- 119 to Zero Inc, Calgary, AB, Canada
- 2Community Health Sciences, University of Calgary, Calgary, AB, Canada
- 3School of Public Health and Health Policy, The City University of New York, New York, NY, United States
Introduction: The COVID-19 pandemic has greatly impacted the way that the world views vaccines. While safe and effective, COVID-19 vaccines were, and continue to be met with hesitancy and misinformation. We aimed to understand public perceptions and trust in COVID-19 vaccinations and how the pandemic has impacted perceptions of non-COVID-19 vaccines.
Methods: Survey data were collected between August 7, 2023–August 16, 2023, from 7,000 respondents aged 18 years and older from the United States (n = 1,000); Nigeria (n = 1,000); United Kingdom (n = 1,000); France (n = 1,000); Canada (n = 1,000); Brazil (n = 1,000); and India (n = 1,000).
Results: Trust in COVID-19 vaccines was highest in Brazil (84.6%) and India (80.4%) and lowest in the United States (63.5%) and France (55.0%). 47.5% of respondents agreed that they trust traditional protein-based vaccines more than mRNA vaccines, 13.5% disagree and 39.0% are neutral about their trust in protein-based versus mRNA vaccines. Overall, 53.9% of respondents reported that the COVID-19 pandemic impacted their perceptions of vaccines with half of these respondents (51.7%) reporting that the pandemic made them think that other vaccines are more important as they understand how critical vaccines can be at preventing serious illnesses.
Discussion: These data can be used by health system decision makers, public health and researchers to understand how vaccine trust impacts perceptions of COVID-19 and influenza vaccines globally and develop tailored interventions that address local concerns.
1 Introduction
Public trust in the safety and efficacy of vaccines is essential to the success of immunization programs globally (1). Trust is often described as the key influence on vaccine acceptance (2) and it impacts not only personal health outcomes, but also the broader landscape of public health (2, 3). Vaccine trust extends beyond individual confidence in the safety and efficacy of a vaccine; rather it includes trust in the institutions that oversee its development, regulation, and administration. The interplay between perceived vaccine quality and safety, coupled with the credibility of the institutions endorsing the vaccine, significantly impact an individual’s likelihood of receiving a vaccine (2). A strong foundation of trust can bolster vaccine uptake, contributing to the achievement of herd immunity and the prevention of widespread infectious diseases. Interpersonal trust refers to the confidence an individual has in those directly responsible for communicating about and administering the vaccine. Personal characteristics including race, socioeconomic status, level of education, and religion profoundly affect interpersonal trust. These attributes influence who an individual interacts with to obtain information regarding vaccines and further shapes their views. This often results in increased interaction with those who validate their own perspectives (2). Trust in health care providers and trust in government confidence are strong drivers of vaccine acceptance across multiple countries and regions (4).
The global landscape of vaccine trust is characterized by a myriad of factors, including cultural, socioeconomic, political, and historical influences (5). Each country presents a unique set of circumstances that can either foster or challenge public confidence in vaccination efforts. Understanding these nuances can help tailor public health communication. Previous COVID-19 global surveys have shown large variation in vaccine acceptance across countries ranging from 47.9% in South Africa to 98.3% in India (6). Despite the disproportionate challenges in vaccine availability and distribution faced by low- and middle-income countries (LMICs), these countries tend to exhibit lower levels of vaccine hesitancy and higher acceptance rates than higher-income countries (7). Previous research shows that perceived susceptibility to COVID-19 infection, severity of complications, and believed benefit are associated with a higher intention to vaccinate (8). Meanwhile, people with concerns about the efficacy and side effects of COVID-19 vaccines are less likely to have a positive vaccination intent (8). As the world grapples with the challenges posed by COVID-19, understanding the dynamics of vaccine trust becomes paramount, not only for this virus but also in shaping broader attitudes toward other respiratory vaccinations, such as influenza vaccines. A recent review indicated that COVID-19 has increased intention to get influenza vaccinations (9). However, there are also reports of decreased influenza vaccination in healthcare personnel throughout the COVID-19 pandemic which is hypothesized to be due to COVID-19 vaccination campaigns leading to less emphasis on influenza vaccination or vaccine fatigue (10). Investigating the interconnectedness of vaccine trust and its repercussions on broader immunization initiatives can shed light on the potential ripple effects of building or eroding public trust.
Various research indicates that trust is integral to vaccine confidence; however, what type of trust has been up for debate. Trust in experts, scientists, medical authorities and medical professionals appears to have a small to moderate effect (11–13). Trust in government shows variation in the effect with a 25-sample study finding non-significant effects on vaccine confidence (13). Interestingly, a 19-country study (14) and an 8-country study (12) found significant effects of trust in government on vaccine acceptance. Finally, Rozek et al.’s (15) 17 country survey, found that trust in health institutions is significant but no effect for trust in political leaders.
The Vaccine Trust Gauge was developed from a previous scoping review (5) to create a standardized approach to measuring trust in vaccines (4). This validated and reliable tool includes perception of vaccine safety, efficacy, and importance, while also inquiring about trust in information sources (16). This paper uses the vaccine trust gauge to delve into the intricate interplay of vaccine trust on a global scale, with a specific focus on COVID-19 and influenza vaccines. By examining patterns of trust across different countries, including Canada, Brazil, France, India, the United States, the United Kingdom, and Nigeria, we aim to unravel the factors influencing public perception.
2 Methods
We conducted an observational cross-sectional survey to explore how vaccine trust differs across countries and the relationship between overall vaccine trust and perceptions of COVID-19 and influenza vaccines.
2.1 Survey instruments
The survey instrument contained 4 parts: (1) demographic questions; (2) the Vaccine Trust Gauge (4); (3) COVID-19 vaccine related questions and (4) Influenza vaccines-related questions. Demographic questions included sex, age, education, and average yearly income. The Vaccine Trust Gauge is a series of questions that measure overall vaccine trust levels and has a high internal reliability (Cronbach’s alpha =0.947) (16). Additional questions related to perceptions about COVID-19 and influenza vaccines were also included in the survey instrument based on the recommendations of public health and infectious disease physicians. These questions were adapted from previous studies and focused on the knowledge, perceived safety and efficacy and intention to receive COVID-19 and influenza vaccinations (6, 14, 17). The full survey instrument can be found in the Supplementary material.
2.2 Recruitment and data collection
Survey data were collected between 7 August - 16 August 2023 from N = 7,000 respondents aged 18 years and older from the United States (n = 1,000); Nigeria (n = 1,000); United Kingdom (n = 1,000); France (n = 1,000); Canada (n = 1,000); Brazil (n = 1,000); and India (n = 1,000). An online opt-in panel of participants was provided by Consensus Strategies and participants were recruited by telephone contact, social media outreach and direct email solicitation. Social media outreach was complete by posting recruitment materials on social media platforms (X and Instagram) where participants were directed toward the online survey. The online survey was available in English, French, Portuguese and Hindi based on the predominant languages in each country. A stratum-based sample design was implemented based on age, gender, statistical regions, median income, and levels of education for each country; a minimum of 50 participants was set for each stratum, with target enrollment calculated to reflect the distribution of each subgroup in the general population of each country. This survey was administered by Emerson College, located in Boston, U.S.A. No personally identifiable information was collected or stored. This approach has been utilized in previous literature to recruit a random sample of the population (6). This project was reviewed and deemed exempt from research by Emerson College’s Institutional Review Board (protocol number 22-019-F-X).
2.3 Analysis
Descriptive statistics were calculated for all variables. Similar to previous papers using the Vaccine Trust Gauge (4), scores from the Vaccine Trust Gauge survey questions were aggregated and then converted to a 0.0–1.0 scale with 0.0 representing no trust at all and 1.0 representing complete trust. The Vaccine Trust Gauge scores were then categorized into high, medium and low trust levels using 0.33 intervals. Multinomial logistic regressions were conducted to explore the association between demographic characteristics, COVID-19 vaccine perspectives and influenza vaccine perspectives and vaccine trust levels. Responses for COVID-19 and influenza vaccine perspectives were categorized as agree (strongly agree and somewhat agree), neutral, and disagree (strongly disagree and somewhat disagree). All analyses were conducted in SAS version 9.4 software.
3 Results
3.1 Demographics
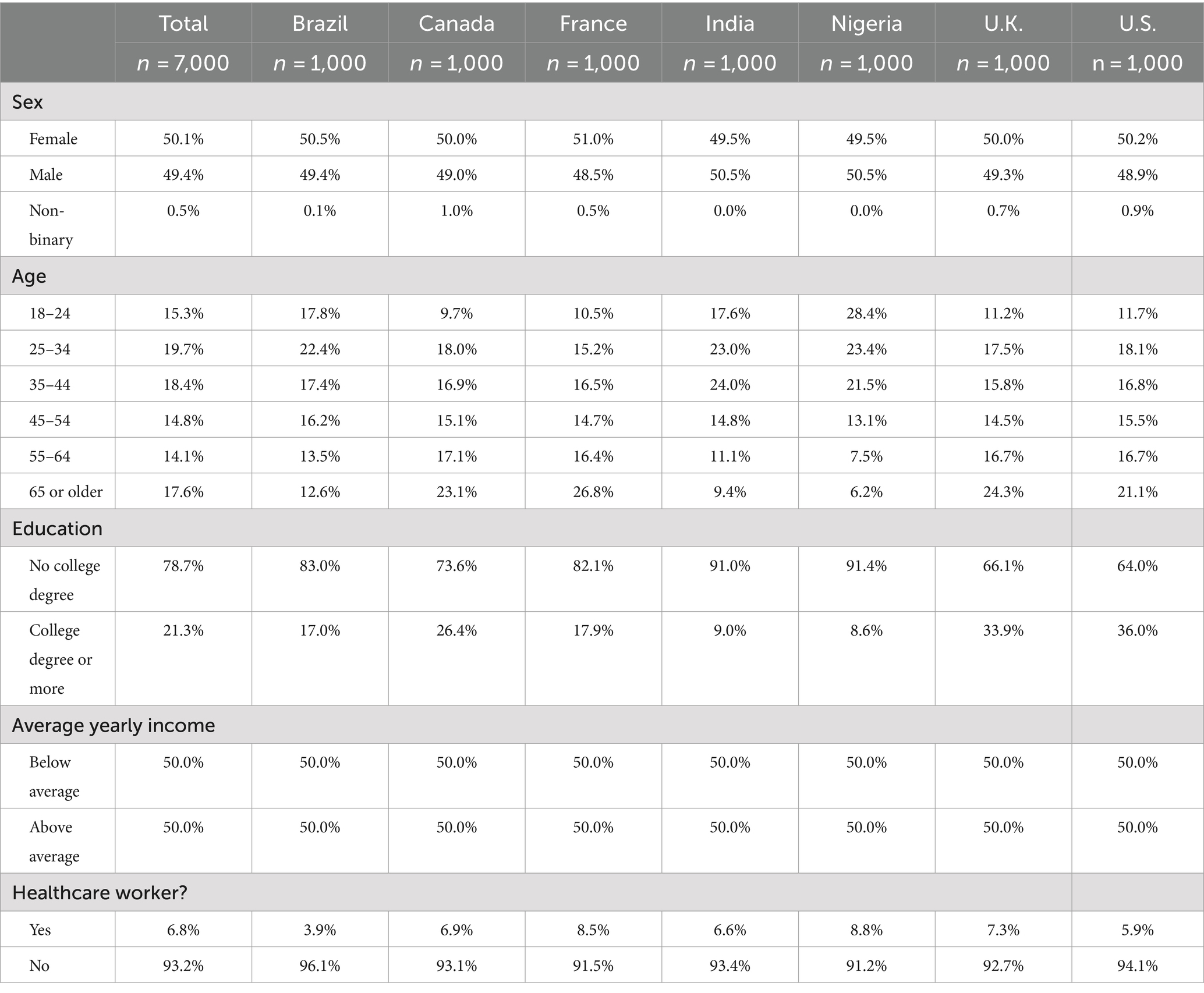
A total of 7,000 people responded to the survey including 1,000 people from Brazil, Canada, France, India, Nigeria, the United Kingdom, and the United States. Women comprised 50.1% of the study population, and 50.0% of all participants earned less than the average median income while 50% earned above the median income. One in five participants had a university degree. Respondent characteristics by country are listed in Table 1.
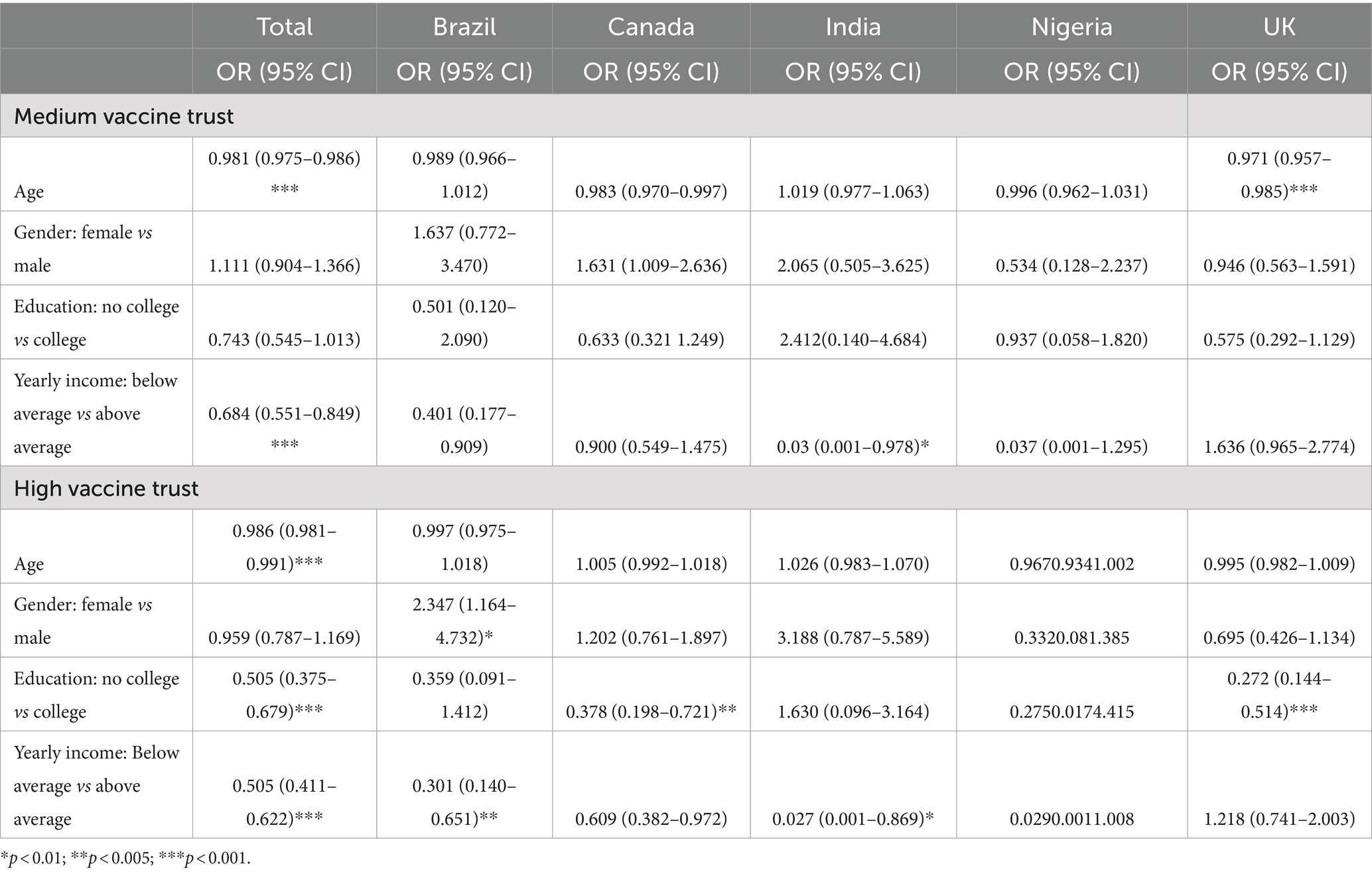
Multinomial logistic regression showed the association between demographic characteristics and vaccine trust levels. The odds of having high vaccine trust decrease by 1.4% for every one-year increase in age (p < 0.001). Having no college education significantly decreases the odds of having high vaccine trust by 49.5% (p < 0.001). Having below-average yearly income significantly decreases the odds of having high vaccine trust by 49.5% (p < 0.001). The odds ratios (OR) and 95% confidence intervals (CI) for each predictor variable are presented in Table 2.
3.2 Vaccine trust levels by country
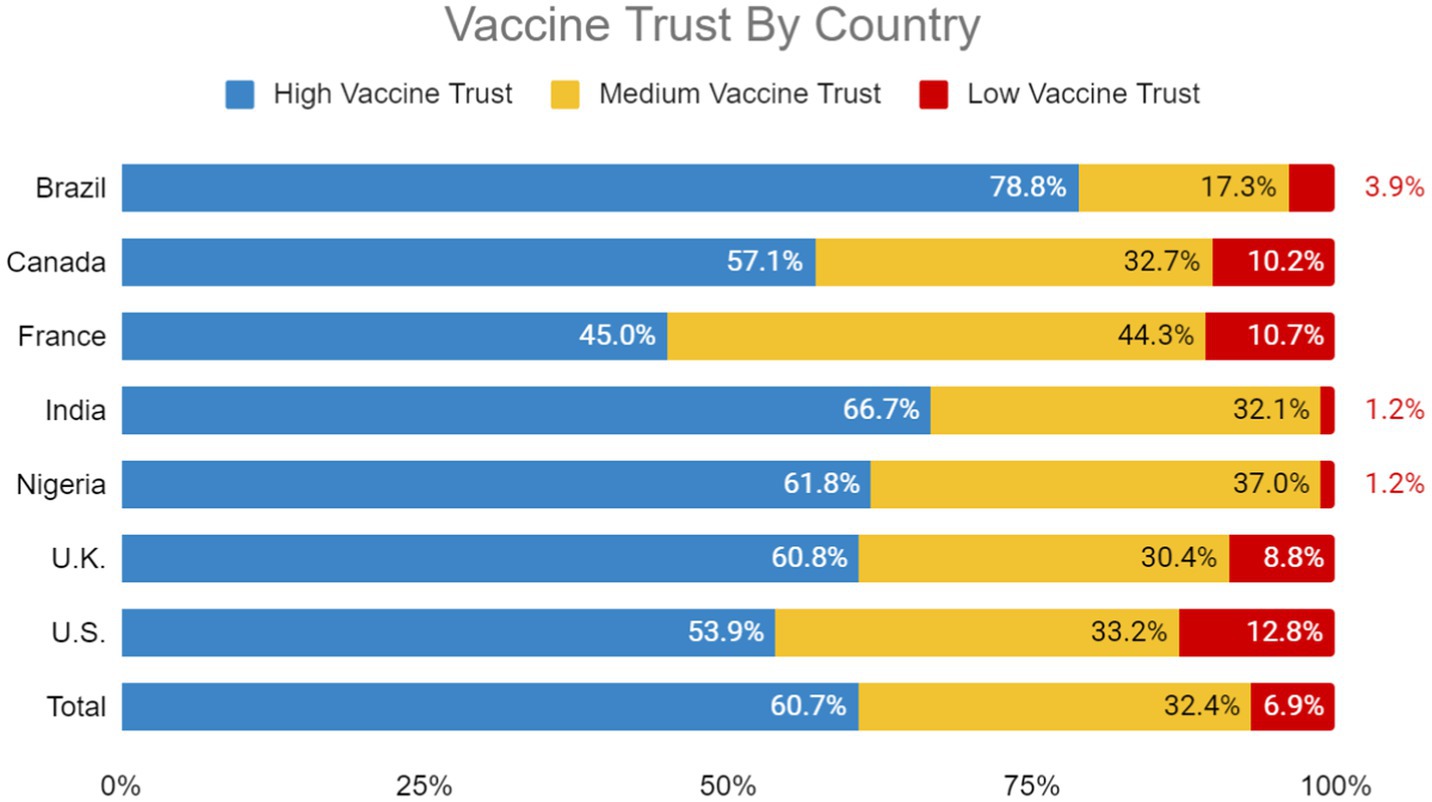
Brazil (78.8%), India (66.7%), Nigeria (61.8%) and the UK (60.8%) had the highest proportion of participants with high vaccine trust. The United States (12.8%), France (10.7%), Canada (10.2%) and the UK (8.8%) had the highest proportion of participants with low vaccine trust (Figure 1).
3.3 COVID-19 vaccine perceptions
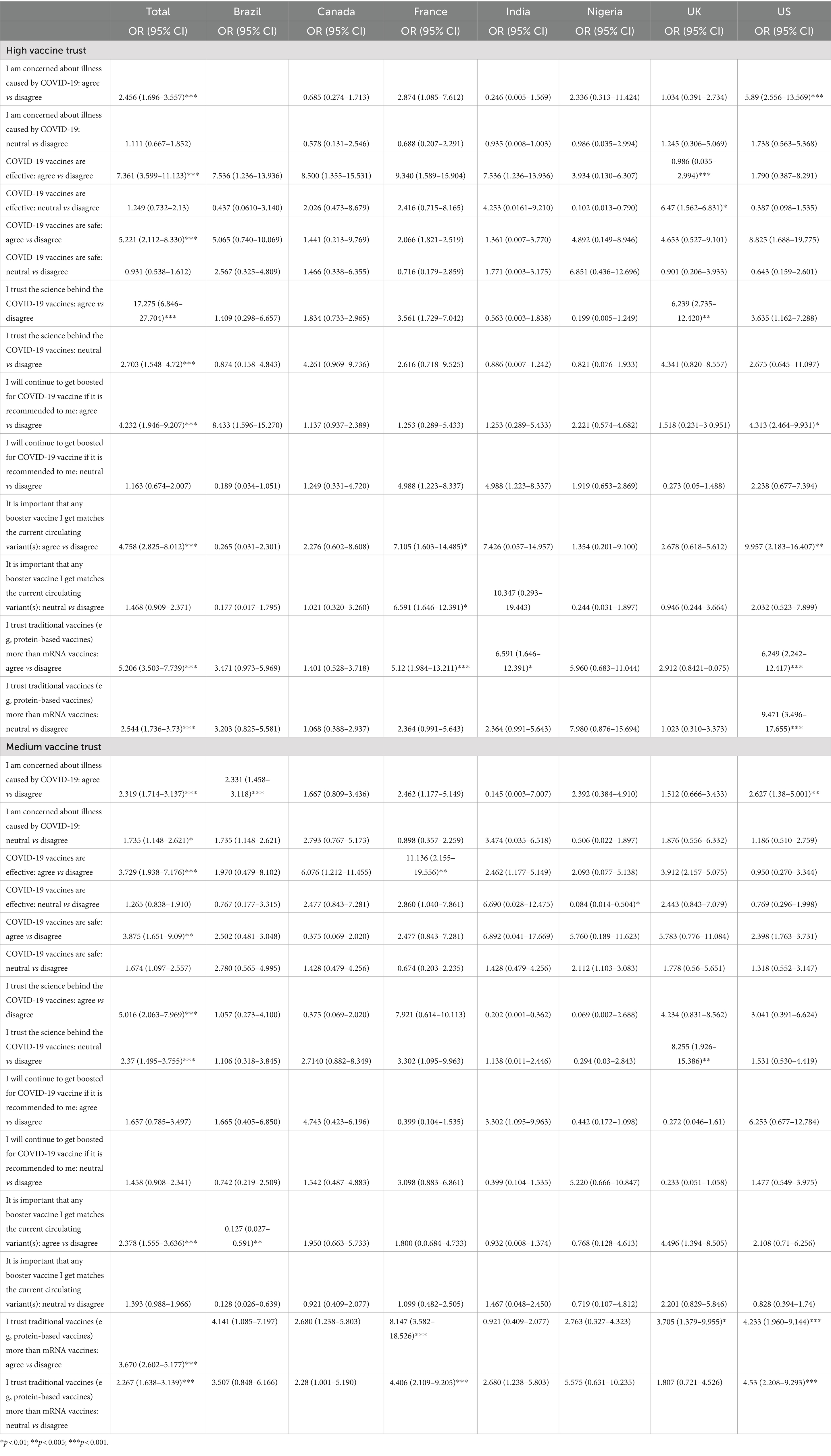
Overall, Brazil (82.9%), Nigeria (68.6%) and India (56.8%) were the countries most concerned about illnesses caused by COVID-19. They were also the countries with the most trust in the safety, efficacy and science behind COVID-19 vaccines. Brazil and India had the highest proportion of people who would continue to receive additional booster vaccines and reported the importance of ensuring the booster matches the current strain of COVID-19. 47.5% of respondents agreed that they trust traditional protein-based vaccines more than mRNA vaccines, 13.5% disagree and 39.0% are neutral about their trust in protein-based versus mRNA vaccines (Figure 2).
India (80.2%), Brazil (71.6%) and Nigeria (67.8%) were the countries that most reported that the pandemic and their knowledge of COVID-19 vaccines changed their perspectives on other vaccines. Of those that said the pandemic impacted their perception of vaccines, increased concerns about the efficacy (72.7%) and safety (68.2%) of vaccines were the main impact of the pandemic on vaccine perceptions.
Table 3 shows the association between vaccine trust levels and COVID-19 perspectives. Those with high vaccine trust were more likely to agree with COVID-19 vaccine confidence sentiments regardless of country. Participants who were concerned about illness caused by COVID-19 had 2.5 times higher odds of having high vaccine trust compared to those who disagreed (p < 0.001). Participants who believe that COVID-19 vaccines are effective had 7.4 times higher odds of having high vaccine trust compared to those who disagreed (p < 0.001). Participants who agreed that COVID-19 vaccines are safe had 5.2 times higher odds of having high vaccine trust compared to those who disagreed (p < 0.001). Participants who agreed that they trust the science behind COVID-19 vaccines had 5.2 times higher odds of having high vaccine trust compared to those who disagreed (p < 0.001). Participants who agreed that they trust traditional vaccines more than mRNA vaccines had 17.2 times higher odds of having high vaccine trust compared to those who disagreed (p < 0.001).
3.4 Influenza vaccine perceptions
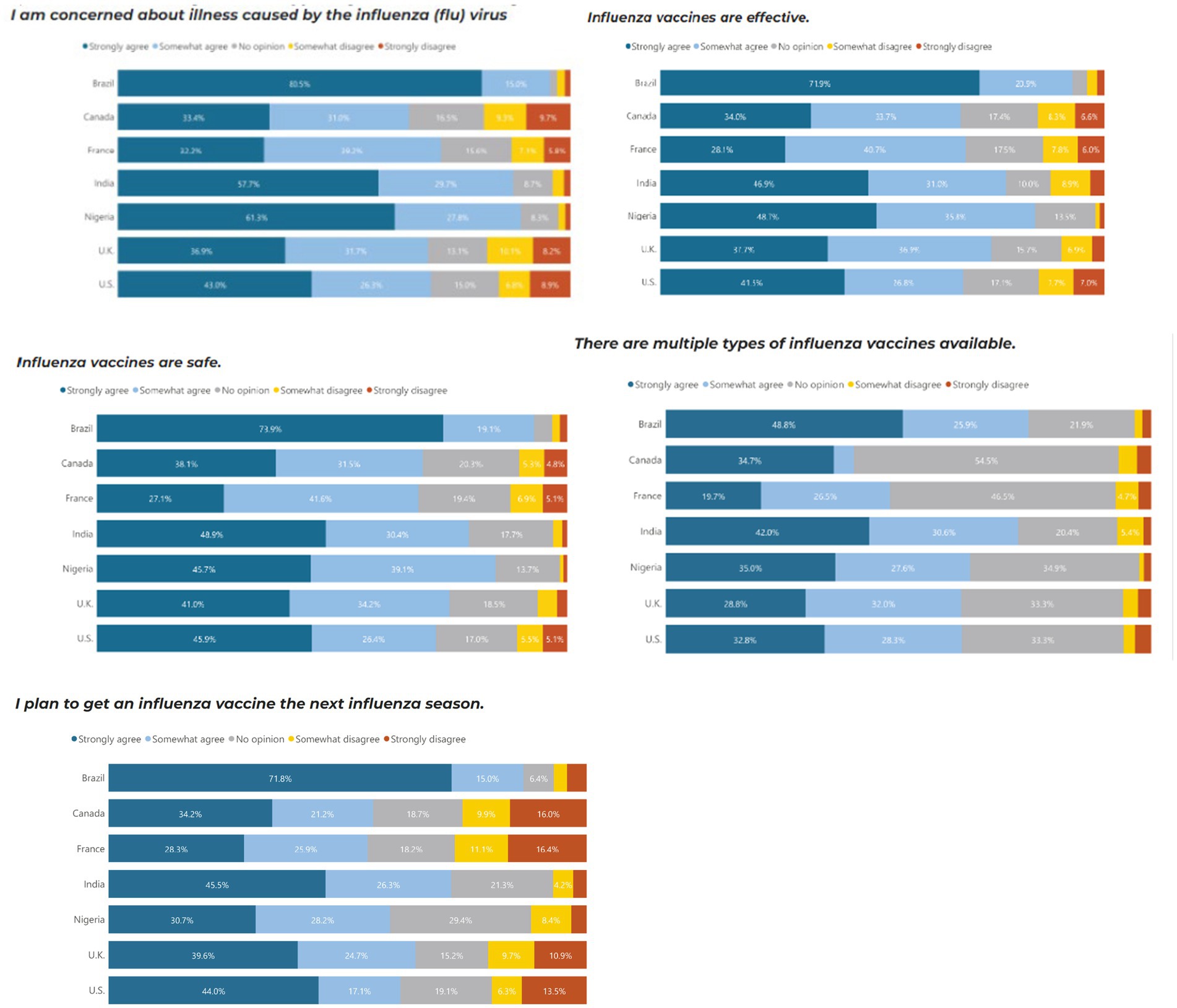
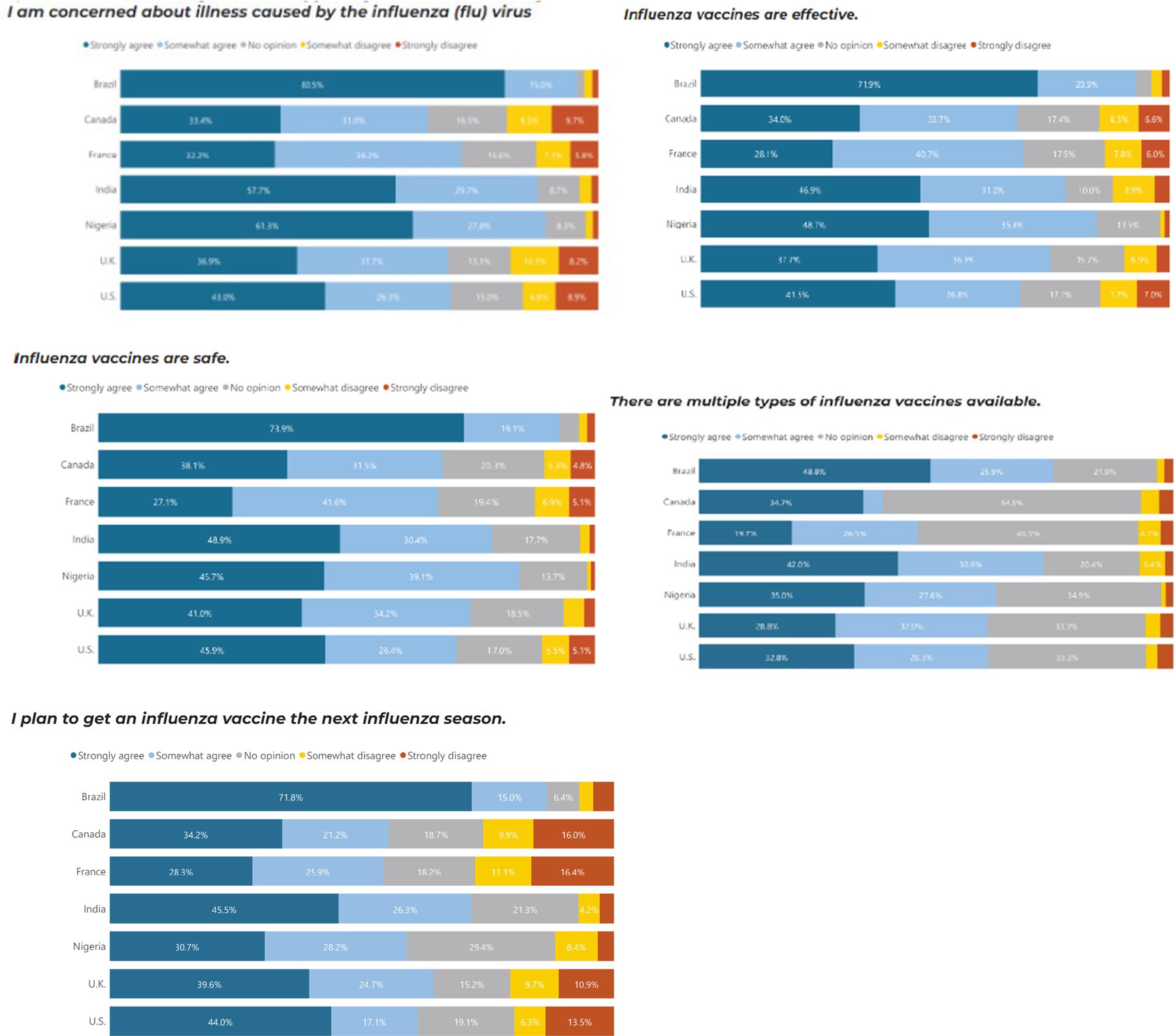
Similar to COVID-19, Brazil (80.5%), Nigeria (61.3%) and India (57.5%) were the countries most concerned about illnesses caused by influenza, and they were also the countries with the most trust in the safety, efficacy and science behind COVID-19 vaccines. In comparison, Brazil and Nigeria, the UK and the US were marginally more concerned about COVID-19 than influenza. Brazil and India had the highest proportion of people who would continue to receive additional booster vaccines and reported the importance of ensuring the booster matches the current strain of COVID-19 (Figure 3).
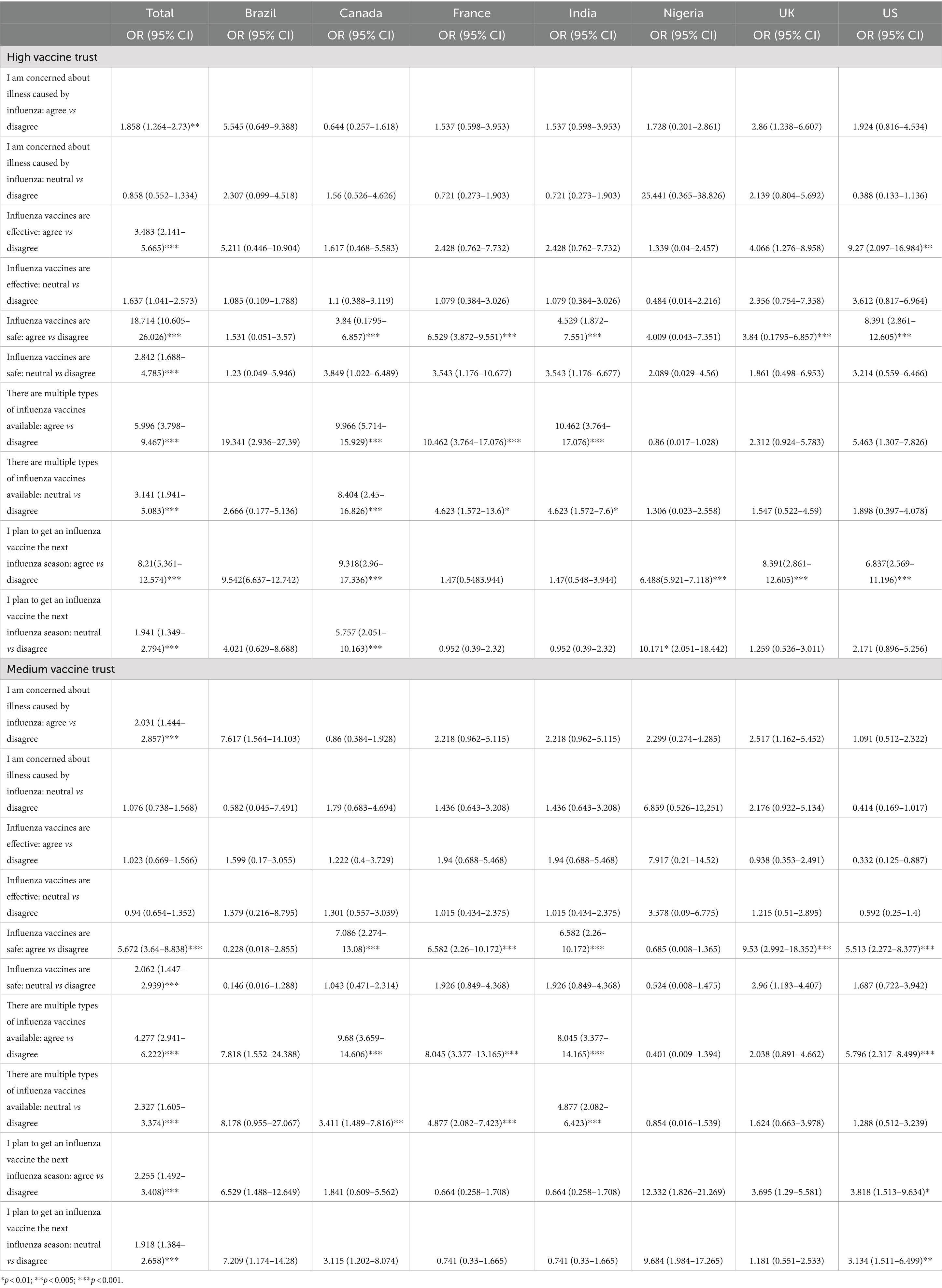
Those with high vaccine trust were more likely to agree with influenza vaccine confidence sentiments regardless of country. Individuals with a high level of concern about illness caused by the influenza virus have 1.9 times higher odds of having high vaccine trust compared to those with low vaccine trust (p = 0.002). Individuals who agree that influenza vaccines are effective have 3.5 times higher odds of having high vaccine trust compared to those with low vaccine trust (p < 0.001). Individuals who agree that influenza vaccines are safe have 18.7 times higher odds of having high vaccine trust compared to those with low vaccine trust (p < 0.001). Individuals who agree that they plan to get an influenza vaccine in the next season have significantly higher odds of having high vaccine trust compared to those who disagree (p < 0.001) (Table 4).
4 Discussion
We found a wide range of variation in vaccine trust across Brazil, Canada, France, India, Nigeria, the United Kingdom, and the United States. Like previous global surveys on vaccine confidence (14), lower- and middle-income countries like India, Nigeria and Brazil tended to have high vaccine trust which was strongly associated with positive perspectives of COVID-19 and influenza vaccines. We found variations in the strength of these associations between countries. A country’s income level often correlates with vaccine uptake since perceptions of vaccine efficacy can vary significantly depending on the country’s economic context. Within high-income countries, elevated distrust in vaccine efficacy may be attributed to the belief in conspiracy theories, institutional distrust in vaccine administration, distribution, and marketing (7).
Our results parallel other research that shows that demographic characteristics like older age, people with no college education and those with lower incomes were less likely to have high vaccine trust (6, 18). These intersecting personal attributes that shape an individual’s perceptions are a main driving force in vaccine acceptability and likelihood of opting to get vaccinated (2). Our findings align with previous research, showcasing that individuals with greater levels of education, minimal financial hardship, and firsthand experience with COVID-19 demonstrate greater inclination to trust science, which is associated with a higher likelihood of vaccine uptake (19). Understanding these associations can aid in tailoring immunization campaigns to specific population characteristics and needs For example, understanding vaccine trust in older age groups may help to better tailor immunization programs to older adult populations who may be at higher risk of being hospitalized if they contracted COVID-19, influenza or other respiratory illnesses (20). Given that populations exhibit varying levels of trust in vaccines, when factoring in dimensions such as race, ethnicity, political affiliation, and religion, it is essential to recognize the heterogeneity within these groups (21). The diverse intersections of identities within these subpopulation can result in a variety of perspectives on vaccination, further underscoring the importance for tailored interventions that address the specific nuances of these communities.
Data suggests that intent to get a COVID-19 booster vaccine decreased from 87.9 to 71.6% in 2023 which is a cause for concern across the globe (17). However, the same study also showed that about 60% of people are more willing to get vaccinated for other non-COVID-19 vaccines due to their experiences throughout the pandemic (17). Our study showed that a higher level of concern about influenza, a strong belief in the effectiveness and safety of influenza vaccines, awareness of multiple vaccine types, and intention to get vaccinated are all associated with significantly higher odds of having high vaccine trust. A low-risk perception of COVID-19 was seen within underserved communities. It’s hypothesized that navigating the COVID-19 infodemic has led to misconceptions, and negative attitudes toward vaccination which have impacted underserved communities (22). A lack of access to trustworthy information coupled with socio-economic challenges present within underserved communities may hinder health literacy and reduce trust in public health efforts. As a result, underserved communities are disproportionately susceptible to misinformation and less inclined to recognize the advantages associated with vaccination (23, 24).
Almost half of all respondents agreed that they trust traditional protein-based vaccines more than mRNA vaccines, and around 40% are neutral. While both mRNA and traditional vaccines have been found to be safe and efficacious for COVID-19 (25), there is still a public preference for protein-based vaccines or no preference at all. Leveraging people’s concerns about COVID-19 and influenza instead of focusing on vaccine technology has been suggested as more beneficial (26). Differences in vaccine preferences can be attributed to overall availability in vaccines within an individual’s respective country. These protein-based vaccines are more prevalent in LMICs due to mRNA vaccines requiring specific infrastructure to adhere to cold-chain protocols (27). This poses a challenge to rural areas as they may not have the capacity to utilize mRNA vaccines on a widespread scale (27). Further expanding on the educational efforts to provide supplemental information regarding mRNA vaccines to individuals residing in lower-income countries is vital in increasing overall vaccine uptake when available (28).
Our results raise crucial questions about the determinants and potential implications for public health strategies. Increasing overall vaccine trust may be the key to improving respiratory vaccine uptake (17). Instead of focusing on marketing individual respiratory vaccines, efforts spent promoting overall vaccine trust may have positive implications for improving COVID-19 and influenza vaccine trust. Utilizing community-based interventions to build mutualistic relationships between vaccine providers and their associated community can provide trust building opportunities, and result in greater rates of vaccine uptake (29). Identifying specific concerns, building trust in healthcare systems, and improving communication strategies may also contribute to fostering a positive perception of vaccines. Furthermore, lessons can be learned from countries with high vaccine trust to inform best practices and potential strategies for enhancing public confidence in vaccinations.
Our study has limitations given that the survey was taken at one point in time in August 2023, after the World Health Organization officially declared the COVID-19 pandemic “over” and does not reflect the changing landscape of vaccinations. However, the survey was conducted in all seven countries at the same time, which allows us to compare the different perspectives at the same point in time. A strength of the project was using a stratum-based sample design which resulted in a sample that best represents the entire population of each country.
5 Conclusion
These findings show that there are differences in vaccine trust across the world. Therefore, tailoring information to the individual context may be valuable for public health immunization programs. Additionally, we found that overall vaccine trust levels are associated with confidence in COVID-19 and influenza vaccines. By understanding variations across countries, public health officials can develop targeted and culturally sensitive messaging that enhances the likelihood of successful vaccine uptake within specific communities. These differences highlight the importance of context-specific considerations and the need for comprehensive cross-cultural analysis to refine public health strategies and interventions tailored to each country’s unique circumstances. Tailoring interventions can ultimately contribute to achieving higher vaccination rates and fostering a more resilient and responsive global health landscape.
Data availability statement
All relevant data are presented in the article/Supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The requirement of ethical approval was waived by Emerson College Human Research Subjects Committee/Institutional Review Board (IRB) for the studies involving humans because this project was reviewed and deemed exempt from research by Emerson College’s Institutional Review Board (protocol number 22-019-F-X). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this project was reviewed and deemed exempt from research by Emerson College’s Institutional Review Board (protocol number 22-019-F-X).
Author contributions
CD: Data curation, Formal analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. MF: Conceptualization, Writing – original draft, Writing – review & editing. JH: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. KR: Writing – original draft, Writing – review & editing. SR: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this project was provided by a research grant from Investigator-Initiated Studies Program of Merck Canada Inc. The opinions expressed in this paper are those of the authors and do not represent those of Merck Canada Inc.
Acknowledgments
We would like to thank the team at Emerson College Polling for conducting the data collection for this project.
Conflict of interest
CD, MF, and JH are employed by 19 to Zero Inc., the sponsors of this special topic.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1406861/full#supplementary-material
References
1. Cooper, LZ, Larson, HJ, and Katz, SL. Protecting public trust in immunization. Pediatrics. (2008) 122:149–53. doi: 10.1542/peds.2008-0987
2. Adhikari, B, Cheah, PY, and Von Seidlein, L. Trust is the common denominator for COVID-19 vaccine acceptance: a literature review. Vaccine X. (2022) 12:100213. doi: 10.1016/j.jvacx.2022.100213
3. Sapienza, A, and Falcone, R. The role of trust in COVID-19 vaccine acceptance: considerations from a systematic review. Int J Environ Res Public Health. (2022) 20:665. doi: 10.3390/ijerph20010665
4. Masoud, D, Pierz, A, Rauh, L, Cruz, AK, Palmedo, C, and Ratzan, S. Vaccine trust gauge: mixed methods research to measure and understand vaccine trust. Vaccine. (2023) 41:4616–24. doi: 10.1016/j.vaccine.2023.06.025
5. Larson, HJ, Clarke, RM, Jarrett, C, Eckersberger, E, Levine, Z, Schulz, WS, et al. Measuring trust in vaccination: a systematic review. Hum Vaccin Immunother. (2018) 14:1599–609. doi: 10.1080/21645515.2018.1459252
6. Lazarus, JV, Wyka, K, White, TM, Picchio, CA, Gostin, LO, Larson, HJ, et al. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat Med. (2023) 29:366–75. doi: 10.1038/s41591-022-02185-4
7. Arce, JSS, Warren, SS, Meriggi, NF, Scacco, A, McMurry, N, Voors, M, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. (2021) 27:1385–94. doi: 10.1038/s41591-021-01454-y
8. Mahmud, I, Al Imam, MH, Vinnakota, D, Kheirallah, KA, Jaber, MF, Abalkhail, A, et al. Vaccination intention against COVID-19 among the unvaccinated in Jordan during the early phase of the vaccination drive: a cross-sectional survey. Vaccine. (2022) 10:1159. doi: 10.3390/vaccines10071159
9. Kong, G, Lim, NA, Chin, YH, Ng, YPM, and Amin, Z. Effect of COVID-19 pandemic on influenza vaccination intention: a meta-analysis and systematic review. Vaccine. (2022) 10:606. doi: 10.3390/vaccines10040606
10. Lymon, H. Declines in influenza vaccination coverage among health care personnel in acute care hospitals during the COVID-19 pandemic—United States, 2017–2023. MMWR Morb Mortal Wkly Rep. (2023) 72:1244–7. doi: 10.15585/mmwr.mm7245a6
11. Allington, D, McAndrew, S, Moxham-Hall, V, and Duffy, B. Coronavirus conspiracy suspicions, general vaccine attitudes, trust and coronavirus information source as predictors of vaccine hesitancy among UK residents during the COVID-19 pandemic. Psychol Med. (2023) 53:236–47. doi: 10.1017/S0033291721001434
12. Lindholt, MF, Jørgensen, F, Bor, A, and Petersen, MB. Public acceptance of COVID-19 vaccines: cross-national evidence on levels and individual-level predictors using observational data. BMJ Open. (2021) 11:e048172. doi: 10.1136/bmjopen-2020-048172
13. Kerr, JR, Schneider, CR, Recchia, G, Dryhurst, S, Sahlin, U, Dufouil, C, et al. Correlates of intended COVID-19 vaccine acceptance across time and countries: results from a series of cross-sectional surveys. BMJ Open. (2021) 11:e048025. doi: 10.1136/bmjopen-2020-048025
14. Lazarus, JV, Ratzan, SC, Palayew, A, Gostin, LO, Larson, HJ, Rabin, K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. (2021) 27:225–8. doi: 10.1038/s41591-020-1124-9
15. Rozek, LS, Jones, P, Menon, A, Hicken, A, Apsley, S, and King, EJ. Understanding vaccine hesitancy in the context of COVID-19: the role of trust and confidence in a seventeen-country survey. Int J Public Health. (2021) 66:636255. doi: 10.3389/ijph.2021.636255
16. Pierz, M. P. H., Masoud, M. P. H., Cruz, M. S., Kate, A., Ratzan, M. D., and Rauh, M. P. H. (2022). Scale for trust and intention to vaccinate (strive): mixed methods research to understand the role of Trust in Vaccine Decision-Making. In 16th vaccine congress (Vol. 2).
17. Lazarus, JV, White, TM, Wyka, K, Ratzan, SC, Rabin, K, Larson, HJ, et al. Influence of COVID-19 on trust in routine immunization, health information sources and pandemic preparedness in 23 countries in 2023. Nat Med. (2024) 30:1559–63. doi: 10.1038/s41591-024-02939-2
18. Nguyen, KH, Chen, Y, Huang, J, Allen, JD, Beninger, P, and Corlin, L. Who has not been vaccinated, fully vaccinated, or boosted for COVID-19? Am J Infect Control. (2022) 50:1185–9. doi: 10.1016/j.ajic.2022.05.024
19. Carrieri, V, Guthmuller, S, and Wübker, A. Trust and COVID-19 vaccine hesitancy. Sci Rep. (2023) 13:9245. doi: 10.1038/s41598-023-35974-z
20. Marín-Hernández, D, Schwartz, RE, and Nixon, DF. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J Med Virol. (2021) 93:64–5. doi: 10.1002/jmv.26120
21. Keselman, A, Smith, CA, Wilson, AJ, Leroy, G, and Kaufman, DR. Cognitive and cultural factors that affect general vaccination and COVID-19 vaccination attitudes. Vaccine. (2022) 11:94. doi: 10.3390/vaccines11010094
22. Kawuki, J, Chen, S, Fang, Y, Liang, X, Chan, PS, and Wang, Z. COVID-19 vaccine acceptance, attitude and perception among slum and underserved communities: a systematic review and Meta-analysis. Vaccine. (2023) 11:886. doi: 10.3390/vaccines11050886
23. Hooper, MW, Nápoles, AM, and Pérez-Stable, EJ. No populations left behind: vaccine hesitancy and equitable diffusion of effective COVID-19 vaccines. J Gen Intern Med. (2021) 36:2130–3. doi: 10.1007/s11606-021-06698-5
24. Newman, PA, Dinh, DA, Nyoni, T, Allan, K, Fantus, S, Williams, CC, et al. Covid-19 vaccine hesitancy and under-vaccination among marginalized populations in the United States and Canada: a scoping review. J Racial Ethn Health Disparities. (2023) 1–22. doi: 10.1007/s40615-023-01882-1
25. Sandoval, C, Guerrero, D, Muñoz, J, Godoy, K, Souza-Mello, V, and Farías, J. Effectiveness of mRNA, protein subunit vaccine and viral vectors vaccines against SARS-CoV-2 in people over 18 years old: a systematic review. Expert Rev Vaccines. (2023) 22:35–53. doi: 10.1080/14760584.2023.2156861
26. Moore, R, Purvis, RS, Hallgren, E, Willis, DE, Hall, S, Reece, S, et al. Motivations to vaccinate among hesitant adopters of the COVID-19 vaccine. J Community Health. (2022) 47:237–45. doi: 10.1007/s10900-021-01037-5
27. Fox, AM, Choi, Y, and Lin, L. Substantial disparities in COVID-19 vaccine uptake and unmet immunization demand in low- and middle-income countries. Health Aff. (2023) 42:1697–705. doi: 10.1377/hlthaff.2023.00729
28. Lupu, D, and Tiganasu, R. Does education influence COVID-19 vaccination? A global view. Heliyon. (2024) 10:e24709. doi: 10.1016/j.heliyon.2024.e24709
29. Barrett, KA, Feldman, J, Trent, J, Weerasinghe, A, Lebovic, G, Yan, S, et al. COVID-19 vaccine confidence in Ontario and strategies to support capability, opportunity, and motivation among at risk populations. JournaL Science Briefs of the Ontario COVID-19 Science Advisory Table 2. (2021) 47. doi: 10.47326/ocsat.2021.02.47.1.0
Keywords: vaccine trust, COVID-19, vaccine confidence, immunization, mRNA
Citation: D’Silva C, Fullerton MM, Hu J, Rabin K and Ratzan SC (2024) A global survey to understand general vaccine trust, COVID-19 and influenza vaccine confidence. Front. Public Health. 12:1406861. doi: 10.3389/fpubh.2024.1406861
Edited by:
Tsitsi Masvawure, Worcester Polytechnic Institute, United StatesReviewed by:
Monica Lynn Schoch-Spana, Johns Hopkins University, United StatesAbu-Hena Kamal, Khulna University of Engineering & Technology, Bangladesh
Copyright © 2024 D’Silva, Fullerton, Hu, Rabin and Ratzan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chelsea D’Silva, Q2hlbHNlYS5kc2lsdmFAMTl0b3plcm8uY2E=
 Chelsea D’Silva
Chelsea D’Silva Madison M. Fullerton1,2
Madison M. Fullerton1,2 Jia Hu
Jia Hu Kenneth Rabin
Kenneth Rabin Scott C. Ratzan
Scott C. Ratzan