- 1Department of Clinical Nutrition, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Health Management Center, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Shanghai Pudong New Area Caolu Community Health Center, Shanghai, China
- 4Stress Research Institute, Department of Psychology, Stockholm University, Stockholm, Sweden
- 5School of Public Health, North China University of Science and Technology, Tangshan, China
- 6Department of Nutrition, College of Health Science and Technology, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Objectives: This study aimed to fill the data gap of the course of renal function decline in old age and explore changes in renal function across different health states with increasing age.
Methods: This observational, retrospective, single-center cohort study included 5,112 Chinese older adults (3,321 men and 1,791 women, range 60–104 years). The individual rate of estimated glomerular filtration rate (eGFR) decline was analyzed using linear mixed-effects model to account for repeated measures over the years.
Results: The median age was 66 years, median BMI was 24.56 kg/m2, and median eGFR was 89.86 mL/min.1.73 m2. For every 1-year increase in age, women’s eGFR decreased by 1.06 mL/min/1.73 m2 and men’s by 0.91 mL/min/1.73 m2. We observed greater age-related eGFR decline in men and women with high systolic blood pressure (SBP). Men with high triglyceride (TG), high low-density lipoprotein cholesterol (LDL-C), and low high-density lipoprotein cholesterol (HDL-C), had greater age-related eGFR decline. In women, different BMI groups showed significant differences in age-related eGFR decline, with the highest decline in those with obesity. Additionally, participants with normal baseline eGFR had a faster age-related decline than those with low baseline eGFR.
Conclusion: The eGFR declined linearly with age in Chinese older adults, with women exhibiting a slightly faster decline than men. Both men and women should be cautious of SBP. Older adults with normal baseline renal function experienced a faster eGFR decline. Men with high TG, LDL-C, and low HDL-C levels, as well as obese women, should be vigilant in monitoring renal function.
1 Introduction
The global population’s aging is the most important medical and social demographic problem worldwide (1). In recent decades, increased average life expectancy has led to a higher proportion of older adults individuals worldwide, accompanied by a rise in non-communicable diseases, including chronic kidney disease (CKD). Aging is a progressive and inevitable biological process, characterized by structural and functional changes in all organs, including the kidneys. The decline in kidney function with advancing age primarily manifests as a decrease in glomerular filtration rate (GFR) (2, 3), the most widely used parameter for measuring kidney function. Although the decrease in GFR with aging has been recognized, the exact estimation of the magnitude of the renal function decline with healthy aging is not yet well-established.
Most studies (4, 5) investigating GFR decline in older adults have relied on cross-sectional designs, which may complicate interpretation due to interindividual modeling across different age groups, disregarding individual trajectories over time.
As the previous study confirmed (6), the generation change in the cross-sectional study and the eGFR decline rate in the longitudinal study are different. Moreover, few studies have included community-dwelling individuals, limiting their ability to reflect real-world decline in a representative older adult population (7). Longitudinal studies with repeated eGFR measurements to model age-related trajectory in old age are scarce. Our research tried to fill the data gap of the course of renal function decline in the older adults population in China, which country has the world’s largest older population (8). Gold standard GFR measurement methods, such as inulin clearance or plasma clearance using radiolabeled or non-radiolabeled exogenous markers, are laborious, expensive, and not feasible for routine clinical use (9). Therefore, estimated glomerular filtration rate (eGFR) is a more accessible approach for evaluating kidney function in daily clinical practice.
Considering the frequent comorbidities, such as obesity (10), diabetes (11, 12) and cardiovascular diseases (13), prevalent in the geriatric population and their potential impact on kidney function, we conducted further analyses to examine changes in renal function among the older adults across different health states with increasing age. Investigating the interaction between health status and age on the renal function decline among the older adults is essential for developing targeted interventions that enhance health status and quality of life, promoting healthy aging.
2 Materials and methods
2.1 Study cohort
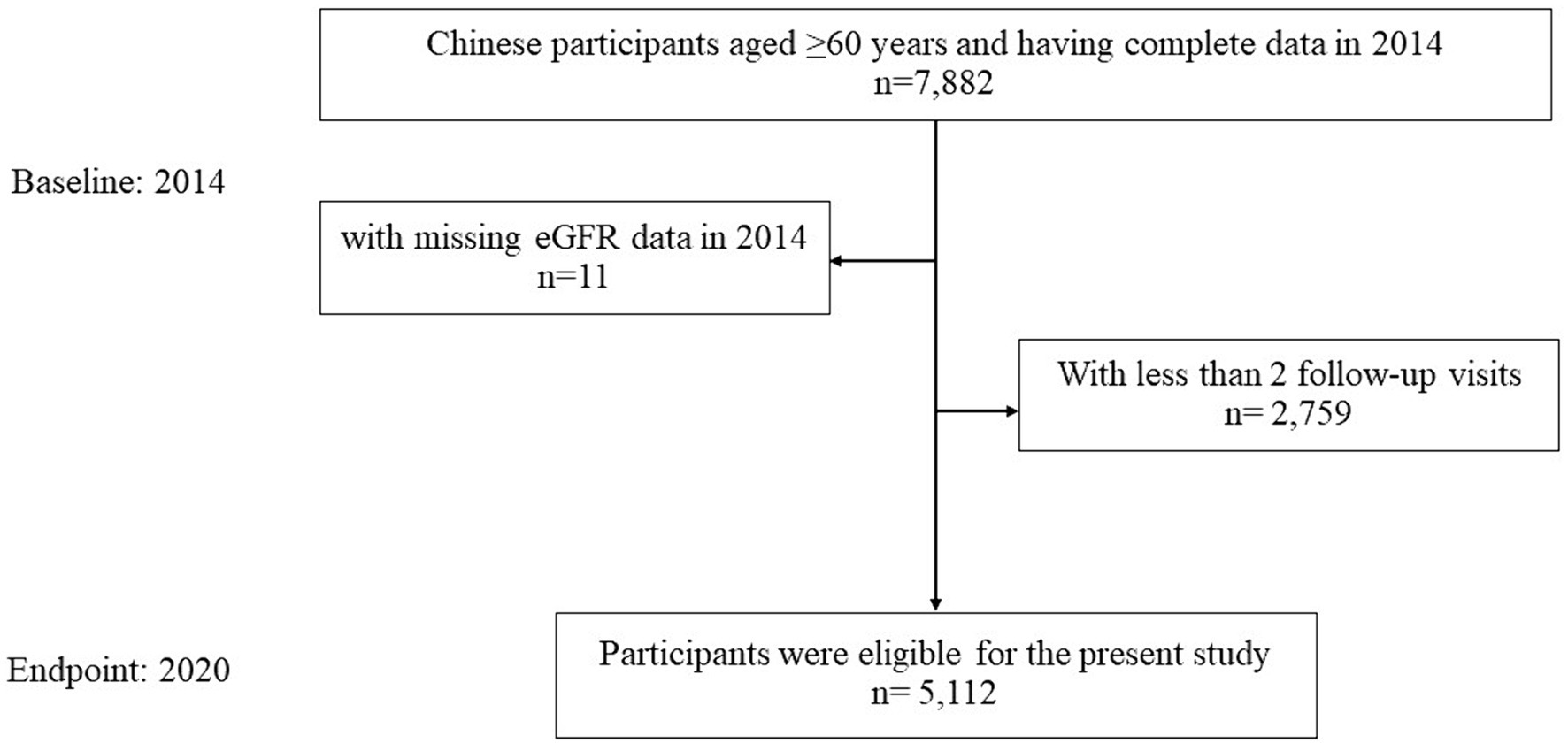
This study was an observational, retrospective, single-center cohort study, which derived from a total number of 7,871 Chinese older participants who had received a health check at the Health Management Center of Ren Ji hospital in year 2014, with available eGFR information. Participants were followed annually to 2020. After excluding participants with less than 1 follow-up visits (n = 2,759) after 2014, the sample size was 5,112 Chinese older adults (3,321 men and 1,791 women, range 60–104 years), with 22,072 observations (Figure 1). Throughout the study period, the proportion of missing participants in annual follow-up examinations increased progressively, starting from baseline as follows: 0, 18.6, 30.6, 42.2, 51.2, 59.5, 66.1%. Notably, 1,059 participants completed every follow-up throughout the entire cohort study period.
2.2 Estimated glomerular filtration rate
All biochemical tests were performed on venous blood taken after fasting for at least 8 h. The eGFR was calculated by the CKD-EPI equation based on age, sex, and serum creatinine concentration (14). We annually measured serum level of creatinine for each participant through the study (2014–2020).
2.3 Clinical information
Clinical information was abstracted from medical records. In brief, height and body weight were measured by trained nurses. According to body mass index (BMI), participants were classified as underweight (< 18.5 kg/m2), normal weight (18.5 to 23.9 kg/m2), overweight (24.0 to 27.9 kg/m2), or obese (≥ 28.0 kg/m2) based on Chinese criteria (15). Blood pressure was measured using an automatic blood pressure monitor (HBP-9020, Omron, China) after at least 10-min rest. Venous blood samples were drawn in the morning after participants were fasted for at least 8 h. All the blood samples were analyzed in the same clinical laboratory center. Serum creatinine was analyzed using the enzymatic method by Roche (CREP2; Roche Diagnostics, Mannheim, Germany), fasting blood glucose (FBG) was analyzed using the hexokinase method, total cholesterol (TC) and total triglycerides (TG) were analyzed using the enzymatic colorimetric method, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were analyzed using the homogeneous enzymatic colorimetric assay. These six indicators were analyzed on Roche Cobas C701 module. Hemoglobin was measured by an automatic hematology analyzer (XN-10, Sysmex, Japan). The classified criteria were as follows: systolic blood pressure (SBP) ≥ 140 mmHg (high SBP), diastolic blood pressure (DBP) ≥ 90 mmHg (high DBP) (16), FBG ≥ 7.0 mmoL/L (high FBG) (17), TC ≥ 6.2 mmol/L (high TC), TG ≥ 2.3 mmol/L (high TG), LDL-C ≥ 4.1 mmol/L (high LDL-C), HDL-C < 1.0 mmol/L (low HDL-C) (18), eGFR <60 mL/min/1.73 m2 (low eGFR). Anemia was diagnosed if hemoglobin was <130 g/L in men while <120 g/L in women (19).
2.4 Statistical analysis
In the descriptive statistics, continuous variables were tested for normal distribution, and if deviating, represented using medians with interquartile ranges (25th-75th quartile). Categorical variables were shown as percentage. The individual rate of eGFR decline (i.e., the slope; ml/min/1.73m2/year) was analyzed using linear mixed-effects model to account for repeated measures over the years. Missing data were accounted for by using the mixed-effects model (20).
To quantify the age-dependent eGFR, our main analyses tested the association between age and eGFR, while omitting the interaction between age and follow-up time. This was because there was no interaction between age and follow-up duration observed. In contrast, as noticing a significant difference between the sexes, all the analyses were performed among men and women separately (Table 1). We then tested the additive interaction between age and the baseline health status, indicated by other biomarkers on eGFR (Table 2). In the end, sensitivity analyses were conducted to repeat the abovementioned analyses on the 1,059 participants who had undergone all follow-ups, for testing the existence of selection bias.
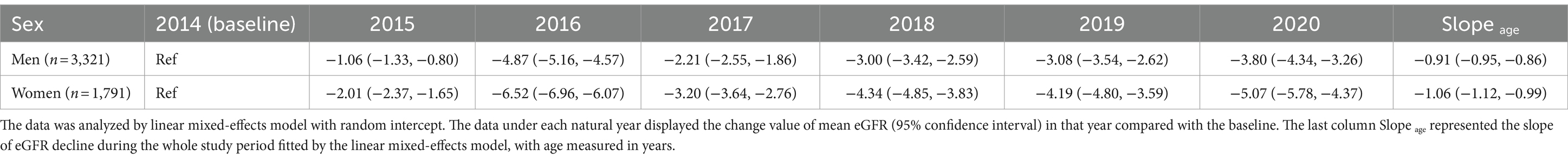
Table 1. Age-related change of eGFR (ml/min/1.73 m2) in 5,112 Chinese older adults during the whole cohort study.
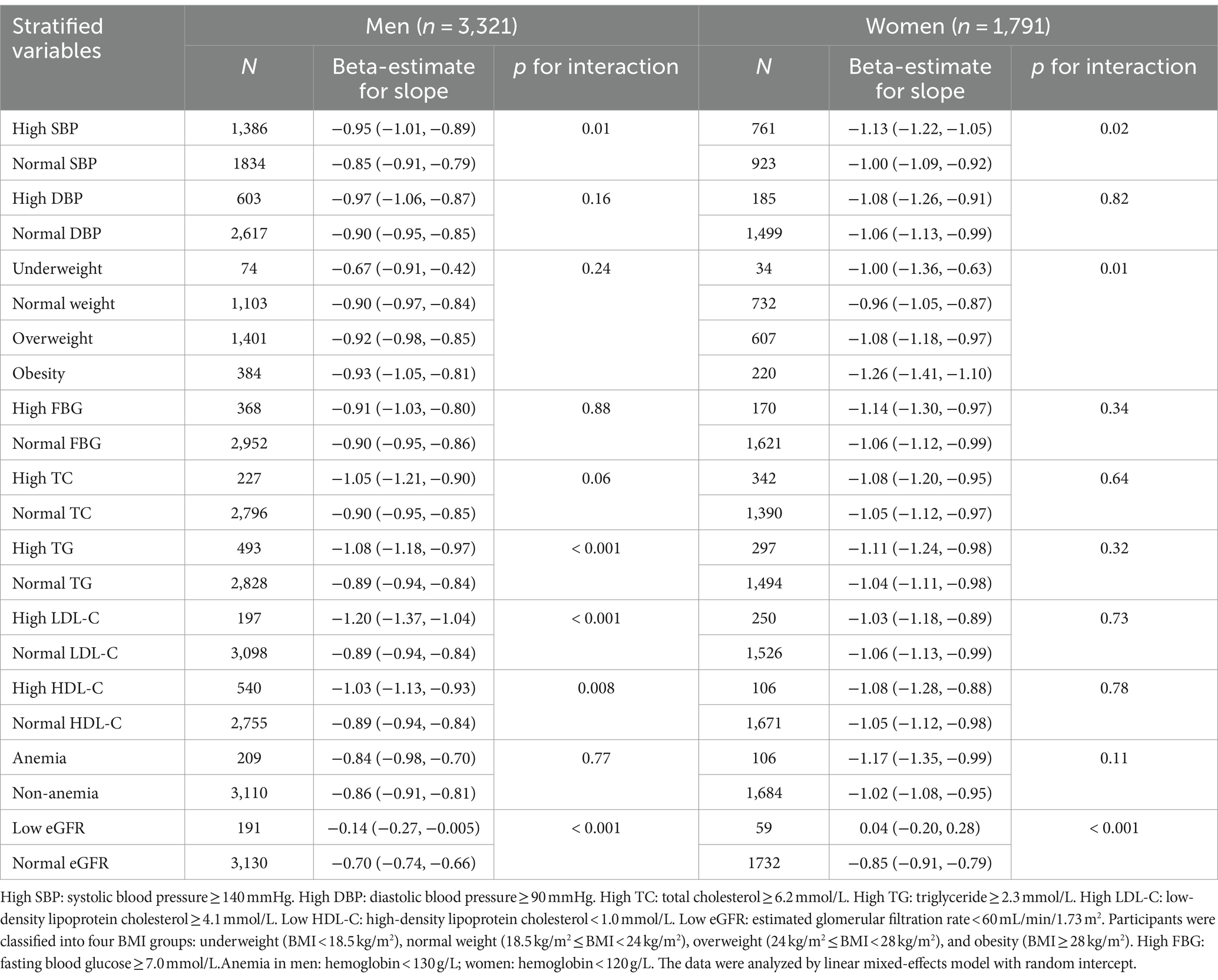
Table 2. Additive interactions between baseline health indicators and age on eGFR among 5,112 Chinese older adults.
All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). Confidence intervals (CI) were determined at 95% level.
3 Results
3.1 Main baseline characteristics
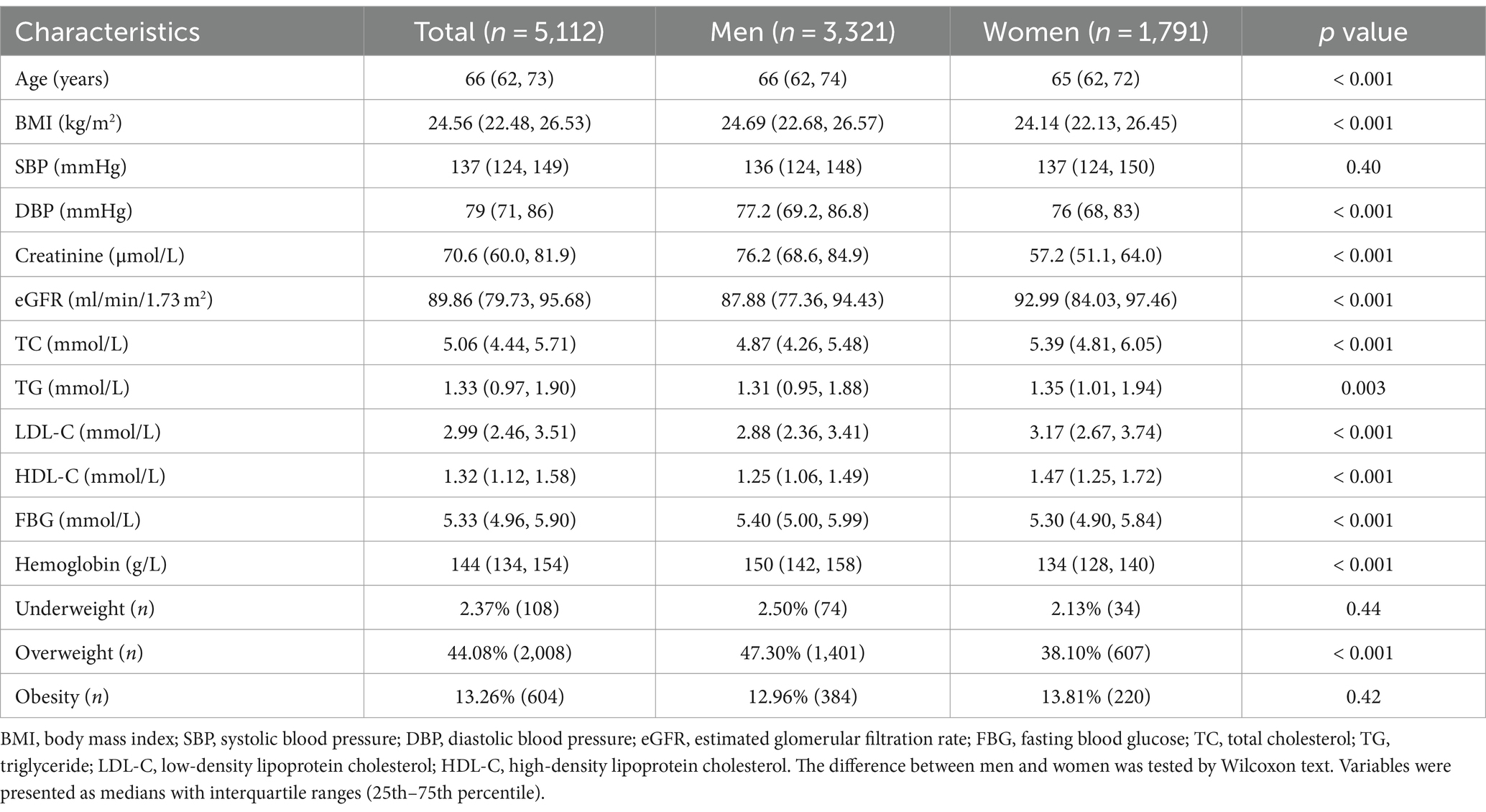
Among 5,112 participants in 2014, mean eGFR was 87.88 (77.36, 94.43) ml/min/1.73 m2 among men and 92.99 (84.03, 97.46) ml/min/1.73 m2 among women. The baseline characteristics were presented in Table 3. Baseline characteristics of the study participants showed that men had higher serum creatinine levels, lower eGFR, lower levels of total cholesterol, triglycerides, LDL-C, and HDL-C compared to women. Slightly higher DBP and FBG were observed in males, but no significant difference was found in SBP. The prevalence of underweight and obesity was similar between males and females, but the rate of overweight was higher in males (47.3% versus 38.1%) compared to females.
3.2 Age-related change of eGFR
The slope of age obtained from the linear mixed-effects model represents the change in eGFR for every 1-year increase in age. As shown in Table 1, for every 1-year increase in age, eGFR decreased by 1.06 mL/min/1.73 m2 (0.99, 1.12) in women, while it decreased by 0.91 mL/min/1.73 m2 (0.86, 0.95) in men. Specific trajectory according to age groups were presented in Figure 2. We analyzed eGFR decline in healthy older adults without hypertension, hyperglycemia, dyslipidemia, low eGFR, and obesity. In line with the main analysis, the declining trend in the healthy older adults appears to be consistent, albeit at a slower rate of decline (Supplementary Table 1).
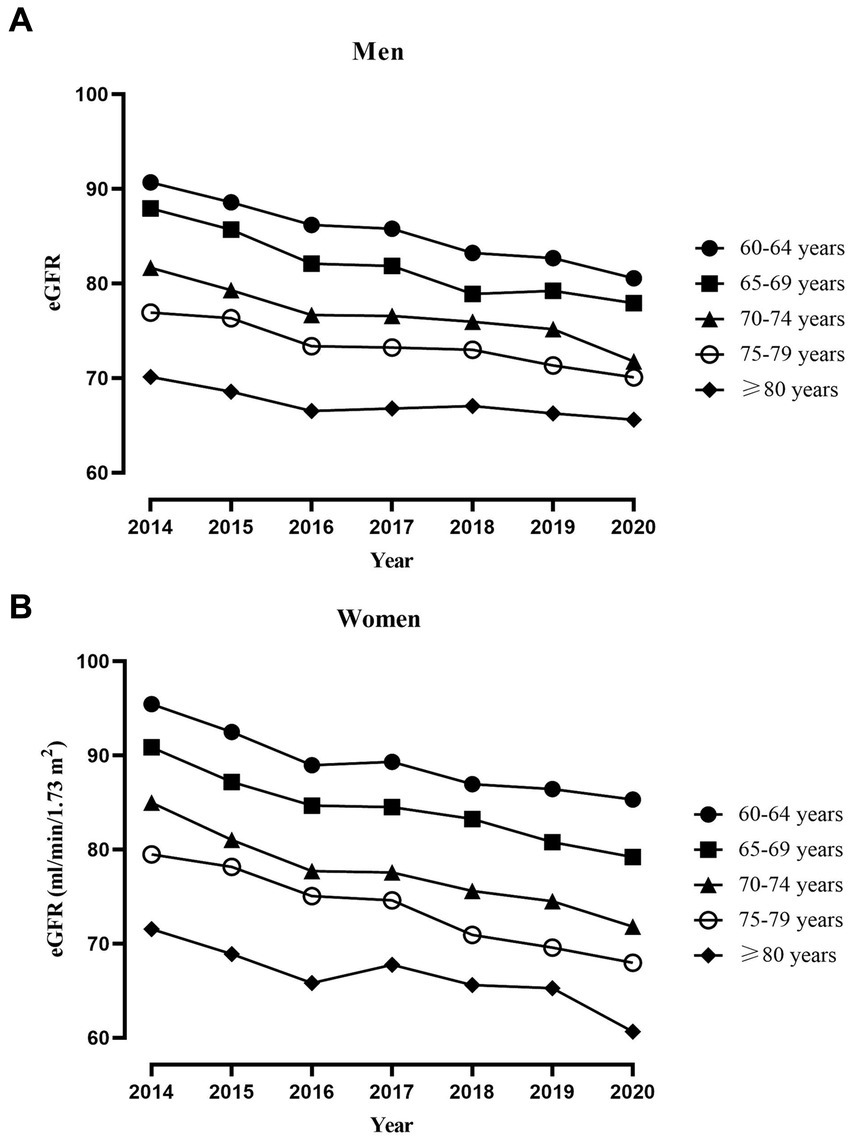
Figure 2. Trajectory of average eGFR in different age groups of older adults individuals throughout the study period. (A) Trajectory of average eGFR in 3,321 men. (B) Trajectory of average eGFR in 1,791 women.
3.3 Additive interactions between health indicators and age on eGFR
In Table 2, we observed greater age-related eGFR decline in men and women with high SBP compared to normal SBP (p interaction = 0.01 and 0.02). Men with high TG and high LDL-C also had greater age-related eGFR decline compared to normal TG and LDL-C (both p interaction < 0.001). Additionally, men with low HDL-C had larger age-related eGFR decline compared to normal HDL-C (p interaction = 0.006). In women, there was a significant difference in the age-related decline of eGFR among different BMI groups, with the highest decline observed in obese women and the slowest decline observed in women with normal weight (p interaction = 0.01). Furthermore, there was no significant difference in age-related decline of eGFR between anemic individuals, irrespective of gender, and non-anemic individuals. In both men and women with normal baseline eGFR, the age-related decline was significantly faster than in those with low baseline eGFR.
3.4 Sensitivity analyses
The sensitivity analysis of 1,059 participants who completed every follow-up revealed that estimated values in Supplementary Table 2 closely mirrored those in the main analyses. Confidence intervals in Supplementary Table 3 consistently overlapped with the main analyses. Discrepancies in p-values across certain groups may be attributed to varying sample sizes, with selection bias less likely to influence outcomes.
4 Discussion
We investigated the course of kidney function decline in the cohort of older participants using repeat GFR estimates and observed the following main findings. First, we found that the eGFR, determined by serum creatinine, declined linearly with age in Chinese older adults. For every 1-year increase in age, eGFR decreased by 1.06 mL/min/1.73 m2 (0.99, 1.12) in women, while it decreased by 0.91 mL/min/1.73 m2 (0.86, 0.95) in men.
The discovery of linear decline was like the longitudinal German population-based cohort in persons aged 70 and above with a repeated estimation of GFR over a median of 6.1 years of follow-up (7). The possible reason is that aging leads to notable kidney structural and functional changes, even without age-related health issues. Kidney cortical volume decreases, surface roughness increases, and simple renal cysts grow with age. Additionally, histologic signs of nephrosclerosis increase with age, as does the decline in nephron number and whole-kidney GFR (21). Moreover, a recent longitudinal analysis of older adults stage 4 and 5 CKD showed similar linear decline, but opposite gender differences compared to our study (22). This difference may be due to higher comorbidity burden and sex differences in advanced CKD patients. For instance, male participants had twice the myocardial infarction rate of female participants. One of the possible reasons for the gender difference may be caused by a sex-dependent decrease in muscle mass with age, biasing our eGFRs.
Second, we found that older adults individuals with poor baseline renal function experienced a slower decline in age-related glomerular filtration rate compared to those with normal renal function. A large Japanese longitudinal study revealed for the first time that eGFR decline rate in healthy subjects depended mainly on eGFR at baseline, but not on age (6). The results of this study supported our findings, which were different from those of previous studies. For example, a study more than 10 years ago showed that eGFR decline was faster with a lower baseline eGFR (23). The discrepancy in research findings was primarily attributed to the previous study measuring serum creatinine only twice over a ten-year period, while our study conducted annual measurements for 6 years. Furthermore, distinct fitting models were utilized for statistical analysis. The reason why the rate of eGFR decline was slower with a lower baseline eGFR is unclear, but a compensatory mechanism might work as kidney function decreases.
Furthermore, we observed greater age-related eGFR decline both in men and women with high SBP compared to normal SBP. A Japanese cohort study also showed that a difference in SBP, but not DBP, is independently associated with a rapid eGFR decline in the general Japanese population, including older subjects (24). Various mechanisms might contribute to kidney injuries in older adults hypertensive patients, including the renin-angiotensin-aldosterone system, oxidative stress, endothelial dysfunction, and genetic and epigenetic factors (25).
Finally, we observed that men with high triglyceride, high LDL-C, or low HDL-C had a faster age-related eGFR decline. Additionally, in BMI groups, obese women experienced the fastest age-related eGFR decline. Other studies (26, 27) also found that high triglyceride and low HDL-C predicted renal function decline. A 7-year cohort study of the older adults found that baseline BMI was associated with an increased risk of rapid eGFR loss (28). Pathways through which obesity might cause renal damage were not well understood. Potential mechanisms included sympathetic nervous and renin-angiotensin-aldosterone systems activation, mechanical stress, hormonal imbalance, and production of inflammatory cytokines (29). Some scholars proposed that renal lipid accumulation could cause structural and functional changes in mesangial cells, podocytes, and proximal tubule cells, affecting nephron function (30). Irrespective of the underlying pathophysiological mechanisms, it was crucial for older adults with obesity or hyperlipidemia to closely monitor their renal function. Further research and clinical trials were needed to assess the efficacy of these interventions in this vulnerable population.
The study’s longitudinal design with a large cohort and multiple biomarkers enhances its uniqueness. It improves our understanding of age-related kidney function decline and provides valuable insights into eGFR decline patterns in old age. It addresses the data gap on renal function decline in China’s older adults population across different health conditions. This study has practical implications for clinical practice and future gerontology research, facilitating the development of targeted interventions to enhance the health status and quality of life of the older adults, promoting healthy aging.
Our study did not include the endogenous biomarker cystatin C, which is potentially more suitable for assessing renal function in older individuals compared to creatinine, as creatinine levels can be affected by sarcopenia (31). This retrospective observational study may include both healthy and sick individuals. Limited data on proteinuria (24), etiological diagnosis of CKD, comorbidities (10, 12, 13), and medication (32) makes it impossible to exclude the impact of diseases and medication on renal function. These uncontrolled confounding factors restrict the generalizability of the study results. Additionally, there may be other factors, such as socioeconomic status (33), dietary habits (34, 35), and physical activity (36), that could influence the risk of renal function decline. However, information regarding these factors was not available in our study.
5 Conclusion
The eGFR, determined by serum creatinine, declined linearly with age in Chinese older adults. Women exhibited a slightly faster age-related decline in eGFR compared to men. Additionally, older adults with good baseline renal function experienced a more rapid eGFR decline than those with lower baseline eGFR. Both men and women should be cautious of SBP. Men with high TG, LDL-C, and low HDL-C levels, as well as obese women, should be vigilant in monitoring renal function.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Committee of Ren Ji Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YJ: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Formal analysis, Data curation, Conceptualization. QC: Writing – review & editing, Validation, Supervision, Resources, Project administration, Investigation, Data curation. WH: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Formal analysis, Conceptualization. TX: Writing – review & editing, Writing – original draft, Software, Methodology, Data curation. MT: Writing – original draft, Visualization, Software, Methodology, Data curation. YL: Writing – review & editing, Visualization, Supervision, Conceptualization. RX: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1392903/full#supplementary-material
Abbreviations
CKD, Chronic kidney disease; GFR, Glomerular filtration rate; eGFR, Estimated glomerular filtration rate; BMI, Body mass index; FBG, Fasting blood glucose; TC, Total cholesterol; TG, Triglyceride; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; SBP, Systolic blood pressure; DBP, Diastolic blood pressure.
References
1. Rudnicka, E, Napierała, P, Podfigurna, A, Męczekalski, B, Smolarczyk, R, and Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas. (2020) 139:6–11. doi: 10.1016/j.maturitas.2020.05.018
2. Noronha, IL, Santa-Catharina, GP, Andrade, L, Coelho, VA, Jacob-Filho, W, and Elias, RM. Glomerular filtration in the aging population. Front Med. (2022) 9:769329. doi: 10.3389/fmed.2022.769329
3. Denic, A, Glassock, RJ, and Rule, AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. (2016) 23:19–28. doi: 10.1053/j.ackd.2015.08.004
4. Eriksen, BO, Palsson, R, Ebert, N, Melsom, T, van der Giet, M, Gudnason, V, et al. GFR in healthy aging: an individual participant data meta-analysis of Iohexol clearance in European population-based cohorts. J Am Soc Nephrol. (2020) 31:1602–15. doi: 10.1681/ASN.2020020151
5. Wetzels, JF, Kiemeney, LA, Swinkels, DW, Willems, HL, and den Heijer, M. Age-and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen biomedical study. Kidney Int. (2007) 72:632–7. doi: 10.1038/sj.ki.5002374
6. Baba, M, Shimbo, T, Horio, M, Ando, M, Yasuda, Y, Komatsu, Y, et al. Longitudinal study of the decline in renal function in healthy subjects. PLoS One. (2015) 10:e0129036. doi: 10.1371/journal.pone.0129036
7. Schaeffner, ES, Ebert, N, Kuhlmann, MK, Martus, P, Mielke, N, Schneider, A, et al. Age and the course of GFR in persons aged 70 and above. Clin J Am Soc Nephrol. (2022) 17:1119–28. doi: 10.2215/CJN.16631221
8. Chen, X, Giles, J, Yao, Y, Yip, W, Meng, Q, Berkman, L, et al. The path to healthy ageing in China: a Peking University-lancet commission. Lancet. (2022) 400:1967–2006. doi: 10.1016/S0140-6736(22)01546-X
9. Raman, M, Middleton, RJ, Kalra, PA, and Green, D. Estimating renal function in old people: an in-depth review. Int Urol Nephrol. (2017) 49:1979–88. doi: 10.1007/s11255-017-1682-z
10. Chen, Y, Dabbas, W, Gangemi, A, Benedetti, E, Lash, J, Finn, PW, et al. Obesity management and chronic kidney disease. Semin Nephrol. (2021) 41:392–402. doi: 10.1016/j.semnephrol.2021.06.010
11. Thomas, MC, Cooper, ME, and Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. (2016) 12:73–81. doi: 10.1038/nrneph.2015.173
12. Garla, V, Kanduri, S, Yanes-Cardozo, L, and Lién, LF. Management of diabetes mellitus in chronic kidney disease. Minerva Endocrinol. (2019) 44:273–87. doi: 10.23736/S0391-1977.19.03015-3
13. Teo, BW, Chan, GC, Leo, CCH, Tay, JC, Chia, YC, Siddique, S, et al. Hypertension and chronic kidney disease in Asian populations. J Clin Hypertens. (2021) 23:475–80. doi: 10.1111/jch.14188
14. Stevens, LA, Claybon, MA, Schmid, CH, Chen, J, Horio, M, Imai, E, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. (2011) 79:555–62. doi: 10.1038/ki.2010.462
15. Zhou, BF . Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases--report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed Environ Sci. (2002) 15:245–52.
16. Joint Committee for Guideline Revision . 2018 Chinese guidelines for prevention and treatment of hypertension-a report of the revision Committee of Chinese Guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. (2019) 16:182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014
17. American Diabetes Association Professional Practice Committee . Classification and diagnosis of diabetes: standards of medical Care in Diabetes-2022. Diabetes Care. (2022) 45:S17–s38. doi: 10.2337/dc22-S002
18. Joint Committee for Developing Chinese guidelines on prevention and treatment of Dyslipidemia in adults . Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. (2007) 35:390–419. doi: 10.3760/j.issn:0253-3758.2007.05.003
19. Katsumi, A, Abe, A, Tamura, S, and Matsushita, T. Anemia in older adults as a geriatric syndrome: a review. Geriatr Gerontol Int. (2021) 21:549–54. doi: 10.1111/ggi.14183
20. Molenberghs, G, Thijs, H, Jansen, I, Beunckens, C, Kenward, MG, Mallinckrodt, C, et al. Analyzing incomplete longitudinal clinical trial data. Biostatistics. (2004) 5:445–64. doi: 10.1093/biostatistics/kxh001
21. Hommos, MS, Glassock, RJ, and Rule, AD. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol. (2017) 28:2838–44. doi: 10.1681/ASN.2017040421
22. Chesnaye, NC, Dekker, FW, Evans, M, Caskey, FJ, Torino, C, Postorino, M, et al. Renal function decline in older men and women with advanced chronic kidney disease-results from the EQUAL study. Nephrol Dial Transplant. (2021) 36:1656–63. doi: 10.1093/ndt/gfaa095
23. Imai, E, Horio, M, Yamagata, K, Iseki, K, Hara, S, Ura, N, et al. Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res. (2008) 31:433–41. doi: 10.1291/hypres.31.433
24. Hirayama, A, Konta, T, Kamei, K, Suzuki, K, Ichikawa, K, Fujimoto, S, et al. Blood pressure, proteinuria, and renal function decline: associations in a large community-based population. Am J Hypertens. (2015) 28:1150–6. doi: 10.1093/ajh/hpv003
25. Mennuni, S, Rubattu, S, Pierelli, G, Tocci, G, Fofi, C, and Volpe, M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. (2014) 28:74–9. doi: 10.1038/jhh.2013.55
26. Tozawa, M, Iseki, K, Iseki, C, Oshiro, S, Ikemiya, Y, and Takishita, S. Triglyceride, but not total cholesterol or low-density lipoprotein cholesterol levels, predict development of proteinuria. Kidney Int. (2002) 62:1743–9. doi: 10.1046/j.1523-1755.2002.00626.x
27. Muntner, P, Coresh, J, Smith, JC, Eckfeldt, J, and Klag, MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. (2000) 58:293–301. doi: 10.1046/j.1523-1755.2000.00165.x
28. de Boer, IH, Katz, R, Fried, LF, Ix, JH, Luchsinger, J, Sarnak, MJ, et al. Obesity and change in estimated GFR among older adults. Am J Kidney Dis. (2009) 54:1043–51. doi: 10.1053/j.ajkd.2009.07.018
29. Schwartz, P, Capotondo, MM, Quaintenne, M, Musso-Enz, GM, Aroca-Martinez, G, and Musso, CG. Obesity and glomerular filtration rate. Int Urol Nephrol. (2023) 56:1663–8. doi: 10.1007/s11255-023-03862-0
30. de Vries, AP, Ruggenenti, P, Ruan, XZ, Praga, M, Cruzado, JM, Bajema, IM, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. (2014) 2:417–26. doi: 10.1016/S2213-8587(14)70065-8
31. Dalrymple, LS, Katz, R, Rifkin, DE, Siscovick, D, Newman, AB, Fried, LF, et al. Kidney function and prevalent and incident frailty. Clin J Am Soc Nephrol. (2013) 8:2091–9. doi: 10.2215/CJN.02870313
32. Wan, EYF, Yu, EYT, Chan, L, Mok, AHY, Wang, Y, Chan, EWY, et al. Comparative risks of nonsteroidal anti-inflammatory drugs on CKD. Clin J Am Soc Nephrol. (2021) 16:898–907. doi: 10.2215/CJN.18501120
33. Lunyera, J, Stanifer, JW, Davenport, CA, Mohottige, D, Bhavsar, NA, Scialla, JJ, et al. Life course socioeconomic status, Allostatic load, and kidney health in black Americans. Clin J Am Soc Nephrol. (2020) 15:341–8. doi: 10.2215/CJN.08430719
34. Liu, Y, Kuczmarski, MF, Miller, ER 3rd, Nava, MB, Zonderman, AB, Evans, MK, et al. Dietary habits and risk of kidney function decline in an urban population. J Ren Nutr. (2017) 27:16–25. doi: 10.1053/j.jrn.2016.08.007
35. Rubio-Aliaga, I, and Krapf, R. Phosphate intake, hyperphosphatemia, and kidney function. Pflugers Arch. (2022) 474:935–47. doi: 10.1007/s00424-022-02691-x
Keywords: aged, glomerular filtration rate, obesity, blood pressure, dyslipidemias
Citation: Jiang Y, Cao Q, Hong W, Xu T, Tang M, Li Y and Xu R (2024) Age and estimated glomerular filtration rate in Chinese older adults: a cohort study from 2014 to 2020. Front. Public Health. 12:1392903. doi: 10.3389/fpubh.2024.1392903
Edited by:
Matthew Lohman, University of South Carolina, United StatesReviewed by:
Xiang Xiao, The First Affiliated Hospital of Chengdu Medical College, ChinaSidy Seck, Gaston Berger University, Senegal
Copyright © 2024 Jiang, Cao, Hong, Xu, Tang, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renying Xu, NzIxMDAxNzM1QHNoc211LmVkdS5jbg==
†ORCID: Renying Xu, orcid.org/0000-0003-2608-5586
†These authors have contributed equally to this work and share first authorship
 Ying Jiang1†
Ying Jiang1† Renying Xu
Renying Xu