- 1The University of Sydney Dental School, The University of Sydney, Sydney, NSW, Australia
- 2Westmead Applied Research Centre, The University of Sydney, Westmead, NSW, Australia
- 3School of Health Sciences, Oral Health, The University of Newcastle, Ourimbah, NSW, Australia
Objective: To identify and describe the impact of current oral health education programmes provided to patients in cardiology hospital wards and outpatient clinics.
Methods: This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis for Scoping Reviews statement. Searches were conducted using electronic databases: Cochrane, Medline, and Scopus, as well as grey literature searching.
Results: Three eligible studies were identified. All included studies reported generalised poor oral health in their participants at baseline, with significant improvement at follow-up. They all reported significant reductions in plaque deposits and gingival bleeding. One study reported significantly less bacteria on participant tongues, as well as fewer days with post-operative atrial fibrillation in the intervention group. Furthermore, in this study, one patient in the intervention group developed pneumonia, whilst four patients in the control group did.
Conclusion: Oral health education for patients with cardiovascular disease is limited and many have poor oral health. Educational programmes to improve oral health behaviours in patients with cardiovascular disease can improve both oral and general health outcomes.
Implications for public health: Oral disease is a modifiable risk factor for cardiovascular disease. Integrating oral health education into cardiology hospital settings is a simple strategy to improve access to oral health information and improve both oral and cardiovascular outcomes.
1 Introduction
Cardiovascular disease (CVD) is a global public health issue. It is the leading cause of death world-wide (1) and whilst modifiable risk factors smoking status, healthy diet, active lifestyle, and alcohol intake are well known (2, 3); one that is rarely publicised is poor oral health. Up to 90% of any population with at least one tooth are living with periodontal disease, a preventable oral condition that plays an integral role in oral as well as systemic health. Preventing this and other oral diseases begins with oral health education and involves oral hygiene instructions.
Optimal oral hygiene practices involve toothbrushing for at least 2 mins twice daily (4, 5) and cleaning interdentally once a day (6). Manually removing dental biofilm from oral tissues with these habits are the most efficient way to ensure good oral health (7) and lower both local and systemic inflammation (8). Traditionally oral health education is delivered within dental settings. However, just under half of adults world-wide do not attend a dentist regularly (9) and as such, do not receive this important messaging.
The health outcomes of those living with CVD can be impacted by poor oral hygiene as never or rarely brushing teeth has been shown to significantly increase the risk of a CVD event (10). The mechanism behind this is a result of poor oral hygiene allowing dental biofilm to remain stagnant on oral tissues, initiating an immune response and involves vasodilation of gingival tissues to allow rapid movement of immune cells to the site (11). As such, even in healthy individuals, poor oral hygiene can lead to elevation of inflammatory markers high-sensitive C-reactive protein (hsCRP) and interleukin (IL)-6 in as little as 3 weeks (12). For those living with CVD, an elevation of these markers puts them at an increased risk of a future cardiac event (13).
Vasodilation of gingival tissues also gives pathogenic oral bacteria within the dental biofilm access to the body and its systems via the blood stream (14–16). Once in the bloodstream these pathogens and their secretions can lodge in distant organs such as the lungs, kidneys, brain (14), and heart (17) where they can initiate a localised inflammatory response (18). In the heart these pathogens have been shown to invade vessel walls and adhere to atherosclerotic lesions, leading to atherosclerosis (19); the primary cause of CVD (20).
Many barriers exist that prevent individuals from attending the dental clinic such as cost, ease of access (21), and anxiety (22) to name a few, highlighting the need to expand oral health education to other areas of healthcare. Research has shown that increased oral hygiene in hospital wards decreases the incidence of non-ventilator hospital acquired pneumonia (23) and shortens hospital stays (24). Within this setting, however, oral care is delegated to nurses who can face many challenges to providing this care (25). As such, oral health practitioners (OHP) would be an appropriate alternative to take on this responsibility (26).
Equally, an emerging educational tool within healthcare has been the use of digital devices (27). Their ability to deliver health messages across all literacy levels (28, 29) has improved patient quality of life (QoL) (30, 31); and digitally delivered health information within patient waiting rooms has been shown to improve oral hygiene practices (32) and promote healthy lifestyle behaviours in patients with CVD (33). Whether face-to-face or digitally delivered, oral health education provided in cardiology settings has the potential to improve oral health and reduce the risk profile of patients living with CVD.
1.1 Aims
This review aims to identify and describe oral health education programmes provided to patients living with CVD within hospital wards and outpatient clinics; as well as discuss any effect they had on health outcomes.
2 Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis for Scoping Reviews (PRISMA-ScR) statement (34). See Supplementary material 1. The review protocol is registered in the Open Science Framework (OSF) registry.1
2.1 Search strategy
The initial search commenced 21st December 2022 and was repeated on 14th August 2023. The final search occurring 7th May 2024. Electronic databases used include Cochrane, Medline (via Ovid), and Scopus. No limitations were placed on language or publication period, and no human filter was applied. Grey literature included phrase searching via Google Scholar, as well as reviewing citation lists of relevant studies. The search strategy included a combination of the following terms “cardiovascular disease, heart disease, education relating to dental health, oral health, health promotion, digital education, video education, patient education, health knowledge, oral hygiene instruction, hospital, oral health, oral care, dental care, preventative dentistry, video recording, video-audio media.” Boolean operators (AND/OR), medical subject headings (MeSH), and truncations were also utilised. For the full search see Supplementary material 2. Every attempt was made to retrieve studies that were inaccessible including web searches, using The University of Sydney library resources, and lastly, attempting to contact corresponding authors.
2.2 Inclusion and exclusion criteria
The population comprised of adults ≥18 years who had been or were recently hospitalised as a result of cardiovascular disease. The intervention included digital and/or traditional oral health education being delivered to these patients within hospital wards or out-patient clinics. Any studies where oral hygiene education took place outside of the hospital environment, or within a dental setting were excluded. Only peer-reviewed publications of randomised controlled trials, quasi-randomised controlled trials, observational studies, including cohort, case–control and cross-sectional studies were eligible for inclusion. Conference abstracts, case-studies/series, letters to the editors, and editorials were excluded. Any non-English or animal studies were manually excluded.
2.3 Screening and data extraction
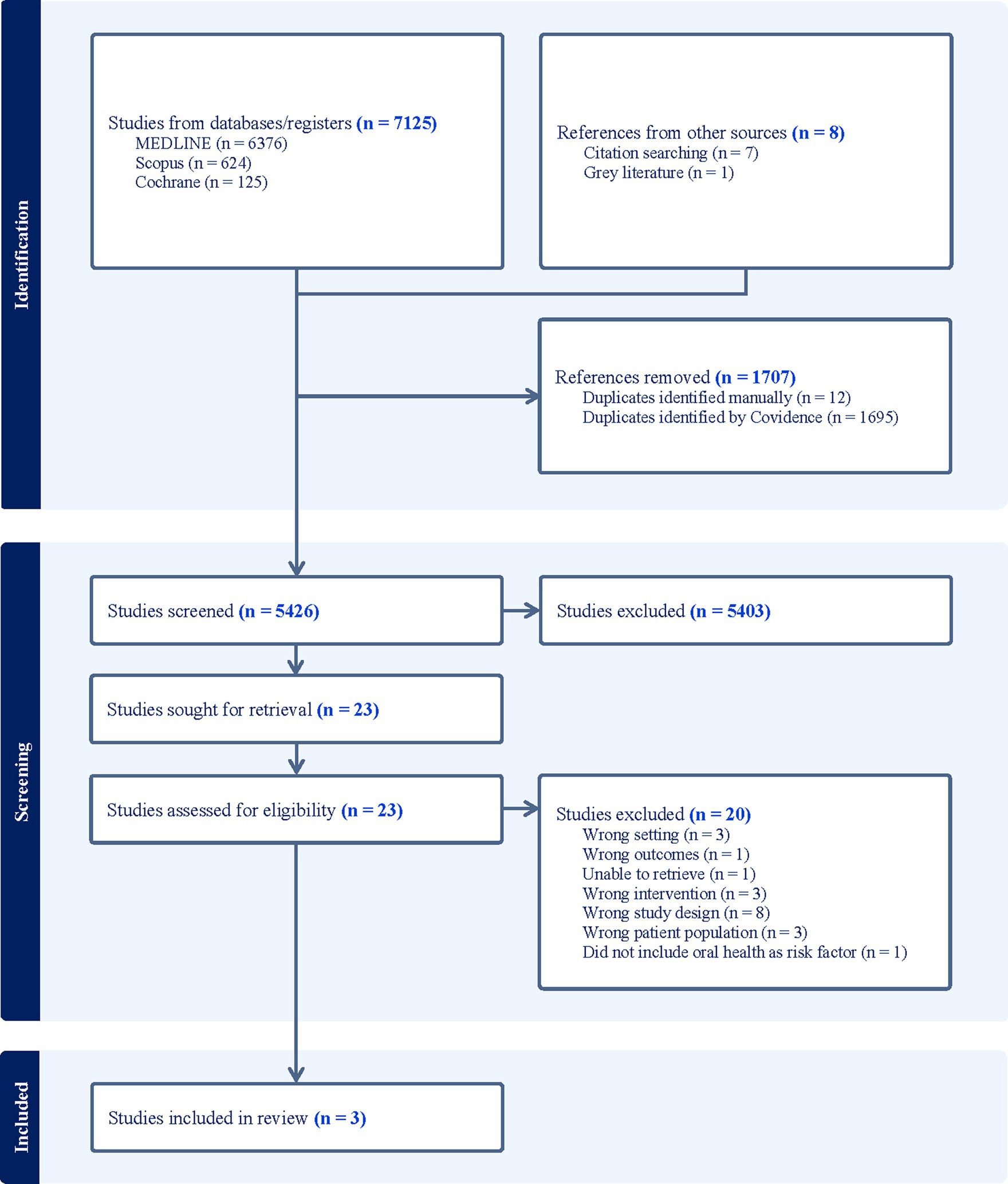
Using Covidence, a systematic review software (35), 7,133 identified citations were imported into the programme where 1,695 duplicates were automatically removed. A further 12 duplicates were manually removed leaving a total of 5,426 for screening. Titles and abstracts were screened independently by LC and one of four alternate reviewers (LR, AT, LQ, FX). Most results did not relate to oral health education, CVD, adults, and/or were not based within a hospital setting. As such they were deemed irrelevant. Twenty-three articles were identified for further assessment, however after full text screening, a total of 3 studies [1 quasi-randomised (36) and 2 randomised controlled trials (37, 38)] met the inclusion criteria (see Figure 1). Any conflicts arising during the screening process were resolved via group discussion. A data extraction tool developed within Covidence was completed independently first by LC, followed by either LR, FX, AT, or LQ. Key characteristics extracted were author(s), publication year, country, study design, aim(s), setting, mean age, sex, outcomes measures, a comparison of oral hygiene intervention, intervention duration, and outcomes. Due to the heterogeneity of the studies, data synthesis has been presented narratively with reference to supporting material.
3 Results
3.1 Characteristics
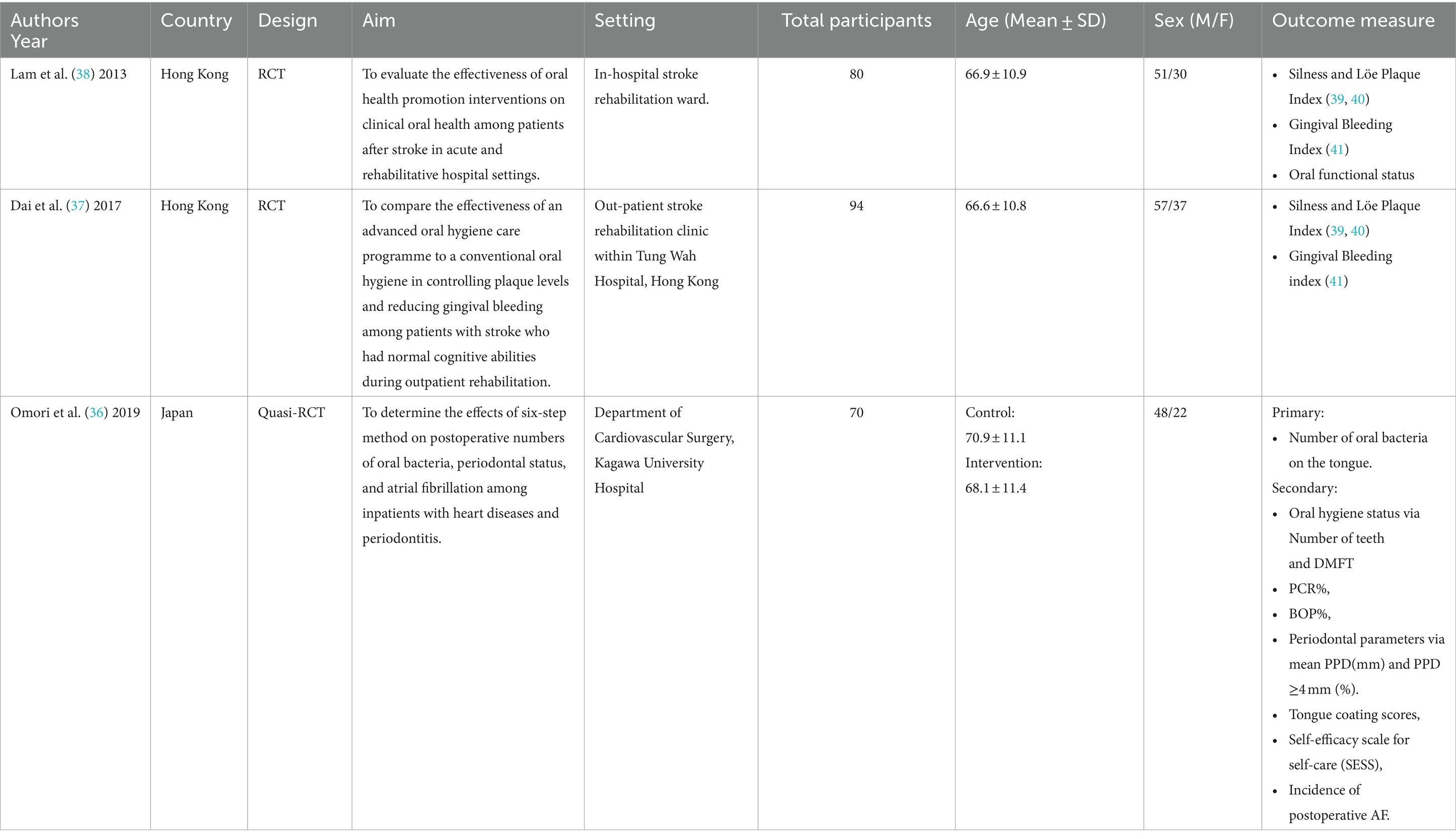
Three studies were identified (36–38), and included a total of 245 participants. See Table 1. The studies took place in either Hong Kong or Japan and spanned between 2013 and 2019. Participants recruited into the studies were patients who were within either a cardiac surgical or stroke rehabilitation hospital ward or were attending a hospital out-patient rehabilitation clinic. Across all studies, the lowest mean age of participants was 66.6 ± 10.8, the highest 70.9 ± 11.1; 60.6–68% were male. Whilst Omori et al. (36) did not discuss employment status, two thirds of Dai et al.’s (37) (housewife: 12.8%, retired: 51.1%, and unemployed: 2.1%), and close to three quarters of Lam et al.’s (38) subjects were not working (53.1% retired, 19.8% homemaker). All studies reported a lack of regular oral hygiene practices at baseline (36–38), and reported no significant difference in oral hygiene status between intervention groups at baseline.
3.2 Outcome measures
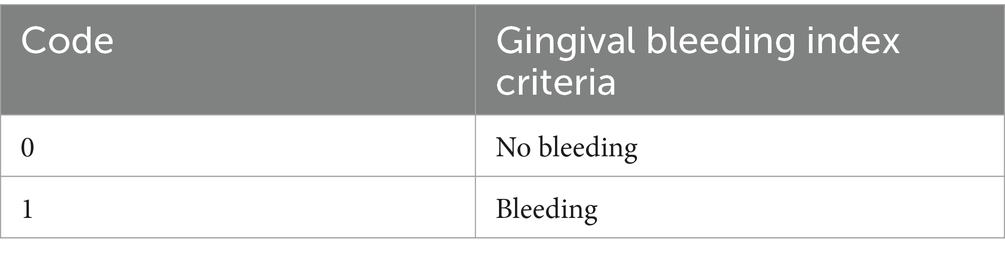
The primary outcome measures for two studies (37, 38) included measures of oral hygiene status using the Silness and Löe plaque index (39, 40) and the gingival bleeding index (41). See Tables 2, 3 for indices criteria. Secondary outcomes included gingival bleeding at 6 months (37) or oral functional status, assessed by patients’ ability to perform toothbrushing and insert/remove their dentures (38). The outcome measures for one study (36) was the number of oral bacteria on the tongue, followed by oral hygiene status, periodontal parameters, tongue coating scores, self-efficacy scale for self-care (SESS) scores, and the incidence of postoperative atrial fibrillation (AF). This study also assessed plaque score by using O’Leary’s plaque control record method (43).
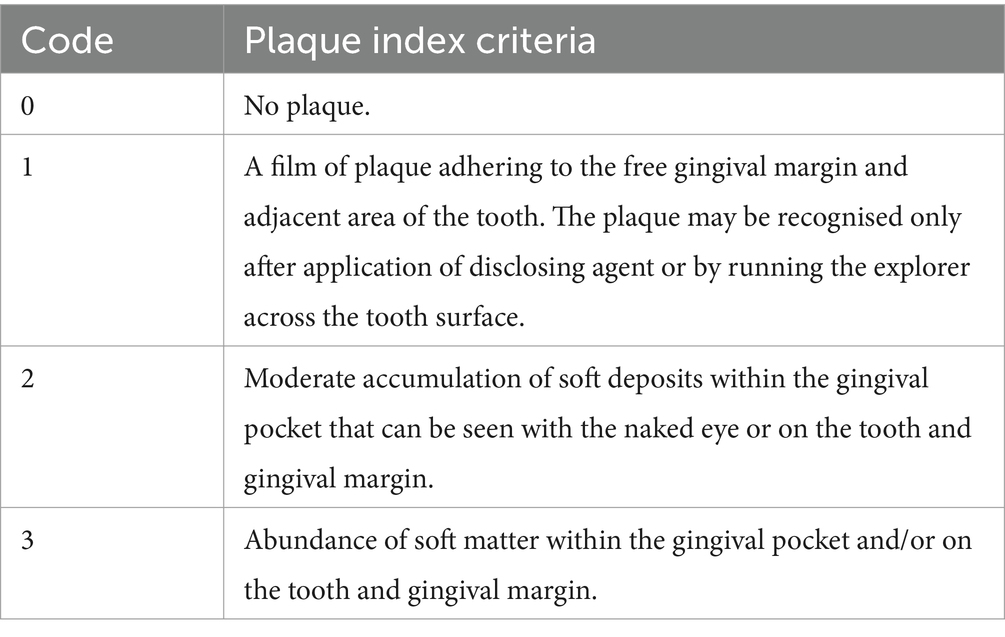
Table 2. Criteria for Silness and Löe plaque index and gingival bleeding index (42).
3.3 Interventions
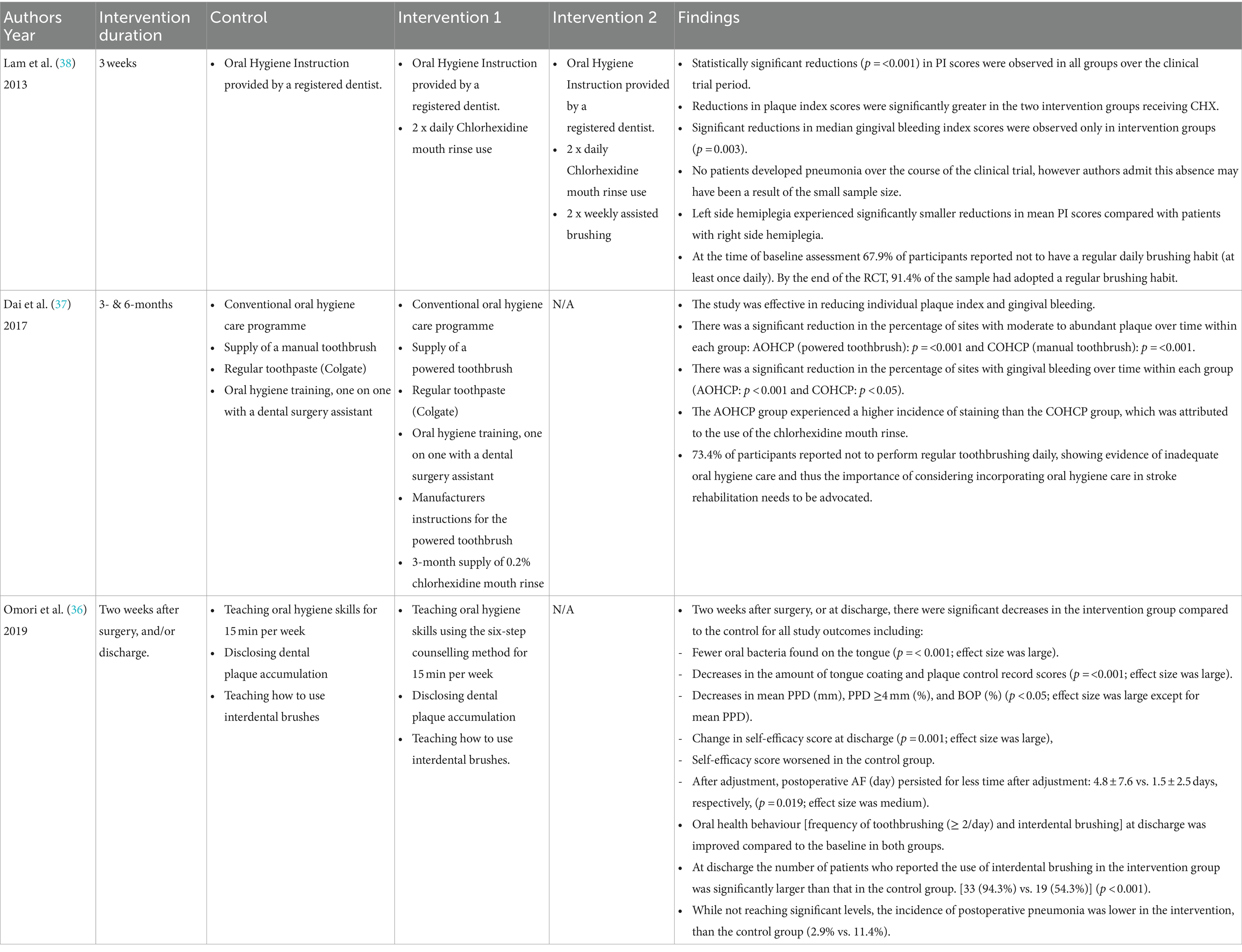
The education provided in each trial focused on oral hygiene instruction and was delivered by oral health practitioners or dental assistants. Each intervention differed in its methods and materials, with follow ups of 3-weeks (38), 3- and 6-months (37), and discharge (approximately 1 month) (36). See Table 4 for intervention details. In the first study, stroke patients attending their outpatient rehabilitation were placed into one of two arms control: conventional oral hygiene care programme (COHCP) or intervention advanced oral hygiene care programme (AOHCP). The control arm receiving an oral hygiene care programme, manual toothbrush, regular toothpaste (Colgate), and one on one oral hygiene instruction with a dental assistant. Whilst the intervention arm received the care programme, toothpaste, and one on one instruction however, also received an electric toothbrush with manufacturer’s instructions and a 3-month supply of chlorhexidine (CHX) mouth rinse (37).
Similarly, investigators from another study involving CVD patients within a surgical ward (36), placed them into one of two arms. Both arms received similar interventions including oral hygiene instruction, using disclosing solution, interdental brush use delivered by certified dental hygienists. The hygienists also provided post-operative oral care to a small number of participants in each group. However, the teaching method differed between the groups. The control group received skills-based teaching, the intervention arm received oral hygiene instruction via a modified behavioural six-step method (44). See Table 5 for this method.

Table 5. Six-step method adapted from included study (36).
The final study (38) included three arms: one control and two interventions. Each arm received oral hygiene instruction, whilst the intervention arms also received chlorhexidine (CHX) mouth rinse alone or in combination with 2 x weekly assisted brushing. The hygiene instruction was performed by a registered dentist and CHX was administered by ward nurses. The intervention arm receiving assisted brushing, had this performed by trained ward nurses. Training involved a 30-min education session run by dental hygienists. The authors deemed it unethical to include a negative control group due to their high risk of developing aspirational pneumonia (45).
3.4 Findings
All 3 studies reported a lack of regular oral hygiene practices at baseline (36–38). At their conclusion, all found an improvement in toothbrushing habits, whilst one (36) reported a significant increase of interdental brush use. All study arms had significant reductions in oral hygiene measures including plaque scores (p = <0.001) (36–38), improved periodontal parameters, and tongue coating scores (36). Gingival bleeding was also reduced in all arms of two studies (p 0.004) (36), (p < 0.001) (37), however, one (38) reported significance in the intervention groups only (p = 0.032). One study assessed tongue bacterial numbers (36), and reported significantly less bacteria (x107cfu/mL) on participant tongues (p < 0.02), as well as fewer days with post-operative AF in the intervention group (1.5 ± 2.8 vs. 4.8 ± 7.6 p = 0.019). It also reported that 5 patients (4 control and 1 intervention) developed pneumonia (36), whereas no patients developed pneumonia in the other studies (37, 38). A 6-month follow-up was conducted in one study and they reported the continuation of plaque reduction (p = 0.05) and a further reduction in bleeding on probing (p < 0.01) in their intervention arm (37). SESS was measured in one study, showing worsened scores in the control arm (36).
4 Discussion
This review assessed the current evidence in relation to oral hygiene education programmes provided to patients within cardiology wards and/or outpatient clinics and found that oral health education is rarely provided in these settings. Poor oral health is a significant global public health issue (46). Essential to preventing poor oral health is oral health education. However, as patients can have major barriers to overcome when accessing dental care (47), oral health education urgently needs to expand beyond the dental clinic. Incorporating this education and improving oral health within other areas of health would greatly benefit everyone and would have a profound positive effect on patients living with CVD (3–6).
At baseline, participants of all included studies had poor oral health as well as suboptimal hygiene habits (36–38). At the end of the study periods, the intervention groups saw significant improvement in both clinical oral health status and self-reported oral hygiene habits. These findings mirror other studies using oral health practitioners (OHP) to educate patients in non-dental settings including residential aged care (48, 49) and mental health facilities (50–53).
Omori et al. (36) specifically illustrated a significant increase of interdental brush use in their study [(94.3%) intervention vs. (54.3%) control]. The improvement of interdental brush use, as well as other outcome parameters within the intervention arm is likely due to the six-step teaching method. This method has been shown to improve health outcomes as clinicians collaborate with patients to set achievable goals (44). Skills-only based health education is more paternalistic in nature, excluding patients’ prior beliefs or understanding, and removing autonomy (54). As the control arm received this type skills-based education, it could be related to the worsening of SESS score in this group.
The improvement of oral health reported in the included studies also had a positive impact on post-operative health outcomes (36–38) such as a reduction or absence of common CVD post-operative complications: pneumonia and post-operative AF; which can lengthen hospital stays or cause premature death (55–57). Although good oral hygiene has been shown to reduce systemic inflammation (58) none of the included studies reported on inflammatory markers such as hsCRP or IL-6.
4.1 Current education strategies
The education strategies employed in all included studies involved traditional face-to-face education (36–38) and many of the excluded studies provided this education to nursing staff only (59–63). Nurses can face many challenges when providing oral care. From a personal level, barriers can include staffing issues, lack of time or training, or aversion to this care (25). This is reflected by a study where nurses admitted to ceasing toothbrushing altogether after the study period, even after oral hygiene was proven to eliminate ventilator assisted pneumonia due to the lack of time and because oral care was of low priority (61). However, challenges can also arise at the organisational level where training, resources and/or appropriate staff numbers are not provided (25).
Furthermore, patients themselves can prevent nurses from providing oral care with aggressive behaviour, care refusal, communication issues, or where oral health is not prioritised (25). As such OHPs including dentists, dental hygienists, and oral health therapists, could be an appropriate alternative to help ease this burden on nursing staff (26). The FDI World Dental Federation also recognises the benefit OHPs would have within primary healthcare settings, calling for them to be integrated into these settings globally by 2030 (64). Currently however, resulting from a lack of political leadership, low oral health prioritisation on political agendas, as well as staffing, infrastructure, and funding obstacles (65), few countries are taking steps to utilise them in this way (66).
4.2 Changing the status quo
One cost-effective way to bring oral health education to patients in these settings is through the use of digital technology. Digital CVD education programmes have been used to improve heart health outcomes (30, 33), including text-message health tips post cardiac event (67). Whilst currently oral health messages are not included, they could easily be incorporated into these existing education packages. Another solution could be providing oral health training to non-dental clinicians such as pharmacists, general practitioners, and other allied health professionals, as many individuals visit these clinicians when they have a dental issue (68).
A long-term solution at the organisational level could be a collaboration between universities and hospitals forming placements for student OHPs. Placements such as this have shown to benefit both students, hospital staff, and patients alike (69). Students placed in hospital settings would provide oral health education, assist with oral care, and with mobile dental units becoming more readily available, urgent treatment could be completed bedside (70). Additionally, referral pathways within the hospital system could also be created, utilising hospital dental clinics where applicable.
4.3 Gaps in the literature
The number of eligible studies that involved the direct provision of oral health education to patients with CVD within hospital settings were limited and predominately located in Hong Kong (37, 38) or Japan (36) between 2013 and 2019. These findings highlight the need to conduct more studies in different global communities. All included studies reported generalised poor oral health in their participants at baseline, similar to recent research within a Romanian emergency hospital (71) and an Australian cardiac rehabilitation clinic (72). Both concluding further oral health education in these spaces are needed (71, 72).
A lack of resources and funding means OHPs are absent from hospital multidisciplinary teams (57, 58). Poor oral health is a modifiable risk factor for CVD however, Appropriately, priority is given to patients’ heart health in cardiology wards however, current literature acknowledges poor awareness of the links between oral and heart health in patients within cardiology wards and outpatient clinics (58, 59). This could be related to the absence of an OHP within these settings and compounded by the limited oral health messages in CVD education (25, 60–62).
This review has discussed oral health education programmes provided to patients with CVD in hospital settings. It has highlighted gaps where an OHP and/or digital technologies would be ideally placed to bridge them. As such, there are opportunities for future research and implementation of oral health education programmes for patients with CVD within hospital settings. Preferably oral health education should form part of primary prevention strategies for good general health, however incorporating it as part of secondary prevention strategies should also be a priority.
4.4 Clinical significance
This review has highlighted the significant role oral health education plays in improving the long-term oral health in patients within hospital settings, as well as lowering the risk of common post-operative and post-stroke complications. Despite the important role oral health can play in cardiovascular health, this review has highlighted a lack of oral health education available to patients with CVD and proposed simple strategies to deliver these messages. The implementation and standardisation of programmes such as these may help to empower at-risk patients at their most vulnerable to improve their oral health for better general health.
4.5 Strengths and limitations
The small number of eligible studies was a major limitation for this review. The three included studies took place in Hong Kong or Japan and thus may not be generalisable to CVD patients globally. However, a strength of this study is it is the first known review to analyse oral health education programmes provided to patients with CVD in hospital settings, highlighting a lack of oral health education in these spaces.
5 Conclusion
This review concludes there is a need for further development and evaluation of oral health education programmes within hospital settings in different countries. Many patients with CVD have poor oral hygiene which can increase their risk of a recurrent cardiac event. The provision of basic oral health education provided directly to patients significantly improved oral hygiene, minimised the risk of post-operative pneumonia, and lowered post-operative days with AF.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. LR: Formal analysis, Writing – review & editing. FX: Formal analysis, Writing – review & editing. LQ: Formal analysis, Writing – review & editing. AT: Formal analysis, Writing – review & editing. JW: Conceptualization, Writing – review & editing. SK: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The post-doctoral research position of SK and LCs PhD scholarship is supported by donations from the Bella-Schwarz Foundation. This foundation had no influence or involvement with the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1389853/full#supplementary-material
Footnotes
References
1. Roth, GA, Mensah, GA, Johnson, CO, Addolorato, G, Ammirati, E, Baddour, LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. The World Health Organisation. World Health Organisation - cardiovascular diseases (CVDs). (2021) [cited 2024 Apr 11]. Cardiovascular diseases (CVDs). Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
3. Prevent Heart Disease. cdc.gov. [cited 2022 Sep 22]. (2024). Available from: https://www.cdc.gov/heart-disease/prevention/?CDC_AAref_Val=https://www.cdc.gov/heartdisease/prevention.htm
4. Gallagher, A, Sowinski, J, Bowman, J, Barrett, K, Patel, K, Bosma, ML, et al. The effect of brushing time and dentifrice on dental plaque removal in vivo. J Dent Hyg. (2009) 83:111–6.
5. Janakiram, C, Taha, F, and Joe, J. The efficacy of plaque control by various Toothbrushing techniques-a systematic review and Meta-analysis. J Clin Diagn Res. (2018) 12:ZE01–6. doi: 10.7860/JCDR/2018/32186.12204
6. Worthington, H, MacDonald, L, Poklepovic Pericic, T, Sambunjak, D, Johnson, T, Imai, P, et al. Home use of interdental cleaning devices, in addition to toothbrushing, for preventing and controlling periodontal diseases and dental caries. Cochrane Database Syst Rev. (2019) 2020:CD012018. doi: 10.1002/14651858.CD012018.pub2
7. Wilkins, E. Patient learning for health behavioral change In: Clinical practice of the dental hygienist. In: E Wilkins, editor. 11th ed. Philadelphia: Lippincott Williams & Wilkins (2013). 363–76.
8. Moon, MG, Kang, SH, Kim, SH, Park, SY, Seol, YJ, Yoon, CH, et al. Association between toothbrushing and cardiovascular risk factors: a cross-sectional study using Korean National Health and nutrition examination survey 2015–2017. BMC Oral Health. (2024) 24:4. doi: 10.1186/s12903-023-03775-5
9. Reda, SM, Krois, J, Reda, SF, Thomson, WM, and Schwendicke, F. The impact of demographic, health-related and social factors on dental services utilization: systematic review and meta-analysis. J Dent. (2018) 75:1–6. doi: 10.1016/j.jdent.2018.04.010
10. de Oliveira, C, Watt, R, and Hamer, M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish health survey. BMJ. (2010) 340:c2451. doi: 10.1136/bmj.c2451
11. Nield-Gehrig, J, and Willman, D. Host immune response to periodontal pathogens In: Foundations of periodontics for the dental hygienist. In: J Nield-Gehrig and D Willman, editors. 3rd ed. Philadelphia: Wolters Kluwer | Lippincott WIlliams & Wilkins (2011). 157–70.
12. Eberhard, J, Grote, K, Luchtefeld, M, Heuer, W, Schuett, H, Divchev, D, et al. Experimental gingivitis induces systemic inflammatory markers in Young healthy individuals: a single-subject interventional study. PLoS One. (2013) 8:e55265. doi: 10.1371/journal.pone.0055265
13. Pant, S, Deshmukh, A, GuruMurthy, GS, Pothineni, NV, Watts, TE, Romeo, F, et al. Inflammation and atherosclerosis—revisited. J Cardiovasc Pharmacol Ther. (2014) 19:170–8. doi: 10.1177/1074248413504994
14. Bui, FQ, Almeida-da-Silva, CLC, Huynh, B, Trinh, A, Liu, J, Woodward, J, et al. Association between periodontal pathogens and systemic disease. Biom J. (2019) 42:27–35. doi: 10.1016/j.bj.2018.12.001
15. Wojtkowska, A, Zapolski, T, Wysokińska-Miszczuk, J, and Wysokiński, AP. The inflammation link between periodontal disease and coronary atherosclerosis in patients with acute coronary syndromes: case–control study. BMC Oral Health. (2021) 21:5. doi: 10.1186/s12903-020-01356-4
16. Gupta, M, Chaturvedi, R, and Jain, A. Role of cardiovascular disease markers in periodontal infection: understanding the risk: official publication of Indian Society for Dental Research. Indian J Dent Res. (2015) 26:231–6. doi: 10.4103/0970-9290.162873
17. Schenkein, HA, and Loos, BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Clin Periodontol. (2013) 40:S51–69. doi: 10.1111/jcpe.12060
18. Hasturk, H, and Kantarci, A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontol. (2015) 69:255–73. doi: 10.1111/prd.12105
19. Hayashi, C, Gudino, CV, Gibson, FC III, and Genco, CA. REVIEW: pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol Oral Microbiol. (2010) 25:305–16. doi: 10.1111/j.2041-1014.2010.00582.x
20. Bauersachs, R, Zeymer, U, Brière, JB, Marre, C, Bowrin, K, and Huelsebeck, M. Burden of coronary artery disease and peripheral artery disease: a literature review. Cardiovasc Ther. (2019) 2019:1–9. doi: 10.1155/2019/8295054
21. Oral Health Monitoring Group. Healthy mouths healthy lives - Australia’s National Oral Health Plan 2015–2024. (2015); Available from: https://www.health.gov.au/sites/default/files/documents/2022/04/healthy-mouths-healthy-lives-australia-s-national-oral-health-plan-2015-2024-australia-s-national-oral-health-plan-2015-2024.pdf
22. Muneer, MU, Ismail, F, Munir, N, Shakoor, A, Das, G, Ahmed, AR, et al. Dental anxiety and influencing factors in adults. Health. (2022) 10:2352. doi: 10.3390/healthcare10122352
23. Giuliano, KK, Penoyer, D, Middleton, A, and Baker, D. Original research: Oral care as prevention for nonventilator hospital-acquired pneumonia: a four-unit cluster randomized study. AJN Am J Nurs. (2021) 121:24–33. doi: 10.1097/01.NAJ.0000753468.99321.93
24. Kaga, A, Ikeda, T, Tachibana, K, Tanaka, R, Kondo, H, Kawabata, T, et al. An innovative oral management procedure to reduce postoperative complications. JTCVS Open. (2022) 10:442–53. doi: 10.1016/j.xjon.2022.01.021
25. Bonetti, D, Hampson, V, Queen, K, Kirk, D, Clarkson, J, and Young, L. Improving oral hygiene for patients. Nurs Stand. (2014) 29:44–50. doi: 10.7748/ns.29.19.44.e9383
26. Lupi, SM, Pascadopoli, M, Maiorani, C, Preda, C, Trapani, B, Chiesa, A, et al. Oral hygiene practice among hospitalized patients: an assessment by dental hygiene students. Health. (2022) 10:115. doi: 10.3390/healthcare10010115
27. Nguyen Hai, T, Meyer, L, McGuire, H, Nguyen Thi Hong, H, and Nguyen, TL. Frontier digital technology: transforming noncommunicable disease prevention among youth. Lancet Reg Health – West Pac. (2022) 29:100590. doi: 10.1016/j.lanwpc.2022.100590
28. Tait, AR, Voepel-Lewis, T, Chetcuti, SJ, Brennan-Martinez, C, and Levine, R. Enhancing patient understanding of medical procedures: evaluation of an interactive multimedia program with in-line exercises. Int J Med Inform. (2014) 83:376–84. doi: 10.1016/j.ijmedinf.2014.01.011
29. Rossi, MJ, Guttmann, D, MacLennan, MJ, and Lubowitz, JH. Video informed consent improves knee arthroscopy patient comprehension. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. (2005) 21:739–43. doi: 10.1016/j.arthro.2005.02.015
30. Oudkerk Pool, MD, Hooglugt, JLQ, Schijven, MP, Mulder, BJM, Bouma, BJ, de Winter, RJ, et al. Review of digitalized patient education in cardiology: a future ahead? Cardiology. (2021) 146:263–71. doi: 10.1159/000512778
31. Timmers, T, Janssen, L, van der Weegen, W, Das, D, Marijnissen, WJ, Hannink, G, et al. The effect of an app for day-to-day postoperative care education on patients with Total knee replacement: randomized controlled trial. JMIR Mhealth Uhealth. (2019) 7:e15323. doi: 10.2196/15323
32. McNab, M, and Skapetis, T. Why video health education messages should be considered for all dental waiting rooms. PLoS One. (2019) 14:e0219506. doi: 10.1371/journal.pone.0219506
33. McIntyre, D, Thiagalingam, A, Klimis, H, Huben, AV, Marschner, S, and Chow, CK. Education on cardiac risk and CPR in cardiology clinic waiting rooms: a randomised clinical trial. Heart. (2021) 107:1637–43. doi: 10.1136/heartjnl-2021-319290
34. Tricco, AC, Lillie, E, Zarin, W, O’Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
36. Omori, C, Ekuni, D, Ohbayashi, Y, Miyake, M, and Morita, M. Quasi-randomized trial of effects of perioperative Oral hygiene instruction on inpatients with heart diseases using a behavioral six-step method. Int J Environ Res Public Health. (2019) 16:4252. doi: 10.3390/ijerph16214252
37. Dai, R, Lam, OLT, Lo, ECM, Li, LSW, and McGrath, C. A randomized clinical trial of oral hygiene care programmes during stroke rehabilitation. J Dent. (2017) 61:48–54. doi: 10.1016/j.jdent.2017.04.001
38. Lam, OL, AS, MM, Samaranayake, LP, Li, LS, and McGrath, C. Randomized clinical trial of Oral health promotion interventions among patients following stroke. Arch Phys Med Rehabil. (2013) 94:435–43. doi: 10.1016/j.apmr.2012.10.024
39. Silness, J, and Löe, H. Periodontal disease in pregnancy II. Correlation between Oral hygiene and periodontal condition. Acta Odontol Scand. (1964) 22:121–35. doi: 10.3109/00016356408993968
40. Löe, H. The gingival index, the plaque index and the retention index systems. J Periodontol. (1967) 38:610–6. doi: 10.1902/jop.1967.38.6.610
41. Ainamo, J, and Bay, I. Problems and proposals for recording gingivitis and plaque. Int Dent J. (1975) 25:229–35.
42. Wyche, C. Indices and scoring methods In: E Wilkins, editor. Clinical practice of the dental hygienist. 11th ed. Philadelphia: Lippincott Williams & Wilkins (2013). 311–35.
43. O’Leary, TJ, Drake, RB, and Naylor, JE. The plaque control record. J Periodontol. (1972) 43:38–8. doi: 10.1902/jop.1972.43.1.38
44. Bandura, A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. (1977) 84:191–215. doi: 10.1037/0033-295X.84.2.191
45. Heuschmann, PU, Kolominsky-Rabas, PL, Misselwitz, B, Hermanek, P, Leffmann, C, Janzen, RWC, et al. Predictors of in-hospital mortality and attributable risks of death after ischemic stroke: the German stroke registers study group. Arch Intern Med. (2004) 164:1761–8. doi: 10.1001/archinte.164.16.1761
46. Global oral health status report. Towards universal health coverage for oral health by 2030. Geneva: World Health Organisation (2022).
47. Duckett, S, Cowgill, M, and Swerissen, H. Filling the gap: A universal dental care scheme for Australia - Grattan Institute. Australia: Grattan Institute (2019).
48. Girestam Croonquist, C, Dalum, J, Skott, P, Sjögren, P, Wårdh, I, and Morén, E. Effects of domiciliary professional Oral Care for Care-Dependent Elderly in nursing homes – Oral hygiene, gingival bleeding, root caries and nursing Staff’s Oral health knowledge and attitudes. Clin Interv Aging. (2020) 15:1305–15. doi: 10.2147/CIA.S236460
49. Amerine, C, Boyd, L, Bowen, DM, Neill, K, Johnson, T, and Peterson, T. Oral health champions in long-term care facilities-a pilot study. Spec Care Dentist. (2014) 34:164–70. doi: 10.1111/scd.12048
50. Almomani, F, Williams, K, Catley, D, and Brown, C. Effects of an Oral health promotion program in people with mental illness. J Dent Res. (2009) 88:648–52. doi: 10.1177/0022034509338156
51. Kuo, MW, Yeh, SH, Chang, HM, and Teng, PR. Effectiveness of oral health promotion program for persons with severe mental illness: a cluster randomized controlled study. BMC Oral Health. (2020) 20:290–11. doi: 10.1186/s12903-020-01280-7
52. Silverstein, LS, Haggerty, C, Sams, L, Phillips, C, and Roberts, MW. Impact of an oral health education intervention among a group of patients with eating disorders (anorexia nervosa and bulimia nervosa). J Eat Disord. (2019) 7:29–9. doi: 10.1186/s40337-019-0259-x
53. Yoshii, H, Kitamura, N, Akazawa, K, and Saito, H. Effects of an educational intervention on oral hygiene and self-care among people with mental illness in Japan: a longitudinal study. BMC Oral Health. (2017) 17:81–1. doi: 10.1186/s12903-017-0372-7
54. Cross, R, Davis, S, and O’Niel, I. Theoretical perspectives In: Health communication: Theoretical and critical perspectives. In: R Cross, S Davis, and I O’Neil, editors. 1st ed: Cambridge, United Kingdom: Polity Press (2017). 11–71.
55. Duchnowski, P, and Śmigielski, W. Risk factors of postoperative hospital-acquired pneumonia in patients undergoing cardiac surgery. Medicina (Mex). (2023) 59:1993. doi: 10.3390/medicina59111993
56. Grossmann, I, Rodriguez, K, Soni, M, Joshi, PK, Patel, SC, Shreya, D, et al. Stroke and pneumonia: mechanisms, risk factors, management, and prevention. Cureus. (2021) 13:e19912. doi: 10.7759/cureus.19912
57. Lopes, LA, and Agrawal, DK. Post-operative atrial fibrillation: current treatments and etiologies for a persistent surgical complication. J Surg Res. (2022) 5:159–72. doi: 10.26502/jsr.10020209
58. Moon, MG, Kang, SH, Kim, SH, Park, SY, Seol, YJ, Yoon, CH, et al. Association between toothbrushing and cardiovascular risk factors: a cross-sectional study using Korean National Health and Nutrition Examination Survey 2015–2017. BMC Oral Health. (2024) 24:4.
59. Happell, B, Platania-Phung, C, Scott, D, and Hanley, C. Access to dental care and dental ill-health of people with serious mental illness: views of nurses working in mental health settings in Australia. Aust J Prim Health. (2015) 21:32–7. doi: 10.1071/PY13044
60. Alageel, S, Gulliford, MC, Wright, A, Khoshaba, B, and Burgess, C. Engagement with advice to reduce cardiovascular risk following a health check programme: a qualitative study. Health Expect Int J Public Particip Health Care Health Policy. (2020) 23:193–201. doi: 10.1111/hex.12991
61. Fields, LB. Oral care intervention to reduce incidence of ventilator-associated pneumonia in the neurologic intensive care unit. J Neurosci Nurs. (2008) 40:291–8. doi: 10.1097/01376517-200810000-00007
62. Gosney, M, Martin, MV, and Wright, AE. The role of selective decontamination of the digestive tract in acute stroke. Age Ageing. (2006) 35:42–7. doi: 10.1093/ageing/afj019
63. Charteris, P, and Kinsella, T. The Oral care link nurse: a facilitator and educator for maintaining oral health for patients at the Royal Hospital for neuro-disability. Spec Care Dentist. (2001) 21:68–71. doi: 10.1111/j.1754-4505.2001.tb00228.x
64. Glick, M, Williams, D, Yahya, I, Cheung, W, Clark, P, Jagait, C, et al. Vision 2030: Delivering optimal Oral health for all. Geneva: FDI World Dental Federation; (2021), 71, 3–4.
65. Harnagea, H, Couturier, Y, Shrivastava, R, Girard, F, Lamothe, L, Bedos, CP, et al. Barriers and facilitators in the integration of oral health into primary care: a scoping review. BMJ Open. (2017) 7:e016078. doi: 10.1136/bmjopen-2017-016078
66. Jiang, CM, Chu, CH, Duangthip, D, Ettinger, RL, Hugo, FN, Kettratad-Pruksapong, M, et al. Global perspectives of Oral health policies and Oral healthcare schemes for older adult populations. Front Oral Health. (2021) 2:703526–6. doi: 10.3389/froh.2021.703526
67. Klimis, H, Thiagalingam, A, and Chow, CK. Text messages for primary prevention of cardiovascular disease: the TextMe2 randomised controlled trial protocol. BMJ Open. (2020) 10:e036767. doi: 10.1136/bmjopen-2020-036767
68. Barnett, T, Hoang, H, Stuart, J, Crocombe, L, and Bell, E. Utilisation of oral health services provided by non-dental health practitioners in developed countries: a review of the literature. Community Dent Health. (2014) 31:224–33. doi: 10.1922/CDH_3465Hoang10
69. Wallace, JP, Taylor, JA, Wallace, LG, and Cockrell, DJ. Student focused oral health promotion in residential aged care facilities. Int J Health Promot Educ. (2010) 48:111–4. doi: 10.1080/14635240.2010.10708193
70. Galeotti, A, Ciribè, M, Matarazzo, G, Antonielli, G, Festa, P, Inserra, A, et al. Dental and periodontal Care at the Bedside Using a portable dental unit in hospitalized special needs patients: the experience of an Italian pediatric hospital. Int J Environ Res Public Health. (2021) 18:7987. doi: 10.3390/ijerph18157987
71. Lazureanu, PC, Popescu, FG, Stef, L, Focsa, M, Vaida, MA, and Mihaila, R. The influence of periodontal disease on Oral health quality of life in patients with cardiovascular disease: a Cross-sectional observational single-center study. Med Kaunas Lith. (2022) 58:584. doi: 10.3390/medicina58050584
Keywords: oral health, cardiovascular disease, health promotion, oral health education, prevention
Citation: Church LA, Robins L, Xu F, Qin L, Tran A, Wallace JP and King S (2024) Oral health education strategies for patients living with cardiovascular disease within hospital settings: a scoping review. Front. Public Health. 12:1389853. doi: 10.3389/fpubh.2024.1389853
Edited by:
Verónica Miksztowicz, National Scientific and Technical Research Council (CONICET), ArgentinaReviewed by:
Fahad Hanna, Torrens University Australia, AustraliaElisa Vanesa Macri, Universidad de Buenos Aires, Argentina
Harsh Priya, All India Institute of Medical Sciences, India
Copyright © 2024 Church, Robins, Xu, Qin, Tran, Wallace and King. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: L. A. Church, bGF1cmVuLmNodXJjaEBzeWRuZXkuZWR1LmF1
 L. A. Church
L. A. Church L. Robins1
L. Robins1 S. King
S. King