- 1Department of Anatomy and Histology, School of Preclinical Medicine, Chengdu University, Chengdu, Sichuan, China
- 2Clinical Medical College & Affiliated Hospital of Chengdu University, Chengdu, Sichuan, China
- 3The First Affiliated Hospital of Xi'an Jiaotong University, Xi’an, Shannxi, China
- 4Department of Pediatrics, Zhongshan Hospital of Xiamen University, Xiamen University, Xiamen, Fujian, China
Background: The aim of this study was to investigate the accuracy of self-tested SARS-CoV-2 rapid antigen tests.
Methods: Databases of Pubmed, Embase, and Cochrane Library were searched for original studies investigating accuracy of self-tested SARS-CoV-2 rapid antigen tests, with RT-PCR as “gold standard.”
Results: Forty-five eligible studies were found after database searching and screening using pre-defined criteria. The accuracy results from 50,897 suspected COVID-19 patients were pooled, and the overall sensitivity, specificity and diagnostic odds ratio were 0.77, 1.00, and 625.95, respectively. Subgroup analysis showed higher sensitivity of rapid antigen tests in subgroups of Abbott Panbio, self-collected nasal swab samples, and use of nasopharyngeal or oropharyngeal swab and lower Ct cutoff value in RT-PCR.
Conclusion: Fully self-performed SARS-CoV-2 rapid antigen tests showed overall high accuracy compared to “gold standard,” and are reliable surrogates for the standard test of COVID-19 using nasopharyngeal or oropharyngeal samples and RT-PCR.
1 Introduction
During the COVID-19 pandemic, the rapidly spreading of the disease has casted a significant burden on healthcare systems, including the fast-growing numbers of patients in hospitals and overwhelming need for SARS-CoV-2 testing. The gold standard testing method of SARS-CoV-2 is reverse transcription-polymerase chain reaction (RT-PCR), which requires trained professional personnel to perform. In order to ease the overwhelming COVID-19 testing burden on healthcare systems, governments of many countries worldwide recommended the use of rapid antigen tests (1). Different from RT-PCR, rapid antigen tests require minimal training, and therefore allow self-testing by suspected patients. Using a long nasal swab, suspected COVID-19 patients are allowed to self-collect a fluid sample. After being dissolved in reaction buffer, the sample is then added into a test cassette containing antibody-coated nitrocellulose membrane, and visually detectable results could be obtained in less than 30 min (2). This is much shorter than RT-PCR which usually takes a couple of hours to finish. These advantages of rapid antigen tests made them very useful during the COVID-19 pandemic, and potentially in possible future disease pandemics.
Although with many advantages, the testing accuracy of rapid antigen tests has not been fully validated. This has attracted the interest of researchers. Many rapid antigen tests have been tested for their accuracy performance compared to the gold standard (RT-PCR), including Abbott BinaxNOW assay (3), Abbott Panbio COVID-19 Ag Test (4), Access Bio CareStart COVID-19 Antigen Test (5), Boson Rapid SARS-CoV-2 antigen test card (6), Roche-SD Biosensor Rapid SARS-CoV-2 Antigen Test (7), QuickNavi-COVID19 Ag kit (8), Quidel Sofia SARS IFA antigen assay (9), FAST COVID-19 SARS-CoV-2 Antigen Rapid Test Kit (10), INDICAID COVID-19 rapid antigen test (11), and etc. A previous systemic review and meta-analysis by Xie et al. analyzed the diagnostic accuracy of rapid antigen tests for SARS-CoV-2 from data of 166,943 suspected COVID-19 patients, and reported sensitivity of 0.76 and specificity of 1.00 (12). However, the sample types for SARS-Cov-2 testing in this meta-analysis involved nasopharyngeal, nasal, and other types of samples. It is known that collection of nasopharyngeal samples requires professional personnel, and could be not finished solely by the suspected COVID-19 patients themselves. Strictly speaking, rapid antigen tests using nasopharyngeal samples lack one of the key advantages of rapid antigen tests: allow self-testing, and are therefore not so useful in disease pandemics since they cannot truly ease the testing burden of healthcare systems. In addition, Xie’s meta-analysis also included LumiraDx SARS-CoV-2 Antigen Test, which requires special equipment to read out results (13), and therefore does not allow self-testing. In this systemic review and meta-analysis, only nasal samples (allow self-testing) and rapid antigen tests with no requirement of special equipment were involved, and the aim of this study was to analyze the accuracy performance of fully self-tested SARS-CoV-2 rapid antigen tests, which could hopefully help guide the large population of suspected COVID-19 patients when they intend to use SARS-CoV-2 rapid antigen tests.
2 Materials and methods
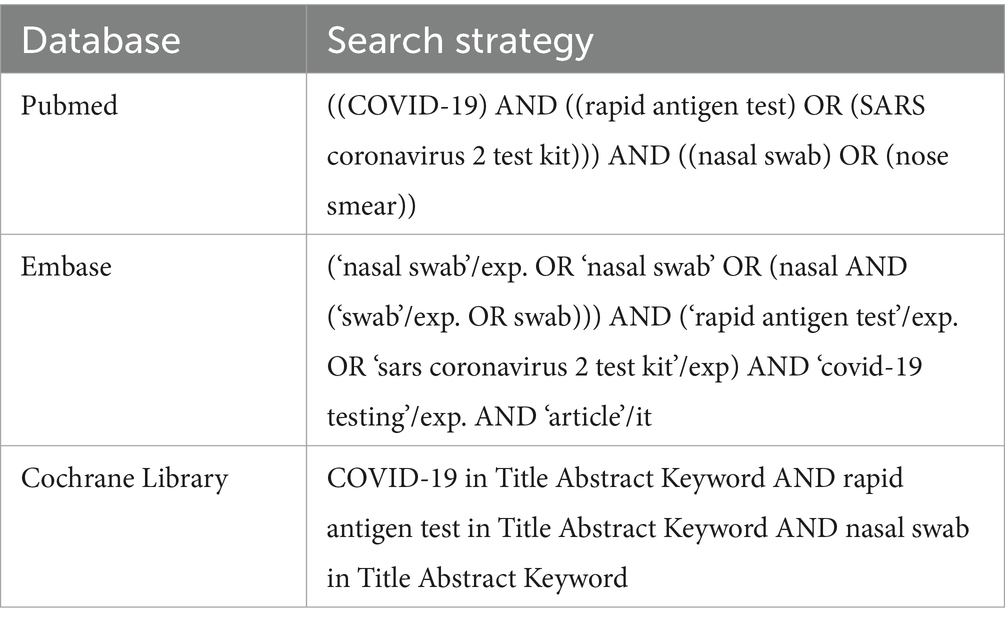
2.1 Literature searching and selection of publication
Literature search was performed by JW and XY in April 2023. Online databases (Pubmed, Embase, and Cochrane Library) were searched using the following keywords: “COVID-19,” “rapid antigen test,” “SARS coronavirus 2 test kit,” and “nasal swab,” with alternative spelling and abbreviations included (Table 1). Articles were firstly imported to Endnote software (Thomson-Reuters) and duplicated articles were removed. The rest studies were screened using the following criteria. Inclusion criteria: all original articles investigating accuracy of self-tested SARS-CoV-2 rapid antigen tests, with RT-PCR as the “gold standard.” Exclusion criteria: (1) not a human study; (2) not testing SARS-CoV-2; (3) not a rapid antigen test; (4) not self-tested; (5) not using RT-PCR as the reference method; (6) un-interpretable data. From the resulting eligible articles, accuracy data were extracted, including true positive, true negative, false positive, and false negative. The following information was also extracted: sample collector, assessed rapid antigen test, sample collection time after symptoms onset, sample type (nasal, nasopharyngeal/oropharyngeal, or combined) for rapid antigen tests and RT-PCR, region of the study, percentage of patients with symptoms, and Ct values used to define positive/negative of RT-PCR results. Quality assessment of diagnostic accuracy studies 2 (QUADAS-2) was used to evaluate each eligible study (14). Search results of JW and XY were compared and discussed by the two researchers. Any disagreement between JW and XY which could not solved was then solved by PC.
2.2 Statistical analysis
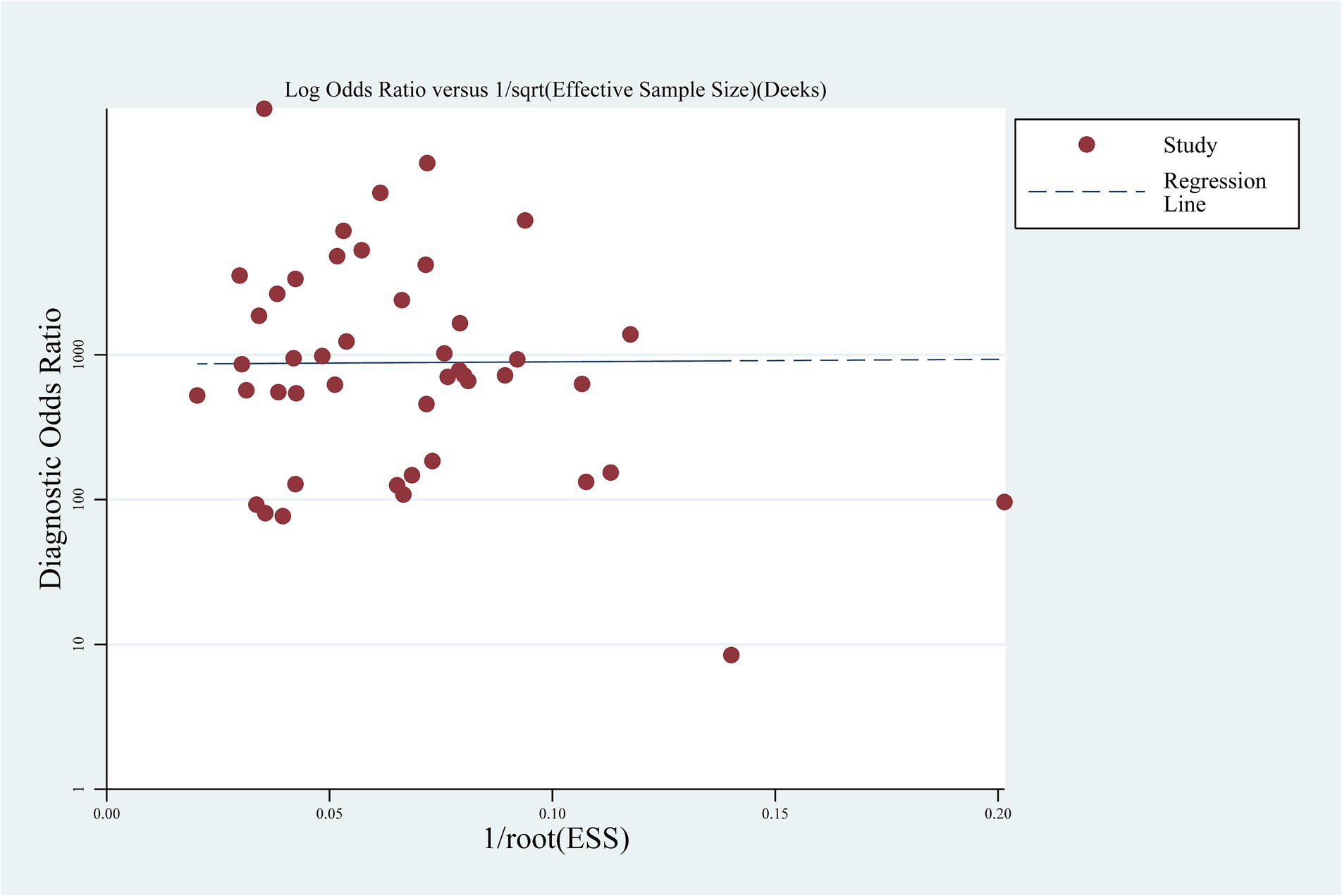
From the accuracy data from each eligible study, sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under curve (AUC) of summary receiver operating characteristic (SROC) curve were pooled using Meta-DiSc 1.4 (15). When significant heterogeneity was observed during the pooling (I2 ≥ 50% and p ≤ 0.05), random effects model was used. Otherwise, fixed effects model was used for the pooling. If significant heterogeneity was observed, threshold analysis and meta-regression were further performed. Potential publication bias in the eligible studies was assessed by Deek’s funnel plot asymmetry test using STATA 12.0 (STATA Corp.). Results were considered statistically significant if p < 0.05.
3 Results
3.1 Search results
As shown in Figure 1, after searching the online databases, 493 publications were identified (Pubmed: 92; Embase: 398; Cochrane Library: 3), with 19 duplicated literatures. Titles and abstracts of the rest 474 publications were screened, and another 402 publications were excluded. Full-texts of the rest 72 articles were then assessed for eligibility, and another 27 articles were further excluded. Some of these 27 articles investigated tests which require special equipment to read out results (e.g., LumiraDx™ SARS-CoV-2 Antigen Test), while other articles had un-interpretable data or used samples taken from nasopharynx or throat which cannot be done by the suspected patients themselves. Accuracy data and other information were extracted from the 45 eligible studies, and meta-analysis was further performed.
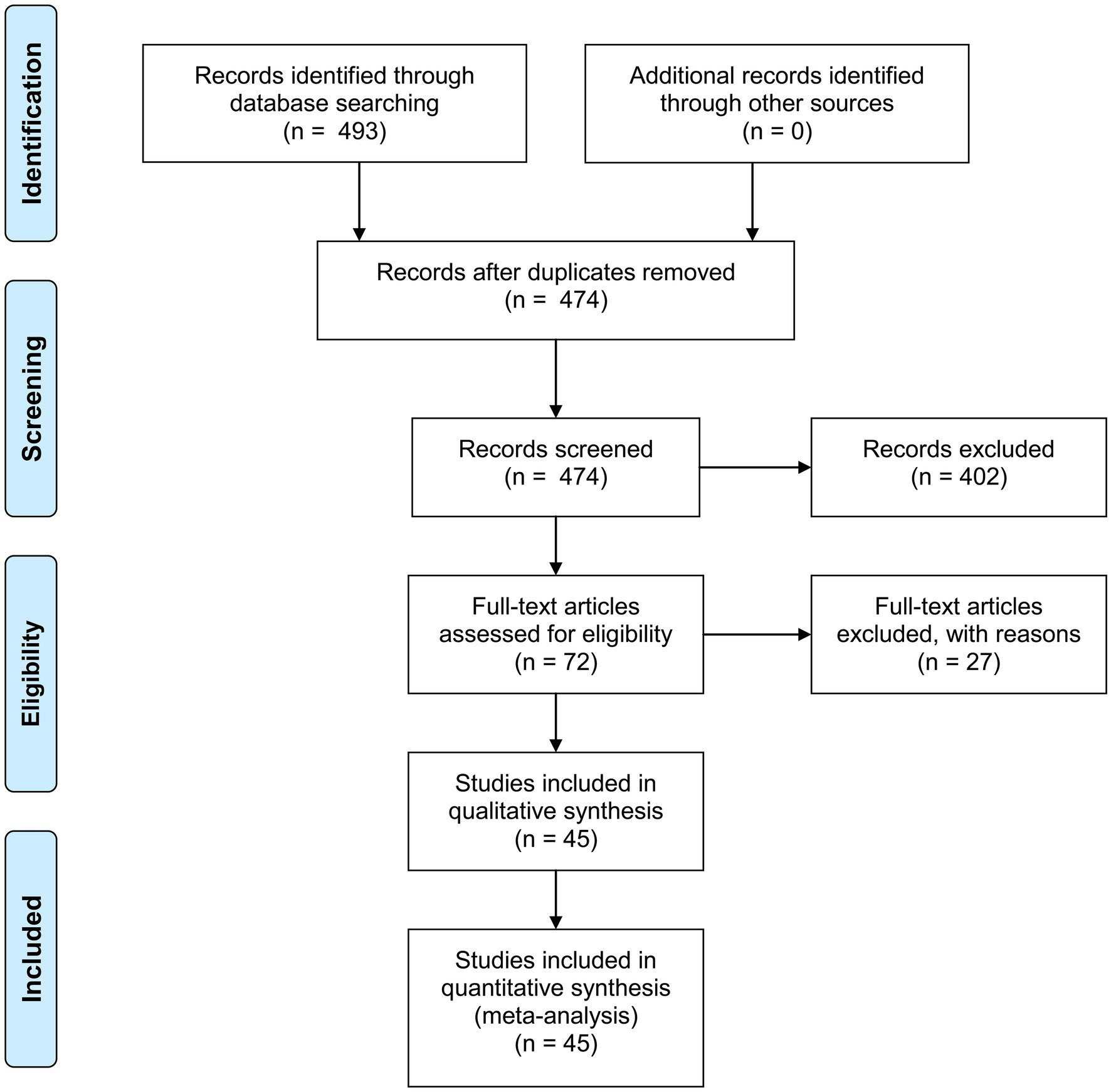
Figure 1. PRISMA 2009 flow diagram. From Moher et al. (55).
3.2 Review of eligible publications
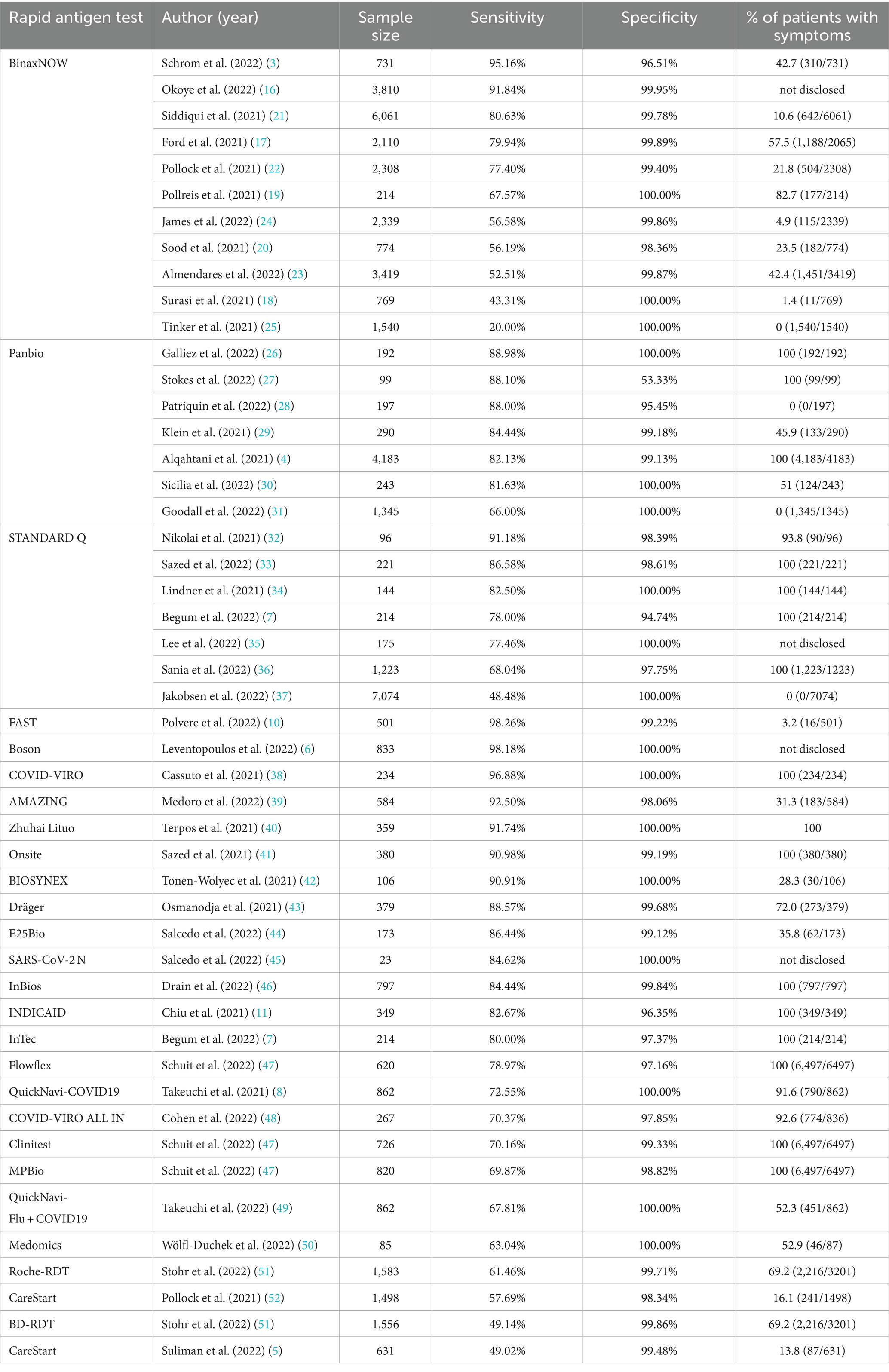
In the 45 eligible studies, three rapid antigen tests were intensively studied, including BinaxNOW, Panbio, and STANDARD Q. Other than these 3 tests, 23 other rapid antigen tests have also been assessed in one or two studies.
3.2.1 BinaxNOW
In all, there were 11 articles investigating Abbott BinaxNOW COVID-19 antigen self test (3, 16–25). In all the studies, BinaxNOW showed high specificity (ranging from 96.51% (3) to 100% (18, 19, 25)). Sensitivity of BinaxNOW varied greatly among different studies, ranging from 20% (25) to 95.16% (3). In the two studies showing relatively low sensitivity (<50%), percentages of suspected patients with symptoms were both low (1.4% (18) and 0% (25)).
3.2.2 Panbio
Seven articles reported the accuracy performance of Abbott Panbio COVID-19 Ag Rapid Test (4, 26–31). All the studies reported high specificity (from 95.45 to 100%), except one which reported specificity of 53.33%. This might be due to the relatively small sample size: 99 suspected patients, in which only 15 had negative results in RT-PCR. Sensitivity reported in these 7 articles ranged from 66 to 88.98%, in which two studies with 0% suspected patients with symptoms showed sensitivity of 66 and 88%.
3.2.3 STANDARD Q
The performance of SD Biosensor STANDARD Q COVID-19 Ag test was assessed in 6 articles (32–37). Specificity of STANDARD Q ranged from 94.74 to 100%. The highest sensitivity reported for STANDARD Q was 91.18%. The lowest sensitivity was 48.48%, which was reported in a study with 0% suspected patients with symptoms.
3.2.4 Other rapid antigen tests
Other than the above-mentioned rapid antigen tests, several other rapid antigen tests were also assessed in only one or two studies, including FAST COVID-19 SARS-CoV-2 Antigen Rapid Test Kit (JOYSBIO Biotechnology, China) (10), Boson Rapid SARS−CoV−2 antigen test card (Xiamen Boson, China) (6), COVID-VIRO (AAZ, France) (38), AMAZING COVID-19 Antigen Sealing Tube Test Strip (Amazing Biotech, China) (39), Zhuhai Lituo Biotechnology COVID-19 antigen detection kit (Zhuhai Lituo, China) (40), Onsite® Rapid Test (CTK Biotech, USA) (41), BIOSYNEX Antigen Self-Test COVID-19 Ag + (Biosynex Swiss, Switzerland) (42), Dräger Antigen Test SARS-CoV-2 (Dräger Safety, Germany) (43), E25Bio Rapid antigen tests (E25Bio, Inc., USA) (44), SARS-CoV-2 N rapid antigen test (self-developed) (45), SCoV-2 Ag Detect Rapid Self-Test (InBios International, Inc., USA) (46), INDICAID COVID-19 rapid antigen test (Rhino Diagnostics, USA) (11), InTec Rapid SARS-CoV − 2 Antigen Test (InTec, China) (7), Flowflex (Acon Laboratories, USA) (47), QuickNavi-COVID19 Ag kit (Denka, Japan) (8), COVID VIRO ALL IN (AAZ, France) (48), Clinitest (Siemens-Healthineers, Germany) (47), MPBio (MP Biomedicals, USA) (47), QuickNavi-Flu+COVID19 Ag kit (Denka, Japan) (49), Medomics SARS-CoV-2 antigen test (Jiangsu Medomics Medical Technology, China) (50), Roche-RDT self-testing kit (Roche Diagnostics, Switzerland) (51), CareStart COVID-19 Antigen Test (Access Bio, USA) (5, 52), and BD-RDT self-testing kit (BD Veritor, USA) (51). Similarly, specificity of these rapid antigen tests was also high, ranging from 96.35% (11) to 100% (6, 8, 38, 40, 42, 45, 49, 50). Sensitivity of these studies ranged from 49.02% (5) to 98.26% (10). Detailed sensitivity and specificity of each rapid antigen test were summarized in Table 2.
3.3 Quality assessment of eligible studies
QUADAS-2 was used to assess the quality of the 45 eligible studies (Table 3). In the assessment of risk of bias, high risk was observed in 3 studies (2 in patient selection, 1 in both index test and reference standard). Percentage of low risk ranged from 2% (n = 1, index test) to 78% (n = 35, flow and timing). In the assessment of application concerns, high risk was observed in 3 studies (2 in patient selection, and 2 in index test), and percentage of low risk ranged from 93% (n = 42, index test) to 100% (n = 45, reference standard).
3.4 Meta-analysis
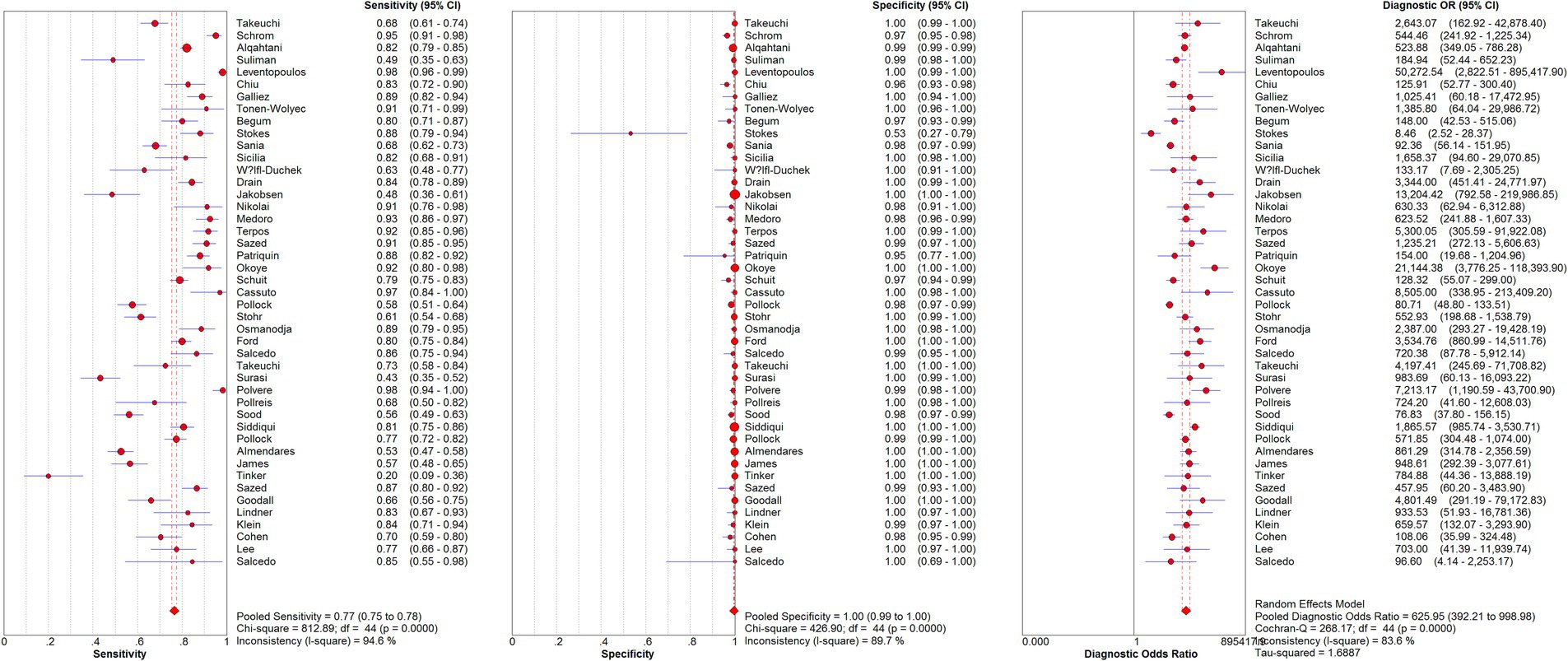
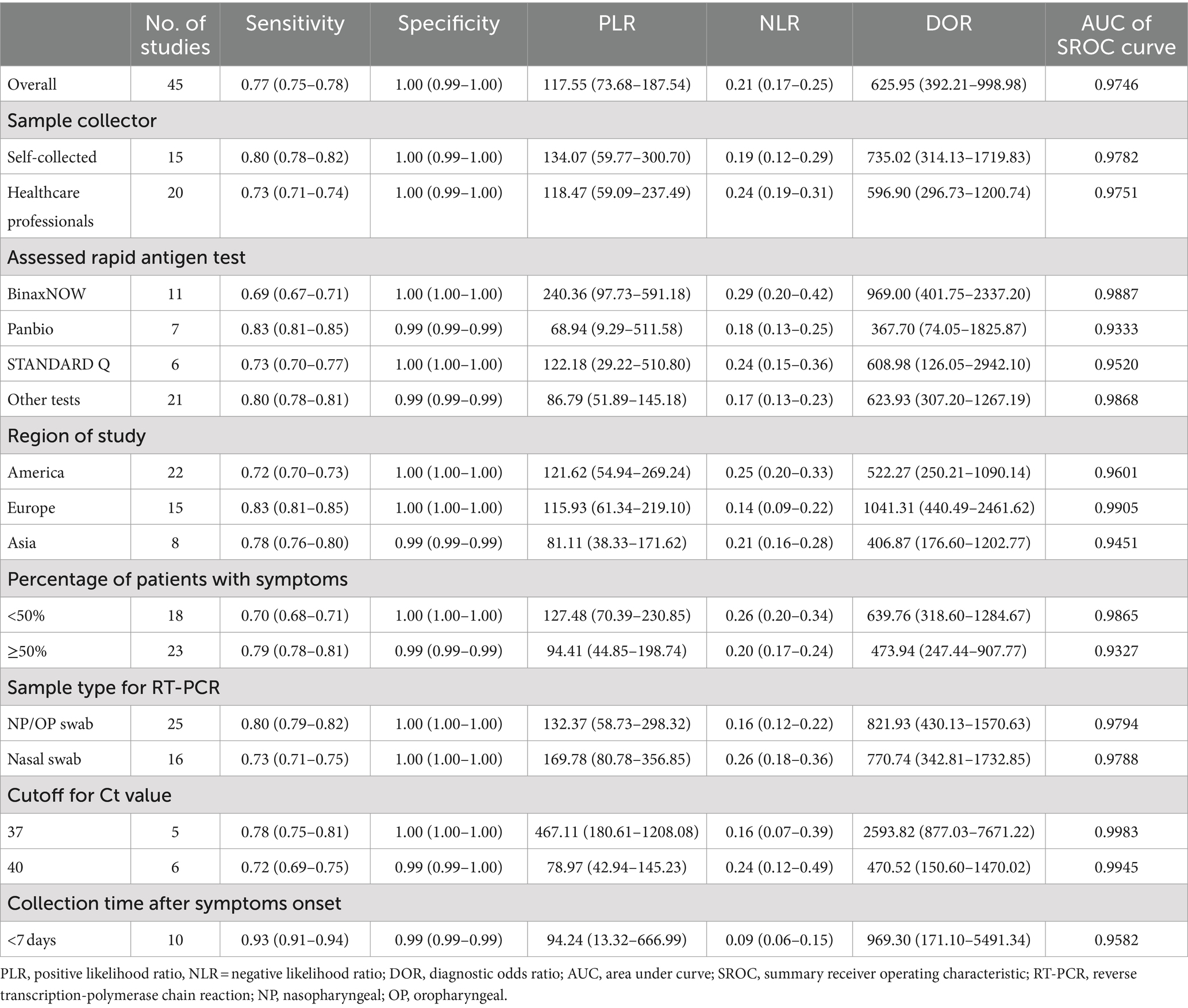
From the 45 eligible studies, we pooled the paired SARS-Cov-2 testing results of rapid antigen tests and RT-PCR from 50,897 suspected COVID-19 patients (see Figure 2 and Table 4). The pooled sensitivity, specificity, and DOR were 0.77 [95% confidence interval (CI): 0.75–0.78], 1.00 (95%CI: 0.99–1.00), and 625.95 (95%CI: 392.21–998.98). The AUC of SROC curve was 0.9746.
During the pooling, significant heterogeneity was observed (see Figure 2). Possible sources of the heterogeneity were then investigated. Diagnostic threshold analysis indicated no significant threshold effect (Spearman correlation coefficient: 0.279, p = 0.063). Further meta-regression showed that inter-study heterogeneity was not associated with collector of samples (p = 0.7627), percentage of patients with symptoms (p = 0.0797), type of rapid antigen kits (p = 0.8037), sample type for RT-PCR (p = 0.7907), Ct values used to define positive/negative of RT-PCR results (p = 0.7744), region of studies (p = 0.1831), or sample collection time after symptoms onset (p = 0.2550). Sample type for rapid antigen tests was not included in the analysis because all the studies used nasal samples for rapid antigen tests.
Subgroup analysis were further performed based on sample collector, assessed rapid antigen test, region of study, percentage of patients with symptoms, and sample type for RT-PCR. Since the pooled specificity was quite high (1.00, 95%CI: 0.99–1.00), we focused more on the pooled sensitivity and DOR of the rapid antigen tests. Although all the rapid antigen tests involved in this systemic review could be self-performed, in some of the studies, sample collection were performed by healthcare professionals, while in other studies, samples were self-collected. After pooling the accuracy results of the two subgroups, as shown in Table 4, in the 15 studies which used self-collected samples for rapid antigen tests, the pooled sensitivity and DOR (0.80 and 735.02) were higher than the 20 studies using samples collected by healthcare professionals (0.73 and 596.90). This is an interesting result because procedures performed by healthcare professionals are normally considered to be more accurate than non-professionals. In the assessed rapid antigen tests, Abbott BinaxNOW showed the lowest pooled sensitivity (0.69) but the highest DOR (969.00). Panbio, another rapid antigen test from Abbott, showed the highest pooled sensitivity (0.83) but the lowest DOR (367.70). Regarding the region of study, studies from Europe showed the highest sensitivity (0.83) and DOR (1041.31), compared to studies from America or Asia. Studies with high percentage (≥ 50%) of patients with symptoms showed higher pooled sensitivity (0.79) but lower pooled DOR (473.94) than studies with low percentage (<50%) of patients with symptoms. For the sample type for RT-PCR, studies using nasopharyngeal or oropharyngeal samples showed higher pooled sensitivity (0.80) and DOR (821.93) compared to studies using nasal samples. This is also an interesting result because the accuracy of tests is usually considered to be higher when the same sample type is used (e.g., nasal samples used for both rapid antigen tests and RT-PCR). Since only two studies used combined nasal and nasopharyngeal/oropharyngeal samples, they were not included in the subgroup analysis.
Majority of the studies (25 in 45) did not report the cutoff for Ct value. The rest studies used cutoff values of 30, 35, 37, 38, 40, 41, and 42. A study by Terpos (40) reported accuracy results when different cutoff for Ct values (25, 33, 37, and), and the results for 37 cutoff value was used because this cutoff value was more commonly used in the studies involved in this meta-analysis. When 37 was used as cutoff for Ct value, the results showed higher sensitivity (0.78) and DOR (2593.82), compared to 40 as the cutoff value (0.72 and 470.52, respectively). The rest cutoff values (30, 35, 38, 41, 42) were not included in the subgroup analysis due to limited numbers of studies using these cutoff values. Instead, we analyzed the relationship between cutoff Ct value of RT-PCR and sensitivity of rapid antigen test using a scatter plot. As shown in Figure 3, the sensitivity of rapid antigen test showed an overall decreasing trend alongwith the increase in the cutoff values of RT-PCR.
For the collection time, studies using samples collected from suspected patients within 7 days after symptoms onset showed surprisingly high sensitivity (0.93). Studies using other collection time (<5 days, <11 days, or < 14 days) were also not included in the subgroup analysis due to limited numbers of studies in these subgroups. Several studies did not report the range of collection time after symptoms onset or only reported mean and standard deviation of collection time. These studies were not included in the subgroup analysis due to the difficulty in comparing these mean ± standard deviation data with the time range.
Deek’s funnel plot was used to assess publication bias, and the result indicated no significant publication bias (p = 0.973) (Figure 4).
4 Discussion
The rapid antigen tests for SARS-CoV-2 require no healthcare professionals to perform and have short turnaround time, and were therefore recommended by the governments in face of the overwhelming need for SARS-CoV-2 test during the COVID-19 pandemic (1). The accuracy of these tests compared to the “gold standard” were however unclear. Several previous studies have assessed the accuracy of rapid antigen tests, including a previous systemic review and meta-analysis by Xie et al. (12). However, Xie’s study included nasopharyngeal swab samples which have to be collected by healthcare professionals. In addition, a type of rapid antigen test included in Xie’s meta-analysis (LumiraDx SARS-CoV-2 Antigen Test) requires special equipment to read out results. Therefore, the results reported in Xie’s meta-analysis did not represent the performance of fully-self-performed rapid antigen tests. In this systematic review and meta-analysis, we focused on the performance of self-tested SARS-CoV-2 rapid antigen tests using nasal swab samples which allow fully self-testing of COVID-19.
After database searching, we identified 45 eligible studies. After pooling the performance results from 50,897 suspected COVID-19 patients, the SARS-CoV-2 rapid antigen tests showed overall moderate sensitivity (0.77) and high specificity (1.00), DOR (625.95) and AUC of SROC curve (0.9746). Xie’s meta-analysis reported similar sensitivity for nasal samples (0.79). In the subgroup analysis, samples collected by the suspected patients themselves showed higher sensitivity (0.80) and DOR (735.02) compared to samples collected by healthcare professionals (0.73 and 596.90, respectively). These results indicate that rapid antigen tests are reliable when samples are self-collected by the suspected patients, which further support the usefulness of rapid antigen tests during disease pandemics. In the three rapid antigen tests which have been intensively assessed (BinaxNOW, Panbio, and STANDARD Q), Panbio showed the highest sensitivity (0.83) and BinaxNOW showed the lowest sensitivity (0.69). Similar observations were shown in Xie’s study (12), except that all the pooled sensitivity was slightly lower (0.65, 0.73, and 0.70 for BinaxNOW, Panbio, and STANDARD Q, respectively).
In Xie’s study, the subgroup analysis involved different Ct cutoff values (<20, 20–25, 25–30, and >30). In our study, however, almost all the Ct cutoff values reported in the eligible studies were > 30. Therefore, we performed subgroup analysis on each specific Ct cutoff value (e.g., 37 and 40 as shown in Table 4). Similar as Xie’s study, higher Ct cutoff value (40) showed lower sensitivity (0.72), compared to cutoff value of 37 (sensitivity: 0.78). Compared to our study, in Xie’s study, the sensitivity of rapid antigen tests was much lower (0.24) when Ct cutoff values of >30 were used (12). For samples collected less than 7 days after symptoms onset, the subgroup analysis results showed high pooled sensitivity (0.93), which was higher than the reported sensitivity in the corresponding subgroup (≤ 7 days after symptom onset) in Xie’s study.
Region of study, percentage of patients with symptoms, and sample types for RT-PCR were not included in the subgroup analysis in Xie’s study (12). In our study, the subgroup analysis showed that compared to other regions (America and Asia), studies from Europe showed both higher sensitivity (0.83) and DOR (1041.31). Studies with higher percentage (≥ 50%) of patients with symptoms had higher pooled sensitivity (0.79) than studies with lower percentage (< 50%) of patients with symptoms, indicating better accuracy of rapid antigen tests in patients with symptoms.
Nasopharyngeal or oropharyngeal swabs are commonly used for the testing of SARS-CoV-2 using RT-PCR. In several studies assessing the accuracy of rapid antigen tests, however, nasal swabs were used for RT-PCR (3, 5, 11, 16–19, 21, 22, 24, 25, 31, 44–46, 52). A previous study showed that when self-collected nasal swabs were used, the sensitivity and specificity of SARS-CoV-2 testing using RT-PCR were 90.32 and 100%, respectively, compared to nasopharyngeal swabs (53), indicating existence of false negative when nasal swabs were used. In our study, the pooled sensitivity of rapid antigen tests was 0.80 when nasopharyngeal or oropharyngeal swabs were used for RT-PCR. When nasal swabs were used for RT-PCR, the sensitivity of rapid antigen tests dropped to 0.73, which might be caused by higher risk of false negative in RT-PCR results when nasal swabs were used. Since two separate nasal swab samples were usually collected for RT-PCR and rapid antigen test (3, 5, 11, 16, 17, 19, 22, 24, 25, 46, 52), failure in collecting COVID-19 virus in either of the two samples would lead to either false positivity or false negativity when RT-PCR is used as gold standard. The increase in the number of false negative samples may resulted in decreased sensitivity as observed in our results, while increase in the number of false positive samples did not change the specificity much due to the large number of true negative samples. These results indicate that nasopharyngeal or oropharyngeal swabs are preferable samples for RT-PCR while assessing the accuracy of rapid antigen tests.
In summary, results of this systemic review and meta-analysis showed overall high accuracy of self-performed SARS-CoV-2 rapid antigen tests. Compared to BinaxNOW and STANDARD Q, Abbott Panbio had the highest sensitivity and therefore more recommended. The results supported the collection of nasal swab by the suspected COVID-19 patients themselves. In addition, sensitivity of rapid antigen tests was higher in patients with symptoms. For the performing of “gold standard” RT-PCR, the standard sample type, nasopharyngeal or oropharyngeal swab, is recommended, and use of lower Ct cutoff value could help increase the sensitivity of rapid antigen tests. Samples collected within 7 days after symptoms onset showed high sensitivity (0.93). Limitation of this study may be the small number of studies in some subgroups of collection time after symptoms onset and cutoff for Ct value. In addition, many rapid antigen tests were only assessed in one or two studies, which cannot be individually analyzed in the subgroup analysis. More studies are required to further validate the results of this study and show more details in the subgroup analysis. This study only involved nasal swab sample results, while there are other sample types for COVID-19 rapid antigen test, e.g., saliva, throat swab which were relatively less studied. In a previous systemic review and meta-analysis, saliva and throat swab samples showed lower sensitivity (68 and 69%, respectively) than nasal swab (83%) (54). More investigations are required to further clarify the performance of rapid antigen tests using these samples types.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
PC: Conceptualization, Funding acquisition, Writing – original draft. JW: Data curation, Methodology, Writing – original draft. PY: Conceptualization, Data curation, Formal analysis, Writing – review & editing. YZ: Data curation, Writing – original draft. MW: Data curation, Formal analysis, Writing – original draft. RG: Conceptualization, Writing – review & editing. HZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. A project supported by Center for Early Childhood Education Research, Sichuan (CECER-2022-B01); A Project Supported by Center for Early Childhood Education Research, Sichuan (grant number CECER-2023-C02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1402949/full#supplementary-material
References
1. Dinnes, J, Deeks, JJ, Adriano, A, Berhane, S, Davenport, C, Dittrich, S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. (2020) 8:CD013705. doi: 10.1002/14651858.CD013705
2. Velavan, TP, Pallerla, SR, and Kremsner, PG. How to (ab)use a COVID-19 antigen rapid test with soft drinks? Int J Infect Diseases. (2021) 111:28–30. doi: 10.1016/j.ijid.2021.08.023
3. Schrom, J, Marquez, C, Pilarowski, G, Wang, CY, Mitchell, A, Puccinelli, R, et al. Comparison of SARS-CoV-2 reverse transcriptase polymerase chain reaction and BinaxNOW rapid antigen tests at a community site during an omicron surge: a cross-sectional study. Ann Intern Med. (2022) 175:682–90. doi: 10.7326/M22-0202
4. Alqahtani, M, Abdulrahman, A, Mustafa, F, Alawadhi, AI, Alalawi, B, and Mallah, SI. Evaluation of rapid antigen tests using nasal samples to diagnose SARS-CoV-2 in symptomatic patients. Front Public Health. (2022) 9:728969. doi: 10.3389/fpubh.2021.728969
5. Suliman, S, Matias, WR, Fulcher, IR, Molano, FJ, Collins, S, Uceta, E, et al. Evaluation of the access bio CareStart rapid SARS-CoV-2 antigen test in asymptomatic individuals tested at a community mass-testing program in Western Massachusetts. Sci Rep. (2022) 12:21338. doi: 10.1038/s41598-022-25266-3
6. Leventopoulos, M, Michou, V, Papadimitropoulos, M, Vourva, E, Manias, NG, Kavvadas, HP, et al. Evaluation of the boson rapid ag test vs RT-PCR for use as a self-testing platform. Diagn Microbiol Infect Dis. (2022) 104:115786. doi: 10.1016/j.diagmicrobio.2022.115786
7. Begum, MN, Jubair, M, Nahar, K, Rahman, S, Talha, M, Sarker, MS, et al. Factors influencing the performance of rapid SARS-CoV-2 antigen tests under field condition. J Clin Lab Anal. (2022) 36:e24203. doi: 10.1002/jcla.24203
8. Takeuchi, Y, Akashi, Y, Kato, D, Kuwahara, M, Muramatsu, S, Ueda, A, et al. Diagnostic performance and characteristics of anterior nasal collection for the SARS-CoV-2 antigen test: a prospective study. Sci Rep. (2021) 11:10519. doi: 10.1038/s41598-021-90026-8
9. Mitchell, SL, Orris, S, Freeman, T, Freeman, MC, Adam, M, Axe, M, et al. Performance of SARS-CoV-2 antigen testing in symptomatic and asymptomatic adults: a single-center evaluation. BMC Infect Dis. (2021) 21:1071. doi: 10.1186/s12879-021-06716-1
10. Polvere, I, Voccola, S, D'Andrea, S, Zerillo, L, Varricchio, R, Madera, JR, et al. Evaluation of FAST COVID-19 SARS-CoV-2 antigen rapid test kit for detection of SARS-CoV-2 in respiratory samples from mildly symptomatic or asymptomatic patients. Diagnostics. (2022) 12:650. doi: 10.3390/diagnostics12030650
11. Chiu, RYT, Kojima, N, Mosley, GL, Cheng, KK, Pereira, DY, Brobeck, M, et al. Evaluation of the INDICAID COVID-19 rapid antigen test in symptomatic populations and asymptomatic community testing. Microbiol Spectr. (2021) 9:e0034221. doi: 10.1128/Spectrum.00342-21
12. Xie, JW, He, Y, Zheng, YW, Wang, M, Lin, Y, and Lin, LR. Diagnostic accuracy of rapid antigen test for SARS-CoV-2: a systematic review and meta-analysis of 166,943 suspected COVID-19 patients. Microbiol Res. (2022) 265:127185. doi: 10.1016/j.micres.2022.127185
13. Krüger, LJ, Klein, JAF, Tobian, F, Gaeddert, M, Lainati, F, Klemm, S, et al. Evaluation of accuracy, exclusivity, limit-of-detection and ease-of-use of LumiraDx: an antigen-detecting point-of-care device for SARS-CoV-2. Infection. (2022) 50:395–406. doi: 10.1007/s15010-021-01681-y
14. Whiting, PF, Rutjes, AW, Westwood, ME, Mallett, S, Deeks, JJ, Reitsma, JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
15. Zamora, J, Abraira, V, Muriel, A, Khan, K, and Coomarasamy, A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. (2006) 6:31. doi: 10.1186/1471-2288-6-31
16. Okoye, GA, Kamara, HI, Strobeck, M, Mellman, TA, Kwagyan, J, Sullivan, A, et al. Diagnostic accuracy of a rapid diagnostic test for the early detection of COVID-19. J Clin Virol. (2022) 147:105023. doi: 10.1016/j.jcv.2021.105023
17. Ford, L, Whaley, MJ, Shah, MM, Salvatore, PP, Segaloff, HE, Delaney, A, et al. Antigen test performance among children and adults at a SARS-CoV-2 community testing site. J Pediatric Infect Diseases Soc. (2021) 10:1052–61. doi: 10.1093/jpids/piab081
18. Surasi, K, Cummings, KJ, Hanson, C, Morris, MK, Salas, M, Seftel, D, et al. Effectiveness of Abbott BinaxNOW rapid antigen test for detection of SARS-CoV-2 infections in outbreak among horse racetrack workers, California, USA. Emerg Infect Dis. (2021) 27:2761–7. doi: 10.3201/eid2711.211449
19. Pollreis, RE, Roscoe, C, Phinney, RJ, Malesha, SS, Burns, MC, Ceniseros, A, et al. Evaluation of the Abbott BinaxNOW COVID-19 test ag card for rapid detection of SARS-CoV-2 infection by a local public health district with a rural population. PLoS One. (2021) 16:e0260862. doi: 10.1371/journal.pone.0260862
20. Sood, N, Shetgiri, R, Rodriguez, A, Jimenez, D, Treminino, S, Daflos, A, et al. Evaluation of the Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection in children: implications for screening in a school setting. PLoS One. (2021) 16:e0249710. doi: 10.1371/journal.pone.0249710
21. Siddiqui, ZK, Chaudhary, M, Robinson, ML, McCall, AB, Peralta, R, Esteve, R, et al. Implementation and accuracy of BinaxNOW rapid antigen COVID-19 test in asymptomatic and symptomatic populations in a high-volume self-referred testing site. Microbiol Spect. (2021) 9:e0100821. doi: 10.1128/Spectrum.01008-21
22. Pollock, NR, Jacobs, JR, Tran, K, Cranston, AE, Smith, S, O’Kane, CY, et al. Performance and implementation evaluation of the Abbott BinaxNOW rapid antigen test in a high-throughput drive-through community testing site in Massachusetts. J Clin Microbiol. (2021) 59:e00083-21. doi: 10.1128/JCM.00083-21
23. Almendares, O, Prince-Guerra, JL, Nolen, LD, Gunn, JKL, Dale, AP, Buono, SA, et al. Performance characteristics of the Abbott BinaxNOW SARS-CoV-2 antigen test in comparison to real-time reverse transcriptase PCR and viral culture in community testing sites during November 2020. J Clin Microbiol. (2022) 60:e0174221. doi: 10.1128/JCM.01742-21
24. James, AE, Gulley, T, Kothari, A, Holder, K, Garner, K, and Patil, N. Performance of the BinaxNOW coronavirus disease 2019 (COVID-19) antigen card test relative to the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay among symptomatic and asymptomatic healthcare employees. Infect Control Hosp Epidemiol. (2022) 43:99–101. doi: 10.1017/ice.2021.20
25. Tinker, SC, Szablewski, CM, Litvintseva, AP, Drenzek, C, Voccio, GE, Hunter, MA, et al. Point-of-care antigen test for SARS-CoV-2 in asymptomatic college students. Emerg Infect Dis. (2021) 27:2662–5. doi: 10.3201/eid2710.210080
26. Galliez, RM, Bomfim, L, Mariani, D, Leitão, IC, Castiñeiras, ACP, Gonçalves, CCA, et al. Evaluation of the Panbio COVID-19 antigen rapid diagnostic test in subjects infected with omicron using different specimens. Microbiol Spectr. (2022) 10:e0125022. doi: 10.1128/spectrum.01250-22
27. Stokes, W, Berenger, BM, Scott, B, Szelewicki, J, Singh, T, Portnoy, D, et al. One swab fits all: performance of a rapid, antigen-based SARS-CoV-2 test using a nasal swab, nasopharyngeal swab for nasal collection, and RT-PCR confirmation from residual extraction buffer. J Appl Lab Med. (2022) 7:834–41. doi: 10.1093/jalm/jfac004
28. Patriquin, G, LeBlanc, JJ, Williams, C, Hatchette, TF, Ross, J, Barrett, L, et al. Comparison between nasal and nasopharyngeal swabs for SARS-CoV-2 rapid antigen detection in an asymptomatic population, and direct confirmation by RT-PCR from the residual buffer. Microbiol Spectr. (2022) 10:e0245521. doi: 10.1128/spectrum.02455-21
29. Klein, JAF, Krüger, LJ, Tobian, F, Gaeddert, M, Lainati, F, Schnitzler, P, et al. Head-to-head performance comparison of self-collected nasal versus professional-collected nasopharyngeal swab for a WHO-listed SARS-CoV-2 antigen-detecting rapid diagnostic test. Med Microbiol Immunol. (2021) 210:181–6. doi: 10.1007/s00430-021-00710-9
30. Sicilia, P, Castro, G, Fantilli, AC, Gierotto, R, López, L, Barbás, MG, et al. Rapid screening of SARS-CoV-2 infection: good performance of nasopharyngeal and nasal mid-turbinate swab for antigen detection among symptomatic and asymptomatic individuals. PLoS One. (2022) 17:e0266375. doi: 10.1371/journal.pone.0266375
31. Goodall, BL, LeBlanc, JJ, Hatchette, TF, Barrett, L, and Patriquin, G. Investigating the sensitivity of nasal or throat swabs: combination of both swabs increases the sensitivity of SARS-CoV-2 rapid antigen tests. Microbiol Spectr. (2022) 10:e0021722. doi: 10.1128/spectrum.00217-22
32. Nikolai, O, Rohardt, C, Tobian, F, Junge, A, Corman, VM, Jones, TC, et al. Anterior nasal versus nasal mid-turbinate sampling for a SARS-CoV-2 antigen-detecting rapid test: does localisation or professional collection matter? Infect Dis. (2021) 53:947–52. doi: 10.1080/23744235.2021.1969426
33. Sazed, SA, Kibria, MG, Zamil, MF, Hossain, MS, Khan, JZ, Juthi, RT, et al. Direct nasal swab for rapid test and saliva as an alternative biological sample for RT-PCR in COVID-19 diagnosis. Microbiol Spectr. (2022) 10:e0199822. doi: 10.1128/spectrum.01998-22
34. Lindner, AK, Nikolai, O, Rohardt, C, Kausch, F, Wintel, M, Gertler, M, et al. Diagnostic accuracy and feasibility of patient self-testing with a SARS-CoV-2 antigen-detecting rapid test. J Clin Virol. (2021) 141:104874. doi: 10.1016/j.jcv.2021.104874
35. Lee, S, Widyasari, K, Yang, HR, Jang, J, Kang, T, and Kim, S. Evaluation of the diagnostic accuracy of nasal cavity and nasopharyngeal swab specimens for SARS-CoV-2 detection via rapid antigen test according to specimen collection timing and viral load. Diagnostics. (2022) 12:710. doi: 10.3390/diagnostics12030710
36. Sania, A, Alam, AN, Alamgir, ASM, Andrecka, J, Brum, E, Chadwick, F, et al. Rapid antigen testing by community health workers for detection of SARS-CoV-2 in Dhaka, Bangladesh: a cross-sectional study. BMJ Open. (2022) 12:e060832. doi: 10.1136/bmjopen-2022-060832
37. Jakobsen, KK, Jensen, JS, Todsen, T, Kirkby, N, Lippert, F, Vangsted, AM, et al. Accuracy of anterior nasal swab rapid antigen tests compared with RT-PCR for massive SARS-CoV-2 screening in low prevalence population. APMIS. (2022) 130:95–100. doi: 10.1111/apm.13189
38. Cassuto, NG, Gravier, A, Colin, M, Theillay, A, Pires-Roteira, D, Pallay, S, et al. Evaluation of a SARS-CoV-2 antigen-detecting rapid diagnostic test as a self-test: diagnostic performance and usability. J Med Virol. (2021) 93:6686–92. doi: 10.1002/jmv.27249
39. Medoro, A, Davinelli, S, Voccola, S, Cardinale, G, Passarella, D, Marziliano, N, et al. Assessment of the diagnostic performance of a novel SARS-CoV-2 antigen sealing tube test strip (colloidal gold) as point-of-care surveillance test. Diagnostics. (2022) 12:1279. doi: 10.3390/diagnostics12051279
40. Terpos, E, Ntanasis-Stathopoulos, I, and Skvarc, M. Clinical application of a new SARS-CoV-2 antigen detection kit (colloidal gold) in the detection of COVID-19. Diagnostics. (2021) 11:995. doi: 10.3390/diagnostics11060995
41. Sazed, SA, Kibria, MG, Hossain, MS, Zamil, MF, Adhikary, PC, Hossain, ME, et al. Clinical evaluation of a new antigen-based COVID-19 rapid diagnostic test from symptomatic patients. Diagnostics. (2021) 11:2300. doi: 10.3390/diagnostics11122300
42. Tonen-Wolyec, S, Dupont, R, Awaida, N, Batina-Agasa, S, Hayette, MP, and Belec, L. Evaluation of the practicability of Biosynex antigen self-test COVID-19 AG+ for the detection of SARS-CoV-2 Nucleocapsid protein from self-collected nasal mid-turbinate secretions in the general public in France. Diagnostics. (2021) 11:2217. doi: 10.3390/diagnostics11122217
43. Osmanodja, B, Budde, K, Zickler, D, Naik, MG, Hofmann, J, Gertler, M, et al. Accuracy of a novel SARS-CoV-2 antigen-detecting rapid diagnostic test from standardized self-collected anterior nasal swabs. J Clin Med. (2021) 10:2099. doi: 10.3390/jcm10102099
44. Salcedo, N, Sena, BF, Qu, X, and Herrera, BB. Comparative evaluation of rapid isothermal amplification and antigen assays for screening testing of SARS-CoV-2. Viruses. (2022) 14:468. doi: 10.3390/v14030468
45. Salcedo, N, Reddy, A, Gomez, AR, Bosch, I, and Herrera, BB. Monoclonal antibody pairs against SARS-CoV-2 for rapid antigen test development. PLoS Negl Trop Dis. (2022) 16:e0010311. doi: 10.1371/journal.pntd.0010311
46. Drain, PK, Bemer, M, Morton, JF, Dalmat, R, Abdille, H, Thomas, KK, et al. Accuracy of 2 rapid antigen tests during 3 phases of SARS-CoV-2 variants. JAMA Netw Open. (2022) 5:e2228143. doi: 10.1001/jamanetworkopen.2022.28143
47. Schuit, E, Venekamp, RP, Hooft, L, Veldhuijzen, IK, van den Bijllaardt, W, Pas, SD, et al. Diagnostic accuracy of covid-19 rapid antigen tests with unsupervised self-sampling in people with symptoms in the omicron period: cross sectional study. BMJ. (2022) 378:e071215. doi: 10.1136/bmj-2022-071215
48. Cohen, R, Aupiais, C, Filleron, A, Cahn-Sellem, F, Romain, O, Béchet, S, et al. Diagnostic accuracy of SARS-CoV-2 rapid antigen test from self-collected anterior nasal swabs in children compared to rapid antigen test and RT-PCR from nasopharyngeal swabs collected by healthcare workers: a multicentric prospective study. Front Pediatr. (2022) 10:980549. doi: 10.3389/fped.2022.980549
49. Takeuchi, Y, Akashi, Y, Kiyasu, Y, Terada, N, Kurihara, Y, Kato, D, et al. A prospective evaluation of diagnostic performance of a combo rapid antigen test QuickNavi-flu+COVID19 ag. J Infect Chemother. (2022) 28:840–3. doi: 10.1016/j.jiac.2022.02.027
50. Wölfl-Duchek, M, Bergmann, F, Jorda, A, Weber, M, Müller, M, Seitz, T, et al. Sensitivity and specificity of SARS-CoV-2 rapid antigen detection tests using Oral, anterior nasal, and nasopharyngeal swabs: a diagnostic accuracy study. Microbiol Spectr. (2022) 10:e0202921. doi: 10.1128/spectrum.02029-21
51. Stohr, J, Zwart, VF, Goderski, G, Meijer, A, Nagel-Imming, CRS, Kluytmans-van den Bergh, MFQ, et al. Self-testing for the detection of SARS-CoV-2 infection with rapid antigen tests for people with suspected COVID-19 in the community. Clin Microbiol Infect. (2022) 28:695–700. doi: 10.1016/j.cmi.2021.07.039
52. Pollock, NR, Tran, K, Jacobs, JR, Cranston, AE, Smith, S, O’Kane, CY, et al. Performance and operational evaluation of the access bio CareStart rapid antigen test in a high-throughput drive-through community testing site in Massachusetts. Open Forum Infect Dis. (2021) 8:ofab243. doi: 10.1093/ofid/ofab243
53. Mannan, N, Raihan, R, Parvin, US, Fazle Akbar, SM, Reza, MS, Islam, S, et al. Detection of SARS-CoV-2 RNA by reverse transcription-polymerase chain reaction (RT-PCR) on self-collected nasal swab compared with professionally collected nasopharyngeal swab. Cureus. (2022) 14:e25618. doi: 10.7759/cureus.25618
54. Khalid, MF, Selvam, K, Jeffry, AJN, Salmi, MF, Najib, MA, Norhayati, MN, et al. Performance of rapid antigen tests for COVID-19 diagnosis: a systematic review and Meta-analysis. Diagnostics. (2022) 12:110. doi: 10.3390/diagnostics12010110
Keywords: COVID-19, self-test, SARS-CoV-2, rapid antigen test, diagnostic accuracy
Citation: Cai P, Wang J, Ye P, Zhang Y, Wang M, Guo R and Zhao H (2024) Performance of self-performed SARS-CoV-2 rapid antigen test: a systematic review and meta-analysis. Front. Public Health. 12:1402949. doi: 10.3389/fpubh.2024.1402949
Edited by:
Roger Nlandu Ngatu, Kagawa University, JapanReviewed by:
Io Cheong, Shanghai Jiao Tong University, ChinaSoegianto Ali, Atma Jaya Catholic University of Indonesia, Indonesia
Copyright © 2024 Cai, Wang, Ye, Zhang, Wang, Guo and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongying Zhao, emhhb2hvbmd5aW5nNzcwODA0QDE2My5jb20=; Ronglian Guo, MTgwMzAyMjgwNTdAMTYzLmNvbQ==
†Theses authors have contributed equally to this work
 Peiling Cai
Peiling Cai Junren Wang1,2†
Junren Wang1,2† Peng Ye
Peng Ye Mengping Wang
Mengping Wang Hongying Zhao
Hongying Zhao