
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 13 March 2024
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1374515
Background: Globally, loss to follow-up (LTFU) remains a significant public health concern despite the rapid expansion of antiretroviral medication programs. It is a significant cause of treatment failure and threatens the enhancement of HIV treatment outcomes among patients on antiretroviral therapy (ART). However, there is a paucity of evidence on its incidence and predictors in Ethiopia. Thus, this study aimed to examine the incidence and predictors of LTFU among adult HIV patients receiving ART at hospitals in Central Ethiopia.
Methods: A multi-centered facility-based retrospective cohort study was conducted among 432 randomly selected adult patients who received antiretroviral therapy. Data were entered into EpiData version 3.1 and exported to Stata version 14 for analysis. The Kaplan–Meier failure function was employed to determine the overall failure estimates, and the log-rank test was used to compare the probability of failure among the different categories of variables. The Cox proportional hazard model was used to identify independent predictors of LTFU.
Results: Overall, 172 (39.8%) study participants were lost to follow-up over the 10-year follow-up period with an incidence rate of 8.12 (95% CI: 7.11, 9.09) per 1,000 person-months. Undisclosed HIV status (AHR: 1.96, 95% CI: 1.14, 3.36), not able to work (AHR: 1.84, 95% CI: 1.13, 2.22), opportunistic infections (AHR: 3.13, 95% CI: 2.17, 4.52), CD4 < 200 cell/mL (AHR: 1.95, 95% CI: 1.18, 3.21), not receiving isoniazid preventive therapy (IPT) (AHR: 2.57, 95% CI: 1.62, 4.06), not participating in clubs (AHR: 1.68, 95% CI: 1.10, 2.22), side effects of drugs (AHR: 1.44, 95% CI: 1.02, 2.04), and high viral load (AHR: 3.15, 95% CI: 1.81, 5.47) were identified as significant predictors of loss to follow-up.
Conclusion: In this study, the incidence of LTFU was high. The focus should be on creating awareness and prevention programs that aim to reduce loss to follow-up by continuing counseling, especially on the negative effects of loss to follow-up and the benefits of ART care.
With advancements and expansion in antiretroviral therapy and the growing access to and use of human immunodeficiency virus (HIV) medical services over almost two decades, a notable positive change has been observed worldwide in people living with HIV/AIDS (PLWH) (1). HIV infection with successful antiretroviral treatment can now be considered a manageable chronic disease in most settings (2).
One of the main obstacles to successful HIV patient care and treatment is the loss to follow-up (LTFU) from antiretroviral therapy (ART) care (3). LTFU is defined as the absence of an ART refill or follow-up for 3 months or longer since their last follow-up schedule (4).
Studies have shown that the incidence of LTFU varies worldwide. A study conducted in Nigeria reported an overall incidence of 55.6 per 100 person-years (5). A similar study conducted in southern Uganda reported an overall incidence rate of 26.7 per 100 person-years (6). However, Ethiopia differs at regional and facility levels. Previous studies conducted in the Amhara region (7) and Arba Minch General Hospital (8) showed that the overall incidence of LTFU was 13.45 per 100 person-years and 5.3 per 100 person-years, respectively.
Different studies have identified factors that predict LTFU from ART clinics, including male sex, age group 15–24, poor/fair adherence, WHO clinical stage III/IV, low CD4, not receiving isoniazid preventive therapy (IPT), and cotrimoxazole preventive therapy (CPT). Similarly, studies have shown that alcohol and substance abuse, TB and other opportunistic infections, stigma, rural residence, demotivation of the service, increased transportation cost, long distance, and waiting time were other contributors to LTFU (9–14).
The loss to follow-up can have negative consequences for patients with HIV. If patients’ LTFU interrupt ART, their immunological and clinical conditions can deteriorate and lead to treatment failure and eventually to increased AIDS mortality. Additionally, it has detrimental effects such as medication resistance, drug toxicity, and potential transmission of the virus. Furthermore, it is a significant obstacle for program implementers as it suggests a poor utilization of limited resources, such as treatment (14, 15). However, there is a paucity of evidence on its incidence and predictors in Ethiopia in general, and there is no specific evidence on it in the study area. Thus, this study aimed to examine the incidence and predictors of LTFU among adult human immunodeficiency virus (HIV) patients receiving ART in Central Ethiopia.
We conducted a multi-centered facility-based retrospective cohort study in hospitals of Kembata zone and Tembaro Special District in Central Ethiopia. The Kembata zone is located 340 km southwest of the capital city of Ethiopia, Addis Ababa, and 130 km from Hawassa. According to the 2019 Zonal Report, the estimated total population was approximately 941,313, of which 498,896 were men and 442,417 were women. The Kembata zone has four governmental hospitals (Dr Bogalech Gebre Memorial General Hospital, Doyogana Primary Hospital, Shinshicho Primary Hospital, and Angecha Primary Hospital), while Tembaro Special District has one hospital (Mudula Primary Hospital). All of these hospitals provide ART services, except Angecha Primary Hospital. According to the Kembata zone Health Management Information System (HMIS) 2020 Report, these hospitals provided antiretroviral (ARV) services to 1,301 patients, and out of which 1,194 were adults. Patients’ medical records from January 2010 to December 2020 were extracted from 1 June 2022 to 30 June 2022.
All HIV-positive adult patients who had at least one treatment follow-up from 1 January 2010 to 31 December 2020 in the hospitals of Kembata zone and Tembaro Special District were considered as the source population, while all adults with advanced and non-advanced clinical stages at ART enrolment from 1 January 2010 to 31 December 2020 in the hospitals of Kembata zone and Tembaro Special District were considered as the study population.
All HIV-positive adult patients aged ≥15 years who were enrolled in ART between January 2010 and December 2020 at the selected hospitals were included in this study. However, patients whose registration records did not show the initiation date, whose baseline record data were unavailable, who had undefined outcomes, and who had been transferred in with incomplete baseline data were excluded from this study.
The sample size was determined using a double population proportion formula considering the advanced stage with a proportion of 0.29 vs. non-advanced stage with a proportion of 0.16 as an independent predictor (13). We set α = 5, power = 80%, and a withdrawal rate of 10% for incomplete data, with an allocation ratio of 1:1 and a standard deviation of 0.5. The estimated sample size for the survivor function was 432. Of these, 216 had advanced WHO clinical stages, and 216 had non-advanced WHO disease stages.
The participants were selected from all public hospitals that provided antiretroviral therapy services in the Kembata zone and Tembaro Special District. A list of potential participants was obtained from the adult ART register of each hospital. The total number of ART patient medical records and charts in these hospitals from January 2010 to December 2020 was 894, of which 467 were recruited at non-advanced disease stages. The number of patients who were recruited from each hospital were proportionally allocated based on the patient load to obtain the final sample size. In addition, the sample was collected using a simple random sampling method by taking the list of all ART patients’ medical records and charts and using their unique ART number in each hospital as a sampling frame.
Event: LTFU is defined as the absence of an ART refill or follow-up for 3 months or longer since the last follow-up schedule and not reported or recorded as dead or transferred on the patient’s logbook or medical cards (4).
Censored: Patients who did not LTFU until the study ended were recorded as dead and were transferred out (16).
Survival time refers to the total time in months from the initiation of antiretroviral therapy to the occurrence of an event or censored (16).
Advanced disease stage is defined as adults and adolescents with CD4 cell counts of less than 200 cells/mm3 or WHO stage 3 or 4 events (14).
Good adherence is defined as patients who took ≥95% of doses or missed ≤2 doses out of 30 doses per month and less than or equal to 3 doses out of 60 doses per month.
Poor adherence is defined as patients taking less than 85% of doses or missing more than five doses out of 30 doses per month and more than nine doses out of 60 doses per month (14).
Appointment spacing model (ASM) or differentiated service delivery (DSD) is a 6-month multi-month dispensing (6-MMD) model in which stable ART patients visit health facilities twice a year for clinical evaluation and laboratory testing when needed; during this visit, they receive 6 months’ worth of ART (14).
Data were extracted using an English checklist based on nationally standardized ART intake, follow-up, and ART registers. Four health professionals (two BSc nurses and two public health officers) experienced in ART were recruited as data collectors. The patients received 2 days of training on the objectives of the study, the contents of the checklist, and how to review patients’ documents. Two supervisors (one public health expert and one data clerk) participated during the data collection period to supervise the overall data collection process. The entire data collection process was closely monitored daily by supervisors and the principal investigators.
After data collection, the investigator coded, checked, and cleaned the data. The data were entered into EpiData version 3.1 and then exported to Stata version 14.0 for data processing and analysis. The outcome variables were re-coded as dichotomous outcomes: respondents were lost or not lost. Descriptive statistics such as median, percentage, and frequency were computed and were presented using text, tables, and graphs. A life table was used to estimate the probability of LTFU every 12, 24, 36, 48, 60, 72, 84, 96, or more months. The Kaplan–Meier failure curve was used to estimate the cumulative probability of LTFU after ART initiation. The log-rank test was used to compare significant differences between the groups and was considered statistically significant at p < 0.05.
A bivariable Cox regression analysis was performed to determine candidate variables for the multivariable Cox regression model. Variables with a p < 0.25 in the bivariable Cox regression model were entered into the multivariable Cox regression model to identify the predictors of incidence of LTFU. The Schoenfeld residuals test (both global and scaled) and graphical (log–log plot of survival) methods were used to check the proportional hazard (PH) assumption, and the overall full model did not violate the proportional hazard assumption. In addition, the most parsimonious model was selected using the log-likelihood ratio, Akaike information criteria (AIC), and Bayesian information criteria (BIC), and the presence of multicollinearity was checked using the variance inflation factor. A p < 0.05 was used to declare statistical significance in the multivariable Cox regression model, and an adjusted hazard ratio (AHR) with its 95% confidence interval was computed to show the strength of the association. Moreover, the goodness of fit of the model was assessed using the Cox–Snell residual technique.
A total of 432 medical records of adults who received ART between January 2010 and December 2020 were reviewed. The median age of the participants was 32 years, with an interquartile range (IQR) of 28–40 years, and nearly 40% of the participants were in the age group of 25 to 34 years. More than half of the study participants (54.4%) were women, 58.8% of the participants were urban dwellers, and 56.5% of the participants were married. Furthermore, nearly two-thirds of the study participants had attended primary school and above (Table 1).
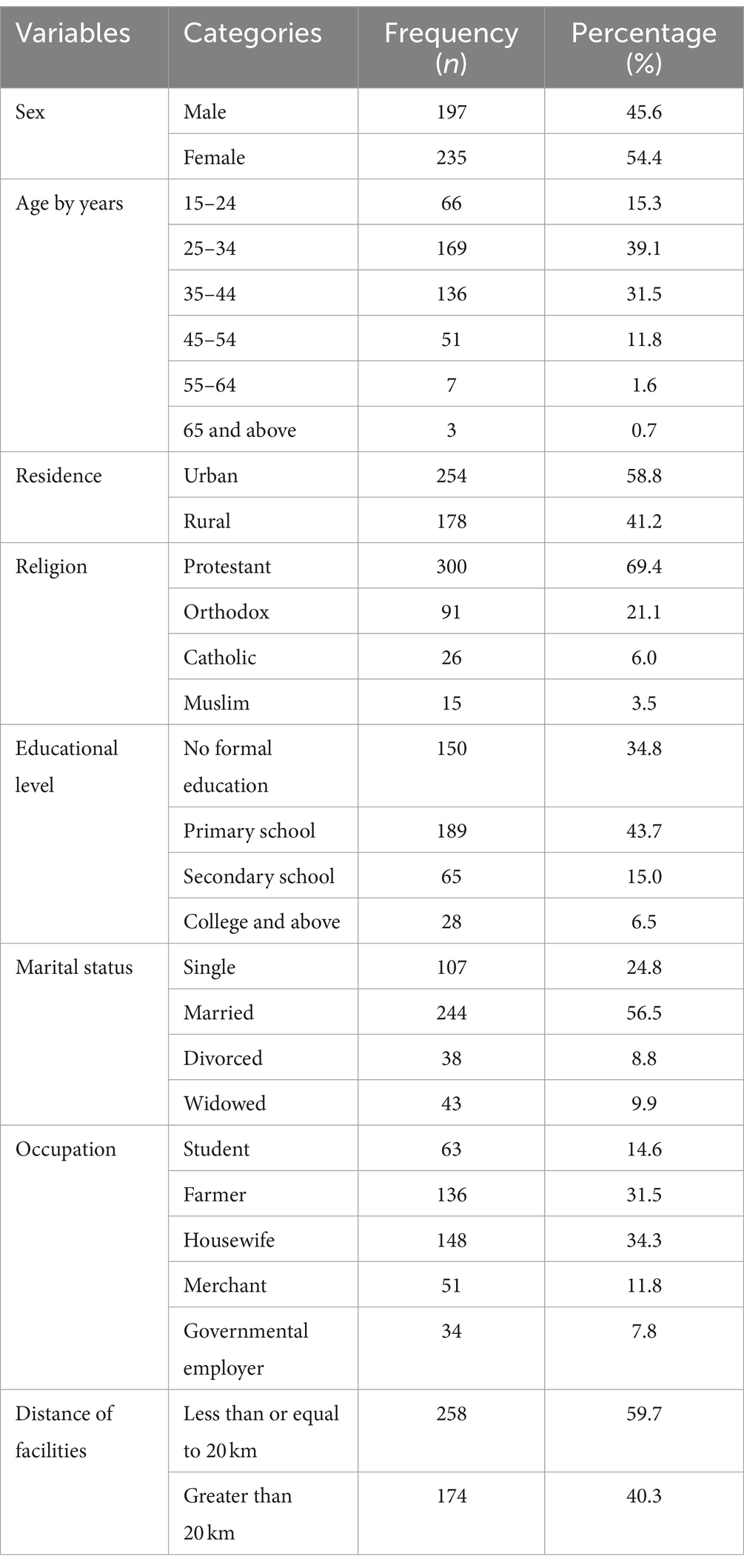
Table 1. Sociodemographic characters of HIV-positive adults receiving ART at hospitals of Kembata zone and Tembaro special district in Central Ethiopia from 1 January 2010 to 31 December 2020 (n = 432).
In this study, 46.3% of participants had other HIV-positive family members, while the majority (59.3%) of the participants were living with their parents. Approximately 56.9% of participants disclosed their HIV status. More than three-fourths (80.6%) of the study participants had no history of alcohol use, while 94.0% of participants had no history of smoking cigarettes (Table 2).
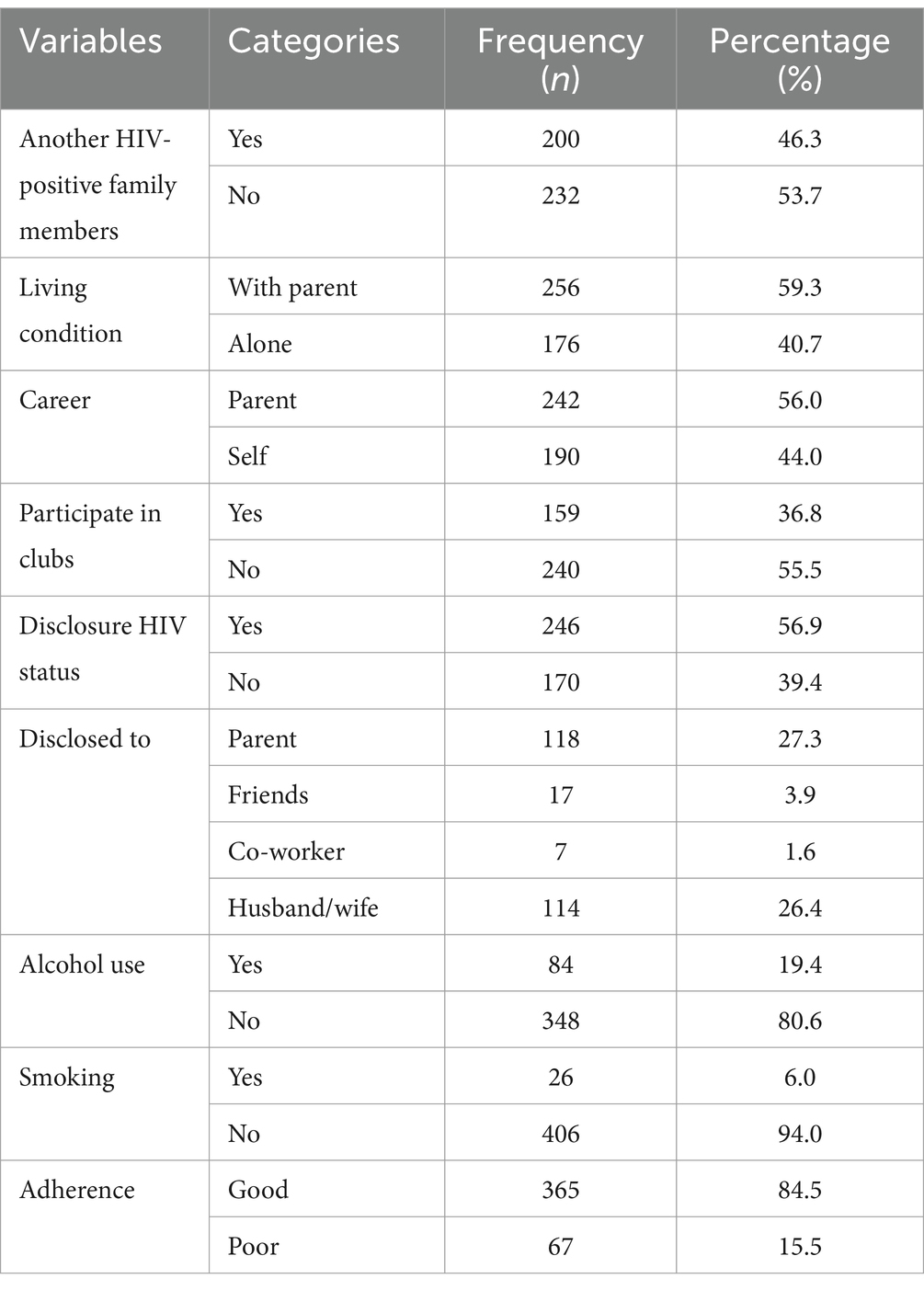
Table 2. Psychosocial and behavioral characteristics of HIV-positive adults using ART at hospitals of Kembata zone and Tembaro special district in Central Ethiopia from 1 January 2010 to 31 December 2020 (n = 432).
The majority (62.0%) of the participants had a normal body mass index (BMI), and 87.5% of the participants had a working functional status. More than half (55.9%) of the participants had CD4 counts 350 cell/mm3 and above. Most (84.3%) of the participants had taken the isoniazid therapy. More than half (53.2) of the participants had at least one opportunistic infection, and 23.8, 19.8, and 10.9% of the participants developed tuberculosis, pneumonia, and zoster, respectively (Table 3).

Table 3. Clinical and treatment-related characteristics of HIV-positive adults using ART at hospitals of Kembata zone and Tembaro special district in Central Ethiopia from 1 January 2010 to 31 December 2020 (n = 432).
In this study, all (432) participants contributed a total of 21,175 person-months, of which 11,227 person-months contributed to patients with advanced disease. In this study, the incidence rate of LTFU was 8.12 (95% CI: 7.11–9.09) per 1,000 person-months. The incidence rate of LTFU with advanced disease stage was 10.15 (95% CI: 9.21–11.19) per 1,000 person-months. Similarly, the incidence rate of LTFU with non-advanced disease stage was 5.83 (95% CI: 4.72–6.91) per 1,000 person-months. The total proportion of the patients with LTFU was 172 (39.8, 95% CI: 35.3–44.5); of these, 114 (52.8, 95% CI: 37.2–56.5) and 58 (26.85, 95% CI: 32.3–40.2) of them had advanced and non-advanced disease stages, respectively. The overall probability of LTFU in the non-advanced disease stage cohort was significantly different from that in the advanced disease stage cohort. This means that the risk of LTFU was higher in the advanced disease stage cohort than in the non-advanced disease stage cohort (log-rank = 0.003) (Figure 1).
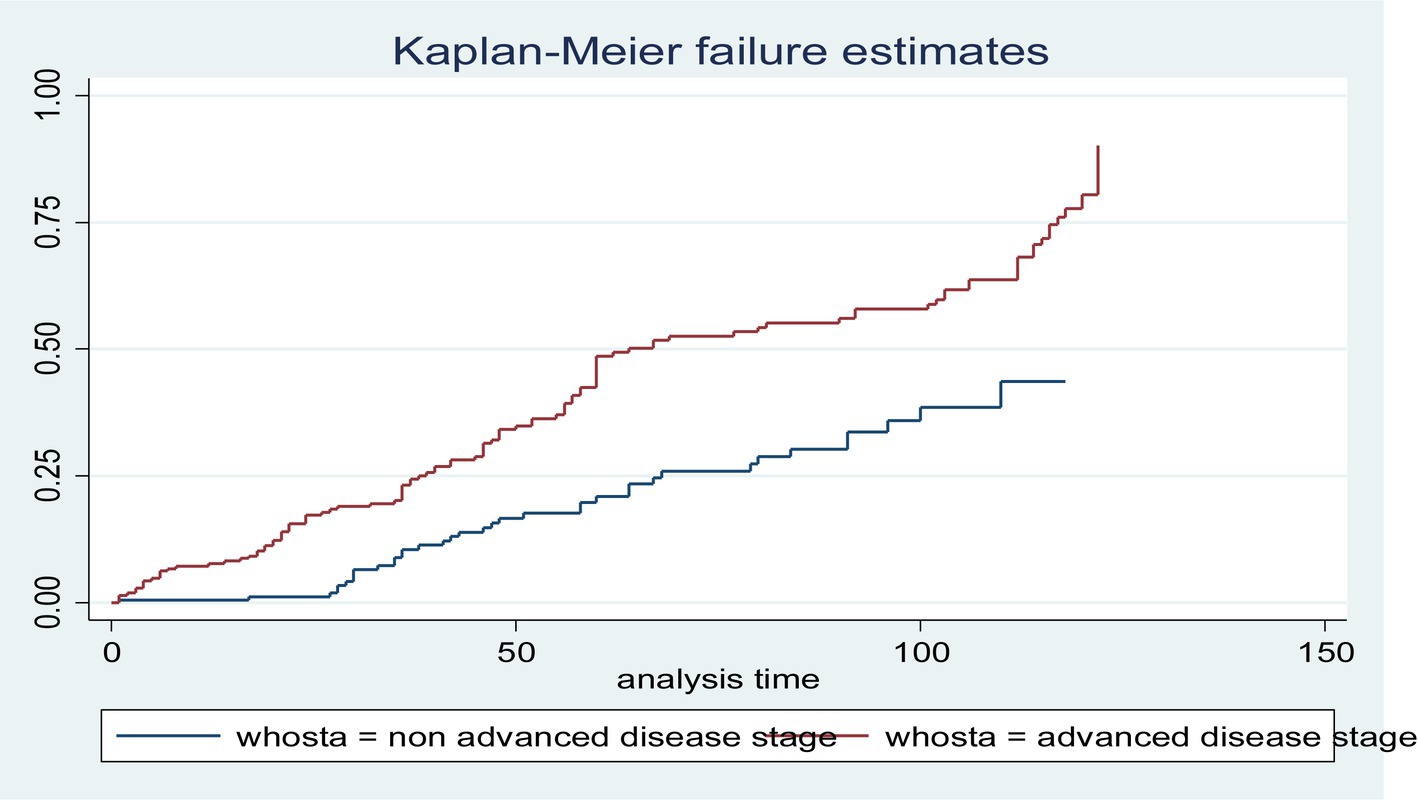
Figure 1. The Kaplan–Meier failure function of loss to follow-up among HIV-positive adults using ART over their WHO stage categories from 1 January 2010 to 31 December 2020 (n = 432).
The overall graph of the Kaplan–Meier failure function shows that the likelihood of LTFU increased over a follow-up period (Figure 2).
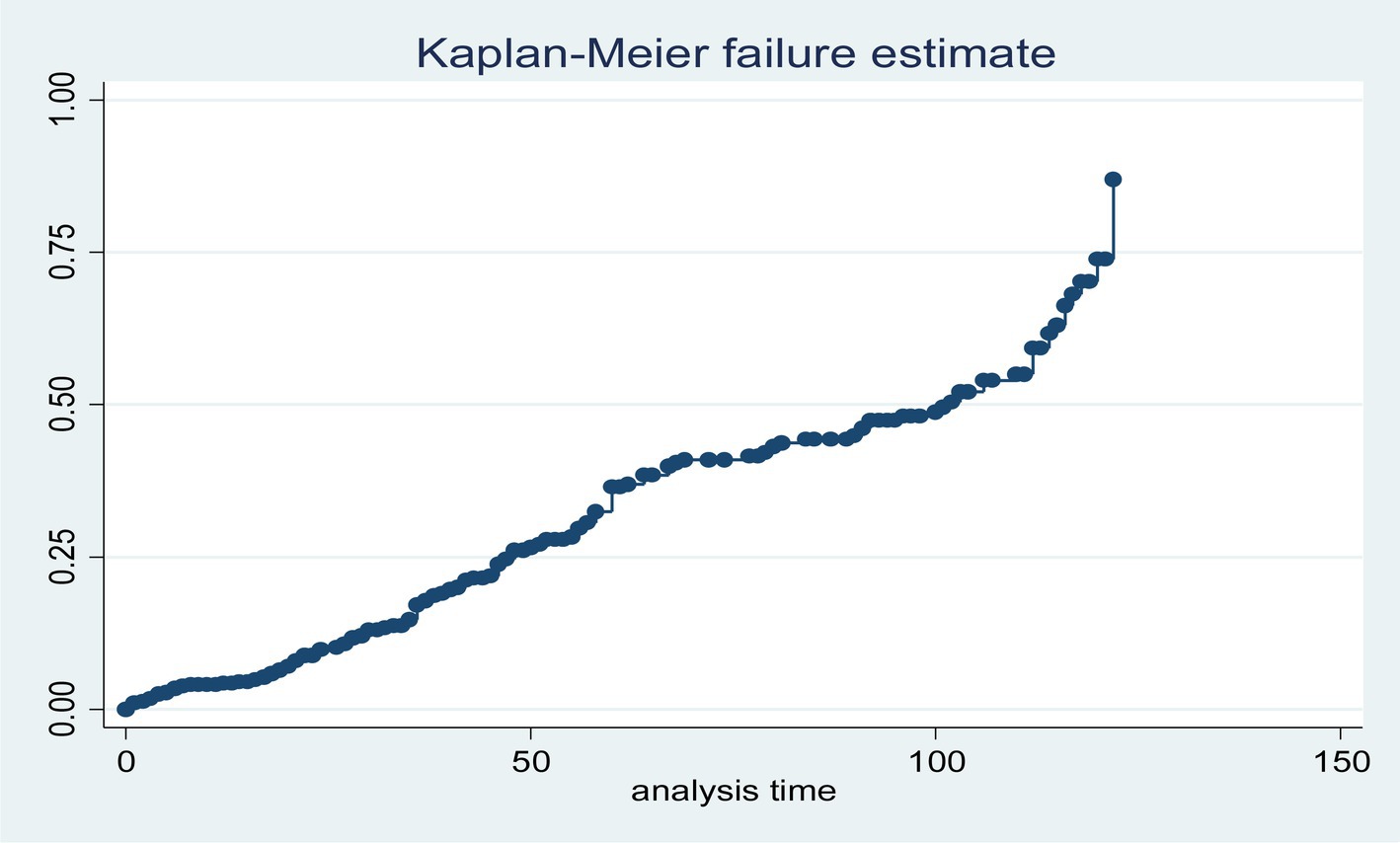
Figure 2. Cumulative failure function of loss to follow-up among HIV-positive adults using ART at hospitals of Kembata zone and Tembaro Special District in Sentral Ethiopia, from 1 January 2010 to 31 December 2020 (n = 432).
The median time for patients to be LTFU receiving ART treatments was 14.25 months. However, the findings of this study revealed that there were differences in the median time of LTFU between advanced and non-advanced disease stage patients, with 16.78 and 12.67 months, respectively.
To determine the predictors of LTFU, variables from bivariate Cox regression analysis (p < 0.25) were received as candidate variables for multivariate Cox regression analysis. Having other HIV-positive family members, living alone, being a self-caregiver, participating in clubs, disclosing HIV status, drug adherence status, functional status, BMI, viral load >1,000 copies/ml, follow-up type, OI, CD4 count <200 cells/mm3, not receiving IPT, and drug-related side effects were significantly associated with LTFU at p < 0.25.
In multivariable Cox regression analysis, HIV status disclosure, participation in clubs, functional status, viral load >1,000 copies/ml, presence of OI, CD4 count <200 cell/mm3, presence of drug-related side effects, and not receiving IPT were significant predictors of an increased risk of LTFU. The risk of LTFU among patients who did not disclose their HIV status was 1.94 (95% CI: 1.12–3.36) times higher than that among those who disclosed their HIV status. Patients with non-working functional status were 1.84 (95% CI: 1.13–2.22) times more likely to have LTFU than those with working functional status. Furthermore, patients who did not receive IPT were more likely to be LTFU than those who received IPT (AHR = 2.53, 95% CI: 1.62–4.06).
In addition, the risk of LTFU was higher in patients with CD4 counts ≤200 cells/ mm3 compared to those with CD4 counts ≥350 cells/mm3 (AHR = 1.95, 95% CI: 1.19–3.22). Similarly, patients who developed opportunistic infection were 3.13 (95%CI: 2.08–4.30) times at risk of loss from care than those who did not develop any opportunistic infection. Furthermore, the risk of LTFU was increased by 44% among patients who developed drug-related side effects as compared with patients who did not develop drug-related side effects (AHR = 1.44, 95% CI: 1.02–2.04). Conversely, patients who did not participate in clubs were 1.69 (95% CI, 1.09–2.62) times more at risk of loss from care than patients participating in clubs. Furthermore, patients with a high viral load had a 3.145 times increased risk of LTFU compared to patients with a viral load of less than 1,000 copies/ml (AHR = 3.145, 95% CI: 1.808–5.469) (Table 4).
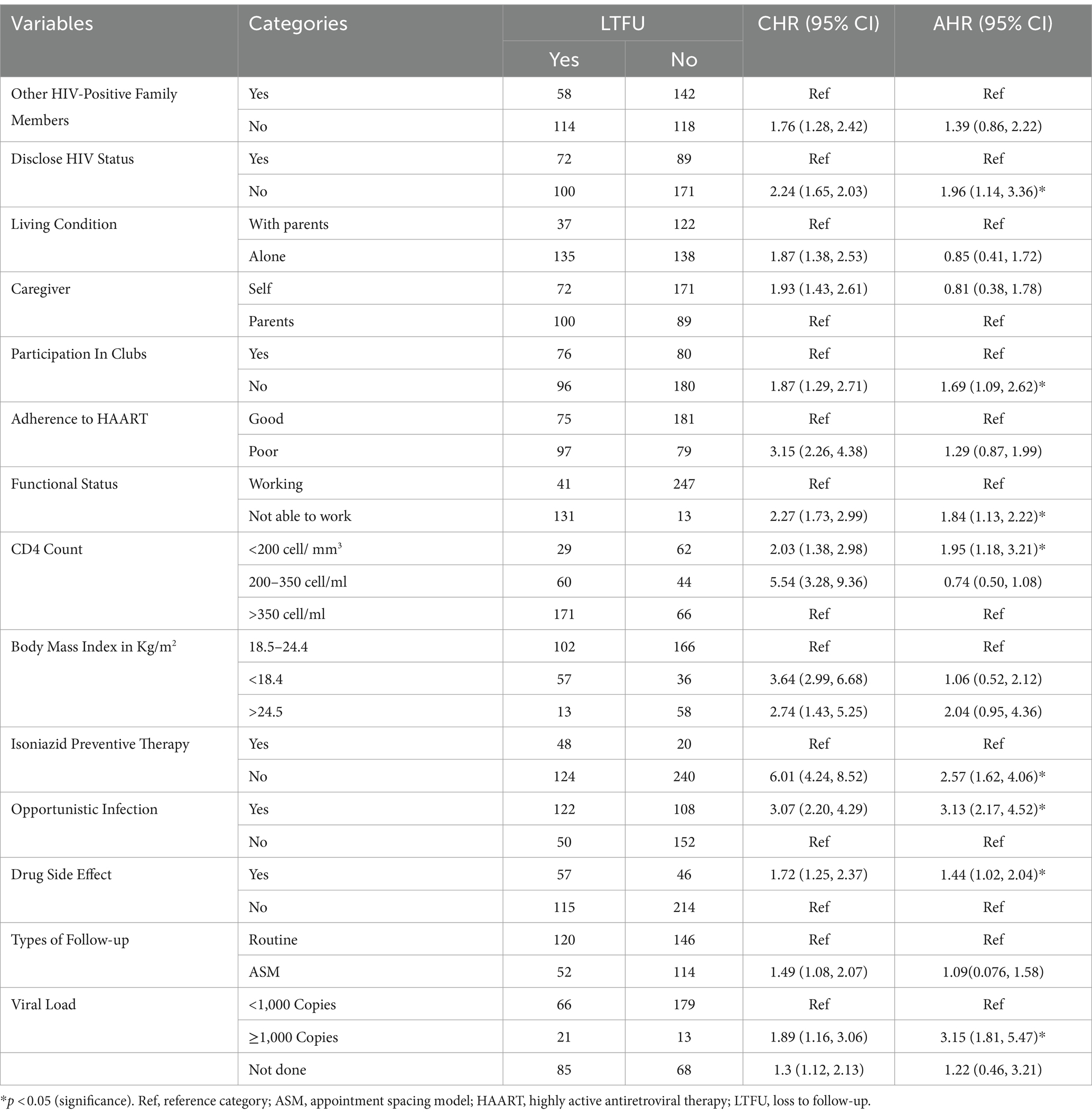
Table 4. Predictors of LTFU among HIV-positive adults using ART at hospitals of Kembata zone and Tembaro special district in Central Ethiopia, 2022 (n = 432).
This study aimed to examine the incidence and predictors of LTFU among adult human immunodeficiency virus (HIV) patients receiving ART at hospitals in Central Ethiopia. The finding revealed that the incidence rate of LTFU was 9.74 per 100 person-years (8.12 per 1,000 person-months) of adult observation in PLHIV receiving ART. Ambulatory functional status, CD4 count less than 200 cells/mm3, not participating in clubs, not disclosing HIV status, opportunistic infections (OI), not receiving isoniazid preventive therapy (IPT), developed drug-related side effects, and viral load >1,000 copies/mm3 were identified as predictors of LTFU.
The incidence rate in this study was consistent with studies conducted in Gondar, Ethiopia (10.90 per 100-person years) (17), Bahir Dar City, Northwest Ethiopia (9.7 per 100 person-years of observation) (18), Debre birhan, Northeast Ethiopia (8.9 per 100 adult years observation) (19), Gonder, Ethiopia (6.7 per 100 person-years) (20) and Cameroon (9.46 per 100 person-years) (21). On the other hand, this finding was lower compared to a study conducted in other African countries: Uganda (21 per 1,000 person-months) (22), Malawi (26.0 per 100 person-years) (23), Pawi, and Northwest Ethiopia (11.6 per 100-person-years) (24).
In contrast, the findings of this study were higher than those studies conducted in different areas of Ethiopia, Mekele (4.5 per 100 person-years) (25), Debre Markos (3.7 per 100 person-years) (26) and Arba Minch (5.3 per 100 person-years) (8). The difference could be attributed to the difference in total follow-up periods, lifestyles of the communities, socioeconomic differences, variations in sample size, variations in health-seeking behavior, differences in patients’ self-transfer behavior to other health institutions without a standardized form or inappropriate recording, and variations in early death report which could be considered as LTFU.
The current study revealed that adult patients who did not disclose their HIV status were 1.96 times more at risk of LTFU than those who disclosed it. This result is in line with studies conducted in Woldian town (27), Kaffa, and Bench Maji zones; individuals who did not disclose their HIV status had more than twice (AHR = 2.28) the risk of LTFU from ART clinics than those who disclosed their HIV status (28). This variation might be because patients who did not disclose their HIV status could not attend the clinic at the expected level, could not feel safe to pick their medication as scheduled, or could not have enough family support due to the fear of discrimination and stigma, which might lead to LTFU. In addition, there is a fear of rejection and violence by partners, family members, the community, and even in the workplace.
Adult patients whose baseline functional status was ambulatory were 1.84 times more at risk of LTFU than those whose baseline functional status was working. This finding is similar to studies conducted in the Hadiya zone, Ethiopia, where the risk of LTFU in ambulatory patients was higher than that in patients with working functional status (AHR = 2.4) (13). These discrepancies might be because most ambulatory patients were economically dependent. In addition, they required close family and social support, and patients’ self-care activities might be interrupted.
The risk of LTFU was nearly two times higher among patients receiving ART with baseline CD4 counts less than 200 cells/mm3 than patients with a baseline CD4 count greater than 350 cells/mm3. This finding is in agreement with studies conducted in South Africa (28), Ethiopia (29) and Mizan-Aman General Hospital (30). The reason for this result might be that those with lower CD4 counts have low immunity and therefore develop multiple opportunistic infections and might develop immune reconstitution inflammatory syndrome (IRIS).
HIV-positive patients receiving ART who did not receive isoniazid preventive therapy were 2.6 times more likely to be LTFU than those who received therapy. This result was concordant with a study conducted at Gondar in 2019, which showed that participants with no isoniazid (INH) prophylaxis were 2.47 times more at risk of being LTFU compared to those who took INH (12). Similarly, a study conducted in Mekele on isoniazid preventive therapy (IPT) (AHR = 0.085) reported a protective effect against LTFU (25). This variation might be because those who did not take IPT were at risk of developing active TB, drug toxicity, and drug–drug interactions.
Adult HIV-positive patients with at least one opportunistic infection were three times more likely to be LTFU than those without any opportunistic infection. This finding is more consistent with studies conducted in sub-Saharan Africa (16), Cameron (31) and Gondar Hospital (12) which shows that those who had opportunistic infection were 2.5, 4.75, and 1.74 times more likely to be LTFU, respectively, compared to those who had no opportunistic infection. This discrepancy might be due to illness severity and increased drug-related pill burden as well as drug–drug interactions, toxicity, and adherence of patients to ARV.
Adult patients receiving ART with a high viral load of ≥1,000 copies/mL were 3.15 (95% CI: 1.808–5.469) times at risk of LTFU compared to those with a low viral load of <1,000 copies/mL. Possible explanations for treatment failure include the development of multiple opportunistic infections and the pill burden.
Similarly, adults receiving ART who developed any drug-related side effects were 1.44 (95% CI: 1.02–2.04) times more likely to be LTFU compared to those who had free drug side effects. A possible explanation might be the severity of the illness, fear of stigma, and discrimination. Furthermore, adults who did not participate in any clubs/associations were 1.7 (95% CI: 1.09–2.62) times more at risk of LTFU compared to those who participated in clubs. This discrepancy might also be due to the fear of stigma and discrimination. Therefore, for these three variables, high viral loads, drug-related side effects, and clubs/associations did not show similar results and were not found in the reviewing literature. However, this finding requires further investigation.
This study has some limitations: the study was conducted on secondary data and might have some secondary data limitations, and retrospective analysis can limit more predictive variables of LTFU on the records than on the patient’s side.
In this study, the incidence of LTFU was high. Patients who did not disclose HIV status, ambulatory functional status, CD4 count less than 200 cells/mm3, not participating in clubs, having opportunistic infections, isoniazid preventive therapy, having a viral load greater than 1,000 copies/mL, and those who developed drug-related side effects were significant predictors of LTFU.
Integration should be considered with multiple sectors and civil society, including partners and organizations, to create awareness and prevention programs that target LTFU. They should provide continuous counseling and health education targeting the negative effects of LTFU and the benefits of ART care. Hospitals should strengthen ART monitoring and follow-up systems to decrease the LTFU and loss tracking methods. In addition, effective patient monitoring systems should be developed, particularly for those with high viral loads, to prevent adverse drug effects.
Future studies should be conducted using prospective and qualitative designs to predict factors of LTFU among PLWH and the outcome of LTFU.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Haramaya University College of Health and Medical Sciences Institutional Health Research ethics review committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
AA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FA: Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology. AC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Bogalech Gebre Memorial General Hospital as part of the MPH study for AA. The funder has no role in the study design, collection, management, analysis, and interpretation of data or in the decision to submit for publication.
The authors acknowledge Haramaya University for ethical clearance and Bogalech Gebre Memorial General Hospital for financial support. They also thank the data collectors and the study participants for their willingness to participate in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AHR, adjusted hazard ratio; ART, antiretroviral therapy; ARV, antiretroviral; ASM, appointment spacing model; CHR, crude hazard ratio; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; LTFU, loss to follow-up; PLWH, people living with HIV; SNNPR, South Nations, Nationalities and Peoples’ Region
1. WHO . World AIDS Day (2015). Available at: https://www.who.int/southeastasia (Accessed June 5, 2023).
2. Reynolds, L . HIV as a chronic disease consideration for service planning in resource-poor settings. J Globalization Health Med Sci. (2011) 7:35–6. doi: 10.1186/1744-8603-7-35
3. McNairy, ML , Lamb, MR , Carter, RJ , Fayorsey, R , Tene, G , Mutabazi, V, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda and Tanzania. J Acquir Immune Defic Syndr. (2013) 62:e70:–e81. doi: 10.1097/QAI.0b013e318278bcb0
4. WHO . Global Report on Early Warning Indicators of HIV Drug Resistance. (2016). Available at: https://apps.who.int/iris/handle/10665/246219 (Accessed June 3, 2023).
5. Balogun, M , Meloni, ST , Igwilo, UU , Roberts, A , Okafor, I , Sekoni, A, et al. Status of HIV-infected patients classified as lost to follow-up from a large antiretroviral program in Southwest Nigeria. PLoS One. (2019):14:e0219903. doi: 10.1371/journal.pone.0219903(7):e0219903
6. Edwin, E , Boniface, AE , Lumori, RM , Michael Kanyesigye, AA , Muyindike, W , and Muzoora, C . Incidence and predictors of early loss to follow up among patients initiated on protease inhibitor-based second-line antiretroviral therapy in southwestern Uganda. AIDS Res Ther. (2021) 18:7. doi: 10.1186/s12981-021-00331-5
7. Telayneh, AT , Tesfa, M , Woyraw, W , Temesgen, H , Alamirew, NM , Haile, D, et al. Time to lost to follow-up and its predictors among adult patients receiving antiretroviral therapy retrospective follow-up study Amhara Northwest Ethiopia. Sci Rep. (2022) 12:2916. doi: 10.1038/s41598-022-07049-y
8. Gebremichael, MA , Gurara, MK , Weldehawaryat, HN , Mengesha, MM , and Berbada, DA . Predictors of loss to follow-up among HIV-infected adults after initiation of the first-line antiretroviral therapy at Arba Minch general hospital, southern Ethiopia: a 5-year retrospective cohort study. Biomed Res Int. (2021) 2021:8659372. doi: 10.1155/2021/8659372
9. Birhanu, MY , Leshargie, CT , Alebel, A , Wagnew, F , Siferih, M , Gebre, T, et al. Incidence and predictors of loss to follow-up among HIV-positive adults in Northwest Ethiopia: a retrospective cohort study. Trop Med Health. (2020) 48:1–10. doi: 10.1186/s41182-020-00266-z
10. Fisiha Kassa, S , Zemene Worku, W , Atalell, KA , and Agegnehu, CD . Incidence of loss to follow-up and its predictors among children with HIV on antiretroviral therapy at the University of Gondar Comprehensive Specialized Referral Hospital: a retrospective data analysis. HIV AIDS Res Palliat Care. (2020) 12:525–33. doi: 10.2147/HIV.S269580
11. Megerso, A , Garoma, S , Eticha, T , Workineh, T , Daba, S , Tarekegn, M, et al. Predictors of loss to follow-up in antiretroviral treatment for adult patients in the Oromia region, Ethiopia. HIV AIDS Res Palliat Care. (2016) 8:83–92. doi: 10.2147/HIV.S98137
12. Mekonnen, N , Abdulkadir, M , Shumetie, E , Baraki, AG , and Yenit, MKJBID . Incidence and predictors of loss to follow-up among HIV infected adults after initiation of first line anti-retroviral therapy at University of Gondar Comprehensive Specialized Hospital Northwest Ethiopia, 2018: Retrospective follow up study. BMC Res Notes. (2019) 12:1–7. doi: 10.1186/s13104-019-4154-y
13. Bikoro, B , Oljira, L , Gobena, T , and Erkalo, D . Incidence and predictors of loss to follow-up among human immunodeficiency virus-infected adult patients on anti-retroviral therapy at Hadiya zone public hospitals, southern Ethiopia: a retrospective cohort study. J Public Health. (2020) 30:1–12. doi: 10.1007/s10389-020-01268-1
14. FMOH . National Consolidated Guidelines for Comprehensive HIV Prevention, Care and Treatment. (2018) pp. 228. Available at: https://www.afro.who.int/publications/national-consolidated-guidelines-comprehensive-hiv-prevention-care-and-treatment:38 (Accessed March 1, 2023).
15. Mberi, MN , Kuonza, LR , Dube, NM , Nattey, C , Manda, S , and Summers, R . Determinants of loss to follow-up in patients on antiretroviral treatment, South Africa, 2004–2012: a cohort study. BMC Health Ser Res. (2015) 15:1–11. doi: 10.1186/s12913-015-0912-2
16. Kebede, HK , Mwanri, L , Ward, P , and Gesesew, HA . Predictors of loss to follow up from antiretroviral therapy among adults in sub-Saharan Africa: a systematic review and meta-analysis. Infect Dis Poverty. (2021) 10:1–18. doi: 10.1186/s40249-021-00822-7
17. Teshale, AT , and Tsegaye, WHF . Incidence and predictors of loss to follow up among adult HIV patients on antiretroviral therapy in University of Gondar Comprehensive Specialized Hospital: a competing risk regression modeling. PLoS. (2020) 15:e0227473. doi: 10.1371/journal.pone.0227473
18. Bantie, B , Seid, A , Kerebeh, G , Alebel, A , and Dessie, G . Loss to follow-up in “test and treat era” and its predictors among HIV-positive adults receiving ART in Northwest Ethiopia: institution-based cohort study. Front Public Health. (2022) 10:876430. doi: 10.3389/fpubh.2022.876430
19. Shiferaw, WS , Belete, AM , Adela, A , Getnet, M , and Aynalem, YA . Incidence and predictors of loss to follow-up among adult HIV-infected patients taking antiretroviral therapy at north Shewa zone public hospitals, Northeast Ethiopia: a retrospective follow-up study. Afr Health Sci. (2022) 22:12–26. doi: 10.4314/ahs.v22i2.3
20. Zeleke, S , Demis, S , Tesfahun, Y , Munye, T , Eshetie, Y , Kefale, D, et al. Incidence and predictors of loss to follow-up among adults on antiretroviral therapy in South Gondar governmental hospitals, Ethiopia: Retrospective Cohort Study. J Multidiscip Healthc. (2023) 16:1737–48. doi: 10.2147/JMDH.S414194
21. Bekolo, CE , Webster, J , Batenganya, M , Sume, GE , and Kollo, B . Trends in mortality and loss to follow-up in HIV care at the Nkongsamba Regional Hospital, Cameroon. J BMC Res Notes. (2013) 6:1–16. doi: 10.1186/1756-0500-6-512
22. Opio, D , Semitala, FC , Kakeeto, A , Sendaula, E , Okimat, P , Nakafeero, B, et al. Loss to follow-up and associated factors among adult people living with HIV at public health facilities in Wakiso district, Uganda: a retrospective cohort study. J BMC Health Ser Res. (2019) 19:1–10. doi: 10.1186/s12913-019-4474-6
23. Tweya, H , Oboho, IK , Gugsa, ST , Phiri, S , Rambiki, E , Banda, R, et al. Loss to follow-up before and after initiation of antiretroviral therapy in HIV facilities in Lilongwe, Malawi. PLoS One. (2018) 13:e0188488. doi: 10.1371/journal.pone.0188488
24. Assemie, MA , Muchie, KF , and Ayele, TA . Incidence and predictors of loss to follow up among HIV-infected adults at Pawi general hospital, Northwest Ethiopia: competing risk regression model. J BMC Res Notes. (2018) 11:287–6. doi: 10.1186/s13104-018-3407-5
25. Gezae, AHT , and Gebretsadik, LG . Incidence and predictors of LTFU among adults with TB/HIV co-infection in two governmental hospitals, Mekelle, Ethiopia, 2009–2016: survival model approach. BMC Infect Dis. (2019) 19:107. doi: 10.1186/s12879-019-3756-2
26. Yigzaw, M , Birhanu, CTL , Alebe, A , Wagnew, F , Siferih, M , Gebre, T, et al. Incidence and predictors of loss to follow-up among HIV-positive adults in Northwest Ethiopia: a retrospective cohort study. Trop Med Health. (2020) 48:78.
27. Dejen, D , Jara, D , Yeshanew, F , Fentaw, Z , Mengie Feleke, T , Girmaw, F, et al. Attrition and its predictors among adults receiving first-line antiretroviral therapy in Woldia town public health facilities, Northeast Ethiopia: a retrospective cohort study. J HIV AIDS Res Palliat Care. (2021) 13:445–54. doi: 10.2147/HIV.S304657
28. Bengura, P , and Managa, MA . Accelerated failure time modelling of tuberculosis predictors in HIV/AIDS patients in Albert Luthuli municipality of South Africa. Res Sq. (2020). doi: 10.21203/rs.3.rs-71793/v1
29. Tiruneh, YM , Galárraga, O , Genberg, B , and Wilson, IB . Retention in care among HIV-infected adults in Ethiopia, 2005–2011: a mixed-methods study. PLoS One. (2016) 11:e0156619. doi: 10.1371/journal.pone
30. Berheto, TM , Haile, DB , and Mohammed, S . Predictors of loss to follow-up in patients living with HIV/AIDS after initiation of antiretroviral therapy. N Am J Med Sci. (2014) 6:453–9. doi: 10.4103/1947-2714.141636
Keywords: adults, incidence, antiretroviral therapy, LTFU, predictors
Citation: Anulo A, Girma A, Tesfaye G, Asefa F, Cheru A and Lonsako AA (2024) Incidence and predictors of loss to follow-up among adult patients receiving antiretroviral therapy in Central Ethiopia: a multi-center retrospective cohort study. Front. Public Health. 12:1374515. doi: 10.3389/fpubh.2024.1374515
Received: 22 January 2024; Accepted: 22 February 2024;
Published: 13 March 2024.
Edited by:
Alberto Cagigi, Nykode Therapeutics ASA, NorwayReviewed by:
Eustachio Cuscianna, University of Bari Aldo Moro, ItalyCopyright © 2024 Anulo, Girma, Tesfaye, Asefa, Cheru and Lonsako. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arega Abebe Lonsako, YXJlZ2FhYmViZTE1N0BnbWFpbC5jb20=; Asfaw Anulo, YXNmYXdhbnVsbzk5NUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.