
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Public Health, 12 April 2024
Sec. Public Mental Health
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1371063
This article is part of the Research TopicNew Insights into Social Isolation and LonelinessView all 11 articles
The experience of loneliness is universal and may have an adverse effect on neurocognitive functioning even at a younger age. Using a comprehensive neurocognitive functioning test (NCFT) battery, we examined the possible negative effects of loneliness on neurocognitive functioning in young adults. The high-loneliness and low-loneliness groups were screened using the UCLA Loneliness Scale v. 3, and measures pertaining to the domains of intelligence, attention, memory, executive function, and psychomotor functioning were tested and compared. As depression and anxiety were significantly higher in the high-loneliness group, an analysis of covariance was conducted. As a result, the high-loneliness group showed significantly poor performance on measures of executive function and attention prior to controlling for depression and anxiety, and executive function retained its significance even after controlling for these variables. Additional analysis showed that depression and anxiety did not significantly mediate the relationship between loneliness and neurocognitive functioning. Such results suggest that loneliness is likely to negatively affect executive functioning and attention in early adulthood and then progressively spread to other domains of cognitive functioning, as reported in the older adult population. The limitations and implications of the present study were considered and addressed.
Positive emotional exchange with others is a source of happiness for most people. However, when this exchange does not sufficiently meet our needs and expectations, we often feel frustrated and lonely. In some cases, loneliness can accompany physiological changes and even somatic symptoms associated with depression (1). However, the impact of loneliness may be more profound, as past studies on the mostly older adult population have consistently reported that its negative effect may even extend to neurocognitive functioning (2). Vulnerability toward loneliness, however, is universal and exposure to chronic loneliness may have an adverse effect on neurocognitive functioning even at a younger age. Accordingly, we examined the possible negative effects of loneliness on neurocognitive functioning in the young adult population.
Loneliness has been found to be a significant risk factor for cognitive decline, such that the severity of loneliness was found to be inversely related to performance on cognitive tests (3). In a prospective study on the older adult population, those with high levels of loneliness showed significantly higher cognitive deficits compared with those with low levels of loneliness (4). Similarly, both chronic and transient loneliness were predictive of the negative consequences for cognitive functioning and the health of the brain in the older adult population (5). The cognitive domains adversely affected by loneliness in the older adult population included memory, attention, language, and executive function (6, 7). Some inconsistencies in the results, nonetheless, are present such that some studies [(e.g., 8)] have reported a bidirectional relationship between loneliness and cognition, while a prospective study by McHugh Power et al. (9) found that attention may affect loneliness but not vice versa. A recent meta-analysis of older adults without dementia (10) found that loneliness was associated with poorer global cognition, episodic memory, working memory, visuospatial function, processing speed, and semantic verbal fluency.
As described above, most past studies on the association between loneliness and neurocognitive functioning deficits have focused mostly on the older adult population. For example, a recent systematic review (11) that examined “the impact of social isolation and loneliness on memory in middle- and older-aged adults” in PubMed, Scopus, and PsycINFO databases until January 2022 found 11 studies whose minimum age of participants was 50 years and only 1 study with the age of the participants being ≥45 years. More recently, a few studies that extended their investigations to include middle-aged populations in their 40s have reported significant findings on the relationship between loneliness and cognitive functioning (12–16). Specifically, loneliness was linked with impairments in executive functioning (16) and memory (12, 13, 15, 16) but not in global cognition, verbal learning, and fluency (12). In this population, persistent loneliness has been found to be associated with smaller parietal, temporal, and hippocampus volumes, which are responsible for memory and executive dysfunction (16). In addition, a higher level of education has been identified as the mediating factor (12, 15) supporting the view that cognitive reserve may serve as a protective factor (17).
In contrast, the effect of loneliness on the neurocognitive functioning of the young adult population has not been extensively examined, even though this age group may be particularly vulnerable to loneliness (18). Loneliness in the younger population is likely to show significant relationships with a narrow band of deficits in neurocognitive functioning compared with the middle-aged counterpart because of the progressive nature of the deficits in cognitive functions (19). A study based on college students has reported the negative effect of loneliness on their social cognition, which caused biased information processing about social relationships and their outcomes (20). It is, however, unclear whether loneliness holds implications for other cognitive functions as found in their middle-aged counterparts.
The negative effect of depression has been examined more extensively in the younger population and may provide some insights since loneliness has been closely linked with depression (21). In general, the domains of attention, verbal memory, visual memory, verbal reasoning/knowledge, and IQ were found to be affected by depression (22). In a recent longitudinal study, depression and anger symptoms were found to be associated with declines in episodic memory and executive functioning (23). Such cognitive domains should be more vulnerable to the adverse effects of loneliness than others in this population, although the relationship between loneliness and depression is likely to be bidirectional (24). However, it should also be mentioned here that some studies have demonstrated that loneliness does not always lead to depression. For example, variables such as self-disgust have been identified to mediate between loneliness, depression, and anxiety (25), and positive coping styles have also been found to alleviate the effect of loneliness on depression (26, 27). Accordingly, in order to delineate the pure effect of loneliness on neurocognitive functioning in a young population, it may be essential to address the effect of depression and anxiety, which may mediate between loneliness and performance on neurocognitive functioning test (NCFT) battery (28, 29).
We, therefore, conducted a preliminary study on the effect of chronic loneliness in a university student population using a comprehensive neurocognitive functioning test battery, which included measures of general IQ, memory, attention, executive functioning, and psychomotor speed. These cognitive domains largely overlap those suggested by the American Psychiatric Association (30) to be considered when assessing cognitive functioning in mental disorders. And since the performance on these cognitive tasks is invariably affected by the emotional state of the subjects (31), we have controlled for depression and anxiety using an ANCOVA. In addition, we also carried out a post-hoc mediational analysis to examine the possible influence of those variables on the association between loneliness and cognitive deficits.
The study was conducted on an initial pool of 365 undergraduate students residing in Gwangju, Korea. The participants completed the initial survey, which included demographic information and psychological scales, including Russell’s UCLA Loneliness Scale v. 3 [RULS v.3: (32, 33)]. Then, 2 months later, they were asked to complete RULS v.3 again. Only those who scored in either the highest or lowest quartiles of this scale at both times were asked to participate in additional NCFT, whereby three people were excluded because they were no longer in the top quartile. As a result, 33 (male = 45%) out of 99 (male = 41%, age = 20.90, SD = 2.29; RULS v.3 mean = 22.09, SD = 11.39, range 4 ~ 24) participants in the lowest quartile and 21 (male = 35%) out of 101 (male = 40%, age = 21.16, SD = 2.66; RULS v.3 mean = 38.24, SD = 7.52, range 27 ~ 57) participants in the highest quartile at the second measurement phase agreed to further procedure, respectively. There was no significant difference in terms of age, education, and gender ratio (χ2 = 0.28, n.s.) between the highest- and the lowest-quartile groups who took the NCFT (see Table 1). As a note, in the lowest quartile, there was no significant difference in the RULS v.3 score between those who agreed and those who refused to participate in the NCF testing (t = 1.66, n.s.), but in the highest quartile, those who agreed to NCF testing had a significantly lower RULS v.3 score than those who refused (t = 2.53*). There was no significant difference between those who agreed and those who refused to participate in age for both the highest quartile (t = −0.12, n.s.) and the lowest quartile (t = 0.83, n.s.), respectively. None of the participants reported being under treatment or medication for any psychiatric problems, and all were right-handed.
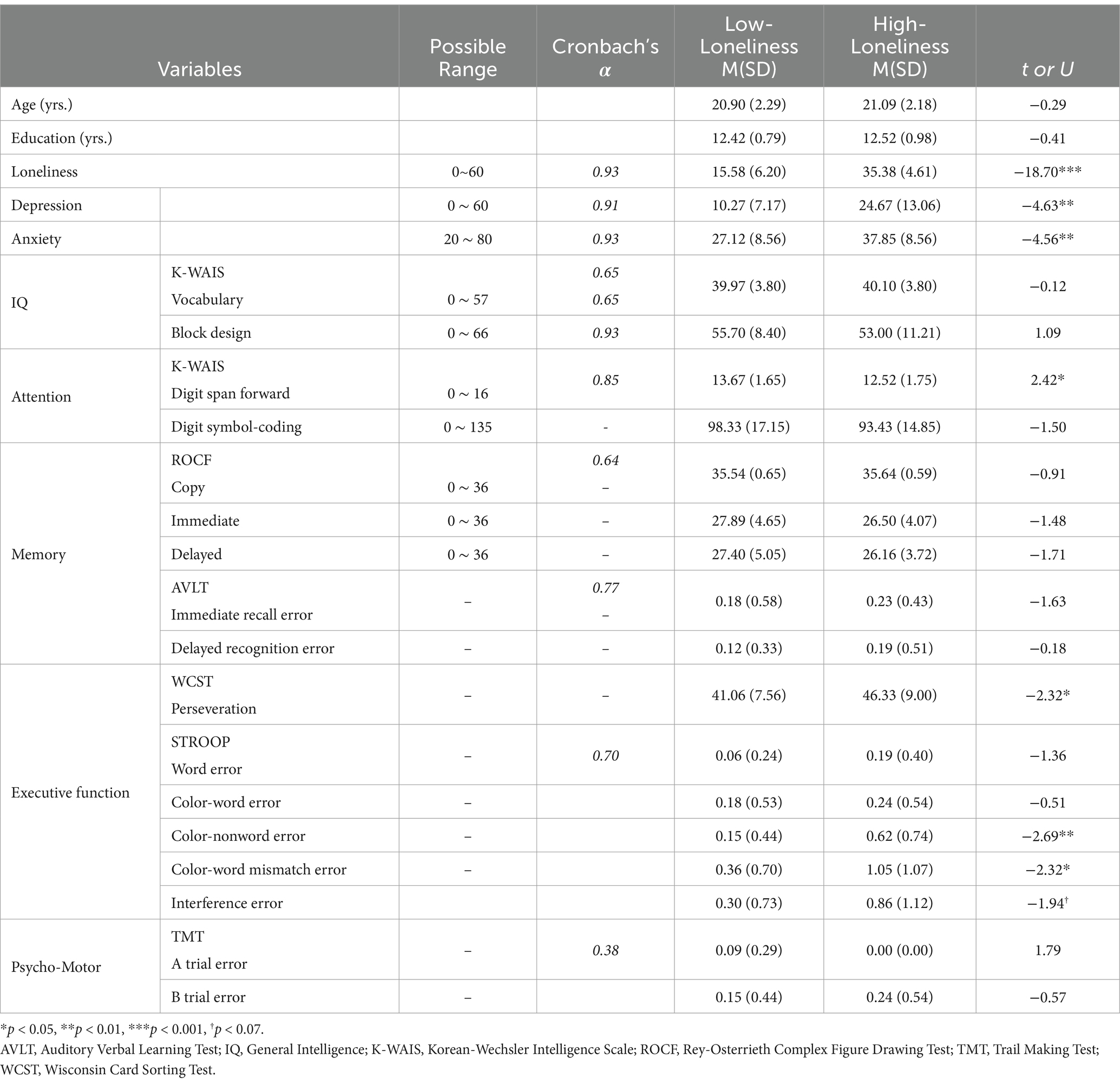
Table 1. Group differences in Neuro-cognitive measures according to independent groups t-test or Mann-Whitney U test.
This study was conducted with the approval of the research ethics committee of Chonnam National University (IRB No.: 1040198-160422-HR-027-03), and all procedures were administered after the participants had signed the written informed consent form.
Each participant first completed self-reported measures of depression and anxiety and subjective loneliness for a preliminary validation study on the RULS v.3 (32, 33). For the measure of depression, the Center for Epidemiologic Studies Depression Scale [CES-D: (34)], consisting of 20 items scored on a 4-point scale with higher scores indicating more severe levels of depression was used. The internal reliability of Cronbach’s α = 0.93 was obtained in our study. Anxiety was measured with the State–Trait Anxiety Inventory-X-2 [STAI-X-2: (35)], composed of 20 items rated on a 4-point scale, with a higher score indicating higher levels of trait anxiety. We obtained Cronbach’s α = 0.91 in our study. Finally, subjective loneliness was measured twice using RULS v.3 in a 2-month interval, whereby Cronbach’s α = 0.95 and α = 0.93 were obtained, respectively. Only the participants with loneliness scores in the top and bottom quartiles in both measurement phases were asked to participate in the neurocognitive testing procedure. Those who agreed to participate were individually tested within 2 weeks using the neurocognitive functioning test battery, which consisted of and was sequenced as follows: (1) Block Design, (2) Auditory Verbal Learning Test [AVLT: (36)], (3) Rey-Osterrieth Complex Figure Drawing Test [ROCF: (37)], (4) Digit Span, (5) Trail Making Test-A and B [TMT-A, B: (38)], (6) Stroop Task (39), (7) Vocabulary, (8) Digit Symbol Coding Test, and (9) Wisconsin Card Sorting Test [WCST: (40)]. Among the test battery, the Block Design, Digit Span, Vocabulary, and Symbol Writing tests were taken from the Korean-Wechsler Intelligence Scale – fourth edition [K-WAIS-IV: (41)]. (For a detailed description of the NCFT battery and its normative data on the outcomes by groups, refer to Supplementary materials 1 and 2, respectively).
First, descriptive statistics were carried out on the performance of each group (Table 1), and the normality of the outcome measures was examined (see Supplementary material 2). For the measures with an abnormal distribution according to the Shapiro–Wilk test (42), we applied the Mann–Whitney U-test. Otherwise, we applied the independent group t-test. Accordingly, we found the high-loneliness group to have significantly higher scores in depression (t = −4.63, p < 0.001) and anxiety (t = −4.56, p < 0.001) than their low-loneliness counterparts (see Table 1). Hence, we further examined the group differences in the normally distributed variables found to be significant by carrying out the analysis of covariance (ANCOVA) controlling for depression, anxiety, and both, respectively. For those that did not meet the assumption of normality, we carried out the non-parametric Quade’s ANCOVA (43). In addition, we carried out a post-hoc mediation analysis to examine the role of depression and anxiety in the association of loneliness and outcome variables that exhibited significant differences between the groups by using Hayes Process Macro (44) for normally distributed variables. As for the variables that did not meet the assumptions of normality, the robust bootstrap test ROBMED for mediation analysis was used (45) since it is less sensitive to deviations from model assumptions such as outliers and heavily tailed distributions. All statistical analyses were carried out with SPSS 28.0 (IBM SPSS, Armonk, NY).
Besides anxiety and depression, we found significant group differences in a number of neurocognitive measures. As shown in Table 1, the high-loneliness group showed poorer performance in the K-WAIS digit span forward trial compared with the low-loneliness counterpart (t = 2.42*), which is related to attentional functioning. The high-loneliness group also showed significantly more WCST perseveration responses (t = −2.32*), Stroop color/non-word trial errors (U = −2.69**), and color/word mismatch trial errors (U = −2.32*). These measures are largely associated with executive functioning.
For the significant variable whose assumption of normality was met (i.e., K-WAIS Digit Span Forward and WCST perseveration), we carried out an ANCOVA controlling for depression and anxiety on the neurocognitive variables. For non-normal measures (i.e., Stroop color-non-word error and color-word mismatch error), we applied Quade’s non-parametric ANCOVA (43). As shown in Table 2, when controlling for depression, WCST perseveration and Stroop color/non-word error maintained their statistical significance. Controlling for anxiety, all variables retained their statistical significance except for the Stroop color/word mismatch error. Finally, when both depression and anxiety were controlled as covariates, WCST perseveration and Stroop color/non-word error still maintained their statistical significance.
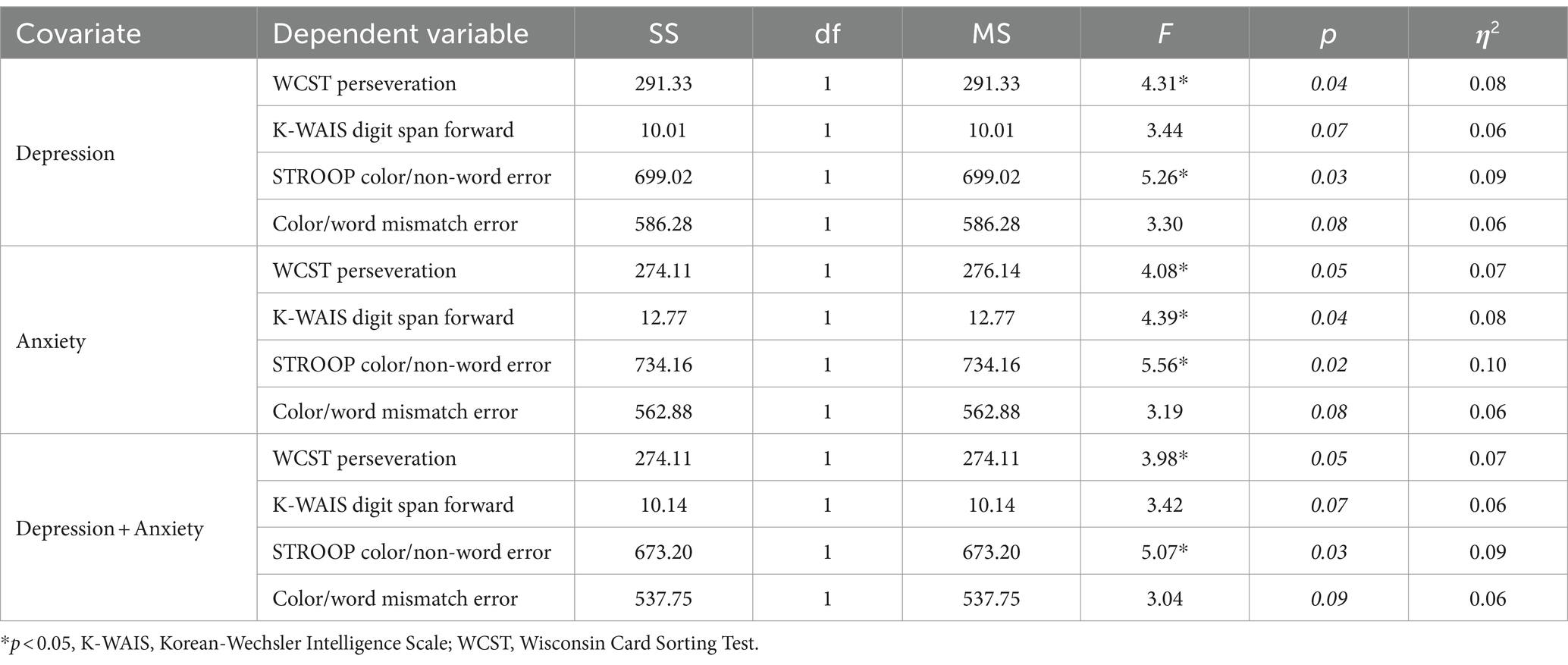
Table 2. Analysis of covariance (ANCOVA) or Quade’s non-parametric ANCOVA on neurocognitive measures controlling for depression and/or anxiety.
As a result of conducting a post-hoc mediation analysis of depression and anxiety on the variables with significant group differences, we did not uncover any significant mediation effects of either variable on the relationships between loneliness and significant neurocognitive measures, respectively.
In this study, we examined whether loneliness may have significant implications on the mental functioning of a young population by using a comprehensive NCF test battery. In the initial analysis, the high-loneliness group showed more severe levels of depression and anxiety as well as poorer performance in measures related to executive functioning and attention, which was in line with previous findings on cognitive decline attributed to loneliness (7, 46). Even when controlling for depression and anxiety as covariates, the high-loneliness group showed significantly poorer performance in tasks related to executive functioning than their low-loneliness counterparts.
The neurocognitive variables that significantly differed between the high- and low-loneliness groups prior to controlling for depression and anxiety were K-WAIS Digit Span forward, WCST perseverative response, Stroop color-non-word error, and color/word mismatch error. These measures involve attentional functioning, which has also been reported to show deficits in depression (47).
The high-loneliness group showed significantly poorer performance in the WCST perseverative response and Stroop color-non-word error, even when depression and anxiety were controlled as covariates. The perseverative response in WCST reflects difficulty in set-shifting or an inability to recognize changes in the selection rule. The Stroop test, on the other hand, generally reflects accuracy in the processing of mismatching cues and controlled behavioral inhibition. The reason for the color/word mismatch error losing its statistical significance when controlled for depression and/or anxiety can be attributed to the limited sample and design of the study, besides the presence of their negative effects on performance. Our overall results suggest that young people high in loneliness may be more vulnerable to problems related to impulsive and addictive behaviors (48).
While we included measures of IQ, memory, and psychomotor functioning in our test battery, we did not obtain any significant group differences in these measures. Hence, it can be suggested that the negative impact of loneliness on cognitive functioning during early adulthood may begin with executive functioning and attention and then progressively spread to other domains of cognitive functioning, as reported in the older adult populations (6, 7). Future studies should apply more comprehensive measures of executive functioning and attention to various age groups to confirm our results.
In addition, we have controlled for depression and anxiety through the ANCOVA and carried out a separate mediational analysis on the effects of both variables on the association between loneliness and neurocognitive functioning, using non-parametric tests where appropriate. The results consistently confirmed that loneliness has a direct effect on the measures of executive functioning and attention, although the lack of a significant mediational effect of depression and anxiety should be confirmed in future research with a larger sample. Furthermore, studies to identify possible mediating variables between loneliness and neurocognitive functioning deficits may provide valuable implications for interventions to alleviate the negative effects of loneliness on cognitive functioning in the young adult population.
Finally, our study was one of the first investigations into the link between loneliness and cognitive functioning in a relatively young population using a comprehensive neurocognitive functioning test battery. Nonetheless, there are a few limitations of this preliminary study that should be considered when interpreting the results. First, our results should be confirmed using larger samples of different age groups and demographic backgrounds to ensure generalizability. Second, this study is cross-sectional in design, so caution should be taken when inferring causality between loneliness and neurocognitive functioning until further longitudinal studies have been conducted. Third, the measure of loneliness that we used is largely a subjective measure; hence, more objective measures of social isolation should be applied in future studies to validate our results. Finally, some measures in our battery may be overlapped and reflect more than one functional domain, e.g., the K-WAIS Digit Symbol Coding task may reflect both psychomotor speed and visual working memory. Future studies should aim to apply more refined measures of neurocognitive functioning to confirm and expand our results.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the research Ethics Committee of Chonnam National University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
EJ: Data curation, Formal analysis, Investigation, Writing – original draft. SH: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The data and part of the results were obtained from the master’s thesis (54) by the first author under the supervision by the second. No part of the thesis has been published elsewhere.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is ++not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1371063/full#supplementary-material
1. House, JS, Landis, KR, and Umberson, D. Social relationships and health. Science. (1988) 241:540–5. doi: 10.1126/science.3399889
2. Cacioppo, S, Balogh, S, and Cacioppo, JT. Implicit attention to negative social, in contrast to nonsocial, words in the Stroop task differs between individuals high and low in loneliness: evidence from event-related brain microstates. Cortex. (2015) 70:213–33. doi: 10.1016/j.cortex.2015.05.032
3. Wilson, RS, Krueger, KR, Arnold, SE, Schneider, JA, Kelly, JF, Barnes, LL, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. (2007) 64:234–40. doi: 10.1001/archpsyc.64.2.234
4. Tilvis, RS, Kähönen-Väre, MH, Jolkkonen, J, Valvanne, J, Pitkala, KH, and Strandberg, TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol Ser A Biol Med Sci. (2004) 59:M268–74. doi: 10.1093/gerona/59.3.m268
5. Zhong, B-L, Chen, S-L, and Conwell, Y. Effects of transient versus chronic loneliness on cognitive function in older adults: findings from the Chinese longitudinal healthy longevity survey. Am J Geriatr Psychiatry. (2016) 24:389–98. doi: 10.1016/j.jagp.2015.12.009
6. Cacioppo, JT, and Cacioppo, S. Social relationships and health: the toxic effects of perceived social isolation. Soc Personal Psychol Compass. (2014) 8:58–72. doi: 10.1111/spc3.12087
7. DiNapoli, EA, Wu, B, and Scogin, F. Social isolation and cognitive function in Appalachian older adults. Res Aging. (2014) 36:161–79. doi: 10.1177/0164027512470704
8. Yin, J, Lassale, C, Steptoe, A, and Cadar, D. Exploring the bidirectional associations between loneliness and cognitive functioning over 10 years: the English longitudinal study of ageing. Int J Epidemiol. (2019) 48:1937–48. doi: 10.1093/ije/dyz085
9. McHugh Power, JE, Hannigan, C, Carney, S, Feeney, J, Kenny, RA, Kee, F, et al. Lonely SARTs: loneliness and sustained attention in the Irish longitudinal study of aging. Aging Neuropsychol Cognit. (2019) 27:197–06. doi: 10.1080/13825585.2019.1602705
10. Harrington, KD, Vasan, S, Kang, JE, Sliwinski, MJ, and Lim, MH. Loneliness and cognitive function in older adults without dementia: a systematic review and meta-analysis. Journal of Alzheimers Disorder. (2023) 91:1243–59. doi: 10.3233/JAD-220832
11. Kang, JW, and Oremus, M. Examining the combined effects of social isolation and loneliness on memory: a systematic review. Arch Gerontol Geriatr. (2023) 104:104801. doi: 10.1016/j.archger.2022.104801
12. Estrella, M, Tarraf, W, Wu, B, Gallo, L, Marquine, M, Perreira, K, et al. Psychosocial factors associated with changes in cognition among middle-aged and older Hispanics/Latinos: findings from the HCHS/SOL and the sociocultural and SOL-investigation of neurocognitive aging (SOL-INCA) ancillary studies. Alzheimers Dement. (2021) 17 (Suppl. 10). doi: 10.1002/alz.056157
13. Estrella, ML, Tarraf, W, Kuwayama, S, Gallo, LC, Wu, B, Marquine, MJ, et al. Psychosocial factors associated with 7-year change in cognition among middle-aged and older Hispanics/Latinos: the Hispanic community health study/study of Latinos-investigation of neurocognitive aging (SOL-INCA) and sociocultural ancillary studies. Alzheimers Dement. (2023) 20:1137–48. doi: 10.1002/alz.13527
14. Hajek, A, Riedel-Heller, SG, and König, H. Perceived social isolation and cognitive functioning. Longitudinal findings based on the German ageing survey. Int J Geriatr Psychiatry. (2020) 35:276–81. doi: 10.1002/gps.5243
15. Kyröläinen, A-J, and Kuperman, V. The effect of loneliness on cognitive functioning among healthy individuals in mid- and late-adulthood: evidence from the Canadian longitudinal study on aging (CLSA). Front Psychol. (2021) 12:701305. doi: 10.3389/fpsyg.2021.701305
16. Tao, Q, Akhter-Khan, SC, Ang, TFA, DeCarli, C, Alosco, ML, Mez, J, et al. Different loneliness types, cognitive function, and brain structure in midlife: findings from the Framingham heart study. eClinicalMedicine. (2022) 53:101643. doi: 10.1016/j.eclinm.2022.101643
17. Shankar, A, Hamer, M, McMunn, A, and Steptoe, A. Social isolation and loneliness: relationships with cognitive function during 4 years of follow-up in the English longitudinal study of ageing. Psychosom Med. (2013) 75:161–70. doi: 10.1097/PSY.0b013e31827f09cd
18. Luhmann, M, and Hawkley, LC. Age differences in loneliness from late adolescence to oldest old age. Dev Psychol. (2016) 52:943–59. doi: 10.1037/dev0000117
19. Boyle, PA, Yu, L, Wilson, RS, Leurgans, SE, Schneider, JA, and Bennett, DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. (2018) 83:74–83. doi: 10.1002/ana.25123
20. Lodder, GM, Scholte, RH, Clemens, IA, Engels, RC, Goossens, L, and Verhagen, M. Loneliness and hypervigilance to social cues in females: an eye-tracking study. PLoS One. (2015) 10:1–17. doi: 10.1371/journal.pone.0125141
21. Cacioppo, JT, Hawkley, LC, and Thisted, RA. Perceived social isolation makes me sad: five year crosslagged analyses of loneliness and depressive symptomatology in the Chicago health, aging, and social relations study. Psychol Aging. (2010) 25:453–63. doi: 10.1037/a0017216
22. Goodall, J, Fisher, C, Hetrick, S, Phillips, L, Parrish, EM, and Allott, K. Neurocognitive functioning in depressed young people: a systematic review and meta-analysis. Neuropsychol Rev. (2018) 28:216–31. doi: 10.1007/s11065-018-9373-9
23. Lindert, J, Paul, KC, Lachman, ME, Ritz, B, and Seeman, TE. Depression-, anxiety-, and anger and cognitive functions: findings from a longitudinal prospective study. Front Psych. (2021) 12:665742. doi: 10.3389/fpsyt.2021.665742
24. Luo, M. Social isolation, ioneliness, and depressive symptoms: a twelve-year population study of temporal dynamics. J Gerontol B Psychol Sci Soc Sci. (2023) 78:280–90. doi: 10.1093/geronb/gbac174
25. Ypsilanti, A, Lazuras, L, Powell, P, and Overton, P. Self-disgust as a potential mechanism explaining the association between loneliness and depression. J Affect Disord. (2019) 243:108–15. doi: 10.1016/j.jad.2018.09.056
26. Zhang, X, Lv, L, Min, G, Wang, Q, Zhao, Y, and Li, Y. Overview of the complex figure test and its clinical application in neuropsychiatric disorders, including copying and recall. Front Neurol. (2021) 12:680474. doi: 10.3389/fneur.2021.680474
27. Zhang, Y, Huang, L, Luo, Y, and Ai, H. The relationship between state loneliness and depression among youths during COVID-19 lockdown: coping style as mediator. Front Psychol. (2021) 12:514. doi: 10.3389/fpsyg.2021.701514
28. Cardona, M, and Andrés, P. Are social isolation and loneliness associated with cognitive decline in ageing? Front Aging Neurosci. (2023) 15:1075563. doi: 10.3389/fnagi.2023.1075563
29. Suddell, S, Mahedy, L, Skirrow, C, Penton-Voak, IS, Munafò, MR, and Wootton, RE. Cognitive functioning in anxiety and depression: results from the ALSPAC cohort. R Soc Open Sci. (2023) 10:221161. doi: 10.1098/rsos.221161
30. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC, London, England: American Psychiatric Association (2013).
31. Gatz, JL, Tyas, SL, St John, P, and Montgomery, P. Do depressive symptoms predict Alzheimer's disease and dementia? J Gerontol Ser A. (2005) 60:744–7. doi: 10.1093/gerona/60.6.744
32. Jin, E, and Hwang, SS. The validity of the Korean-UCLA loneliness scale version 3. Kor J Youth Stud. (2019) 26:53–80. doi: 10.21509/KJYS.2019.10.26.10.53
33. Russell, DW. UCLA loneliness scale (version 3): reliability, validity, and factor structure. J Pers Assess. (1996) 66:20–40. doi: 10.1207/s15327752jpa6601_2
34. McDowell, I, and Newell, C. “The Center for Epidemiologic Studies Depression Scale (CES-D)” Measuring Health: A Guide to Rating Scales and Questionnaires. (1996) New York: Oxford University Press. 254–258.
35. Spielberger, CD, Edwards, C, Mantoun, J, and Lushene, R. The State-trait anxiety inventory Windsor. UK: NFER-Nelson. (1987).
36. Cheong, SS, Woo, JM, Kim, E, Yeon, BK, and Hong, KS. Development of Korean auditory verbal learning test. J Korean Neuropsychiatr Assoc. (1999) 38:1016–25.
38. Lezak, MD, Howieson, DB, Bigler, ED, and Tranel, D. Neuropsychological assessment. 5th. ed. New York: Oxford University Press (2012).
39. Stroop, JR. Studies of interference in serial verbal reactions. J Exp Psychol. (1935) 18:643–62. doi: 10.1037/h0054651
40. Grant, DA, and Berg, EA. Wisconsin card sorting test J Exp Psychol. (1993) 38, 404. doi: 10.1037/t31298-000
41. Choe, AY, Hwang, S, Kim, JH, Park, K, Chey, J, and Hong, SH. Validity of the K-WAIS-IV short forms. Korean J Clin Psychol. (2014) 33:413–28. doi: 10.15842/kjcp.2014.33.2.011
42. Mishra, P, Pandey, CM, Singh, U, Gupta, A, Sahu, C, and Keshri, A. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth. (2019) 22:67–72. doi: 10.4103/aca.ACA_157_18
43. Quade, D. Rank analysis of covariance. J Am Stat Assoc. (1967) 62:1187–00. doi: 10.1080/01621459.1967.10500925
44. Hayes, AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York, London: Guilford Press (2013).
45. Alfons, A, Ates, NY, and Groenen, PJF. A robust bootstrap test for mediation analysis. Organ Res Methods. (2022) 25:591–617. doi: 10.1177/1094428121999096
46. Yu, J, Lam, CL, and Lee, TM. Perceived loneliness among older adults with mild cognitive impairment. Int Psychogeriatr. (2016) 28:1681–5. doi: 10.1017/S1041610216000430
47. Wang, X, Zhou, H, and Zhu, X. Attention deficits in adults with major depressivedisorder: a systematic review and meta-analysis. Asian J Psychiatr. (2020) 53:102359. doi: 10.1016/j.ajp.2020.102359
48. Li, W, Zhang, W, Xiao, L, and Nie, J. The association of internet addiction symptoms with impulsiveness, loneliness, novelty seeking and behavioral inhibition system among adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. (2016) 243:357–64. doi: 10.1016/j.psychres.2016.02.020
49. Jensen, AR. Vocabulary and general intelligence. Behav Brain Sci. (2001) 24:1109–10. doi: 10.1017/S0140525X01280133
50. Montoliu, T, Hidalgo, V, and Salvador, A. The relationship between loneliness and cognition in healthy older men and women: the role of cortisol. Psychoneuroendocrinology. (2019) 107:270–9. doi: 10.1016/j.psyneuen.2019.05.024
51. Negri, A, Castiglioni, M, Caldiroli, CL, and Barazzetti, A. Language and intelligence: a relationship supporting the embodied cognition hypothesis. J Intelligence. (2022) 10:42. doi: 10.3390/jintelligence10030042
52. Schmidt, M. Rey auditory and verbal learning test. A handbook. Los Angeles: Western Psychological Association (1996).
53. Tombaugh, TN. Trail making test a and B: normative data stratified by age and education. Arch Clin Neuropsychol. (2004) 19:203–14. doi: 10.1016/S0887-6177(03)00039-8
54. Jin, E. The validity of the UCLA Loneliness Scale version 3 and the effects of loneliness on neurocognitive functions in university students [Master’s Thesis, Chonnam National University]. (2017). Available at: https://dcollection.jnu.ac.kr/public_resource/pdf/000000057433_20240327005620.pdf
Keywords: loneliness, neurocognitive functioning, executive function, attention, depression, young adults
Citation: Jin E and Hwang SS-H (2024) A preliminary study on the neurocognitive deficits associated with loneliness in young adults. Front. Public Health. 12:1371063. doi: 10.3389/fpubh.2024.1371063
Received: 15 January 2024; Accepted: 01 March 2024;
Published: 12 April 2024.
Edited by:
Yuka Kotozaki, Iwate Medical University, JapanReviewed by:
Corine S. M. Wong, The University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2024 Jin and Hwang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel Suk-Hyun Hwang, aHdhbnNhbWFAaGFubWFpbC5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.