- 1Instituto de Efectividad Clínica y Sanitaria (IECS-CONICET), Buenos Aires, Argentina
- 2Centro de Investigaciones Epidemiológicas y Salud Pública (CIESP-IECS), CONICET, Emilio Ravignani 2024 (C1414CPV), Buenos Aires, Argentina
- 3National Influenza Centre PAHO/WHO, Servicio Virosis Respiratorias, Departamento Virología, Instituto Nacional de Enfermedades Infecciosas, Buenos Aires, Argentina
- 4Hospital Nacional de Pediatría, ‘Dr. Juan P. Garrahan’, Buenos Aires, Argentina
- 5Servicio de Aislamiento, Hospital Nacional de Niños "Dr. Carlos Sáenz Herrera", Caja Costarricense de Seguro Social (CCSS), San José, Costa Rica
- 6Facultad de Medicina, Universidad de Ciencias Médicas (UCIMED), San José, Costa Rica
- 7Instituto de Investigación en Ciencias Médicas (IICIMED), San José, Costa Rica
- 8Barcelona Institute for Global Health, Hospital Clínic-Universitat de Barcelona, Barcelona, Spain
- 9Vaccine Medical, Pfizer SAS, Bogotá, Colombia
- 10Pfizer Inc, New York, NY, United States
Background: Respiratory Syncytial Virus (RSV) is a common cause of lower respiratory tract infections (LRTI) and hospitalization worldwide. The impact of RSV in Latin America and the Caribbean (LAC) including expensive treatment options, such as palivizumab, have been extensively discussed. However, publications on the impact of RSV disease burden in the region are scarce. This systematic review aimed to determine the incidence and prevalence of RSV in LAC by age and RSV subtype.
Methods: We conducted a systematic review following Cochrane methods to evaluate the disease burden of RSV in LAC countries. We searched studies from January 2012 to January 2023 in literature databases and grey literature without language restrictions. We included guidelines, observational, economic, and surveillance studies from LAC countries. Pairs of reviewers independently selected, and extracted data from included studies. The risk of bias was assessed using the Study Quality Assessment Tools (NHLBI) and AGREE-II. We performed proportion meta-analyses using methods to stabilize the variance. The protocol was registered in PROSPERO (CRD42023393731).
Results: We included 156 studies, mainly from Brazil (25%), Colombia (14.5%), and Argentina (13.8%), as well as four clinical practice guidelines. Most studies were cross-sectional (76.9%) and were classified as low risk of bias (52.6%). The majority included inpatients (85.6%), pediatric (73.7%), and normal-risk patients (67.1%). The highest pooled prevalence was estimated in patients <1 year old (58%), with type A and B prevalence of 52 and 34%, respectively. The RSV-LRTI incidence was 15/100 symptomatic infants aged <2 years old, and the ICU admission was 42%. The RSV-LRTI lethality was 0.6, 3% in patients aged <2 and 0–5 years old, respectively, and 23% among >65 years old high-risk patients. The identified guidelines lack methodological rigor and have limitations in their applicability. The seasonality was more evident in South America than in Central America and The Caribbean, with a clear gap during the pandemic.
Conclusion: This is the most exhaustive and updated body of evidence describing a significant burden of RSV in LAC, particularly at the extremes of life, and its seasonality patterns. Our findings could contribute could contribute facilitating effective prevention and treatment strategies for this significant public health problem.
Systematic review registration: PROSPERO CRD UK (registration number: CRD42023393731).
1 Introduction
Respiratory syncytial virus (RSV) is a leading cause of acute lower respiratory tract infections (LRTIs), among children, old people, and those with underlying comorbidities (1, 2). RSV is the most common cause of viral pneumonia and bronchiolitis in infants, causing 28% of all cases of LRTI, and 13–22% of related mortality in children aged 0–59 months, resulting in a considerable disease burden worldwide (3). Hospitalized infants with RSV bronchiolitis and pneumonia represent a higher healthcare use of resources (4). These include frequent admission to the Pediatric Intensive Care Unit (PICU) and the use of non-invasive respiratory support or mechanical ventilation. While risk factors like prematurity, lower age, and comorbidities such as congenital heart disease (CHD) and chronic lung disease contribute to RSV hospitalization, it is noteworthy that the majority of hospitalized infants are without comorbidities, highlighting the widespread impact of the virus on otherwise healthy infants (5, 6).
In adults, principally individuals aged 65 and above in high-income countries and older than 60 years old in lower-income countries, RSV is an important cause of acute respiratory infection (ARI), hospitalization, and death (7). Among US adults, an estimated 177,000 hospitalizations and 14,000 deaths are associated with RSV infections annually (8, 9).
RSV infection generates a substantial economic burden in the infant and adult populations on a global scale. To analyze the potential benefits of introducing preventive and therapeutic interventions, it is essential to have estimates of the RSV disease epidemiology and economic burdens in Latin America and the Caribbean (LAC). Previous systematic reviews highlighted the evidence gaps about the burden of RSV in Latin America (10, 11). Additionally, data are scarce by age groups, RSV subtype, coinfection, or population risk, and rarely considered seasonality aspects in children and adult patients (≥ 18 years) in the LAC region. A rigorous systematic review is needed to cover these gaps about the disease burden of RSV in LAC.
The objective of this systematic review and meta-analysis was primarily to determine the incidence and prevalence of RSV in LAC. Also, to determine RSV severity and complications in subgroups of interest, the seasonality and RSV outbreaks, and describe the results over time,.
2 Methods
We present here part of a broader systematic review that also included the use of resources and direct/indirect costs associated with RSV disease in LAC. The economic findings were published elsewhere in another publication (63). We conducted a systematic literature review and meta-analysis following the Cochrane Manual of Systematic Reviews (12), the PRISMA (13, 14) statement for reporting systematic reviews and meta-analyses, and the MOOSE guidelines (specific for reviews of observational studies) (15). The protocol was registered in PROSPERO (registration number: CRD42023393731).
2.1 Inclusion criteria
Studies of any epidemiological design in Spanish, English, or Portuguese and surveillance reports published since January 1st, 2012, and January 4th, 2023, which included LAC patients of any age or risk group, were eligible for inclusion. We considered that previously published studies could make it difficult to estimate the current burden of RSV. We planned to include observational studies such as cohorts, case–control studies, and representative case series (involving at least 50 laboratory-confirmed RSV cases or at least 10 RSV patients with complications), the control arms of the randomized clinical trials (RCTs) and quasi-RCTs, controlled before-and-after studies (CBAs) and uncontrolled before-and-after studies (UBAs), interrupted time series (ITSs), controlled ITSs (CITSs) that meet the inclusion criteria of the Cochrane COPD group (16). We also included clinical practice guidelines and consensus documents that addressed immunoprophylaxis. We considered systematic reviews and meta-analyses only as sources of primary studies.
2.2 Search strategy for identification of studies and data sources
We searched records published during the study period in the following databases: PubMed, LILACS, Embase, CINAHL, Cochrane Library, Web of Science, Tufts Economic Database, and EconLIT. The detailed search strategy is described in the Supplementary material. For studies with multiple publications, the one with the largest sample size and/or the most recent publication was considered the primary reference; secondary references were used to complement the data. Reference lists of included articles were hand-searched for additional information. If necessary, authors of relevant articles were consulted for missing or clarifying information. The search for gray literature was performed in the following sources: databases of proceedings of regional and international congresses, websites of major medical regional and international societies and associations related to the topic, and the Virtual Health Library. We also contacted members of the PAHO, who gave us access to the database of RSV cases reported from LAC countries between 2017 and 2023. Generic internet search and metasearch engines (Google) were also searched. The complete search strategy is detailed in the Supplementary material.
2.3 Selection of articles and data extraction
Selection, data extraction, and risk of bias assessment of each article were performed independently by pairs of reviewers, and discrepancies were resolved by consensus of the entire team. All phases of study selection were carried out using COVIDENCE®, a web-based platform designed for the systematic review process (17) and data extraction used a previously piloted data extraction form (based on five studies). From eligible articles, the research team extracted the following study information: publication and study characteristics (type of publication, year published, authors, geographic location, study design including domains for risk of bias assessment), study population characteristics (age, sex, sample size, population risk, inclusion, and exclusion criteria), and outcomes [incidence, prevalence, case fatality rate, hospitalization rate, intensive care unit (ICU) admission rate, disease complications of RSV, and RSV seasonality and outbreaks].
2.4 Risk of bias assessment
We evaluated the risk of bias of the included studies using a checklist developed by the U.S. National Heart, Lung, and Blood Institute, which classifies studies as high risk of bias (poor), uncertain (fair), and low risk of bias (good) (18). For cohort studies and cross-sectional studies assessment, the tool comprises 14 items, while case series studies are assessed based on nine items. The clinical practice guidelines were assessed by the AGREE-II instrument (19). The AGREE-II instrument consists of 23 items, grouped into six domains: (1) Scope and objectives, (2) Participation of decision-makers, (3) Methodological rigor, (4) Clarity of presentation, (5) Applicability, and (6) Editorial independence. A narrative and tabular synthesis were conducted of the available recommendations from clinical practice guidelines and consensus issued by scientific societies and health authorities at the national level.
2.5 Statistical analysis
We reported narrative and structured information alongside descriptive statistics to characterize results. To analyze our data, we conducted proportion meta-analyses using the R software (meta-package) for all analyses. The pooled proportion was calculated as the back-transformation of the weighted mean of the transformed proportions, using inverse arcsine variance weights for both the fixed and random effects models, and reported with a 95% confidence interval (95% CI) (20). We applied DerSimonian-Laird weights for the random effects model where heterogeneity between studies was found (21, 22). The I2 statistic was calculated as a measure of the proportion of the overall variation attributable to between-study heterogeneity (23). Funnel plots were used to explore publication bias across at least 10 studies, although the usefulness for non-intervention studies is uncertain. Selective reporting within studies was assessed by comparing available protocols with the reports. We described the seasonality pattern by geographic region from 2017 to 2013 based on PAHO surveillance data (24).
2.5.1 Subgroup analysis, sensitivity analysis, and investigation of heterogeneity
If the number of studies allowed, we performed subgroup analyses based on to study design, dataset year, age group (<6 months, 6 to 12 months, 1–2 years, 3 to 5 years, 6 to 14 years, 15 to 65 years, and > 65 years), and the risk level of the population (no risk conditions/average risk, underlying medical conditions/risk factor). If these groups were only reported in wider age groups (i.e., 0–5 years) we also presented them. Additionally, we conducted a sensitivity analysis to assess the impact of risk of bias on the results of the primary analyses by limiting the analysis to studies with low risk of bias in the main domains.
3 Results
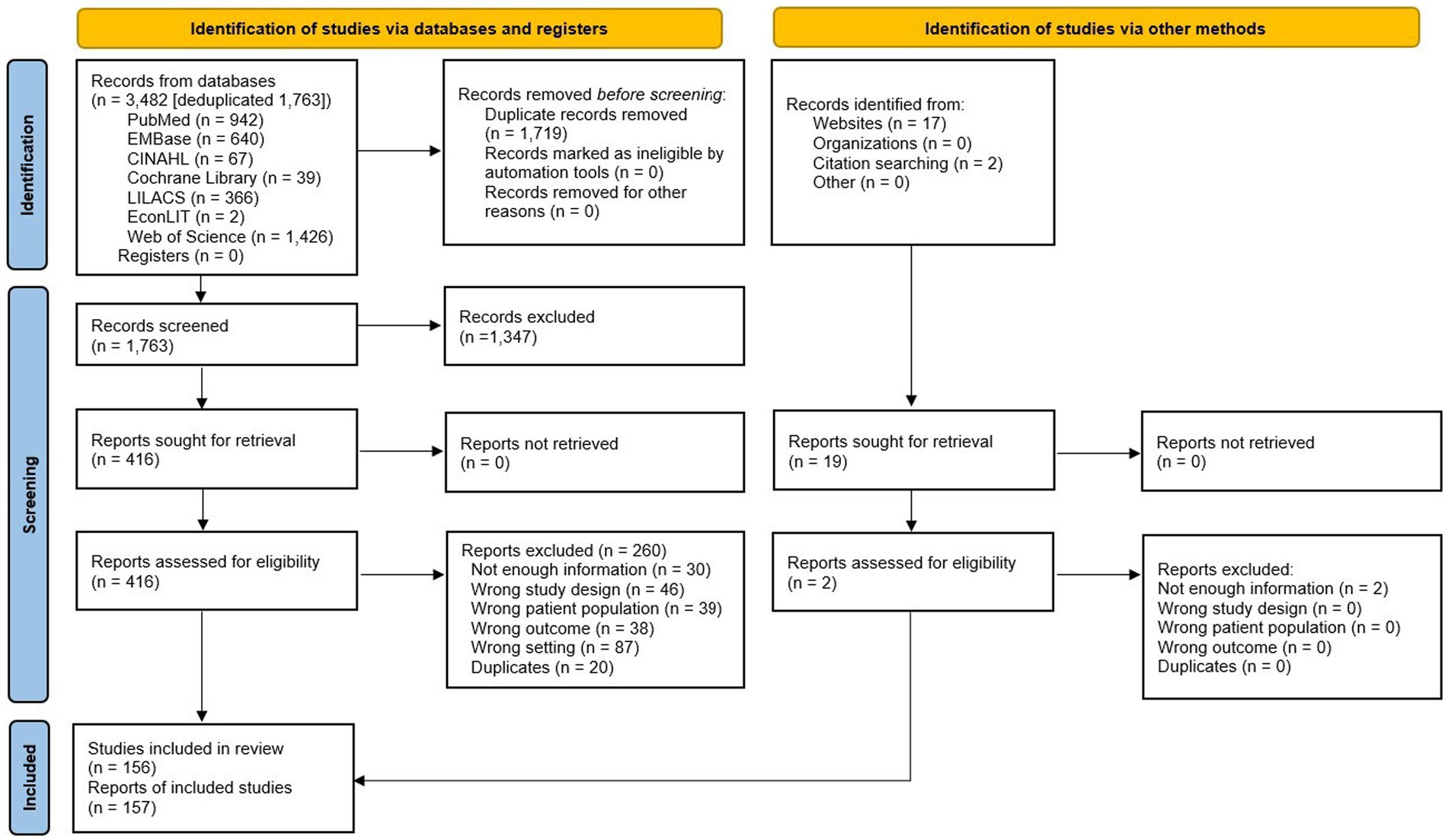
We identified 3,482 records in seven different databases, and after eliminating duplicates, we screened the 1,763 remaining records by title and abstract and 416 potentially eligible reports through full-text assessment. Finally, 156 studies (157 records) met the inclusion criteria (Figure 1; Supplementary Table 1). The list of studies excluded and their exclusion reasons are available in the Supplementary Table 2.
3.1 Characteristics of the included studies
Supplementary Table 1 summarizes the characteristics of the included studies. We included 156 studies: 125 full texts, 21 congress abstracts, and 6 theses. Among the studies included, there were 4 guidelines. Regarding the methodological design, there were 117 cross-sectional studies, 31 case series, 3 prospective cohorts, and 1 case–control study. The studies were carried out in 20 countries, and the most represented were Brazil (25), Colombia (22), Argentina (21), Chile (15), Peru (9), and Mexico (8). Nine studies included multiple countries from LAC. The articles were published between 2013 and 2022, and the inclusion period of participants was from 2000 to 2022, although we only included data from participants recruited from the year 2012 onwards. A total of 16 (10.5%) studies included participants during the years 2019–2020 of the COVID-19 pandemic. The reported duration of the studies ranged from one to 165 months.
Regarding the study population, 112 (73.7%) studies included pediatric patients, 22 (14.5%), both pediatric and adults, 10 (6.6%) only adult patients, and 8 (5.2%) studies did not report the age of the participants. Only 76 (50%) studies reported the clinical risk of the participants (e.g., prematurity, chronic conditions, immunocompromised), with 51 (67.1%) studies that included normal-risk participants, 18 (23.7%) high-risk, and 7 (9.2%) a mix of participants. Regarding the setting, 113 (85.6%) studies included inpatients, 17 (12.9%) included outpatients, and 2 (1.5%) included both.
A total of 1,445,198 samples, with 18% testing positive for RSV, along with epidemiological data from 69,891 patients, were included in the analysis. The diagnostic methods used were polymerase chain reaction (PCR) in 76 (50%) studies, Immunofluorescence (IF) in 36 (23.7%), direct (DIF) in 11 and indirect (IFI) in 17, immunochromatographic rapid test in nine (5.9%), mixed methods in eight (5.3%). Only 27 (17.8%) studies evaluated the type of RSV (A or B).
3.2 Risk of bias of the included studies
For cross-sectional and cohort studies, 57 (47.5%) were assessed as being at low risk (good quality), 56 (46.7%) were assessed as moderate risk (fair quality), and only seven (5.8%) as high risk (poor quality). The most frequent domains that did not meet appropriateness were related to sample size justification, the evaluation of exposures more than once or different levels of exposure, and the participation rate of eligible persons. It should be noted that many domains did not apply to the objectives of the included studies, such as blinding of the evaluators, assessment of exposure before the outcome, sufficiency of timeframe, percentage of loss to follow-up, and evaluation of potential confounding variables. For case series studies, 23 (74.2%) were rated as low risk, 6 (19.4%) as moderate risk, and only two (6.4%) as high risk. The domain that did not meet appropriateness more frequently was whether the cases were consecutive. Only one case–control study was included and rated as moderate risk due to a lack of information about the selection of participants, the use of concurrent controls, the blinding of the assessors, and because potential confounding variables were not measured. The risk of bias assessment by study design is presented in Supplementary Tables 3–5.
3.3 RSV burden of disease
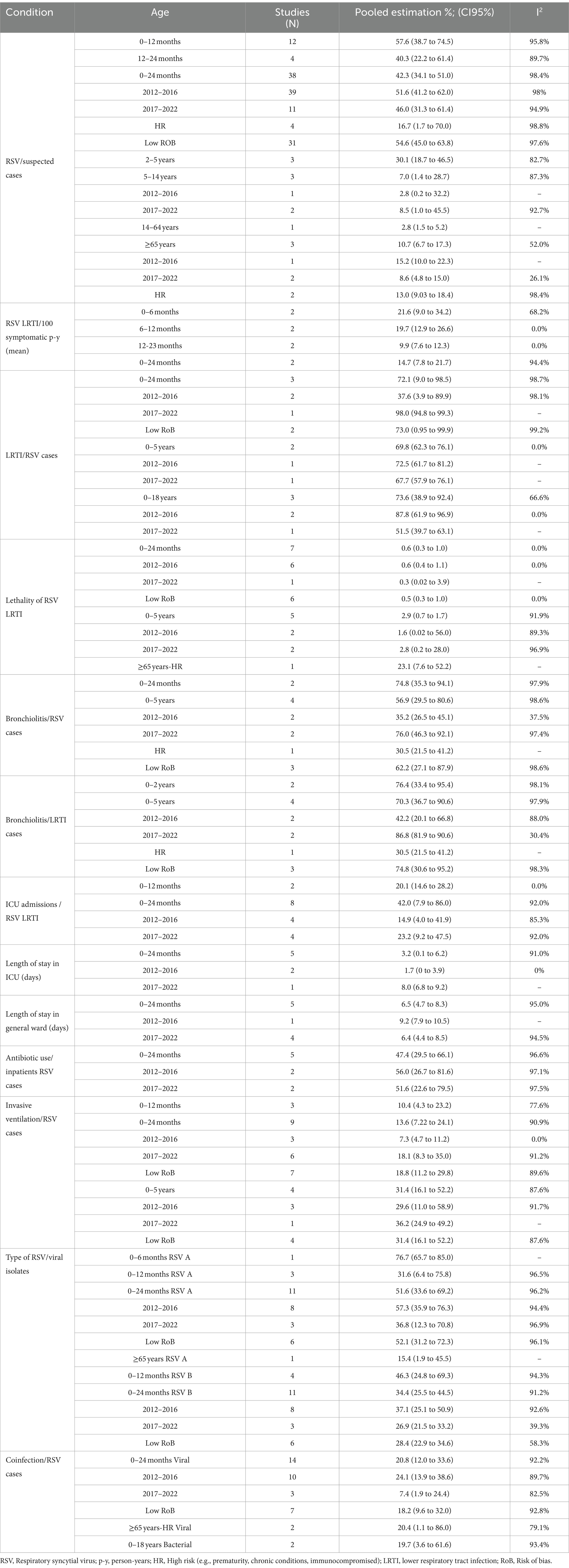
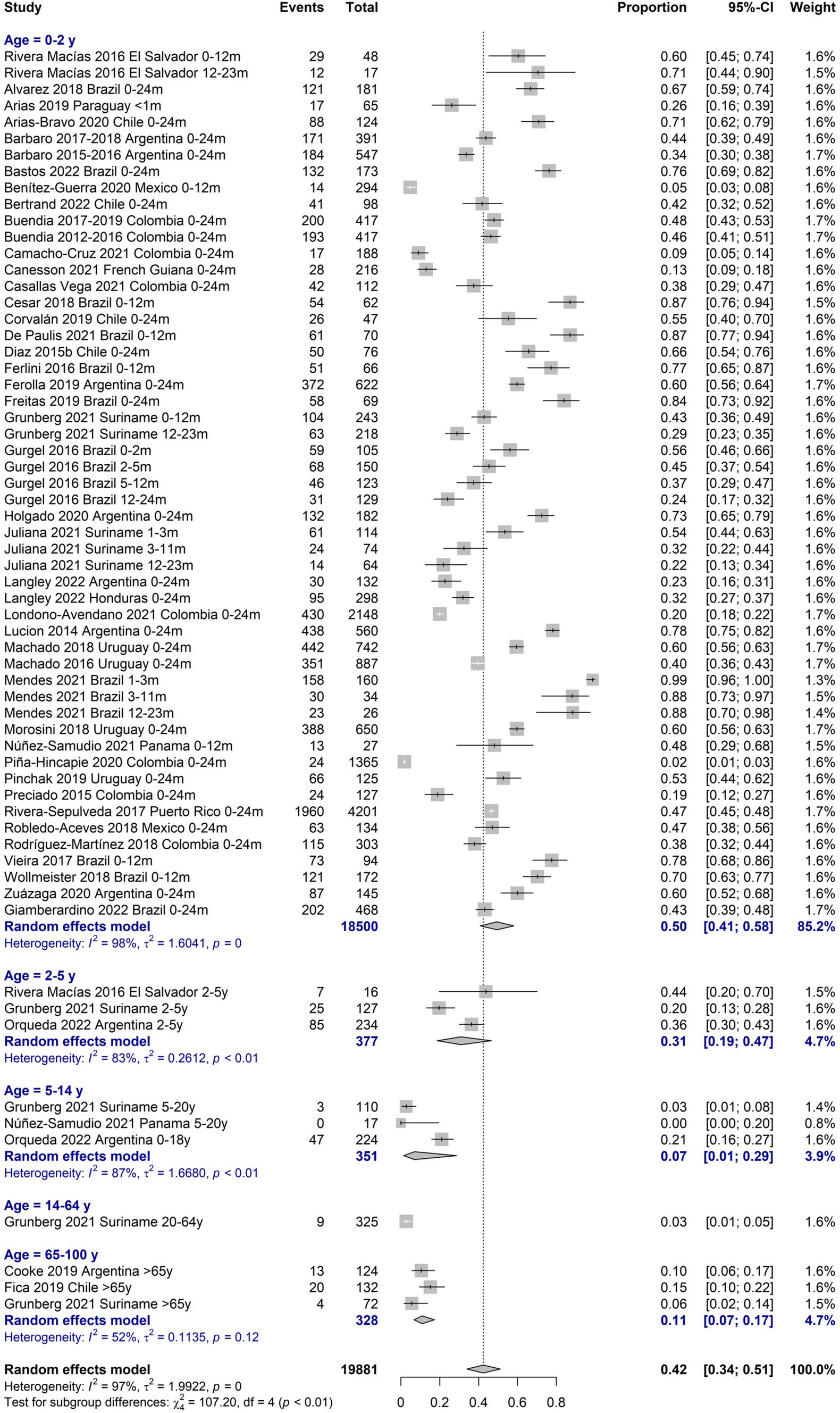
Table 1 describes the main findings of RSV burden of disease by age groups with separate estimations for periods 2012–2016 and 2017–2022, for low-risk of bias (RoB) studies, and for high-risk patients when available. Regarding the prevalence of RSV infections, the age group most represented among the included studies was 0–24 months (38 studies), mostly presenting a low RoB. The positivity prevalence among suspected cases in this age group was 42.3% (95%CI 34.1–51.0), with the highest prevalence of 57.6% (95%CI 38.7–74.5) among 0-12-month-old infants. The age group of ≥65 years was poorly represented (3 studies), with a prevalence of 10.7% (95%CI 6.7–17.3; Figure 2).
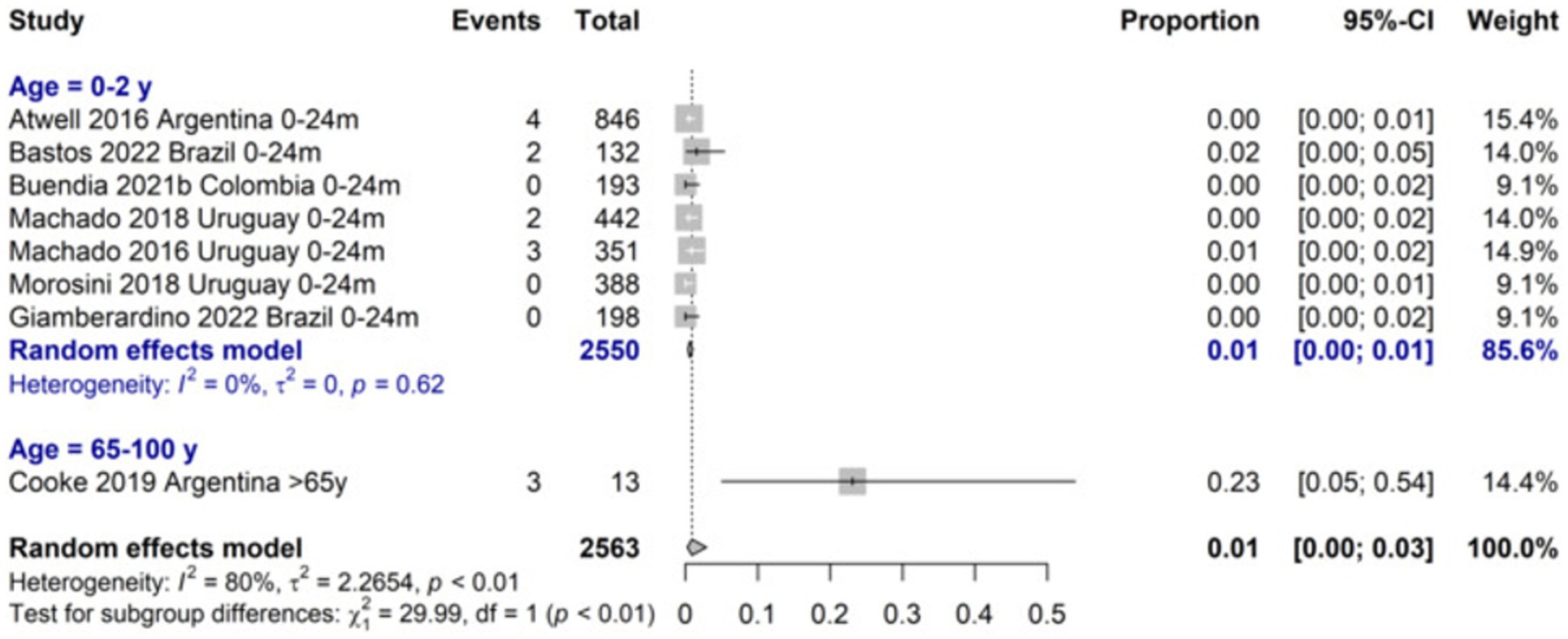
The incidence of LRTI due to RSV was 14.7 (95%CI 7.8–21.7) per 100 symptomatic 0–24 months-old infants and higher for the 0–6 months age group (21.6%; 95%CI 9.0–34.2). The proportion of LRTI among RSV cases among 0–24 months-old infants was 72.1 (95%CI 9.0 to 98.5) and was similar for children aged 0 to 18 years old. The RSV-LRTI lethality was 0.6% (95%CI 0.3–1.0) in the 0–24 months and 2.9% (95%CI 0.7–1.7) in the 0–5 years old group, respectively. The ≥65-year lethality was estimated to be 23.1% (7.6 to 52.2) based on a single study on a high-risk population (Figure 3). Among RSV-LRTI cases, studies reporting bronchiolitis showed 76.4% (95%CI 33.4 to 95.4) and 70.3% (95%CI 36.7 to 90.6) in the 0–24 months and 0–5 years old, respectively.
The highest proportion of ICU admissions among RSV-LRTI was 42.0% (95%CI 7.9–86.0) for the 0–24 months age group, with a mean length of stay at ICU of 3.2 days (0.1 to 6.2) and general ward of 6.5 days (4.7 to 8.3), respectively. Antibiotic use in children aged 0–24 months was 47.4, and 13.6% of this age group required invasive ventilation.
RSV type A presented a higher proportion than type B from 0 to 24 months (51.6% vs. 34.4, respectively), being more markedly at lower age. The most frequent co-infections were viral with similar percentages in younger and older patients, 20.8% between 0 and 24 months and 20.4% in ≥65 years, with more coinfections detected in the youngest during 2012–2016 compared to 2017–2022 (24.1% vs. 7.4%). The summary estimates reported in Table 1 are derived from meta-analyses, which forest plots are available in the Supplementary Figures 1–5. The heterogeneity was high for most meta-analyses.
3.4 Immunoprophylaxis recommendations: evaluation of guidelines
Based on our literature search, we identified four guidelines for treating RSV in the LATAM region. The guidelines were developed in Argentina (26), Honduras (27), Brazil (28), and Chile (29). Three guidelines focused on the treatment and prevention of RSV-related bronchiolitis, while one focused on bronchiolitis of any viral cause (27). The assessed guidelines show notable methodological limitations. They are narrative reviews without systematic searches, clear methodologies, external expert reviews, and a proposal to update existing guidelines. They lack clear overall objectives (26, 27), and only one guideline specifies the aim of reducing RSV hospitalizations (28). Beneficiaries and outcomes are vaguely described (26, 27). Target populations are not mentioned consistently throughout the guidelines. While one outlines it briefly (26), another specifies it in detail (27). No guideline provides a specific health question. Treatment recommendations are included for premature infants. However, the infants’ age is presented ambiguously (26, 29) or not specified (27, 28). Author details are only fully provided in one guideline (29). No guidelines included views of the target population or provided information on literature searches. Three of the four guidelines do not mention the treatment benefits, side effects, and risks (27–29). Recommendations vary in specificity from general (27) to specific (26, 28, 29). In three guidelines, these recommendations are linked to clinical evidence from previously conducted studies (26, 27, 29). Facilitators, barriers, and stakeholders are not described. All guidelines lack implementation guidance and resource implications. Cost-effectiveness and economic data are missing, and monitoring criteria are not presented. Editorial independence is mentioned in only one guideline (27). The complete evaluation of the assessment of guidelines is in Supplementary Table 6.
These four guidelines provide recommendations for palivizumab use in vulnerable populations, offering insights into inclusion criteria, prescription procedures, post-administration serum levels, and epidemiological data related to RSV infection. There is consensus recommending palivizumab in premature babies less than 29 weeks, without chronic pulmonary disease in premature babies younger than 12 months old at the start of RSV station. Also, to infants under 12 months with hemodynamically significant heart disease or infants under 24 months of age undergoing heart transplant during RSV season.
3.5 RSV seasonality
The Figure 4 shows the seasonality pattern of RSV isolates from 2017 to 2023 across three geographical regions: South America, Central/North America, and the Caribbean. The time series, from PAHO surveillance, data show a clear cyclical pattern for each region. South America exhibits pronounced peaks in RSV isolates each year in the winter season, having the highest number of isolates; the intensity of these peaks seems to be diminishing slightly over the years. Central America’s RSV isolates also follow a seasonal trend. Still, the peaks are less pronounced than those in South America, relatively consistent over the years, with no notable increase or decrease in the number of isolates. The Caribbean shows the least pronounced peaks among the three regions and is more spread out, with a noticeable increase in recent years. Of note, there was a clear gap during the pandemic (2020–2021).
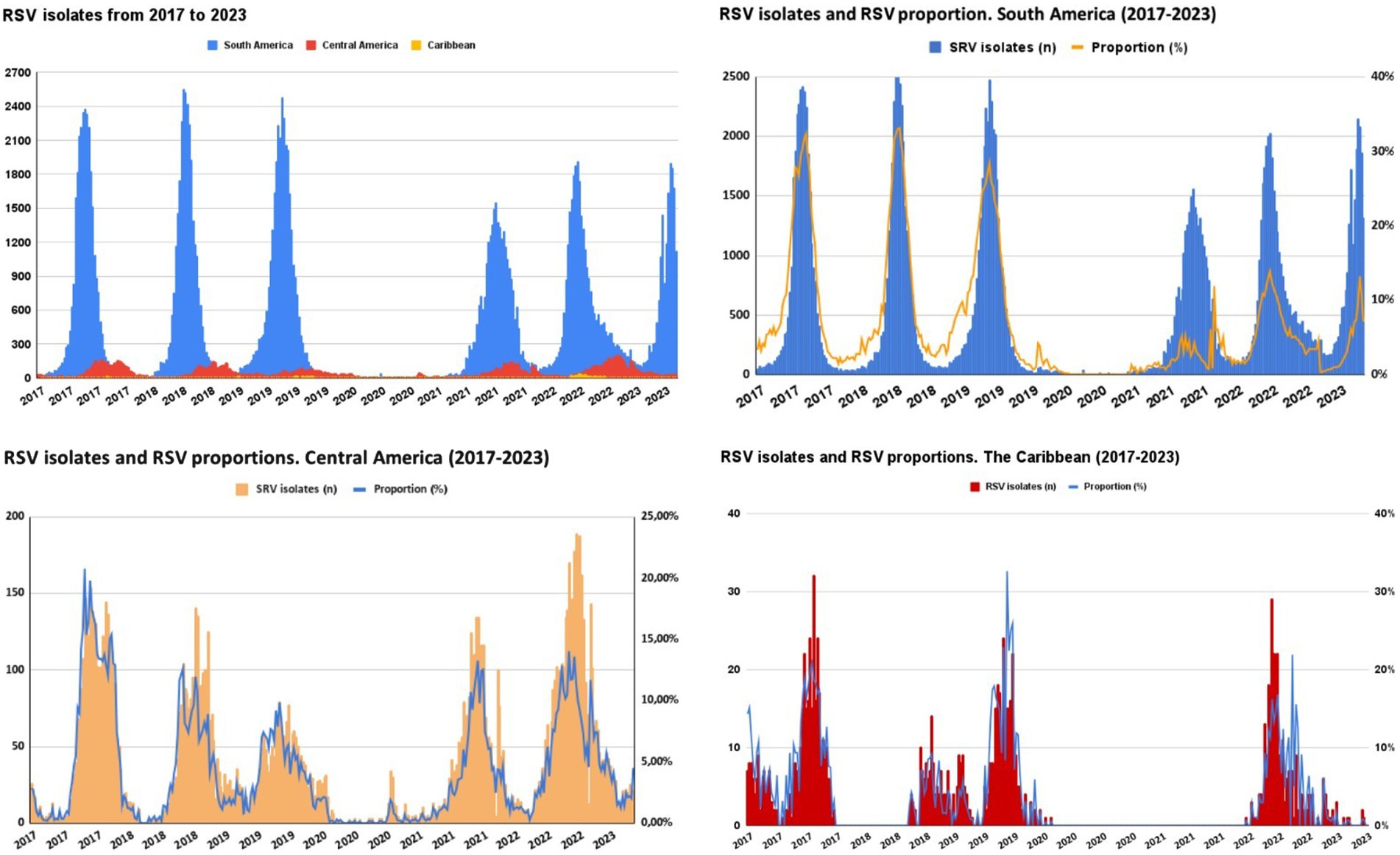
Figure 4. Seasonality pattern of RSV isolates 2017–2023 in South Americaa, Central/North Americab, and the Caribbeanc. aArgentina, Bolivia, Brazil, Chile, Colombia, Ecuador, Paraguay, Perú, Uruguay, and Venezuela. bCosta Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, and Panama. cCuba, Dominican Republic, and Haiti.
4 Discussion
Our systematic review updates a previous review (10) to evaluate the burden of disease of RSV in the Latin American pediatric and adult population, and, as far as we know, includes the largest number of studies and patients in our region. A total of 156 studies and epidemiological data from 69,891 patients from LAC were included in this study. We identified mainly cross-sectional studies with low risk of bias, with Brazil, Colombia, and Argentina being the most represented countries in the region. National reports from many LAC countries have limited information, likely due to the absence of mandatory reporting and passive surveillance of RSV. In agreement with our review, most systematic reviews reported data on pediatric inpatients and generally involved healthy individuals (3, 30–33). In the countries of LAC, the RSV laboratory surveillance program is predominantly conducted in pediatric patients requiring hospitalization (34), consequently resulting in a higher publication rate of studies focusing on this patient group. Consistently with this pattern, we found only one review assessing the epidemiology of RSV in adults and the older adults in LAC (11).
RSV is the primary cause of bronchiolitis and viral community-acquired pneumonia in infants, and LRTI in the older population. It can lead to life-threatening respiratory disease, particularly in patients with risk factors such as prematurity, young age, and comorbidities such as hemodynamically significant congenital heart disease and chronic lung disease of prematurity (5, 6). A long-term effect following RSV infection in infants is the risk of subsequent wheezing in children, especially if the initial infection requires hospitalization, and 20 to 40% experience recurrent episodes of asthma (35–37). We observed a high pooled RSV prevalence in children under 2 years of age, especially in those under 6 months, with a higher proportion of serotype A infection compared to serotype B. Similar findings have been reported globally (38).
A national study in the US revealed that infants with high-risk comorbidities hospitalized due to RSV during the period from 1997 to 2012 had a fivefold increase in mechanical ventilation requirement compared to infants without high-risk conditions. RSV imposes a significant disease burden on pediatric patients, with hospitalization rates three times higher than those associated with other respiratory viruses (25, 39).
We found that RSV-LRTI ICU admission was 42% and the requirement of invasive ventilation was 14% among 0-2-year-old patients. The RSV-LRTI lethality was 0.6, 3%, and in patients aged <2, 0–5 years, respectively, and 23% among >65 years old high-risk patients. However, the elevated risk of this older adult population explains the high rate reported by a single study. Li et al. estimated that the adjusted hospitalization rate for adults aged 65 years and older was 347 per 100,000 (95%CI 203–595) with an age-dependent increase, ranging from 231 per 100,000 in adults aged 65–74 years to 692 per 100,000 in adults aged 85 years or more (8). Ali et al. reported a high rate of hospitalization due to RSV-infected Mexican adults with influenza-like illness (40.9–69.9%). Liu Li et al. reported, the in-hospital case fatality ratio (CFR) for RSV was 6.1% (95%CI 3.3–11.0) (8) and Savic et al. (5) 7.1% (95%CI 5.4–9.4) among individuals aged 60 years or older (7). Worldwide, RSV claims the lives of over 100,000 children annually, with approximately half of these deaths occurring in infants under 6 months of age. The majority of these fatalities take place in countries with limited resources (3). Our CFR estimations highlighted the magnitude of the problem for a very frequent disease.
Regarding resource utilization, we found an average length of stay of 3.2 days and 6.5 days in the ICU and the general ward in patients aged under 24 months. These findings are aligned with previous reports (40–47). In the group under 2 years of age, RSV infection leads to increased antimicrobial use. A study reported that up to one-third of children with RSV LRTI, without co-bacterial infections, receive unnecessary antibiotic treatment (48). Similarly, we reported viral coinfection occurring in both the group under 2 years and in those over 65, at approximately 21%. Other pediatric cohort studies exhibit a greater rate of viral co-detections at 55% (49), falling between the 34% reported in the US EPIC study (50), focused on viral detection in children under 5 years old with pneumonia, and the 61% documented by the ORAACLE Study Group, a Norwegian clinical cohort, that investigated the length of stay of hospitalized patients with bronchiolitis (51). The variations in these findings may be attributed to the precision of the novel molecular detection methods employed.
Differences in seasonality were previously reported worldwide (52) and in LAC (10, 53). In the Southern Hemisphere, RSV circulates primarily during the late fall or early winter season, characterized by marked peaks in circulation. In contrast, Central American countries exhibit a seasonal trend, but with less pronounced peaks. These observed differences likely stem from variations in climatic patterns that are linked to the behavior of RSV in each region. During the first year of the pandemic of SARS-CoV-2, a gap in RSV circulation was observed in the countries of the region. It may be attributed to the new virus infection displacing the opportunity for RSV circulation. This, combined with strict isolation measures and the initial closure of schools and daycare centers implemented during the pandemic, could have contributed to the observed phenomenon. Numerous studies worldwide evaluate the effectiveness of non-pharmacological interventions (NPIs) in containing the spread of respiratory viruses, primarily SARS-CoV-2 and Influenza (54–58). NPIs aimed at limiting the spread of SARS-CoV-2 appear to have affected the circulation of other respiratory viruses. An increase in the percentage of positivity and the diversity of respiratory viruses was observed as the degree of restriction decreased (59).
Four guidelines addressing the use of palivizumab for the prevention of RSV infection in high-risk populations in Latin America were identified (26–29). However, all of them present serious methodological limitations which limit their ability to offer definitive conclusions regarding the effectiveness and clinical applicability of palivizumab in the region. Therefore, it is highlighted that further work is needed to develop high quality guidelines and recommendations using the GRADE approach (60) to enhance RSV infection prevention in Latin America.
Our study presents some limitations. We did not find population-based cohort studies, which provide the best burden of disease estimates. The limited number of reports conducted in the adult population, as well as in outpatients, concerning the burden of RSV indicates a gap in information in these populations. Due to the different testing methods employed, it is expected variability in RSV estimates, particularly for the adult population, where certain testing methods are known to have lower sensitivity. Consequently, the results may underestimate the true burden of RSV in adults. Standardizing testing approaches could provide more accurate estimates of RSV prevalence. Unfortunately, the data for this age group was scarce avoiding subgroup analysis by testing method. The literature search was conducted in January 2023, therefore some latest published studies could be missed and findings cannot be extrapolated beyond this date. Finally, most meta-analyses showed high levels of heterogeneity. Meta-analysis from observational studies usually exhibits greater heterogeneity in both the frequency and magnitude than pooling experimental studies. This inherent variability stems from the real-world nature of epidemiological research, which encompasses diverse populations, environmental exposures, and healthcare settings. However, we partially addressed this issue by employing the random-effects model that yields broader and more conservative confidence intervals. When faced with high levels of heterogeneity, these confidence intervals provide a more reliable estimation than the point estimates (61). On the other side, our systematic review also has relevant strengths. We conducted exhaustive searches across multiple databases and found more studies than any published systematic review on this topic in LAC. We independently screened, extracted, and assessed the risk of bias in each study by pairs of reviewers, ensuring a comprehensive evaluation of the available evidence, and performed proportion meta-analyses. In summary, we followed a rigorous methodology that also considered RSV types, population risks, seasonality, time variations, and the risk of bias in included studies. Finally, we need to be well prepared in Latin America in terms of knowing the epidemiology, impact, disease burden, associated costs, and microbiological aspects of RSV in both children and adults to achieve a smoother road for new monoclonal antibodies and vaccine introductions (62). The findings from this systematic review provide crucial insights for physicians and health policymakers by highlighting the significant burden of RSV in LAC, particularly among infants under one-year-old and high-risk older adult patients. Policymakers should consider integrating RSV vaccination programs, especially targeting the most vulnerable populations. Furthermore, the observed seasonality patterns of RSV, more pronounced in South America, suggest that timing vaccination and immunoprophylaxis efforts to align with peak RSV seasons could enhance their effectiveness. Recommendations include the establishment of robust surveillance systems to continuously monitor RSV incidence and prevalence. These strategies will help reduce the healthcare burden and improve patient outcomes in Latin America.
5 Conclusion
This comprehensive body of evidence thoroughly describes the significant impact of RSV in LAC, especially among the very young and older adult populations, along with its seasonal patterns. Our findings have the potential to inform and enhance strategies for the effective prevention and treatment of this critical public health issue. Additionally, there are some implications for research, including the need for population-based cohort studies that better represent the extremes of life and outpatient settings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
AC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MP: Data curation, Investigation, Writing – original draft, Writing – review & editing. MS: Investigation, Data curation, Writing – original draft, Writing – review & editing. EB: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. SR: Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. RU-G: Investigation, Writing – original draft, Writing – review & editing. KS: Data curation, Investigation, Writing – original draft, Writing – review & editing. SR: Data curation, Investigation, Writing – original draft, Writing – review & editing. JC: Formal analysis, Writing – original draft, Writing – review & editing. JL: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. RA: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported, in whole, by an independent grant from Pfizer Argentina. The sponsor had no role in study design, data collection, data analysis, data interpretation, or report writing.
Acknowledgments
The authors would like to thank Daniel Comande, the librarian at the Institute for Clinical Effectiveness and Health Policy, for his contribution to the bibliographic searches.
Conflict of interest
JL works for Vaccine Medical, Pfizer SAS, Bogotá, Colombia and RA works for Pfizer Inc., New York, New York, United States. RU-G has participated recently in scientific events sponsored by Pfizer and AstraZeneca, received honoraria as a speaker by Pfizer, and in advisory boards by MSD, all related to RSV prevention and treatment.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1377968/full#supplementary-material
References
1. Suh, M, Movva, N, Jiang, X, Bylsma, LC, Reichert, H, Fryzek, JP, et al. Respiratory syncytial virus is the leading cause of United States infant hospitalizations, 2009-2019: a study of the national (Nationwide) inpatient sample. J Infect Dis. (2022) 226:S154–63. doi: 10.1093/infdis/jiac120
2. Shi, T, Denouel, A, Tietjen, AK, Campbell, I, Moran, E, Li, X, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and Meta-analysis. J Infect Dis. (2020) 222:S577–83. doi: 10.1093/infdis/jiz059
3. Shi, T, McAllister, DA, O’Brien, KL, Simoes, EAF, Madhi, SA, Gessner, BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. (2017) 390:946–58. doi: 10.1016/S0140-6736(17)30938-8
4. Ledbetter, J, Brannman, L, Wade, SW, Gonzales, T, and Kong, AM. Healthcare resource utilization and costs in the 12 months following hospitalization for respiratory syncytial virus or unspecified bronchiolitis among infants. J Med Econ. (2020) 23:139–47. doi: 10.1080/13696998.2019.1658592
5. Doucette, A, Jiang, X, Fryzek, J, Coalson, J, McLaurin, K, and Ambrose, CS. Trends in respiratory syncytial virus and bronchiolitis hospitalization rates in high-risk infants in a United States nationally representative database, 1997-2012. PLoS One. (2016) 11:e0152208. doi: 10.1371/journal.pone.0152208
6. Rha, B, Curns, AT, Lively, JY, Campbell, AP, Englund, JA, Boom, JA, et al. Respiratory syncytial virus–associated hospitalizations among young children: 2015–2016. Pediatrics. (2020) 146:e20193611. doi: 10.1542/peds.2019-3611
7. Savic, M, Penders, Y, Shi, T, Branche, A, and Pirçon, J-Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Other Respir Viruses. (2023) 17:e13031. doi: 10.1111/irv.13031
8. Li, Y, Kulkarni, D, Begier, E, Wahi-Singh, P, Wahi-Singh, B, Gessner, B, et al. Adjusting for case under-ascertainment in estimating RSV hospitalisation burden of older adults in high-income countries: a systematic review and modelling study. Infect Dis Ther. (2023) 12:1137–49. doi: 10.1007/s40121-023-00792-3
9. Jajosky, R. 2019 changes to the National Notifiable Diseases Surveillance System (NNDSS) and other relevant updates. (2018). Available at: https://stacks.cdc.gov/view/cdc/132380 (Accessed December 12, 2023).
10. Bardach, A, Rey-Ares, L, Cafferata, ML, Cormick, G, Romano, M, Ruvinsky, S, et al. Systematic review and meta-analysis of respiratory syncytial virus infection epidemiology in Latin America. Rev Med Virol. (2014) 24:76–89. doi: 10.1002/rmv.1775
11. Ali, A, Lopardo, G, Scarpellini, B, Stein, RT, and Ribeiro, D. Systematic review on respiratory syncytial virus epidemiology in adults and the elderly in Latin America. Int J Infect Dis. (2020) 90:170–80. doi: 10.1016/j.ijid.2019.10.025
12. Higgins, J, and Welch, V. Cochrane handbook for systematic reviews of interventions. Available at: http://www.training.cochrane.org/handbook (Accessed October 01, 2023).
13. Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gøtzsche, PC, Ioannidis, JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
14. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
15. Stroup, DF, Berlin, JA, Morton, SC, Olkin, I, Williamson, GD, Rennie, D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
16. EPOC. Cochrane effective practice organisation of care group. Data collection checklist. Available athttps://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/datacollectionchecklist.pdf (Accessed October 07, 2023).
17. Babineau, J. Product review: Covidence (systematic review software). J Can Health Libr Assoc. (2014) 35:68–71. doi: 10.5596/c14-016
18. Study Quality Assessment Tools. NHLBI, NIH. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (Accessed October 01, 2023).
19. Brouwers, MC, Kho, ME, Browman, GP, Burgers, JS, Cluzeau, F, Feder, G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. (2010) 182:E839–42. doi: 10.1503/cmaj.090449
20. Freeman, MF, and Tukey, JW. Transformations related to the angular and the square root. aoms. (1950) 21:607–11. doi: 10.1214/aoms/1177729756
21. Higgins, JPT, and Green, S. Cochrane handbook for systematic reviews of interventions Wiley (2008). 672 p. Available at: http://www.training.cochrane.org/handbook (Accessed October 01, 2023).
22. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
23. Higgins, JPT, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. PAHO/WHO. Weekly update: influenza, SARS-CoV-2, RSV and other respiratory viruses - Epidemiological Weeks. Available at: https://www.paho.org/en/documents/topics/influenza-sars-cov-2-rsv-and-other-respiratory-viruses (Accessed December 12, 2023).
25. Suh, M, Movva, N, Bylsma, LC, Fryzek, JP, and Nelson, CB. A systematic literature review of the burden of respiratory syncytial virus and health care utilization among United States infants younger than 1 year. J Infect Dis. (2022) 226:S195–212. doi: 10.1093/infdis/jiac201
26. Fernández Jonusas, SF, Maubett, DA, Satragno, D, Cattaino, A, Alonso, MM, Rubio, C, et al. Recommendations for palivizumab use. Update 2015. Arch Argent Pediatr. (2016) 114:84–8. doi: 10.5546/aap.2016.84
27. Martínez, LMC, and Márquez, MAC. Uso de Palivizumab en Recién Nacidos Prematuros. Act Ped Hond. (2019) 9:953–61. doi: 10.5377/pediatrica.v9i2.8790
28. Ribeiro, JGL, and Chaimowicz, A. Respiratory syncytial Vírus (RSV) infection prevention: palivizumab use. Rev Assoc Med Minas Gerais. (2016) 26:S66–S68. doi: 10.5935/2238-3182.20160026
29. Fuentes, SC, Cornejo, CG, and Bustos, BR. Actualización en el tratamiento de bronquiolitis aguda: menos es más. Neumol Pediatr. (2016) 11:65–70. doi: 10.51451/np.v11i2.303
30. Scotta, MC, Chakr, V, de Moura, A, Becker, RG, de Souza, APD, Jones, MH, et al. Respiratory viral coinfection and disease severity in children: a systematic review and meta-analysis. J Clin Virol. (2016) 80:45–56. doi: 10.1016/j.jcv.2016.04.019
31. Diggikar, S, Paul, A, Razak, A, Chandrasekaran, M, and Swamy, RS. Respiratory infections in children born preterm in low and middle-income countries: a systematic review. Pediatr Pulmonol. (2022) 57:2903–14. doi: 10.1002/ppul.26128
32. Wang, X, Li, Y, Mei, X, Bushe, E, Campbell, H, and Nair, H. Global hospital admissions and in-hospital mortality associated with all-cause and virus-specific acute lower respiratory infections in children and adolescents aged 5-19 years between 1995 and 2019: a systematic review and modelling study. BMJ Glob Health. (2021) 6:e006014. doi: 10.1136/bmjgh-2021-006014
33. Stein, RT, Bont, LJ, Zar, H, Polack, FP, Park, C, Claxton, A, et al. Respiratory syncytial virus hospitalization and mortality: systematic review and meta-analysis. Pediatr Pulmonol. (2017) 52:556–69. doi: 10.1002/ppul.23570
34. PAHO/WHO. Influenza, SARS-CoV-2, VSR y otros virus respiratorios. Available at: https://www.paho.org/es/temas/influenza-sars-cov-2-vsr-otros-virus-respiratorios (Accessed December 14, 2023).
35. Piedimonte, G, and Perez, MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. (2014) 35:519–30. doi: 10.1542/pir.35.12.519
36. Driscoll, AJ, Arshad, SH, Bont, L, Brunwasser, SM, Cherian, T, Englund, JA, et al. Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? Critical review of the evidence and guidance for future studies from a World Health Organization-sponsored meeting. Vaccine. (2020) 38:2435–48. doi: 10.1016/j.vaccine.2020.01.020
37. Simões, EAF, Chirikov, V, Botteman, M, Kwon, Y, and Kuznik, A. Long-term assessment of healthcare utilization 5 years after respiratory syncytial virus infection in US infants. J Infect Dis. (2020) 221:1256–70. doi: 10.1093/infdis/jiz278
38. Cantú-Flores, K, Rivera-Alfaro, G, Muñoz-Escalante, JC, and Noyola, DE. Global distribution of respiratory syncytial virus a and B infections: a systematic review. Pathog Glob Health. (2022) 116:398–409. doi: 10.1080/20477724.2022.2038053
39. Gentile, A, Lucion, MF, Del Valle, JM, Areso, MS, Bakir, J, Viegas, M, et al. Burden of respiratory syncytial virus disease and mortality risk factors in Argentina: 18 years of active surveillance in a children’s hospital. Pediatr Infect Dis J. (2019) 38:589–94. doi: 10.1097/INF.0000000000002271
40. Buendía, JA, Acuña-Cordero, R, and Rodriguez-Martinez, CE. The cost-utility of early use of high-flow nasal cannula in bronchiolitis. Heal Econ Rev. (2021) 11:41–8. doi: 10.1186/s13561-021-00339-7
41. Buendía, JA, Patino, DG, and Sinisterra, D. Cost of illness of RSV infection in a middle-income tropical country. Turk J Pediatr. (2021) 63:673–82. doi: 10.24953/turkjped.2021.04.015
42. Buendía, JA, and Patiño, DG. Costs of respiratory syncytial virus hospitalizations in Colombia. PharmacoEconomics - open. (2021) 5:71–6. doi: 10.1007/s41669-020-00218-7
43. Buendía, JA, Acuña-Cordero, R, and Rodriguez-Martinez, CE. Budget impact analysis of high-flow nasal cannula for infant bronchiolitis: the Colombian National Health System perspective. Curr Med Res Opin. (2021) 37:1627–32. doi: 10.1080/03007995.2021.1943342
44. Comas-García, A, Aguilera-Martínez, JI, Escalante-Padrón, FJ, Lima-Rogel, V, Gutierrez-Mendoza, LM, and Noyola, DE. Clinical impact and direct costs of nosocomial respiratory syncytial virus infections in the neonatal intensive care unit. Am J Infect Control. (2020) 48:982–6. doi: 10.1016/j.ajic.2020.04.009
45. Jara, JH, Azziz-Baumgartner, E, De Leon, T, Luciani, K, Brizuela, YS, Estripeaut, D, et al. Costs associated with acute respiratory illness and select virus infections in hospitalized children, El Salvador and Panama, 2012-2013. J Inf Secur. (2019) 79:108–14. doi: 10.1016/j.jinf.2019.05.021
46. Rodríguez-Martínez, CE, Sossa-Briceño, MP, and Nino, G. Emergency department-initiated home oxygen for viral bronchiolitis: a cost-effectiveness analysis. Pediatr Pulmonol. (2022) 57:2154–60. doi: 10.1002/ppul.26018
47. Cintra, MA. Avaliação do custo efetividade da profilaxia do vírus sincicial respiratório em lactentes com cardiopatia congênita. (2013). Available at: https://www.arca.fiocruz.br/handle/icict/9313 (Accessed August 08, 2023).
48. van Houten, CB, Naaktgeboren, C, Buiteman, BJM, van der Lee, M, Klein, A, Srugo, I, et al. Antibiotic overuse in children with respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. (2018) 37:1077–81. doi: 10.1097/INF.0000000000001981
49. Petat, H, Gajdos, V, Angoulvant, F, Vidalain, P-O, Corbet, S, Marguet, C, et al. High frequency of viral co-detections in acute bronchiolitis. Viruses. (2021) 13:990. doi: 10.3390/v13060990
50. Jain, S, Williams, DJ, Arnold, SR, Ampofo, K, Bramley, AM, Reed, C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. (2015) 372:835–45. doi: 10.1056/NEJMoa1405870
51. Skjerven, HO, Megremis, S, Papadopoulos, NG, Mowinckel, P, Carlsen, K-H, Lødrup Carlsen, KC, et al. Virus type and genomic load in acute bronchiolitis: severity and treatment response with inhaled adrenaline. J Infect Dis. (2016) 213:915–21. doi: 10.1093/infdis/jiv513
52. Li, Y, Reeves, RM, Wang, X, Bassat, Q, Brooks, WA, Cohen, C, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health. (2019) 7:E1031–45. doi: 10.1016/s2214-109x(19)30264-5
53. Baumeister, E, Duque, J, Varela, T, Palekar, R, Couto, P, Savy, V, et al. Timing of respiratory syncytial virus and influenza epidemic activity in five regions of Argentina, 2007-2016. Influenza Other Respir Viruses. (2019) 13:10–7. doi: 10.1111/irv.12596
54. Bo, Y, Guo, C, Lin, C, Zeng, Y, Li, HB, Zhang, Y, et al. Effectiveness of non-pharmaceutical interventions on COVID-19 transmission in 190 countries from 23 January to 13 April 2020. Int J Infect Dis. (2021) 102:247–53. doi: 10.1016/j.ijid.2020.10.066
55. Redlberger-Fritz, M, Kundi, M, Aberle, SW, and Puchhammer-Stöckl, E. Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J Clin Virol. (2021) 137:104795. doi: 10.1016/j.jcv.2021.104795
56. Oh, D-Y, Buda, S, Biere, B, Reiche, J, Schlosser, F, Duwe, S, et al. Trends in respiratory virus circulation following COVID-19-targeted nonpharmaceutical interventions in Germany, January - September 2020: analysis of national surveillance data. Lancet Reg Health Eur. (2021) 6:100112. doi: 10.1016/j.lanepe.2021.100112
57. Chow, EJ, Uyeki, TM, and Chu, HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. (2023) 21:195–210. doi: 10.1038/s41579-022-00807-9
58. El-Heneidy, A, Ware, RS, Robson, JM, Cherian, SG, Lambert, SB, and Grimwood, K. Respiratory virus detection during the COVID-19 pandemic in Queensland, Australia. Aust N Z J Public Health. (2022) 46:10–5. doi: 10.1111/1753-6405.13168
59. Cobos, M, Nannini, EC, Balbuena, JP, Crocci, EE, Schreiner, D, Garcia, EA, et al. Impact of population mobility on the behavior of respiratory viruses during the COVID-19 pandemic. Medicina. (2023) 83:719–26.
60. Alonso-Coello, P, Oxman, AD, Moberg, J, Brignardello-Petersen, R, Akl, EA, Davoli, M, et al. GRADE evidence to decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: clinical practice guidelines. Gac Sanit. (2017) 32:167.e1–167.e10. doi: 10.1016/j.gaceta.2017.03.008
61. Ciapponi, A, Glujovsky, D, Virgilio, SA, and Bardach, AE. Conducting and disseminating epidemiological systematic reviews in Latin America and the Caribbean: pitfalls and lessons learned. Value Health Reg Issues. (2017) 14:64–72. doi: 10.1016/j.vhri.2017.07.011
62. Ulloa-Gutierrez, R. Virus respiratorio sincicial en niños y adultos de Latinoamérica: ¿Cómo nos preparamos para el 2024? Rev Chilena Infectol. (2023) 40:583–7. doi: 10.4067/s0716-10182023000600583
Keywords: respiratory syncytial virus, bronchiolitis, lower respiratory tract infection, prevalence, incidence, mortality, seasonality, Latin America and the Caribbean
Citation: Ciapponi A, Palermo MC, Sandoval MM, Baumeister E, Ruvinsky S, Ulloa-Gutierrez R, Stegelmann K, Ruesjas SA, Cantos J, LaRotta J, de Almeida RS and Bardach A (2024) Respiratory syncytial virus disease burden in children and adults from Latin America: a systematic review and meta-analysis. Front. Public Health. 12:1377968. doi: 10.3389/fpubh.2024.1377968
Edited by:
Ashish Kakkar, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Seyed Aria Nejadghaderi, Kerman University of Medical Sciences, IranYolanda Penders, GlaxoSmithKline, Belgium
Copyright © 2024 Ciapponi, Palermo, Sandoval, Baumeister, Ruvinsky, Ulloa-Gutierrez, Stegelmann, Ruesjas, Cantos, LaRotta, de Almeida and Bardach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agustín Ciapponi, YWNpYXBwb25pQGllY3Mub3JnLmFy
†These authors have contributed equally to this work
‡ORCID: Agustin Ciapponi, orcid.org/0000-0001-5142-6122
Rolando Ulloa-Gutierrez, orcid.org/0000-0002-9157-9227
Ariel Bardach, orcid.org/0000-0003-4437-0073
 Agustín Ciapponi
Agustín Ciapponi María Carolina Palermo1†
María Carolina Palermo1† María Macarena Sandoval
María Macarena Sandoval Elsa Baumeister
Elsa Baumeister Silvina Ruvinsky
Silvina Ruvinsky Rolando Ulloa-Gutierrez
Rolando Ulloa-Gutierrez Rodrigo Sini de Almeida
Rodrigo Sini de Almeida Ariel Bardach
Ariel Bardach