- 1Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
- 219 to Zero Inc., Calgary, AB, Canada
- 3Alberta Cervical Cancer Screening Program, Alberta Health Services, Calgary, AB, Canada
- 4Population and Public Health, Alberta Health Services, Calgary, AB, Canada
- 5Community Health Sciences, University of Calgary, Calgary, AB, Canada
- 6Women’s College Hospital Institute of Virtual Care and Systems Solutions, Toronto, ON, Canada
- 7Department of Family and Community Medicine and Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada
- 8Faculty of Nursing, University of Calgary, Calgary, AB, Canada
Introduction: Human papillomavirus (HPV) testing as a method of cervical cancer screening can be performed by healthcare providers or by patients through self-sampling directly in the community, removing several barriers experienced by under screened populations. The objective of this scoping review was to determine which HPV self-sampling implementation and engagement strategies have been used to engage under screened populations (i.e., Indigenous, newcomer, and rural and remote communities) in cervical cancer screening.
Methods: A scoping review was conducted searching MEDLINE, CINAHL, EMBASE, Cochrane Library, and SocINDEX from inception to August 2023. The inclusion criteria were: (1) Indigenous, newcomer, and rural and remote communities; (2) countries identified as members of the Organization for Economic Co-operation and Development; and (3) intervention included HPV self-sampling. The review was registered prior to conducting the search (https://osf.io/zfvp9).
Results: A total of 26 studies out of 2,741 studies met the inclusion criteria. In-person engagement with trusted community leaders was the most widely used and accepted recruitment and engagement strategy across all three populations. Six out of seven studies with Indigenous communities distributed HPV self-sampling kits to eligible participants in person in a clinical setting for collection on site or at home. Similarly, nine of the identified studies that engaged newcomers recruited participants in person through the community, where eligible participants were either given a kit (n = 7) or received one in the mail (n = 2). Lastly, of the 10 identified studies engaging rural and remote participants in HPV self-sampling, six recruited eligible participants in person at various community locations and four used electronic medical records or registries to identify and mail kits to participants.
Discussion: HPV self-sampling through in person kit distribution and mail out of HPV self-sampling kits is an effective way to increase participation rates amongst under screened populations.
1 Introduction
With the global call to eradicate cervical cancer, human papillomavirus (HPV) testing has been identified as one of the action pillars to increase cervical cancer screening participation (1, 2). People who develop cervical cancer are typically those that are under screened or have never been screened before (3). This includes populations such as those who do not have access to a general practitioner, ethnic minorities, new immigrants and refugees (i.e., newcomers), those of lower socioeconomic status, those who live in rural and remote areas, or identify as Indigenous (i.e., individuals who inhabited a geographical region before or during the arrival of different cultures or ethnic origins) (4–10).
Traditional cervical cancer screening uses liquid-based cytology, collected by clinicians via Papanicolaou (Pap) tests, at regular screening intervals (11). However, several barriers have been identified when receiving a Pap test such as discomfort, difficulty making an appointment, embarrassment, lack of time, and limited access to transportation (12). Therefore, HPV testing removes several of these barriers to screening as the sample can be collected by the patient themselves, either in a clinical setting or at home (i.e., HPV self-sampling). Furthermore, it has been shown that HPV testing can identify cervical pre-cancer earlier than screening with the Pap test and may lower the likelihood of developing cervical cancer (13).
Previous research has tested the feasibility and acceptability of HPV self-sampling among under screened or never screened populations, reporting that HPV self-sampling is easy to understand, complete, and participants would be willing to use this method again (14–16). Furthermore, in Canada, recent pilot projects indicate widespread acceptance of HPV testing and self-sampling among never- and under-screened (9, 13, 17) and First Nations populations (7, 18). As a result, HPV testing has gained global acceptance as the leading approach to cervical cancer screening to improve patient outcomes (19). This strategy has been adopted by countries around the world (20) including Australia (21), the United Kingdom (22), and the Netherlands (23) and, in 2024, Canadian provinces such as British Columbia introduced HPV testing as part of their provincial screening program making it available for all residents (24).
As other regions prepare to pilot or fully implement HPV testing as part of their screening programs, it is essential to understand and consider the needs of never- and under screened populations to increase cervical cancer screening participation rates. For example, the Canadian province of Alberta is preparing to pilot HPV self-sampling in 2024 amongst Indigenous, newcomer, and rural and remote communities, as these populations were identified through a series of geospatial and population specific studies conducted in Alberta to identify hot spots where cancer screening rates are the lowest (25). However, there is limited synthesized evidence on best practices to meaningfully engage these communities in HPV self-sampling. Therefore, to inform the pilot of HPV self-sampling in the Canadian province of Alberta, this scoping review aimed to answer the following questions:
1. What HPV self-sampling pilot and program implementation strategies have been used to recruit or engage Indigenous, newcomer, and rural and remote populations in cervical cancer screening?
2. What are the key considerations (i.e., barriers, facilitators, etc.) for implementing HPV self-sampling among Indigenous, newcomer, and rural and remote populations?
2 Methods
A scoping review was conducted following the methodological framework by the Joanna Briggs Institute (26). A comprehensive published protocol can be accessed1 and we reported our process according to the PRISMA Extension for Scoping Reviews (27) (Supplementary Material Table 1).
2.1 Search strategy
After reviewing the search terms used in previous reviews, as well as the key words from relevant peer-reviewed articles on HPV self-sampling, key search terms were piloted in three databases (MEDLINE, CINAHL, Embase) to identify seed articles relevant to the research question. The seed articles were then analyzed for index terms and text terms to ensure comprehensiveness and used to construct a final list of keywords and subject headings for the formal scoping review. A search was conducted in August 2023 from inception of the following five electronic databases: MEDLINE, CINAHL, EMBASE, Cochrane Library, and SocINDEX, to identify articles that meet the inclusion criteria (Supplementary Material Table 2). The researchers also scanned the reference lists of eligible studies, and those of relevant review articles to yield relevant articles.
2.2 Inclusion and exclusion criteria
Once the searches were complete, the datasets were uploaded on EndNote 20 (Berkeley, California, United States), and two researchers screened a random 10% sample of the articles in tandem for eligibility criteria. Any conflicts were resolved through discussion to reach consensus. Upon reaching inter-user agreement and consistency, the researchers then screened titles and abstract in duplicate. Conflicts were resolved through discussion, and when required, a third researcher was engaged to resolve any remaining disagreements. After screening the titles and abstracts, the researchers conducted a full text review of the articles, including hand searching the reference lists to identify additional relevant articles. Study eligibility was determined by the following criteria, guided by the PICO (Patient, Intervention, Comparison, Outcome) Framework (28):
∙ Population: Studies were included if their population included adults (18+), were conducted in one of the 38 countries identified as members of the Organization for Economic Co-operation and Development (OECD) (29), and from one of the following populations:
◦ Indigenous: Individuals who inhabited a geographical region before or during the arrival of different cultures or ethnic origins (10).
◦ Newcomer: ‘Newcomer’ is a heterogeneous term used to define individuals who are recent immigrants or refugees to a country (30).
◦ Rural and remote: Individuals living outside of the urban commuting zone and isolated from neighboring communities, respectively (31).
∙ Intervention: Studies that tested HPV self-sampling or physician-supported self-sampling via opt-in (i.e., eligible individuals are mailed a letter prompting them to register for HPV self-sampling) and opt-out (i.e., eligible individuals are directly sent an HPV self-sampling kit) for at-home testing, provider clinics, community centers, gatherings, or Community Health Ambassadors (CHA) were included. Studies that tested physician-collected samples or non-HPV cervical screening (i.e., Pap tests, etc.) were excluded.
∙ Comparator: Any comparator was considered appropriate for inclusion in this review (i.e., opt in vs. opt out, physician HPV testing, Pap tests, no testing, etc.).
∙ Outcome: Study outcomes were broad and could include testing uptake, program acceptance, participation and testing rates, identification of barriers, facilitators, attitudes, and behaviors toward self-sampling. Studies that measured cost effectiveness or non-patient-centered outcomes were excluded (i.e., health system impacts).
∙ Study Design: The researchers included the following study designs: Experimental and quasi-experimental study designs (i.e., randomized controlled trials, non-randomized controlled trials); observational studies (i.e., prospective and retrospective cohort studies); case–control studies and analytical cross-sectional studies; descriptive observational study designs (i.e., case series, individual case reports and descriptive cross-sectional studies); qualitative studies; editorials; gray literature including conference abstracts, reports from health authorities and healthcare organizations in Canada and other global leaders. Secondary studies—i.e., systematic reviews, meta-analyses, scoping reviews and literature reviews—were excluded.
2.3 Data extraction
Two researchers conducted a pilot data extraction on 10% of the articles to reach agreement and consistency. Once these parameters were agreed upon, the researchers divided the remaining articles in half, and independently extracted the following data: Title; Authorship; Year of publication; Country where the study was conducted in; Population and sample size; Research methods; Study aim/research questions; Description of intervention; Outcomes (primary and secondary); Key findings relevant to the research questions (Supplementary Material Table 3).
3 Results
A total of 26 studies met the inclusion criteria. Seven studies specifically addressed Indigenous engagement while nine and 10 addressed newcomer and rural/remote communities, respectively (Figure 1).
3.1 Indigenous population HPV self-sampling studies
3.1.1 Population characteristics
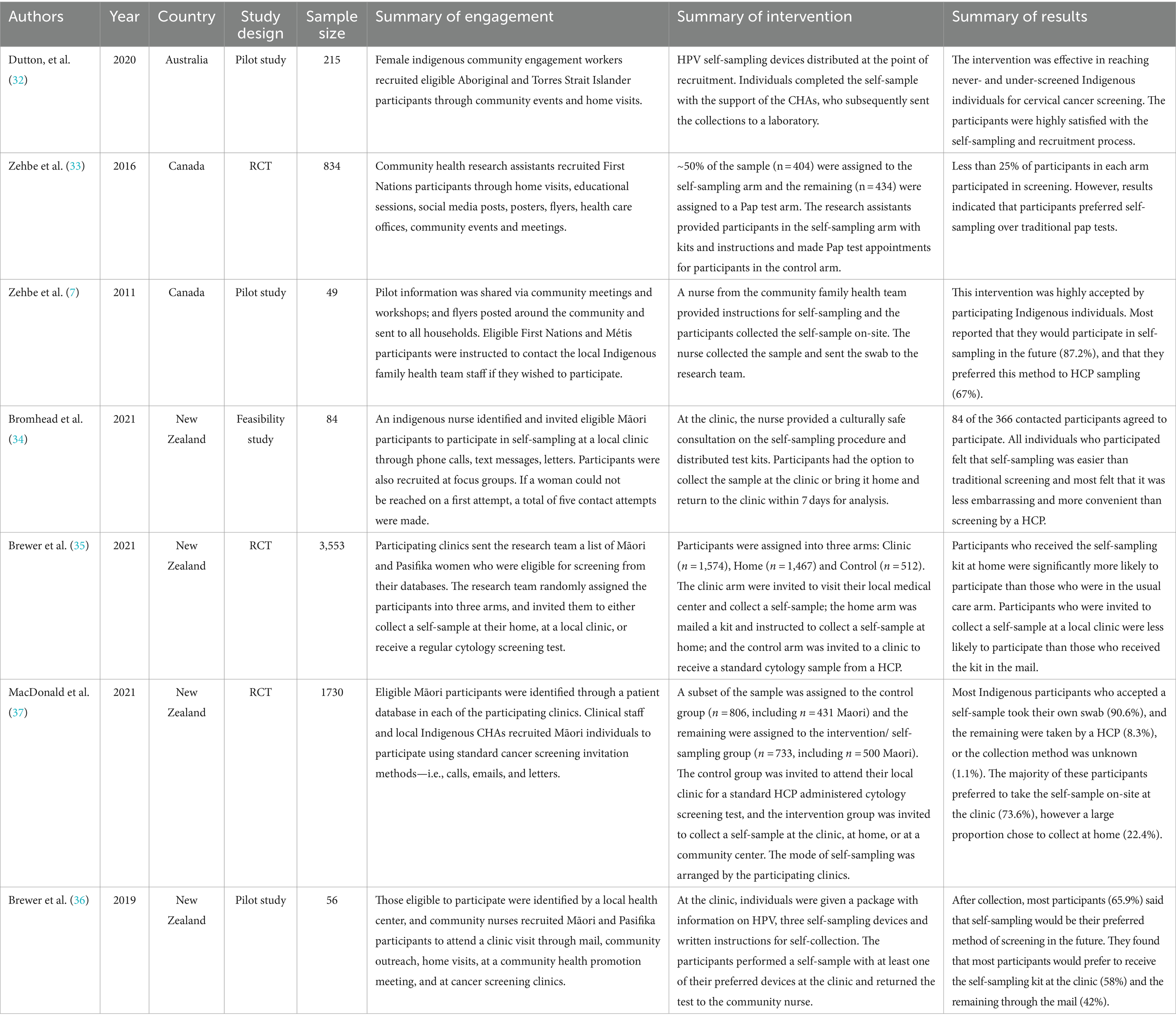
Seven studies recruited Indigenous populations to participate in HPV self-sampling programs (n = 49–3,553 participants/study; Table 1) (7, 32–37). These studies were conducted in Australia (n = 1), Canada (n = 2) and New Zealand (n = 4). Eligible people came from Māori (n = 4), First Nations (n = 2), Métis (n = 1), and Indigenous Australian (n = 1) communities. Participants’ ages varied across studies but ranged from 25 to 69 years old.
3.1.2 Engagement and recruitment strategies
Several studies (n = 4) identified eligible participants through health registries or/databases, and clinical patient records (34–37). Once identified, these participants were recruited to participate in HPV self-sampling in a variety of ways. Four studies called, texted, or sent letters to eligible individuals inviting them to participate (34–37). Two of these studies also used door-to-door recruitment strategies to recruit participants (36, 37), with one study using trusted indigenous community healthcare workers to invite individuals to participate (37). In these studies, interested participants could be enrolled immediately by members of the research team if they expressed interest (36, 37).
The remaining studies (n = 3) identified eligible participants through a community search strategy (7, 32, 33). Two studies used community events to recruit eligible participants in real-time (32, 33). However, in combination with community event recruitment, these two studies also recruited through home visits (32, 33). One study recruited participants by distributing flyers at community events such as parenting workshops; and through newsletters delivered directly to households in the community (7). Interested participants were asked to contact relevant CHAs to enroll in the study (7).
3.1.3 HPV self-sampling interventions
One study included a mailed component for HPV self-sampling kits as part of their study, where one group of participants were mailed kits to participants in an opt-out model and were instructed to self-sample and return their kits to the research team by mail (35).
All six remaining studies distributed HPV self-sampling kits to participants in person (7, 32–34, 36, 37). In total, five studies provided participants with a self-sampling kit in a clinical setting (7, 33–37). Four studies requested that participants collect the sample at the clinic and return the kit to the healthcare provider (HCP) immediately following collection (7, 33, 35, 36); and two studies gave participants the option to collect either at the clinic, or at home and return their sample to researchers later (34, 37).
Only one study provided self-sampling kits to participants at home or in community settings for immediate collection (32). These participants were given a self-sampling kit by female community engagement workers appointed by the Local Aboriginal Land Council, and were instructed to self-collect in their home, or at the community setting where they were recruited (32).
Two studies offered participants in their intervention groups the choice of a Pap test instead of an HPV self-sampling test (33, 37), however the remaining studies provided self-sampling as the only option to the intervention groups (7, 34–36).
3.1.4 Participation rates
In the two studies that gave participants a choice between HPV self-sampling kits and a Pap test, self-sampling was chosen more often (33, 37). In one study, significantly more Māori participants chose self-sampling (73.6%) over Pap testing (13.9%; p < 0.001); and across this study population, individuals who were offered a self-sampling kit were 2.8 times more likely to participate in screening than those who were not offered (p < 0.001) (37). Similarly, another study found that uptake for self-sampling was slightly higher (20.6%) than Pap testing (16.0%), when both were offered to Indigenous participants, though results were not significant (p = 0.694, 33).
The one study that used mailed-out kits found that participation rates in Māori individuals that were mailed a kit to their homes were significantly higher than those offered regular care (35). They determined that Māori individuals who are mailed a self-sampling kit were 10 times more likely to participate in screening than those who were assigned regular care (i.e., Pap test; Odds Ratio = 9.7; 95%CI 3.0-31.5) (35).
Four studies explored outcomes that were not directly related to participation rates (32–34, 36). These studies were not designed to report on participation rates, because all individuals enrolled in the study completed a self-sample (32–34, 36). Instead these studies assessed factors like acceptability (32, 34, 36), positivity rates (33), and feasibility (34), which were not captured in the scope of this review.
3.1.5 Barriers and facilitators to self-sampling
HPV self-sampling was generally accepted as a screening tool for cervical cancer. In several studies, participants reported that self-sampling was an easy and convenient way to screen for cervical cancer (7, 32, 34, 36). Three studies found that participants would consider mail-out options to be acceptable as a means for reducing any barriers to accessing a clinic (32, 35, 36). In addition, four studies identified the importance of having an Indigenous healthcare worker or community ambassador to help build trust among study participants (7, 33, 34, 37).
3.2 Newcomer population HPV self-sampling studies
3.2.1 Population characteristics
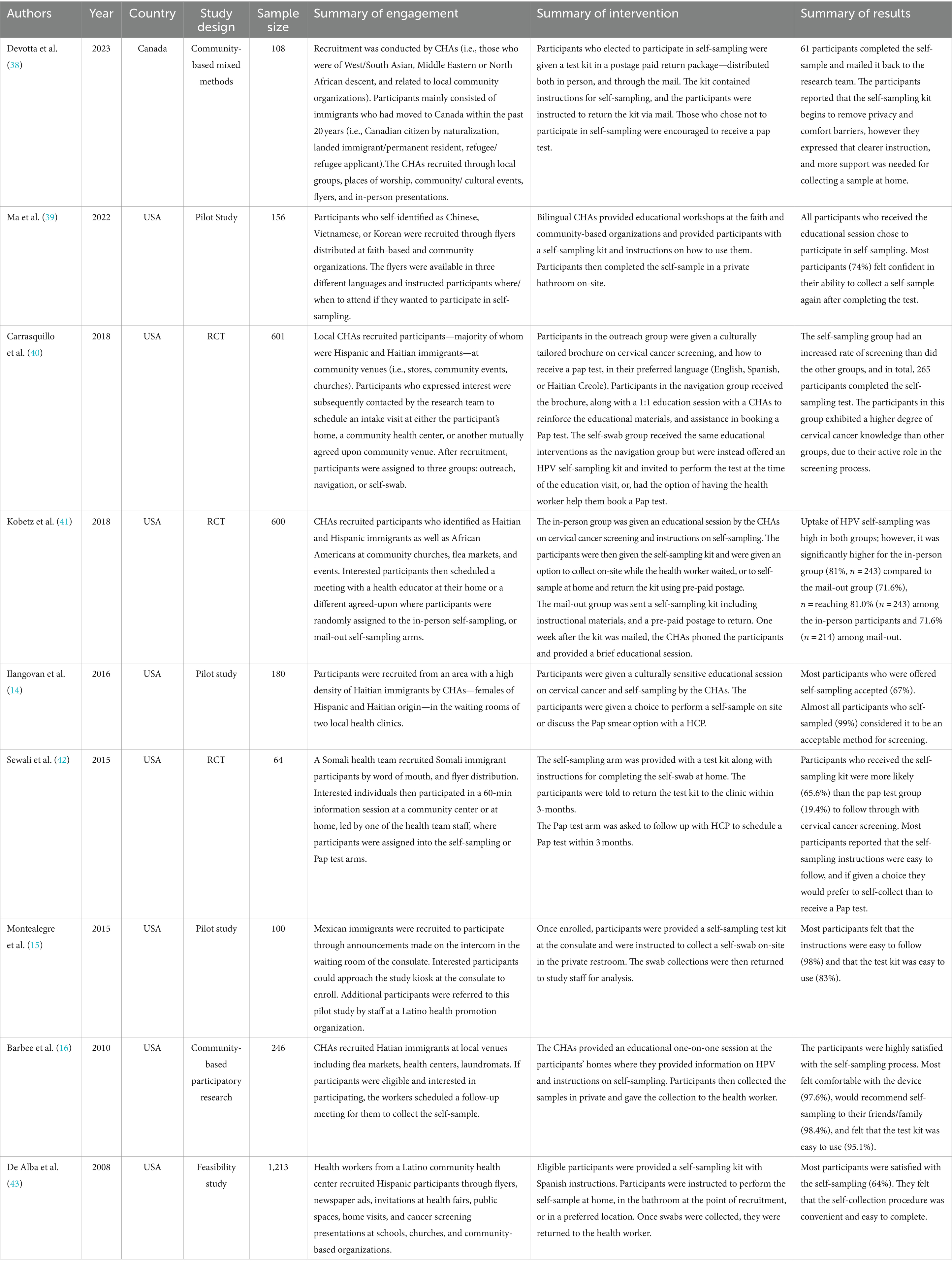
Nine studies were identified where the target population were newcomers who were eligible for cervical cancer screening (n = 64–1,213 participants/ study; Table 2) (14–16, 38–43). Participants were living in the United States (n = 8) or Canada (n = 1). Study participants identified as Haitian, Hispanic, Chinese, Vietnamese, Korean, Middle Eastern, North African, Black, and Somali.
3.2.2 Engagement and recruitment strategies
Of the studies that looked at HPV self-sampling amongst newcomer communities, all nine used in-person engagement to identify and recruit eligible participants. Eight studies used individuals who were familiar or identified with the communities they served—classified as “community champions” (38), “community health workers” (14, 16, 39, 40, 42, 43), or “community health educators” (41)—to recruit eligible participants. In comparison, one study recruited participants through staff and public announcements in the waiting area of a Consulate (15).
Through CHAs, several approaches were used to engage newcomers including direct (i.e., presentations, door-to-door, canvassing, etc.), or passive (i.e., flyers, posters, advertisements in the newspaper, etc.) recruitment at various locations in the community such as neighborhood associations, tea parties, parent groups (38); community events like health fairs (38–43); places of worship (38–41); stores or other public spaces (i.e., laundromats) (16, 40, 42, 43); public health clinics (14, 16); and flea markets (16, 41).
3.2.3 HPV self-sampling interventions
After identifying eligible participants, CHAs then provided education on HPV self-sampling and cervical cancer at locations including: the homes of participants, community organizations, or other mutually agreed upon locations. At these locations, participants were given the option to either immediately self-collect with the support of a CHA (16, 39, 40), self-collect later (i.e., at home) (38, 42), or both (40, 43).
In addition to distributing the kits in-person, two studies also sent HPV self-sampling kits directly to the homes of eligible participants who were identified through various engagement strategies and opted-in to participate (38, 41). Furthermore, in the study by Kobetz et al. (2018), participants who received their HPV self-sampling kit in the mail also received HPV education over the phone by a CHA. Those that self-collected at home were instructed to either return the HPV self-sampling kits in a prepaid envelope via mail (38, 41) or return it to the CHA (42, 43), for laboratory processing.
Two studies recruited participants at either a Consulate (15) or public health clinics (14), and immediately provided eligible participants with the HPV self-sampling kit for collection on-site. When participants were recruited through the public health clinics, the CHA identified as either Latina or Haitian and were instructed to only recruit Latina or Haitian patients (14).
Education and materials provided to participants about HPV self-sampling were provided in multiple languages and the CHAs were able to verbally translate and communicate with participants (15, 16, 38–42).
3.2.4 Participation rates
Overall, in-person community education and recruitment through CHAs were highly effective at engaging eligible participants in HPV self-sampling. A study by Kobetz et al. found that when newcomers were engaged and given an HPV self-sampling kit in-person, participation rates were higher (81.0%) than for those that were sent a kit in the mail after being engaged in person (71.6%; p < 0.01, 41). Carrasquillo et al. reported 64.0% participation when newcomers were recruited at locations in the community such as stores and places of worship (40). One study by Ilangovan et al. reported 67.0% participation when CHAs were used to recruit participants of the same ethnicity at a public health clinic (14). Carrasquillo et al. also reported participation rates of 76.0% amongst a group of participants who were not allocated to the HPV self-sampling arm of the study but were given the option to participate once the study was over (40). Studies by De Alba et al. and Ma et al. reported that 1,213 and 156 newcomers participated in HPV self-sampling, respectively, through in-person recruitment at community events, places of worship, etc., but did not report the number of people who were invited to participate (39, 43). Therefore, we are unable to assess the participation rate.
When comparing HPV self-sampling participation rates to Pap testing, Sewali et al. found that newcomers were more likely to participate in cervical cancer screening via self-sampling (65.6%) compared to Pap testing (19.4%) within a clinical setting (p = 0.0002) (42). Similarly, Devotta et al. found that 61 participants mailed back their sample and only 23.6% of the participants who chose not to participate in self-sampling went for a Pap test (38). Lastly, studies by Montealegre et al. and Barbee et al. were not designed to report the participation rates (15, 16).
3.2.5 Barriers and facilitators to self-sampling
Overall, the use of CHAs made participants more confident to perform self-collection and likely resulted in high participation rates (14, 16, 38, 41). Participants also expressed that through the education sessions they gained a greater knowledge of cervical cancer and the importance of screening (40), and appreciated the convenience and privacy of the test compared to the Pap test (15).
However, a few barriers were noted which included worry that the test would be uncomfortable, concerns of how their partner (i.e., spouse) would react (38); and fear of sampling incorrectly (15, 16, 38). The study by Kobetz et al. also found that participants were uncomfortable mailing their samples at government-run post offices due concerns around immigration status (41).
3.3 Rural and remote population HPV self-sampling studies
3.3.1 Population characteristics
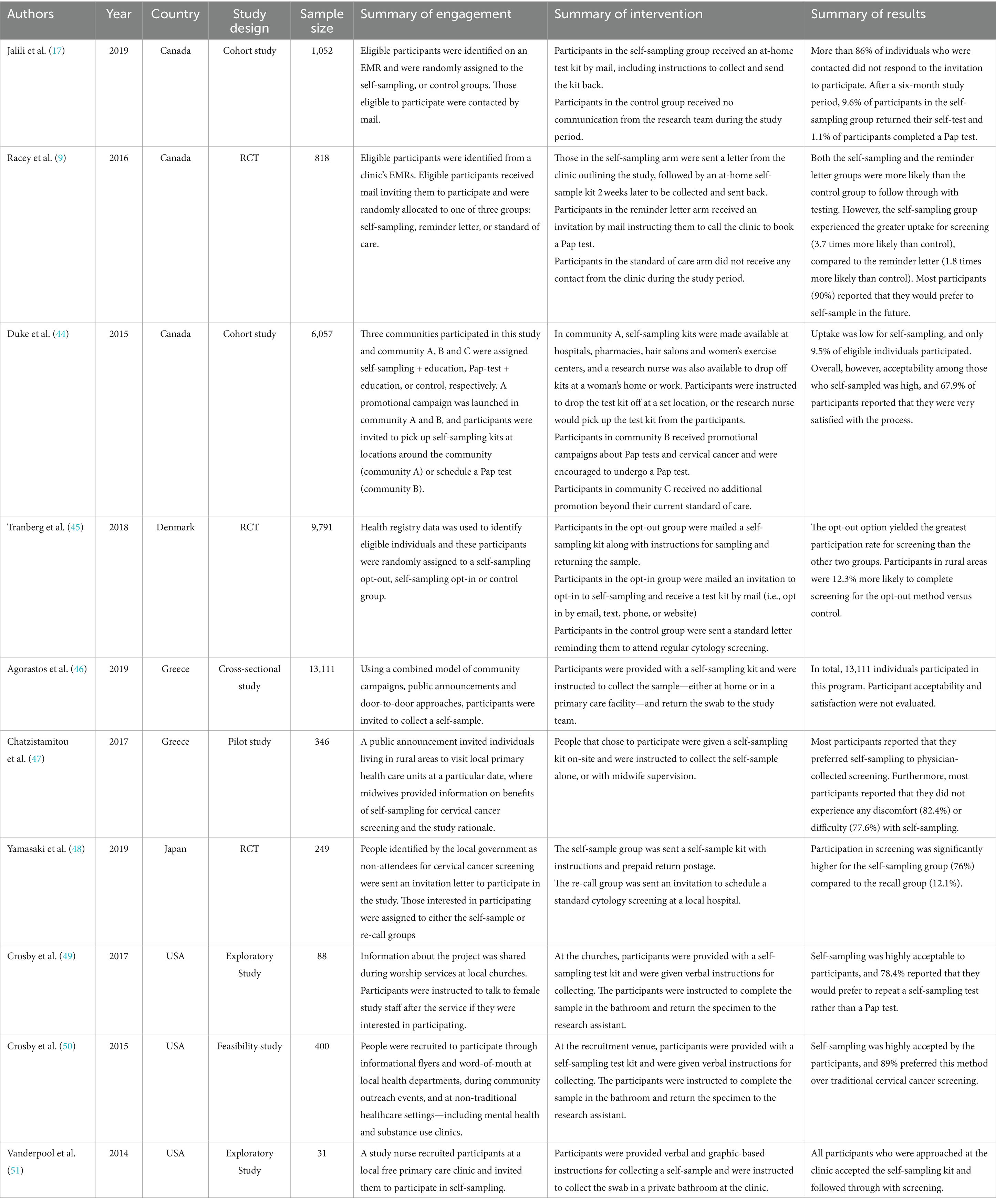
In total, 10 studies were identified where the target population were individuals eligible for cervical cancer screening residing in rural and/or remote areas in Canada (n = 3), Denmark (n = 1), Greece (n = 2), Japan (n = 1), and the United States (n = 3) (n = 31–13,111 participants per study; Table 3) (9, 17, 44–51). Researchers defined the study population’s eligibility as those living in rural or semi-rural area codes and regions, those who are geographically isolated, or those living in remote areas.
3.3.2 Engagement and recruitment strategies
Half of the included studies (n = 5) used a form of in-person community engagement to identify and recruit eligible participants for HPV self-sampling (44, 46, 47, 49, 50). Methods of community engagement varied across studies, but these strategies included education and handing out kits through midwives (46) or nurses (44); visiting the homes of people in rural and remote areas (44); recruitment during worship services at rural and remote churches (49); during community outreach events and in non-traditional healthcare settings such as mental health clinics and substance treatment centers (50); at community settings like hair salons and women’s exercise centers, hospitals and pharmacies (50); and public messaging or campaigns to notify eligible participants of where they could go to access an HPV self-sampling kit (i.e., local primary healthcare units) were also used to recruit participants (46, 47).
Four studies also identified eligible people to participate in HPV self-sampling through electronic medical records (EMRs) or registry data (9, 17, 45, 48). These studies all used mail-out options (opt-in or opt-out) to invite individuals to participate. The least used engagement strategy among studies of those living in rural or remote areas was recruitment through a primary health care facility (n = 1, 51). Participants in this study were approached by a nurse during their visit to a free health care facility and were asked to self-administer an HPV test.
3.3.3 HPV self-sampling interventions
The same studies that recruited participants through EMRs or registries (9, 17, 45, 48) provided HPV self-sampling kits by mail and instructed them to return these kits through pre-paid postage (n = 4). However, these studies varied in their approaches–where some sent kits to all eligible individuals in an opt-out method without prior communication (9, 17, 45), and others sent invitations for participants to opt-in to this sampling method by providing consent through the mail or registering for a HPV self-sampling kit via email, text message, phone, or through a website (45, 48).
In contrast, many studies (n = 6) provided participants with the self-sampling kits at the point of recruitment and/or care (44, 46, 47, 49–51). In five studies, the participants were provided a self-sample kit by the person recruiting them and were instructed to self-collect at home (46), in public or private washrooms (49, 50), or at a clinic (47, 51). In one study, participants were invited to attend a clinic where the self-sampling kit was provided to them to self-collect under supervision of a midwife, or privately in the bathroom (47). Under these circumstances, the self-collected swabs were immediately given back to researchers for subsequent laboratory analysis. However, in contrast, one study that provided self-sample kits at the point of recruitment did so by distributing kits in public areas for participants to bring home, self-collect, and mail back to the research team for analysis (44).
3.3.4 Participation rates
Mailed-out self-sample kits consistently yielded greater participation rates than control groups who received routine care (i.e., those who were prompted to participate in a Pap test). One study found that when compared with a reminder to receive a Pap test, or no reminders at all, participants who received an HPV self-sample kit in the mail were 3.7 times more likely to participate in screening (9). Likewise, another study found that in comparison to Pap test participation (1.1%), mailed out HPV self-sample kits yielded a higher participation rate (9.6%); note that this rate reflects participation in both urban and rural areas however 27/50 participants total were from rural areas (17). Furthermore, one study found that in comparison to participation rates for individuals who received standard Pap test recall letters (12.6%), individuals who received the HPV self-sample kits in the mail were more likely to participate (76%) (48).
When provided with HPV self-sampling kits at locations such as hospitals, pharmacies, hair salons, and women’s exercise centers, 20.1% of those who obtained kits from these locations mailed them back to the research team (44). One study found that in comparison to the control group, an opt-out model yielded 12.3% higher participation rates, and in addition, participation rates in the opt-out model were 6.6% higher than an opt-in model (45). Similarly, another study reported high participation rates where 97.5% of identified individuals were eligible and participated in HPV self-sampling (46). Four studies were not designed to report on participation rates. Two of these, all individuals enrolled in the study completed a self-sample (47, 51) while the other two studies, recruited participants via community outreach and other non-traditional healthcare settings (49, 50).
3.3.5 Barriers and facilitators to self-sampling
Overall, participant-reported barriers to HPV self-sampling were limited across the included studies. Three studies reported that a subset of individuals in their sample populations felt pain or discomfort during the self-sampling procedure (47, 49, 50). One study also noted that some of their participants had minimal trust in the healthcare system, which could serve as a barrier for uptake (51). However, the acceptance for self-sampling was generally high among participants in the included studies (9, 17, 44–51).
4 Discussion
This scoping review examined several HPV self-sampling engagement and implementation strategies to increase cervical cancer screening participation among Indigenous, newcomer, and remote communities. For all three populations, in person recruitment (~70% of studies) was highly successful at identifying eligible participants; however, registries and EMRs were also used to identify eligible participants from Indigenous and rural and remote communities (~30% of studies; Tables 1–3). Regardless of the strategy used to identify participants, in-person kit distribution, mail-outs, or a combination of both methods were used among all three populations regardless of sample size. Additionally, in-person distribution resulted in generally greater uptake than mail-outs—especially when CHAs were used to engage directly with participants. Overall, participants among the identified studies were receptive to HPV self-sampling and would complete this form of screening again in the future.
4.1 Key considerations when engaging indigenous, newcomer, and remote communities in HPV self-sampling
4.1.1 Indigenous populations
HPV self-sampling has been identified as an easy, convenient, and an acceptable way to screen for cervical cancer among Indigenous populations (Table 3) (7, 32, 34, 36). Like newcomer and rural and remote engagement, included HPV self-sampling studies that utilized tailored engagement and education carried out by trusted voices from the community had successful participation rates and cited CHAs as an important channel for delivering the program (7, 33, 34, 37). Indigenous communities face several barriers when accessing health services such as geographical isolation, low health literacy, health system biases and racism (52); thus, CHAs have been proposed as an effective medium to reduce barriers and facilitate trusted health conversations (53). The use of CHAs is considered highly important for building and maintaining trust among underserved populations, and this peer-to-peer model has been proposed to increase the uptake of other cancer screening programs—such as breast and colorectal—in Indigenous communities (53). Additionally, while few studies have explored mailed-out approaches with Indigenous communities (36), participants indicated that mailing kits directly to their homes would be an acceptable way to reach them as it reduces access barriers by eliminating the need for clinic visits, which are mandatory for current screening practices (i.e., Pap test) (34–36). Ultimately, future HPV self-sampling studies (i.e., embedded within screening programs) should consider a combination of both in person engagement through CHAs as health advocates and explore participation through mail-out approaches which may be an acceptable way of engaging Indigenous communities in HPV self-sampling.
4.1.2 Newcomer populations
Unlike Indigenous and rural and remote participant identification, in-person engagement was the only method used to identify and recruit eligible newcomers to participate in HPV self-sampling in the identified studies - medical registries and EMRs were not used, which may be a function of how immigration data is stored and managed. For example, in Canada, the delivery of health services (i.e., provincial screening programs) is the responsibility of provincial governments which have their own health databases (54). In comparison, immigration data is managed nationally by the Immigration, Refugees and Citizenship Canada (IRCC); and unfortunately, not every province can link health and immigration data (54). As a result, it can be challenging to systematically identify newcomers who are eligible for cancer screening and may provide one explanation for why the identified studies in this review used direct engagement in the community to identify and recruit newcomers to participate in HPV self-sampling. Additionally, in-person engagement reduces several barriers faced by newcomers when accessing screening services such as limited access to primary care services, limited social support, and lack of knowledge on cervical cancer screening (55, 56). Therefore, it is not surprising that the implementation of CHAs to support and educate newcomers on HPV self-sampling resulted in participants feeling more confident to perform self-collection and resulted in high participation rates (14, 16, 38, 41). A recent study by Lofters et al., found that CHAs were highly successful at supporting and encouraging eligible newcomers to participate in HPV self-sampling by serving as trusted voices within their communities, conversing in first languages and having healthcare backgrounds (57). Like Indigenous engagement in cancer screening, CHAs have also been successful at engaging newcomers and other underserved populations in breast and colorectal cancer screening (58, 59). This provides important considerations when designing future HPV self-sampling engagement strategies that aim to eliminate barriers to screening such as language barriers which continue to be a main factor inhibiting newcomers from participating in cancer screening services (60). Ultimately, better integration and utilization of community and social services is crucial for supporting and increasing newcomer engagement in HPV self-sampling.
4.1.3 Rural and remote populations
Acceptance of HPV self-sampling was generally high among rural and remote communities, and participation rates were even higher when eligible individuals were engaged directly in the community compared to indirect approaches (i.e., mail-outs; Table 2). Other than one study with an Indigenous population (36), indirect approaches for kit distribution (i.e., direct mailouts) were only used with rural and remote populations within the identified studies. Interestingly, in-person community engagement was the most widely used strategy to recruit and distribute self-sampling kits to eligible rural and remote participants (44, 46, 47, 49–51). Like the identified studies with Indigenous and newcomer populations, the use of CHAs enabled direct and immediate education of cervical cancer screening and ease of access to self-sampling kits by utilizing pre-existing infrastructures in the community (i.e., churches, hair salons, etc.), making it easily accessible and convenient to be screened. As mentioned, CHAs are highly effective at improving the timely completion of breast, colorectal, and cervical cancer screening among underserved communities such as individuals living in rural and remote regions by bridging the gap between community needs and access to health services (61, 62). This may explain why the use of CHAs yielded higher participation rates compared to mail-out methods with rural and remote populations, however this requires further investigation. Regardless, both in person community engagement and mail-out options reduce key barriers faced by rural and remote communities when accessing health services such as barriers associated with geographical isolation and limited transportation (63). Like future work with Indigenous communities, future HPV self-sampling projects with rural and remote populations should consider ways to utilize the trusted voices of CHAs combined with mail-out approaches as alternatives to Pap testing (64).
4.2 Strengths and limitations
The scoping review has several strengths. Five databases were included in the search and most notably, two reviewers independently screened the articles and confirmed inter-rater reliability. A scoping review was selected for this review as it is a useful method for examining and mapping emerging evidence by identifying knowledge gaps, types of available evidence, understanding how research on a specific topic is conducted, etc. (65). Unlike systematic reviews, for example, scoping reviews do not necessarily provide a comprehensive analysis of the strength of evidence or the effectiveness of the interventions (65), however, that was not the purpose of this review.
The scoping review has several limitations. Most notably, Indigenous, newcomer, and rural and remote communities have varying definitions globally; and although these communities make up a large majority of the underscreened populations in some OECD countries, this may not be the case across all OECD countries. Another important limitation is that while this review focused on the strategies used to engage underscreened communities in HPV self-sampling, there are several other key considerations along the screening pathway (i.e., communication of results, clinical follow-up, etc.) that are important to understand when tailoring and implementing HPV self-sampling among diverse communities. Other limitations such as the inclusion of both qualitative and quantitative studies, make it harder to draw clear comparisons or conclusions across included studies. Lastly, this review was limited to papers written in English, which may have excluded key papers that focus on populations who speak several first languages.
5 Conclusion
Overall, Indigenous, newcomer, and rural and remote communities are accepting of HPV self-sampling as it reduces several barriers they experience when accessing health services such as cancer screening programs. Regardless of the method used to distribute the self-sampling kits (i.e., in person versus mail-out), the use of CHAs and pre-existing community events and infrastructures (i.e., churches, hair salons, clinics) was highly successful at increasing cervical cancer screening participation rates among all three populations. Evidently, CHAs are an important consideration in the implementation of HPV self-sampling; however, it is important to highlight that CHAs are regularly tasked to educate on multiple health topics at once and rarely have time dedicated to a single health topic (66). This may have implications on the integration of CHAs delivering health services directly in the community outside of pilot programs where dedicated resources and support are allocated to CHAs, emphasizing the importance of building capacity for health systems to support their integration (61). Additionally, this review highlights the importance of meaningful community engagement through tailored cervical cancer screening education that is culturally relevant and available in first languages. Future HPV self-sampling projects should take the time to understand the unique needs and barriers experienced by the populations they wish to engage and tailor their approaches accordingly. For example, distributing self-sampling kits in person, through mail-outs, or a combination of both approaches may all be appropriate strategies depending on the population and their previous experiences with screening services. Overall, participant engagement and recruitment is one of several important steps in the cancer screening pathway for achieving equitable participation and outcomes for underscreened populations through HPV self-sampling.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CF: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. CD'S: Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. BC: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. S-IO: Project administration, Writing – original draft, Writing – review & editing. HD: Project administration, Writing – original draft, Writing – review & editing. HY: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. JC: Supervision, Writing – original draft, Writing – review & editing. NI: Supervision, Writing – original draft, Writing – review & editing. SD: Methodology, Supervision, Writing – original draft, Writing – review & editing. JH: Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank our community, academic, and health system partners for their contributions in sharing their experiences implementing HPV self-sampling and supporting knowledge translation of this review.
Conflict of interest
MF, CF, CD’S, and JH were employed by the company 19 to Zero Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1430968/full#supplementary-material
Footnotes
References
1. World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. (2020). Available at: https://books.google.ca/books?hl=en&lr=&id=hsNqEAAAQBAJ&oi=fnd&pg=PA5&dq=World+Health+Organization.+Global+strategy+to+accelerate+the+elimination+of+cervical+cancer+as+a+public+health+problem.+World+Health+Organization%3B+2020+Dec+31&ots=Uv6RP_pK6O&sig=jBIn515EtHg4JWZ_ApcTiI5jleg#v=onepage&q=World%20Health%20Organization.%20Global%20strategy%20to%20accelerate%20the%20elimination%20of%20cervical%20cancer%20as%20a%20public%20health%20problem.%20World%20Health%20Organization%3B%202020%20Dec%2031&f=false (Accessed on 2024 May 3).
2. Daponte, N, Valasoulis, G, Michail, G, Magaliou, I, Daponte, AI, Garas, A, et al. HPV-based self-sampling in cervical Cancer screening: an updated review of the current evidence in the literature. Cancer. (2023) 15:1669. doi: 10.3390/cancers15061669
3. Spence, AR, Goggin, P, and Franco, EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. (2007) 45:93–106. doi: 10.1016/j.ypmed.2007.06.007
4. Ferdous, M, Lee, S, Goopy, S, Yang, H, Rumana, N, Abedin, T, et al. Barriers to cervical cancer screening faced by immigrant women in Canada: a systematic scoping review. BMC Womens Health. (2018) 18:165–13. doi: 10.1186/s12905-018-0654-5
5. Flores, Y, Shah, K, Lazcano, E, Hernández, M, Bishai, D, Ferris, DG, et al. Design and methods of the evaluation of an HPV-based cervical cancer screening strategy in Mexico: the Morelos HPV study the Morelos HPV study. Salud Publica Mex. (2002) 44:335–44. doi: 10.1590/S0036-36342002000400007
6. Lofters, A, Devotta, K, Prakash, V, and Vahabi, M. Understanding the acceptability and uptake of HPV self-sampling amongst women under- or never-screened for cervical Cancer in Toronto (Ontario, Canada): an intervention study protocol. Int J Environ Res Public Health. (2021) 18:9114. doi: 10.3390/ijerph18179114
7. Zehbe, I, Moeller, H, Severini, A, Weaver, B, Escott, N, Bell, C, et al. Feasibility of self-sampling and human papillomavirus testing for cervical cancer screening in first nation women from Northwest Ontario, Canada: a pilot study. BMJ Open. (2011) 1:e000030. doi: 10.1136/bmjopen-2010-000030
8. Mcdonald, JT, and Kennedy, S. Cervical Cancer screening by immigrant and minority women in Canada. J Immigr Minor Health. (2007) 9:323–34. doi: 10.1007/s10903-007-9046-x
9. Racey, CS, and Gesink, DC. Barriers and facilitators to cervical Cancer screening among women in rural Ontario, Canada: the role of self-collected HPV testing. J Rural Health. (2016) 32:136–45. doi: 10.1111/jrh.12136
10. United Nations. (2024). Who are indigenous peoples? Available at: https://www.un.org/esa/socdev/unpfii/documents/5session_factsheet1.pdf (Accessed May 3, 2024).
11. Canadian Task Force on Preventive Health Care. Recommendations on screening for cervical cancer. CMAJ Canadian Med Assoc J. (2013) 185:35–45. doi: 10.1503/cmaj.121505
12. Waller, J, Bartoszek, M, Marlow, L, and Wardle, J. Barriers to cervical cancer screening attendance in England: a population-based survey. J Med Screen. (2009) 16:199–204. doi: 10.1258/jms.2009.009073
13. Ogilvie, GS, Van Niekerk, D, Krajden, M, Smith, LW, Cook, D, Gondara, L, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. (2018) 320:43–52. doi: 10.1001/jama.2018.7464
14. Ilangovan, K, Kobetz, E, Koru-Sengul, T, Marcus, EN, Rodriguez, B, Alonzo, Y, et al. Acceptability and feasibility of human papilloma virus self-sampling for cervical Cancer screening. J Women's Health. (2016) 25:944–51. doi: 10.1089/jwh.2015.5469
15. Montealegre, JR, Mullen, PDL, Jibaja-Weiss, M, Vargas Mendez, MM, and Scheurer, ME. Feasibility of cervical Cancer screening utilizing self-sample human papillomavirus testing among Mexican immigrant women in Harris County, Texas: a pilot study. J Immigr Minor Health. (2015) 17:704–12. doi: 10.1007/s10903-014-0125-5
16. Barbee, L, Kobetz, E, Menard, J, Cook, N, Blanco, J, Barton, B, et al. Assessing the acceptability of self-sampling for HPV among haitian immigrant women: CBPR in action. Cancer Causes Control. (2010) 21:421–31. doi: 10.1007/s10552-009-9474-0
17. Jalili, F, O’Conaill, C, Templeton, K, Lotocki, R, Fischer, G, Manning, L, et al. Assessing the impact of mailing self-sampling kits for human papillomavirus testing to unscreened non-responder women in Manitoba. Curr Oncol. (2019) 26:167–72. doi: 10.3747/co.26.4575
18. Dick, A, Holyk, T, Taylor, D, Wenninger, C, Sandford, J, Smith, L, et al. Highlighting strengths and resources that increase ownership of cervical cancer screening for indigenous communities in northern British Columbia: community-driven approaches. Int J Gynecol Obstet. (2021) 155:211–9. doi: 10.1002/ijgo.13915
19. Chrysostomou, AC, Stylianou, DC, Constantinidou, A, and Kostrikis, LG. Cervical Cancer screening programs in Europe: the transition towards HPV vaccination and population-based HPV testing. Viruses. (2018) 10:729. doi: 10.3390/v10120729
20. Canadian Partnership Against Cancer. (2023). HPV primary screening and abnormal screen follow-up for cervical cancer. Available at: https://www.partnershipagainstcancer.ca/topics/hpv-primary-screening-environmental-scan/#:~:text=Several%20countries%2C%20including%20Australia%2C%20the,do%20the%20same%20in%20Canada (Accessed May 3, 2024).
21. Cancer Council Australia. National cervical screening program. (2024). Available at: https://www.health.gov.au/our-work/national-cervical-screening-program?language=und (Accessed May 15, 2024).
22. NHS England. (2023). Cervical screening: programme and colposcopy management. Available at: https://www.gov.uk/government/publications/cervical-screening-programme-and-colposcopy-management (Accessed May 3, 2024).
23. National Institute for Public Health and the Environment. (2024). FRamework for the execution of cervical Cancer population screening. Available at: https://www.rivm.nl/documenten/framework-for-execution-of-cervical-cancer-population-screening (Accessed May 3, 2024).
24. BC Cancer. (2024). Cervix Screening for Health Professionals. Available at: http://www.bccancer.bc.ca/screening/health-professionals/cervix#:~:text=About%20the%20Program&text=BC%20is%20transitioning%20from%20cytology,to%20have%20a%20Pap%20test (Accessed May 3, 2024).
25. Jessiman-Perreault, G, Law, J, Adhikari, K, Machado, AA, Moysey, B, Xu, L, et al. Geospatial analysis and participant characteristics associated with colorectal cancer screening participation in Alberta, Canada: a population-based cross-sectional study. BMC Health Serv Res. (2023) 23:1454–16. doi: 10.1186/s12913-023-10486-8
26. Munn, Z, Tufanaru, C, and Aromataris, E. Data extraction and synthesis. Am J Nurs. (2014) 114:49–54. doi: 10.1097/01.NAJ.0000451683.66447.89
27. Tricco, AC, Lillie, E, Zarin, W, O’Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
28. Schardt, C, Adams, MB, Owens, T, Keitz, S, and Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. (2007) 7:1–6. doi: 10.1186/1472-6947-7-16
29. Organization for Economic Co-operation and Development. (2024). Our global reach. Available at: https://www.oecd.org/about/members-and-partners/ (Accessed May 3, 2024).
30. Government of Canada. (2023). Newcomers to Canada. Available at: https://www.canada.ca/en/revenue-agency/services/forms-publications/publications/t4055/t4055-newcomers-canada.html (Accessed May 3, 2024).
31. Government of Canada. (2023). Legal aid service delivery in rural and remote communities across Canada: Issues and perspectives in the context of COVID-19. Available at: https://www.justice.gc.ca/eng/rp-pr/jr/laid-daide/summary-sommaire.html#:~:text=Remote%20communities%3A%20Those%20that%20have,is%20a%20significant%20determining%20factor (Accessed May 3, 2024).
32. Dutton, T, Marjoram, J, Burgess, S, Montgomery, L, Vail, A, Callan, N, et al. Uptake and acceptability of human papillomavirus self-sampling in rural and remote aboriginal communities: evaluation of a nurse-led community engagement model. BMC Health Serv Res. (2020) 20:1–9. doi: 10.1186/s12913-020-05214-5
33. Zehbe, I, Jackson, R, Wood, B, Weaver, B, Escott, N, Severini, A, et al. Community-randomised controlled trial embedded in the Anishinaabek cervical Cancer screening study: human papillomavirus self-sampling versus Papanicolaou cytology. BMJ Open. (2016) 6:e011754. doi: 10.1136/bmjopen-2016-011754
34. Bromhead, C, Wihongi, H, Sherman, SM, Crengle, S, Grant, J, Martin, G, et al. Human papillomavirus (Hpv) self-sampling among never-and under-screened indigenous māori, pacific and asian women in aotearoa New Zealand: a feasibility study. Int J Environ Res Public Health. (2021) 18:10050. doi: 10.3390/ijerph181910050
35. Brewer, N, Bartholomew, K, Grant, J, Maxwell, A, McPherson, G, Wihongi, H, et al. Acceptability of human papillomavirus (HPV) self-sampling among never- and under-screened indigenous and other minority women: a randomised three-arm community trial in Aotearoa New Zealand. Lancet Reg Health West Pac. (2021) 16:100265. doi: 10.1016/j.lanwpc.2021.100265
36. Brewer, N, Foliaki, S, Bromhead, C, Viliamu-Amusia, I, Pelefoti-Gibson, L, Jones, T, et al. Acceptability of human papillomavirus self-sampling for cervical-cancer screening in under-screened Māori and Pasifika women: a pilot study. N Z Med J. (2019) 132:21–31.
37. MacDonald, EJ, Geller, S, Sibanda, N, Stevenson, K, Denmead, L, Adcock, A, et al. Reaching under-screened/never-screened indigenous peoples with human papilloma virus self-testing: a community-based cluster randomised controlled trial. Aust N Z J Obstet Gynaecol. (2021) 61:135–41. doi: 10.1111/ajo.13285
38. Devotta, K, Vahabi, M, Prakash, V, and Lofters, A. Reach and effectiveness of an HPV self-sampling intervention for cervical screening amongst under- or never-screened women in Toronto, Ontario Canada. BMC Womens Health. (2023) 23:36–16. doi: 10.1186/s12905-023-02174-w
39. Ma, GX, Zhu, L, Zhai, S, Lin, TR, Tan, Y, Johnson, C, et al. Empowering low-income Asian American women to conduct human papillomavirus self-sampling test: a community-engaged and culturally tailored intervention. Cancer Control. (2022) 29:1–8. doi: 10.1177/10732748221076813
40. Carrasquillo, O, Seay, J, Amofah, A, Pierre, L, Alonzo, Y, McCann, S, et al. HPV self-sampling for cervical Cancer screening among ethnic minority women in South Florida: a randomized trial. J Gen Intern Med. (2018) 33:1077–83. doi: 10.1007/s11606-018-4404-z
41. Kobetz, E, Seay, J, Koru-Sengul, T, Bispo, JB, Trevil, D, Gonzalez, M, et al. A randomized trial of mailed HPV self-sampling for cervical cancer screening among ethnic minority women in South Florida. Cancer Causes Control. (2018) 29:793–801. doi: 10.1007/s10552-018-1055-7
42. Sewali, B, Okuyemi, KS, Askhir, A, Belinson, J, Vogel, RI, Joseph, A, et al. Cervical cancer screening with clinic-based pap test versus home HPV test among Somali immigrant women in Minnesota: a pilot randomized controlled trial. Cancer Med. (2015) 4:620–31. doi: 10.1002/cam4.429
43. De Alba, I, Anton-Culver, H, Hubbell, FA, Ziogas, A, Hess, JR, Bracho, A, et al. Self-sampling for human papillomavirus in a community setting: feasibility in Hispanic women. Cancer Epidemiol Biomarkers Prev. (2008) 17:2163–8. doi: 10.1158/1055-9965.EPI-07-2935
44. Duke, P, Godwin, M, Ratnam, S, Dawson, L, Fontaine, D, Lear, A, et al. Effect of vaginal self-sampling on cervical cancer screening rates: a community-based study in Newfoundland. BMC Womens Health. (2015) 15:1–9. doi: 10.1186/s12905-015-0206-1
45. Tranberg, M, Bech, BH, Blaakær, J, Jensen, JS, Svanholm, H, and Andersen, B. Hpv self-sampling in cervical cancer screening: the effect of different invitation strategies in various socioeconomic groups – a randomized controlled trial. Clin Epidemiol. (2018) 10:1027–36. doi: 10.2147/CLEP.S164826
46. Agorastos, T, Chatzistamatiou, K, Tsertanidou, A, Mouchtaropoulou, E, Pasentsis, K, Kitsou, A, et al. Implementation of HPV-based cervical Cancer screening combined with self-sampling using a midwifery network across rural Greece: the Grecoself study. Cancer Prev Res. (2019) 12:701–10. doi: 10.1158/1940-6207.CAPR-19-0192
47. Chatzistamatiou, K, Chatzaki, E, Constantinidis, T, Nena, E, Tsertanidou, A, and Agorastos, T. Self-collected cervicovaginal sampling for site-of-care primary HPV-based cervical cancer screening: a pilot study in a rural underserved Greek population. J Obstet Gynaecol (Lahore). (2017) 37:1059–64. doi: 10.1080/01443615.2017.1323197
48. Yamasaki, M, Abe, S, Miura, K, and Masuzaki, H. The effect of self-sampled HPV testing on participation in cervical cancer screening on a remote island. Acta Med Nagasaki. (2019) 62:55–61. doi: 10.11343/amn.62.55
49. Crosby, RA, Hagensee, ME, Fisher, R, Stradtman, LR, and Collins, T. Self-collected vaginal swabs for HPV screening: an exploratory study of rural black Mississippi women. Prev Med Rep. (2017) 7:227–31. doi: 10.1016/j.pmedr.2016.12.014
50. Crosby, RA, Hagensee, ME, Vanderpool, R, Nelson, N, Parrish, A, Collins, T, et al. Community-based screening for cervical cancer: a feasibility study of rural appalachian women. Sex Transm Dis. (2015) 42:607–11. doi: 10.1097/OLQ.0000000000000365
51. Vanderpool, RC, Jones, MG, Stradtman, LR, Smith, JS, and Crosby, RA. Self-collecting a cervico-vaginal specimen for cervical cancer screening: an exploratory study of acceptability among medically underserved women in rural Appalachia. Gynecol Oncol. (2014) 132:S21–5. doi: 10.1016/j.ygyno.2013.10.008
52. Nguyen, NH, Subhan, FB, Williams, K, and Chan, CB. Barriers and mitigating strategies to healthcare access in indigenous communities of Canada: a narrative review. Health. (2020) 8:112. doi: 10.3390/healthcare8020112
53. James, A, Chamberlain, D, Azar, D, and Sewell, L. Talking about health: community ambassadors as a health promotion strategy to increase breast and bowel cancer screening in regional Australia. Health Promotion J Australia. (2023) 34:246–54. doi: 10.1002/hpja.635
54. Urquia, ML, Walld, R, Wanigaratne, S, Eze, ND, Azimaee, M, McDonald, JT, et al. Linking national immigration data to provincial repositories: the case of Canada. Int J Popul Data Sci. (2021) 6:19. doi: 10.23889/ijpds.v6i1.1412
55. Szarewski, A, Cadman, L, Ashdown-Barr, L, and Waller, J. Exploring the acceptability of two self-sampling devices for human papillomavirus testing in the cervical screening context: a qualitative study of Muslim women in London. J Med Screen. (2009) 16:193–8. doi: 10.1258/jms.2009.009069
56. Oelke, ND, and Vollman, AR. “Inside and outside”: Sikh Women’s perspectives on cervical Cancer screening. Canadian J Nurs Res Archive. (2007) 39:175–90.
57. Lofters, A, Prakash, V, Devotta, K, and Vahabi, M. The potential benefits of “community champions” in the healthcare system. Healthcare Manag Forum. (2023) 36:382–7. doi: 10.1177/08404704231179911
58. Dunn, SF, Lofters, AK, Ginsburg, OM, Meaney, CA, Ahmad, F, Moravac, MC, et al. Cervical and breast Cancer screening after CARES: a community program for immigrant and marginalized women. Am J Prev Med. (2017) 52:589–97. doi: 10.1016/j.amepre.2016.11.023
59. Feltner, FJ, Ely, GE, Whitler, ET, Gross, D, and Dignan, M. Effectiveness of community health Workers in Providing Outreach and Education for colorectal Cancer screening in Appalachian Kentucky. Soc Work Health Care. (2012) 51:430–40. doi: 10.1080/00981389.2012.657296
60. Marshall, S, Vahabi, M, and Lofters, A. Acceptability, feasibility and uptake of HPV self-sampling among immigrant minority women: a focused literature review. J Immigr Minor Health. (2019) 21:1380–93. doi: 10.1007/s10903-018-0846-y
61. Roland, KB, Milliken, EL, Rohan, EA, Degroff, A, White, S, Melillo, S, et al. Use of community health workers and patient navigators to improve Cancer outcomes among patients served by federally qualified health centers: a systematic literature review. Health Equity. (2017) 1:61–76. doi: 10.1089/heq.2017.0001
62. Balcazar, H, Lee Rosenthal, E, Nell Brownstein, J, Rush, CH, Matos, S, and Hernandez, L. Community health workers can be a public health force for change in the United States: three actions for a new paradigm. Am J Public Health. (2011) 101:2199–203. doi: 10.2105/AJPH.2011.300386
63. Thorn, H, and Olley, R. Barriers and facilitators to accessing medical services in rural and remote Australia: a systematic review. Asia Pacific J Health Manag. (2023) 18:20–9. doi: 10.24083/apjhm.v18i1.1755
64. Kerner, J, Liu, J, Wang, K, Fung, S, Landry, C, Lockwood, G, et al. Canadian Cancer screening disparities: a recent historical perspective. Curr Oncol. (2015) 22:156–63. doi: 10.3747/co.22.2539
65. Munn, Z, Peters, MDJ, Stern, C, Tufanaru, C, McArthur, A, and Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. (2018) 18:143–7. doi: 10.1186/s12874-018-0611-x
66. Basu, P, Mahajan, M, Patira, N, Prasad, S, Mogri, S, Muwonge, R, et al. A pilot study to evaluate home-based screening for the common non-communicable diseases by a dedicated cadre of community health workers in a rural setting in India. BMC Public Health. (2019) 19:1–12. doi: 10.1186/s12889-018-6350-4
Keywords: human papillomavirus, self-sampling, cervical cancer screening, scoping review, under screened populations, community engagement
Citation: Fullerton MM, Ford C, D’Silva C, Chiang B, Onobrakpor S-I, Dievert H, Yang H, Cabaj J, Ivers N, Davidson S and Hu J (2024) HPV self-sampling implementation strategies to engage under screened communities in cervical cancer screening: a scoping review to inform screening programs. Front. Public Health. 12:1430968. doi: 10.3389/fpubh.2024.1430968
Edited by:
Redhwan Ahmed Al-Naggar, National University of Malaysia, MalaysiaReviewed by:
Jennifer McIntosh, The University of Melbourne, AustraliaSally Rose, University of Otago, New Zealand
Copyright © 2024 Fullerton, Ford, D’Silva, Chiang, Onobrakpor, Dievert, Yang, Cabaj, Ivers, Davidson and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madison M. Fullerton, TWFkaXNvbi5mdWxsZXJ0b25AdWNhbGdhcnkuY2E=
 Madison M. Fullerton
Madison M. Fullerton Caitlin Ford2
Caitlin Ford2 Chelsea D’Silva
Chelsea D’Silva Jia Hu
Jia Hu