- 1Department of Nursing, Kunming Municipal Hospital of Traditional Chinese Medicine, Kunming, China
- 2Department of Clinical Psychology, Yunnan Provincial Hospital of Infectious Disease, Kunming, China
- 3Cardiology Department, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 4Psychiatric Department, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 5ICU in Geriatric Department, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 6Urology Department, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 7Neurosurgery Department, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 8General Surgery Department, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 9Department of Nursing, The First Affiliated Hospital of Kunming Medical University, Kunming, China
Background: Families of children with congenital heart disease (CHD) face tremendous stressors in the process of coping with the disease, which threatens the health of families of children with CHD. Studies have shown that nursing interventions focusing on family stress management can improve parents’ ability to cope with illness and promote family health. At present, there is no measuring tool for family stressors of CHD.
Methods: The items of the scale were generated through qualitative interviews and a literature review. Initial items were evaluated by seven experts to determine content validity. Factor analysis and reliability testing were conducted with a convenience sample of 670 family members. The criterion-related validity of the scale was calculated using scores on the Self-Rating Anxiety Scale (SAS).
Results: The CHD Children’s Family Stressor Scale consisted of six dimensions and 41 items. In the exploratory factor analysis, the cumulative explained variance of the six factors was 61.085%. In the confirmatory factor analysis, the six factors in the EFA were well validated, indicating that the model fits well. The correlation coefficient between CHD Children’s Family Stressor Scale and SAS was r = 0.504 (p < 0.001), which indicated that the criterion-related validity of the scale was good. In the reliability test, Cronbach’s α coefficients of six sub-scales were 0.774–0.940, and the scale-level Cronbach’s α coefficient value was 0.945.
Conclusion: The study indicates that the CHD Children’s Family Stressor Scale is valid and reliable, and it is recommended for use in clinical practice to assess CHD children’s family stressors.
Background
Congenital heart disease (CHD) is the most common birth defect worldwide, accounting for almost 30% of all major congenital disabilities, and the mean prevalence of CHD globally was 8.2 per thousand (1, 2). CHD has become the leading cause of death from birth defects in infants and young children (3–5), and the global age-standardized mortality rate of CHD in 2017 was 3.9/100,000 (5). Compared with normal children, children with CHD are prone to recurrent disease with many serious complications. Some children with complex CHD often need multiple palliative or corrective surgery, continuous nutritional support, and drug treatment (6–8). Furthermore, children with CHD might have lower cardiorespiratory endurance and physical activity, impaired growth and personality development, intellectual and learning disabilities, and psychiatric disorders such as anxiety, depression, and attention deficit (9), all of which cause a huge burden to the family. It has been evidenced that parents of children with CHD have to deal with the stress of changing parental roles, parenting burdens and balancing responsibilities, communication problems with healthcare providers, insufficient support network, financial issues and balancing work and family responsibilities (10). As for the children with CHD, they might suffer peer bullying and isolation, difficulties with academic achievement, distressing inability to participate in sports and disturbed body image (6). In addition, for the siblings of children with CHD, their education, social activities, and physical and mental health are affected as well (11, 12). All the above suggest that families of children with CHD are under great pressure, which will lead to adverse health outcomes for family members and family function as well, and ultimately reduce the family health level (13–15). Therefore, it is particularly important to take action to relieve the stress of families of children with CHD. Research has shown that nursing measures focusing on family stress management can reduce parents’ distress, improve their ability to cope with the disease (16–18), and promote family health. The first step to conduct family stress management is an accurate diagnosis of family stressors. Therefore, the development of the stressors scale for families of children with CHD can help healthcare providers accurately and efficiently assess the stressors in children’s families, and based on this, interventions and educational strategies can be established to help families effectively cope with stress, thereby promoting family health (19).
A wide variety of measures, including general and disease-specific scales, have been used to assess parental stressors. Streisand et al. (20) developed the Pediatric Inventory for Parents (PIP) to measure parental stressors consisting of 42 items and four dimensions. PIP have been applied in many chronic diseases, including cancer, inflammatory bowel disease and type I diabetes, and demonstrated good reliability and validity (21). Katie et al. (22) developed the Pediatric Parenting Stress Inventory (PPSI) based on a literature review and clinical experience to identify problems and disease-related stressors experienced by parents of children with severe illness, which consists of 45-item and five dimensions. Cronbach’s α of the scale is 0.94 and has good validity. Miles et al. (23) developed the Parental Stressor Scale: Pediatric Intensive Care Unit (PSS:PICU) to measure the stressors of parents when their children were admitted to the intensive care unit, which includes 79 items and seven dimensions. Cronbach’s α of the scale ranges from 0.69 to 0.95 for each dimension and has good validity. Margaret et al. (24) developed the Parental Stressor Scale: Neonatal Intensive Care Unit, which is designed to measure parental perceptions of stressors arising from the physical and psychosocial environment of the neonatal intensive care unit. The scale includes 26 items and three dimensions, and Cronbach’s α of the scale ranges from 0.89 to 0.94. Miles et al. (25) also developed the Parental Stressor Scale: Infant Hospitalization (PSS:IH) based on Margaret’s NICU parental stressor scale to measure the stressors of parents of children in the ICU or general pediatric ward. It includes 22 items and three dimensions, and Cronbach’s α of the scale ranges from 0.87 to 0.90. However, the above scales focused on the parents instead of the family, ignoring the systemic responses of the family as a whole. Burke et al. (26) obtained The Burke Assessment Guide to Stressors and Tasks in Families with a Child with a Chronic Condition by studying nine families for 10 years and is gradually perfected by compiling the family’s description of the problems dealt with in the past and the problems dealt with now. Finally, 52 concerns of families of children with chronic diseases were summarized, including 11 groups of different stressors, which were used to evaluate the stressors of families with chronic diseases. However, it was only used as an evaluation tool and did not form a scale, and its reliability and validity were not yet clear. So far, there is no tool to assess stressors in families of children with CHD. The lack of a well-validated and reasonably comprehensive scale to measure the stressors of families with children diagnosed with CHD may be a key barrier to implement family stress management for these families. The purpose of this study was to develop and validate a scale to assess the stressors of families with children diagnosed with CHD using the family as the research unit.
Methods
Study design
The scale was developed using a sequential exploratory mixed research method combining qualitative research with a quantitative procedure in three phases: Phase 1 for generation of the item pool through interviewing family members of children with CHD and reviewing the relevant literature; Phase 2 for item improvement and content validity evaluation by expert judgments; Phase 3 for evaluation of the psychometric properties, such as construct validity, internal consistency reliability and criterion-related validity. The study was approved by the Kunming Medical University Research Ethics Committee. Participants in both qualitative and quantitative research understood the purpose of the research, participated voluntarily in this research, and had the right to withdraw from the research at any time.
Phase 1: Generation of the item pool
Procedure and participants
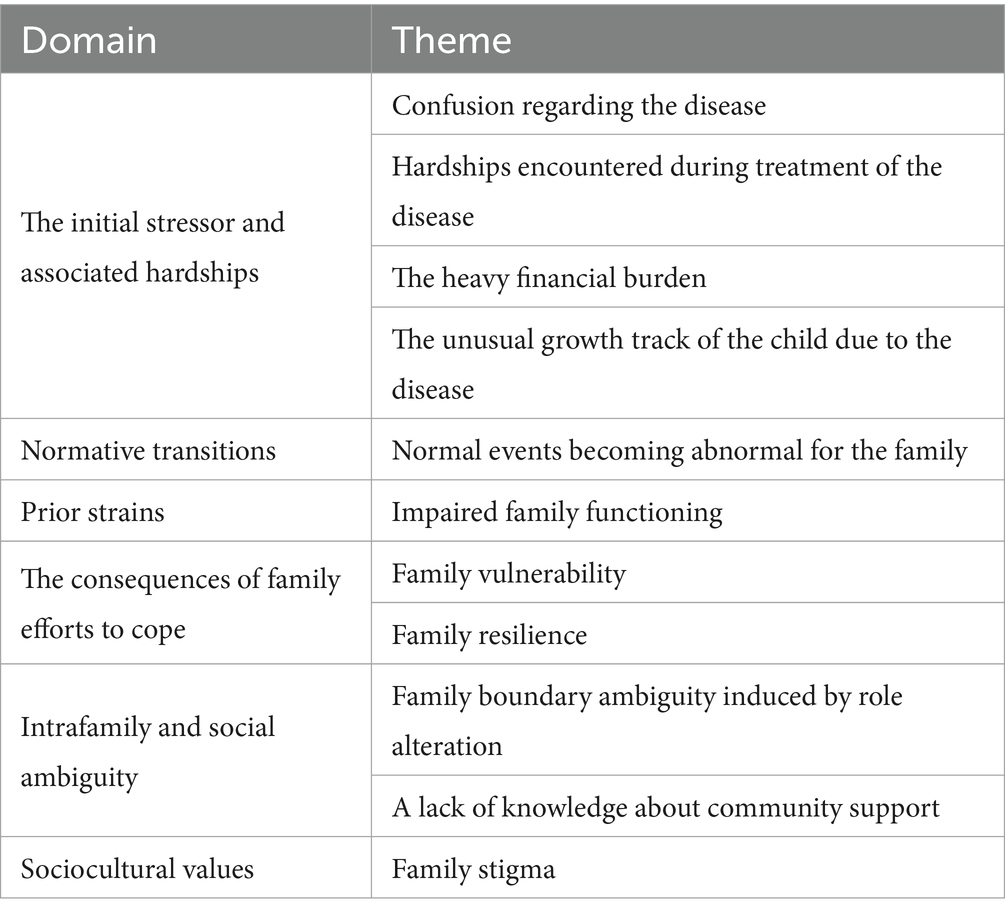
A qualitative study using in-depth, face-to-face interviews was applied to explore the stressors in families of children with CHD. We used a purposive sampling method to select family members of CHD children in a tertiary referral hospital in Yunnan Province, China. Participants were stratified according to the types of surgery (interventional medical and surgery operation) of CHD children and then sampled into subgroups divided according to the different stages of the disease (diagnosis, preoperative, postoperative, and follow-up). The family members were selected based on (1) having a son or daughter with CHD who was between 0 and 14 years of age, (2) having the ability to understand and express their own experiences and ideas in Chinese, and (3) being able to participate in the study with a signed informed consent form. The exclusion criteria were as follows: family members with complicated conditions such as severe diseases of other systems except for heart or other serious diseases or already being in end-stage CHD. A total of 21 participants (five fathers and 16 mothers) completed semi-structured interviews. Examples of guiding questions were as follows: (a) What are you worried about since the child was diagnosed? (b) What are the differences in your family life before and after your child has got the illness? (c) Apart from the illness of the child, what are other difficulties for your family? (d) What makes you feel stressed in the process of treating the disease and caring for the child? (e) What difficulties do family members face when working and socializing? (f) Please describe the most memorable difficulties you experienced after your child was diagnosed. The data were analyzed using directed content analysis according to the double ABCX model of family stress and adaptation (27, 28). In the model, the stressors of the family are cumulative and include five aspects: the initial stressors and their hardship, normative transitions, the prior strains, the consequences of a family effort to cope, and ambiguity, both intra-family and social, which could inform the initial direction of qualitative data analysis without limiting the identification of new themes (28, 29).
Content analysis was selected as the preferred data analysis method to obtain themes. The themes obtained from qualitative research analysis provided a theoretical basis for the formation of the scale’s structure and were combined with relevant literature to compile the scale’s item pool. To eliminate the risk of bias, the interviewer (ZY) and corresponding author (MF), who had expertise in qualitative data, analyzed the data independently. Finally, 10 themes emerged from qualitative research; Table 1 shows the themes of the qualitative study.
DeVellis (30) suggested that the item should avoid double-barreled, ambiguous pronoun references and exceptionally lengthy items to assure its clarity and reading difficulty level. A combination of forward and reverse entries was used to avoid an acquiescence, affirmation or agreement bias (30). Entries retained a degree of redundancy so that items that did not behave as expected could be removed at a later stage of validation (30). A total of 85 items were generated based on the results of qualitative research and literature review.
Phase 2: Item improvement and content validity evaluation
Procedure and participants
Two rounds of item improvement were performed during this phase. The first round applied expert group meetings. We invited two experts who had been engaged in children’s heart treatment and worked for 15 years, one expert in the field of children’s nursing who had worked for 15 years, one expert in the field of child psychology with PhD qualification and worked for 15 years, one expert in the field of CHD health management and worked for 20 years, one social worker working for CHD relief programmer and worked for a year to hold an expert consensus meeting. Each expert indicated their own suggestions (to add, delete, and modify) on whether the items were clearly stated, repeated, reflected the stressors of families with children diagnosed with CHD, and were suitable for the Chinese cultural background. The second round used expert consultation. An e-mail consultation questionnaire was sent separately to two experts in the field of children’s heart treatment and five experts in the field of children’s heart nursing. These experts included one pediatric cardiac surgery specialist with PhD qualification who worked for 10 years; one pediatric cardiology specialist with a bachelor’s degree and who worked for more than 20 years; two CHD nursing specialists with master’s qualifications who worked for 15 years; three nursing specialists with bachelor’s degree and worked for more than 20 years in CHD caring. The experts were asked to rate the items on the scale using a 4-point evaluation scale (1 = not relevant, 2 = somewhat relevant, 3 = relevant, and 4 = highly relevant) (31). There is an opinion column in the consultation questionnaire, and experts can recommend additions, deletions or revisions to dimensions and items. Through calculating the inter-rater agreement (IR); content validity index for items (I-CVI); scale-level content validity index and universal agreement calculation (S-CVI/UA); scale-level content validity index, averaging calculation method (S-CVI/Ave), and the content validity of the scale was evaluated (31, 32). Items with the I-CVI value of ≥0.78 were retained (31).
After the expert consensus meeting discussion, the initial item pool was revised from 85 to 76, eight items were deleted, four items were modified, and one item was added. In the expert consultation, the content validity of the calculation scale is as follows: 63 items with the I-CVI of 1.00, 12 with the I-CVI of 0.71, and 1 with the I-ICV of 0.57. Finally, 13 items with the I-CVI of ≤0.78 were deleted according to the evaluation criteria of I-CVI. Based on the results of the expert consultation, no additions, deletions, or revisions to the scale items were required IR = 0.83, indicating good agreement among experts; The S-CVI/UA was 0.829, and the S-CVI/Ave was 0.949, which meant items had good correlation and representativeness, suggesting the scale can be applied in practice. Responses were given a 5-point Likert scale (0 = no effect, 4 = extremely affected) to reflect the impact of the items that occurred in the family, and the non-occurrence in the scale options with a score of 0 indicates that the item does not occur. A total of 20 family members of children with CHD were selected to conduct a pilot study in the form of interviews to find out whether the difficulty level of the item was appropriate and whether the expression was accurate and clear. They generally responded that the scale was easy to fill in, and the items were clear and unambiguous.
Phase 3: Psychometrics properties of the questionnaire
Questionnaire
The questionnaire is divided into four parts. The first part is the introduction, which explains the research purpose, confidentiality principle, and ethical principle. The second part is the general data questionnaire, which collects relevant demographic data, including age, gender, role of family members, educational level, diagnosis of the child, and type of surgery. The third part is the 63-item CHD Children’s Family Stressor Scale. The fourth part is the Self-Rating Anxiety Scale (SAS). Studies have shown that the more stressors people have, the more serious their anxiety (33) are. Therefore, in this study, the anxiety level of the children’s family members was used to calculate the scale’s calibration-related validity.
Sample
We gave out the CHD Children’s Family Stressor Scale to family members of children with CHD based on the following inclusion and exclusion criteria. The inclusion criteria are as follows: (1) children with CHD are less than 14 years old; (2) children’s stem family members, such as parents, grandparents, and siblings (over 18 years old); (3) living with the child; (4) agree to participate in this study; (5) were not diagnosed with psychiatric, with basic listening, speaking, reading, and writing skills, and being able to read Chinese. The exclusion criteria are as follows: (1) history of mental illness or cognitive impairment, such as psychotic, mood, or anxiety disorder; (2) children with complicated conditions, such as severe diseases of systems other than the heart or end-stage CHD; (3) communication barriers (unable to communicate in Chinese). The sample size was calculated based on an item (an item is a question within the questionnaire) to participant ratio of 1:5–1:10, and an average attrition rate of 20% in questionnaire responses was considered (30).
Data collection
The survey was conducted in three tertiary hospitals in China from December 2021 to March 2022. These tertiary hospitals are located in cities southwest China, such as Kunming, Chengdu and Guangzhou. The researchers collected data through paper questionnaires and the Questionnaire Star app. The paper questionnaire and the electronic questionnaire were sent by the researcher to the participants to fill in. The researchers will explain the purpose of the study and the rules for filling it out to participants. Questionnaire completion was voluntary and anonymous. Each questionnaire was completed independently by participants. A total of 800 questionnaires were sent to the participants, and 748 questionnaires were returned. The response rate of the questionnaire was 93.5%. After excluding unqualified questionnaires, a total of 670 questionnaires were recovered and analyzed. The criteria for excluding are as follows: (1) missing items are greater than or equal to one item, (2) all items have the same score, and (3) select two or more options for the same item.
Data analysis
The database was established by Excel and imported into R language, SPSS 26.0 statistical software, and AMOS 24.0 statistical software for data analysis. First, exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) were used to test the construct validity of the scale. The entire sample was randomly divided into two sub-samples (A and B). Sub-sample A (n = 335) was used for EFA, and sub-sample B (n = 335) for CFA. Before using EFA, the Keizer–Meyer–Olkin (KMO) measure of sampling adequacy and Bartlett’s sphere test were conducted to check whether the data were suitable for EFA. Data factorability was determined by a KMO value of at least 0.60 and the significance of Bartlett’s test of sphericity value (p < 0.05) (34). A Monte Carlo parallel factor analysis was performed using R language to determine the number of factors. SPSS 26.0 was used to perform principal axis factor analysis and oblimin rotation method to identify meaningful factor dimensions (29, 35). The appropriate items were selected based on the factor loading more than 0.4 (36, 37). Sub-sample B (n = 355) was used for CFA to verify the feasibility of factor results from EFA with AMOS 24.0. Chi-square/degrees of freedom (CMIN/DF), incremental fit index (IFI), comparative fit index (CFI), goodness-of-fit index (GFI), root of mean square residual (RMR), and root-mean-square error of approximation (RMSEA) was adopted as goodness-of-fit indicators to evaluate the degree of interpretation of the constructed factor structure to the sample (38, 39). The scale’s calibration-related validity was calculated using scores on the Self-Rating Anxiety Scale (SAS). Cronbach’s α coefficient was used to evaluate the internal consistency reliability, and the acceptable level should be greater than 0.7 (40). The configural invariance, metric invariance and scalar invariance models were performed to verify the measurement invariance of the scale with AMOS 24.0.
Results
Demographic data
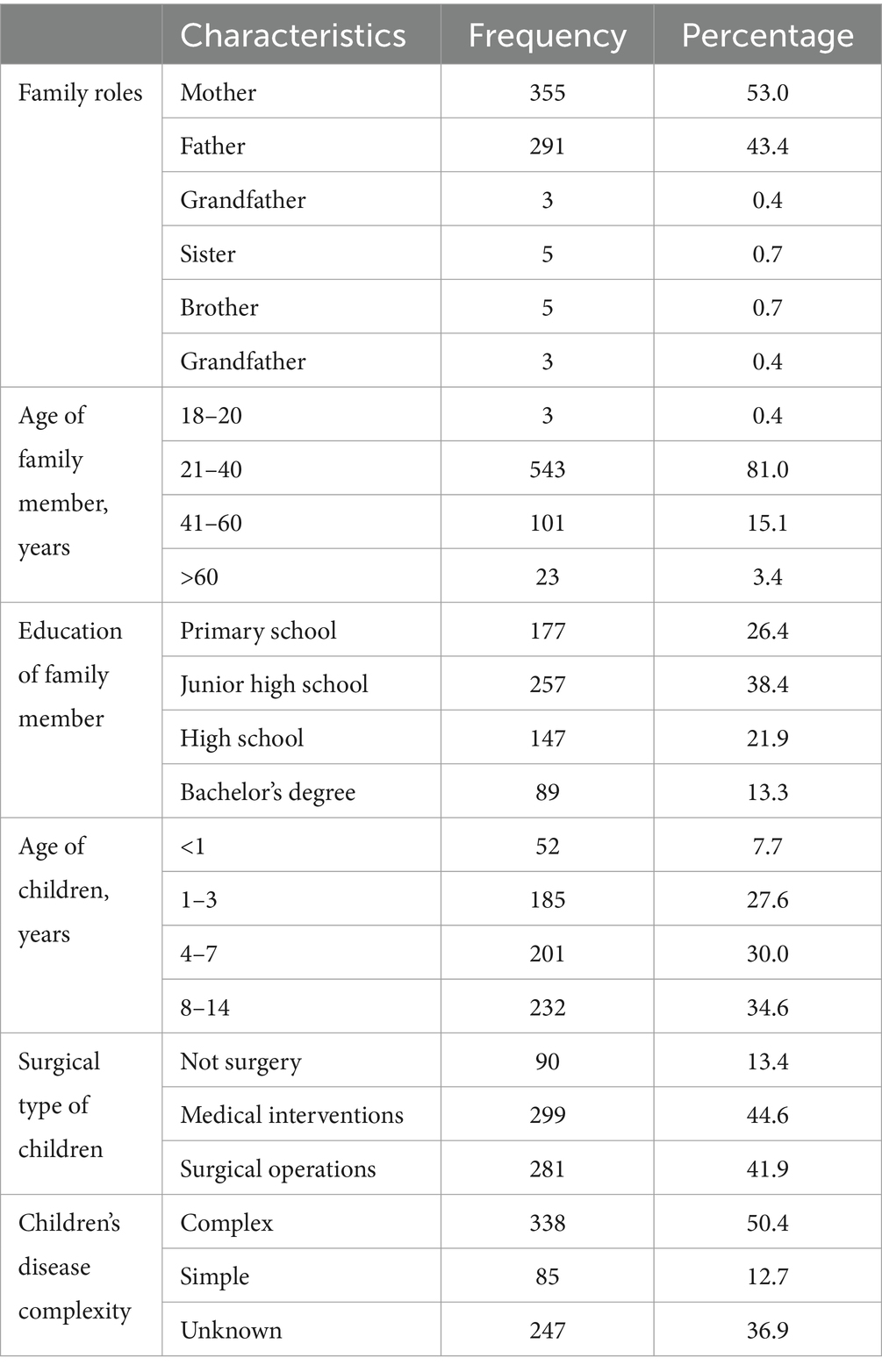
Of the 670 family members who submitted eligible questionnaires, 291 (43.4%) were fathers, 355 (53.0%) were mothers, 10 (1.5%) were grandmothers, three (0.4%) were grandfathers, five (0.7%) were brothers and five (0.7%) were sisters. The age ranged from 18 to 60 years old, with an average age of 33.43 years. A total of 89 (13.3%) had baccalaureate degrees or above, and 581 (86.7%) had associate degrees or below. In total, 90 (13.4%) children had not received surgery, 299 (44.6%) underwent medical intervention operation, and 281 (41.9%) received surgery. The age of children ranged from 0.2 to 14 years old, with an average age of 5.9 years. Overall, 85 (12.7%) children had complex CHD, 338 (50.4%) had simple CHD and 247 (36.9%) were not clear about their specific diagnosis. Table 2 shows the characteristics of the study participants.
Exploratory factor analysis
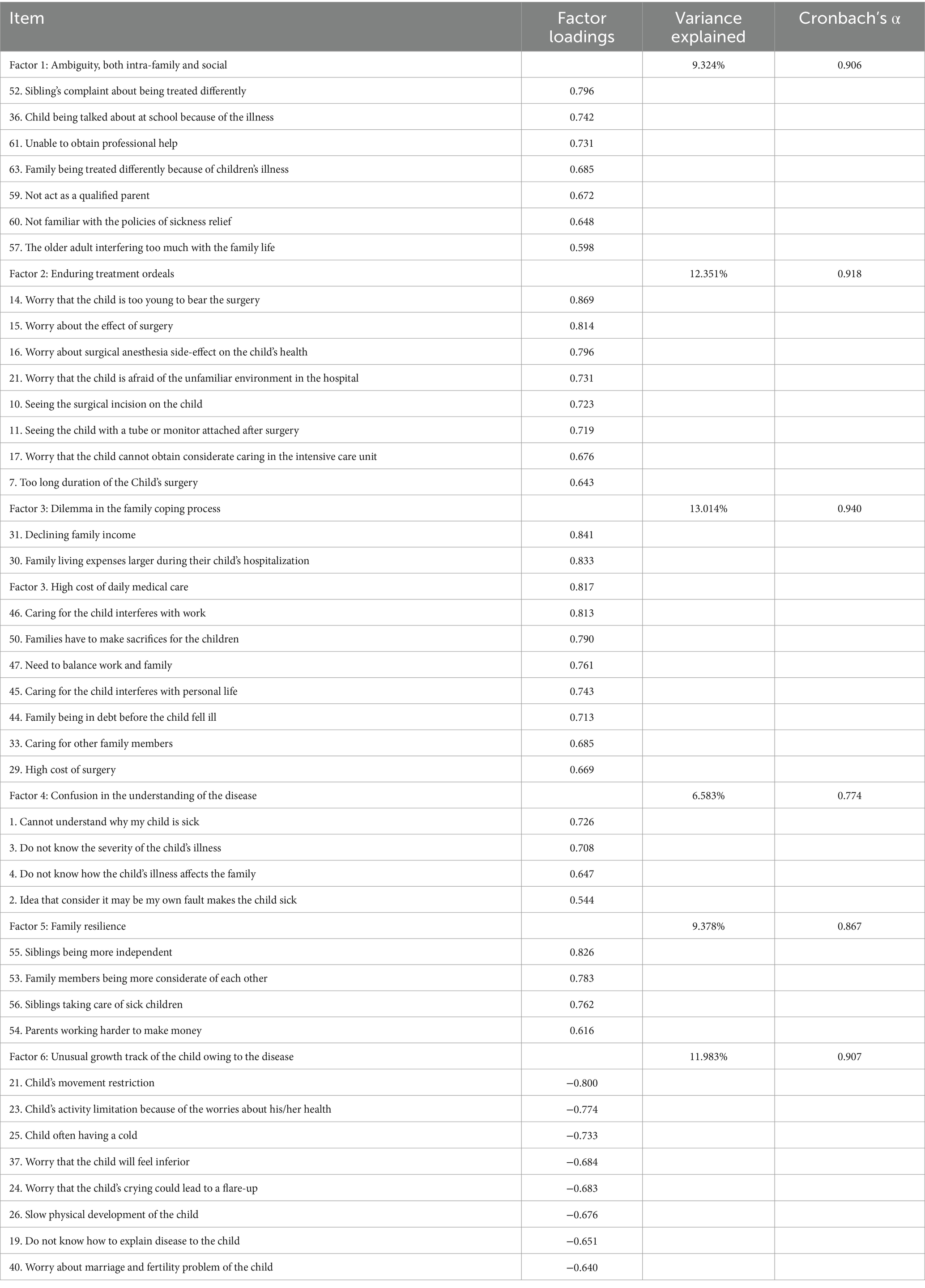
EFA was performed on 63 items. In the EFA group, all the KMO values were more than 0.6, and the p-values for Bartlett’s test of sphericity were < 0.001, suggesting the data were appropriate for factor analysis (34). A six-factor solution including 41 items and explaining 61.085% of the total item variance in the database was obtained. Factor 1 (seven items), “ambiguity, both intra-family and social” accounted for 9.324% of the variance; Factor 2 (eight items), “enduring treatment ordeals” accounted for 12.351%; Factor 3 (10 items), “dilemma in the family coping process” accounted for 13.014%; Factor 4 (four items), “confusion in the understanding of the disease” accounted for 6.583%; Factor 5 (four items), “family resilience” accounted for 9.378%; Factor 6 (eight items), “unusual growth track of the child owing to the disease” accounted for 11.983%. Table 3 shows the loadings of items in each factor.
Confirmatory factor analysis
The CFA with the maximum-likelihood estimation method was used to verify the model. The fitness indices of the model were as follows: CMIN/DF = 2.067, RMSEA = 0.057, RMR = 0.095, CFI = 0.921, IFI = 0.921, TLI = 0.913, which indicated a good fit between the model and the data (38, 39, 41). All indices provided confirmatory evidence for the factor structure.
Criterion-related validity
The correlation coefficient between CHD Children’s Family Stressor Scale and SAS was r = 0.504 (p < 0.001), which indicated that the criterion-related validity of CHD Children’s Family Stressor Scale was good.
Internal consistency reliability
Cronbach’s α coefficient value of the scale level is 0.945, and Cronbach’s α coefficient values of the six dimensions in the scale are 0.906, 0.918, 0.940, 0.907, 0.774, and 0.867, respectively, which indicate a satisfactory internal consistency reliability of the scale as a whole and each dimension (40).
Measurement invariance
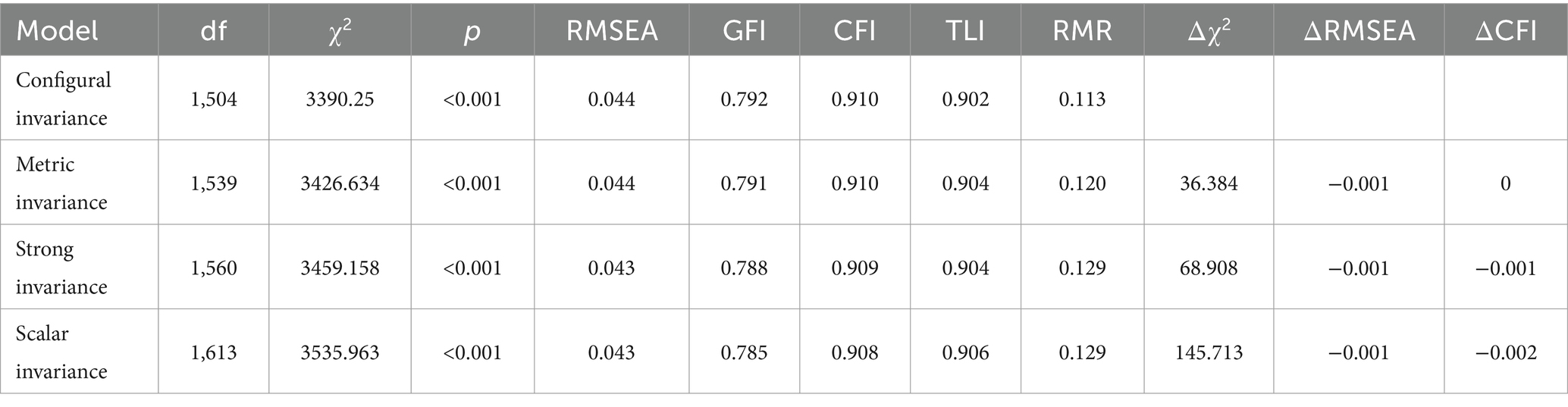
The model fit indices of all baseline models, for fathers and mothers separately, are summarized in Table 4. The invariance model is considered acceptable when the value of the CFI difference (ΔCFI) is below 0.010 (42). The tests of metric invariance (ΔCFI = 0), strong invariance (ΔCFI = −0.001) and scalar invariance (ΔCFI = −0.002) showed good fit, which indicated that the scale factor structure reached measurement invariance across the sample of fathers and mothers.
Discussion
To the knowledge of the authors, this is the first study both to develop and undertake a detailed validation of a scale to assess the stressors of families with children diagnosed with CHD. It consists of 41 items, which are organized into six subscales (ambiguity, both intra-family and social, enduring treatment ordeals, dilemma in the family coping process, confusion in the understanding of the disease, and family resilience and unusual growth track of the child owing to the disease). When constructing the item pool, qualitative research was conducted to clarify the structural composition of the scale and operationally define the concepts measured guided by the double ABCX model of family stress and adaptation. Previous related studies on parental stressors of children with CHD (10, 43, 44) and related stressor scales (20, 22, 24–26, 45, 46) were combined to develop the items. These items were generated from the perspective of family members of children with CHD and verified by the published literature in the field of expertise. This approach not only conforms to the principles of measurement tool development (16, 47) but also ensures the content validity of the tool. The items were verified using the expert consensus meeting method and the expert consultation method. Experts from different majors in the field of CHD health management provided suggestions for item optimization from different perspectives, which strengthens the face validity and content validity of the scale.
In assessing the psychometric properties of the CHD children’s family stressor scale, exploratory and confirmatory factor analyses supported the evidence for the construct validity of the scale. This scale extracted six factors through EFA, and the cumulative explanatory variables of the six factors were 61.085%. The obtained model was basically consistent with the double ABCX model of family stress and adaptation. The model was tested using CFA, which showed a good fit between the model and the data. An RMSEA less than 0.06 is considered a close fit (41), the CFI, IFI, and TLI of the obtained model are all greater than 0.90, and the values of CMIN/DF, RMSEA, and RMR also supported the acceptable fit of the model (48). The correlation coefficient between CHD children’s family stressor scale and SAS was r = 0.504 (p < 0.001), which indicated that the criterion-related validity of CHD children’s family stressor scale was good. Cronbach’s α coefficient of each dimension in this scale is greater than 0.7, and Cronbach’s α coefficient value of the scale level is greater than 0.9, which proves that the scale has relatively ideal internal consistency reliability. By sub-analyses, we found that there was no statistical difference in the mean scores of the CHD Children’s Family Stressor Scale among different sample groups, which indicated that the scale can be used across populations (i.e., fathers and mothers).
From the above results, it can be seen that the CHD children’s family stressor scale is an effective and reliable tool for evaluating the stressors of CHD children’s families. Numerous studies have shown that families of children with CHD suffer from physical, psychological and economic torment during the long process from diagnosis to surgery, and they face stressors from different aspects (49). How to better manage stress is one of the important issues faced by families of children with CHD. In the practice of stress management in families with CHD children, the scale can be utilized as an effective assessment tool for healthcare professionals to understand the stressors of families with CHD children and provide targeted interventions to relieve family stress.
Limitations
This study has several limitations. First, participants were mainly recruited primarily from hospitals in the southwest of China, so these data may not be generalizable nationwide. Second, the recruited participants were mainly parents of children with CHD, lacking the perspectives of other family members, which may lead to limitations in the study content. Finally, the scale has only large-scale investigation once, and its convergent/divergent validity and test–retest reliability were not examined, and the effect practical application of the scale can be further verified in future studies. Therefore, in future studies, it is necessary to expand the sample collection area and include other family members of children with CHD, such as siblings and grandparents.
Conclusion
We finally obtained the CHD Children’s Family Stressor Scale, with six dimensions and 41 items. The six dimensions are ambiguity, both intra-family and social, enduring treatment orders, dilemma in the family coping process, confusion in the understanding of the disease, family resilience and unusual growth track of the child owing to the disease. The scale has been tested to be a reliable and valid tool for assessing the stressors of families with CHD children. Although the above limitations, preliminary findings suggest that this newly developed tool has good reliability and validity. In addition, criterion validity based on correlation with other validated tools is also appropriate. Therefore, it is recommended that this tool be used in clinical practice to assess stressors in families of children with CHD and provide targeted interventions for stress management in families with children with CHD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YZ: Data curation, Formal analysis, Methodology, Software, Writing – original draft. HZ: Formal analysis, Methodology, Writing – review & editing. YB: Data curation, Investigation, Writing – review & editing. ZC: Data curation, Writing – review & editing. YW: Investigation, Writing – review & editing. QH: Formal analysis, Methodology, Writing – review & editing. MY: Supervision, Writing – review & editing. WW: Writing – review & editing. LD: Supervision, Writing – review & editing. FM: Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (72364022).
Acknowledgments
The authors would like to express their appreciation to the CHD family who participated in this study and sincerely share their experiences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu, Y, Chen, S, Zühlke, L, Black, GC, Choy, MK, Li, N, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. (2019) 48:455–63. doi: 10.1093/ije/dyz009
2. Lee, JY. Global burden of congenital heart disease: experience in Korea as a potential solution to the problem. Korean Circulation J. (2020) 50:691–4. doi: 10.4070/kcj.2020.0216
3. Lopez, KN, Morris, SA, Sexson Tejtel, SK, Espaillat, A, and Salemi, JL. US mortality attributable to congenital heart disease across the lifespan from 1999 through 2017 exposes persistent racial/ethnic disparities. Circulation. (2020) 142:1132–47. doi: 10.1161/CIRCULATIONAHA.120.046822
4. GBD 2017 Congenital Heart Disease Collaborators. Global, regional, and national burden of congenital heart disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc Health. (2020) 4:185–200. doi: 10.1016/S2352-4642(19)30402-X
5. Wu, W, He, J, and Shao, X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990-2017. Medicine. (2020) 99:e20593. doi: 10.1097/MD.0000000000020593
6. Chong, LSH, Fitzgerald, DA, Craig, JC, Manera, KE, Hanson, CS, Celermajer, D, et al. Children's experiences of congenital heart disease: a systematic review of qualitative studies. Eur J Pediatr. (2018) 177:319–36. doi: 10.1007/s00431-017-3081-y
7. Dalir, Z, Heydari, A, Kareshki, H, and Manzari, ZS. Coping with caregiving stress in families of children with congenital heart disease: A qualitative study. Int J Community Based Nurs Midwifery. (2020) 8:127–39. doi: 10.30476/IJCBNM.2020.83029.1113
8. Poh, PF, Lee, JH, Loh, YJ, Tan, TH, and Cheng, KKF. Readiness for hospital discharge, stress, and coping in mothers of children undergoing cardiac surgeries: A single-center prospective study. Pediatr Crit Care Med. (2020) 21:e301–10. doi: 10.1097/PCC.0000000000002276
9. Gonzalez, VJ, Kimbro, RT, Cutitta, KE, Shabosky, JC, Bilal, MF, Penny, DJ, et al. Mental health disorders in children with congenital heart disease. Pediatrics. (2021) 147:e20201693. doi: 10.1542/peds.2020-1693
10. Sood, E, Karpyn, A, Demianczyk, AC, Ryan, J, Delaplane, EA, Neely, T, et al. Mothers and fathers experience stress of congenital heart disease differently: recommendations for pediatric critical care. Pediatr Crit Care Med. (2018) 19:626–34. doi: 10.1097/PCC.0000000000001528
11. Connor, JA, Kline, NE, Mott, S, Harris, SK, and Jenkins, KJ. The meaning of cost for families of children with congenital heart disease. J Pediatr Health Care. (2010) 24:318–25. doi: 10.1016/j.pedhc.2009.09.002
12. Mughal, AR, Sadiq, M, Hyder, SN, Qureshi, AU, A Shah, SS, Khan, MA, et al. Socioeconomic status and impact of treatment on families of children with congenital heart disease. J College of Physicians and Surgeons--Pakistan: JCPSP. (2011) 21:398–402.
13. Ni, ZH, Lv, HT, Ding, S, and Yao, WY. Home care experience and nursing needs of caregivers of children undergoing congenital heart disease operations: A qualitative descriptive study. PLoS One. (2019) 14:e0213154. doi: 10.1371/journal.pone.0213154
14. Lisanti, AJ. Parental stress and resilience in CHD: a new frontier for health disparities research. Cardiol Young. (2018) 28:1142–50. doi: 10.1017/S1047951118000963
15. Bruce, E, Lilja, C, and Sundin, K. Mothers' lived experiences of support when living with young children with congenital heart defects. J Spec Pediatr Nurs. (2014) 19:54–67. doi: 10.1111/jspn.12049
16. Flint, A, Farrugia, C, Courtney, M, and Webster, J. Psychometric analysis of the Brisbane practice environment measure (B-PEM). J Nurs Scholarship: Official Pub Sigma Theta Tau Int Honor Society of Nurs. (2010) 42:76–82. doi: 10.1111/j.1547-5069.2009.01328.x
17. Lumsden, MR, Smith, DM, and Wittkowski, A. Coping in parents of children with congenital heart disease: A systematic review and Meta-synthesis. J Child Fam Stud. (2019) 28:1736–53. doi: 10.1007/s10826-019-01406-8
18. Kauffmann, E, Harrison, MB, Burke, SO, and Wong, C. Stress-point intervention for parents of children hospitalized with chronic conditions. Pediatr Nurs. (1998) 24:362–6.
19. Senger, BA, Ward, LD, Barbosa-Leiker, C, and Bindler, RC. Stress and coping of parents caring for a child with mitochondrial disease. Appl Nurs Res. (2016) 29:195–201. doi: 10.1016/j.apnr.2015.03.010
20. Streisand, R, Braniecki, S, Tercyak, KP, and Kazak, AE. Childhood illness-related parenting stress: the pediatric inventory for parents. J Pediatr Psychol. (2001) 26:155–62. doi: 10.1093/jpepsy/26.3.155
21. Larson, MR, Latendresse, SJ, Teasdale, A, and Limbers, CA. The pediatric inventory for parents: development of a short-form in fathers of children with type 1 diabetes (T1D). Child Care Health Dev. (2020) 46:468–84. doi: 10.1111/cch.12769
22. Devine, KA, Heckler, CE, Katz, ER, Fairclough, DL, Phipps, S, Sherman-Bien, S, et al. Evaluation of the psychometric properties of the pediatric parenting stress inventory (PPSI). Health Psychol. (2014) 33:130–8. doi: 10.1037/a0032306
23. Miles, MS, Carter, MC, Spicher, C, and Hassanein, R. Maternal and paternal stress reactions when a child is hospitalized in a pediatric intensive care unit. Issues Compr Pediatr Nurs. (1984) 7:333–42. doi: 10.3109/01460868409009770
24. Miles, MS, Funk, SG, and Carlson, J. Parental Stressor Scale: neonatal intensive care unit. Nurs Res. (1993) 42:148–52. doi: 10.1097/00006199-199305000-00005
25. Miles, MS, and Brunssen, SH. Psychometric properties of the parental stressor scale: infant hospitalization. Adv Neonatal Care. (2003) 3:189–96. doi: 10.1016/S1536-0903(03)00138-3
26. Burke, SO, Kauffmann, E, Harrison, MB, and Wiskin, N. Assessment of stressors in families with a child who has a chronic condition. MCN American J Maternal Child Nurs. (1999) 24:98–106. doi: 10.1097/00005721-199903000-00010
27. Hsieh, HF, and Shannon, SE. Three approaches to qualitative content analysis. Qual Health Res. (2005) 15:1277–88. doi: 10.1177/1049732305276687
28. McCubbin, HI, and Patterson, JM. The family stress process: the double ABCX model of adjustment and adaptation. Marriage Fam Rev. (1983) 6:7–37. doi: 10.1300/J002v06n01_02
29. Bai, Y, Li, J, Bai, Y, Ma, W, Yang, X, and Ma, F. Development and validation of a questionnaire to evaluate the factors influencing training transfer among nursing professionals. BMC Health Serv Res. (2018) 18:107. doi: 10.1186/s12913-018-2910-7
30. DeVellis, RF. Scale development: Theory and applications. London: Sage Publications Ltd. (2003).
31. Polit, DF, Beck, CT, and Owen, SV. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res Nurs Health. (2007) 30:459–67. doi: 10.1002/nur.20199
32. Lynn, MR. Determination and quantification of content validity. Nurs Res. (1986) 35:382–5. doi: 10.1097/00006199-198611000-00017
33. Yang, L. Stressors and coping strategies in children with chronic illness and their parents: Effects of an educational interventon in children and their parents. Peking Union Medical College (2008).
35. Voss, U, Müller, H, and Schermelleh-Engel, K. Towards the assessment of adaptive vs. rigid coping styles: validation of the Frankfurt monitoring blunting scales by means of confirmatory factor analysis. Personal Individ Differ. (2006) 41:295–306. doi: 10.1016/j.paid.2005.12.021
38. Bentler, PM, and Bonett, DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. (1980) 88:588–606. doi: 10.1037/0033-2909.88.3.588
39. Iacobucci, D. Structural equations modeling: fit indices, sample size, and advanced topics. J Consum Psychol. (2010) 20:90–8. doi: 10.1016/j.jcps.2009.09.003
40. Terwee, CB, Bot, SD, de Boer, MR, van der Windt, DAWM, Knol, DL, Dekker, J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. (2007) 60:34–42. doi: 10.1016/j.jclinepi.2006.03.012
41. Schreiber, JB, Nora, A, Stage, FK, Barlow, EA, and King, J. Reporting structural equation modeling and confirmatory factor analysis results: A review. J Educ Res. (2006) 99:323–38. doi: 10.3200/JOER.99.6.323-338
42. Marzorati, C, Monzani, D, Mazzocco, K, Masiero, M, Pavan, F, Monturano, M, et al. Validation of the Italian version of the abbreviated expanded prostate Cancer index composite (EPIC-26) in men with prostate Cancer. Health Qual Life Outcomes. (2019) 17:147. doi: 10.1186/s12955-019-1214-x
43. Hoffman, MF, Karpyn, A, Christofferson, J, Neely, T, McWhorter, LG, Demianczyk, AC, et al. Fathers of children with congenital heart disease: sources of stress and opportunities for intervention. Pediatr Crit Care Med. (2020) 21:e1002–9. doi: 10.1097/PCC.0000000000002388
44. Lisanti, AJ, Allen, LR, Kelly, L, and Medoff-Cooper, B. Maternal stress and anxiety in the pediatric cardiac intensive care unit. Am J Crit Care. (2017) 26:118–25. doi: 10.4037/ajcc2017266
45. Alzawad, Z, Lewis, FM, and Li, M. Content validity of parental stressor scale: pediatric intensive care unit (PSS:PICU). West J Nurs Res. (2021) 43:381–91. doi: 10.1177/0193945920951223
46. Masri, S, Charafeddine, L, Tamim, H, Naamani, M, Jammal, T, and Akoury-Dirani, L. Validation of the Arabic version of the parental stressor scale: neonatal intensive care unit (PSS: NICU). J Clin Psychol Med Settings. (2020) 27:593–602. doi: 10.1007/s10880-019-09643-1
47. Kong Wong, K-FS. The impact on families with hospitalized children: development of a hospitalization impact and coping scale on families (HICS). (2010).
48. Priest, HM. Essentials of nursing research: methods, appraisal, and utilization. Nurs Res. (2006) 13:91–2. doi: 10.7748/nr.13.4.91.s11
Keywords: congenital heart disease, validity, reliability, family, stressor
Citation: Zhang Y, Zhou H, Bai Y, Chen Z, Wang Y, Hu Q, Yang M, Wei W, Ding L and Ma F (2024) Development and validation of a questionnaire to measure the congenital heart disease of children’s family stressor. Front. Public Health. 12:1365089. doi: 10.3389/fpubh.2024.1365089
Edited by:
Lutchmie Narine, Syracuse University, United StatesReviewed by:
Berta Paz-Lourido, University of the Balearic Islands, SpainJohn Pascoe, Wright State University, United States
Copyright © 2024 Zhang, Zhou, Bai, Chen, Wang, Hu, Yang, Wei, Ding and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Ma, cmViZWNjYW1hbGVpQDEyNi5jb20=
 Yi Zhang
Yi Zhang Hang Zhou2
Hang Zhou2