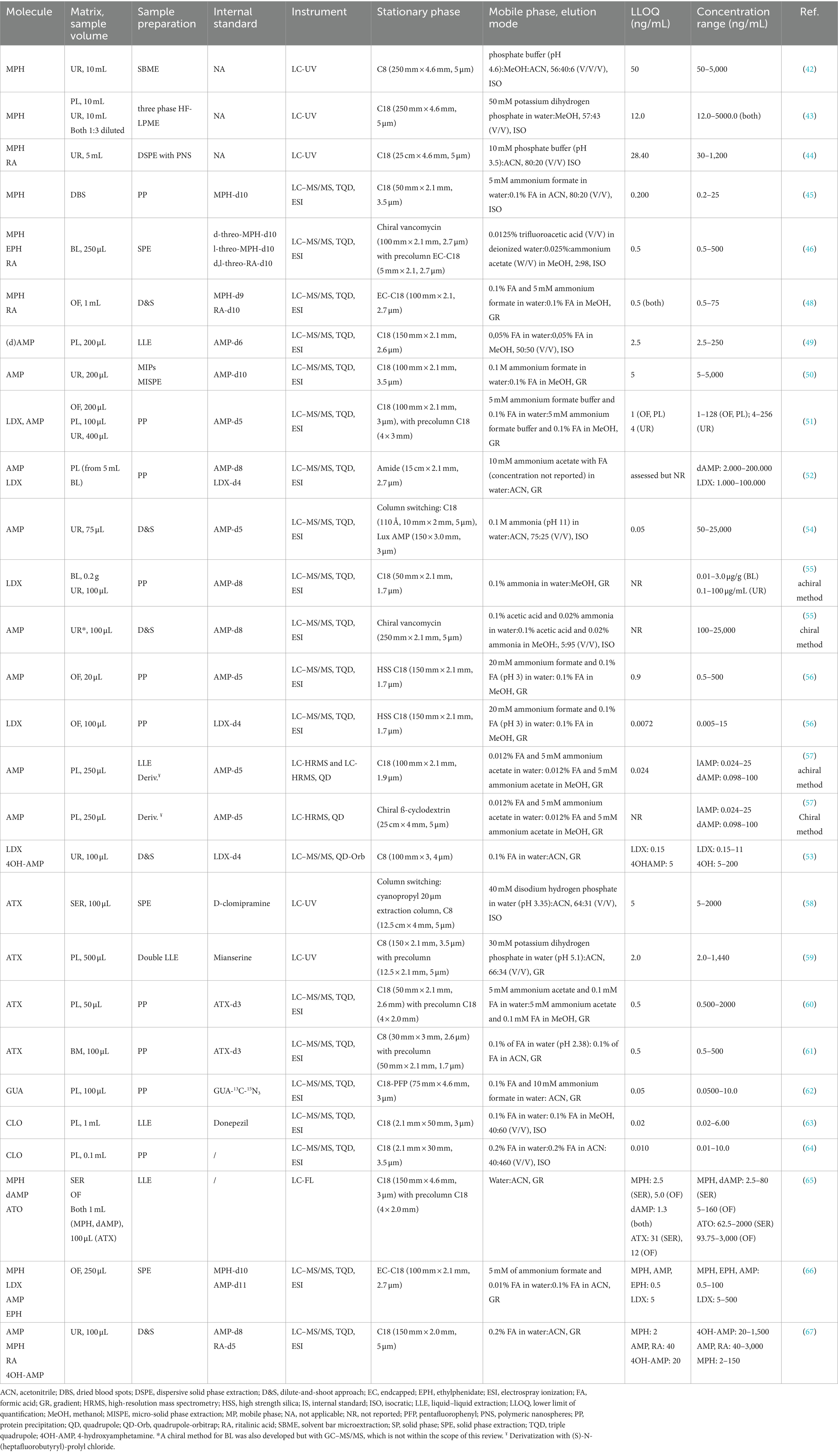
Table 2. Studies retrieved with quantitative liquid chromatographic methods for all included medication.
2 Liquid chromatographic methods for the quantification of ADHD medication in biological samples
2.1 Methylphenidate
Six studies that report on a quantitative assay for MPH were retrieved (Table 2), of which three papers used a LC-UV method for the quantification of MPH in urine. All three focused on the development of alternative sample preparation methods, i.e., solvent bar microextraction (SBME) (42), three phase hollow fiber liquid phase microextraction (HF-LPME) (43), and dispersive solid-phase extraction (DSPE) (44). The latter method also allowed the quantification of the inactive metabolite ritalinic acid (RA), which is advantageous for drug screening or patient compliance purposes as MPH has a short half-life (~2.5 h). All three methods required a large sample volume (3–10 mL) and the obtained detection limits were relatively high for sensitive analysis in low volume biological samples.
Two LC–MS/MS methods were developed for MPH quantification in blood. Ghandi et al. (45) developed a method for analyses of dried blood spots (DBS) in pharmacokinetic studies, although the method was not tested on real samples. This approach would be interesting for testing infant blood levels as it only requires low volumes (5 or 10 μL). They used a simple protein precipitation (PP) step. Smith et al. (46) applied mixed-mode solid-phase extraction (SPE) followed by chiral LC–MS/MS for quantification of the enantiomers of MPH and its metabolites ethylphenidate (EPH) and RA in blood samples. EPH is formed by transesterification with alcohol when administered concomitantly (47). A chiral stationary phase with vancomycin as chiral selector was used, thereby excluding the necessity of derivatization reagents to separate the enantiomers. The deuterated internal standards were able to compensate for the significant matrix effects observed (46).
Mulet et al. (48) developed a dilute-and-shoot LC–MS/MS method for MPH and RA quantification in oral fluid. The quick method facilitates non-invasive sampling and monitoring of MPH levels in patients with ADHD. Using stable isotope labeled internal standards (SIL-ISs) was recommended because ion suppression was observed. The authors also recommend storing the oral fluid samples at −20°C and analyzing them as soon as possible, particularly in the case of low sample concentrations (48).
2.2 (lis)(dex)amphetamine
Few methods are published for the quantification of AMP or LDX levels in light of ADHD treatment (Table 2). Most often, they are analyzed together with a large panel of other analytes in the context of drug abuse. These methods have not been included in this review as ranges differ significantly. Herbrink et al. (49) developed a LC–MS/MS method to quantify dAMP in plasma using double liquid–liquid extraction (LLE) (plasma-organic and organic-water) combined with snap-freezing. The applicability of the method was shown in clinical studies. El-Beqqali et al. (50) developed a molecularly imprinted polymer-sol–gel (MIPs) deposited on a tablet of polyethylene substrate for micro-solid phase extraction and LC–MS/MS analysis of AMP in urine samples. Each tablet could be used at least 20 times without changes in extraction efficiency.
Two LC–MS/MS methods were described for the simultaneous quantification of LDX and its metabolite AMP. The first method by Comiran et al. (51) analyzed both compounds in oral fluid, plasma and urine after a simple sample preparation step, comprising dilution, PP and filtration. Interestingly, LDX was detected in all three matrices 2 h after administration of 70 mg LDX. The use of matrix-matched calibration curves was important to minimize the impact of the matrix effect, but the method would further benefit from adding a SIL-IS for LDX in oral fluid, as the used IS AMP-d5 did not compensate. The second method by Rizea-Savu et al. (52) used PP as sample preparation to assess both levels of LDX and AMP in plasma in a bioequivalence study. Absolute numbers for LDX plasma concentrations were not provided but were in accordance (1 h after administration of LDX) with the study of Comiran et al. (51), although the dose was not provided.
It is well-documented that many ADHD patients struggle with addiction to drugs of abuse. Thevis et al. (53) proposed a sensitive LC–MS/MS method able to quantify low LDX levels (LLOQ: 0.05 ng/mL) in urine to distinguish LDX use from AMP abuse in doping control, as approximately 2% of the prodrug is eliminated intact into urine. LDX could be quantified up to 6 h and detected up to 11 h after administering a low therapeutic dose. Four papers (54–57) describe the use of chiral LC–MS/MS analysis of clinical samples to monitor ADHD medication compliance and abuse of AMP concomitantly (Table 2). In AMP treatment, single dAMP is often administered, as it has stronger stimulant properties than lAMP, which presents more cardiovascular and peripheral effects (54). By quantifying both enantiomers, the use of AMP for ADHD could therefore be distinguished from AMP abuse. Hädener et al. (54) described a column-switching LC–MS/MS method for AMP in urine, combining online sample purification on a C18 trapping column and chiral separation on a polysaccharide-based chiral column. Chermá et al. (55) showed that LDX only converts into dAMP and not in lAMP in the blood circulation. Only dAMP concentrations should therefore be detected in patients taking LDX. A similar approach was used by Böttcher et al. (56). First, an achiral analysis of AMP and LDX in oral fluid was performed. Secondly, illicit drug use was assessed using a qualitative chiral method for AMP. Instead of analyzing on a chiral stationary phase, Leis et al. (57) derivatized AMP with (S)-N-(heptafluorobutyryl)-prolyl chloride to form diastereomers that could be separated on an achiral stationary phase. They compared their newly developed LC-high resolution MS method for the enantioselective analysis of AMP in plasma with their previously developed GC method (57) and obtained comparable results.
2.3 Atomoxetine
Four quantification methods for determining ATX in biological samples were published (Table 2). Two LC-UV methods were described for quantifying ATX in serum (58) and plasma (59), with LLOQs of 5 and 2 ng/mL, respectively. The method of Ruppert et al. (58) included PP as sample pretreatment step, while a double LLE was performed in (59) leading to a preconcentration of approximately 3 times. Xia and Guo (60) developed a LC–MS/MS method to quantify ATX in 50 μL plasma samples using PP as sample pretreatment. LC–MS/MS for simultaneous analysis of 19 drugs and metabolites in 100 μL breast milk, among which ATX, was described by Monfort et al. (61). PP was used as sample pretreatment as this is more generic compared to SPE. However, the addition of SIL-ISs was necessary to correct for matrix effects. The method is intended to be used in studies to obtain knowledge on drug transfer into breast milk, but results on ATX have not yet been published.
2.4 Viloxazine
No studies describing assays in human matrices were retrieved.
2.5 Guanfacine
Only one study was retrieved, reporting a LC–MS/MS able to quantify GUA in plasma (100 μL) for a bioequivalence study of extended release tablets for ADHD (62) (Table 2). A simple PP was used to purify the samples and determine GUA quantitatively within a range of 0.05–10 ng/mL with sufficient recovery and no significant influence of matrix effects due to the inclusion of GUA-13C-15N3 as an internal standard.
2.6 Clonidine
Two papers regarding the quantification of CLO in plasma with LC–MS/MS were found (Table 2). In the first paper (63), plasma was extracted with diethylether, evaporated and reconstituted in ACN with formic acid. The matrix effects for CLO were negligible, but ion suppression was observed for the IS donepezil. This confirms that using SIL-ISs is the method of choice to efficiently correct for matrix effects. The second paper (64) used PP as sample pretreatment for analysis in plasma. Although no IS was included, the validation characteristics seem to comply. However, the paper lacked sufficient information. Both methods were used in bioequivalence studies for transdermal patches (63) and tablets (64).
2.7 Bupropion
No methods for the quantification of BUP regarding ADHD treatment have been published.
2.8 Multidrug assays
In this section, methods are described that were developed for the analysis of multiple ADHD medications at a time are described (Table 2). Stegmann et al. (65) developed a method to simultaneously quantify MPH, dAMP and ATX in serum and oral fluid. They used LC with fluorescence detection (FL) as it is more cost-effective than LC–MS/MS and as many routine quantification methods still use a HPLC system with a UV or FL detector. However, after LLE of the samples an additional derivatization with 4-(4,5-diphenyl-1H-imidazol-2-yl) benzoyl chloride (DIB-Cl) was needed for fluorescent labeling. No clear correlation between serum and oral fluid could be found for MPH (n = 13) and dAMP (n = 4), even though it was detected in all patients. In addition, ATX (n = 1) was not detected in oral fluid (LOD 5.9 ng/mL). However, the sample size (n = 18) was relatively small, and no final conclusions could be made about the correlation between serum and oral fluid. Later, Smith et al. (66) reported an LC–MS/MS method for oral fluid, capable of quantifying AMP, LDX, MPH and its transesterification metabolite ETH. They used SPE combining hydrophobic and cation exchange extraction. Matrix effects were significant, but the different SIL-ISs were able to adequately correct for this. The correlation between oral fluid and blood concentrations was not assessed. A LC–MS/MS method for the simultaneous quantification of MPH and its metabolite ritalinic acid (RA), and AMP and its metabolite 4-hydroxyamphetamine (4-OHAMP) in urine was developed and validated by Kwon et al. (67). The urine samples were centrifuged, diluted with water, the IS was added and the sample was then injected. The applicability of the method was shown for detecting ADHD medication abuse instead of treatment improvement.
3 Discussion
In general, recent methods to quantify ADHD medication with liquid chromatography in biological matrices are limited. Most papers retrieved during this literature review are focused on forensic analysis, drug abuse, or other treatments with doses that are unrepresentative for ADHD patients. The included papers mostly reported LC–MS/MS methods, emphasizing their importance in biological sample analysis. In five cases, LC-UV was used as an analysis technique (42–44, 58, 59), but LLOQ values were always higher than for LC–MS/MS methods. LC coupled to fluorescence detection was used once for the simultaneous determination of MPH, dAMP and ATX (65). Although increased sensitivity was obtained, an additional sample preparation step, i.e., derivatization, was required next to LLE to obtain fluorescent molecules. Therefore, LC–MS/MS remains the method of choice for developing a sensitive and selective method for monitoring ADHD medication transfer to breast milk.
During the development of LC–MS/MS methods, the presence of possible matrix effects should be carefully considered. As observed in most studies, these matrix effects are adequately corrected when using matrix-matched calibration curves and the addition of SIL-ISs. Only two LC–MS/MS methods (63, 64) did not include a SIL-IS. Only once a 13C-15N IS was selected (62), in all other methods deuterated forms were used as SIL-ISs, probably because of their easier availability and lower cost.
Depending on the sample type, different sample pretreatment options are available and/or needed. For urine and oral fluid samples, a dilute-and-shoot approach is possible, but for plasma samples PP is minimally needed (51, 52, 60, 62, 64), especially in LC–MS/MS methods. PP is frequently used due to its simplicity, and the mass spectrometer adds an additional level of selectivity. However, sometimes additional sample preparation is needed (49, 57, 59, 63). Classically, LLE or SPE are used, both having their advantages and disadvantages. Interestingly, novel micro-extraction techniques including SBME (42), HF-LPME (43), and DSPE (44) have been investigated. Combining them with more sensitive LC–MS/MS methods would be interesting to further evaluate their potential for volume-limited samples. While high selectivity is offered by MIPs, this may limit the number of compounds that can be used for extraction, making them less ideal for TDM (50). The single paper on breast milk used PP (61), while SPE seems the preferred method to efficiently remove lipids, proteins and salts and reduce matrix effects. Moreover, the addition of SIL-ISs compensates for matrix effects.
Almost all achiral methods used a C18 column, although one paper used an amide column (52). Some studies used C8 columns. For example, Monfort and colleagues (61) specifically chose a C8 column as an analytical column to avoid too many phospholipids in breast milk sticking to the column and deteriorating the chromatographic performance. Typically, conventional or narrowbore columns have been used, indicating that sensitivity can still be improved by using miniaturized systems, which would be beneficial for breast milk analysis, since we expect lower medication levels than in maternal plasma. Enantioselective analysis of AMP can also be important (46, 54–57) to distinguish between use and abuse of ADHD medication, but this is not necessary for determining the concentration in breast milk.
Most published articles reported methods for MPH, AMP and ATX. Only one method for GUA and two for CLO in plasma were found. Other methods for CLO were applied in hypertensive (68–73) or analgesic treatment (74, 75) or on mouse plasma (76). No methods to quantify VLX and BUP for treating ADHD in human matrices were published. However, one study described a chiral LC–MS/MS analysis of VLX in rat plasma (77). After PP, the enantiomers were separated on a cellulose tris-(3,5-dichlorophenylcarbamate) column. Methods retrieved for BUP were published for the treatment of depression or smoking cessation, which is reasonable as BUP is widely used off-label for ADHD (3).
When developing an analysis method for TDM, it preferably enables the quantification of multiple ADHD medications at the same time, as a considerable part of patients uses more than one type of ADHD medication (12). Some methods with multiple ADHD medications were published (65–67), but no method exists that considers all available ADHD medications. An ideal method should be able to detect a wide range of levels, so it is applicable to both patients and their offspring, the latter who are expected to exhibit much lower plasma levels. Such an analytical method is currently lacking. Moreover, a method combining medication for ADHD and comorbidities, such as depression or anxiety (78), requires further research.
To date, only one method in breast milk regarding the treatment of ADHD (i.e., ATX) has been published in the last 10 years. This method is simple and has a high sensitivity, providing a promising first step to increase knowledge regarding the transfer of ADHD medication into breast milk. Also, two LC–MS/MS methods to assess AMP abuse in breast milk were published (79, 80).
As it is not ethical to perform multiple invasive sampling in infants, and because of its simplicity, oral fluid or urine testing might be a non-invasive alternative for TDM (65, 81). This however requires a computational modeling of the correlation between these matrices and plasma, serum or breast milk, about which further research is still necessary. Only one of the included papers (65) investigated a correlation between serum and oral fluid with no clear conclusions. Comiran et al. however reported a study (82) using their developed method (51) and found a statistically significant correlation of 0.87 between plasma and oral fluid for AMP but not LDX. They also observed a high variation of AMP concentrations in urine, which might be due to the lack of pH control. They also observed low recovery of intact LDX.
In conclusion, this review highlights the need for continued research to refine our understanding of medication transfer into breast milk and potential risks, and to develop clinical guidelines to support mothers with ADHD in making informed choices regarding medication use during pregnancy and lactation. To know how much the (unborn) child is exposed and to support the decision to (re)start ADHD medication, clinical practice is still in need of a fast and specific TDM method for different biological matrices. Furthermore, it is unclear whether a correlation exists between maternal blood and fetal blood, between maternal blood and breast milk, and between blood and oral fluid (65, 81). This makes evidence-based decisions regarding ADHD medication use during the reproductive period challenging.
Author contributions
LH: Conceptualization, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. CC: Writing – review & editing. MP: Writing – review & editing. DM: Methodology, Writing – review & editing. AE: Conceptualization, Methodology, Project administration, Supervision, Visualization, Writing – original draft. ET: Conceptualization, Methodology, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors received support for publication of the Universitaire Stichting of Belgium. No funding was received to assist with the preparation of this manuscript. No funding was received for conducting this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Psychiatric Association. Diagnostic and statistical Mannual of mental disorders. 5th ed. Virginia, US: American Psychiatric Association (2013).
2. Ramos-Quiroga, JA, Montoya, A, Kutzelnigg, A, Deberdt, W, and Sobanski, E. Attention deficit hyperactivity disorder in the European adult population: prevalence, disease awareness, and treatment guidelines. Curr Med Res Opin. (2013) 29:1093–104. doi: 10.1185/03007995.2013.812961
3. Kooij, JJS, Bijlenga, D, Salerno, L, Jaeschke, R, Bitter, I, Balázs, J, et al. Updated European consensus statement on diagnosis and treatment of adult ADHD. Eur Psychiatry. (2019) 56:14–34. doi: 10.1016/j.eurpsy.2018.11.001
4. Sibley, MH, Mitchell, JT, and Becker, SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. (2016) 3:1157–65. doi: 10.1016/S2215-0366(16)30190-0
5. Faraone, SV, Banaschewski, T, Coghill, D, Zheng, Y, Biederman, J, Bellgrove, MA, et al. The world federation of ADHD international consensus statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. (2021) 128:789–818. doi: 10.1016/j.neubiorev.2021.01.022
6. Chan, AYL, Ma, TT, Lau, WCY, Ip, P, Coghill, D, Gao, L, et al. Attention-deficit/hyperactivity disorder medication consumption in 64 countries and regions from 2015 to 2019: a longitudinal study. EClinicalMedicine. (2023) 58:101780. doi: 10.1016/j.eclinm.2022.101780
7. Findling, RL, Candler, SA, Nasser, AF, Schwabe, S, Yu, C, Garcia-Olivares, J, et al. Viloxazine in the management of CNS disorders: A historical overview and current status. CNS Drugs. (2021) 35:643–53. doi: 10.1007/s40263-021-00825-w
8. Eurostat. How many children were born in the EU in 2022? Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20240307-1 (Accessed Jul 26, 2024).
9. United States Centers for Disease Control and Prevention. FastStats - births and Natality. Available at: https://www.cdc.gov/nchs/fastats/births.htm (Accessed Jul 26, 2024).
10. Hanley, GE, and Mintzes, B. Patterns of psychotropic medicine use in pregnancy in the United States from 2006 to 2011 among women with private insurance. BMC Pregnancy Childbirth. (2014) 14:242. doi: 10.1186/1471-2393-14-242
11. Haervig, KB, Mortensen, LH, Hansen, AV, and Strandberg-Larsen, K. Use of ADHD medication during pregnancy from 1999 to 2010: a Danish register-based study. Pharmacoepidemiol Drug Saf. (2014) 23:526–33. doi: 10.1002/pds.3600
12. Cohen, JM, Srinivas, C, Furu, K, Cesta, CE, Reutfors, J, and Karlstad, Ø. Prevalence trends and individual patterns of ADHD medication use in pregnancy in Norway and Sweden, 2010–2019. Eur J Clin Pharmacol. (2023) 79:173–80. doi: 10.1007/s00228-022-03428-6
13. Anderson, KN, Ailes, EC, Danielson, M, Lind, JN, Farr, SL, Broussard, CS, et al. Attention-deficit/hyperactivity disorder medication prescription claims among privately insured women aged 15–44 years — United States, 2003–2015. MMWR Morb Mortal Wkly Rep. (2018) 67:66–70. doi: 10.15585/mmwr.mm6702a3
14. Anderson, KN, Dutton, AC, Broussard, CS, Farr, SL, Lind, JN, Visser, SN, et al. ADHD medication use during pregnancy and risk for selected birth defects: National Birth Defects Prevention Study, 1998-2011. J Atten Disord. (2020) 24:479–89. doi: 10.1177/1087054718759753
15. Walsh, CJ, Rosenberg, SL, and Hale, EW. Obstetric complications in mothers with ADHD. Front Reprod Health. (2022) 4:1040824. doi: 10.3389/frph.2022.1040824
16. Marraccini, ME, Weyandt, LL, Gudmundsdottir, BG, Oster, DR, and McCallum, A. Attention-deficit hyperactivity disorder: clinical considerations for women. J Midwifery Womens Health. (2017) 62:684–95. doi: 10.1111/jmwh.12671
17. Ornoy, A. Pharmacological treatment of attention deficit hyperactivity disorder during pregnancy and lactation. Pharm Res. (2018) 35:2323. doi: 10.1007/s11095-017-2323-z
18. Li, L, Sujan, AC, Butwicka, A, Chang, Z, Cortese, S, Quinn, P, et al. Pregnancy-related and offspring outcomes: A systematic review. CNS Drugs. (2021) 34:731–47. doi: 10.1007/s40263-020-00728-2
19. Poulton, AS, Armstrong, B, and Nanan, RK. Perinatal outcomes of women diagnosed with attention-deficit/hyperactivity disorder: an Australian population-based cohort study. CNS Drugs. (2018) 32:377–86. doi: 10.1007/s40263-018-0505-9
20. Ornoy, A, and Koren, G. The effects of drugs used for the treatment of attention deficit hyperactivity disorder (ADHD) on pregnancy outcome and breast-feeding: A critical review. Curr Neuropharmacol. (2021) 19:1794–804. doi: 10.2174/1570159X18666201127164000
21. Kittel-Schneider, S, Quednow, BB, Leutritz, AL, McNeill, RV, and Reif, A. Parental ADHD in pregnancy and the postpartum period – A systematic review. Neurosci Biobehav Rev. (2020) 2021:63–77. doi: 10.1016/j.neubiorev.2021.01.002
22. Baker, AS, and Freeman, MP. Management of Attention Deficit Hyperactivity Disorder during Pregnancy. Obstet Gynecol Clin N Am. (2018) 45:495–509. doi: 10.1016/j.ogc.2018.04.010
23. Andrade, C. Risk of major congenital malformations associated with the use of methylphenidate or amphetamines in pregnancy. J Clin Psychiatry. (2018) 79:12136. doi: 10.4088/JCP.18f12136
24. Abdulhakim, M, Van Kernebeek, MW, Santermans, L, and Crunelle, FM. Risico’s bij gebruik van illegale middelen tijdens de zwangerschap. Verslaving Herstel Zwangerschap. (2022) 2:12–7.
25. Jiang, H, Zhang, X, Jiang, CM, and Fu, HB. Maternal and neonatal outcomes after exposure to ADHD medication during pregnancy: A systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. (2019) 28:288–95. doi: 10.1002/pds.4716
26. Koren, G, Barer, Y, and Ornoy, A. Fetal safety of methylphenidate—A scoping review and meta analysis. Reprod Toxicol. (2020) 93:230–4. doi: 10.1016/j.reprotox.2020.03.003
27. Besag, FMC. ADHD treatment and pregnancy. Drug Saf. (2014) 37:397–408. doi: 10.1007/s40264-014-0168-5
28. UNICEF. Breastfeeding. Available at: https://data.unicef.org/topic/nutrition/breastfeeding/#status. (Accessed Jan 26, 2024).
29. Srinivas, C, Karlstad, Ø, Stigum, H, Furu, K, Cesta, CE, Reutfors, J, et al. Trajectories of ADHD medication use before, during, and after pregnancy: A population-based study from Norway and Sweden. Pharmacoepidemiol Drug Saf. (2023) 32:1152–60. doi: 10.1002/pds.5654
31. Collin-Lévesque, L, El-Ghaddaf, Y, Genest, M, Jutras, M, Leclair, G, Weisskopf, E, et al. Infant exposure to methylphenidate and duloxetine during lactation. Breastfeed Med. (2018) 13:221–5. doi: 10.1089/bfm.2017.0126
32. Hackett, L, Ilett, K, Kristensen, J, Kohan, R, and Hale, T. Infant dose and safety of breastfeeding for dexamphetamine and methylphenidate in mothers with attention deficit hyperactivity disorder 40. Ther Drug Monit. (2005) 27:220–1. doi: 10.1097/00007691-200504000-00056
33. Spigset, O, Brede, WR, and Zahlsen, K. Excretion of methylphenidate in breast milk. Am J Psychiatry. (2007) 164:348. doi: 10.1176/ajp.2007.164.2.348
34. Ilett, KF, Hackett, LP, Kristensen, JH, and Kohan, R. Transfer of dexamphetamine into breast milk during treatment for attention deficit hyperactivity disorder. Br J Clin Pharmacol. (2007) 63:371–5. doi: 10.1111/j.1365-2125.2006.02767.x
35. Boutroy, MJ, Gisonna, CR, and Legagneur, M. Clonidine: placental transfer and neonatal adaption. Early Hum Dev. (1988) 17:275–86. doi: 10.1016/0378-3782(88)90015-1
36. Hartikainen-Sorri, AL, Heikkinen, JE, and Koivisto, M. Pharmacokinetics of clonidine during pregnancy and nursing. Obstet Gynecol. (1987) 69:598–600.
37. Sevrez, C, Lavocat, MP, Mounier, G, Elefant, E, Magnin, S, Teyssier, G, et al. Transplacental or breast milk intoxication to clonidine: a case of neonatal hypotonia and drowsiness. Arch Pediatr. (2014) 21:198–200. doi: 10.1016/j.arcped.2013.11.004
38. Linda, H, and Chaudron, CJS. Bupropion levels in breast Milk fir 4 mother-infant pairs: more answers to lingering questions. J Clin Psychiatry. (2009) 70:297–8. doi: 10.4088/jcp.07l03133
39. Haas, JS, Kaplan, CP, Barenboim, D, Jacob, P, and Benowitz, NL. Bupropion in breast milk: an exposure assessment for potential treatment to prevent post-partum tobacco use. Tob Control. (2004) 13:52–6. doi: 10.1136/tc.2003.004093
40. Briggs, GG, Samson, JH, Ambrose, PJ, and Schroeder, DH. Excretion of bupropion in breast Milk. Ann Pharmacother. (1993) 27:431–3. doi: 10.1177/106002809302700406
41. Chaudron, LH, and Schoenecker, CJ. Bupropion and breastfeeding: A case of a possible infant seizure. J Clin Psychiatry. (2004) 65:881–2. doi: 10.4088/JCP.v65n0622f
42. Maddadi, S, Qomi, M, and Rajabi, M. Extraction, preconcentration, and determination of methylphenidate in urine sample using solvent bar microextraction in combination with HPLC–UV: optimization by experimental design. J Liq Chromatogr Relat Technol. (2017) 40:806–12. doi: 10.1080/10826076.2017.1369994
43. Miraee, SN, Qomi, M, Shamshiri, F, and Raoufi, P. Hollow-Fiber liquid-phase microextraction followed by high performance liquid chromatography for the determination of trace amounts of methylphenidate hydrochloride in biological fluids. Biomed Pharmacol J. (2014) 7:715–25. doi: 10.13005/bpj/546
44. Taghvimi, A, Jahed, FS, Dastmalchi, S, and Javadzadeh, Y. Clinical application study of polymeric Nanospheres network in methylphenidate extraction from urine samples by dispersive solid phase extraction adsorbent. Adv Pharm Bull. (2022) 12:561–7. doi: 10.34172/apb.2022.054
45. Gandhi, A, Beekman, C, Parker, R, Fang, L, Babiskin, A, and Matta, MK. Novel and rapid LC-MS/MS method for quantitative analysis of methylphenidate in dried blood spots. Bioanalysis. (2018) 10:839–50. doi: 10.4155/bio-2018-0024
46. Smith, CR, and Swortwood, MJ. Chiral separation and quantification of d,l-methylphenidate, d,l-ethylphenidate and ritalinic acid in blood by LC-MS/MS. Forensic Chem. (2022) November 2021:100400. doi: 10.1016/j.forc.2022.100400
47. Patrick, KS, Williard, RL, VanWert, AL, Dowd, JJ, Oatis, JE, and Middaugh, LD. Synthesis and pharmacology of ethylphenidate enantiomers: the human transesterification metabolite of methylphenidate and ethanol. J Med Chem. (2005) 48:2876–81. doi: 10.1021/jm0490989
48. Mulet, CT, Arroyo-Mora, LE, Leon, LA, Gnagy, E, and DeCaprio, AP. Rapid quantitative analysis of methylphenidate and ritalinic acid in oral fluid by liquid chromatography triple quadrupole mass spectrometry (LC-QqQ-MS). J Chromatogr B Analyt Technol Biomed Life Sci. (2018) 1092:313–9. doi: 10.1016/j.jchromb.2018.06.025
49. Herbrink, M, Thijssen, B, Hillebrand, MJX, Rosing, H, Schellens, JHM, Nuijen, B, et al. Development and validation of a high-performance liquid chromatography-tandem mass spectrometry assay for the quantification of dexamphetamine in human plasma. J Pharm Biomed Anal. (2018) 30:259–64. doi: 10.1016/j.jpba.2017.10.009
50. El-Beqqali, A, Andersson, LI, Jeppsson, AD, and Abdel-Rehim, M. Molecularly imprinted polymer-sol-gel tablet toward micro-solid phase extraction: II. Determination of amphetamine in human urine samples by liquid chromatography–tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. (2017) 1063:130–5. doi: 10.1016/j.jchromb.2017.08.027
51. Comiran, E, Barreto, F, Meneghini, LZ, Carlos, G, Fröehlich, PE, and Limberger, RP. Method validation and determination of lisdexamfetamine and amphetamine in oral fluid, plasma and urine by LC–MS/MS. Biomed Chromatogr. (2017) 31:1–8. doi: 10.1002/bmc.3812
52. Rizea-Savu, S, Duna, SN, Panagiotopoulos, D, and Sandulovici, RC. Single dose comparative bioavailability study of Lisdexamfetamine dimesylate as Oral solution versus reference hard capsules in healthy volunteers. Front Pharmacol. (2022) 13:881198. doi: 10.3389/fphar.2022.881198
53. Thevis, M, Sigmund, G, Thomas, A, Vogel, M, Walpurgis, K, Kwiatkowska, D, et al. Isotope-dilution mass spectrometric quantification of the prodrug lisdexamfetamine in human urine in doping control analysis. Rapid Commun Mass Spectrom. (2014) 28:781–6. doi: 10.1002/rcm.6844
54. Hädener, M, Bruni, PS, Weinmann, W, Frübis, M, and König, S. Accelerated quantification of amphetamine enantiomers in human urine using chiral liquid chromatography and on-line column-switching coupled with tandem mass spectrometry. Anal Bioanal Chem. (2017) 409:1291–300. doi: 10.1007/s00216-016-0056-1
55. Chermá, MD, Nilsson, GH, Johansson, A, Jönsson, AK, and Ahlner, J. Use of Lisdexamfetamine or amphetamine? Interpretation of chiral amphetamine analyses. J Anal Toxicol. (2022) 46:10–6. doi: 10.1093/jat/bkaa170
56. Böttcher, M, Kühne, D, and Beck, O. Compliance testing of patients in ADHD treatment with lisdexamphetamine (Elvanse®) using oral fluid as specimen. Clin Mass Spectr. (2019) 14:99–105. doi: 10.1016/j.clinms.2019.04.002
57. Leis, HJ, Fauler, G, and Windischhofer, W. Enantioselective quantitative analysis of amphetamine in human plasma by liquid chromatography/high-resolution mass spectrometry. Anal Bioanal Chem. (2014) 406:4473–80. doi: 10.1007/s00216-014-7850-4
58. Ruppert, K, Geffert, C, Clement, HW, Bachmann, C, Haberhausen, M, Schulz, E, et al. Therapeutic drug monitoring of atomoxetine in children and adolescents with attention-deficit/ hyperactivity disorder: a naturalistic study. J Neural Transm. (2022) 129:945–59. doi: 10.1007/s00702-022-02483-8
59. Teichert, J, Rowe, JB, Ersche, KD, Skandali, N, Sacher, J, Aigner, A, et al. Determination of atomoxetine or escitalopram in human plasma by HPLC: applications in neuroscience research studies. Int J Clin Pharmacol Ther. (2020) 58:426–38. doi: 10.5414/CP203705
60. Xia, Y, Guo, HL, Hu, YH, Long, JY, Chen, J, Chen, F, et al. Determination of atomoxetine levels in human plasma using LC-MS/MS and clinical application to Chinese children with ADHD based on CPIC guidelines. Anal Methods. (2021) 13:2434–41. doi: 10.1039/D1AY00521A
61. Monfort, A, Jutras, M, Martin, B, Boucoiran, I, Ferreira, E, and Leclair, G. Simultaneous quantification of 19 analytes in breast milk by liquid chromatography-tandem mass spectrometry (LC-MS/MS). J Pharm Biomed Anal. (2021) 204:114236. doi: 10.1016/j.jpba.2021.114236
62. Jinying Wang, QZ. Development and validation of A high-performance liquid chromatography-mass spectroscopy assay for the bioequivalence study of Guanfacine extended release (GXR) tablet in human plasma. Curr Pharm Anal. (2022) 18:871–80. doi: 10.2174/1573412918666220614144710
63. Zhuang, J, Chen, J, Wang, X, Pang, Y, Bi, H, Huang, L, et al. Validation of a HPLC-ESI MS/MS method for the determination of clonidine in human plasma and its application in a bioequivalence study in Chinese healthy volunteers. Biomed Chromatogr. (2015) 29:1506–13. doi: 10.1002/bmc.3450
64. Danafar, H, and Hamidi, M. LC-MS method for studying the pharmacokinetics and bioequivalence of clonidine hydrochloride in healthy male volunteers. Avicenna J Med Biotechnol. 8:91–8.
65. Stegmann, B, Dörfelt, A, and Haen, E. Quantification of methylphenidate, dexamphetamine, and Atomoxetine in human serum and Oral fluid by HPLC with fluorescence detection. Ther Drug Monit. (2016) 38:98–107. doi: 10.1097/FTD.0000000000000245
66. Smith, CR, and Swortwood, MJ. Analysis of methylphenidate, ethylphenidate, lisdexamfetamine, and amphetamine in oral fluid by liquid chromatography-tandem mass spectrometry. J Forensic Sci. (2022) 67:669–75. doi: 10.1111/1556-4029.14977
67. Kwon, W, Suh, S, In, MK, and Kim, JY. Simultaneous determination of methylphenidate, amphetamine and their metabolites in urine using direct injection liquid chromatography-tandem mass spectrometry. Mass Spectr Lett. (2014) 5:104–9. doi: 10.5478/MSL.2014.5.4.104
68. Coache, D, Friciu, M, Gaelle Roullin, V, Boule, M, Forest, JM, and Leclair, G. Stability evaluation of compounded clonidine hydrochloride oral liquids based on a solidphase extraction HPLC-UV method. PLoS One. (2021) 16:279. doi: 10.1371/journal.pone.0260279
69. De Nicolò, A, Avataneo, V, Rabbia, F, Sciandra, M, Tosello, F, Cusato, J, et al. UHPLC–MS/MS method with sample dilution to test therapeutic adherence through quantification of ten antihypertensive drugs in urine samples. J Pharm Biomed Anal. (2017) 142:279–85. doi: 10.1016/j.jpba.2017.05.018
70. De Nicolò, A, Avataneo, V, Rabbia, F, Bonifacio, G, Cusato, J, Tomasello, C, et al. UHPLC–MS/MS method with protein precipitation extraction for the simultaneous quantification of ten antihypertensive drugs in human plasma from resistant hypertensive patients. J Pharm Biomed Anal. (2016) 129:535–41. doi: 10.1016/j.jpba.2016.07.049
71. Avataneo, V, Fanelli, E, De Nicolò, A, Rabbia, F, Palermiti, A, Pappaccogli, M, et al. A non-invasive method for detection of antihypertensive drugs in biological fluids: the salivary therapeutic drug monitoring. Front Pharmacol. (2022) 12:755184. doi: 10.3389/fphar.2021.755184
72. Mamina, O, and Kabachny, V. Identification and quantitative determination of clonidine by HPLC method. ScienceRise: pharmaceutical. Science. (2020) 27:30–6. doi: 10.15587/2519-4852.2020.215101
73. Qin, J, Wang, L, Wu, L, Chen, J, Shen, T, Li, Y, et al. Development of an LC-MS/MS method for determining the pharmacokinetics of clonidine following oral administration of Zhenju antihypertensive compound. Biomed Chromatogr. (2015) 29:1068–75. doi: 10.1002/bmc.3393
74. Tang, F, Bada, H, Ng, CM, and Leggas, M. Validation of a HPLC/MS method for simultaneous quantification of clonidine, morphine and its metabolites in human plasma. Biomed Chromatogr. (2019) 33:4527. doi: 10.1002/bmc.4527
75. Veigure, R, Aro, R, Metsvaht, T, Standing, JF, Lutsar, I, Herodes, K, et al. A highly sensitive method for the simultaneous UHPLC–MS/MS analysis of clonidine, morphine, midazolam and their metabolites in blood plasma using HFIP as the eluent additive. J Chromatogr B Analyt Technol Biomed Life Sci. (2017) 1052:150–7. doi: 10.1016/j.jchromb.2017.03.007
76. AlRabiah, H, Attia, SM, Al-Shakliah, NS, and Mostafa, GAE. Development and validation of an HPLC-UV detection assay for the determination of clonidine in mouse plasma and its application to a pharmacokinetic study. Molecules. (2020) 25:109. doi: 10.3390/molecules25184109
77. Rayala, VVS, Panda, SR, and Radhakrishnanand, P. Pharmacokinetic, protein binding, and tissue distribution investigations of Viloxazine enantiomers in rat plasma by HPLC–MS/MS using polysaccharide-based immobilized chiral column: A preclinical approach to possible chiral switch. Chromatographia. (2023) 86:751–64. doi: 10.1007/s10337-023-04293-w
78. Katzman, MA, Bilkey, TS, Chokka, PR, Fallu, A, and Klassen, LJ. Adult ADHD and comorbid disorders: Clinical implications of a dimensional approach. BMC Psychiatry. (2017) 17:302. doi: 10.1186/s12888-017-1463-3
79. Accioni, F, García-Gómez, D, Girela, E, and Rubio, S. SUPRAS extraction approach for matrix-independent determination of amphetamine-type stimulants by LC-MS/MS. Talanta. (2018) 182:574–82. doi: 10.1016/j.talanta.2018.02.039
80. López-García, E, Mastroianni, N, Postigo, C, Valcárcel, Y, González-Alonso, S, Barceló, D, et al. Simultaneous LC–MS/MS determination of 40 legal and illegal psychoactive drugs in breast and bovine milk. Food Chem. (2017) 245:159–67. doi: 10.1016/j.foodchem.2017.10.005
81. Marchei, E, Farré, M, Pardo, R, Garcia-Algar, O, Pellegrini, M, Pacifici, R, et al. Correlation between methylphenidate and ritalinic acid concentrations in oral fluid and plasma. Clin Chem. (2010) 56:585–92. doi: 10.1373/clinchem.2009.138396
Keywords: ADHD, biological liquids, breast milk, liquid chromatography, quantification, dexamphetamine, methylphenidate, atomoxetine
Citation: De Hondt L, Cosemans C, Plusquin M, Mangelings D, Van Eeckhaut A and Tommelein E (2024) Quantification of ADHD medication in biological fluids of pregnant and breastfeeding women with liquid chromatography: a comprehensive review. Front. Public Health. 12:1437328. doi: 10.3389/fpubh.2024.1437328
Edited by:
Michelle Juarez, Stony Brook University, United StatesReviewed by:
Suyash Prasad, Independent Researcher, San Francisco, United StatesCopyright © 2024 De Hondt, Cosemans, Plusquin, Mangelings, Van Eeckhaut and Tommelein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lena De Hondt, TGVuYS5EZS5Ib25kdEB2dWIuYmU=
†These authors have contributed equally to this work and share last authorship
 Lena De Hondt
Lena De Hondt Charlotte Cosemans
Charlotte Cosemans Michelle Plusquin
Michelle Plusquin Debby Mangelings3
Debby Mangelings3 Ann Van Eeckhaut
Ann Van Eeckhaut Eline Tommelein
Eline Tommelein