- 1Paris School of Economics, Paris, France
- 2CNRS, Paris, France
- 3PSL Research University, Université Paris Dauphine, LEDA, UMR CNRS, Paris, France
- 4IRIS, Inserm/EHESS/CNRS, Aubervilliers, France
- 5CESP UMR, Université Paris-Saclay, APHP, le Kremlin-Bicêtre, France
Background: Testing for COVID-19 has been strongly recommended for individuals experiencing COVID-19-like symptoms or those with a close relative who tested positive. In France, tests were free of charge until mid-October 2021 and became widely available after June 2020. Our main objective was to investigate whether access to COVID-19 testing in France was associated with socio-economic conditions, considering gender and ethno-racial status.
Methods: A random population-based cohort survey was conducted in France in May 2020 and November 2020, including 95,388 participants aged 18 and over. We used logistic regressions to identify how having been tested in 2020 was associated with socio-economic status and exposure factors among two groups of individuals. The first group consisted of individuals who had no close relative test positive but reported experiencing COVID-19-like symptoms, such as cough, fever, dyspnea, or sudden onset of ageusia, dysgeusia, or anosmia (N = 12,729). The second group included individuals, with or without symptoms, who reported that a close relative had tested positive for COVID-19 (N = 5,360).
Findings: In both groups, testing was more frequent among individuals living in urban areas. For individuals who had no close relative test positive but reported COVID-19-like symptoms, women were 1.04 (95%CI [1.01–1.06]) more likely than men to be tested, and testing decreased with age up to 35. Individuals holding a university degree were (1.08 [1.04–1.12]) more likely than those who only completed high school to be tested, as well as those in one of the three top income deciles with reference to the bottom decile (OR 1.07, [1.02–1.13] for the top decile). Ethno-racial status was not significantly associated with testing. For respondents who had a close relative test positive, testing was strongly associated with having experienced some symptoms, especially after September 1 (OR 1.34, 95%CI [1.30–1.39]). However, there was no link between testing and income, education, or ethno-racial status.
Interpretation: When an individual experiences symptoms but has no close relative who is positive, the motivation to test to avoid transmitting the virus to relatives by isolating oneself is particularly strong. This strategy makes sense when there are actual possibilities to isolate, which may explain why higher income and education positively impact the propensity to test. For individuals who had a close relative test positive, most socio-economic variables were no longer related to testing once infection risk was controlled for. The availability of tests at no cost is not sufficient in itself to eliminate socio-economic inequalities in testing.
Introduction
In an epidemic context, effective clinical management and outbreak control hinge on the detection of as many cases as possible. As early as March 2, 2020, the WHO emphasized that “Rapid collection and testing of appropriate specimens from patients meeting the suspect case definition for COVID-19 is a priority for clinical management and outbreak control” (1). Indeed, testing is the cornerstone of the test-treat-track strategy to help contain the COVID-19 pandemic. The decision to test an individual should be based solely on an assessment of the likelihood of infection, independent of sex, income, education, or other socio-economic characteristics. However, if certain social groups do not have equal access to tests, this raises significant concerns regarding both equity and outbreak control. Investigating this issue empirically is crucial, particularly as socio-economic inequalities have played a secondary role in the design of policy interventions against COVID-19 (2).
Few studies have examined socio-economic inequalities in SARS-CoV-2 testing. Although resource shortages, documented in various contexts, likely affected different socio-economic groups differently (3), assessing inequalities in access to testing has been challenging due to insufficient data (4). Some studies, using surveillance data matched with local socio-economic information, revealed lower testing uptake among minority ethno-racial groups and residents of deprived areas in Liverpool (5), undocumented migrants and homeless individuals in Geneva (6), less affluent communities in Massachusetts (7), and in Switzerland (8) and France (9). Interestingly, both Riou et al. and Vandentorren et al., though in different contexts, found that raw testing rates were actually higher among the most deprived groups; only after adjustments (for age, sex, state, and wave for Riou et al.; for population density for Vandentorren et al.) did it become apparent that access to testing was lower among those more exposed to infection risk. To our knowledge, Trevisan et al. (10) is the only study using survey data, conducted in Italy, and it found that older age was associated with lower access to testing but did not document other inequalities.
Surveillance systems do not collect data on socio-economic variables at the individual level and instead use local information as proxies, failing to provide direct insights into individual behavior. Additionally, since only individuals who got tested are registered, it is impossible to observe those who were exposed to risk but did not get tested, even though health authorities recommended it. Using survey data collected in France, our study aims to fill this gap.
France presents an interesting case for the study of testing policies and behaviors. To eliminate financial barriers, tests were free of charge and fully covered by social security for all residents. However, at the onset of the pandemic in March 2020, test supplies were very limited, and during the first half of 2020, testing was restricted to health professionals and symptomatic patients with a medical prescription. The total number of daily tests remained low, below one per thousand people.1 To increase supply, biological labs were offered a generous fee of 73.59€ per test. After July 24, the priority remained for symptomatic patients, but a medical prescription was no longer required. The number of tests increased steadily during summer 2020, fluctuating between 2.5 and 5.5 daily tests per thousand individuals in the fall. Testing was never mandatory but was recommended by health authorities in two situations: when a person learned that a close relative tested positive, and when a person experienced symptoms suggestive of SARS-CoV-2 infection. Disparities in testing access among these “higher risk” situations across socio-economic groups raise several issues: they contradict the ethical principle of equitable access to healthcare, hinder disease control, and may exacerbate health inequalities.
Our survey data allows us to identify individuals who have encountered one of these situations and, among them, those who have been tested or not. When a person experiences symptoms but has no known positive relative, they are at risk of both being infected and transmitting the virus to household members. A positive relative increases the salience of the virus and awareness of infection risk, but motivations for testing may vary. Testing behavior may follow different social determinants in these two scenarios.
In this paper, we analyze whether, despite the absence of out-of-pocket costs, socio-economic conditions still influence testing behaviors in France. We contrast the two situations of interest: having a close relative who is positive and experiencing symptoms without a positive relative, to detect different mechanisms at play.
Materials and methods
Study design and participants
The EpiCoV (Epidémiologie et Conditions de Vie) cohort was established in April 2020 with the primary aim of understanding the key epidemiological, socio-economic, and behavioral aspects related to the COVID-19 epidemic in France. The survey received approval from the CNIL (French Data Protection Authority) on April 25, 2020 (ref: MLD/MFI/AR205138) and the “Comité de protection des personnes” (the French equivalent of the Research Ethics Committee) on April 24, 2020. It also obtained certification from the “Comité du Label de la statistique publique,” ensuring its adherence to statistical quality standards.
The cohort protocol, detailed in another publication (11), involved a random sample of 135,000 individuals aged 15 and over, drawn from the tax database of the National Institute of Statistics and Economic Studies (INSEE). This database covers 96% of the population living in France, excluding those in institutional settings. The first wave of the study occurred in May 2020 when COVID-19 tests were not widely available. A second wave was conducted in November and December 2020, which included questions on access to testing. In total, 107,808 respondents participated in this second wave, representing 81.7% of the first wave participants. Respondents completed the questionnaire online, with phone interviews available for those without internet access. Additionally, 10% of internet-accessible participants were randomly selected for phone interviews to account for potential method collection effects.
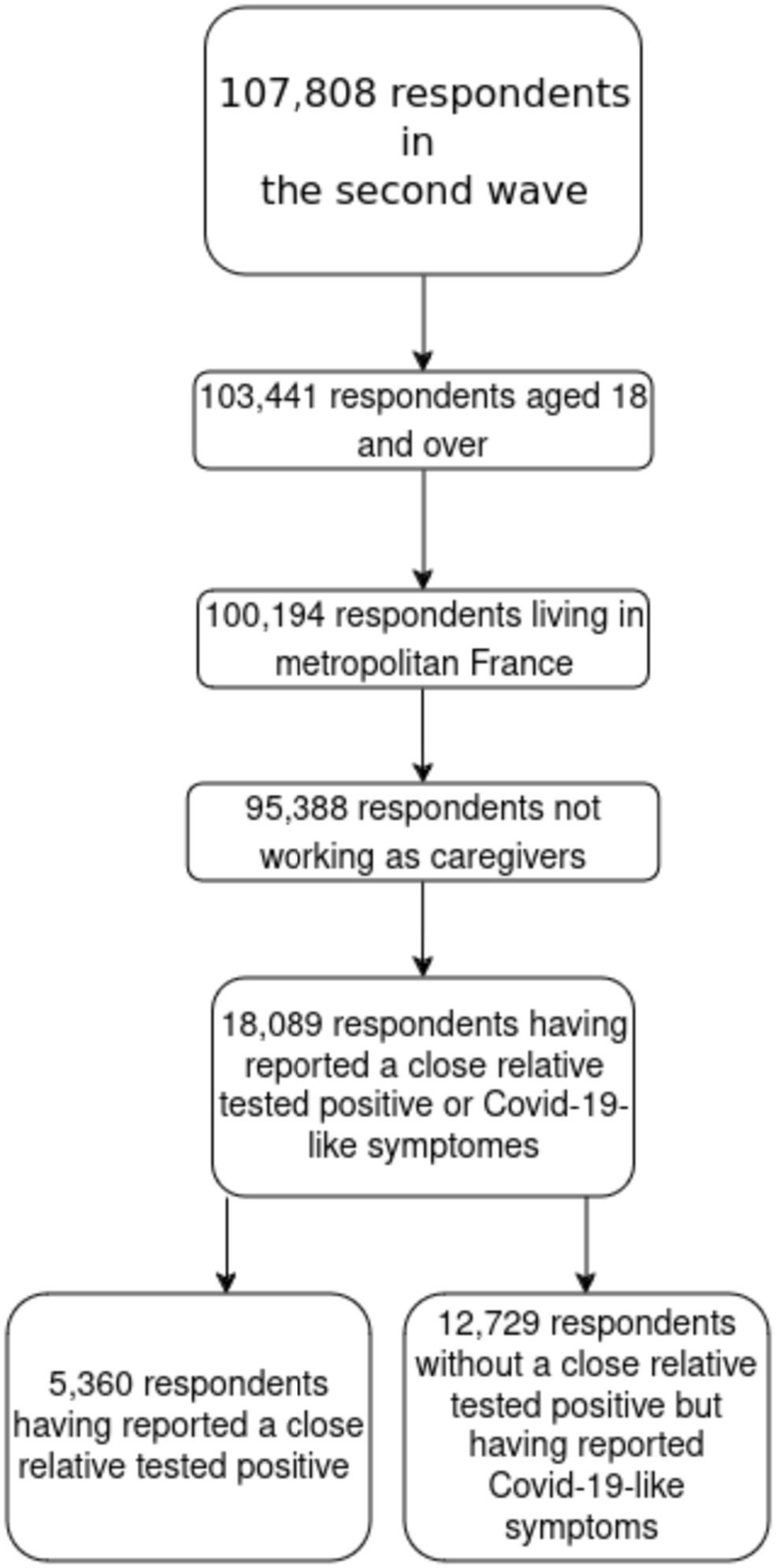
This study is based on the second wave of EpiCoV, including 107,808 respondents. We excluded individuals aged 17 or younger (4,544 observations). We also excluded individuals living in overseas territories (3,247 observations) due to significant differences in healthcare systems with metropolitan France (continental France and Corsica). Caregivers were also excluded (4,806 observations) as they could be tested at their workplace. The final sample comprised 95,388 individuals (see Figure 1).
Outcome
Participants were asked the following question: “Have you been tested for the coronavirus with a nasal swab or a saliva test?” with response options: “Yes, once/Yes, more than once/Never.” Therefore, the outcome variable should be interpreted as “the participant has already been tested at least once since the beginning of the pandemic.”
Socio-economic variables
We considered six socio-economic variables: sex, age, education, income, and ethno-racial status. Sex was a binary variable (male/female). Age was categorized into seven brackets: 18–24, 25–34, 35–44, 45–54, 55–64, 65–74, and 75 or older. Education levels were measured according to the hierarchical grid of diplomas in France: no degree, professional degree, completed high school, college degree, and higher degree. Income deciles were obtained from fiscal data matched with the sample. Ethno-racial status distinguished the mainstream population (individuals residing in metropolitan France who are neither immigrants nor natives of French Overseas Departments, nor descendants of immigrants or DOM natives) from minority populations, with distinctions between first-generation (immigrants) and second-generation (descendants of immigrants) based on country of origin. The term “racialized” refers to individuals from the Maghreb, Turkey, Asia, and Sub-Saharan Africa.
Exposure risk factors
We considered five main risk factors for COVID-19 exposure:
1 Household size: single, 2, 3, or 4 or more persons.
2 Population density: defined by Eurostat methodology with urban centers (areas with more than 50,000 inhabitants and a population density of at least 1,500 inhabitants per km2) and urban clusters (areas with more than 5,000 inhabitants and a population density of at least 350 inhabitants per km2). The survey contains information about the city of residence of each participant, and this information was matched with outside information from INSEE about the density of each city (more precisely, each “commune”) in France.
3 Workplace exposure: respondents reported their work status during the 7 days before the interview (not worked, worked only from home, worked both from home and outside, or worked exclusively outside). Since this question was asked during both the first and second waves of the survey, we use the responses collected from both waves. We therefore categorized this variable in four categories:
1. those who had not worked during any wave;
2. those who worked exclusively from home during at least one of the two waves;
3. those who worked part time outside home during at least one of the two waves;
4. those who have worked full time outside home during both waves.
4 Geographical variations in prevalence: regions were categorized as Grand Est, Hauts-de-France, Ile-de-France, Auvergne-Rhône-Alpes, Bourgogne-Franche-Comté, Provence-Alpes-Côte d’Azur, and less exposed regions.
5 Chronic disease: respondents indicated whether they had at least one chronic disease.
Statistical methods
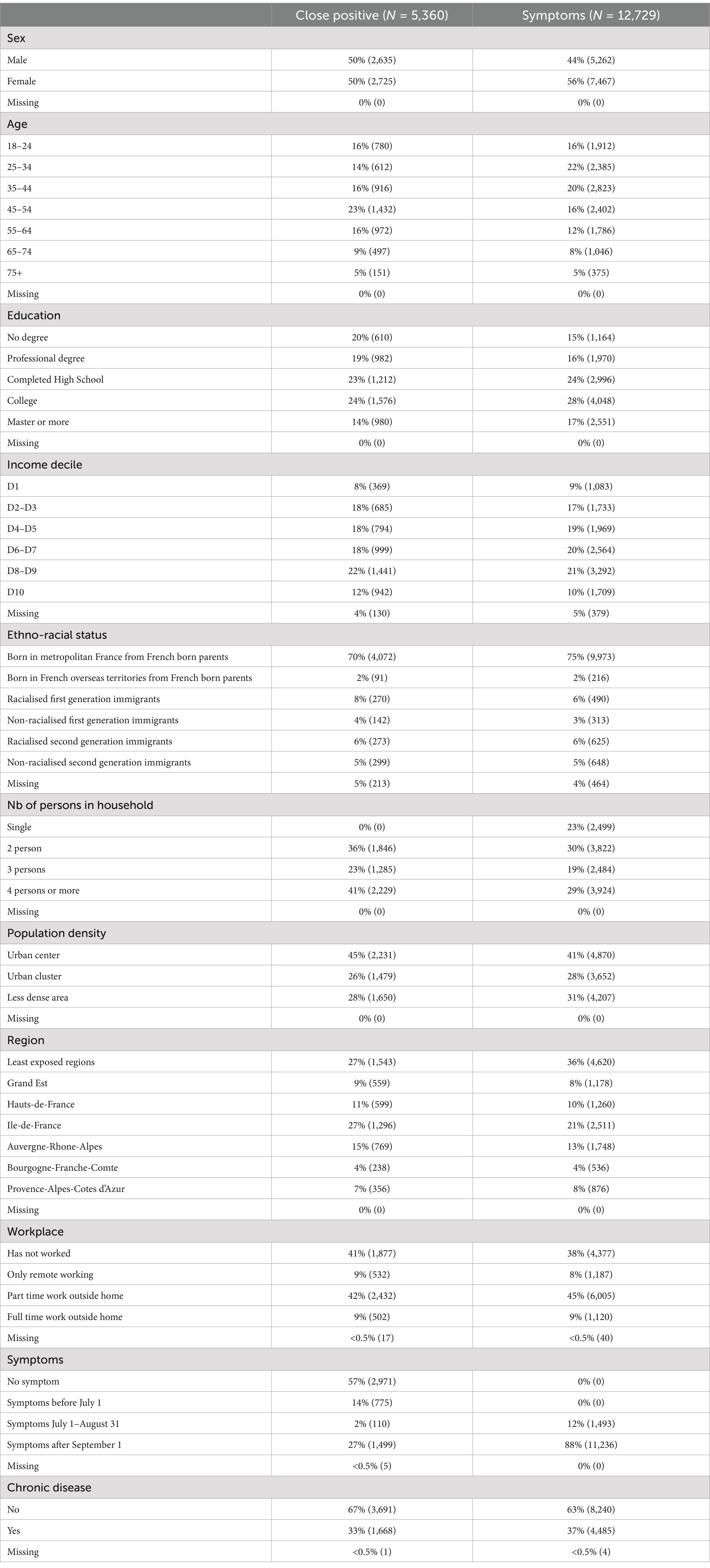
Statistical analysis was adjusted using weights established by INSEE to produce representative estimators of the population. We focused on two high-risk situations and stratified the sample accordingly. The first stratum included individuals who responded affirmatively to the question: “Since the beginning of the epidemic (February 2020), has another person living with you tested positive for the coronavirus through a nasal swab or a blood test (antibodies)?” This group comprised 5,360 individuals. The second stratum included 12,729 individuals who reported experiencing COVID-19-like symptoms, defined by the European Centre for Disease Prevention as at least one of the following: cough, fever, dyspnea, or sudden onset of ageusia, dysgeusia, or anosmia, between July 1 and August 21, 2022, or after September 1 (Table 1).
The socio-economic and risk exposure variables selected were those who showed some association with risk of infection or protective behavior in previous studies (12, 13).
We first describe the socio-economic and health characteristics of those tested, for each stratified subsample. The share of individuals who go tested was estimated for each subsample, with the associated 95% confidence interval. Logistic regressions were then performed to identify characteristics associated with testing, independent of other variables. We first estimated crude odds-ratios for each variable, and then performed a multinomial regression to estimate adjusted odds-ratios. Tables 2, 3 below report these estimates, with their 95% confidence interval and the associated p-value.
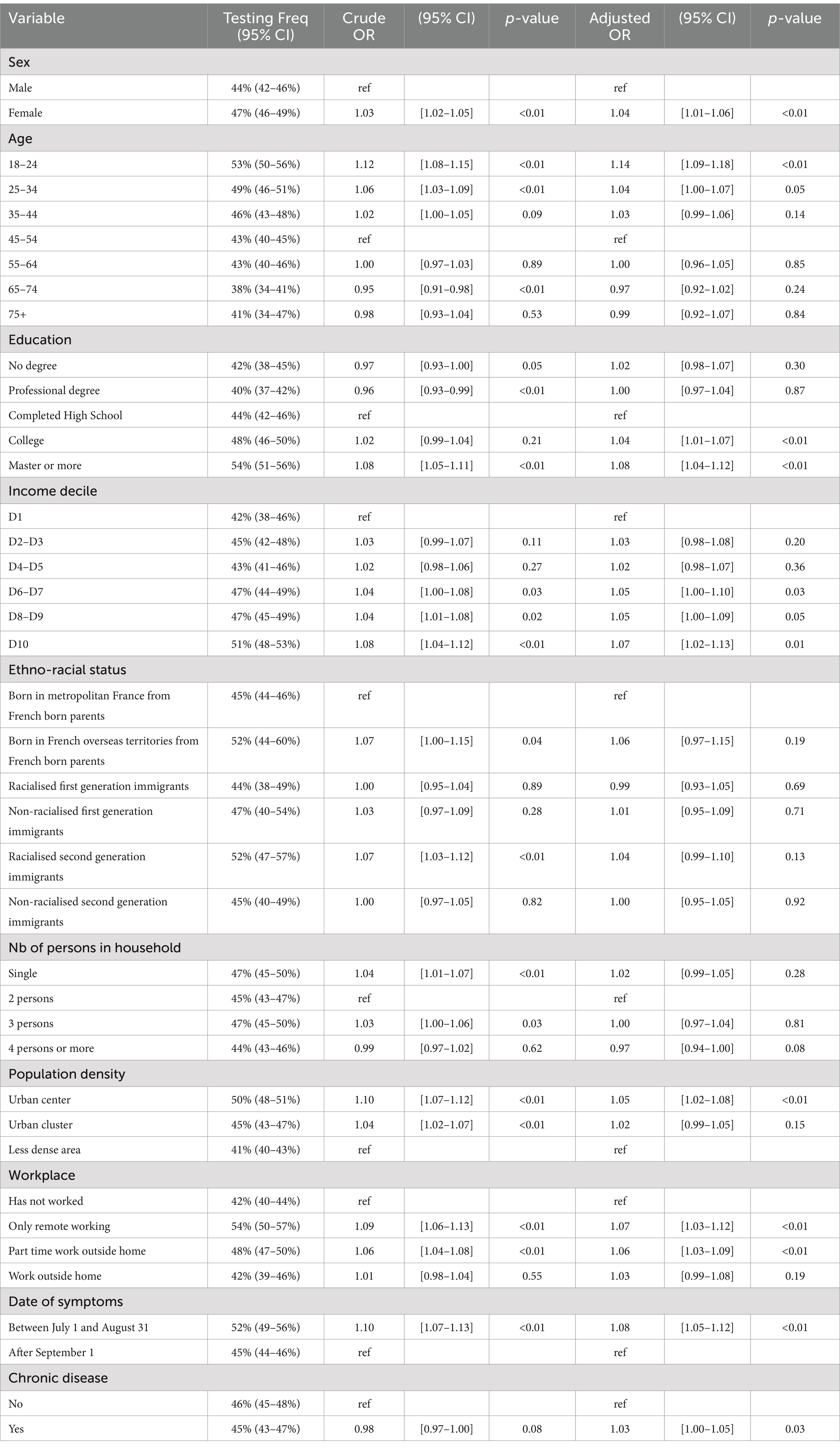
Table 2. COVID-19 testing for individuals who have experienced COVID-19-like symptoms but did not report having a close relative test positive (N = 12,729).
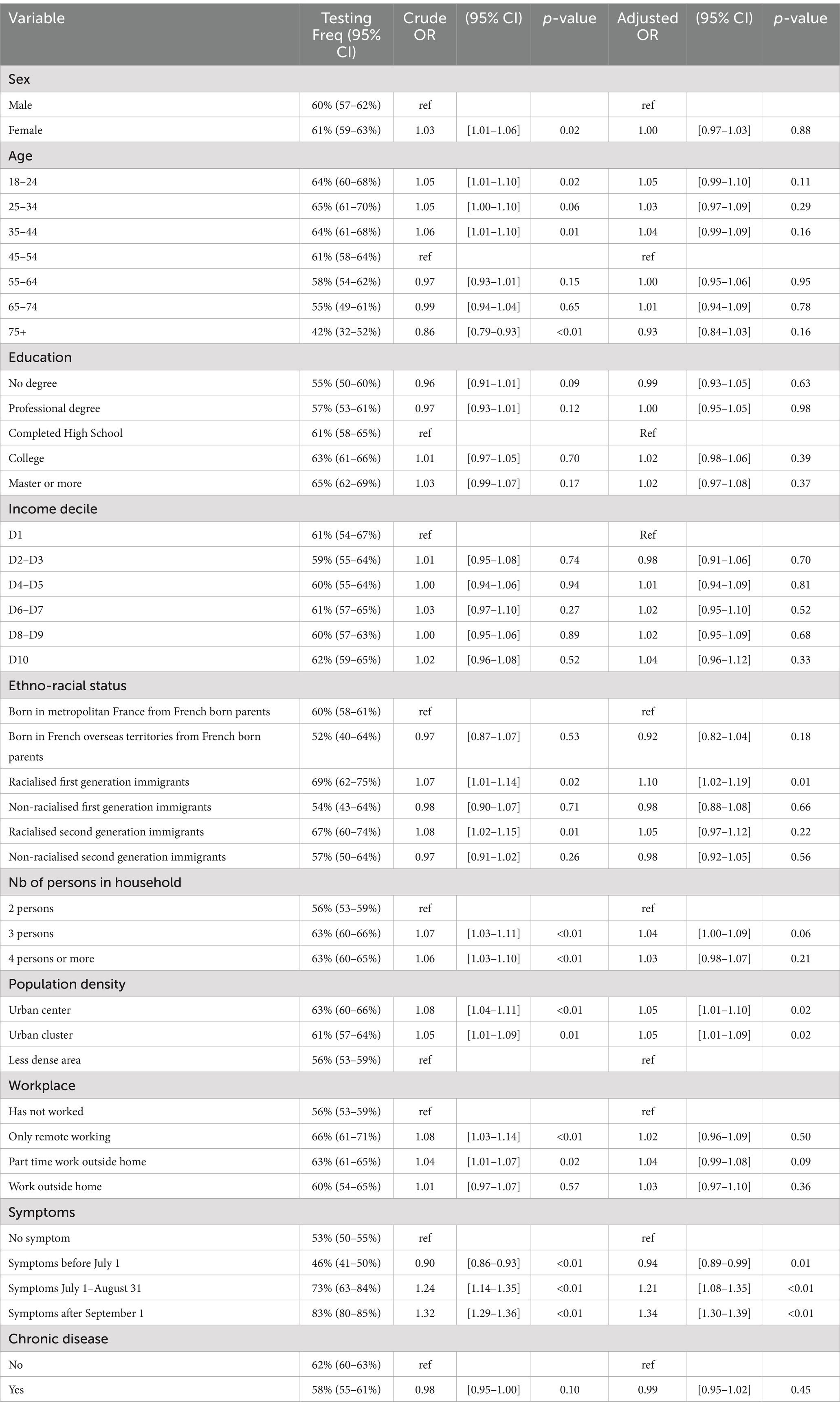
Table 3. COVID-19 testing for individuals who reported having a close relative test positive (N = 5,360).
Observations with missing values on key socio-economic and exposure variables were excluded from the multivariable analysis (in all 15%). All analyses were conducted using R software (version 4.2.3). Data wrangling was performed with the help of the tidyverse (14), weighted analyses with the survey package (15) and some summaries were produced using the gtsummary package (16). Percentages presented are weighted to account for the sample design. Multivariable analyses are also weighted. Given the sample size, observed differences were consistently statistically significant, so no tests are presented for univariate analyses (Figure 1).
Results
The following table provides descriptive statistics for the two stratified samples. For each variable category, we present the share in the weighted sample, the unweighted number of observations, and the 95% confidence interval (CI) of this share. Additionally, for each variable, we include the (unweighted) number of missing values and their (weighted) share with the associated confidence interval.
Tables 2, 3 below present the results of the regression analysis. The outcome variable is coded as 1 if the individual has undergone testing. For each variable, the tables display the weighted proportion of individuals who have been tested along with the 95% confidence interval (CI), the associated odds ratio with its CI, and the p-value. This information is provided for both the univariate and multivariable regression analyses. The multivariable analysis also includes controls for the region of residence, which are detailed in the appendix.
Among the 12,729 individuals who had no close relative test positive but reported COVID-19-like symptoms after July 1, 2020, 45.8% underwent testing. Women were more likely than men to get tested, with an odds ratio (OR) of 1.04 (95% CI [1.01–1.06], p < 0.01) in the multivariable analysis. Testing likelihood also decreased with age up to 35 years: compared to those aged 45–55 years, individuals aged 18–24 were 1.14 (95% CI [1.09–1.18]) times more likely to test, and those aged 25–34 were 1.04 (95% CI [1.00–1.07]) times more likely to test. Education level was positively associated with testing: compared to those who only completed high school, individuals with a college degree were 1.04 (95% CI [1.01–1.07]) times more likely to test, and those with a master’s degree were 1.08 (95% CI [1.04–1.12]) times more likely to test. Testing was also positively associated with income: compared to individuals in the lowest income decile, those in the sixth decile or above were 1.05 times more likely to test (95% CI [1.00–1.09]). No significant difference in testing was observed based on the country of origin once income and education were controlled for. Living in a household with four or more persons was negatively associated with testing (OR = 0.97, 95% CI [0.94–1.00] compared to households with two persons). Living in an urban center was associated with a higher likelihood of testing compared to living in a low-density area (OR = 1.05, 95% CI [1.02–1.08]). Individuals who worked exclusively from home during the first and second waves of the survey were more likely to test (OR = 1.07, 95% CI [1.03–1.12]), as were those who worked partly from home (OR = 1.06, 95% CI [1.03–1.09]). The timing of symptoms also played a role, with those experiencing symptoms before August 31 being 1.08 times more likely to test (95% CI [1.05–1.12]). Finally, suffering from a chronic disease slightly increased the likelihood of testing (OR = 1.03, 95% CI [1.00–1.05]).
Overall among the 5.360 individuals who had a close relative test positive, approximately 60% had been tested. There was no significant difference in testing likelihood between women and men. When controlling for all variables, the multivariate regression did not reveal any significant effects of age, income, or education on the likelihood of testing (at the 5% significance level).
However, compared to individuals born in metropolitan France to French parents, racialized first-generation immigrants were 1.10 times more likely to be tested (95% CI [1.02–1.19]). Individuals living in urban areas (either centers or clusters) were also more likely to be tested, with odds ratios of 1.05 (95% CI [1.00–1.10] for centers) and 1.05 (95% CI [1.00–1.09] for clusters). Living in a three-person household increased the likelihood of testing by 1.04 times (95% CI [1.00–1.09]) compared to living in a two-person household.
Among those who had a close relative test positive and also experienced symptoms, testing likelihood varied with the timing of symptoms. Individuals who experienced symptoms before July 1 were less likely to test compared to those with no symptoms (OR = 0.94, 95% CI [0.89–0.99]). Conversely, those who experienced symptoms between July 1 and August 31 were 1.21 times more likely to test (95% CI [1.08–1.35]), and those with symptoms after September 1 were 1.34 times more likely to test (95% CI [1.30–1.39]). Notably, among individuals who had a close relative test positive and experienced symptoms after September 1, 83% underwent testing. This sharp increase in testing rates during the summer of 2020 reflects the transition from a period of limited testing availability to widespread access.
Discussion
Our analysis of testing behavior, based on a representative random survey with detailed data on socio-economic variables and exposure to COVID-19 risk, reveals that the association between socio-economic conditions and testing behavior varies across different risk situations.
In the first scenario, where individuals have experienced symptoms but no household member has tested positive, we found that women were more likely than men to get tested. Testing was positively associated with being younger, wealthier, more educated, and living in an urban area. This aligns with previous studies (5, 7–9) which utilized administrative data and ecological variables to find similar trends.
Conversely, in the second scenario, where a household member had tested positive, the likelihood of testing was much higher overall, but in this case, the only socio-economic variable positively associated with testing was population density. Testing was strongly correlated with having experienced symptoms, particularly when symptoms occurred during periods of increased testing availability. These findings underscore the value of survey data in complementing administrative sources and highlight the importance of considering exposure risk when analyzing socio-economic inequalities in testing.
Nevertheless, this study has some limitations. First, while we observe socio-economic conditions, exposure to risk, and testing behavior at the individual level, we lack precise data on the timing of exposure and testing. We cannot determine whether a test was taken before or after a higher risk situation. Second, the survey collected only proxy information on ethno-racial status. Given that individuals from ethno-racial minorities are more likely to work in high-risk jobs (13), exploring this dimension could provide additional insights. Third, like many national population-based surveys, our study does not capture highly vulnerable groups such as undocumented migrants and homeless individuals, who are particularly affected by the pandemic (13). Additionally, people living in institutions like nursing homes were excluded from the sampling design. Finally, since the outcome under consideration is quite frequent in each subsample, adjusted odds-ratios may overestimate the association (17, 19).
Given these limitations, our findings should be interpreted with caution. Although we refrain from causal interpretations, some mechanisms can be suggested. As Bevan et al. (18) highlighted, a key motivation for testing is a sense of solidarity—testing to protect others by isolating oneself if the test result is positive. This motivation is stronger when the individuals needing protection are household members. When no close relative has tested positive, an individual testing positive risks introducing the virus to their household, which may increase their motivation to test. In contrast, if a close relative has already tested positive, isolation may be less effective as other family members might have already been exposed. Therefore, the motivation to test is likely stronger in the symptom-only scenario compared to the close-contact scenario.
Isolation also requires resources. Wealthier individuals are more likely to have the means to isolate themselves, such as living in larger homes or owning secondary properties. Lower-income individuals, who often live in more crowded conditions, may find it more challenging to isolate themselves, reducing the incentive to test. Additionally, Bajos et al. (12) found that social discrimination can weaken solidarity motives in vaccination, which could also apply to testing. Since poorer and less educated individuals experience more discrimination, this might further explain their lower testing rates. In summary, socio-economic status influences not only the ability to act on a positive test result, but also the solidarity motive for testing.
Other findings are more straightforward. Population density is positively associated with testing, possibly due to closer proximity to testing facilities in urban areas compared to rural ones. The positive association between education and testing in the “symptoms” group may reflect greater awareness and adherence to health recommendations. Additionally, having a close relative test positive may increase one’s awareness of infection risk.
Overall, our study provides moderate evidence that testing is associated with socio-economic variables. While the multivariable analysis showed no significant links between testing and income, education, socio-professional group, or country of origin in the “close contact” group, socio-economic status still influenced testing behavior in the “symptoms” group. This suggests that even if tests were available at no financial cost, socio-economic inequalities in access to testing were not entirely eliminated.
Generalizability
France’s approach to free COVID-19 testing differentiates it from many other countries where individuals incur out-of-pocket costs. This unique aspect of the French context may limit the generalizability of our findings to other countries. While testing for other infections, such as HIV or hepatitis, is also free in France in some cases without a medical prescription, the specific visibility, awareness, and susceptibility associated with COVID-19 might lead to different patterns for other infectious diseases.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: available on request to the Epicov team. Requests to access these datasets should be directed to ZnJlZGVyaWMucm9iZXJnZWF1QGluc2VybS5mcg==.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
P-YG: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. FJ: Formal analysis, Investigation, Writing – review & editing. AS: Formal analysis, Methodology, Writing – review & editing. JW: Conceptualization, Methodology, Writing – review & editing. NB: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing.
The EPICOV study group
The members of EPICOV study group: Nathalie Bajos (co-principal investigator), Josiane Warszawski (co-principal investigator), Guillaume Bagein, Emilie Counil, Florence Jusot, Claude Martin, Laurence Meyer, Philippe Raynaud, Alexandra Rouquette, Ariane Pailhé, Delphine Rahib, Patrick Sicard, Alexis Spire.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant agreement No. 856478).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. Interim guidance. (2020). Available at: https://iris.who.int/bitstream/handle/10665/331329/WHO-COVID-19-laboratory-2020.4-eng.pdf?sequence=1&isAllowed=y (Accessed December 27, 2024).
2. Richard, Z, Chabrol, F, Gautier, L, Zinszer, K, and Ridde, V. Considering social inequalities in health in COVID-19 response: insights from a French case study. Health Promot Int. (2023) 38:1–11. doi: 10.1093/heapro/daac173
3. Savard, A, Gabet, M, Richard, Z, Oliveira, SRDA, Coulibaly, A, Cazarin, G, et al. A descriptive comparison of mass testing during the COVID-19 pandemic in Montreal, Paris, Bamako, and Recife. Int J Public Health. (2022) 67:1604992. doi: 10.3389/ijph.2022.1604992
4. Raeesi, A, Kiani, B, Hesami, A, Goshayeshi, L, Firouraghi, N, Ebrahimi, SM, et al. Access to the COVID-19 services during the pandemic - a scoping review. Geospat Healt. (2022) 17. doi: 10.4081/gh.2022.1079
5. Green, MA, García-Fiñana, M, Barr, B, Burnside, G, Cheyne, CP, Hughes, D, et al. Evaluating social and spatial inequalities of large scale rapid lateral flow SARS-CoV2 antigen testing in COVID-19 management: an observational study of Liverpool, UK (November 2020 to January 2021). Lancet Reg Health Europe. (2021) 6:100107. doi: 10.1016/j.lanepe.2021.100107
6. Baggio, S, Jacquerioz, F, Salamun, J, Spechbach, H, and Jackson, Y. Equity in access to COVID-19 testing for undocumented migrants and homeless persons during the initial phase of the pandemic. J Migrat Health. (2021) 4:100051. doi: 10.1016/j.jmh.2021.100051
7. Dryden-Peterson, S, Velásquez, GE, Stopka, TJ, Davey, S, Lockman, S, and Ojikutu, BO. Disparities in SARS-CoV2 testing in Massachusetts during the COVID-19 pandemic. JAMA Netw Open. (2021) 4:e2037067. doi: 10.1001/jamanetworkopen.2020.37067
8. Riou, J, Panczak, R, Althaus, CL, Junker, C, Perisa, D, Schneider, K, et al. Socioeconomic position and the COVID-19 care cascade from testing to mortality in Switzerland: a population-based analysis. Lancet Public Health. (2021) 6:e683–91. doi: 10.1016/S2468-2667(21)00160-2
9. Vandentorren, S, Smaïli, S, Chatignoux, E, Maurel, M, Alleaume, C, Neufcourt, L, et al. The effect of social deprivation on the dynamic of SARS-CoV2 infection in France: a population-based analysis. Lancet Public Health. (2022) 7:e240–9. doi: 10.1016/S2468-2667(22)00007-X
10. Trevisan, C, Pedone, C, Maggi, S, Noale, M, di, M, Sojic, A, et al. Accessibility to SARS-CoV-2 swab test during the COVID-19 pandemic: did age make the difference? Health Policy. (2021) 125:1580–6. doi: 10.1016/j.healthpol.2021.10.002
11. Warsawski, J, Bagein, G, Derohyon, T, and Bajos, NEpiCoV study group. EpiCoV: a large population-based cohort in COVID-19 times: methodological issues. Journées de méthodologie statistique de l’INSEE. (2022) 14, 1–28.
12. Bajos, N, Spire, A, Silberzan, L, Sireyjol, A, Jusot, F, Meyer, L, et al. When lack of trust in the government and in scientists reinforces social inequalities in vaccination against COVID-19. Front Public Health. (2022) 10:908152. doi: 10.3389/fpubh.2022.908152
13. Gosselin, A, Warszawski, J, and Bajos, NEpiCoV Study Group. Higher risk, higher protection: COVID-19 risk among immigrants in France-results from the population-based EpiCoV survey. Eur J Pub Health. (2022) 32:655–63. doi: 10.1093/eurpub/ckac046
14. Wickham, H, Averick, M, Bryan, J, Chang, W, McGowan, L, François, R, et al. Welcome to the tidyverse. J Open Source Softw. (2019) 4:1686. doi: 10.21105/joss.01686
15. Lumley, T. Complex surveys: a guide to analysis using R. (2010). Wiley, Hoboken, New Jersey, USA.
16. G/Daniel, D, Sjoberg,, Whiting, K, Curry, M, G/Jessica, J, Lavery,, et al. Reproducible summary tables with the gtsummary package. R J. (2021) 13:570–80. doi: 10.32614/RJ-2021-053
17. Barros, AJ, and Hirakata, VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. (2003) 3:21. doi: 10.1186/1471-2288-3-21
18. Bevann, I, Ba14xter, MS, Stagg, HR, and Street, A. Knowledge, attitudes, and behavior related to COVID-19 testing: a rapid scoping review. Diagnostics (Basel). (2021) 11:1685. doi: 10.3390/diagnostics11091685
19. Yorlets, RR, Lee, Y, and Gantenberg, JR. Calculating risk and prevalence ratios and differences in R: developing intuition with a hands-on tutorial and code. Ann Epidemiol. (2023) 86:104–9. doi: 10.1016/j.annepidem.2023.08.001. Erratum in: Ann Epidemiol. 2024;99:47. doi: 10.1016/j.annepidem.2024.09.003
Keywords: COVID-19, socio-economic inequalities, testing, random survey, France
Citation: Geoffard P-Y, Jusot F, Sireyjol A, Warszawski J and Bajos N (2025) Socio-economic inequalities in access to COVID-19 tests in France in 2020: evidence from the EPICOV socio-epidemiological cohort. Front. Public Health. 12:1434370. doi: 10.3389/fpubh.2024.1434370
Edited by:
Hilde Langseth, Cancer Registry of Norway, NorwayReviewed by:
Irina Kislaya, Bernhard Nocht Institute for Tropical Medicine (BNITM), GermanyKai-Wen Cheng, Governors State University, United States
Copyright © 2025 Geoffard, Jusot, Sireyjol, Warszawski and Bajos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pierre-Yves Geoffard, cGllcnJlLXl2ZXMuZ2VvZmZhcmRAcHNlbWFpbC5ldQ==
 Pierre-Yves Geoffard
Pierre-Yves Geoffard Florence Jusot3
Florence Jusot3 Antoine Sireyjol
Antoine Sireyjol Nathalie Bajos
Nathalie Bajos