- 1U.S. Environmental Protection Agency, Office of Research and Development, Center for Public Health and Environmental Assessment, Research Triangle Park, NC, United States
- 2Gillings Global School of Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 3Division of Environmental Health Sciences, College of Public Health, The Ohio State University, Columbus, OH, United States
- 4Division of Cardiology, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 5Department of Family Medicine, University of Maryland Medical Center, Baltimore, MD, United States
- 6Division of Cardiology, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
- 7Department of Cardiovascular Medicine, Heart and Vascular Institute, University of Pittsburgh Medical Center, Pittsburgh, PA, United States
Background: Sudden death accounts for approximately 10% of deaths among working-age adults and is associated with poor air quality. Objectives: To identify high-risk groups and potential modifiers and mediators of risk, we explored previously established associations between fine particulate matter (PM2.5) and sudden death stratified by potential risk factors.
Methods: Sudden death victims in Wake County, NC, from 1 March 2013 to 28 February 2015 were identified by screening Emergency Medical Systems reports and adjudicated (n = 399). Daily PM2.5 concentrations for Wake County from the Air Quality Data Mart were linked to event and control periods. Potential modifiers included greenspace metrics, clinical conditions, left ventricular hypertrophy (LVH), and neutrophil-to-lymphocyte ratio (NLR). Using a case-crossover design, conditional logistic regression estimated the OR (95%CI) for sudden death for a 5 μg/m3 increase in PM2.5 with a 1-day lag, adjusted for temperature and humidity, across risk factor strata.
Results: Individuals having LVH or an NLR above 2.5 had PM2.5 associations of greater magnitude than those without [with LVH OR: 1.90 (1.04, 3.50); NLR > 2.5: 1.25 (0.89, 1.76)]. PM2.5 was generally less impactful for individuals living in areas with higher levels of greenspace.
Conclusion: LVH and inflammation may be the final step in the causal pathway whereby poor air quality and traditional risk factors trigger arrhythmia or myocardial ischemia and sudden death. The combination of statistical evidence with clinical knowledge can inform medical providers of underlying risks for their patients generally, while our findings here may help guide interventions to mitigate the incidence of sudden death.
1 Introduction
Among adults aged 18–64 in the United States, non-accidental sudden deaths unrelated to previous or obvious causes account for as much as 10% of deaths and 2 million years of productive life lost (1). Estimates of sudden death vary vastly across the scientific literature, depending on the definition of sudden death used. Most definitions include timing and situational restrictions that result in the under-reporting of many cases (e.g., within 1 h of witnessed or 24 h of unwitnessed events) (2). In order to increase the likelihood of identifying missed deaths, avoid assumptions of coronary artery disease causality, and include unwitnessed deaths (3), population-level assessments seem necessary to develop more effective interventions to reduce sudden deaths.
There is an extensive body of literature causally linking ambient exposure to particulate matter less than 2.5 micrometers in aerodynamic diameter (PM2.5), which acts through inflammatory and oxidative mechanisms, to overall mortality, respiratory morbidity and mortality, and cardiovascular and cardiometabolic morbidity and mortality (4–19). However, there remain many uncertainties surrounding air pollution exposures and health outcomes, including the potential for different underlying etiologies for cause-specific subtypes of deaths (20–22) and the impacts of existing social and health conditions or concurrent exposures on air pollution-health associations (23–25).
We previously observed increased odds of sudden death with higher acute exposure to particulate matter less than 2.5 micrometers in aerodynamic diameter (PM2.5). In other analyses of this population, inflammation, left ventricular hypertrophy (LVH), stroke, chronic respiratory disease, coronary artery disease, metabolic syndrome, and lower values for greenspace metrics were associated with increased risk for sudden death (1, 26–29). Beyond our data, LVH and neutrophil-to-lymphocyte ratio (NLR) are of particular interest as they are important contributors to cardiovascular-related morbidity and mortality (30–34). Building on this information, we adapted a theoretical framework for air pollution associations with sudden death described by Cascio (35), p. 35 to highlight how the underlying conditions of individuals and their external surroundings might alter responses to ambient air pollutant exposures (Figure 1).
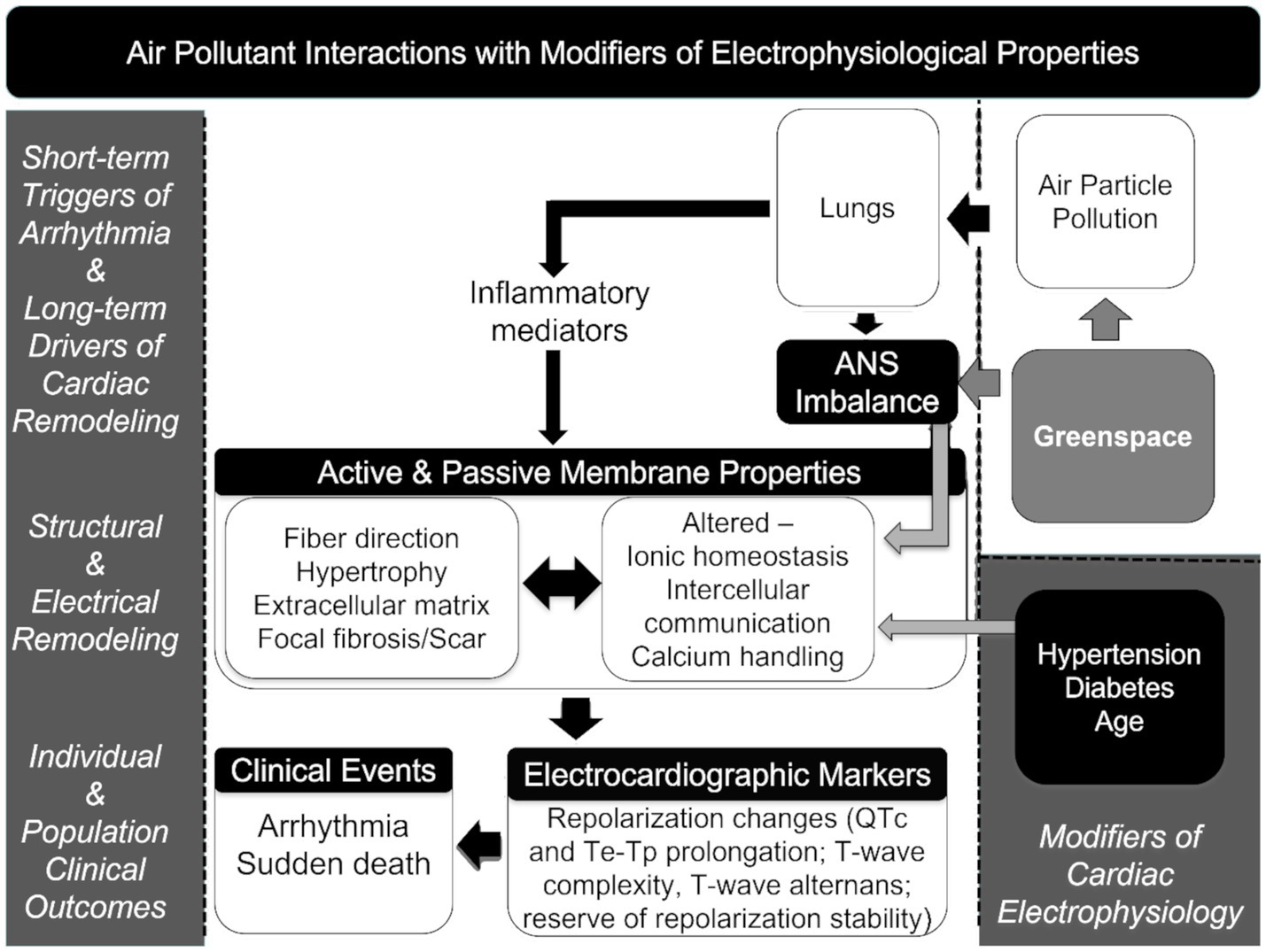
Figure 1. Theoretical framework connecting air pollution exposures and mortality, including electrophysiological properties (35).
The goal of this analysis is to investigate potential modifiers of the PM2.5-sudden death association (i.e., where the response to PM varies across levels of other factors). To identify high-risk groups, we explored previously established associations between PM2.5 and sudden death stratified by individual and area-level factors that may modify the association between PM2.5 and sudden death. Defining clinical and environmental factors that either increase risk or decrease risk for sudden death might better serve to identify characteristics of an individual or a population that increase vulnerability to exposure susceptibility to a sudden death outcome. Knowledge of such clinical and environmental risks and salutary factors might improve interventional strategies to reduce sudden death in the face of an increasing likelihood of detrimental environmental exposures such as wildland fire smoke.
2 Methods
2.1 Study population and base exposure-outcome analysis
For this analysis, the study population, primary exposure, outcome, covariates, and base analysis are the same as in Rappazzo et al. (36). Briefly, data on sudden deaths in Wake County, NC, in the United States from 1 March 2013 to 28 February 2015 (n = 399) (1, 37, 38) was linked to PM2.5 concentrations, temperature, and relative humidity data acquired from the Environmental Protection Agency’s Air Quality System (39, 40). Hourly measurements of PM2.5, humidity, and temperature were downloaded from the single central site monitor in Wake County and averaged to daily 24-h periods (midnight to midnight) for linkage. All individuals included in this analysis experienced an out-of-hospital death that was defined as a sudden pulseless condition in the absence of terminal disease or overdose at the time of death. Emergency medical service (EMS) records were screened; following this, EMS and medical records, death certificates, medical examiner, and toxicology reports were obtained, and a panel of cardiologists adjudicated the cases to identify 399 victims of sudden death living in Wake County during this time period (26).
This study was conducted with a base population from Wake County, North Carolina. Wake County is a highly populated (approximately 1,000,000 people during the study period) and fast-growing area, with a highly educated population (53% with a bachelor’s degree compared to 32% for the United States), higher proportion of Black and African-American population (21% compared to 13% nationally), and lower poverty and disability than the United States average (7.4% vs. 11.4% poverty and 6% vs. 9% disability) (41, 42).
We used a case-crossover design with a time-stratified referent selection approach (43–47). In case-crossover designs, each individual serves as their own control, with the analytic focus on the question of when the event of interest occurs rather than whether it occurs. Control periods are in time-stratified design and are selected bi-directionally in the same calendar month-year to maximize exchangeability between event and referent periods.
Referent (control) days were selected within the same month and calendar year of the recorded death and on all the same days of the week (e.g., all Mondays within the event month/year if death occurred on a Monday). Odds ratios (ORs) and 95% confidence intervals (95%CIs) for mortality with a 5 μg/m3 increase in PM2.5 were estimated using conditional logistic regression models adjusted for temperature and relative humidity on the day of death/referent day (lag 0) and preceding lag days (lags 1 to 3) (natural cubic splines). Temperature and humidity are adjusted for as they are time-varying factors that may be related to the outcome; other factors are not adjusted for as the case-crossover approach accounts for non-time-varying factors by design. In the previous analysis, associations were elevated with exposure at a single day lag (lag 1); therefore, the analyses in this study use that as the base, unstratified, association.
2.2 Pathophysiologic framework and individual-level modifiers and mediators
As shown in Figure 1, the a priori concept driving the hypothesis tested in the study is based on a theoretical framework for air pollution associated with sudden death described by Cascio (35) in which the exposure to PM2.5 affects changes in inflammatory mediators and autonomic balance affecting electrophysiological properties that can be augmented in the presence of structural changes in the heart muscle (e.g., LVH) as a consequence of age and chronic hypertension increasing the risk of arrhythmia and sudden death (35). Greenspace metrics are incorporated into the framework as possible salutary factors that have the potential to reduce PM2.5 exposure and also modify the autonomic response to stresses.
Hypothesized individual-level modifiers and mediators investigated include those related to clinical markers of inflammation and arrhythmia risk and clinical conditions. Overweight/obesity (48) and NLR (31, 32) were chosen as markers of stress and inflammation LVH as a marker for a substrate for ventricular arrhythmia and sudden death (34). These markers were chosen due to their availability in the sudden death case registry data and their assumed place on the direct causal pathway to sudden death due to myocardial ischemia and infarction and spontaneous ventricular tachycardia and fibrillation, respectively. Specific variables were body mass index (BMI), which may be a flag for high levels of metabolic stress and inflammation related to diabetes and sleep apnea and may indicate a higher susceptibility to the impacts of air pollutants dichotomized at a BMI of less than or equal to 25 or above 25; LVH identified through review of existing echocardiograms, electrocardiographs, and autopsy reports, and classified as present in any of the three sources or absent in all, or as unknown if the subject lacked source records or if the subject had only electrocardiographs available that were negative for LVH (due to low sensitivity); and NLR dichotomized at greater than or equal to 2.15—a level that has previously been shown to be associated with increased mortality (30).
The following chronic conditions and risk factors associated with higher mortality were included in the analysis coronary artery disease, chronic respiratory disease, chronic kidney disease, diabetes, dyslipidemia, hypertension, and stroke. For analysis purposes, these conditions are stratified by the presence or absence of the condition. In addition to stratification by individual condition diagnoses, individuals were stratified by those having no clinical conditions, one clinical condition, or more than one clinical condition.
2.3 Area-level modifiers
We also considered greenspace and income metrics as potential area-level modifiers, as we had previously observed associations between greenspace metrics and sudden death across census tracts (28). These greenspace metrics were examined because they may promote physical activity and reduce stress, potentially act as a filter for air pollution, or act as a general buffer for environmental hazards. In this analysis, we linked the census tract greenspace metrics of greenway density, forest cover, urban land, and average tree canopy to individuals and stratified by median value across Wake County, NC. Greenspace metric details are described fully elsewhere (28, 49). Briefly, greenway density is the total length of greenways, trails, and multi-use trails in a census tract divided by the total area of that census tract. Information on greenways and trails was obtained from the GIS division of Wake County Government, North Carolina (28). Forest cover and urban land were both estimated using the National Land Cover Dataset 2011 with a 30 m spatial resolution (50). Area of forest cover (land use codes 41, 42, and 43) and urban land (land use codes 22, 23, and 24) were calculated for each census tract and divided by the total area of that census tract for a percentage metric (49). Finally, the average tree canopy was estimated using the National Land Cover Dataset 2011 Cartographic Canopy dataset (51), summing the percentage of tree canopy in each pixel across census tracts divided by the total number of pixels in the census tract. Median values across Wake County census tracts were calculated for each greenspace metric and stratified as above or below the median to signify “high” or “low” area greenspace. Area-level income has also been previously associated with sudden death (52) and was investigated here with an annual median household income at the census tract level from the 2010 Census, stratified at above or below the median for Wake County.
2.4 Statistical analysis
A case-crossover design cannot test non-temporal interaction effects in models. Therefore, we investigated stratified effects, in which the population is a subset to those only having or not having a particular condition, to identify potential modifiers of the base PM2.5-mortality association. Strata effects cannot be directly compared, so we use descriptive methods to determine differences, i.e., if stratified effect estimates appear to separate from the unstratified/base effect estimate in opposite directions, such that the stratified effect estimates would be different. For example, a base effect of 1.25 with stratified effects of 1.05 and 1.50. In addition, we performed sensitivity analyses on a population excluding individuals with chronic kidney disease and stroke (n = 62), as these groups were extreme in their clinical characteristics. All analyses were performed in SAS 9.4 (Cary, NC). Figures were created using R 3.5.3–4.2.3 (53) and Rstudio (54), with the tidyverse package (55).
This research was approved by the University of North Carolina at Chapel Hill’s Office of Human Research Ethics and has been approved yearly by administrative review (#14–2036). The Environmental Protection Agency’s Human Subjects Research Officer also reviewed this study and declared it non-human subjects research as all individuals were deceased at the time of data collection.
3 Results
3.1 Descriptive results
Characteristics of the study population and study area are presented in Tables 1, 2, with maps of area-level characteristics presented in Supplementary material (Supplementary Figures S1–S5). The study population is approximately two-thirds male, and one-third of Black or African-American, with a median age of 55 years. The prevalence of clinical conditions ranged from 7% (stroke) to 56% (hypertension). For the census tracts in the study area, median household income ranged from $17,000 to $169,000, with a median of $56,000 and a mean of $62,000. Greenspace metrics ranged broadly, with some census tracts having limited to no tree canopy or forest cover, whereas in other census tracks the majority of the land was classified as tree canopy or forest cover. Over the study period, PM2.5 ranged from 1.82 to 31.14, with a median of 10.25 and an IQR of 5.20. Seasonal distributions of PM2.5, relative humidity, and temperature are shown in Supplementary Table S1.
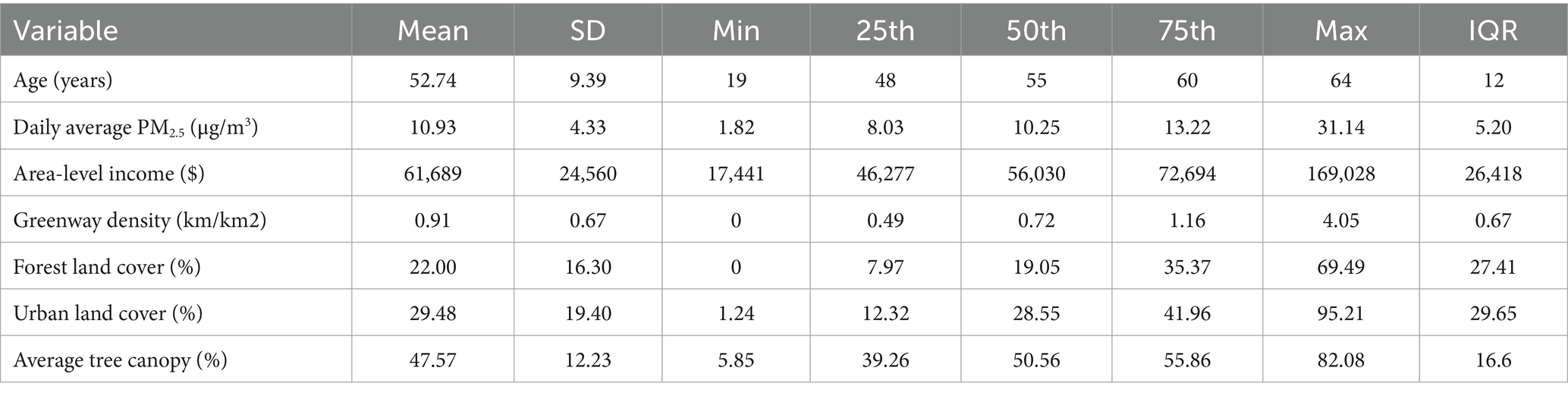
Table 2. Distributions of continuous variables across study population (age), study period (PM2.5), or study area (income and greenspace).
The unstratified estimate for a 5 μg/m3 increase in PM2.5 is 1.18 (0.98, 1.41). Stratified ORs are compared to this estimate. Results are presented for the main analysis in Figure 2; Supplementary Table S2, and for the population subset sensitivity analysis in Supplementary Table S2; Supplementary Figure S6.
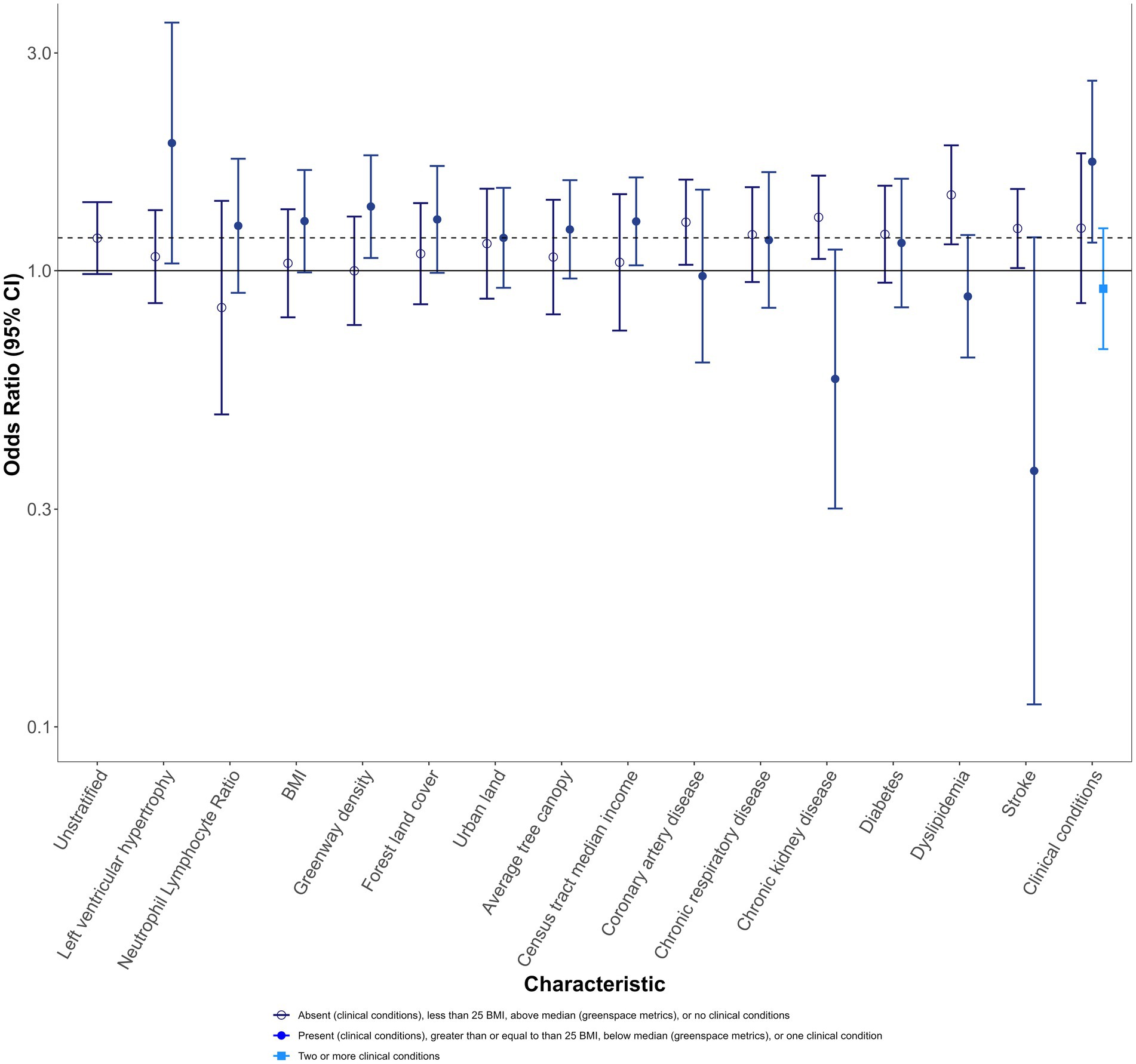
Figure 2. Mortality odds ratios and 95% CIs for 5 μg/m3 increase in PM2.5 1 day before recorded sudden death, stratified by individual and area-level characteristics, for the full population. Darker blue open circle OR: absent (clinical and specific clinical conditions), less than 25 BMI, above the median (greenspace metrics), or no clinical conditions. Medium blue closed circle OR: present (clinical and specific clinical conditions), greater than or equal to 25 BMI, below the median (greenspace metrics), or one clinical condition. Light blue square OR: two or more conditions.
3.2 Main analysis
Individuals with LVH had ORs elevated from the unstratified effect estimate (1.90 (1.04, 3.50)), while those without LVH had effects below the unstratified effect estimate (1.07 (0.85, 1.36)). Individuals with NLR followed a similar pattern as those with LVH, though ORs were less divergent for those with NLR. Individuals with higher BMI also had ORs elevated from the unstratified OR.
For greenspace metrics, effect estimates for those living in areas with lower greenway density, forest land cover, or average tree canopy were higher in magnitude than the overall population association. In particular, greenway density exhibited distinct separation (OR with more greenways: 1.00 (0.76, 1.31); OR with fewer greenways: 1.38 [1.07, 1.79)]. Results for census tract median income followed a similar pattern, with greater odds of sudden death for those living in below-median income areas and no evidence of effect for those living in above-median income areas.
ORs were typically higher for those without clinical conditions, with the exception of chronic respiratory disease and diabetes. When examining the number of conditions, those with no clinical conditions were similar to the unstratified effect estimate [1.24 (0.85, 1.81)], while those with a single clinical condition had an OR elevated from that effect [1.73 (1.15, 2.61)], and those with more than one clinical condition had a lower OR [0.91 (0.67, 1.24)].
3.3 Sensitivity analysis
Removal of individuals with stroke or kidney disease shifted stratified ORs away from the null compared to the full population, but overall patterns remained similar (Supplementary Table S2; Supplementary Figure S6). The difference between no clinical conditions and at least one clinical condition became similar to one another, suggesting that the reduction in OR observed in the main analysis was largely due to individuals with past stroke or kidney disease.
4 Discussion
Individuals with LVH or an abnormal NLR had a disproportionately higher likelihood of PM2.5-related death than those without these conditions, suggesting that they may be particularly susceptible to the impacts of air pollution exposures. Similarly, individuals with one clinical condition had higher PM2.5 ORs than the full population, though this did not hold for individuals with multiple conditions. PM2.5 was generally less impactful for individuals living in areas with higher levels of greenspace.
Our findings suggest that LVH and NLR may serve as clinical markers in a causal pathway whereby PM2.5 exposures increase the risk of sudden death. Both abnormalities may lead to cardiac repolarization changes (56) potentiating PM2.5 exposures that may result in ventricular fibrillation and sudden death. For example, animal evidence suggests that PM exposure may result in left ventricular remodeling (57), human evidence suggest that living near traffic may alter left ventricular mass (58, 59); long-term PM impacts these sub-clinical conditions, and that the presence of these conditions may lead to increased susceptibility to acute PM2.5 exposures. LVH and inflammation may be the final step in the mechanism whereby poor air quality and traditional risk factors trigger arrhythmia or myocardial ischemia and sudden death. Existing theoretical frameworks of the connection between PM2.5 exposure and mortality (Figure 1) may offer more insight into potential mechanisms and provide points of reference for future research and interventions (35).
4.1 Potential mechanisms/modes of action
Greenspace might affect PM2.5-mortality associations through a variety of pathways. Possibilities include exposure reduction, either due to lower air pollution emissions in those areas with higher levels of greenspace (56) or through the filtration and removal of air pollutants by vegetation (60–62). Higher levels of greenspace might also impact air pollution-related mortality by buffering through the salutary effects of stress reduction (63), improved social cohesion (64), or increased physical activity (65, 66). Greenspace metrics may also signify individuals living in wealthier areas with more opportunity to access resources, that we posit would act as an additional buffer to the negative effects of air pollution exposures.
Previous studies show that chronic conditions may increase the risk of the harmful health effects of air pollution, as they pose a more susceptible biological state; however, studies of the effects of short-term PM2.5 exposures on mortality in at-risk populations have produced mixed results (19). In an examination of the Nurses’ Health Study population, Puett et al. (67), observed potential differences in risk for those with and without hypertension, with all-cause mortality risks being somewhat lower in those with hypertension while fatal coronary heart disease risk was much higher in those with hypertension (67). Individuals with hypercholesteremia and higher BMIs evidenced higher risks for PM2.5-related all-cause mortality; but there were no differences across diabetes, median house value, or median household income strata (67). Wellenius et al. (68) found no differences in acute stroke risk with short-term PM2.5 exposures according to the presence of comorbid diabetes, hypertension, atrial fibrillation, or history of stroke (68). In our study, most chronic conditions appeared to confer a protective effect; this effect may have occurred due to the interaction of multiple chronic conditions or medical treatment. For example, those with cardiovascular disease and hypertension may be taking medications or behaving in ways that reduce the risk of heart attack and may counteract the detrimental effects of PM2.5 exposure (19, 69–71). By definition, individuals included in this study have out-of-hospital deaths, and it may be that those with these conditions are more likely to go to the hospital and experience within-hospital deaths, which would result in attenuated effects due to population selection. Alternatively, there may also be mechanistic pathways that are only observable, with specific causes of death not differentiated here.
4.2 Study limitations
This analysis has several potential limitations. The population is likely underpowered for full identification of potential modifiers because of the small number of subjects within each stratum. However, our sample size represents one of the largest population samples of all causes of sudden death among working-age adults. In addition, we are cautious in our interpretations of observed stratified effects and use them to identifying factors of potential mediators of air pollution on sudden death. A single site monitor was used to assign exposure and small area differences in air pollution, as between areas with and without greenspace, are not captured; though given the self-controlled design, this should not strongly impact results unless the response to PM is highly non-linear. The case-crossover design, while reducing the likelihood of unmeasured confounding, means that we cannot directly examine interaction effect estimates that would more directly identify modifying factors. Some of the potential modifiers are at the area level rather than individual level, and we cannot determine how individuals would have interacted with these, only that they lived in a census tract with those characteristics. Relatedly, we can only examine acute exposures, and there is potential for chronic air pollution to contribute to underlying conditions and susceptibility to acute air pollution exposures.
4.3 Study strengths
The analysis also has numerous strengths. The detailed demographic, geographic, clinical, and mortality data allowed us to explore potential modifiers of the PM2.5-mortality association, particularly LVH and NLR, potential direct causes of sudden death. We were also able to examine a diverse set of greenspace metrics from the National Land Cover Dataset, as the metrics analyzed may reflect different aspects of greenspace and are unavailable in the commonly used normalized difference vegetation index (28). In addition to these, other strengths include the control for confounding through study design and corresponding low expectation of residual confounding, the thorough case ascertainment that reduces the likelihood of potential selection bias, and the case definition that is expanded from the traditional definition and leads to the study populations being more racially and economically diverse than in previous studies of sudden death (36).
4.4 Conclusion
Here, we highlight the value of merging complex, environmental, clinical information, and geocoded events with newer, more sophisticated stratified analysis techniques. Our research approach should be generalizable to other complex clinical and research problems in cardiology, possibly to the study of the interaction of risk factors for atrial fibrillation, ischemic heart disease, heart failure, and common cardiovascular conditions with attendant morbidity, disability, and mortality. A better understanding of these complex interactions may support effective prevention efforts for targeted clinical syndromes.
Most importantly, our analysis supports the causal model of Cascio (35) that ventricular hypertrophy and inflammation are steps on a causal pathway leading from air pollution to sudden death (35). Further, our analysis supports the emerging mechanistic model that sudden death should be considered a syndrome, with causation from atherosclerosis, but, in addition, from primary arrhythmia related to structural abnormities in the myocardium, particularly ventricular hypertrophy (72). Further articulating the causal pathways leading to sudden death will help improve specificity in interventions and guide future research. We hope this research motivates more work in this field with larger cohorts. For the present, the causal pathway we outlined may serve as a guide for preliminary environmental and clinical intervention programs to mitigate sudden death. We believe our findings to be of import in light of the mounting evidence of PM as a driver of atherosclerosis progression, ischemic heart disease, heart failure, and arrhythmia. Understanding those who are most at-risk may aid in providing information on the means to limit exposures for those individuals.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Access to underlying health data may be permitted after appropriate review and approvals. Requests to access these datasets should be directed to RS, cm9zc19zaW1wc29uQG1lZC51bmMuZWR1.
Ethics statement
The studies involving humans were approved by University of North Carolina at Chapel Hill Institutional Review Board (#14–2036). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
KR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. NE: Data curation, Writing – review & editing. JW: Data curation, Visualization, Writing – review & editing. AC: Data curation, Writing – review & editing. GJ: Data curation, Writing – review & editing. SK: Data curation, Writing – review & editing. WC: Supervision, Writing – review & editing. RS: Investigation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The SUDDEN project is funded by private donations from the Heart and Vascular Division of the University of North Carolina at Chapel Hill and the McAllister Heart Institute in Chapel Hill, North Carolina. The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant Award Number 1 UL1TR001111. This effort was performed as part of normal duties at the U.S. Environmental Protection Agency.
Acknowledgments
The authors would like to recognize the contributions of Sanjana Thota for data assistance, Sarah Zelasky for figure assistance, Sarah Chen and Laura Jackson for comments and suggestions, and Thomas Luben and Stephanie Deflorio-Barker for their reviews. The authors would like to thank the North Carolina Office of the Chief Medical Examiner, the SUDDEN team of researchers, and the North Carolina Translational and Clinical Sciences Institute at the University of North Carolina at Chapel Hill.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author Disclaimer
The research described in this article has been reviewed by the Center for Public Health and Environmental Assessment, US EPA, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use. The Wake County EMS Data System supports, maintains, and monitors EMS service delivery, patient care, and disaster preparedness for the Wake County, NC community at large. This manuscript has been reviewed by Wake County EMS Data System investigators for scientific content and consistency of data interpretation with previous Wake County EMS Data System publications. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1367416/full#supplementary-material
Abbreviations
μg/m3, Micrograms per cubic meter; BMI, Body mass index; CI, Confidence interval; EMS, Emergency medical services; km/km2, Kilometer per square kilometer; LVH, Left ventricular hypertrophy; NC, North Carolina; NLR, Neutrophil-to-lymphocyte ratio; OR, Odds ratio; PM, particulate matter; PM2.5, particulate matter under 2.5 microns in aerodynamic diameter/fine particulate matter.
References
1. Mirzaei, M, Joodi, G, Bogle, B, Chen, S, and Simpson, RJ. Years of life and productivity loss because of adult sudden unexpected death in the United States. Med Care. (2019) 57:498–502. doi: 10.1097/MLR.0000000000001129
2. Sefton, C, Keen, S, Tybout, C, Lin, F-C, Jiang, H, Joodi, G, et al. Characteristics of sudden death by clinical criteria. Medicine. (2023) 102:e33029. doi: 10.1097/MD.0000000000033029
3. Lewis, ME, Lin, F-C, Nanavati, P, Mehta, N, Mounsey, L, Nwosu, A, et al. Estimated incidence and risk factors of sudden unexpected death. Open Heart. (2016) 3:e000321. doi: 10.1136/openhrt-2015-000321
4. Atkinson, R, Kang, S, Anderson, H, Mills, I, and Walton, H. Epidemiological time series studies of PM2. 5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. (2014) 69:660–5. doi: 10.1136/thoraxjnl-2013-204492
5. Dai, J, Chen, R, Meng, X, Yang, C, Zhao, Z, and Kan, H. Ambient air pollution, temperature and out-of-hospital coronary deaths in Shanghai. Environ Pollut. (2015) 203:116–21. doi: 10.1016/j.envpol.2015.03.050
6. Dockery, DW, Pope, CA 3rd, Xu, X, Spengler, JD, Ware, JH, Fay, ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. (1993) 329:1753–9. doi: 10.1056/NEJM199312093292401
7. Forastiere, F, Stafoggia, M, Picciotto, S, Bellander, T, D’Ippoliti, D, Lanki, T, et al. A case-crossover analysis of out-of-hospital coronary deaths and air pollution in Rome, Italy. Am J Respir Crit Care Med. (2005) 172:1549–55. doi: 10.1164/rccm.200412-1726OC
8. Hoek, G, Krishnan, RM, Beelen, R, Peters, A, Ostro, B, Brunekreef, B, et al. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. (2013) 12:43. doi: 10.1186/1476-069X-12-43
9. Marchini, T. Redox and inflammatory mechanisms linking air pollution particulate matter with cardiometabolic derangements. Free Radic Biol Med. (2023) 209:320–41. doi: 10.1016/j.freeradbiomed.2023.10.396
10. Münzel, T, Hahad, O, Sørensen, M, Lelieveld, J, Duerr, GD, Nieuwenhuijsen, M, et al. Environmental risk factors and cardiovascular diseases: a comprehensive expert review. Cardiovasc Res. (2022) 118:2880–902. doi: 10.1093/cvr/cvab316
11. Pope, CA, and Dockery, DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage Assoc. (2006) 56:709–42. doi: 10.1080/10473289.2006.10464485
12. Rajagopalan, S, Al-Kindi, SG, and Brook, RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. (2018) 72:2054–70. doi: 10.1016/j.jacc.2018.07.099
13. Rosenthal, FS, Kuisma, M, Lanki, T, Hussein, T, Boyd, J, Halonen, JI, et al. Association of ozone and particulate air pollution with out-of-hospital cardiac arrest in Helsinki, Finland: evidence for two different etiologies. J Expo Sci Environ Epidemiol. (2013) 23:281–8. doi: 10.1038/jes.2012.121
14. Samet, JM, Dominici, F, Curriero, FC, Coursac, I, and Zeger, SL. Fine particulate air pollution and mortality in 20 US cities, 1987–1994. N Engl J Med. (2000) 343:1742–9. doi: 10.1056/NEJM200012143432401
15. Schwartz, J. What are people dying of on high air pollution days? Environ Res. (1994) 64:26–35. doi: 10.1006/enrs.1994.1004
16. Schwartz, J. Assessing confounding, effect modification, and thresholds in the association between ambient particles and daily deaths. Environ Health Perspect. (2000) 108:563–8. doi: 10.1289/ehp.00108563
17. Serinelli, M, Vigotti, MA, Stafoggia, M, Berti, G, Bisanti, L, Mallone, S, et al. Particulate matter and out-of-hospital coronary deaths in eight Italian cities. Occup Environ Med. (2010) 67:301–6. doi: 10.1136/oem.2009.046359
18. U.S. EPA. Integrated science assessment (ISA) for particulate matter (final report, Dec 2009). In: Agency USEP. Washington, DC (2009).
19. U.S. EPA. Integrated science assessment (ISA) for particulate matter (final report, Dec 2019). Washington, DC: U.S. Environmental Protection Agency (2019).
20. Kaufman, JD, Adar, SD, Barr, RG, Budoff, M, Burke, GL, Curl, CL, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the multi-ethnic study of atherosclerosis and air pollution): a longitudinal cohort study. Lancet. (2016) 388:696–704. doi: 10.1016/S0140-6736(16)00378-0
21. Pope, CA, Ezzati, M, Cannon, JB, Allen, RT, Jerrett, M, and Burnett, RT. Mortality risk and PM 2.5 air pollution in the USA: an analysis of a national prospective cohort. Air Qual Atmos Health. (2018) 11:245–52. doi: 10.1007/s11869-017-0535-3
22. Yu, Y, Yao, S, Dong, H, Wang, L, Wang, C, Ji, X, et al. Association between short-term exposure to particulate matter air pollution and cause-specific mortality in Changzhou, China. Environ Res. (2019) 170:7–15. doi: 10.1016/j.envres.2018.11.041
23. Hajat, A, Hsia, C, and O’Neill, MS. Socioeconomic disparities and air pollution exposure: a global review. Curr Environ Health Rep. (2015) 2:440–50. doi: 10.1007/s40572-015-0069-5
24. Sacks, JD, Stanek, LW, Luben, TJ, Johns, DO, Buckley, BJ, Brown, JS, et al. Particulate matter–induced health effects: who is susceptible? Environ Health Perspect. (2011) 119:446–54. doi: 10.1289/ehp.1002255
25. Solomon, GM, Morello-Frosch, R, Zeise, L, and Faust, JB. Cumulative environmental impacts: science and policy to protect communities. Annu Rev Public Health. (2016) 37:83–96. doi: 10.1146/annurev-publhealth-032315-021807
26. Joodi, G, Maradey, JA, Bogle, B, Mirzaei, M, Sadaf, MI, Pursell, I, et al. Coronary artery disease and atherosclerotic risk factors in a population-based study of sudden death. J Gen Intern Med. (2020) 35:531–7. doi: 10.1007/s11606-019-05486-6
27. Sadaf, MI, Caldwell, M, Young, LA, Mirzaei, M, Chen, S, Joodi, G, et al. High prevalence of diabetes mellitus and mental illness among victims of sudden death. South Med J. (2021) 114:86–91. doi: 10.14423/SMJ.0000000000001213
28. Wu, J, Rappazzo, KM, Simpson, RJ, Joodi, G, Pursell, IW, Mounsey, JP, et al. Exploring links between greenspace and sudden unexpected death: a spatial analysis. Environ Int. (2018) 113:114–21. doi: 10.1016/j.envint.2018.01.021
29. Capone, A, Rabii, K, Joodi, G, Choi, WS, Lin, FC, Pursell, IW, et al. P6409High prevalence of left ventricular hypertrophy among all-cause sudden unexpected death victims. Eur Heart J. (2017) 38:409. doi: 10.1093/eurheartj/ehx493.P6409
30. Kim, S, Eliot, M, Koestler, DC, Wu, WC, and Kelsey, KT. Association of Neutrophil-to-Lymphocyte Ratio with Mortality and Cardiovascular Disease in the Jackson heart study and modification by the Duffy antigen variant. JAMA Cardiol. (2018) 3:455–62. doi: 10.1001/jamacardio.2018.1042
31. Koza, Y. What is the clinical benefit of neutrophil-lymphocyte ratio in cardiovascular patients? J Cardiovasc Thoracic Res. (2014) 6:131–2. doi: 10.5681/jcvtr.2014.028
32. Koza, Y. Neutrophil–Lympocyte ratio and cardiovascular diseases: An update. SAGE Publications Sage CA: Los Angeles, CA, pp. 105–106. (2016).
33. Spirito, P, Bellone, P, Harris, KM, Bernabò, P, Bruzzi, P, and Maron, BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. (2000) 342:1778–85. doi: 10.1056/NEJM200006153422403
34. Stewart, MH, Lavie, CJ, Shah, S, Englert, J, Gilliland, Y, Qamruddin, S, et al. Prognostic implications of left ventricular hypertrophy. Prog Cardiovasc Dis. (2018) 61:446–55. doi: 10.1016/j.pcad.2018.11.002
35. Cascio, WE. Proposed pathophysiologic framework to explain some excess cardiovascular death associated with ambient air particle pollution: insights for public health translation. Biochim Biophys Acta. (2016) 1860:2869–79. doi: 10.1016/j.bbagen.2016.07.016
36. Rappazzo, KM, Joodi, G, Hoffman, SR, Pursell, IW Jr, Mounsey, JP, Cascio, WE, et al. A case-crossover analysis of the relationship of air pollution with out-of-hospital sudden unexpected death in Wake County, North Carolina (2013-2015). Sci Total Environ. (2019) 694:133744. doi: 10.1016/j.scitotenv.2019.133744
37. Nanavati, PP, Mounsey, JP, Pursell, IW, Simpson, RJ Jr, Lewis, ME, Mehta, ND, et al. Sudden unexpected death in North Carolina (SUDDEN): methodology review and screening results. Open Heart. (2014) 1:e000150. doi: 10.1136/openhrt-2014-000150
38. Patel, S, Conover, MM, Joodi, G, Chen, S, Simpson, RJ Jr, and Deyo, ZM. Medication use in women and men with sudden unexpected death. Ann Pharmacother. (2018) 52:868–75. doi: 10.1177/1060028018771061
39. U.S. EPA. Air data: Air quality data collected at outdoor monitors across the US (2023). Available at: https://www.epa.gov/outdoor-air-quality-data.
40. U.S. EPA. Air quality system (AQS) API. (2020). Available at: https://aqs.epa.gov/aqsweb/documents/data_api.html.
41. U.S. Census Bureau. Quick facts Wake County, North Carolina (2021). Available at: https://www.census.gov/quickfacts/fact/table/wakecountynorthcarolina/PST045221.
42. U.S. Census Bureau. Quick facts United States (2021). Available at: https://www.census.gov/quickfacts/fact/table/US/PST045221.
43. Jaakkola, JJ. Case-crossover design in air pollution epidemiology. Eur Respir J Suppl. (2003) 40:81s–5s. doi: 10.1183/09031936.03.00402703
44. Janes, H, Sheppard, L, and Lumley, T. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology. (2005) 16:717–26. doi: 10.1097/01.ede.0000181315.18836.9d
45. Maclure, M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. (1991) 133:144–53. doi: 10.1093/oxfordjournals.aje.a115853
46. Maclure, M, and Mittleman, MA. Should we use a case-crossover design? Annu Rev Public Health. (2000) 21:193–221. doi: 10.1146/annurev.publhealth.21.1.193
47. Maclure, M, and Mittleman, MA. Case-crossover designs compared with dynamic follow-up designs. Epidemiology. (2008) 19:176–8. doi: 10.1097/EDE.0b013e318162afb9
48. Powell-Wiley, TM, Poirier, P, Burke, LE, Després, J-P, Gordon-Larsen, P, Lavie, CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. (2021) 143:e984–e1010. doi: 10.1161/CIR.0000000000000973
49. Wu, J, and Jackson, L. Association of land use and its change with beach closure in the United States, 2004-2013. Sci Total Environ. (2016) 571:67–76. doi: 10.1016/j.scitotenv.2016.07.116
50. Homer, C, Dewitz, J, Yang, L, Jin, S, Danielson, P, Xian, G, et al. Completion of the 2011 National Land Cover Database for the conterminous United States–representing a decade of land cover change information. Photogramm Eng Remote Sens. (2015) 81:345–54. doi: 10.1016/S0099-1112(15)30100-2
51. MRLC. Multi-resolution land characteristics consortium. Data (2023). Available at: https://www.mrlc.gov/data.
52. Mounsey, LA, Lin, F-C, Pursell, I, Joodi, G, Lewis, ME, Nwosu, A, et al. Relation of household income to incidence of sudden unexpected death in Wake County, North Carolina. Am J Cardiol. (2017) 119:1030–5. doi: 10.1016/j.amjcard.2016.11.061
53. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2021).
54. RStudio Team. RStudio: Integrated development environment for R. Boston, MA: RStudio, Inc. (2019).
55. Wickham, H, Averick, M, Bryan, J, Chang, W, McGowan, LDA, François, R, et al. Welcome to the Tidyverse. J Open Source Softw. (2019) 4:1686. doi: 10.21105/joss.01686
56. Rosen, MR, and Janse, MJ. Concept of the vulnerable parameter: the Sicilian gambit revisited. J Cardiovasc Pharmacol. (2010) 55:428–37. doi: 10.1097/FJC.0b013e3181bfaddc
57. Wold, LE, Ying, Z, Hutchinson, KR, Velten, M, Gorr, MW, Velten, C, et al. Cardiovascular remodeling in response to long-term exposure to fine particulate matter air pollution. Circ Heart Fail. (2012) 5:452–61. doi: 10.1161/CIRCHEARTFAILURE.112.966580
58. Gill, EA, Curl, CL, Adar, SD, Allen, RW, Auchincloss, AH, O’Neill, MS, et al. Air pollution and cardiovascular disease in the multi-ethnic study of atherosclerosis. Prog Cardiovasc Dis. (2011) 53:353–60. doi: 10.1016/j.pcad.2011.02.001
59. Van Hee, VC, Adar, SD, Szpiro, AA, Barr, RG, Bluemke, DA, Diez Roux, AV, et al. Exposure to traffic and left ventricular mass and function: the multi-ethnic study of atherosclerosis. Am J Respir Crit Care Med. (2009) 179:827–34. doi: 10.1164/rccm.200808-1344OC
60. Baldauf, R. Roadside vegetation design to improve local, near-road air quality. Transp Res D Transp Environ. (2017) 52:354–61. doi: 10.1016/j.trd.2017.03.013
61. Brantley, HL, Hagler, GS, Deshmukh, PJ, and Baldauf, RW. Field assessment of the effects of roadside vegetation on near-road black carbon and particulate matter. Sci Total Environ. (2014) 468-469:120–9. doi: 10.1016/j.scitotenv.2013.08.001
62. Markevych, I, Schoierer, J, Hartig, T, Chudnovsky, A, Hystad, P, Dzhambov, AM, et al. Exploring pathways linking greenspace to health: theoretical and methodological guidance. Environ Res. (2017) 158:301–17. doi: 10.1016/j.envres.2017.06.028
63. Egorov, AI, Griffin, SM, Converse, RR, Styles, JN, Klein, E, Scott, J, et al. Greater tree cover near residence is associated with reduced allostatic load in residents of Central North Carolina. Environ Res. (2020) 186:109435. doi: 10.1016/j.envres.2020.109435
64. Jennings, V, and Bamkole, O. The relationship between social cohesion and urban green space: an avenue for health promotion. Int J Environ Res Public Health. (2019) 16:452. doi: 10.3390/ijerph16030452
65. Astell-Burt, T, Feng, X, and Kolt, GS. Green space is associated with walking and moderate-to-vigorous physical activity (MVPA) in middle-to-older-aged adults: findings from 203 883 Australians in the 45 and up study. Br J Sports Med. (2014) 48:404–6. doi: 10.1136/bjsports-2012-092006
66. Coombes, E, Jones, AP, and Hillsdon, M. The relationship of physical activity and overweight to objectively measured green space accessibility and use. Soc Sci Med. (2010) 70:816–22. doi: 10.1016/j.socscimed.2009.11.020
67. Puett, RC, Hart, JE, Yanosky, JD, Paciorek, C, Schwartz, J, Suh, H, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ health study. Environ Health Perspect. (2009) 117:1697–701. doi: 10.1289/ehp.0900572
68. Wellenius, GA, Burger, MR, Coull, BA, Schwartz, J, Suh, HH, Koutrakis, P, et al. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. (2012) 172:229–34. doi: 10.1001/archinternmed.2011.732
69. Tong, H, Rappold, AG, Caughey, M, Hinderliter, AL, Bassett, M, Montilla, T, et al. Dietary supplementation with olive oil or fish oil and vascular effects of concentrated ambient particulate matter exposure in human volunteers. Environ Health Perspect. (2015) 123:1173–9. doi: 10.1289/ehp.1408988
70. Zhong, J, Karlsson, O, Wang, G, Li, J, Guo, Y, Lin, X, et al. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc Natl Acad Sci USA. (2017) 114:3503–8. doi: 10.1073/pnas.1618545114
71. Zhong, J, Trevisi, L, Urch, B, Lin, X, Speck, M, Coull, BA, et al. B-vitamin supplementation mitigates effects of fine particles on cardiac autonomic dysfunction and inflammation: a pilot human intervention trial. Sci Rep. (2017) 7:45322. doi: 10.1038/srep45322
Keywords: particulate matter, sudden death, modification, greenspace, left ventricular hypertrophy, inflammation, arrhythmia risk
Citation: Rappazzo KM, Egerstrom NM, Wu J, Capone AB, Joodi G, Keen S, Cascio WE and Simpson RJ Jr (2024) Fine particulate matter-sudden death association modified by ventricular hypertrophy and inflammation: a case-crossover study. Front. Public Health. 12:1367416. doi: 10.3389/fpubh.2024.1367416
Edited by:
Worradorn Phairuang, Kanazawa University, JapanReviewed by:
Yaowatat Boongla, Thammasat University, ThailandTimoteo Marchini, University Heart Center Freiburg, Germany
Copyright © 2024 Rappazzo, Egerstrom, Wu, Capone, Joodi, Keen, Cascio and Simpson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristen M. Rappazzo, UmFwcGF6em8ua3Jpc3RlbkBlcGEuZ292
 Kristen M. Rappazzo
Kristen M. Rappazzo Nicole M. Egerstrom2
Nicole M. Egerstrom2 Jianyong Wu
Jianyong Wu Wayne E. Cascio
Wayne E. Cascio