- 1National and Local Joint Engineering Research Center of Biodiagnosis and Biotherapy, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 2Department of Infectious Diseases, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 3School of Mathematics and Statistics, Xi'an Jiaotong University, Xi'an, China
- 4Center for Infectious Diseases, The Second Affiliated Hospital of Air Force Medical University, Xi'an, China
- 5Department of Clinical Medicine, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 6Provincial Research Center, Shaanxi Provincial Clinical Medical Research Center of Infectious Diseases, Xi'an, China
- 7Global Health Institute, School of Public Health, Xi'an Jiaotong University Health Science Center, Xi'an, China
- 8Key Laboratory of Surgical Critical Care and Life Support (Xi'an Jiaotong University), Ministry of Education, Xi'an, China
- 9Department of Infectious Diseases, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Background: We aimed to determine the trend of TB-related deaths during the COVID-19 pandemic.
Methods: TB-related mortality data of decedents aged ≥25 years from 2006 to 2021 were analyzed. Excess deaths were estimated by determining the difference between observed and projected mortality rates during the pandemic.
Results: A total of 18,628 TB-related deaths were documented from 2006 to 2021. TB-related age-standardized mortality rates (ASMRs) were 0.51 in 2020 and 0.52 in 2021, corresponding to an excess mortality of 10.22 and 9.19%, respectively. Female patients with TB demonstrated a higher relative increase in mortality (26.33 vs. 2.17% in 2020; 21.48 vs. 3.23% in 2021) when compared to male. Female aged 45–64 years old showed a surge in mortality, with an annual percent change (APC) of −2.2% pre-pandemic to 22.8% (95% CI: −1.7 to 68.7%) during the pandemic, corresponding to excess mortalities of 62.165 and 99.16% in 2020 and 2021, respectively; these excess mortality rates were higher than those observed in the overall female population ages 45–64 years in 2020 (17.53%) and 2021 (33.79%).
Conclusion: The steady decline in TB-related mortality in the United States has been reversed by COVID-19. Female with TB were disproportionately affected by the pandemic.
1 Introduction
Tuberculosis (TB) is one of the leading causes of disease burden and death worldwide. The global cost of TB control is up to US $1 billion annually (1). In 2014, the World Health Organization (WHO) proposed a strategy to combat TB, which strives to achieve a 95% reduction in TB mortality from 2015 to 2035, and to reduce individual and systemic healthcare costs related to TB (2). Between 2015 and 2018, the number of global TB deaths fell by 11%, which fell short of the projected decrease represented by this WHO goal (3). In 2019, there were an estimated 10 million new TB cases worldwide. As a low-burden TB country, the United States has a stabile TB incidence at ~30 new cases per 1 million people after years of slow incidence decline (4). However, this incidence still does not reach the goal of a 20% drop in incidence between 2015 and 2020 set forth by the WHO TB strategy (2).
Unfortunately, the COVID-19 pandemic has had a devastating impact on TB control, and in many aspects reversed the global progress made in the fight against TB. Many areas of the world have seen declines in TB testing and reporting of cases, and some have even observed the first increase in TB-related mortality in the past decade (5–7). In countries with a high burden of TB, the disruption of healthcare services due to reduced outbreaks, redistribution of human and material resources, and reduced social support could lead to a projected 20% increase in the number of TB-related deaths in the next 5 years (6, 8–10). Despite efforts by some TB centers to keep healthcare services fully operational during the pandemic, it has proven impossible to avoid a large decrease in the number of diagnosed cases and an increased number of patients lost to follow-up or dying during this time (11).
The substantial impact of the COVID-19 pandemic on TB control is highlighted by a sharp decline in case reporting and the squeeze on medical resources, which ultimately lead to a decline in the ability to provide timely diagnosis and treatment resulting in poor outcomes and increased TB-related mortality (12–14). On the other hand, co-infection of tuberculosis and COVID-19 was associated with an increased risk of unfavorable clinical outcomes, as reported by Aiello et al., which may have been related to immune responses (15). Particularly, the decreased immune response in older adults may have limited the ability to contain pathogen growth (16). As of yet, there has been little evidence published on the impact of the COVID-19 pandemic on TB in the United States, particularly with respect to possible gender differences. Therefore, we aimed to assess the impact of the COVID-19 pandemic on TB mortality in the United States with focus on sex differences using nationally representative data.
2 Methods
2.1 Data sources
The current study represented a population-based time series analysis combined with predictive analysis performed on data from decedents in the United States. Data were analyzed from decedents who had died from January 1, 2006 to December 31, 2021. Data were obtained from the National Vital Statistics System (NVSS) dataset through the Center for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC-WONDER) website. This database contained annual death data of more than 99% of decedents in 50 U.S. states and the District of Columbia. The most recent data available were used (updated most recently on April 16, 2022). We also collected demographic data including age, sex, and race/ethnicity. Since all data from NVSS were publicly available and completely deidentified, the study did not seek approval from the Institutional Review Board. The study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
2.2 Definition
The targeted population was decedents aged 25 years or older with TB as one of the causes of death. The study period was defined between January 1, 2006 and December 31, 2021. We identified TB and COVID-19 cases using criteria detailed in the 10th Edition of the International Classification of Diseases (ICD-10). The stratified analyses were classified by age (25–44, 45–64, and ≥65 years) and sex (male and female). We also stratified the data by racial/ethnic groups defined by the U.S. Office of Management and Budget, including non-Hispanic Alaska Indians/American Natives, non-Hispanic Asians, non-Hispanic Blacks, Hispanics, and non-Hispanic Whites. In 2021, the racial/ethnic component on the CDC Miracle website changed when the new subgroup “multiple races” was introduced. Thus, the subgroup analysis by race/ethnicity included only data through 2020.
2.3 Statistical analysis
We used age-standardized mortality rates (ASMR), using the 2010 U.S. Census as a reference, to ensure comparability across study years. Crude mortality rate (number of TB-related deaths/total population) was calculated and age-standardized using direct methods. In the direct approach, we stratified the number of TB-related deaths and the reference population by 10 years (from 25 to 64 years old, then ≥65 years old).
Using mortality data available from 2006 and 2019, TB-related mortality and general population mortality were predicted to determine the expected ASMR between 2020 and 2021. Autoregressive moving average model (ARMA), autoregressive integrated moving average model (ARIMA), polynomial regression, and linear regression were tested. Given the trend in mortality from 2006 to 2019, we chose polynomial regression with least squares for fitting. Model fitness was assessed using R square (Supplementary Table 1). The impact of the pandemic was determined by calculating the percentage difference between the expected and observed mortality rates, such that the excess mortality rate was defined as:
A time-trend analysis was performed using a connected-point analysis via piecewise regression with Monte Carlo permutation tests to estimate the direction, magnitude, and significance of trends, and to determine the segmentation of trends. All analyses were performed using Joint Point Trend Analysis software (version 4.9.1.0; National Cancer Institute, Bethesda, MD), PyCharm3.9.0 (prediction analysis), and R 4.0.2 software (all other analyses and data cleaning). The significance threshold was defined as a two-tailed p-value of < 0.05.
3 Results
3.1 Population and characteristics of analyzed deaths
A total of 18,628 TB-related deaths among adults aged 25 years or older were recorded between 2006 and 2021 (Table 1). The older adults group (≥65 years) represented the greatest percentage of these deaths at 65.61%, followed by 28.21% in the 45–64 years group, with the lowest percentage occurring in the 25–44 years age group (6.18%). With respect to sex, the mortality of males was greater than that of females (63.18 vs. 36.82%, respectively). Finally, with respect to race/ethnicity, nearly half of the analyzed TB-related deaths occurred in non-Hispanic Whites (8,008; 46.6%), followed by non-Hispanic Blacks (3,153; 18.4%), non-Hispanic Asians (2,882; 16.8%), and Hispanics (2,709; 15.8%). American Indian/Alaska Natives represented only 2.45% of the deaths (Table 1).
3.2 The impact of the COVID-19 pandemic on mortality in patients with TB and in the general population
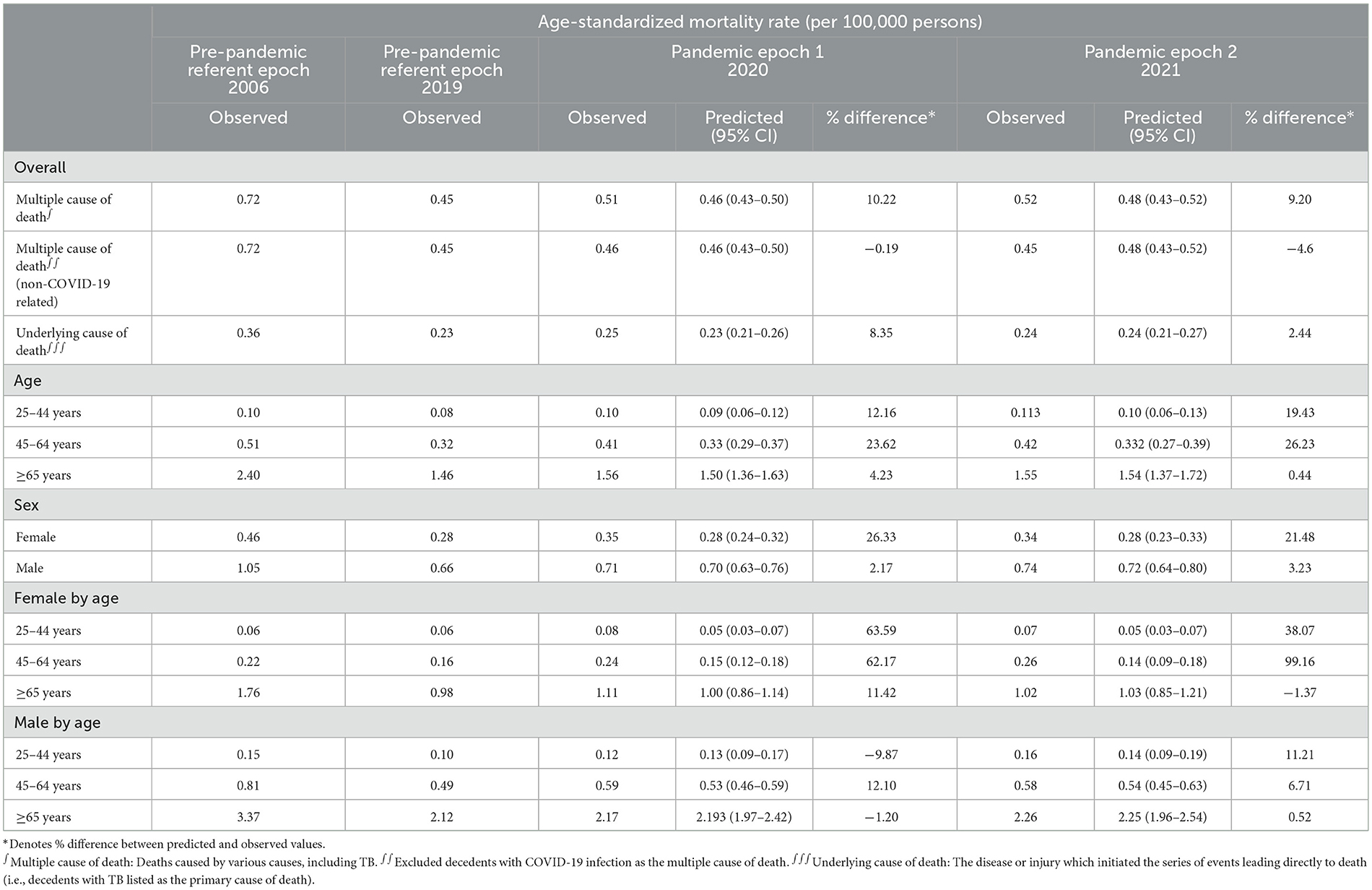
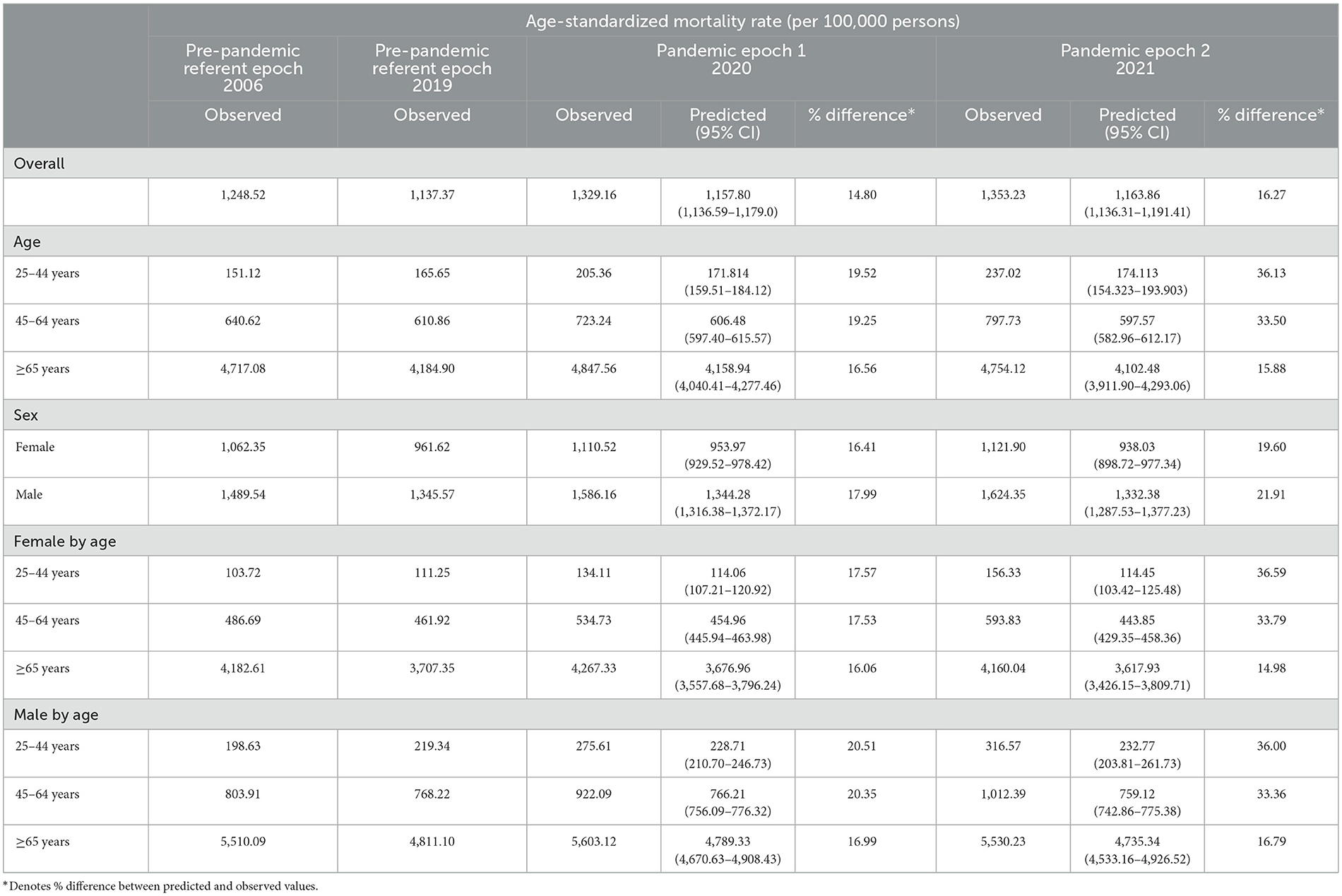
The TB-related ASMR was 0.72 per 100,000 persons in 2006, which decreased to 0.45 per 100,000 persons by 2019 (Figure 1 and Table 2). A rise in mortality rate was observed during the pandemic, with an ASMR of 0.51 per 100,000 persons in 2020 and 0.52 per 100,000 persons in 2021, corresponding to excess mortality of 10.22 and 9.20%, respectively. The overall ASMRs of adults in the U.S. general population stratified by age and sex are shown in Table 3 and Supplementary Figure 1.
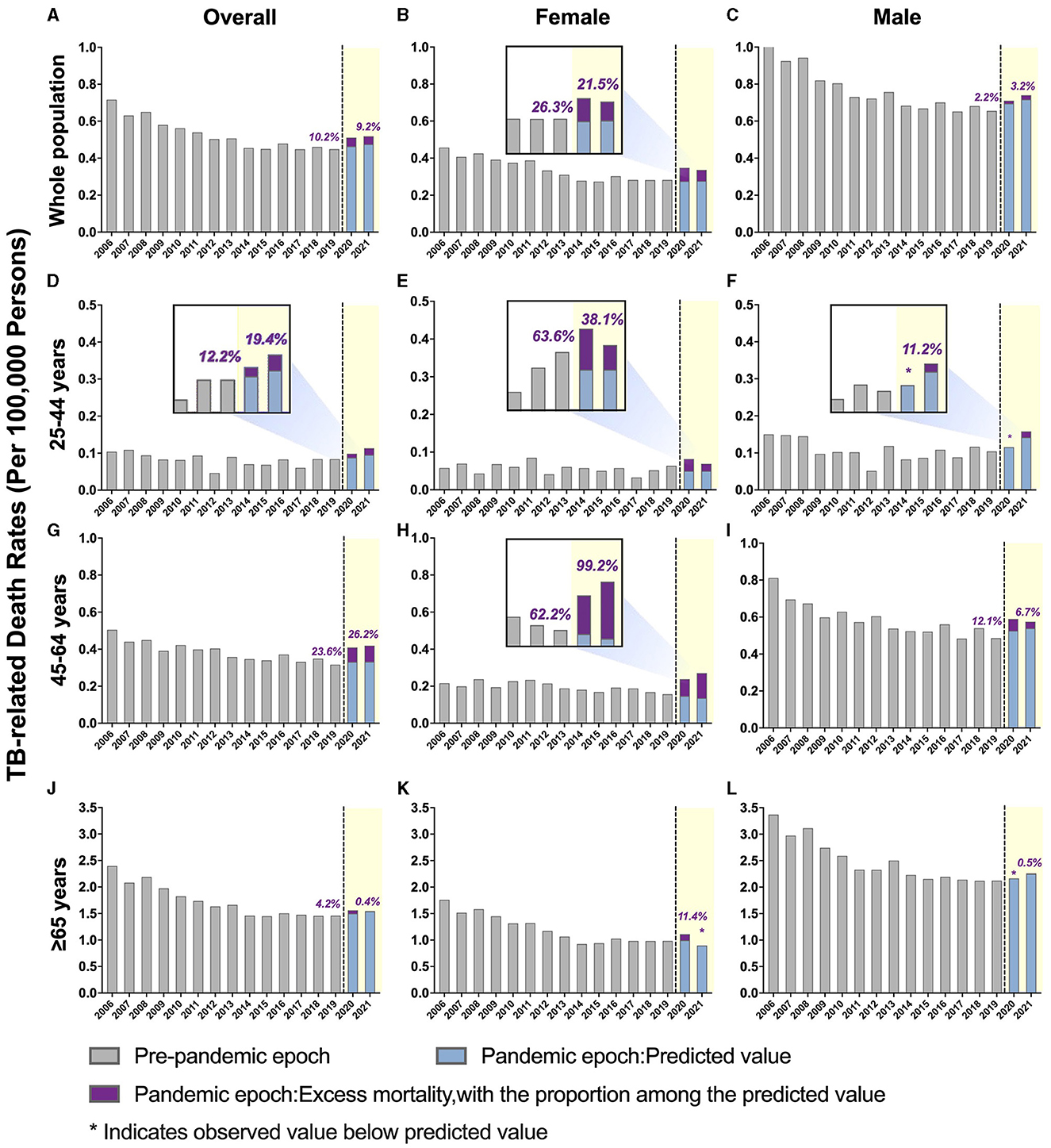
Figure 1. Temporal trends of all-cause mortality in people with tuberculosis (TB) and excess mortality during the COVID-19 pandemic by sex, further stratified by age. (A–C) Overall TB-related mortality: (A) males and females; (B) females only; (C) males only. (D–F) TB-related mortality, ages 25–44 years: (D) male and female; (E) female only; (F) male only. (G–I) TB-related mortality, ages 45–64 years: (G) male and female; (H) female only; (I) male only. (J–L) TB-related mortality, ages ≥ 65 years: (J) males and females; (K) females only; (L) males only. The blue bars represent the predicted mortality rate. Purple bars represent the excess mortality associated with COVID-19.
3.3 Sex disparities in the impact of the COVID-19 pandemic on TB-related deaths
A downward trend in TB-related mortality rate in males was evident prior to the pandemic, dropping from 1.05 per 100,000 persons in 2006 to 0.66 per 100,000 persons in 2019. This rate increased during the pandemic, with an annual percent change (APC) of 6.7% (predicted 95% CI: −6.5 to 21.7%; p > 0.05). The ASMRs were 0.710 per 100,000 persons in 2020 and 0.740 per 100,000 persons in 2021, with excess mortality of 2.17 and 3.23%, respectively (Figure 1 and Supplementary Table 2).
In contrast, the TB-related mortality rates in females increased during the pandemic, with ASMRs of 0.35 per 100,000 persons in 2020 and 0.336 per 100,000 persons in 2021 (Table 2 and Supplementary Figure 2). Notably, the percent difference between the observed and predicted mortality in female patients with TB during the pandemic was higher than in the general female population in 2020 (26.325 vs. 16.41%, respectively) and 2021 (21.48 vs. 19.60%, respectively; Tables 2, 3).
3.4 By age group
The pre-pandemic TB-related ASMR of the 45–64 years age group showed a significant downward trend, decreasing from 0.51 per 100,000 persons in 2006 to 0.32 per 100,000 persons in 2019 (APC: −2.8%; p < 0.05). However, during the pandemic, there was an upward trend in mortality with an APC of 16.8% (95% CI: −2.2 to 39.5%; p > 0.05). The observed mortality rates were 0.41 per 100,000 persons in 2020 and 0.42 per 100,000 persons in 2021, corresponding to excess mortality of 23.62 and 26.23%, respectively (Figure 1, Table 2, and Supplementary Table 2). Furthermore, the excess mortality observed in this group was higher in 2020 compared to the general population (23.62 vs. 19.25%, respectively; Tables 2, 3). Mortality rates within the 25–44 and ≥ 65 years age groups also decreased before the pandemic, but the trend was not significant (Figure 1 and Table 2).
3.5 By sex-age subgroup
Females aged 45–64 years showed a significant decrease in mortality pre-pandemic (APC −2.2%; p < 0.05), and mortality surged with an APC of 22.8% (95% CI: −1.7 to 68.7%; p > 0.05) during the pandemic (Supplementary Table 3). The observed mortality rates of female patients with TB in this age group were 0.24 per 100,000 persons and 0.26 per 100,000 persons in 2020 and 2021, respectively (Figures 1, 2, and Table 2). These data represent excess mortalities of 62.17 and 99.16% in 2020 and 2021, respectively, Which were higher than the excess mortalities in the general female population aged 45–64 years in 2020 (17.53%) and 2021 (33.79%; Tables 2, 3). Female patients with TB and aged 25–44 years also showed high excess mortalities in 2020 (63.59%) and 2021 (38.07%), both higher than that in general female population of the same age group (Figures 1, 2, Tables 2, 3). The increase in male mortality was modest throughout the pandemic, and the mortality rate observed across all ages for males was within the 95% CI of the predicted mortality rate (Figure 2).
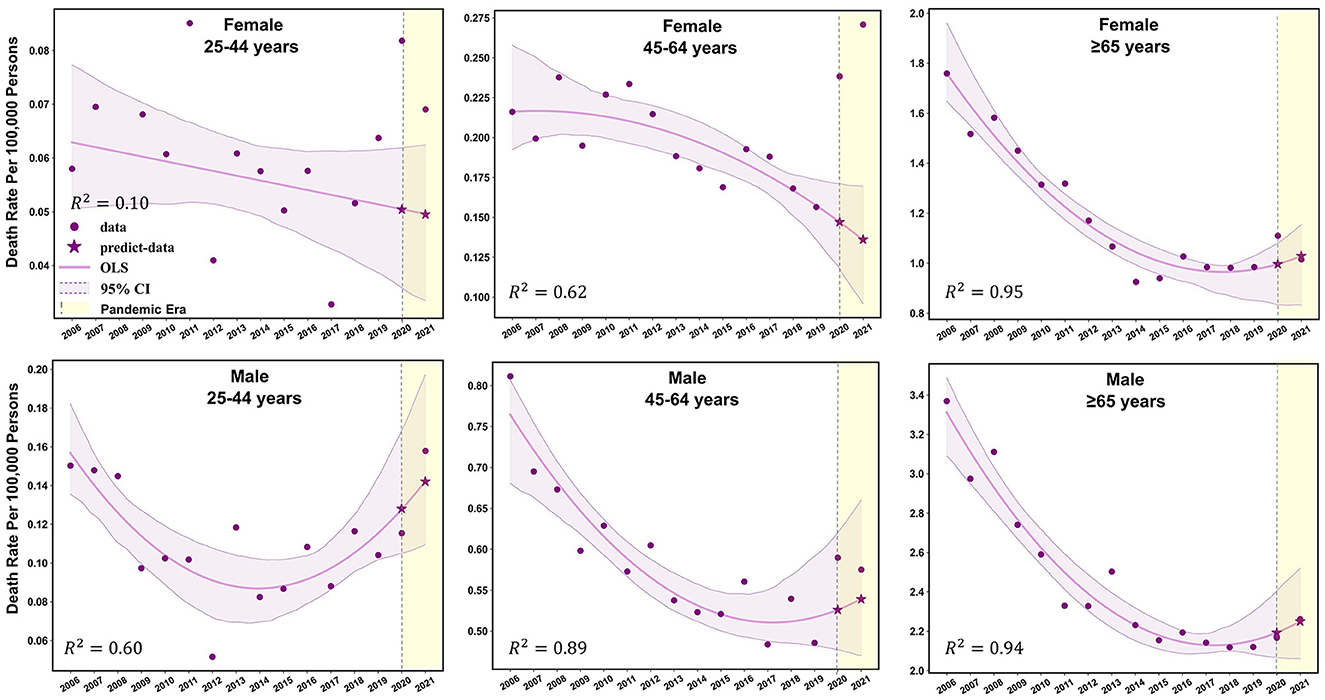
Figure 2. Observed and predicted age-standardized mortality rates for TB-related deaths before and during the COVID-19 pandemic among different sex-age subgroups.
3.6 By race/ethnicity group
Overall mortality rates decreased across all racial/ethnic groups prior to the pandemic and demonstrated a mild increase during the pandemic (Supplementary Figure 3). In 2020, the observed mortality rate increased significantly in the non-Hispanic white subgroups to higher than the predicted mortality rate, representing an excess mortality of 10.29%. Observed mortality rates for racial/ethnic subgroups of non-Hispanic black, Hispanic, non-Hispanic Asian, and non-Hispanic American Indian/Alaska Native were within the 95% CI of predicted mortality rates (Supplementary Table 4 and Supplementary Figure 3).
4 Discussion
This study analyzed 18,628 TB-related deaths occurring between 2006 and 2021 in the United States, and in doing so demonstrated the significant impact of the COVID-19 pandemic with respect to the progress made in eliminating TB in this country. During the pandemic, TB-related mortality rates significantly increased for the first time since 2006, and the observed TB-related mortality rates were significantly higher than the projected mortality rates for all age and sex subgroups in 2020 and 2021. Our data support the theory that since the early 2020's, the disruption of TB management has shifted healthcare priorities to COVID-19, which has led to the reversal of the decline of TB-related mortality. When further stratified by age and sex, there was a profound and steep increase in TB-related mortality among females aged 45–64 years, and a more moderate increase of the same in females aged 25–44 years, both of which exceeded mortality in the same female age groups of the general population. This phenomenon was observed despite the overall excess mortality being lower during the pandemic (10.22 vs. 14.800% in 2020 and 9.194 vs. 16.271% in 2021). Taken together, the precipitous increase in mortality rates suffered by young and middle-aged females with TB highlights the need for additional resources to address the underlying social and economic burden experienced by female patients and to mitigate the effects that will undoubtedly linger for years following the pandemic.
The challenges associated with TB control have been recognized from the start of the pandemic. Recent studies have shown that TB patients utilized healthcare services less during the pandemic than in previous years. The implementation of emergency measures such as home quarantine, cancellation of public transport, closure of public places, TB hospitals being designated as COVID-19 hospitals, and allocation of medical staff to COVID-19 wards has led to the interruption of health services, resulting in a significant decrease in TB diagnosis and reporting and an increase in TB-associated mortality (17–22). We quantified excess mortality in patients with TB during the pandemic and found a higher number of excess deaths in the female TB patient population, suggesting that more cases and opportunities for disease management were being missed in female TB patients. Soko et al. also found that females were most affected by TB service disruption and had the most pronounced decline in TB case notifications (20). Although service disruptions led to decreased disease detection, the proportion of females in the TB patient population increased more than before the pandemic (23). In addition to the impact of healthcare disruption, fear and stigma surrounding COVID-19, lack of access to adequate healthcare, and social determinants like gender inequality also likely contributed to the decrease in TB diagnosis and the increase in mortality among female TB patients.
We also observed an upward trend in deaths in male TB patients, but this was not significant as the observed mortality rate was within the 95% CI of the model predicted value. There are data suggesting that male gender is an independent risk factor for death related to TB and COVID-19 coinfection (24). In addition to male sex, the increased mortality was also associated with the presence of TB sequelae (25). These data suggest that the risk of death after COVID-19 infection is increased for both old and new TB cases. The risk of death was 1.51-fold in TB patients with COVID-19 diagnosed before the pandemic and 2.7-fold in newly-diagnosed TB patients with COVID-19 (26). Certainly, there is also evidence that the impact of COVID-19 coinfection on the TB clinical course seems modest, except for in cases of death where no serious clinical deterioration was observed (27). This seems to be explained by the fact that the effects of COVID-19 on TB disease course can be controlled through appropriate clinical care. In fact, COVID-19 mortality and incidence rates are low in the U.S. as well as in many Asian countries with high TB burden, which may be associated with immunity produced by long-term TB infection (28). Ahmed et al. reported that established pulmonary TB may have a protective effect against COVID-19, which may also support this speculation (29). However, TB has also been suggested as a risk factor for disease progression in COVID-19 (30), as evidenced by the higher mortality, shorter time to death, and longer recovery time after acquisition of previous TB infection compared with patients without TB (31). The reports by Petrone et al. and Najafi et al. also indicated that the inability of the immune system to in vitro respond to SARS-CoV-2 and M. tuberculosis antigens in TB-COVID-19 co-infected subjects (32, 33).
Furthermore, vaccination as a key social prevention strategy for TB may also have implications for COVID-19. A Japanese study found that routine infant BCG coverage was protective against the transmission of COVID-19 (34). This may be related to the effect of BCG vaccination on COVID-19-related immunity (35), but there has been controversy whether BCG vaccination plays any role. Lerm et al. suggest that COVID-19 does not cause serious disease in most unvaccinated young people and healthy people, and that high-quality studies on the effects of BCG vaccination on COVID-19 incidence, disease course, and transmission are needed to determine whether and how BCG could help control the outbreak (36).
In any case, the disruption of TB services due to medical and social measures put in place for management of COVID-19 is one of the main reasons for the sharp increase in TB-related deaths, with females being most affected. The U.S. government is a very important force in the global fight against TB, and it also provides the largest funds for global tuberculosis control in the world. Currently, TB is a greater long-term threat than COVID-19, and the government must strengthen services and support for TB patients, especially for disproportionately affected groups.
The main strength of our study is the use of a nationwide database to quantify the impact of the COVID-19 pandemic on the TB mortality rate in the United States and associated sex differences. The database we used had comprehensive records of deaths such that our selection bias was low. Secondly, a suitable model was utilized to predict general mortality rates during the pandemic, which provided the basis for further identification of excess mortality. In addition, our subgroup analysis highlighted the differences in excess mortality between sexes, ages, and ethnic groups. Our main limitation is that the death data are susceptible to biases such as underreporting, which could lead to underestimation of the excess mortality rate. For all analyses, data associated to clinical characteristics, cs, including occupation, education, and income, were not available. Therefore, additional studies are needed to investigate correlates and potential determinants of the trends in excess mortality in female patients aged 45–64 year found in our analyses of vital statistics. Furthermore, no data on race/ethnicity were used from 2021 because a new category was added (more than one race) during this year.
5 Conclusion
The steady decline in TB-related mortality in the United States has unfortunately been reversed by COVID-19. Female patients with TB were disproportionately affected during the pandemic, likely owing to care gaps and health disparities experienced by females that were accentuated during the pandemic. TB is a more substantial long-term threat to global health than COVID-19; as such, effective public health infrastructure and concrete policy changes are instrumental to ensuring an equitable and resilient health care delivery system to help prevent the deaths of vulnerable populations with TB.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because Since all data from NVSS were publicly available and completely de-identified, the study did not seek approval from the institutional review board.
Author contributions
HD: Methodology, Writing – review & editing, Writing – original draft, Data curation. YL: Data curation, Formal analysis, Writing – original draft. FL: Writing – original draft, Methodology, Data curation. XL: Writing – original draft, Formal analysis, Data curation. MQ: Writing – original draft, Formal analysis, Data curation. YB: Writing – original draft, Formal analysis, Data curation. SQ: Writing – original draft, Formal analysis, Data curation. XH: Writing – original draft, Methodology, Data curation. FJ: Writing – review & editing, Supervision, Project administration, Investigation, Conceptualization. Q-LZ: Investigation, Project administration, Supervision, Writing – review & editing. NG: Writing – review & editing, Supervision, Project administration, Investigation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
FJ: Speaker: Gilead Sciences, MSD, and Ascletis. Consulting/advisory board: Gilead and MSD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1413604/full#supplementary-material
References
1. Organization WH. Global Tuberculosis Report 2022. (2022). Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report (accessed June 14, 2023).
2. Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. Who's new end TB strategy. Lancet. (2015) 385:1799–801. doi: 10.1016/S0140-6736(15)60570-0
3. Organization WH. Global Tuberculosis Report 2018. Available online at: https://www.who.int/publications/i/item/9789241565646 (accessed June 14, 2023).
4. Salinas JL, Mindra G, Haddad MB, Pratt R, Price SF, Langer AJ. Leveling of tuberculosis incidence—United States, 2013-2015. Morb Mortal Wkly Rep. (2016) 65:273–8. doi: 10.15585/mmwr.mm6511a2
5. Dheda K, Perumal T, Moultrie H, Perumal R, Esmail A, Scott AJ, et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir Med. (2022) 10:603–22. doi: 10.1016/S2213-2600(22)00092-3
6. Bagcchi S. Dismal global tuberculosis situation due to COVID-19. Lancet Infect Dis. (2021) 21:1636. doi: 10.1016/S1473-3099(21)00713-1
7. Migliori GB, Thong PM, Alffenaar JW, Denholm J, Tadolini M, Alyaquobi F, et al. Gauging the impact of the COVID-19 pandemic on tuberculosis services: a global study. Eur Respir J. (2021) 58:2021. doi: 10.1183/13993003.01786-2021
8. Hogan AB, Jewell BL, Sherrard-Smith E, Vesga JF, Watson OJ, Whittaker C, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. (2020) 8:e1132–e41. doi: 10.1016/S2214-109X(20)30288-6
9. Cilloni L, Fu H, Vesga JF, Dowdy D, Pretorius C, Ahmedov S, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine. (2020) 28:100603. doi: 10.1016/j.eclinm.2020.100603
10. The Lancet Infectious D. Tuberculosis and malaria in the age of COVID-19. Lancet Infect Dis. (2021) 21:1. doi: 10.1016/S1473-3099(20)30946-4
11. Magro P, Formenti B, Marchese V, Gulletta M, Tomasoni LR, Caligaris S, et al. Impact of the SARS-CoV-2 epidemic on tuberculosis treatment outcome in Northern Italy. Eur Respir J. (2020) 56:2002665. doi: 10.1183/13993003.02665-2020
12. Gupta N, Ish P, Gupta A, Malhotra N, Caminero JA, Singla R, et al. A profile of a retrospective cohort of 22 patients with COVID-19 and active/treated tuberculosis. Eur Respir J. (2020) 56:2003408. doi: 10.1183/13993003.03408-2020
13. Khurana AK, Aggarwal D. The (in)significance of TB and COVID-19 co-infection. Eur Respir J. (2020) 56:2002105. doi: 10.1183/13993003.02105-2020
14. Motta I, Centis R, D'Ambrosio L, García-García JM, Goletti D, Gualano G, et al. Tuberculosis, COVID-19 and migrants: preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. (2020) 26:233–40. doi: 10.1016/j.pulmoe.2020.05.002
15. Aiello A, Najafi-Fard S, Goletti D. Initial immune response after exposure to mycobacterium tuberculosis or to SARS-CoV-2: similarities and differences. Front Immunol. (2023) 14:1244556. doi: 10.3389/fimmu.2023.1244556
16. Grifoni A, Alonzi T, Alter G, Noonan DM, Landay AL, Albini A, et al. Impact of aging on immunity in the context of COVID-19, HIV, and tuberculosis. Front Immunol. (2023) 14:1146704. doi: 10.3389/fimmu.2023.1146704
17. Crowder R, Geocaniga-Gaviola DM, Fabella RA, Lim A, Lopez E, Kadota JL, et al. Impact of shelter-in-place orders on TB case notifications and mortality in the Philippines during the COVID-19 pandemic. J Clin Tuberc Other Mycobact Dis. (2021) 25:100282. doi: 10.1016/j.jctube.2021.100282
18. Louie JK, Agraz-Lara R, Romo L, Crespin F, Chen L, Graves S. Tuberculosis-associated hospitalizations and deaths after COVID-19 shelter-in-place, San Francisco, California, USA. Emerg Infect Dis. (2021) 27:2227–9. doi: 10.3201/eid2708.210670
19. Barrenechea-Pulache A, Portocarrero-Bonifaz A, Rojas-Roque C, Gamboa-Unsihuay JE, Hernández-Vásquez A. Forgetting other communicable diseases during the COVID-19 pandemic: tuberculosis mortality in Peru. Lancet Reg Health Am. (2022) 9:100226. doi: 10.1016/j.lana.2022.100226
20. Soko RN, Burke RM, Feasey HRA, Sibande W, Nliwasa M, Henrion MYR, et al. Effects of coronavirus disease pandemic on tuberculosis notifications, Malawi. Emerg Infect Dis. (2021) 27:1831–9. doi: 10.3201/eid2707.210557
21. Nabity SA, Han E, Lowenthal P, Henry H, Okoye N, Chakrabarty M, et al. Sociodemographic characteristics, comorbidities, and mortality among persons diagnosed with tuberculosis and COVID-19 in close succession in California, 2020. J Am Med Assoc Netw Open. (2021) 4:e2136853. doi: 10.1001/jamanetworkopen.2021.36853
22. Chiang CY, Islam T, Xu C, Chinnayah T, Garfin AMC, Rahevar K, et al. The impact of COVID-19 and the restoration of tuberculosis services in the Western Pacific Region. Eur Respir J. (2020) 56:2003054. doi: 10.1183/13993003.03054-2020
23. Srivastava S, Jaggi N. TB positive cases go up in ongoing COVID-19 pandemic despite lower testing of TB: an observational study from a hospital from Northern India. Ind J Tuberc. (2022) 69:157–60. doi: 10.1016/j.ijtb.2021.04.014
24. TB/COVID-19 Global Study Group. Tuberculosis and COVID-19 co-infection: description of the global cohort. Eur Respir J. (2022) 59:2102538. doi: 10.1183/13993003.02538-2021
25. Tadolini M, Codecasa LR, García-García JM, Blanc FX, Borisov S, Alffenaar JW, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. (2020) 56:2002328. doi: 10.1183/13993003.02328-2020
26. Western Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa. Risk Factors for Coronavirus Disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. (2021) 73:e2005–e2015. doi: 10.1093/cid/ciaa1198
27. Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian Reference Hospital. Eur Respir J. (2020) 56:2001708. doi: 10.1183/13993003.01708-2020
28. Inoue K, Kashima S. Association of the past epidemic of mycobacterium tuberculosis with mortality and incidence of COVID-19. PLoS ONE. (2021) 16:e0253169. doi: 10.1371/journal.pone.0253169
29. Ahmed N, Hamid S, Memon MA. Relationship of prior pulmonary tuberculosis with the occurrence of COVID-19 pneumonia: review of 500 plus HRCT chest scans from two different centres of Sindh, Pakistan. J Ayub Med Coll Abbottabad. (2021) 33:368–75.
30. Gao Y, Liu M, Chen Y, Shi S, Geng J, Tian J. Association between tuberculosis and COVID-19 severity and mortality: a rapid systematic review and meta-analysis. J Med Virol. (2021) 93:194–6. doi: 10.1002/jmv.26311
31. Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis. (2020) 52:902–7. doi: 10.1080/23744235.2020.1806353
32. Petrone L, Petruccioli E, Vanini V, Cuzzi G, Gualano G, Vittozzi P, et al. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int J Infect Dis. (2021) 113(Suppl.1):S82–S7. doi: 10.1016/j.ijid.2021.02.090
33. Najafi-Fard S, Aiello A, Navarra A, Cuzzi G, Vanini V, Migliori GB, et al. Characterization of the immune impairment of patients with tuberculosis and COVID-19 coinfection. Int J Infect Dis. (2023) 130(Suppl.1):S34–S42. doi: 10.1016/j.ijid.2023.03.021
34. Kinoshita M, Tanaka M. Impact of routine infant BCG vaccination on COVID-19. J Infect. (2020) 81:625–33. doi: 10.1016/j.jinf.2020.08.013
35. Ozdemir C, Kucuksezer UC, Tamay ZU. Is BCG vaccination affecting the spread and severity of COVID-19? Allergy. (2020) 75:1824–7. doi: 10.1111/all.14344
Keywords: tuberculosis, COVID-19, age-standardized mortality rate, excess mortality, female
Citation: Deng H, Liu Y, Lv F, Li X, Qi M, Bo Y, Qiu S, He X, Ji F, Zeng Q-L and Gao N (2024) Sex disparities of the effect of the COVID-19 pandemic on mortality among patients living with tuberculosis in the United States. Front. Public Health 12:1413604. doi: 10.3389/fpubh.2024.1413604
Received: 07 April 2024; Accepted: 03 June 2024;
Published: 18 June 2024.
Edited by:
Delia Goletti, National Institute for Infectious Diseases “Lazzaro Spallanzani” IRCCS, ItalyReviewed by:
Alessandra Aiello, National Institute for Infectious Diseases Lazzaro Spallanzani-IRCCS, ItalyChiara Farroni, National Institute for Infectious Diseases Lazzaro Spallanzani (IRCCS), Italy
Copyright © 2024 Deng, Liu, Lv, Li, Qi, Bo, Qiu, He, Ji, Zeng and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanpu Ji, jifanpu1979@163.com; infection@xjtu.edu.cn; Qing-Lei Zeng, zengqinglei2009@163.com; Ning Gao, gaohuining1@163.com
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share last authorship
 Huan Deng1,2†
Huan Deng1,2† Xinyuan He
Xinyuan He Fanpu Ji
Fanpu Ji Qing-Lei Zeng
Qing-Lei Zeng Ning Gao
Ning Gao