- 1Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 2Department of Laboratory Sciences, Bahir Dar Health Sciences College, Bahir Dar, Ethiopia
Background: Food-borne infections continue to be a major public health problem at the international level. The issue becomes more serious in developing countries like Ethiopia.
Objective: This study aimed to examine the prevalence of Salmonella and Shigella species and intestinal parasites, as well as antimicrobial resistance patterns and associated factors among food handlers at the University of Gondar cafeteria in northwest Ethiopia.
Methods: An institutional-based cross-sectional study was conducted from February to June 2021 in the University of Gondar cafeterias. Data related to the socio-demographic characteristics and hygienic practices of study participants were collected using structured questionnaires. A total of 290 stool samples were collected from food handlers. Culture and conventional biochemical tests were used to isolate the Salmonella and the Shigella species. Wet mount, Formol-ether concentration, and Kato Katz techniques were applied to identify intestinal parasites. Additionally, drug susceptibility tests were performed using the disk diffusion method. Statistical analysis was done using SPSS version 26.
Results: Of 290 food handlers’ stool samples analyzed, Twenty-seven 27 (9.3%) were positive for both Salmonella and Shigella species. The prevalence of Salmonella and Shigella species was 16 (5.5%) and 11 (3.8%), respectively. Most of the isolated pathogens were resistant to tetracycline 19 (70.4%), and trimethoprim/sulphamethoxazole 19 (70.4%). The overall rate of multi-drug resistant Shigella and Salmonella isolate was 59.3%. Besides, Fifty-seven 57 (19.7%) of the participants were positive for one or more intestinal parasites. The most prevalent intestinal Parasitosis was E. histolytica/dispar 22 (7.6%), followed by G. lamblia 13 (4.5%), and Ascaris lumbricoides 11 (3.8) not washing hands after using the toilet (AOR: 4.42, 95% CI: 1.57, 10.56), and consuming unpasteurized milk (AOR: 3.14, 95% CI: 1.65, 3.96), were factors significantly associated with the prevalence of Salmonella, and Shigella infection. Similarly, not washing hands after using the toilet (AOR: 2.19, 95% CI: 1.0, 1.4), and consuming unpasteurized milk (AOR: 10.4, 95% CI: 3.8, 28.8), were factors significantly associated with the prevalence of intestinal parasites infection.
Conclusion: The prevalence of intestinal parasites, Salmonella, and Shigella species was high. Therefore, it is imperative to implement a public health policy that includes ongoing microbiological surveillance.
Introduction
Food-borne diseases (FBD) refer to illnesses caused by consuming pathogenic microorganisms, such as bacteria, fungus, viruses, and parasites, or their toxins in the case of bacteria and fungi (1–3). Additionally, FBD is caused by unprotected food handling and processing, and poor Sanitary conditions (4). It has recently become a worldwide and local health problem. Every year, two million deaths from food-borne illnesses are reported, affecting around one-third of the world’s population (5). Across both developed and developing countries, it is a major public health concern (5). The World Health Organization (WHO) states that 600 million people worldwide become severely ill each year, with 420,000 dying as a result of food contamination. FBD affects an estimated 48 million people in the United States each year, resulting in 128,000 hospitalizations and 3,000 fatalities (6, 7). Food-borne and waterborne infections induce diseases that are both short-term (such as nausea, vomiting, and diarrhea) and long-term (such as cancer, kidney or liver failure, tissue damage, brain disorders, and neurological abnormalities) and are also projected to cause approximately 700,000 deaths each year in Africa (8).
Gastrointestinal parasitic infections are prevalent in both developed and developing countries, with the largest frequency in less developed countries as a result of socioeconomic, demographic, and health-related behaviors, as well as inadequate personal hygiene and environmental sanitation (9). The most common way for intestinal parasitic infections to spread is through contaminated food and water, but they can be further transmitted from person to person through feco-oral contact (10). Nearly one-third of the populations in affluent countries suffer intestinal infections caused by parasites (11). Ascaris lumbricoides, Trichuris trichiura, hookworm, Entamoeba histolytica, and Giardia lamblia infect an estimated 1.2 billion, 795 million, 740 million, 500 million, and 2.8 million people worldwide, respectively (12, 13). In Ethiopia, the burden of intestinal parasites (IPs) is extremely high. A third (26 million), a quarter (21 million), and one in every eight (11 million) Ethiopians are infected with Ascaris lumbricoides, Trichuris trichiura, and hookworm, respectively (14–16). As a result, Ethiopia has the second, third, and fourth largest burdens of ascariasis, hookworm, and trichuriasis, in sub-Saharan Africa, respectively (17).
Food-borne disease is also caused by enteric bacterial infections (EBIs), including those caused by the genus Shigella (which causes Shigellosis) and Salmonella (which causes Salmonellosis). They thus remain important public health concerns. Moreover, vaccinations do not produce immunity in young infants, and the development of antibiotic resistance makes clinical prevention and control of typhoid fever challenging, especially in Africa (18). Every year, Salmonella species cause 93.8 million episodes of gastroenteritis worldwide, resulting in 155,000 fatalities. Of these instances, 80.3 million were thought to be food-borne (19). Shigella species are more common in temperate and tropical areas. Shigella species cause an estimated 80–165 million cases of disease and 600,000 deaths worldwide each year (20). Salmonella and Shigella species resistant to commonly prescribed antibiotics are a threat to children and the general community (21). Ethiopia has been reported to exhibit considerable rates of resistance for Shigella and Salmonella species to tetracycline (52.5, 82.4%), trimethoprim/sulphamethoxazole (37.5, 76.5%), and ampicillin (60, 47.1%), respectively (22).
Food handlers are those who work in the food preparation and serving industry. If they have bacterial or parasite diseases in their gastrointestinal tract and maintain poor personal hygiene, they may pose a major risk of spreading IPs and EBIs to the general public. Asymptomatic food handlers are known to play an essential role in the spread of infections and continue to pose a hazard to the public’s health (23–26). Previous research has shown that food prepared in large quantities by a variety of food handlers at higher education institutions is frequently prone to contamination by infectious microorganisms or asymptomatic carriers, which can cause outbreaks of food-borne diseases (24, 25). Furthermore, due to insufficient laboratory infrastructure that impedes effective detection and antibiotic susceptibility testing, Shigella, and Salmonella species isolation in most African laboratories, including Ethiopia, continues to be difficult (27). As a result, there is a dearth of current information on Shigella, and Salmonella species, and their patterns of antibiotic susceptibility, as well as the prevalence of intestinal parasites among Ethiopian food handlers. Therefore, the purpose of this study was to evaluate the frequency of intestinal parasites, Shigella, and Salmonella species in the University of Gondar cafeteria, as well as the patterns of antimicrobial resistance of the isolates along with associated factors.
Methods and materials
Study setting and period
An institutional-based cross-sectional study was carried out among food handlers working at the University of Gondar Hospital and Student Cafeteria, northwest, Ethiopia from February to June 2021. The University of Gondar is located in Gondar at a distance of 175 from Bahir Dar and 740 km from Addis Ababa. Currently, the University of Gondar Hospital and Student Cafeteria serve meals to over 37,000 students and staff.
Study population, sample size, and sampling technique
The source populations were all food handlers at the University of Gondar Hospital and the student Cafeteria, handled food. The study populations were food handlers who worked in the cafeteria at the University of Gondar, were available at the time of data collection, and gave their consent. Food handlers who used antibiotics and/or anthelminthic drugs and were not have willingness to give stool samples were also excluded during sample collection.
The sample size was determined by using a single population proportion formula considering the following assumptions: Zα/2 = 1.96 for the standard scale of 95% level of confidence, level of precision (d) = 3%, p = expected prevalence don by Mama and Alemu (6.9%) (28).
With a 5% non-response rate = 290. As a result, 290 food handlers from all campus cafeterias were included in the study. A simple random sampling method was used to select the study participants. A stratified random sampling technique was used to recruit 290 study participants from the sampling frame of food handlers The number of food handlers chosen from each cafeteria was as follows: Hospital (n = 120), College of Medicine and Health Science campus (n = 108), Atse tewodros campus (n = 138), Maraki campus (n = 186), Fasile campus (n = 52), and Tseda campus (n = 45). To select representative participants, the final sample size was proportionally allocated to each stratum using the formula donated below. Where n = total sample size to be selected, N = total population, Ni = total population of each stratum, and ni = sample size from each strata. Then the final sample size chosen from each cafeteria from the hospital, College of Medicine and Health Science campus, Atse Tewodros campus, Maraki campus, Fasile campus, and Tseda campus were 54, 48, 83, 62, 23, and 20 food handlers, respectively.
Data collection methods and tools
Socio-demographic data collection
Using a standardized questionnaire, data regarding socio-demographic characteristics and associated factors such as gender, age, education level, marital status, service years, medical checkups, the practice of hand washing after the toilet, hand washing using soap, fingernail status, wearing hair cover, the practice of drinking unpasteurized milk, eating raw meat, and eating raw vegetables were collected. The questionnaires’ validity was checked for accuracy and completeness by conducting a pretest at Wogera prison center.
Specimen collection, handling, and transportation
Using a labeled, clean, wide-mouth plastic container and a clean, wooden applicator stick, 3 g of fresh stool samples were collected from food handlers per the recommended procedures (28). Then the samples were subsequently preserved in Cary Blair media (for culture) and a 10% (V/V) formalin solution (for wet mount and parasite concentration) was used. The samples were then promptly transported to the laboratory using a sample carrier.
Specimen processing and examination
Parasite identification
To identify intestinal protozoa, each sample was immediately analyzed with normal saline (0.85% NaCl) and Lugol’s iodine. A Formol-ether concentration method and Kato Katz methods were employed to enhance the detection of intestinal parasites (28).
Isolation and identification of Shigella and Salmonella species
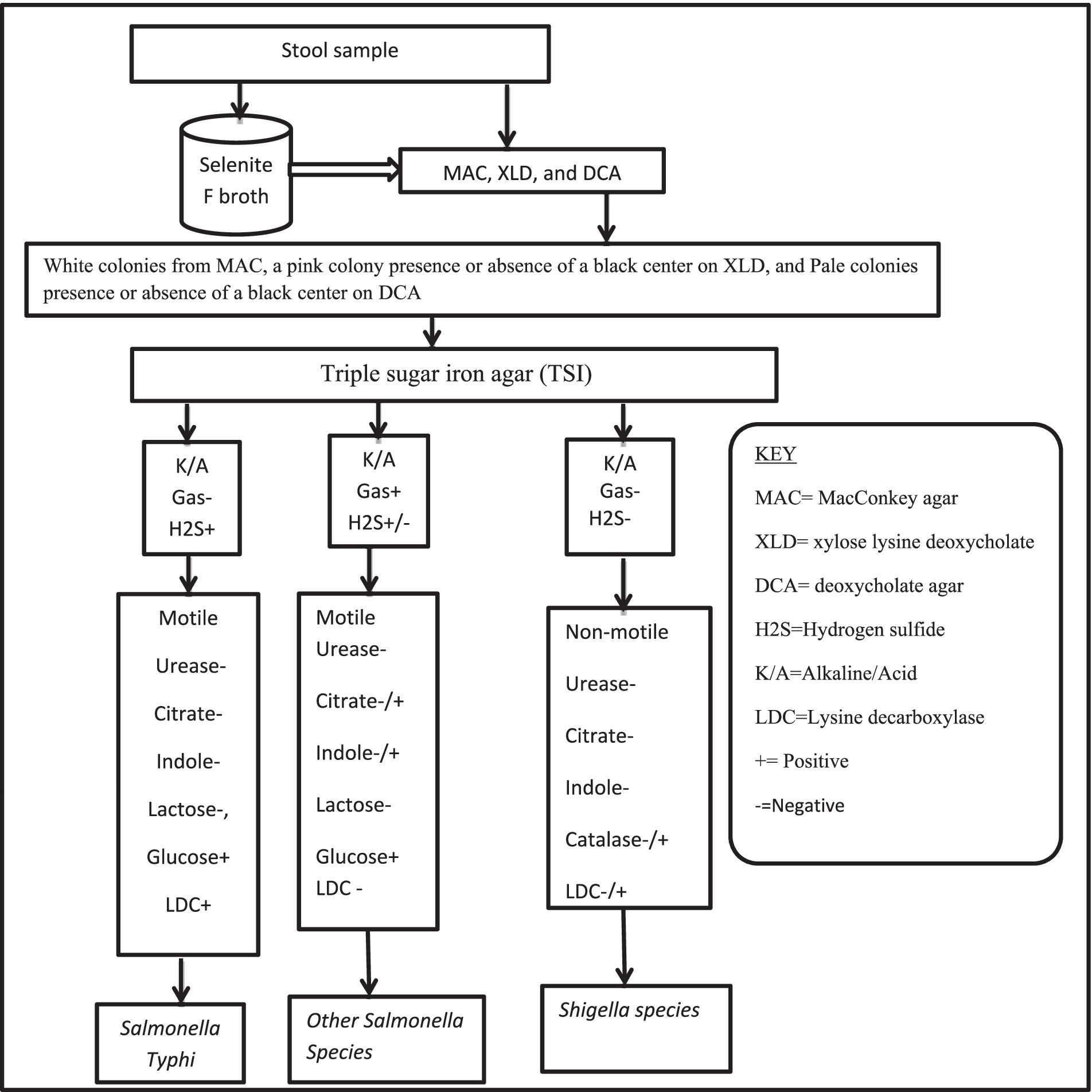
Using the standardized technique, Salmonella, and Shigella species were characterized and isolated (29). To enrich the bacteria, one milliliter of a stool sample was mixed with nine milliliters of Selenite F broth after being transferred from the Cary Blair medium (Oxoid, Ltd. UK) and incubated at 37°C for 24 h. MacConkey agar (21) (Oxoid, Ltd., UK), deoxycholate agar (DCA), and xylose lysine deoxycholate (XLD) agar (Oxoid, Ltd., UK) were used to subculture an inoculum from selenite F broth. After 24 h of incubation at 37°C, the growth of Salmonella and Shigella species was differentiated by their colony characteristic appearance on DCA agar (Shigella species shows pale colonies in color, Salmonella species black center pale color colonies) and XLD agar (Shigella has red color colonies, Salmonella species red with a presence of black color center). Discreet pure colonies with a characteristic of Salmonella- and/or Shigella-like species were further inoculated into standard biochemical tests such as Triple Sugar Iron Agar (TSI) (Oxoid, Ltd., UK), Sulfide Indole Motility Agar (SIM) (Oxoid, Ltd., UK), Urea Agar (Oxoid, Ltd., UK), Simmons Citrate Agar (Oxoid, Ltd., UK), and Lysine iron Agar (Oxoid, Ltd., UK) for species identification. Subsequently, according to the established technique, the results of the biochemical test results consistent with Salmonella and/or Shigella species were reported (Figure 1) (30, 31). Then discreet pure colonies presence or absence of black centered on DCA or XLD were picked and suspended in sterile normal saline (0.85% NaCl) for an antimicrobial susceptibility test (AST).
Antimicrobial susceptibility testing
Once the bacteria had been identified, the isolates’ ASTs were carried out using a modified Kirby-Bauer disk diffusion technique following the recommendation of the Clinical and Laboratory Standard Institute (CLSI), 2020. The bacterial suspension was prepared by emulsifying pure colonies from a young culture in 0.85% sterile normal saline. This suspension was then compared to 0.5 McFarland turbidity standards (32). Then, using the lawn culture method, the bacterial suspension was inoculated onto Muller-Hinton agar (MHA, Oxoid, Ltd., UK). The following antibiotics disks were used as Amoxicillin/clavulanic acid (20/10 μg), Cefotaxime (30 μg), Ceftazidime (30 μg), Ceftriaxone (30 μg), and Cefoxitin (30 μg), Gentamicin (10 μg), Meropenem (10 μg), Tetracycline (30 μg), Ciprofloxacin (30 μg), Chloramphenicol (30 μg), and Trimethoprim/Sulphamethoxazole (5 μg) (BD USA). After that, the plates were incubated for 24 h at 37°C. Following overnight incubation, the zone of inhibition was measured and classified according to CLSI, 2020 as sensitive, intermediate, or resistant (33). The isolates’ multi-drug resistance patterns were categorized using the standards established by Magiorakos et al. (34).
Quality control
Before data collection, the questionnaire was pretested and the data collectors were given training to guarantee the accuracy of the data. The collected data was reviewed daily for consistency and accuracy. Participants were given instructions on how to appropriately collect samples. Additionally, we checked the culture media, antibiotic discs, and other reagents’ expiration dates ahead of time. By inoculating well-known strains E. coli American type culture collection (ATCC) 25922, S. typhimurium ATCC 14028 (35), and S. flexnerii ATCC 12022 (36), the culture media’s quality was evaluated. Every laboratory procedure was carried out per standard operating protocols (37).
Statistical analysis
At first, all data were coded and reviewed for accuracy. After coding, the data were entered using EPI-info version 7. For further analysis, the data was transferred to SPSS version 26. For categorical variables, frequency distributions and percentages were calculated. The socio-demographic factors and the prevalence of Salmonella, Shigella, or intestinal parasites among the study participants were analyzed using Bivariable logistic regression. To determine factors that are statistically significantly associated with the presence of Salmonella, Shigella, or intestinal parasite infections, variables with p ≤ 0.2 in the Bivariable logistic regression analysis have proceeded to the multivariable logistic regression analysis. A p-value of <0.05 was considered statistically significant at a 95% confidence level.
Ethical consideration
Ethical clearance was obtained from the Ethical Review Committee of the School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. A support letter was obtained from the Department of Medical Microbiology at the University of Gondar with reference number SBLS/02/25/2021. Written informed consent was obtained from each study participant. Strict confidentiality was maintained during the interview process and anonymity was kept during data processing and report writing. For the proper parasitic and antimicrobial treatments, food handlers who tested positive for enteric infections (bacterial and parasite) were linked healthcare facility at the University of Gondar.
Results
Socio-demographic characteristics of the study participants
In this study, 290 food handlers participated with a 100% response rate. The average age of the study participants was 32.1 years (standard deviation: ±8.7 years), with the majority of them 240 (82.8%) falling between the ages of 20 and 40. The majority 242 (76.9%) of these were females. Regarding educational status, 135 (46.6%) completed secondary school and above. Of the total respondents, 116 (40%), do not have a practice of hand washing after the toilet. 181 (62.4%) of the total respondents had medical checks. Approximately over 71 (24.5%) of the participants in the study had a practice of consuming unpasteurized milk, while 59 (20.3%) and 148 (51%) regularly consumed raw meat and vegetables, respectively (Table 1).
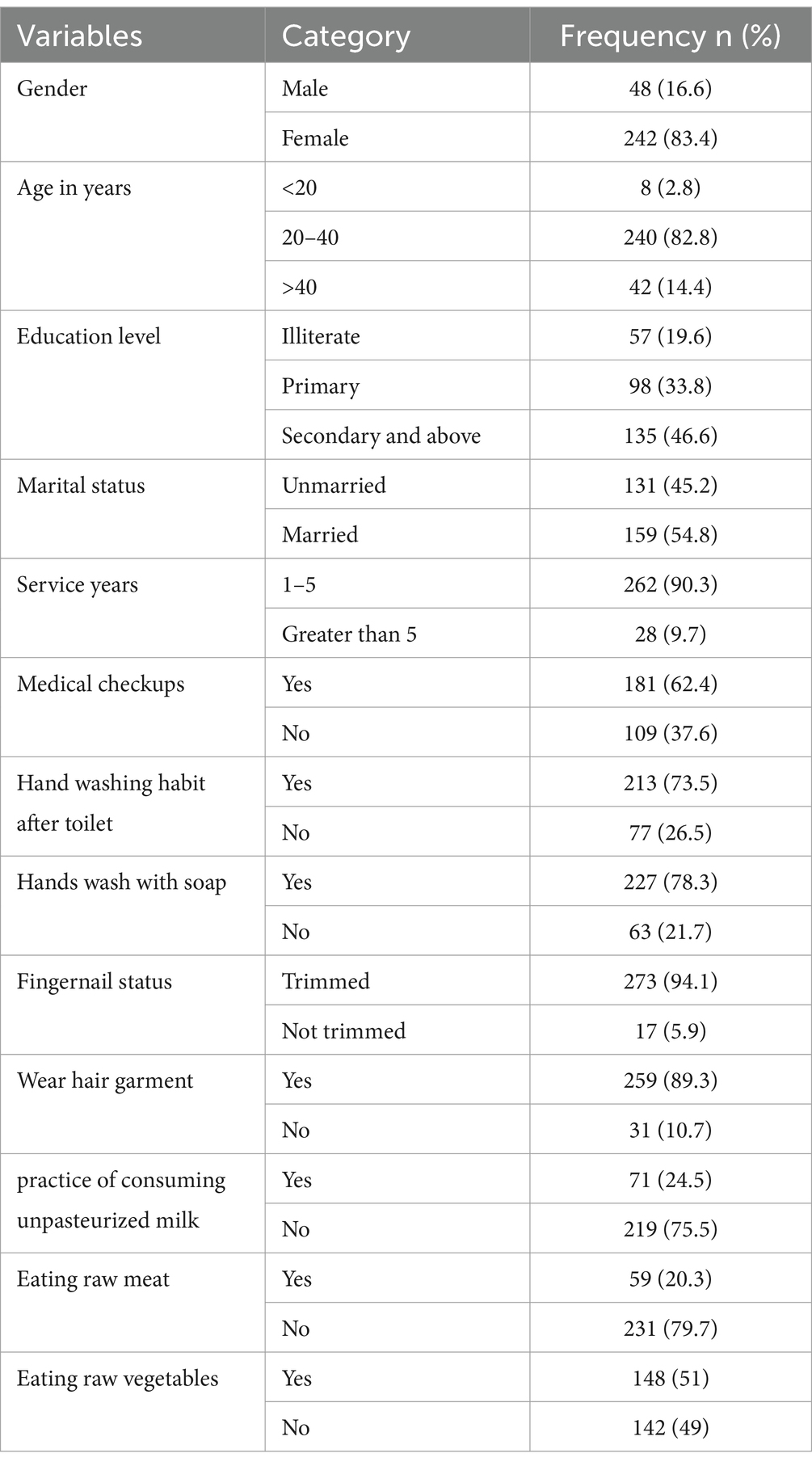
Table 1. Socio-demographic characteristics of the study participants at the University of Gondar Cafeterias, Northwest Ethiopia, 2021.
The magnitude of Salmonella, and Shigella species, and intestinal parasites in stool samples from food handlers
The overall combined magnitude of Salmonella and Shigella isolates was 27 (9.3%). The magnitude of Salmonella and Shigella species in this study was 16 (5.5%) and 11 (3.8%), respectively (Figure 2). In this study, there was at least one intestinal parasite in 290 of the samples analyzed 57 (19.65%). The most detected intestinal Parasitosis was E. histolytica/dispar 22 (7.6%), followed by G. lamblia 13 (4.5%) Ascaris lumbricoides 11 (3.8%), and the least detected intestine parasitosis was 2 (0.69%) Trichuris trichiura and Enterobius vermicularis. The mixed infections of E. histolytica/dispar and G. lamblia were observed in 7% of the samples (Figure 3).
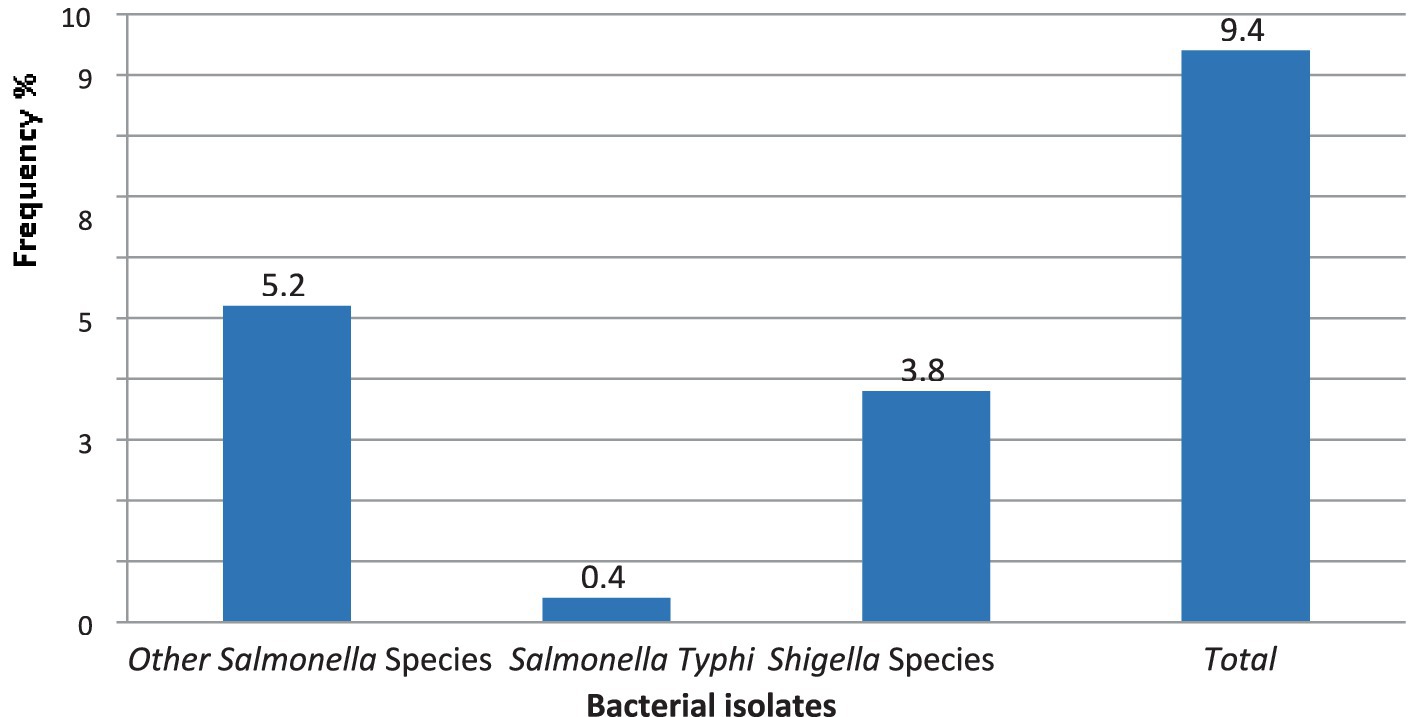
Figure 2. The magnitude of Salmonella and Shigella species in stool samples from food handlers at the University of Gondar cafeterias, Northwest Ethiopia, 2021.
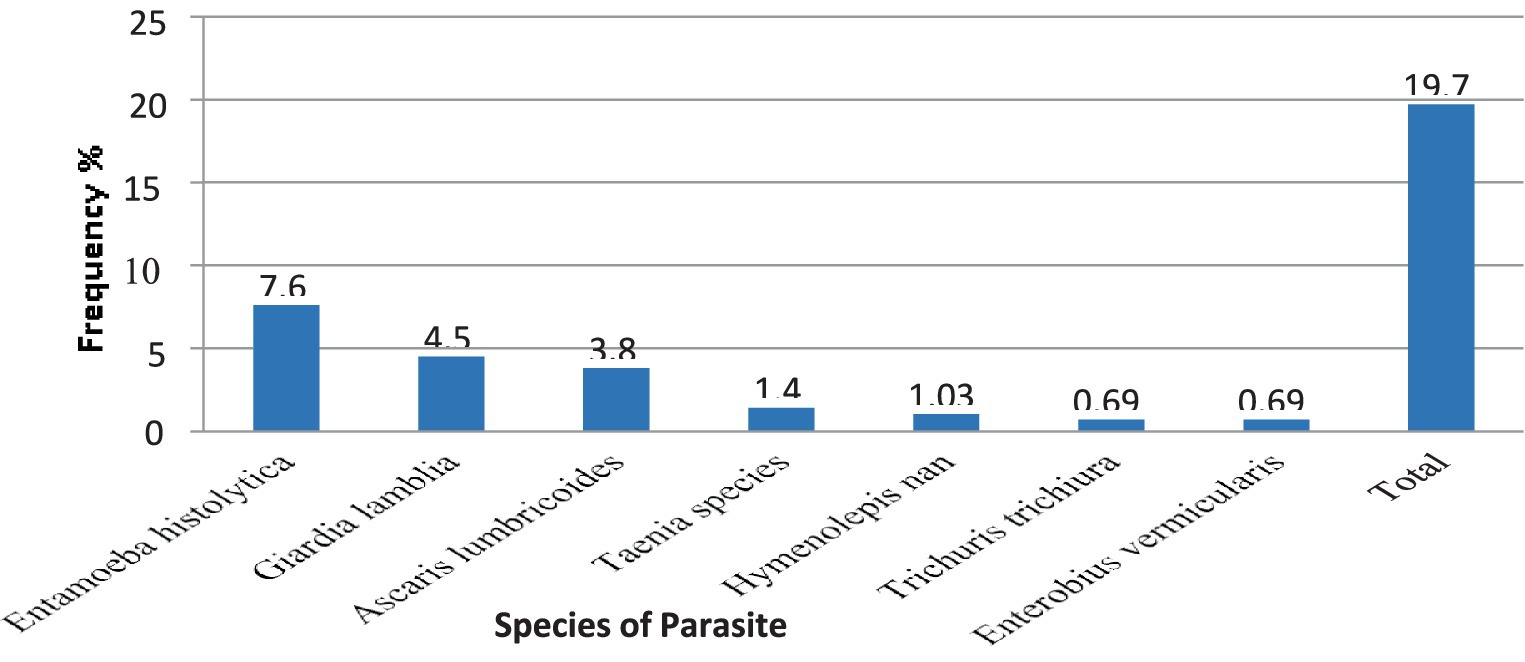
Figure 3. The magnitude of intestinal Parasites in stool samples from food handlers at the University of Gondar Cafeterias, Northwest Ethiopia, 2021.
Associated risk factors of Salmonella and Shigella, and intestinal parasite infection
After bivariate logistic regression analysis, the following factors were added to a multivariate logistic regression analysis: the practice of washing hands after using a toilet (p ≤ 0.001), the practice of consumption of unpasteurized milk (p ≤ 0.000), and consumption of raw vegetables (p ≤ 0.012). The practice of not washing hands after using the toilet (AOR: 4.42, 95% CI: 1.57, 10.56), and the practice of consuming unpasteurized milk (AOR: 3.14, 95% CI: 1.65, 3.96), were factors significantly associated with the prevalence of Salmonella, and Shigella infection (Table 2). After bivariate logistic regression analysis, the following factors were added to a multivariate logistic regression analysis: the practice of washing hands after using a toilet (p ≤ 0.007), the practice of consumption of unpasteurized milk (p ≤ 0.000), and consumption of raw vegetables (p ≤ 0.015). The practice of not washing hands after using the toilet (AOR: 2.19, 95% CI: 1.0, 1.4), and the practice of consuming unpasteurized milk (AOR: 10.4, 95% CI: 3.8, 28.8), were factors significantly associated with the prevalence of intestinal parasites infection (Table 3).
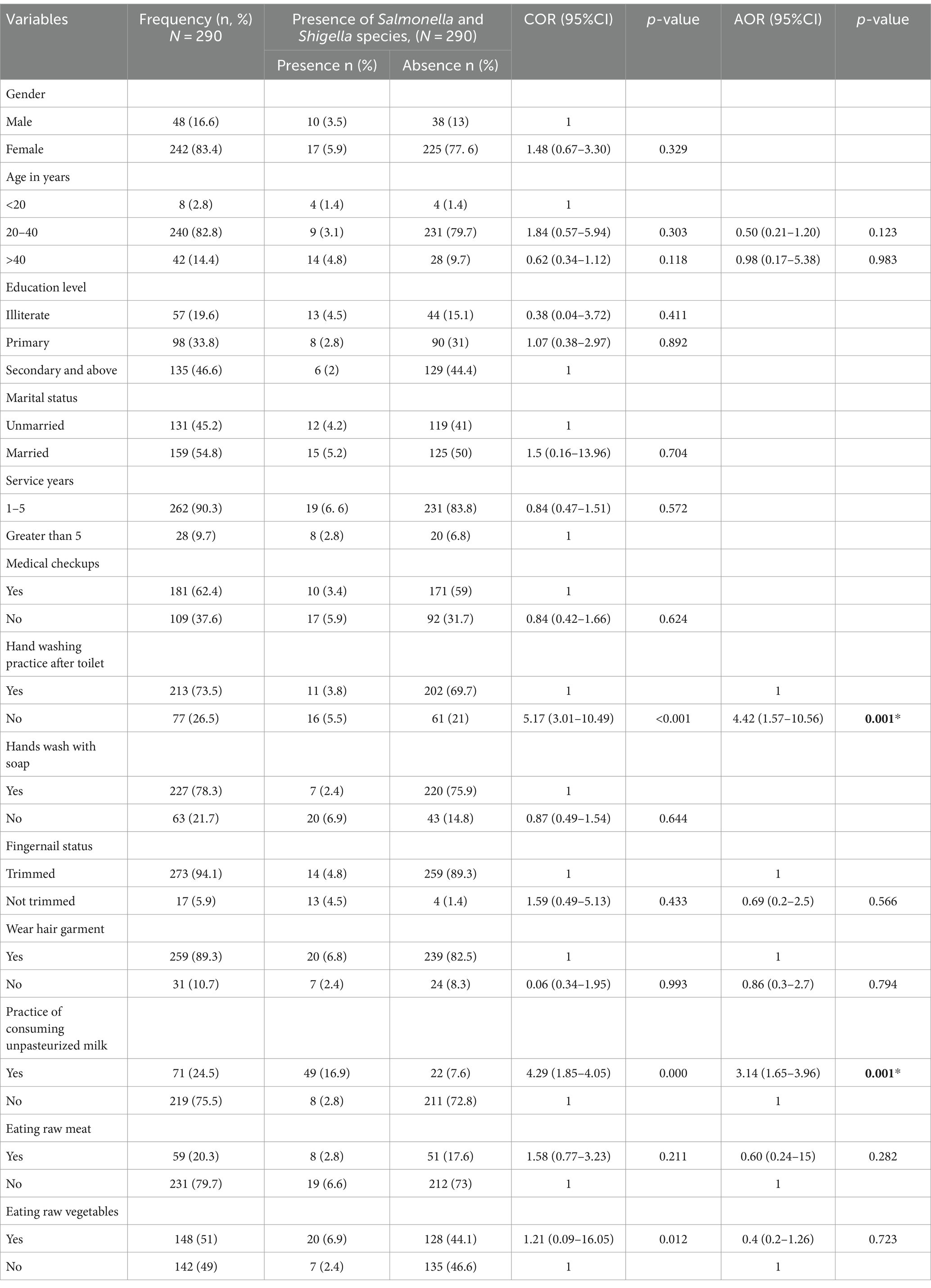
Table 2. Associated risk factors of Salmonella and Shigella infection among the study participants at the University of Gondar Cafeterias, Northwest Ethiopia, 2021.
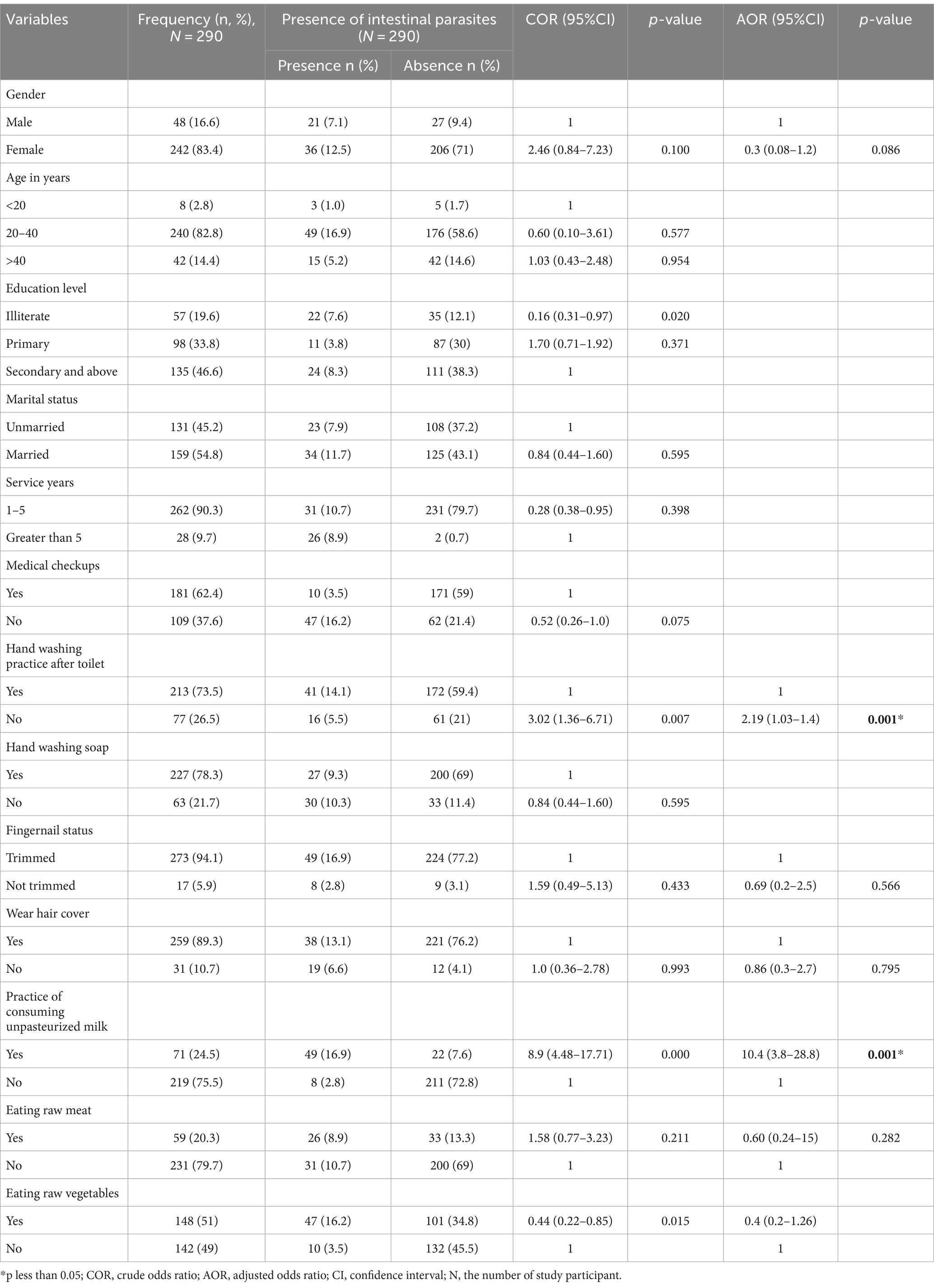
Table 3. Associated risk factors of intestinal parasite infection among the study participants at the University of Gondar Cafeterias, Northwest Ethiopia, 2021.
Antimicrobial resistance profiles of Salmonella and Shigella isolates
The following antibiotics were tested against Salmonella and Shigella isolates Amoxicillin/clavulanic acid, Cefotaxime, Ceftazidime, Ceftriaxone, and Cefoxitin, Gentamicin, Meropenem, and were used to assess the susceptibility profile of 16 Salmonella and 11 Shigella isolates and the results revealed that all isolates were susceptible to Meropenem 11 (100%). Of the Shigella isolates, 11 (100%) to tetracycline and Trimethoprim/Sulphamethoxazole were resistant, followed by 10 (91%) to Amoxicillin/clavulanic acid and 9 (82%) to gentamycin. Furthermore, the Salmonella isolates were resistant to tetracycline; Trimethoprim/Sulphamethoxazole and Amoxicillin/clavulanic acid were 7 (46.7%) (Table 4).
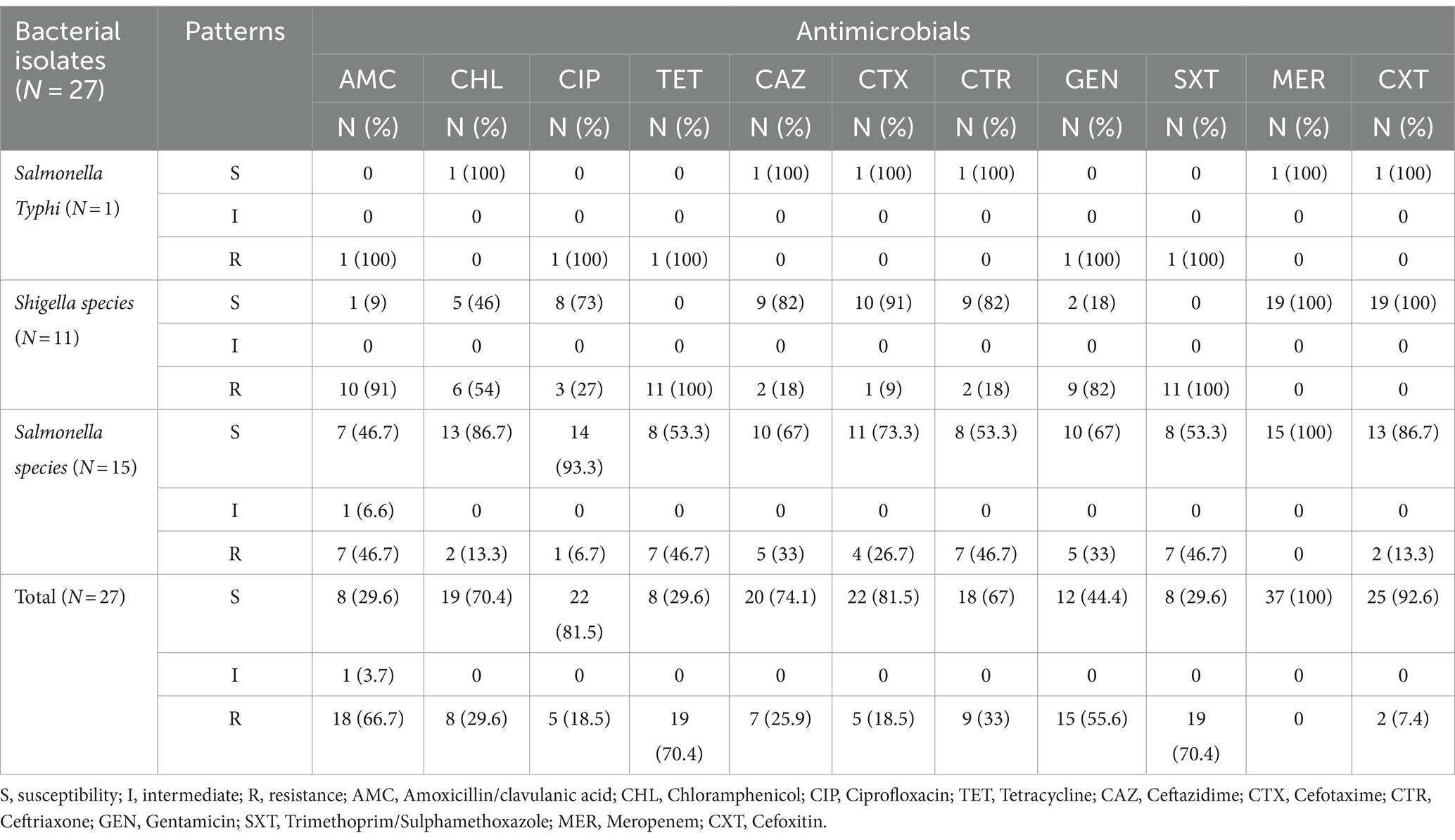
Table 4. Antimicrobial resistance profile of Salmonella and Shigella isolates among study participants at the University of Gondar Cafeterias, Northwest Ethiopia, 2021.
In this study, drug resistance to at least three different classes of antimicrobial agents is referred to as multidrug resistance, the overall multidrug resistance was 16 (59.3%), and out of the 27 isolates, 8 (53.3%) Salmonella and 7 (63.6%) Shigella species were multidrug-resistant isolates (Table 5).
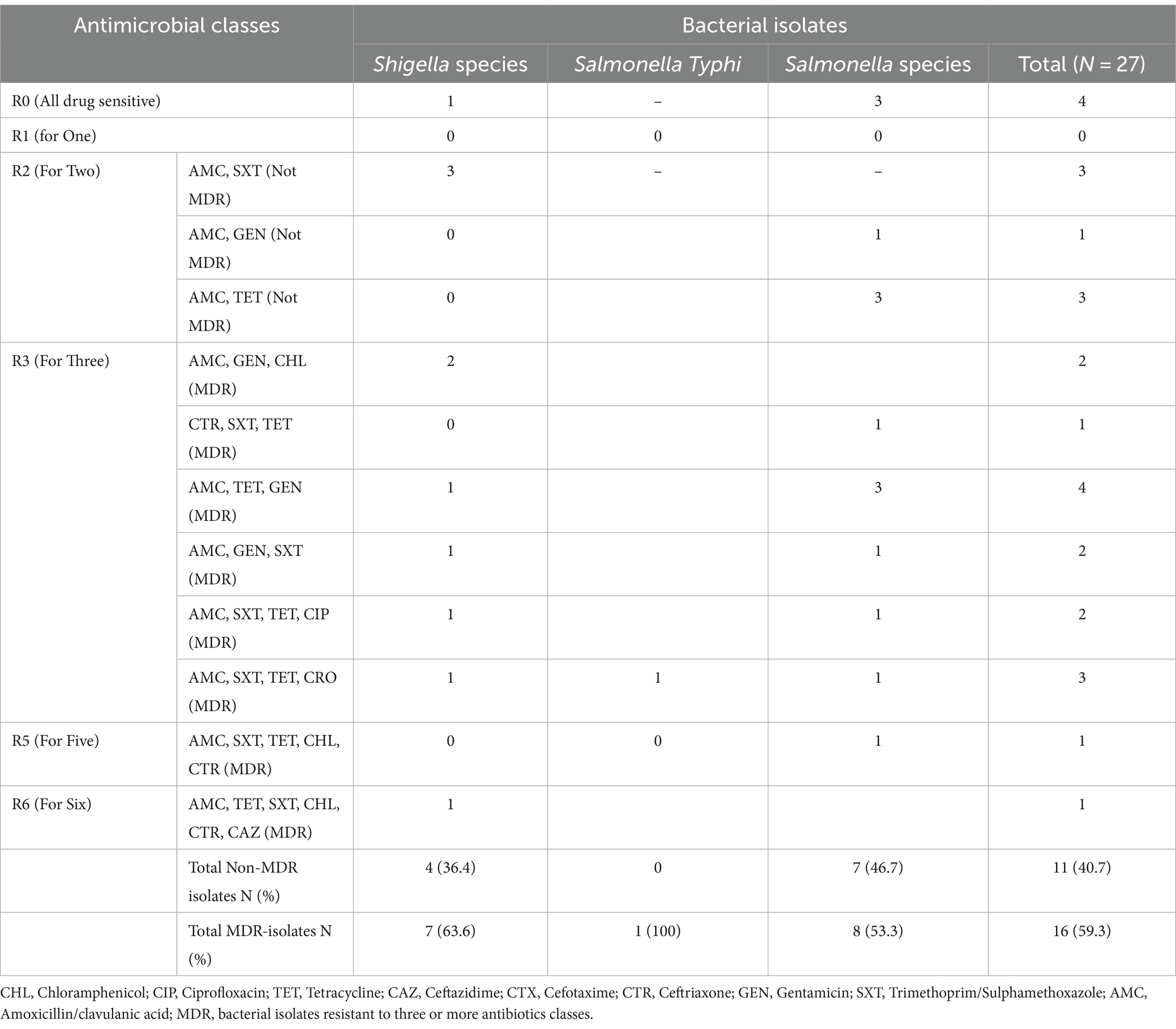
Table 5. The magnitude of Multidrug-resistant Salmonella and Shigella isolates among study participants at the University of Gondar Cafeterias, Northwest Ethiopia, 2021.
Discussion
Poor food handling practices can cause food contamination, which in turn may result in food-borne diseases that could represent a risk to the health of the general population. Food handlers play a major role in the spread of pathogens (38–40).
In our study, 27 (9.3%) of Salmonella or Shigella species were detected in food handlers. This finding is similar to those of previous studies performed in Ethiopia in which the reported isolation rate was 5.9% in Debre Markos University, Ethiopia (41) and 10% in Arba Minch, Ethiopia (42). But, our result is lower than those reported from Nigeria (62.6%) (43), and Sudan (30.1%) (44), and higher than that reported from Ethiopia in Gondar 3.1% (45) and 3.5% in Addis Ababa (46), and in Japan (0.032%) (47). The discrepancies may be due to differences in sample sizes, research participant characteristics, and pathogen isolation techniques. This incidence of enteric pathogens in the current investigation suggests that the food handlers’ cleanliness standards were put to the test because enteric pathogens like Shigella and Salmonella species were detected from stool cultures (41). The cornerstone of limiting the spread of infections from food handlers to consumers is appropriate hygiene habits, and knowledge status, both individually and when handling food (42, 45, 48).
In this study, the magnitudes of Salmonella species among food handlers were 16 (5.5%). This was comparable to studies carried out in Northern Ethiopia, Adigrat (7.3%) (39), Southern, Ethiopia, Arba Minch (6.9%) (42), and Nigeria, (5.5%) (49). However, our result was higher than the studies reported from Ethiopia, Addis Ababa (3.5%) (46), Bahir Dar (1.6%) (23), and Gondar (3.1%) (50). However, our results were lower than those of research conducted in Ethiopia, Dilla (9.5%) (21), Addis Ababa (10.5%) (22), and Nigeria (42.3%) (43). The discrepancy could be explained by the research sites’ varying levels of environmental sanitation and inadequate sanitary conditions.
In this study, the isolation rate of Shigella species was 3.8%; it can be a sign of inadequate food handler cleanliness and result in bacillary dysentery epidemics among the population of students. The results of the research are supported by the findings in Ethiopia, Arba Minch (3%) (42), Dilla (3.2%) (21), and Gondar (2.7–3.1%) (45, 51). Nevertheless, less than the results of other research done in Nigeria (15.5%) (43). These might result from the food handlers’ irregular training in food preparation, handling, and cleanliness procedures, as well as variations in study years.
In this study, the frequency of parasite infection was found to be 19.65%. This agrees with studies conducted in Ghana (21.6%) (10); however, it is higher than what was found in research carried out in Sudan (6.9%) (44) and Iran (11.9%) (52). Nevertheless, it is lower compared to the findings in Ethiopia, Bahir Dar (41.1%) (23) and Dilla (38.6%) (21), in Anatolia (52.2%) (53), in Nigeria (38.1%,) (43), and India (29.3%) (54). The discrepancy could be explained by variations in study years, sociodemographic characteristics, Study area, sanitary conditions, environmental sanitation, safe water supply, health promotion practices, food hygiene and safety training, and awareness of intestinal parasite transmission and prevention.
In this investigation, E. histolytica/dispar was the main parasite found in 7.6% of the stool samples analyzed. This is consistent with earlier research that was done in Ethiopia, where this parasite was found most frequently (15, 55). E. histolytica is one of the typical protozoan parasites that cause human gastroenteritis, along with Giardia lamblia 4.5% (56). It spreads mostly by fecal-oral contact, and in areas where poor hygiene standards are prevalent, contaminated hands play a significant part in its transmission (57, 58).
In this study, Food handlers who did not have a hand-washing practice after the toilet were two times more likely to be positive for intestinal parasites and Shigella and Salmonella species compared to those who had a hand-washing habit after using the toilet. Food handlers who drank unpasteurized milk were 10 times more likely to be positive for intestinal parasites and Shigella and Salmonella species compared to those who drank pasteurized milk. Food handlers this means that most of the intestinal parasitosis, Salmonellosis, and Shigellosis were transmitted by lack of hand washing practice after toilet and drunken unpasteurized milk. This study is a consistent study with Ethiopia, Gondar (51), Bahir Dar (59), Motta (60), Dilla (21), and Mekelle (61). Poor hygienic environments, inadequate toilets, and a lack of readily available facilities for hand washing practices all contribute to the spread of Salmonella and Shigella species. The majority of the university’s food handlers reported only washing their hands with water, and some of them admitted to skipping the step entirely after using the bathroom (62).
In this study, regarding antimicrobial susceptibility test results, a high rate of isolate susceptibility was observed to a few of the antimicrobial agents which is comparable to the study conducted at Debre Markos University, Ethiopia (41). However, Shigella and Salmonella isolates showed a high resistance rate to tetracycline, Trimethoprim/Sulphamethoxazole, and Augmentin which is concordant with the previous report from Gondar Town Health Institution, Ethiopia (63). This may have been caused by the extensive and unnecessary use of antibiotics. In the current practice of Ethiopia, it is simple for the general people to walk into a pharmacy and purchase antibiotics off the market. The high resistance rates seen could be explained by this indiscriminate usage of antibiotics (64).
In this study, Shigella species were highly resistant to Tetracycline and Trimethoprim/Sulphamethoxazole (100%), Chloramphenicol (54%), and Amoxicillin/clavulanic acid (91%). This is similar to the study conducted in Ethiopia, Haramaya, that reported resistance of Shigella species to Tetracycline (76.2%), Chloramphenicol (66.7%) (25), and in Tigrai that reported resistance of Shigella species to Tetracycline (50%), Amoxicillin/clavulanic acid (100%), and Chloramphenicol (50%) (58). Additionally, it was comparable research from Arbaminch, Ethiopia found that the Shigella species were 40% of Amoxicillin/clavulanic acid (65). Another study conducted at the Gondar University cafeteria also showed that 75% of tetracycline and chloramphenicol, followed by Trimethoprim/Sulphamethoxazole (50%) and Gentamicin (25%) (66) and at catering establishments at Debre Markos University the result showed that 62.5% of Tetracycline, 25% Chloramphenicol, and (37.5%) Trimethoprim/Sulphamethoxazole (41).
The Salmonella species were also resistant to many of the antibiotics in the present study: 46.7% to Tetracycline, Amoxicillin/clavulanic acid, and Trimethoprim/Sulphamethoxazole. This is comparable with a study done in Ethiopia, Gondar (51), Debre Markos University (41), Tigrai (58), and Dilla (21), which indicated that antimicrobial resistance of Salmonella species is an increasing concern. These results suggested that Ethiopian antimicrobial resistance is still a problem that has to be addressed. Antibiotic resistance has emerged as a significant global public health problem, necessitating coordinated action (67). Antibiotic resistance is mostly caused by changes in bacterial genomes, inappropriate antibiotic use, poor drug regulation rules, incorrect drug prescriptions, and disobedience to prescriptions (68).
In this study, Salmonella isolates were highly susceptible to Ceftriaxone which was comparable with previous reports in Ethiopia (21, 69). The magnitude of MDR Shigella and Salmonella species in this study was 63.6 and 53.3%, respectively, which is comparable with the previous findings in Ethiopia (21, 69) but lower than 100% resistance in Addis Ababa, Ethiopia (46). The high MDR rate of Salmonella and Shigella isolates for most of the antibiotics currently used could limit our antibiotic options for empirical therapy.
Limitations
Due to a lack of available materials, the Shigella and Salmonella isolates in our investigation could only be identified at the species level. Furthermore, due to financial constraints, it was not possible to differentiate between the Entamoeba complex species (E. dispar and E. histolytica) by using molecular methods.
Conclusion and recommendations
In this study, the overall prevalence of Salmonella species and Shigella species was high as compared to the previous reports. Salmonella species was highly resistant to Trimethoprim/Sulphamethoxazole, amoxicillin-clavulanic acid, and Tetracycline, however highly susceptible to Meropenem, Ciprofloxacin, Cefoxitine, and Chloramphenicol. Shigella species also show a high resistance rate to Trimethoprim/Sulphamethoxazole, Tetracycline, amoxicillin-clavulanic acid, and Gentamicin, and showed the least resistance to Ceftazidime, and Cefotaxime. Among the total Salmonella and Shigella isolates, multidrug resistance was documented in 59.3% of all bacterial isolates. The overall prevalence of different intestinal parasites from stool samples was 19.65%. Among those, the most prevalent intestinal parasitosis was E. histolytica/dispar, followed by G. lamblia. Regarding risk factors, consumption of unpasteurized milk, and no hand washing practice after the toilet showed high significant correlation with intestinal parasites, Shigella, or salmonella infection. Through continuing health education and training programs, the level of knowledge, as well as the standard of conduct of food handlers at cafeteria facilities, must be improved. To improve the personal hygiene of the food handlers, it is also advised that the required facilities be provided, such as a clean toilet and a safe water supply. Additionally, to prevent microbial infections in food handlers, it is recommended that consumers drink pasteurized milk, and constant epidemiological surveillance is recommended.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Review Committee of the School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AzA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. SE: Methodology, Supervision, Validation, Writing – review & editing. DK: Investigation, Software, Supervision, Validation, Visualization, Writing – review & editing. AyA: Data curation, Investigation, Writing – original draft. WA: Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. FM: Conceptualization, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors acknowledge the University of Gondar, student cafeteria managers, and all study participants for their cooperation during sample collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AST, Antimicrobial Susceptibility Test; ATCC, American type culture collection; CLSI, Clinical and Laboratory Standard Institute; DCA, deoxycholate agar; EBIs, enteric bacterial infections; IPs, intestinal parasites; MAC, MacConkey agar; MDR, Multidrug resistance; XLD, xylose lysine deoxycholate
References
1. Girma, A, and Aemiro, A. Evaluation of soil streptomyces isolates from North-Western Ethiopia as potential inhibitors against spoilage and foodborne bacterial pathogens. J Chem. (2022) 2022:1–12. doi: 10.1155/2022/5547406
2. Ameme, DK, Abdulai, M, Adjei, EY, Afari, EA, Nyarko, KM, Asante, D, et al. Foodborne disease outbreak in a resource-limited setting: a tale of missed opportunities and implications for response. Pan Afr Med J. (2016) 23:69. doi: 10.11604/pamj.2016.23.69.7660
3. Grace, D, Alonso, S, Mutua, FK, Roesel, K, Lindahl, JF, and Amenu, K. Food safety investment expert advice: Burkina Faso, Ethiopia, Nigeria. (2018). Available at: https://cgspace.cgiar.org/items/7579bbc3-09c9-4c74-a169-5ae5076ae407
4. World Health Organization . Food safety- key facts. (2022). Available at: https://www.who.int/news-room/factsheets/detail/food-safety.
5. Hendriksen, RS, Vieira, AR, Karlsmose, S, Lo Fo Wong, DM, Jensen, AB, Wegener, HC, et al. Global monitoring of Salmonella serovar distribution from the World Health Organization global foodborne infections network country data Bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. (2011) 8:887–900. doi: 10.1089/fpd.2010.0787
6. World Health Organization . WHO estimates of the global burden of foodborne diseases: Foodborne disease burden epidemiology reference group 2007–2015 World Health Organization (2015). Available at: https://www.who.int/publications/i/item/9789241565165
7. Scharff, RL . Economic burden from health losses due to foodborne illness in the United States. J Food Prot. (2012) 75:123–31. doi: 10.4315/0362-028X.JFP-11-058
8. WHO G . National food safety systems in Africa: a situation analysis. (2005). Available at: https://www.afro.who.int/publications/national-food-safety-systems-africa-situation-analysis
9. Norhayati, M, Fatmah, M, Yusof, S, and Edariah, A. Intestinal parasitic infections in man: a review. Med J Malays. (2003) 58:296–305.
10. Ayeh-Kumi, P, Quarcoo, S, Kwakye-Nuako, G, Kretchy, J, Osafo-Kantanka, A, and Mortu, S. Prevalence of intestinal parasitic infections among food vendors in Accra, Ghana. J. Trop. Med. Parasitol. (2009) 32:1–8.
11. Tulu, B, Taye, S, and Amsalu, E. Prevalence and its associated risk factors of intestinal parasitic infections among Yadot primary school children of south eastern Ethiopia: a cross-sectional study. BMC Res Notes. (2014) 7:848–7. doi: 10.1186/1756-0500-7-848
12. De Silva, NR, Brooker, S, Hotez, PJ, Montresor, A, Engels, D, and Savioli, L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. (2003) 19:547–51. doi: 10.1016/j.pt.2003.10.002
13. Pham Duc, P, Nguyen-Viet, H, Hattendorf, J, Zinsstag, J, Dac Cam, P, and Odermatt, P. Risk factors for Entamoeba histolytica infection in an agricultural community in Hanam province, Vietnam. Parasites Vect. (2011) 4:1–9. doi: 10.1186/1756-3305-4-102
14. Yimam, Y, Woreta, A, and Mohebali, M. Intestinal parasites among food handlers of food service establishments in Ethiopia: a systematic review and meta-analysis. BMC Public Health. (2020) 20:1–12. doi: 10.1186/s12889-020-8167-1
15. Kebede, E, Seid, A, and Akele, S. Prevalence and associated risk factors of intestinal parasitic infections among asymptomatic food handlers in Wollo University student’s cafeteria, Northeastern Ethiopia. BMC Res Notes. (2019) 12:1–6. doi: 10.1186/s13104-019-4182-7
16. Girma, A, and Aemiro, A. Prevalence and associated risk factors of intestinal parasites and enteric bacterial infections among selected region food handlers of Ethiopia during 2014–2022: a systematic review and meta-analysis. Sci World J. (2022) 2022:1–14. doi: 10.1155/2022/7786036
17. Deribe, K, Meribo, K, Gebre, T, Hailu, A, Ali, A, Aseffa, A, et al. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasit Vectors. (2012) 5:1–15. doi: 10.1186/1756-3305-5-240
18. Martin, LB, Simon, R, MacLennan, CA, Tennant, SM, Sahastrabuddhe, S, and Khan, MI. Status of paratyphoid fever vaccine research and development. Vaccine. (2016) 34:2900–2. doi: 10.1016/j.vaccine.2016.03.106
19. Majowicz, SE, Musto, J, Scallan, E, Angulo, FJ, Kirk, M, O’Brien, SJ, et al. The global burden of NontyphoidalSalmonellaGastroenteritis. Clin Infect Dis. (2010) 50:882–9. doi: 10.1086/650733
20. Crump, JA, Luby, SP, and Mintz, ED. The global burden of typhoid fever. Bull World Health Organ. (2004) 82:346–53.
21. Diriba, K, Awulachew, E, and Ashuro, Z. Prevalence and antimicrobial resistance pattern of Salmonella, Shigella, and intestinal parasites and associated factor among food handlers in Dilla University student cafeteria, Dilla, Ethiopia. Int J Microbiol. (2020) 2020:1–10. doi: 10.1155/2020/3150539
22. Mengistu, G, Mulugeta, G, Lema, T, and Aseffa, A. Prevalence and antimicrobial susceptibility patterns of Salmonella serovars and Shigella species. J Microb Biochem Technol. (2014) 6:S2–S006. doi: 10.4172/1948-5948.S2-006
23. Abera, B, Biadegelgen, F, and Bezabih, B. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Bahir Dar town, Northwest Ethiopia. Ethiop J Health Dev. (2010) 24:46–50. doi: 10.4314/ejhd.v24i1.62944
24. Birhaneselassie, M, and Williams, D. A study of Salmonella carriage among asymptomatic food-handlers in southern Ethiopia. Int J Nutr Food Sci. (2013) 2:243–5. doi: 10.11648/j.ijnfs.20130205.15
25. Marami, D, Hailu, K, and Tolera, M. Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella species among asymptomatic food handlers working in Haramaya University cafeterias, Eastern Ethiopia. BMC Res Notes. (2018) 11:1–6. doi: 10.1186/s13104-018-3189-9
26. Bekele, F, Tefera, T, Biresaw, G, and Yohannes, T. Parasitic contamination of raw vegetables and fruits collected from selected local markets in Arba Minch town, Southern Ethiopia. Infect Dis Poverty. (2017) 6:1–7. doi: 10.1186/s40249-016-0226-6
27. Sosa, AJ, Byarugaba, DK, Amábile-Cuevas, CF, Hsueh, P-R, Kariuki, S, and Okeke, IN. Antimicrobial resistance in developing countries Springer (2010). Available at: https://link.springer.com/content/pdf/10.1007/978-0-387-89370-9.pdf
28. Centers for Diseases control and Prevention . DPD–laboratory identification of parasites of public health concern. (2016). Available at: https://www.cdc.gov/dpdx/diagnosticprocedures/stool/specimenproc.html (Accessed May 28, 2020).
29. Mikoleit, M. Biochemical identification of Salmonella and Shigella using an abbreviated panel of tests. Geneva, Switzerland: WHO (2010). Available at: 20_07-gfn-biochem-v002-final-16oct2015.pdf
30. Brown, DF, Wootton, M, and Howe, RA. Antimicrobial susceptibility testing breakpoints and methods from BSAC to EUCAST. J Antimicrob Chemother. (2016) 71:3–5. doi: 10.1093/jac/dkv287
31. Tang, Y-W, Stratton, CW, and Tang, Y-W. Advanced techniques in diagnostic microbiology Springer (2013).
32. Zapata, A, and Ramirez-Arcos, S. A comparative study of McFarland turbidity standards and the Densimat photometer to determine bacterial cell density. Curr Microbiol. (2015) 70:907–9. doi: 10.1007/s00284-015-0801-2
33. Weinstein, MP, and Lewis, JS. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J Clin Microbiol. (2020) 58:e01864-19. doi: 10.1128/JCM.01864-19
34. Magiorakos, A-P, Srinivasan, A, Carey, RB, Carmeli, Y, Falagas, M, Giske, C, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
35. Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute: Wayne, PA. (2017). p. 106–112.
36. Daligault, H, Davenport, K, Minogue, T, Bishop-Lilly, K, Broomall, S, Bruce, D, et al. Genome assembly of Shigella flexneri ATCC 12022, a quality control reference strain. Genome Announc. (2014) 2:01052-14. doi: 10.1128/genomeA.01052-14
37. Basu, S, Pal, A, and Desai, P. Quality control of culture media in a microbiology laboratory. Indian J Med Microbiol. (2005) 23:159–63. doi: 10.1016/S0255-0857(21)02585-8
38. Assefa, T, Tasew, H, Wondafrash, B, and Beker, J. Contamination of bacteria and associated factors among food handlers working in the student cafeterias of Jimma University Main Campus, Jimma, South West Ethiopia. Altern Integr Med. (2015) 2015:1–8. doi: 10.4172/2376-0214.1000345
39. Legese, H, Kahsay, T, Gebrewahd, A, Berhe, B, Fseha, B, Tadesse, S, et al. Prevalence, antimicrobial susceptibility pattern, and associated factors of Salmonella and Shigella among food handlers in Adigrat University student’s cafeteria, northern Ethiopia, 2018. Trop Dis Travel Med Vacc. (2020) 6:1–9. doi: 10.1186/s40794-020-00119-x
40. Tagar, S, and Ahmed, N. An assessment of hygiene Indicator Bacteria and MDR Salmonella on poultry butchers’ hands and rinsing water at XDR Salmonella struck areas. Egypt J Vet Sci. (2022) 53:307–22. doi: 10.21608/ejvs.2022.123764.1329
41. Mengist, A, Mengistu, G, and Reta, A. Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella among food handlers in catering establishments at Debre Markos university, Northwest Ethiopia. Int J Infect Dis. (2018) 75:74–9. doi: 10.1016/j.ijid.2018.08.008
42. Mama, M, and Alemu, G. Prevalence, antimicrobial susceptibility patterns and associated risk factors of Shigella and Salmonella among food handlers in Arba Minch University, South Ethiopia. BMC Infect Dis. (2016) 16:1–7. doi: 10.1186/s12879-016-2035-8
43. Ifeadike, C, Ironkwe, O, Nnebue, CC, Nwabueze, SA, Ubajaka, CF, Adogu, POU, et al. Prevalence and pattern of bacteria and intestinal parasites among food handlers in the Federal Capital Territory of Nigeria. Niger Med J. (2012) 53:166–71. doi: 10.4103/0300-1652.104389
44. Saeed, HA, and Hamid, HH. Bacteriological and parasitological assessment of food handlers in the Omdurman area of Sudan. J Microbiol Immunol Infect. (2010) 43:70–3. doi: 10.1016/S1684-1182(10)60010-2
45. Andargie, G, Kassu, A, Moges, F, Tiruneh, M, and Huruy, K. Prevalence of bacteria and intestinal parasites among food-handlers in Gondar town, Northwest Ethiopia. J Health Popul Nutr. (2008) 26:451–5. doi: 10.3329/jhpn.v26i4.1887
46. Aklilu, A, Kahase, D, Dessalegn, M, Tarekegn, N, Gebremichael, S, Zenebe, S, et al. Prevalence of intestinal parasites, salmonella and shigella among apparently health food handlers of Addis Ababa university student’s cafeteria, Addis Ababa, Ethiopia. BMC Res Notes. (2015) 8:1–6. doi: 10.1186/s13104-014-0967-x
47. Murakami, K, Ishihara, T, Horikawa, K, and Oda, T. Features of Salmonella serovars among food handlers in Kyushu, Japan. Microbiologica-quarterly journal of. Microbiol Sci. (2007) 30:155–60.
48. Reta, MA, Lemma, MT, Gemeda, AA, and Lemlem, GA. Food handling practices and associated factors among food handlers working in public food and drink service establishments in Woldia town, Northeast Ethiopia. Pan Afr Med J. (2021) 40:PAMJ - 40(128). doi: 10.11604/pamj.2021.40.128.19757
49. Mobolaji, O, and Olubunmi, O. Assessment of the hygienic practices and the incidence of enteric bacteria in food handlers in small businesses in an urban area in Abeokuta. Int J Microbiol Res. (2014) 5:41–9. doi: 10.14303/irjm.2014.015
50. Garedew-Kifelew, L, Wondafrash, N, and Feleke, A. Identification of drug-resistant Salmonella from food handlers at the University of Gondar, Ethiopia. BMC Res Notes. (2014) 7:1–6. doi: 10.1186/1756-0500-7-545
51. Dagnew, M, Tiruneh, M, Moges, F, and Gizachew, M. Bacterial profile and antimicrobial susceptibility pattern among food handlers at Gondar University cafeteria, Northwest Ethiopia. J Infectious Dis Ther. (2013) 6:1–6. doi: 10.4172/2332-0877.1000105
52. Kheirandish, F, Tarahi, M, Haghighi, A, Nazemalhosseini-Mojarad, E, and Kheirandish, M. Prevalence of intestinal parasites in bakery workers in Khorramabad, Lorestan Iran. Iran J Parasitol. (2011) 6:76.
53. Simsek, Z, Koruk, I, Copur, AC, and Gürses, G. Prevalence of Staphylococcus aureus and intestinal parasites among food handlers in Sanliurfa, Southeastern Anatolia. J Public Health Manag Pract. (2009) 15:518–23. doi: 10.1097/PHH.0b013e3181aa2814
54. Ghosh, A, Mishra, PP, and Sharma, VP. Prevalence of parasitic infestations amongst the food handlers in a City of north eastern region of India. Natl J Integr Res Med. (2014) 5:15–18.
55. Mama, M, and Alemu, G. Prevalence and factors associated with intestinal parasitic infections among food handlers of southern Ethiopia: cross sectional study. BMC Public Health. (2015) 16:1–7. doi: 10.1186/s12889-016-2790-x
56. Gaafar, MR . Evaluation of enzyme immunoassay techniques for diagnosis of the most common intestinal protozoa in fecal samples. Int J Infect Dis. (2011) 15:e541–4. doi: 10.1016/j.ijid.2011.04.004
57. Alum, A, Rubino, JR, and Ijaz, MK. The global war against intestinal parasites—should we use a holistic approach? Int J Infect Dis. (2010) 14:e732–8. doi: 10.1016/j.ijid.2009.11.036
58. Mardu, F, Negash, H, Legese, H, Berhe, B, Tesfay, K, Haileslasie, H, et al. Assessment of knowledge, practice, and status of food handlers toward Salmonella, Shigella, and intestinal parasites: a cross-sectional study in Tigrai prison centers, Ethiopia. PLoS One. (2020) 15:e0241145. doi: 10.1371/journal.pone.0241145
59. Abera, B, Yitayew, G, and Amare, H. Salmonella serotypeTyphi, Shigella, and intestinal parasites among food handlers at Bahir Dar University, Ethiopia. J Infection Dev Countries. (2016) 10:121–6. doi: 10.3855/jidc.6890
60. Yesigat, T, Jemal, M, and Birhan, W. Prevalence and associated risk factors of Salmonella, Shigella, and intestinal parasites among food handlers in Motta town, north West Ethiopia. Can J Infect Dis Med Microbiol. (2020) 2020:1–11. doi: 10.1155/2020/6425946
61. Nigusse, D, and Kumie, A. Food hygiene practices and prevalence of intestinal parasites among food handlers working in Mekelle university student’s cafeteria, Mekelle. Glob Adv Res J Soc Sci. (2012) 1:65–71.
62. Amegah, KE, Addo, HO, Ashinyo, ME, Fiagbe, L, Akpanya, S, Akoriyea, SK, et al. Determinants of hand hygiene practice at critical times among food handlers in educational institutions of the Sagnarigu municipality of Ghana: a cross-sectional study. Environ Health Insights. (2020) 14:117863022096041. doi: 10.1177/1178630220960418
63. Demissie, TA, Wubie, T, Yehuala, FM, Fetene, M, and Gudeta, A. Prevalence and antimicrobial susceptibility patterns of Shigella and Salmonella species among patients with diarrhea attending Gondar town health institutions, Northwest Ethiopia. Sci J Pub Health. (2014) 2:469–75. doi: 10.11648/j.sjph.20140205.24
64. Mengist, A, Kannan, H, and Abdissa, A. Prevalence and antimicrobial susceptibility pattern of anorectal and vaginal group B streptococci isolates among pregnant women in Jimma, Ethiopia. BMC Res Notes. (2016) 9:1–5. doi: 10.1186/s13104-016-2158-4
65. Webb, MJ, Kauer, SD, Ozer, EM, Haller, DM, and Sanci, LA. Does screening for and intervening with multiple health compromising behaviours and mental health disorders amongst young people attending primary care improve health outcomes? A systematic review. BMC Family Pract. (2016) 17:1–12. doi: 10.1186/s12875-016-0504-1
66. Mulat, D, Moges, T, Feleke, M, and Mucheye, G. Infectious diseases & therapy bacterial profile and antimicrobial susceptibility pattern among food handlers at Gondar University cafeteria, Northwest Ethiopia. J Infect Dis Th. (2013) 1:2–7.
67. Ibrahim, RA, Teshal, AM, Dinku, SF, Abera, NA, Negeri, AA, Desta, FG, et al. Antimicrobial resistance surveillance in Ethiopia: implementation experiences and lessons learned. Afr J Lab Med. (2018) 7:1–4. doi: 10.4102/ajlm.v7i2.770
68. Ndihokubwayo, JB, Yahaya, AA, Desta, AT, Ki-Zerbo, G, Odei, EA, Keita, B, et al. Antimicrobial resistance in the African region: issues, challenges and actions proposed. Afr Health Monitor. (2013) 16:27–30.
69. Solomon, FB, Wada, FW, Anjulo, AA, Koyra, HC, and Tufa, EG. Burden of intestinal pathogens and associated factors among asymptomatic food handlers in South Ethiopia: emphasis on salmonellosis. BMC Res Notes. (2018) 11:1, 502–506. doi: 10.1186/s13104-018-3610-4
Keywords: Salmonella species, Shigella species, intestinal parasites, multi-drug resistant, food handler, Gondar
Citation: Amare A, Eshetie S, Kasew D, Amare A, Abebe W and Moges F (2024) Prevalence of Salmonella spp., Shigella spp., and intestinal parasites among food handlers working in University of Gondar student’s cafeteria, Northwest Ethiopia. Front. Public Health. 12:1370338. doi: 10.3389/fpubh.2024.1370338
Edited by:
Ponsiano Ocama, Makerere University, UgandaReviewed by:
Melese Abate Reta, University of Pretoria, South AfricaOkon Okwong Kenneth, Federal Medical Center Makurdi, Nigeria
Copyright © 2024 Amare, Eshetie, Kasew, Amare, Abebe and Moges. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Azanaw Amare, YXphbmF3MDNAZ21haWwuY29t
 Azanaw Amare
Azanaw Amare Setegn Eshetie
Setegn Eshetie Desie Kasew
Desie Kasew Ayenew Amare2
Ayenew Amare2