- 1National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Center of Gerontology and Geriatrics, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, Sichuan, China
Background: In 2022, the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO) launched a consensus on the diagnostic methods for sarcopenic obesity (SO). The study aimed to identify the prevalence and diagnostic agreement of SO using different diagnostic methods in a cohort of subjects from West China aged at least 50 years old.
Methods: A large multi-ethnic sample of 4,155 participants from the West China Health and Aging Trend (WCHAT) study was analyzed. SO was defined according to the newly published consensus of the ESPEN/EASO. Furthermore, SO was diagnosed as a combination of sarcopenia and obesity. The criteria established by the Asian Working Group for Sarcopenia 2019 (AWGS2019) were used to define sarcopenia. Obesity was defined by four widely used indicators: percent of body fat (PBF), visceral fat area (VFA), waist circumference (WC), and body mass index (BMI). Cohen’s kappa was used to analyze the diagnostic agreement of the above five diagnostic methods.
Results: A total of 4,155 participants were part of the study, including 1,499 men (63.76 ± 8.23 years) and 2,656 women (61.61 ± 8.20 years). The prevalence of SO was 0.63–7.22% with different diagnostic methods. The diagnosis agreement of five diagnostic methods was poor-to-good (κ: 0.06–0.67). The consensus by the ESPEN/EASO had the poorest agreement with other methods (κ: 0.06–0.32). AWGS+VFA had the best agreement with AWGS+WC (κ = 0.67), and consensus by the ESPEN/EASO had the best agreement with AWGS+ PBF (κ = 0.32).
Conclusion: The prevalence and diagnostic agreement of SO varies considerably between different diagnostic methods. AWGS+WC has the highest diagnostic rate in the diagnosis of SO, whereas AWGS+BMI has the lowest. AWGS+VFA has a relatively good diagnostic agreement with other diagnostic methods, while the consensus of the ESPEN/EASO has a poor diagnostic agreement. AWGS+PBF may be suitable for the alternative diagnosis of the 2022 ESPEN/EASO.
Introduction
Sarcopenia is an age-related skeletal muscle disorder characterized by a decrease in muscle mass, strength, and function. Sarcopenic obesity (SO) is a condition characterized by the coexistence of sarcopenia and obesity (1). Obesity and sarcopenia have synergistic and reinforcing effects (2). Patients with sarcopenia experience a decrease in total energy expenditure, which promotes ectopic fat deposition. In addition, obesity can lead to oxidative stress, inflammation, increased insulin resistance, and the exacerbation of muscle metabolism and breakdown (3, 4). SO is associated with increased body fat and decreased muscle volume and function, which reduces the likelihood of an individual with SO engaging in exercise. A lack of exercise is both the cause and the result of SO (5). However, most treatments for obesity, including factors such as diet, surgery, and imbalanced nutritional structure, inevitably lead to a loss of skeletal muscle mass (SMM), resulting in weight loss characterized by a decrease in SMM (6–8). In addition, having high body fat may lead to a decrease in relative SMM (skeletal muscle mass/body weight, SMM/W) in individuals with obesity, but due to their greater body mass, these individuals exert more physical effort during daily activities, which may preserve absolute SMM (9). Similarly, overall muscle function and muscle contractile quality are conserved in individuals with mild obesity (10). This has made the diagnosis, treatment, and standard formulation of SO difficult.
In previous research, SO was diagnosed by the combination of sarcopenia and obesity. Generally, the standard criteria for sarcopenia established by the Asian Working Group for Sarcopenia (AWGS) or the European Working Group on Sarcopenia in Older People are used to define sarcopenia. However, the diagnostic criteria for SO vary as a result of different methods for diagnosing obesity, such as body mass index (BMI), waist circumference (WC), percent of body fat (PBF), and visceral fat area (VFA) (11, 12). Due to the absence of unified standards for obesity-related diagnosis, it is difficult to correlate the results of various research teams. The establishment of diagnostic criteria for SO assessment is important for identifying patients with SO, the precise treatment of SO, and the evaluation of SO-related results. In 2022, the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO) launched a consensus of SO diagnostic methods based on skeletal muscle function and body composition (12). The consistency of traditional diagnostic methods and newly released consensuses remain unclear, with important implications for the diagnosis/monitoring of SO.
This article aimed to compare the prevalence and consistency of different assessment methods in a natural population cohort of individuals aged over 50 years. In addition, we further explored the basal metabolic profiles of each group of patients with SO, which may provide a basis for exploring the optimal diagnosis for SO. We hypothesized that the new SO consensus would yield the best diagnostic efficiency.
Methods
Study population
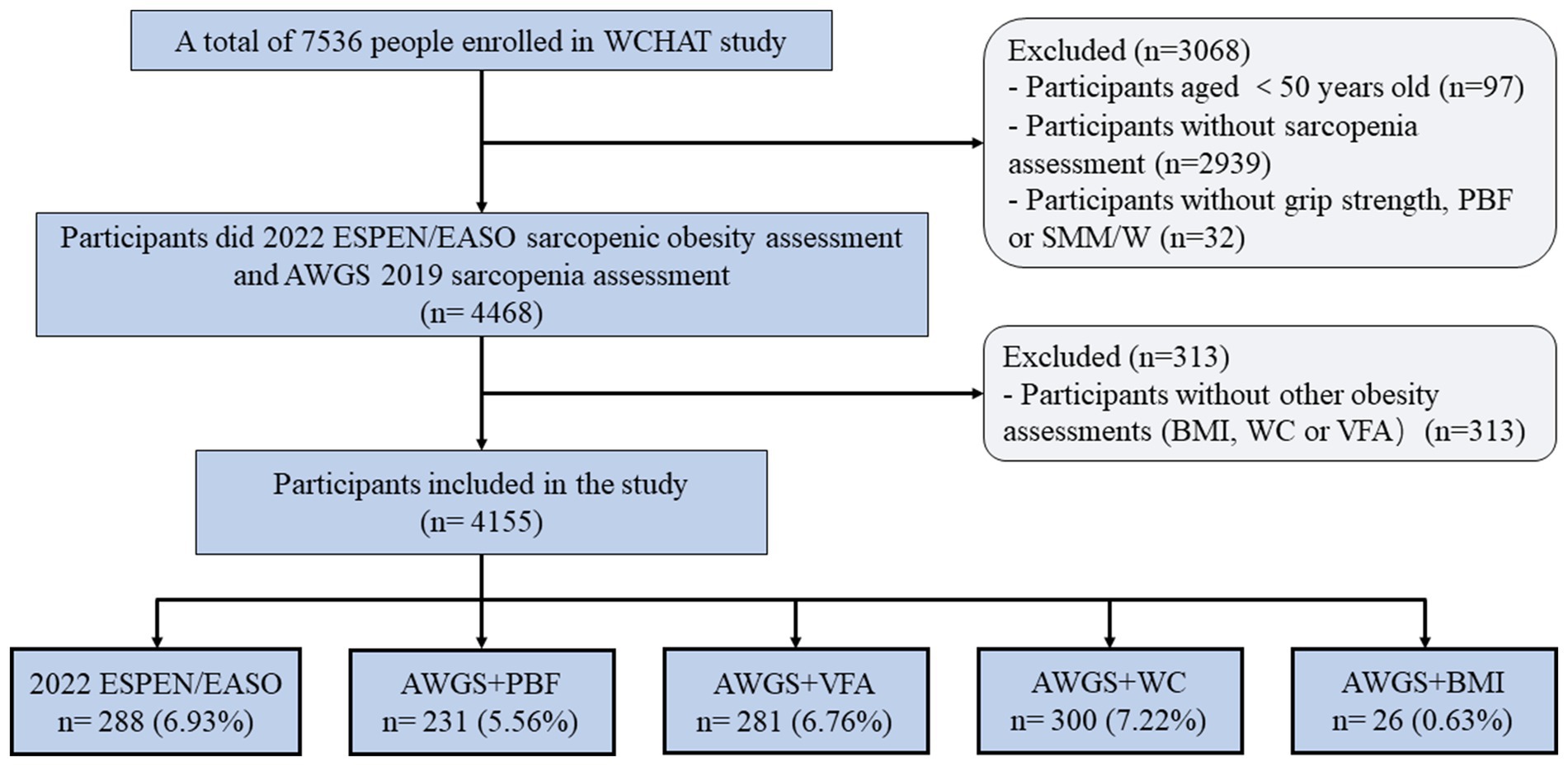
This study used the baseline data from the West China Health and Aging Trend (WCHAT) study. Previous studies have published details of the study design and questionnaires used to generate data (13). In this study, 7,536 participants were enrolled at first. Out of these, only 4,500 participants aged 50 years and above finished sarcopenia assessment. Furthermore, 32 subjects were excluded as they did not have information on handgrip strength (HS), PBF, or SMM /W. In addition, 313 subjects were excluded as they did not have information on obesity measurements like BMI, WC, PBF, or VFA. Finally, 4,155 participants were included in the current study (Figure 1).
Measurements of sarcopenia
According to the Asian Working Group for Sarcopenia 2019 (AWGS2019) consensus criteria, sarcopenia was defined as low muscle mass in the presence of either low HS or slow gait speed (14). The Inbody 770 instrument (Biospace, Seoul, Korea) was used to assess the muscle mass. The cut-off values of appendicular skeletal muscle mass index (ASMI) were 7.0 kg/m2 for males and 5.7 kg/m2 for females. The HS was measured with a grip dynamometer (EH101; Camry, Zhongshan, China). The test was repeated twice and the highest value was recorded (15). Low muscle strength was defined as HS <28 kg in males and < 18 kg in females. The four-meter gait speed was tested using an infrared sensor (16). During the test, participants were required to walk at their usual pace. The acceleration and deceleration phases were excluded. The cut-off value of gait speed was 1.0 m/s (17).
Measurements of obesity
The indicators of obesity included PBF, VFA, WC, and BMI. PBF and VFA were measured using Bioelectric Impedance Analysis. WC was measured with a flexible, non-elastic tape at the midpoint between the ribs and ilium in the standing position. BMI was calculated by dividing weight by the square of height (CSTF-ST, Qinghuatongfang, China). The cutoff values of obesity indicators were as follows: (1) PBF ≥ 41% for females and PBF ≥ 29% for males; (2) VFA > 100 cm2 (18); (3) WC ≥ 80 cm for females and WC ≥ 90 cm for males (19), and (4) BMI ≥ 28 kg/m2 (20).
Definitions of SO
According to the 2022 ESPEN/EASO consensus, participants with decreased muscle strength (HS < 28 kg for males and < 18 kg for females), low muscle mass (SMM/W < = 37% for males, ≤ 27.6% for females), and high-fat mass (>29% for male, > 41% for female) were defined as SO (12). Further, SO was also diagnosed as a combination of the above four different diagnostic criteria of obesity and sarcopenia.
Laboratory examinations
Fasting blood samples were obtained from the antecubital vein after an overnight fast. Complete blood count, blood glucose, and lipid profile were tested. Inflammatory biomarkers were further calculated, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII). SII was calculated using the following formula: SII = peripheral platelets* neutrophils/ lymphocyte counts (21).
Statistical analysis
Continuous variables are presented as mean ± standard deviation. Categorical variables are presented as numbers (percentages). Differences between groups were evaluated with the unpaired t-test and Mann–Whitney U test for continuous data with a normal and non-normal distribution, respectively. A comparison of categorical variables was conducted with chi-square tests. We also assessed the sex-stratified and age-stratified prevalence of SO. Binary logistic regression analysis was used to examine the association of age and sex with SO. A diagnostic agreement of SO between different diagnostic methods was evaluated using Cohen’s kappa score. The interpretations of κ value were as follows: poor agreement = 0.00–0.20, fair agreement = 0.21–0.40, moderate agreement = 0.41–0.60, good agreement = 0.61–0.80, and very good agreement = 0.81–1.00 (22). Statistical analyses were performed using Stata v16.0 (Stata Corp, College Station, TX, USA) software programs. Two-sided p-values of <0.05 were considered statistically significant.
Results
Characteristics of participants
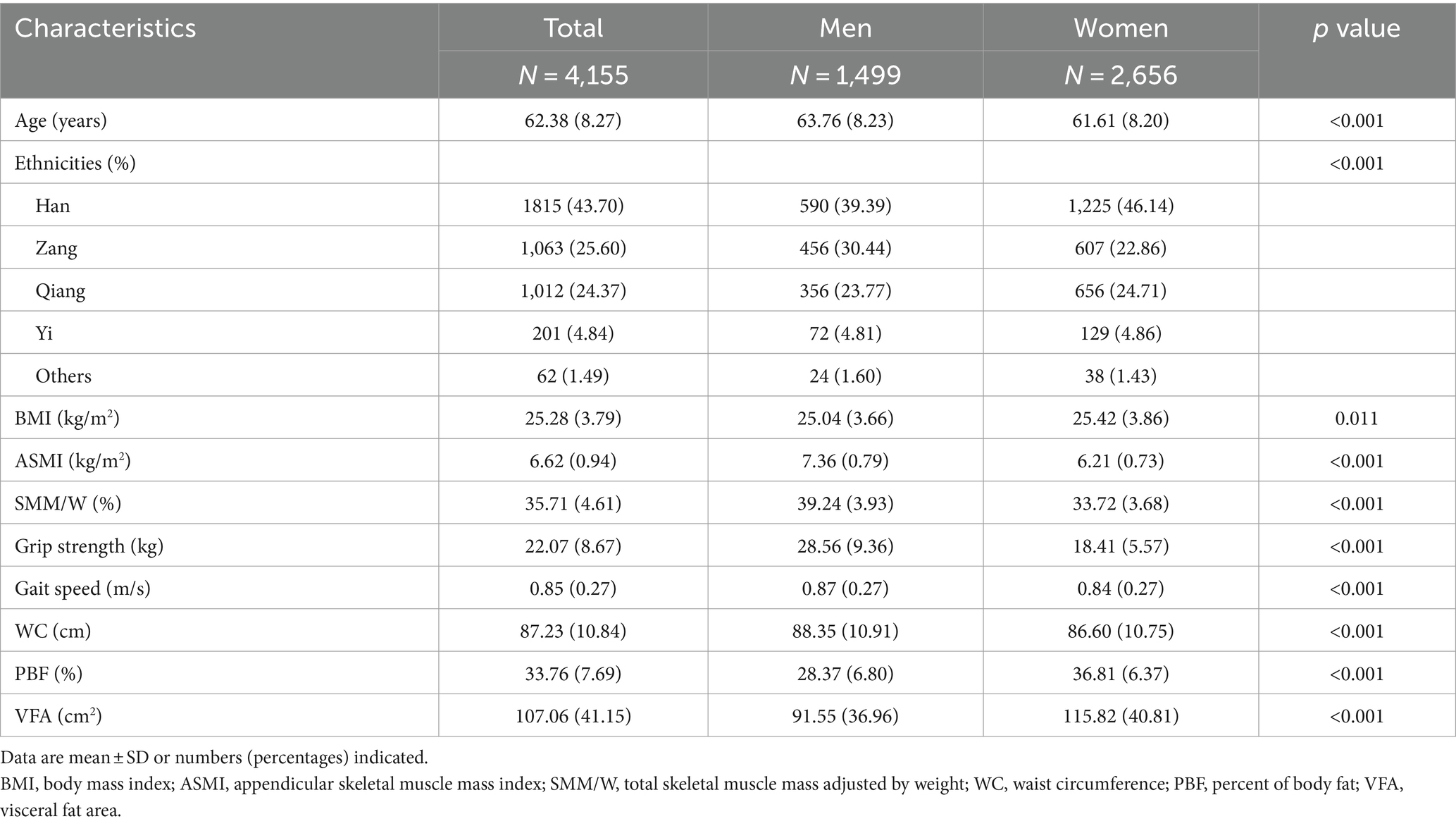
The characteristics of our participants are presented in Table 1. A total of 4,155 participants were included in our study, including 1,499 men (63.76 ± 8.23 years) and 2,656 women (61.61 ± 8.20 years). Indicators related to obesity, including BMI, PBF, and VFA, were all significantly higher in women than in men (p < 0.05). However, the indicators related to sarcopenia, including ASMI, HS, and gait speed, were all significantly higher in men than in women (p < 0.05). As compared to women, men had a higher WC and weight-adjusted SMM (p < 0.05).
Prevalence of SO
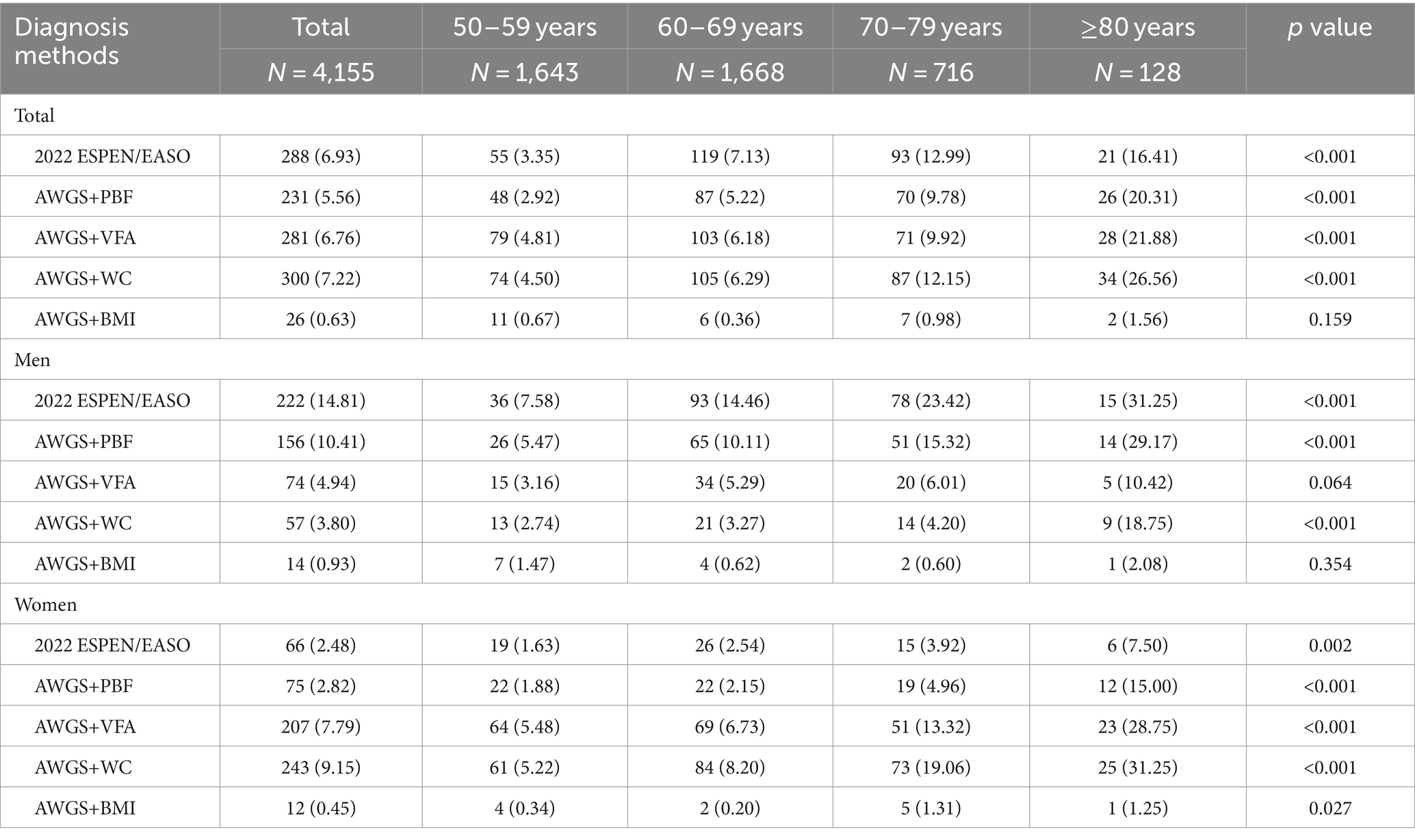
The prevalence of SO varied across different diagnostic methods, with rates of 6.93, 5.56, 6.76, 7.22, and 0.63% according to the 2022 ESPEN/EASO, AWGS+PBF, AWGS+VFA, AWGS+WC, and AWGS+BMI criteria, respectively (see Table 2). In particular, the prevalence of SO diagnosed by AWGS+BMI was much lower than with other diagnostic methods. Except for AWGS+BMI (p = 0.159), the prevalence of SO diagnosed by other methods was significantly different among four age groups (p < 0.05), and the prevalence increased with age. Stratified by sex, according to the 2022 ESPEN/EASO and AWGS+PBF, the prevalence of SO was significantly higher in men (14.81 and 10.41%) than in women (2.48 and 2.82%). However, when using AWGS+VFA and AWGS+WC as diagnostic criteria, the prevalence of SO in males was 4.94 and 3.80%, and in females was 7.79 and 9.15%, respectively. In the case of AWGS+BMI, the detection rate of SO in men (0.93%) was similar to that in women (0.45%).
The correlations between SO and age/sex are shown in Supplementary Table S1. Participants were divided into 4 groups based on age: 50–59, 60–69, 70–79, and ≥ 80 years. Compared with the youngest age group, the odds ratio (OR) for SO diagnosed by 2022 ESPEN/EASO was 2.35 (95%CI: 1.67–3.32), 5.05 (95%CI: 3.46–7.36), and 10.04 (95%CI: 5.57–18.12) for 60–69, 70–79, and ≥ 80 years groups, respectively. Similar correlations between age groups and SO diagnosed by AWGS+PBF, AWGS+VFA, and AWGS+WC were detected. Female was negatively associated with SO when diagnosed by 2022 ESPEN/EASO (OR = 0.17, 95%CI: 0.12–0.22) and AWGS+PBF (OR = 0.28, 95%CI: 0.21–0.38). In contrast, a positive association between females and SO was detected according to AWGS+VFA (OR = 1.92, 95%CI: 1.45–2.53) and AWGS+WC (OR = 3.06, 95%CI: 2.26–4.14).
Agreement between different SO diagnostic methods
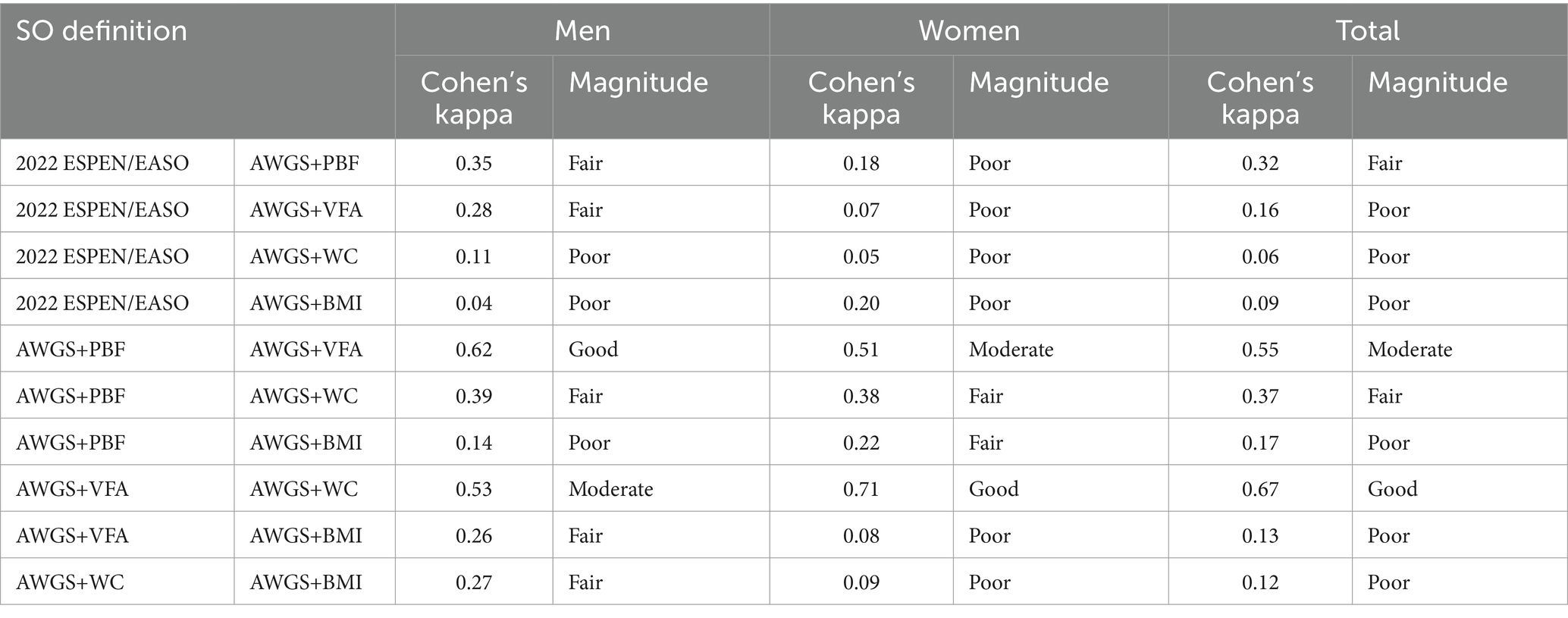
The agreement between different diagnostic methods is shown in Table 3. The agreement between different diagnostic methods for SO varied, with poor agreement observed between 2022 ESPEN/EASO and AWGS+VFA (κ = 0.16), AWGS+WC (κ = 0.06), and AWGS+BMI (κ = 0.09), while fair agreement was found between 2022 ESPEN/EASO and AWGS+PBF (κ = 0.32). Among the other four diagnostic methods, AWGS+VFA and AWGS+WC showed good agreement (κ = 0.67). Meanwhile, AWGS+VFA was moderately consistent with AWGS+VFA (κ = 0.55). Stratified by sex, there was good agreement between AWGS+VFA and AWGS+PBF in males (κ = 0.62) and between AWGS+VFA and AWGS+WC in females (κ = 0.71).
Metabolic and inflammatory profiles of SO diagnosed by different methods
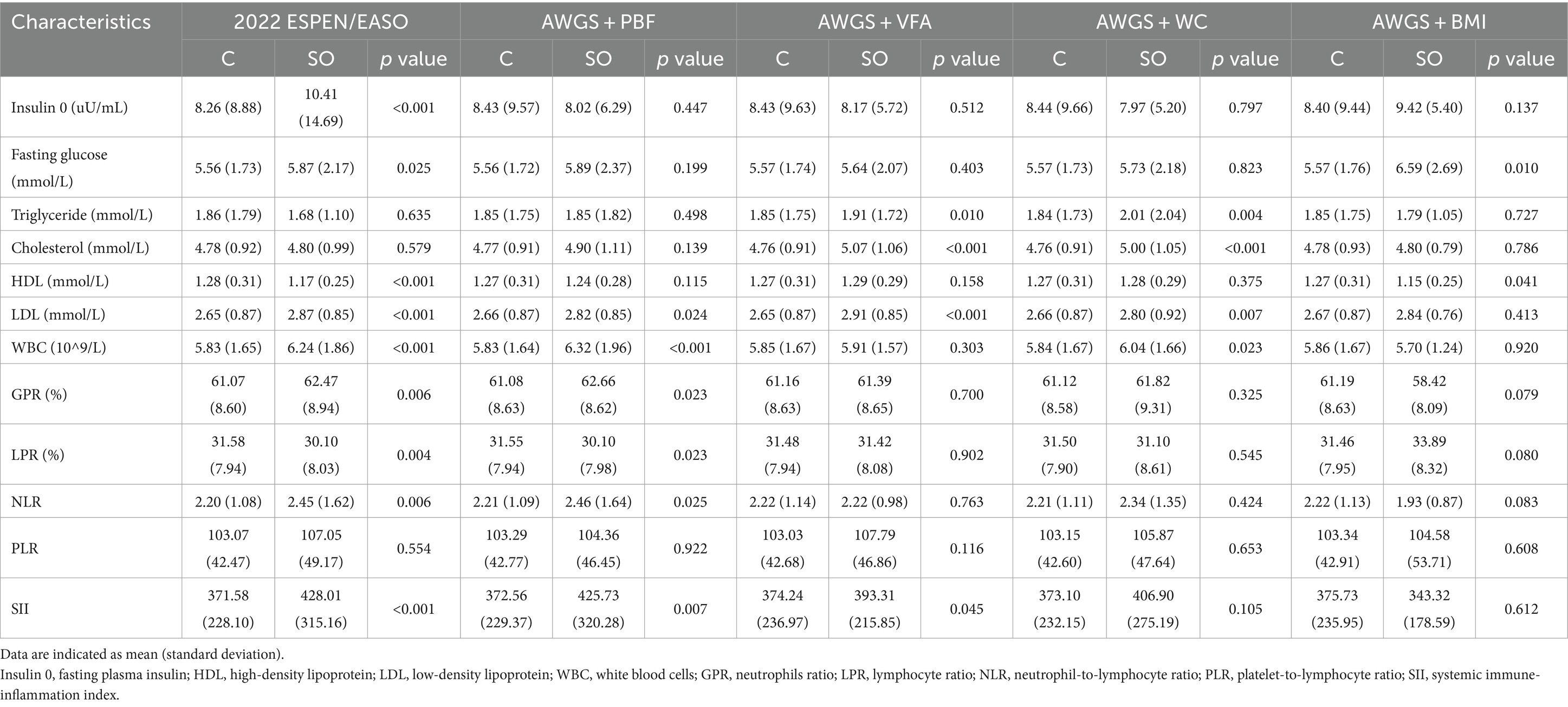
The fasting plasma insulin was significantly higher in the SO group diagnosed by the 2022 ESPEN/EASO (10.41 ± 14.69) as compared to the non-SO group (8.26 ± 8.88) (Table 4). Fasting glucose was significantly higher in SO groups diagnosed by the 2022 ESPEN/EASO and AWGS+BMI (p < 0.05 in both). Triglyceride and cholesterol were significantly higher in SO groups diagnosed by AWGS+VFA and AWGS+WC (p < 0.05 in both). High-density lipoprotein (HDL) in SO groups diagnosed by ESPEN/EASO and AWGS+BMI was significantly lower than that in control groups (both p < 0.05). Meanwhile, except for the SO group diagnosed by AWGS+BMI, the low-density lipoprotein (LDL) of all SO groups was significantly increased (all p < 0.05).
Indicators related to inflammation, including neutrophils ratio (GPR) and NLR were significantly higher in SO groups diagnosed by the 2022 ESPEN/EASO and AWGS+PBF (both p < 0.05). White blood cells (WBC) were significantly higher in SO groups diagnosed by the 2022 ESPEN/EASO, AWGS+PBF, and AWGS+WC (all p < 0.05). The lymphocyte ratio was significantly lower in SO groups diagnosed by the 2022 ESPEN/EASO and AWGS+PBF (p < 0.05 in both). Furthermore, SII was significantly higher in SO groups diagnosed by the 2022 ESPEN/EASO, AWGS+PBF, and AWGS+VFA (all p < 0.05).
Discussion
Using five different diagnostic methods, we compared the prevalence of SO among a multiethnic community-dwelling population of individuals over 50 years old living in western China. Our study revealed that AWGS+VFA had a relatively good diagnostic agreement, while the consensus of ESPEN/EASO had a poor diagnostic agreement with other diagnostic methods. Considering that the traditional diagnosis is the combination of sarcopenia and obesity, it is not surprising that the consistency between ESPEN/EASO and the other four traditional proposals using AWGS 2019 is not high. However, the traditional diagnostic criteria have been questioned, as growing evidence shows that SO is not only a combination of the two conditions but also a specific condition on its own (23). The unique metabolic and inflammatory profiles of patients with SO diagnosed by ESPEN/EASO further emphasized this issue.
Although BMI has been widely used to define SO, our findings indicated that BMI is considerably less sensitive than the other four identified criteria. This finding was in accordance with previous studies (11, 24). This suggests that BMI may not be suitable as an indicator of obesity according to the definition of SO in older Asian adults. According to previous studies, BMI cannot account for age-related changes in body fat composition, loss of lean body mass, or variations in body fat distribution (4). This is important because compared with peripheral fat deposition, central obesity could lead to increased mortality (25).
Our results on the prevalence of SO were consistent with those of previous studies. A previous study reported that the prevalence of SO among community-dwelling older adults in China varied greatly (0.1–7.9%) when different obesity diagnostic methods were combined with the AWGS 2019 criteria (11). Similarly, two other studies reported that the prevalence of SO ranged from 0.5 to 10.5% when using the AWGS 2014 criteria in combination with different obesity diagnostic methods (24, 26). Interestingly, in our study, we found that WC-defined obesity had the highest prevalence of SO, which was 7.22%. It is possible that most of the multiethnic population in western China, especially the Zang ethnic group, has central obesity resulting from their dietary habits. BMI-defined obesity was associated with the lowest prevalence of SO (0.63%). This might be because the cutoff value for obesity of 28 kg/m2 was slightly high for older people diagnosed with sarcopenia. Furthermore, the proportion of body fat increases and decreases in muscle mass with age. However, these changes are not well reflected in height, weight, or BMI (27). Furthermore, when SO was diagnosed using AWGS+VFA and AWGS+PBF, the prevalence of SO was similar (6.76 and 5.56%, respectively), and the agreement between those measurements was moderate (κ = 0.55). These findings were consistent with those of previous studies (11, 24).
For gender differences, we found a large variation in the prevalence of SO between males and females. This might be related to hormonal changes. Gender-specific alterations in body composition are partly attributable to age-related changes in sex hormone levels. In women, menopause causes weight gain, which is characterized by an increase in fat mass, mostly located in the visceral area (28). This redistribution of fat leads to an increase in WC and a concomitant loss of muscle mass (29). In men, testosterone plays a crucial role in promoting muscle regeneration by activating satellite cells (30). In addition, testosterone enhances muscle protein synthesis and increases androgen receptor expression (31). Decreasing testosterone levels during aging may negatively affect muscle mass and fat distribution in older adults (32). Interestingly, in our study, we found that there was a negative association between females and SO when the 2022 ESPEN/EASO and AWGS+PBF diagnostic criteria were used. However, females were positively associated with SO when the AWGS+VFA and AWGS+WC diagnostic criteria were used, which was consistent with the findings of previous studies (11). Longitudinal studies are needed to confirm the relationship between sex and SO. Furthermore, given the large differences in the prevalence of SO between sexes when diagnosed using the 2022 ESPEN/EASO criteria, further studies are needed to identify the optimal cutoff points for diagnosing SO to be considered in research and clinical practice.
In addition, it seems that old age was a confirmed risk factor for developing SO according to all five diagnostic methods. With age, many factors are related to changes in body composition. Etiological factors including reduced physical activity, decreased mitochondrial volume, and diminished oxidative capacity, could lead to a decrease in the resting metabolic rate (33). Furthermore, reductions in the resting metabolic rate, the thermic effect of food, and participation in physical activity result in a reduction in total energy expenditure, which may lead to a progressive increase in body fat (34). Body fat has been reported to increase until the age of 70 years (35), while muscle mass decreases after 40 years of age, resulting in weight gain in older adults being primarily in the form of fat rather than lean mass (36). In addition, vertebral compression can lead to height loss, thereby affecting BMI (37). In other words, various factors could underlie the association between aging and SO.
It is well known that both muscle and adipose tissue play important roles in metabolic regulation. Previous studies have reported that SO is associated with metabolic syndrome (38–40), and an increased risk of developing metabolic syndrome may manifest decades before the development of SO (41). In our study, we observed that participants with SO were more likely to have metabolic dysfunction, characterized by increased fasting plasma insulin, fasting glucose, triglyceride, cholesterol, and LDL levels, and decreased HDL levels, which was consistent with previous evidence (40, 41). Insulin resistance serves as the central mechanism underlying the development of SO (42). As the largest insulin-sensitive tissue, skeletal muscle plays a crucial role in modulating insulin resistance. Thus, loss of muscle mass exacerbates insulin resistance. Furthermore, the accumulation of fat within muscle tissue triggers a proinflammatory cascade and oxidative stress, leading to mitochondrial dysfunction, impaired insulin sensitivity, and muscle atrophy (42). Therefore, emerging evidence suggests a link between SO and a hyperinflammatory state (43). Our study also revealed that patients diagnosed with SO using the 2022 ESPEN/EASO and AWGS+PBF criteria were more likely to exhibit dysfunctional inflammatory profiles, characterized by elevated WBC counts, GPR, NLR, and SII. Considering the diagnostic agreement and similar metabolic and inflammatory profiles between AWGS+PBF and 2022 ESPEN/EASO, AWGS+PBF may be suitable for the alternative diagnosis of 2022 ESPEN/EASO.
Several limitations should be noted when interpreting our results. First, our study design was cross-sectional, which limits our ability to establish causality. Second, our results included only people from western China, so the generalizability of our findings to other Asian populations may be limited. Third, the proportion of very old adults in our study was relatively small, and the majority of participants were in good health. Furthermore, we excluded 3,381 individuals from the 7,536 participants due to a lack of important diagnostic data. These may introduce some bias into the analysis and should be taken into consideration when interpreting the results. Future research should include non-Chinese populations and encompass more diverse and heterogeneous groups of older adults. Additionally, longitudinal studies that examine the trajectory of SO are necessary.
Conclusion
There is considerable variation in the prevalence of SO across definitions, with agreement between them ranging from low to good. Our results indicated that AWGS+WC has the highest diagnostic rate in diagnosing SO, while AWGS+BMI has the lowest. AWGS+VFA has a relatively good diagnostic agreement with other diagnostic methods, while the consensus of ESPEN/EASO has poor diagnostic consistency. Individuals with SO diagnosed by the 2022 ESPEN/EASO method were more likely to exhibit dysfunctional metabolic and inflammatory profiles. Sex-specific cutoffs of ESPEN/EASO should be further explored to enable accurate and early characterization of SO in older Asian populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of West China Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. GZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. ZX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. ZZ: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – review & editing. NH: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – review & editing. MG: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – review & editing. XL: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing. BD: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 92248304, No. 82101653), the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD20010), the China Postdoctoral Science Foundation (2021M692297, 2023M732473), and the National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2023YY003, Z2021JC003). The sponsors had no roles in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Acknowledgments
We thank all the volunteers for their participation and the personnel for their contribution to the WCHAT study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1356878/full#supplementary-material
References
1. Bahat, G, Kilic, C, Ozkok, S, Ozturk, S, and Karan, MA. Associations of sarcopenic obesity versus sarcopenia alone with functionality. Clin Nutr. (2021) 40:2851–9. doi: 10.1016/j.clnu.2021.04.002
2. Schoufour, JD, Tieland, M, Barazzoni, R, Ben Allouch, S, van der Bie, J, Boirie, Y, et al. The relevance of diet, physical activity, exercise, and persuasive technology in the prevention and treatment of sarcopenic obesity in older adults. Front Nutr. (2021) 8:661449. doi: 10.3389/fnut.2021.661449
3. Li, CW, Yu, K, Shyh-Chang, N, Jiang, Z, Liu, T, Ma, S, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. (2022) 13:781–94. doi: 10.1002/jcsm.12901
4. Batsis, JA, and Villareal, DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. (2018) 14:513–37. doi: 10.1038/s41574-018-0062-9
5. Alizadeh, PH . Exercise therapy for people with sarcopenic obesity: myokines and adipokines as effective actors. Front Endocrinol (Lausanne). (2022) 13:811751. doi: 10.3389/fendo.2022.811751
6. Molero, J, Olbeyra, R, Flores, L, Jiménez, A, de Hollanda, A, Andreu, A, et al. Prevalence of low skeletal muscle mass following bariatric surgery. Clin Nutr ESPEN. (2022) 49:436–41. doi: 10.1016/j.clnesp.2022.03.009
7. Prokopidis, K, Cervo, MM, Gandham, A, and Scott, D. Impact of protein intake in older adults with sarcopenia and obesity: a gut microbiota perspective. Nutrients. (2020) 12:12. doi: 10.3390/nu12082285
8. McCarthy, D, and Berg, A. Weight loss strategies and the risk of skeletal muscle mass loss. Nutrients. (2021) 13:13. doi: 10.3390/nu13072473
9. Tomlinson, DJ, Erskine, RM, Morse, CI, Winwood, K, and Onambélé-Pearson, G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology. (2016) 17:467–83. doi: 10.1007/s10522-015-9626-4
10. Muollo, V, Rossi, AP, Zignoli, A, Teso, M, Milanese, C, Cavedon, V, et al. Full characterisation of knee extensors' function in ageing: effect of sex and obesity. Int J Obes. (2021) 45:895–905. doi: 10.1038/s41366-021-00755-z
11. Mo, YH, Yang, C, Su, YD, Dong, X, Deng, WY, Liu, BB, et al. Prevalence and diagnostic agreement of sarcopenic obesity with different definitions among Chinese community-dwelling older adults. Age Ageing. (2022) 51:51. doi: 10.1093/ageing/afab272
12. Donini, LM, Busetto, L, Bischoff, SC, Cederholm, T, Ballesteros-Pomar, MD, Batsis, JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin Nutr. (2022) 41:990–1000. doi: 10.1016/j.clnu.2021.11.014
13. Hou, L, Liu, X, Zhang, Y, Zhao, W, Xia, X, Chen, X, et al. Cohort profile: West China health and aging trend (WCHAT). J Nutr Health Aging. (2021) 25:302–10. doi: 10.1007/s12603-020-1530-1
14. Chen, LK, Woo, J, Assantachai, P, Auyeung, TW, Chou, MY, Iijima, K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–07.e2. doi: 10.1016/j.jamda.2019.12.012
15. He, H, Pan, L, Wang, D, Liu, F, Du, J, Pa, L, et al. Normative values of hand grip strength in a large unselected Chinese population: evidence from the China national health survey. J Cachexia Sarcopenia Muscle. (2023) 14:1312–21. doi: 10.1002/jcsm.13223
16. Jung, HW, Roh, HC, Kim, SW, Kim, S, Kim, M, and Won, CW. Cross-comparisons of gait speeds by automatic sensors and a stopwatch to provide converting formula between measuring modalities. Ann Geriatr Med Res. (2019) 23:71–6. doi: 10.4235/agmr.19.0016
17. Lee, S, Kim, S, Kim, M, Yoo, J, Kim, B, Yoo, M, et al. An optimal questionnaire representing slow gait speed(<1m/s) in community-dwelling older adults: the Korean frailty and aging cohort study (KFACS). J Nutr Health Aging. (2019) 23:648–53. doi: 10.1007/s12603-019-1213-y
18. Liu, C, Wong, PY, Chung, YL, Chow, SK, Cheung, WH, Law, SW, et al. Deciphering the “obesity paradox” in the elderly: a systematic review and meta-analysis of sarcopenic obesity. Obes Rev. (2023) 24:e13534. doi: 10.1111/obr.13534
19. Alberti, KG, Zimmet, P, and Shaw, J. Metabolic syndrome--a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
20. Zhang, W, Chen, Y, and Chen, N. Body mass index and trajectories of the cognition among Chinese middle and old-aged adults. BMC Geriatr. (2022) 22:613. doi: 10.1186/s12877-022-03301-2
21. Chen, JH, Zhai, ET, Yuan, YJ, Wu, KM, Xu, JB, Peng, JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. (2017) 23:6261–72. doi: 10.3748/wjg.v23.i34.6261
22. Basha, MAA, Metwally, MI, Gamil, SA, Khater, HM, Aly, SA, El Sammak, AA, et al. Comparison of O-RADS, GI-RADS, and IOTA simple rules regarding malignancy rate, validity, and reliability for diagnosis of adnexal masses. Eur Radiol. (2021) 31:674–84. doi: 10.1007/s00330-020-07143-7
23. Barazzoni, R, Cederholm, T, Zanetti, M, and Gortan, CG. Defining and diagnosing sarcopenia: is the glass now half full? Metabolism. (2023) 143:155558. doi: 10.1016/j.metabol.2023.155558
24. Khor, EQ, Lim, JP, Tay, L, Yeo, A, Yew, S, Ding, YY, et al. Obesity definitions in sarcopenic obesity: differences in prevalence, agreement and association with muscle function. J Frailty Aging. (2020) 9:37–43. doi: 10.14283/jfa.2019.28
25. Lee, YS, Biddle, S, Chan, MF, Cheng, A, Cheong, M, Chong, YS, et al. Health promotion board-ministry of health clinical practice guidelines: obesity. Singapore Med J. (2016) 57:292–300. doi: 10.11622/smedj.2016103
26. Liu, X, Hao, Q, Yue, J, Hou, L, Xia, X, Zhao, W, et al. Sarcopenia, obesity and sarcopenia obesity in comparison: prevalence, metabolic profile, and key differences: results from WCHAT study. J Nutr Health Aging. (2020) 24:429–37. doi: 10.1007/s12603-020-1332-5
27. Rothman, KJ . BMI-related errors in the measurement of obesity. Int J Obes. (2008) 32:S56–9. doi: 10.1038/ijo.2008.87
28. Trémollieres, FA, Pouilles, JM, and Ribot, CA. Relative influence of age and menopause on total and regional body composition changes in postmenopausal women. Am J Obstet Gynecol. (1996) 175:1594–600. doi: 10.1016/s0002-9378(96)70111-4
29. Sowers, M, Zheng, H, Tomey, K, Karvonen-Gutierrez, C, Jannausch, M, Li, X, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. (2007) 92:895–901. doi: 10.1210/jc.2006-1393
30. Bondanelli, M, Ambrosio, MR, Margutti, A, Franceschetti, P, Zatelli, MC, and Degli Uberti, EC. Activation of the somatotropic axis by testosterone in adult men: evidence for a role of hypothalamic growth hormone-releasing hormone. Neuroendocrinology. (2003) 77:380–7. doi: 10.1159/000071310
31. LeBlanc, ES, Wang, PY, Lee, CG, Barrett-Connor, E, Cauley, JA, Hoffman, AR, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab. (2011) 96:3855–63. doi: 10.1210/jc.2011-0312
32. Yeap, BB . Are declining testosterone levels a major risk factor for ill-health in aging men? Int J Impot Res. (2009) 21:24–36. doi: 10.1038/ijir.2008.60
33. Conley, KE, Esselman, PC, Jubrias, SA, Cress, ME, Inglin, B, Mogadam, C, et al. Ageing, muscle properties and maximal O(2) uptake rate in humans. J Physiol. (2000) 526:211–7. doi: 10.1111/j.1469-7793.2000.00211.x
34. Cannon, B, and Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. (2011) 214:242–53. doi: 10.1242/jeb.050989
35. Heo, M, Faith, MS, Pietrobelli, A, and Heymsfield, SB. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999-2004. Am J Clin Nutr. (2012) 95:594–602. doi: 10.3945/ajcn.111.025171
36. Abizanda, P, Romero, L, Sánchez-Jurado, PM, Ruano, TF, Ríos, SS, and Sánchez, MF. Energetics of aging and frailty: the FRADEA study. J Gerontol A Biol Sci Med Sci. (2016) 71:787–96. doi: 10.1093/gerona/glv182
37. Xu, W, Perera, S, Medich, D, Fiorito, G, Wagner, J, Berger, LK, et al. Height loss, vertebral fractures, and the misclassification of osteoporosis. Bone. (2011) 48:307–11. doi: 10.1016/j.bone.2010.09.027
38. Kang, SY, Lim, GE, Kim, YK, Kim, HW, Lee, K, Park, TJ, et al. Association between sarcopenic obesity and metabolic syndrome in postmenopausal women: a cross-sectional study based on the Korean national health and nutritional examination surveys from 2008 to 2011. J Bone Metab. (2017) 24:9–14. doi: 10.11005/jbm.2017.24.1.9
39. Scott, D, Cumming, R, Naganathan, V, Blyth, F, Le Couteur, DG, Handelsman, DJ, et al. Associations of sarcopenic obesity with the metabolic syndrome and insulin resistance over five years in older men: the concord health and ageing in men project. Exp Gerontol. (2018) 108:99–105. doi: 10.1016/j.exger.2018.04.006
40. Siervo, M, Rubele, S, Shannon, OM, Prado, CM, Donini, LM, Zamboni, M, et al. Prevalence of sarcopenic obesity and association with metabolic syndrome in an adult Iranian cohort: the Fasa PERSIAN cohort study. Clin Obes. (2021) 11:e12459. doi: 10.1111/cob.12459
41. Ma, J, Hwang, SJ, McMahon, GM, Curhan, GC, McLean, RR, Murabito, JM, et al. Mid-adulthood cardiometabolic risk factor profiles of sarcopenic obesity. Obesity (Silver Spring). (2016) 24:526–34. doi: 10.1002/oby.21356
42. Hong, SH, and Choi, KM. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci. (2020) 21:21. doi: 10.3390/ijms21020494
43. Karanth, SD, Washington, C, Cheng, TD, Zhou, D, Leeuwenburgh, C, Braithwaite, D, et al. Inflammation in relation to sarcopenia and sarcopenic obesity among older adults living with chronic comorbidities: results from the national health and nutrition examination survey 1999-2006. Nutrients. (2021) 13:13. doi: 10.3390/nu13113957
Keywords: sarcopenic obesity, prevalence, diagnostic agreement, WCHAT, obesity
Citation: Hu F, Zhang G, Xu Z, Zuo Z, Huang N, Ge M, Liu X and Dong B (2024) The diagnostic agreement of sarcopenic obesity with different definitions in Chinese community-dwelling middle-aged and older adults. Front. Public Health. 12:1356878. doi: 10.3389/fpubh.2024.1356878
Edited by:
Sheikh M. Alif, Federation University Australia, AustraliaReviewed by:
Valentina Muollo, University of Verona, ItalyMarcelo Coertjens, Federal University of Piauí, Brazil
Copyright © 2024 Hu, Zhang, Xu, Zuo, Huang, Ge, Liu and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolei Liu, xiaolei1823@163.com; Birong Dong, birongdong123@outlook.com
†These authors have contributed equally to this work
 Fengjuan Hu
Fengjuan Hu Gongchang Zhang1,2†
Gongchang Zhang1,2† Zhiliang Zuo
Zhiliang Zuo Meiling Ge
Meiling Ge Xiaolei Liu
Xiaolei Liu Birong Dong
Birong Dong