- 1PinneyAssociates, Inc., Pittsburgh, PA, United States
- 2Center of Excellence for the Acceleration of Harm Reduction (CoEHAR), University of Catania, Catania, Italy
- 3Unit of Pediatric Clinic, AOU “Policlinico”, PO “G. Rodolico”, Department of Clinical & Experimental Medicine, University of Catania, Catania, Italy
- 4Department of Clinical & Experimental Medicine, University of Catania, Catania, Italy
This perspective discusses how to best define “e-cigarette use” among youth in a way that is relevant to individual and human health. Commonly-used definitions of youth e-cigarette use have been adapted from measures validated for tobacco cigarette smoking among adults, but may not carry the same meaning for a different product (with a much lower risk profile and very different patterns of use) and a different population (whose use is more often transient and experimental, rather than frequent and persistent). We discuss strengths and weaknesses of different definitions, and recommend improvements in defining youth e-cigarette use. We find that current literature employs a range of definitions of e-cigarette use, from lifetime use (“even a puff”) to daily use. More lenient measures capture more potentially at-risk youth, but much of this is transient experimentation that has negligible risks in and itself, if not persistent. More stringent measures such as daily use are more relevant to individual and public health. Future research should examine possible improvements to definitions which include intensity of use (e.g., number of puffs per day) and persistence/duration of use, either via self-report or technology-assisted data capture.
1 Introduction
E-cigarettes are a lower-risk nicotine product that can benefit adults who smoke and are unlikely to quit entirely (1–4), but there are ongoing concerns about youth e-cigarette use. Continued surveillance of youth e-cigarette use is needed, especially considering that use patterns continue to change with the evolving product market. For example, e-cigarettes were introduced into the US market in 2007, but adult current use prevalence remained low (<2%) through at least 2012 (5), after which it fluctuated through 2018 at approximately 3–4% (6). Retail data broadly corroborate these trends, with low sales prior to 2013, and the e-cigarette market increased with Blu in 2013, Vuse in 2014, and JUUL in 2017 (7). Since 2017, US retail trends (primarily reflecting purchases by adults, who comprise a greater share of the population (see 8)); e.g., have shifted toward high-nicotine content e-cigarettes (9), and the most common brands in 2022 (Vuse, JUUL, Elf Bar, NJOY, and Breeze Smoke) (10) utilize nicotine-salt formulations, which provide higher nicotine delivery (11). This is beneficial for adult smokers wanting to switch to e-cigarettes but has raised concerns about these products’ addictiveness for youth.
Over this time frame, e-cigarettes have become the most common nicotine product among US youth (12–15) as cigarette smoking reached historic lows (16–20). Youth prevalence of any e-cigarette use in the past 30 days (P30D) peaked in 2019 in the US; this was primarily of JUUL (21, 22). Out of concern over this unacceptably high rate of youth use, Juul Labs, Inc. voluntary discontinued its non-tobacco, non-menthol-flavored products, followed shortly by the U.S. Food and Drug Administration’s (FDA’s) announcement to prioritize enforcement against non-tobacco, non-menthol-flavored pod/cartridge e-cigarettes (23). Subsequently, youth e-cigarette use shifted to sweet- and fruit-flavored disposable products (21) such as Puff Bar in 2021 and 2022 (24, 25) and more recently, Elf Bar (13). Fortunately, youth P30D use has also fallen substantially has fallen by >60% in 2023, compared to its 2019 peak (22), and correspondingly, youth use of JUUL as usual brand fell from 16.3% of all high school students and 5.7% of all middle school students in 20191 (22) to <0.3% of all youth in 20232 (13).
Given that cigarettes are at the most harmful end of the continuum of risk (1–4) and evidence that the two products are substitutes (21, 26–28), it is also important to monitor youth cigarette smoking as e-cigarette us trends change. A related concern is dual use, especially with cigarettes, given the possibility of combined exposures to multiple products. However, reassuringly, accompanying the peak-and-decline in P30D youth e-cigarette use, youth P30D cigarette smoking fell to the all-time low of 1.5% (22). Similarly, P30D use of 2+ products has declined along with overall P30D e-cigarette use, among both high school (from 10.2% in 2020 to 3.9% in 2023) and middle school (from 4.0% in 2020 to 2.5% in 2023) students (13, 29). Several other countries also show a concomitant rise in e-cigarette use and a rapid decline in smoking, including Canada, England, New Zealand, and Germany (30–33). Nevertheless, ongoing surveillance of youth nicotine use is warranted, especially for e-cigarettes, as the most commonly-used product currently.
A necessary element of youth surveillance, as well as comparability of research, is defining “e-cigarette use” consistently across studies and using a measure that has external validity (i.e., relevance to public and individual health). There is currently no clear consensus on how best to define “use,” and the research field would benefit from explicitly weighing different definitions. Here we discuss trends in different current definitions of “e-cigarette use” and corresponding strengths and weaknesses, and make recommendations.
1.1 Historical context
Commonly-used metrics for measuring e-cigarette use in both youth and adults seem to have been adapted from those used for cigarette smoking in adults, which have been validated against both biochemical markers of exposure (e.g., cotinine or carbon monoxide) and clinical health outcomes. Self-reported measures of smoking – especially measures of daily consumption such as cigarettes per day (CPD) – are generally strongly correlated with biochemical markers of exposure (e.g., cotinine or carbon monoxide) in adults (34, 35), which in turn are associated with adverse health outcomes (36–38). Importantly, however, the concordance between self-reported smoking and exposure levels varies widely across studies, and partly depends on how smoking status is defined (34). Specifically, many light and occasional smokers (e.g., <10 CPD) have similar exposure levels to tobacco-naïve individuals (35, 39), prompting recommendations to define positive smoking status using daily-consumption criteria (e.g., 10+ CPD) to prevent misclassification that could obscure the true impact of regular smoking on health (39).
On the other hand, duration of smoking and/or cumulative exposure (e.g., pack-years) are more strongly associated with clinical outcomes in adults (e.g., lung cancer, coronary artery disease, and severity of chronic obstructive pulmonary disease) than is CPD alone (40). In fact, one study concluded that “smoking at a lower intensity for longer duration is more deleterious than smoking at a higher intensity for a shorter duration” (41).
These validated measures of adult cigarette smoking have been adapted in two separate ways without rigorously evaluating whether these adaptations alter their utility: first, to a different product (from cigarettes to e-cigarettes), and second, to a different population (from adults to youth). Regarding the first adaptation – from cigarettes to e-cigarettes – complications may arise from the fact that e-cigarettes have a much lower risk profile than cigarettes (2), which seems to indicate a higher-threshold definition is warranted to measure an equivalent level of health risk. Additionally, e-cigarettes and cigarettes involve different patterns of use (see below), and thus a given definition of use may be incomparable between the products. Additionally, despite the recommendations from the adult smoking literature to measure quantity and/or duration of cigarette smoking (39–41), not all nationally-representative US surveys collect such information for e-cigarette use (42, 43), limiting the available measures to only current use and resulting in a more lenient definition.
Regarding the adaptation from adults to youth, there are additional complications stemming from the fact that youth use is not typically as heavy or prolonged as adult use, and is more often transient and experimental. For example, smoking is likely to be underreported by underage youth – especially when they have privacy concerns when providing survey responses (44) – which may explain why self-reported nonsmoking individuals can have above-threshold exposure levels (39, 45). Another explanation for this type of discrepancy, suggested by a Statistics Canada publication, is that “smoking initiation or experimentation in this period may have resulted in some cases being inappropriately classified… particularly among respondents aged 12 to 19” (35) – the implication being that mere initiation or experimentation should not be considered as true smoking. Additionally, there are notable exposure differences in how one smokes; youth who did not inhale into their lungs more often had below-threshold exposure levels (45).
Despite the importance of accounting for intensity and/or duration when defining “use,” youth use is often measured using more loosely, defining “current smoking” as any smoking (even a puff) in P30D. This low threshold is likely motivated by the fact that “no amount of smoking is safe” (46), and youth cigarette smoking – even low amounts – can be associated with nicotine dependence (47, 48) and potentially lead to long-tern use (49, 50). While little to date is known about how often infrequent e-cigarette use leads to long-term chronic use, it could plausibly be expected to be less likely for e-cigarettes than cigarettes, considering that dependence on e-cigarettes is lower than on cigarettes (51, 52). Relatedly, youth measures of smoking are typically less stringent than typical measures of adult use, in that youth do not (yet) meet the criteria for “established” use (i.e., cumulative 100 cigarettes/lifetime) or daily consumption (e.g., 10+ CPD) typically used among adults (53), since youth have had less time to accrue this level of use. Thus, adopting a “lower bar/threshold” for measuring youth tobacco cigarette use is often considered appropriate.
While surveillance of cigarette and e-cigarette use is often presented equivalently between youth and adults as “current use” (13, 53), the specific questions are different: adult current use is standardly assessed as use on “some days” or “every day” (vs. “not at all”) (42, 43, 54, 55) while youth current use is standardly assessed as “any use, even a puff, in the past 30 days (P30D)” (13, 15, 56). The two measures are largely consistent with each other, but there is some notable discrepancy: for example, a comparison of the two metrics in young adults found that the standard youth definition yields higher prevalence estimates than the standard adult definition (34.4% vs. 27.3% for “any use in P30D” vs. “some day or every day use,” respectively) (57).
In summary, measures developed and validated for adult cigarette smoking have been adapted in two ways – from cigarettes to e-cigarettes, and from adults to youth – both of which introduce separate sets of complications. These adaptations raise the question of whether these measures remain valid, and call for re-evaluation and, if necessary, improvement of standard metrics for e-cigarette use that are relevant to individual and public health.
1.2 Metrics for measuring youth e-cigarette use
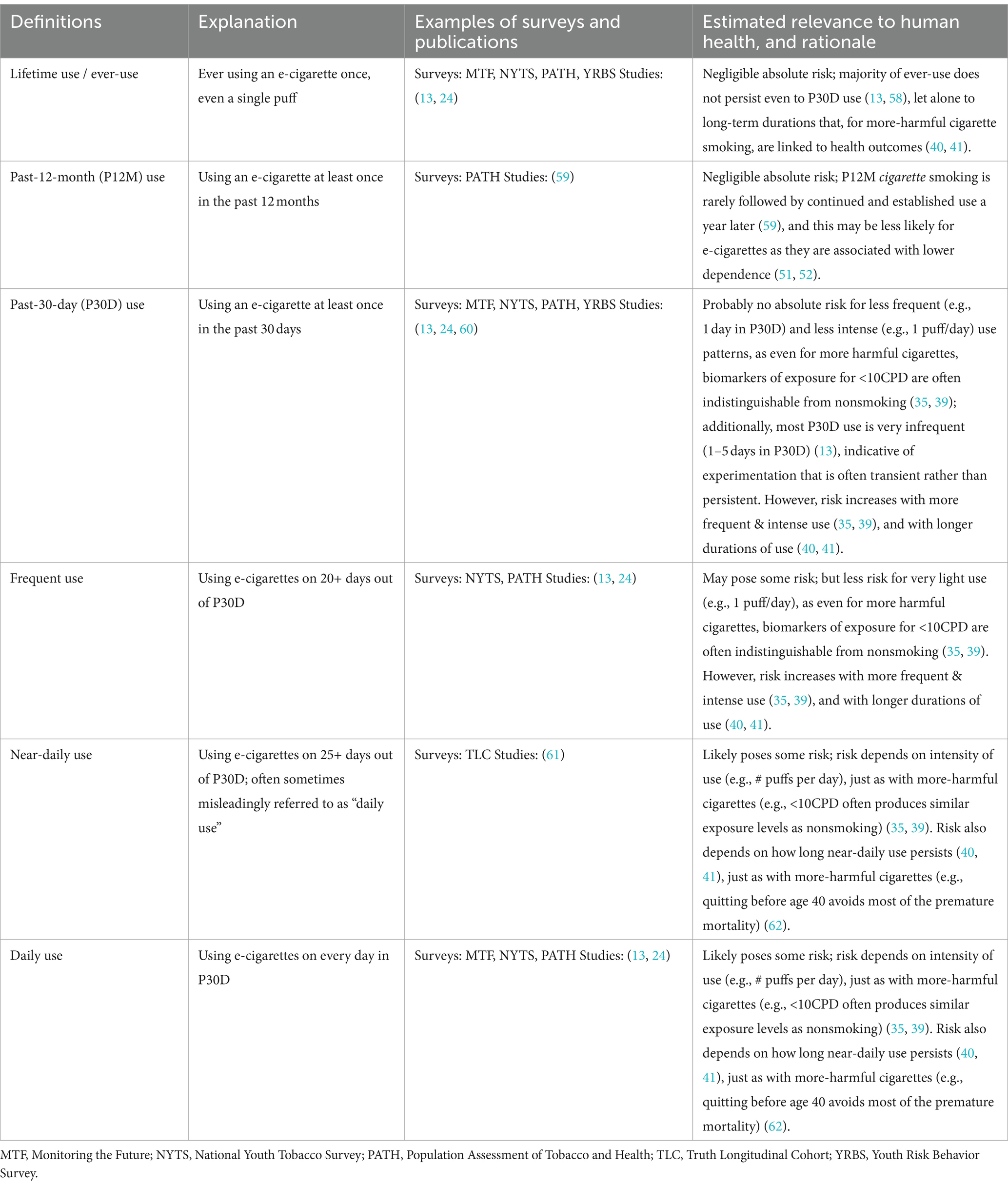
Table 1 presents the common definitions of e-cigarette use, which range from lifetime use (i.e., ever had even a single puff) to daily use. While there is no clear consensus in the literature, the most standard measures in the literature are lifetime use, past-12-month (P12M) use, and P30D use, which are used in several US national youth surveys. Also fairly common are frequency-based measures such as use on 20+ days out of P30D and daily use. The exact measure used is important as it can lead to different interpretations; for example, King cites NYTS data, switching between percentages (“in 2019, current (past-30-day) e-cigarette prevalence reached a peak among middle-school (10.5%) and high-school (27.5%) students”) and raw numbers (“nonetheless, in 2021, more than 2 million US middle- and high-school students used e-cigarettes”) (63), which obscures the magnitude of the decline after 2019.
2 Discussion
2.1 Strengths and weaknesses of existing measures of use
Broadly, the main distinction between the common definitions presented in Table 1 is the frequency of use. Note that these measures do not include information on daily consumption/intensity of use (e.g., # puffs per day), which is often not captured at all in surveys.
On one hand, lenient definitions (e.g., lifetime use, P12M use) have both conceptual and practical advantages: as noted above for cigarette smoking, the first use of an e-cigarette is not harmful in and of itself, but could lead to long-term and problematic use (49, 50), which could motivate capturing all youth potentially at risk. Practically, lenient definitions capture greater numbers of youth, making statistical analyses easier, as opposed to more stringent definitions yielding too few youth to statistically analyze (Table 2), even in large nationally-representative surveys (59).
The drawback of using lenient definitions of use is that they capture a large fraction of experimental use that does not evolve into long-term use and (if not) poses negligible harms to human health. For example, data from NYTS 2022 and 2023 show that less than half of the youths who ever used e-cigarettes persisted in using them in the P30D (13, 58). P30D use also includes some level of experimental use, especially if one-time experimentation occurs in the month preceding the survey. Among youths who reported using e-cigarettes in the P30D in NYTS 2023, more (46.1%) used e-cigarettes on only 1–5 days in the P30D period than used them frequently (i.e., on 20+ days out of the P30D period; 34.7%) (13). Few used on intermediate number of days (19.1% used on 6–19 days), confirming the bimodal frequency distribution observed for nicotine product use (64). This suggests that near-daily (sometimes misleadingly referred to as “daily” use (61)) or daily P30D use, rather than any P30D use, is more relevant to health risks.
Additionally, any P30D use often does not lead to continued/persistent use over time. For example, an analysis of product-use transitions in PATH study showed that approximately one-quarter of youths who exclusively used e-cigarettes in the P30D were not using either e-cigarettes or cigarettes the following year (65). In a more recent study of youth and young adults (ages 15–24) in Ohio, US, very infrequent use (i.e., on ≤5 days in P30D) was found to be highly stable over time, with 76.8% maintaining the same behavior 12 months later (66). In fact, using on ≤5 days in P30D was at least as stable as more frequent use (i.e., on 6+ days in P30D): the probability of maintaining ≤5 vs. 6+ use days over 4 months was 81.5% vs. 73.1%, though the significance of this difference was not tested (66). Definitions that include information on persistence or continued use were proposed by Sun et al. (59) in the context of cigarette smoking (Table 2), and could reasonably be extended to e-cigarette use. The first definition is rather lenient, capturing initiation in the P12M, and subsequent definitions are increasingly strict. The number of youth captured by each additional criterion drops rapidly; even adding one additional lenient criterion of P12M use 1 year later drops the number of youth meeting criteria for “use” by ~40%. Arguably, the most stringent definition (use at multiple timepoints, leading to established and lifetime use) is the most indicative of problematic patterns of use; however, its prevalence is vanishingly small, comprising only 3% of youth captured by the most lenient definition, and is too few to statistically analyze (59).
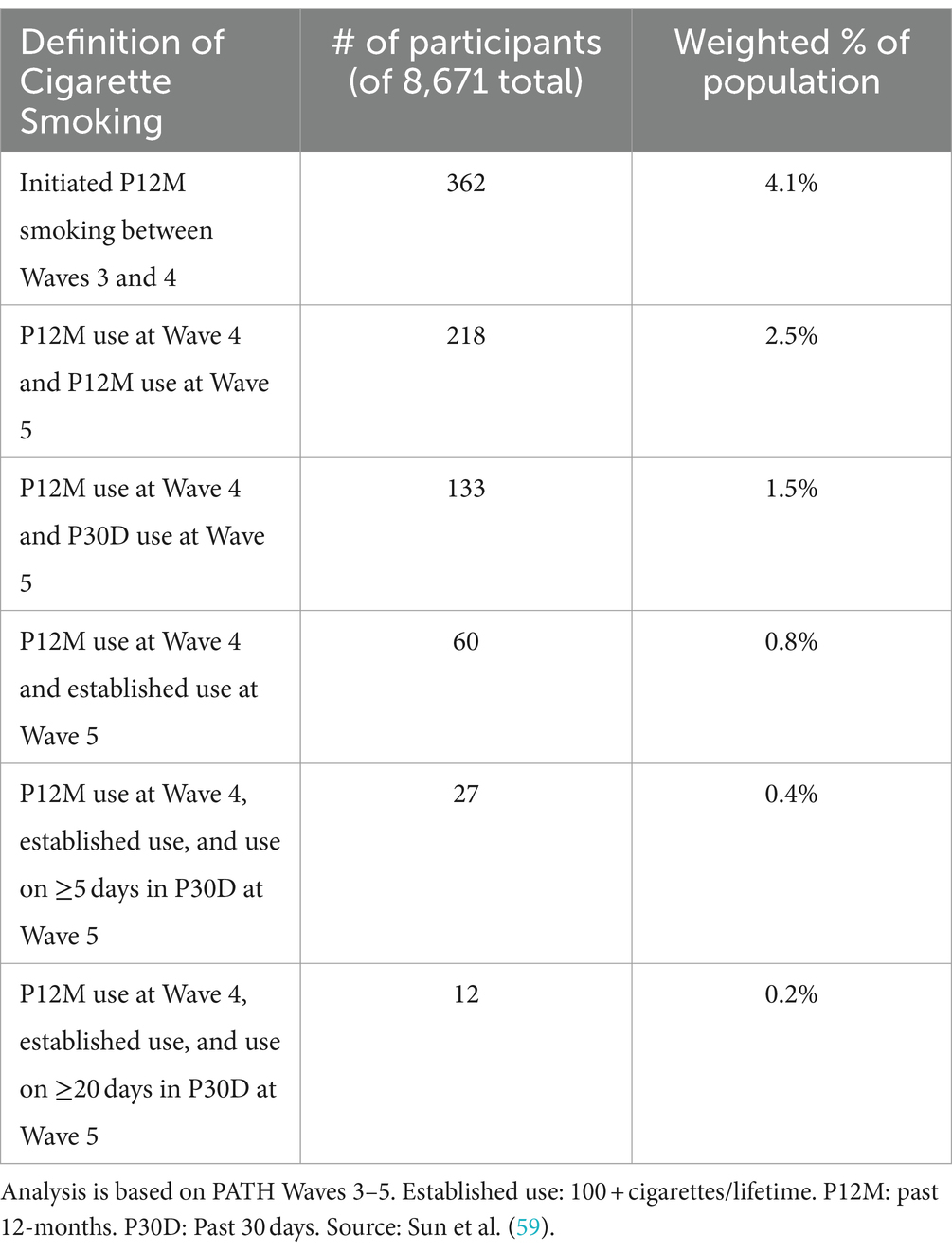
Table 2. Examples of increasingly stringent measures of use, from Sun et al. (59).
Overall, more stringent measures such as daily and persistent use better isolate truly problematic use patterns. The concerns about long-term health effects and nicotine dependence (24, 67) are moot if initial experimentation does not evolve into regular, long-term use. Even for tobacco cigarettes – which pose significantly greater risks than e-cigarettes (1, 2) – stopping smoking before the age of 40 has been shown to substantially reduce risks of dying from smoking-related diseases (62).
2.2 Recommendations for future research
Ongoing research is needed on which measures of youth e-cigarette use may best distinguish transient, experimental use from truly problematic patterns of use (i.e., high daily consumption, frequent use, and/or long duration of use). Table 2 shows the importance of assessing continuous e-cigarette use over long time periods; however, many youth surveys are cross-sectional in nature, and cannot prospectively assess persistent use. Future research could examine the accuracy of retrospective self-reported duration or persistent use. Additionally, alternative definitions could include measures of e-cigarette daily use intensity, such as number of puffs or puffing sessions per day. Xie et al. recently validated number of puffs per month against cravings and low intention to quit (68); future research is needed to further validate against dependence scales and subsequent use patterns (especially frequent and persistent use), and on how to most accurately collect intensity data (e.g., self-reports vs. data collected with digital tracking tools).
Another consideration is that use patterns differ between cigarettes and e-cigarettes, which may impact the relevance of different measures of use. For example, a “use occasion” for a cigarette is typically finishing an entire cigarette, but e-cigarettes are often consumed in smaller amounts but more frequently – a pattern that has been described as “grazing” (69, 70). These different use patterns, along with the above inability to consistently distinguish exposure levels of low-level smoking vs. non-use, demonstrate that measures of use should not be assumed to be equivalent across products. Similarly, dependence measures cannot be assumed to be equivalent across products, and in fact in some cases are shown to be incomparable (71).
2.3 Limitations
There are many considerations, sometimes conflicting, in how to best assess “use.” For example, validation against biochemical exposures vs. clinical outcomes identifies different self-report variables as important (CPD vs. duration, respectively). Measures of use are probabilistic and imperfect: even daily use (which we identify as likely relevant to health outcomes) will capture some youth who will not persist to established, long-term use; and will miss others who do at a later point in time. Further complications arise from standardizing measures of use across the diversity of e-cigarette products, such as differences in nicotine delivery and possible harmful exposures due to product characteristics (e.g., freebase nicotine vs. nicotine salts, different nicotine concentration, device power, and flavors). Much remains unknown about the validity of different definitions, and pros and cons must be weighed – which we aim here to elucidate.
3 Conclusion
It is regrettable that the metrics currently employed to evaluate youth e-cigarette usage have been directly borrowed from those used for cigarette smoking in adults, without re-evaluating whether their validity holds for a different product (with different use patterns and a much lower risk profile) and in a different population (whose use patterns are more often transient and experimental). Definitions of use that are more indicative of truly problematic measures of use must include criteria for continuous use over some time, cumulative lifetime use, and frequent use. Methods offering objective and precise data collection about the intensity of e-cigarette use (e.g., # puffs) like digital tracking tools, mobile applications and sensor technology are likely to be most valuable, though additional validation work is needed. More generally, the wide range of measures currently used has a correspondingly wide range of prevalence estimates; low thresholds have greater “capture” and may evoke emotional responses that are not grounded in quantification of the actual risks to individual and public health. Forthcoming research, therefore, would benefit from providing a “data interpretation guide” that specifies the relevance of each study’s selected measure(s) of use to individual and public health.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
AS: Writing – review & editing, Writing – original draft, Project administration, Investigation, Conceptualization. MR: Writing – review & editing. RP: Writing – review & editing, Supervision, Investigation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
AS is an employee of PinneyAssociates, Inc., which provides consulting services on tobacco harm reduction to Juul Labs, Inc (JLI). AS also individually provides consulting services on behavioral science to the Center of Excellence for the Acceleration of Harm Reduction (CoEHAR) through ECLAT Srl, which received funding from the Foundation for a Smoke-Free World (FSFW). JLI and FSFW had no role in this manuscript. MR is a full tenured professor of Pediatrics at the University of Catania (Italy) and Director of the Pediatric Clinic and the Postgraduate Training Program in Pediatrics at the same University; he is also Director of the Regional Referral Centre for Expanded Newborn Screening (ENS), for Neurometabolic Diseases and for Rare Diseases of the Nervous System in Childhood at the University Hospital of Catania [AOU “Policlinico”, PO “G. Rodolico”]; he is also the current President of the Italian Society of Pediatric Neurology (Società Italiana di Neurologia Pediatrica, SINP) and Delegate of the National Council of the Italian Society of Pediatrics (Società Italiana di Pediatria, SIP). He has received grants from the Italian Ministry of Health (PON, POS-T4), the National Institute of Health (Bethesda, US), the Medical Research Council (MRC, Oxford, UK), and the National Health System (NHS, Oxford, UK); he also received grants from Alexion, Sanofi, and Takeda. He received fees, as member of advisory boards and consulting services from Alexion, Sanofi, and Jazz pharma. He received textbooks royalties from Springer Nature Group and Elsevier and from EDRA. He is also a pro bono advisor for the Italian Lay Groups for Neurofibromatosis [ANF, Associazione NeuroFibromatosi, Parma, Italy], Tuberous Sclerosis [AST, Associazione Sclerosi Tuberosa, Rome, Italy], and Sturge-Weber syndrome [Associazione Struge-Weber Italia, Turin, Italy], and for Hypomelanosis of Ito [Ito Foundation, UK]. RP is a full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same University. He has received grants from U-BIOPRED and AIR-PROM, Integral Rheumatology & Immunology Specialists Network (IRIS), Foundation for a Smoke Free World, Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, Merk Sharp & Dohme, Boehringer Ingelheim, Novartis, Arbi Group Srl., Duska Therapeutics, Forest Laboratories, Ministero dell Universita’ e Ricerca (MUR) Bando PNRR 3277/2021 (CUP E63C22000900006) and 341/2022 (CUP E63C22002080006), funded by NextGenerationEU of the European Union (EU), and the ministerial grant PON REACT-EU 2021 GREEN- Bando 3411/2021 by Ministero dell Universita' e (MUR) – PNRR EU Community. He is the founder of the Center for Tobacco Prevention and Treatment (CPCT) at the University of Catania and of the Center of Excellence for the Acceleration of Harm Reduction at the same university. He receives consultancy fees from Pfizer, Boehringer Ingel- heim, Duska Therapeutics, Forest Laboratories, CV Therapeutics, Sermo Inc., GRG Health, Clarivate Analytics, Guidepoint Expert Network, and GLG Group. He receives textbooks royalties from Elsevier. He is also involved in a patent application for ECLAT Srl. He is a pro bono scientific advisor for Lega Italiana Anti Fumo (LIAF) and the International Network of Nicotine Consumers Organizations (INNCO); and he is Chair of the European Technical Committee for Standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4). These funders had no role in this manuscript.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Estimated as: 27.5% of high school students who used e-cigarettes in P30D × 59.1% of P30D users who listed JUUL as usual brand; and 10.5% of middle school students who used e-cigarettes in P30D × 54.1% who listed JUUL as usual brand (22).
2. ^Estimated as 7.7% of all youth who used e-cigarettes in P30D × 3.4% of P30D users who listed JUUL as usual brand (13).
References
1. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems . Public health consequences of E-cigarettes. Washington, DC: The National Academies Press (2018).
2. Office for Health Improvement and Disparities (UK) . Nicotine vaping in England: 2022 evidence update main findings. United Kingdom: Office for Health Improvement and Disparities (2022).
3. Toll, BA, Smith, TT, and King, BA. Nicotine e-cigarettes: considerations for healthcare providers. Nat Med. (2024) 4:7. doi: 10.1038/s41591-024-02926-7
4. Balfour, DJK, Benowitz, NL, Colby, SM, Hatsukami, DK, Lando, HA, Leischow, SJ, et al. Balancing consideration of the risks and benefits of E-cigarettes. Am J Public Health. (2021) 111:1661–72. doi: 10.2105/AJPH.2021.306416
5. Levy, DT, Yuan, Z, Li, Y, Mays, D, and Sanchez-Romero, LM. An examination of the variation in estimates of E-cigarette prevalence among U.S. Int J Environ Res Public Health. (2019) 16:3164. doi: 10.3390/ijerph16173164
6. Dai, H, and Leventhal, AM. Prevalence of e-cigarette use among adults in the United States, 2014-2018. JAMA. (2019) 322:1824–7. doi: 10.1001/jama.2019.15331
7. Huang, J, Duan, Z, Kwok, J, Binns, S, Vera, LE, Kim, Y, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control. (2019) 28:146–51. doi: 10.1136/tobaccocontrol-2018-054382
8. Wang, Y, Duan, Z, Weaver, SR, Popova, L, Spears, CA, Ashley, DL, et al. Consumption of JUUL vs. other E-cigarette brands among U.S. E-cigarette users: evidence from wave 5 of the PATH study. Int J Environ Res Public Health. (2022) 19:837. doi: 10.3390/ijerph191710837
9. Ali, FRM, Seaman, EL, Crane, E, Schillo, B, and King, BA. Trends in US E-cigarette sales and prices by nicotine strength, overall and by product and flavor type, 2017-2022. Nicotine Tob Res. (2023) 25:1052–6. doi: 10.1093/ntr/ntac284
10. Ali, FRM, Seidenberg, AB, Crane, E, Seaman, E, Tynan, MA, and Marynak, K. E-cigarette unit sales by product and flavor type, and top-selling brands, United States, 2020-2022. MMWR Morb Mortal Wkly Rep. (2023) 72:672–7. doi: 10.15585/mmwr.mm7225a1
11. Christen, SE, Hermann, L, Bekka, E, Vonwyl, C, Hammann, F, van der Velpen, V, et al. Pharmacokinetics and pharmacodynamics of inhaled nicotine salt and free-base using an e-cigarette: a randomized crossover study. Nicotine Tob Res. (2024) 10:ntae074. doi: 10.1093/ntr/ntae074
12. Action on Smoking and Health . (2023). Use of e-cigarettes (vapes) among young people in Great Britain. Available at: https://ash.org.uk/uploads/Use-of-vapes-among-young-people-GB-2023-v2.pdf
13. Birdsey, J, Cornelius, M, Jamal, A, Park-Lee, E, Cooper, MR, Wang, J, et al. Tobacco product use among U.S. middle and high school students - National Youth Tobacco Survey, 2023. MMWR Morb Mortal Wkly Rep. (2023) 72:1173–82. doi: 10.15585/mmwr.mm7244a1
14. Asthma and Respiratroy Foundation of New Zealand . A 2021 report into youth vaping: The ARFNZ/SPANZ vaping in NZ youth survey. Wellington: Asthma and Respiratroy Foundation of New Zealand (2001).
15. Miech, RA, Johnston, LD, Patrick, ME, O'Malley, PM, and Bachman, JG. Monitoring the future national survey results on drug use, 1975–2023: Secondary school students. Ann Arbor, MI: Institute for Social Research, University of Michigan (2023).
16. Levy, DT, Warner, KE, Cummings, KM, Hammond, D, Kuo, C, Fong, GT, et al. Examining the relationship of vaping to smoking initiation among US youth and young adults: a reality check. Tob Control. (2019) 28:629–35. doi: 10.1136/tobaccocontrol-2018-054446
17. Meza, R, Jimenez-Mendoza, E, and Levy, DT. Trends in tobacco use among adolescents by grade, sex, and race, 1991-2019. JAMA Netw Open. (2020) 3:27465. doi: 10.1001/jamanetworkopen.2020.27465
18. Selya, AS, and Foxon, F. Trends in electronic cigarette use and conventional smoking: quantifying a possible 'diversion' effect among US adolescents. Addiction. (2021) 116:1848–58. doi: 10.1111/add.15385
19. Sokol, NA, and Feldman, JM. High school seniors who used E-cigarettes may have otherwise been cigarette smokers: evidence from monitoring the future (United States, 2009-2018). Nicotine Tob Res. (2021) 23:1958–61. doi: 10.1093/ntr/ntab102
20. Wagner, LM, and Clifton, SM. Modeling the public health impact of e-cigarettes on adolescents and adults. Chaos. (2021) 31:113137. doi: 10.1063/5.0063593
21. Selya, A, Shiffman, S, and Hannon, MJ. Youth patterns of use of electronic nicotine delivery systems (ENDS), population assessment of tobacco and health (PATH) waves 4-5.5. Addict Behav. (2023) 145:107783. doi: 10.1016/j.addbeh.2023.107783
22. Cullen, KA, Gentzke, AS, Sawdey, MD, Chang, JT, Anic, GM, Wang, TW, et al. E-cigarette use among youth in the United States, 2019. JAMA. (2019) 322:2095–103. doi: 10.1001/jama.2019.18387
23. US Food and Drug Administration . (2020). Enforcement priorities for electronic nicotine delivery systems (“ENDS”) and other deemed products on the market without premarket authorization 2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-priorities-electronic-nicotine-delivery-system-ends-and-other-deemed-products-market
24. Cooper, M, Park-Lee, E, Ren, C, Cornelius, M, Jamal, A, and Cullen, KA. Notes from the field: E-cigarette use among middle and high school students - United States, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1283–5. doi: 10.15585/mmwr.mm7140a3
25. Park-Lee, E, Ren, C, Sawdey, MD, Gentzke, AS, Cornelius, M, Jamal, A, et al. Notes from the field: E-cigarette use among middle and high school students - National Youth Tobacco Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1387–9. doi: 10.15585/mmwr.mm7039a4
26. Abouk, R, Courtemanche, C, Dave, D, Feng, B, Friedman, AS, Maclean, JC, et al. Intended and unintended effects of e-cigarette taxes on youth tobacco use. J Health Econ. (2023) 87:102720. doi: 10.1016/j.jhealeco.2022.102720
27. Cotti, C, Courtemanche, C, Maclean, JC, Nesson, E, Pesko, MF, and Tefft, NW. The effects of e-cigarette taxes on e-cigarette prices and tobacco product sales: evidence from retail panel data. J Health Econ. (2022) 86:102676. doi: 10.1016/j.jhealeco.2022.102676
28. Pesko, MF, Courtemanche, CJ, and Maclean, JC. The effects of traditional cigarette and e-cigarette tax rates on adult tobacco product use. J Risk Uncertain. (2020) 60:229–58. doi: 10.1007/s11166-020-09330-9
29. Gentzke, AS, Wang, TW, Jamal, A, Park-Lee, E, Ren, C, Cullen, KA, et al. Tobacco product use among middle and high school students - United States, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1881–8. doi: 10.15585/mmwr.mm6950a1
30. Levy, DT, Cadham, CJ, Yuan, Z, Li, Y, Gravely, S, and Cummings, KM. Comparison of smoking prevalence in Canada before and after nicotine vaping product access using the SimSmoke model. Can J Public Health. (2023) 114:992–1005. doi: 10.17269/s41997-023-00792-3
31. Levy, DT, Sánchez-Romero, LM, Li, Y, Yuan, Z, Travis, N, Jarvis, MJ, et al. England SimSmoke: the impact of nicotine vaping on smoking prevalence and smoking-attributable deaths in England. Addiction. (2021) 116:1196–211. doi: 10.1111/add.15269
32. Levy, DT, Sánchez-Romero, LM, Travis, N, Yuan, Z, Li, Y, Skolnick, S, et al. US nicotine vaping product SimSmoke simulation model: the effect of vaping and tobacco control policies on smoking prevalence and smoking-attributable deaths. Int J Environ Res Public Health. (2021) 18:4876. doi: 10.3390/ijerph18094876
33. Walker, N, Parag, V, Wong, SF, Youdan, B, Broughton, B, Bullen, C, et al. Use of e-cigarettes and smoked tobacco in youth aged 14-15 years in New Zealand: findings from repeated cross-sectional studies (2014-19). Lancet Public Health. (2020) 5:e204–12. doi: 10.1016/S2468-2667(19)30241-5
34. Patrick, DL, Cheadle, A, Thompson, DC, Diehr, P, Koepsell, T, and Kinne, S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. (1994) 84:1086–93. doi: 10.2105/AJPH.84.7.1086
35. Wong, SL, Shields, M, Leatherdale, S, Malaison, E, and Hammond, D. Assessment of validity of self-reported smoking status. Health Rep. (2012) 23:47–53.
36. Eisner, MD, Balmes, J, Yelin, EH, Katz, PP, Hammond, SK, Benowitz, N, et al. Directly measured secondhand smoke exposure and COPD health outcomes. BMC Pulm Med. (2006) 6:1–11. doi: 10.1186/1471-2466-6-12
37. Lei, T, Li, M, Zhu, Z, Yang, J, Hu, Y, and Hua, L. Comprehensive evaluation of serum cotinine on human health: novel evidence for the systemic toxicity of tobacco smoke in the US general population. Sci Total Environ. (2023) 892:164443. doi: 10.1016/j.scitotenv.2023.164443
38. Theilen, LH, McNeil, RB, Hunter, S, Grobman, WA, Parker, CB, Catov, JM, et al. Serum cotinine and adverse cardiovascular outcomes: a cross-sectional secondary analysis of the nuMoM2b heart health study. Am J Perinatol. (2021) 40:1311–20. doi: 10.1055/a-1580-3155
39. Petitti, DB, Friedman, GD, and Kahn, W. Accuracy of information on smoking habits provided on self-administered research questionnaires. Am J Public Health. (1981) 71:308–11. doi: 10.2105/AJPH.71.3.308
40. Pleasants, RA, Rivera, MP, Tilley, SL, and Bhatt, SP. Both duration and pack-years of tobacco smoking should be used for clinical practice and research. Ann Am Thorac Soc. (2020) 17:804–6. doi: 10.1513/AnnalsATS.202002-133VP
41. Lubin, JH, and Caporaso, NE. Cigarette smoking and lung cancer: Modeling Total exposure and intensity. Cancer Epidemiol Biomarkers Prev. (2006) 15:517–23. doi: 10.1158/1055-9965.EPI-05-0863
42. Centers for Disease Control and Prevention . (2022). Behavioral risk factor surveillance system (BRFSS) survey data and documentation. Available at: https://www.cms.gov/about-cms/agency-information/omh/resource-center/hcps-and-researchers/data-tools/sgm-clearinghouse/brfss
43. Statistics NCIH . (2023). National Health Interview Survey (NHIS) questionnaire. Available at: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Survey_Questionnaires/NHIS/2023/EnglishQuest-508.pdf
44. Currivan, D, Nyman, AL, Turner, CF, and Biener, L. Does telephone audio computer-assisted self-interviewing improve the accuracy of prevalence estimates of youth smoking? Evidence from the UMass tobacco study. Public Opin Q. (2004) 68:542–64. doi: 10.1093/poq/nfh039
45. Dolcini, MM, Adler, NE, Lee, P, and Bauman, KE. An assessment of the validity of adolescent self-reported smoking using three biological indicators. Nicotine Tob Res. (2003) 5:473–83. doi: 10.1080/1462220031000118586
46. Control CfD, Prevention . Vital signs: current cigarette smoking among adults aged< GT>/= 18 years---United States, 2005--2010. MMWR. Morb Mortal Wkly Rep. (2011) 2011:102–3. doi: 10.1016/j.ypdi.2011.01.006
47. Dierker, L, and Mermelstein, R. Early emerging nicotine-dependence symptoms: a signal of propensity for chronic smoking behavior in adolescents. J Pediatr. (2010) 156:818–22. doi: 10.1016/j.jpeds.2009.11.044
48. Zhan, W, Dierker, LC, Rose, JS, Selya, A, and Mermelstein, RJ. The natural course of nicotine dependence symptoms among adolescent smokers. Nicotine Tob Res. (2012) 14:1445–52. doi: 10.1093/ntr/nts031
49. Dierker, L, Hedeker, D, Rose, J, Selya, A, and Mermelstein, R. Early emerging nicotine dependence symptoms in adolescence predict daily smoking in young adulthood. Drug Alcohol Depend. (2015) 151:267–71. doi: 10.1016/j.drugalcdep.2015.03.009
50. Selya, AS, Dierker, L, Rose, JS, Hedeker, D, and Mermelstein, RJ. Early-emerging nicotine dependence has lasting and time-varying effects on adolescent smoking behavior. Prev Sci. (2016) 17:743–50. doi: 10.1007/s11121-016-0673-0
51. Kaplan, B, Alrumaih, F, Breland, A, Eissenberg, T, and Cohen, JE. A comparison of product dependence among cigarette only, ENDS only, and dual users: findings from wave 3 (2015–2016) of the PATH study. Drug Alcohol Depend. (2020) 217:108347. doi: 10.1016/j.drugalcdep.2020.108347
52. Shiffman, S, and Sembower, MA. Dependence on e-cigarettes and cigarettes in a cross-sectional study of US adults. Addiction. (2020) 115:1924–31. doi: 10.1111/add.15060
53. Cornelius, ME, Loretan, CG, Jamal, A, Davis Lynn, BC, Mayer, M, Alcantara, IC, et al. Tobacco product use among adults - United States, 2021. MMWR Morb Mortal Wkly Rep. (2023) 72:475–83. doi: 10.15585/mmwr.mm7218a1
54. National Cancer Institute . (2021). Tobacco use supplement to the current population survey (TUS-CPS) data dictionary. Available at: https://cancercontrol.cancer.gov/brp/tcrb/tus-cps
55. National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), Center for Tobacco Products (CTP) at Food and Drug Administration (FDA) . (2004). Population assessment of tobacco and health (PATH) study series. Available at: https://www.icpsr.umich.edu/web/NAHDAP/series/606.
56. Kasza, KA, Hammond, D, Reid, JL, Rivard, C, and Hyland, A. Youth use of e-cigarette flavor and device combinations and brands before vs after FDA enforcement. JAMA Netw Open. (2023) 6:e2328805. doi: 10.1001/jamanetworkopen.2023.28805
57. Delnevo, CD, Lewis, MJ, Kaufman, I, and Abatemarco, DJ. Defining cigarette smoking status in young adults: a comparison of adolescent vs adult measures. Am J Health Behav. (2004) 28:374–80. doi: 10.5993/AJHB.28.4.9
58. Park-Lee, E, Ren, C, Cooper, M, Cornelius, M, Jamal, A, and Cullen, KA. Tobacco product use among middle and high school students - United States, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1429–35. doi: 10.15585/mmwr.mm7145a1
59. Sun, R, Méndez, D, and Warner, KE. Association of Electronic Cigarette use by US adolescents with subsequent persistent cigarette smoking. JAMA Netw Open. (2023) 6:e234885. doi: 10.1001/jamanetworkopen.2023.4885
60. Stanton, CA, Bansal-Travers, M, Johnson, AL, Sharma, E, Katz, L, Ambrose, BK, et al. Longitudinal e-cigarette and cigarette use among US youth in the PATH study (2013-2015). J Natl Cancer Inst. (2019) 111:1088–96. doi: 10.1093/jnci/djz006
61. Hair, EC, Do, EK, Liu, SM, Tulsiani, S, Vallone, DM, and Pierce, JP. Patterns of daily cigarette and E-cigarette use among United States youth and young adults: insights from the truth longitudinal cohort between 2018 and 2019. Prev Med Rep. (2023) 36:102416. doi: 10.1016/j.pmedr.2023.102416
62. Doll, R, Peto, R, Boreham, J, and Sutherland, I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. (2004) 328:1519. doi: 10.1136/bmj.38142.554479.AE
63. King, BA . Flavors remain a major driver of youth E-cigarette use. Am J Public Health. (2022) 112:999–1000. doi: 10.2105/AJPH.2022.306895
64. Villanti, AC, Pearson, JL, Glasser, AM, Johnson, AL, Collins, LK, Niaura, RS, et al. Frequency of youth E-cigarette and tobacco use patterns in the United States: measurement precision is critical to inform public health. Nicotine Tob Res. (2017) 19:1345–50. doi: 10.1093/ntr/ntw388
65. Brouwer, AF, Jeon, J, Jimenez-Mendoza, E, Land, SR, Holford, TR, Friedman, AS, et al. Changing patterns of cigarette and ENDS transitions in the USA: a multistate transition analysis of youth and adults in the PATH study in 2015–2017 vs 2017–2019. Tob Control. (2023) 28:57905. doi: 10.1136/tc-2022-057905
66. Roberts, ME, Singer, JM, Lu, B, Wagner, DD, Wold, LE, Qiang, R, et al. The case of young people who use e-cigarettes infrequently: who is this population? What becomes of them? Drug Alcohol Depend. (2024) 259:111316. doi: 10.1016/j.drugalcdep.2024.111316
67. Zeller, M . Evolving “the real cost” campaign to address the rising epidemic of youth e-cigarette use. Am J Prev Med. (2019) 56:S76–8. doi: 10.1016/j.amepre.2018.09.005
68. Xie, C, Jeffers, AM, and Winickoff, JP. Categorizing vaping intensity among youth. Nicotine Tob Res. (2024). doi: 10.1093/ntr/ntae003
69. Dawkins, L, Turner, J, Roberts, A, and Soar, K. ‘Vaping’profiles and preferences: an online survey of electronic cigarette users. Addiction. (2013) 108:1115–25. doi: 10.1111/add.12150
70. Dowd, AN, John, L, Betts, JM, Belsare, P, Sazonov, E, and Tiffany, ST. An examination of objective and self-report measures of ad libitum electronic cigarette use: identifying patterns of puffing behavior and evaluating self-report items. Nicotine Tob Res. (2023) 25:1391–9. doi: 10.1093/ntr/ntad037
Keywords: adolescents, behavior, e-cigarettes, electronic nicotine delivery systems, nicotine use, surveillance
Citation: Selya A, Ruggieri M and Polosa R (2024) Measures of youth e-cigarette use: strengths, weaknesses and recommendations. Front. Public Health. 12:1412406. doi: 10.3389/fpubh.2024.1412406
Edited by:
Susan M. Snyder, Georgia State University, United StatesReviewed by:
Vijay Sivaraman, North Carolina Central University, United StatesCopyright © 2024 Selya, Ruggieri and Polosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arielle Selya, YXNlbHlhQHBpbm5leWFzc29jaWF0ZXMuY29t
 Arielle Selya
Arielle Selya Martino Ruggieri
Martino Ruggieri Riccardo Polosa
Riccardo Polosa