- 1Department of Psychology, University of Nevada, Las Vegas, NV, United States
- 2Department of Psychiatry, Yale School of Medicine, New Haven, CT, United States
Background: Methadone is an effective and widely used medication for opioid use disorder (MOUD). Within in the United States (US), older adults represent an increasing proportion of those receiving MOUD, yet little is known about characteristics of older individuals in these programs.
Objectives: To evaluate mental and physical health characteristics of younger and older adults receiving MOUD and test whether age moderates the relation between physical and mental health variables.
Methods: Data for this secondary analysis are drawn from a cross-sectional survey of a convenience sample of individuals seeking methadone dosing as part of MOUD at four opioid treatment programs in two regions of the US. Descriptive statistics and correlational and moderation analyses examined outcomes of pain severity, pain interference, self-rated health, physical activity, depression, and anxiety across younger (18-49) and older (50+ years) participants.
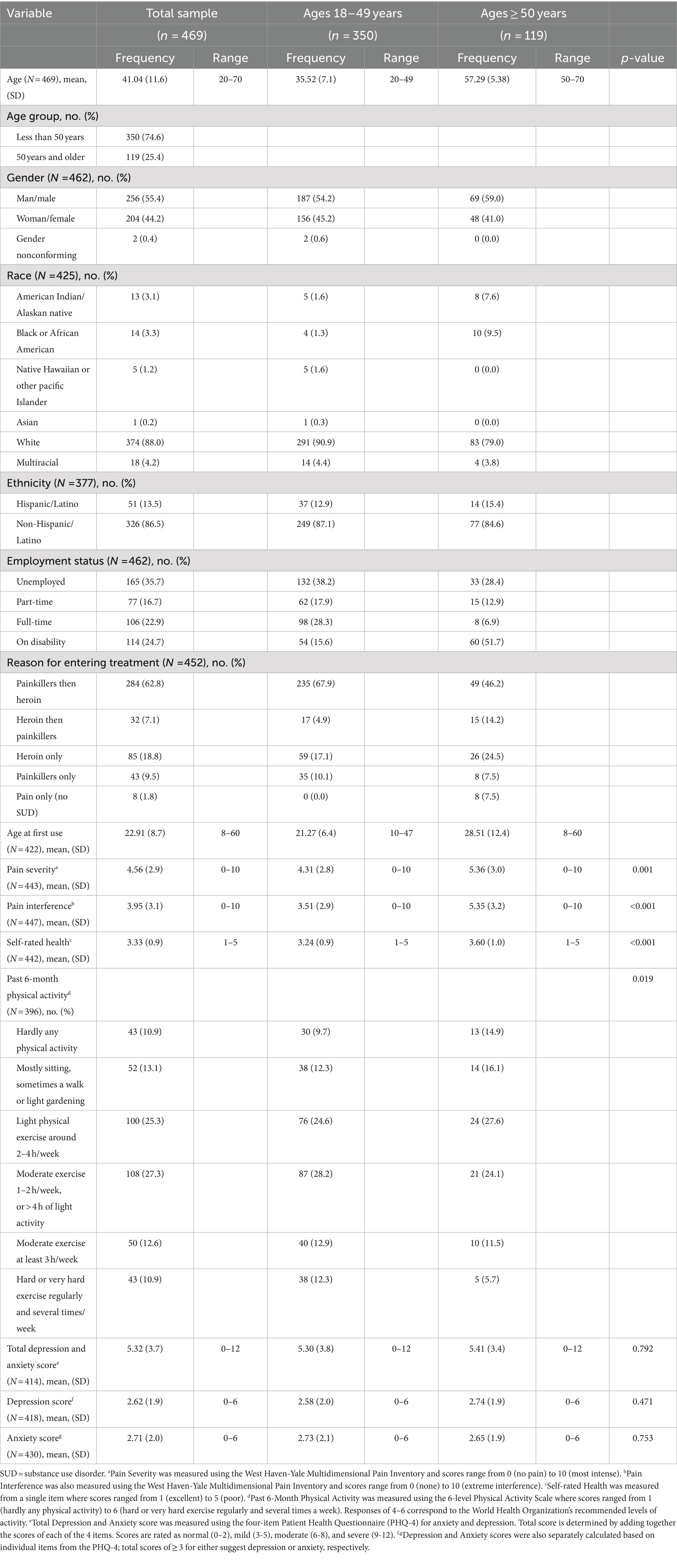
Results: Analyses included 469 participants (mean [range] age, 41.01 [20–70] years). Older participants reported higher pain severity and interference, less physical activity, and worse self-rated health than those in the younger age group (ps < 0.05). Associations between mental and physical health variables were generally weak-to-moderate in the older age group (r = 0.26 to r = 0.44, p < 0.01), and weak in the younger age group, although age did not moderate associations.
Conclusion: Clinically significant mental and physical health variables are associated among individuals receiving methadone for MOUD, with older adults facing unique challenges.
Clinical implications: Opioid use treatment should include not only pain management but also assessment and treatment of depression and anxiety and optimization of other health behaviors (e.g., physical activity) across age groups. Pain management and health promotion are particularly relevant targets for aging individuals receiving MOUD.
1 Introduction
The opioid epidemic in the United States (US) is steadily increasing with opioid-involved overdose deaths escalating from 47,000 in 2018 to almost 70,000 in 2020 (1). Methadone is an effective and widely used medication for opioid use disorder (MOUD) typically offered through opioid treatment programs (OTPs) (2). As in many facets of healthcare, there is a corresponding surge in the number of older adults receiving OUD (3–5). This demographic shift is driven in part by cohort effects such as increased heroin availability and use in the 1960s and 1970s and the beginning of the opioid epidemic in the 1990s (6, 7) coupled with improved access to healthcare and treatment services, which has afforded longer lifespans (8). Treatment can be initiated at any age and some individuals may require long-term—even lifelong—maintenance. Thus, older adults receiving MOUD reflect both individuals who have aged with OUD and those newly acquiring OUD in middle or later life. Despite elevated OUD among adults 50 and older, medical and mental health comorbidities and treatment considerations among older adults with OUD have historically received little attention (5, 9–12).
Both older and younger individuals utilizing methadone in MOUD programs tend to have high levels of disease burden and poor general health compared to population norms (13–15). Depressive and anxiety symptoms are especially common among younger and middle aged individuals (16, 17) and older adults (15, 18) with substance use disorders (SUDs), including OUD and those utilizing methadone in MOUD, relative to average population prevalence. These co-occurring conditions are associated with lower initiation and retention in treatment and an increased risk for relapse across adult populations (19). However, evidence is limited and inconclusive regarding age differences in health status among individuals using MOUD. While older adults in the general US population often have better mental health than their younger counterparts, including lower prevalence of diagnosable (“clinical”) mental health conditions (20), some examinations of individuals prescribed methadone in MOUD showed no differences between older and younger adults in terms of endorsement of psychiatric symptoms (14, 21). Even subsyndromal depressive symptoms in older adults can impart functional decline, greater comorbidity, frailty, cognitive decline, and greater demand for healthcare services (22–24). Mental health problems among older adults are often and disproportionally associated with physical health concerns (25) and this relationship becomes more complex as individuals age and face increasing health concerns (26). Age is an important predictor of a poorer course of depressive (27) and anxiety (28) disorders for other reasons, including loneliness and loss of social support in addition to associated health vulnerabilities such as pain, cognitive impairment, and comorbid chronic disease and related inflammation (27). These consistent findings illustrate that the biological underpinnings linking physical, cognitive, and mental health are pronounced in aging and may suggest a stronger relationship between mental and physical health with age.
Despite the US demography shifts and evidence that older adults are more likely to need and seek treatment for substance use disorders, including OUD, much of the substance use literature focuses on alcohol use and excludes older adult participants (29). The field has largely failed to examine older adults with OUD and those receiving MOUD. Several age-related factors, including co-occurring chronic disease and declining health, chronic pain, functional ability, social isolation, and stigma may influence an individual’s success or satisfaction with methadone in MOUD (30). Older adults are more likely to report greater pain severity (31), and pain during OUD treatment is associated with poorer treatment outcomes like relapse and lower retention in OTPs (32). Conversely, older age is associated with a greater likelihood of retention in OTPs (33), which is a leading predictor of positive treatment outcomes, including reduction of relapse and high-risk behaviors (34). Until very recently, the existing literature on older adults in OTPs receiving methadone was generally limited to single site data collection (e.g., from one clinic in a US city) and characterized by small sample size (e.g., 24–54 participants) (12). Work by Rosen and colleagues (15, 35) included larger samples (i.e., 140 individuals aged 50 and older), albeit from one US city. Although preliminary, this body of work has documented a high prevalence of mental health disorders (e.g., major depressive disorder, generalized anxiety disorder), prevalent chronic physical disease (e.g., arthritis, hypertension), and subjective self-perception of having fair to poor physical health among the majority of the older adult respondents. More recently, a pilot study comparing older adults receiving methadone to a national population-based cohort revealed significantly higher prevalence of conditions including visual and hearing impairment, incontinence, chronic pain, and falls than the national population-based cohort (13).
Aging individuals who have used heroin, other opiates, and opioids experience an accelerated aging process and have complex accumulated health needs. Additionally, long-term opioid use for chronic pain can develop into OUD (36), and chronic pain is often experienced in older adults and individuals on MOUD. However, comparisons between age groups and a lifespan perspective on these clinical profiles is lacking. A better understanding of the unique profiles of older vs. younger adults utilizing methadone in MOUD may help researchers and practitioners develop strategies to engage and effectively treat these individuals.
The current study had two primary aims: (1) to describe mental and physical health characteristics of younger and older adults utilizing methadone in MOUD and test the hypothesis that older adults will report worse mental and physical health characteristics than younger adults; and (2) to conduct an exploratory test of whether age moderated relationships between physical and mental health variables among adults utilizing methadone in MOUD. Although we expect mental and physical health to be positively related in both younger and older groups, we propose to test a moderation model to identify whether the strength of this relationship varies by age group. The dearth of literature on aging adults in MOUD—and among substance use in older adults generally—calls for more examination into potential effect modifiers to better understand risk and resilience and inform targeted needs for assessment and intervention.
2 Method
2.1 Design, participants, and procedures
Data were drawn for secondary analysis from a cross-sectional study of adults enrolled in OTPs, conducted in two US regions in 2017. Reporting follows the STROBE statement guidelines (37). The original study enrolled 484 participants who were recruited from four OTPs in Southern New England (n = 384) and the Pacific Northwest (n = 100). Using convenience sampling, clinic patients were invited by the investigators and clinical staff to complete a self-administered paper survey assessing sociodemographic and health information, substance use, psychosocial functioning, and MOUD characteristics and outcomes. Individuals were excluded from participation if they were younger than 18 years old (and therefore were not able to receive methadone) or were not fluent in English. Participants provided informed consent and the study was approved by the Institutional Review Boards of Dartmouth College and Spectrum Health Services.
To answer the research questions of this secondary analysis, we removed 16 individuals with no age data from the original sample. Compared to the included sample (N = 469), the 16 excluded individuals had greater pain interference (M = 3.95 vs. 5.75, p = 0.048) and lower levels of past 6-month physical activity (M = 2.36 vs. 3.50, p = 0.010). Those included vs removed from analysis on the basis of missing age data did not differ on other outcomes of interest.
2.2 Measures
2.2.1 Participant characteristics and sample classification
2.2.1.1 Sociodemographic characteristics
Participants self-reported basic demographic information including age (in years), gender (man/male, woman/female, or gender nonconforming), race (American Indian/Alaskan Native, Black or African American, Native Hawaiian or other Pacific Islander, Asian, White, or multiracial), ethnicity (Hispanic/Latino or Non-Hispanic/Latino), and employment status (Unemployed, part-time, full-time, or on disability).
2.2.1.2 Treatment variables
Participants self-reported the option which best described why they began an OTP (I was FIRST addicted to painkillers and then became addicted to heroin; I was FIRST addicted to heroin and then became addicted to painkillers; I was addicted to painkillers but never used heroin; I was addicted to heroin but never abused painkillers; I was NEVER addicted, I take methadone ONLY for pain).
2.2.2 Health-related variables
2.2.2.1 Pain
Two items assessing pain intensity/severity (0 = no pain to 10 = most intense) and pain interference (0 = none to 10 = extreme interference) were drawn from the West Haven-Yale Multidimensional Pain Inventory (38). This measure has demonstrated reliability and convergent validity (39).
2.2.2.2 Self-rated health
A single item (“In general, would you say your health is…) measured self-rated health from 1 (excellent) to 5 (poor). This measure is valid, reliable, and widely used in epidemiological and survey research (40).
2.2.2.3 Physical activity
Self-reported physical activity was measured using the 6-level physical activity scale, “How physically active do you think you have been during the last six months?” (41). Physical activity was classified by one of the following responses, 1 = hardly any physical activity through 6 = hard or very hard exercise regularly and several times a week. Responses between 4–6 correspond to the World Health Organization’s recommended levels of physical activity (42). This scale has demonstrated good psychometric characteristics, including among older adults and others with lower levels of physical activity (43).
2.2.2.4 Mental health
The presence and frequency of depression and anxiety symptoms were assessed using the 4-item Patient Health Questionnaire (PHQ-4) (44). The PHQ-4 is a self-report instrument consisting of two core depression items from the PHQ-9 (45) and two core anxiety items from the Generalized Anxiety Disorder (GAD-7) scale (46). Responses to each item ranged from 0 (not at all) to 3 (nearly every day). The PHQ-4 is commonly used in survey research to briefly screen for the presence of depressive and anxiety symptoms and has demonstrated reputable internal reliability, construct validity, and discriminant validity (44). Separate depression and anxiety symptom scales were calculated by summing the two depression or anxiety items, respectively. Additionally, total depression and anxiety symptoms (hereinafter referred to as mental health) were determined for each participant by adding together the scores of all 4 items. Higher scores indicate higher levels of depressive symptoms, anxiety symptoms, and mental health symptoms.
2.3 Statistical analysis
Given the accelerated aging and premature mortality associated with substance use, there is no current consensus regarding the cutoff for “older” adults in the substance use literature (8, 47). We compared participants between ages 18 and 49 years (“younger adults”) to participants ages 50 years and older (“older adults”) based on prior studies of SUDs in older adulthood that generally include adults ages 50 years and older (48, 49). Given the difference in sample sizes between younger and older adults, we used Levene’s test to assess homogeneity of variance in all outcome variables between the two age groups. All outcome variables met the threshold for equal variances assumed (p > 0.05) with the exception of anxiety symptoms as measured by the GAD-2 (p = 0.04). GAD-2 scores were thus subjected to square root transformation to equalize or stabilize variance; once transformed, Levene’s test supported equality of variance (p = 0.06). All statistical tests described below were analyzed using both the raw GAD-2 continuous score and the transformed GAD-2 score; results did not change. For the purpose of parsimony and ease of interpretation, the results of the untransformed (raw) GAD-2 measure are reported throughout.
Prior to all analyses, the data were reviewed for other general assumptions of parametric tests (50). Normality was assessed by skewness and kurtosis values between −2 and + 2 and aided by visual inspection of univariate normal Q-Q plots and histograms. For all regression analyses (moderation analyses, see below), normality was further assessed via inspection of normal P–P plot of regression standardized residuals and linearity assessed via scatterplot of standardized residuals. Data on all outcomes were sufficiently linear. Outliers were infrequent and such cases were retained as analysis with and without these values did not affect the results. Summary statistics described the sample. Correlations between the primary variables of interest (i.e., age group, pain, mental health variables, self-rated health, and physical activity) were calculated using Pearson’s correlation for continuous variables, Spearman’s correlations for ordinal variables, and Pearson’s chi-square for categorical variables. Strength of correlations were interpreted according to existing guidelines (51) as 0.0 to 0.2 = very weak, 0.2 to 0.4 = weak, 0.4 to 0.6 = moderate, 0.6 to 0.8 = strong (absolute values). 95% confidence intervals (CI) were added to all correlations in brackets.
Moderation analyses tested the effect of age on the associations of physical health and pain variables with depression symptoms, anxiety symptoms, and mental health. Specifically, the moderating effect of age was tested for the following associations: (1) self-rated health and depression, (2) self-rated health and anxiety, (3) self-rated health and aggregate mental health, (4) physical activity and depression, (5) physical activity and anxiety, (6) physical activity and mental health, (7) pain severity and depression, (8) pain severity and anxiety, (9) pain severity and mental health, (10) pain interference and depression, (11) pain interference and anxiety, and (12) pain interference and mental health. Age was kept as a continuous variable for moderation analyses to increase sensitivity for detecting a significant effect. All statistical analyses were performed using IBM SPSS Statistics version 28.0 and version 4.0 of the PROCESS macro for SPSS (52).
G power analyses for linear regression were performed post hoc to calculate observed power to detect a moderation effect of age in our sample (53). Observed statistical power across models was >0.95.
3 Results
3.1 Participant characteristics
Participant characteristics are presented in Table 1. The mean age of the sample was 41 years (SD = 11.61), and 74.60% of the sample were less than 50 years old (n = 350). Over half of the sample identified as men (55.60%, n = 266) and White (87.60%, n = 383). Compared to those younger than 50 years old, participants in the older age group were more likely to be on disability (15.6% vs. 51.7%, p < 0.001) and less likely to have entered initial treatment with a painkiller addiction before acquiring a heroin addiction (67.9% vs. 46.2%, p < 0.001). Older adults also reported higher pain severity (M = 4.32 vs. M = 5.36, p = 0.001), higher pain interference (M = 3.51 vs. M = 5.35, p < 0.001), worse self-rated health (M = 3.24 vs. M = 3.60, p < 0.001), and less physical activity in the past 6 months (M = 3.59 vs. M = 3.18, p = 0.019), compared to their younger counterparts in the sample.
The overall sample reported mild-to-moderate symptoms of depression and anxiety on the PHQ-4 (M = 5.34, SD = 3.7). Endorsement of depressive symptoms (younger: M = 2.58 [SD = 2.0] vs. older: M = 2.74 [SD = 1.9], p = 0.471) and anxiety symptoms (younger: M = 2.73 [SD = 2.1] vs. older: M = 2.65 [SD = 1.9], p = 0.75) was not significantly different across age groups.
3.2 Associations between health-related variables
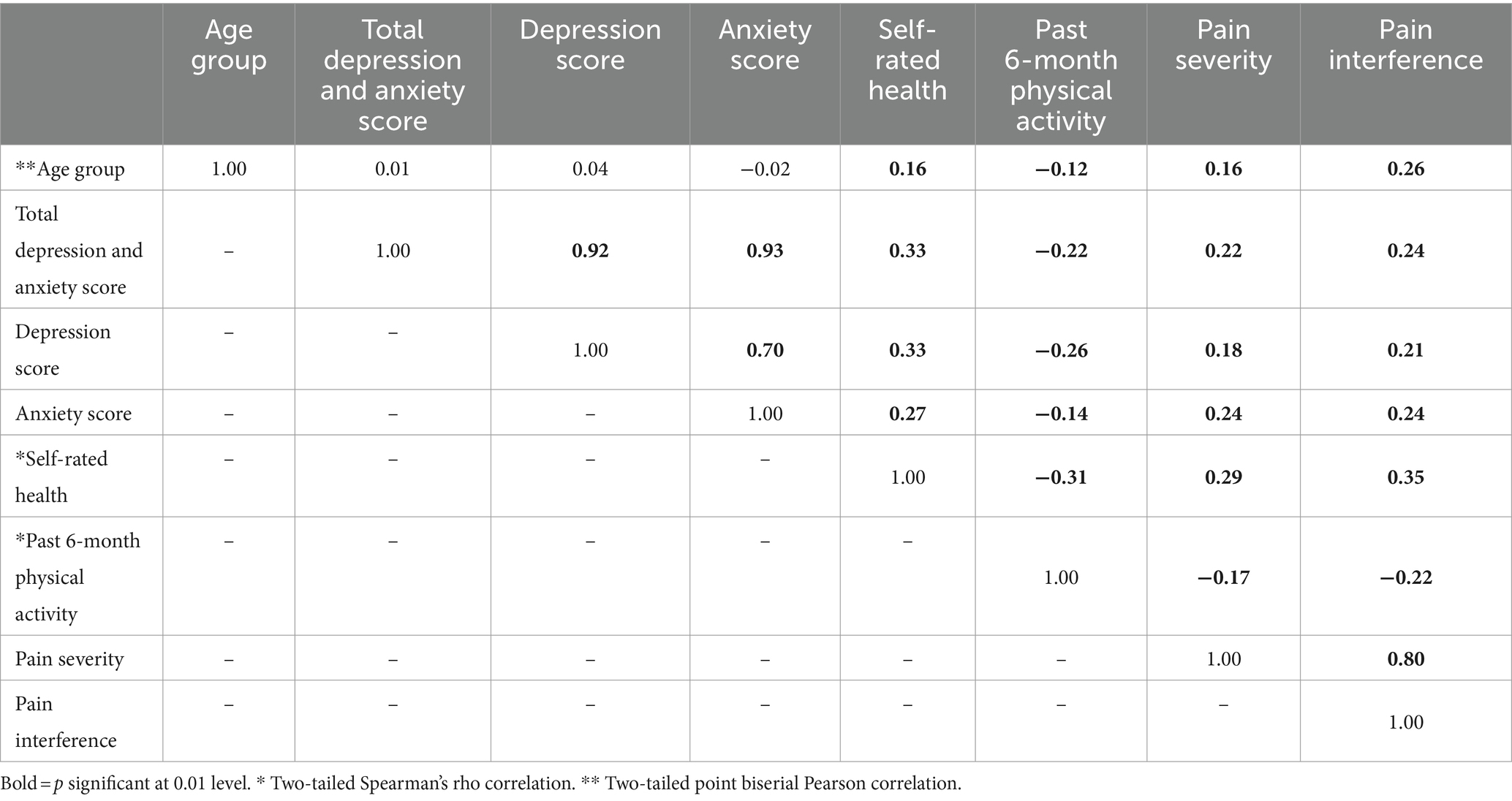
Main correlations are presented in Table 2. Collapsing across the entire sample, bivariate correlations revealed a weak-to-moderate association between worse self-rated health and greater depression (r = 0.33 [0.27, 0.44]), greater anxiety (r = 0.27 [0.19, 0.37]), higher pain severity (r = 0.29 [0.22, 0.40]), and higher pain interference (r = 0.37 [0.29, 0.45]); all ps < 0.001. Similarly, less physical activity was weakly associated with greater depression (r = −0.26 [−0.35, −0.16]), greater anxiety (r = −0.14 [−0.23, − 0.04]), higher pain severity (r = −0.16 [−0.27, −0.08]), and higher pain interference (r = −0.21 [−0.31, −0.12]) all ps < 0.01. Correlation analyses also showed that higher pain severity and higher pain interference were weak-to-moderately associated with greater depression (r = 0.18 [0.08, 0.27] and r = 0.21 [0.12, 0.30], both ps < 0.001) and greater anxiety (r = 0.24 [0.14, 0.32] and r = 0.24 [0.15, 0.33], both ps < 0.001). Finally, greater mental health symptomatology demonstrated positive weak associations with pain severity (r = 0.22 [0.12, 0.31]) and pain interference (r = 0.24 [0.15, 0.33], both ps < 0.001).
Among older adults, worse mental health was associated with greater pain severity (r = 0.26 [0.05, 0.45], p = 0.016) and pain interference (r = 0.42 [0.23, 0.58], p < 0.001), worse self-rated health (r = 0.44 [0.26, 0.59], p < 0.001), and less physical activity (r = −0.26 [−0.45, −0.04], p = 0.023). These associations were weak for younger adults in the sample (mental health and pain severity: r = 0.20 [0.10, 0.31], p < 0.001; pain interference: r = 0.20 [0.09, 0.30], p < 0.001; self-rated health r = 0.33 [0.23, 0.43], p < 0.001; physical activity r = − 0.21 [−0.31, −0.10], p < 0.001). For the older adults in this sample, worse self-rated health was associated with less past 6-month physical activity (r = −0.26 [−0.45, −0.05], p = 0.016), greater pain severity (r = 0.33 [0.14, 0.50], p < 0.001), and greater pain interference (r = 0.42 [0.25, 0.57], p < 0.001). Younger adults also evidenced a weak relationship between worse self-rated health and less past 6-month physical activity (r = −0.31 [−0.41, −0.21], p < 0.001), greater pain severity (r = 0.28 [0.18, 0.38], p < 0.001), and pain interference (r = 0.32 [0.22, 0.41], p < 0.001).
3.3 Moderating effect of age
Moderation analyses were performed to assess the indirect effect of age on the relationships between self-rated health, past 6-month physical activity, pain severity, pain interference, and depression symptoms, anxiety symptoms, and mental health. Age did not moderate any of these relations between variables (all p-values >0.10; Table 3).
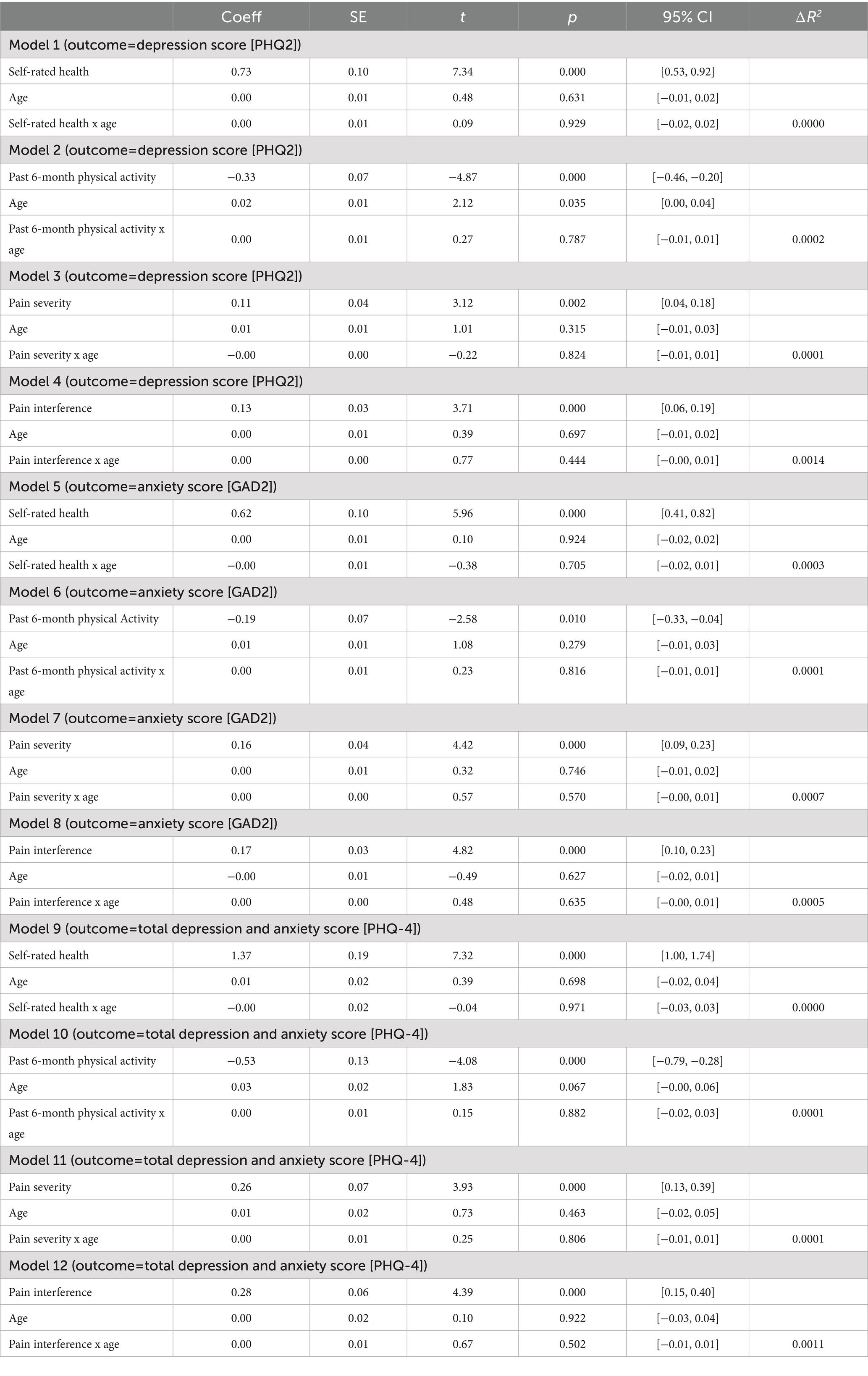
Table 3. Results of regression models testing moderating effect of age on physical health and mental health variables.
4 Discussion
The present study assessed the distinct profile of older adults in an OTP by describing mental and physical health characteristics across age groups and testing whether older adults differed from their younger counterparts in our sample on health-related variables. We also tested whether age moderated the relationships between these characteristics. Older adults in our sample reported significantly worse overall physical health, pain severity, and pain interference compared to younger adults. Across the sample, worse self-rated health and less physical activity were associated with greater depression and anxiety and higher pain severity and interference. Higher pain severity and interference were also significantly associated with greater depression and anxiety symptoms. Finally, the relationships between worse mental health and worse self-rated health, less physical activity, greater pain severity, and greater pain interference were generally of weak-to-moderate strength in the older adult group and weak among the younger adult group. However, age did not moderate the associations between variables.
We found support for the hypothesis that older adults receiving MOUD (that is, those who are aging with long-term use of heroin and other narcotics) would be in worse physical health than younger adults receiving MOUD. The sample on average endorsed mild-to-moderate symptoms of depression and anxiety; however, differences in mental health between younger and older participants in our sample were not significant. This extends prior works on adults with substance use disorders, which have primarily focused on alcohol use, to address the unique facets of older adults with OUD maintained on methadone (29, 54). Findings from the current study align with prior work demonstrating that older adults with OUD had poorer physical health compared to younger adults (8, 14, 55). We also found that older adults had greater pain severity and interference compared to the younger group, which may be encapsulated in poorer self-rated health ratings among older adults. These findings are important given that the presence of pain (32) and mental health symptoms (19) have been associated with lower substance use treatment retention, while older age has been associated with higher treatment retention (33). Future work should examine whether older age may mitigate the relationships between physical and mental health and treatment retention.
Despite the lack of statistical significance in the moderation analyses, it is possible—and worthy of future research—that the relationship between mental and physical health characteristics may be stronger among older vs. younger adults receiving MOUD. One possible explanation for this finding is that perceptions of health may vary by age, such that older adults may attribute mental health concerns more strongly to physical health problems compared to younger adults. Mental health presentations in older adults may be subtle or nonspecific and thus overlooked (56). Mental and physical health difficulties also vary in presentation, complexity, and experience across the life course, with older adults more likely to have medical and psychiatric comorbidity and overlapping symptom presentation. The physical health burden of mental health symptoms is magnified across the lifespan, with vulnerabilities of frailty and functional decline more likely among older than younger adults. An example of this in our sample was the moderate relation between worse mental health and pain interference (a self-report assessment of functioning) in older adults compared to the weak correlation found in the younger sample. The allostatic load framework refers to the accumulation of physiological disturbances (e.g., activation of the hypothalamic–pituitary–adrenal [HPA] axis) in response to stress and the long-term effects of such dysregulation on mental and physical health (57). This presents one possible pathway though which mental and physical health are linked, perhaps more strongly in older adults with greater allostatic load. The presence of co-occurring SUD further complicates this clinical picture; however, the intersection of age and SUD is severely understudied. An older review of 20 leading journals in substance and abuse and gerontology (10 each) found a paucity of articles with a joint focus on older adults and substance abuse (broadly considered; less than 1% of all articles) (48). Principles of geriatric medicine have yet to be incorporated into substance use treatment on a large scale, and geriatric providers are often not focused on or feel ill-equipped to treat SUDs in older adults. To adequately prepare clinicians for working with older adults who use substances, including those with OUD, further research on age-related factors in risk and treatment, as well as a coordinated effort between disciplines and practitioners, is crucial.
4.1 Limitations
This study provides further insights into the distinct characteristics of individuals with OUD who are maintained on methadone across age groups; however, several limitations should be carefully considered. This was a cross-sectional study, prohibiting causal associations. Future studies should consider whether these findings hold over time and across birth cohorts (e.g., among those 65 years and older and older versus 50-64-year-olds). These data were drawn from a convenience sample and may not represent the general adult population in OTPs. For example, those with more severe mental health conditions may be missing from the sample. Related, mental and physical health data are based on self-report rather than clinical data, which may have introduced bias. Future studies may consider methodology such as chart review to include a more representative sample and collect objective measures, such as diagnostic interviewing and standard physical health markers. We do not have data on survey response rates or whether respondents were representative of the OTP clinic clientele census, nor do we have data on how mental and physical health variables related to key outcomes such as treatment retention or substance use among those receiving methadone for MOUD. The majority of the sample were non-Hispanic White individuals, limiting generalizability. However, this study included a larger sample than most previous studies of older adults in OTPs, and utilized data collected from multiple cities across two distinct US regions. Despite these shortcomings, these findings contribute to the growing knowledge of the diverse characteristics and corresponding needs across individuals in treatment for OUD and lay a foundation for future research to continue to build on the profile of older adults in OTPs.
4.2 Summary
Significant associations between mental and physical health characteristics exist for individuals utilizing methadone for medication for OUD, although age does not appear to moderate these effects in this sample. Older adults utilizing methadone have some similarities (similar mental health symptoms) and differences (more pain, worse self-rated health) compared to their younger adult counterparts, highlighting the need to better understanding these characteristics in aging individuals in OTPs to maximize intervention effectiveness and engagement.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: available upon request from Brandi P. Cotton (YnJhbmRpLmNvdHRvbkB5YWxlLmVkdQ==).
Ethics statement
The studies involving humans were approved by Institutional Review Boards of Dartmouth College and Spectrum Health Services. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TW: Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. RM: Writing – original draft. SK: Writing – review & editing. BC: Data curation, Investigation, Methodology, Resources, Validation, Writing – review & editing. BR: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by the National Institute of Mental Health (T32 MH073553) and the National Institute of General Medical Sciences (P20 GM103440). The publication fees for this article were supported by the Graduate and Professional Student Association Open Access Article Fund at the University of Nevada, Las Vegas, United States.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. National Institute on Drug Abuse. National Institute on Drug Abuse. (2020) (cited 2022 Dec 16). Overdose death rates. Available at: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates
2. Substance Abuse and Mental Health Services Administration. Substance Abuse and Mental Health Services Administration. (2022). Methadone. Available at: https://www.samhsa.gov/medication-assisted-treatment/medications-counseling-related-conditions/methadone#:~:text=Methadone%20is%20a%20medication%20approved,methadone%20is%20safe%20and%20effective
3. Cotton, BP, Bryson, WC, and Bruce, ML. Methadone maintenance treatment for older adults: cost and logistical considerations. PS. (2018) 69:338–40. doi: 10.1176/appi.ps.201700137
4. European Monitoring Centre for Drugs and Drug Addiction. Health and Social Responses to Drug Problems: A European Guide. (2017). Health and social responses to drug problems: A European guide. Available at: https://www.emcdda.europa.eu/publications/health-and-social-responses-a-european-guide_en
5. Han, B, Polydorou, S, Ferris, R, Blaum, CS, Ross, S, and McNeely, J. Demographic trends of adults in new York City opioid treatment programs—an aging population. Subst Use Misuse. (2015) 50:1660–7. doi: 10.3109/10826084.2015.1027929
6. Cicero, TJ, Ellis, MS, Surratt, HL, and Kurtz, SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. (2014) 71:821. doi: 10.1001/jamapsychiatry.2014.366
7. DeWeerdt, S. Tracing the US opioid crisis to its roots. Nature. (2019) 573:S10–2. doi: 10.1038/d41586-019-02686-2
8. Carew, AM, and Comiskey, C. Treatment for opioid use and outcomes in older adults: a systematic literature review. Drug Alcohol Depend. (2018) 182:48–57. doi: 10.1016/j.drugalcdep.2017.10.007
9. Blanco, C, and Volkow, ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. (2019) 393:1760–72. doi: 10.1016/S0140-6736(18)33078-2
10. Doukas, N. Older adults in methadone maintenance treatment: a literature review. J Soc Work Pract Addict. (2011) 11:230–44. doi: 10.1080/1533256X.2011.596456
11. Konakanchi, JS, and Sethi, R. The growing epidemic of opioid use disorder in the elderly and its treatment. Prim Care Comp J Clin Psychiatry. (2023) 25:44998. doi: 10.4088/PCC.21r03223
12. Rosen, D, Hunsaker, A, Albert, SM, Cornelius, JR, and Reynolds, CF. Characteristics and consequences of heroin use among older adults in the United States: a review of the literature, treatment implications, and recommendations for further research. Addict Behav. (2011) 36:279–85. doi: 10.1016/j.addbeh.2010.12.012
13. Han, BH, Cotton, BP, Polydorou, S, Sherman, SE, Ferris, R, Arcila-Mesa, M, et al. Geriatric conditions among middle-aged and older adults on methadone maintenance treatment: a pilot study. J Addict Med. (2022) 16:110–3. doi: 10.1097/ADM.0000000000000808
14. Lofwall, MR, Brooner, RK, Bigelow, GE, Kindbom, K, and Strain, EC. Characteristics of older opioid maintenance patients. J Subst Abus Treat. (2005) 28:265–72. doi: 10.1016/j.jsat.2005.01.007
15. Rosen, D, Smith, ML, and Reynolds, CF. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. (2008) 16:488–97. doi: 10.1097/JGP.0b013e31816ff35a
16. Smith, JP, and Book, SW. Comorbidity of generalized anxiety disorder and alcohol use disorders among individuals seeking outpatient substance abuse treatment. Addict Behav. (2010) 35:42–5. doi: 10.1016/j.addbeh.2009.07.002
17. Stein, MD, Herman, DS, Bailey, GL, Straus, J, Anderson, BJ, Uebelacker, LA, et al. Chronic pain and DepressionAmong primary care patients treated with buprenorphine. J Gen Intern Med. (2015) 30:935–41. doi: 10.1007/s11606-015-3212-y
18. Wu, LT, and Blazer, DG. Substance use disorders and psychiatric comorbidity in mid and later life: a review. Int J Epidemiol. (2014) 43:304–17. doi: 10.1093/ije/dyt173
19. Slayter, EM. Disparities in access to substance abuse treatment among people with intellectual disabilities and serious mental illness. Health Soc Work. (2010) 35:49–59. doi: 10.1093/hsw/35.1.49
20. Renn, BN, Areán, PA, and Unützer, J. Epidemiology of selected mental disorders in later life. In: Handbook of mental health and aging [internet]. 3rd ed. Elsevier; (2020) p. 7–22. Available at https://linkinghub.elsevier.com/retrieve/pii/B9780128001363000028
21. Firoz, S, and Carlson, G. Characteristics and treatment outcome of older methadone-maintenance patients. Am J Geriatr Psychiatry. (2004) 12:539–41. doi: 10.1097/00019442-200409000-00015
22. Alexopoulos, GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. (2019) 9:188. doi: 10.1038/s41398-019-0514-6
23. Kaup, AR, Byers, AL, Falvey, C, Simonsick, EM, Satterfield, S, Ayonayon, HN, et al. Trajectories of depressive symptoms in older adults and risk of dementia. JAMA Psychiatry. (2016) 73:525–31. doi: 10.1001/jamapsychiatry.2016.0004
24. Soysal, P, Veronese, N, Thompson, T, Kahl, KG, Fernandes, BS, Prina, AM, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev. (2017) 36:78–87. doi: 10.1016/j.arr.2017.03.005
25. Fiske, A, Ebert, AR, Fenstermacher, EA, and Owsiany, MT. Mood Disorders in Later Life. In: Comprehensive clinical psychology [internet]. Elsevier; (2022). p. 161–179. Available at: https://linkinghub.elsevier.com/retrieve/pii/B9780128186978000431
26. Bergua, V, Blanchard, C, and Amieva, H. Depression in older adults: do current DSM diagnostic criteria really fit? Clin Gerontol. (2023) 1–38:1–38. doi: 10.1080/07317115.2023.2274053
27. Schaakxs, R, Comijs, HC, Lamers, F, Kok, RM, Beekman, ATF, and Penninx, BWJH. Associations between age and the course of major depressive disorder: a 2-year longitudinal cohort study. Lancet Psychiatry. (2018) 5:581–90. doi: 10.1016/S2215-0366(18)30166-4
28. Lenze, EJ, and Wetherell, JL. A lifespan view of anxiety disorders. Dialogues Clin Neurosci. (2011) 13:381–99. doi: 10.31887/DCNS.2011.13.4/elenze
29. Rosen, D, Engel, RJ, Beaugard, C, Davis, N, and Cochran, G. Baby Boomer’s substance abuse and researcher indifference. J Gerontol Soc Work. (2019) 62:16–28. doi: 10.1080/01634372.2018.1530715
30. Han, BH, Orozco, MA, Miyoshi, M, Doland, H, Moore, AA, and Jones, KF. Experiences of aging with opioid use disorder and comorbidity in opioid treatment programs: a qualitative analysis. J Gen Intern Med. (2024) 39:1673–80. doi: 10.1007/s11606-024-08676-z
31. Voon, P, Hayashi, K, Milloy, MJ, Nguyen, P, Wood, E, Montaner, J, et al. Pain among high-risk patients on methadone maintenance treatment. J Pain. (2015) 16:887–94. doi: 10.1016/j.jpain.2015.06.003
32. Nordmann, S, Vilotitch, A, Lions, C, Michel, L, Mora, M, Spire, B, et al. Pain in methadone patients: time to address undertreatment and suicide risk (ANRS-Methaville trial). PLoS One. (2017) 12:e0176288. doi: 10.1371/journal.pone.0176288
33. O’Connor, AM, Cousins, G, Durand, L, Barry, J, and Boland, F. Retention of patients in opioid substitution treatment: a systematic review. PLoS One. (2020) 15:e0232086. doi: 10.1371/journal.pone.0232086
34. Sheikh Fathollahi, M, Torkashvand, F, Najmeddin, H, and Rezaeian, M. Predictors of one-year retention in methadone maintenance treatment (MMT) in Iran, Rafsanjan. Int J High Risk Behav Addict. (2016) 5:e29121. doi: 10.5812/ijhrba.29121
35. Rosen, D. Factors associated with illegal drug use among older methadone clients. The Gerontologist. (2004) 44:543–7. doi: 10.1093/geront/44.4.543
36. Huhn, AS, Strain, EC, Tompkins, DA, and Dunn, KE. A hidden aspect of the U.S. opioid crisis: rise in first-time treatment admissions for older adults with opioid use disorder. Drug Alcohol Depend. (2018) 193:142–7. doi: 10.1016/j.drugalcdep.2018.10.002
37. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, Vandenbroucke, JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. (2007) 147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010
38. Kerns, RD, Turk, DC, and Rudy, TE. The west haven-Yale multidimensional pain inventory (WHYMPI). Pain. (1985) 23:345–56. doi: 10.1016/0304-3959(85)90004-1
39. Bernstein, IH, Jaremko, ME, and Hinkley, BS. On the utility of the west haven-Yale multidimensional pain inventory. Spine. (1995) 20:956–63. doi: 10.1097/00007632-199504150-00014
40. Bombak, AE. Self-rated health and public health: a critical perspective. Front Public Health. (2013):1. doi: 10.3389/fpubh.2013.00015/abstract
41. Grimby, G. Physical activity and muscle training in the elderly. Acta Med Scand Suppl. (1986) 711:233–7.
42. Elfving, B, Andersson, T, and Grooten, WJ. Low levels of physical activity in back pain patients are associated with high levels of fear-avoidance beliefs and pain catastrophizing. Physiother Res Int. (2007) 12:14–24. doi: 10.1002/pri.355
43. Frändin, K, and Grimby, G. Assessment of physical activity, fitness and performance in 76-year-olds. Scand J Med Sci Sports. (1994) 4:41–6. doi: 10.1111/j.1600-0838.1994.tb00404.x
44. Kroenke, K, Spitzer, RL, Williams, JBW, and Löwe, B. An ultra-brief screening scale for anxiety and depression: the PHQ–4. Psychosomatics. (2009) 50:613–21. doi: 10.1176/appi.psy.50.6.613
45. Kroenke, K, Spitzer, RL, and Williams, JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
46. Spitzer, RL, Kroenke, K, Williams, JBW, and Löwe, B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092. doi: 10.1001/archinte.166.10.1092
47. Mayock, P, and Butler, S. “I’m always hiding and ducking and diving”: the stigma of growing older on methadone. Drugs Educ Prev Policy. (2022) 29:139–49. doi: 10.1080/09687637.2021.1886253
48. Rosen, D, Engel, RJ, Hunsaker, AE, Engel, Y, Detlefsen, EG, and Reynolds, CF. Just say know: an examination of substance use disorders among older adults in Gerontological and substance abuse journals. Soc Work Public Health. (2013) 28:377–87. doi: 10.1080/19371918.2013.774668
49. Schepis, TS, Simoni-Wastila, L, and McCabe, SE. Prescription opioid and benzodiazepine misuse is associated with suicidal ideation in older adults. Int J Geriatr Psychiatry. (2019) 34:122–9. doi: 10.1002/gps.4999
50. Tabachnick, BG, Fidell, LS, and Ullman, JB. Using multivariate statistics. Seventh ed. NY, NY: Pearson (2019). 832 p.
51. Akoglu, H. User’s guide to correlation coefficients. Turkish J Emerg Med. (2018) 18:91–3. doi: 10.1016/j.tjem.2018.08.001
52. Hayes, AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. Third ed. New York, NY: The Guilford Press (2022) (Methodology in the social sciences).
53. Faul, F, Erdfelder, E, Buchner, A, and Lang, AG. Statistical power analyses using G*power 3.1: tests for correlation and regression analyses. Behav Res Methods. (2009) 41:1149–60. doi: 10.3758/BRM.41.4.1149
54. Han, BH. Aging, multimorbidity, and substance use disorders: the growing case for integrating the principles of geriatric care and harm reduction. Int J Drug Policy. (2018) 58:135–6. doi: 10.1016/j.drugpo.2018.06.005
55. Rajaratnam, MDR, Sivesind, PDD, Todman, PDM, Roane, MDD, and Seewald, MDR. The aging methadone maintenance patient: treatment adjustment, long-term success, and quality of life. J Opioid Manag. (2018) 5:27–37. doi: 10.5055/jom.2009.0004
56. Rao, R, Crome, I, Crome, P, and Iliffe, S. Substance misuse in later life: challenges for primary care: a review of policy and evidence. Prim Health Care Res Dev. (2019) 20:e117. doi: 10.1017/S1463423618000440
Keywords: opioids, older adults, depression, anxiety, self-rated health, medication for opioid use disorder, methadone
Citation: Walker TJ, Mohankumar R, Kraus SW, Cotton BP and Renn BN (2024) Mental and physical health characteristics of older and younger adults receiving medication for opioid use disorder. Front. Public Health. 12:1418690. doi: 10.3389/fpubh.2024.1418690
Edited by:
Juan Carlos Sepúlveda-Arias, Technological University of Pereira, ColombiaReviewed by:
Jose Erik Alvarez Contino, Juan Bruno Zayas Cifuentes University Polyclinic, CubaLandhing Moran, National Institute on Drug Abuse (NIH), United States
Copyright © 2024 Walker, Mohankumar, Kraus, Cotton and Renn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brenna N. Renn, YnJlbm5hLnJlbm5AdW5sdi5lZHU=
†ORCID: Brenna N. Renn, https://orcid.org/0000-0002-8746-171X
 Teresa J. Walker
Teresa J. Walker Rakshitha Mohankumar
Rakshitha Mohankumar Shane W. Kraus
Shane W. Kraus Brandi P. Cotton2
Brandi P. Cotton2 Brenna N. Renn
Brenna N. Renn