- 1Clinical Pharmacology and Pharmacotherapy, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium
- 2Child & Youth Institute, KU Leuven, Leuven, Belgium
- 3Drug Delivery and Disposition, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium
- 4Teratology Information Service, Netherlands Pharmacovigilance Centre Lareb, ‘s-Hertogenbosch, Netherlands
- 5BioNotus GCV, Niel, Belgium
- 6Department of Development and Regeneration, KU Leuven, Leuven, Belgium
- 7Neonatal Intensive Care Unit, University Hospitals Leuven, Leuven, Belgium
- 8Department of Hospital Pharmacy, Erasmus University Medical Center, Rotterdam, Netherlands
Introduction: While breastfeeding is recommended, knowledge regarding medicine transfer to human milk and its safety for nursing infants is limited. Only one paper has previously described dimethyl fumarate (DMF) transfer during breastfeeding in two patients at 5 and 6 months postpartum, respectively. The current case report describes maternal pharmacokinetic data of monomethyl fumarate (MMF), the active metabolite of DMF, and infant exposure estimations of MMF at 3 months postpartum.
Methods: A 32-year-old Caucasian woman started DMF therapy (120 mg, 2x/day) for multiple sclerosis at 3 months postpartum, after weaning her infant from breastfeeding. On day 99 after birth, the patient collected four milk samples over 24 h after 6 days of treatment at the initial dose. Additionally, a single maternal blood sample was collected to calculate the milk-to-plasma (M/P) ratio. The samples were analyzed using liquid chromatography coupled with the mass spectrometry method.
Results: A wide range of measured steady-state concentrations of MMF (5.5-83.5 ng/mL) was observed in human milk samples. Estimated daily infant dosage values for MMF, calculated with 150 and 200 mL/kg/day human milk intake, were 5.76 and 7.68 μg/kg/day, and the relative infant doses were 0.16 and 0.22%. The observed mean M/P ratio was 0.059, similar to the M/P ratio predicted using the empirical Koshimichi model (0.06).
Discussion: Combining this case report with the two previously described cases, the estimated infant exposure is low, albeit with relevant intra- and inter-patient variabilities. Research should further focus on infant exposure and safety.
1 Introduction
Dimethyl fumarate (DMF), a methyl ester of fumaric acid, is an oral immunomodulatory compound indicated for relapsing–remitting multiple sclerosis (MS). Patients taking DMF have a decreased rate of disability progression, and their annual relapse rate is reduced by half (20% after DMF vs. 40% after placebo intake). It lowers the levels of T cells, dendritic cells, and B cells and induces a shift toward anti-inflammatory activity through the Nrf2 pathway, among others. DMF (molecular weight: 144.13 g/mol) in the gastro-resistant formulation is mainly absorbed in the small intestines. Esterases quickly hydrolyze DMF to monomethyl fumarate (MMF), which has a peak concentration of 2–2.5 h after administration. The plasma protein binding of MMF varies from 27 to 40% in adults, and DMF is later metabolized through the tricarboxylic acid cycle to carbon dioxide (CO2), glucose, fumaric acid, and citric acid. Excretion occurs mainly as CO2 through exhalation (60%) and secondary by renal (15.5%) and fecal (0.9%) excretion. MMF has a terminal phase half-life of approximately 1 h, and in most adults, circulating MMF is not present anymore at 24 h (1–4). Although MMF is a small, slightly lipophilic (LogP 0.34) molecule with relatively low protein binding, transfer to the slightly more acidic human milk (pH 7.1–7.2) is expected to be low since weak acids such as MMF (pKa 3.31) will concentrate in the plasma (pH 7.4) due to ion trapping (5–7).
After the first large multinational prospective study ‘Pregnancy in MS (PRIMS)’, it is no longer believed that pregnancy could deteriorate the progression of MS in women. On the contrary, a decrease in relapse rate was observed during pregnancy without pharmacotherapy (8, 9). However, during the first 3–4 months postpartum, an increase in the risk of relapses was reported, while exclusive breastfeeding is considered to play a possible protective role in this relapse risk. This protective factor might be explained by the fact that mothers with higher disease intensity are more likely to opt for formula feeding to restart pharmacotherapy (8).
Despite this higher disease activity after delivery and the protective impact of exclusive breastfeeding, limited data are known on the safety of MS pharmacotherapy during lactation. Interferon beta and glatiramer acetate are considered compatible with breastfeeding, although based on limited evidence. At present, DMF is not recommended during breastfeeding, and discontinuation of breastfeeding is advised (8, 9). Only one article has described the transfer of MMF into human milk. Ciplea et al. described a case series of two women taking DMF during breastfeeding: a 35-year-old woman who restarted oral DMF therapy at 6 months postpartum after weaning her infant from breastfeeding, and a 36-year-old patient who discontinued breastfeeding at 5 months to restart DMF treatment. Both women continued to pump so that human milk samples could be collected on the 8th day of using 240 mg DMF, twice daily. Human milk concentrations of MMF (average concentration: 7.5 and 2.7 ng/mL) and relative infant dose (RID: 0.019 and 0.007%) were defined and seemed to be low (10).
Breastfeeding is associated with multiple health benefits for maternal and infant health (11). Therefore, the World Health Organization’s (WHO)1 recommendations state that infants should be exclusively breastfed up to 6 months of age and partially breastfed, combined with complementary foods, until a minimum of 2 years of age. Overall, the use and safety of medicines in breastfeeding mothers and the transfer of medicines to their milk have been largely understudied (12). As a result, medicines are often used off-label in this population, and infants can be exposed to unknown risks (13). Furthermore, mothers sometimes choose to postpone their pharmacotherapy and ignore their own health or to interrupt or quit breastfeeding. This case report is part of the UmbrelLACT study established within the Innovative Medicine Initiative (IMI) ConcePTION,2 with the aim of limiting uncertainties regarding the effects and exposure of specific medicines during pregnancy and lactation for which knowledge is currently marginally present (14).
The objective of this case report was to determine the concentration of MMF in human milk at 3 months postpartum, to calculate the milk-to-plasma (M/P) ratio of MMF, and to estimate the exposure to a 3-month-old infant. This article is written following the CARE guidelines and ‘The guidelines for reporting cases of medication use during lactation’ (15, 16).
2 Methods
This brief research report describes a patient included in the UmbrelLACT protocol (NCT06042803), for which the methods have been described earlier in more detail (14). Approval of the Ethics Committee Research UZ/KU Leuven (S67204) and biobank has been received (14). The patient signed the written informed consent form.
Study information regarding this clinical lactation study is shared via multiple channels, i.e., University hospitals in Leuven, the Belgian pregnancy registry (BELpREG), or other health facilities. After inclusion, clinical data of the mother, e.g., anthropometry, comorbidities, pregnancy and breastfeeding data, and medicine intake during the 3 days before samples were collected using a self-reporting questionnaire.
2.1 Sample collection
The human milk samples were collected over a period of 24 h in a steady state. Sampling occurred at home with an electrical breast pump. For each expression session, the total milk volume was reported, and a sample (±10 mL) was taken for analysis. The milk samples were stored in the fridge at the patient’s home (4°C) for a maximum of 24 h.
A maternal blood sample was taken after collecting the last milk sample, in an Ethylenediaminetetraacetic acid (EDTA) tube.
The samples were transported on ice and were subsequently stored at −80°C. Bioanalysis of the samples was conducted using the liquid chromatography with mass spectrometry (LC–MS/MS) method.
2.2 Bioanalysis method for monomethyl fumarate (MMF)
Mass spectrometry measurements were performed using a Dionex UltiMate 3,000 LC System (Thermo Scientific Bremen, Germany) coupled via heated electrospray ionization to a Q Exactive Orbitrap mass spectrometer (Thermo Scientific).
Milk or plasma samples of 150 μL were extracted in 80/20 methanol/water with 13C5-D5-15N Glutamic acid as the internal standard. The same procedure was applied to the spiked blank human milk samples used for the calibration curve. These were left overnight at −80°C and then centrifuged for 10 min at 13000 rpm. A volume of 200 μL of supernatant was added to 800 μL of chloroform, after which the samples were vortexed, left to separate at 4°C for 30 min, then vortexed and left to separate again at 4°C for 30 min. Subsequently, 100 μL of the upper phase was transferred to an MS vial and injected into a 15-cm C-18 column (Acquity UPLC-HSS T3 1. 8 μm; 2.1 × 150 mm, Waters). A step gradient was carried out using solvent A (10 mM TBA and 15 mM acetic acid in Milli-Q) and solvent B (100% methanol). The gradient started with 5% of solvent B and 95% of solvent A and remained at 5% B until 2-min post-injection. A linear gradient to 37% B was carried out until 7 min and increased to 41% until 14 min. Between 14 and 26 min, the gradient increased to 95% B and remained at 95% B for 4 min. At 30 min, the gradient returned to 5% B. The chromatography was stopped at 40 min. The flow was kept constant at 0.25 mL/min, and the column was maintained at 40°C throughout the analysis. The HESI source was operated at negative polarity mode using a spray voltage of 4.8 kV, sheath gas at 40, auxiliary gas at 10, and the latter heated to 260°C. The ion transfer capillary temperature was 300°C. The m/z ratio is 129.0 Da for MMF (at negative ESI ionization) and 157.1 Da for the internal standard. The mass spectrometer operated in full scan (range [70.0000–1050.0000]), and the AGC target was set at 3.0E+006 using a resolution of 140,000. Data collection was performed using the Xcalibur software (Thermo Scientific). The data analyses were performed by integrating the peak areas (El-Maven – Polly – Elucidata).
Concentrations were calculated by dividing the abundances, the integrated area of the peak of the compound of interest (MMF), by the slope of the calibration curve (0–2,000 nM) prepared in blank milk (Figure 1). The limit of detection (LOD) was set to correspond to three times the signal in blank human milk without the MMF. The concentration in blank human milk (2.74 ng/mL) was subtracted from the concentrations found in human milk samples and from the LOD, yielding a blank-corrected LOD of 5.48 ng/mL. The blank-corrected limit of quantification (LOQ) was 24.7 ng/mL, which implies that certain sample concentrations might have a larger margin on quantification accuracy.
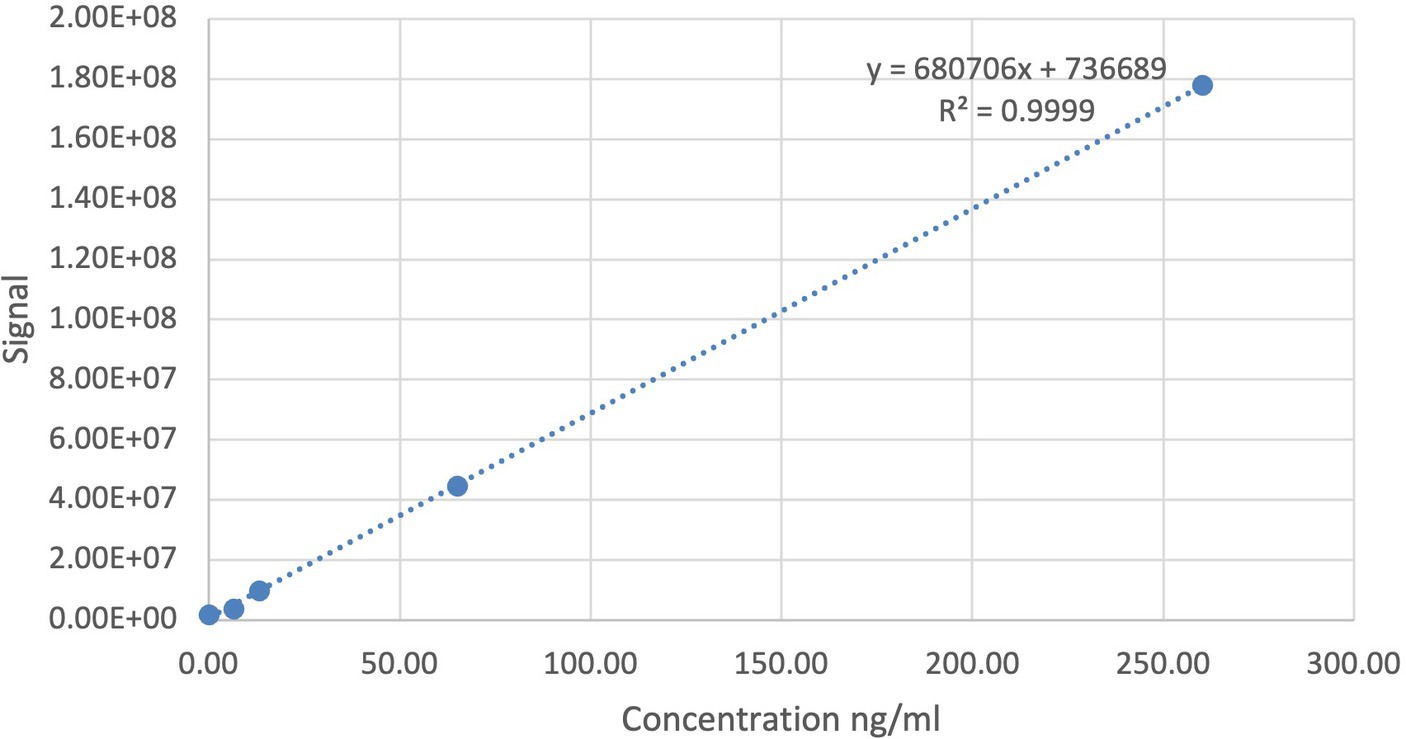
Figure 1. Calibration curve (0–2,000 nM) of the bioanalysis method for monomethyl fumarate (MMF) prepared in blank human milk.
Additionally, it was observed that DMF added to blank human milk samples produces MMF, which suggests that enzymatic conversion or spontaneous transfer of a methyl by DMF, e.g., to proteins, occurs in human milk, likely reflecting esterase activity in human milk. This esterase activity is hardly relevant for clinical in vivo data, as DMF is already metabolized before reaching the systemic circulation.
3 Results
3.1 Case description: patient information
This case report describes a mother–infant pair, where the infant was weaned before the start of maternal DMF treatment. Therefore, only maternal samples were collected in this case study, and the intake by the infant was estimated. Consequently, there is no observed exposure or safety data in the infant during MMF exposure. The mother is a 32-year-old, primigravida, Caucasian woman who discontinued her treatment since early pregnancy, due to safety concerns during the pregnancy. At the re-initiation of treatment at 3 months postpartum, the patient’s weight was 62 kg and height was 1.87 m, resulting in a body mass index of 17.7 kg/m2. While gradually tapering milk production after weaning the infant, samples were collected 6 days after restarting the DMF therapy at the starting dose (2 × 120 mg/day in the first week). The patient had several comorbidities, more specifically Hashimoto’s thyroiditis and thalassemia. Kidney or liver function problems were not reported by the patient. She did not smoke or use recreational drugs, nor did she drink alcohol. The patient followed a pork-free diet.
3.2 Timeline
The time points of the sampling day are illustrated in Figure 2.
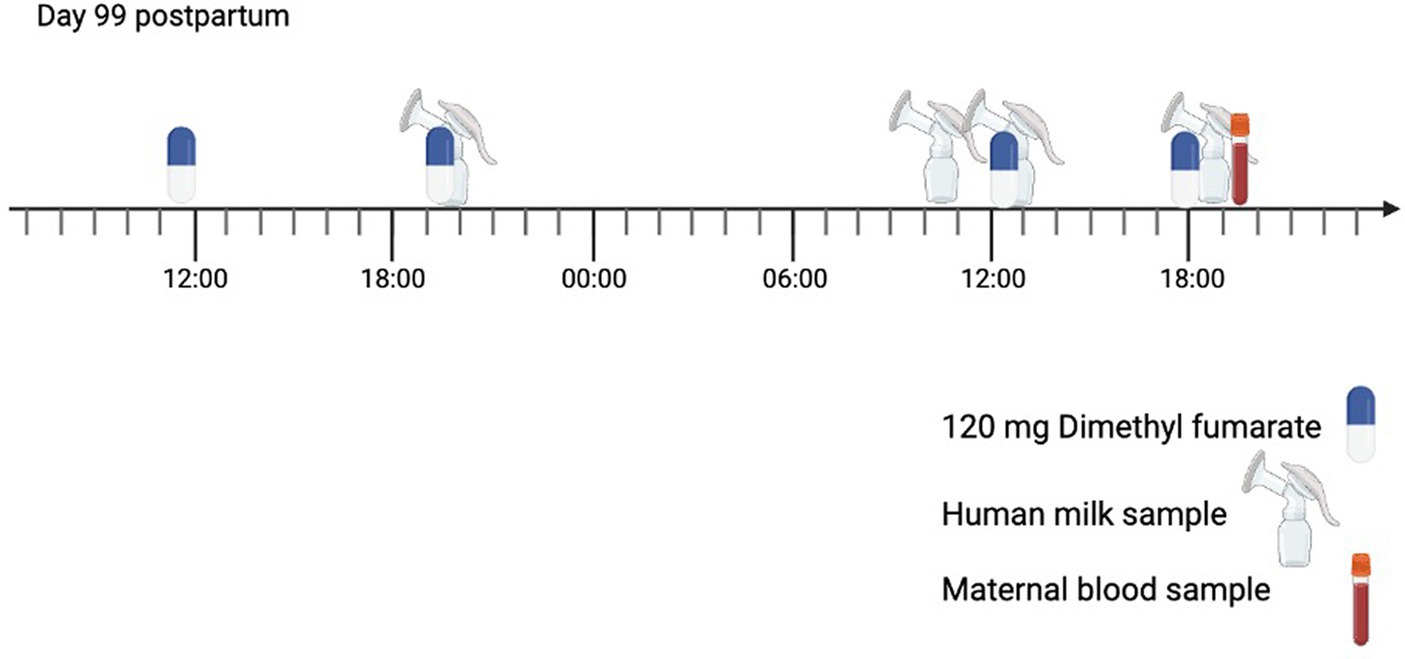
Figure 2. Timeline of the sampling day (start on day 99 postpartum). The mother was taking dimethyl fumarate (DMF) 120 mg twice per day for multiple sclerosis (MS). The mother collected a human milk sample during four expression sessions.
3.3 Therapeutic interventions
The patient used DMF polpharma 120 mg gastro-resistant hard capsules (Polpharma SA Pharmaceutical works, Starogard Gdański, Poland) orally twice daily after a meal. Furthermore, she combined this treatment with Hemafer (357 mg ferric hydroxide polymaltose complex 1x/day, tablets, Fol), Möller’s forte omega-3 (1x/day, capsules), vitamin C (1,000 mg, 1x/d), and vitamin D3 (2000 IU, 1x/d). On day 100 after delivery, the patient did not take vitamin C. These supplements and co-medications are not expected to interact with DMF or MMF.
3.4 Maternal data
Clinical data of the mother were collected using a self-reporting questionnaire. The patient reported facial flushing as a common side effect of DMF, which is mentioned in the summary of product characteristics (SmPCs) with an incidence of ≥10% (3).
3.5 Samples
Human milk samples were collected over a period of 24 h, starting from the intake of DMF in the evening of day 99 after delivery. These samples were collected at a steady state since the DMF treatment restarted 6 days prior. As the patient’s milk production was decreasing, limited total milk volumes and number of samples were expressed from both breasts during the sampling day.
A maternal blood sample was taken 75 min after collecting the last milk sample. A summary of the human milk sample characteristics is described in Table 1.
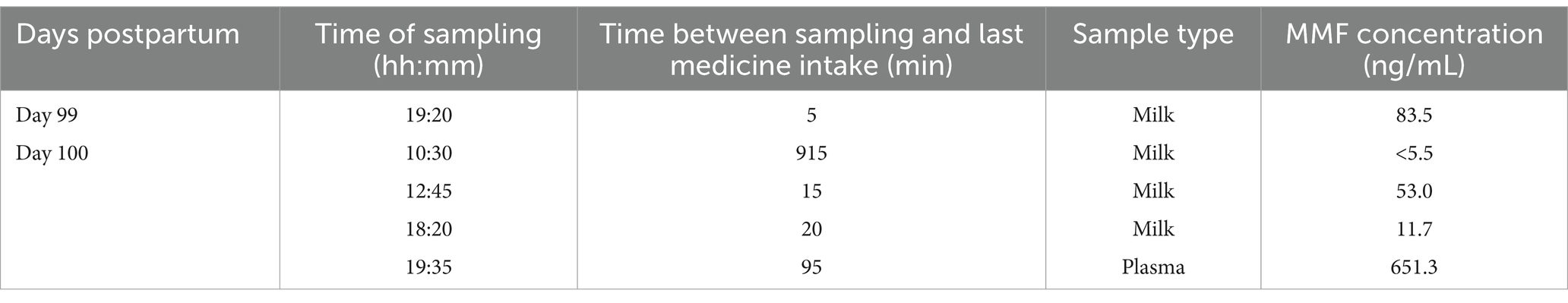
Table 1. Overview of the timing and the type of the collected samples along with the measured milk concentrations of monomethyl fumarate (MMF).
3.6 Human milk pharmacokinetics
A total of 4 human milk samples were collected by the patient. The concentration of MMF in the milk samples ranged from 83.5 ng/mL to a concentration under the detection limit of 5.5 ng/mL 2 h prior to the next dose. The concentrations found in our case report are presented in Figure 3 and pooled with the concentrations of the case series previously reported at 5 and 6 months postpartum (data extracted with Webplotdigitizer3) (10). All samples in these three pooled cases were collected at steady state, albeit with different daily doses (2×120 mg/day in the current case report and 2×240 mg/day as described by Ciplea et al., respectively).
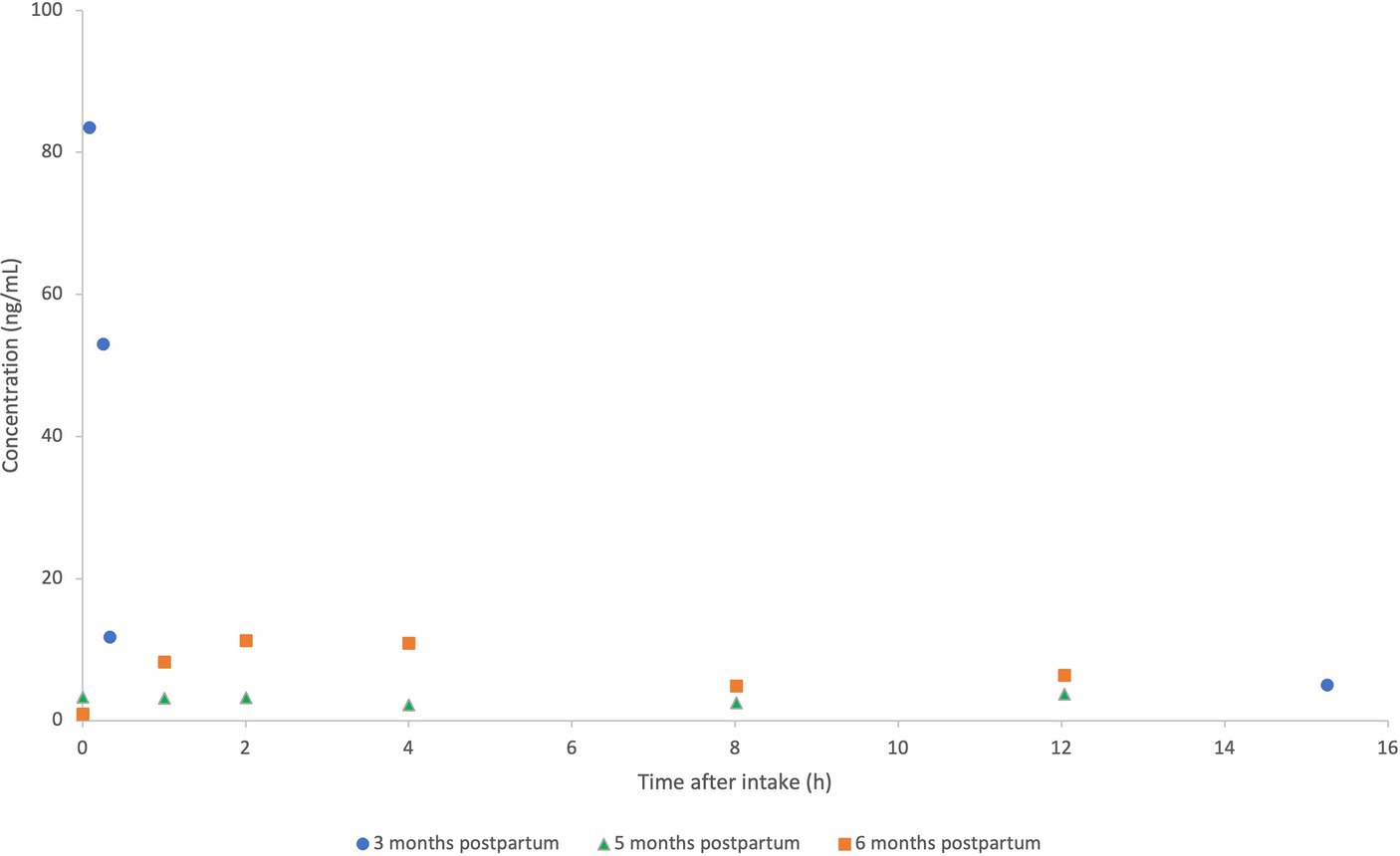
Figure 3. Pooled concentration-time profiles of monomethyl fumarate (MMF) in human milk at 3 months (2×120 mg/day; current case report), compared to concentrations of MMF at 5–6 months postpartum (2×240 mg/day in previously published case series) (10). MMF concentrations (ng/mL) of the patient at 3 months (current case report) (•), vs. 5 months (Δ) and 6 months (⎕) postpartum (case series as previously published by Ciplea et al.).
The concentration of MMF in the blood sample, 95 min after the last DMF intake, was 651.3 ng/mL.
3.7 Estimation of infant exposure
The daily infant dose (DID) for a breastfeeding infant after intake of DMF by the mother was calculated using the standardized milk intake of 150 mL/kg/day and milk intake of 200 mL/kg/day to estimate the infant dose in early infancy (Eq. 1).
In this case, the estimated DIDs of MMF were 5.76 and 7.68 μg/kg/day for a milk intake of 150 mL/kg/day and 200 mL/kg/day, respectively. To calculate the DID, the average steady-state milk concentration in this case report was based on the mean of the four milk samples collected by the patient, as we could not determine an area under the curve (AUC) due to the limited time points.
Calculating the RID was executed by comparing the DID of MMF through human milk, converted to the DID of DMF, and the DMF daily maternal dosage, using 62 kg as the maternal weight (Eq. 2).
The RID for DMF based on the DID with a standardized milk intake was 0.16%, and the RID calculating the intake in early infancy (200 mL/kg/day) was 0.22%.
As the efficacy and safety of DMF have not been established in pediatric patients under 10 years of age, the relative infant therapeutic dose (RIDtherapeutic) to compare the DID through human milk to the daily dosages for therapeutic goals, could not be calculated.
3.8 Milk-to-plasma (M/P) ratio
We compared the MMF concentration of all four milk samples to the concentration in the maternal plasma sample. These M/P ratios, ranging from 0.01 to 0.13, had a mean of 0.059. In addition, the predicted M/P ratio based on the empirical method developed by Koshimichi et al. was 0.06 (17). This empirical model predicts the M/P ratio based on the physicochemical parameters of the compound of interest, such as lipophilicity (LogP 0.34 and LogD 7.4 −3.09) and molecular weight (130.1 g/mol) (17, 18). These parameters for MMF were extracted from PubChem4 and via Chemaxon MarvinSketch (version Europium 7). However, this prediction is less reliable for medicines with a short half-life (5).
4 Discussion
This case report presents the concentrations of MMF as observed in human milk, the calculated M/P ratio, and the estimated child exposure to MMF at 3 months postpartum. This case report hereby adds to the very limited literature currently available, as only two cases of MMF transfer to human milk have been previously reported at 5 and 6 months after delivery (10). Furthermore, this is the first time the M/P ratio of MMF was calculated based on observed data and compared to the predicted M/P ratio according to the empirical method by Koshimichi et al. (17).
When comparing the human milk concentrations in our study, we noticed high intra-individual variability during the first hour after intake at a steady state (Figure 3). Notably, high inter-individual variability was also seen between both patients in the previous case series of Ciplea et al., with an AUC of 90 and 33 ng*h/mL, respectively. It is reported that the intake of DMF in combination with food, especially fatty food intake, can delay the peak concentration, but it is reported not to have a relevant effect on DMF subsequent exposure (2, 3). Intake with food is also recommended to reduce flushing, which means that this combination reflects common practices, as in this case.
Even though our patient was taking half the daily dose of the previously published case series, the concentrations found in the human milk of this case are considerably higher, with the highest measured concentration of 83.5 ng/mL (5 min after intake) compared to a Cmax of 11.2 and 3.7 ng/mL (2 h after intake) in Ciplea et al. This trend is also observed in the DID and RID. In this case report, the calculated DID for the standardized human milk intake (150 mL/kg/day) is 6- to 16-fold higher than the DIDs found in the previous case series. The RID, calculated with a human milk intake of 150 mL/kg/day, is 10-fold higher in our patient (10). In addition, the plasma levels in our patient were high compared to previous observations in patients using 240 mg of DMF twice daily (19).
We can only hypothesize on the underlying causes of these differences and the inter-individual variability. This might be explained by the physiological changes during the postpartum period in women. Some changes during postpartum, e.g., endocrine-driven metabolic clearance, are not completely understood and need further research (20). MMF metabolism by esterases in the gastrointestinal tract, tissues, and blood might be altered postpartum. For instance, the effect of rocuronium, a competitor of acetylcholine, is prolonged during the postpartum period, suggesting lower esterase activity. However, this prolongation can also be explained by the increased body weight, as this effect was not seen when doses were calculated by lean body mass (21). Gastric emptying rate could also play a factor in the absorption pattern of this compound (22), although this is not always observed postpartum (23). The peak concentration of MMF is postponed when the intake of DMF is combined with a fatty meal (3). This delayed absorption could contribute to the observed variability. Furthermore, the possibility of decreased elimination of MMF due to a change in the tricarboxylic acid cycle between 3 and 5–6 months postpartum is highly unlikely. Additionally, the risk of variability in DMF exposure due to pharmacogenetics is considered to be low and therefore, not a probable cause of these results (24). The hypothesis regarding the effect of postpartum physiological changes is further supported by the high concentration in plasma at 95 min after DMF intake compared to the literature (19).
Another hypothesis for the higher concentrations in our patient might be the accumulation of MMF in human milk, as this is a slightly lipophilic metabolite (LogP 0.34). Since our patient expressed milk four times during 24 h, compared to 6 times during 12 h in the case series (10), there might be more accumulation of MMF in the lipid fraction of human milk (5). However, the computed fraction unbound in human milk (fu,m 1.00) counters this hypothesis (17).
Despite the higher values in our study, the RIDs calculated with a human milk intake of 150 mL/kg/day and 200 mL/kg/day are 0.16 and 0.22%, respectively, which is far below the arbitrary threshold for acceptable risk for infants of 10% (25). In line with previous reports, these new findings show no new evidence for concern for DMF therapy during lactation. Furthermore, DMF is not expected to be present in human milk as it is rapidly metabolized into its metabolites, such as MMF. Nonetheless, next to the low RIDs, other aspects should be acknowledged regarding the safety of infants, such as oral bioavailability and potential developmental toxicity (10). Therefore, more data should be obtained to adequately advise DMF treatment to breastfeeding mothers, as high inter- and intra-individual variability has been observed and the maturational physiology of infants and its consequences are not yet completely understood. Moreover, the general health of breastfed infants whose mothers take DMF and possible infant side effects, e.g., flushing and gastrointestinal discomfort, should be studied as well (3). Unfortunately, data on infants’ tolerance during lactation-related DMF exposure were lacking both in this case report and the previously published case series (10).
Due to the nature of clinical lactation studies, it is uncertain if the patient managed to follow the instructions perfectly, a limitation related to the pragmatic design of these studies. For example, it is possible that milk was not fully expressed. This would mean that these calculations are based on concentrations found in foremilk. As foremilk has a considerably lesser fat content than hindmilk and MMF is (somewhat) lipophilic, the concentration measured with fully expressed milk might be higher than in this case report. As the patient had weaned her infant from breastfeeding, the DID could not be calculated using the exact intake volume for this infant. Additionally, the limited number of human milk samples did not allow us to calculate the AUC of MMF in human milk, and the Cmax could not be determined. Moreover, as all three mothers restarted their treatment after weaning their infant from breastfeeding, the effect of MMF in human milk could not be assessed in the infant, which is another knowledge gap that has to be addressed in the future. However, this report describes the earliest case of DMF treatment during lactation and hence, adds pieces to the puzzle to provide relevant information for evidence-based shared-decision making between health care professionals and patients. Due to the observed variability, future studies are warranted to further determine infant exposure to DMF through human milk. The development of PBPK models is recommended for this compound, as only few data are needed to additionally assess the extent of exposure to this compound in nursing infants (18).
In summary, the estimation of infant exposure is low in our case and the two other previously described cases, despite the relevant intra- and inter-individual observed variability. Research is needed to further assess the exposure and safety of the infant.
Data availability statement
The datasets presented in this article are not readily available because of concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to MVN, bWFydGplLnZhbm5lc3RlQGt1bGV1dmVuLmJl.
Ethics statement
The studies involving humans were approved by Ethics Committee Research UZ/KU Leuven (S67204). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MVN: Conceptualization, Data curation, Methodology, Project administration, Writing – original draft, Writing – review & editing. NN: Conceptualization, Methodology, Project administration, Writing – review & editing. MC: Conceptualization, Methodology, Project administration, Writing – review & editing. BC: Conceptualization, Data curation, Methodology, Writing – review & editing. PA: Conceptualization, Methodology, Project administration, Writing – review & editing, Supervision. AS: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. KA: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The ConcePTION project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement no. 821520. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and the EFPIA. Nina Nauwelaerts also received a PhD scholarship from the Research Foundation – Flanders (1S50721N). The research activities of Anne Smits are supported by a Senior Clinical Investigatorship of the Research Foundation – Flanders (FWO) (18E2H24N). The research activities of Michael Ceulemans are supported by the Internal Funds KU Leuven (PDMt1/23/020).
Acknowledgments
The authors thank the patient for collecting human milk samples and participating in this study. The research project leading to these results was conducted as part of the ConcePTION consortium. This paper only reflects the personal views of the stated authors. The authors are grateful for the contributions of Kristel Van Calsteren and An Eerdekens in developing the UmbrelLACT protocol. The authors thank Sam De Craemer and Bart Ghesquiere from the VIB Metabolomics Core Facility Leuven for the bio-analyses. The authors are grateful to the KU Leuven, YouReCa, and the Flemish Government for supporting the IdeaLab project ‘Momsmed’, in which most of the authors were involved, and which boosted the interdisciplinary collaboration on clinical lactation research within our institute. The figures were created using BioRender.com.
Conflict of interest
PA is co-owner of the company BioNotus GCV.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Several authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^https://www.who.int/health-topics/breastfeeding#tab=tab_1
2. ^https://www.imi-conception.eu/
References
1. Callegari, I, Derfuss, T, and Galli, E. Update on treatment in multiple sclerosis. Presse Med. (2021) 50:104068. doi: 10.1016/j.lpm.2021.104068
2. Bresciani, G, Manai, F, Davinelli, S, Tucci, P, Saso, L, and Amadio, M. Novel potential pharmacological applications of dimethyl fumarate—an overview and update. Front Pharmacol. (2023) 14:1264842. doi: 10.3389/fphar.2023.1264842
3. European Medicine Agency. Dimethyl fumarate Polpharma: Summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/dimethyl-fumarate-polpharma-epar-product-information_en.pdf (Accessed December 1, 2023); (2023).
4. Gold, R, Kappos, L, Arnold, DL, Bar-Or, A, Giovannoni, G, Selmaj, K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. (2012) 367:1098–107. doi: 10.1056/NEJMoa1114287
6. Almas, S, Vance, J, Baker, T, and Hale, T. Management of multiple sclerosis in the breastfeeding mother. Mult Scler Int. (2016) 2016:1–10. doi: 10.1155/2016/6527458
7. Atkinson, HC, Begg, EJ, and Darlow, BA. Drugs in human milk. Clinical pharmacokinetic considerations. Clin Pharmacokinet. (1988) 14:217–40. doi: 10.2165/00003088-198814040-00003
8. Vukusic, S, Michel, L, Leguy, S, and Lebrun-Frenay, C. Pregnancy with multiple sclerosis. Rev Neurol. (2021) 177:180–94. doi: 10.1016/j.neurol.2020.05.005
9. Varytė, G, Arlauskienė, A, and Ramašauskaitė, D. Pregnancy and multiple sclerosis: an update. Curr Opin Obstet Gynecol. (2021) 33:378–83. doi: 10.1097/GCO.0000000000000731
10. Ciplea, AI, Datta, P, Rewers-Felkins, K, Baker, T, Gold, R, Hale, TW, et al. Dimethyl fumarate transfer into human milk. Ther Adv Neurol Disord. (2020) 13:1756286420968414. doi: 10.1177/1756286420968414
11. del Ciampo, LA, and del Ciampo, IRL. Breastfeeding and the benefits of lactation for women’s health. Rev Bras Ginecol Obstet. (2018) 40:354–9. doi: 10.1055/s-0038-1657766
12. Fomina, YY, Byrne, JJ, and Spong, CY. Evaluating strength of recommendations for commonly administered medications in lactating women. J Matern Fetal Neonatal Med. (2023) 36:2163626. doi: 10.1080/14767058.2022.2163626
13. Ren, Z, Bremer, AA, and Pawlyk, AC. Drug development research in pregnant and lactating women. Am J Obstet Gynecol. (2021) 225:33–42. doi: 10.1016/j.ajog.2021.04.227
14. Van Neste, M, Nauwelaerts, N, Ceulemans, M, Van, CK, Eerdekens, A, Annaert, P, et al. Determining the exposure of maternal medicines through breastfeeding: the UmbrelLACT study protocol-a contribution from the ConcePTION project. BMJ Paediatr Open. (2024) 8:e002385. doi: 10.1136/bmjpo-2023-002385
15. Gagnier, JJ, Kienle, G, Altman, DG, Moher, D, Sox, H, and Riley, D. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. (2013) 2013:bcr2013201554. doi: 10.1136/bcr-2013-201554
16. Anderson, PO. Guidelines for reporting cases of medication use during lactation. Breastfeed Med. (2022) 17:93–7. doi: 10.1089/bfm.2021.0357
17. Koshimichi, H, Ito, K, Hisaka, A, Honma, M, and Suzuki, H. Analysis and prediction of drug transfer into human milk taking into consideration secretion and reuptake clearances across the mammary epithelia. Drug Metab Dispos. (2011) 39:2370–80. doi: 10.1124/dmd.111.040972
18. Nauwelaerts, N, Macente, J, Deferm, N, Bonan, RH, Huang, MC, Van Neste, M, et al. Generic workflow to predict medicine concentrations in human Milk using physiologically-based pharmacokinetic (PBPK) modelling—a contribution from the ConcePTION project. Pharmaceutics. (2023) 15:1469. doi: 10.3390/pharmaceutics15051469
19. Edwards, KR, Kamath, A, Button, J, Kamath, V, Mendoza, JP, Zhu, B, et al. A pharmacokinetic and biomarker study of delayed-release dimethyl fumarate in subjects with secondary progressive multiple sclerosis: evaluation of cerebrospinal fluid penetration and the effects on exploratory biomarkers. Mult Scler Relat Disord. (2021) 51:102861. doi: 10.1016/j.msard.2021.102861
20. Van Neste, M, Bogaerts, A, Nauwelaerts, N, Macente, J, Smits, A, Annaert, P, et al. Challenges related to Acquisition of Physiological Data for physiologically based pharmacokinetic (PBPK) models in postpartum, lactating women and breastfed infants—a contribution from the ConcePTION project. Pharmaceutics. (2023) 15:2618. doi: 10.3390/pharmaceutics15112618
21. Gin, T, Chan, MT, Chan, KL, and Yuen, PM. Prolonged neuromuscular block after Rocuronium in postpartum patients. Anesth Analg. (2002) 94:686–9. doi: 10.1097/00000539-200203000-00038
22. Gin, T, Cho, AMW, Lew, JK, Lau, GS, Yuen, PM, Critchley, JA, et al. Gastric emptying in the postpartum period. Anaesth Intensive Care. (1991) 19:521–4. doi: 10.1177/0310057X9101900405
23. Howle, R, Sultan, P, Shah, R, Sceales, P, Van de Putte, P, and Bampoe, S. Gastric point-of-care ultrasound (PoCUS) during pregnancy and the postpartum period: a systematic review. Int J Obstet Anesth. (2020) 44:24–32. doi: 10.1016/j.ijoa.2020.05.005
24. Xu, L, Peng, CC, Dawson, K, Stecher, S, Woodworth, J, and Prakash, C. Metabolism, pharmacokinetics and excretion of [14C]dimethyl fumarate in healthy volunteers: an example of xenobiotic biotransformation following endogenous metabolic pathways. Xenobiotica. (2023) 53:163–72. doi: 10.1080/00498254.2023.2217506
Keywords: case report, breastfeeding, human milk, lactation, pharmacokinetics, multiple sclerosis, dimethyl fumarate, monomethyl fumarate
Citation: Van Neste M, Nauwelaerts N, Ceulemans M, Cuppers B, Annaert P, Smits A and Allegaert K (2024) Very low monomethyl fumarate exposure via human milk: a case report—a contribution from the ConcePTION project. Front. Public Health. 12:1393752. doi: 10.3389/fpubh.2024.1393752
Edited by:
Michelle Plusquin, University of Hasselt, BelgiumReviewed by:
Douglas De Souza, State University of Campinas, BrazilLena De Hondt, Vrije University Brussels, Belgium
Copyright © 2024 Van Neste, Nauwelaerts, Ceulemans, Cuppers, Annaert, Smits and Allegaert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martje Van Neste, bWFydGplLnZhbm5lc3RlQGt1bGV1dmVuLmJl
 Martje Van Neste
Martje Van Neste Nina Nauwelaerts
Nina Nauwelaerts Michael Ceulemans
Michael Ceulemans Benedikte Cuppers4
Benedikte Cuppers4 Pieter Annaert
Pieter Annaert Anne Smits
Anne Smits Karel Allegaert
Karel Allegaert