- 1Department of Epidemiology and Biostatistics, School of Public Health, College of Health Sciences and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- 2School of Public Health, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia
Background: The effect of dolutegravir (DTG)-based regimens on reducing attrition from care among women enrolled in the prevention of mother-to-child transmission (PMTCT) care program is unknown. Therefore, this study aimed to compare the incidence of attrition among women exposed to DTG-based with those exposed to efavirenz (EFV)-based first-line antiretroviral therapy (ART) in Ethiopia.
Methods: An uncontrolled before-and-after study was conducted involving 932 women (with 466 on EFV-based and 466 on DTG-based regimens) who were enrolled in the PMTCT care program from September 2015 to February 2023. The outcome variable was attrition (i.e., maternal death or loss to follow-up before their infants' final HIV status was determined). A Kaplan–Meier estimator was employed to estimate the probability of attrition. The Cox proportional hazards regression model was fitted to identify predictor variables. The adjusted hazard ratio (aHR) with the corresponding 95% confidence interval (CI) was calculated to examine the risk difference in the comparison groups.
Results: The cumulative incidence of attrition among women was 5.2% (3.0% for those placed in the DTG-based regimen arm and 7.3% for those placed in the EFV-based regimen arm). Women on DTG-based regimens had a 57% (aHR: 0.43; 95% CI: 0.23–0.80) lower risk of attrition from care compared to those on EFV-based regimens. Women who delivered their infants at home (aHR: 2.35; 95% CI: 1.14–4.85), had poor/fair adherence (aHR: 3.23; 95% CI: 1.62–6.45), had unsuppressed/unknown viral load status (aHR: 2.61; 95% CI: 1.42–4.79), and did not disclose their status to partners (aHR: 2.56; 95% CI: 1.34–4.92) had a higher risk of attrition from PMTCT care compared to their counterparts.
Conclusion: The cumulative incidence of attrition among women receiving PMTCT care is optimal. In addition, the risk of attrition among women receiving DTG-based regimens is lower than that among women receiving EFV-based regimens. Thus, DTG-based first-line ART regimen supplementation should be sustained to achieve a national retention target of 95% and above.
1 Introduction
Mother-to-child transmission (MTCT) of the human immunodeficiency virus (HIV) is the most common cause of infection in children (1). Globally, at the end of 2022, approximately 1.5 million children were living with HIV and 130,000 acquired the virus (2). Antiretroviral therapy (ART) and other interventions can reduce the risk of transmission to <5% in breastfeeding women and to <2% in non-breastfeeding women (3).
Ethiopia adopted the World Health Organization's (WHO) Option B plus recommendations as the preferred strategy for the prevention of mother-to-child transmission (PMTCT) of HIV in 2013 (4). Option B plus involves lifelong ART for all HIV-infected pregnant and breastfeeding women, irrespective of their immunological status and WHO clinical staging (3). This strategy is designed to deliver high-quality service to improve the quality of maternal life and reduce the incidence of MTCT of HIV among exposed infants (5). In 2022, 82% of pregnant women with HIV had access to ART to prevent the transmission of the virus to their infants during pregnancy and childbirth and to protect their own health (6). However, the effectiveness and quality of Option B plus depend on retaining women throughout the continuum of care during pregnancy and breastfeeding (7). In addition, attrition poses a major challenge in the PMTCT program aimed at ending Acquired Immune Deficiency Syndrome (AIDS) as a public health threat by 2030 (8).
The WHO updated different treatment guidelines, and Ethiopia adopted the WHO protocol to prevent new pediatric HIV infections and improve the survival of mothers and their infants (3, 5, 9). Accordingly, as of the end of 2018, a dolutegravir (DTG)-based regimen is recommended as the first-line treatment for people living with HIV, and this recommendation expanded to include pregnant and breastfeeding women as of July 2019 (10, 11). However, failure to retain women in care hinders the benefits of the drug in reducing the MTCT of HIV.
According to the targets set by the United Nations Program on HIV/AIDS (UNAIDS), Ethiopia has aimed to achieve a 95% retention rate (i.e., patients being alive and receiving ART) in care since 2020 (8, 12). However, various studies in Africa, including Ethiopia, indicate that the retention rate is below this global target of 95% (13–18). Previous studies have focused on the magnitude and risk factors of attrition among women on efavirenz (EFV)-based regimens (13, 14, 16, 17, 19, 20). Despite the preference for DTG-based regimens due to their high efficacy, better tolerability, high genetic resistance barrier, low cost, rapid viral suppression, and fewer side effects compared to other antiretroviral drugs, their effectiveness in retaining women in PMTCT care over the previously used EFV-based regimens has not been well investigated (3, 11, 21). Therefore, this study aims to compare the incidence of attrition and its risk factors among women receiving DTG-based with those receiving EFV-based first-line ART in Ethiopia.
2 Materials and methods
2.1 Study design and period
An uncontrolled before-and-after study was conducted among women enrolled in the PMTCT care program from September 2015 to February 2023. Data were retrieved from the records between 10 March 2023 and 25 May 2023.
2.2 Study setting and population
The study was conducted in two regions of Ethiopia: Central Ethiopia and South Ethiopia. Currently, these regions consist of 140 health facilities (49 hospitals and 91 health centers) that provide ART and PMTCT services to 1,236 women in the PMTCT care program (675 in South Ethiopia and 561 in Central Ethiopia). Among 72 facilities that have been providing both PMTCT and ART services since 2015 when the study started, 34 facilities (20 hospitals and 14 health centers) were selected. The source population for the unexposed (before) group comprised all pregnant and breastfeeding women on EFV-based first-line ART, while that for the exposed (after) group consisted of all pregnant and breastfeeding women on DTG-based first-line ART in Ethiopia. All eligible women who enrolled in PMTCT care from September 2015 onward and received only EFV-based regimens until discharge were recruited for the unexposed (before) group, whereas all eligible women who received only DTG-based regimens during the entire PMTCT period until discharge were recruited for the exposed (after) group.
2.3 Sample size determination and sampling technique
The sample size was calculated using the double population proportion formula with G Power statistical software version 3.1.9.7. A significance level (alpha) of 5%, a power of 80%, an 84.6% retention rate in care among the unexposed group (17), and a ratio of unexposed to exposed of 1 were considered. We used an effect size of 0.2 (which is a small effect size that is conventionally used since there is no evidence supporting the magnitude of attrition among women exposed to DTG-based regimens). After considering 20% of missing data, the total sample size was 932 (466 for DTG-based regimens and 466 for EFV-based regimens). The final sample size is allocated proportionally to the number of pregnant and breastfeeding women enrolled in PMTCT care across all 34 health facilities. All eligible women in the selected facilities were included in the study until the expected sample size was reached in both groups.
2.4 Operational definitions
Exposed group refers to pregnant and breastfeeding women who have received DTG-based first-line ART until discharge from the PMTCT program.
Linked to ART refers to a woman who has transferred from Option B plus PMTCT care to the ART unit for continuity of care after her infant's final HIV status is determined.
Lost to follow-up (LTFU) refers to a woman who has not been seen within 28 days of the last scheduled clinic appointment and is not registered as dead or transferred to another clinic before the infant's final HIV status is determined (22).
Attrition refers to a woman enrolled in Option B plus PMTCT care who either died or is LTFU before the infant's final HIV status is determined (9).
Retention refers to a situation whereby a woman who is alive and on Option B plus PMTCT care until her infant's final HIV status is determined (9).
Non-retention refers to a situation in which women are not in the PMTCT program due to death, LTFU, or linkage to ART.
Transfer out refers to a woman who started ART in one facility but transferred to another facility for continuity of care with a standard transfer-out form (3).
Unexposed group refers to pregnant and breastfeeding women who are on EFV-based first-line ART until discharge from the PMTCT program.
2.5 Study variables
The outcome variable was maternal attrition from care among women on Option B plus PMTCT care. Women who remained in care until their infants' final HIV status was determined were categorized as censored, whereas those who died or were LTFU before their infants' final HIV status was determined were categorized as attrition, i.e., the event of interest. The exposure variable was the ART regimen the mother was receiving. Covariates in the present study were maternal sociodemographic, obstetric, and drug- and clinical-related variables. Mothers' ART adherence was categorized as poor, fair, or good (3, 22, 23). A viral load measurement not detected or below 50 copies/ml throughout the follow-up period is indicated as a suppressed viral load status, whereas a viral load measurement above 50 copies/ml at any time of the follow-up period is indicated as an unsuppressed viral load status (3, 22, 24). Details of the measurement of the variables are provided elsewhere (25).
2.6 Data management and analysis
Data were retrieved from the PMTCT registration book, Smart Care (a computer-based data registry found at the ART unit of respective facilities), and women's hospital records, which included the intake forms and follow-up cards. The data were collected using Open Data Kit (ODK) version 2.4 and then exported to Stata 14.0 (StataCorp, College Station, Texas, U.S.A.) for analysis. The event (i.e., death or LTFU) was coded as 1, and the censor (i.e., retention in care until the infant's final outcome was determined) was coded as 0. The Kaplan–Meier estimates were used to calculate the probabilities of attrition at the 6th, 12th, and 18th months of enrollment. A log-rank test was used to determine the difference in survival experiences between the two categories. The proportional hazard assumption was assessed using the log–log plot. In addition, the Cox–Snell residual and global tests were conducted, and the model was found to be fit (p = 0.2669). A bivariable Cox regression analysis was conducted on covariates that fulfilled the proportional hazards assumption. Covariates with a p-value of < 0.25 in the unadjusted analyses were selected as candidate variables for multivariate analysis. Then, a multivariable Cox proportional hazards regression model was fitted to identify the effect of DTG-based regimens and other predictor variables on attrition from care. The adjusted hazard ratio (aHR) with 95% confidence interval (CI) was computed, and all predictors that were associated with the outcome variable with a p-value of ≤ 0.05 were declared as a significant predictor of the outcome variable. Stratification was performed for occupation, facility type, CD4 count, and enrollment type that violated the proportional hazards assumption. However, there was no significant difference in the risk of attrition among women on DTG-based regimens.
3 Results
3.1 Sociodemographic characteristics
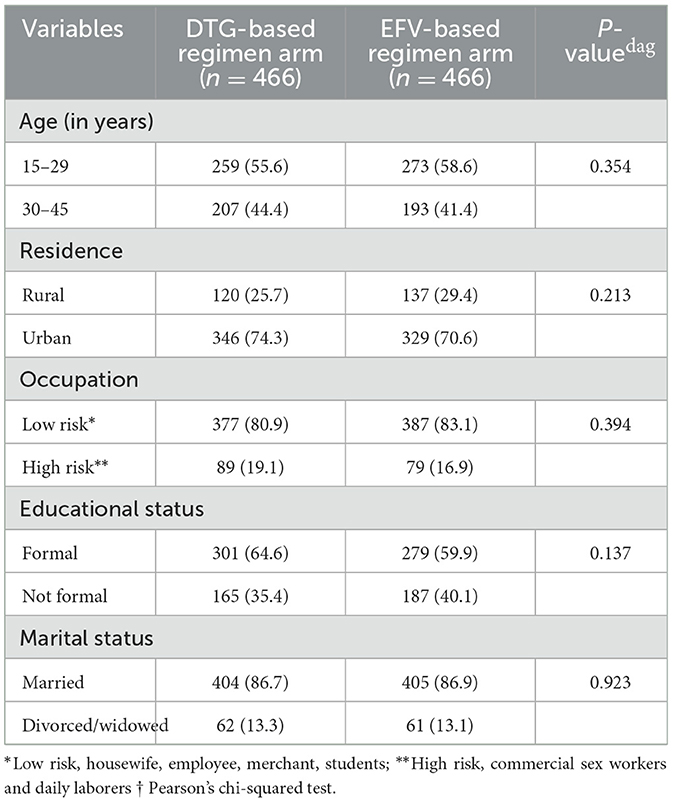
A total of 956 women's documents were reviewed and 932 study subjects were included in our study, achieving a response rate of 97.5%. In this study, 44.4% of women in the DTG-based regimen arm and 41.4% of women in the EFV-based regimen arm were over the age of 30 years. Additionally, 19.1% of women in the DTG-based regimen arm and 16.9% of women in the EFV-based regimen arm had high-risk occupations. On the other hand, 35.4% of women in the DTG-based regimen arm and 40.1% in the EFV-based regimen arm did not have a formal education (Table 1).
3.2 Obstetric characteristics
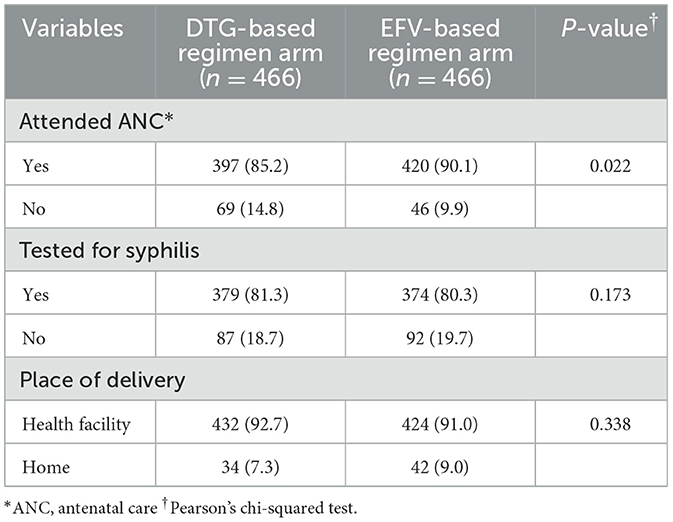
In our study, 85.2% of women in the DTG-based regimen arm and 90.1% of women in the EFV-based regimen arm had attended antenatal care during their pregnancy. However, 7.3% of women in the DTG-based regimen arm and 9.0% of women in the EFV-based regimen arm delivered their infants at home (Table 2).
3.3 Drug- and clinical-related characteristics
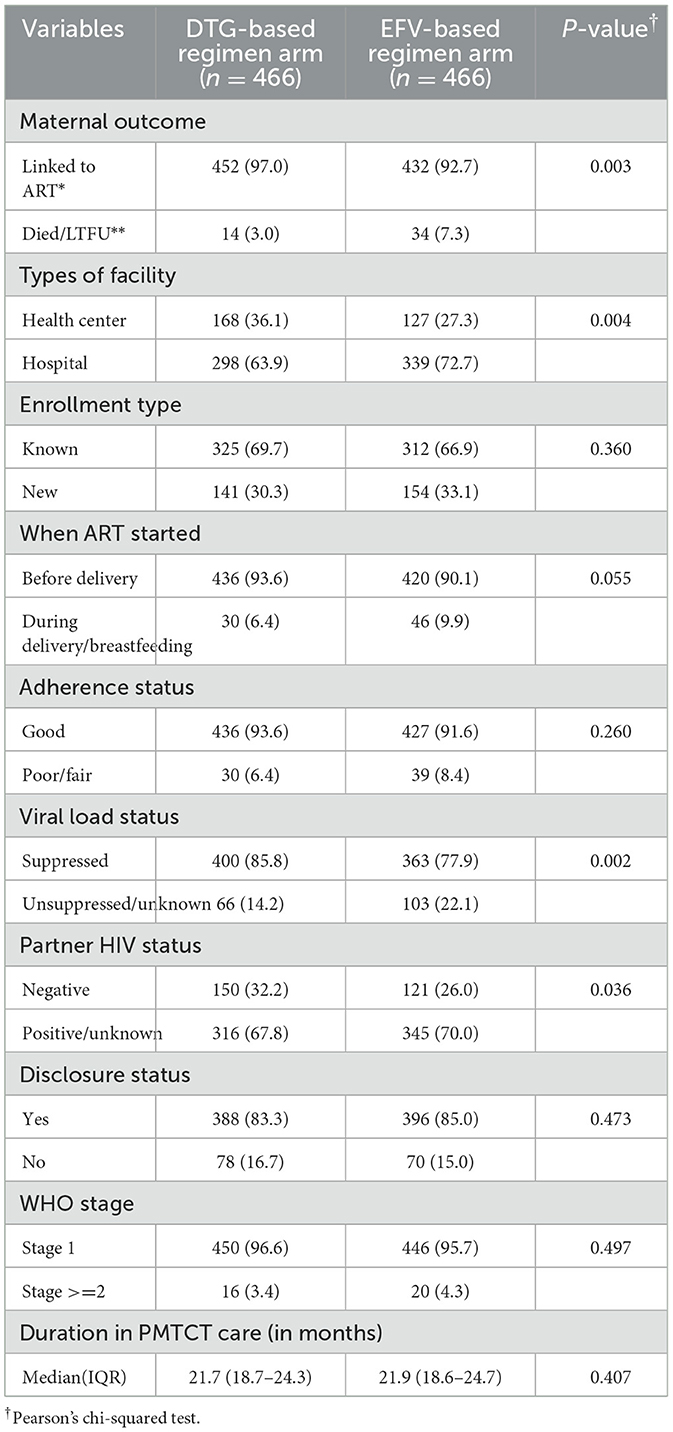
In this study, 48 (5.2%) women (3.0% in the DTG-based regimen arm and 7.3% in the EFV-based regimen arm) died or were LTFU from PMTCT care. In addition, 30.3% of women in the DTG-based regimen arm and 33.1% of women in the EFV-based regimen arm were newly enrolled in PMTCT care; 6.4% of them in the DTG-based regimen arm and 9.9% in the EFV-based regimen arm started ART during their delivery or breastfeeding period. Moreover, 6.4% of women in the DTG-based regimen arm and 8.4% in the EFV-based regimen arm had poor/fair adherence to ART during the PMTCT period. Furthermore, 16.7% of women in the DTG-based regimen arm and 15.0% of women in the EFV-based regimen arm did not disclose their HIV status to their partners. The median [interquartile range (IQR)] maternal PMTCT duration was 21.7 (18.7–24.3) months in the DTG-based regimen arm and 21.9 (18.6–24.7) months in the EFV-based regimen arm (Table 3).
3.4 Survival estimates
The Kaplan–Meier survival estimates indicated that women in the DTG-based regimen arm had a higher survival rate (retention in care) than those in the EFV-based regimen arm (Figure 1).
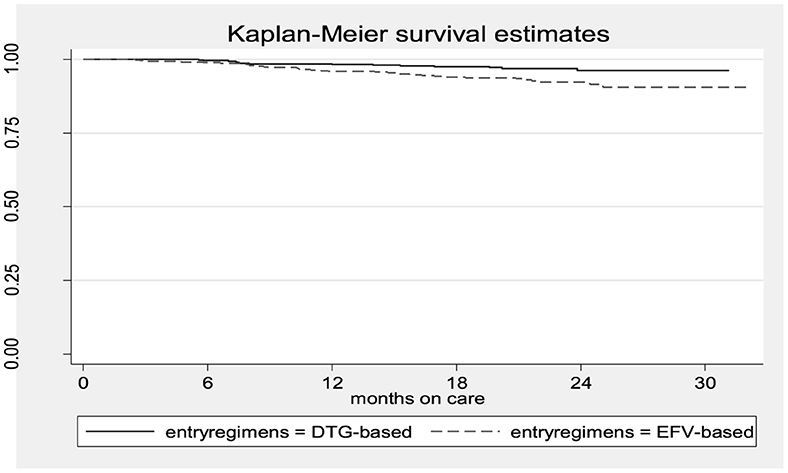
Figure 1. The Kaplan–Meier survival estimates of women who received DTG-based vs. those who received EFV-based first-line ART.
In addition, women who did not disclose their HIV status to their sexual partners, had unsuppressed/unknown viral load status, had poor/fair adherence to ART, and delivered their infants at home had a lower survival rate than their counterparts (Figure 2).
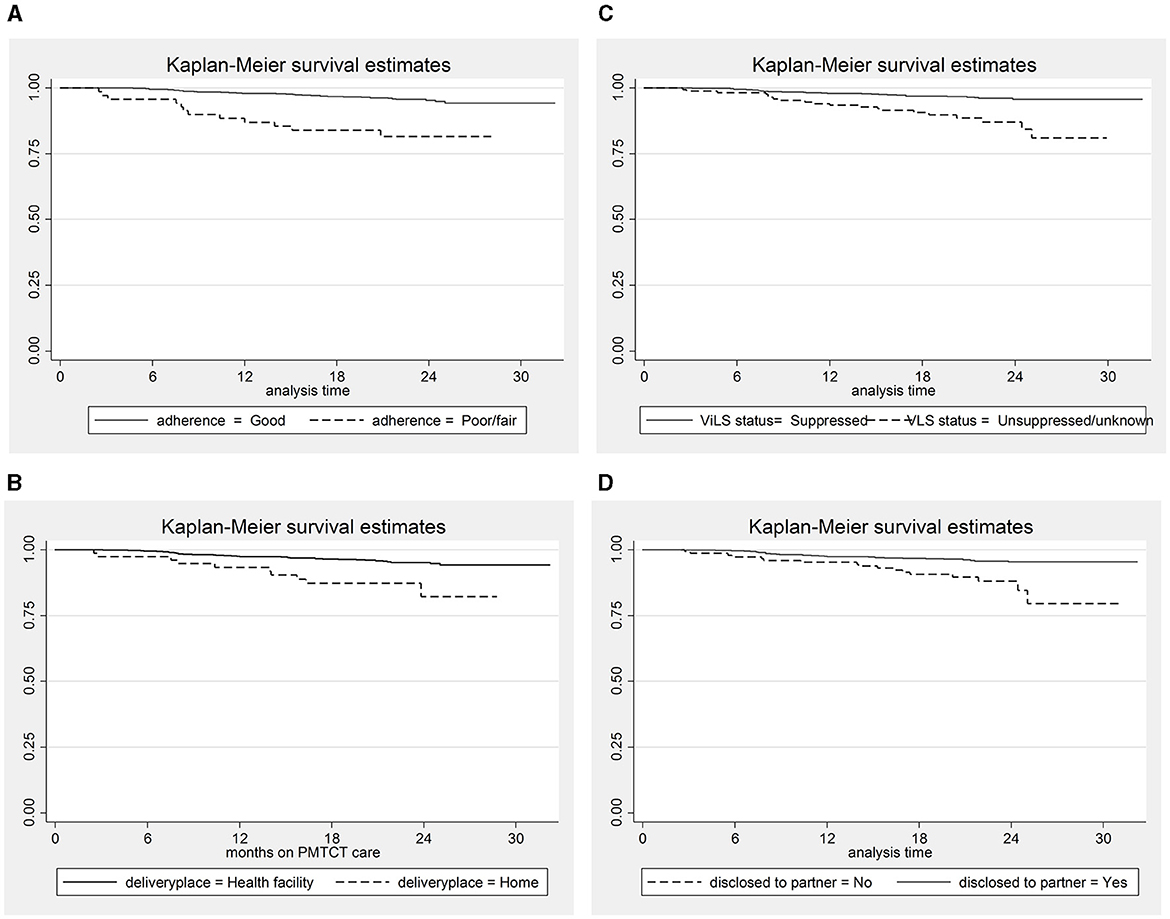
Figure 2. The Kaplan–Meier survival estimates for predictor variables. (A) Adherence status; (B) place of delivery; (C) Viral load suppression status; (D) Disclosure status.
3.5 Maternal attrition status
The cumulative incidence of attrition from PMTCT care among women is 5.2% [95% CI: 3.9%−6.8%; (3.0% in the DTG-based regimen arm and 7.3% in the EFV-based regimen arm)]. There was a lower non-retention rate among women in the DTG-based regimen arm compared to those in the EFV-based regimen arm at the 6th, 12th, and 18th months (Figure 3).
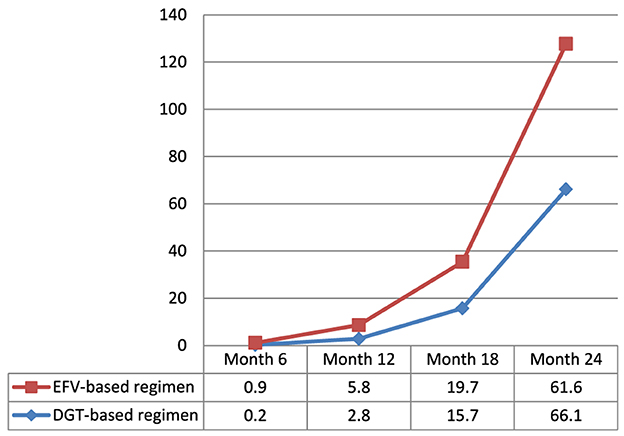
Figure 3. Magnitude of non-retention status in PMTCT care among women exposed to DTG-based compared to those exposed to EFV-based first-line antiretroviral therapy.
3.6 Effect of DTG-based regimens on attrition status
In a multivariable Cox proportional hazards regression analysis, mothers who were on a DTG-based regimen had approximately 57% (aHR: 0.43; 95% CI: 0.23–0.80) lesser hazard of attrition from PMTCT care than those who were on an EFV-based regimen. In addition, mothers who delivered their infants at home were 2.35 times (aHR: 2.35; 95% CI: 1.14–4.85) more likely to experience attrition from PMTCT care than their counterparts. Similarly, those who exhibited poor/fair adherence to ART were 3.23 times (aHR: 3.23; 95% CI: 1.62–6.45) more likely to do so. Mothers who had unsuppressed/unknown viral load status were 2.61 times (aHR: 2.61; 95% CI: 1.42–4.79) more likely to drop out, and those who did not disclose their status to partner were 2.56 times (aHR: 2.56; 95% CI: 1.34–4.92) more likely to experience attrition from PMTCT care than their counterparts (Table 4).
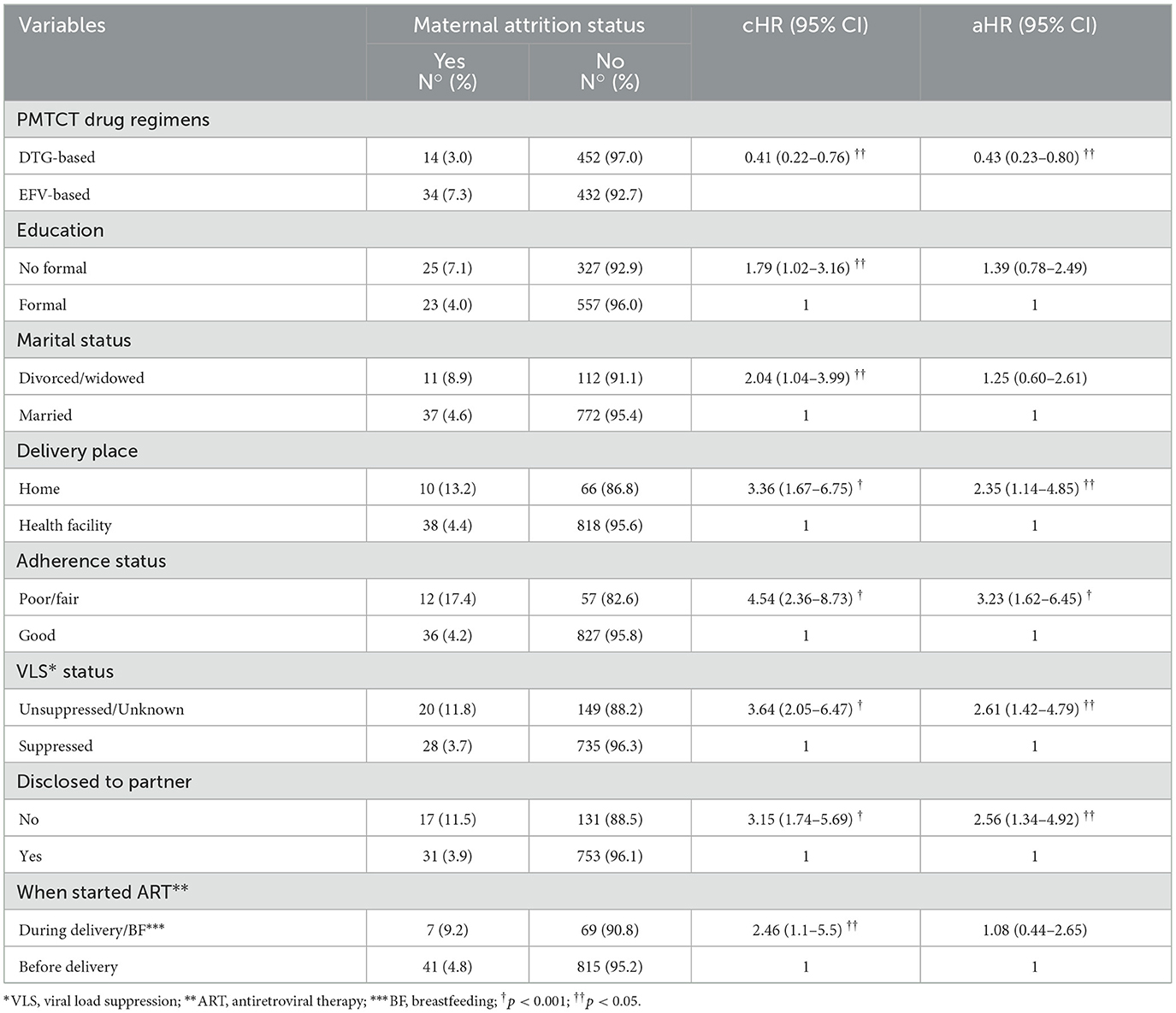
Table 4. Effect of DTG-based first-line ART regimens and other covariates on maternal attrition in PMTCT care.
4 Discussion
In this study, women on DTG-based regimens have lower attrition rates than those on EFV-based regimens. Delivery at home, poor/fair maternal adherence to medication, unsuppressed/unknown viral load status, and lack of disclosure to male partners were the identified risk factors for attrition among women in PMTCT care.
Furthermore, the cumulative incidence of attrition among women in PMTCT care was found to be 5.2% (3.0% in the DTG-based regimen and 7.3% in the EFV-based regimen). This finding in this study is lower than the results of the studies conducted in Uganda (14, 15, 26–28), Zimbabwe (16), Cameroon (17), Mozambique (29), and Ethiopia (18, 30–32) and the systematic review and meta-analysis conducted in Africa (13). This difference in the results can be attributed to better tolerability, higher efficacy, and effectiveness of the DTG-based regimen that were included in the study unlike the previous studies that did not include the DTG-based regimen (3, 11, 21). In addition, the differences in operational definitions adopted in the studies may have led to the differing results; these previous studies defined LTFU as women not being seen within 90 days of their last scheduled clinic appointment, whereas our study used 28 days as a cutoff point for LTFU. Women who were not retained in PMTCT care may develop drug resistance, have high viral copies, and transmit HIV to uninfected sexual partners and their infants (3, 22). A mechanism for retaining and tracing patients LTFU should be strengthened in all facilities that provide PMTCT services (9).
In our study, the place of delivery was significantly associated with attrition status. Women who had opted for delivery at home had a 2.35 times higher risk of attrition from PMTCT care compared to those who delivered at health facilities. Women who enrolled in the PMTCT program after delivery faced a higher risk of attrition from care than those who enrolled in the program during pregnancy (33). This finding could be because mothers who enrolled in the PMTCT program after delivery are more likely to have delivery at home and may not have received adequate counseling on the importance of regular clinic visits and the consequences of attrition from care for themselves and their infants (34). Therefore, efforts should focus on mobilizing and creating awareness among pregnant women about the benefits of attending institutional delivery.
Women with poor/fair adherence to ART were found to have a 3.23 times higher risk of attrition from PMTCT care compared to those having good ART adherence. This finding is consistent with the results of studies conducted in different parts of Ethiopia, such as Nekemte (33), East Gojam (35), University of Gondar (36), and Oromia region (37). The possible reasons for poor adherence may include conflicts with religious beliefs, seeking traditional healers, lack of partner support, fear of stigma and discrimination, lack of knowledge on the importance of adherence, and fear of the side effects of drugs, all of which may lead patients to miss their medication (33, 35).
Women who did not disclose their HIV status to their male partners had a 2.56 times higher risk of attrition than those who disclosed it. This finding is consistent with the results of the studies conducted in Uganda (14, 15), Cameroon (17), and Ethiopia (33). Women who disclosed their HIV status were more likely to have no fear of negative consequences from their partners, such as divorce, and received support from their spouse for retention in care (38). In addition, disclosing their HIV status to partners may reduce discrimination and fear associated with accessing HIV care services, which may make their stay on ART care comfortable (15, 39). Therefore, health workers in the PMTCT unit should strengthen counseling services for women on the importance of disclosure of HIV status to sexual partners throughout their care.
The study identified viral load suppression status as another risk factor for attrition. The risk of attrition among women who had unsuppressed/unknown viral load status was 2.61 times higher than those who had suppressed viral load status. This finding aligns with the result of the study conducted at the University of Gondar (36). The possible explanation is that women with unsuppressed viral load status may discontinue care due to the fear of providers' reaction to high viral loads (3, 23). Conversely, women with known viral load status may receive more attention and follow-up care from the providers, leading to better retention in care compared to those with unknown viral load status. Failure to achieve viral suppression augments the possibility of transmitting HIV to an infant through breastfeeding and puts children at risk of perinatal infection. Therefore, special attention should be given to women with unknown and unsuppressed viral load status to achieve global and national targets of zero new infections among HIV-exposed infants (40).
This study had a larger sample size and covered a wider geographic area. However, it had certain limitations. First, women who were LTFU might have continued their care at other facilities without documented referral. This might result in overestimation of the incidence of attrition. Second, women who transferred out from care were not traced, and they were excluded from the analysis, which may lead to underestimation of the attrition rate. Third, the information was based on routinely recorded data, which could include measurement and recording errors due to the nature of secondary data.
5 Conclusion
In this study, the cumulative incidence of attrition among women after the implementation of DTG-based regimens was found to be optimal. In addition, the risk of attrition from PMTCT care among women on DTG-based regimens is lower compared to those on EFV-based regimens. Poor adherence, non-disclosure of HIV status to partner, delivery at home, and unsuppressed/unknown viral load status were identified as risk factors for attrition in the study area.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
WF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TT: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing. EW: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing. AA: Conceptualization, Data curation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research work was supported by the Wolaita Sodo University.
Acknowledgments
The authors acknowledge the staff of the South and Central Ethiopia Regional Health Bureau for their technical and logistical support. The authors also sincerely thank all the data collectors and staff working in the PMTCT and ART units at the surveyed health facilities for their patience and cooperation during the entire data collection period.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ART, Antiretroviral therapy; ARV, Antiretroviral; aHR, adjusted hazard ratio; CI, confidence interval; cHR, crude hazard ratio; 3TC, Lamivudine; DTG, Dolutegravir; EFV, Efavirenz; HIV, Human Immunodeficiency Virus; IQR, Interquartile range; MTCT, Mother-to-child transmission; PMTCT, Prevention of mother-to-child transmission; TDF, Tenofovir; WHO, World Health Organization.
References
1. WHO. HIV and AIDS, Key facts. (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed July 13, 2023).
2. WHO. HIV statistics, globally and by WHO region. (2023). Available online at: https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/j0294-who-hiv-epi-factsheet-v7.pdf (accessed June 17, 2024).
3. FMoH. National comprehensive PMTCT/MNCH integrated training manual. Addis Ababa: FMoH (2021). p. 368–70.
4. WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Geneva: WHO Press. (2013). p. 251.
5. FMoH. National comprehensive and integrated prevention of mother-to-child transmission of HIV guide. Addis Ababa: FMoH (2017). p. 97.
6. UNAIDS. Global HIV statistics. (2023). Available online at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed June 17, 2024).
7. FMoH. National strategic plan for triple elimination of transmission of HIV, Syphilis, and Hepatitis B virus 2021-2025. Addis Ababa (2021).
8. WHO. The Global AIDS strategy 2021-2026. UNAIDS. (2020). p. 160. Available online at: https://www.unaids.org/en/Global-AIDS-Strategy-2021-2026 (accessed March 25, 2021).
9. FMoH. National Guideline for Prevention of Mother-to-child Transmission of HIV, Syphilis and Hepatitis B Virus. Addis Ababa (2021). p. 145.
10. WHO. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. Geneva: WHO (2018).
11. USAID. Tenofovir, Lamivudine and Dolutegravir (TLD) Transition. (2019). p. 2. Available online at: https://www.fhi360.org/sites/default/files/media/documents/linkages-tld-transition-information.pdf
12. UNAIDS. Fast-Track: Ending the AIDS epidemic by 2030. (2015). p. 10. Available online at: https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf (accessed June 17, 2024).
13. Knettel BA, Cichowitz CN, James SK, Chumba LN, Mmbaga BT, Watt MH. Retention in HIV care during pregnancy and the postpartum period in the option B+ era: systematic review and meta-analysis of studies in Africa. J Acquir Immune Defic Syndr. (2018) 77:427–38. doi: 10.1097/QAI.0000000000001616
14. Gerald O, Ruth M, Fredrick M. Rate and associated factors of non-retention of mother-baby pairs in HIV care in the elimination of mother-to-child transmission programme, Gulu-Uganda: a cohort study. BMC Health Serv Res. (2017) 17:1–10. doi: 10.1186/s12913-017-1998-5
15. Kiwanuka G, Kiwanuka N, Muneza F, Nabirye J, Oporia F, Odikro M, et al. Retention of HIV infected pregnant and breastfeeding women on option B+ in Gomba District, Uganda: a retrospective cohort study. BMC Infect Dis. (2018) 18:1–11. doi: 10.1186/s12879-018-3450-9
16. Chimwaza A, Tweya H, Mugurungi O, Mushavi A, Mukungunugwa S, Sithole N, et al. Early retention among pregnant women on ‘Option B+” in urban and rural Zimbabwe. AIDS Res Ther. (2021) 18:1–8. doi: 10.1186/s12981-021-00333-3
17. Penda C, Serge B, Essome H, Walter K, Paul N. Determinants of retention on the prevention of mother-to-child HIV transmission (option B+) in cameroon. J Ped Moth Care. (2019) 4:120.
18. Almado GA, King JE. Retention in care and health outcomes of HIV-exposed infants in a prevention of mother-to-child transmission of HIV (PMTCT) cohort in Addis Ababa, Ethiopia. HIV/AIDS - Res Palliat Care. (2021) 13:171–9. doi: 10.2147/HIV.S286347
19. Hoffman R, Phiri K, Parent J, Grotts J, Elashoff D, Kawale P, et al. Factors associated with retention in Option B+ in Malawi: a case control study. J Int AIDS Soc. (2017) 20:1–8. doi: 10.7448/IAS.20.01.21464
20. Lumbantoruan C, Kelaher M, Kermode M, Budihastuti E. Pregnant women's retention and associated health facility characteristics in the prevention of mother-to-child HIV transmission in Indonesia: cross-sectional study. BMJ Open. (2020) 10:1–6. doi: 10.1136/bmjopen-2019-034418
21. WHO. WHO recommends dolutegravir as preferred HIV treatment option in all populations. (2019). Available online at: https://www.who.int/news/item/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations (accessed July 22, 2019).
22. FMoH. National comprehensive HIV prevention, care, and treatment training participant manual. FMoH. (2021). p. 158.
23. FMoH. National guidelines for comprehensive HIV prevention, care and treatment. Addis Ababa: FMoH (2022). p. 262.
24. FMoH. National Comprehensive HIV Prevention, Care and Treatment Training for Health care Providers Participant Manual. Addis Ababa: FMoH (2022). p. 256–7.
25. Facha W, Tadesse T, Wolka E. Effect of dolutegravir-based first-line antiretroviral therapy on mother-to-child transmission of HIV among HIV-exposed infants in ethiopia : a before-and-after study. HIV/AIDS – Res Palliat Care. (2024) 16:203–15. doi: 10.2147/HIV.S456261
26. Koss CA, Natureeba P, Kwarisiima D, Ogena M, Clark TD, Olwoch P, et al. Viral suppression and retention in care up to 5 years after initiation of lifelong art during pregnancy (Option B+) in rural Uganda. J Acquir Immune Defic Syndr. (2017) 74:279–84. doi: 10.1097/QAI.0000000000001228
27. Kamacooko O, Mayanja Y, Bagiire D, Namale G, Hansen CH, Seeley J. Predictors of lost to follow-up in a “test and treat” programme among adult women with high-risk sexual behavior in Kampala, Uganda. BMC Public Health. (2020) 20:353. doi: 10.1186/s12889-020-8439-9
28. Muhumuza S, Akello E, Kyomugisha-Nuwagaba C, Baryamutuma R, Sebuliba I, Lutalo I, et al. Retention in care among HIV-infected pregnant and breastfeeding women on lifelong antiretroviral therapy in Uganda: A retrospective cohort study. PLoS ONE. (2017) 12:1–9. doi: 10.1371/journal.pone.0187605
29. Jara L, Philip W, Michael H, Manuel M, Jochen E, Olivia K, et al. Retention in care of HIV-infected pregnant and lactating women starting ART under Option B+ in rural mozambique. Trop Med Int Health. (2016) 01:1–10. doi: 10.1111/tmi.12728
30. Atanga PN, Ndetan HT, Achidi EA, Meriki HD, Hoelscher M, Kroidl A. Retention in care and reasons for discontinuation of lifelong antiretroviral therapy in a cohort of Cameroonian pregnant and breastfeeding HIV-positive women initiating ‘Option B+' in the South West Region. Trop Med Int Health. (2017) 22:161–70. doi: 10.1111/tmi.12816
31. Mitiku I, Arefayne M, Mesfin Y, Gizaw M. Factors associated with loss to follow-up among women in Option B+ PMTCT programme in northeast Ethiopia: a retrospective cohort study. J Int AIDS Soc. (2016) 19:1–8. doi: 10.7448/IAS.19.1.20662
32. Demissie D, Bayissa M, Maru H, Michael T. Assessment of loss to follow-up (LTFU) and associated factors among pregnant women initiated antiretroviral under option B+ in selected health facilities of West Zone Oromia, Ethiopia. EC Gynaecol. (2019) 8:314–21.
33. Tolossa T, Kassa GM, Chanie H, Abajobir A, Mulisa D. Incidence and predictors of lost to follow-up among women under Option B+ PMTCT program in western Ethiopia: a retrospective follow-up study. BMC Res Notes. (2020) 13:1–7. doi: 10.1186/s13104-019-4882-z
34. FMoH. National Antenatal Care guidelines. Addis Ababa: FMoH (2022). p. 1–80. Available online at: http://repository.iifphc.org/bitstream/handle/123456789/1647/ANC-GUIDELINE_Feb-24-2022.pdf (accessed June 17, 2024).
35. Telayneh A, Tesfa M, Woyraw W, Temesgen H, Alamirew N, Haile D, et al. Time to lost to follow-up and its predictors among adult patients receiving antiretroviral therapy retrospective follow-up study Amhara Northwest Ethiopia. Sci Rep. (2022) 12:1–11. doi: 10.1038/s41598-022-07049-y
36. Azanaw MM, Baraki AG, Yenit MK. Incidence and predictors of loss-to-follow-up among pregnant and breastfeeding women on option B+ PMTCT program in northwest Ethiopia: a retrospective follow-up study. Res Square. (2019) 10:1–28. doi: 10.21203/rs.3.rs-60540/v1
37. Megerso A, Garoma S, Eticha T, Workineh T, Daba S, Tarekegn M, et al. Predictors of loss to follow-up in antiretroviral treatment for adult patients in the Oromia region, Ethiopia. HIV/AIDS - Res Palliat Care. (2016) 8:83–92. doi: 10.2147/HIV.S98137
38. Mpinganjira S, Tchereni T, Gunda A, Mwapasa V. Factors associated with loss-to-follow-up of HIV-positive mothers and their infants enrolled in HIV care clinic: a qualitative study. BMC Public Health. (2020) 20:1–10. doi: 10.1186/s12889-020-8373-x
39. Getaneh T, Dessie G. Experiences and reasons of attrition from option B + among mothers under prevention of mother to child transmission program in northwest Ethiopia : qualitative study. HIV/AIDS - Res Palliat Care. (2021) 13:851–9. doi: 10.2147/HIV.S314306
40. WHO. Global Health Sector Strategy on HIV 2016-2021. World Health Organization (2016). p. 60. Available online at: http://apps.who.int/iris/bitstream/10665/246178/1/WHO-HIV-2016.05-eng.pdf?ua=1 (accessed May 03, 2016).
Keywords: attrition, lost to follow-up, retention, dolutegravir, efavirenz, Ethiopia
Citation: Facha W, Tadesse T, Wolka E and Astatkie A (2024) Attrition from care and its predictors among women exposed to dolutegravir- and efavirenz-based first-line antiretroviral therapy in Ethiopia: a before-and-after study. Front. Public Health 12:1385441. doi: 10.3389/fpubh.2024.1385441
Received: 12 February 2024; Accepted: 17 June 2024;
Published: 02 July 2024.
Edited by:
Marc Jean Struelens, Université libre de Bruxelles, BelgiumReviewed by:
Kamlendra Singh, University of Missouri, United StatesVicente Estrada, San Carlos University Clinical Hospital, Spain
Copyright © 2024 Facha, Tadesse, Wolka and Astatkie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wolde Facha, d29sZGllLmZhY2hhQHdzdS5lZHUuZXQ=; d29sZGllZmFjaGFAZ21haWwuY29t
†These authors have contributed equally to this work
‡ORCID: Wolde Facha orcid.org/0000-0002-7463-524X
 Wolde Facha
Wolde Facha Takele Tadesse1†
Takele Tadesse1† Ayalew Astatkie
Ayalew Astatkie