
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 24 April 2024
Sec. Injury Prevention and Control
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1358010
 Hayoung Choi1†
Hayoung Choi1† Jungeun Shin2†
Jungeun Shin2† Jin-Hyung Jung3
Jin-Hyung Jung3 Kyungdo Han4
Kyungdo Han4 Wonsuk Choi5
Wonsuk Choi5 Han Rim Lee2,6
Han Rim Lee2,6 Jung Eun Yoo7
Jung Eun Yoo7 Yohwan Yeo8
Yohwan Yeo8 Hyun Lee9*†
Hyun Lee9*† Dong Wook Shin10,11*†
Dong Wook Shin10,11*†Purpose: Tuberculosis (TB) is linked to sustained inflammation even after treatment, and fracture risk is higher in TB survivors than in the general population. However, no individualized fracture risk prediction model exists for TB survivors. We aimed to estimate fracture risk, identify fracture-related factors, and develop an individualized risk prediction model for TB survivors.
Methods: TB survivors (n = 44,453) between 2010 and 2017 and 1:1 age- and sex-matched controls were enrolled. One year after TB diagnosis, the participants were followed-up until the date of fracture, death, or end of the study period (December 2018). Cox proportional hazard regression analyses were performed to compare the fracture risk between TB survivors and controls and to identify fracture-related factors among TB survivors.
Results: During median 3.4 (interquartile range, 1.6–5.3) follow-up years, the incident fracture rate was significantly higher in TB survivors than in the matched controls (19.3 vs. 14.6 per 1,000 person-years, p < 0.001). Even after adjusting for potential confounders, TB survivors had a higher risk for all fractures (adjusted hazard ratio 1.27 [95% confidence interval 1.20–1.34]), including hip (1.65 [1.39–1.96]) and vertebral (1.35 [1.25–1.46]) fractures, than matched controls. Fracture-related factors included pulmonary TB, female sex, older age, heavy alcohol consumption, reduced exercise, and a higher Charlson Comorbidity Index (p < 0.05). The individualized fracture risk model showed good discrimination (concordance statistic = 0.678).
Conclusion: TB survivors have a higher fracture risk than matched controls. An individualized prediction model may help prevent fractures in TB survivors, especially in high-risk groups.
Tuberculosis (TB) is the most common communicable respiratory disease worldwide. It is a major public health issue in the Republic of Korea, with an estimated incidence of 31 cases per 100,000 people in 2023 (1, 2). Even after completing effective treatment, TB infections may affect TB survivors’ health by chronically elevating systemic inflammatory cytokines (3), leading to various complications other than respiratory diseases, such as ischemic heart disease (4) and dementia (5).
Osteoporotic fractures, including hip and vertebral fractures, are a major cause of morbidity and mortality in the aging population (6), with an increasing burden on the healthcare system (7). Studies have found that proinflammatory cytokines induce osteoclast bone resorption, consequently increasing the risk of osteoporotic fractures (8). Thus, chronic inflammatory disorders, such as chronic obstructive pulmonary disease (COPD), rheumatoid disease, and inflammatory bowel disease, are associated with osteoporosis and increased fracture risk (9).
While increased fracture risk is related to chronic inflammatory disease, data on the association between TB and the incidence of fractures are limited. A Taiwanese retrospective cohort study compared the incidence of osteoporosis and osteoporotic fractures between TB survivors and matched controls. After adjusting for variables including sociodemographic (income level and urbanization) and co-morbidities, TB survivors showed around two-fold higher risk of osteoporosis and osteoporotic fracture than matched controls (10). However, this study was limited in that important osteoporosis-related factors, including body mass index (BMI), smoking habits, and alcohol consumption, need to be adjusted for, as nutritional status affects osteoporosis incidence (11). A Korean study that further adjusted for BMI, smoking status, and alcohol consumption revealed an approximately 1.3-fold higher risk of osteoporosis and osteoporosis-related fractures in TB survivors than in matched controls (12); however, the study lacked a prediction model for fractures in TB survivors. The development of an individualized prediction model for estimating future fracture risk would be a cost-effective strategy for preventing fractures in TB survivors.
Hence, this study aimed to assess the association between a previous TB infection history and the risk of fractures while comprehensively adjusting for confounders, identifying fracture-related risk factors, and developing a fracture prediction model for TB survivors.
The National Health Insurance Service (NHIS) in the Republic of Korea is a mandatory, universal public health insurance system that covers approximately 97% of the Korean population and provides medical aid to 3% of the lowest-income population. All medical services supplied by healthcare providers are reimbursed by the NHIS.
The NHIS database includes (1) a qualification database containing information on age, sex, income, region, and eligibility type; (2) a claim database providing general information on specifications and statements on diagnosis defined by the International Classification of Disease 10th revision (ICD-10), consultation, and prescription; (3) a health screening database; and (4) death information (13–18). The health screening database encompasses data from annual or biennial health screening examination programs for all adults; details are provided in previous studies (13–18).
To establish a TB survivor cohort, among the 231,984 patients diagnosed with TB between 2010 and 2017, 26,928 diagnosed with multidrug-resistant TB or treated for less than 156 days were excluded. Of the identified 205,056 TB survivors, this study initially included 95,294 who underwent health screening within 2 years before the date of TB diagnosis. After excluding 32,260 participants aged <50 years, 854 participants with missing information, 7,941 with a history of fracture before TB diagnosis, 1,213 with an occurrence of fracture within 1 year of TB diagnosis, and 1,456 who died within 1 year of TB diagnosis, 51,570 TB survivors remained. Of the 51,570 TB survivors, 44,453 were eligible for 1:1 age and sex matching (Figure 1).
To establish a control group, we performed 1:1 matching sequentially by year to match TB survivors diagnosed with TB in a specific year to eligible living control participants in the same year based on age (≥ 50 years) and sex. Ages of study participants were changed to integers, and then the ages of the TB survivors and controls were exactly matched. Control participants were allotted an index date identical to the date of TB diagnosis of matched TB survivors. Finally, 44,453 TB survivors and 44,453 matched controls were included in this study (Figure 1).
This study was approved by the Institutional Review Board of the Hanyang University Hospital (application no. HYUH-2019-10-047). The review board waived the requirement for written informed consent because the data were publicly available and anonymized under confidentiality guidelines.
The primary exposure in this study was TB, and the inclusion criteria were as follows: (1) patients with at least two healthcare uses, including outpatient department visits, emergency department visits, or hospitalizations, with ICD-10 codes for TB (A15-19) and the specific NHIS codes for TB (V206, V246, and V000); and (2) patients who were prescribed two or more of the following anti-TB drugs for at least 90 days. More details are provided in a previous study (4, 5, 13, 16, 19). TB was classified as pulmonary TB (A15, A16, or A19) or extrapulmonary TB (A17 or A18 without A15, A16, or A19).
The primary endpoint was the occurrence of new fractures during the follow-up period. Fracture incidence was defined using the ICD-10 code for each fracture type (7, 20–22). Vertebral fractures were defined by ICD-10 codes S220, S221, S320, S327, M484, and M485 and at least two related outpatient clinic visits (23), whereas hip fractures were defined by ICD-10 codes S720 and S721 and related hospitalizations (7). All other fractures were defined by ICD-10 codes S420, S422, S423, S525, S526, S823, S825, and S826, and more than two related visits to an outpatient clinic. This study followed the participants from 1 year after the index date to the date of the fracture event, death, or until the last follow-up date (December 31, 2018), whichever came first.
Socioeconomic position (location of residence and level of income), lifestyle factors (smoking, alcohol consumption, and physical activity), and BMI were considered potential covariates (24–32). Smoking status was categorized into three groups: never, former, and current smokers. Alcohol consumption was divided into non-, mild to moderate (< 30 g of alcohol/day), and heavy (≥ 30 g of alcohol/day) drinkers (33). Regular exercise was defined as ≥30 min of moderate physical activity ≥ five times per week or ≥ 20 min of vigorous physical activity ≥ three times per week (34, 35). BMI was calculated by dividing the weight (kg) by the height in meters squared (m2). Participants’ BMIs were divided into the following groups: underweight (<18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), and obese (≥ 25.0 kg/m2) following the recommendations for Asians by the World Health Organization (36). The overall comorbidity load was evaluated using the Charlson Comorbidity Index (CCI) (37, 38).
The baseline characteristics of TB survivors were compared with those of matched controls using a two-tailed Student’s t-test for continuous variables and a χ2-test for categorical variables. The cumulative incidence of fractures was calculated by dividing the number of incident cases by the total follow-up duration (1,000 person-years). A cumulative incidence plot was used to compare the incidence of fractures between TB survivors and matched controls, and a log-rank test was used to evaluate significant differences between the two groups. Cox proportional hazards models were used to evaluate fracture risk in TB survivors and explore related factors. Model 1 was an unadjusted model, and Model 2 was adjusted for age, sex, BMI, smoking status, alcohol consumption, socioeconomic position (income level and residential area), and regular exercise.
To develop a fracture prediction model for TB survivors, we applied 10 variables (TB type, age, sex, BMI, smoking, alcohol consumption, regular exercise, income level, residential area, and CCI) as weighted risk scores based on the beta coefficients of each variable in the Cox proportional hazards model by assigning scores (0–100). The total score ranged from 0 to 270. Model selection was conducted, including all selected variables considered significant in the univariable analysis. Model discrimination was evaluated using the concordance statistic (c-statistic). SAS version 9.4 (SAS Institute Inc., Cary, NC, United States) was used for statistical analyses, and p-values <0.05 were considered statistically significant.
The mean age of the TB survivors was 64.5 years, and 60.8% were male. The proportions of participants in the lowest income quantile (19.8% vs. 18.6%) and ever smokers (43.4% vs. 40.2%) were higher in TB survivors than those in the matched controls, whereas the proportions of alcohol drinkers (36.8% vs. 38.5%) and regular exercisers (19.4% vs. 23.4%) were lower in TB survivors than those in the matched controls (p < 0.001 for all). Additionally, TB survivors showed significantly lower BMI (mean, 22.4 kg/m2 vs. 24.1 kg/m2) and a higher CCI (2.7 vs. 1.6) than the matched controls (p < 0.001 for both) (Table 1).
The median duration of follow-up after 1 year of lag time was 3.3 (interquartile range, 1.5–5.2) years and 3.5 (interquartile range, 1.6–5.4) years for TB survivors and their matched controls, respectively. During the follow-up period, 6.6% (2,933/44,453) of TB survivors and 5.2% of the matched controls (2,329/44,453) experienced total fracture incidents, with an incidence rate of 19.3 and 14.6 per 1,000 person-years, respectively. Figure 2A shows a significant difference in the cumulative incidence probability of all fractures over 7 years between TB survivors and matched controls (log-rank p < 0.001). In line with this, TB survivors had a higher fracture risk [adjusted hazard ratio (aHR) 1.27, 95% confidence interval (CI) 1.20–1.34] compared to their matched controls (Table 2).
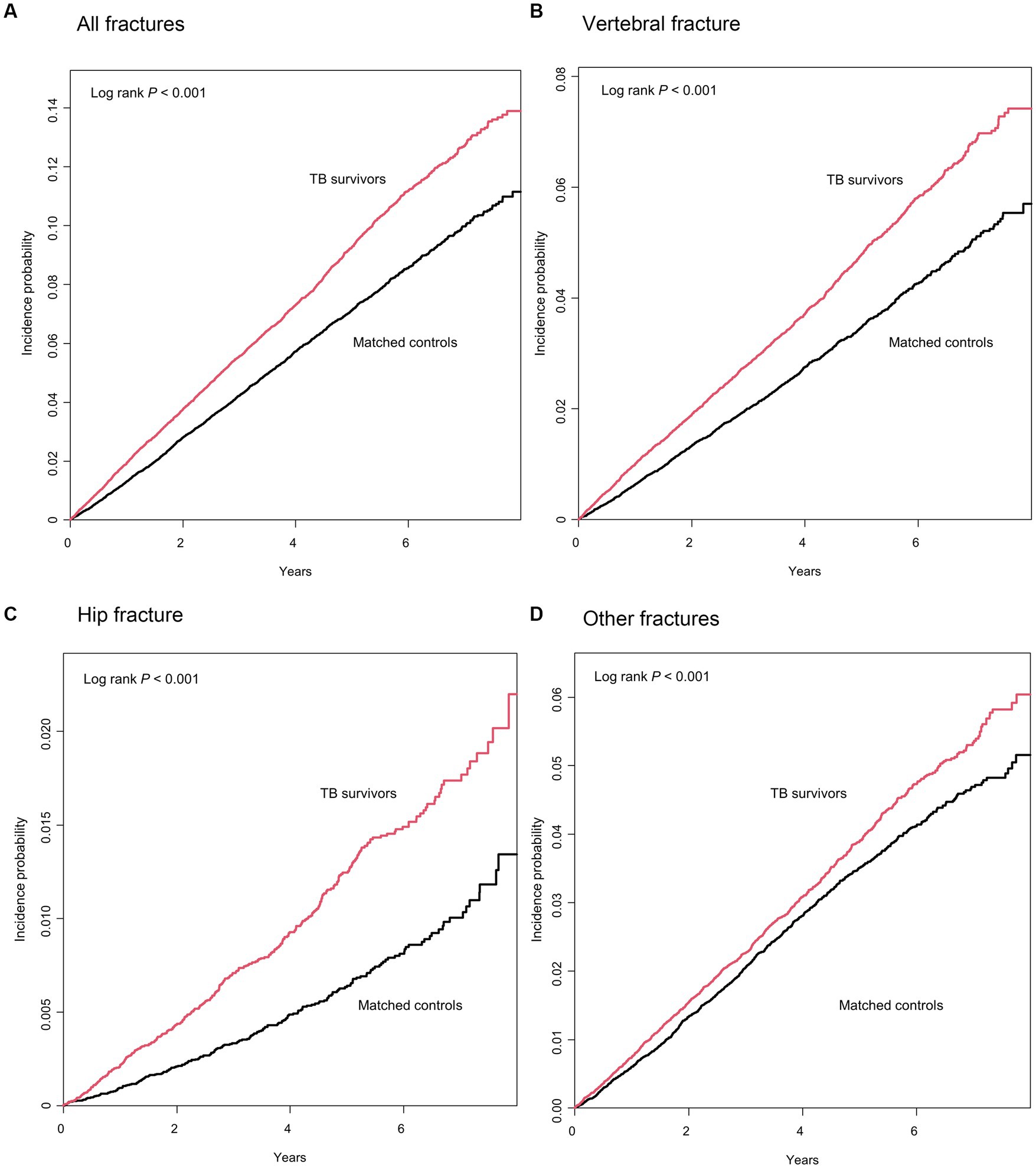
Figure 2. Cumulative incidence probability (%) of fracture in tuberculosis (TB) survivors versus matched controls. Year 0 indicates 1 year after TB diagnosis in TB survivors and 1 year after the time of being matched in matched controls, respectively.
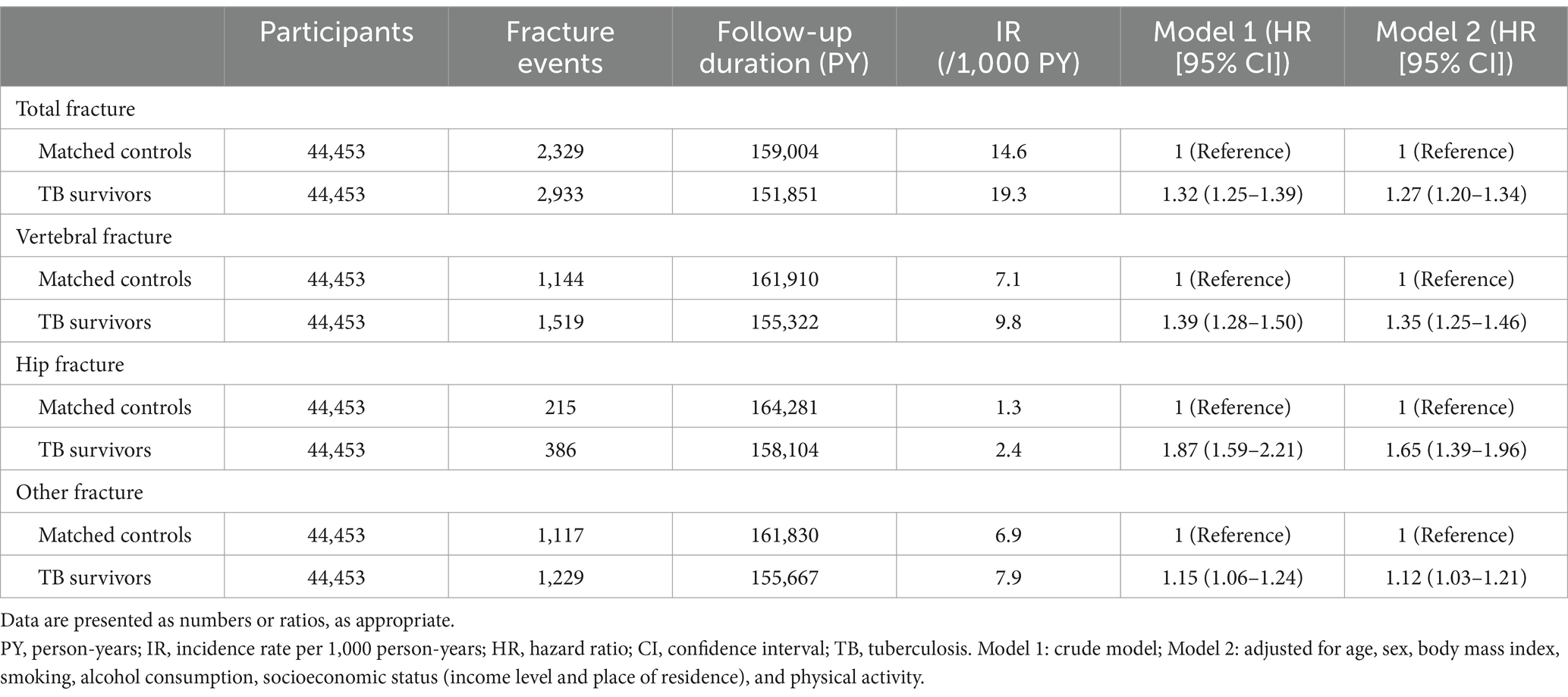
Table 2. Hazard ratios and 95% confidence intervals for the incidence of osteoporotic fractures in tuberculosis survivors compared to the matched control group.
When categorizing the fractures into three groups by location—hip, vertebrae, and other fractures, TB survivors had a higher risk of vertebral fracture (aHR 1.35, 95% CI 1.25–1.46), hip fracture (aHR 1.65, 95% CI 1.39–1.96), and other fractures (aHR 1.12, 95% CI 1.03–1.21) (Table 2 and Figures 2B–D).
Figure 3 shows the various stratified analyses regarding the risk of fracture in TB survivors and their matched controls after adjusting for potential confounding variables in all subgroups. TB survivors exhibited a higher risk of fractures across subgroups.
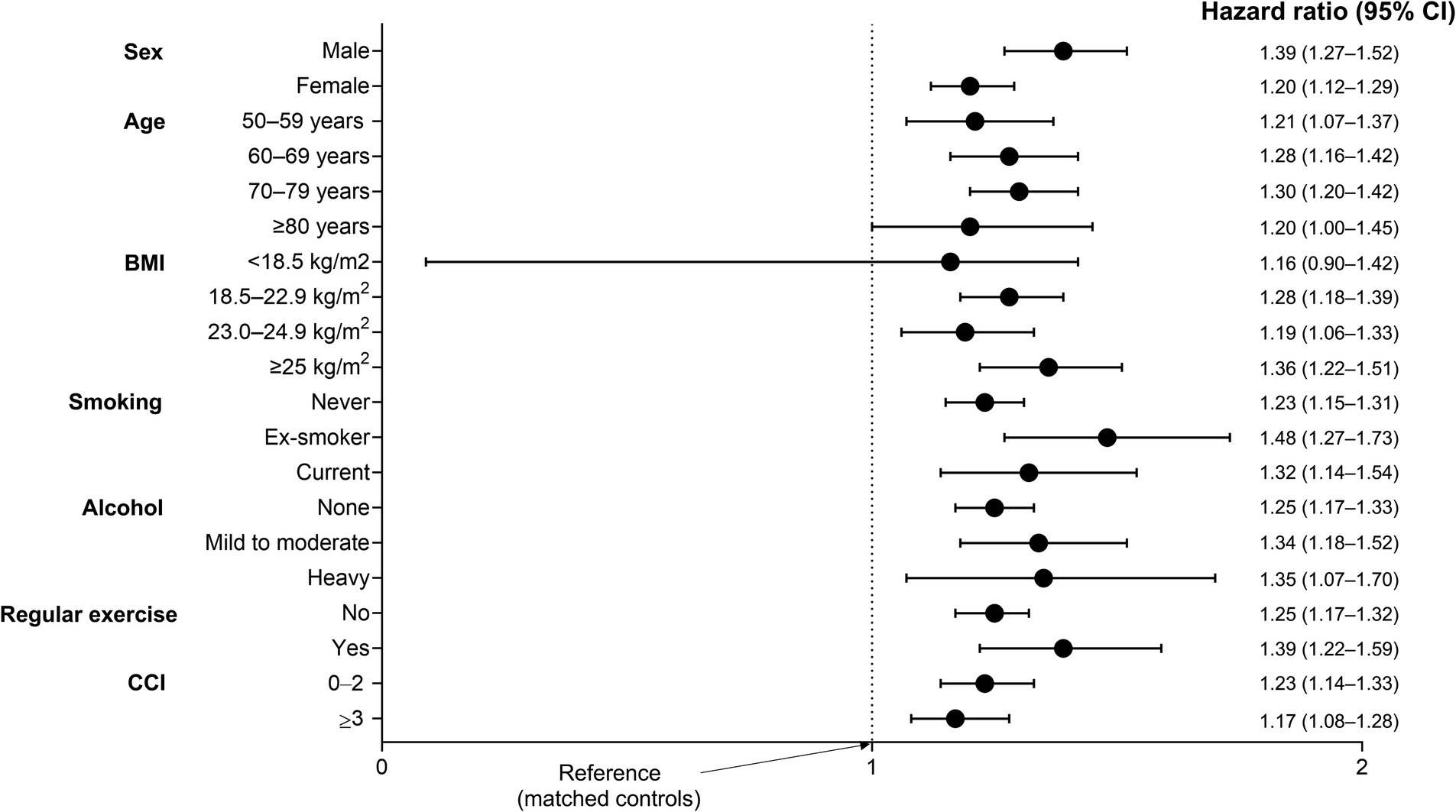
Figure 3. Subgroup analysis for the incidence of fracture in TB survivors compared to matched controls. Adjusted for age, sex, income level, residential area, smoking, alcohol consumption, regular physical activity, body mass index, and Charlson Comorbidity Index. CCI, Charlson Comorbidity Index; CI, confidence interval.
Table 3 depicts factors associated with all fractures in TB survivors; factors associated with increased risk of all fractures were pulmonary TB vs. extrapulmonary TB (aHR 1.15, 95% CI 1.02–1.29), female vs. male sex (aHR 2.08, 95% CI 1.87–2.31), older age (aHR 2.05, 95% CI 1.86–2.26 for ages 60–74 years; aHR 3.68, 95% CI 3.29–4.12 for ages ≥75 years vs. ages 50–59 years), heavy alcohol consumption vs. none (aHR 1.26, 95% CI 1.08–1.48), lower income level (aHR 1.13, 95% CI 1.03–1.24 for income level Q1 [lowest] and aHR 1.11, 95% CI 1.01–1.22 for income level Q3 vs. income level Q4 [highest]), and CCI ≥ 2 vs. CCI = 0 (aHR 1.40, 95% CI 1.22–1.61). By contrast, regular physical activity was associated with a reduced risk of all fractures (aHR 0.89, 95% CI 0.80–0.98).
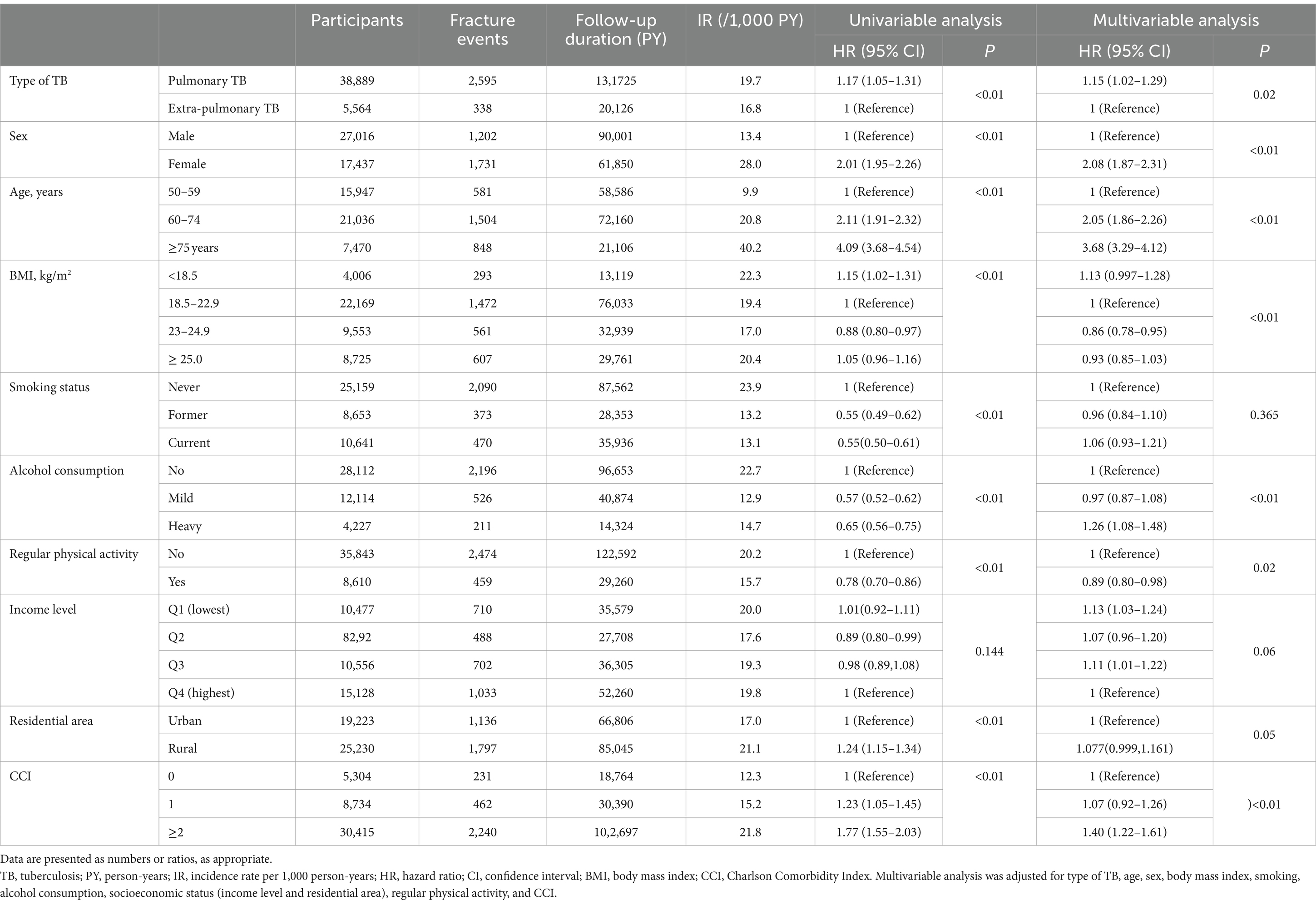
Table 3. Hazard ratios and 95% confidence intervals for the incidence of osteoporotic fractures in TB survivors.
The nomogram for the fracture risk in TB survivors is shown in Figure 4. The fracture risk prediction model showed good discrimination (C-statistic = 0.678; 95% CI = 0.668–0.689).
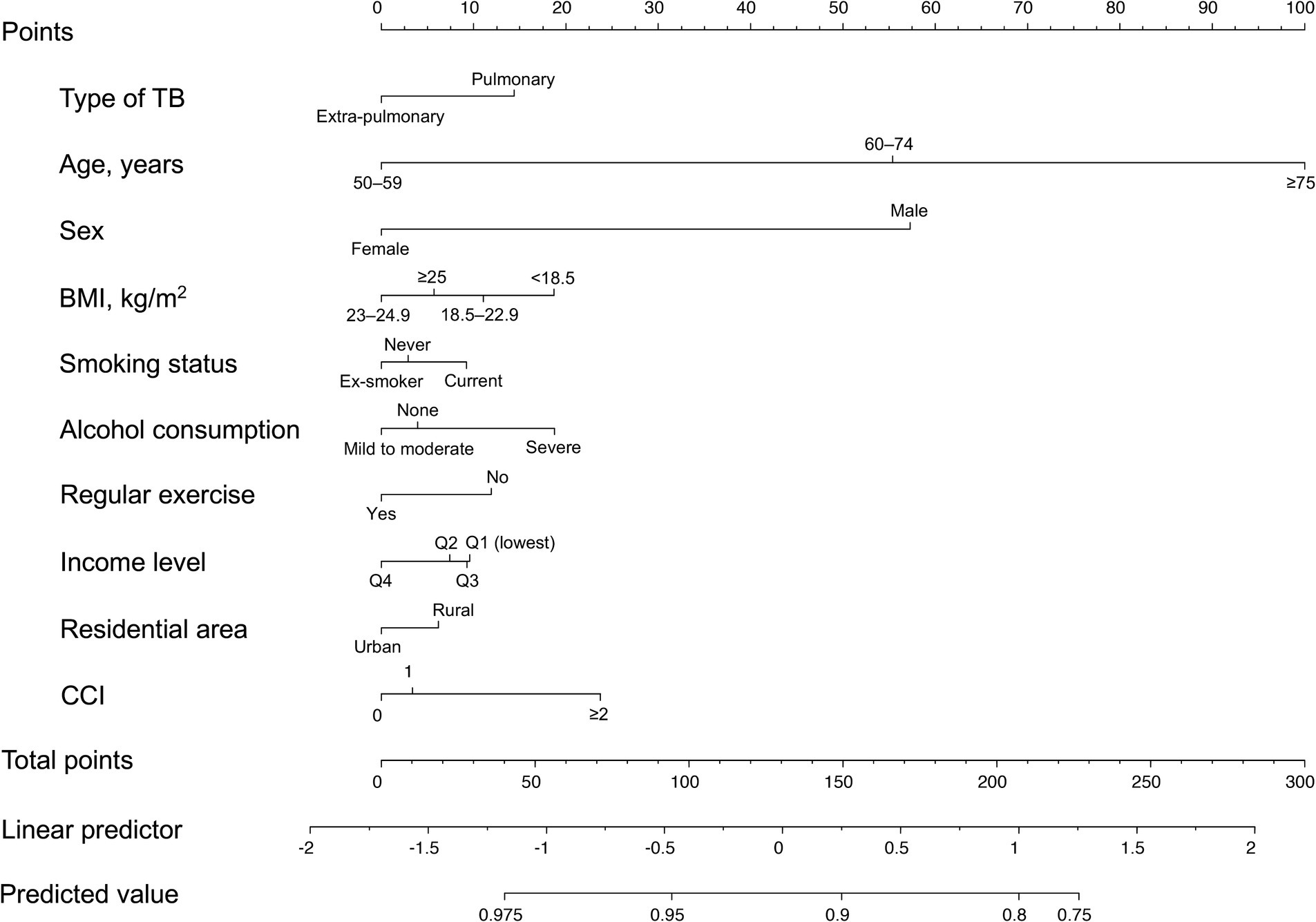
Figure 4. Nomogram for the prediction model of fracture probability. TB, tuberculosis; BMI, body mass index; CCI, Charlson Comorbidity Index.
To our knowledge, this study is the first to evaluate fracture risk in TB survivors while comprehensively adjusting for potential confounders, including nutritional status, lifestyle habits, and comorbidities, and to develop an individualized risk prediction model for fractures in TB survivors. The fracture risk was significantly higher in TB survivors than in matched controls at all locations. Furthermore, a risk-prediction model for fractures based on related factors was reliable among TB survivors.
The pathogenesis of fractures in TB survivors is not fully understood, but three potential mechanisms are as follows. The first possible mechanism is chronic inflammatory reaction induced by TB infection. Studies have suggested that pro-inflammatory markers such as IL-6, IL-1, and TNF-alpha act as modulators of osteoblasts and osteoclasts, leading to up-regulation of osteoclast activation and osteoporosis (9, 39). It has been suggested that TB infection chronically elevates systemic inflammatory cytokines resulting from non-resolving inflammation as an outcome of intracellular survival strategies (3, 40). Remarkably, these inflammatory reactions persist even after the completion of anti-TB treatment, with chronic inflammation acting as an osteoclastic stimulator in TB survivors, resulting in increased fracture risk (39, 41).
The second presumable mechanism is weight loss caused by TB infection. TB survivors suffer from anorexia and weight loss and remain underweight compared with healthy controls (42, 43). In addition, TB survivors have a higher prevalence of sarcopenia than those without TB infection (44). Several studies have shown that low body weight and BMI increase osteoporosis and fracture risks (11), so the low BMI of TB survivors could contribute to an elevated fracture incidence in this population.
The third potential mechanism is Vitamin D deficiency. In previous studies, low serum vitamin D levels (≤ 25 nmol/L) exhibited a significant association with an increased risk of TB infection. Even after completing TB treatment, survivors demonstrate a higher incidence of vitamin D deficiency than the general population (45–47). Vitamin D deficiency in TB survivors can alter calcium absorption, causing a negative calcium balance and bone reabsorption, leading to increased osteoporosis and fracture risks (48).
In our study, the hazard of hip fractures in TB survivors relative to matched controls was higher than that of vertebral and other fractures. The mechanism is not fully understood; however, it is suspected that TB survivors undergo increased pro-inflammatory cytokine and osteoclastic cell activities, resulting in net resorption and a decrease in the endocortical surface by 25–40% at the femoral neck and distal radius (49). In addition, altered calcium absorption due to vitamin D deficiency may lead to decreased bone reabsorption in the cortical bone, weakening the cortical bone of the hip more frequently than the vertebral bones (48).
A highlight of our study is that we speculated on the risk factors for fractures in TB survivors and developed an individualized estimation prediction model. Although there is an increased fracture risk in TB survivors compared with the general population, no studies have attempted to develop risk estimation models for TB survivors. The major advantage of our study is that, to the best of our knowledge, we have developed a fracture risk estimation model based on clinical variables that can be easily obtained from real-world clinics. Accordingly, our model can be applied to TB survivors in real-world clinics.
Our study had some limitations. First, our follow-up period (median 3.4 years after a 1-year lag period) was relatively short, considering it takes a long time to develop osteoporosis. Second, information on vitamin D levels was unavailable due to the use of claims data, limiting the ability to conclude a direct correlation. Third, this study could not include all possible confounding factors, such as previous falls and physical function status, because of the use of claims data. In addition, the causes of the fractures were incomplete, and fractures resulting from other causes, such as traffic accidents or other sources of trauma, were not excluded. However, fractures caused by traffic accidents or violence were not covered by the NHIS and were not included in this study. Despite these limitations, our study has several strengths, including the use of a large and representative database of the entire Korean population, near-complete follow-up, and adjustments for lifestyle variables, including BMI, smoking, alcohol consumption, and regular physical activities.
This study clearly demonstrated TB survivors are a high-risk group for fractures, so they would benefit from following clinicians’ attempts to reduce fracture risk. First, effective and complete TB treatment is necessary to reduce the inflammatory cytokines induced by TB infection. Second, physicians need to pay attention to weight gain in TB survivors and provide proper nutritional advice during and even after TB treatment. Third, considering TB survivors’ high risk of vitamin D deficiency, constructive suggestions for sustained bone mineral density in postmenopausal women or those aged over 50 years for TB survivors are necessary. Future studies are warranted to investigate how such strategies can be incorporated into our daily practice to monitor and prevent osteoporotic fractures in TB survivors effectively.
In conclusion, this nationwide population-based cohort study demonstrated that TB survivors have a higher fracture risk than the general population. Future studies are necessary to understand better the precise mechanisms underlying the association between TB and fractures.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board of the Hanyang University Hospital (application no. HYUH-2019-10-047). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The review board waived the requirement for written informed consent because the data were publicly available and anonymized under confidentiality guidelines.
HC: Data curation, Formal analysis, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. JS: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. J-HJ: Data curation, Formal analysis, Resources, Writing – original draft, Writing – review & editing. KH: Conceptualization, Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. WC: Data curation, Formal analysis, Methodology, Resources, Writing – original draft, Writing – review & editing. HRL: Investigation, Software, Writing – original draft, Writing – review & editing. JY: Investigation, Project administration, Resources, Software, Writing – original draft, Writing – review & editing. YY: Data curation, Formal analysis, Investigation, Resources, Software, Validation, Writing – original draft, Writing – review & editing. HL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing. DS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Basic Science Research Program of the Korean Ministry of Education (No. 2021R1I1A3052416). The funder had no role in the design of the study, data collection and analysis, or the preparation of the manuscript.
This study was performed using a database from the National Health Insurance System, and the results do not necessarily represent the opinion of the National Health Insurance Corporation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Noh, CS, Kim, HI, Choi, H, Kim, Y, Kim, CH, Choi, JH, et al. Completion rate of latent tuberculosis infection treatment in patients aged 65 years and older. Respir Med. (2019) 157:52–8. doi: 10.1016/j.rmed.2019.09.004
2. Korea Centers for Disease Control and Prevention . Annual report on the notified tuberculosis in the Repulic of Korea (2023) Available at: https://tbzero.kdca.go.kr/tbzero/main.do (Accessed February 04, 2024).
3. Kaufmann, SH, and Dorhoi, A. Inflammation in tuberculosis: interactions, imbalances and interventions. Curr Opin Immunol. (2013) 25:441–9. doi: 10.1016/j.coi.2013.05.005
4. Lee, HR, Yoo, JE, Choi, H, Han, K, Lim, YH, Lee, H, et al. Tuberculosis and the risk of ischemic heart disease: a Nationwide cohort study. Clin Infect Dis. (2023) 76:1576–84. doi: 10.1093/cid/ciac946
5. Yeo, Y, Yoo, JE, Han, K, Jung, JH, Choi, H, Park, J, et al. Risk of dementia in survivors of active tuberculosis in Korea: a nationwide cohort study. J Infect Public Health. (2024) 17:286–92. doi: 10.1016/j.jiph.2023.12.006
6. Ahmed, LA, Center, JR, Bjørnerem, A, Bluic, D, Joakimsen, RM, Jørgensen, L, et al. Progressively increasing fracture risk with advancing age after initial incident fragility fracture: the Tromsø study. J Bone Miner Res. (2013) 28:2214–21. doi: 10.1002/jbmr.1952
7. Kang, HY, Yang, KH, Kim, YN, Moon, SH, Choi, WJ, Kang, DR, et al. Incidence and mortality of hip fracture among the elderly population in South Korea: a population-based study using the national health insurance claims data. BMC Public Health. (2010) 10:230. doi: 10.1186/1471-2458-10-230
8. McLean, RR . Proinflammatory cytokines and osteoporosis. Curr Osteoporos Rep. (2009) 7:134–9. doi: 10.1007/s11914-009-0023-2
9. Redlich, K, and Smolen, JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. (2012) 11:234–50. doi: 10.1038/nrd3669
10. Chen, YY, Feng, JY, Ting, WY, Yen, YF, Chuang, PH, Pan, SW, et al. Increased risk of incident osteoporosis and osteoporotic fracture in tuberculosis patients: a population-based study in a tuberculosis-endemic area. Osteoporos Int. (2017) 28:1711–21. doi: 10.1007/s00198-017-3939-x
11. Burger, H, de Laet, CE, van Daele, PL, Weel, AE, Witteman, JC, Hofman, A, et al. Risk factors for increased bone loss in an elderly population: the Rotterdam study. Am J Epidemiol. (1998) 147:871–9. doi: 10.1093/oxfordjournals.aje.a009541
12. Park, SH, Yoon, SR, Nam, JY, Ahn, JY, Jeong, SJ, Ku, NS, et al. Impact of tuberculosis on the incidence of osteoporosis and osteoporotic fractures: a nationwide population-based cohort study. Public Health. (2023) 216:13–20. doi: 10.1016/j.puhe.2022.12.009
13. Choi, H, Han, K, Jung, JH, Park, SH, Kim, SH, Kang, HK, et al. Long-term mortality of tuberculosis survivors in Korea: a population-based longitudinal study. Clin Infect Dis. (2023) 76:e973–81. doi: 10.1093/cid/ciac411
14. Choi, H, Han, K, Yang, B, Shin, DW, Sohn, JW, and Lee, H. Female reproductive factors and incidence of nontuberculous mycobacterial pulmonary disease among postmenopausal women in Korea. Clin Infect Dis. (2022) 75:1397–404. doi: 10.1093/cid/ciac134
15. Choi, H, Han, K, Jung, JH, Park, J, Kim, BG, Yang, B, et al. Impact of rheumatoid arthritis and Seropositivity on the risk of non-cystic fibrosis bronchiectasis. Chest. (2024). doi: 10.1016/j.chest.2024.01.001
16. Moon, SM, Choi, H, Kim, SH, Kang, HK, Park, DW, Jung, JH, et al. Increased lung Cancer risk and associated risk factors in tuberculosis survivors: a Korean population-based study. Clin Infect Dis. (2023) 77:1329–39. doi: 10.1093/cid/ciad373
17. Kim, Y, Yoon, JH, Ryu, J, Yang, B, Chung, SJ, Kang, HK, et al. Gastroesophageal reflux disease increases susceptibility to nontuberculous mycobacterial pulmonary disease. Chest. (2023) 163:270–80. doi: 10.1016/j.chest.2022.08.2228
18. Shin, DW, Cho, J, Park, JH, and Cho, B. National General Health Screening Program in Korea: history, current status, and future direction. Precis. Future Med. (2022) 6:9–31. doi: 10.23838/pfm.2021.00135
19. Yoo, JE, Choi, H, Han, K, Park, SH, Park, J, Lee, H, et al. Tuberculosis and risk of Parkinson's disease: a nationwide cohort study. Pulmonology. (2023) 29:250–2. doi: 10.1016/j.pulmoe.2022.10.002
20. Lim, S, Koo, BK, Lee, EJ, Park, JH, Kim, MH, Shin, KH, et al. Incidence of hip fractures in Korea. J Bone Miner Metab. (2008) 26:400–5. doi: 10.1007/s00774-007-0835-z
21. Park, C, Ha, YC, Jang, S, Jang, S, Yoon, HK, and Lee, YK. The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J Bone Miner Metab. (2011) 29:744–51. doi: 10.1007/s00774-011-0279-3
22. Lee, YK, Yoon, BH, and Koo, KH. Epidemiology of osteoporosis and osteoporotic fractures in South Korea. Endocrinol. Metab. (2013) 28:90–3. doi: 10.3803/EnM.2013.28.2.90
23. Lee, YK, Jang, S, Jang, S, Lee, HJ, Park, C, Ha, YC, et al. Mortality after vertebral fracture in Korea: analysis of the National Claim Registry. Osteoporos Int. (2012) 23:1859–65. doi: 10.1007/s00198-011-1833-5
24. Christianson, MS, and Shen, W. Osteoporosis prevention and management: nonpharmacologic and lifestyle options. Clin Obstet Gynecol. (2013) 56:703–10. doi: 10.1097/GRF.0b013e3182a9d15a
25. Taehee, K, Hyun, L, Cho Yun, J, Sang Woo, Y, Bo-Guen, K, Tai Sun, P, et al. Does COVID-19 vaccination increase the risk of ILD in a population level? ERJ Open Res. (2024) 10. doi: 10.1183/23120541.00690-2023
26. Kim, BG, Lee, H, Jeong, CY, Yeom, SW, Park, DW, Park, TS, et al. Risk of newly diagnosed interstitial lung disease after COVID-19 and impact of vaccination: a nationwide population-based cohort study. Front Public Health. (2023) 11:1295457. doi: 10.3389/fpubh.2023.1295457
27. Kim, BG, Lee, H, Yeom, SW, Jeong, CY, Park, DW, Park, TS, et al. Increased risk of new-onset asthma after COVID-19: a Nationwide population-based cohort study. J Allergy Clin Immunol Pract. (2024) 12:120–32.e5. doi: 10.1016/j.jaip.2023.09.015
28. Kim, T, Choi, H, Lee, H, Han, K, Park, DW, Park, TS, et al. Impact of allergic disease on the risk of mycobacterial disease. J Allergy Clin Immunol Pract. (2023) 11:2830–8.e4. doi: 10.1016/j.jaip.2023.04.044
29. Yang, B, Kim, BG, Han, K, Jung, JH, Kim, JH, Park, DW, et al. Systemic sclerosis and risk of bronchiectasis: a nationwide longitudinal cohort study. Arthritis Res Ther. (2023) 25:209. doi: 10.1186/s13075-023-03189-2
30. Lee, H, Kim, BG, Jeong, CY, Park, DW, Park, TS, Moon, JY, et al. Long-term impacts of COVID-19 on severe exacerbation and mortality in adult asthma: a nationwide population-based cohort study. J Allergy Clin Immunol Pract. (2024). doi: 10.1016/j.jaip.2024.03.035
31. Cho, MH, Cho, JH, Eun, Y, Han, K, Jung, J, Cho, IY, et al. Rheumatoid arthritis and risk of lung Cancer: a Nationwide cohort study. J Thorac Oncol. (2024) 19:216–26. doi: 10.1016/j.jtho.2023.10.006
32. Kim, BG, Lee, H, Kang, MG, Kim, JS, and Moon, JY. Risk of ischemic heart disease in chronic obstructive pulmonary disease: a Nationwide cohort study. J Korean Med Sci. (2023) 38:e344. doi: 10.3346/jkms.2023.38.e344
33. Kim, SH, Han, K, Park, J, Park, DW, Moon, JY, Sim, YS, et al. Association between non-cystic fibrosis bronchiectasis and the risk of incident dementia: a nationwide cohort study. Chron Respir Dis. (2023) 20:14799731231222282. doi: 10.1177/14799731231222282
34. Lee, H, Ryu, J, Chung, SJ, Park, DW, Park, TS, Moon, JY, et al. Overall and respiratory mortality reduction with physical activity in subjects with and without asthma. Allergy. (2023) 78:1677–80. doi: 10.1111/all.15638
35. Yang, B, Lee, H, Ryu, J, Park, DW, Park, TS, Chung, JE, et al. Impacts of regular physical activity on hospitalisation in chronic obstructive pulmonary disease: a nationwide population-based study. BMJ Open Respir Res. (2024) 11:e001789. doi: 10.1136/bmjresp-2023-001789
36. WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363:157–63. doi: 10.1016/s0140-6736(03)15268-3
37. Khan, NF, Perera, R, Harper, S, and Rose, PW. Adaptation and validation of the Charlson index for read/OXMIS coded databases. BMC Fam Pract. (2010) 11:1. doi: 10.1186/1471-2296-11-1
38. Lee, H, Kim, SH, Lee, SK, Choi, H, Chung, SJ, Park, DW, et al. Impact of air pollution on healthcare utilization in patients with bronchiectasis. Front Med. (2023) 10:1233516. doi: 10.3389/fmed.2023.1233516
39. Amarasekara, DS, Yun, H, Kim, S, Lee, N, Kim, H, and Rho, J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. (2018) 18:e8. doi: 10.4110/in.2018.18.e8
40. Malherbe, ST, Shenai, S, Ronacher, K, Loxton, AG, Dolganov, G, Kriel, M, et al. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med. (2016) 22:1094–100. doi: 10.1038/nm.4177
41. Deveci, F, Akbulut, HH, Turgut, T, and Muz, MH. Changes in serum cytokine levels in active tuberculosis with treatment. Mediat Inflamm. (2005) 2005:256–62. doi: 10.1155/MI.2005.256
42. Ko, Y, Kim, C, Park, YB, Mo, EK, and Moon, JW. Changes in nutritional status in pulmonary tuberculosis: longitudinal changes in BMI according to acid-fast Bacilli smear positivity. J Clin Med. (2020) 9:4082. doi: 10.3390/jcm9124082
43. Ockenga, J, Fuhse, K, Chatterjee, S, Malykh, R, Rippin, H, Pirlich, M, et al. Tuberculosis and malnutrition: the European perspective. Clin Nutr. (2023) 42:486–92. doi: 10.1016/j.clnu.2023.01.016
44. Shin, MK, Choi, JY, Kim, SY, Kim, EY, Lee, SH, Chung, KS, et al. Association of protein consumption and energy intake on sarcopenia in tuberculosis survivors. Ther Adv Chronic Dis. (2021) 12:20406223211056712. doi: 10.1177/20406223211056712
45. Zeng, J, Wu, G, Yang, W, Gu, X, Liang, W, Yao, Y, et al. A serum vitamin D level <25 nmol/l pose high tuberculosis risk: a meta-analysis. PLoS One. (2015) 10:e0126014. doi: 10.1371/journal.pone.0126014
46. Iftikhar, R, Kamran, SM, Qadir, A, Haider, E, and Bin, UH. Vitamin D deficiency in patients with tuberculosis. J Coll Physicians Surg Pak. (2013) 23:780–3.
47. Huaman, MA, Sterling, TR, Shepherd, BE, and Fiske, CT. 25-Hydroxyvitamin D levels after recovery from tuberculosis: insights into pathogenesis. Tuberculosis (Edinb). (2014) 94:51–4. doi: 10.1016/j.tube.2013.10.009
48. Brincat, M, Gambin, J, Brincat, M, and Calleja-Agius, J. The role of vitamin D in osteoporosis. Maturitas. (2015) 80:329–32. doi: 10.1016/j.maturitas.2014.12.018
Keywords: tuberculosis, survivor, fractures, epidemiology, risk factor
Citation: Choi H, Shin J, Jung J-H, Han K, Choi W, Lee HR, Yoo JE, Yeo Y, Lee H and Shin DW (2024) Tuberculosis and osteoporotic fracture risk: development of individualized fracture risk estimation prediction model using a nationwide cohort study. Front. Public Health. 12:1358010. doi: 10.3389/fpubh.2024.1358010
Received: 08 January 2024; Accepted: 08 April 2024;
Published: 24 April 2024.
Edited by:
Jingwen Hu, University of Michigan, United StatesReviewed by:
Basheerahmed Abdulaziz Mannasaheb, Almaarefa University, Saudi ArabiaCopyright © 2024 Choi, Shin, Jung, Han, Choi, Lee, Yoo, Yeo, Lee and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Wook Shin, ZHdzaGluLm1kQGdtYWlsLmNvbQ==; Hyun Lee, bmFtdWhhbmF5ZXlvQG5hdmVyLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.