
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health , 18 December 2024
Sec. Aging and Public Health
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1424613
This article is part of the Research Topic Frailty- and Age-Associated Diseases: Possibilities For Intervention (Volume 2) View all 7 articles
 Xiao-Ming Wang1,2†
Xiao-Ming Wang1,2† Yuan-Hui Zhang1†
Yuan-Hui Zhang1† Chen-Chen Meng1
Chen-Chen Meng1 Lu Fan1
Lu Fan1 Lei Wei1
Lei Wei1 Yan-Yang Li3*
Yan-Yang Li3* Xue-Zheng Liu1*
Xue-Zheng Liu1* Shi-Chao Lv1*
Shi-Chao Lv1*As the population ages, the prevalence of age-related frailty increases sharply, which increases the risk of poor health status of older adults, such as disability, falls, hospitalization, and death. Across the globe, frailty is moving toward the forefront of health and medical research. Currently, frailty is believed to be preventable and reversible, so the early identification of frailty is critical. However, there are neither precise biomarkers of frailty nor definitive laboratory tests and corresponding clinical testing techniques and equipment in clinical practice. As a result, the clinical identification of frailty is mainly achieved through the widely used frailty scale, which is an objective, simple, time-saving, effective, economical, and feasible measurement tool. In this narrative review, we summarized and analyzed the various existing frailty scales from different perspectives of screening and evaluation, aiming to provide a reference for clinical researchers and practitioners to judge and manage frail older people accurately.
As the life expectancy of the global population gradually increases with the advancement of medical treatment and the improvement of living standards, the problem of population aging is becoming increasingly serious, and how to face population aging positively has become the most important medical and social issue in the world (1). One of the major challenges facing an aging population is the increasing prevalence of age-related frailty, which is a state of reduced ability to cope with stimuli due to age-related declines in the physiological reserve capacity and function of multiple systems and organs (2). Several prospective cohort studies have shown that frailty is strongly associated with poor health and that frail older people are more likely to experience death (3), disability (4–6), falls (7), and hospitalization (2) than non-frail older adults. Although frailty poses a significant risk of adverse health outcomes in older adults, it is a dynamic and reversible disease, which means that it is preventable and controllable and that early recognition and interventions of frailty can halt its progression (2, 8). Early identification and diagnosis of frailty will help to maximize the reversal of its further progression, alleviate or delay underlying symptoms, control adverse clinical health outcomes such as recurrent hospitalization and death, maintain their functional status, and enhance their quality of life. Several studies have shown that timely recognition and intervention of frailty in the clinical setting or daily life can contribute to benefits for older adults (9, 10), and may even delay the onset of death in 3 to 5% of older adults (11). Clinical practice guidelines developed by the International Conference of Frailty and Sarcopenia Research (ICFSR) also recommend that adults 65 years old and older should be screened for frailty using a simple, validated, and rapid screening tool appropriate for the specific scenario and that all older adults considered to be frail or pre-frail should be further assessed for frailty (12). However, there are no standardized criteria regarding the selection of screening and assessment tools for frailty. Therefore, the development of efficient and practical screening and assessment tools for frailty should be a top priority in the field of frailty research. This paper provides an overview of the current state of research on screening and assessment tools for age-related frailty and the characteristics of commonly used frailty scales, with the aim of helping clinical researchers and practitioners to accurately judge and fine-tune the management of frail older adults.
The search database was PubMed. The retrieval time node ranged from January 2000 to December 2023. The retrieval strategy was optimized with the use of Boolean logical operators. The retrieval formula is ((“Frailty/diagnosis”[Mesh] OR “Frailty/epidemiology”[Mesh]) OR ((Frailties[Title/Abstract]) OR (Frailness[Title/Abstract])OR (Frailty Syndrome[Title/Abstract]) OR (Debility[Title/Abstract]) OR (Debilities[Title/Abstract]))) AND ((“2000/01/01”[Date - Publication]: “2023/12/31”[Date - Publication])). Then, we imported the transcript of all retrieved literature into Endnote Application. After briefly reading the title and abstract, articles not related to the screening or assessment of frailty were excluded. The specific inclusion criteria were defined as follows: (1) papers covered a population of older adults aged ≥60 years; (2) the paper’s main topic was screening and assessment of frailty; (3) the full text was accessible; (4) paper was presented in English. The exclusion criterion was that the paper was on the pathogenesis or interventions for frailty or its association with other diseases. The process of screening the literature was done independently by two researchers, followed by cross-checking. In case of disagreement between the two researchers, a third person was consulted to assist in the judgment. Next, we read the remaining literature and used an Excel sheet to record the screening or assessment scales addressed in each paper, selecting those that appeared ≥150 times. Finally, we read the literature pertaining to the above scales carefully and used another Excel sheet to document the content, focus, measurement patterns, and application scenarios of each scale for subsequent categorization and summarization.
At present, studies on screening and assessment tools for frailty have mostly focused on two areas: frailty-related biological markers and frailty-related scales. The development of frailty involves multiple complex pathophysiological processes such as chronic inflammatory responses, imbalances in energy metabolism, nutritional deficiencies, immune disorders, oxidative stress, and so on (13). In older frail patients, the levels of biological factors involved in these pathophysiologic processes are altered accordingly and can be biomarkers of frailty. To date, biomarkers of age-related frailty can be categorized as inflammatory response-related biomarkers (C-reactive protein, interleukin-6, tumor necrosis factor) (14–17), metabolism-related biomarkers (muscle growth inhibitor, 25-hydroxyvitamin D, insulin-like growth factor 1) (18–21), immune-related biomarkers (neutrophils/lymphocytes ratio, platelet/lymphocyte ratio and systemic immune-inflammatory index) (22), Oxidative stress-related biomarkers (8-dihydro-2′-eoxyguanosine, reactive oxygen species, and superoxide dismutase) (23, 24), and nutrient-related biomarkers (docosahexaenoic acid and vitamin B12) (25, 26). However, biomarkers are susceptible to a variety of factors, making these indicators potentially less stable. For example, the circulating level of insulin-like growth factor 1 can be affected by nutritional levels and genetic factors, and the ratio of neutrophil/lymphocyte is susceptible to factors such as acute illnesses and infections. In addition, some frailty biomarkers have gender specificity, such as C-reactive protein, interleukin-6, and muscle growth inhibitors, which makes it necessary to take gender into account when selecting biomarkers. Therefore, although the changes in biomarkers precede the appearance of the organism’s phenotype, and the objectivity and sensitivity of biomarkers are superior, their specificity, precision, stability, and reliability are weaker than those of frailty-related scales (27). In fact, due to the lack of definitive laboratory tests and appropriate clinical testing techniques and equipment (28), the frailty-related scales have become the most commonly used clinical tool for the identification and assessment of frailty (29).
The frailty scales are mostly based on the three conceptual models of frailty and are established by incorporating the clinical symptoms, signs and subjective feelings of the patient, which have the advantages of simplicity, time-saving, validity, economy and feasibility, such as: Fried Frailty Phenotype (FFP), Frailty Index (FI), Groningen Frailty Indicator (GFI), and so on. Three of the conceptual models described above are the Biological Phenotype, the Cumulative Health Deficit Model, and the Frailty Integral Model. In 2001, Fried et al. (30) proposed the concept of the Fried Frailty Phenotype (FFP) based on the theory of the Biological Phenotype, stating that frailty is a syndrome that meets three or more of the five phenotypic criteria, which is mostly centered on physical deterioration, together with a decrease in physical performance and muscular strength. The Cumulative Health Deficit Model suggests that the more health deficits are accumulated, the more severe the degree of frailty. Based on the Cumulative Health Deficit Model, Rockwood et al. (31) proposed the concept of the Frailty Index (FI) in 2005, which considered frailty as a complex unity of physiological, psychological, and social functioning, and a risk condition that develops as a result of the accumulation of multiple disorders due to multiple factors. The above two models are commonly used in existing studies and have been generally confirmed. Based on these two models, Gobbens et al. (32) proposed the Integral Model of Frailty (IMF), which further defines the operational definition of frailty as a dynamic and continuous process that includes somatic, psychological, and social aspects. Recently, WHO has proposed a new concept of “intrinsic capacity” based on healthy aging, which emphasizes the physiological and psychological dimensions of the individual, and is a longitudinal assessment that follows a trajectory rather than the traditional assessment of frailty at a cross-section or cut-off point. In a sense, intrinsic capacity evolves from frailty, and frailty is one of the components of the decline trajectory of intrinsic capacity (33). Another hybrid concept analysis of frailty described frailty as a dynamic and fluctuating inability to manage biopsychosocial and environmental stimuli that involves a decline in functioning and life changes, leading to a loss of autonomy and motivation, or poor health outcomes (34). Based on the above concepts, the frailty scales should address multiple dimensions such as somatic, psychological as well as social conditions. Frailty scales can be categorized as screening scales and assessment scales, and the two are often conflated in clinical practice. In fact, screening tools are not identical to assessment tools, and the emphasis of the two is not the same. The frailty screening scales focus on their operationalization, efficiency, and high sensitivity in order to screen older patients at risk of frailty or in a stage of frailty in a very short period of time, while the frailty assessment scales is more complex, focusing on high precision and support by reasonable biological indicators in order to determine more precisely the stage of frailty in which they are placed, and then to develop different treatment plannings according to their stages and risks (35).
Besides, the different screening and assessment tools count the different prevalence rates of frailty (36). A Meta-analysis showed that the prevalence of frailty within the same group was 12% using the FFP versus 24% using the FI (37). Another cross-sectional study among older Brazilians showed that the prevalence of frailty was 0.3% when assessed only in the physical domain of the Tilburg Frailty Indicator (TFI), 2.9% when assessed in both the physical and social domains, and 52% when assessed in a combination of all three domains: physical, social and psychological (38). Although a variety of geriatric frailty scales have been developed, there is still no recognized gold scale for assessing geriatric frailty, and translation from research to clinical practice remains a challenge in the future (39).Next, this article summarizes and analyzes the existing commonly used frailty scales in terms of their screening and assessment roles, and classifies each scale according to its content, focus, measurement mode, and application scenarios, so that clinicians or researchers can use the most appropriate frailty scales according to their characteristics, thus achieving the goal of early screening, early assessment, and early intervention of frailty, and reducing a series of adverse outcomes.
The purpose of frailty screening is primarily to make a quick diagnosis, which is performed in all groups of older people, to identify those who are at high risk of frailty or already in a state of frailty through the use of simple tests. Here, we summarize and generalize the advantages and disadvantages of some commonly used screening scales for age-related frailty.
The FS is a clinically applicable self-screening scale for frail older adults proposed by the experts of the International Academy on Nutrition and Aging (IANA) (40). FS is a simple patient self-reported questionnaire containing only 5 items as follows: fatigue, increased sense of resistance, decreased activity, multimorbidity co-morbidity, and weight loss. It quickly categorizes the state of an older person into 3 types, among which those who meet 3 or more items are frail, those who meet 1 to 2 items are pre-frail, and those who do not have any of the 1 items are in a healthy state (41). FS can be easily mastered by healthcare professionals and has a high degree of maneuverability and screening efficacy, which has been translated into many languages and widely used worldwide (42–44). In addition, FS has high specificity and sensitivity. A survey of the Chinese version of the adaptation of FS among 1,235 Chinese community-dwelling older adults showed that the sensitivity of FS was as high as 86.96% while the specificity was as high as 85.64% (42). Another study, which conducted on 308 Chinese older patients aged 60 years and above, showed that FS had a sensitivity of 85.9% and a specificity of 72.5%, and noted that FS was convenient and time-saving, which could be used for the initial screening of frail patients to improve work efficiency (45). A cross-sectional study conducted by Aprahamian et al. (46) in a geriatric outpatient clinic showed that the sensitivity of FS was 54% and the specificity was 73% and suggested that FS could be selected as a screening tool for frailty because of its significant time and cost benefits. FS can be used not only to screen for frailty but also to predict adverse outcomes in older adults. FS is a valid predictor of mortality in older adults over 10 years, according to a cohort study among older adults aged 65 years and older (47). In a longitudinal study of women’s health in middle age in Australia, FS predicted the incidence of disability in women from middle age to old age over the next 15 years (48).
FS is entirely self-reported, without any objective measures, and can even be completed by telephone without face-to-face inspection, which makes it simple and easy to administer (49). The simplicity and ease of FS increases the convenience and completion rate of frailty screening, reduces the cost of screening, helps to carry out the development of frailty review, and is worthy of clinical application. However, FS suffers from a certain amount of information bias due to its complete reliance on patient self-reported outcomes, and special attention should be paid to this point in clinical applications.
The CFS is a frailty screening tool developed in 2005 by Rockwood et al. (31) for use in the Canadian Health and Aging Study. The original CFS contained 4 dimensions: physical activity, mobility, physical function, and energy status, which categorized older adults’ health status into 7 levels. With further research on geriatric frailty, the scope of CFS was expanded to co-morbidities, functional status, and cognitive ability domains, increasing the classification to 9 (50). The specific levels of CFS are as follows: Very Fit, Fit, Managing Well, Living with Very Mild Frailty, Living with Mild Frailty, Living with Moderate Frailty, Living with Severe Frailty, Living with Very Severe Frailty, and Terminally Ill, wherein levels 5 and above are defined exactly as a frailty state. A follow-up study of 210 acutely hospitalized older patients with adverse health outcomes found that both CFS and FS could identify older adults at risk for hospitalized adverse health outcomes and could be used as an easy screening tool for frailty; however, CFS demonstrated higher sensitivity than FS (89.6% vs. 54.6%) (51). Another cross-sectional study conducted in China also confirmed that the sensitivity of CFS was superior to FS as a screening tool for age-related frailty, both in all patients (94.1% vs. 63.0%) and in patients from different wards (91.8–98.5% vs. 58.0–65.7%) (52). In their study of the association between CFS and in-hospital mortality in patients with Corona Virus Disease 2019 (COVID-19), Sablerolles et al. (53) found that the in-hospital mortality was significantly higher in frailty patients (CFS 6–9) than in healthy patients (CFS 1–3) and that the grade of CFS was negatively correlated with the health status of the patient. A meta-analysis indicated that CFS could predict in-hospital mortality in acutely ill older patients and is a reliable predictor of short-term mortality in older patients presenting to the emergency department (54).
CFS combines clinical judgment with objective measures and can be used not only to predict the need for institutional care or the incidence of death, but also to assess specific domains including co-morbidities, functioning, and cognition, making it a widely used screening tool for frailty (55). Because of its simplicity, rapidity, and accurate ability to predict adverse outcomes, CFS is often considered the most desirable tool for geriatric frailty screening in emergency medicine (56). In addition, CFS is highly sensitive to symptoms associated with frailty syndrome, which makes it also useful for assessing and stratifying the management of frailty (57). However, the completion of CFS needs to be based on clinical diagnosis combined with the interpretation of clinical parameters, which requires that the user should be a medical staff with a certain medical knowledge base, which limits the popularization and application of CFS to a certain extent.
The EFS is a multidimensional screening scale for frailty developed by ROLFSON et al. (58) for non-specialists without specialized training in geriatrics based on the traditional frailty phenotype. The EFS consists of 9 dimensions and 11 entries as follows: (1) Cognitive function (unable to complete the clock drawing test successfully); (2) Functional performance (needing help from others in daily activities); (3) General health (self-assessment of health and the number of hospitalizations in the last year); (4) Independence (unable to complete manual labor alone, unable to walk 2 flights of stairs or walk 1,000 m); (5) Social support (unable to seek outside support successfully when encountering problems); (6) Medication status (being on 5 or more prescription medications at the same time, forgetting to take medication); (7) Mental status (depression); (8) Nutritional aspects (unintentional and significant weight loss recently); (9) Self-control (urinary and fecal incontinence). The EFS has a maximum score of 17, with a score of 0 to 4 indicating no frailty, 5 to 6 indicating sensitive individuals prone to frailty, 7 to 8 indicating mild frailty, 9 to 10 indicating moderate frailty, and 11 or more indicating severe frailty. EFS covers all domains of frailty and is highly correlated with other frailty scales (59). A study of the differences in the prevalence of frailty calculated by five frailty screening tools showed that the prevalence of frailty screened by the EFS was 25.2%, which was most similar to the prevalence of frailty of 27.6% after integrating the five frailty screening tools mentioned above, suggesting that the accuracy of EFS screening was high (52). In addition, the above study found that the EFS had the highest specificity for the assessment of frailty in surgical wards at 98.1%. EFS is commonly used in the identification of geriatric frailty prior to surgery and helps to stratify the risks and identify potentially modifying factors, which makes it have a higher feasibility rating in the surgical setting (60). A prospective study in people aged 70 years and older undergoing major abdominal surgery showed that the EFS has good reliability and validity and can be used as a preoperative assessment tool to predict the risk of surgical complications in older adults (61). In a study conducted by McIsaac et al. (62), it was found that although the accuracy of assessments of postoperative risks using the modified Fried Index (mFI) and the EFS was similar, the EFS had the advantage of a shorter time-consuming and greater patient acceptance, and should be recommended for clinical use.
EFS can be completed within 5 min, with high acceptance by both investigators and respondents. It is easy to operate and can be used by professionals or even non-professionals in multiple departments. It has a wide range of applications venues, which can be used in medical settings such as emergency, outpatient, and hospital wards, as well as in non-medical settings such as the community and the home, making it a reliable screening tool for geriatric frailty (63). However, EFS uses only one question to assess the specifics of the social support domain, disputing the comprehensiveness of the social frailty screen.
FFP is a phenotype derived by Fried et al. (30) from observing and tracking the follow-up to validate adverse outcomes in 5317 older adults aged 65 years or older who participated in the U.S. Longitudinal Cardiovascular Health Study, which explains why FFP was also called the Cardiovascular Health Study Index (CHS). The entries of FFP consist of 5 self-reported symptoms combined with biologically measured signs. The details of its entries are as follows: significant loss of body mass (unintentional weight loss of more than 4.5 kg or more in the past 1 year), weakness (low grip strength in both hands), fatigue (self-reported to be more easily fatigued in the last 6 months), slowness of the body (significant slowing of the walking speed), and physical inactivity (sedentary and physically inactive). Among them, those who fulfill 3 or more are defined as frail, those who have 1 or 2 are defined as pre-frail, and those who do not have any of the above 5 are defined as non-frail. FFP can not only measure the physical frailty status of older adults but also reflect the mental health status of the older adults and obtain more objective and accurate data, which is the most popular and widely used frailty measurement tool in the clinic (12). Results of a survey conducted among cancer patients showed that FFP had a sensitivity of 92% and a specificity of 41% for screening for frailty (64). FFP is widely applicable and its reliability and validity have been validated many times (65). FFP has now been shown to have predictive value for adverse health outcomes in a diverse range of older adults, including hospitalized and general older adults (66).
FFP is excellent for initial stratification for risks of the older population based on different characteristics (i.e., robust, pre-frail, and frail), without the need for an initial clinical assessment, and can be applied at the first patient contact (67). However, FFP focuses on the physiological level of assessment, lacks social, psychological, environmental, and multiple disease factors, and the implementation of some items (e.g., grip strength, step speed, etc.) requires trained personnel and specialized tools (68). The characteristics of FFP described above make it inappropriate for older adults with cognitive impairment, psychiatric disorders, impaired functioning, or in the acute phase of illness, which also make its applicability limited to hospitals, communities, and nursing facilities. In addition, the 5 phenotypic criteria of frailty allow for different ways of measuring them, and many previous studies have adapted their measurements, which have been confirmed to lead to differences in measurement effects (69). Therefore, future studies should report all the details about how the phenotypic criteria of frailty are measured in order to facilitate the interpretation of the results.
In recent years, more and more frailty screening tools have been developed as a result of the progress of frailty research. For example, in 2007 Ensrud et al. (70) found that data collected using the Study of Osteoporotic Fractures (SOF) Index was independently associated with FFP-predicted frailty-related adverse health outcomes, and subsequently, the SOF index became one of the screening tools for frailty; the Kihon Checklist (KCL) was proposed by the Japanese government for the implementation of the long-term care insurance system (71); and the Simple Self-Assessment Screening Tool—the Vulnerable Elders Survey-13 (VES-13) was created by Saliba et al. (72). The characteristics of common frailty screening scales are summarized in Table 1 and compared from nine perspectives, including entries, time required, content, and so on (30, 31, 40, 58, 70–76).
Rapid screening should be followed by further precise assessment of all older adults in pre-frail and frail states. An accurate assessment to evaluate which state of frailty a frail older adult is in can help to predict poor health outcomes better and facilitate the development of individualized treatment and management plans for frailty patients. Recently, there have been a large number of studies devoted to the development of objective quantitative frailty assessment tools (77). Since these assessment tools are not limited to questionnaires and there are differences in the consistency of the assessment tools, places of application, populations administered, and dimensions assessed, the results of the assessment of frailty cannot yet be judged uniformly. Therefore, we will next summarize the characteristics of the commonly used assessment scales for age-related frailty.
FI is a classic tool for assessing frailty in older adults developed by Mitnitski et al. (78) based on the cumulative deficit model. It covers multiple dimensions such as physical, psychology, cognition, and social functioning, and contains 30 to 70 evaluation items, the specific content of which is variable. Since there are no standardized criteria for the content of its items, researchers can choose their own entries according to their own research purposes. Although FI lacks specific variables that are uniformly standardized, the stability of FI is supported by the fact that FI consisting of different numbers and types of deficient items yields similar assessment results in different populations or research settings. Generally speaking, when FI ≥ 0.25, it implies frailty; when FI < 0.12, it implies non-frailty; when FI is between 0.12 and 0.25, it implies that the older adults are in the pre-frail stage (79). The sensitivity and specificity of FI in identifying frailty was 94.8 and 87.0% in all patients, 96.4 and 88.8% in patients on the cardiology ward, 95.9 and 81.1% in patients on the non-surgical ward and were 89.6 and 89.5% on the surgical ward (52). FI can be used not only for the screening of debilitation but also for the assessment of debilitation. FI is the first tool to successfully quantify the frailty state of older adults and has been widely used in several countries due to its good reliability and validity (80). FI is strongly associated with negative health-related outcomes (including mortality) and with deterioration in disease-specific health status, which makes it a good predictor of clinical prognosis (81, 82). It has been found that FI can be utilized to evaluate the role of musculoskeletal disorders on frailty, rather than just being a categorical variable (83). In addition, FI has important applications in reflecting health service needs, public health management, and interventions. During the period of the COVID-19 pandemic, the use of an electronic version of the FI to assess frailty helped clinicians make decisions by identifying patients most likely to require ICU (intensive care unit) admission and those with a poor prognosis (84).
By focusing on the cumulative number of individual health deficits and integrating multiple complex health information into a single indicator, FI breaks through the limitation of a single variable describing the functional status, and can better assess the overall health status of older adults. With its advantages of multidimensionality, continuity, and objectivity, FI is suitable for frailty assessment in almost all environments. However, the establishment of FI requires a large amount of clinical information, while obtaining a large amount of clinical information is laborious, extremely cumbersome, and time-consuming, which is a major challenge in the use of FI for the assessment of frailty.
CGA was conceptualized for the development of a scientific rehabilitation training program for older adults in the 1940s by Marjory Warren (85). As the population ages, the application of CGA continues to extend and becomes a common method of assessing and treating older patients with frailty or loss of function (86). CGA focuses on comprehensive assessments of somatic function, cognitive function, psychology, and social/environmental factors in older adults, thereby identifying and quantifying the degree of frailty, and providing the basis for subsequent frailty intervention strategies and comprehensive care, which is conducive to the early reversal of frailty, the slowing down of the deterioration process of frailty, and the improvement of health outcomes (87). A systematic evaluation that included 22 studies involving 10,315 patients showed that patients in the group that took interventions based on CGA had a lower likelihood of death or worsening of their condition and a higher likelihood of cognitive improvement compared to the group that took conventional medical care (88). Lee et al. (87) found that a CGA-based intervention program for a frail population could potentially promote healthy aging in community-dwelling older adults, with sustained health benefits of up to 1 year for them. Mazya et al. (89) conducted a trial of dynamic geriatric assessment-frailty intervention, in which the control group of the study received conventional treatment and humanistic care while the intervention group received dynamic assessment by CGA and multidisciplinary team interventions (including medication adjustments, exercise, and dietary advice, etc.) in addition to conventional care. After the 24-month intervention, the proportion of patients in the intervention group who were pre-frail was significantly higher than in the control group, suggesting that more patients with chronic diseases or co-morbidities in the intervention group moved from frailty to pre-frailty or strong than that in the control group. With the help of CGA, a comprehensive and scientific assessment of frailty can be made and a personalized medical intervention plan for the older adults can be developed to slow down the process of frailty by healthcare professionals.
CGA can accurately judge the health status of older adults, assess the degree or stage of frailty, identify its causes or triggers, and provide suggestions for its preventive or therapeutic measures, which can help in making risk stratification and clinical decisions for frailty. However, as the CGA is a multidimensional and interdisciplinary diagnostic and therapeutic process that emphasizes a multidimensional and comprehensive risk factor exploration and assessment, it requires a large amount of manpower, energy, and time, which is inconvenient in practice and is only applicable to the hospital healthcare environment (90). Furthermore, despite the fact that many studies have shown a large advantage of CGA for the assessment of frailty, most of these studies were conducted on small samples of older adults within a single institution or region, resulting in poor accuracy of the results of these studies, which still need to be further validated (91).
GFI is a widely used frailty screening tool developed by Steverink et al. (92) in 2001. The GFI consists of the physical dimension (mobility, multiple health problems, physical fatigue, vision and hearing), the psychological dimension (depressed mood and anxiety), the cognitive dimension (cognitive dysfunction), and the social dimension (emotional isolation), with a total of 15 entries. The 15 entries of the GFI are all dichotomous questions, with each score set at 0 or 1. Higher scores on the GFI indicate more severe frailty, with those scoring ≥4 diagnosed as moderately or severe frailty (93). A study comparing the ability of four frailty screening tools to predict frailty-related adverse outcomes showed that the sensitivity of the GFI in predicting the frailsty adverse outcomes of death and hospitalization was 76.2 and 63.9%, respectively, and the specificity was 42.1 and 50.3%, which suggests that the GFI has a higher sensitivity and a poorer specificity (94). When the Chinese version of the GFI was used to screen for pre-frailty and frailty in 350 Chinese community-dwelling older adults, it demonstrated good internal consistency, with a Cronbach’s alpha coefficient of 0.87, a re-test reliability of 0.87, and the concurrent validity between the GFI and the Fried frailty phenotype of 0.76. suggesting that the GFI is a reliable and valid tool for pre-frailty and frailty screening in community-dwelling older adults (95). In addition, GFI can accurately predict total healthcare costs for the following year and can help healthcare professionals allocate healthcare resources (96).
GFI has been widely used in many countries such as Germany, Italy, France, and so on, and it is suitable for use in different assessment environments, such as communities, nursing homes, and healthcare facilities (97). However, compared with other scales, GFI focuses more on physical indicators such as physical strength, functioning, and health status, and does not adequately take into account psychological, cognitive, and social aspects, which may make the results of the GFI assessment slightly less integrated and comprehensive.
As the prevalence of frailty increases, more and more frailty assessment tools are being developed to make individualized and precise interventions for frail patients. For example, the Comprehensive Frailty Assessment Instrument (CFAI), which was first included in environmental assessment, was developed (98) and the Rapid Geriatric Assessment (RGA), which improves on the cumbersome assessment process of the CGA, was also created (99). A comparison of various common frailty assessment scales is shown in Table 2 (78, 86, 92, 98–101).
Frailty is an emerging global health burden with significant implications for clinical practice and public health. With the rapid growth of an aging population, the prevalence of frailty increases year by year. Frailty is a dynamically changing clinical state, with the pre-frailty phase showing potential reversibility. The early recognition of frailty and appropriate interventions for it can help slow or even reverse the process of it and reduce the risk of adverse outcomes. In order to achieve “healthy aging” centered on wellness, we need to improve the rate of the early identification of frailty for its early precise intervention. Although there are approximately 67 screening and assessment tools for frailty internationally and and there is a trend toward an increase in the number of such tools (77), different screening and assessment tools have more significant differences in conceptual basis, clinical utility, program content, and place of application. As a result, there is still considerable debate as to what is the best scale for screening and assessing frailty.
Strictly speaking, frailty screening scales and assessment scales have different requirements and should not be confused. Screening scales need to be simple, quick, and highly sensitive to frailty, which allows clinicians to recognize frailty quickly. Assessment scales require high accuracy, utility, and support of sound biological theories, which allows clinicians to identify the stages of frailty in older adults accurately and predict the occurrence of adverse health events in frail older adults, such as falls, cognitive deficits, loss of mobility, and death. Through reading a large amount of literature, we have differentiated and reviewed the most common frailty scales for screening and assessment in order to help researchers as well as healthcare professionals to select the most appropriate frailty scales for its identification and assessment. Based on our summaries and generalizations, we roughly design the process of screening and assessment for the frailty in different types of older people, which is shown in Figure 1. The high-risk groups and older people without frailty symptoms are screened out through a rapid screening scale, and the high-risk groups should go to the hospital for treatment. Older people without frailty symptoms should adopt self-screening and regular community screening once every 6 months to prevent the emergence of frailty. For older hospitalized patients, high-risk groups are screened out through the doctor’s simple judgment (whether it needs to be evaluated directly), and then a detailed and comprehensive evaluation can make them quickly benefit from the follow-up personalized intervention treatment.
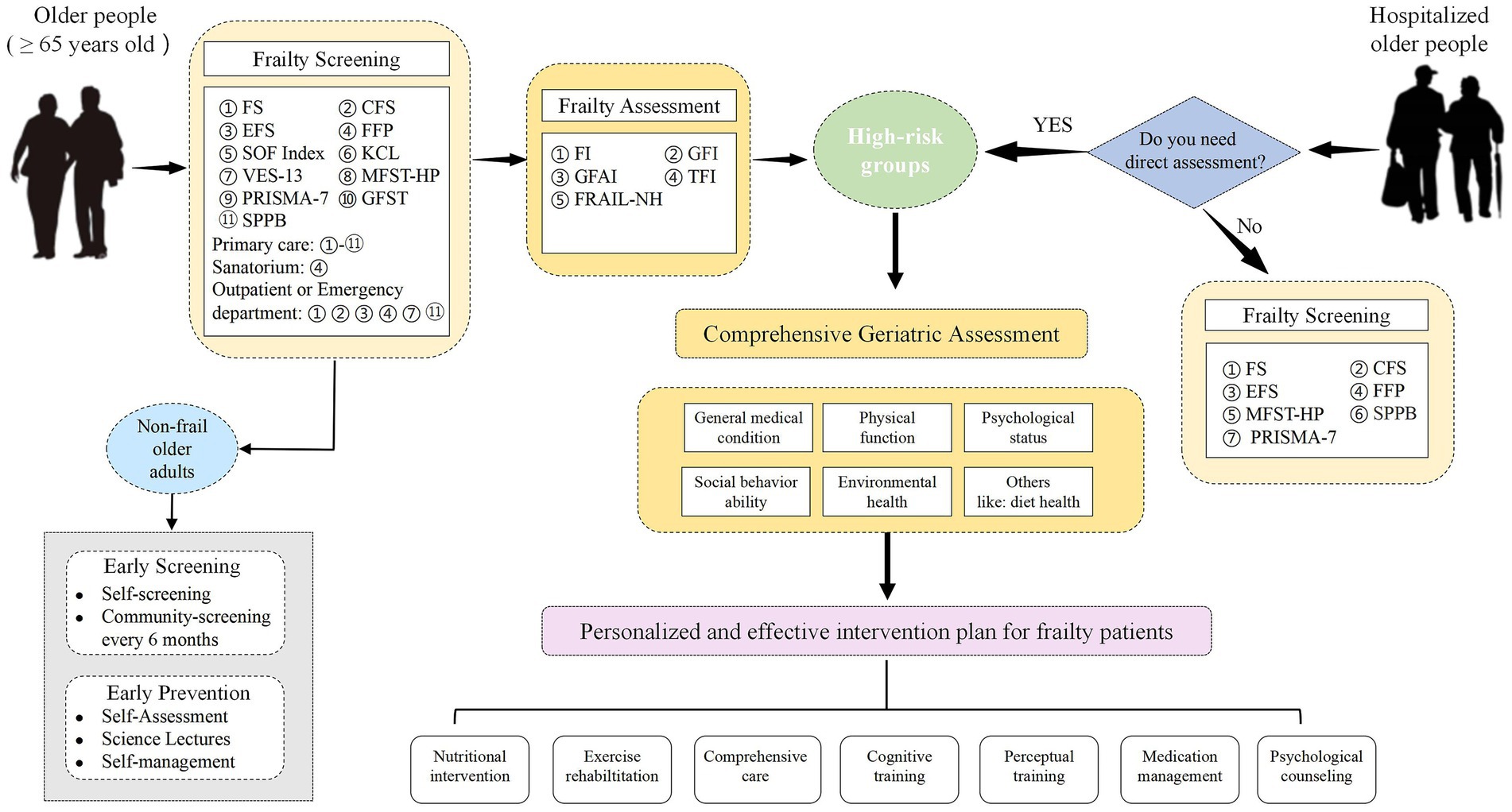
Figure 1. The process of screening and assessment for the frailty in different types of older people. The development of frailty can be slowed or even reversed by the early recognition, accurate assessment, and timely interventions for frailty, which will reduce the strain of frailty on health care systems around the world and promote healthy aging of the global population. In the future, we still need to explore objective, simple, time-saving, effective, economical, and feasible scales that can accurately identify and assess frailty in clinical practice.
The development of frailty can be slowed or even reversed by early recognition, accurate assessment, and timely interventions for frailty, which will reduce the strain of frailty on healthcare systems around the world and promote healthy aging of the global population. Although we have provided insights into potential solutions for early identification and assessment of frailty by reviewing a large body of literature, however, there are some limitations to our study. Firstly, we searched only one database and limited our search to one language, which may have led to the omission of relevant articles. Secondly, our review lacked a critical assessment of the included articles, which resulted in the variable quality of the articles we included. Finally, we only summarized scales that have been studied frequently and are relatively well-established, which is somewhat one-sided. In the future, we still need to explore objective, simple, time-saving, effective, economical, and feasible scales that can accurately identify and assess frailty in clinical practice.
X-MW: Writing – original draft. Y-HZ: Writing – original draft. C-CM: Conceptualization, Writing – review & editing. LF: Conceptualization, Writing – review & editing. LW: Conceptualization, Writing – review & editing. Y-YL: Formal analysis, Writing – review & editing. X-ZL: Funding acquisition, Supervision, Writing – review & editing. S-CL: Supervision, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported, at least in part, by the Science & Technology Development Fund of Tianjin Education Commission for Higher Education (2022ZD044).
We thank our reviewers and editor for critical feedback that enhanced this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rudnicka, E, Napierała, P, Podfigurna, A, Męczekalski, B, Smolarczyk, R, and Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas. (2020) 139:6–11. doi: 10.1016/j.maturitas.2020.05.018
2. Hoogendijk, EO, Afilalo, J, Ensrud, KE, Kowal, P, Onder, G, and Fried, LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394:1365–75. doi: 10.1016/S0140-6736(19)31786-6
3. Fan, J, Yu, C, Guo, Y, Bian, Z, Sun, Z, Yang, L, et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health. (2020) 5:e650–60. doi: 10.1016/S2468-2667(20)30113-4
4. Liu, HX, Ding, G, Yu, WJ, Liu, TF, Yan, AY, Chen, HY, et al. Association between frailty and incident risk of disability in community-dwelling elder people: evidence from a meta-analysis. Public Health. (2019) 175:90–100. doi: 10.1016/j.puhe.2019.06.010
5. He, B, Ma, Y, Wang, C, Jiang, M, Geng, C, Chang, X, et al. Prevalence and risk factors for Frailty among community-dwelling older people in China: A systematic review and Meta-analysis. J Nutr Health Aging. (2019) 23:442–50. doi: 10.1007/s12603-019-1179-9
6. Nascimento, CM, Ingles, M, Salvador-Pascual, A, Cominetti, MR, Gomez-Cabrera, MC, and Viña, J. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. (2019) 132:42–9. doi: 10.1016/j.freeradbiomed.2018.08.035
7. Cheng, M-H, and Chang, S-F. Frailty as a risk factor for falls among community dwelling people: evidence from a Meta-analysis. J Nurs Scholarsh Off Publ Sigma Theta Tau Int Honor Soc Nurs. (2017) 49:529–36. doi: 10.1111/jnu.12322
8. Boreskie, KF, Hay, JL, Boreskie, PE, Arora, RC, and Duhamel, TA. Frailty-aware care: giving value to frailty assessment across different healthcare settings. BMC Geriatr. (2022) 22:13. doi: 10.1186/s12877-021-02722-9
9. Faber, MJ, Bosscher, RJ, Chin A Paw, MJ, and van Wieringen, PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: A multicenter randomized controlled trial. Arch Phys Med Rehabil. (2006) 87:885–96. doi: 10.1016/j.apmr.2006.04.005
10. Huang, C-Y, Mayer, PK, Wu, M-Y, Liu, D-H, Wu, P-C, and Yen, H-R. The effect of tai chi in elderly individuals with sarcopenia and frailty: A systematic review and meta-analysis of randomized controlled trials. Ageing Res Rev. (2022) 82:101747. doi: 10.1016/j.arr.2022.101747
11. Shamliyan, T, Talley, KMC, Ramakrishnan, R, and Kane, RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. (2013) 12:719–36. doi: 10.1016/j.arr.2012.03.001
12. Dent, E, Morley, JE, Cruz-Jentoft, AJ, Woodhouse, L, Rodríguez-Mañas, L, Fried, LP, et al. Physical Frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. (2019) 23:771–87. doi: 10.1007/s12603-019-1273-z
13. Cohen, CI, Benyaminov, R, Rahman, M, Ngu, D, and Reinhardt, M. Frailty: A multidimensional biopsychosocial syndrome. Med Clin North Am. (2023) 107:183–97. doi: 10.1016/j.mcna.2022.04.006
14. Samson, LD, Boots, AMH, Verschuren, WMM, Picavet, HSJ, Engelfriet, P, and Buisman, A-M. Frailty is associated with elevated CRP trajectories and higher numbers of neutrophils and monocytes. Exp Gerontol. (2019) 125:110674. doi: 10.1016/j.exger.2019.110674
15. Semmarath, W, Seesen, M, Yodkeeree, S, Sapbamrer, R, Ayood, P, Malasao, R, et al. The association between Frailty indicators and blood-based biomarkers in early-old community dwellers of Thailand. Int J Environ Res Public Health. (2019) 16:3457. doi: 10.3390/ijerph16183457
16. Nascimento, CMC, Zazzetta, MS, Gomes, GAO, Orlandi, FS, Gramani-Say, K, Vasilceac, FA, et al. Higher levels of tumor necrosis factor β are associated with frailty in socially vulnerable community-dwelling older adults. BMC Geriatr. (2018) 18:268. doi: 10.1186/s12877-018-0961-6
17. Kochar, BD, Cai, W, and Ananthakrishnan, AN. Inflammatory bowel disease patients who respond to treatment with anti-tumor necrosis factor agents demonstrate improvement in pre-treatment Frailty. Dig Dis Sci. (2022) 67:622–8. doi: 10.1007/s10620-021-06990-8
18. Chew, J, Tay, L, Lim, JP, Leung, BP, Yeo, A, Yew, S, et al. Serum Myostatin and IGF-1 as gender-specific biomarkers of Frailty and low muscle mass in community-dwelling older adults. J Nutr Health Aging. (2019) 23:979–86. doi: 10.1007/s12603-019-1255-1
19. Spira, D, Buchmann, N, König, M, Rosada, A, Steinhagen-Thiessen, E, Demuth, I, et al. Sex-specific differences in the association of vitamin D with low lean mass and frailty: results from the Berlin aging study II. Nutr Burbank Los Angel Cty Calif. (2019) 62:1–6. doi: 10.1016/j.nut.2018.11.020
20. Sousa-Santos, AR, Afonso, C, Santos, A, Borges, N, Moreira, P, Padrão, P, et al. The association between 25(OH)D levels, frailty status and obesity indices in older adults. PLoS One. (2018) 13:e0198650. doi: 10.1371/journal.pone.0198650
21. Doi, T, Makizako, H, Tsutsumimoto, K, Hotta, R, Nakakubo, S, Makino, K, et al. Association between insulin-like growth Factor-1 and Frailty among older adults. J Nutr Health Aging. (2018) 22:68–72. doi: 10.1007/s12603-017-0916-1
22. Zhang, H, Hao, M, Hu, Z, Li, Y, Jiang, X, Wang, J, et al. Association of immunity markers with the risk of incident frailty: the Rugao longitudinal aging study. Immun Ageing A. (2022) 19:1. doi: 10.1186/s12979-021-00257-6
23. Liang, Y-D, Liu, Q, Du, M-H, Liu, Z, Yao, S-M, Zheng, P-P, et al. Urinary 8-oxo-7,8-dihydroguanosine as a potential biomarker of frailty for elderly patients with cardiovascular disease. Free Radic Biol Med. (2020) 152:248–54. doi: 10.1016/j.freeradbiomed.2020.03.011
24. Bernabeu-Wittel, M, Gómez-Díaz, R, González-Molina, Á, Vidal-Serrano, S, Díez-Manglano, J, Salgado, F, et al. Oxidative stress, telomere shortening, and apoptosis associated to sarcopenia and Frailty in patients with multimorbidity. J Clin Med. (2020) 9:2669. doi: 10.3390/jcm9082669
25. Soh, Y, and Won, CW. Association between frailty and vitamin B12 in the older Korean population. Medicine. (2020) 99:e22327. doi: 10.1097/MD.0000000000022327
26. Kim, D, Won, CW, and Park, Y. Association between erythrocyte levels of n-3 polyunsaturated fatty acids and risk of Frailty in community-dwelling older adults: the Korean Frailty and aging cohort study. J Gerontol A Biol Sci Med Sci. (2021) 76:499–504. doi: 10.1093/gerona/glaa042
27. Saedi, AA, Feehan, J, Phu, S, and Duque, G. Current and emerging biomarkers of frailty in the elderly. Clin Interv Aging. (2019) 14:389–98. doi: 10.2147/CIA.S168687
28. Anabitarte-García, F, Reyes-González, L, Rodríguez-Cobo, L, Fernández-Viadero, C, Somonte-Segares, S, Díez-Del-Valle, S, et al. Early diagnosis of frailty: technological and non-intrusive devices for clinical detection. Ageing Res Rev. (2021) 70:101399. doi: 10.1016/j.arr.2021.101399
29. Faller, JW, Pereira, D, de Souza, S, Nampo, FK, Orlandi, FS, and Matumoto, S. Instruments for the detection of frailty syndrome in older adults: A systematic review. PLoS One. (2019) 14:e0216166. doi: 10.1371/journal.pone.0216166
30. Fried, LP, Tangen, CM, Walston, J, Newman, AB, Hirsch, C, Gottdiener, J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A. (2001) 56:M146–57. doi: 10.1093/gerona/56.3.M146
31. Rockwood, K, Song, X, MacKnight, C, Bergman, H, Hogan, DB, McDowell, I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. (2005) 173:489–95. doi: 10.1503/cmaj.050051
32. Gobbens, RJJ, Luijkx, KG, Wijnen-Sponselee, MT, and Schols, JMGA. In search of an integral conceptual definition of Frailty: opinions of experts. J Am Med Dir Assoc. (2010) 11:338–43. doi: 10.1016/j.jamda.2009.09.015
33. Belloni, G, and Cesari, M. Frailty and intrinsic capacity: two distinct but related constructs. Front Med. (2019) 6:133. doi: 10.3389/fmed.2019.00133
34. Fan, X, Li, Y, Xu, L, Li, C, and Song, R. A hybrid concept analysis of Frailty among older adults living in the community. J Adv Nurs. doi: 10.1111/jan.16508
35. Martin, FC, and O’Halloran, AM. Tools for assessing Frailty in older people: general concepts. Adv Exp Med Biol. (2020) 1216:9–19. doi: 10.1007/978-3-030-33330-0_2
36. Andrade, LEL, New York, BS, Gonçalves, RSSA, Fernandes, SGG, and Maciel, ÁCC. Mapping instruments for assessing and stratifying frailty among community-dwelling older people: a scoping review. BMJ Open. (2021) 11:e052301. doi: 10.1136/bmjopen-2021-052301
37. O’Caoimh, R, Sezgin, D, O’Donovan, MR, Molloy, DW, Clegg, A, Rockwood, K, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. (2021) 50:96–104. doi: 10.1093/ageing/afaa219
38. Santiago, LM, and Mattos, IE. Prevalência e fatores associados à fragilidade em idosos institucionalizados das regiões Sudeste e Centro-Oeste do Brasil. Rev Bras Geriatr E Gerontol. (2014) 17:327–37. doi: 10.1590/S1809-98232014000200010
39. Ambagtsheer, RC, Visvanathan, R, Dent, E, Yu, S, Schultz, TJ, and Beilby, J. Commonly used screening instruments to identify Frailty among community-dwelling older people in a general practice (primary care) setting: A study of diagnostic test accuracy. J Gerontol A Biol Sci Med Sci. (2020) 75:1134–42. doi: 10.1093/gerona/glz260
40. Van Kan, GA, Rolland, Y, Bergman, H, Morley, JE, Kritchevsky, SB, and Vellas, B. On behalf of the geriatric advisory panel. The I.A.N.A. task force on frailty assessment of older people in clinical practice. J Nutr Health Aging. (2008) 12:29–37. doi: 10.1007/BF02982161
41. Abellan van Kan, G, Rolland, YM, Morley, JE, and Vellas, B. Frailty: toward a clinical definition. J Am Med Dir Assoc. (2008) 9:71–2. doi: 10.1016/j.jamda.2007.11.005
42. Dong, L, Qiao, X, Tian, X, Liu, N, Jin, Y, Si, H, et al. Cross-cultural adaptation and validation of the FRAIL scale in Chinese community-dwelling older adults. J Am Med Dir Assoc. (2018) 19:12–7. doi: 10.1016/j.jamda.2017.06.011
43. Jung, H-W, Yoo, H-J, Park, S-Y, Kim, S-W, Choi, J-Y, Yoon, S-J, et al. The Korean version of the FRAIL scale: clinical feasibility and validity of assessing the frailty status of Korean elderly. Korean J Intern Med. (2016) 31:594–600. doi: 10.3904/kjim.2014.331
44. Rosas-Carrasco, O, Cruz-Arenas, E, Parra-Rodríguez, L, García-González, AI, Contreras-González, LH, and Szlejf, C. Cross-cultural adaptation and validation of the FRAIL scale to assess Frailty in Mexican adults. J Am Med Dir Assoc. (2016) 17:1094–8. doi: 10.1016/j.jamda.2016.07.008
45. Hou, P, Xue, H, Li, Y, Mao, X, Sun, K, Xue, L, et al. Performance of the FRAIL scale in screening Frailty among elderly patients with coronary heart disease. Chin Gen Pract. (2019) 22:1052–6.
46. Aprahamian, I, Cezar, NOC, Izbicki, R, Lin, SM, Paulo, DLV, Fattori, A, et al. Screening for Frailty with the FRAIL scale: A comparison with the phenotype criteria. J Am Med Dir Assoc. (2017) 18:592–6. doi: 10.1016/j.jamda.2017.01.009
47. Thompson, MQ, Theou, O, Tucker, GR, Adams, RJ, and Visvanathan, R. FRAIL scale: predictive validity and diagnostic test accuracy. Australas J Ageing. (2020) 39:e529–36. doi: 10.1111/ajag.12829
48. Susanto, M, Hubbard, RE, and Gardiner, PA. Validity and responsiveness of the FRAIL scale in middle-aged women. J Am Med Dir Assoc. (2018) 19:65–9. doi: 10.1016/j.jamda.2017.08.003
49. Morley, JE, Malmstrom, TK, and Miller, DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. (2012) 16:601–8. doi: 10.1007/s12603-012-0084-2
50. Rockwood, K, and Theou, O. Using the clinical Frailty scale in allocating scarce health care resources. CGJ. (2020) 23:254–9. doi: 10.5770/cgj.23.463
51. Chong, E, Ho, E, Baldevarona-Llego, J, Chan, M, Wu, L, and Tay, L. Frailty and risk of adverse outcomes in hospitalized older adults: A comparison of different Frailty measures. J Am Med Dir Assoc. (2017) 18:638.e7–638.e11. doi: 10.1016/j.jamda.2017.04.011
52. Liang, Y-D, Zhang, Y-N, Li, Y-M, Chen, Y-H, Xu, J-Y, Liu, M, et al. Identification of Frailty and its risk factors in elderly hospitalized patients from different wards: A cross-sectional study in China. Clin Interv Aging. (2019) 14:2249–59. doi: 10.2147/CIA.S225149
53. Sablerolles, RSG, Lafeber, M, van Kempen, JAL, van de Loo, BPA, Boersma, E, Rietdijk, WJR, et al. Association between clinical Frailty scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev. (2021) 2:e163–70. doi: 10.1016/S2666-7568(21)00006-4
54. Lee, JH, Park, YS, Kim, MJ, Shin, HJ, Roh, YH, Kim, JH, et al. Clinical Frailty scale as a predictor of short-term mortality: A systematic review and meta-analysis of studies on diagnostic test accuracy. Acad Emerg Med Off J Soc Acad Emerg Med. (2022) 29:1347–56. doi: 10.1111/acem.14493
55. Church, S, Rogers, E, Rockwood, K, and Theou, O. A scoping review of the clinical Frailty scale. BMC Geriatr. (2020) 20:393. doi: 10.1186/s12877-020-01801-7
56. Fehlmann, CA, Nickel, CH, Cino, E, Al-Najjar, Z, Langlois, N, and Eagles, D. Frailty assessment in emergency medicine using the clinical Frailty scale: a scoping review. Intern Emerg Med. (2022) 17:2407–18. doi: 10.1007/s11739-022-03042-5
57. Chen, Y, Yang, R, Li, C, Cao, W, Hou, H, and Dong, W. Different Frailty screening tools and the influential factors in elderly hospitalized patients. Int J Geriatr. (2021) 42:133–8.
58. Rolfson, DB, Majumdar, SR, Tsuyuki, RT, Tahir, A, and Rockwood, K. Validity and reliability of the Edmonton frail scale. Age Ageing. (2006) 35:526–9. doi: 10.1093/ageing/afl041
59. Perna, S, Francis, MD, Bologna, C, Moncaglieri, F, Riva, A, Morazzoni, P, et al. Performance of Edmonton frail scale on frailty assessment: its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. (2017) 17:2. doi: 10.1186/s12877-016-0382-3
60. Richards, SJG, Frizelle, FA, Geddes, JA, Eglinton, TW, and Hampton, MB. Frailty in surgical patients. Int J Color Dis. (2018) 33:1657–66. doi: 10.1007/s00384-018-3163-y
61. He, Y, Li, LW, Hao, Y, Sim, EY, Ng, KL, Lee, R, et al. Assessment of predictive validity and feasibility of Edmonton frail scale in identifying postoperative complications among elderly patients: a prospective observational study. Sci Rep. (2020) 10:14682. doi: 10.1038/s41598-020-71140-5
62. McIsaac, DI, Taljaard, M, Bryson, GL, Beaulé, PE, Gagné, S, Hamilton, G, et al. Frailty as a predictor of death or New disability after surgery: A prospective cohort study. Ann Surg. (2020) 271:283–9. doi: 10.1097/SLA.0000000000002967
63. Jankowska-Polańska, B, Uchmanowicz, B, Kujawska-Danecka, H, Nowicka-Sauer, K, Chudiak, A, Dudek, K, et al. Assessment of frailty syndrome using Edmonton frailty scale in polish elderly sample. Aging Male Off J Int Soc Study Aging Male. (2019) 22:177–86. doi: 10.1080/13685538.2018.1450376
64. Cheung, DST, Ho, M-H, Chau, PH, Yu, DSF, Chan, WL, Soong, SI, et al. Screening for Frailty using the FRAIL scale in older Cancer survivors: A cross-sectional comparison with the Fried phenotype. Semin Oncol Nurs. (2024) 40:151617. doi: 10.1016/j.soncn.2024.151617
65. Varan, HD, Deniz, O, Çöteli, S, Doğrul, RT, Kızılarslanoğlu, MC, and Göker, B. Validity and reliability of Fried frailty phenotype in Turkish population. J Med Sci. (2022) 52:524–7. doi: 10.55730/1300-0144.5318
66. Thompson, MQ, Theou, O, Tucker, GR, Adams, RJ, and Visvanathan, R. Recurrent measurement of Frailty is important for mortality prediction: findings from the north West Adelaide health study. J Am Geriatr Soc. (2019) 67:2311–7. doi: 10.1111/jgs.16066
67. Cesari, M, Gambassi, G, van Kan, GA, and Vellas, B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. (2014) 43:10–2. doi: 10.1093/ageing/aft160
68. Dent, E, Kowal, P, and Hoogendijk, EO. Frailty measurement in research and clinical practice: A review. Eur J Intern Med. (2016) 31:3–10. doi: 10.1016/j.ejim.2016.03.007
69. Theou, O, Cann, L, Blodgett, J, Wallace, LMK, Brothers, TD, and Rockwood, K. Modifications to the frailty phenotype criteria: systematic review of the current literature and investigation of 262 frailty phenotypes in the survey of health, ageing, and retirement in Europe. Ageing Res Rev. (2015) 21:78–94. doi: 10.1016/j.arr.2015.04.001
70. Ensrud, KE, Ewing, SK, Taylor, BC, Fink, HA, Stone, KL, Cauley, JA, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. (2007) 62:744–51. doi: 10.1093/gerona/62.7.744
71. Suzuki, N, Makigami, K, Goto, A, Yokokawa, H, and Yasumura, S. Comparison of ability-based and performance-based IADL evaluation of community-dwelling elderly using the Kihon checklist and TMIG index of competence. Nihon Ronen Igakkai Zasshi Jpn J Geriatr. (2007) 44:619–26. doi: 10.3143/geriatrics.44.619
72. Saliba, D, Elliott, M, Rubenstein, LZ, Solomon, DH, Young, RT, Kamberg, CJ, et al. The vulnerable elders survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. (2001) 49:1691–9. doi: 10.1046/j.1532-5415.2001.49281.x
73. Warnier, RMJ, van Rossum, E, van Leendert, JAA, Pijls, NAT, Mulder, WJ, Schols, JMGA, et al. Screening for Frailty in Hospitalized Older Adults: Reliability and Feasibility of the Maastricht Frailty Screening Tool for Hospitalized Patients (MFST-HP). Res Gerontol Nurs (2016) 9:243–251. doi: 10.3928/19404921-20160906-01
74. Raîche, M, Hébert, R, and Dubois, M-F. PRISMA-7: a case-finding tool to identify older adults with moderate to severe disabilities. Arch Gerontol Geriatr (2008) 47:9–18. doi: 10.1016/j.archger.2007.06.004
75. Subra, J, Gillette-Guyonnet, S, Cesari, M, Oustric, S, Vellas, B, and Team, Platform. The integration of frailty into clinical practice: preliminary results from the Gérontopôle. J Nutr Health Aging (2012) 16:714–720. doi: 10.1007/s12603-012-0391-7
76. Guralnik, JM, Simonsick, EM, Ferrucci, L, Glynn, RJ, Berkman, LF, Blazer, DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol (1994) 49:M85–94. doi: 10.1093/geronj/49.2.m85
77. Buta, BJ, Walston, JD, Godino, JG, Park, M, Kalyani, RR, Xue, Q-L, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. (2016) 26:53–61. doi: 10.1016/j.arr.2015.12.003
78. Mitnitski, AB, Mogilner, AJ, and Rockwood, K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. (2001) 1:323–36. doi: 10.1100/tsw.2001.58
79. Malmstrom, TK, Miller, DK, and Morley, JE. A comparison of four frailty models. J Am Geriatr Soc. (2014) 62:721–6. doi: 10.1111/jgs.12735
80. Brousseau, A-A, Dent, E, Hubbard, R, Melady, D, Émond, M, Mercier, É, et al. Multinational emergency department study. Identification of older adults with frailty in the emergency department using a frailty index: results from a multinational study. Age Ageing. (2018) 47:242–8. doi: 10.1093/ageing/afx168
81. Shi, SM, Olivieri-Mui, B, McCarthy, EP, and Kim, DH. Changes in a Frailty index and association with mortality. J Am Geriatr Soc. (2021) 69:1057–62. doi: 10.1111/jgs.17002
82. Kojima, G, Iliffe, S, and Walters, K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. (2018) 47:193–200. doi: 10.1093/ageing/afx162
83. Cattaneo, F, Buondonno, I, Cravero, D, Sassi, F, and D’Amelio, P. Musculoskeletal diseases role in the Frailty syndrome: A case-control study. Int J Environ Res Public Health. (2022) 19:11897. doi: 10.3390/ijerph191911897
84. Bellelli, G, Rebora, P, Valsecchi, MG, Bonfanti, P, and Citerio, G. COVID-19 Monza team members. Frailty index predicts poor outcome in COVID-19 patients. Intensive Care Med. (2020) 46:1634–6. doi: 10.1007/s00134-020-06087-2
86. Rubenstein, LZ, Siu, AL, and Wieland, D. Comprehensive geriatric assessment: toward understanding its efficacy. Aging Milan Italy. (1989) 1:87–98. doi: 10.1007/BF03323881
87. Lee, H, Lee, E, and Jang, I-Y. Frailty and comprehensive geriatric assessment. J Korean Med Sci. (2020) 35:e16. doi: 10.3346/jkms.2020.35.e16
88. Ellis, G, Whitehead, MA, Robinson, D, O’Neill, D, and Langhorne, P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. (2011) 343:d6553. doi: 10.1136/bmj.d6553
89. Mazya, AL, Garvin, P, and Ekdahl, AW. Outpatient comprehensive geriatric assessment: effects on frailty and mortality in old people with multimorbidity and high health care utilization. Aging Clin Exp Res. (2019) 31:519–25. doi: 10.1007/s40520-018-1004-z
90. Veronese, N, Custodero, C, Demurtas, J, Smith, L, Barbagallo, M, Maggi, S, et al. Comprehensive geriatric assessment in older people: an umbrella review of health outcomes. Age Ageing. (2022) 51:afac104. doi: 10.1093/ageing/afac104
91. Palmer, K, and Onder, G. Comprehensive geriatric assessment: benefits and limitations. Eur J Intern Med. (2018) 54:e8–9. doi: 10.1016/j.ejim.2018.02.016
92. Steverink, N, Slaets, J, Schuurmans, H, Lis van, M, and Frailty, M. Development and testing of the Groningen Frailty Indicator (GFI). Gerontologist. (2001) 41:236–7. doi: 10.13401/j.cnki.jsumc.2023.02.014
93. Peters, LL, Boter, H, Buskens, E, and Slaets, JPJ. Measurement properties of the Groningen Frailty Indicator in home-dwelling and institutionalized elderly people. J Am Med Dir Assoc. (2012) 13:546–51. doi: 10.1016/j.jamda.2012.04.007
94. Op Het Veld, LPM, Beurskens, AJHM, de Vet, HCW, van Kuijk, SMJ, Hajema, K, Kempen, GIJM, et al. The ability of four frailty screening instruments to predict mortality, hospitalization and dependency in (instrumental) activities of daily living. Eur J Ageing. (2019) 16:387–94. doi: 10.1007/s10433-019-00502-4
95. Huang, EYZ, Cheung, J, Liu, JYW, Kwan, RYC, and Lam, SC. Groningen Frailty Indicator-Chinese (GFI-C) for pre-frailty and frailty assessment among older people living in communities: psychometric properties and diagnostic accuracy. BMC Geriatr. (2022) 22:788. doi: 10.1186/s12877-022-03437-1
96. Peters, LL, Burgerhof, JGM, Boter, H, Wild, B, Buskens, E, and Slaets, JPJ. Predictive validity of a frailty measure (GFI) and a case complexity measure (IM-E-SA) on healthcare costs in an elderly population. J Psychosom Res. (2015) 79:404–11. doi: 10.1016/j.jpsychores.2015.09.015
97. Theou, O, Brothers, TD, Mitnitski, A, and Rockwood, K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. (2013) 61:1537–51. doi: 10.1111/jgs.12420
98. De Witte, N, Gobbens, R, De Donder, L, Dury, S, Buffel, T, Schols, J, et al. The comprehensive frailty assessment instrument: development, validity and reliability. Geriatr Nurs N Y N. (2013) 34:274–81. doi: 10.1016/j.gerinurse.2013.03.002
99. Morley, JE, and Adams, EV. Rapid geriatric assessment. J Am Med Dir Assoc. (2015) 16:808–12. doi: 10.1016/j.jamda.2015.08.004
100. Gobbens, RJJ, van Assen, MALM, Luijkx, KG, Wijnen-Sponselee, MT, and Schols, JMGA. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc (2010) 11:344–355. doi: 10.1016/j.jamda.2009.11.003
Keywords: older people, frailty, screening, assessment, scale
Citation: Wang X-M, Zhang Y-H, Meng C-C, Fan L, Wei L, Li Y-Y, Liu X-Z and Lv S-C (2024) Scale-based screening and assessment of age-related frailty. Front. Public Health. 12:1424613. doi: 10.3389/fpubh.2024.1424613
Received: 29 April 2024; Accepted: 15 November 2024;
Published: 18 December 2024.
Edited by:
Consuelo Borras, University of Valencia, SpainReviewed by:
Ashwin Subramaniam, Peninsula Health, AustraliaCopyright © 2024 Wang, Zhang, Meng, Fan, Wei, Li, Liu and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-Yang Li, MTU1MTA5Nzc2NzVAMTYzLmNvbQ==; Xue-Zheng Liu, ZHJoYXJlMTk3NUAxNjMuY29t; Shi-Chao Lv, ZHJfbHZAZm94bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.