- 1Department of Geriatric Psychiatry, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Alzheimer's Disease and Related Disorders Center, Shanghai Jiao Tong University, Shanghai, China
- 3Division of Psychotic Disorders, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Clinical Center for Psychotic Disorders, National Center for Mental Disorders, Shanghai, China
Introduction: Apolipoprotein E (APOE) epsilon 4 is regarded as the most significant genetic contributor linked to mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Daily life elements might also influence cognitive abilities to some extent. This research aimed to investigate whether carrying APOE ε4 alters the effects of lifestyle on cognitive ability.
Methods: The research included 1871 senior community members with APOE gene data, all participating in clinical, neuropsychological, and daily living factor assessments. Based on their APOE ε4 status, they were categorized into two groups: the APOE ε4 group (n = 362) and the non-APOE ε4 group (n = 1,509). Subsequently, a multivariate logistic regression analysis was employed to investigate the link between cognitive deficits and APOE ε4, along with lifestyle patterns.
Results: Our research revealed a reduced incidence of MCI (OR = 0.745, 95% CI: 0.587–0.945, p = 0.015) and dementia (OR = 0.422, 95% CI: 0.259–0.688, p = 0.001) in the non-APOE ε4 carriers. Furthermore, the general linear regression analysis revealed a notable interplay between APOE ε4 and sleep disturbances, potentially impacting cognitive deterioration together (F = 6.817, p = 0.001).
Conclusions: The research indicates that possessing APOE ε4 alters the impact of everyday life factors on cognitive decline. In addition, there is a significant interaction between APOE ε4 and sleep disorders, which may jointly lead to the appearance of cognitive impairment.
1 Introduction
Despite numerous extensive genome-wide association studies (GWAS) and meta-analyses, the ε4 allele in the APOE gene continues to be the primary genetic contributor to sporadic Alzheimer’s disease (AD) (1). The presence of APOE ε4 elevates the likelihood of AD development and concurrently diminishes the onset age of AD (2). Increasingly, data suggests that APOE ε4 heightens the likelihood of Alzheimer’s disease development through amplified toxic impacts and diminished protective capabilities (3). Beyond Alzheimer’s disease, APOE ε4 is also linked to mild cognitive impairment (MCI) (4, 5), typical aging (6, 7), and other neurodegenerative conditions like Lewy body dementia (LBD) (8) and vascular dementia (VD) (9). Yet, the precise function of APOE ε4 in these illnesses remains unclear.
Because dementia is thought to begin decades before clinical symptoms appear, interventions targeting risk factors in non-Alzheimer’s and even middle-aged adults may prevent or delay the onset of cognitive decline (10). Previous studies have explored the link between the Mediterranean diet and Alzheimer’s disease, with diets low in salt, fat, fruits and vegetables thought to be associated with cognitive status (11, 12). In addition to diet, other modifiable lifestyle, such as smoking, depression, hypertension, diabetes mellitus, physical inactivity, low educational attainment, and obesity in middle age, may also play a significant role in the development of dementia (13, 14). Therefore, we propose a comprehensive theory that a “healthy lifestyle” such as a Mediterranean diet, non-smoking, light to moderate alcohol consumption (1–15 g/day for women; 1–30 g/day for men), moderate or vigorous exercise, and active treatment of chronic diseases such as hypertension, diabetes, and depression are associated with cognitive function. An unhealthy lifestyle of high salt, high fat, high sugar diet, sedentary, excessive smoking, excessive alcohol consumption, poor blood sugar and blood pressure control can lead to cognitive decline (15). Similarly, even if all of the above healthy lifestyle patterns or unhealthy lifestyle patterns are not realized, individual or isolation factors may be related to cognitive function. It must be clarified here that the present study was intended only to examine some aspects of the theory, not all.
However, it is not known whether lifestyle influences cognitive function vary by carrying different APOE genotypes. In Licher et al.’s (16) study, they demonstrated that favorable modifiable risk profiles were associated with a lower risk of dementia compared to poor genetic risk in individuals with moderate to low genetic risk. In Rosenich et al. (17) study, they found that aging and APOE ε4 were associated with an increased rate of hippocampal volume (HV) loss and decline in episodic memory (EM). However, the Rodriguez et al. (18) study found that in the general population aged 40–79, the APOE gene’s dementia risk variation did not alter the relationship between lifestyle factors and cognitive performance. Large differences between epidemiological studies can be attributed to possible common factors such as differences in sample size, demographic characteristics of participants (including age and sex), method design, and comorbidities (19).
In this study, we used data from the Shanghai Brain Health Cohort Study to investigate whether APOE gene changes the impact of lifestyles (e.g., smoking, drinking alcohol, tea drinking, eating habits, characteristic food consumption habits and chronic diseases) on cognitive function. We hypothesized that (1) certain lifestyle patterns (such as smoking, and alcohol consumption) may have an impact on cognitive function; (2) APOE ε4 genotype may alter the impact of certain lifestyle factors on cognitive function.
2 Methods
All participants were from the Shanghai Brain Health Cohort Study, which has been described in detail in our previous studies (20). Simply put, 1,871 community older adults with APOE gene data were included in the current study. Of these, 649, or 34.7 percent, were men. Their average age was 69.31 ± 7.87 years, average years of schooling was 10.56 ± 3.64 years, and mean body mass index (BMI) was 23.97 ± 3.37 kg/m2. All subjects underwent comprehensive physical examinations, daily habits surveys, clinical data collection, and neuropsychological evaluation. They will also be asked to fill out a standardized questionnaire that includes daily living habits (such as smoking, alcohol drinking, tea drinking, surfing the Internet, etc.), eating habits (such as whether they eat fruit, whether they eat ginger, etc.), and surveys of chronic diseases (such as hypertension, diabetes, depression, sleep duration, etc.).
The research program complies with the Helsinki Declaration ethical guidelines and was approved by the Medical Committee of the Shanghai Mental Health Center. Written informed consent was obtained from all participants.
2.1 Clinical assessment
Clinical assessments in all subjects were conducted by two experienced attending physicians. MCI diagnosis was based on Petersen’s criteria: (1) subjective cognitive complaints and were best narrated by family members; (2) neuropsychological tests showing objective cognitive impairment; (3) the ability to maintain daily life; and (4) without dementia (21). Dementia was diagnosed on the basis of Diagnostic and Statistical Manual of Mental Disorders IV (DSM IV) (22, 23): (1) impaired ability to work or perform daily activities; (2) reduced function and performance compared to before; (3) cognitive decline that cannot be explained by delirium or other mental illness; and (4) cognitive or behavioral disorders involving at least two aspects: memory, executive function, visuospatial abilities, speech functions, personality, and behavior changes. The final diagnosis was based on information obtained from semi-structured interviews with the participant’s medical history, standard physical, neurological, and psychiatric examinations.
2.2 Neuropsychological tests
2.2.1 Montreal cognitive assessment (MoCA)
Montreal Cognitive Assessment (MoCA) is a screening instrument developed by Kang et al. (24) to detect cognitive impairment. MoCA has a total score of 30, including memory, visuospatial ability, executive ability, language ability, orientation, etc. Based on previous studies, the optimal thresholds for the MoCA comprehensive index of screening for MCI were ≤75 years old, education ≤6 years was 19.5; >75 years old, education ≤6 years was 15.5; and education >6 years was 24.5 (25).
2.3 APOE genotyping
All participants stopped eating after 9 pm and collected peripheral blood between 7 am and 9 am the following day. Genomic DNA was extracted from blood cells using the Blood Genome DNA Extraction kit (Qiagen NV, Venlo, Netherlands) (after high-speed centrifugation), and APOE genotypes were determined by polyploid-amplified-refractory mutation system polymerase chain reaction (PCR). According to method above (26), APOE ε4 types include ε2/ε4, ε3/ε4, and ε4/ε4, while the class of non-APOE ε4 types includes ε2/ε2, ε2/ε3, and ε3/ε3.
2.4 Lifestyle assessment
Trained interviewers collect data through face-to-face interviews and complete ongoing training before the study begins. Lifestyles we surveyed included smoking, alcohol drinking, tea drinking, eating habits (such as fruit and ginger), surfing the Internet, and information about chronic disease (such as hypertension, diabetes, depression). These variables were selected because previous literature has indicated that all of these factors are strongly associated with cognitive function (27–32). In general, smoking, alcohol consumption, chronic diseases such as hypertension, diabetes, depression, etc., were linked to poorer cognitive function, while tea, vegetables, ginger consumption and surfing the internet were linked to better cognitive performance.
In the current study, smokers were defined as those who smoked more than 100 cigarettes a day in their lifetime, while non-smokers were defined as those who never smoked or smoked fewer than 100 cigarettes in their lifetime (33). A standard unit of alcohol is defined as 14 g of pure alcohol, and five units a day is considered light to moderate alcohol consumption. Non-drinkers are defined as those who never or rarely drink alcohol (for example, holiday drinking, drinking less than one standard unit at a time) (34). Tea drinker was defined whether respondents drank tea more than three times a week. The question is that “over the past month, you have typically had tea several times a week” (35). Since our previous studies have only shown that green tea could help with cognition, in the current study, we are only looking at the effects of green tea on cognitive function (35, 36). Through the Food Frequency Questionnaire (FFQ) (37), we obtained information on participants’ dietary consumption habits and frequency of consumption of fruits and ginger. Investigators would ask, “Do you eat fruit?.” Responses were recorded as daily (people who ate fruit at least once a day), weekly (people who ate fruit 1–6 times a week) or monthly (for those who ate fruit less than once a week). Those who ate fruit at least once a week are considered fruit eaters. The same true of ginger consumption. In the current study, ginger consumption was defined as eating ginger (as a dish) at least once a week, primarily as a food, not a mere flavoring agent. Ginger was listed as a separate variable because previous studies have suggested it may have cognitive protection (38, 39). Surfing the Internet was defined as whether respondents used the Internet and/or e-mail. The question is “Do you use the internet or email?” How often do you surf the Internet?” People who use the internet less than once every 3 months or never go online will be classed as non-internet users (40). Sleep duration surveys are conducted primarily through self-reporting, including: too little sleep (less than 5 h), too much sleep (more than 8 h), and normal sleep (5–8 h). The question is “How many hours of sleep do you get each night.” Diabetes status is determined based on self-reported diagnosis by a physician or treatment with insulin and/or oral hypoglycemic agents. Fasting blood glucose level was ≥7.0 mmol/L (126 mg/dL) or oral glucose tolerance test 2-h value was ≥11.1 mmol/L may also be considered for T2DM (41). Hypertension status was based on self-reported or oral treatment with antihypertensive drugs. Their average systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg was also considered hypertension (42). In addition, depression status was based on self-reporting or oral antidepressant treatment.
3 Statistical analysis
The representation of continuous variables was in the form of mean ± standard deviation (SD), while categorical variables were denoted as percentages. Subjects were categorized into groups with APOE ε4 carriers (n = 362) and those without (n = 1,509), depending on their APOE ε4 gene status. Initially, the study employed ANOVA to examine continuous factors like age, education, BMI, and MoCA. Subsequently, the chi-square test evaluated categorical elements including gender, smoking habits, alcohol consumption, tea use, dietary patterns, fruit intake, ginger consumption, surfing the internet, sleep length, hypertension, diabetes, depression, and cognitive categorization between the two groups. The Bonferroni method was employed to account for the effects of multiple tests. Subsequently, we employed multivariate logistic regression to evaluate the link between a solitary lifestyle element and cognitive abilities (MCI and dementia), considering APOE status, age, education, BMI, and gender as additional variables. Additionally, to examine if the APOE ε4 genotype influenced the link between cognitive abilities and lifestyle, multivariate adjusted logistic regression models were employed in subgroup studies. Ultimately, the impact of APOE ε4 and lifestyle interplay on cognitive abilities was examined using the general linear regression analysis model. Every analysis utilized IBM SPSS Statistics for Windows 22.0, with all p-values determined at a two-tailed significance threshold of 0.05.
4 Results
4.1 Characteristics of the participants
Table 1 displays the features of the research samples. The present study encompassed 1,871 individuals, comprising 362 carriers of APOE ε4 and 1,509 carriers of non-APOE ε4. Within this group, 649 individuals were males, making up 34.7 percent of the overall count. The average age stood at 69.31 ± 7.87 years, with an average educational duration of 10.56 ± 3.64 years, and a mean body mass index of 23.97 ± 3.37 kg/m2. People diagnosed with APOE ε4 showed a reduced tendency to consume ginger, decreased internet usage, a higher probability of experiencing sleep issues, and a greater chance of mild cognitive impairment or dementia (p < 0.05).
4.2 Relationship between single lifestyle factors and cognitive impairment
Table 2 displayed the correlation between ginger consumption, surfing the internet, and issues with sleep length with cognitive deficits. Upon adjusting for factors like age, gender, education, APOE ε4, and BMI, it was discovered that insufficient sleep significantly correlated with an increased likelihood of MCI (Odds Ratio = 1.275, 95% confidence interval: 1.106–1.600, p-value = 0.036). Likewise, our findings indicate a significant correlation between insufficient sleep and an increased likelihood of developing dementia (OR = 1.880, 95% confidence interval: 1.094–3.232, p = 0.022). Nonetheless, the revised model revealed no notable links between ginger consumption or surfing the internet, and the presence of MCI or dementia.
4.3 Association between APOE ε4 genotypes and lifestyles and cognitive impairment
The multivariable logistic regression analysis, treating diagnosis as the dependent factor and APOE ε4 classification as the independent, revealed that individuals without APOE ε4 had a reduced likelihood of developing MCI (OR = 0.745, 95% confidence interval: 0.587–0.945, p = 0.015) and dementia (OR = 0.422, 95% confidence interval: 0.259–0.688, p = 0.001). Our examination of subgroups with the APOE ε4 gene revealed that educational levels (OR = 0.871, 95% confidence interval: 0.810–0.938, p < 0.001), BMI (OR = 0.913, 95% confidence interval: 0.847–0.985, p = 0.019), and tea consumption (OR = 0.548, 95% confidence interval: 0.339–0.886, p = 0.014) correlated with a reduced likelihood of MCI; age (OR = 1.145, 95% confidence interval: 1.061–1.236, p = 0.001) was linked to an increased risk of dementia, whereas hypertension (OR = 0.157, 95% confidence interval: 0.047–0.527, p = 0.003) correlated with a decreased risk of dementia.
Our examination of subgroups lacking the APOE ε4 gene revealed a correlation between age (OR = 1.016, 95% confidence interval: 1.001–1.032, p = 0.031) and an increased likelihood of MCI, whereas education (OR = 0.093, 95% confidence interval: 0.874–0.933, p < 0.001) correlated with a reduced probability of MCI. Factors such as age (Odds Ratio = 0.004, 95% Confidence Interval: 1.017–1.093, p-value = 0.004), insufficient sleep (Odds Ratio = 2.612, 95% Confidence Interval: 1.508–4.522, p-value = 0.001), and depression (Odds Ratio = 7.410, 95% Confidence Interval: 2.706–20.291, p-value<0.001) correlated with an increased likelihood of dementia. Conversely, education (Odds Ratio = 0.887, 95% Confidence Interval: 0.828–0.950, p-value = 0.001), tea consumption (Odds Ratio = 0.506, 95% Confidence Interval: 0.277–0.925, p-value = 0.027), and fruits consumption (Odds Ratio = 0.309, 95% Confidence Interval: 0.143–0.666, p-value = 0.003) were linked to a reduced risk of dementia. The findings are displayed in Tables 3, 4.
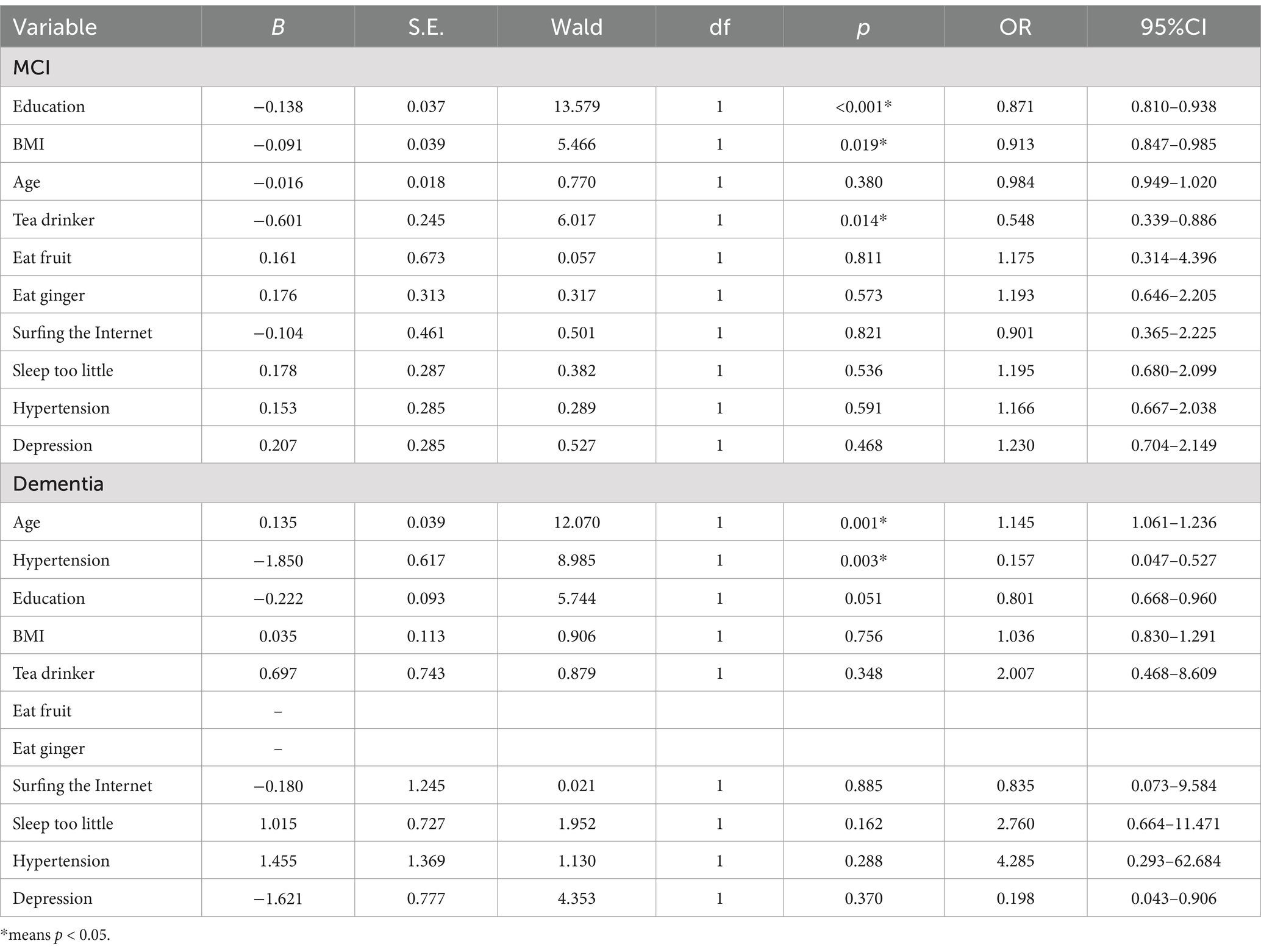
Table 3. Association between multiple lifestyle factors and cognitive impairment in participants with the APOE ε4 gene.
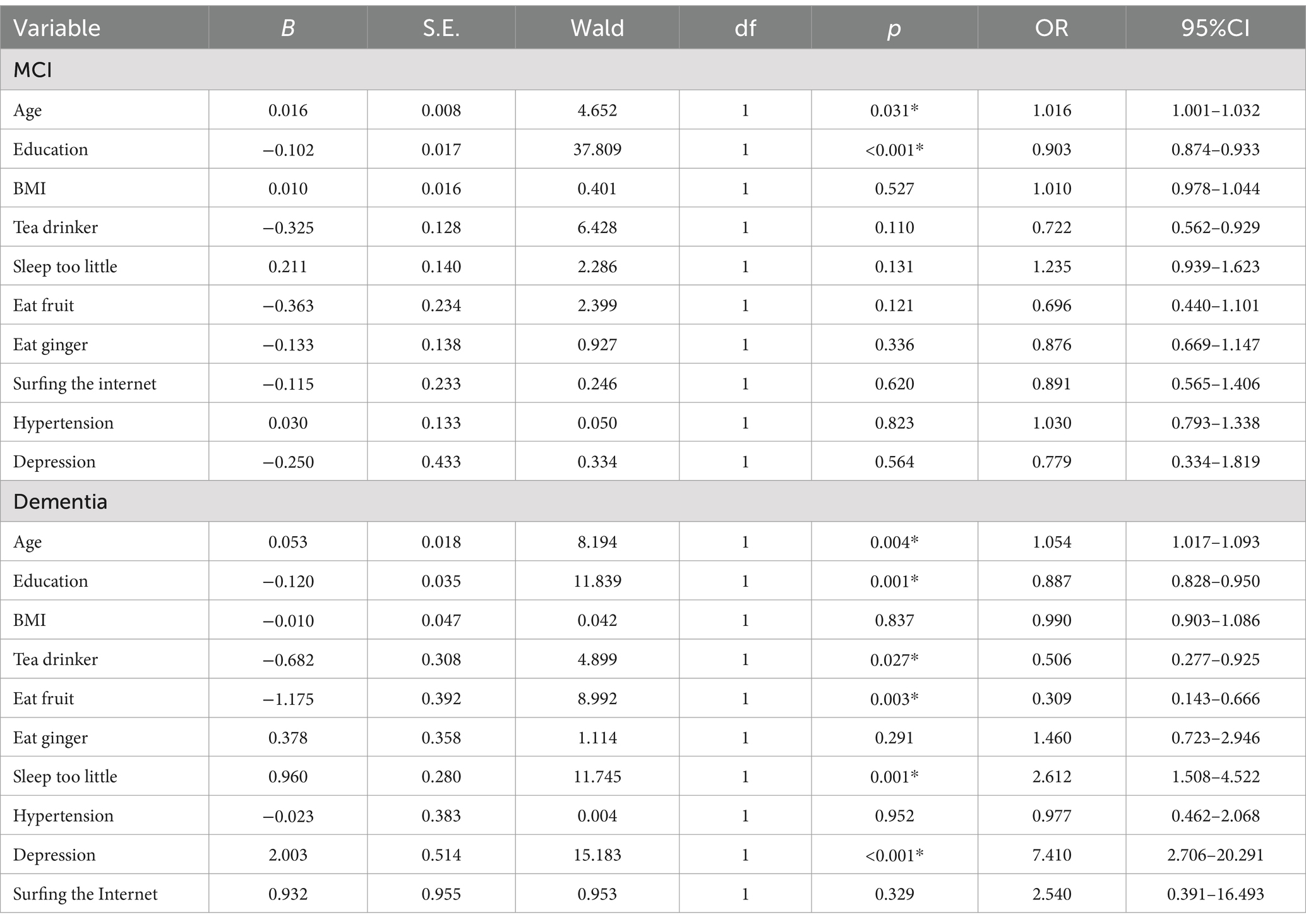
Table 4. Association between multiple lifestyle factors and cognitive impairment in participants without the APOE ε4 gene.
Ultimately, a general linear regression model was employed to explore how the interaction of APOE ε4 genotype with sleep duration issues impacts general cognitive abilities. This general linear regression model was executed with insufficient sleep as the sole lifestyle factor, as it was the only one significantly linked to both MCI and dementia in the logistic regression studies. Within this framework, the aggregate MoCA score serves as the dependent variable, whereas issues related to sleep duration and the APOE ε4 genotype are categorized as a constant element. Ultimately, the findings revealed a notable interplay between the APOE ε4 genotype and issues with sleep duration, potentially impacting cognitive deterioration (F = 6.817, p = 0.001), and the existence of APOE ε4 coupled with “insufficient sleep” indicated a potential reduction in MoCA scores (notably, APOE ε4 by itself had no impact on MoCA scores). Due to the lack of significant positive outcomes (p > 0.05) for other factors like ginger consumption and surfing the internet in the general linear regression model, the aforementioned results were omitted.
5 Discussion
In the current study, we investigated the relationship between APOE ε4 genotype, lifestyle and cognitive decline. Unlike other studies, we added some new variables, such as eating habits, eating ginger, sleep duration problems, and internet use, etc. The reason for adding the above variables is that our previous studies have suggested that these variables are associated with cognitive function (43–46). At the same time, ethnic differences are an important factor to consider. Compared with the older adult in Europe and America, the older adult in China may also have different lifestyles (such as drinking tea, eating ginger, and lunch break), leading to differences in dementia incidence and influencing factors. The incidence of dementia, for example, varies by race, as high as 8.5 percent Latin America and 5.14 percent China (47). A meta-analysis found that the APOE ε4 and ε2 affect blacks differently form other races (48). Asians have the highest burden of physical and memory impairments and are more likely to show impaired language fluency due to low education (49). In addition, other studies have shown that birth can lead to differences between ethnic groups in Asia, and that social factors may have a different impact on the development of dementia (50). Therefore, through the epidemiological investigation of the older adult in China, we can find new influencing factors of Alzheimer’s disease and provide new theoretical basis for the prevention and treatment of Alzheimer’s disease. Through multiple logistics regression analysis, we finally found that (1) the risk of mild cognitive impairment and dementia was significantly reduced in people who did not carry the APOE ε4 allele; (2) APOE ε4 carrying status may affect the impact of everyday life on cognitive decline; and (3) there was a significant interaction between APOE ε4 and sleep duration problems, both of which may contribute to cognitive impairment.
The apolipoprotein (APOE) epsilon 4 allele is the most common genetic risk factor for Alzheimer’s disease (AD), accounting for about 4% of Alzheimer’s disease variation (51). The relationship between APOE ε4 genotype and cognitive impairment is well studied. For example, a meta-analysis showed that APOE ε4 was strongly associated with susceptibility to vascular dementia in Chinese population (52). Another meta-analysis showed that APOE ε2+ was neither a risk factor nor a prevention factor for Parkinson’s disease dementia (PDD), while APOE ε4+ was a risk factor for PDD (53). In addition, many studies have also shown that APOE ε4 may adversely affect MCI (54–56). Therefore, our conclusions are consistent. There are several theories about the effects of the APOE ε4 allele on the brain. One theory is that different APOE variants have different effects on the accumulation of amyloid-beta in the brain (57). Another theory is that different APOE alleles differ in binding and clearance of Aβ (58). In addition, the study further suggests that e4 variants increase blood–brain barrier damage (59).
Our results showed that the carrying status of APOE ε4 could change the influence of daily lifestyle on cognitive decline, and the influencing factors of MCI and dementia were significantly different with APOE ε4 gene carrying status. For example, in our current study, we found that education, BMI and tea drinker were associated with lower MCI odds among participants with the APOE ε4 gene, while age was associated with higher MCI odds, and education was associated with lower MCI odds among non-APOE ε4 carriers. However, we found only a significant interaction between APOE ε4 and sleep duration problems on cognitive impairment, and not no interaction between APOE ε4 and other variables, such as tea drinking, Internet use, diabetes, hypertension, depression, etc.
Aging is characterized by changes in sleep quality and structure. When sleep changes become apparent, they can produce or accelerate cognitive decline, even in the absence of significant pathological factors (60). Aging changes sleep duration and quality, which is also common in Alzheimer’s disease. The increased in Aβ production and decrease in reduced Aβ clearance were due to the close interplay of Aβ, sleep disturbance and wakefulness. In addition to Aβ, sleep deprivation found in AD may be associated with the effects of tau pathology (61). There is strong evidence linking sleep quality to the onset and development of dementia (62, 63). For example, a cross-sectional study conducted in China showed that poor suboptimal sleep duration and quality in non-institutionalized adults aged 45 years and older (n = 10,768) were associated with poor cognitive ability. A state-of-the-art review shows a four-pronged link between sleep disruption and quality of life of people with dementia: physical, social/behavioral, emotional health, and cognition (64). In addition, a systematic study suggested that up to 90 percent of people with Lewy body dementia (LBD) have at least one sleep disorder, such as subjectively poor sleep quality, excessive daytime sleepiness (EDS), and rapid eye movement behavior disorder (RBD) (65).
Interestingly, sleep quality appears to be intrinsically linked to APOE genetic polymorphism. For example, a pilot study showed that healthy older adults with a risk allele (APOE ε4+) have more sleep complaints or objective evidence of sleep disruption than healthy older adults without a risk allele (APOE ε4−) (66). An integrative review have shown that APOE ε4 was associated with poor sleep quality in terms of sleep efficiency, sleep latency, rapid eye movement, wake after sleep onset, 24-h total sleep time, and the deterioration of nighttime total sleep time in people with mild cognitive impairment (MCI) or AD (67). In addition, a population-based study of older adults in rural China showed a link between sleep problems and dementia and AD, mainly in people with APOE ε4 (68). Therefore, our conclusions are consistent.
We have to admit that our study has some limitations. First, it is only a cross-sectional study and cannot establish a causal link between APOE ε4, a variable of daily life, and cognitive decline; Second, long-term follow-up of these populations is needed to observe the long-term effects of APOE ε4 and daily life variables on cognitive function; Third, we were not able to uncover the mechanism by which APOE ε4 interacts with sleep duration problems to influence cognitive decline; Finally, this cross-sectional study includes people with MCI and dementia, and the exposure variable (participation in lifestyles) is collected using self-reported information. Thus, recall bias is present and can interfere with the findings.
In summary, we found that the carrying state of APOE ε4 can change the influence of daily life variables on cognitive impairment, and there may be a significant interaction between APOE ε4 and sleep disorders, which jointly promote the occurrence of cognitive impairment. Although many previous studies have pointed out that daily lifestyle may have a certain impact on cognitive function, they have not paid special attention to the regulatory role of APOE4 on the above effects. Therefore, in this sense, our study has made further extension and expansion to reveal the possible mechanism of APOE ε4’s regulation of cognitive function.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Medical Committee of the Shanghai Mental Health Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WL: Writing – original draft. XW: Formal analysis, Writing – original draft. LS: Formal analysis, Methodology, Writing – original draft. LY: Software, Supervision, Writing – original draft. SX: Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from This work was supported by the China Ministry of Science and Technology (STI2030-Major Projects-2022ZD0213100), Shanghai public health projects (GWVI-11.2-XD24), Clinical Research Plan of SHDC (No. SHDC2020CR1038B), the National Natural Science Foundation of China (82101564, 82001123, and 82271607), the clinical research center project of Shanghai Mental Health Center (CRC2017ZD02), Shanghai Clinical Research Center for Mental Health (19MC1911100), the Feixiang Program of Shanghai Mental Health Center (2020-FX-03), Chinese Academy of Sciences (XDA12040101), Shanghai Clinical Research Center for Mental Health (SCRC-MH, 19MC1911100), the Shanghai Science and Technology Committee (20Y11906800).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kahya, M, Vidoni, E, Burns, JM, Thompson, AN, Meyer, K, and Siengsukon, CF. The relationship between apolipoprotein ε4 carrier status and sleep characteristics in cognitively Normal older adults. J Geriatr Psychiatry Neurol. (2017) 30:273–9. doi: 10.1177/0891988717720301
2. Sando, SB, Melquist, S, Cannon, A, Hutton, ML, Sletvold, O, Saltvedt, I, et al. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer's disease; a case control study from Central Norway. BMC Neurol. (2008) 8:9. doi: 10.1186/1471-2377-8-9
3. Liu, CC, Liu, CC, Kanekiyo, T, Xu, H, and Bu, G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. (2013) 9:106–18. doi: 10.1038/nrneurol.2012.263
4. Valero, S, Marquié, M, de Rojas, I, Espinosa, A, Moreno-Grau, S, Orellana, A, et al. Interaction of neuropsychiatric symptoms with APOE ε4 and conversion to dementia in MCI patients in a memory clinic. Sci Rep. (2020) 10:20058. doi: 10.1038/s41598-020-77023-z
5. Venneri, A, McGeown, WJ, Biundo, R, Mion, M, Nichelli, P, and Shanks, MF. The neuroanatomical substrate of lexical-semantic decline in MCI APOE ε4 carriers and non-carriers. Alzheimer Dis Assoc Disord. (2011) 25:230–41. doi: 10.1097/WAD.0b013e318206f88c
6. Serrano-Pozo, A, Li, Z, Noori, A, Nguyen, HN, Mezlini, A, Li, L, et al. Effect of APOE alleles on the glial transcriptome in normal aging and Alzheimer's disease. Nature Aging. (2021) 1:919–31. doi: 10.1038/s43587-021-00123-6
7. Löwe, LC, Gaser, C, and Franke, K. The effect of the APOE genotype on individual BrainAGE in Normal aging, mild cognitive impairment, and Alzheimer's disease. PLoS One. (2016) 11:e0157514. doi: 10.1371/journal.pone.0157514
8. Borroni, B, Grassi, M, Costanzi, C, Archetti, S, Caimi, L, and Padovani, A. APOE genotype and cholesterol levels in lewy body dementia and Alzheimer disease: investigating genotype-phenotype effect on disease risk. Am J Geriatr Psychiatry. (2006) 14:1022–31. doi: 10.1097/01.JGP.0000225088.29353.08
9. Treves, TA, Bornstein, NM, Chapman, J, Klimovitzki, S, Verchovsky, R, Asherov, A, et al. APOE-epsilon 4 in patients with Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. (1996) 10:189–91. doi: 10.1097/00002093-199601040-00003
10. Zhang, XX, Tian, Y, Wang, ZT, Ma, YH, Tan, L, and Yu, JT. The epidemiology of Alzheimer's disease modifiable risk factors and prevention. J Prev Alzheimers Dis. (2021) 8:313–21. doi: 10.14283/jpad.2021.15
11. Solch, RJ, Aigbogun, JO, Voyiadjis, AG, Talkington, GM, Darensbourg, RM, O'Connell, S, et al. Mediterranean diet adherence, gut microbiota, and Alzheimer’s or Parkinson’s disease risk: a systematic review. J Neurol Sci. (2022) 434:120166. doi: 10.1016/j.jns.2022.120166
12. Guasch-Ferré, M, and Willett, WC. The Mediterranean diet and health: a comprehensive overview. J Intern Med. (2021) 290:549–66. doi: 10.1111/joim.13333
13. Kivipelto, M, Mangialasche, F, and Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. (2018) 14:653–66. doi: 10.1038/s41582-018-0070-3
14. Baumgart, M, Snyder, HM, Carrillo, MC, Fazio, S, Kim, H, and Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. (2015) 11:718–26. doi: 10.1016/j.jalz.2015.05.016
15. Dhana, K, Franco, OH, Ritz, EM, Ford, CN, Desai, P, Krueger, KR, et al. Healthy lifestyle and life expectancy with and without Alzheimer’s dementia: population based cohort study. BMJ. (2022) 377:e068390. doi: 10.1136/bmj-2021-068390
16. Licher, S, Ahmad, S, Karamujić-Čomić, H, Voortman, T, Leening, MJG, Ikram, MA, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med. (2019) 25:1364–9. doi: 10.1038/s41591-019-0547-7
17. Rosenich, E, Bransby, L, Yassi, N, Fripp, J, Laws, SM, Martins, RN, et al. Differential effects of APOE and modifiable risk factors on hippocampal volume loss and memory decline in Aβ- and Aβ+ older adults. Neurology. (2022) 98:e1704–15. doi: 10.1212/WNL.0000000000200118
18. Rodriguez, FS, Schroeter, ML, Arélin, K, Witte, AV, Baber, R, Burkhardt, R, et al. APOE e4-genotype and lifestyle interaction on cognitive performance: results of the LIFE-adult-study. Health Psychol. (2018) 37:194–205. doi: 10.1037/hea0000515
19. Angelopoulou, E, Paudel, YN, Papageorgiou, SG, and Piperi, C. APOE genotype and Alzheimer's disease: the influence of lifestyle and environmental factors. ACS Chem Neurosci. (2021) 12:2749–64. doi: 10.1021/acschemneuro.1c00295
20. Li, W, Yue, L, Sun, L, and Xiao, S. An increased aspartate to alanine aminotransferase ratio is associated with a higher risk of cognitive impairment. Front Med. (2022) 9:780174. doi: 10.3389/fmed.2022.780174
21. Petersen, RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. (2004) 256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x
22. McKhann, G, Drachman, D, Folstein, M, Katzman, R, Price, D, and Stadlan, EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. (1984) 34:939–44. doi: 10.1212/WNL.34.7.939
23. McKhann, GM, Knopman, DS, Chertkow, H, Hyman, BT, Jack, CR Jr, Kawas, CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. (2011) 7:263–9. doi: 10.1016/j.jalz.2011.03.005
24. Kang, JM, Cho, YS, Park, S, Lee, BH, Sohn, BK, Choi, CH, et al. Montreal cognitive assessment reflects cognitive reserve. BMC Geriatr. (2018) 18:261. doi: 10.1186/s12877-018-0951-8
25. Zhang, S, Qiu, Q, Qian, S, Lin, X, Yan, F, Sun, L, et al. Determining appropriate screening tools and cutoffs for cognitive impairment in the Chinese elderly. Front Psych. (2021) 12:773281. doi: 10.3389/fpsyt.2021.773281
26. Donohoe, GG, Salomäki, A, Lehtimäki, T, Pulkki, K, and Kairisto, V. Rapid identification of apolipoprotein E genotypes by multiplex amplification refractory mutation system PCR and capillary gel electrophoresis. Clin Chem. (1999) 45:143–6. doi: 10.1093/clinchem/45.1.143
27. Rehm, J, Hasan, OSM, Black, SE, Shield, KD, and Schwarzinger, M. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. (2019) 11:1. doi: 10.1186/s13195-018-0453-0
28. Junger, AL, de Sousa Romeiro, AM, Noll, M, de Oliveira, C, and Silveira, EA. Impact of type, intensity, frequency, duration and volume of physical activity on dementia and mild cognitive impairment in older adults: protocol for a systematic review and meta-analysis. BMJ Open. (2023) 13:e074420. doi: 10.1136/bmjopen-2023-074420
29. Xu, W, Wang, H, Wan, Y, Tan, C, Li, J, Tan, L, et al. Alcohol consumption and dementia risk: a dose-response meta-analysis of prospective studies. Eur J Epidemiol. (2017) 32:31–42. doi: 10.1007/s10654-017-0225-3
30. Jones, A, Ali, MU, Kenny, M, Mayhew, A, Mokashi, V, He, H, et al. Potentially modifiable risk factors for dementia and mild cognitive impairment: an umbrella review and Meta-analysis. Dement Geriatr Cogn Disord. (2024) 53:91–106. doi: 10.1159/000536643
31. Vishwanath, S, Qaderi, V, Steves, CJ, Reid, CM, Hopper, I, and Ryan, J. Cognitive decline and risk of dementia in individuals with heart failure: a systematic review and Meta-analysis. J Card Fail. (2022) 28:1337–48. doi: 10.1016/j.cardfail.2021.12.014
32. Waziry, R, Claus, JJ, and Hofman, A. Dementia risk following ischemic stroke: a systematic review and Meta-analysis of factors collected at time of stroke diagnosis. J Alzheimers Dis. (2022) 90:1535–46. doi: 10.3233/JAD-220317
33. Zhang, XY, Liang, J, Chen, DC, Xiu, MH, He, J, Cheng, W, et al. Cigarette smoking in male patients with chronic schizophrenia in a Chinese population: prevalence and relationship to clinical phenotypes. PLoS One. (2012) 7:e30937. doi: 10.1371/journal.pone.0030937
34. Vinader-Caerols, C, Duque, A, Montañés, A, and Monleón, S. Blood alcohol concentration-related lower performance in immediate visual memory and working memory in adolescent binge drinkers. Front Psychol. (2017) 8:1720. doi: 10.3389/fpsyg.2017.01720
35. Li, W, Yue, L, and Xiao, S. Prospective associations of tea consumption with risk of cognitive decline in the elderly: a 1-year follow-up study in China. Front Nutr. (2022) 9:752833. doi: 10.3389/fnut.2022.752833
36. Xu, H, Fiocco, AJ, Liu, X, Wang, T, Li, G, and Xiao, S. Association between tea consumption and cognitive function in cognitively healthy older adults and older adults with mild cognitive impairment. Gen Psychiatr. (2021) 34:e100512. doi: 10.1136/gpsych-2021-100512
37. Xu, X, Xiao, S, Rahardjo, TB, and Hogervorst, E. Tofu intake is associated with poor cognitive performance among community-dwelling elderly in China. J Alzheimers Dis. (2015) 43:669–75. doi: 10.3233/JAD-141593
38. Zarei, M, Uppin, V, Acharya, P, and Talahalli, R. Ginger and turmeric lipid-solubles attenuate heated oil-induced oxidative stress in the brain via the upregulation of NRF2 and improve cognitive function in rats. Metab Brain Dis. (2021) 36:225–38. doi: 10.1007/s11011-020-00642-y
39. Jittiwat, J, and Wattanathorn, J. Ginger pharmacopuncture improves cognitive impairment and oxidative stress following cerebral ischemia. J Acupunct Meridian Stud. (2012) 5:295–300. doi: 10.1016/j.jams.2012.09.003
40. LaMonica, HM, English, A, Hickie, IB, Ip, J, Ireland, C, West, S, et al. Examining internet and eHealth practices and preferences: survey study of Australian older adults with subjective memory complaints, mild cognitive impairment, or dementia. J Med Internet Res. (2017) 19:e358. doi: 10.2196/jmir.7981
41. Gao, Y, Xiao, Y, Miao, R, Zhao, J, Zhang, W, Huang, G, et al. The characteristic of cognitive function in type 2 diabetes mellitus. Diabetes Res Clin Pract. (2015) 109:299–305. doi: 10.1016/j.diabres.2015.05.019
42. Bundy, JD, and He, J. Hypertension and related cardiovascular disease burden in China. Ann Glob Health. (2016) 82:227–33. doi: 10.1016/j.aogh.2016.02.002
43. Li, W, Sun, L, Yue, L, and Xiao, S. Associations between afternoon napping, left amygdala volume and cognitive performance in elderly with normal cognitive function. Sleep Med. (2024) 113:232–7. doi: 10.1016/j.sleep.2023.11.032
44. Li, W, Sun, L, Yue, L, and Xiao, S. The left temporal transverse cortex is affected by poor sleep quality, which in turn contributes to depressive symptoms in older adults. Heliyon. (2023) 9:e20751. doi: 10.1016/j.heliyon.2023.e20751
45. Li, W, Yue, L, and Xiao, S. Association between internet use, cognitive function, and Globus pallidus volumes: a study among the elderly in Chinese communities. Front Public Health. (2022) 10:886974. doi: 10.3389/fpubh.2022.886974
46. Li, W, Sun, L, Yue, L, Li, G, and Xiao, S. The association between eating green vegetables every day and mild cognitive impairment: a community-based cross-sectional study in Shanghai. Neuropsychiatr Dis Treat. (2019) 15:3213–8. doi: 10.2147/NDT.S221074
47. Jia, J, Wang, F, Wei, C, Zhou, A, Jia, X, Li, F, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. (2014) 10:1–9. doi: 10.1016/j.jalz.2013.01.012
48. Qin, W, Li, W, Wang, Q, Gong, M, Li, T, Shi, Y, et al. Race-related association between APOE genotype and Alzheimer's disease: a systematic review and Meta-analysis. J Alzheimers Dis. (2021) 83:897–906. doi: 10.3233/JAD-210549
49. Wang, K, Zhu, Z, and Qi, X. Socioeconomic status disparities in cognitive and physical functional impairment among older adults: comparison of Asians with other major racial/ethnic groups. J Urban Health. (2023) 100:839–51. doi: 10.1007/s11524-023-00768-1
50. Hayes-Larson, E, Fong, J, Mobley, TM, Gilsanz, P, Whitmer, RA, Gee, GC, et al. The role of nativity in heterogeneous dementia incidence in a large cohort of three Asian American groups and white older adults in California. Alzheimers Dement. (2022) 18:1580–5. doi: 10.1002/alz.12563
51. Davies, G, Harris, SE, Reynolds, CA, Payton, A, Knight, HM, Liewald, DC, et al. A genome-wide association study implicates the APOE locus in non-pathological cognitive ageing. Mol Psychiatry. (2014) 19:76–87. doi: 10.1038/mp.2012.159
52. Liu, X, Li, L, Liu, F, Deng, S, Zhu, R, and Li, Q. ApoE gene polymorphism and vascular dementia in Chinese population: a meta-analysis. J Neural Transm. (2012) 119:387–94. doi: 10.1007/s00702-011-0714-6
53. Pang, S, Li, J, Zhang, Y, and Chen, J. Meta-analysis of the relationship between the APOE gene and the onset of Parkinson's disease dementia. Parkinsons Dis. (2018) 2018:1–12. doi: 10.1155/2018/9497147
54. Wang, X, Zhou, W, Ye, T, Lin, X, and Zhang, J. Sex difference in the association of APOE4 with memory decline in mild cognitive impairment. J Alzheimers Dis. (2019) 69:1161–9. doi: 10.3233/JAD-181234
55. Fei, M, and Jianhua, W. Apolipoprotein ε4-allele as a significant risk factor for conversion from mild cognitive impairment to Alzheimer's disease: a meta-analysis of prospective studies. J Mol Neurosci. (2013) 50:257–63. doi: 10.1007/s12031-012-9934-y
56. Jiang, Y, He, T, Deng, W, and Sun, P. Association between apolipoprotein E gene polymorphism and mild cognitive impairment: a meta-analysis. Clin Interv Aging. (2017) 12:1941–9. doi: 10.2147/CIA.S143632
57. Hultman, K, Strickland, S, and Norris, EH. The APOE ɛ4/ɛ4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer's disease patients. J Cereb Blood Flow Metab. (2013) 33:1251–8. doi: 10.1038/jcbfm.2013.76
58. Deane, R, Sagare, A, Hamm, K, Parisi, M, Lane, S, Finn, MB, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. (2008) 118:4002–13. doi: 10.1172/JCI36663
59. Zipser, BD, Johanson, CE, Gonzalez, L, Berzin, TM, Tavares, R, Hulette, CM, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. (2007) 28:977–86. doi: 10.1016/j.neurobiolaging.2006.05.016
60. Casagrande, M, Forte, G, Favieri, F, and Corbo, I. Sleep quality and aging: a systematic review on healthy older people, mild cognitive impairment and Alzheimer's disease. Int J Environ Res Public Health. (2022) 19:8457. doi: 10.3390/ijerph19148457
61. Uddin, MS, Tewari, D, Mamun, AA, Kabir, MT, Niaz, K, Wahed, MII, et al. Circadian and sleep dysfunction in Alzheimer's disease. Ageing Res Rev. (2020) 60:101046. doi: 10.1016/j.arr.2020.101046
62. Irwin, MR, and Vitiello, MV. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. (2019) 18:296–306. doi: 10.1016/S1474-4422(18)30450-2
63. Wennberg, AMV, Wu, MN, Rosenberg, PB, and Spira, AP. Sleep disturbance, cognitive decline, and dementia: a review. Semin Neurol. (2017) 37:395–406. doi: 10.1055/s-0037-1604351
64. Petrovsky, DV, McPhillips, MV, Li, J, Brody, A, Caffeé, L, and Hodgson, NA. Sleep disruption and quality of life in persons with dementia: a state-of-the-art review. Geriatr Nurs. (2018) 39:640–5. doi: 10.1016/j.gerinurse.2018.04.014
65. Elder, GJ, Lazar, AS, Alfonso-Miller, P, and Taylor, JP. Sleep disturbances in Lewy body dementia: a systematic review. Int J Geriatr Psychiatry. (2022) 37:5814. doi: 10.1002/gps.5814
66. Drogos, LL, Gill, SJ, Tyndall, AV, Raneri, JK, Parboosingh, JS, Naef, A, et al. Evidence of association between sleep quality and APOE ε4 in healthy older adults: a pilot study. Neurology. (2016) 87:1836–42. doi: 10.1212/WNL.0000000000003255
67. Wei, W, Wang, K, Shi, J, and Li, Z. The relationship between sleep disturbance and apolipoprotein E ε4 in adults with mild cognitive impairment and Alzheimer's disease dementia: an integrative review. Biol Res Nurs. (2022) 24:327–37. doi: 10.1177/10998004221081044
Keywords: APOE ε4 , ways of living, mild cognitive impairment, dementia, community
Citation: Li W, Wang X, Sun L, Yue L and Xiao S (2024) Correlation between the APOE ε4 genotype, lifestyle habits, and cognitive deficits in Chinese adults over 60: a cross-sectional analysis in China. Front. Public Health. 12:1417499. doi: 10.3389/fpubh.2024.1417499
Edited by:
Daniel Velázquez Díaz, AdventHealth, United StatesReviewed by:
Efthimios M. C. Skoulakis, Alexander Fleming Biomedical Sciences Research Center, GreeceRachel Amelia Clark Cole, The University of Iowa, United States
Copyright © 2024 Li, Wang, Sun, Yue and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, ODIyMjAzODY3QHFxLmNvbQ==; Ling Yue, YmVsbGludGhlbW9vbkBob3RtYWlsLmNvbQ==; Shifu Xiao, eGlhb3NoaWZ1QG1zbi5jb20=
†These authors have contributed equally to this work
 Wei Li
Wei Li XiaoLiang Wang
XiaoLiang Wang Lin Sun
Lin Sun Ling Yue
Ling Yue Shifu Xiao
Shifu Xiao