
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 25 July 2024
Sec. Environmental Health and Exposome
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1415028
This article is part of the Research Topic Toxicity Mechanisms of Environmental Pollutants and Health Risk Assessment View all 21 articles
Objective: To investigate the association between exposure to atmospheric pollutants and preterm birth in a river valley-type city and its critical exposure windows.
Methods: A retrospective cohort study was used to collect data from the medical records of preterm and full-term deliveries in two hospitals in urban areas of a typical river valley-type city from January 2018 to December 2019. A total of 7,288 cases were included in the study with general information such as pregnancy times, the number of cesarean sections, occupation, season of conception and regularity of the menstrual cycle. And confounding factors affecting preterm birth were inferred using the chi-square test. The effects of exposure to each pollutant, including particulate matter 2.5 (PM2.5), particulate matter 10 (PM10), nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO) and ozone (O3), during pregnancy on preterm birth and the main exposure windows were explored by establishing a logistic regression model with pollutants introduced as continuous variables.
Results: Maternal age, pregnancy times, number of births, number of cesarean sections, season of conception, complications diseases, comorbidities diseases, hypertension disorder of pregnancy and neonatal low birth weight of the newborn were significantly different between preterm and term pregnant women. Logistic regression analysis after adjusting for the above confounders showed that the risk of preterm birth increases by 0.9, 0.6, 2.4% in T2 and by 1.0, 0.9, 2.5% in T3 for each 10 μg/m3 increase in PM2.5, PM10, NO2 concentrations, respectively. The risk of preterm birth increases by 4.3% in T2 for each 10 μg/m3 increase in SO2 concentrations. The risk of preterm birth increases by 123.5% in T2 and increases by 188.5% in T3 for each 10 mg/m3 increase in CO concentrations.
Conclusion: Maternal exposure to PM2.5, PM10, NO2, CO was associated with increased risk on preterm birth in mid-pregnancy (T2) and late pregnancy (T3), SO2 exposure was associated with increased risk on preterm birth in mid-pregnancy (T2).
There are approximately 13.4 million preterm births globally in 2020, accounting for more than one-tenth of all newborns (1). Although the number of preterm births has declined compared to 2010, there has been no measurable change in the global preterm birth rate during this decade (1). In China, the status of preterm birth is not encouraging. Research data show that China’s preterm birth rate is 12.0% in 2014, the second highest in the world (2). With the opening of China’s two-child policy in 2016, the preterm birth rate has shown a trend of gradual increase (3), and the incidence is not balanced between regions (4).
Rising rates of preterm birth are accompanied by an increase in the number of children under the age of five who die from preterm birth complications, with statistics indicating that approximately 900,000 children worldwide have died from preterm birth complications in 2019 (5). The infants who survive from preterm birth events also face great risks, such as lifelong disabilities, and these surviving preterm infants are prone to comorbidities such as cerebral palsy, progressive developmental lag, chronic lung disease or neurological sequelae (6, 7), which can impose a heavy burden on both families and society in terms of mental and economic aspects. Preterm labor is considered to be triggered by multiple mechanisms, including infection or inflammation, uteroplacental ischemia or hemorrhage, uterine overstretching, stress, oxidative stress, and other immune-mediated processes (8, 9). Besides, there is evidence that preterm birth is the result of the interaction of multiple risk factors (10), and in addition to well-known risk factors such as maternal demographics (11, 12), psychological characteristics (13), pregnancy comorbidities (14), and genetic characteristics (15), epidemiological studies have suggested that preterm birth is associated with atmospheric pollutants (16–18).
In China, with the rapid economic development of industrialization and urbanization in the past decades, environmental problems have become increasingly serious (19). These are dominated by increasing atmospheric pollution and particulate matter in the environment, with PM2.5, PM10, SO2, O3, NO2 and CO being the main air pollutants. A study on air pollutants conducted in 2015 found that the rate of air pollution and persistent air pollution in northern China is much higher than that in the south, especially in cities in the Bohai Rim and Xinjiang Province (20). It can be seen that there is spatial heterogeneity in air pollutant levels in different cities, especially in the northern cities of China. Therefore, a typical river valley city located in Northwest China was selected for this study, which develops on the axis of the Weihe Plain and is dominated by mountains and hills, with a slightly more complex geological structure than other surrounding cities. The city has a long heating period due to cold winters, which increases the amount of coal and carbon consumed, and the pollutants released from coal combustion are not easily dispersed due to the unique geographic characteristics of the city. It is also due to the frequent occurrence of unfavorable weather such as fog and inversions, which further contribute to the increase in pollutant concentrations.
This study investigates the association between exposure to pollutants and the occurrence of preterm birth in river basin cities and the main exposure windows, with a view to inform potential risk factors of preterm births.
We collect information on all pregnant women with preterm and full-term births from January 2018 to December 2019 from two hospitals in Baoji city. This includes general maternal information (name, age, date of admission, occupation, gestational address), current pregnancy (pregnancy times, number of births, number of cesarean sections, last menstrual period, season, regularity of menstrual cycle, mode of delivery in this case), pregnancy outcome, neonatal information [neonatal date of birth, gestational age (gestational age was usually calculated from the first day of the mother’s last menstrual period), weight (g), number of births], complications diseases, hypertensive disorder of pregnancy, comorbidity diseases and passive smoking.
Pregnancy comorbidities are a condition in which a pregnant woman develops other diseases in addition to the symptoms associated with pregnancy, i.e., a state in which a pregnant woman is comorbid with one or more diseases. Including combined cardiovascular disease, combined hematological disease, combined respiratory disease, combined gastrointestinal disease, combined urological disease, combined endocrine disease, combined infectious disease and combined neoplasm.
Pregnancy complications refer to a variety of conditions that occur during pregnancy that may have some impact on the health of the mother and the births. These include placenta previa, placental abruption, premature rupture of membranes, low amniotic fluid, fetal distress and so on.
Inclusion criteria: ① local residence for more than 1 year and detailed address; ② no assisted conception (exclusion of fertility achieved through unnatural conception and with the help of medical technology); ③ age greater than 18 years old; ④ no acute or chronic diseases; ⑤ the births was born as a single live birth; ⑥ no communication barriers (communication barriers, including hearing or visual impairments, neurological disorders or psychological disorders, etc.). Exclusion criteria: ① not residing in Baoji city during pregnancy or residing locally for less than 1 year; ② assisted conception (e.g., in vitro fertilization (IVF)); ③ unmarried women; ④ ectopic pregnancy; ⑤ births born as twin or multiple births.
In the case information collected in this retrospective cohort study, a total of 7,288 cases were included in the study, of which 372 cases were preterm pregnant women and 6,916 cases were full-term pregnant women. Preterm births were selected from those delivered at 28 weeks to less than 37 weeks of gestation from the first day of the last menstrual period, and term births were selected from those delivered at 37 weeks of gestation.
The questionnaire used to collect information on maternity was designed with a clear research objective combined with existing research findings and expertise. A small-scale pre-test was conducted before its official use, and the questionnaire was further revised and improved based on the test results. Validity analyses were also conducted to assess the reasonableness of the questionnaire design as well as to verify the reliability of the questionnaire through reliability analyses. Finally, in order to ensure the high quality of the information collected, all investigators were required to receive professional training before entering the above hospitals, obtaining the case records of all participants, and completing the questionnaire.
A total of 7 air monitoring stations have been set up in Baoji city, the distribution of which can be seen at Figure 1. The actual straight-line distance between the pregnant women eventually included in the study and each of the above air monitoring stations was calculated based on the latitude and longitude of their main residential address during pregnancy, and then the nearest monitoring station to each of the pregnant women’s residential address was selected. The air pollutant concentrations monitored at that station were used as the exposure of air pollutants for that pregnant woman during her pregnancy period. The air pollutant data was obtained from the National Urban Air Quality Real-Time Distribution System (https://air.cnemc.cn:18007/) of the China Environmental Monitoring General Station of the Ministry of Environmental Protection (MEP), and included the concentrations of six pollutants, namely, PM2.5, PM10, O3, SO2, CO and NO2. The PM2.5, PM10, SO2, CO and NO2 are 24 h moving averages, and O3 is the maximum 8 h moving average. To determine the pollutant exposure window, we divided the course of pregnancy into three stages: 0–12 weeks of gestation was defined as early pregnancy (T1), 13–27 weeks as mid-pregnancy (T2), and 28 weeks to the end of pregnancy as late pregnancy (T3) (21, 22). According to the time period corresponding to the different exposure windows of each pregnant women, the daily moving average of each pollutant in that time period was found separately, and after averaging, the air pollutant exposure level of the pregnant women in a certain exposure window was modelled accordingly, so as to determine the susceptibility windows for various air pollutants during pregnancy.
Data was collated using Excel and analyzed using SPSS 20.0. Normality test was performed for pollutants and the levels of exposure to pollutants in different exposure windows were described using mean, standard deviation (SD), median, and interquartile range (IQR). General maternal data was compared using one-way chi-square test to determine whether there is a difference between pregnant women with preterm versus term births. If they do, they can be considered and evaluated as potential confounders and were introduced as covariates in the subsequent logistic regression model. After the test, confounding factors are maternal age, pregnancy times, number of births, number of cesarean sections, season of conception, complications disease, comorbidity diseases, hypertensive disorder complicating pregnancy and low birth weight. For missing data, it was treated as discrete missing values 999 in SPSS software. The relationship between each pollutant and preterm birth and the main exposure windows were explored by logistic regression modelling, adjusting for confounding factors after introducing the pollutant as a continuous variable (α = 0.05).
The air pollutant exposure window for some of the pregnant women who gave birth in 2018 was in 2017, so 2017 was included when describing the air pollution profile. And we compared the concentration of each pollutant with the national secondary standard (Level II), respectively. The national secondary standards of PM2.5, PM10, SO2, NO2, CO and O3 are respectively 35 μg/m3, 70μg/m3, 60μg/m3, 40μg/m3, 4mg/m3, 200μg/m3 (Table 1). During the period of 2017–2019, PM2.5 and PM10 concentrations were significantly higher than the national secondary standards most of the time; SO2 and CO concentrations were always lower than the national secondary standards; NO2 concentrations showed an unstable situation of being sometimes higher and sometimes lower than the national secondary standards; and O3 concentrations, although they were almost always lower than the national secondary standards, showed a different pattern from the other pollutants: with peaks in the summer and drops in the winter (Figure 2).
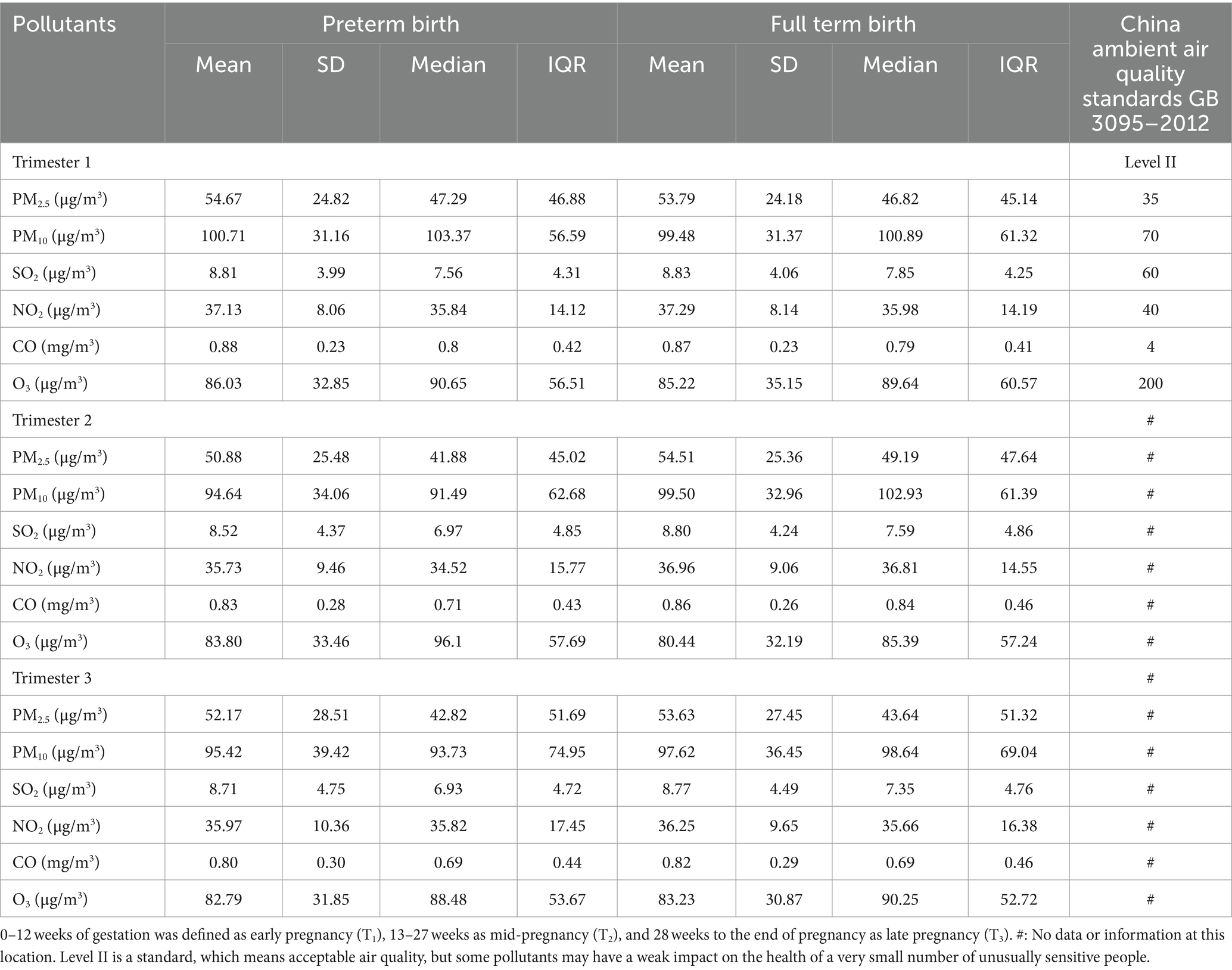
Table 1. Description of exposure levels in preterm and term women across exposure windows in 2017–2019.
Analysis of the general data of pregnant women revealed that: pregnant women older than 30 years are more likely to have preterm births; pregnant women with more than one pregnancy times, births and cesarean sections are prone to have preterm births; compared with full-term pregnant women, season of conception of preterm pregnant women is concentrated in winter (29.3%) and spring (27.7%); the proportion of preterm pregnant women suffering from pregnancy complications (93.3%), hypertensive disorders of pregnancy (18.8%), and pregnancy comorbidities (77.7%) is higher than that of full-term pregnant women. At the same time, preterm births are more likely to result in low birth weight (46.2%).
Maternal age, pregnancy times, number of births, number of cesarean sections, season of conception, complications diseases, comorbidities diseases, hypertension disorder of pregnancy, and neonatal low birth weight of the newborn were significantly (P < 0.05) different between preterm and term pregnant women. Maternal occupation, regularity of the menstrual cycle and passive smoking did not differ significantly between preterm and term pregnant women. And we did not model newborn sex and other variables, such as mother’s education and maternal smoking during pregnancy, because of the difficulty to obtain the data in retrospective cohort study and large number of missing values (Table 2).
In early pregnancy, mid-pregnancy and late pregnancy, the mean levels of exposure concentrations of PM2.5 and PM10 for preterm and full-term pregnant women were higher than the national secondary standard (Level II), while the concentrations of other pollutants were lower than the national secondary standard.
In early pregnancy, the mean levels of PM2.5, PM10, CO and O3 exposure of preterm pregnant women were 54.67 μg/m3, 100.71 μg/m3, 0.88 mg/m3 and 86.03 μg/m3, respectively, which were slightly higher than those of term pregnant women. The mean levels of SO2 and NO2 exposure were higher in term than in preterm women. In mid-pregnancy, the mean levels of PM2.5, PM10, SO2, NO2 and CO exposure for preterm pregnant women were lower than those for term pregnant women, and only the mean level of O3 exposure was higher than that for term pregnant women, at 83.80 μg/m3. In the late pregnancy, the mean levels of PM2.5, PM10, SO2, NO2, CO and O3 exposure of preterm women were all lower than those of term pregnant women. Differences in pollutant exposure levels in the three exposure windows between the two groups may be due to the fact that full-term pregnant women are generally exposed for a longer period of time in late pregnancy than preterm pregnant women, which results in higher exposure levels of each pollutant for full-term pregnant women than for preterm pregnant women in late pregnancy (Table 1).
Before adjustment, only PM2.5, PM10, NO2, CO exposure was associated with increased risk on preterm birth in mid-pregnancy, and exposure to the other pollutants had no association with preterm birth in each exposure window. In multivariate analyses of single-pollutant models, exposure to PM2.5, PM10, NO2, CO was associated with increased risk on preterm birth in mid-pregnancy (T2) and late pregnancy (T3), SO2 exposure was associated with increased risk on preterm birth in mid-pregnancy (T2). The risk of preterm birth increased by 0.9, 0.6, 2.4% in T2 and by 1.0, 0.9, 2.5% in T3 for each 10 μg/m3 increase in PM2.5, PM10, NO2 concentrations, respectively. The risk of preterm birth increased by 4.3% in T2 for each 10 μg/m3 increase in SO2. The risk of preterm birth increased by 123.5% in T2 and by 188.5% in T3 for each 10 mg/m3 increase in CO concentrations. Other pollutants were not associated with increased risk on preterm birth in 3 exposure windows (Table 3).
In this retrospective cohort study, we investigated the association between air pollutants and preterm birth in Baoji city in 2018–2019. The study showed that preterm births were conceived more often in spring and winter compared to full-term births. This may be due to the fact that Baoji city has a warm-temperate monsoon climate with cold and dry winters, so coal combustion increases during the collective heating phase and pollutants are released, which results in higher concentrations of air pollutants in spring and winter than in other seasons. There were significant differences between preterm births and full-term births with respect to maternal age, pregnancy times, number of births, number of cesarean sections, season of conception, complications diseases, comorbidities diseases, hypertension disorder of pregnancy and neonatal low birth weight. In multivariate single pollutant models, exposure to PM2.5, PM10, NO2, CO was associated with increased risk on preterm birth in mid-pregnancy and late pregnancy, and SO2 exposure was associated with increased risk on preterm birth in mid-pregnancy. The risk of preterm birth increased by 0.9, 0.6, 2.4% in T2 and by 1.0, 0.9, 2.5% in T3 for each 10 μg/m3 increase in PM2.5, PM10, NO2 concentrations, respectively. The risk of preterm birth increased by 4.3% in T2 for each 10 μg/m3 increase in SO2. The risk of preterm birth increased by 123.5% in T2 and by 188.5% in T3 for each 10 mg/m3 increase in CO concentrations. There was no association between exposure to O3 and preterm birth in any stage of pregnancy.
These results are consistent with findings from other studies. The exposure of PM2.5 in T1, T2, T3 and E can increase the risk of preterm birth (23). And the strongest association was observed in the second trimester (24). Exposure to high concentrations of PM10 increases the risk of preterm birth (25, 26), the study (25) also suggested that the risk may vary according to the clinical subtypes of preterm birth and the time window of exposure. Significant association was found between NO2 exposure and preterm birth (27, 28) and NO2 exposure in only the 3rd trimester was positively associated with PTB (29). It is showed that (30) exposure to PM2.5, PM10, and NO2 for 1 week prior to delivery increased the risk of preterm birth. One study (31), also conducted in a river valley type city, showed that PM10, O3 exposure in late pregnancy, SO2 in mid pregnancy, and SO2 exposure in late pregnancy were all likely to increase the risk of preterm birth. Although the above studies have shown that exposure to PM2.5, PM10, NO2, CO and SO2 during pregnancy increases the risk of preterm birth, the key exposure windows and associated intensities are not the same, which may be attributed to different pollution levels, specific study periods and study populations, or other factors.
In our study, we did not observe any association for O3, however other studies showed that O3 was associated with preterm birth. A study (32) showed that exposure to O3 during pregnancy increased the risk of preterm birth. Another study in China (33) also illustrated that O3 was one of the risk factors for the occurrence of preterm birth, and the susceptibility window was late in pregnancy at T3. These inconsistencies may be due to differences in the locations of the studies, differences in the experimental design of the studies, or differences in the methods of exposure assessment and statistical analyses.
Although this study showed that exposures to certain pollutants in later pregnancy is associated with preterm birth, there is no clear understanding of the molecular mechanism of the occurrence of preterm birth induced by air pollutants. It has been suggested that it is fetal growth and development within the placenta that is more sensitive and vulnerable to exposure to air pollutants (34), thereby predisposing to preterm birth or other adverse pregnancy outcomes. Prenatal exposure to air pollution has also been found to be associated with nitrosative stress and epigenetic alterations in the placenta (35). Air pollution particles may be transferred into and across the placental barrier, leading to placental oxidative and nitrosative stress due to the ability of pollution particles to produce reactive oxygen species/reactive nitrogen species (ROS/RNS) in a direct or indirect manner; another important target of the early life effects of air pollution is the induction of epigenetic alterations of the placenta, including DNA methylation, histone and noncoding RNA modifications, and changes in chromatin remodeling. The Developmental Origins of Health and Disease (DOHaD) hypothesis similarly suggests an association between perinatal complications induced by prenatal exposure to air pollutants (preterm birth or fetal growth restriction), and placental epigenomics (36). In contrast, another study found that the onset of preterm birth inversely enhances the toxicity of air pollutants through oxidative stress and placental function (37), which means that air pollution particles affect the anatomical structure and/or physiological function of the developing lungs and related systems through oxidative stress, which also contributes to placental aging leading to preterm birth, and that the occurrence of preterm birth during this critical period may further enhance the ensuing alterations in lung function and physiology.
The strength of this study is that all data was collected from two of the largest hospitals in the city, which are the top hospitals in China’s healthcare system, with a high level of medical technology and quality of service. As a result, large amounts of medical data can be collected and the data tends to be more accurate and reliable, contributing to more accurate conclusions. This study also has some limitations. First, using ambient monitoring data, we could not account for the differences in pollutant concentrations between the daily living and working environments of the study subjects. And assessing pollutant exposure levels of the study subjects based only on their home addresses may lead to some errors and may bias the association; Secondly, although the nearest monitoring site method is able to obtain more accurate data than the global average method used in previous studies, it still has some limitations. For example, in some cases, the quality of data collected may be poor due to errors in monitoring equipment, improper maintenance, etc., affecting the accuracy of the analyzed results. In addition, the large number of missing important confounders, such as child sex, maternal education level, and marital status is an important limitation of this study.
In conclusion, we observed an association between exposures to PM2.5, PM10, NO2, CO in mid-pregnancy and late pregnancy and increased risk on preterm birth, but little evidence of associations with O3.
In light of importance of air quality on maternal health and birth outcomes, measures to improve air quality, its monitoring and health educations for women especially in reiver valley cities need to be public health priorities.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Medical School of Yan’an University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. JL: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. LL: Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. MC: Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. XT: Data curation, Methodology, Writing – original draft, Writing – review & editing. ZW: Data curation, Methodology, Writing – original draft, Writing – review & editing. JH: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 41761100); and the Natural Science Basic Research Program of Shaanxi (No. 2018JQ4013); and the 2022 National Innovation and Entrepreneurship Training Program for College Students of Yan’an University (No. 202210719043).
The authors thank the National Natural Science Foundation of China (41761100), the Natural Science Basic Research Program of Shaanxi (2018JQ4013).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ohuma, EO, Moller, AB, Bradley, E, Chakwera, S, Hussain-Alkhateeb, L, Lewin, A, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. (2023) 402:1261–71. doi: 10.1016/S0140-6736(23)00878-4
2. Chawanpaiboon, S, Vogel, JP, Moller, AB, Lumbiganon, P, Petzold, M, Hogan, D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7:e37–46. doi: 10.1016/S2214-109X(18)30451-0
3. Deng, K, Liang, J, Mu, Y, Liu, Z, Wang, Y, Li, M, et al. Preterm births in China between 2012 and 2018: an observational study of more than 9 million women. Lancet Glob Health. (2021) 9:e1226–41. doi: 10.1016/S2214-109X(21)00298-9
4. Zou, L, Wang, X, Ruan, Y, Li, G, Chen, Y, and Zhang, W. Preterm birth and neonatal mortality in China in 2011. Int J Gynaecol Obstet. (2014) 127:243–7. doi: 10.1016/j.ijgo.2014.06.018
5. Perin, J, Mulick, A, Yeung, D, Villavicencio, F, Lopez, G, Strong, KL, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. (2022) 6:106–15. doi: 10.1016/S2352-4642(21)00311-4
6. Liu, L, Johnson, HL, Cousens, S, Perin, J, Scott, S, Lawn, JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. (2012) 379:2151–61. doi: 10.1016/S0140-6736(12)60560-1
7. Mwaniki, MK, Atieno, M, Lawn, JE, and Newton, CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. (2012) 379:445–52. doi: 10.1016/S0140-6736(11)61577-8
8. Burris, HH, Baccarelli, AA, Wright, RO, and Wright, RJ. Epigenetics: linking social and environmental exposures to preterm birth. Pediatr Res. (2016) 79:136–40. doi: 10.1038/pr.2015.191
9. Proietti, E, Röösli, M, Frey, U, and Latzin, P. Air pollution during pregnancy and neonatal outcome: a review. J Aerosol Med Pulm Drug Deliv. (2013) 26:9–23. doi: 10.1089/jamp.2011.0932
10. Goldenberg, RL, Culhane, JF, Iams, JD, and Romero, R. Epidemiology and causes of preterm birth. Lancet. (2008) 371:75–84. doi: 10.1016/S0140-6736(08)60074-4
11. Torchin, H, and Ancel, PY. Epidemiology and risk factors of preterm birth. J Gynecol Obstet Biol Reprod. (2016) 45:1213–30. doi: 10.1016/j.jgyn.2016.09.013
12. Ye, CX, Chen, SB, Wang, TT, Zhang, SM, Qin, JB, and Chen, LZ. Risk factors for preterm birth: a prospective cohort study. Zhongguo Dang Dai Er Ke Za Zhi. (2021) 23:1242–9. doi: 10.7499/j.issn.1008-8830.2108015
13. Barfield, WD. Public health implications of very preterm birth. Clin Perinatol. (2018) 45:565–77. doi: 10.1016/j.clp.2018.05.007
14. Jiang, M, Mishu, MM, Lu, D, and Yin, X. A case control study of risk factors and neonatal outcomes of preterm birth. Taiwan J Obstet Gynecol. (2018) 57:814–8. doi: 10.1016/j.tjog.2018.10.008
15. Crawford, N, Prendergast, D, Oehlert, JW, Shaw, GM, Stevenson, DK, Rappaport, N, et al. Divergent patterns of mitochondrial and nuclear ancestry are associated with the risk for preterm birth. J Pediatr. (2018) 194:40–46.e4. doi: 10.1016/j.jpeds.2017.10.052
16. Alman, BL, Stingone, JA, Yazdy, M, Botto, LD, Desrosiers, TA, Pruitt, S, et al. Associations between PM2.5 and risk of preterm birth among liveborn infants. Ann Epidemiol. (2019) 39:46–53.e2. doi: 10.1016/j.annepidem.2019.09.008
17. Hansen, C, Neller, A, Williams, G, and Simpson, R. Maternal exposure to low levels of ambient air pollution and preterm birth in Brisbane, Australia. BJOG. (2006) 113:935–41. doi: 10.1111/j.1471-0528.2006.01010.x
18. Wilhelm, M, and Ritz, B. Local variations in CO and particulate air pollution and adverse birth outcomes in Los Angeles County, California, USA. Environ Health Perspect. (2005) 113:1212–21. doi: 10.1289/ehp.7751
19. Hu, LW, Lawrence, WR, Liu, Y, Yang, BY, Zeng, XW, Chen, W, et al. Ambient air pollution and morbidity in Chinese. Adv Exp Med Biol. (2017) 1017:123–51. doi: 10.1007/978-981-10-5657-4_6
20. Zhan, D, Kwan, MP, Zhang, W, Wang, S, and Yu, J. Spatiotemporal variations and driving factors of air pollution in China. Int J Environ Res Public Health. (2017) 14:1538. doi: 10.3390/ijerph14121538
21. Chen, Q, Ren, Z, Liu, Y, Qiu, Y, Yang, H, Zhou, Y, et al. The association between preterm birth and ambient air pollution exposure in Shiyan, China, 2015–2017. Int J Environ Res Public Health. (2021) 18:4326. doi: 10.3390/ijerph18084326
22. Ha, S, Hu, H, Roussos-Ross, D, Haidong, K, Roth, J, and Xu, X. The effects of air pollution on adverse birth outcomes. Environ Res. (2014) 134:198–204. doi: 10.1016/j.envres.2014.08.002
23. Zhang, X, Fan, C, Ren, Z, Feng, H, Zuo, S, Hao, J, et al. Maternal PM2.5 exposure triggers preterm birth: a cross-sectional study in Wuhan, China. Glob Health Res. Policy. (2020) 5:17. doi: 10.1186/s41256-020-00144-5
24. Jiang, P, Li, Y, Tong, MK, Ha, S, Gaw, E, Nie, J, et al. Wildfire particulate exposure and risks of preterm birth and low birth weight in the Southwestern United States. Public Health. (2024) 230:81–8. doi: 10.1016/j.puhe.2024.02.016
25. Zhao, N, Qiu, J, Zhang, Y, He, X, Zhou, M, Li, M, et al. Ambient air pollutant PM10 and risk of preterm birth in Lanzhou, China. Environ Int. (2015) 76:71–7. doi: 10.1016/j.envint.2014.12.009
26. Han, Y, Jiang, P, Dong, T, Ding, X, Chen, T, Villanger, GD, et al. Maternal air pollution exposure and preterm birth in Wuxi, China: effect modification by maternal age. Ecotoxicol Environ Saf. (2018) 157:457–62. doi: 10.1016/j.ecoenv.2018.04.002
27. Bhardwaj, N, Nigam, A, De, A, and Gupta, N. Ambient air pollution: a new intrauterine environmental toxin for preterm birth and low birth weight. J Obstet Gynaecol India. (2023) 73:25–9. doi: 10.1007/s13224-023-01790-8
28. Ahn, TG, Kim, YJ, Lee, G, You, YA, Kim, SM, Chae, R, et al. Association between individual air pollution (PM10, PM2.5) exposure and adverse pregnancy outcomes in Korea: a multicenter prospective cohort, air pollution on pregnancy outcome (APPO) study. J Korean Med Sci. (2024) 39:e131. doi: 10.3346/jkms.2024.39.e131
29. Ju, L, Li, C, Yang, M, Sun, S, Zhang, Q, Cao, J, et al. Maternal air pollution exposure increases the risk of preterm birth: evidence from the meta-analysis of cohort studies. Environ Res. (2021) 202:111654. doi: 10.1016/j.envres.2021.111654
30. Siddika, N, Rantala, AK, Antikainen, H, Balogun, H, Amegah, AK, Ryti, NRI, et al. Short-term prenatal exposure to ambient air pollution and risk of preterm birth—a population-based cohort study in Finland. Environ Res. (2020) 184:109290. doi: 10.1016/j.envres.2020.109290
31. He, J, Cao, N, Hei, J, Wang, H, He, J, Liu, Y, et al. Relationship between ambient air pollution and preterm birth: a retrospective birth cohort study in Yan’an, China. Environ Sci Pollut Res Int. (2022) 29:73271–81. doi: 10.1007/s11356-022-20852-4
32. Siddika, N, Rantala, AK, Antikainen, H, Balogun, H, Amegah, AK, Ryti, NRI, et al. Synergistic effects of prenatal exposure to fine particulate matter (PM2.5) and ozone (O3) on the risk of preterm birth: a population-based cohort study. Environ Res. (2019) 176:108549. doi: 10.1016/j.envres.2019.108549
33. Wang, X, Wang, X, Gao, C, Xu, X, Li, L, Liu, Y, et al. Relationship between outdoor air pollutant exposure and premature delivery in China-systematic review and meta-analysis. Int J Public Health. (2023) 68:1606226. doi: 10.3389/ijph.2023.1606226
34. Tosevska, A, Ghosh, S, Ganguly, A, Cappelletti, M, Kallapur, SG, Pellegrini, M, et al. Integrated analysis of an in vivo model of intra-nasal exposure to instilled air pollutants reveals cell-type specific responses in the placenta. Sci Rep. (2022) 12:8438. doi: 10.1038/s41598-022-12340-z
35. Saenen, ND, Martens, DS, Neven, KY, Alfano, R, Bové, H, Janssen, BG, et al. Air pollution-induced placental alterations: an interplay of oxidative stress, epigenetics, and the aging phenotype? Clin Epigenetics. (2019) 11:124. doi: 10.1186/s13148-019-0688-z
36. Lapehn, S, and Paquette, AG. The placental epigenome as a molecular link between prenatal exposures and Fetal health outcomes through the DOHaD hypothesis. Curr Environ Health Rep. (2022) 9:490–501. doi: 10.1007/s40572-022-00354-8
Keywords: preterm birth, air pollution, environmental exposure, risk assessment, public health
Citation: Gu J, Li J, Liu L, Cao M, Tian X, Wang Z and He J (2024) Exploring the association between atmospheric pollutants and preterm birth risk in a river valley city. Front. Public Health. 12:1415028. doi: 10.3389/fpubh.2024.1415028
Received: 09 April 2024; Accepted: 15 July 2024;
Published: 25 July 2024.
Edited by:
Giovanna Deiana, University of Sassari, ItalyReviewed by:
Octavio Jiménez-Garza, University of Guanajuato, MexicoCopyright © 2024 Gu, Li, Liu, Cao, Tian, Wang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinwei He, aG90cmVkXzcxNEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.