
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 02 May 2024
Sec. Digital Public Health
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1381328
Predicting, issuing early warnings, and assessing risks associated with unnatural epidemics (UEs) present significant challenges. These tasks also represent key areas of focus within the field of prevention and control research for UEs. A scoping review was conducted using databases such as PubMed, Web of Science, Scopus, and Embase, from inception to 31 December 2023. Sixty-six studies met the inclusion criteria. Two types of models (data-driven and mechanistic-based models) and a class of analysis tools for risk assessment of UEs were identified. The validation part of models involved calibration, improvement, and comparison. Three surveillance systems (event-based, indicator-based, and hybrid) were reported for monitoring UEs. In the current study, mathematical models and analysis tools suggest a distinction between natural epidemics and UEs in selecting model parameters and warning thresholds. Future research should consider combining a mechanistic-based model with a data-driven model and learning to pursue time-varying, high-precision risk assessment capabilities.
Unnatural epidemics (UEs) are caused by human intervention and may be deliberate or accidental releases of naturally occurring or altered pathogens (1). UEs cause substantial harm due to their wide range of infectiousness, rapid transmission, insidious processes, and multiple transmission methods (2). The intricacy of crowd behaviors and uncertainty regarding time and geography in UE outbreaks make prevention and control difficult (3).
This emphasizes the importance of early identification, prediction, and warning in UEs (4). Mathematical models and analysis tools can help combat UEs in prediction, early warning, and risk assessment (5). A study has proposed a model for labeling abnormal outbreak patterns to predict the development trends of complex pathogens (6). To achieve automatic early warning and response to UEs, some scholars have proposed real-time surveillance and aberration detection algorithms for enhanced outbreak monitoring (7). In addition, analysis tools can use simple scoring rules to complete a risk assessment of UEs. Scholars can use the analysis tools to assess the needs of applied epidemiology and training programs to develop greater capacity (8) and rank highly hazardous microorganisms. To better integrate various mathematical models and analysis tools for disease prediction, early warning, and risk assessment, disease surveillance systems have emerged as practical application tools. Current disease surveillance systems require early warning mechanisms that can detect a significant increase in confirmed cases by analyzing historical data. However, these methods cannot estimate outbreak size or detect new UEs. The data on UEs are scarce, making projections less accurate. Existing mathematical models and analysis tools for prediction, early warning, and risk assessment of UEs lack comprehensive review.
Therefore, this study aims to address the following research questions: What kinds of mathematical models or analysis tools have been developed for risk assessment of UEs? How should the suitability of the mathematical models and analysis tools be verified? What is the application of mathematical models and analysis tools embedded in surveillance systems? By reviewing relevant literature, this study will provide insights into mathematical models and analysis tools used for quantifying the risk of UEs. The findings will assist public health officials in making timely projections and decisions about UEs.
This scoping review was conducted using the recommended PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) guidelines published in 2018 (9). Supplementary material S1 provides the PRISMA-ScR Checklist.
A rigorous literature search was conducted from inception to 31 December 2023 using various online databases, such as PubMed, Web of Science, Scopus, and Embase. A combination of three types of keywords for the issue is necessary: subjects (words such as bioterrorism), purposes (words such as predict), and methods (words such as model) (10). These search terms are limited to the title, abstract, and keywords of the articles. Logical operators “AND” and “OR” were used to combine terms to meet PubMed, Web of Science, Scopus, and Embase standards. Appropriate filters and keywords were selected for the four databases. Supplementary material S2 provides detailed search strategies.
All peer-reviewed sources were reviewed independently by two authors. A third author resolved disagreements. A total of 1992 articles were reviewed, and after de-duplication using Endnote X9 (Clarivate Analytics, United States), 1,103 articles met the nadir criteria to enter the data extraction stage. Seventy-one articles were screened for initial inclusion based on the constraints of the selected metrics in this study. Twelve articles were chosen using snowball sampling. Seventeen of the eighty-three articles were eliminated because the necessary metrics could not be derived. Finally, 66 articles were reviewed in this study (Figure 1). The study selection form is shown in Supplementary material S3.
Data were extracted from all identified studies using a predefined format. Variables included year of publication, country, first author, journal, and so on (10). Supplementary material S4 provides the data extraction form.
Based on the current literature, risk assessment of UEs began to attract scholars from all fields in the 21st century, with more than 60% of the articles coming from the United States, which dominates research on mathematical models and analysis tools for risk assessment. Sixteen other countries have also published corresponding articles. Thirty-five journals contributed sixty-six articles to the study. With six articles, Emerging Infectious Diseases, Morbidity and Mortality Weekly Report Supplements, and Risk Analysis contributed the most articles. Supplementary material S5 summarizes the characteristics of the included studies. Supplementary material S6 displays the articles published in risk assessment studies using mathematical models and analysis tools.
This scoping review used descriptive-analytical methods. The graphs were designed using R statistical software (version 4.1.3).
Data-driven models are commonly used in statistical or machine learning methods such as support vector machines. Based on goodness-of-fit rather than mode-of-action or mechanism, data-driven models use correlations to find the optimal input variables to predict desired outputs. Therefore, their structure is “driven by the data” (11). Table 1 summarizes data-driven models for risk assessment of UEs.
Data-driven models have important applications in prediction. The first application is to detect outbreaks of UEs. The function of mutual information (12) uses daily anti-influenza drug sales to determine detection limits (thresholds) of UEs to complete early warning. A first-order model (13) can identify outbreaks 1–2 weeks before respiratory disease events. This model uses patient monitoring data, including age, complaints, and discharge diagnoses. Structural model (14) predicts the outbreak level 2 weeks after the alert using real and simulated anthrax exposure counting with noise. This model can detect and predict partially observed epidemics. Modified cyclical regression model (15) uses ambulance dispatch data for outbreak detection to measure influenza mortality and report the alarm threshold. Support vector regression (16) links dengue cases with weather parameters and predicts 20-week dengue trends. Cumulative sum (CUSUM) method (17–19) and exceedance method by Farrington (FARR) (18) are often used for early warning. A “signal” is generated in the system when the observed and expected count disparities exceed a threshold. CUSUM is widely used to report the epidemic severity of regional outbreaks in a system. UEs can also be tracked by the moving average of numerous data cycles (20, 21). Furthermore, data-driven models can track public opinions during outbreaks. Using supervised machine learning algorithms (47), researchers can identify anthrax-related tweets. Plotting data over time helped determine if an event was detected (based on a spike in the number of tweets occurring three times). This shows that machine learning can use not only conventional clinical data but also public opinion data for outbreak detection.
The second application is directed at predicting the size and time of attacks. Bayesian methods and networks (22–26) can predict simulated anthrax attacks using case counts and attack time. Bayesian methods can quickly assess the extent and time of a biological error attack and predict how many people will develop symptoms and need medical care. The What’s Strange About Recent Events (WSARE) algorithm (27, 28) reports anomalous patterns based on time, location, and population at risk to construct an outbreak risk profile. The recursive least square adaptive filter (29–31) detects short-term outbreak signals because it emphasizes recent past data while calculating forecast counts. Space–time scan statistics (32–35) prioritize temporal and geographical partitions based on attack size and time. Furthermore, generalized linear mixed models (36) estimate the infection probability of being a case for a surveillance day in a setting area. Time series analysis, such as the modified exponentially weighted moving average technique (37), the seasonal autoregressive integrated moving average model (ARIMA) (38), trimmed-mean seasonal models (39, 40) and ARIMA (39), is frequently used to examine epidemic trends. It uses count data to predict outbreaks and detect abnormal epidemics early.
With the emergence of more and more mathematical models for the risk assessment of UEs, there is a growing focus on evaluating the performance of these models. Some studies (34, 41) use a single input data or multivariate aggregated data to check the sensitivity of the model prediction performance. The results prove that two-stream surveillance is superior to one-stream surveillance. Model performance can also be measured by model practice effectiveness. Decision-analytic model (42) predicts lives, quality-adjusted life years (QALYs), and costs for a series of simulated bioterrorist attacks. However, false positives of the model were also noticed. G-/P-surveillance methods (43) that use only incidence case count data set the time to 21–30 days or 1 year to detect a relatively sudden increase in incidence. It greatly reduces false alarms but decreases sensitivity. Control chart and confidence interval methods (44) use call data and incubation periods for early warning of outbreaks. In comparison, the control chart for false alarm control is stricter. On top of having case data, different models incorporate additional data based on factors affecting the issue. Recombinant temporal aberration detection algorithms (46) also employ outpatient diagnoses, volume, and other relevant factors to decompose existing temporal aberration detection algorithms into two consecutive phases. This method investigates the impact of each phase on outbreak detection performance by reporting outbreak signals. In addition, the algorithms enhance the model’s detection of UEs.
Mechanistic-based models use mechanisms and algorithms regardless of data availability (50, 51). Data are used to fit these mechanistic-based models and enable their operations. They aim to describe causation, although they typically contain empirical components as well (11). Table 2 summarizes mechanistic-based models for risk assessment of UEs.
Mechanistic-based models for risk assessment of UEs mainly rely on infectious disease transmission mechanisms to construct a skeleton-like framework and commonly include Susceptible-Exposed-Infected-Removed (SEIR) models (52, 54), disease propagation differential equations, and other models (64, 65). Unlike data-driven models, mechanistic-based models emphasize fitting the model to the data. Mechanistic-based models can predict the size of UEs. A probabilistic anthrax model (55) uses a Markov chain Monte Carlo sampling algorithm to estimate the outbreak size. It evaluates post-anthrax release mitigating measures to better estimate unnatural outbreaks. Most mechanistic-based models (53, 56–59) predict cumulative infections, daily numbers of infections, and the size of UEs based on pathogen transmission in the target population. These models create rapid reaction systems and procedures by identifying and geospatially analyzing UEs. A few mechanistic-based models focus on the impact of dose–response mechanisms on the risk assessment of UEs. Input indicators are crucial to dose–response models (48, 49, 60–63). The input indicators can be included in clinical indicators (e.g., human respiration rate), environmental indicators (e.g., indoor room area), and pathogen dispersal indicators (e.g., spore dispersal rate). Dose–response models can estimate the size and timing of UEs for airborne pathogen infections, using human, environmental, and pathogen data to assess infection risk. Dose–response models can provide a reliable reference for risk assessors and healthcare decision-makers.
In addition to data-driven and mechanistic-based models, analysis tools are often applied to UEs. Analysis tools, including qualitative and quantitative tools, enable decision-makers to quickly and reliably differentiate between natural epidemics and UEs (66). Scores are assigned based on answers, similar to questionnaire scoring. Finally, these characteristics are scored to reflect the risk of UEs. Table 3 provides analysis tools for risk assessment of UEs.
A qualitative tool (67) presents epidemiological clues that highlight features of an epidemic that may suggest an unnatural attack. Another class of quantitative tools (68–77, 79–81) can calculate the risk factor scores to differentiate between natural epidemics and UEs. If the risk factor scores exceed the threshold, the event is likely unnatural. Such a simple and useful scoring method enables rapid differentiation between biological attacks and other epidemics, shortens the time for decision-makers to report the epidemic situation, and develops appropriate responses. Some scoring tools (8, 78) also assess biological weapon danger.
Model validation checks if a “model reliably reproduces the crucial behavior and quantities of interest within the intended context of use” (82). Model validation enhances model performance and applicability. The general analytical perspective of model validation starts with model uncertainty. Model uncertainty requires further refinement or tests of the model itself to affect final results. The validity of models and tools is increased by calibration (12, 14), improvement (74–76), and comparison (13, 55, 59, 63) under model uncertainty. Table 4 shows the model validation.
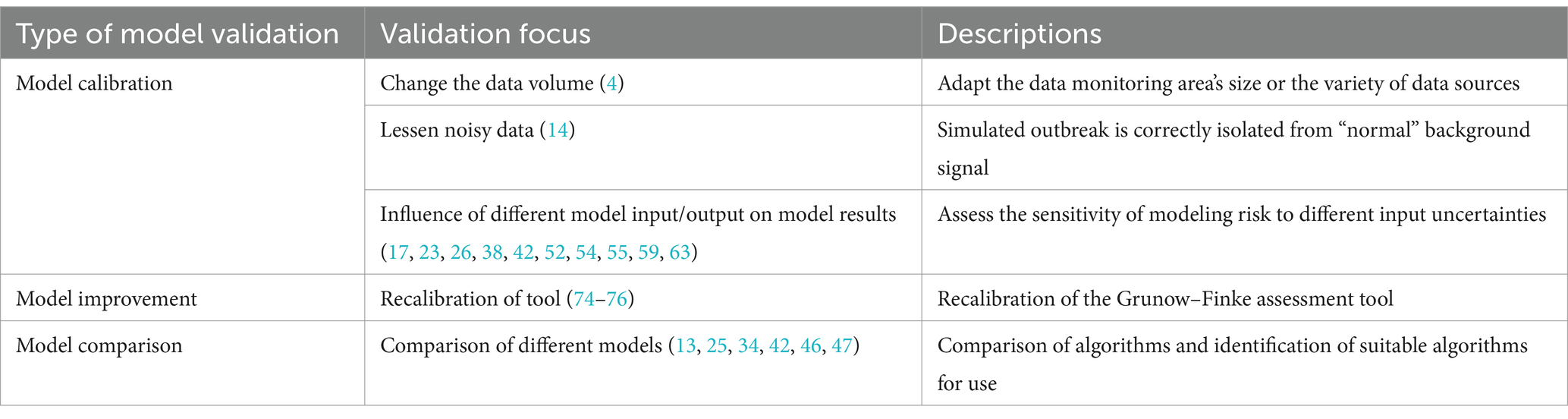
Table 4. Validation of mathematical models and analysis tools for risk assessment of unnatural epidemics.
Model calibration is a performance test of the model that has been built. Model calibration, which adjusts model parameters to match experimental data, affects a model’s ability to predict the future (83). This approach is particularly useful when a model involves multiple inputs or parameters (84). This study summarizes three directions for model validation.
First, it involves changing various inputs or model parameters (17, 23, 26, 38, 42, 52, 54, 55, 59, 63). Different model input–output combinations are tested to see how they affect results. Modeling risk sensitivity to input uncertainties is commonly studied. Then, method validation analyses the same scenario for different combinations of inputs and is assessed with evaluation indicators, such as R-squared, root mean square error (RMSE), mean square error (MSE), mean absolute error (MAE), mean absolute percentage error (MAPE), sensitivity, and receiver operating characteristic (ROC) curve. In addition to modifying model inputs and outputs, model misspecifications can affect model estimate accuracy. Legrand et al. (55) replicated the process of estimating anthrax spread in a model but intentionally misspecified parameter values, data, or model structure. The results suggest this can affect model estimation accuracy. Second, it involves reducing the influence of noisy data on genuine data. Background subtraction can test the multiple forward predictions of the established structural model (14). The final model was able to correctly isolate the simulated epidemic from the “normal” background signal. Third, it involves changing data sources to test model applicability. One approach (12) is to expand the monitored area by collecting data on daily sales of disease-related drugs. Researchers can then check drug purchase records for abnormalities to assess whether an epidemic has occurred. To test the sensitivity of the model’s prediction ability, another approach (34, 41) involves using a single input data set and multivariate aggregated data. Model calibration is common when a system is poorly understood or measured (83). However, model calibration is difficult because quantitative epidemic prediction models have many parameters to calibrate. Thus, poor parameter determination is the main obstacle to model prediction accuracy.
Model improvement extends beyond method validation. It involves calibrating the model to increase event risk assessment accuracy. This process typically leads to improved accuracy of a single model. The original technique is insensitive to unnatural infectious diseases and is weak at spotting previous epidemics with known causes. The Grunow-Finke tool (GFT) is the best-known tool for such differentiation. The recalibration of GFT for identifying UEs (74–76) involved removing criteria from the old GFT. In addition, it also adjusts weighting factors for additional criteria and reintroduces significant ones. It sets evaluation tools that identify the thresholds of natural epidemics and UEs, too. Model improvement focuses on the lower sensitivity and specificity of the model or tool to improve the broad applicability of them. Model improvement involves reviewing the original model’s assumptions and determining if existing changes conflict with existing data.
Knowing the right model’s structure may make it more interesting to determine its absolute performance. This situation involves model comparison for various defined models (83). Model comparison involves comparing and observing the results of different methods for the same problem. It compares multiple models to prove its performance. Building multiple models for the same problem and comparing their predictions help determine the best model and algorithm for forecasting unnatural outbreaks (13, 25, 34, 42, 46, 47). In general, when assessing model outbreak thresholds, three or more standard deviations from the baseline model can be used. Exponentially weighted moving averages (EWMA) (13) can be used as a method to modify the forecast based on recent errors. Finally, various models are compared to the baseline model to assess its predictive power. Accuracy, recall, F1-score, sensitivity, specificity, ROC, and p-value measure model efficacy. When attempting to predict UEs, not all models can be assessed for efficacy using the same set of evaluation indicators. This is where attention must be paid to model comparison for risk assessments of UEs.
Mathematical models and analysis tools are used for UEs in our review, but they can also be used to determine the occurrence of natural epidemics. The difference exists between natural epidemics and UEs when using mathematical models and analytical tools. The values for natural epidemics and UEs are not the same in the choice of model parameters (60, 62, 63) and warning thresholds (19). However, some studies (13, 20, 44) have also pointed out that some models and tools used for natural epidemics are currently unlikely to provide early detection of UEs. In the current study, data-driven models and mechanistic-based models are less likely to mention the difference between natural epidemics and UEs in terms of model use.
Several syndrome surveillance systems have been created recently to detect natural epidemics and UEs. Mathematical modeling of surveillance systems has two general directions. One approach simulates various UEs. The second approach is to evaluate warning algorithms for reported event outbreaks using surveillance system data. Currently, the system’s mission is to detect historical outbreaks and determine when the disease will be detected. The systems used in this study are divided into three types: event-based surveillance systems, indicator-based surveillance systems, and hybrid surveillance systems. Supplementary material S7 shows the mathematical models and analysis tools applied in the surveillance systems.
Event-based surveillance systems use unstructured data from non-health-sector sources (85). Frequently, data seen daily in society, such as drug sales and absence data, are used as early warning signals (86). It is a common way for such surveillance systems to conduct risk assessment with mathematical models, such as the National Health Service Clinical Assessment System (NHSCAS) and Early Alerting and Reporting (EAR) project. NHSCAS (44, 45) provides early warning of outbreaks caused by UEs or more common infections. The syndrome surveillance system overlays cryptosporidiosis epidemic data onto a statistical model of health hotline (NHS Direct) call data to test it. It calculates the upper limit of prediction for the proportion of diarrhea calls using the confidence interval and control chart methods. A scored risk assessment tool is used in the EAR project (72). It is based on the etiology, health effects, clinical presentation, and epidemiology of the project’s event or outbreak, as well as the sources of information and the potential for intentional release.
Indicator-based surveillance systems generally analyze data routinely collected from healthcare facilities through institutional disease reporting (87). WSARE algorithm (27, 28) detects outbreak-related anomalies. Date-indexed biomonitoring data (e.g., emergency department data) feeds the algorithm. By comparing date events, statistically significant abnormalities are found and analyzed for early warning. Demographic and hospital monitoring data will alert systems to UEs (19, 20, 29, 30). If a signal exceeds the statistical distortion threshold, an alarm is raised. Commonly used models for this type of system are time series methods (moving average method), CUSUM, and recursive least square adaptive filter. The WHO recommends event-based surveillance to supplement indicator-based surveillance. Indicator-based surveillance may not uncover outbreaks and major public health problems. Additionally, current techniques cannot detect rare but significant outbreaks such as Ebola and avian influenza, as well as novel diseases (86).
Hybrid surveillance systems use both event and indicator variables to construct the model. Mostashari et al. (15) monitor UEs using ambulance dispatch calls. They also employ statistical techniques to measure retrospective data on influenza-like illness (ILI) call types. An alarm was raised when the observed ILI rate exceeded the expected upper confidence limit by 1 day. A system that uses a dengue prediction model using support vector regression (16) relates cases to weather parameters (i.e., temperature, humidity, and rainfall). Even with a small amount of data training, the system can capture dengue trends. The national bioterrorism syndromic surveillance demonstration program (32, 33) combines incidence counts and zip codes to predict the number of episodes of illness using space–time scan statistics. Absenteeism rates (21) are used in system data to track abnormal occurrences. A multi-data surveillance system (18) uses CUSUM and FARR by chief complaints, NHS call types, and the number of records assigned to each syndrome. They detect syndrome numbers rising slowly, acutely, and locally. Time series analysis employed by Early Notification of Community-Based Epidemics (ESSENCE) II (37) for early warning reveals that high-profile events may change detection and alert thresholds.
This study involves two models and a class of tools with established theoretical and computational background. Data-driven models solve biosafety challenges and self-learn for epidemic prediction and monitoring, while mechanistic-based models use extensive knowledge of disease transmission mechanisms to forecast epidemics. This makes disease development more traceable. For decision-makers addressing biosecurity events, the analysis tools in this study are user-friendly and adaptable to multiple diseases. They facilitate quick collaboration among agencies to combat UEs. Machine learning methods are increasingly preferred by researchers in data-driven models. With massive data, machine learning is being applied in UEs. Machine learning enhances tracking and real-time reporting of highly hidden, short-spread, and rapidly spreading unnatural outbreaks.
Although there are many variations of algorithms in data-driven models in terms of technical means, there are certain requirements for data. The data quality tries to be complete and accurate. If the quality of the data is not satisfactory, it is often necessary to find a way out of the algorithms. Therefore, data-driven models increasingly favor machine learning algorithms. Model parameter fitting makes mechanistic-based model prediction harder. The use of analysis tools, although simple, is generally not superior to the above two types of models in terms of accuracy for event identification.
Mathematical models and analysis tools have advanced, but challenges remain. First, the difficulty of data collection; Complete and accurate data are crucial for data-driven models but limited for UEs. Mechanistic-based models are not influenced by external data, but parameters must be fitted multiple times to improve risk assessment. Second, updating the models is also challenging due to uncertain biological factors. Transmission characteristics of viruses and bacteria need to be accurately identified. Mechanistic-based models struggle with spatial predictions. At the same time, syndromic surveillance solutions typically target one illness. It has a limited ability to distinguish between outbreaks of individual diseases with similar syndromes.
Fully digitized demographic and health data and efficient testing and reporting systems are crucial for effective disease control. Mathematical models and analysis tools play a vital role in controlling epidemics by providing risk assessment. Future applications will focus on improving model parameters to consider social exposure, human activity, economic impact, and environmental impact. Machine learning algorithms will play a significant role in case detection, contact tracking, and intervention responses, narrowing risk assessment to smaller scales and shorter timeframes.
UEs generate social and work disruptions, mortality, and economic losses due to their covert spread. Current research in this field focuses on analyzing the risk of UEs using symptoms and social data for prediction, early warning, and risk assessment. Mathematical models and analysis tools can be very useful for risk assessment of UEs. Now, using data-driven models combined with non-medical data (such as opinion data) for risk assessment of UEs is indeed becoming more prevalent. Most approaches for differentiating from natural epidemics and UEs globally use analysis tools. However, there are limitations to existing mathematical models and analysis tools, such as limited data availability and the inability to update models in real time. Risk analysis tools rely on data inputs such as data source validity and specified illness parameters of recognized pathogens, which is a limitation. Furthermore, the data used to generate the risk scores was gathered from multiple sources and at various points in time, which may be out of date or contain biases in some circumstances. Data and monitoring errors may also arise during actual events. Most importantly, the included studies, while showing that the models they examined were used for UEs, can also be used to identify the occurrence of natural epidemics. In the current study, mathematical models and analysis tools suggest a distinction between natural epidemics and UEs in the selection of model parameters and warning thresholds.
This review summarizes mathematical models and analysis tools for risk assessment of UEs. If data limitations persist, subsequent model development should focus on model structure or data. For example, the model structure can be improved by replacing the model calculation method to reduce data integrity dependence (e.g., combining a mechanism model with a data-driven approach). In terms of used data, data cleaning can interpolate missing values or consider noisy data. For model factors, the selection will affect the methods of model validation. High-quality surveillance data and indicators are necessary for future surveillance systems. Future model development should consider combining a mechanistic-based model with a data-driven model and learning in the pursuit of time-varying, high-precision risk assessment capabilities.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
JL: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. YL: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. ZM: Data curation, Formal analysis, Writing – review & editing. ZL: Data curation, Formal analysis, Writing – review & editing. GZ: Conceptualization, Supervision, Writing – review & editing. CC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key R&D Program of China [2021YFC260050401].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1381328/full#supplementary-material
1. Jansen, HJ, Breeveld, FJ, Stijnis, C, and Grobusch, MP. Biological warfare, bioterrorism, and biocrime. Clin Microbiol Infect. (2014) 20:488–96. doi: 10.1111/1469-0691.12699
2. Lee, S, and Kim, Y. Predictors of bioterrorism preparedness among clinical nurses: a cross-sectional study. Nurse Educ Today. (2023) 122:105727. doi: 10.1016/j.nedt.2023.105727
3. Khardori, N . Bioterrorism and bioterrorism preparedness: historical perspective and overview. Infect Dis Clin N Am. (2006) 20:179–211. doi: 10.1016/j.idc.2006.03.007
4. Ministry of Government LegislationShin, GR . The definition of terrorism concept reflecting the change of terrorism in the anti-terrorism act. Gachon law. Review. (2020) 13:101–34. doi: 10.15335/GLR.2020.13.3.004
5. Mykhalovskiy, E, and Weir, L. The global public health intelligence network and early warning outbreak detection: a Canadian contribution to global public health. Can J Public Health. (2006) 97:42–4. doi: 10.1007/BF03405213
6. MacIntyre, CR . Biopreparedness in the age of genetically engineered pathogens and open access science: an urgent need for a paradigm shift. Mil Med. (2015) 180:943–9. doi: 10.7205/MILMED-D-14-00482
7. Zhang, H, Wang, L, Lai, S, Li, Z, Sun, Q, and Zhang, P. Surveillance and early warning systems of infectious disease in China: from 2012 to 2014. Health Plan Manag. (2017) 32:329–38. doi: 10.1002/hpm.2434
8. Tomuzia, K, Menrath, A, Frentzel, H, Filter, M, Weiser, AA, Bräunig, J, et al. Development of a comparative risk ranking system for agents posing a bioterrorism threat to human or animal populations. Biosecur Bioterr. (2013) 11:S3–S16. doi: 10.1089/bsp.2012.0070
9. Tricco, AC, Lillie, E, Zarin, W, O’Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
10. Fleuren, LM, Klausch, TLT, Zwager, CL, Schoonmade, LJ, Guo, T, Roggeveen, LF, et al. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. (2020) 46:383–400. doi: 10.1007/s00134-019-05872-y
11. Ellis, JL, Jacobs, M, Dijkstra, J, Van Laar, H, Cant, JP, Tulpan, D, et al. Review: synergy between mechanistic modelling and data-driven models for modern animal production systems in the era of big data. Animal. (2020) 14:s223–37. doi: 10.1017/S1751731120000312
12. Kobari, T, Iwaki, K, Nagashima, T, Ishii, F, Hayashi, Y, and Yajima, T. Detection limit used for early warning in public health surveillance. Anal Sci. (2009) 25:795–800. doi: 10.2116/analsci.25.795
13. Brillman, JC, Burr, T, Forslund, D, Joyce, E, Picard, R, and Umland, E. Modeling emergency department visit patterns for infectious disease complaints: results and application to disease surveillance. BMC Med Inform Decis Mak. (2005) 5:4. doi: 10.1186/1472-6947-5-4
14. Cheng, KE, Crary, DJ, Ray, J, and Safta, C. Structural models used in real-time biosurveillance outbreak detection and outbreak curve isolation from noisy background morbidity levels. J Am Med Inform Assoc. (2013) 20:435–40. doi: 10.1136/amiajnl-2012-000945
15. Mostashari, F, Fine, A, Das, D, Adams, J, and Layton, M. Use of ambulance dispatch data as an early warning system for communitywide influenzalike illness. New York City J Urban Health. (2003) 80:i43–9. doi: 10.1007/PL00022314
16. Ali, MA, Ahsan, Z, Amin, M, Latif, S, Ayyaz, A, and Ayyaz, MN. ID-viewer: a visual analytics architecture for infectious diseases surveillance and response management in Pakistan. Public Health. (2016) 134:72–85. doi: 10.1016/j.puhe.2016.01.006
17. Yuan, CM, Love, S, and Wilson, M. Syndromic surveillance at hospital emergency departments--southeastern Virginia. MMWR Suppl. (2004) 53:56–8. doi: 10.2307/23315691
18. Meyer, N, McMenamin, J, Robertson, C, Donaghy, M, Allardice, G, and Cooper, D. A multi-data source surveillance system to detect a bioterrorism attack during the G8 summit in Scotland. Epidemiol Infect. (2008) 136:876–85. doi: 10.1017/S0950268807009132
19. Rhee, C, Burkom, H, Yoon, C, Stewart, M, Elbert, Y, Katz, A, et al. Syndromic surveillance system for Korea–US joint biosurveillance portal: design and lessons learned. Health Secur. (2016) 14:152–60. doi: 10.1089/hs.2015.0067
20. Dembek, ZF, Carley, K, Siniscalchi, A, and Hadler, J. Hospital admissions syndromic surveillance--Connecticut, September 200-November 2003. MMWR Suppl. (2004) 53:50–2.
21. Lawson, BM, Fitzhugh, EC, Hall, SP, Franklin, C, Hutwagner, LC, Seeman, GM, et al. multifaceted syndromic surveillance in a public health department using the early aberration reporting system. J Public Health Manag Pract. (2005) 11:274–81. doi: 10.1097/00124784-200507000-00003
22. Walden, J, and Kaplan, EH. Estimating time and size of bioterror attack. Emerg Infect Dis. (2004) 10:1202–5. doi: 10.3201/eid1007.030623
23. Hogan, WR, Cooper, GF, Wallstrom, GL, Wagner, MM, and Depinay, JM. The Bayesian aerosol release detector: an algorithm for detecting and characterizing outbreaks caused by an atmospheric release of Bacillus anthracis. Stat Med. (2007) 26:5225–52. doi: 10.1002/sim.3093
24. Shen, Y, Adamou, C, Dowling, JN, and Cooper, GF. Estimating the joint disease outbreak-detection time when an automated biosurveillance system is augmenting traditional clinical case finding. J Biomed Inform. (2008) 41:224–31. doi: 10.1016/j.jbi.2007.11.002
25. Izadi, M, Buckeridge, D, Okhmatovskaia, A, and Tu, SW. A Bayesian network model for analysis of detection performance in surveillance systems. In: Amia Annual Symposium Proceedings Amia Symposium (2009):2009:276–280
26. Ray, J, Marzouk, YM, and Najm, HN. A Bayesian approach for estimating bioterror attacks from patient data. Statist Med. (2011) 30:101–26. doi: 10.1002/sim.4090
27. Wong, WK, Moore, A, Cooper, G, and Wagner, M. Wsare: What’s strange about recent events? J Urban Health. (2003) 80:i66–75. doi: 10.1007/PL00022317
28. Kaufman, Z, Wong, WK, Peled-Leviatan, T, Cohen, E, Lavy, C, Aharonowitz, G, et al. Evaluation of a syndromic surveillance system using the WSARE algorithm for early detection of an unusual, localized summer outbreak of influenza B: implications for bioterrorism surveillance. Isr Med Assoc J. (2007) 9:3–7.
29. Tsui, Fu-Chiang, Espino, Jeremy U., Wagner, Michael M., Gesteland, Per, Ivanov, Oleg, Olszewski, Robert T., et al. Data, network, and application: technical description of the Utah RODS winter Olympic biosurveillance system. In: Proceedings Amia Symposium (2002): 815–819.
30. Gesteland, PH, Gardner, RM, Tsui, FC, Espino, JU, Rolfs, RT, James, BC, et al. Automated syndromic surveillance for the 2002 winter Olympics: Table 1. J Am Med Inform Assoc. (2003) 10:547–54. doi: 10.1197/jamia.M1352
31. Tsui, FC, Espino, JU, Dato, VM, Gesteland, PH, Hutman, J, and Wagner, MM. Technical description of RODS: a real-time public health surveillance system. J Am Med Inform Assoc. (2003) 10:399–408. doi: 10.1197/jamia.M1345
32. Yih, WK, Caldwell, B, Harmon, R, Kleinman, K, Lazarus, R, Nelson, A, et al. National bioterrorism syndromic surveillance demonstration program. MMWR Suppl. (2004) 53:43–9.
33. Nordin, JD, Goodman, MJ, Kulldorff, M, Ritzwoller, DP, Abrams, AM, Kleinman, K, et al. Simulated Anthrax attacks and syndromic surveillance. Emerg Infect Dis. (2005) 11:1394–8. doi: 10.3201/eid1109.050223
34. Kleinman, KP, Abrams, A, Mandl, K, and Platt, R. Simulation for assessing statistical methods of biologic terrorism surveillance. MMWR Suppl. (2005) 54:101–8.
35. Kulldorff, M, Mostashari, F, Duczmal, L, Katherine Yih, W, Kleinman, K, and Platt, R. Multivariate scan statistics for disease surveillance. Stat Med. (2007) 26:1824–33. doi: 10.1002/sim.2818
36. Kleinman, K . A generalized linear mixed models approach for detecting incident clusters of disease in small areas, with an application to biological terrorism. Am J Epidemiol. (2004) 159:217–24. doi: 10.1093/aje/kwh029
37. Lombardo, J, Burkom, H, Elbert, E, Magruder, S, Lewis, SH, Loschen, W, et al. A systems overview of the electronic surveillance system for the early notification of community-based epidemics (ESSENCE II). J Urban Health. (2003) 80:i32–42. doi: 10.1007/PL00022313
38. Buckeridge, DL, Switzer, P, Owens, D, Siegrist, D, Pavlin, J, and Musen, M. An evaluation model for syndromic surveillance: assessing the performance of a temporal algorithm. MMWR Suppl. (2005) 54:109–15.
39. Reis, BY, and Mandl, KD. Time series modeling for syndromic surveillance. BMC Med Inform Decis Mak. (2003) 3:2. doi: 10.1186/1472-6947-3-2
40. Reis, BY, Pagano, M, and Mandl, KD. Using temporal context to improve biosurveillance. Proc Natl Acad Sci USA. (2003) 100:1961–5. doi: 10.1073/pnas.0335026100
41. Reis, BY, and Mandl, KD. Integrating syndromic surveillance data across multiple locations: effects on outbreak detection performance. In: Amia. Annual Symposium Proceedings. Amia Symposium (2003): 2003:549–553
42. McBrien, KA, Kleinman, KP, Abrams, AM, and Prosser, LA. Use of outcomes to evaluate surveillance systems for bioterrorist attacks. BMC Med Inform Decis Mak. (2010) 10:25. doi: 10.1186/1472-6947-10-25
43. Wallenstein, S, and Naus, J. Scan statistics for temporal surveillance for biologic Terroris. MMWR Suppl. (2004) 53:74–8.
44. Cooper, DL, Verlander, NQ, Smith, GE, Charlett, A, Gerard, E, Willocks, L, et al. Can syndromic surveillance data detect local outbreaks of communicable disease? A model using a historical cryptosporidiosis outbreak. Epidemiol Infect. (2006) 134:13–20. doi: 10.1017/S0950268805004802
45. Smith, GE, Cooper, DL, Loveridge, P, Chinemana, F, Gerard, E, and Verlander, N. A national syndromic surveillance system for England and Wales using calls to a telephone helpline. Eur Secur. (2006) 11:9–10. doi: 10.2807/esm.11.12.00667-en
46. Murphy, SP, and Burkom, H. Recombinant temporal aberration detection algorithms for enhanced biosurveillance. J Am Med Inform Assoc. (2008) 15:77–86. doi: 10.1197/jamia.M2587
47. Miller, M, Romine, W, and Oroszi, T. Public discussion of Anthrax on twitter: using machine learning to identify relevant topics and events. JMIR Public Health Surveill. (2021) 7:e27976. doi: 10.2196/27976
48. Nicas, M, and Hubbard, A. A risk analysis for airborne pathogens with low infectious doses: application to respirator selection against Coccidioides immitis spores. Risk Anal. (2002) 22:1153–63. doi: 10.1111/1539-6924.00279
49. Nicas, M, and Hubbard, A. A risk analysis approach to selecting respiratory protection against airborne pathogens used for bioterrorism. AIHA J. (2003) 64:95–101. doi: 10.1080/15428110308984797
50. Liu, X, Zheng, X, and Balachandran, B. COVID-19: data-driven dynamics, statistical and distributed delay models, and observations. Nonlin Dyn. (2020) 101:1527–43. doi: 10.1007/s11071-020-05863-5
51. Lessler, J, Azman, AS, Grabowski, MK, Salje, H, and Rodriguez-Barraquer, I. Trends in the mechanistic and dynamic modeling of infectious diseases. Curr Epidemiol Rep. (2016) 3:212–22. doi: 10.1007/s40471-016-0078-4
52. Legrand, J, Viboud, C, Boelle, PY, Valleron, AJ, and Flahault, A. Modelling responses to a smallpox epidemic taking into account uncertainty. Epidemiol Infect. (2004) 132:19–25. doi: 10.1017/S0950268803001390
53. Chitnis, N, Hyman, JM, and Valle, SYD. Mathematical models of contact patterns between age groups for predicting the spread of infectious diseases. MBE. (2013) 10:1475–97. doi: 10.3934/mbe.2013.10.1475
54. Ohkusa, Y, Taniguchi, K, and Okubo, I. Prediction of smallpox outbreak and evaluation of control-measure policy in Japan, using a mathematical model. J Infect Chemother. (2005) 11:71–80. doi: 10.1007/s10156-005-0373-3
55. Legrand, J, Egan, JR, Hall, IM, Cauchemez, S, Leach, S, and Ferguson, NM. Estimating the location and spatial extent of a covert Anthrax release. R Brookmeyer , editor. PLoS Comput Biol. (2009); 5:e1000356, doi: 10.1371/journal.pcbi.1000356
56. Meltzer, M . Modeling potential responses to smallpox as a bioterrorist weapon. Emerg Infect Dis. (2001) 7:959–69. doi: 10.3201/eid0706.010607
57. Egan, JR, Legrand, J, Hall, IM, Cauchemez, S, Ferguson, NM, and Leach, S. Re-assessment of mitigation strategies for deliberate releases of anthrax using a real-time outbreak characterization tool. Epidemics. (2010) 2:189–94. doi: 10.1016/j.epidem.2010.06.001
58. Zenihana, T, and Ishikawa, H. Effectiveness assessment of countermeasures against bioterrorist smallpox attacks in Japan using an individual-based model. Environ Health Prev Med. (2010) 15:84–93. doi: 10.1007/s12199-009-0111-z
59. Rainisch, G, Meltzer, MI, Shadomy, S, Bower, WA, and Hupert, N. Modeling tool for decision support during early days of an Anthrax event. Emerg Infect Dis. (2017) 23:46–55. doi: 10.3201/eid2301.151787
60. McClellan, G, Coleman, M, Crary, D, Thurman, A, and Thran, B. Human dose-response data for Francisella tularensis and a dose- and time-dependent mathematical model of early-phase fever associated with tularemia after inhalation exposure: human dose-response data for Francisella tularensis. Risk Anal. (2018) 38:1685–700. doi: 10.1111/risa.12995
61. Reshetin, VP, and Regens, JL. Simulation modeling of Anthrax spore dispersion in a bioterrorism incident. Risk Anal. (2003) 23:1135–45. doi: 10.1111/j.0272-4332.2003.00387.x
62. Fennelly, KP, Davidow, AL, Miller, SL, Connell, N, and Ellner, JJ. Airborne infection with Bacillus anthracis —from Mills to mail. Emerg Infect Dis. (2004) 10:996–1001. doi: 10.3201/eid1006.020738
63. Hong, T, and Gurian, PL. Updating a B. anthracis risk model with field data from a bioterrorism incident. Environ Sci Technol. (2015) 49:6701–11. doi: 10.1021/acs.est.5b00010
64. Vyhmeister, E, Provan, G, Doyle, B, Bourke, B, Castane, GG, and Reyes-Bozo, L. Comparison of time series and mechanistic models of vector-borne diseases. Spat Spatiotemporal Epidemiol. (2022) 41:100478. doi: 10.1016/j.sste.2022.100478
65. Miyama, T, Jung, S, Hayashi, K, Anzai, A, Kinoshita, R, Kobayashi, T, et al. Phenomenological and mechanistic models for predicting early transmission data of COVID-19. MBE. (2021) 19:2043–55. doi: 10.3934/mbe.2022096
66. Lesmanawati, DAS, Veenstra, P, Moa, A, Adam, DC, and MacIntyre, CR. A rapid risk analysis tool to prioritise response to infectious disease outbreaks. BMJ Glob Health. (2020) 5:e002327. doi: 10.1136/bmjgh-2020-002327
67. Treadwell, TA . Epidemiologic clues to bioterrorism. Public Health Rep. (2003) 118:92–8. doi: 10.1093/phr/118.2.92
68. Grunow, R, and Finke, EJ. A procedure for differentiating between the intentional release of biological warfare agents and natural outbreaks of disease: its use in analyzing the tularemia outbreak in Kosovo in 1999 and 2000. Clin Microbiol Infect. (2002) 8:510–21. doi: 10.1046/j.1469-0691.2002.00524.x
69. Dembek, ZF, Kortepeter, MG, and Pavlin, JA. Discernment between deliberate and natural infectious disease outbreaks. Epidemiol Infect. (2007) 135:353–71. doi: 10.1017/S0950268806007011
70. Radosavljevic, V, and Belojevic, G. A new model of bioterrorism risk assessment. Biosecur Bioterr. (2009) 7:443–51. doi: 10.1089/bsp.2009.0016
71. Radosavljevic, V, and Belojevic, G. Unusual epidemic events: a new method of early orientation and differentiation between natural and deliberate epidemics. Public Health. (2012) 126:77–81. doi: 10.1016/j.puhe.2011.11.006
72. Riccardo, F, Shigematsu, M, Chow, C, McKnight, CJ, Linge, J, Doherty, B, et al. Interfacing a biosurveillance portal and an international network of institutional analysts to detect biological threats. Biosecur Bioterr. (2014) 12:325–36. doi: 10.1089/bsp.2014.0031
73. Radosavljević, V, Finke, EJ, and Belojević, G. Analysis of Escherichia Coli O104:H4 outbreak in Germany in 2011 using differentiation method for unusual epidemiological events. Cent Eur J Public Health. (2016) 24:9–15. doi: 10.21101/cejph.a4255
74. Lin, M, Chen, H, Jia, L, Yang, M, Qiu, S, Song, H, et al. Using a grey relational analysis in an improved Grunow–Finke assessment tool to detect unnatural epidemics. Risk Anal. (2022)
75. Chen, X, Chughtai, AA, and MacIntyre, CR. Recalibration of the Grunow-Finke assessment tool to improve performance in detecting unnatural epidemics: recalibration of the Grunow-Finke assessment tool. Risk Anal. (2019) 39:1465–75. doi: 10.1111/risa.13255
76. Chen, X, Chughtai, AA, and MacIntyre, CR. Application of a risk analysis tool to Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in Saudi Arabia. Risk Anal. (2020) 40:915–25. doi: 10.1111/risa.13472
77. Radosavljevic, V . Analysis of COVID-19 outbreak origin in China in 2019 using differentiation method for unusual epidemiological events. Open Med. (2021) 16:955–63. doi: 10.1515/med-2021-0305
78. Pappas, G, Panagopoulou, P, and Akritidis, N. Reclassifying bioterrorism risk: are we preparing for the proper pathogens? J Infect Public Health. (2009) 2:55–61. doi: 10.1016/j.jiph.2009.03.002
79. Farkas, CB, Dudás, G, Babinszky, GC, and Földi, L. Analysis of the virus SARS-CoV-2 as a potential bioweapon in light of international literature. Mil Med. (2023) 188:531–40. doi: 10.1093/milmed/usac123
80. Cieslak, TJ, Kortepeter, MG, Wojtyk, RJ, Jansen, HJ, Reyes, RA, Smith, JO, et al. Beyond the dirty dozen: a proposed methodology for assessing future bioweapon threats. Mil Med. (2018) 183:e59–65. doi: 10.1093/milmed/usx0004
81. Rotz, LD, Khan, AS, Lillibridge, SR, Ostroff, SM, and Hughes, JM. Public health assessment of potential biological terrorism agents. Emerg Infect Dis. (2002) 8:225–30. doi: 10.3201/eid0802.010164
82. Rykiel, EJ . Testing ecological models: the meaning of validation. Ecol Model. (1996) 90:229–44. doi: 10.1016/0304-3800(95)00152-2
83. Gross, F, and MacLeod, M. Prospects and problems for standardizing model validation in systems biology. Prog Biophys Mol Biol. (2017) 129:3–12. doi: 10.1016/j.pbiomolbio.2017.01.003
84. Karnon, J . Model validation: has it’s time come? PharmacoEconomics. (2016) 34:829–31. doi: 10.1007/s40273-016-0415-y
85. Nelson, NP, Yang, L, Reilly, AR, Hardin, JE, and Hartley, DM. Event-based internet biosurveillance: relation to epidemiological observation. Emerg Themes Epidemiol. (2012) 9:4. doi: 10.1186/1742-7622-9-4
86. Usman, R, Bakare, L, Akpan, UO, Dalhat, M, Dada, AO, Okudo, I, et al. Establishing event-based surveillance system in Nigeria: a complementary information generating platform for improved public health performance, 2016. Pan Afr Med J. (2022) 42:63. doi: 10.11604/pamj.2022.42.63.29621
Keywords: unnatural epidemics, mathematical models, risk assessment, machine learning, analysis tools
Citation: Li J, Li Y, Mei Z, Liu Z, Zou G and Cao C (2024) Mathematical models and analysis tools for risk assessment of unnatural epidemics: a scoping review. Front. Public Health. 12:1381328. doi: 10.3389/fpubh.2024.1381328
Received: 16 February 2024; Accepted: 09 April 2024;
Published: 02 May 2024.
Edited by:
Camelia Delcea, Bucharest Academy of Economic Studies, RomaniaReviewed by:
Shawn Dolley, Open Global Health, United StatesCopyright © 2024 Li, Li, Mei, Liu, Zou and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunxia Cao, Y2FvY2h1bnhpYUB0anUuZWR1LmNu; Gaofeng Zou, em91Z2FvZmVuZ0B0anUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.